1. Introduction
The discovery of driver mutations has markedly changed the treatment for non-small cell lung cancer (NSCLC) [
1,
2]. Specific molecular targeted therapies (MTTs) have achieved substantial therapeutic effects in patients with driver mutations. In particular, advanced NSCLC patients with driver mutations, including epidermal growth factor receptor (EGFR) mutations and anaplastic lymphoma kinase (ALK) rearrangement, can benefit from treatment with MTTs [
1,
2,
3]. Furthermore, MTTs are preferentially used for NSCLC patients with driver mutations who develop postoperative recurrence, because postoperative recurrence is considered to be a systemic disease [
4].
However, some NSCLC patients have oligo-recurrence, i.e., the recurrence of a limited number of tumor foci [
5]. The concept of oligo-recurrence was originally proposed in 1995 as an intermediate state of cancer spread between localized disease and widespread disease, and was defined as limited recurrent/metastatic foci as well as a controlled primary lesion [
5,
6]. Oligo-recurrence of NSCLC is often treatable by local ablative therapies (LATs), including surgical resection and definitive radiotherapy [
7,
8]. Moreover, a previous study suggested that the use of MTTs for oligo-recurrence in NSCLC patients with EGFR mutations did not improve prognosis after recurrence [
9]. However, very few studies to date have directly compared the efficacy of LAT and MTT in NSCLC patients with driver mutations who have oligo-recurrence, and whether LAT should be chosen over MTT in this population remains controversial. Therefore, in the present study we aimed to investigate the optimal initial treatment strategy for oligo-recurrence in NSCLC patients with driver mutations.
2. Materials and methods
2.1. Study design and patient selection
This retrospective study was approved by the Institutional Review Board of Cancer Institute Hospital, Japanese Foundation for Cancer Research in Tokyo, Japan (study approval no.: 2021-GA-1304), and was conducted in accordance with the amended Declaration of Helsinki. The need for written informed consent was waived because of the retrospective nature of the study and the anonymity of the subjects.
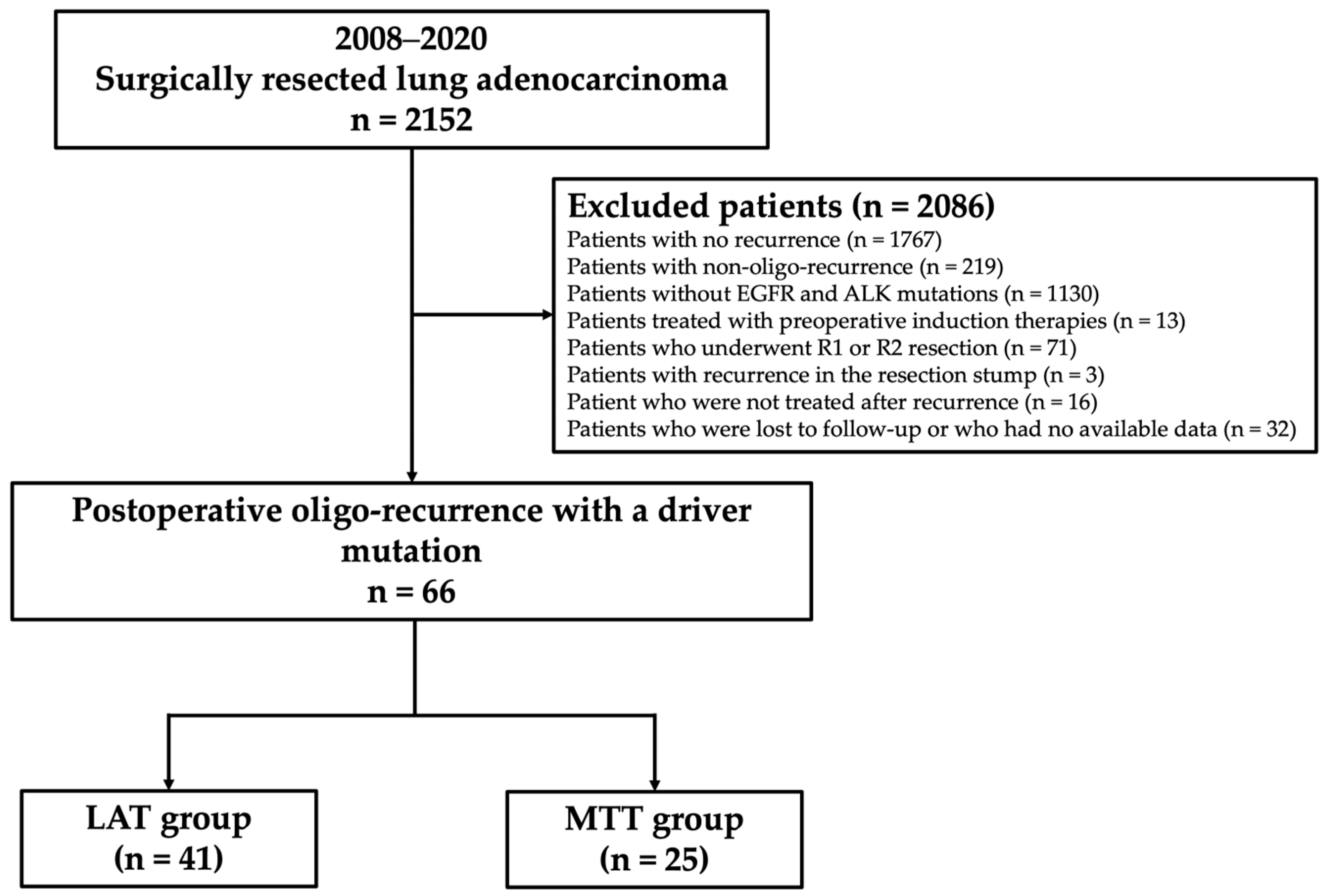
Of the 2,152 consecutive lung adenocarcinoma patients who underwent surgical resection at Cancer Institute Hospital, Japanese Foundation for Cancer Research, Tokyo, Japan, between 2008 and 2020, patients with EGFR mutations or ALK rearrangement who underwent lobectomy or more extensive pulmonary resection with mediastinal lymphadenectomy, and who developed postoperative recurrence of any type were analyzed. Patients who did not have recurrence (n = 1,767), did not have EGFR and ALK mutations (n = 1,130), were treated with preoperative induction therapies (n = 13), underwent R1 or R2 resection (n = 71), had recurrence at the resected stump (n = 3), did not undergo treatment after recurrence (n = 16), or were lost to follow-up or whose data were unavailable (n = 32) were excluded from the study. Initial recurrence was classified into two categories based on the recurrence site, i.e., locoregional recurrence and distant recurrence. Locoregional recurrence was defined as the presence of a tumor within the ipsilateral hemithorax and regional lymph nodes, such as the hilar, mediastinal, and cervical lymph nodes. Distant recurrence was defined as the presence of a tumor in the contralateral lung or outside the hemithorax. Furthermore, recurrence types were classified into two categories, i.e., oligo-recurrence and poly-recurrence. Oligo-recurrence was defined as one to three locoregional and/or distant recurrences as the first recurrence, with a regional lymph node station classified as a single organ. Poly-recurrence was defined as other types of recurrence. Patients with poly-recurrence were excluded from this study. A total of 66 patients with available data were finally evaluated. A flowchart showing the patient selection protocol is shown in
Figure 1.
Using medical records, data of the following clinicopathological characteristics were collected: age, sex, smoking status, pathological stage, tumor size, types of driver mutations, degree of histological differentiation, lymph-vascular invasion, visceral pleural invasion, intrapulmonary metastasis, time to recurrence (TTR) after surgery, time from recurrence to treatment, time from recurrence to initiation of tyrosine kinase inhibitor (TKI) treatment, recurrence pattern, number of recurrence lesions, and initial treatment post-recurrence. Pathological staging was performed according to the 8th edition of the Union for International Cancer Control guidelines [
10]. Degree of histological differentiation of the resected specimens was determined according to the 4th edition of the World Health Organization classification system [
11].
2.2. Postoperative surveillance and definition of recurrence patterns and initial therapies post-recurrence
All patients underwent chest and abdominal computed tomography (CT) and blood examinations twice a year for two years after the initial surgery, and once a year thereafter [
12]. Patients who presented with any symptom or sign of recurrence in these examinations underwent further evaluation, including brain magnetic resonance imaging and positron emission tomography. Recurrence was identified on the basis of the imaging findings, and was histologically and/or cytologically confirmed when possible. Recurrence sites and the number of recurrence lesions were reviewed. The initial treatment post-recurrence was classified into two categories, i.e., LAT and MTT. LAT was defined as treatments performed with curative intent, including complete surgical resection, stereotactic ablation radiotherapy, cerebral stereotactic radiosurgery, other radical radiation therapies of at least 45 Gy, and concurrent chemoradiotherapy. MTT included any kind of EGFR-TKI or ALK-TKI treatment that could be used in clinical practice. The course of treatment for each patient with recurrence was decided by the multidisciplinary thoracic oncology team at our institute based on a comprehensive decision incorporating recurrence patterns, performance status, and the patient’s social background and preference. Stereotactic radiosurgery is the preferred option for cerebral oligo-recurrence [
13]. All patients with recurrence underwent head, chest, and abdominal CT, and blood examinations every three months after the diagnosis of recurrence or the start of post-recurrence therapy. Disease progression was determined in accordance with the Response Evaluation Criteria in Solid Tumor, ver. 1.1 (RECIST 1.1). The prognoses of the two groups of patients who received LATs or MTTs as the post-recurrence initial therapy were compared. Moreover, recurrence patterns and prognoses, including the course of treatment after the initial therapy were investigated. Prognoses after recurrence were assessed using post-recurrence overall survival (PR-OS) and post-recurrence progression-free survival (PR-PFS) rates.
2.3. Detection of driver mutations
EGFR mutational status was determined using Cobas
® EGFR Mutation Test v2 (Roche Molecular Systems, Inc., Pleasanton, CA, USA) [
14]. ALK rearrangement was evaluated by immunohistochemical (IHC) analysis using ALK Detection Kits
® (Nichirei Bioscience, Tokyo, Japan) [
14]. ALK positivity on IHC analysis was defined as tumor cell staining of more than 80%. Positive cases were further examined for confirmation using Vysis
® ALK Break-Apart fluorescence
in situ hybridization (FISH) Probe Kits (Abbott Molecular, Chicago, IL, USA). ALK rearrangement was considered to be positive when more than 15% of the tumor cells demonstrated split signals or single red signals [
15]. All FISH and IHC analyses were performed using 4-µm-thick formalin-fixed paraffin-embedded tissue samples prepared from the surgically resected specimens. The results were evaluated by two experienced pulmonary pathologists (H.N. and Y.I.).
2.4. Statistical analysis
Between-group comparisons of patient characteristics were performed using the Mann-Whitney U test for continuous variables and the Fisher’s exact test for categorical variables. Survival curves were plotted using the Kaplan-Meier method, and compared using the log-rank test. Data of patients who were alive on November 30, 2023, were censored for survival analysis. TTR was calculated as the time from the date of surgery for resection of the primary lesion to the date of diagnosis of recurrence. Time from recurrence to treatment was calculated as the time from the date of diagnosis of recurrence to the date of initial treatment post-recurrence. Time from recurrence to initiation of TKI treatment was calculated as the time from the date of diagnosis of recurrence to the date of initiation of TKI treatment. PR-OS was calculated as the time from the date of disease recurrence to the date of death from any cause. PR-PFS was calculated as the time from the date of disease recurrence to the date of disease progression, death, or the last contact/follow-up if patients remained disease-free. Statistical significance was set at a p-value of less than 0.05. All statistical analyses were performed using the SPSS statistical software program (version 27.0; DDR3 RDIMM, SPSS Inc., Chicago, IL, USA).
3. Results
3.1. Patient characteristics
The clinicopathological characteristics of the enrolled patients are summarized in
Table 1. LATs or MTTs were administered as initial post-recurrence therapies to 41 (initial LAT group) and 25 (initial MTT group) patients. There was a significant difference in pathological stage between the two groups of patients, with the initial MTT group having more advanced disease. The number of recurrence lesions tended to be lower in the initial LAT group than in the initial MTT group.
Comparison of the sites of initial recurrence after the initial treatment in patients with oligo-recurrence with driver mutations are summarized in
Table 2. In the initial LAT group, 23 (56%) patients were also treated with MTTs, and in the initial MTT group, six (24%) patients received LATs as an additional therapy. The median time from recurrence to initiation of TKI treatment was longer in the initial LATs group than in the initial MMTs group (37 months vs. 1 month,
p < 0.001). However, the final number of MTTs used was not significantly different between the two groups.
3.2. Prognostic analyses
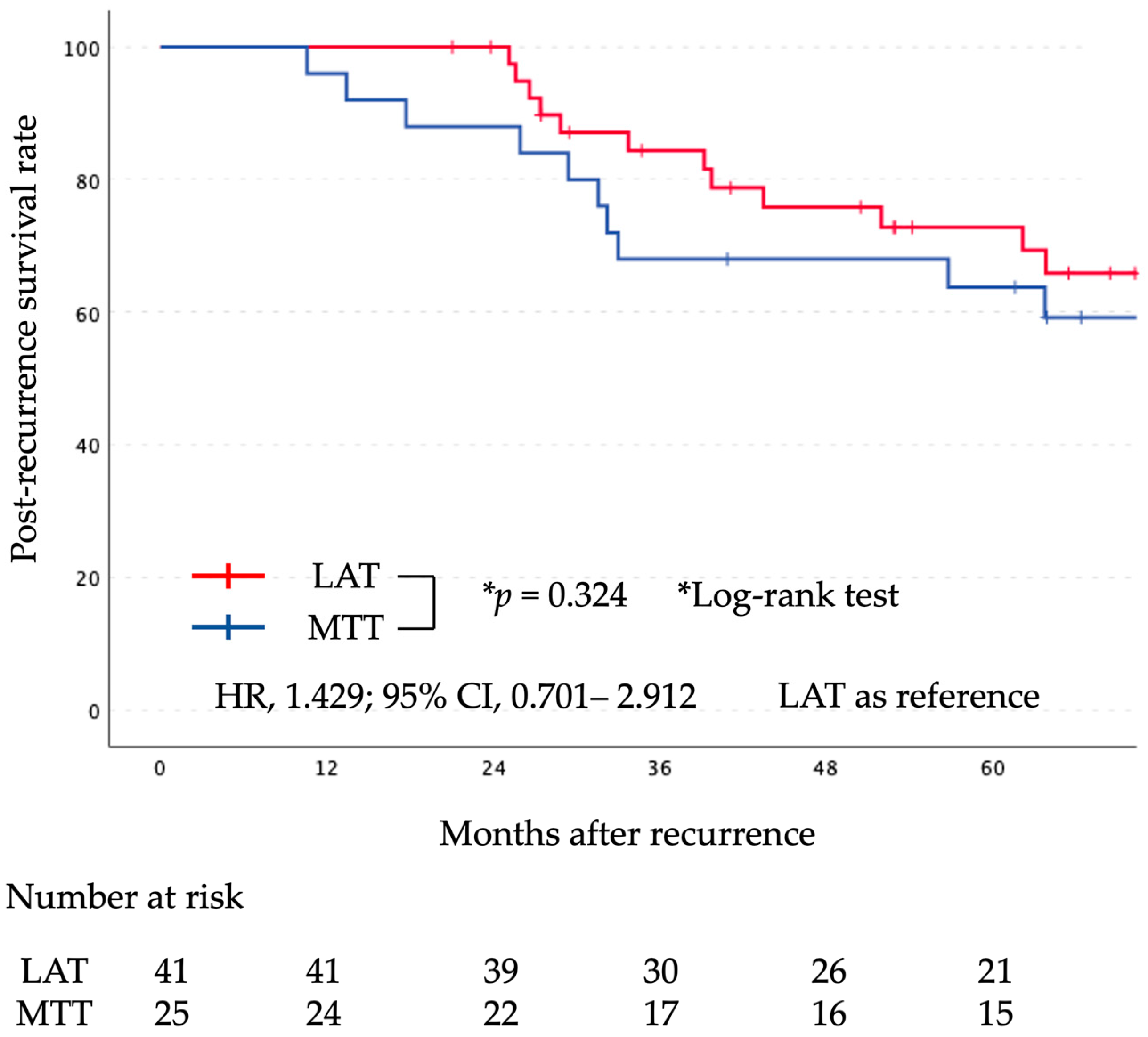
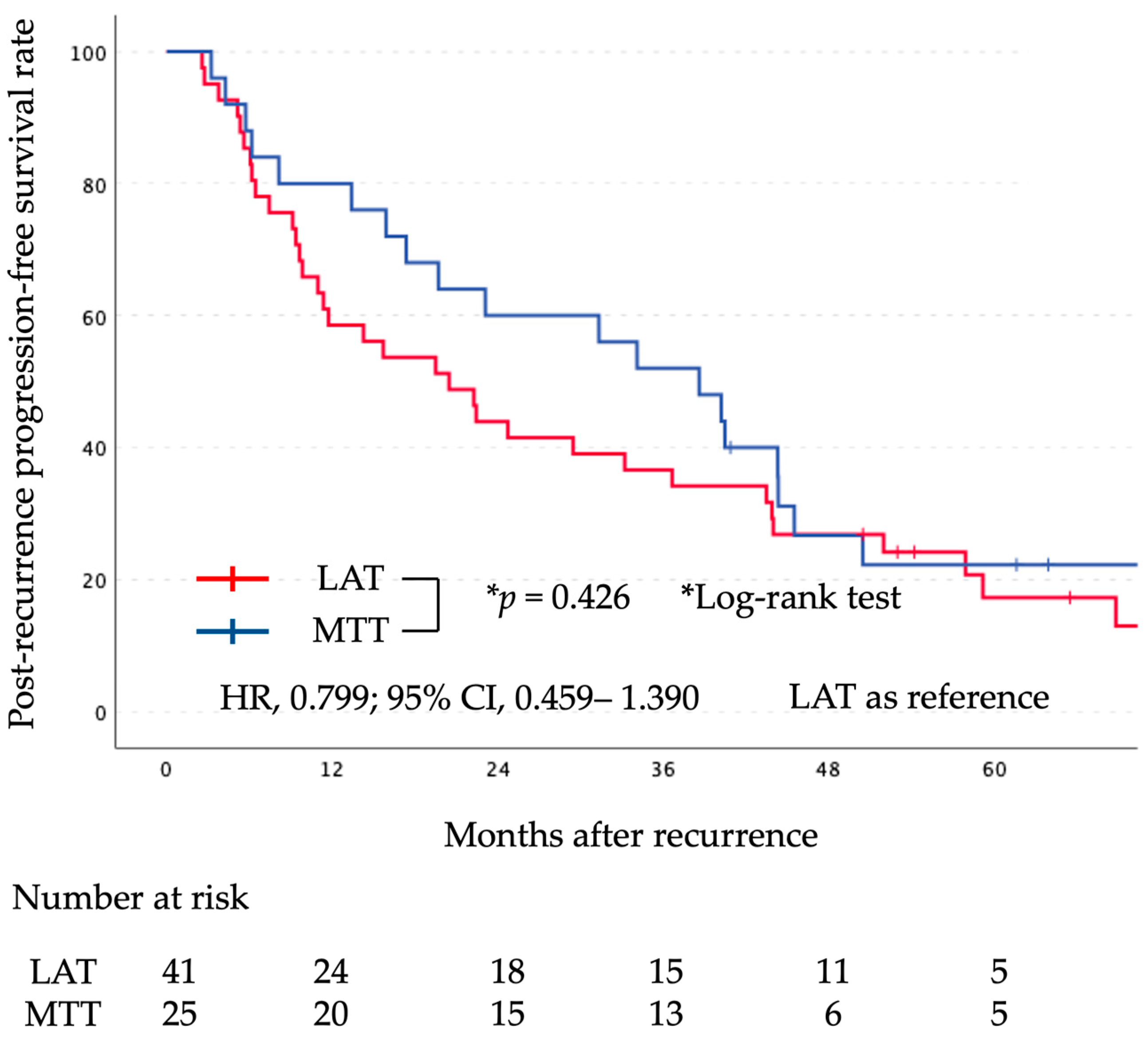
The median follow-up period after surgery was 71 months (range, 20–172 months). Kaplan-Meier curves comparing the two groups for PR-OS and PR-PFS are shown in
Figure 2 and
Figure 3, respectively. There was no significant difference in PR-OS (HR, 1.429; 95% CI, 0.701–2.912; log-rank,
p = 0.324) and PR-PFS (HR, 0.799; 95% CI, 0.459–1.390; log-rank,
p = 0.426) between the initial LAT group and the initial MTT group.
4. Discussion
4.1. Primary findings
The choice of LAT over MTT for NSCLC patients with driver mutations who have oligo-recurrence remains controversial. Our results demonstrated that LAT does not prolong either PR-OS or PFS of patients compared with MTT and it was not possible to show which was more effective compared with TKI. However, to our knowledge, the present study is one of the few studies performed to date investigating treatment strategies for lung cancer patients with driver mutations who have developed oligo-recurrence.
4.2. Post-recurrence therapies for oligo-recurrence patients with driver mutations
LATs have been shown to improve the prognosis of both advanced NSCLC patients with oligo-metastasis who do not show progression after the first-line systemic therapy, and those who develop postoperative oligo-recurrence [
7,
8,
9,
16,
21,
22,
23,
24,
27,
28,
31]. On the other hand, the efficacy of MTTs for postoperative recurrence of NSCLC has been reported [
25,
29,
30]. However, the usefulness of LATs for NSCLC patients with driver mutations has not been adequately investigated. The Randomized Phase III Trial of First-Line Tyrosine Kinase Inhibitor With or Without Radiotherapy for Synchronous Oligometastatic EGFR-Mutated NSCLC demonstrated the effectiveness of radiation therapy (RT) [
32]. A randomized phase II trial of osimertinib, a representative EGFR-tyrosine kinase inhibitor, with or without LAT for patients harboring EGFR mutations with oligo-metastatic NSCLC is currently ongoing [
17]. However, NSCLC patients with driver mutations are often excluded from participating in clinical trials on oligo-metastasis [
18]. This is because most of the driver mutations are adenocarcinomas, and furthermore, MTTs can have substantial therapeutic effects on patients with driver mutations, which are different from the effects on NSCLC patients without driver mutations [
1,
2,
3]. Therefore, it may be difficult to conduct prospective clinical trials regarding an optimal treatment strategy for NSCLC patients with driver mutations who have postoperative oligo-recurrence. We found only 1 study that directly compared the efficacy of LATs and MTTs on oligo-recurrence in patients with EGFR-mutated NSCLC, but this previous study also found no significant difference in PR-OS between LAT and MTT [
26].
4.3. Interpretation of the study findings
The main findings of this study are as follows. First, there was no significant difference between the initial LAT group and the initial MTT group in both PR-OS and PR-PFS, and it is hence not possible to conclude which treatment will result in a more favorable prognosis. Second, in this study, 29 patients (71%) in the initial LAT group were eventually treated with MTTs. However, nine patients (22%) in the initial LAT group had no recurrence after treatment, and were cured. Finally, LAT was found to delay the initiation of MTT. This was thought to delay the development of resistance to MTTs, but no significant difference was found in the final number of MTTs used. On the other hand, LAT has the advantage of reducing the high medical costs of TKI treatment, which is favorable from the point of view of the patients and health care economics. Therefore, our results imply that a combination of LATs and MTTs may be a promising treatment strategy for NSCLC patients with driver mutations who develop oligo-recurrence, but that LATs should be considered first.
4.4. Study limitations
This study has some limitations. First, this was a retrospective observational study at a single institute. The number of cases enrolled was relatively small, which in turn affected the statistical robustness. Second, the criterion of one to three lesions for oligo-recurrence was unique to this study. Regarding oligo-metastasis, the definition has been formulated by a multidisciplinary consensus statement, which is a maximum of five metastases and three organs that can be radically treated for all tumor sites [
19]. No similar consensus has been reached on oligo-recurrence, and the definition of oligo-recurrence is not uniform [
5,
6,
9,
16]. However, the European Society for Therapeutic Radiology and Oncology, the European Organization for Research and Treatment of Cancer and the American Society for Radiation Oncology have jointly published a consensus report on the definition and classification of oligo-metastases, and therapeutic strategies for oligo-metastases based on this consensus definition and classification are being developed [
33]. At the consensus meeting, most researchers agreed that the criterion for oligo-metastases should be a maximum of 2 metastatic organs and 3 metastases [
34]. Third, the post-recurrence treatment strategy was not standardized among patients because of the retrospective study design. In fact, some patients with driver mutations were treated without LAT despite oligo-recurrence. Finally, we did not investigate other gene alterations (e.g., ROS1, RET, BRAF, and MET), which could have also affected the study results. However, the use of broad genomic sequencing may not result in a more favorable prognosis, because no specific inhibitors for these rare driver mutations are currently available [
20]. Further large clinical studies are needed to overcome these limitations. In addition, our findings may be modified with the development of new agents.
5. Conclusions
LAT as a first-line treatment did not show statistically significant differences in post-recurrence survival or progression-free survival compared with molecular targeted therapies. However, local therapies as an initial treatment may be preferable, as they may be effective in curing recurrence, and delay the start of administration of molecular targeted therapies.
Author Contributions
Conceptualization, Y.M.; methodology, Y.M.; software, T.T. and Y.M.; validation, T.T., Y.M., H.N., J.I., M.N., M.N., S.O., N.I, and M.M.; formal analysis, T.T. and Y.M.; investigation, T.T. and Y.M.; resources, T.T. and Y.M.; data curation, T.T, Y.M., H.N., J.I., and M.N.; writing—original draft preparation, T.T. and Y.M.; writing—review and editing, T.T., Y.M., H.N., J.I., M.N., M.N., S.O., N.I, and M.M.; visualization,(( T.T. and Y.M. All authors have read and agreed to the published version of the manuscript.
Funding
The authors received no external funding for this research.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board of the Cancer Institute Hospital of the Japanese Foundation for Cancer Research in Tokyo, Japan (study approval no.: 2021-GA-1304).
Informed Consent Statement
Patient consent was waived due to the retrospective nature of the study and the anonymity of subjects.
Data Availability Statement
The data of this study is available from the authors upon reasonable request.
Acknowledgments
The authors thank Ms. Tomoyo Kakita for providing assistance with the immunohistochemical staining, Mr. Motoyoshi Iwakoshi for tissue slice preparation, Ms. Miyuki Kogure for slide preparation, and Dr. Yuichi Ishikawa for providing valuable advice on the pathological diagnoses.
Conflicts of Interest
The authors declare that they have no conflicts of interest in association with this study.
References
- Lynch, T.J.; Bell, D.W.; Sordella, R. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med, 2004, 350(21), 2129-2139. [CrossRef]
- Paez, J.G.; Jänne, P.A.; Lee, J.C. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science, 2004, 304(5676), 1497-1500. [CrossRef]
- Kwak, E.L.; Bang Y.J, Camidge, D.R. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med, 2010, 363(18), 1693-1703. [CrossRef]
- Socinski, M.A.; Evans, T.; Gettinger, S. Treatment of stage IV non-small cell lung cancer: diagnosis and management of lung cancer, diagnosis and management of lung cancer, 3rd ed. American College of Chest Physicians evidence-based clinical practice guidelines. Chest, 2013, 143(5)(suppl), e341S-e368S. [CrossRef]
- Patel, P.R.; Yoo, D.S.; Niibe, Y. A call for the aggressive treatment of oligometastatic and oligo-recurrent non-small cell lung cancer. Pulm Med, 2012, 480961. [CrossRef]
- Hellman, S.; Weichselbaum, R.R. Oligometastases. J Clin Oncol, 1995, 13(1), 8-10. [CrossRef]
- Gomez, D.R.; Blumenschein G.R.; Lee, J.J. Local consolidative therapy versus maintenance therapy or observation for patients with oligometastatic non-small-cell lung cancer without progression after first-line systemic therapy: a multicentre, randomised, controlled, phase 2 study. Lancet Oncol, 2016, 17(12), 1672-1682. [CrossRef]
- Gomez, D.R.; Tang, C.; Zhang, J. Local consolidative therapy vs. maintenance therapy or observation for patients with oligometastatic non–small-cell lung cancer: long-term results of a multi-institutional, phase II, randomized study. J Clin Oncol, 2019, 37(18), 1558-1565. [CrossRef]
- Hishida, T.; Yoshida, J.; Aokage, K. Postoperative oligo-recurrence of non-small-cell lung cancer: clinical features and survival†. Eur J Cardiothorac Surg, 2016, 49(3), 847-853. [CrossRef]
- Travis, W.D.; Asamura, H.; Bankier, A.A. The IASLC lung cancer staging project: proposals for coding T categories for subsolid nodules and assessment of tumor size in part-solid tumors in the forthcoming eighth edition of the TNM classification of lung cancer. J Thorac Oncol, 2016, 11(8), 1204-1223. [CrossRef]
- Travis, W.D.; Ladanyi, M.; Scagliotti, G. Adenocarcinoma. In: Travis WD, Brambilla E, Burke AP, Marx A, Nicholson AG, eds., WHO Classification of Tumours of the Lung, Pleura, Thymus and Heart. 4th ed, international. Lyon: Agency for Research on Cancer, 2015, 26-37.
- Postmus, P. E.; Kerr, K.M.; Oudkerk, M. Early and locally advanced non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol, 2017, 28(suppl_4), iv1-iv21. [CrossRef]
- Ashworth, A.; Rodrigues, G.; Boldt, G. Is there an oligometastatic state in non-small cell lung cancer? A systematic review of the literature. Lung Cancer, 2013, 82(2), 197-203. [CrossRef]
- Heeke, S.; Benzaquen, J.; Hofman, V. Critical assessment in routine clinical practice of liquid biopsy for EGFR status testing in non–small-cell lung cancer: A single-laboratory experience (LPCE, Nice, France). Clin Lung Cancer, 2020, 21(1), 56-65, e8. [CrossRef]
- Takeuchi, K.; Togashi, Y.; Kamihara, Y. Prospective and clinical validation of ALK immunohistochemistry: results from the phase I/II study of alectinib for ALK-positive lung cancer (AF-001JP study). Ann Oncol, 2016, 27(1), 185-192. [CrossRef]
- Matsuguma, H.; Nakahara, R.; Wakamatsu, I. Definitive local therapy for oligo-recurrence in patients with completely resected non-small cell lung cancer. Am J Clin Oncol, 2020, 43(3), 210-217. [CrossRef]
- Tahsin, M. K.; Emily, A. V.; Saumil G. Osimertinib. Surgery, and radiation therapy in treating patients with stage IIIB or IV non-small cell lung cancer with EGFR mutations (NORTHSTAR). Ann Surg Oncol, 2022, 29(8), 4688-4689. [CrossRef]
- Taichi, M.; Hirotsugu, K.; Hideyuki, H. Phase II Study of Multidisciplinary Therapy using Pembrolizumab for Patients with Synchronous Oligometastatic Stage IV Non-Small Cell Lung Cancer (TRAP OLIGO study) (WJOG11118L). BMC cancer, 2021, 21, 1121.
- Dingemans, A. C.; Hendriks, L.E.L.; Berghmans T. Definition of synchronous oligometastatic non-small cell lung cancer-A consensus report. J Thorac Oncol, 2019, 14(12), 2109-2119. [CrossRef]
- Presley, C. J.; Tang, D.; Soulos, P.R. Association of broad-based genomic sequencing with survival among patients with advanced non-small cell lung cancer in the community oncology setting. JAMA. 2018, 320(5), 469-477. [CrossRef]
- Niibe, Y.; Kazumoto, T. Toita, T. Frequency and characteristics of isolated para-aortic lymph node recurrence in patients with uterine cervical carcinoma in Japan: a multi-institutional study. Gynecol Oncol. 2006, 103, 435-438. [CrossRef]
- Niibe, Y.; Kenjo, M.; Kazumoto, T. Multi-institutional study of radiation therapy for isolated para-aortic lymph node recurrence in uterine cervical carcinoma: 84 subjects of a population of more than 5,000. Int J Radiat Oncol Biol Phys. 2006, 66, 1366–1369. [CrossRef]
- Niibe, Y.; Kuranami, M.; Matsunaga, K. Value of high-dose radiation therapy for isolated osseous metastasis in breast cancer in terms of oligo-recurrence. Anticancer Res. 2008, 28, 3929–3931.
- Niibe, Y.; Hayakawa, K. Oligometastases and oligo-recurrence: the new era of cancer therapy. Jpn J Clin Oncol. 2010, 40, 107-111. [CrossRef]
- Moriya, T.; Hamaji, M.; Yoshizawa, A. Epidermal growth factor receptor (EGFR)-tyrosine kinase inhibitors as a first-line treatment for postoperative recurrent and EGFR- mutated non-small-cell lung cancer. Interact Cardiovasc Thorac Surg. 2022, 34, 416–423. [CrossRef]
- Sonoda, D.; Kondo, Y.; Maruyama R. Examination of the effectiveness of local therapy for oligo-recurrence of EGFR-mutated NSCLC. Thorac Cancer. 2023, 14, 766-772. [CrossRef]
- Sonoda, D.; Matsuura, Y.; Kondo, Y. A Reasonable Definition of Oligo-Recurrence in Non-Small-Cell Lung Cancer. Clinical Lung Cancer. 2022, 23(1), 82-90. [CrossRef]
- Niibe, Y.; Nishimura, T.; Inoue, T. Oligo-recurrence predicts favorable prognosis of brain-only oligometastases in patients with non-small cell lung cancer treated with stereotactic radiosurgery or stereotactic radiotherapy: a multiinstitutional study of 61 subjects. BMC Cancer. 2016, 16:659, 1-11. [CrossRef]
- Mitsudomi, T.; Kosaka, T.; Endoh, Hideki. Mutations of the epidermal growth factor receptor gene predict prolonged survival after gefitinib treatment in patients with non-small-cell lung cancer with postoperative recurrence. J Clin Oncol. 2005, 23(11), 2513-2520. [CrossRef]
- Matsuura, Y.; Ninomiya, H.; Ichinose, J. Prognostic impact and distinctive characteristics of surgically resected anaplastic lymphoma kinase-rearranged lung adenocarcinoma. JTCS. 2022, 163(2), 441-451. [CrossRef]
- Niibe, Y.; Yamamoto, T.; Onishi, H.; Pulmonary oligometastases treated be stereotactic body radiation therapy. Anticancer Res. 2020, 40(1), 393-399. [CrossRef]
- Wang, XS.; Bai, YF.; Verma, V. Randomized Trial of First-Line Tyrosine Kinase Inhibitor With ir Without Radiotherapy for Synchronous Oligometastic EGFR-Mutated Non-Small Cell Lung Cancer. J Natl Cancer Inst. 2023, 115(6), 742-748. [CrossRef]
- Guckenberger, M.; Lievens. Y.; Bouma, AB.; Characterisation and classification of oligometastatic disease. Lancet Oncol. 2020, 21, e18-28. [CrossRef]
- Levy, A.; Hendriks, LEL.; Berghmans, T.; EORTC Lung Cancer Group survey on the definition of NSCLC synchronous oligometastatic disease. Eur J Cancer. 2019, 122, 109-114. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).