Submitted:
27 December 2023
Posted:
28 December 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
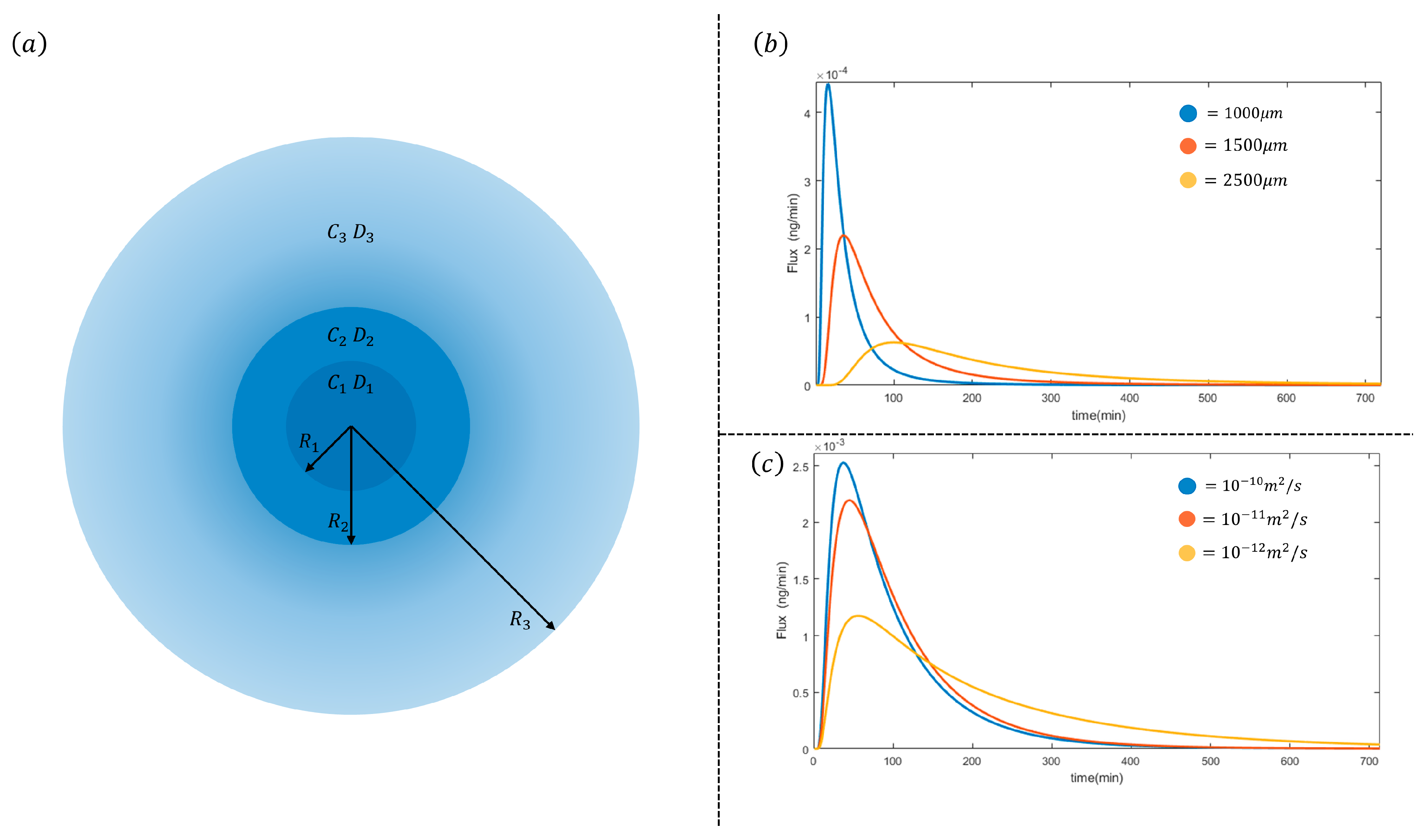
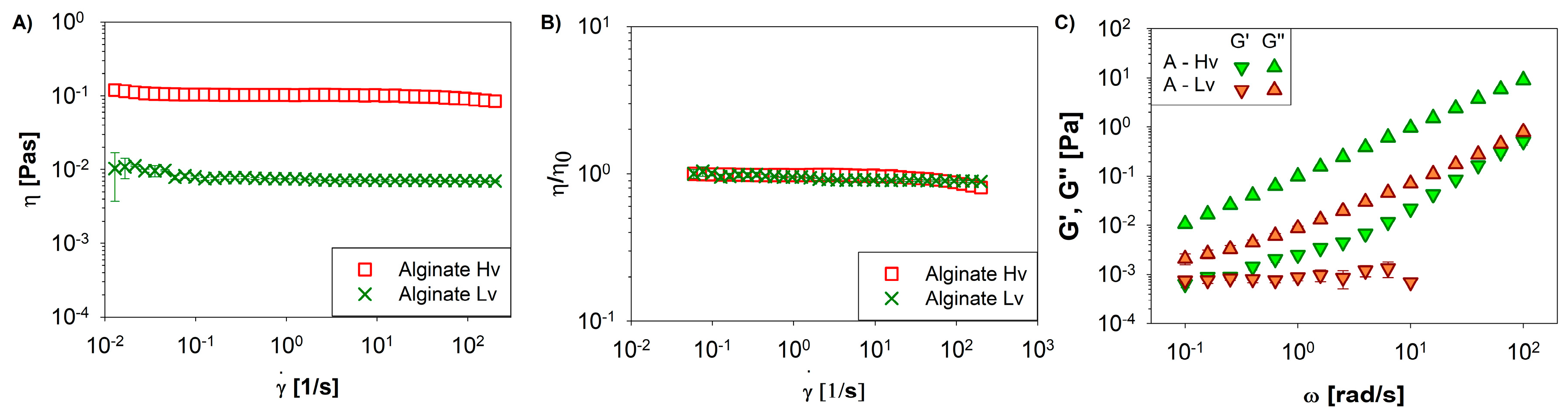
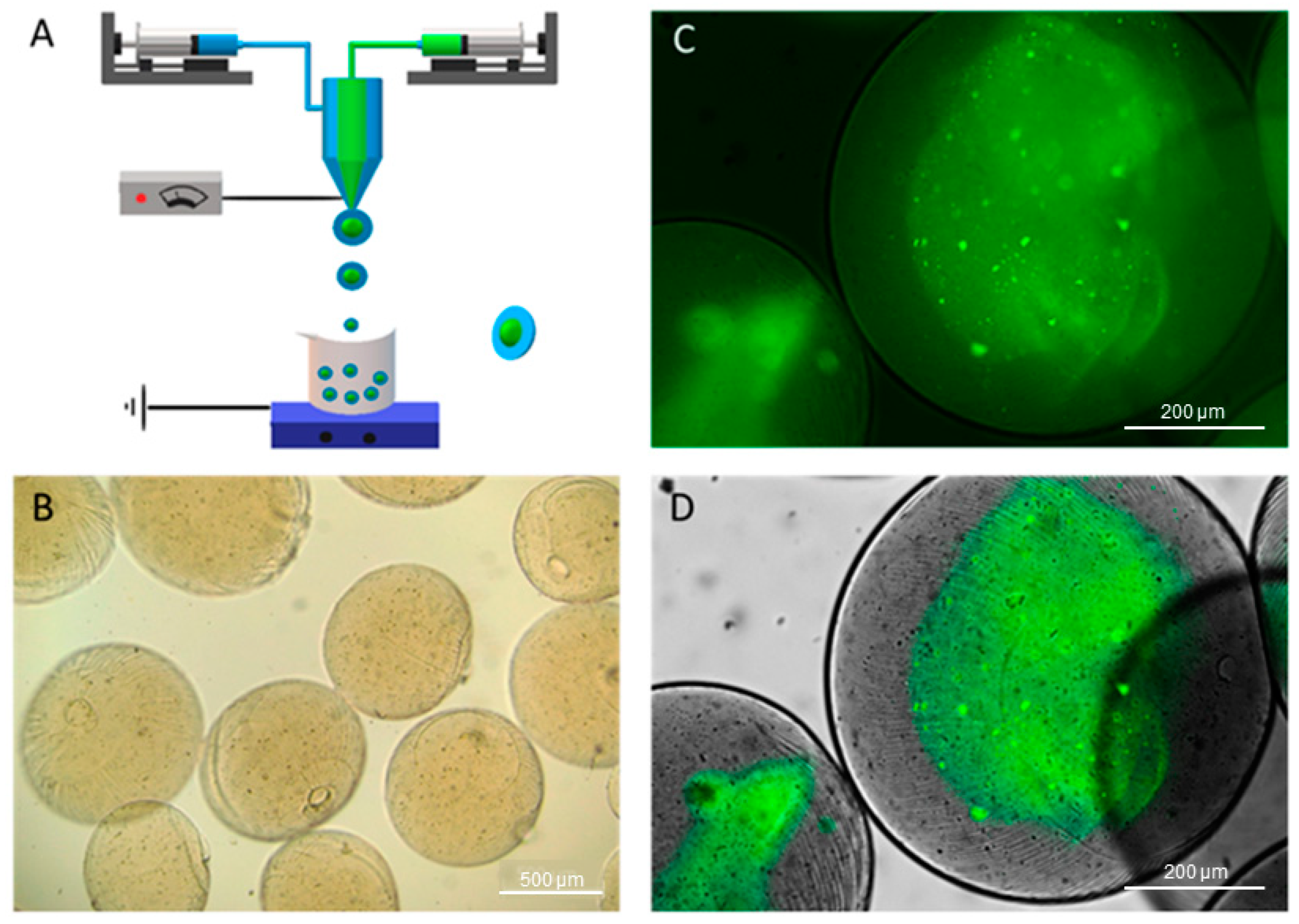
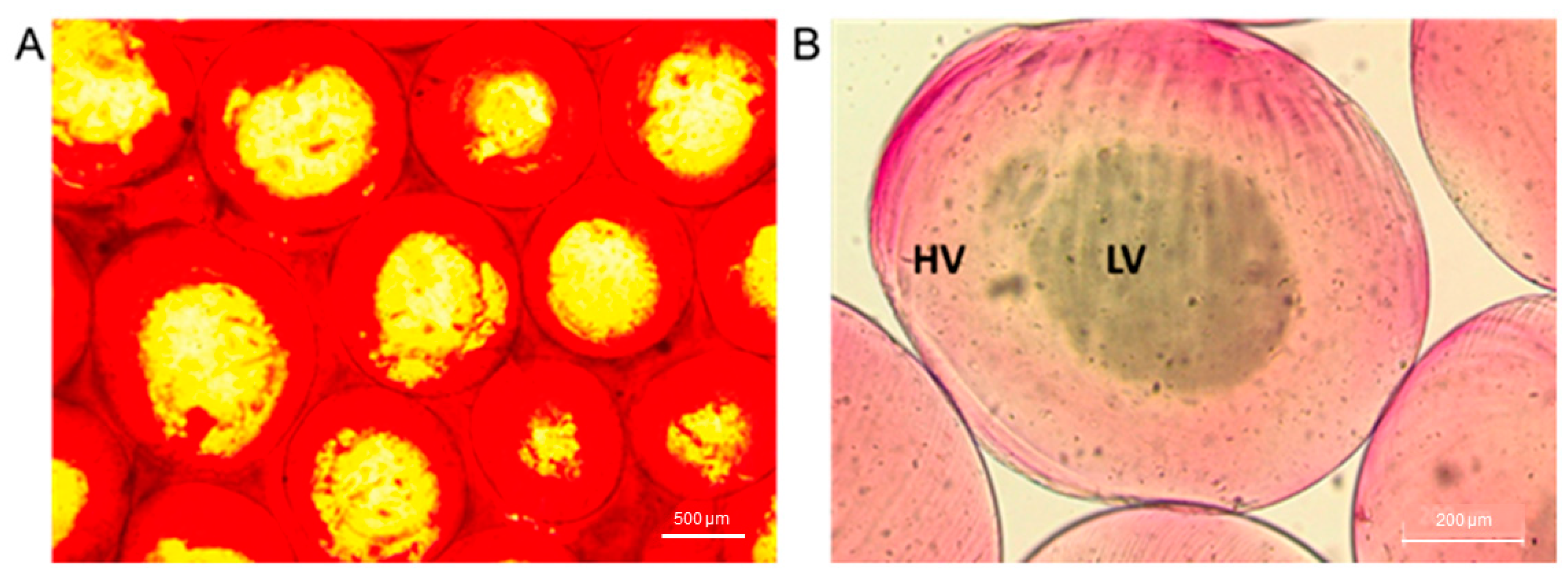
2. Results and Discussion
| Parameter | Value | Units |
|---|---|---|
4. Materials and Methods
2.1. Preparation of Alginate Microparticles
2.2. Microparticles characterization
2.3. Rheological setup
2.4. Modelling
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Faheem, A.M.; Abdelkader, D.H. Novel Drug Delivery Systems. In Engineering Drug Delivery Systems; Seyfoddin, A., Dezfooli, S.M., Greene, C.A., Eds.; Elsevier, 2020; pp. 1–16.
- Romana, B.; Hassan, Md.M.; Sonvico, F.; Garrastazu Pereira, G.; Mason, A.F.; Thordarson, P.; Bremmell, K.E.; Barnes, T.J.; Prestidge, C.A. A Liposome-Micelle-Hybrid (LMH) Oral Delivery System for Poorly Water-Soluble Drugs: Enhancing Solubilisation and Intestinal Transport. European Journal of Pharmaceutics and Biopharmaceutics 2020, 154, 338–347. [Google Scholar] [CrossRef]
- Sultana, A.; Zare, M.; Thomas, V.; Kumar, T.S.S.; Ramakrishna, S. Nano-Based Drug Delivery Systems: Conventional Drug Delivery Routes, Recent Developments and Future Prospects. Med Drug Discov 2022, 15, 100134. [Google Scholar] [CrossRef]
- Liu, R.; Luo, C.; Pang, Z.; Zhang, J.; Ruan, S.; Wu, M.; Wang, L.; Sun, T.; Li, N.; Han, L.; et al. Advances of Nanoparticles as Drug Delivery Systems for Disease Diagnosis and Treatment. Chinese Chemical Letters 2023, 34, 107518. [Google Scholar] [CrossRef]
- Ahmad, A.; Mubarak, N.M.; Jannat, F.T.; Ashfaq, T.; Santulli, C.; Rizwan, M.; Najda, A.; Bin-Jumah, M.; Abdel-Daim, M.M.; Hussain, S.; et al. A Critical Review on the Synthesis of Natural Sodium Alginate Based Composite Materials: An Innovative Biological Polymer for Biomedical Delivery Applications. Processes 2021, 9. [Google Scholar] [CrossRef]
- Prasher, P.; Sharma, M.; Mehta, M.; Satija, S.; Aljabali, A.A.; Tambuwala, M.M.; Anand, K.; Sharma, N.; Dureja, H.; Jha, N.K.; et al. Current-Status and Applications of Polysaccharides in Drug Delivery Systems. Colloid Interface Sci Commun 2021, 42, 100418. [Google Scholar] [CrossRef]
- Agüero, L.; Zaldivar-Silva, D.; Peña, L.; Dias, M.L. Alginate Microparticles as Oral Colon Drug Delivery Device: A Review. Carbohydr Polym 2017, 168, 32–43. [Google Scholar] [CrossRef]
- Hariyadi, D.M.; Islam, N. Current Status of Alginate in Drug Delivery. Adv Pharmacol Pharm Sci 2020, 2020, 8886095. [Google Scholar] [CrossRef] [PubMed]
- Jeyhani, M.; Mak, S.Y.; Sammut, S.; Shum, H.C.; Hwang, D.K.; Tsai, S.S.H. Controlled Electrospray Generation of Nonspherical Alginate Microparticles. ChemPhysChem 2018, 19, 2113–2118. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Wei, W.; Ni, B.-J. Chapter 3 - Algae-Based Alginate Biomaterial: Production and Applications. In Biomass, Biofuels, and Biochemicals; Ngo, H., Guo, W., Pandey, A., Chang, J.-S., Lee, D.-J., Eds.; Elsevier, 2022; pp. 37–66 ISBN 978-0-323-96142-4.
- Altobelli, R.; Guarino, V.; Ambrosio, L. Micro- and Nanocarriers by Electrofludodynamic Technologies for Cell and Molecular Therapies. Process Biochemistry 2016, 51, 2143–2154. [Google Scholar] [CrossRef]
- Guarino, V.; Khodir, W.K.W.A.; Ambrosio, L. Biodegradable Microparticles and Nanoparticles by Electrospraying Techniques. J Appl Biomater Funct Mater 2012, 10, 191–196. [Google Scholar] [CrossRef]
- Yang, J.-S.; Xie, Y.-J.; He, W. Research Progress on Chemical Modification of Alginate: A Review. Carbohydr Polym 2011, 84, 33–39. [Google Scholar] [CrossRef]
- Irvine, J.; Afrose, A.; Islam, N. Formulation and Delivery Strategies of Ibuprofen: Challenges and Opportunities. Drug Dev Ind Pharm 2018, 44, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Yayehrad, A.T.; Wondie, G.B.; Marew, T. Different Nanotechnology Approaches for Ciprofloxacin Delivery Against Multidrug-Resistant Microbes. Infect Drug Resist 2022, 15, 413–426. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhu, Q.; Li, Z.; Yan, H.; Lin, Q. The Molecular Structure and Self-Assembly Behavior of Reductive Amination of Oxidized Alginate Derivative for Hydrophobic Drug Delivery. Molecules 2021, 26. [Google Scholar] [CrossRef] [PubMed]
- Zou, Z.; Zhang, B.; Nie, X.; Cheng, Y.; Hu, Z.; Liao, M.; Li, S. A Sodium Alginate-Based Sustained-Release IPN Hydrogel and Its Applications. RSC Adv 2020, 10, 39722–39730. [Google Scholar] [CrossRef] [PubMed]
- Hariyadi, D.M.; Islam, N. Current Status of Alginate in Drug Delivery. Adv Pharmacol Pharm Sci 2020, 2020, 8886095. [Google Scholar] [CrossRef]
- Abourehab, M.A.S.; Rajendran, R.R.; Singh, A.; Pramanik, S.; Shrivastav, P.; Ansari, M.J.; Manne, R.; Amaral, L.S.; Deepak, A. Alginate as a Promising Biopolymer in Drug Delivery and Wound Healing: A Review of the State-of-the-Art. Int J Mol Sci 2022, 23. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Zuo, X.; Zhou, Z.; Gu, Y.; Zheng, H.; Wang, X.; Wang, G.; Xu, C.; Wang, F. PLGA-Based Micro/Nanoparticles: An Overview of Their Applications in Respiratory Diseases. Int J Mol Sci 2023, 24. [Google Scholar] [CrossRef] [PubMed]
- Pushpamalar, J.; Sathasivam, T.; Gugler, M.C. Hydrogel BeadsBeads of Natural PolymersPolymers as a Potential Vehicle for Colon-Targeted Drug DeliveryDrug Delivery BT - Bio-Carrier Vectors: Methods and Protocols; Narayanan, K., Ed.; Springer US: New York, NY, USA, 2021; pp. 171–182. ISBN 978-1-0716-0943-9. [Google Scholar]
- Gali, L.; Pirozzi, A.; Donsì, F. Biopolymer- and Lipid-Based Carriers for the Delivery of Plant-Based Ingredients. Pharmaceutics 2023, 15. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Zhang, B.; Sun, R.; Liu, W.; Zhu, Q.; Zhang, X.; Wang, R.; Chen, C. PLGA-Based Biodegradable Microspheres in Drug Delivery: Recent Advances in Research and Application. Drug Deliv 2021, 28, 1397–1418. [Google Scholar] [CrossRef]
- Jaworek, A. 1 - Electrohydrodynamic Microencapsulation Technology. In Encapsulations; Grumezescu, A.M., Ed.; Academic Press, 2016; pp. 1–45 ISBN 978-0-12-804307-3.
- Guarino, V.; Ambrosio, L.; Bellini, D. Process for the Preparation of Microspheres Comprising Semisynthetic Polymers 2009.
- Zhao, S.; Huang, C.; Yue, X.; Li, X.; Zhou, P.; Wu, A.; Chen, C.; Qu, Y.; Zhang, C. Application Advance of Electrosprayed Micro/Nanoparticles Based on Natural or Synthetic Polymers for Drug Delivery System. Mater Des 2022, 220, 110850. [Google Scholar] [CrossRef]
- Cruz-Maya, I.; Guarino, V. 3D Scaffolds Fabrication via Bicomponent Microgels Assembly: Process Optimization and In Vitro Characterization. Micromachines (Basel) 2022, 13, 1726. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Maya, I.; Altobelli, R.; Alvarez-Perez, M.A.; Guarino, V. Mineralized Microgels via Electrohydrodynamic Atomization: Optimization and In Vitro Model for Dentin–Pulp Complex. Gels 2023, 9. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Maya, I.; Altobelli, R.; Marrese, M.; Guarino, V. Design of Alginate Based Micro-gels via Electro Fluid Dynamics to Construct Microphysiological Cell Culture Systems. Polym Adv Technol 2021, 32, 2981–2989. [Google Scholar] [CrossRef]
- Rentería-Ortega, M.; Salgado-Cruz, M.D.L.P.; Morales-Sánchez, E.; Alamilla-Beltrán, L.; Farrera-Rebollo, R.R.; Valdespino León, M.; Calderón-Domínguez, G. Effect of Electrohydrodynamic Atomization Conditions on Morphometric Characteristics and Mechanical Resistance of Chia Mucilage-Alginate Particles. CyTA - Journal of Food 2020, 18, 461–471. [Google Scholar] [CrossRef]
- Guarino, V.; Caputo, T.; Calcagnile, P.; Altobelli, R.; Demitri, C.; Ambrosio, L. proce Cellulose-Based Microspheres for Oral Administration of Ketoprofen Lysinate. J Biomed Mater Res B Appl Biomater 2018, 106, 2636–2644. [Google Scholar] [CrossRef]
- Zhang, X.; Qu, Q.; Zhou, A.; Wang, Y.; Zhang, J.; Xiong, R.; Lenders, V.; Manshian, B.B.; Hua, D.; Soenen, S.J.; et al. Core-Shell Microparticles: From Rational Engineering to Diverse Applications. Adv Colloid Interface Sci 2022, 299, 102568. [Google Scholar] [CrossRef] [PubMed]
- Guarino, V.; Altobelli, R.; Caputo, T.; Ambrosio, L.; Caserta, S.; Calcagnile, P.; Demitri, C. Mono- and Bi-Phasic Cellulose Acetate Micro-Vectors for Anti-Inflammatory Drug Delivery. Pharmaceutics 2019, 11. [Google Scholar] [CrossRef] [PubMed]
- Enayati, M.; Ahmad, Z.; Stride, E.; Edirisinghe, M. One-Step Electrohydrodynamic Production of Drug-Loaded Micro- and Nanoparticles. J R Soc Interface 2009, 7, 667–675. [Google Scholar] [CrossRef]
- Siepmann, J.; Siepmann, F. Mathematical Modeling of Drug Delivery. Int J Pharm 2008, 364, 328–343. [Google Scholar] [CrossRef]
- Kim, M.K.; Lee, J.Y.; Oh, H.; Song, D.W.; Kwak, H.W.; Yun, H.; Um, I.C.; Park, Y.H.; Lee, K.H. Effect of Shear Viscosity on the Preparation of Sphere-like Silk Fibroin Microparticles by Electrospraying. Int J Biol Macromol 2015, 79, 988–995. [Google Scholar] [CrossRef]
- Rutkowski, S.; Si, T.; Gai, M.; Frueh, J.; He, Q. Hydrodynamic Electrospray Ionization Jetting of Calcium Alginate Particles: Effect of Spray-Mode, Spraying Distance and Concentration. RSC Adv 2018, 8, 24243–24249. [Google Scholar] [CrossRef]
- Dodero, A.; Vicini, S.; Alloisio, M.; Castellano, M. Rheological Properties of Sodium Alginate Solutions in the Presence of Added Salt: An Application of Kulicke Equation. [CrossRef]
- Dodero, A.; Vicini, S.; Alloisio, M.; Castellano, M. Sodium Alginate Solutions: Correlation between Rheological Properties and Spinnability. J Mater Sci 2019, 54, 8034–8046. [Google Scholar] [CrossRef]
- Miller, C.C.; Walker, J. The Stokes-Einstein Law for Diffusion in Solution. Proceedings of the Royal Society of London. Series A, Containing Papers of a Mathematical and Physical Character 1997, 106, 724–749. [Google Scholar] [CrossRef]
- Lokhande, A.B.; Mishra, S.; Kulkarni, R.D.; Naik, J.B. Influence of Different Viscosity Grade Ethylcellulose Polymers on Encapsulation and in Vitro Release Study of Drug Loaded Nanoparticles. J Pharm Res 2013, 7, 414–420. [Google Scholar] [CrossRef]
- Yan, W.-C.; Tong, Y.W.; Wang, C.-H. Coaxial Electrohydrodynamic Atomization toward Large Scale Production of Core-Shell Structured Microparticles. AIChE Journal 2017, 63, 5303–5319. [Google Scholar] [CrossRef]
- van der Kooij, R.S.; Steendam, R.; Frijlink, H.W.; Hinrichs, W.L.J. An Overview of the Production Methods for Core–Shell Microspheres for Parenteral Controlled Drug Delivery. European Journal of Pharmaceutics and Biopharmaceutics 2022, 170, 24–42. [Google Scholar] [CrossRef]
- Eleftheriadou, D.; Evans, R.E.; Atkinson, E.; Abdalla, A.; Gavins, F.K.H.; Boyd, A.S.; Williams, G.R.; Knowles, J.C.; Roberton, V.H.; Phillips, J.B. An Alginate-Based Encapsulation System for Delivery of Therapeutic Cells to the CNS. RSC Adv 2022, 12, 4005–4015. [Google Scholar] [CrossRef] [PubMed]
- Cu, Y.; Saltzman, W.M. Mathematical Modeling of Molecular Diffusion through Mucus. Adv Drug Deliv Rev 2009, 61, 101–114. [Google Scholar] [CrossRef] [PubMed]
- Thu, B.; Gåserød, O.; Paus, D.; Mikkelsen, A.; Skjåk-Bræk, G.; Toffanin, R.; Vittur, F.; Rizzo, R. Inhomogeneous Alginate Gel Spheres: An Assessment of the Polymer Gradients by Synchrotron Radiation-Induced x-Ray Emission, Magnetic Resonance Microimaging, and Mathematical Modeling. Biopolymers 2000, 53, 60–71. [Google Scholar] [CrossRef]
- Sheth, S.; Barnard, E.; Hyatt, B.; Rathinam, M.; Zustiak, S.P. Predicting Drug Release From Degradable Hydrogels Using Fluorescence Correlation Spectroscopy and Mathematical Modeling. Front Bioeng Biotechnol 2019, 7. [Google Scholar] [CrossRef] [PubMed]
- Axpe, E.; Chan, D.; Offeddu, G.S.; Chang, Y.; Merida, D.; Hernandez, H.L.; Appel, E.A. A Multiscale Model for Solute Diffusion in Hydrogels. Macromolecules 2019, 52, 6889–6897. [Google Scholar] [CrossRef] [PubMed]
- Caccavo, D. An Overview on the Mathematical Modeling of Hydrogels’ Behavior for Drug Delivery Systems. Int J Pharm 2019, 560, 175–190. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Lumpkin, J.A.; Rosenblatt, J. Mathematical Modeling of Drug Release from Hydrogel Matrices via a Diffusion Coupled with Desorption Mechanism. Journal of Controlled Release 1994, 32, 17–25. [Google Scholar] [CrossRef]
- Dembczynski, R.; Jankowski, T. Characterisation of Small Molecules Diffusion in Hydrogel-Membrane Liquid-Core Capsules. Biochem Eng J 2000, 6, 41–44. [Google Scholar] [CrossRef] [PubMed]
- Gabardo, S.; Rech, R.; Ayub, M.A.Z. Determination of Lactose and Ethanol Diffusion Coefficients in Calcium Alginate Gel Spheres: Predicting Values To Be Used in Immobilized Bioreactors. J Chem Eng Data 2011, 56, 2305–2309. [Google Scholar] [CrossRef]
- Puguan, J.M.C.; Yu, X.; Kim, H. Diffusion Characteristics of Different Molecular Weight Solutes in Ca–Alginate Gel Beads. Colloids Surf A Physicochem Eng Asp 2015, 469, 158–165. [Google Scholar] [CrossRef]
- Øyaas, J.; Storrø, I.; Svendsen, H.; Levine, D.W. The Effective Diffusion Coefficient and the Distribution Constant for Small Molecules in Calcium-Alginate Gel Beads. Biotechnol Bioeng 1995, 47, 492–500. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, S.; Shimono, K. Molecular Modeling to Estimate the Diffusion Coefficients of Drugs and Other Small Molecules. Molecules 2020, 25. [Google Scholar] [CrossRef]
- Abrami, M.; Marizza, P.; Zecchin, F.; Bertoncin, P.; Marson, D.; Lapasin, R.; de Riso, F.; Posocco, P.; Grassi, G.; Grassi, M. Theoretical Importance of PVP-Alginate Hydrogels Structure on Drug Release Kinetics. Gels 2019, 5. [Google Scholar] [CrossRef]
- Turco, G.; Donati, I.; Grassi, M.; Marchioli, G.; Lapasin, R.; Paoletti, S. Mechanical Spectroscopy and Relaxometry on Alginate Hydrogels: A Comparative Analysis for Structural Characterization and Network Mesh Size Determination. Biomacromolecules 2011, 12, 1272–1282. [Google Scholar] [CrossRef]
- Offeddu, G.S.; Axpe, E.; Harley, B.A.C.; Oyen, M.L. Relationship between Permeability and Diffusivity in Polyethylene Glycol Hydrogels. AIP Adv 2018, 8, 105006. [Google Scholar] [CrossRef]
- Rehmann, M.S.; Skeens, K.M.; Kharkar, P.M.; Ford, E.M.; Maverakis, E.; Lee, K.H.; Kloxin, A.M. Tuning and Predicting Mesh Size and Protein Release from Step Growth Hydrogels. Biomacromolecules 2017, 18, 3131–3142. [Google Scholar] [CrossRef]
- Diclofenac. Available online: https://go.drugbank.com/drugs/DB00586.
- Gombotz, W.R.; Wee, S.F. Protein Release from Alginate Matrices. Adv Drug Deliv Rev 2012, 64, 194–205. [Google Scholar] [CrossRef]
- Oshi, M.A.; Lee, J.; Kim, J.; Hasan, N.; Im, E.; Jung, Y.; Yoo, J.-W. PH-Responsive Alginate-Based Microparticles for Colon-Targeted Delivery of Pure Cyclosporine A Crystals to Treat Ulcerative Colitis. Pharmaceutics 2021, 13. [Google Scholar] [CrossRef]
- Agarwal, T.; Narayana, S.N.G.H.; Pal, K.; Pramanik, K.; Giri, S.; Banerjee, I. Calcium Alginate-Carboxymethyl Cellulose Beads for Colon-Targeted Drug Delivery. Int J Biol Macromol 2015, 75, 409–417. [Google Scholar] [CrossRef]
- Ferraro, R.; Caserta, S. SLE3S-Water System: A Linear Rheological Characterisation. Rheol Acta 2023, 62, 365–375. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





