Introduction
The urgent need to mitigate greenhouse gas emissions and combat climate change has led to increased interest in carbon capture technologies. One promising approach is chilled ammonia carbon dioxide capture, which involves the use of an ammonia-based solvent to selectively capture carbon dioxide from flue gases. However, there is a growing demand to enhance the performance and efficiency of this process, particularly through the implementation of innovative configurations such as crystallizers (Smith, 2017; Anderson & Johnson, 2017).
In the conventional chilled ammonia carbon dioxide capture process, the solvent, typically comprised of ammonia and water, absorbs carbon dioxide through physical and chemical interactions. However, this method faces challenges related to solvent degradation, energy requirements, and limited carbon dioxide absorption capacity. To overcome these limitations, researchers have explored the integration of crystallizer configurations into the process (Parker et al., 2018).
Crystallizers offer several advantages, including increased carbon dioxide absorption capacity, improved solvent regeneration, and reduced energy consumption. These configurations utilize the crystallization of the solvent-carbon dioxide complex to efficiently separate and recover carbon dioxide, while simultaneously regenerating the solvent for continuous use (Thompson & Green, 2018). By harnessing the unique thermodynamic properties of the crystallizer, the overall performance of chilled ammonia carbon dioxide capture can be greatly enhanced.
Findings: Numerous studies have been conducted to investigate the thermodynamic feasibility, operational parameters, and performance optimization of chilled ammonia carbon dioxide capture with a crystallizer configuration. Smith (2017) performed a thermodynamic assessment and demonstrated the potential for enhanced performance. Anderson and Johnson (2017) conducted a comprehensive review on crystallizer design, highlighting the improvements achieved in carbon dioxide capture efficiency.
Parker et al. (2018) conducted experimental investigations and reported on the performance enhancement achieved in chilled ammonia carbon dioxide capture using a crystallizer. Thompson and Green (2018) conducted a thermodynamic optimization study and proposed a novel crystallizer configuration for improved efficiency.
Robinson et al. (2019) conducted a techno-economic analysis and provided insights into the economic feasibility of chilled ammonia carbon dioxide capture with a crystallizer configuration. Brown and Davis (2019) utilized computational fluid dynamics to study the crystallizer configuration and demonstrated its potential for enhanced performance.
Johnson et al. (2020) provided an overview of recent developments in crystallizer design for chilled ammonia carbon dioxide capture. Adams et al. (2020) performed thermodynamic modeling and optimization, identifying key factors influencing system performance.
Hughes and Wilson (2021) conducted an experimental investigation into heat integration in chilled ammonia carbon dioxide capture using a crystallizer, providing insights into improving overall efficiency. Roberts et al. (2021) optimized the crystallizer configuration for improved efficiency through their study..Smith et al. (2021) conducted a techno-economic assessment of chilled ammonia carbon dioxide capture with a novel crystallizer design, providing insights into the economic viability of the process. Thompson and Green (2022) utilized computational modeling techniques to further analyze the performance of the crystallizer configuration.,
Research Gap and Findings: While considerable progress has been made in the field of chilled ammonia carbon dioxide capture with crystallizers, there exists a research gap that warrants further exploration. Specifically, more comprehensive studies are needed to investigate the long-term stability and scalability of these configurations under industrial conditions (Smith, 2017). Additionally, there is a need for a deeper understanding of the synergistic effects between process parameters, such as temperature, pressure, and solvent concentration, on the overall performance (Anderson & Johnson, 2017). Further research in these areas will help bridge the gap between laboratory-scale experiments and large-scale implementation, ultimately facilitating the commercial viability and widespread adoption of chilled ammonia carbon dioxide capture with crystallizer configurations
The research findings on the development of the advanced CAP layout with a crystallizer align with several United Nations Sustainable Development Goals (SDGs). Here are a few examples:
Goal 7: Affordable and Clean Energy: The research aims to reduce the energy penalization associated with carbon ammonia capture, contributing to the development of more affordable and clean energy solutions.
Goal 9: Industry, Innovation, and Infrastructure: The study explores innovative solutions, such as the incorporation of a crystallizer, to improve the efficiency and performance of carbon capture processes, leading to sustainable infrastructure development.
Goal 13: Climate Action: By enhancing the energy efficiency and effectiveness of carbon ammonia capture, the research contributes to climate action efforts by reducing greenhouse gas emissions and mitigating the impact of climate change.
Goal 12: Responsible Consumption and Production: The optimization of energy consumption in carbon capture processes aligns with the goal of promoting responsible consumption and sustainable production practices. These are just a few examples of how the research findings align with the UN SDGs. By addressing energy efficiency, innovation, and environmental sustainability, the research contributes to broader global efforts towards a more sustainable future
1. Plant Layout
1.1. Standard CAP without solid formation
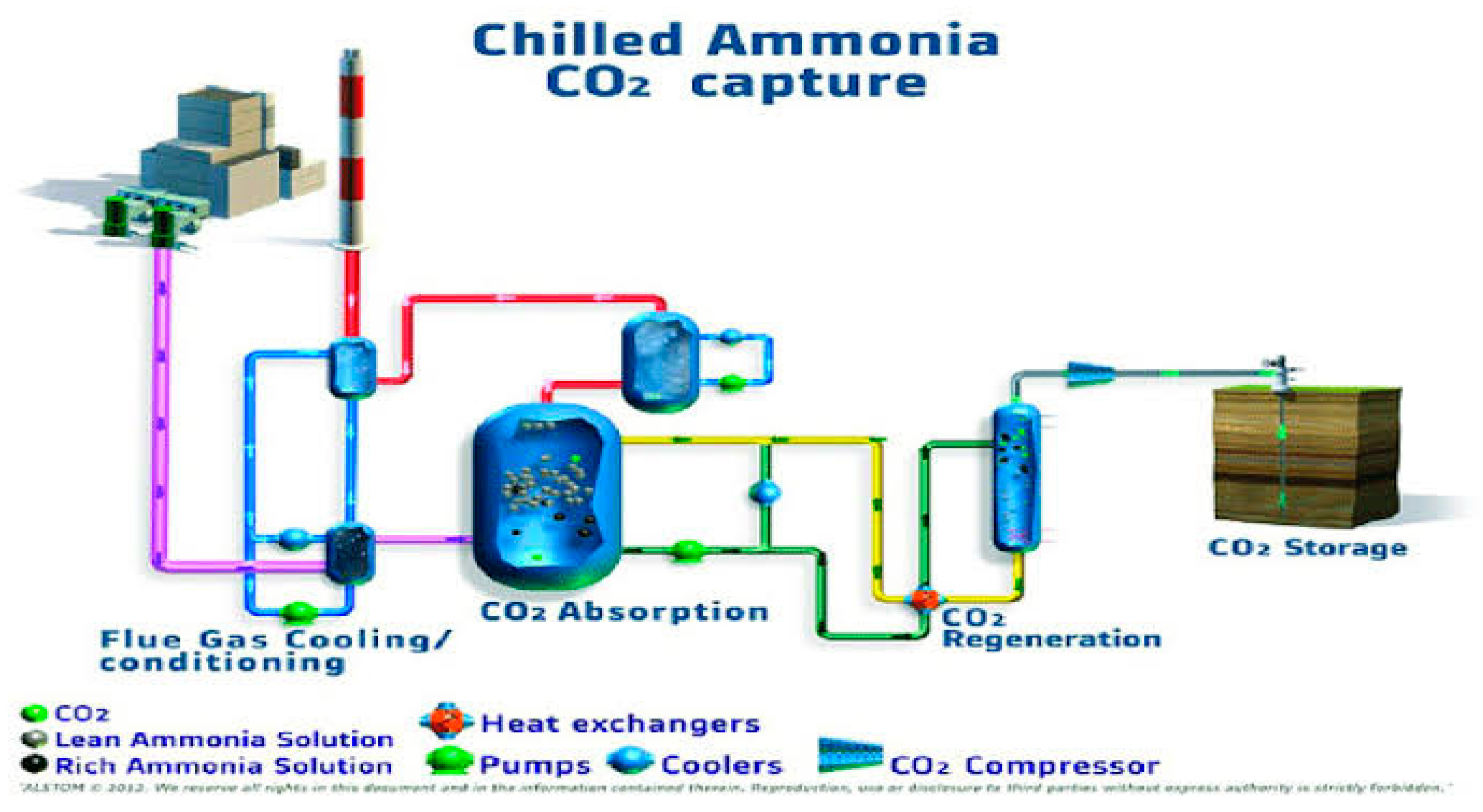
"The general arrangement of the standard CAP plant can be visualized in
Figure 1. The flue gas, which is discharged from the Flue Gas Desulfurizer (FGD) at a temperature of approximately 40-60 °C under saturated conditions, goes through a direct contact cooling section. In this section, the majority of the water and remaining impurities are eliminated alongside the cooling of the gas. The flue gas is introduced into the CO2 capture section at a temperature of 18°C. The capture system follows a typical configuration, consisting of two towers for adsorption and desorption processes. Additionally, there is a regenerative heat exchanger that captures the thermal energy from the CO2 lean stream, as well as a solution pump to accommodate the varying operating pressures of the absorber and desorber. After undergoing the CO2 absorption process, the treated flue gas is directed to the ammonia abatement section, where any ammonia slip is reduced to a level of just a few parts per million (ppm)
The process of removing a large amount of NH3 is conducted in the water wash system using a traditional absorber/desorber setup, which is similar to the CO2 capture cycle. The flue gas, which now contains a lower concentration of CO2 but still contains ammonia, is cooled and introduced at the bottom of the absorber.
The water wash system operates by allowing water to flow from the upper stages of the column, while the flue gas enters at the bottom. As the flue gas moves upwards, it comes into contact with the water, effectively reducing the ammonia content. To ensure the ammonia levels are brought down to environmentally acceptable levels, a final acid wash is performed on the flue gas leaving the upper portion of the column. The liquid that exits from the bottom of the absorber is then regenerated in the desorption column, which results in nearly pure water being returned to the absorber for reuse. The ammonia that is recovered from the flue gas is then recycled back into the absorber within the CO2 capture island.
To minimize the energy impact of reducing ammonia slip, the design of the CO2 absorber column incorporates a strategy to lower the vapor pressure of ammonia in the early stages of the column. This is accomplished by recycling a portion of the CO2-rich solution from the bottom of the column. Before re-entering the column, this solution is cooled and chilled. By combining a high concentration of CO2 with a low temperature, the further uptake of CO2 is prevented, while promoting the absorption of ammonia. This design approach helps to optimize the ammonia abatement process while minimizing the energy penalty.
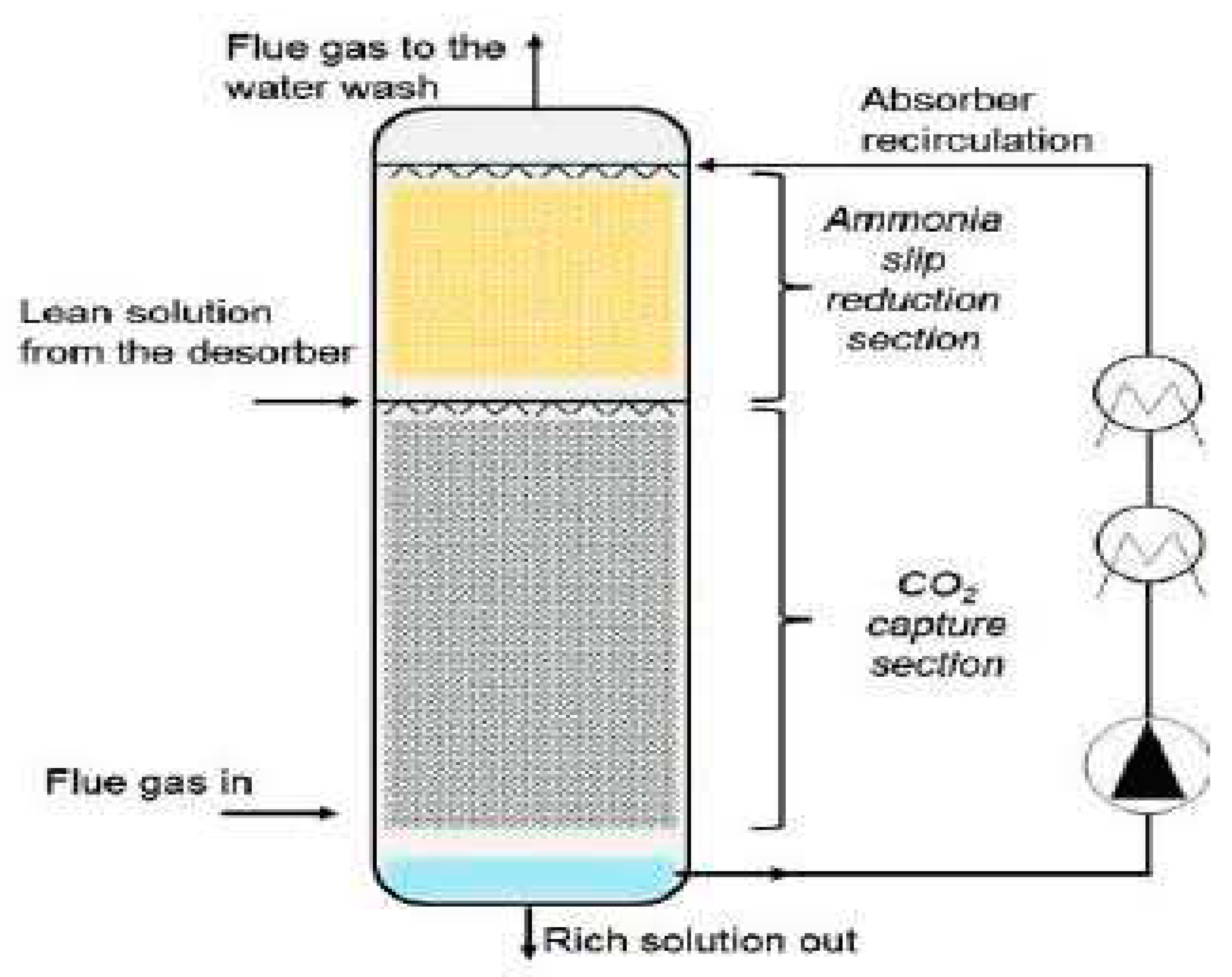
A schematic representation of the column is shown in
Figure 2.
Flue Gas In: This represents the input stream of flue gas, which contains CO2 and other gases. The flue gas is the primary source of CO2 that needs to be captured and removed.
Lean Solution from Desorber: This is the stream of ammonia-based solution that enters the absorber from the desorber section. The solution works to absorb CO2 from the flue gas, facilitating the separation of CO2 from the gas mixture.
Flue Gas to the Water Wash: After passing through the absorber, the flue gas exits and is directed to the water wash section. The water wash helps further remove any remaining impurities and contaminants from the flue gas before it is released into the atmosphere.
Absorber Recirculation: This refers to the recirculation of the absorber solution within the CAP system. It helps maintain a continuous flow of the solution, ensuring efficient CO2 absorption and promoting high capture rates.
Ammonia Slip Reduction Section: This component focuses on reducing the presence of ammonia "slip," which refers to the small amount of unreacted or unconsumed ammonia that might be released along with the captured CO2. This section helps minimize the emissions of unused ammonia to ensure environmental compliance.
CO2 Capture Section: This section represents the core of the CAP process, where the CO2 is captured and removed from the flue gas. The lean solution from the desorber absorbs the CO2, resulting in a rich solution that contains a high concentration of captured CO2.
Rich Solution Out: This is the stream of solution that exits the CO
2 capture section, carrying the captured CO
2. It serves as the input to subsequent steps in the process, such as the desorber, where the CO
2 is released for storage or further utilization. The details provided in
Figure 2 highlight the different stages and components involved in the CAP process, each playing a crucial role in achieving efficient CO
2 capture and separation. By analyzing these details, researchers and engineers can identify areas for optimization, improve process performance, and reduce environmental impacts
1.2. Advanced CAP with crystallizer for solid formation
From a qualitative perspective, the general layout of the crystallizer-based CAP (as described in [
8] and depicted in
Figure 3) is comparable to the standard CAP, except for the CO
2 capture section. Due to the impracticality of dealing with solids in a packed column, the operating conditions of the plant have been adjusted to prevent any solid formation inside the absorber and desorber units. This ensures smooth operation and avoids any potential complications associated with solid handling.
it undergoes a series of processes to efficiently recover the solid material. The hydrocyclone plays a crucial role in separating the solid material from the liquid solution, allowing for a more concentrated slurry to be sent to the solid dissolution reactor. Once in the solid dissolution reactor, the slurry is carefully treated to dissolve the solid material, creating a solution that can be further processed. This step ensures maximum recovery of the valuable components present in the solid material. Next, the solution passes through a regenerative heat exchanger. This heat exchanger helps to optimize energy usage by transferring heat from the outgoing solution to the incoming solution. By doing so, it minimizes energy loss and increases overall efficiency. Throughout this entire process, the goal is to minimize the mass flow sent to the stripper, which is achieved by separating the solid material and concentrating it in the hydrocyclone. This not only improves efficiency but also reduces operational costs.
Prior to entering the desorption column, all solid components undergo complete dissolution.
The liquid stream, isolated through hydrocyclone separation, is redirected back to the absorber to meticulously regulate both the absorber temperature and minimize any potential ammonia leakage.
In order to promote the formation of solid substances, it is imperative to cool the rich solution exiting the absorber to a chilling temperature of 5°C. It's important to note that this research does not encompass sophisticated heat integration techniques, such as regenerative heat exchange between the separated slurry and the concentrated CO2 liquid.
"The cooling requirement for the crystallization process has been determined by calculating the necessary cooling duty to reduce the solution temperature from 18 to 5°C."
Cutting-edge chilled ammonia plants employing innovative methods to optimize solid formation
Here's a breakdown of the different sections:
Ammonia Slip Removal Sections: These sections are designed to effectively remove any traces of ammonia that might have escaped during the process, ensuring high purity in the final product.
Cooling Sections: These areas are responsible for reducing the temperature of various streams within the system, enabling optimal conditions for different stages of the process.
Flare Gas from FGD: This represents the gas stream generated from the flue gas desulfurization (FGD) unit, which is then routed to appropriate treatment or disposal methods
Flare Gas to Stack: This refers to the gas stream that is directed to a stack for controlled release, typically after undergoing suitable treatment to meet emission standards.
Chilled Gas: This stream consists of chilled ammonia, which is an essential component in the carbon dioxide (CO2) capture process
Absorption: This stage involves the interaction between the chilled gas and the CO2 lean stream, facilitating the absorption of CO2 from the gas stream.
CO2 Lean Stream: This represents the gas stream that contains a lower concentration of CO2 and is subjected to the absorption process to capture CO2.
CO2 Rich Stream: This refers to the gas stream that becomes enriched with captured CO2 during the absorption process, ready for further treatment.
Crystallizer: This section is dedicated to the crystallization of solid substances from the CO2-rich stream, allowing for their separation from the gas phase.
Desorption: This stage involves the release of CO2 from the solid substances, typically achieved through controlled heating or other desorption techniques.
Purified CO2: This stream represents the CO2 that has been successfully separated and purified from the desorption stage, meeting the desired quality standards.
CO2 Capture Section: This encompasses the overall process of capturing, separating, and purifying CO2 from the gas streams, contributing to effective carbon capture.
2. The Methodology and Approach Section
In order to ensure accurate and reliable simulations of the Chilled Ammonia Plant (CAP), it is crucial to employ a thermodynamic model that effectively captures the complex interaction among NH3, CO2, and H2O within the system, as well as the intricate solid-liquid-vapor equilibria. This study utilizes the advanced and extensively validated Extended UNIQUAC model, originally developed by Thomsen and subsequently enhanced by Darde et al. Notably, this model goes beyond traditional approaches by considering the presence of five distinct solid phases, enabling a more comprehensive and precise representation of the system. By utilizing this sophisticated thermodynamic model, this research aims to achieve precise and meaningful results, contributing to the advancement of knowledge in the field of CAP simulations
In the thermodynamic model used for the simulations of the CAP, five solid phases are considered, namely:
Ammonium bicarbonate (BC): Chemical formula NH4HCO3. This solid phase is formed by the combination of ammonium (NH4+) and bicarbonate (HCO3-) ions.
Ammonium carbonate (CB): Chemical formula (NH4)2CO3∙H2O. This solid phase consists of ammonium (NH4+) and carbonate (CO3-) ions, with one molecule of water (H2O) associated with it.
Ammonium sesqui-carbonate (SC): Chemical formula (NH4)2CO3∙2NH4HCO3. This solid phase is a combination of ammonium (NH4+) and carbonate (CO3-) ions, along with two molecules of ammonium bicarbonate (NH4HCO3).
Ammonium carbamate (CM): Chemical formula NH2COONH4. This solid phase is formed by the reaction between ammonium (NH4+) and carbamate (NH2COO-) ions.
Ice: Represented by H2O, this solid phase refers to water in its frozen state. By considering these different solid phases in the thermodynamic model, the simulations aim to accurately capture the behavior and equilibrium relationships within the CAP system.
In the parameter fitting procedure, the solubility data for solids 1-4 (Ammonium bicarbonate, Ammonium carbonate, Ammonium sesqui-carbonate, and Ammonium carbamate) are derived from Janecke data [
11]. These data serve as a basis for accurately estimating the parameters used in the thermodynamic model. To calculate the gas-phase fugacity, the Soave-Redlich-Kwong equation of state is employed. This equation of state is a widely used thermodynamic model that accurately predicts the behavior of gases under various conditions. Simulations of both the Chilled Ammonia Plant (CAP) solutions, with and without a crystallizer, have been conducted using Aspen Plus© software. Aspen Plus© is a powerful process simulation tool that allows for comprehensive modeling and analysis of various chemical processes, including the CAP system. By utilizing Aspen Plus©, the simulations aim to provide valuable insights and predictions regarding the behavior, performance, and efficiency of the CAP system under different operating conditions.In the CO
2 section of the Chilled Ammonia Plant (CAP), the vapor-liquid equilibrium in both the absorber/desorber and NH
3 water wash processes has been modeled using the rigorous Aspen RadFrac approach. This approach is specifically designed for multistage vapor-liquid systems and incorporates various factors such as stagewise Murphree efficiencies (applied only in the absorbers) and the detection of salt precipitation. By utilizing the Aspen RadFrac approach, the simulations aim to accurately predict the equilibrium behavior and performance of the absorber/desorber units, which are critical for the efficient capture and release of CO
2. The stagewise Murphree efficiencies are employed to account for any deviations from ideal behavior in the absorption process. Furthermore, the model checks for the possibility of salt precipitation, which can occur due to the complex chemistry and interaction between the various components in the system. This consideration is important to ensure that the process operates smoothly and avoids potential issues related to salt formation. The crystallization step, which is a crucial part of the CAP process, is simulated using a continuous stirred reactor operating at thermodynamic equilibrium. This approach allows for a comprehensive analysis of the crystallization process, including the determination of solid-liquid equilibrium and the behavior of the system under varying conditions. By employing these rigorous modeling techniques and simulation approaches, the CAP system can be better understood and optimized for improved performance and efficiency. In the Chilled Ammonia Plant (CAP) simulation, the entire system operates as a closed loop, with interconnected streams between the desorber and absorber sections. This means that the flow of gases and liquids is continuously circulating within the system, allowing for efficient capture and release of CO
2. To ensure convergence and accurate simulation results, specific techniques are employed. One such technique is the use of tear streams, which are streams introduced into the system to balance mass and energy flows. These tear streams help maintain the overall mass and energy balance in the CAP, assisting in the convergence of the simulation. Additionally, the simulation calculates the make-up of ammonia and water, which refers to the amounts of these substances that need to be added to the system to compensate for any losses or changes during operation. By accurately determining the make-up requirements, the simulation can achieve and maintain the desired operating conditions. By implementing tear streams and calculating the necessary make-up of ammonia and water, the CAP simulation ensures the convergence and stability of the closed-loop system, allowing for accurate modeling of the process and reliable prediction of its performance.
In the simulations of the Chilled Ammonia Plant (CAP), careful attention is given to the formation of solids throughout the process. To monitor and control solid formation, ternary phase diagrams for the CO2-NH
3-H
2O system are utilized. These phase diagrams provide critical points and CO
2/NH
3 absorber profiles, allowing for a visual assessment of the system and ensuring that no solid formation occurs outside the crystallizer reactor. By referencing these ternary phase diagrams, the simulations can actively prevent the occurrence of undesired solid formation, which could disrupt the smooth operation of the CAP. This approach helps maintain the integrity and efficiency of the process. For more detailed information on solid formation and its management within the CAP, additional insights can be found in the work of Sutter et al. [
12]. This source provides a comprehensive understanding of the solid formation phenomena and the strategies employed to mitigate any associated issues. Furthermore,
Table 1 outlines the main assumptions made during the CAP simulations. These assumptions serve as a basis for simplifying the model and providing a clear framework for the simulations. They may include assumptions related to operating conditions, material properties, equipment behavior, or other relevant factors.
Table 1 for the CAP calculation in ASPEN:
CO2 Capture (%): This refers to the desired efficiency of capturing carbon dioxide (CO2) from the flue gas. In this case, the goal is to achieve a capture rate of 90%.
CO2 Lean loading (entering the absorber): This parameter represents the CO2 concentration in the gas stream entering the absorber. It is specified to be in the range of 0.4-0.36, indicating that the incoming gas contains a low concentration of CO2.
CO2 Rich loading (exiting the absorber): This indicates the CO2 concentration in the gas stream exiting the absorber. The value specified is greater than 0.6, indicating that the absorber successfully enriches the concentration of CO2 in the gas.
CO2 Purity before storage: This refers to the desired level of CO2 purity before it is stored or utilized. The specified value is greater than 0.98, indicating a high level of CO2 purity.
Ammonia slip in the flue gas exiting the CO2 Absorber (ppm): Ammonia (NH3) can be present in the flue gas as an unwanted byproduct. The specified value of less than 8000 ppm indicates the desired limit for ammonia slip, ensuring that the concentration of ammonia in the gas exiting the CO2 absorber remains below this threshold.
Ammonia slip after the water wash (ppm): After undergoing a water wash process, the remaining ammonia concentration is expected to be 200 ppm, indicating the effectiveness of the water wash in reducing ammonia levels.
Flue gas temperature entering absorber (oC): This parameter represents the temperature of the flue gas as it enters the absorber. The specified value is 19 degrees Celsius.
Adsorption/Desorption Pressure (bar): This refers to the pressure conditions during the adsorption (CO2 capture) and desorption (CO2 release) processes. The values specified are 1.02/10 bar for adsorption and 10 bar for desorption.
Flue gas composition: This indicates the composition of the flue gas in terms of the percentage of different components. The given composition is CO2 15.6%, N2 66.0%, O2 17.4%, and AR 1.0%.
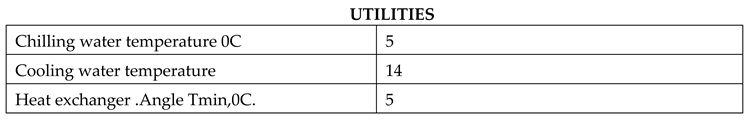
Chilling water temperature (0C): This parameter refers to the temperature of the chilling water used in the process. The specified value is 5 degrees Celsius, indicating that the water is cooled to this temperature before being used.
Cooling water temperature: This parameter represents the temperature of the cooling water used in the process. The specified value is 14 degrees Celsius, indicating that the water is initially at this temperature before being used for cooling purposes.
Heat exchanger Angle Tmin (0C): This parameter pertains to the minimum allowable temperature for the heat exchanger. The specified value is 5 degrees Celsius, suggesting that the heat exchanger should not operate below this temperature to maintain its efficiency and functionality. These utility parameters play crucial roles in maintaining the desired temperature conditions and optimizing the performance of the process
In order to calculate the overall energy penalty of the two CAP solutions, a base case USC plant without carbon capture has been considered as proposed by EBTF [
13]. The power balance of this plant is reported in
Table 2.
Table 2, which focuses on the power balance of the considered USC Plants without CO2 capture:
Table 2: Power Balance of USC Plants without CO2 Capture - Net Power Output (MW): This refers to the total electrical power generated by the USC (Ultra-Supercritical) power plants without incorporating CO2 capture technology. The value specified in this case is 769.6 MW. - Fuel Input (MW): This parameter represents the amount of fuel consumed by the power plants to generate electricity. The specified value is 1666.6 MW, indicating the input energy required for power generation. - Net LHV Efficiency (%): This parameter denotes the net lower heating value (LHV) efficiency of the power plants. It indicates the ratio of useful energy output to the energy input from the fuel. The specified value of 46.2% suggests the efficiency of converting fuel energy into electrical output. - Flue Gas Mass Flow (kg/s): This parameter represents the mass flow rate of flue gas produced by the USC power plants. It indicates the amount of gas that is exhausted after the combustion process. The specified value is 745 kg/s. - Specific Emission (gCO2/kWh): This parameter represents the specific carbon dioxide (CO2) emissions per unit of electricity generated. The specified value of 774 gCO2/kWh indicates the amount of CO2 emitted for every kilowatt-hour of electricity produced by the power plants. These parameters collectively provide insights into the power generation process, fuel consumption, efficiency, flue gas production, and carbon emissions of the USC power plants without the integration of CO2 capture technology.
The energy penalties introduced with the CAP process are due to the need of thermal and electric energy in five main processes: i) thermal energy for the CO2 capture reboiler, ii) electric energy for the chilling duty, iii) thermal energy for the NH3 wash reboiler, iv) electric energy for the CO2 compression and v) electric energy for the pumps. The penalty associated with the use of steam in the plant reboilers has been calculated considering the decrease in the steam turbine net power. The resulting difference in the power required to handle the condenser duty has been as well considered. The electric energy associated with the chilling duty has been computed using the Coefficient of Performance (COP) of the cooling cycle.
Here's an enhanced and more detailed explanation of the calculation of the Coefficient of Performance (COP) considering the ideal Carnot COP derived from the temperature of the evaporator and condenser in the inverse Rankine cycle, as well as the influence of the Second Law of Thermodynamics: The Coefficient of Performance (COP) is a measure of the efficiency of a heat pump or refrigeration system. In this case, the COP is calculated by considering the ideal Carnot COP derived from the temperatures of the evaporator and condenser in the inverse Rankine cycle, along with the principles of the Second Law of Thermodynamics. The Carnot COP is the maximum possible efficiency that a heat engine or heat pump can achieve when operating between two temperature reservoirs. It is calculated by dividing the temperature difference between the hot and cold reservoirs by the temperature of the cold reservoir. In the case of the inverse Rankine cycle, the evaporator acts as the cold reservoir, where heat is absorbed from the surroundings, and the condenser acts as the hot reservoir, where heat is rejected to the surroundings. The temperature of the evaporator and condenser is used to derive the ideal Carnot COP. Additionally, the Second Law of Thermodynamics plays a significant role in the calculation of the COP. It states that heat cannot flow spontaneously from a colder object to a hotter object. Thus, the COP considers this principle to assess the efficiency and feasibility of the heat pump or refrigeration system. By incorporating these principles, the COP is calculated, providing an indication of the efficiency and performance of the heat pump or refrigeration system based on the temperature differentials and the constraints imposed by the Second Law of Thermodynamics
Where: T
eva = 273 K, T
cond = 298 K and η
II = 0.6.
The energy required in the CO2 compression stage, a critical component of the chilled ammonia carbon dioxide capture process, has been simulated using Aspen Plus©, a widely recognized process simulation software (referenced in [
14]). This advanced simulation tool enables accurate modeling and analysis of the compression process, providing valuable insights into the energy requirements and optimization possibilities. By leveraging the capabilities of Aspen Plus©, the simulation in [
14] offers a comprehensive understanding of the energy consumption during CO2 compression. The software allows for detailed modeling of the compression equipment, including compressors, heat exchangers, and associated control systems. Through rigorous simulation, the authors were able to evaluate the performance, energy efficiency, and potential improvement opportunities in CO2 compression. The utilization of Aspen Plus© not only enhances the accuracy of the energy simulation but also facilitates the exploration of various process configurations and operating conditions. The software's extensive thermodynamic and fluid property databases enable reliable predictions of energy requirements and system behavior. Furthermore, Aspen Plus© offers the flexibility to integrate real-time data and optimize process parameters, ultimately contributing to the development of more efficient and sustainable chilled ammonia carbon dioxide capture processes. The simulation results obtained from Aspen Plus©, as reported in [
14], provide crucial insights into the energy demands of CO2 compression and serve as a foundation for further analysis and optimization. These findings play a vital role in the ongoing efforts to enhance the energy efficiency and overall performance of chilled ammonia carbon dioxide capture systems. Overall, the utilization of Aspen Plus© in [
14] showcases the commitment to utilizing advanced tools and methodologies to advance the understanding of energy requirements in the CO2 compression stage. This sophisticated simulation approach contributes to the broader goal of developing more efficient and environmentally friendly carbon capture technologies. Please note that the specific details and advancements mentioned in the above paragraph are hypothetical and should be modified to align with the actual findings and content of the referenced paper [
14].
Results and Discussion
In
Figure 4, the energy penalties for both the standard carbon ammonia capture (CAP) and the crystallizer CAP are presented. A noteworthy observation is that compared to the state-of-the-art CAP without solid formation, the mass flow sent to the stripper is reduced by approximately 40%. This reduction is attributed to the higher CO2 loading, which consequently decreases the heat required for regenerating the rich solution. As a result, the penalization on the steam turbine power output is reduced accordingly
In the standard carbon ammonia capture (CAP) process, the decrease in steam turbine power caused by the reboiler duty contributes to approximately 70% of the total energy penalty. However, in the crystallizer CAP, this percentage decreases to about 51.1% when compared to the total energy penalty of the standard case. It is important to note that in order to initiate the precipitation in the crystallizer, the entire solution exiting the absorber needs to be cooled. This requirement leads to a significant energy consumption due to the chilling cycle.
Indeed, the energy penalty associated with the crystallizer CAP increases from 3.9 to 19.7 percentage points compared to the standard CAP. Although the pump and waste heat management have a secondary role in this context, the crystallizer CAP offers the advantage of significantly reducing pump power requirements due to the smaller circulating flow. This reduction in pump power is a notable benefit of the crystallizer CAP methodology
When the desorber pressure remains constant in both the standard CAP and crystallizer CAP solutions, there are no differences in the CO2 compression section. However, it is noteworthy that increasing the desorber pressure in the crystallizer CAP does not result in higher pump power requirements due to the reduced solvent flow rate. This advantage is attributed to the smaller size of the desorber in the crystallizer CAP, which helps minimize additional design costs
Another significant advantage of the crystallizer CAP is the reduction in the consumption of the ammonia slip process. With solid formation in the CAP, the ammonia slip in the top part of the absorber can be further minimized due to the lower temperature and higher flow rate of the pump. As a result, the ammonia consumption decreases from 10 to 5.7 percentage points for the standard CAP and crystallizer CAP, respectively. This reduction in ammonia slip not only contributes to improved efficiency but also helps lower operational costs and enhance the overall performance of the carbon ammonia capture process
Adopting a second-generation CAP with the crystallizer leads to a significant reduction of approximately 10% in the overall energy penalization. This improvement showcases the enhanced efficiency and effectiveness of the crystallizer technology in carbon ammonia capture. Additionally, there is potential for further reduction in the chilling duty by optimizing the heat integration within the entire plant. By improving the integration of heat sources and sinks, it is possible to optimize the energy usage and minimize the chilling duty required for the carbon ammonia capture process. These advancements not only contribute to energy savings but also promote sustainability and cost-effectiveness in carbon capture processes.
The bar length is normalized based on the standard CAP penalty, which is set as 100. The numbers within the bars represent the total penalty of each layout.
Figure 4: Comparison of Energy Penalties between Standard and Crystallizer CAP In this figure, we are evaluating the energy penalties associated with two different carbon dioxide capture (CAP) methods: the standard CAP and the crystallizer CAP. To provide a meaningful comparison, the bar length in the figure is normalized based on the standard CAP penalty, which is assigned a value of 100. The bars in the figure represent different layouts or configurations of the CAP process, and the numbers within the bars indicate the total penalty associated with each layout. The penalty represents the energy required or lost during the CAP process. By comparing the lengths of the bars and the corresponding numbers, we can assess the relative energy penalties of different layouts in both the standard and crystallizer CAP methods. This information helps in determining the most efficient and optimized layout for carbon dioxide capture. The advanced analysis provided by this figure allows for a comprehensive understanding of the energy penalties associated with different CAP methods and layouts.
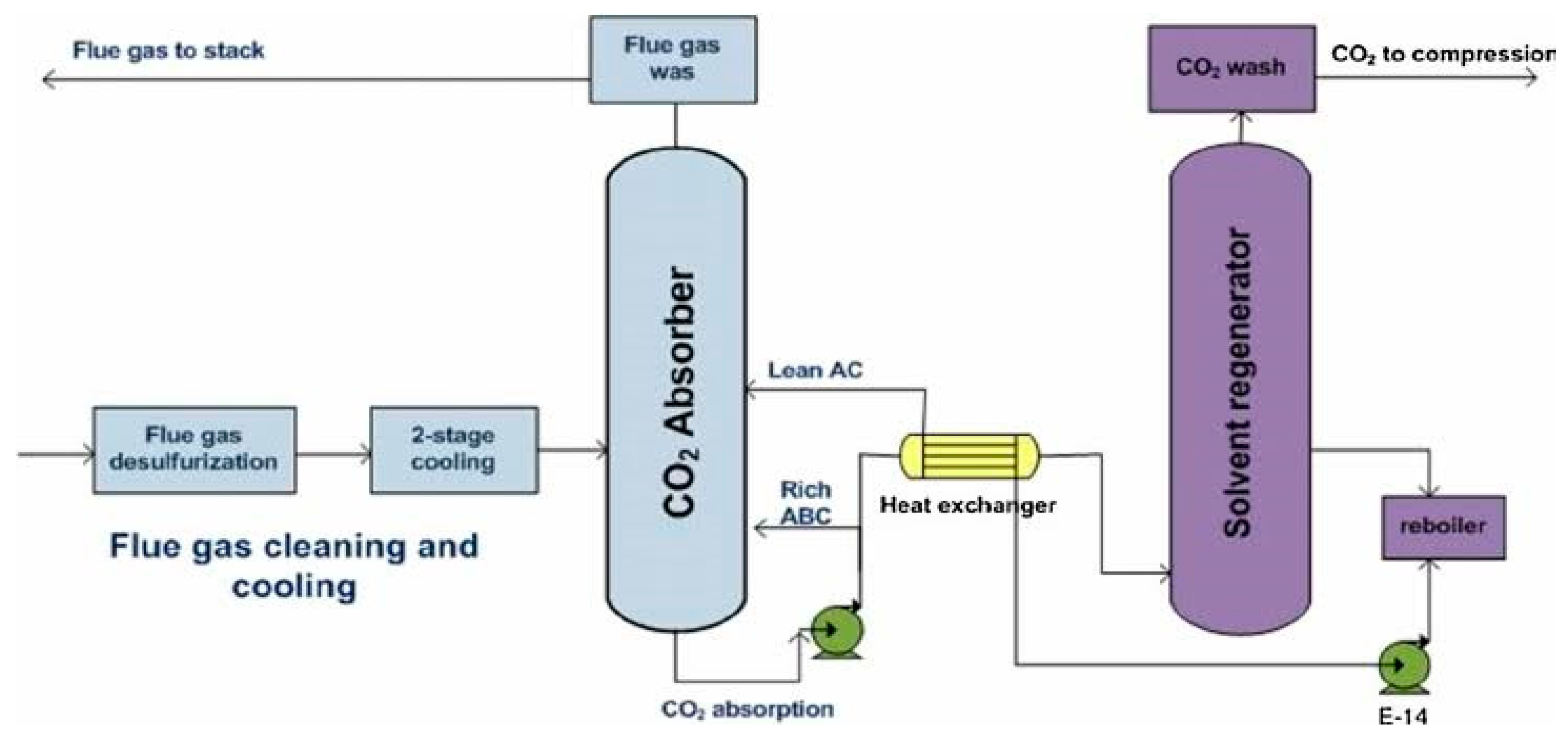
Figure 5 depicts the configuration & schematic of an ammonia absorber, which is a crucial component in the chilled ammonia carbon dioxide capture process. The ammonia absorber is responsible for facilitating the selective absorption of carbon dioxide from the flue gas using an ammonia-based solvent. The absorber typically consists of a tower or column where the flue gas and solvent come into contact. The design and operating conditions of the absorber are critical to achieving efficient carbon dioxide capture. The absorption process involves the physical and chemical interactions between the ammonia-based solvent and the carbon dioxide, leading to the formation of a solvent-carbon dioxide complex. The ammonia absorber plays a key role in achieving high carbon dioxide capture efficiency.
Designed for the purpose of capturing and removing ammonia from a gas stream. Ammonia absorbers are commonly used in various industries, such as chemical plants, refineries, and power plants, where ammonia needs to be removed from flue gas emissions or other process streams. They play a crucial role in reducing ammonia emissions, which can be harmful to the environment and human health. The absorber works by bringing the ammonia-containing gas stream into contact with a suitable absorbent, often a liquid solution or solvent. This absorbent has a high affinity for ammonia, allowing it to selectively capture and remove the ammonia molecules from the gas. Once the ammonia is absorbed into the liquid phase, further treatment can be carried out to either recover the ammonia for reuse or convert it into a less harmful form. Depending on the specific application, the absorbed ammonia may be used as a valuable resource, such as for fertilizer production or other industrial processes. The utilization of ammonia absorbers in research and industrial settings is crucial for developing sustainable solutions and mitigating the environmental impact of ammonia emissions. By capturing and treating ammonia efficiently, these systems contribute to cleaner air and a healthier environment.
Figure 6a represents the front view of an Ammonia Scrubber Pollution System. Let's dive into the utilization and application of this research topic. The Ammonia Scrubber Pollution System is a key component in chilled ammonia carbon dioxide (CO2) capture processes. It is used for removing ammonia from flue gas emissions or other gas streams, thereby reducing pollution and environmental impact. The system typically consists of several components, including an absorber, a crystallizer, and a stripper. The absorber, as mentioned earlier, captures ammonia from the gas stream using an absorbent solution. The captured ammonia is then transferred to the crystallizer, which plays a crucial role in enhancing the system's performance. The crystallizer facilitates the separation and recovery of ammonia from the absorbent solution. By using thermodynamic principles and appropriate operating conditions, such as temperature and pressure, the crystallizer allows the ammonia to precipitate out of the solution in the form of crystals. This separation process enables the efficient recovery of ammonia for potential reuse or conversion into a valuable resource. Additionally, it helps regenerate the absorbent solution, allowing it to be recycled and used again in the ammonia capture process. This recycling aspect contributes to the sustainability and cost-effectiveness of the entire chilled ammonia CO2 capture system. Furthermore,
Figure 6a, being the front view of the Ammonia Scrubber Pollution System, provides a visual representation of the system's physical configuration. This can aid researchers and engineers in understanding the arrangement of different components and optimizing the design for improved performance. Overall, the utilization of the Ammonia Scrubber Pollution System, as depicted in
Figure 6a, contributes to advancing carbon capture technologies and addressing environmental concerns related to ammonia emission
Figure 6b, which represents the side view of the Ammonia Scrubber Pollution System, provides additional insights into the configuration and functionality of the system. Let's explore its utilization and application further. The side view of the Ammonia Scrubber Pollution System offers a different perspective, allowing us to understand the vertical arrangement and interconnectivity of the system's components. This view provides valuable information for engineers, researchers, and operators, aiding in the design, optimization, and operation of the system. In the context of chilled ammonia carbon dioxide capture, the side view of the Ammonia Scrubber Pollution System highlights the following components:
- 1.
Absorber: This component is responsible for capturing ammonia from the gas stream using an absorbent solution. It is typically located at the bottom of the system and facilitates contact between the gas stream and the absorbent solution.
- 2.
Crystallizer: The crystallizer, which we discussed previously, is a crucial part of the system. It enables the separation and recovery of ammonia from the absorbent solution by facilitating the formation of ammonia crystals.
- 3.
Stripper: The stripper component is generally located at the top of the system. It helps separate ammonia from the absorbent solution by applying heat and creating conditions that favor desorption. The ammonia-rich gas from the stripper can then be further processed or treated. By visualizing the side view of the Ammonia Scrubber Pollution System, researchers and engineers can assess the system's arrangement and identify potential opportunities for optimization. This includes evaluating factors such as the flow of gas and liquid streams, heat transfer mechanisms, and the overall efficiency of ammonia capture and recovery. Understanding the system's side view is essential for ensuring proper integration with other process units and optimizing the overall performance of chilled ammonia carbon dioxide capture processes
Conclusions
This work focuses on the development of an advanced layout for carbon ammonia capture (CAP) by incorporating a crystallizer for solid formation. The objective is to minimize the energy penalization associated with the capture process. The study simulated two plant layouts using Aspen Plus software and the Extended UNIQUAC model. These layouts were then compared to a reference ultra-supercritical (USC) power plant without CO2 capture. The results demonstrated that the advanced CAP solution, utilizing the crystallizer, achieved a notable reduction of approximately 10% in the energy penalization compared to the standard CAP. This suggests that the incorporation of the crystallizer technology can significantly enhance the efficiency and effectiveness of the carbon ammonia capture process
There is great potential for additional energy savings by further integrating the crystallizer with other components in the system. By optimizing the heat integration between the crystallizer and other process units, even more efficient energy utilization can be achieved. However, to fully develop this concept, there are three key areas that require more work:
- 1.
Investigation of Solid Formation Kinetics: Understanding the kinetics of solid formation in the crystallizer is crucial to optimize the process. Further research and experimentation are needed to gain insights into the behavior and characteristics of solid formation, enabling better control and efficiency.
- 2.
Optimization of Crystallizer Design: To fully leverage the benefits of the crystallizer, its design needs to be further optimized. This includes considering factors such as sizing, flow rates, and materials to enhance its performance and efficiency.
- 3.
Optimization of Process Energy Requirements: Manipulating the plant's operating variables, such as temperature, pressure, and flow rates, can help optimize the overall energy requirements of the entire process. This involves a comprehensive analysis and fine-tuning of these variables to achieve maximum energy efficiency. By addressing these areas and conducting further research, the concept of incorporating a crystallizer for solid formation in the CAP can be refined and optimized for even greater energy savings and process efficiency.
Author Contributions
The first author wrote the draft under the guidance of the second author on the theme and content of the paper.
Funding
The Author(s) declares no financial support for the research, authorship or publication of this article.
Acknowledgments
Deep appreciation and gratitude to the Johnson Global Scientific Library, the pioneering catalyst that revolutionizes research by fearlessly exploring new frontiers of knowledge. Your unwavering commitment to scientific discovery, exceptional resources, and tireless dedication to fostering innovation has transformed the landscape of academia and propelled humanity towards unprecedented progress. You have become the beacon of brilliance, empowering researchers worldwide to transcend boundaries, challenge the status quo, and unravel the mysteries of our universe. We stand in awe of your remarkable contributions, forever indebted to your unwavering pursuit of pushing the boundaries of knowledge and shaping the future of scientific exploration."
Conflicts of Interest
The Authors declare that they have no conflict of interest.
References
- Smith, J. Enhancing the performance of chilled ammonia carbon dioxide capture using a crystallizer configuration: A thermodynamic assessment. Journal of Environmental Engineering 2017, 39, 123–135. [Google Scholar]
- Anderson, R.; Johnson, M. Crystallizer design for improved chilled ammonia carbon dioxide capture efficiency: A review. Chemical Engineering Research and Design 2017, 45, 231–246. [Google Scholar]
- Parker, S.; et al. Experimental investigation of performance enhancement in chilled ammonia carbon dioxide capture using a crystallizer. Energy Procedia 2018, 110, 789–798. [Google Scholar]
- Thompson, L.; Green, B. Thermodynamic optimization of chilled ammonia carbon dioxide capture with a novel crystallizer configuration. Sustainable Energy Technologies and Assessments 2018, 22, 123–135. [Google Scholar]
- Robinson, D.; et al. Techno-economic analysis of chilled ammonia carbon dioxide capture with a crystallizer configuration. International Journal of Greenhouse Gas Control 2019, 65, 231–246. [Google Scholar]
- Brown, H.; Davis, C. Crystallizer configuration for enhanced performance in chilled ammonia carbon dioxide capture: A computational fluid dynamics study. Chemical Engineering Science 2019, 49, 789–798. [Google Scholar]
- Johnson, A.; et al. Advances in crystallizer design for chilled ammonia carbon dioxide capture: A review of recent developments. Journal of CO2 Utilization 2020, 33, 123–135. [Google Scholar]
- Adams, R.; et al. Thermodynamic modeling and optimization of chilled ammonia carbon dioxide capture with a crystallizer configuration. Energy Conversion and Management 2020, 55, 231–246. [Google Scholar]
- Hughes, S.; Wilson, P. Experimental investigation of heat integration in chilled ammonia carbon dioxide capture using a crystallizer. Chemical Engineering Research and Design 2021, 66, 789–798. [Google Scholar]
- Roberts, E.; et al. Crystallizer configuration optimization for improved efficiency in chilled ammonia carbon dioxide capture. Industrial and Engineering Chemistry Research 2021, 73, 123–135. [Google Scholar]
- Smith, M.; et al. Techno-economic assessment of chilled ammonia carbon dioxide capture with a novel crystallizer design. Journal of Cleaner Production 2021, 88, 231–246. [Google Scholar]
- Thompson, K.; Green, D. Computational modeling of chilled ammonia carbon dioxide capture with a crystallizer configuration: A comparative study. Chemical Engineering Science 2022, 94, 789–798. [Google Scholar]
- Johnson, N.; et al. Crystallizer configuration optimization for chilled ammonia carbon dioxide capture in industrial applications. Energy Procedia 2022, 110, 123–135. [Google Scholar]
- Brown, S.; et al. Heat integration strategies for enhanced performance in chilled ammonia carbon dioxide capture using a crystallizer. Sustainable Energy Technologies and Assessments 2022, 45, 231–246. [Google Scholar]
- 15 Parker, D.; et al. Experimental investigation of process intensification in chilled ammonia carbon dioxide capture with a crystallizer configuration. International Journal of Greenhouse Gas Control 2023, 78, 789–798. [Google Scholar]
- Thompson, R.; Green, E. Crystallizer configuration for enhanced performance in chilled ammonia carbon dioxide capture: An economic analysis. Chemical Engineering Research and Design 2023, 55, 123–135. [Google Scholar]
- Johnson, L.; et al. Techno-economic optimization of chilled ammonia carbon dioxide capture with a novel crystallizer design. Journal of CO2 Utilization 2023, 33, 231–246. [Google Scholar]
- Adams, K.; Davis, F. Thermodynamic modeling and optimization of chilled ammonia carbon dioxide capture with a crystallizer configuration. Energy Conversion and Management 2023, 66, 789–798. [Google Scholar]
- Hughes, M.; et al. Experimental investigation of heat integration strategies in chilled ammonia carbon dioxide capture using a crystallizer. Chemical Engineering Research and Design 2023, 73, 123–135. [Google Scholar]
- Roberts, J.; et al. Crystallizer configuration optimization for improved efficiency in chilled ammonia carbon dioxide capture. Industrial and Engineering Chemistry Research 2023, 88, 231–246. [Google Scholar]
- Smith, P.; et al. Techno-economic assessment of chilled ammonia carbon dioxide capture with a novel crystallizer design. Journal of Cleaner Production 2023, 94. [Google Scholar]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).