1. Introduction
Crassulacean acid metabolism (CAM) is an important photosynthetic pathway in addition to C
3 and C
4, which often exists in species with CO
2 or water-limited environments, such as aquatic habitats, tropical rainforests and arid regions [
1], so plants with CAM pathway typically have higher water use efficiency and greater drought tolerance. Recent studies have revealed the multiple independent origins of the CAM pathway, which have existed in 18,000 species among 400 genera of 40 families minimally [
2,
3,
4], accounting for about 6% [
5,
6,
7] to 7% [
8,
9] vascular plants. Of which about 10% Orchidaceae exercise CAM pathway [
10], with the most CAM species diversity.
Dendrobium Sw. is the second largest genus of Orchidaceae [
11], containing rich CAM resources. However,
Dendrobium CAM plants identification is mainly based on the determination of carbon isotope(δ
13C) value [
12,
13,
14], which cannot distinguish C
3 plants with CAM pathway. Because some C
3 plants subjected to external influences (e.g., drought stress) also activate the CAM pathway, such plants were defined as facultative CAM plants [
2,
15,
16]. So far, studies on the identification of
Dendrobium facultative CAM plants have lagged relatively behind, only a few species have been reported:
D. officinale, D. nobile, D. hercoglossum, D. moniliforme, D. heterocarpum, D. chrysotoxum, D. wardianum, D. findlayanum and two cultivars (
D. nobile 'V1',
D. nobile 'V4') were identified as facultative CAM under drought stress [
17,
18,
19,
20,
21,
22,
23,
24,
25]. Previous studies have shown that the CAM pathway has independently evolved at least eight times in
Dendrobium [
14]. However, due to the lack of identification of different photosynthetic pathways, especially the facultative CAM, the number of CAM origins is more abundant than expected, we still know little about the resources and origins of
Dendrobium CAM.
Chloroplast, the power factories for plant growth and development, is the most critical organelles for plant photosynthesis. In recent years, 153 complete chloroplast genomes of
Dendrobium have been published [
26], research on the them have revealed that: (i) independent absence of
ndh genes in diferent
Dendrobium lineage, e.g. Niu et al. [
27] compared 30
Dendrobium plastids and found that
ndhC, I and
K were lost in all species,
ndhA and
H were also lost and
ndhF was pseudogenized in
D. huoshanense and
D. moniliforme. (ii) the evolution rates of the non-coding regions were diversifed. For instance, different
Dendrobium species combinations showed inconsistent patterns in the top10 mutational hotspots [
27,
28]. However, there is little research on whether these differences are related to the presence of CAM pathways: Xue et al. [
25] compared the chloroplast genomes of
Dendrobium with multiple photosynthetic pathways and concluded that different photosynthetic pathways led to the non-proportional evolution of the chloroplast genomes of
Dendrobium. But there remains a lack of information about the relationship between the evolution of the CAM pathway and the variation among
Dendrobium chloroplast genomes.
Due to the lack of identification of different photosynthetic pathways, there are few studies on the influence of CAM pathway on the genomic evolution of Dendrobium plasmid, and little is known about the origin of CAM pathway. Therefore, in this study, we analyzed the chloroplast genomes of 10 Dendrobium species, including whole-genome sequencing of six species and five previously published genome sequences in NCBI. Different photosynthetic pathways were identified using δ13C values, net photosynthetic rates (Pn) and titratable acidity values. The plastid structure, mutation hotspots, sequence differences and repeat regions were characterized, variable sites were identified, and the phylogenetic tree was constructed. Then we compared the relationship between different photosynthetic pathways and chloroplast genome variation in Dendrobium. The aim of this study was to clarify the chloroplast genome characteristics, enrich CAM plants genome data and provide genetic information from different perspectives for further systematic study of Dendrobium.
2. Results
2.1. Identification of Multiple Photosynthetic Pathways in Dendrobium
The photosynthetic pathways of 10
Dendrobium species were identified in this study. Leaf δ
13C values of them ranged from -30.0‰ (
D. capituliflorum) to -13.6‰ (
D. delacourii). According to previous studies [
29], -20‰ was used as a cutoff to distinguish C
3 plants from constitutive CAM plants, therefore,
D. capituliflorum, D. fimbriatum, D. jiajiangense, D. aphrodite, D. cumulatum, D. fanjingshanense, D. stuposum were identified as C
3 plants preliminarily;
D. aphyllum,
D. delacourii and
D. parishii were identified as constitutive CAM plants (
Table 1).
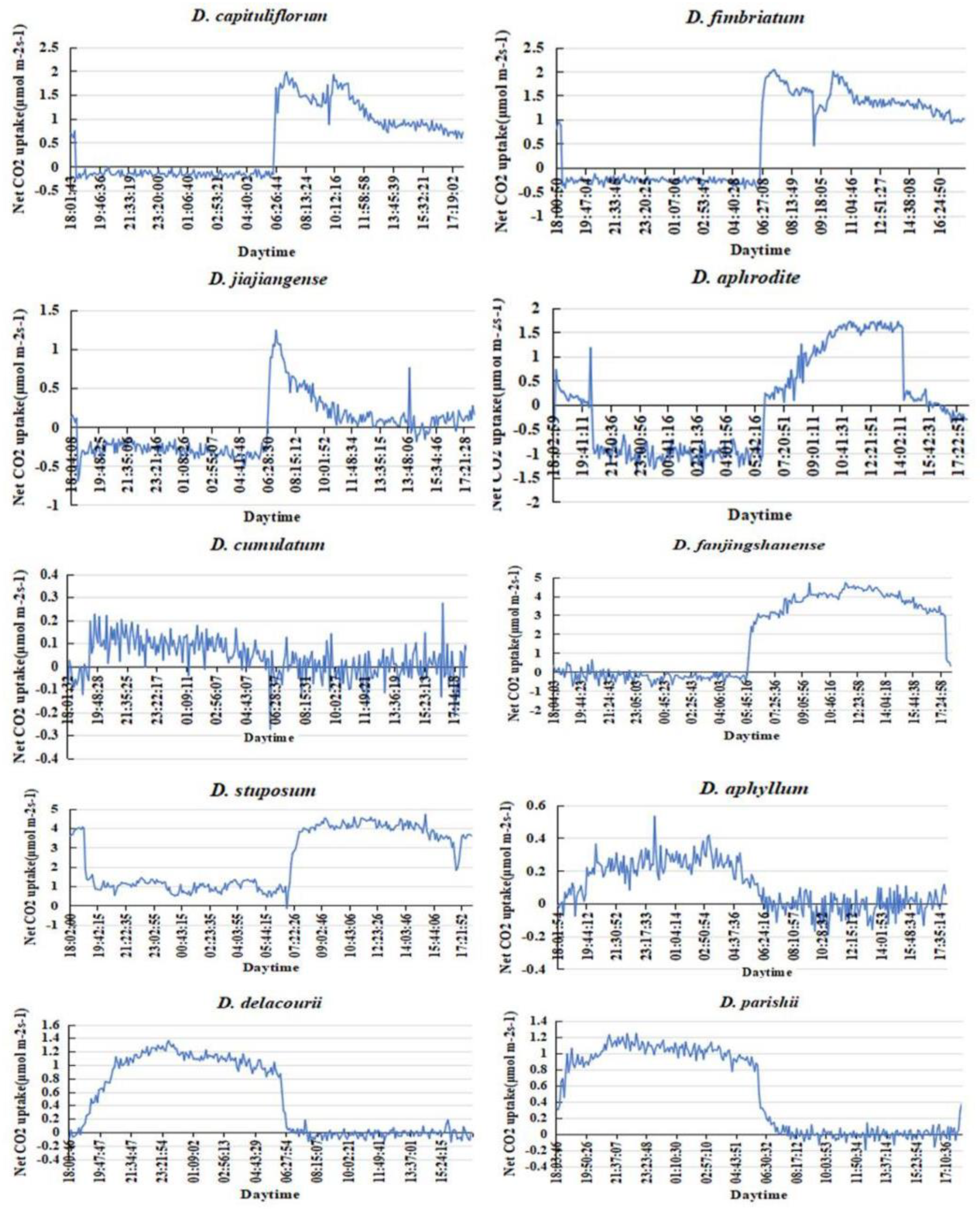
Combined with the net photosynthetic rates (Pn) curve, the obligatory C
3 and facultative CAM in C
3 plants were distinguished. The Pn of
D. aphyllum, D. delacourii and
D. parishii were negative during the day and positive at night, which showed a very significant trend at night, further verified that they were constitutive CAM plants. Among the C
3 plants(δ
13C<-20‰),
D. cumulatum and
D. stuposum had Pn expanded Zero during both day and night, can be determined as facultative CAM. The rest of C
3 plants, their Pn were positive during the day and negative at night, with a non-significant trend at night, which is exact opposite of the constitutive CAM plants (
Figure 1).
Further combined with titratable acid values,all three constitutive CAM species(δ
13C≥-20‰) had significant day-night titratable acid differences. C
3 plants(δ
13C<-20‰) of
D. capituliflorum, D. fimbriatum and
D. jiajiangense had non-significant day-night titratable acid differences, which were determined to be obligate C
3 plants, while
D. aphrodite, D. cumulatum, D. fanjingshanense and
D. stuposum showed significant differences, which clearly were facultative CAM plants (
Table 1).
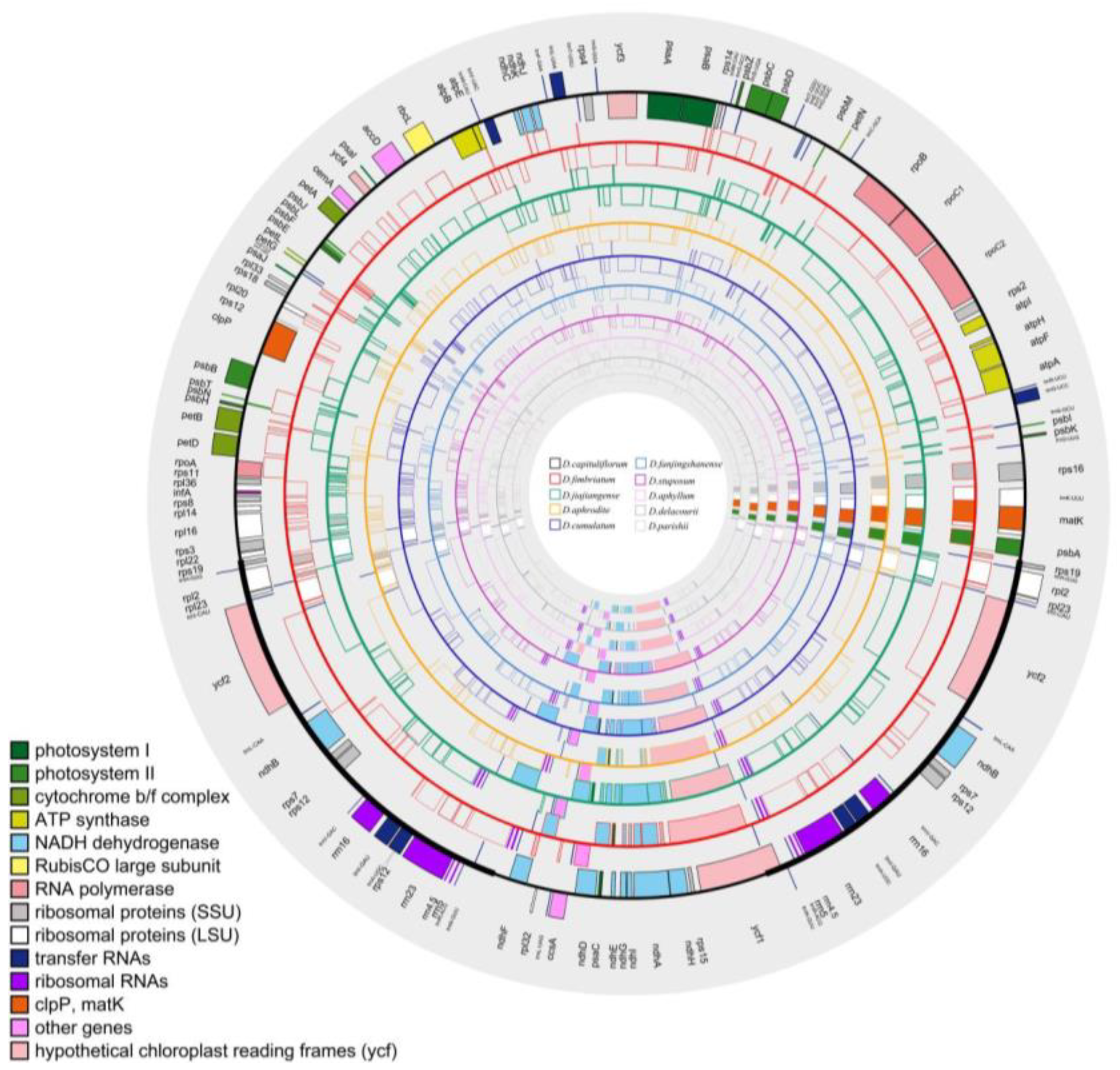
2.2. Characteristics of the Plastome
The chloroplast genomes of the 10
Dendrobium species, all of which showed a typical double-linked tetrad structure, consisted of a small single-copy region (SSC), a large single-copy region (LSC), and two inverted repeat regions (IRa and IRb) (
Figure 2).The SSC ranged from 14,328 bp to 18,479 bp for the obligate C
3, from 13,689 bp to 18,650 bp for the facultative CAM, and from 13,550 bp to 14,396 bp for the constitutive CAM; the LSC ranged from 84,763 bp to 87,938 bp for obligate C
3, from 84,990bp to 87,657 bp for the facultative CAM, and from 84,703 bp to 85,647bp for the constitutive CAM; the IRs ranged from 26,291 bp to 27,081 bp for the obligate C
3, from 26,029 bp to 27,107 bp for the facultative CAM, and from 26,026 bp to 27,040 bp for the constitutive CAM (
Table 2).
The genome sizes of obligate C
3 ranged from 151,673 bp to 160,010 bp, and the GC content ranged from 37.2% to 37.6%; facultative CAM plants ranged from 152,108 bp to 160,375 bp, and the GC content ranged from 37.1% to 37.5%; constitutive CAM plants ranged from 151,689 bp to 152,487 bp, and the GC content ranged from 37.5% to 37.6%; the GC content (43.1%-43.5%) in IRs of 10
Dendrobium species were higher than that in LSC (34.8%-35.3%) and SSC (30.1%-30.9%) (
Table 2).
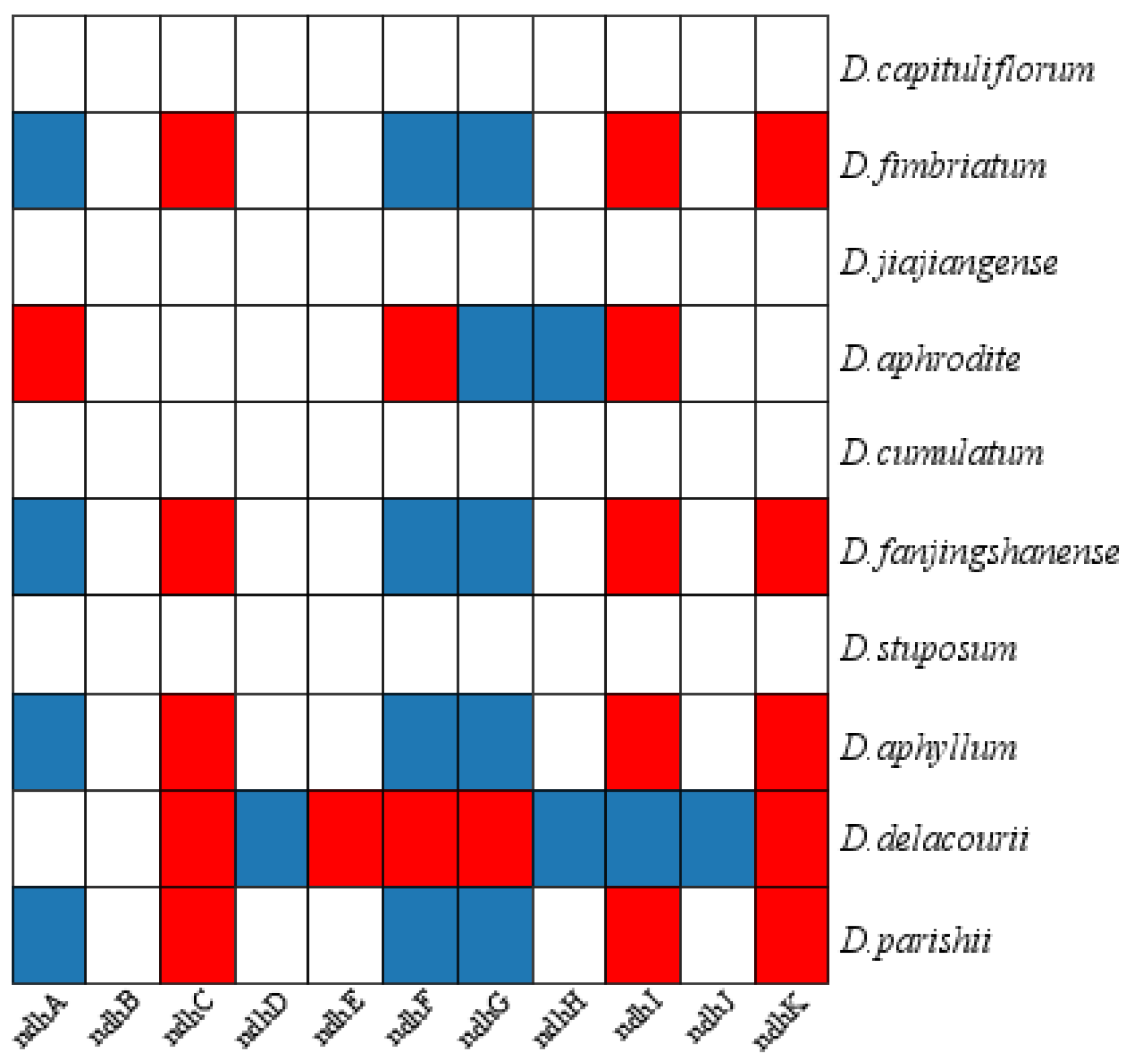
A total of 127-132 genes were encoded (
Supplementary Figure S1), and the difference was the number of
ndh genes. Among the obligate C
3 plants, 11 complete
ndh genes were present in
D. capituliflorum and
D. jiajiangense, while
D. fimbriatum lost
ndhC, ndhI, ndhK and was pseudogenticized in
ndhA, ndhF, and
ndhG; Among the facultative CAM plants, 11 complete
ndh genes were present in
D. cumulatum and
D. stuposum, while
D .aphrodite lost
ndhA, ndhF, ndhI and was pseudogenized with
ndhG and
ndhH, D. fanjingshanense lost
ndhC, ndhI, ndhK and was pseudogenized with
ndhA, ndhF, and
ndhG; Among the constitutive CAM plants, 11 complete
ndh genes were not present,
D. aphyllum and
D. parishii lost
ndhC, ndhI, ndhK and
ndhA, ndhF, ndhG pseudogenized,
D. delacourii lost
ndhC, ndhE, ndhF, ndhG, ndhK and
ndhD, ndhH, ndhI, ndhJ pseudogenized (
Figure 3,
Supplementary Table S2).
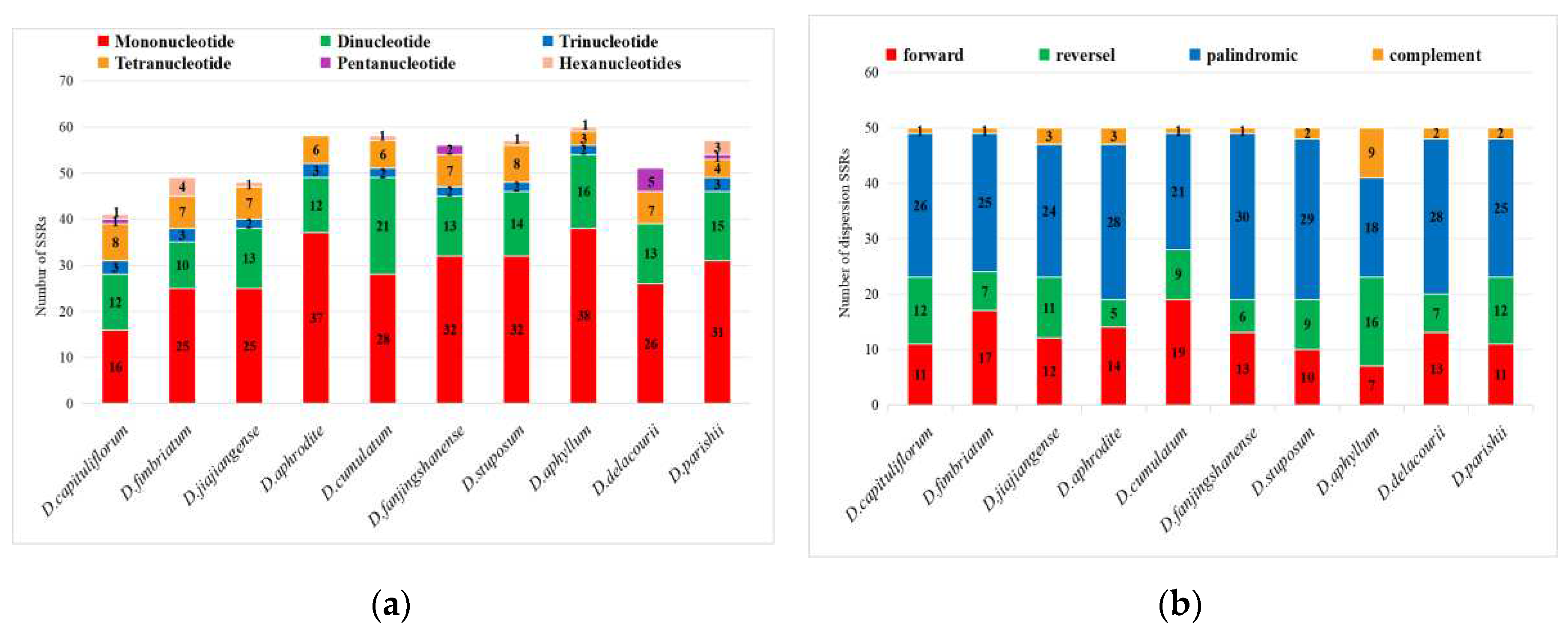
2.3. Repeated Analysis
The total number of SSRs ranged from 41 to 60. Mononucleotide, dinucleotide, and tetranucleotide were the nucleotide types common to the 10
Dendrobium species; trinucleotide did not appear in
D. delacourii, pentanucleotide did not appear in
D. fimbriatum, D. jiajiangense, D. aphrodite, D. cumulatum, D. stuposum, D. aphyllum, and hexanucleotide did not appear in
D. aphrodite, D. fanjingshanense, D. aphrodite (
Figure 4a).
The number of dispersions SSRs of the 10
Dendrobium species varied little, and all of them contained four types of repeat sequences, including forward, reverse, palindrome and complementary; the proportion of palindrome was the highest among the four types of repeat sequences, and complementary accounted for the smallest proportion (
Figure 4b).
2.4. Plastome Sequence Divergence and Barcoding Investigation
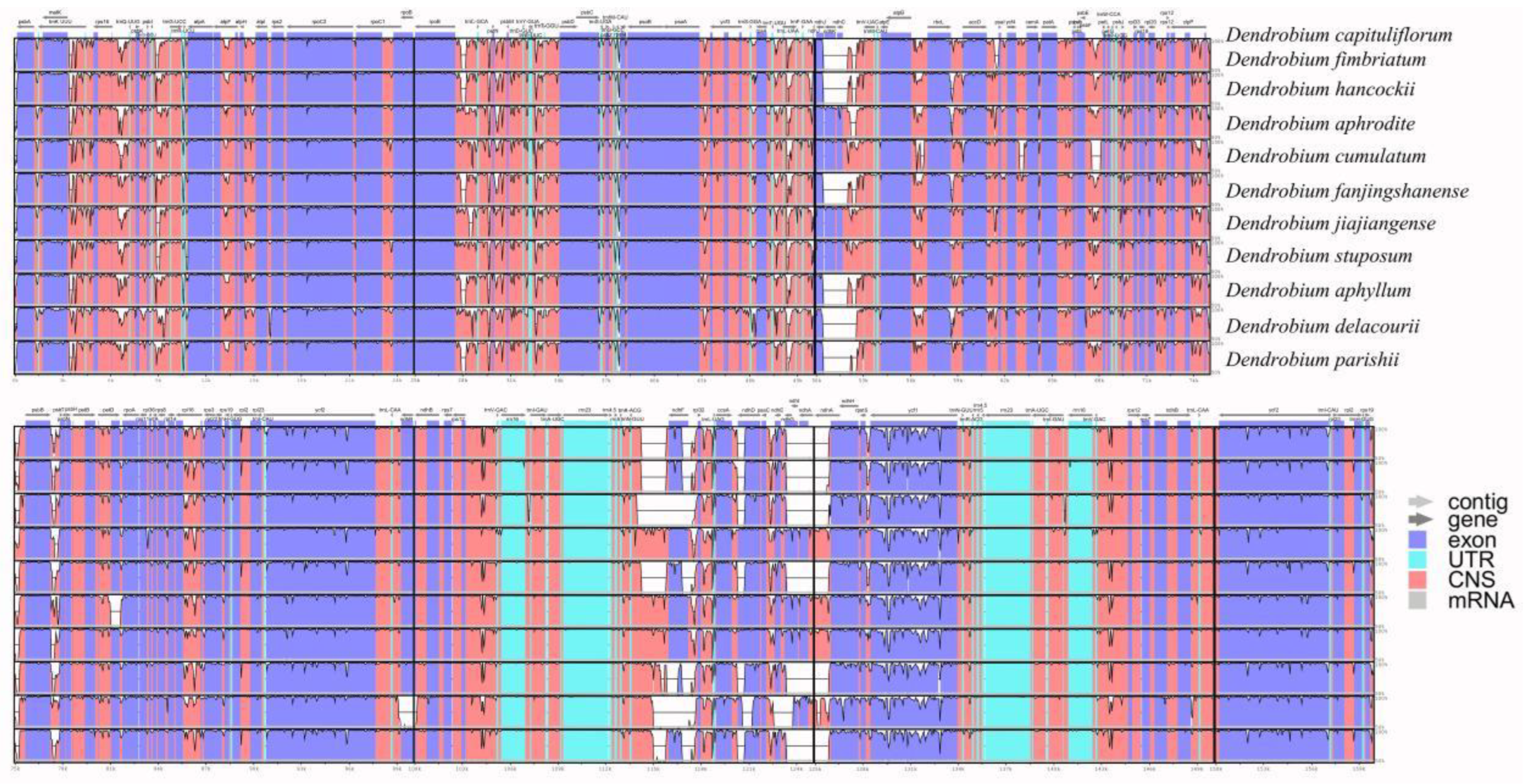
Visualization of chloroplast genome sequence comparison results of 10
Dendrobium species using the online tool mVISTA, which showed the non-coding and coding regions of the 10
Dendrobium chloroplast genomes were almost the same (
Figure 5), and the LSC (from
ndhJ to
trnVUAC, psbF to
petL) region and the SSC (from
trnNGUU to
ndhA) region were found to have the least similarity than the IR regions. Given these results, there are many regions to develop DNA barcodes to differentiate
Dendrobium species.
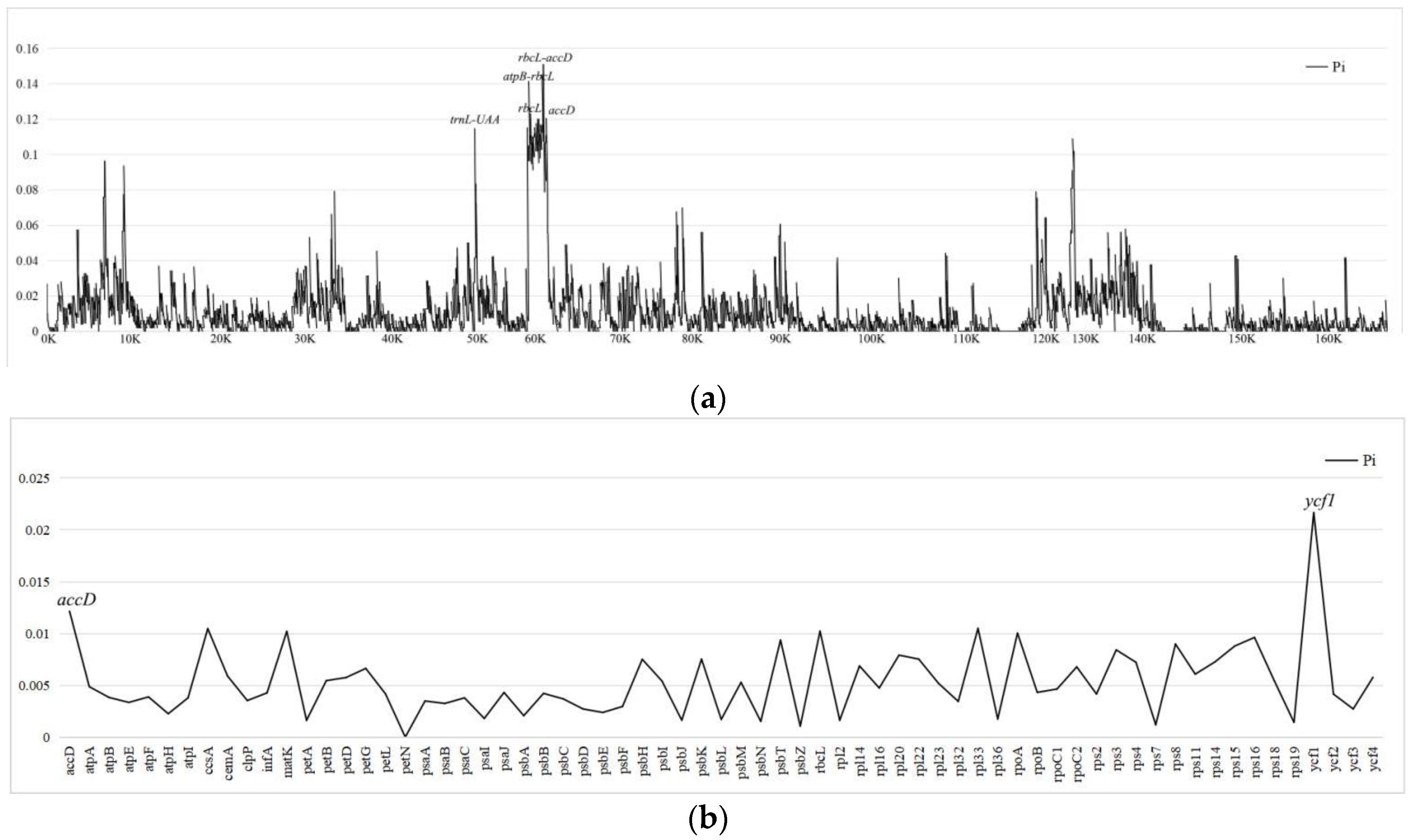
To further analyze mutational hotspots in
Dendrobium plastids, we analyzed nucleotide diversity (Pi) using DnaSP6 for genome-wide comparisons (
Figure 6,
Supplementary Tables S3 and S4). The nucleotide diversity (Pi) values of the 10 plastomes ranged from 0 to 0.15089, We selected 5 mutational hotspots with Pi>0.10 (
rbcL-accD>atpB-rbcL>rbcL>trnLUAA>accD) as candidate barcodes. In protein-coding genes the results showed that at the cutoff point of Pi > 0.12, two coding sequences (
accD>ycf1) had high nucleotide diversity and were suitable for phylogeny (
Figure 6).
2.5. IR Junctions’ Contraction and Expansion
The gene structure and positions of the IR junctions were well-conserved across the 10
Dendrobium species (
Figure 7), with differences mainly in the length. At the adjacent regions of the LSC and IRb (JLB), the
rpl22 genes of the LSC crossed over into IRb. The junction between LSC and lRa (JLA), which were located in the
rps19 genes and
psbA genes, except
D. fanjingshanense and
D. Parishii were located in the
rpl22 genes and
psbA genes. The adjacent regions of SSC and lRb (JSB), which were located in the
ndhF genes, except
D. aphrodite and
D. delacourii were lost the gene. While the
trnN and
ycf1 genes were adjacent to the junction between SSC and IRa (JSA). The
ycf1 genes crossed 25 bp-1,082 bp over into IRa region, the degree of expansion varied greatly.
The main difference between the chloroplast genomes of different carbon assimilation pathways exists in the IR/SSC: in JSB regions, an overlap of ycf1 and ndhF by 23-56 bp in D. fimbriatum (obligate C3), D. fanjingshanense (facultative CAM), D. aphyllum and D. Parishii (constitutive CAM), which showing an increasing trend from C3 to CAM; in JSA regions, an expansion of ycf1 gene to IRa by 25 bp-1082 bp in the rest of species, which showing a decreasing trend from C3 to CAM.
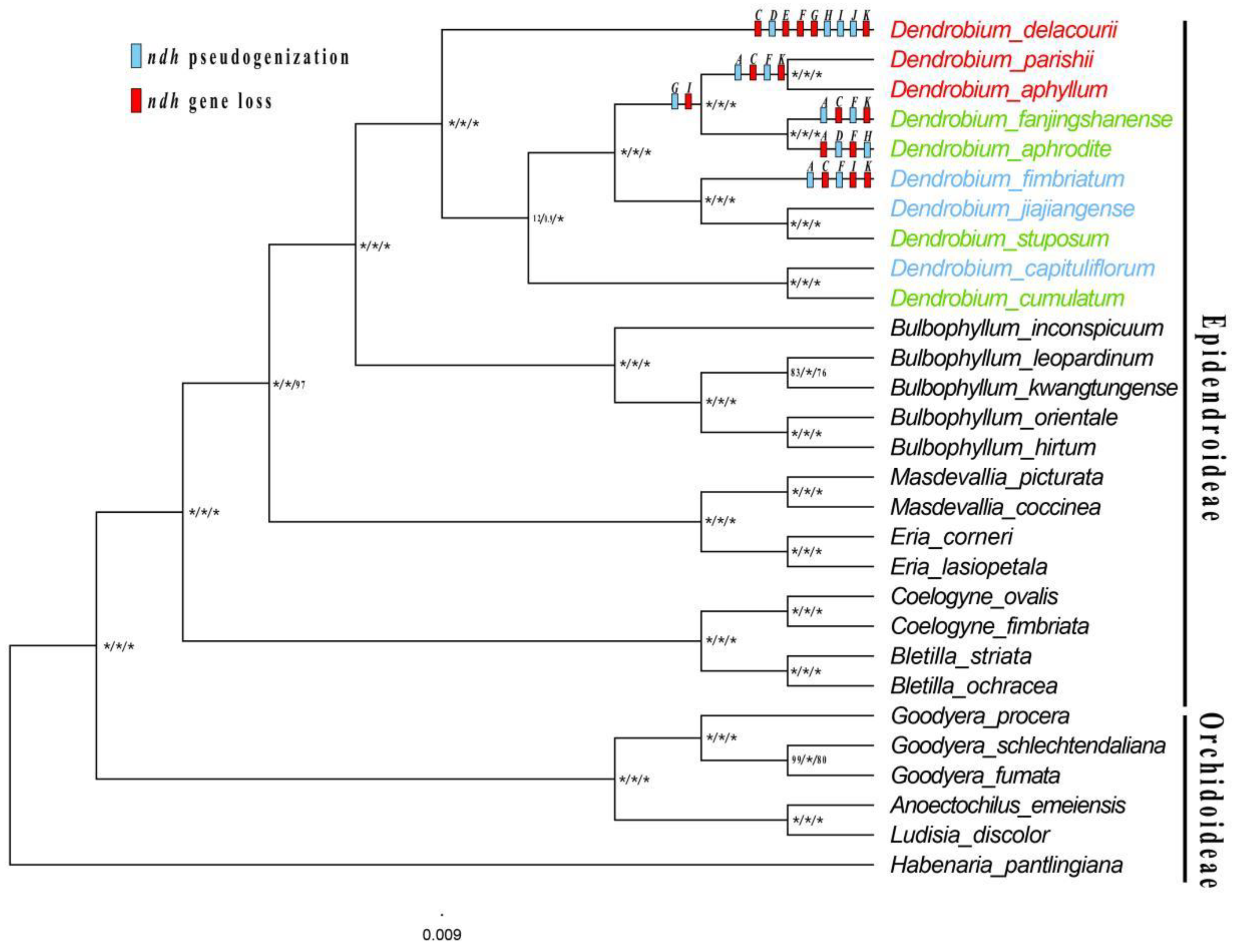
2.6. Phylogenetic Analysis
The phylogenetic relationships inferred by ML, BI and MP analysis of the complete genomes predicted the same topology (
Figure 8). In general, the phylogenetic trees revealed that the orchids have a monophyletic relationship with strong support (BS≥75%, PP≥0.90). Furthermore, the
Dendrobium was monophyletic and was sister to
Bulbophyllum. The constitutive CAM plants appeared independently at least twice in 10
Dendrobium species; the facultative CAM plants were discretedly distributed. Character state reconstructions of
ndh gene status across
Dendrobium revealed a pattern of independent gene loss and pseudogenization (
Figure 8). Character state optimization revealed that the complete
ndh gene family was present in the ancestor of
Dendrobium.
3. Discussion
3.1. Identification the Photosynthetic Pathways Could Provide Vital Information for CAM Research
CAM is an important model of photosynthetic pathway, and plants with CAM pathway usually have higher water use efficiency and stronger drought tolerance [
15,
30].
Dendrobium is almost all epiphytic, with a wide distribution space and a large elevation range. The unique habitat and wide distribution of
Dendrobium resulted in multiple photosynthetic pathways. However, because of the absence of a identification of photosynthetic pathways, the rich CAM resources of
Dendrobium are still to be explored. Recently, whole-tissue carbon isotope ratios (δ
13C) have been used to categorize species as C
3 or CAM predominantly [
14]. However, δ
13C cannot identify species in which CAM was present in C
3 with the presence of stress. Therefore, we measured titratable acidity and net photosynthetic rates (Pn) to distinguish multiple photosynthetic pathways in
Dendrobium more precisely. Based on the result, we confirmed that there were various photosynthetic pathways, including obligate C
3, facultative CAM and constitutive CAM among
Dendrobium species. Based on the Pn in constitutive CAM plants were below zero during the day but enhanced that the night, which showed a very significant trend during nighttime, while in C
3 plants was expanded to zero during daytime but below night. Further combined with titratable acid values, constitutive CAM species had significant day-night titratable acid differences, C
3 plants which had non-significant day-night titratable acid differences were determined to be obligate C
3 plants, which showed significant differences were determined to be facultative CAM plants clearly. Combined with the comparative chloroplast genomic analysis, we believe we can provide new insights into the evolution of CAM.
3.2. The Plastome Characteristics
The size of the plastid genome of Orchidaceae has a large variation ranging from 19,047 bp (
Epipogium roseum) [
31] to 234,657 bp (
Cypripedium lichiangense). The GC content ranged from 33.9% (
C. formosanum) to 37.8% (
C. macranthos) [
32,
33,
34], and the number of genes was mostly around 130. Currently, the published plastids of
Dendrobium members range from 148,431 bp (
D. zhenghuoense) [
35] to 160,123 bp (
D. thyrsiflorum) [
36]. In this study, we obtained the plastome sequences of 10 species of
Dendrobium using next-generation sequencing technology. The plastome size ranges from 151,673 bp (
D. fimbriatum) to 160,375 bp (
D. cumulatum), the GC content ranged from 37.1% to 37.7% and the number of genes ranged from 127 to 132, located within the scope revealed by the reported orchid plastomes previously[
31,
32,
37,
38,
39,
40], but
D. cumulatum will be the largest plastid genome in
Dendrobium.
3.3. Plastome Structural Evolution Under Different Carbon Assimilation Pathways
In most angiosperms, plastid genomes are maternally inherited, thus maintaining a highly conserved structure in closely related species [
41]. The contraction and expansion of IR region is a common phenomenon in the evolution process [
42], which often affect the size of the entire chloroplast genome and is the main driving force for the structural variation of chloroplast genome [
43,
44,
45]. The difference of IR/SC boundary is more significant in Orchids [
46,
47], so Luo et al. [
34] divided chloroplast genomes of orchids into four categoriesand and two evolutionary routes by the differences: (1) the
ycf1 gene within the SSC region expands toward IRa, causing
ycf1 in the IRb region to move toward the border and overlap with the
ndhF gene; (2) the
ycf1 gene within the SSC region continues to move toward the interior of the SSC region, and the portion of the coding region that is located at the border becomes shorter and shorter, after which the
ycf1 gene is completely embedded in the SSC region. In this study, we observed some difference in the IR/SC boundary regions of different carbon assimilation pathways in
Dendrobium plastomes. First, in JSB regions, an overlap of
ycf1 and
ndhF by 23-56 bp, which showing an increasing trend from C
3 to CAM; second, in JSA regions, an expansion of
ycf1 gene to IRa by 25 bp-1082 bp, which showing a decreasing trend from C
3 to CAM, which correspond to the two evolutionary routes of Luo, respectively, these findings suggest that the evolution of the IR/SC boundary is different between different carbon assimilation pathways.
The plastid genome size of structural CAM plants was 151,689 bp to 152,487 bp, which were shorter than that of obligate C
3 plants (151,673 bp-160,010 bp) and facultative CAM plants (152,108 bp-160,375 bp). The main difference is the phenomenon that
ndh gene loss or pseudogenization in constitutive CAM plants. This phenomenon was observed in 6 of 10
Dendrobium species, including 3 constitutive CAM plants (100%), 2 facultative CAM plants (50%), and 1 obligate C
3 plant (33%). Although the
ndh genes have been detected in the mitochondrial (mt) genomes of some orchids, there is no direct evidence whether these genes are related to the loss of
ndh genes in plastids [
33]. Therefore, the mechanism of
ndh gene loss and pseudoplasmaization in orchids needs to be further investigated. In addition, studies have suggested that the loss of
ndh genes in
Littorella (Plantaginaceae) can possibly related to their amphibious lifestyle and partial dependence on CAM photosynthesis [
48]. Liu et al. [
32] studied the Aeridinae were all constitutive CAM plants and all of them were
ndh loss or pseudogenization, what’s more, the constitutive CAM plants in our study also
ndh gene loss or pseudogenization, and this phenomenon accounted for a decreasing proportion of constitutive CAM, facultative CAM, and obligate C
3 plants, it is speculated that the loss of the
ndh gene may be closely related to the CAM pathway.
3.4. The Barcoding Investigation and Phylogenetic Analysis
Nucleotide diversity (Pi) is an important indicator that can be used to assess genetic differences between different species, and the position with higher variability in the nucleic acid sequences can be used to infer evolutionary relationships [
49,
50]. In addition, the genetic loci used for DNA barcoding usually contain enough informative loci to effectively define closely related species[
51], which has been used to identify
Dendrobium species[
52,
53,
54]. In this study, the nucleotide diversity analysis on intact plastids of
Dendrobium, five highly variable regions were identified and protein-coding genes identified two highly variable coding genes. The seven highly variable regions identified in this study can be used as DNA molecular markers to distinguish
Dendrobium relatives, and the results can be used to develop
Dendrobium DNA barcodes.
Repeated sequences have the advantages of rich polymorphism, high repeatability and good co-dominance [
55], which play an important role in species evolution as well as identification of relatives [
56,
57], often used in the study of genetic diversity and systematic relationships of taxa[
58,
59,
60,
61].In orchids, SSR markers have been widely used in
Cymbidium [
62],
Yucca [
63],
Phalaenopsis [
64,
65] and
Dendrobium [
66,
67], which mononucleotides and dinucleotides are prevalent and highly abundant [
68,
69]. In this study, a total of 41–60 SSRs were identified from
Dendrobium plasmids, indicating that the plasmid genome of
Dendrobium retained abundant genetic information. Most of the SSRs are mononucleotide and dinucleotides repeat in these 10
Dendrobium species, in the range of 68% to 90%; similar results are found in most orchids [
70,
71,
72,
73]. Compared with
D. nobile, which has a trinucleotide content of up to 37% [
74], the average trinucleotide content in our study was only about 4%, and even
D. delacourii did not has trinucleotides (
Supplementary Table S5), which is probably due to a more conserved SSR [
75]. The above findings can provide a data basis for further studies on population genetics.
Our results revealed the multiple independent CAM origins exist in
Dendrobium species. Despite being a valuable class of plants in water conservation and drought resistance, there are few studies on the evolution in CAM plants. Orchidaceae family is the most distributed group of CAM pathways, recent studies have shown that the constitutive CAM pathway has evolved at least nine times in
Bulbophyllum [
76,
77], and have evolved independently at least eight times in
Dendrobium [
14]. The identification of facultative CAM plants is relatively difficult, so there is little literature on the evolution of facultative CAM pathways. In our study, the significance of diurnal titrable acid and net photosynthetic rates curve were measured, four facultative CAM plants and three constitutive CAM plants were identified. The results supported that the three constitutive CAM plants originated independently twice in the plastid phylogenetic genomics tree, and facultative CAM plants showed discrete distribution with high support for each branch. And Our results strongly imply that the ancestral
Dendrobium plastome possessed full ORFs for all
ndh genes.
4. Materials and Methods
4.1. Plant Materials
The materials used in this study are the species with complete chloroplast genome data in NCBI (
https://www.ncbi.nlm.nih.gov/) and some ex-situ protected native species. Ten
Dendrobium species were selected, including
D. aphrodite, D. capituliflorum, D. cumulatum, D. delacourii, D. jiajiangense, D. stuposum, D. aphyllum, D. fanjingshanense, D. fimbriatum and D. parishii. They were cultivated in the Fujian Agriculture and Forestry University, Fujian province, China. Their voucher information is given in
Supplementary Table S1.
4.2. Determination of Photosynthetic Pathways
4.2.1. Determination of Whole-tissue Carbon Isotope Ratios
Healthy leaves of each species were sampled about 2g, dried and preserved with silica gel. The dried leaves were put into a 10mL centrifugal tube, crushed with a ball mill (JXFSTPRP-64), and weighed with 4mg for package sample. At the Ecological Stable Isotope Center, College of Forestry, Fujian Agriculture and Forestry University, Stable isotope Mass Spectrometer (IsoPrime100, Limonta, Germany) was used to analyze the samples, and standard samples (acetanilide, L-histidine, D-glutamic acid and glycine) were used for data calibration. The carbon isotope value (δ
13C) of the sample was calculated by the formula:
The accuracy of the measured value is less than 0.2‰.
4.2.2. Determination of Titratable Acidity
The diurnal fluctuation of total organic acid content per unit fresh weight of leaves is one of the indicators reflecting the photosynthetic pathway. Under clear weather conditions, soil hygrometer L99-TWS-1 was used to measure the matrix free water content of potting soil. Plants under drought stress (five days after matrix humidity dropped to 0) were selected as test objects, and the third to fifth mature healthy leaves were sampled. The sampling time was day (between 5:00 and 6:20) and night (between 17:45 and 16:30). Three repeat samples were taken at each time period. After all samples were collected, they were cleaned and disinfected with alcohol, wiped dry, and immediately stored in liquid nitrogen. Total organic acids were determined by indicator titration [
78]. The specific preparation and operation methods are as follows:
Reagent I: 1% phenolphthalein solution, 1g phenolphthalein dissolved in 60ml anhydrous ethanol and 40ml water;
Reagent II: c(NaOH)=0.01mol L-1, 0.04 gNaOH dissolved in 100ml water.
- 2.
Equipment
10ml beaker, Centrifuge, Grinding machine, Manual single channel pipette (10μl~100μl adjustable).
- 3.
Titration steps
Add 1g sample to 10ml water, grind it into homogenate in a grinder, centrifuge at 8000rpm at 4℃ for 10min, add 0.5ml supernatant to 9.5ml water in a small beacher, add 10μl reagent I, titrate with reagent II until the light red color does not disappear, record the volume of NaOH used. The total titrated acid is calculated, and the acid content is expressed as the molar amount of OH
- consumed per unit of fresh weight tissue. The formula is as follows:
N: the consumption of OH- per kilogram of fresh weight tissue (mol kg-1 FW);
V: the volume of NaOH solution consumed at the end of the titration (ml);
C: the concentration of NaOH solution (mol L-1);
W: the mass of the sample (g).
Refer to previous studies [
76,
79]. The mean value and standard deviation of the three repeated values were calculated respectively, and then the difference of the day and night mean value and standard deviation was made in pairs respectively to determine whether there was a significant difference by comparing the size of the two differences: difference of the mean value>sum of the standard deviation, there is a significant difference; on the contrary, there is no significant difference.
4.2.3. Determination of the Net Photosynthetic Rate
The whole experiment process was completed in the artificial climate chamber with controlled conditions: the temperature was 28°C during the day (6:00-18:00, 200 µmol m
-2s
-1 light treatment) and 22°C at night (18:00-6:00, dark treatment) [
80], with an air relative humidity of 40%. The detailed steps are as follows: the volumetric water content of the matrix was determined using the soil hygrometer L99-TWS-1, and the experiment was conducted five days after the drop to 0. The third to fifth mature healthy leaves were selected and the CO
2 exchange rate was measured for 24h by using the portable photosynthetic apparatus Li-6400XT, transparent bottom leaf chamber and 6400-02B red and blue light source. The measuring system is equipped with a 10L air buffer bottle, the gas flow rate is 500μms, the value interval is 5min, and the whole-day photosynthesis curve is drawn. Perform a match correction before each value.
4.3. DNA Extraction and Sequencing
In this study, Six of ten
Dendrobium species were newly sequenced. 2g fresh plant leaves were sampled and snap-frozen in liquid nitrogen and stored in an ultra-low temperature refrigerator at -80°C. The total DNA was isolated using a modified CTAB method [
81]. The total DNA of the samples was extracted by Dneasy Plant Mini Kits (QIAGEN, Germany), and then detected by agarose gel electrophoresis, and the DNA with more than 1μg of extracted amount and obvious main bands was selected. The total DNA from the samples was randomly interrupted to construct a 150 bp Denovo small fragment libraries, which was sequenced by the Beijing Genomics Institute (Shenzhen, China) on the Illumina HiSeq 2500 platform, and 20 Gb of raw data were obtained from each sample, which were filtered to obtain at least 10 Gb the final high-quality data (clean data) for subsequent data analysis.
4.4. Plastome Assembly and Annotation
Plasmid assembly and annotation were performed according to previous methods [
82]. The paired-end reads were assembled using the GetOrganelle pipeline (
https://github.com/Kinggerm/GetOrganelle), and then, the filtered reads were assembled using SPAdes version 3.10 [
83]. Assembled the chloroplast genome using the published plastome of
D. thyrsiflorum(MN306203) as the reference sequence. The gene annotation using DOGMA [
84] based on default parameters and calibrated with Geneious Prime v2021.1.1 [
85]. Genome-wide mapping of chloroplasts using OGDRAW [
86].
4.5. Genome Comparison and Analysis, IR Border and Divergence Analyses
Repeat sequence analysis, diversity analysis and IR boundary analysis
The plastome genomes across the ten species of
Dendrobium were visualized with mVISTA using the LAGAN alignment program [
87] with the sequence of
D. capituliflorum as a reference. The rearrangements of plastomes were detected and plotted using Mauve of ten species [
88]. Contraction and expansion of IR boundaries in the chloroplast genomes of 10
Dendrobium plants were measured and compared using the online program IRscope (
https://irscope.shinyapps.io/irapp/) [
89]. Mutation hotspot regions and genes were aligned to the plastid sequences using MAFFT v7 [
90]. The nucleotide diversity (Pi) of the 10 plastids of
Dendrobium was then calculated using DnaSP v6.12.03 (DNA sequence polymorphism) [
91]. Highly mutated hotspot regions were identified by a sliding window strategy. The step size was set to 25 bp and the window length was 100 bp.
4.6. Repeat Sequence Analysis
Simple Sequence Repeats (SSRs) of 10
Dendrobium species were identified and localized using MISA-web, where the number of mononucleotide, dinucleotide, trinucleotide, tetranucleotide, pentanucleotide and hexanucleotide repeats were set to 10, 5, 4, 3, 3, 3, 3, and 3 respectively [
92]. The online REPuter software (
https://bibiserv.cebitec.uni-bielefeld.de/reputer) was used to identify interspersed nuclear elements (INE), including forward, reverse, palindrome, and complement sequences, setting the maximum number of repetitive sequences to 50 and the minimum repetition size to 8, the hamming distance was set to 3 [
93].
4.7. Phylogenetic Reconstruction
Phylogenetic analysis of 29 species of Orchidaceae using complete plastid genomes. Of these 29 species, 6
Dendrobium species are our newly sequenced and the complete plasmid data of other 23 species were publicly available from NCBI. A list of taxa analyzed with voucher information and GenBank accessions is shown in
Supplemental Table S1. The whole plastome fasta sequences of the genus
Dendrobium and its outgroups were aligned using Geneious Prime v2021.1.1 [
85], then used the CIPRES Science Gateway to construct phylogenetic trees for the maximum likelihood (ML), Maximum Parsimony (MP) and Bayesian Inference (BI) phylogenetic trees[
94]. The ML tree was constructed using the GTRGAT nucleotide substitution model using the Bootstrap algorithm with 1000 repetitions and the rest of the parameters as default [
95]. The MP tree was constructed using a heuristic search and branch exchange algorithm (TBR), where all nucleotide traits were equally weighted and searched with an arbitrary repetition of 1,000 times, and the reliability of the phylogenetic tree was analyzed with a 1,000 repetitions of a self-expansion method (Bootstrap) [
96].The BI tree was constructed using the GTR+I+Γ model with the Markov Chain Monte Carlo (MCMC) method, where each of the four Markov chains was run for 10,000,000 generations, with samples taken every 100 generations, and the top 25% of the trees were discarded as burn-in samples to ensure that each chain reached a stable state (Ronquist et al., 2012). The first 25% of the trees are discarded as burn-in samples to ensure that each chain reaches a steady state, and finally the posterior probability (PP) of each branch is obtained [
97].
5. Conclusions
Our research based on the results of δ13C, net photosynthetic rates (Pn) and titratable acid value, identified 10 Dendrobium species into three photosynthetic pathways. Then the chloroplast whole genome characteristics of them were analyzed which shows that the overall structure and gene content of the plastomes of 10 Dendrobium species are relatively conserved, with only certain differences in genome size, gene content, GC content, repeat sequences. Among multiple photosynthetic pathways, the chloroplast genome size of constitutive CAM plants is shorter because of their ndh gene loss or pseudogenization, the loss of the ndh gene may be closely related to the CAM pathway. What’s more, the evolution of the IR/SC boundary is different between different carbon assimilation pathways. Phylogenetic analyses showed that there are multiple independent CAM origins in selected Dendrobium species, and the ancestral Dendrobium plastome possessed full ORFs for all ndh genes. The study aims to clarify the chloroplast genomic features of different photosynthetic pathways, enrich the genomic data of CAM plants in Dendrobium.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on
Preprints.org.
Author Contributions
Conceptualization, methodology, Y.L., Z.L. and M.L.; Software, formal analysis, visualization, Y.L.; Investigation, resources and data curation, Y.L., S.Z. and J.D.; writing—original draft preparation, Y.L.; writing—review and editing, J.S., D.L., S.L., Z.L. and M.L.; supervision, Y.L. Z.L. and M.L.; project administration, M.L.; funding acquisition M.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the National Key Research and Development Program of China (2023YFD1600504).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Acknowledgments
We acknowledge the technical support of laboratory staff during the conduction of laboratory experiments, Ding-Kun Liu, Xiong-De Tu, and Cheng-Yuan Zhou.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Rodrigues, M. A.; Matiz, A.; Cruz, A. B.; Matsumura, A. T.; Takahashi, C. A.; Hamachi, L.; Fe´lix, L. M.; Pereira, P. N.; LatansioAidar, S. R.; Aidar, M. P. M.; Demarco, D.; Freschi, L.; Mercier, H.; Kerbauy, G. B. Spatial pattems of photosynthesis in thin and thickleaved epiphytic orchids: Unravelling C3-CAM plasticity in an organ-compartmented way. Annals of Botany 2013, 1 12(1): 17_29. [CrossRef]
- Winter, K.; Holtum, J. A. M.; Smith, J. A. C. Crassulacean acid metabolism: a continuous or discrete trait? New Phytologist 2015, 208(1): 73–78. [CrossRef]
- Niechayev, N. A.; Pereira, P. N.; Cushman, J. C. Understanding trait diversity associated with crassulacean acid metabolism (CAM). Current Opinion in Plant Biology 2019, 49: 74–85. [CrossRef]
- Wickell, D.; Kuo, L. Y.; Yang, H. P.; Ashok, A. D.; Irisarri, I.; Dadras, A.; de Vries, S.; de Vries, J.; Huang, Y. M.; Li, Z.; Barker, M. S.; Hartwick, N. T.; Michael, T. P.; Li, F. W. Underwater CAM photosynthesis elucidated by Isoetes genome. Nature Communications 2021, 12: 6348. [CrossRef]
- Silvera, K. M.; Neubig, M.; Whitten, W. M.; Williams, N. H.; Winter, K.; Cushman, J. C. Evolution along the crassulacean acid metabolism continuum. Functional Plant Biology 2010, 37 (11): 995–1010. [CrossRef]
- Cai, J.; Liu, X.; Vanneste, K.; Proost, S.; Tsai, W. C.; Liu, K. W.; Chen, L. J.; He, Y.; Xu, Q.; Bian, C.; Zheng, Z.; Sun, F.; Liu, W.; Hsiao, Y. Y.; Pan, Z. J.; Hsu, C. C.; Yang, Y. P.; Hsu, Y. C.; Chuang, Y. C.; Dievart, A.; Dufayard, J. F.; Xu, X.; Wang, J. Y.; Wang, J.; Xiao, X. J.; Zhao, X. M.; Du, R.; Zhang, G. Q.; Wang, M.; Su, Y. Y.; Xie, G. C.; Liu, G. H.; Li, L. Q.; Huang, L. Q.; Luo, Y. B.; Chen, H. H.; Van de Peer, Y.; Liu, Z. J. The genome sequence of the orchid Phalaenopsis equestris. Nat Genet 2015;47(1):65–72. [CrossRef]
- Yang, X. H.; Cushman, J. C.; Borland, A. M.; Edwards, E. J.; Wullschleger, S. D.; Tuskan, G. A.; Owen, N. A.; Griffiths, H.; Smith, J. A. C.; De, P. L.; Henrique, C.; Weston, D. J.; Cottingham, R.; Hartwell, J.; Davis, S. C.; Silvera, K.; Ming, R.; Schlauch, K.; Abraham, P.; Stewart, J. R.; Guo, H. B.; Albion, R.; Ha, J. M.; Lim, S. D.; Wone, B. W. M.; Yim, W. C.; Garcia, T.; Mayer, J. A.; Petereit, J.; Nair, S. S.; Casey, E.; Hettich, R. L.; Ceusters, J.; Ranjan, P.; Palla, K. J.; Yin, H.; Reyes-García, C.; Andrade, J. L.; Freschi, L.; Beltrán, J. D.; Dever, L. V.; Boxall, S. F.; Waller, J.; Davies, J.; Bupphada, P.; Kadu, N.; Winter, K.; Sage, R. F.; Aguilar, C. N.; Schmutz, J.; Jenkins, J.; Holtum, J. A. M. A roadmap for research on crassulacean acid metabolism (CAM) to enhance sustainable food and bioenergy production in a hotter, drier world. New Phytologist 2015, 207(3): 491–504. [CrossRef]
- Valdez-Hernández, M.; González-Salvatierra, C.; Reyes-García, C.; Jackson, P. C.; Andrade, J. L. Physiological Ecology of Vascular Plants. In: Islebe GA, et al.,editors. Biodiversity and Conservation of the Yucatán Peninsula. International Publishing Switzerland: Springer; 2015. p. 97–129.
- Hermida-Carrera, C.; Fares, M. A.; Font-Carrascosa, M.; Kapralov, M. V.; Koch, M. A.; Mir, A; Molins, A; Ribas-Carbó, M; Rocha, J.; Galmés, J. Exploring molecular evolution of Rubisco in C3 and CAM Orchidaceae and Bromeliaceae. BMC Evol Biol 2020; 20(1): 11. [CrossRef]
- Silvera, K.; Santiago, L. S.; Cushman, J. C.; Winter,K. The incidence of crassulacean acid metabolism in Orchidaceae derived from carbon isotope ratios: a checklist of the flora of Panama and Costa Rica. Botanical Journal of the Linnean Society 2010, 163(2): 194–222. [CrossRef]
- Christenhusz, M. J. M.; Byng, J. W. The number of known plants species in the world and its annual increase. Phytotaxa 2016, 261: 201–217. [CrossRef]
- Winter, K.; Wallace, B. J.; Stocker, G. C.; Roksandic, Z. Crassulacean acid metabolism in Australian vascular epiphytes and some related species. Oecologia 1983, 57(1–2): 129–141. [CrossRef]
- Silvera, K.; Santiago, L. S.; Cushman, J. C.; Winter, K. Crassulacean acid metabolism and epiphytism linked to adaptive radiations in the Orchidaceae. Plant Physiology 2009, 149 (4): 1838–1847. [CrossRef]
- Li, M. H.; Liu, D. K.; Zhang, G. Q.; Deng, H.; Tu, X. D.; Wang, Y.; Lan, S. R.; Liu, Z. J. A perspective on crassulacean acid metabolism photosynthesis evolution of orchids on different continents: Dendrobium as a case study. Journal of Experimental Botany 2019 70 (22): 6611–6619. [CrossRef]
- Schweiger, A. H.; Nürk, N. M.; Beckett, H.; Liede-Schumann, S.; Midgley, G. F.; Higgins, S. I. The eco-evolutionary significance of rainfall constancy for facultative CAM photosynthesis. New Phytologist 2021, 230: 1653–1664. [CrossRef]
- Winter. K. Ecophysiology of constitutive and facultative CAM photosynthesis J. Exp. Bot., 2019, 70(22): 6495-6508. [CrossRef]
- Su, W. H.; Zhang, G. F. Primary Study on Photosynthetic Characteristics of Dendrobium nobile. Traditional Chinese medicinal materials 2003, 26: 157–159.
- Su, W. H.; Zhang, G. F. The photosynthesis pathway in leaves of Dendrobium officinale. Acta Phytoecologica Sinica 2003, 27: 631–637. [CrossRef]
- Ren, J. W.; Wang. Y.; Pen, Z. H.; Hu, Q. Measurement of Malic Acid Diel Fluctuation of Leaves in Three Dendrobia. Acta Agriculturae Universitis Jiangxiensis 2010, 32(3): 547–552.
- Liu, Z. D. The study of transformation mechanism of morphology and physiology in the switch from C3-photosynthesis to Crassulacean acid metabolism of Dendrobium. HuaZhong Agricultural University 2014.
- Qiu, S.; Sultana, S.; Liu, Z. D.; Yin, L. Y.; Wang, C. Y. Identification of obligate C3 photosynthesis in Dendrobium. Photosynthetica: International Journal for Photosynthesis Research 2015, (53-2).
- Zou, L. H.; Wan, X.; Deng, H.; Zheng, B. Q.; Yan, B. J. RNA-seq transcriptomic profiling of crassulacean acid metabolism pathway in Dendrobium catenatum. Scientific Data 2018, 5(1): 180252. [CrossRef]
- Zou, L. H. Crassulacean Acid Metabolism Pathway and Its Key Genes’ Co-expression Networks in Dendrobium catenatum. BeiJing: Chinese Academy of Forestry 2019.
- Zeng, M. Y.; Xu, S. W.; Wang, F. J.; Lin,Y, J.; Zhang, S.;Lan, S. R.; Li, M. H. Measurement of Carbon Assimilation Pathway for the Mainly Native Species of Parents in Nobile Type Dendrobium. Molecular Plant Breeding 2023, https://link.cnki.net/urlid/46.1068.S.20230912.1030.002.
- Xue, Q. Q.; Yang, J. P.; Yu, W. H.; Wang, H. M.; Hou, Z. Y.; Li, C.; Xue, Q. Y.; Liu, W.; Ding, X. Y.; Niu, Z. T. The climate changes promoted the chloroplast genomic evolution of Dendrobium orchids among multiple photosynthetic pathways. BMC Plant Biology 2023, Vol.23(No.1):189. [CrossRef]
- Liu, H. Y.; Liu, L. K.; Wang, Z. L.; Yu L. M.; Li J. P.; Zeng Y. Research Progress on Chloroplast Genome of Orchidaceae. Chinese Wild Plant Resources 2023, Vol. 42 No. 7 Jul: 73-79.
- Niu, Z; Xue, Q; Zhu, S; Jing, S; Liu, W; Ding, X. The complete plastome sequences of four orchid species: insights into the evolution of the Orchidaceae and the utility of plastomic mutational hotspots. Front Plant Sci 2017, 8: 715. [CrossRef]
- Zhu, S; Niu, Z; Xue, Q; Hui, W.; Xie, X.; Ding, X. Accurate authentication of Dendrobium officinale and its closely related species by comparative analysis of complete plastomes. Acta Pharmaceutica Sinica B 2018, 8(06): 969-980. [CrossRef]
- Osmond, C. B.; Allaway, W. G.; Sutton, B. G.; Troughton, J. H.; Winter, K. Carbon isotope discrimination in photosynthesis of CAM plants. Nature 1973, 246(5427): 41–42. [CrossRef]
- Borland, A. M.; Griffiths, H.; Hartwell, J.; Smith, J. A. Exploiting the potential of plants with crassulacean acid metabolism for bioenergy production on marginal lands. Journal of Experimental Botany 2009, 60(10): 2879–2896. [CrossRef]
- Schelkunov, M. I.; Shtratnikova, V. Y.; Nuraliev, M. S.; Selosse, M. A.; Penin, A. A.; Logacheva, M. D. Exploring the limits for reduction of plastid genomes: A case study of the mycoheterotrophic orchids Epipogium aphyllum and Epipogium roseum. Genome Biol. Evol 2015, 7, 1179–1191. [CrossRef]
- Liu, D. K.; Tu, X. D.; Zhao, Z.; Zeng, M. Y.; Zhang, S.; Ma, L.; Zhang, G. Q.; Wang, M. M.; Liu, Z. J.; Lan, S. R.; Li, M. H.; Chen, S. P. Plastid phylogenomic data yield new and robust insights into the phylogeny of Cleisostoma–Gastrochilus clades (Orchidaceae, Aeridinae). Molecular Phylogenetics and Evolution 2020, 145:106729. [CrossRef]
- Lin, C. S.; Chen, J. J.; Huang, Y. T.; Chan, M. T.; Daniel, H.; Chang, W. J.; Hsu, C. T.; Liao, D. C.; Wu, F. H.; Lin, S. Y.; Liao, C. F.; Deyholos, M. K.; Wong, G. K.; Albert, V. A.; Chou, M. L.; Chen, C. Y.; Shih, M. C. The location and translocation of ndh genes of chloroplast origin in the Orchidaceae family. Sci Rep 2015, 5: 9040. [CrossRef]
- Luo, J.; Hou, B. W.; Niu, Z. T.; Liu, W.; Xue, Q. Y.; Ding, X. Y. Comparative chloroplast genomes of photosynthetic orchids: insights into evolution of the Orchidaceae and development of molecular markers for phylogenetic applications. PloS One 2014, 9 (6): e99016. [CrossRef]
- Huang, Y. Z.; Zhuang, L. B.; Zhai, J. W.; Lin, W. J. The complete chloroplast genome sequence of Dendrobium zhenghuoense (Orchidaceae). Mitochondrial DNA Part B 2019, Vol.4(No.2): 3326-3327. [CrossRef]
- Pan, Y. Y.; Li, T. Z.; Chen, J. B.; Huang, J.; Rao, W. H. Complete chloroplast genome of Dendrobium thyrsiflorum (Orchidaceae)(Article). Mitochondrial DNA Part B: Resources 2019, Vol.4(No.2): 3192-3193. [CrossRef]
- Guo, Y. Y; Yang, J. X.; Li, H. K.; Zhao, H. S. Chloroplast genomes of two species of Cypripedium: Expanded genome size and proliferation of AT-biased repeat sequences. Frontiers in Plant Science 2021, 12: 609729. [CrossRef]
- Kim, Y. K.; Jo, S.; Cheon, S. H.; Joo, M. J.; Hong, J. R.; Kwak, M.; Kim, K. J. Plastome Evolution and Phylogeny of Orchidaceae, with 24 New Sequences. Front. Plant Sci 2020, 11, 22. [CrossRef]
- Ye, B. J.; Zhang, S.; Tu, X. D.; Liu, D. K.; Li, M. H. The complete plastid genome of Thrixspermum tsii (Orchidaceae, Aeridinae). Mitochondrial DNA Part B 2020, 5, 384–385. [CrossRef]
- Guo, Y. Y.; Yang, J. X.; Bai, M. Z.; Zhang, G. Q.; Liu, Z. J. The chloroplast genome evolution of Venus slipper (Paphiopedilum): IR expansion, SSC contraction, and highly rearranged SSC regions. BMC Plant Biol 2021, 21, 248. [CrossRef]
- Wicke, S.; Schneeweiss, G. M.; DePamphilis, C. W.; Müller, K. F.; Quandt, D. The evolution of the plastid chromosome in land plants: Gene content, gene order, gene function. Plant Mol. Biol 2011, 76, 273–297. [CrossRef]
- Huang, H.; Shi, C.; Liu, Y.; Mao, S. Y.; Gao, L. Z. Thirteen Camellia chloroplast genome sequences determined by high-throughput sequencing: Genome structure and phylogenetic relationships. BMC Evol. Biol 2014, 14, 151. [CrossRef]
- Kim, K. J. Complete chloroplast genome sequences from Korean ginseng (Panax schinseng Nees) and comparative analysis of sequence evolution among 17 vascular plants. DNA Research 2004, 11(4): 247-261. [CrossRef]
- Raubeson, L. A.; Peery, R.; Chumley, T. W.; Dziubek, C.; Fourcade, H. M.; Boore, J. L.; Jansen, R. K. Comparative chloroplast genomics: Analyses including new sequences from the angiosperms Nuphar advena and Ranunculus macranthus. BMC Genom 2007, 8, 174. [CrossRef]
- Dugas, D. V.; Hernandez, D.; Koenen, E. J.; Schwarz, E.; Straub, S.; Hughes, C. E.; Jansen, R. K.; Nageswara-Rao, M.; Staats, M. Mimosoid legume plastome evolution: IR expansion, tandem repeat expansions, and accelerated rate of evolution in clpP. Sci. Rep 2015, 5, 16958. [CrossRef]
- Wang, W. C.; Chen, S. Y.; Zhang, X. Z. Whole-genome comparison reveals divergent IR Borders and mutation hotspots in chloroplast genomes of herbaceous bamboos (Bambusoideae: Olyreae). Molecules 2018, 23(7):1537. [CrossRef]
- Park, S.; An, B.; Park, S. Reconfiguration of the plastid genome in Lamprocapnos spectabilis: IR boundary shifting, inversion, and intraspecific variation. Scientific Reports 2018, 8(1): 1-14. [CrossRef]
- Mower, J. P.; Guo, W. H.; Partha, R.; Fan, W.; Levsen, N.; Wolff, K.; Nugent, J. M.; González, N. Plastomes from tribe Plantagineae (Plantaginaceae) reveal infrageneric structural synapormorphies and localized hypermutation for Plantago and functional loss of ndh genes from Littorella. Molecular phylogenetics and evolution 2021, 107217. [CrossRef]
- Ding, S.; Dong, X.; Yang, J.; Guo, C.; Cao, B.; Guo, Y.; Hu, G. Complete Chloroplast Genome of Clethra fargesii Franch., an Original Sympetalous Plant from Central China: Comparative Analysis, Adaptive Evolution, and Phylogenetic Relationships. Forests 2021, 12, 441. [CrossRef]
- Abdullah; Mehmood, F.; Shahzadi, I.; Waseem, S.; Mirza, B.; Ahmed, I.; Waheed, M. T. Chloroplast genome of Hibiscus rosasinensis (Malvaceae): Comparative analyses and identification of mutational hotspots. Genomics 2020, 112, 581–591. [CrossRef]
- Chen, J. L.; Wang, F.; Zhou, C. Y.; Ahmad, S.; Zhou, Y. Z.; Li, M. H.; Liu, Z. J.; Peng, D. H. Comparative Phylogenetic Analysis for Aerides (Aeridinae, Orchidaceae) Based on Six Complete Plastid Genomes. International journal of molecular sciences 2023, Vol.24(No.15): 12473. [CrossRef]
- Xu, S. Z.; Li, D, Z.; Li, J. W.; Xiang, X. G.; Jin, W. T.; Huang, W. C.; Jin, X. H.; Huang, L. Q. Evaluation of the DNA barcodes in Dendrobium (Orchidaceae) from mainland Asia. PLoS ONE 2015, 10, e0115168. [CrossRef]
- Niu, Z. T.; Zhu, S. Y.; Pan, J. J.; Li, L. D.; Sun, J.; Ding, X. Y. Comparative analysis of Dendrobium plastomes and utility of plastomic mutational hotspots. Sci. Rep 2017, 7, 2073. [CrossRef]
- Feng, S. G.; Jiang, Y.; Wang, S.; Jiang, M. Y.; Chen, Z.; Ying, Q. C.; Wang, H. Z. Molecular identification of Dendrobium species (Orchidaceae) based on the DNA barcode ITS2 region and its application for phylogenetic study. International Journal of Molecular Sciences 2014, 16, 21975–21988. [CrossRef]
- Sun, Z. X.; Ao, P. X.; Bi, Y. F.; Zhao, Y. Complete Chloroplast Genome Sequence and Characteristics Analysis of Medicago sativa‘Deqin'. Acta Agrestia Sinica 2022, 30(02):320-328. [CrossRef]
- Chen, Y.; Hu, N.; Wu, H. Analyzing and characterizing the chloroplast genome of Salix wilsonii. BioMed Res. Int 2019, 5190425. [CrossRef]
- Khan, A.; Asaf, S.; Khan, A. L.; Al-Harrasi, A.; Al-Sudairy, O.; AbdulKareem, N. M.; Khan, A.; Shehzad, T.; Alsaady, N.; Al-Lawati, A.; Al-Rawahi, A; Shinwari, Z. K. First complete chloroplast genomics and comparative phylogenetic analysis of Commiphora gileadensis and C. foliacea: Myrrh producing trees. PLoS One\ 2019, 14, e0208511. [CrossRef]
- Yu, J.; Dossa, K.; Wang, L.; Zhang, Y.; Wei, X.; Liao, B.; Zhang, X. PMDBase: A database for studying microsatellite DNA and marker development in plants. Nucleic Acids Res 2017, 45, D1046–D1053. [CrossRef]
- Singh, R. B.; Mahenderakar, M. D.; Jugran, A. K.; Singh, R. K.; Srivastava, R. K. Assessing genetic diversity and population structure of sugarcane cultivars, progenitor species and genera using microsatellite (SSR) markers. Gene 2020, 753, 144800. [CrossRef]
- Liu, X.; Xu, D.; Hong, Z.; Zhang, N.; Cui, Z. Comparative and Phylogenetic Analysis of the Complete Chloroplast Genome of Santalum (Santalaceae). Forests 2021, 12, 1303. [CrossRef]
- Hong, Z.; He, W.; Liu, X.; Tembrock, L. R.; Wu, Z.; Xu, D.; Liao, X. Comparative Analyses of 35 Complete Chloroplast Genomes from the Genus Dalbergia (Fabaceae) and the Identification of DNA Barcodes for Tracking Illegal Logging and Counterfeit Rosewood. Forests 2022, 13, 626. [CrossRef]
- Huang, Y.; Li, F.; Chen, K. S. Analysis of diversity and relationships among Chinese orchid cultivars using EST-SSR markers. Biochemical Systematics and Ecology 2010, 38:93–102. [CrossRef]
- Fei, W.; Zhao, W. Z.; Dong, Z. H.; Ma, L. Y.; Li, W. Y.; Li, Z. Y.; Xin, P. Y. Analysis of the Chloroplast Genome Characteristics of 6 Species of Yucca. Bulletin of Botanical Research 2023, 43(01): 109-119. [CrossRef]
- Wu, W. L.; Chung, Y. L.; Kuo, Y. T. Development of SSR Markers in Phalaenopsis Orchids, Their Characterization, Cross-Transferability and Application for Identification. Orchid Biotechnology III 2017: 91-107. [CrossRef]
- Lee, Y. F.; Liu, Y. C.; Jheng, C. F.; Lin, J. Y.;Wu, W. L.; Chang, C. C.; Lin, B. Y.; Chen, T. C.; Chen, T. C. Comparative Chloroplast DNA Analysis of Phalaenopsis Orchids and Evaluation of cpDNA Markers for Distinguishing Moth Orchids. Orchid Biotechnology III 2017: 61-90.
- Zheng, S. G.; Hu, Y. D.; Zhao, R. X.; Yan, S.; Zhang, X. Q.; Zhao, T. M.; Chun, Z. Genomewide researches and applications on Dendrobium. Planta 2018, 248, 769–784. [CrossRef]
- Zhao, T. M.; Zheng, S. G.; Hua, Y. D.; Zhao, R. X.; Lia, H. J.; Zhang, X. Q.; Chun, Z. Classification of interspecific and intraspecific species by genome-wide SSR markers on Dendrobium(Article). South African Journal of Botany 2019: 136-146. [CrossRef]
- Hua, W. P ; Chen, C. Characterization and SSR identify of the complete chloroplast genome of Paphiopedilum concolor (Orchidaceae). Mitochondrial DNA Part B-Resources 2019, Vol.4(No.1): 1074-1076. [CrossRef]
- Fan, J. Z.; Li, X. L.; Li, M. Z.; Bu, Z. Y.; He, J. Z.; Zeng, Y. H. Genomic Characteristics and Phylogenic Analysis of Chloroplast of the Endangered Plant Paphiopedilum venustum. Chinese Journal of Tropical Crops 2023, 44(06): 1097-1105. [CrossRef]
- Li, D. M.; Zhao, C. Y.; Liu, X. F. Complete chloroplast genome sequences of Kaempferia galanga and Kaempferia elegans: Molecular structures and comparative analysis. Molecules 2019, 24, 474. [CrossRef]
- Mo, Z.; Lou, W.; Chen, Y.; Jia, X.; Zhai, M.; Guo, Z.; Xuan, J. The chloroplast genome of Carya illinoinensis: Genome structure, adaptive evolution, and phylogenetic analysis. Forests 2020, 11, 207. [CrossRef]
- Xu, J.; Liu, C.; Song, Y.; Li, M. Comparative Analysis of the Chloroplast Genome for Four Pennisetum Species: Molecular Structure and Phylogenetic Relationships. Front. Genet 2021, 12, 687844. [CrossRef]
- Sun, Y.; Zou, P.; Jiang, N.; Fang, Y.; Liu, G. Comparative analysis of the complete chloroplast genomes of nine Paphiopedilum species. Front. Genet 2022, 12, 772415.75. [CrossRef]
- Lu, J. J.; Kang, J. Y.; Feng, S. G.; Zhao, H. Y.; Liu, J. J.; Wang, H. Z. Transferability of SSR markers derived from Dendrobium nobile expressed sequence tags (ESTs) and their utilizationin Dendrobium phylogeny analysis. Scientia Horticulturae 2013, 158: 8–15. [CrossRef]
- Tang, C. Q.; Qiu, Z. X.; Tan, C.; Qian, Y. M.; Chen, X. Sorbus koehneana (Rosaceae): Its Complete Chloroplast Genome and Phylogenetic Relationship with S. unguiculata. Acta Horticulturea Sinica 2022, 49 (3): 641–654.
- Gamisch, A.; Winter, K.; Fischer, G. A.; Peter, C. H. Evolution of crassulacean acid metabolism (CAM) as an escape from ecological niche conservatism in Malagasy Bulbophyllum (Orchidaceae). New Phytologist 2021, 231(3): 1236–1248. [CrossRef]
- Hu, A.; Gale, S. W.; Liu, Z. J.; Fischer, G. A.; Saunders, R. M. Diversification slowdown in the Cirrhopetalum alliance (Bulbophyllum, Orchidaceae): insights from the evolutionary dynamics of crassulacean acid metabolism. Frontiers in Plant Science 2022, 13: 794171. [CrossRef]
- Lin, M.; Hsu, B. Photosynthetic plasticity of Phalaenopsis in response to different light environments. Journal of Plant Physiology 2004, 161(11): 1259–1268. [CrossRef]
- Silvera, K.; Santiago, L. S.; Winter, K. Distribution of crassulacean acid metabolism in orchids of Panama: evidence of selection for weak and strong modes. Functional Plant Biology 2005, 32(5): 397–407. [CrossRef]
- Winter, K.; Holtum, J. Facultative crassulacean acid metabolism (CAM) plants: powerful tools for unravelling the functional elements of CAM photosynthesis. Journal of Experimental Botany 2014, 65(13): 3425–3441. [CrossRef]
- Li, J.; Wang, S.; Yu, J.; Wang, L.; Zhou, S. A. Modified CTAB Protocol for Plant DNA Extraction. Chin. Bull. Bot 2013, 48, 72–78. [CrossRef]
- Jin, J. J.; Yu, W. B.; Yang, J. B.; Song, Y.; DePamphilis, C. W.; Yi, T. S.; Li, D. Z. GetOrganelle: A fast and versatile toolkit for accurate de novo assembly of organelle genomes. Genome Biol 2020, 21, 241. [CrossRef]
- Bankevich, A.; Nurk, S.; Antipov, D.; Gurevich, A. A.; Dvorkin, M.; Kulikov, A. S.; Lesin, V. M.; Nikolenko, S. I.; Pham, S.; Prjibelski, A. D.; et al. SPAdes: A new genome assembly algorithm and its applications to single-cell sequencing. J. Comput. Biol. J. Comput. Mol. Cell Biol 2012, 19, 455–477. [CrossRef]
- Wyman, S. K.; Jansen, R. K.; Boore, J. L. Automatic annotation of organellar genomes with DOGMA. Bioinformatics 2004, 20, 3252–3255. [CrossRef]
- Kearse, M.; Moir, R.; Wilson, A.; Stones-Havas, S.; Cheung, M.; Sturrock, S.; Buxton, S.; Cooper, A.; Markowitz, S.; Duran, C.; Thierer, T.; Ashton, B.; Meintjes , P.; Drummond, A. Geneious Basic: An integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 2012, 28, 1647–1649. [CrossRef]
- Greiner, S.; Lehwark, P.; Bock, R. OrganellarGenomeDRAW (OGDRAW) version 1.3.1: Expanded toolkit for the graphical visualization of organellar genomes. Nucleic Acids Res 2019, 47, W59–W64. [CrossRef]
- Brudno, M.; Malde, S.; Poliakov, A.; Do, C.B.; Couronne, O.; Dubchak, I.; Batzoglou, S. Glocal alignment: Finding rearrangements during alignment. Bioinformatics 2003, 19 (Suppl. S1), i54–i62. [CrossRef]
- Rissman, A. I.; Mau, B.; Biehl, B. S.; Darling, A. E.; Glasner, J. D.; Perna, N. T. Reordering contigs of draft genomes using the Mauve aligner. Bioinformatics 2009, 25, 2071–2073. [CrossRef]
- Amiryousefi, A.; Hyvonen, J.; Poczai, P. IRscope: An online program to visualize the junction sites of chloroplast genomes. Bioinformatics 2018, 34, 3030–3031. [CrossRef]
- Katoh, K.; Standley, D. M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability Mol. Biol. Evol 2013, 30, 772–780.92. [CrossRef]
- Rozas, J.; Ferrer-Mata, A.; Sánchez-DelBarrio, J. C.; Guirao-Rico, S.; Librado, P.; Ramos-Onsins, S. E.; Sánchez-Gracia, A. DnaSP 6: DNA Sequence Polymorphism Analysis of Large Data Sets. Mol. Biol. Evol 2017, 34, 3299–3302. [CrossRef]
- Beier, S.; Thiel, T.; Münch, T.; Scholz, U.; Mascher, M. MISA-web: A web server for microsatellite prediction. Bioinformatics 2017, 33, 2583–2585. [CrossRef]
- Kurtz, S.; Choudhuri, J. V.; Ohlebusch, E.; Schleiermacher, C.; Stoye, J.; Giegerich, R. REPuter: The manifold applications of repeat analysis on a genomic scale. Nucleic Acids Res 2001, 29, 4633–4642. [CrossRef]
- Miller, M. A.; Pfeiffer, W.; Schwartz, T. Creating the CIPRES Science Gateway for inference of large phylogenetic trees. In Proceedings of the 2010 Gateway Computing Environments Workshop (GCE), New Orleans, LA, USA, 14 November 2010; pp. 1–8. [CrossRef]
- Stamatakis, A.; Hoover, P.; Rougemont, J. A rapid bootstrap algorithm for the RAxML Web servers. Syst. Biol 2008, 57, 758–771. [CrossRef]
- Swofford, D. L. Phylogenetic analysis using parsimony (* and other methods). Sinauer, Sunderland, Massachusetts, USA, 2002.
- Ronquist, F.; Teslenko, M.; Van Der Mark, P.; Ayres, D. L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M. A.; Huelsenbeck, J. P. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol 2012, 61, 539–542.93. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).