1. Introduction
Pesticide contamination has become a growing concern in the tropics due to its adverse effects on a variety of non-targeted organisms. Faunal assemblages of lotic ecosystems are threatened by anthropogenic activities, including pesticide pollution [
1,
2]. Urban and agricultural runoff can transport contaminants and pesticides into streams [
3,
4]. Pesticides, however, are expected to have a much higher effect on aquatic environments because these are the ultimate recipients of contaminants [
5,
6]. The adverse effects of pesticides can vary depending on the organism and the chemical's mode of action. Most ecotoxicological studies have focused on a few selected model species, ignoring the effects on other nontarget organisms that may play important roles in tropical lotic ecosystems [
7,
8].
Organophosphate and pyrethroids pesticides like glyphosate, malathion, and permethrin have been present in many tropical freshwater environments [
9,
10]. For years, glyphosate has been a common organophosphate pesticide that controls weeds in cultivated croplands. However, due to its toxicity to humans, it has been recently banned in several countries [
11]. The primary mode of action is in the shikimate pathway that links primary and secondary metabolisms [
12]. Specifically, glyphosate inhibits the activity of the enzyme 5-enolpyuvylshikimate-3-phosphate synthase (EPSPS) found in plants [
13]. The exposure of glyphosate to nontarget organisms is of great concern to aquatic toxicologists because of their extensive use, high water solubility, and slow degradation rate in freshwater environments.
Permethrin is a synthetic pyrethroid used in agricultural and residential zones to control a wide range of insects. The application of permethrin is increasing because of its use in mosquito control programs [
14]. Toxicological data for permethrin are available for aquatic organisms, but the information is needed to apply to tropical regions [
7,
14]. Synthetic pyrethroids induce a continuous series of nerve impulses that disrupts the normal functioning of the nervous system. The mode of action is related to the inhibition of sodium channels in nerve membranes and acetylcholinesterase (AChE) enzyme activity [
15,
16].
Malathion is a common organophosphate insecticide used to control mosquitoes and fruit flies. As an organophosphate insecticide, malathion inhibits acetylcholinesterase (AChE). Acetylcholinesterase is a critical enzyme for the normal function of the nervous system [
17]. The inhibition of this enzyme can result in the accumulation of the neurotransmitter acetylcholine in the synaptic gap leading to disruption of the nervous system [
16]. Previous studies found that malathion is highly toxic to many fish and aquatic invertebrates [
18,
19,
20,
21,
22]. In addition, the morphology, physiology, and behavior of aquatic invertebrates, such as crustaceans, make them particularly susceptible to toxic chemicals [
23]. Because of those biological characteristics, this study used the widely distributed tropical freshwater shrimp
Xiphocaris elongata [
24] as a model organism to determine the acute toxicity of permethrin, malathion, and glyphosate.
2. Materials and Methods
2.1. Collection and Acclimation of Freshwater Shrimp
Adults of
Xiphocaris elongata (cephalothorax larger than 13.0 mm) were collected at Río Sabana near Sabana Field Research Station (18°19'29" N, 65°43'47" W) in Luquillo (elevation 113 above sea level), Puerto Rico. Freshwater shrimp were collected using an electrofishing backpack (Model 12-B, Smith-Root, Vancouver, Washington, USA)[
25]. Collections consisted of five upstream electrofishing passes in each sampling reach (10 m). Hand nets were used to collect the organisms. The habitats sampled included riffles, runs, pools, and aquatic vegetation. The cephalothorax length (CL) of each shrimp was measured from the post-orbital region to the end of the carapace with a dial caliper (0.01 mm precision). The measurement from the tip of the rostrum was not used because the length of Xiphocaris elongata varies depending on the presence of fish predators. Acclimation was performed where the organisms were maintained for at least one week in aerated water, at 20°C, and with a photoperiod of 12h:12h (light: dark).
2.2. Acute Toxicity Tests
Acute toxicity tests were performed to determine the effect of pesticides on the survival of X. elongata. Toxicities were determined by applying a static procedure without renewal of test solutions. All experiments were conducted in glass aquaria with a 5L capacity to which the pesticides were added at different concentrations. Toxicities were expressed based on the concentration of the active ingredient rather than the formulation.
Ten test concentrations of pesticides (between 0.0 to 0.000040 µg/L) and controls were used for each bioassay. Adult freshwater shrimp were selected from the acclimation tanks and distributed randomly into exposure tanks. The exposure tanks (N= 25) (cubic dimensions: length- 15.4 cm, height 15.4- cm, depth- 15.4 cm) were filled with 2.0 L of dechlorinated water, pesticide, and the shrimp. Dead shrimp were counted and removed from the tanks every 24 hrs (24, 48, 72, 96 hrs); the alive organisms continued in the experiment until we reached 96 hrs. This procedure was repeated every 24 hrs. The control and the exposure tanks were kept under constant aeration during the experiment. If the control tanks' mortality rate reached 10% during the first 24 hrs, the experiments had to be reset. One hundred shrimp were used for each concentration group. The organisms were not fed during the test period. After pesticide exposure, mortality was recorded between 24h and 96h.
2.3. Statistical Analyses
Lethal concentration (LC
50) estimates were determined using probit analyses. Dose–response results were modeled in JMP Pro version 17 Software Software SAS Institute Inc., Cary, NC, USA [
26]. Individual mortality per dose was used in the probit analyses to predict the median lethal concentrations. One-way variance analysis (ANOVA) was used to compare the mortality rates among different test groups. A post hoc Tukey's test was used to identify organophosphate and pyrethroids pesticides test with highly significant differences (P<0.05).
3. Results
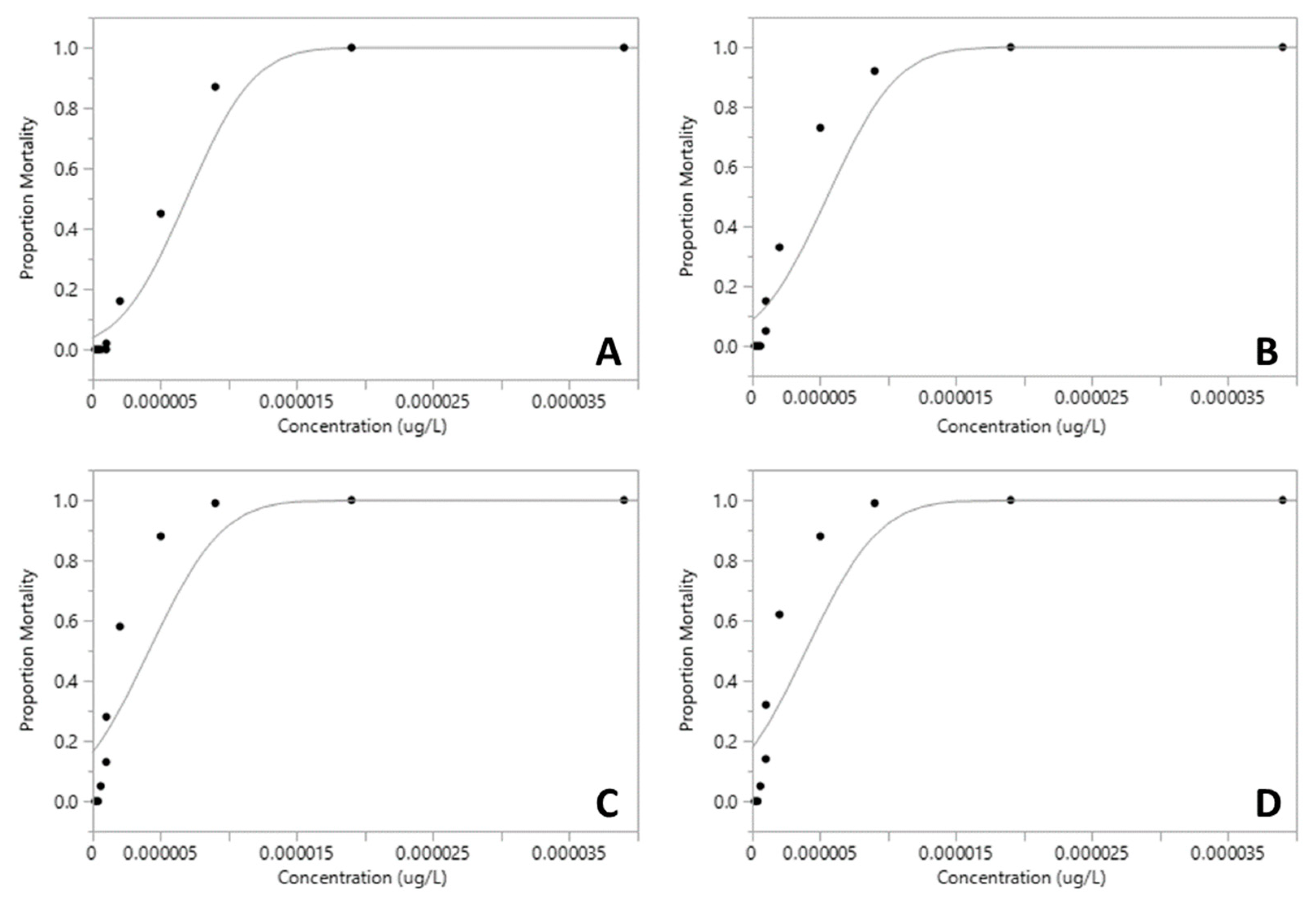
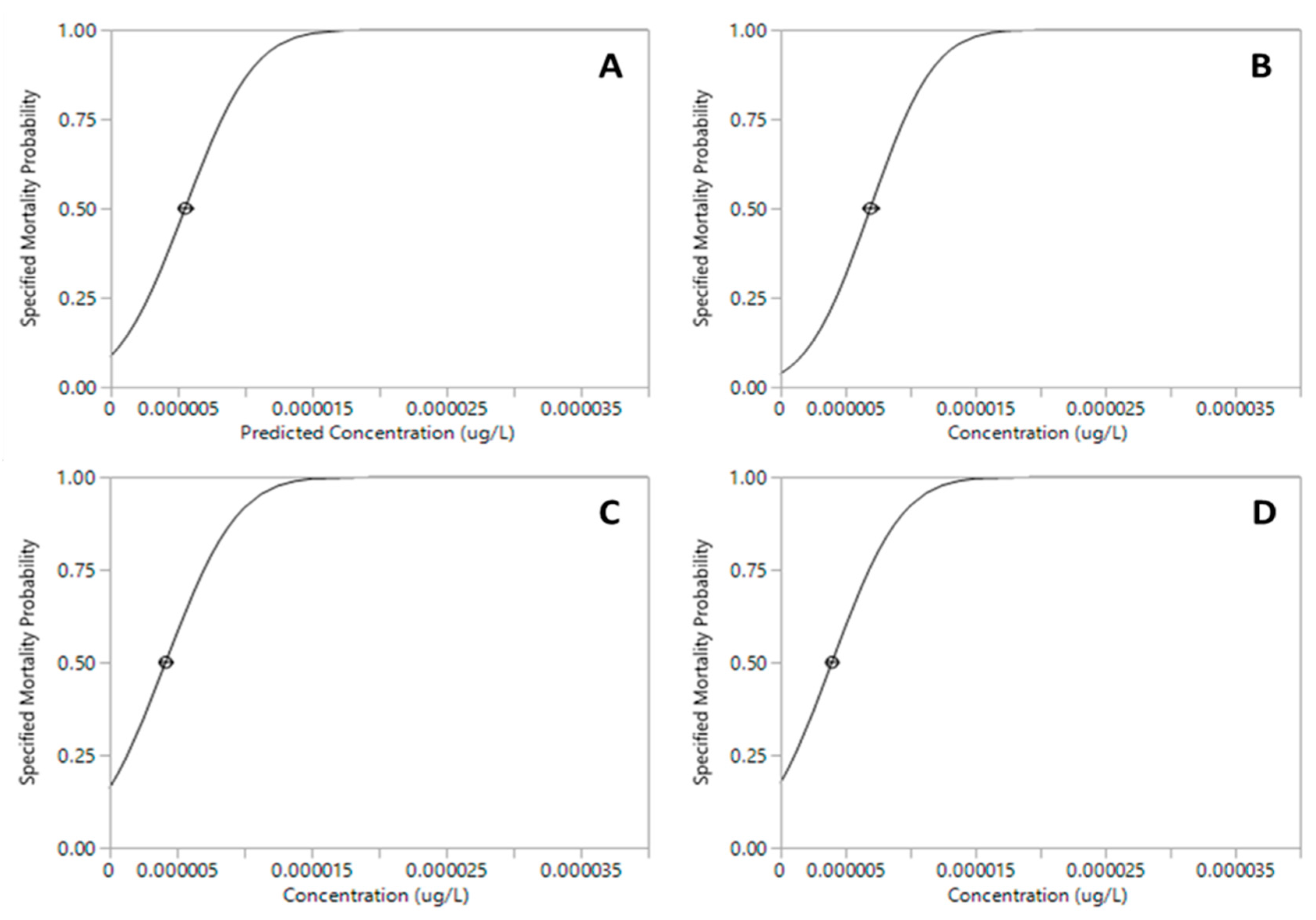
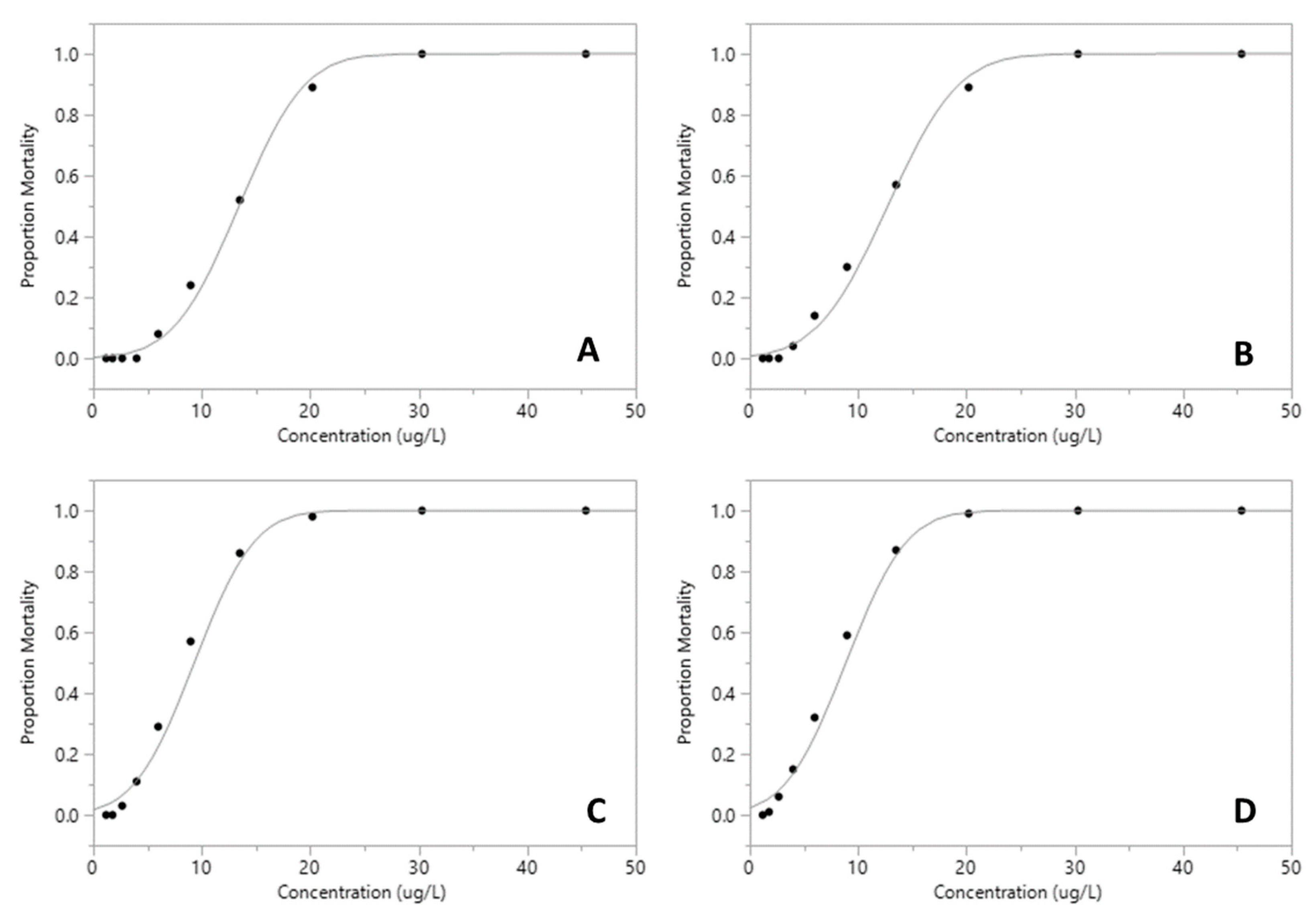
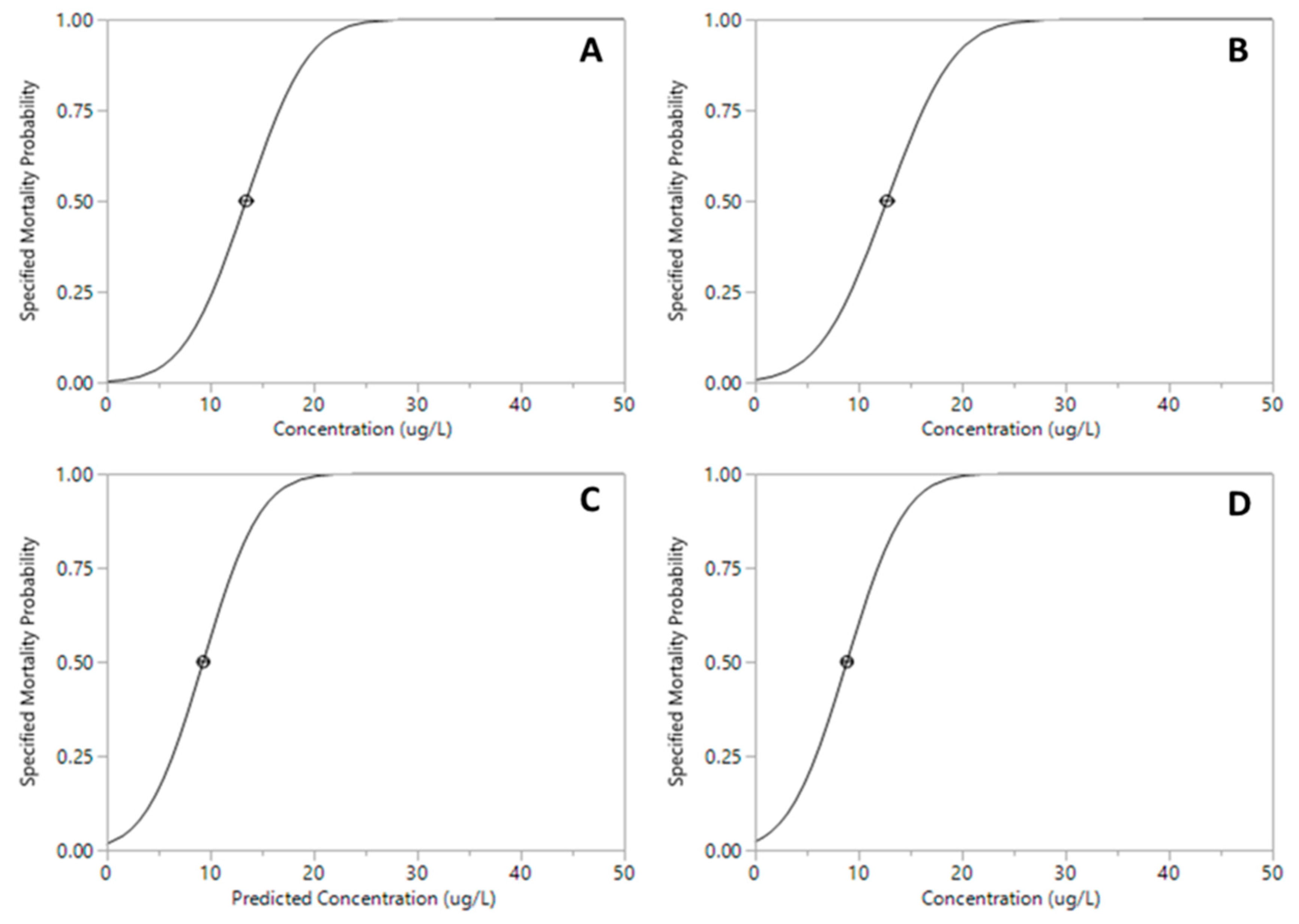
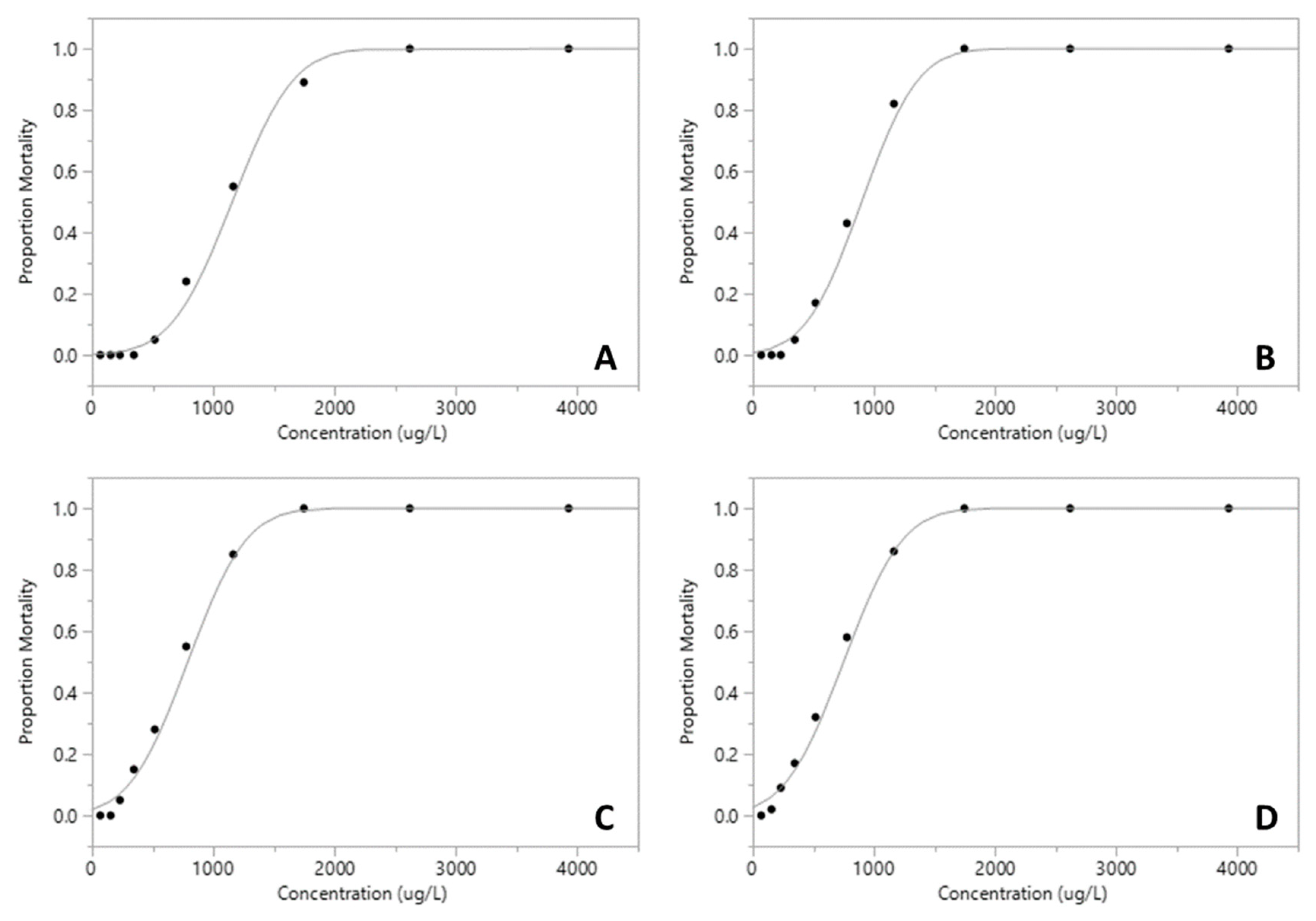
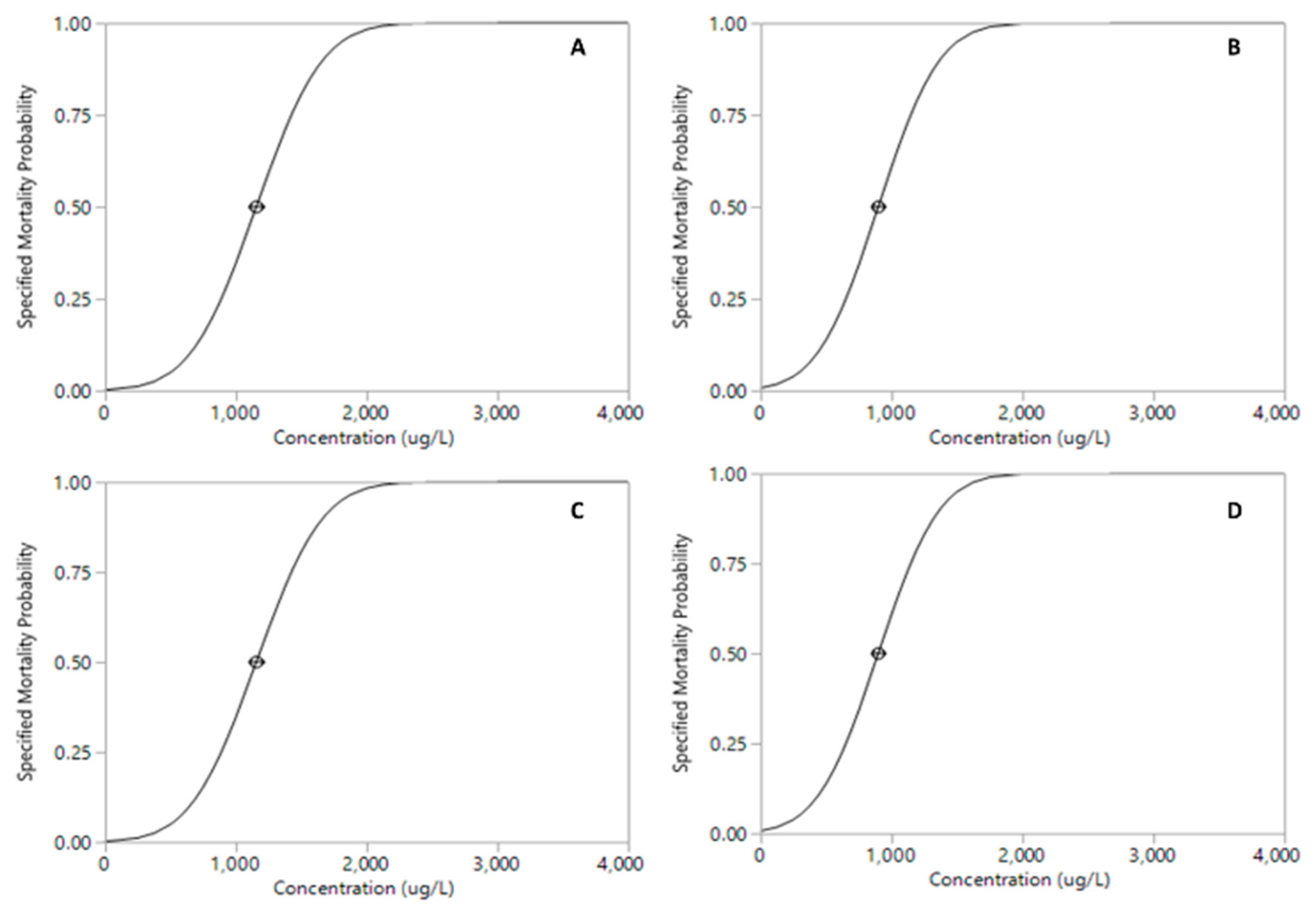
The proportion of mortality of Xiphocaris elongata in each concentration group became progressively higher as the concentration of exposure increased. The mortality observed in acute toxicity tests for permethrin (ANOVA, F(
9,39)=66.13; P < 0.001), malathion (ANOVA, F(
9,39)=84.35; P < 0.001), and glyphosate (ANOVA, F(
9,39)=107.64; P <0.001) were statistically different (
Table 1). Median lethal concentration (LC
50) values for 24h and 96h for permethrin were 6.91x10
-6 µg.L
-1 and 3.96 x10
-6 µg.L
-1, respectively (
Table 1;
Figure 1 and
Figure 2). However, malathion median lethal concentration values for 24 h and 96 h were 13.44 µg.L
-1 and 8.87 µg.L
-1, respectively (
Table 1;
Figure 3 and
Figure 4). The least toxic pesticide was glyphosate with a LC
50 value of 1156.4 μg.L
-1 and 748.92 μg.L
-1 for 24h and 96h, respectively (
Table 1;
Figure 5 and
Figure 6).
4. Discussion
This study assessed the acute toxicity of permethrin, glyphosate, and malathion for the tropical freshwater shrimp
Xiphocaris elongata. To evaluate the biological outcome of such exposure, it is necessary to determinate lethal and sublethal concentrations for organisms. Due to the high costs and long duration of chronic tests, there is a need to do more acute toxicity research. One advantage of using acute toxicity data is that it has been proven to be highly accurate in making long-term predictions [
19]. Permethrin was the most toxic pesticide tested for our model organism, followed by malathion and glyphosate. Similarly, Sánchez-Fortun and Barahona [
27] have found that the aquatic invertebrates most sensitive to pyrethroids pesticides are surface-dwelling insects, mayfly nymphs, and crustaceans. Among crustacean species,
Daphnia magna, Daphnia pulex, Palaemonetes pugio, and
Macrobrachium rosenbergii, have been used as model organisms in aquatic toxicology [
28,
29,
30]. However, the disadvantage of using those species is that some are not present in tropical freshwater environments. In tropical regions, aquatic organisms are often exposed to pesticides and other contaminants, for this reason there is a need to develop model organisms for this geographic region such as we have done with the tropical freshwater shrimp
Xiphocaris elongata.
Shrimp are highly susceptible to pyrethroids pesticides [
31,
32]. Even at sublethal concentrations, significant behavioral changes in shrimp feeding behavior and locomotion activities could affect their survival. In this study, permethrin median lethal concentration (LC
50) value for 96h exposure was 6.91x10-6 µg.L
-1. A study about the effect of permethrin on the grass shrimp
Palaemonetes pugio proved to be acutely toxic at 0.21 µg.L
-1 [
33]. Another study found that the freshwater prawn
Palaemonetes argentinus are susceptible to pyrethroids with a median lethal concentration of 0.0031µg.L
-1 [
34]. This study also determinated that malathion is high to moderate toxic with a 96h LC
50 value of 13.44 µg.L
-1. Similar results were found in a study where malathion exhibited severe toxicity to the grass shrimp
Palaemonetes pugio with a LC
50 of 38.19 µg.L-1 [
35].
Few studies have evaluated the toxicity of glyphosate on freshwater organisms [
36,
37,
38]. In this study, glyphosate median lethal concentration value for 96 h was 748.92 μg.L
-1. Mensah among others [
39] also investigated the acute toxicity of glyphosate on freshwater shrimp
Caridina nilotica. Their results show a median lethal concentration of 25.3 mg.L
-1. The low toxicity of glyphosate exposure may be because the shikimic acid pathway is absent in animals. However, some studies demonstrated that high concentrations could alter animal mitochondrial activity by uncoupling oxidative phosphorylation during cellular respiration [
38].
In summary, the tropical shrimp Xiphocaris elongata is an ecologically important organism in freshwater environments that can be exposed to synthetic pyrethroid and organophosphate pesticides. This study demonstrates that X. elongata is a suitable test or model organism that can be a representative bioindicator of pesticide toxicity in tropical freshwater environments. Similar results were observed in a study where AChE activity were used as biomarker to detect and assess organophosphate and pyrethroid pesticide exposure using X. elongata as model to assess the risk of pesticide contamination in tropical freshwater environment (Torres-Pérez & Pérez-Reyes, 2023). Experiments with this freshwater shrimp species showed a good control performance and reproducibility for the different pesticides tested. Future studies should evaluate sublethal effects using environmentally relevant concentrations to specific pesticides and mixtures.
Author Contributions
Conceptualization, WTP and OPR; methodology, WTP and OPR; formal analysis, WTP and OPR; investigation, WTP and OPR; resources, WTP and OPR; data curation, WTP and OPR; writing—original draft preparation, WTP and OPR; writing—review and editing, WTP and OPR; funding acquisition, OPR. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Science Foundation Grant#-1736019 and Grant # HRD-1547798- NSF CREST.
Acknowledgments
We are especially grateful to our many colleagues, especially Alan P. Covich and Todd A. Crowl, for their collaboration on studies of freshwater shrimp in Puerto Rico.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Schäfer, R. B., Caquet, T., Siimes, K., Mueller, R., Lagadic, L., & Liess, M. Effects of pesticides on community structure and ecosystem functions in agricultural streams of three biogeographical regions in Europe. Science of The Total Environment, 2007, 382(2-3): 272–285. [CrossRef]
- Liess, M., & Von Der Ohe, P. C. Analyzing effects of pesticides on invertebrate communities in streams. Environmental Toxicology and Chemistry, 2005, 24(4): 954 – 965. [CrossRef]
- Schiff, K., & Sutula, M. Organophosphorus pesticides in storm-water runoff from southern California (USA). Environmental Toxicology and Chemistry, 2004, 23(8): 1815- 1821. [CrossRef]
- Zhang, W., Ye, Y., Hu, D., Ou, L., & Wang, X. Characteristics and transport of organochlorine pesticides in urban environment: air, dust, rain, canopy throughfall, and runoff. Journal of Environmental Monitoring, 2010, 12(11): 2153 – 2160. [CrossRef]
- Kolpin, D. W., Thurman, E. M., & Linhart, S. M. The environmental occurrence of herbicides: the importance of degradates in ground water. Archives of Environmental Contamination and Toxicology, 1998, 35(3): 385–390. [CrossRef]
- Willis, G. H., & McDowell, L. L. Pesticides in agricultural runoff and their effects on downstream water quality. Environmental Toxicology and Chemistry, 1982, 1(4): 267–279. [CrossRef]
- Daam, M. A., & Van den Brink, P. J. Implications of differences between temperate and tropical freshwater ecosystems for the ecological risk assessment of pesticides. Ecotoxicology, 2009, 19(1): 24–37. [CrossRef]
- Gunnarsson, J. S., & Castillo, L. E. Ecotoxicology in tropical regions. Environmental Science and Pollution Research, 2018, 25(14): 13203–13206. [CrossRef]
- Buttermore, E., Cope, G. W., Kwak, T.J., Cooney, P.B., Shea, D., & Lazaro, P.R. Contaminants in tropical island streams and their biota. Environmental Research, 2018, 161: 615 – 623. [CrossRef]
- Santiago, X.B., Rivera, D., Pabón, A., & García, A. An examination of the use of pesticides in Puerto Rican agriculture. Rurals, 2016, 10(1): 1 – 10.
- van Bruggen, A. H. C., He, M. M., Shin, K., Mai, V., Jeong, K. C., Finckh, M. R., & Morris, J. G. Environmental and health effects of the herbicide glyphosate. Science of the Total Environment, 2018,616-617: 255–268. [CrossRef]
- Mensah, P. K., Muller, W. J., & Palmer, C. G..Acute toxicity of Roundup® herbicide to three life stages of the freshwater shrimp Caridina nilotica (Decapoda: Atyidae). Physics and Chemistry of the Earth, 2011, 36(14-15): 905–909. [CrossRef]
- Stenersen, J. Chemical Pesticides: Mode of Action and Toxicology. CRC Press, 2004, Boca Raton, Florida, United States of America, p. 296. [CrossRef]
- United States Environmental Protection Agency. 2005. Permethrin, EFED revised risk assessment for the reregistration eligibility decision on permethrin. Docket number 2004-0385-0014.
- De Oliveira, P., Gomes, A. Q., Pacheco, T. R., Vitorino de Almeida, V., Saldanha, C., & Calado, A. Cell-specific regulation of acetylcholinesterase expression under inflammatory conditions. Clinical Hemorheology and Microcirculation, 2012, 51(2), 129-137. [CrossRef]
- Cox, C. Permethrin insecticide fact sheet. Journal of Pesticide Reform, 1998, 18 (2): 1–20.
- Lionetto, M. G., Caricato, R., Calisi, A., & Schettino, T. Acetylcholinesterase inhibition as a relevant biomarker in environmental biomonitoring: new insights and perspectives. Ecotoxicology around the globe, 2011, 87-115. [CrossRef]
- Biehl, L., & Callaghan, A. A comparative study on the relationship between acetylcholinesterase activity and acute toxicity in Daphnia magna exposed to anticholinesterase insecticides. Environmental Toxicology and Chemistry, 2004, 23 (5): 1241–1247. [CrossRef]
- Kumar, A., Correll, R., Grocke, S., & Bajet, C. Toxicity of selected pesticides to freshwater shrimp, Paratya australiensis (Decapoda: Atyidae): Use of time series acute toxicity data to predict chronic lethality. Ecotoxicology and Environmental Safety, 2010, 73(3): 360–369. [CrossRef]
- Kumar, A., & Jawahar, A. Toxic impacts of two organophosphorus pesticides on the acetylcholinesterase activity and biochemical composition of freshwater fairy shrimp Streptocephalus dichotomus. International Journal of Pharma and Bio Sciences, 2013, 4 (2): 966 – 972.
- Printes, L. B., & Callaghan, A. A comparative study on the relationship between acetylcholinesterase activity and acute toxicity in Daphnia magna exposed to anticholinesterase insecticides. Environmental Toxicology and Chemistry, 2004, 23(5): 1241-1247. [CrossRef]
- 22. Printes LB, Callaghan A.. Intraclonal variability in Daphnia acetylcholinesterase activity: the implications for its applicability as a biomarker. Environ Toxicol Chem, 2003, 22(9):2042–2047. [CrossRef]
- Rinderhagen, M., Ritteroff J., Zauke, G.P. Crustaceans as bioindicators. Environmental Research, 2000, 9: 161–194.
- Guérin-Méneville, F.E. Crustáceos. In: de la Sagra, R. (Eds.) Historia física, político y natural de la isla de Cuba, 1856, Paris: Arthus Bertrand.
- Pottier, G., Bargier, N.; Bennevault, Y.; Vigouroux, R.; Azam, D.; Marchand, F.; Nevoux, M. & Roussel, J. M. Optimising electrofishing settings for shrimp and fish in shallow tropical streams. Fisheries Research, 2022, 256, 106457. [CrossRef]
- SAS Institute, (2023) JMP Statistical Discovery LLC.
- Sánchez-Fortún, S., & Barahona, M. V.. Comparative study on the environmental risk induced by several pyrethroids in estuarine and freshwater invertebrate organisms. Chemosphere, 2005, 59(4):553–559. [CrossRef]
- Altshuler, I., Demiri, B., Xu, S., Constantin, A., Yan, N. D., & Cristescu, M. E.. An Integrated Multi-Disciplinary Approach for Studying Multiple Stressors in Freshwater Ecosystems: Daphnia as a Model Organism. Integrative and Comparative Biology, 2011, 51(4): 623–633. [CrossRef]
- Seda, J., & Petrusek, A. Daphnia as a model organism in limnology and aquatic biology: introductory remarks. Journal of Limnology, 2011, 70(2): 337 – 344. [CrossRef]
- Satapornvanit, K., Baird, D. J., & Little, D. C.. Laboratory toxicity test and post-exposure feeding inhibition using the giant freshwater prawn Macrobrachium rosenbergii. Chemosphere, 2009, 74(9): 1209–1215. [CrossRef]
- Hook, S. E., Doan, H., Gonzago, D., Musson, D., Du, J., Kookana, R., Sellars, M., & Kumar, A. The impacts of modern-use pesticides on shrimp aquaculture: An assessment for northeastern Australia. Ecotoxicology and Environmental Safety, 2018, 148: 770–780. [CrossRef]
- Smith, T. M., & Stratton, G. W.. Effects of synthetic pyrethroid insecticides on nontarget organisms. Residue Reviews, 1986, 97: 93–120. [CrossRef]
- De Lorenzo, M. E., Serrano, L., Chung, K. W., Hoguet, J., & Key, P. B. Effects of the insecticide permethrin on three life stages of the grass shrimp, Palaemonetes pugio. Ecotoxicology and Environmental Safety, 2006, 64(2): 122–127. [CrossRef]
- Collins, P., & Cappello, S. Cypermethrin Toxicity to Aquatic Life: Bioassays for the Freshwater Prawn Palaemonetes argentinus. Archives of Environmental Contamination and Toxicology, 2006, 51(1): 79–85. [CrossRef]
- Key, P. B., Fulton, M. H., Scott, G. I., Layman, S. L., & Wirth, E. F. Lethal and sublethal effects of malathion on three life stages of the grass shrimp, Palaemonetes pugio. Aquatic Toxicology, 1998, 40(4): 311–322. [CrossRef]
- Hong, Y., Yang, X., Huang, Y., Yan, G., & Cheng, Y. Assessment of the oxidative and genotoxic effects of the glyphosate-based herbicide roundup on the freshwater shrimp, Macrobrachium nipponensis. Chemosphere, 2018, 210: 896–906. [CrossRef]
- Fiorino, E., Sehonova, P., Plhalova, L., Blahova, J., Svobodova, Z., & Faggio, C. Effects of glyphosate on early life stages: comparison between Cyprinus carpio and Danio rerio. Environmental Science and Pollution Research, 2018, 25(9): 8542–8549. https://link.springer.com/article/10.1007/s11356-017-1141-5.
- Uren, W. T. M., Laing, L. V., Florance, H., & Santos, E. M.. Effects of Glyphosate and its Formulation, Roundup, on Reproduction in Zebrafish (Danio rerio). Environmental Science & Technology, 2014, 48(2):1271–1279. [CrossRef]
- Mensah, P. K., Palmer, C. G., & Odume, O. N.. Ecotoxicology of Glyphosate and Glyphosate-Based Herbicides — Toxicity to Wildlife and Humans. In Toxicity and Hazard of Agrochemicals. 2015, pp. 93 -112. [CrossRef]
- Torres-Pérez, W.X. & Pérez-Reyes, O. Effect of Particle Size and Pesticide Contamination on Preference and Ingestion Rates by the Tropical Freshwater Shrimp Xiphocaris elonga. Open Journal of Ecology. 2023, pp. 183 -198. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).