1. Introduction
Thyroid cancer incidence rates have risen globally over the past few decades, increasing from 95,030 incident cases in 1990 (95% Confidence interval [CI], 90,070-100,720), to 255,490 cases in 2017 (95% CI, 245,710-272,470) [
1]. Incidence rates differ by sex and showing an approximate 3:1 ratio comparing rates between females to males [
2,
3]. Incidence rates have also continued to rise in Taiwan, and the age-standardized rate in females increased from 4.39 in 1993-1996 to 17.29 in 2013-2016 per 100,000 individuals [
4].
Thyroid cancer is a disease with multiple risk factors [
5]. Incidence is three times higher in females compared to males, suggesting female sex hormones may play a role [
6]. Hormone has been found related with pathogenesis of breast, prostate, endometrium, ovary, testis, and thyroid cancer [
7], and estrogen exposure is a verified risk factor for some hormone related cancers, such as breast [
8] and endometrial cancer [
9]. Estrogen was also found a potent growth factor for malignant thyroid cells [
10]. Females with a history of breast cancer were almost three times (95% CI, 0.78-7.9) more likely to develop thyroid cancer [
11]. During pregnancy, the expression of estrogen and its receptors in the mother changes, and pregnancy was found having a protective effect on breast cancer [
12]. In comparison, lower parity of pregnancy was found a risk factor of breast cancer [
13]. We thought that whether similar effects may occur for thyroid cancer is worth investigating.
Thyroid cancer incidence in Taiwan has risen rapidly, especially in females. As exposure to its risk factors has also changed significantly, the impact of these changes on thyroid cancer incidence is still unclear. Thus, this study aims to describe the time-dependent characteristics of female thyroid cancer incidence in Taiwan.
2. Materials and Methods
We used the Taiwan Cancer Registry (TCR) data to calculate thyroid cancer incidence among Taiwanese females from 1995 to 2019, using the following codes; ICD-O-FT: T-193, ICD-O-3: C73. We identified 43,751 cases of thyroid cancer during the study period. The TCR is a highly complete and accurate database with a coverage rate of 98.4% [
14]. According to the TCR Report (
https://www.hpa.gov.tw/Pages/TopicList.aspx?nodeid=269), the proportion of patients with thyroid cancer confirmed by cytology or histopathology has been consistently high, rising from 93.60% in 1995 to 99.61% in 2019. We calculated age-adjusted rates using the 2000 standard world population. Age-specific incidence rates were grouped every five years, from age 30 to 89, for a total of 12 age groups. Diagnosis periods were also grouped every five years, from 1995 to 2019, for a total of 5 periods. Birth cohorts were grouped every five years from 1925 to 1974 for a total of 10 groups. We calculated the annual percentage change (APC) in thyroid cancer incidence rates and associated 95% confidence intervals using the National Cancer Institute’s (NCI) Joinpoint program (version 4.9.1.0).
As Taiwanese law requires citizens to declare and provide birth data, we collected fertility rate data from 1950 to 1999 from the National Development Council and Department of Household Registration websites to examine the relationship between fertility and thyroid cancer incidence. Considering the incubation period between fertility and thyroid cancer, we performed a Spearman rank correlation analysis between thyroid cancer incidence rates among 40-59-year-old females and fertility rates of 20-29-year-old females in the same birth cohort. The closer the r value to 1 or -1 shows greater positive or negative correlation.
The data for correlating thyroid cancer incidence rates and fertility rates across different countries were gathered form cancer incidence in five continents (CI5) and the world bank (
https://data.worldbank.org/indicator/SP.DYN.TFRT.IN?end=2021&name_desc=false&start=1960). For the purpose of conducting international comparisons, we chose to analyze using age-standardized incidence rates and total fertility rates. We utilized data from volumes 4, 9 and 12 of for the 5-year periods age-standardized incidence rates:1973-1977, 1993-1997, and 2013-2017, respectively. The selected countries include Europe (United Kingdom, Sweden, and Finland), North America (United States, SEER), Oceania, (Australia), and Asia (Hong Kong, Japan, and Singapore). Based on the causal continuity of the disease, fertility rates were selected for the years 1960, 1980 and 2010. This research protocol was approved by the Institutional Review Board of Fu-Jen Catholic University (No. C104014).
3. Results
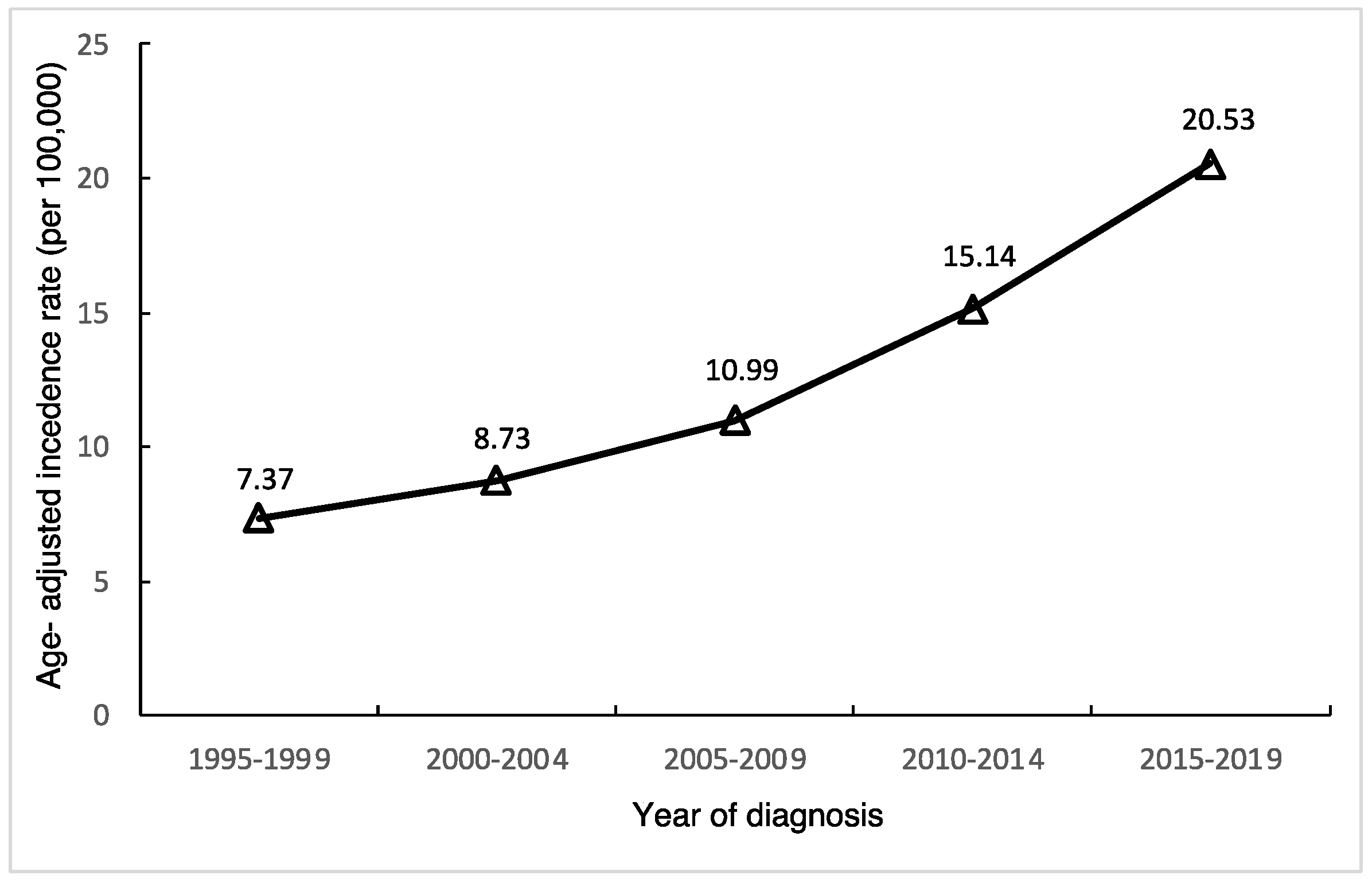
After calculating age-standardized incidence rates of thyroid cancer in Taiwan, we found the age-standardized incidence in females continually rising (
Figure 1), from 7.37 per 100,000 during 1995-1999 to 20.53 per 100,000 during 2015-2019, with an APC of 5.9% (95% CI, 5,3-6.5) as shown in
Table 1. The relative percent change from 1995-2019 was 272.9%.
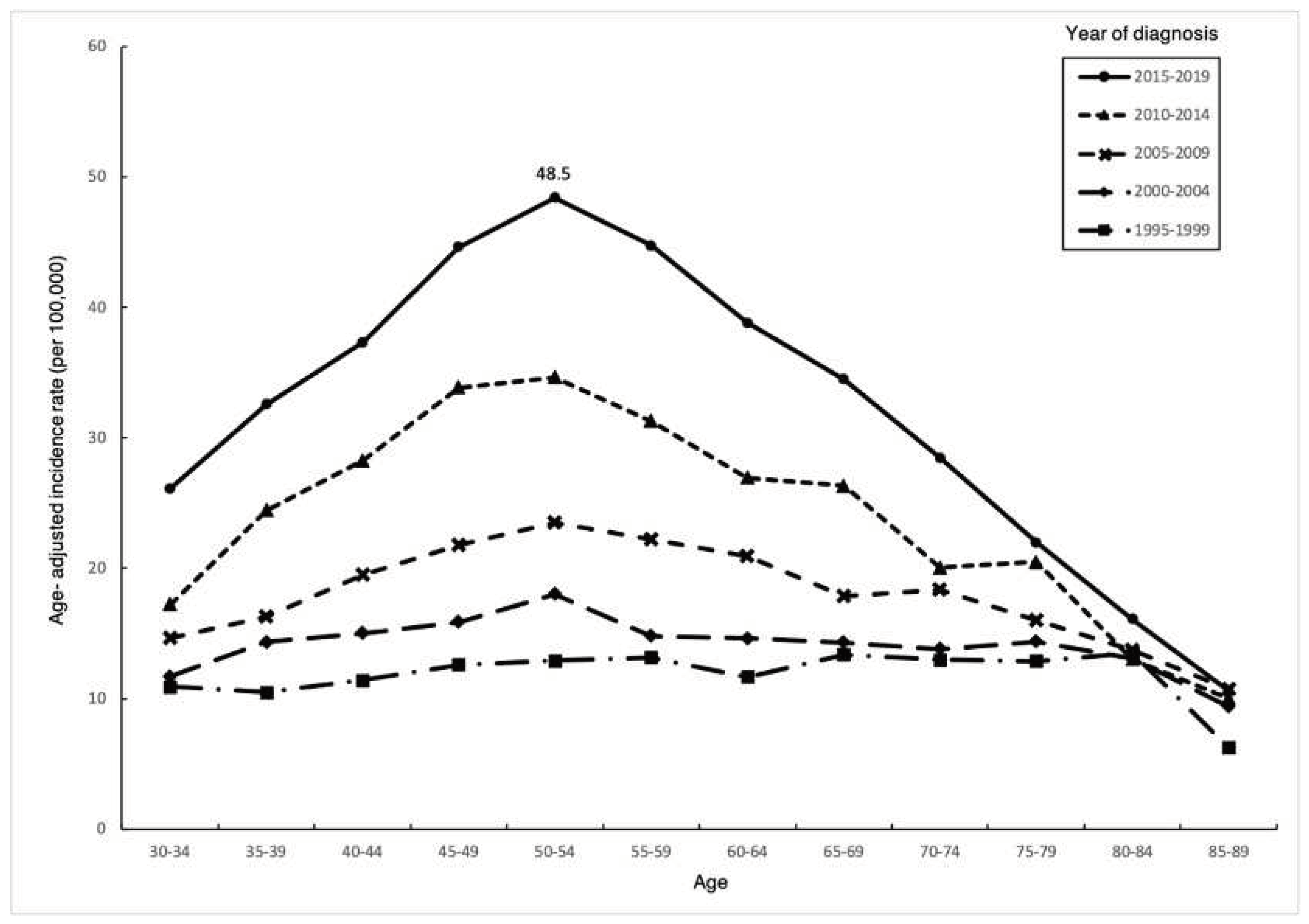
Thyroid cancer incidence increased overtime in all age groups of females (
Figure 2), and the magnitude increased over time. The age-specific incidence generally increased with age, reaching a peak of 48.5/100,000 during 2015-2019 in the 50-54 age group, then rapidly decreasing with age, thereafter, resulting in an overall trend resembling an inverted V shape.
Table 1 shows that the highest APC occurred in the 50-54 age group (6.8%, 95% CI, 6.1-7.5), while the APC in individuals over 75 years old was less than 3%.
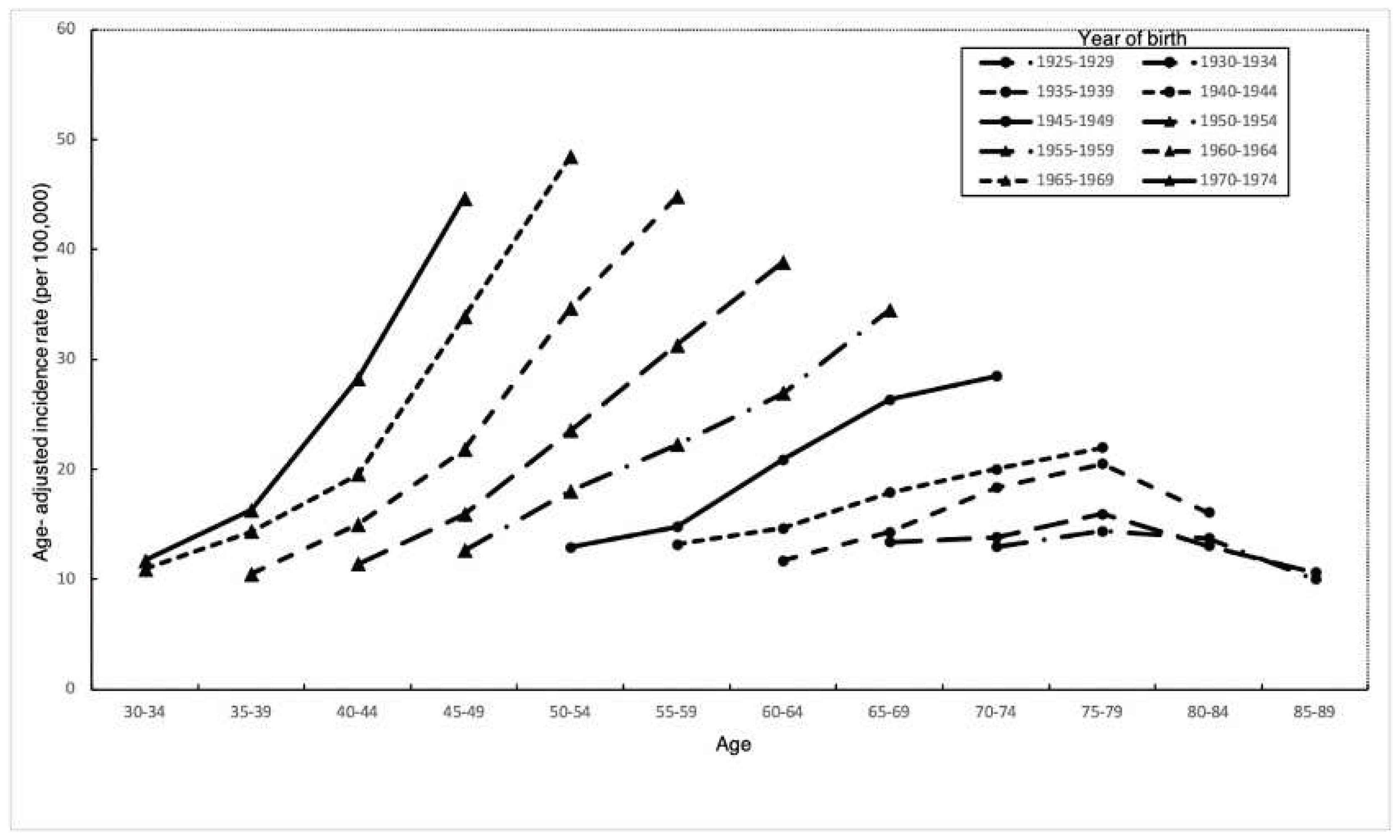
In every birth cohort, thyroid cancer incidence increased with age (
Figure 3). Incidence rates in early birth cohorts increased with age, reached a peak at ages 70-74, and declined thereafter. The highest incidence occurred at increasingly younger ages in later birth cohorts. In addition, we observed a trend of accelerating age-specific incidence rates in different birth cohorts. Individuals born in later birth cohorts were reaching the same incidence rate at earlier points in life. Besides, changes in incidence among cohorts prior to 1944 were all less than changes occurring in cohorts after 1945-1949.
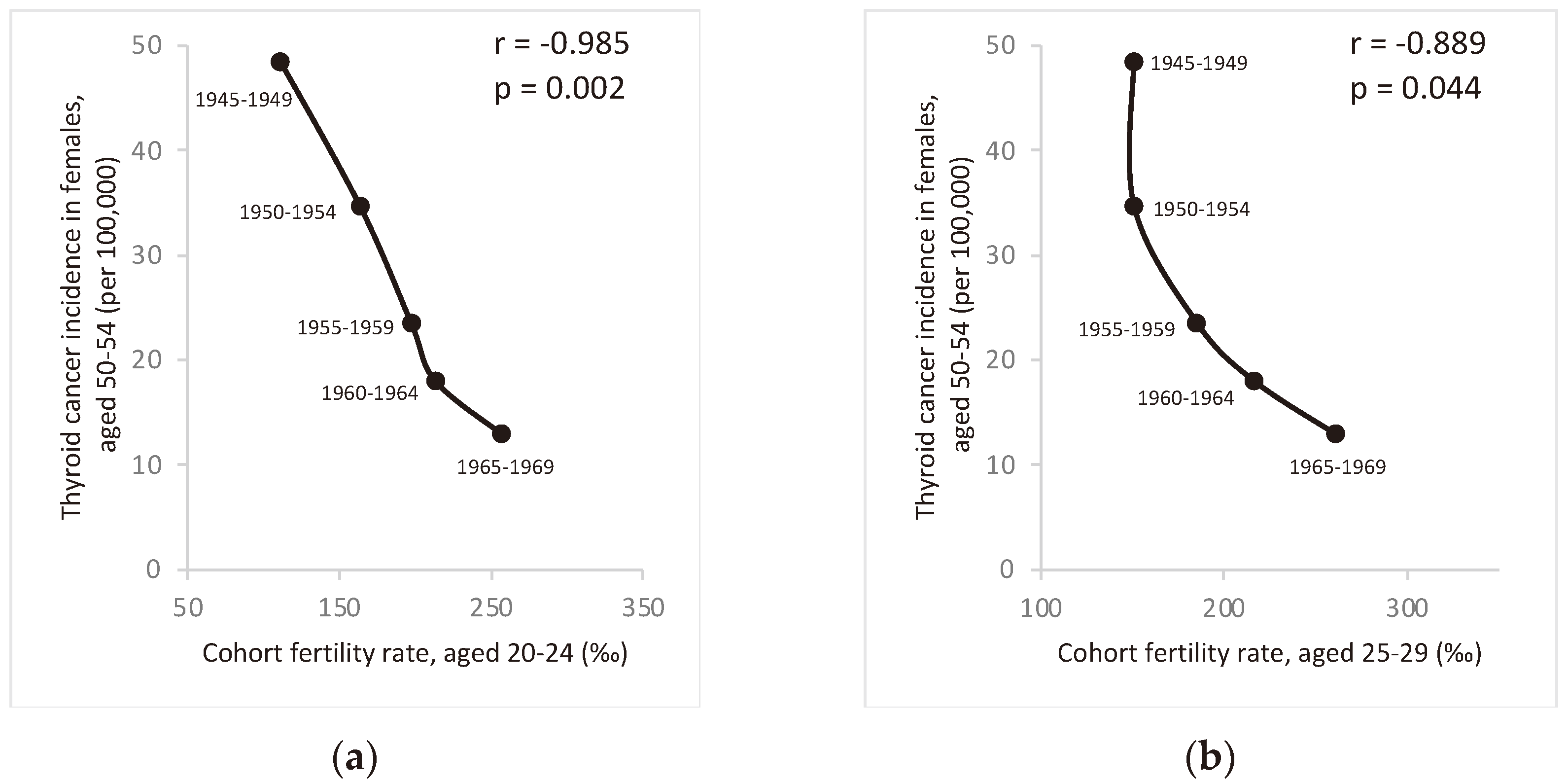
Figure 4 shows a significant negative correlation between thyroid cancer incidence in the 50-54 age group and fertility rates in the 20-29 age group among females from the same birth cohort. This suggests that fertility rates may play a role in the dramatic changes in thyroid cancer incidence among Taiwanese females in recent decades. Similar graphs showing strong correlation between thyroid cancer incidence in females of other age groups (40-59 years) and fertility rates aged 20-29 years from the same cohort can be found in the supplemental figure S1.
The relationship between thyroid cancer incidence rates and fertility rates across different countries was shown in the supplemental figure S2 illustrates. Within each region, there is a consistent pattern of thyroid cancer incidence generally increasing over time, while fertility rates concurrently decrease, indicating a negative correlation between the two variables. Regional trends, however, exhibit some variations: in Asia, there is a rapid decline in fertility rates coupled with a notable increase in thyroid cancer incidence. In the Americas, thyroid cancer incidence has also experienced a rapid rise, although the decline in fertility rate is not as pronounced. Meanwhile, in Europe, changes in both thyroid cancer and fertility rates appear to be relatively gradual.
4. Discussion
Authors should discuss the results and how they can be interpreted from the perspective of previous studies and of the working hypotheses. The findings and their implications should be discussed in the broadest context possible. Future research directions may also be highlighted. Thyroid cancer incidence in Taiwanese female increased during 1995-2019, and the largest increase occurred in the 50-54 age group. After investigating changes in Taiwan’s fertility rates, we found a strong negative correlation between fertility rates and female thyroid cancer incidence. In addition, thyroid cancer incidence increased with later diagnosis years and birth cohorts. We posit that some possible causes for the changing thyroid cancer incidence can be divided into two categories: increases due to overdiagnosis, or actual increases in thyroid cancer incidence. Actual increases may be associated with changes in many risk factors, including fertility, plastic exposure, diabetes, obesity. And these may relate with hormonal and reproductive factors.
Overdiagnosis
As medical technology improving, health examinations and cancer screenings have become increasingly sophisticated. However, thyroid cancer overdiagnosis has been a global health problem [
15]. In many countries, including the United States, South Korea and European countries, overdiagnosis is considered a main reason for the increase in thyroid cancer incidence. Research estimates that during 2003-2007, overdiagnosis accounted for 90% of thyroid cancer cases in South Korea, and 70-80% of cases in the United States [
16]. In the United States, the number of Medicare claims with thyroid ultrasound as an initial imaging test increased 20.9% per year from 2002 to 2013 [
17]. In South Korea, the proportion of new cases diagnosed by ultrasound screening increased rapidly from 13.0% in 1999 to 56.7% in 2008. The increased use of ultrasound correlates with the rapid increase in thyroid cancer incidence in South Korea [
18], and we may be seeing a similar situation in Taiwan. Taiwan’s national health insurance (NHI) program was established in 1995. Although it allows doctors to have access to better tools, such as ultrasound, for more extensive diagnostic capabilities, it also leads to early diagnosis, and possible overdiagnosis. According to one study that randomly sampled one million people from the NHI report (2004-2010), there was a 440.1% increase in the number of individuals who received ultrasound-guided fine needle aspiration biopsy of the thyroid in Taiwan, most of which were ordered by internal medicine physicians (76.4%). During this same time period, the age-standardized incidence rate of thyroid cancer increased by 94.8% [
19]. Tumor size and clinical staging data at the time of diagnosis also showed a trend toward earlier diagnosis; thyroid cancer patients diagnosed during 1993-1998 were diagnosed earlier than those diagnosed during 1979-1992 [
20]. In addition, the change in the proportion of early thyroid cancer diagnoses after the NHI program was established in 1995 is also worth exploring.
Among the four histological subtypes of thyroid cancer, survival rates (globally) from highest to lowest are: papillary, follicular, medullary, and anaplastic carcinoma. According to the TCR report (
https://www.hpa.gov.tw/Pages/TopicList.aspx?nodeid=269), most of the increase in thyroid cancer incidence is due to papillary carcinoma. Out of all thyroid cancers, the proportion of papillary carcinoma for females increased from 76.78% in 1995 to 92.60% in 2019. This change may be due to the increase in the proportion of Taiwanese patients undergoing opportunistic thyroid cancer screening.
Fertility
In previous studies, we found similarities in the impact of fertility on thyroid and breast cancers. Studies found an increased odds of thyroid cancer in females with a late age at first birth [
21]. Compared with females whose first birth took place at < 20 years old, females who experienced their first childbirth at ≥ 35 years old had a 2.7 (1.1–6.8) fold risk of developing thyroid cancer. In comparison, late age at first birth has been found associated with increasing risk of developing breast cancer in many studies [
22,
23], and negative correlation has been found between number of deliveries and breast cancer, with an OR of 0.81 (95% CI, 0.7-0.9) [
24]. Similarly, our results also showed increased thyroid cancer incidence after the age of 40 in a low-fertility female cohort. These results suggest that the recent decline in Taiwan's fertility rates may be correlated with the substantial increase thyroid cancer incidence among Taiwanese females.
In addition, we further speculate that the same phenomenon may also be occurring in other countries with rapid changes in fertility rates, not only in Asia and America, but also Europe, and will be an important health issue in the future. However, to our knowledge, no relevant molecular or biological research results have been found, and we are unable to explain or verify its biological mechanism.
Plastic exposure (Phthalates)
Phthalates are risk factors for thyroid cancer because they can mimic thyroid hormones and disrupt thyroid function [
25]. Di-(2-ethylhexyl) phthalate (DEHP) exposure in mice can cause thyroid cell proliferation and DNA damage, and activates the thyroid stimulating hormone receptor pathway [
26]. Taiwan has a thriving plastic industry. In 2000, the concentrations in the surface water of two major phthalates ranged from ND to 18.5 μg/L for DEHP, and from 1.0–13.5 μg/L for di-
n-butyl phthalate (DBP) [
27]. In comparison, Europe has a relatively low incidence of thyroid cancer, and the total concentration of 8 phthalates (including DHEP, DBP) in 2014 was 1.2μg/L in the Rhone River, a major river crossing France and Switzerland [
28]. The concentration of phthalates in Taiwanese rivers is much higher than in Europe. Therefore, the higher concentration of phthalates Taiwanese environment may be one of factors contributing to the rapid increase in thyroid cancer.
Diabetes mellitus
Individuals with diabetes have an increased risk of thyroid cancer, with an HR of 1.38 (95% CI, 1.13-1.67) for females [
29]. Thus, the rapid increase in diabetic patients in Taiwan may be one explanation for the rising thyroid cancer incidence in females. From 2005-2014, the incidence of diabetes increased by 19%, and the increase was most obvious in patients aged 20-39 years [
30].
Obesity
Obesity is closely related to thyroid and sex hormones. Serum thyroid-stimulating hormone and T3 were found positively associated with BMI [
31]. Body fat distribution and adipocyte differentiation were influenced greatly by sex hormone [
32], and both sex hormone and sex hormone-binding globulin were found strong correlation with obesity [
33]. Obesity was found a risk factor for thyroid cancer, and a meta-analysis of 282,137 people found that obesity has a greater impact on thyroid cancer in females [
34]. (per 5 kg/m
2: HR= 1.14 [95% CI, 1.06–1.23] in females) According to NAHSIT, the rate of obesity (BMI >24) in Taiwanese people has increased from 33.0% in 1993-1996 to 42.8% in 2017-2020 among females.
5. Conclusions
Our results show an increasing incidence of thyroid cancer in females in Taiwan, with overdiagnosis being a main reason for the increase. Notably, we discovered a strong negative correlation between fertility and thyroid cancer rates. Many risk factors for thyroid cancer exist in Taiwan, but with the exception of overdiagnosis, childbirth, and plastic exposure, other risk factors have not risen much. However, the incidence of thyroid cancer in females continues to rise rapidly, especially among aged 50-54. Therefore, we speculate that changes in fertility rates have impacted thyroid cancer incidence, a finding that is rarely discussed in previous studies. The mechanisms behind this finding deserve to be explored in future studies. Decline in fertility is a global issue, and the incidence of thyroid cancer is increasing at different rates in all countries. Therefore, this has important implications as a global demographic issue, and investigating changes in fertility is important for future thyroid cancer research.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org, Figure S1: (a) Correlation between age-specific thyroid cancer incidence rates and cohort fertility rates in Taiwanese females aged 20-24 (b) Correlation between age-specific thyroid cancer incidence rates and cohort fertility rates in Taiwanese females aged 25-29; Figure S2: Incidence rates of thyroid cancer and fertility rates in selected countries; Table S1: Age-specific fertility rates in Taiwanese females, 1950-1999.
Author Contributions
Conceptualization, J.-Y.W. (Jiun-Yan Wu), Y.-C.C. (Yong-Chen Chen) and W.-L.H. (Wan-Lun Hsu); methodology, Y.-C.C., Y.-K.L. (Yu-Kwang Lee) and W.-L.H.; software, J.-Y.W.; validation, Y.-K.S. (Yuh-Kae Shyu); formal analysis, J.-Y.W.; resources, C.-J.C. (Chun-Ju Chiang); data curation, C.-J.C.; writing—original draft preparation, J.-Y.W.; writing—review and editing, J.-Y.W., Y.-C.C., W.-L.H., L.-J.L. (Li-Jen Liao), and S.-L.Y. (San-Lin You); visualization, J.-Y.W. and Y.-C.W. (Yu-Chiao Wang); supervision, Y.-C.C. and W.-L.H.. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Patients or the public were not involved in this study. The data is sourced from the website
https://twcr.tw/. This research protocol was approved by the Institutional Review Board of Fu-Jen Catholic University (No. C104014). The study was performed in accordance with the Declaration of Helsinki.
Informed Consent Statement
Not applicable. The manuscript did not contain individual person’s data. (Patient consent was waived due to this study using a claims database with encrypted personal identities, and the IRB exempted the study from review.
Data Availability Statement
The datasets generated and/or analyzed during the current study are not publicly available in accordance with the policy of the Health and Welfare Data Science Center, Ministry of Health and Welfare, Taiwan, but are available from the corresponding author upon reasonable request.
Acknowledgments
The authors are grateful to the Fu-Jen University Foundation for their helpful comments and recommendations.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Deng, Y., et al., Global burden of thyroid cancer from 1990 to 2017. JAMA Netw. Open, 2020; 3(6), p. e208759-e208759. [CrossRef]
- Rahbari, R., L. Zhang, and E. Kebebew, Thyroid cancer gender disparity. Future Oncol., 2010; 6(11), p. 1771-1779. [CrossRef]
- Kilfoy, B.A., et al., International patterns and trends in thyroid cancer incidence, 1973–2002. Cancer Causes Control, 2009; 20(5), p. 525-531. [CrossRef]
- Huang, Y. and Y. Chen, Cancer incidence characteristic evolution based on the National Cancer Registry in Taiwan. J. Oncol., 2020; 2020. [CrossRef]
- Pellegriti, G., et al., Worldwide increasing incidence of thyroid cancer: update on epidemiology and risk factors. J. Cancer Epidemiol., 2013; 2013. [CrossRef]
- Cordina-Duverger, E., et al., Hormonal and reproductive risk factors of papillary thyroid cancer: A population-based case-control study in France. Cancer Epidemiol., 2017; 48, p. 78-84. [CrossRef]
- Henderson, B.E., et al., Endogenous hormones as a major factor in human cancer. Cancer Res., 1982; 42(8), p. 3232-3239.
- Yager, J.D. and N.E. Davidson, Estrogen carcinogenesis in breast cancer. N. Eng. J. Med., 2006; 354(3), p. 270-282. [CrossRef]
- Henderson, B.E. and H.S. Feigelson, Hormonal carcinogenesis. Carcinogenesis, 2000; 21(3), p. 427-433. [CrossRef]
- Derwahl, M. and D. Nicula, Estrogen and its role in thyroid cancer. Endocr. Relat. Cancer, 2014; 21(5), p. T273-T283. [CrossRef]
- McTiernan, A., N.S. Weiss, and J.R. Daling, Incidence of thyroid cancer in women in relation to known or suspected risk factors for breast cancer. Cancer Res., 1987; 47(1), p. 292-295.
- WIGLE, D.T., Breast cancer and fertility trends in Canada. Am. J. Epidemiol., 1977; 105(5), p. 428-438. [CrossRef]
- Colditz, G.A. and B. Rosner, Cumulative risk of breast cancer to age 70 years according to risk factor status: data from the Nurses' Health Study. Am. J. Epidemiol., 2000; 152(10), p. 950-964. [CrossRef]
- Chiang, C.-J., Y.-W. Wang, and W.-C. Lee, Taiwan’s nationwide cancer registry system of 40 years: past, present, and future. J. Formos. Med. Assoc., 2019; 118(5), p. 856-858.
- Pizzato, M., et al., The epidemiological landscape of thyroid cancer worldwide: GLOBOCAN estimates for incidence and mortality rates in 2020. Lancet Diabetes Endocrinol., 2022; 10(4), p. 264-272. [CrossRef]
- Vaccarella, S., et al., Worldwide thyroid-cancer epidemic? The increasing impact of overdiagnosis. N. Engl. J. Med., 2016; 375(7), p. 614-617. [CrossRef]
- Haymart, M.R., et al., Thyroid ultrasound and the increase in diagnosis of low-risk thyroid cancer. J. Clin. Endocrinol. Metab., 2019; 104(3), p. 785-792. [CrossRef]
- Oh, C.-M., et al., Age-period-cohort analysis of thyroid cancer incidence in Korea. Cancer Res. Treat., 2015; 47(3), p. 362-369. [CrossRef]
- Lee, K.-L., et al., The use of fine needle aspiration and trends in incidence of thyroid cancer in Taiwan. J. Chin. Med. Assoc., 2018; 81(2), p. 164-169. [CrossRef]
- Lin, J.-D., et al., Trends in the clinical characteristics of patients with papillary thyroid carcinoma in Taiwan. Oncology, 2000; 58(4), p. 280-285. [CrossRef]
- Navarro Silvera, S.A., A.B. Miller, and T.E. Rohan, Risk factors for thyroid cancer: a prospective cohort study. Int. J. Cancer, 2005; 116(3), p. 433-438. [CrossRef]
- Iwasaki, M., et al., Role and impact of menstrual and reproductive factors on breast cancer risk in Japan. Eur. J. Cancer Prev., 2007; p. 116-123. [CrossRef]
- Gao, Y.T., et al., Association of menstrual and reproductive factors with breast cancer risk: results from the Shanghai Breast Cancer Study. Int. J. Cancer, 2000; 87(2), p. 295-300. [CrossRef]
- Ng, E.H., et al., Risk factors for breast carcinoma in Singaporean Chinese women: the role of central obesity. Cancer, 1997; 80(4), p. 725-731. [CrossRef]
- Fiore, M., et al., Role of emerging environmental risk factors in thyroid cancer: a brief review. Int. J. Environ. Res. Public Health, 2019; 16(7), p. 1185. [CrossRef]
- Kim, S., et al., Di-2-ethylhexylphthalate promotes thyroid cell proliferation and DNA damage through activating thyrotropin-receptor-mediated pathways in vitro and in vivo. Food Chem. Toxicol., 2019; 124, p. 265-272. [CrossRef]
- Yuan, S., et al., Occurrence and microbial degradation of phthalate esters in Taiwan river sediments. Chemosphere, 2002; 49(10), p. 1295-1299. [CrossRef]
- Paluselli, A., et al., Occurrence of phthalate acid esters (PAEs) in the northwestern Mediterranean Sea and the Rhone River. Prog. Oceanogr., 2018; 163, p. 221-231. [CrossRef]
- Yeo, Y., et al., Diabetes mellitus and risk of thyroid cancer: a meta-analysis. PloS one, 2014; 9(6), p. e98135. [CrossRef]
- Sheen, Y.-J., et al., Trends in prevalence and incidence of diabetes mellitus from 2005 to 2014 in Taiwan. J. Formos. Med. Assoc., 2019; 118, p. S66-S73. [CrossRef]
- Pearce, E.N., Thyroid hormone and obesity. Curr. Opin. Endocrinol. Diabetes Obes., 2012; 19(5), p. 408-413. [CrossRef]
- Lizcano, F. and G. Guzmán, Estrogen deficiency and the origin of obesity during menopause. Biomed Res. Int., 2014; 2014. [CrossRef]
- Tchernof, A. and J.-P. Després, Sex steroid hormones, sex hormone-binding globulin, and obesity in men and women. Horm. Metab. Res., 2000; 32(11/12), p. 526-536. [CrossRef]
- Pappa, T. and M. Alevizaki, Obesity and thyroid cancer: a clinical update. Thyroid, 2014; 24(2), p. 190-199. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).