Submitted:
27 December 2023
Posted:
28 December 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
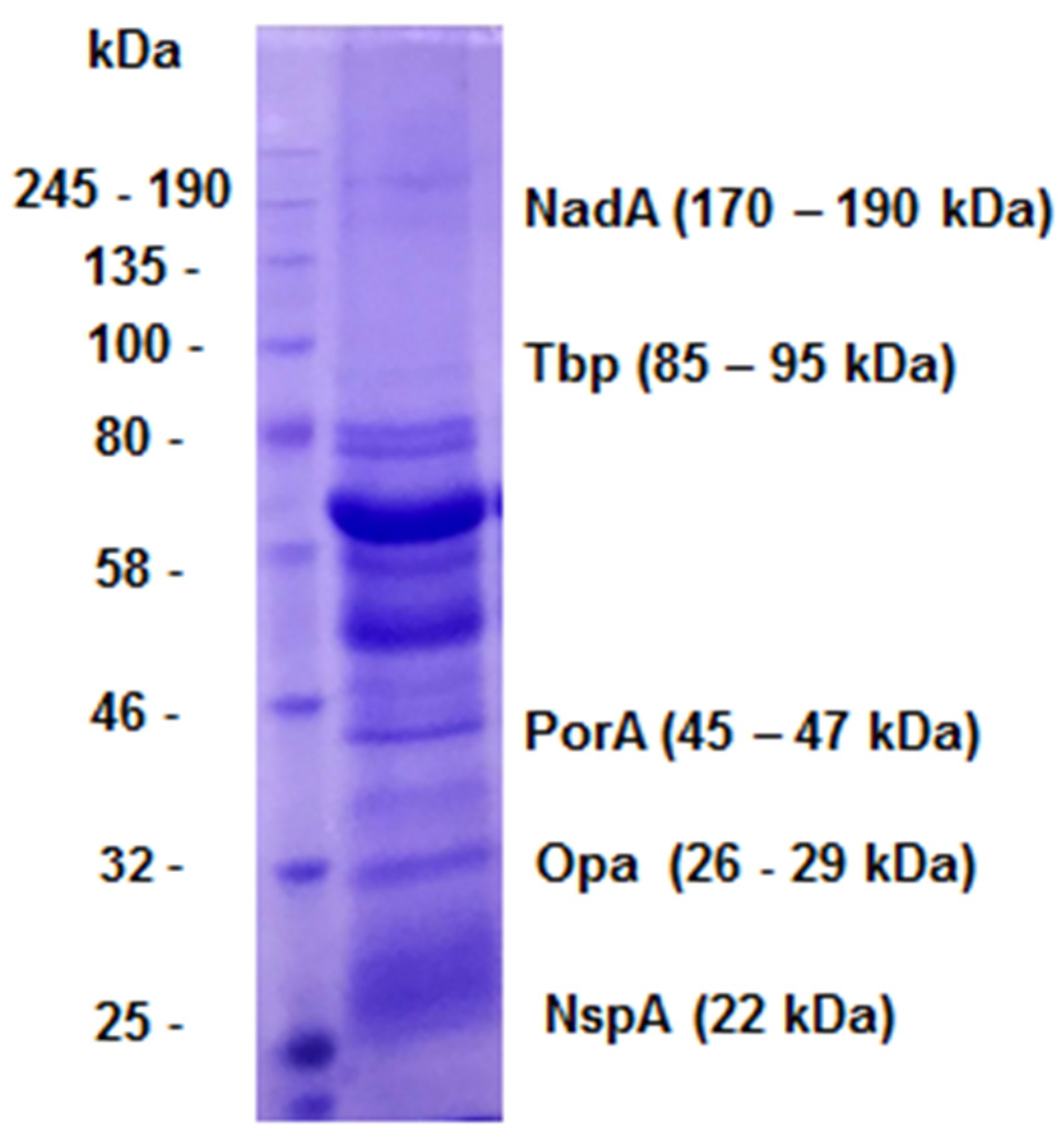
2.1. Antigenic preparations and Serum
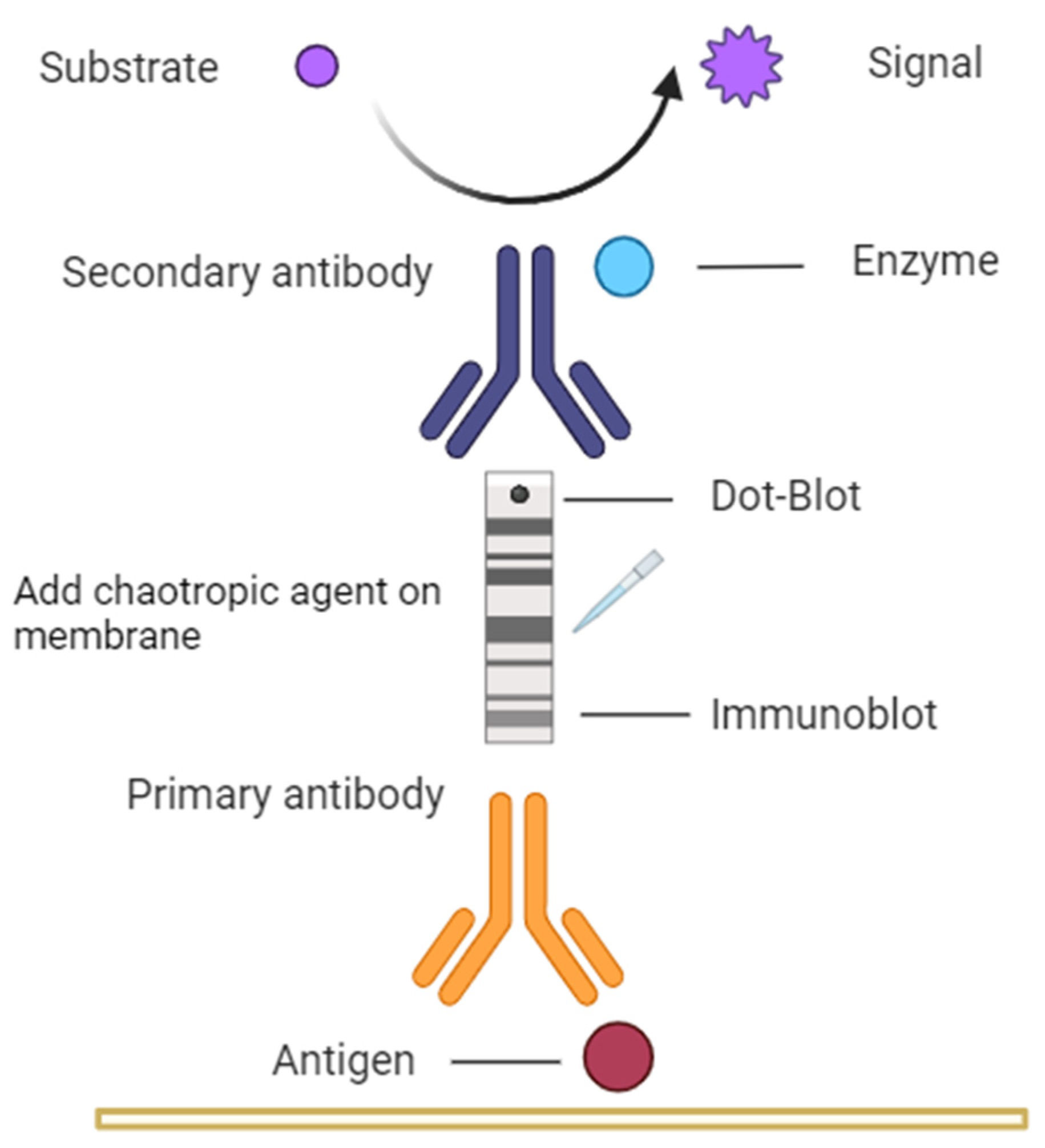
2.2. Immunoblot
2.3. Dot-Blot
2.4. Strategy used in the study to assess avidity on nitrocellulose membranes
2.5. Anti-IgG Antibody Avidity Assessment
3. Results and Discussion
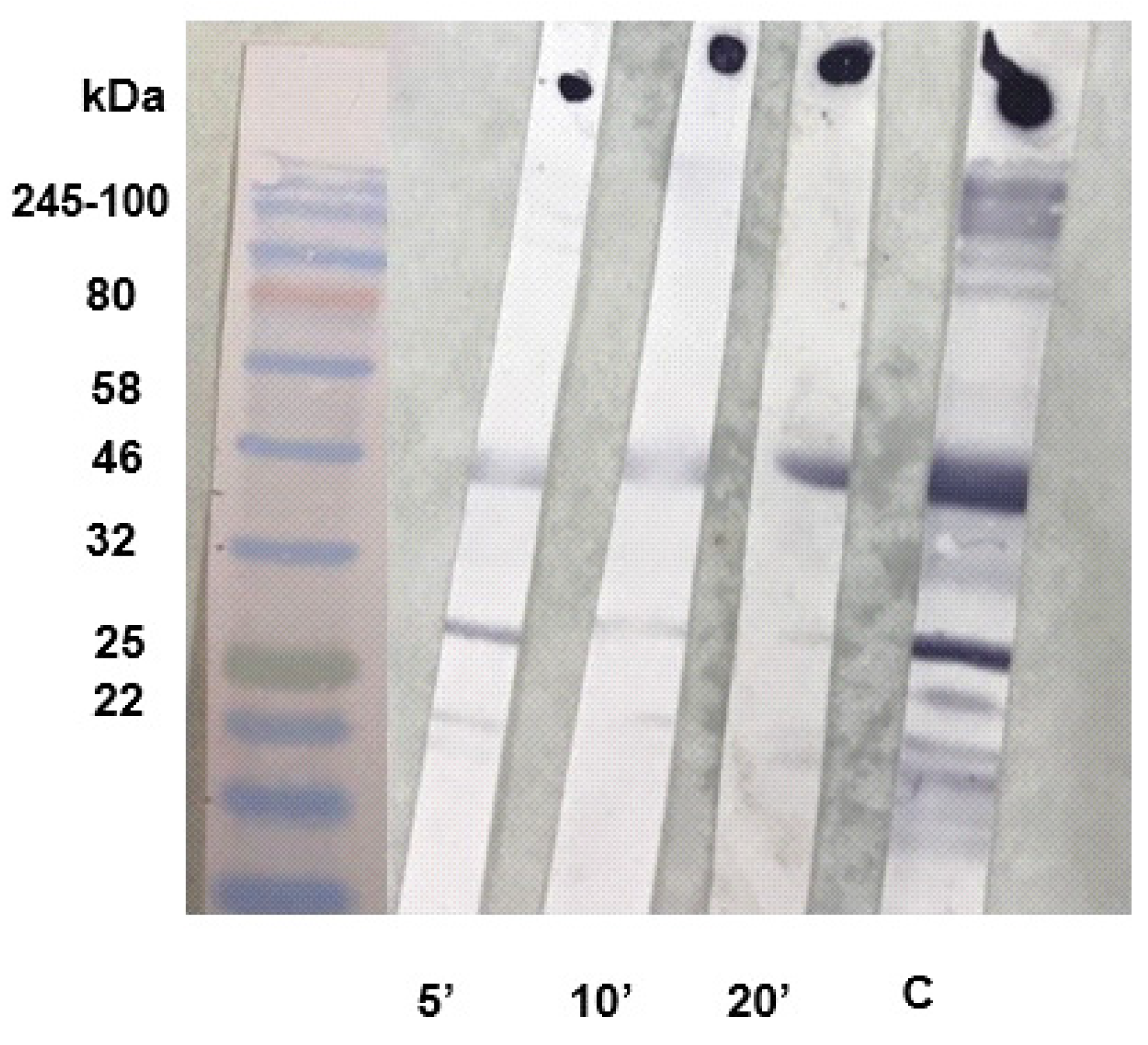
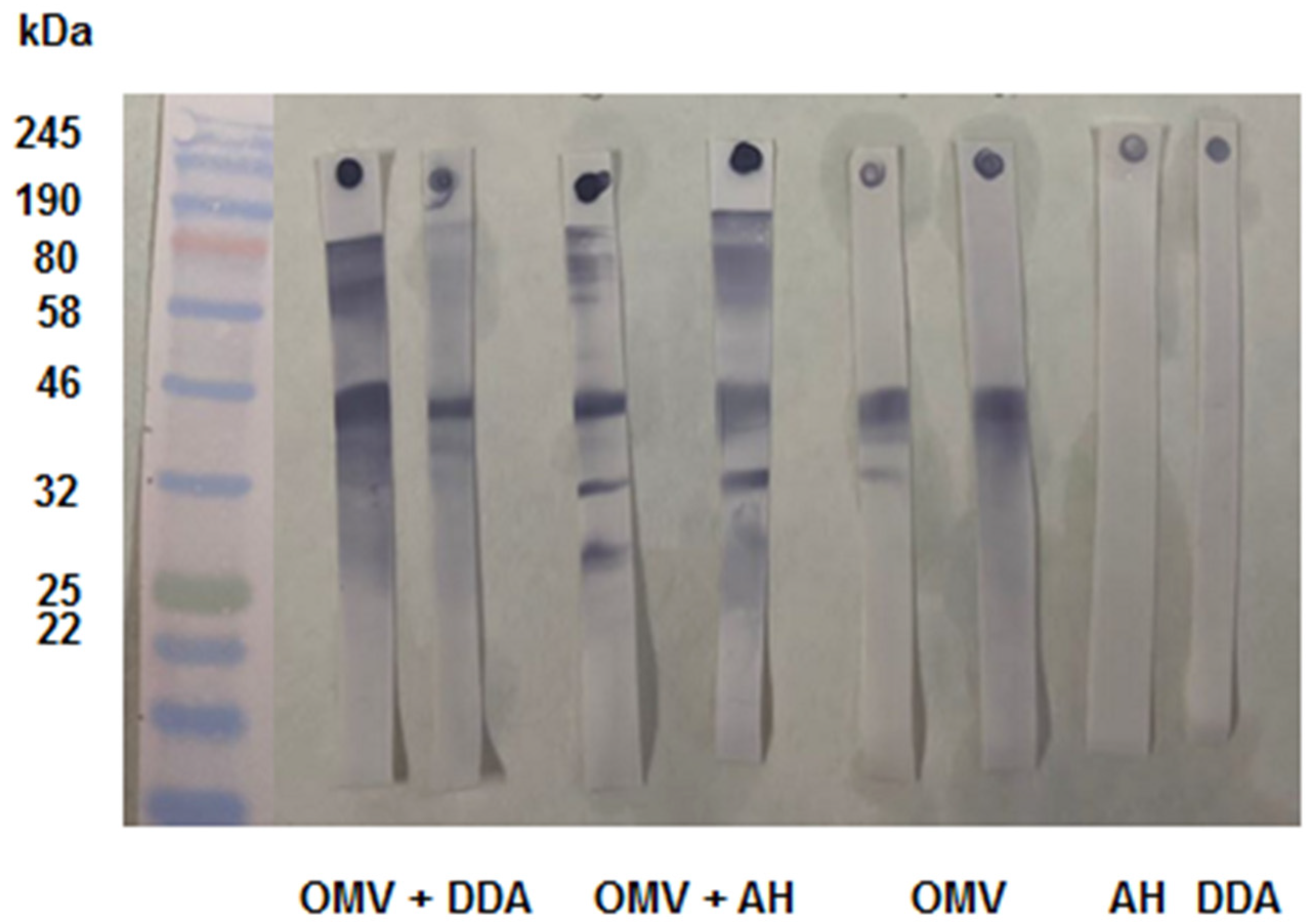
3.1. Immunoblot and Dot-Blot for N. meningitidis OMVs
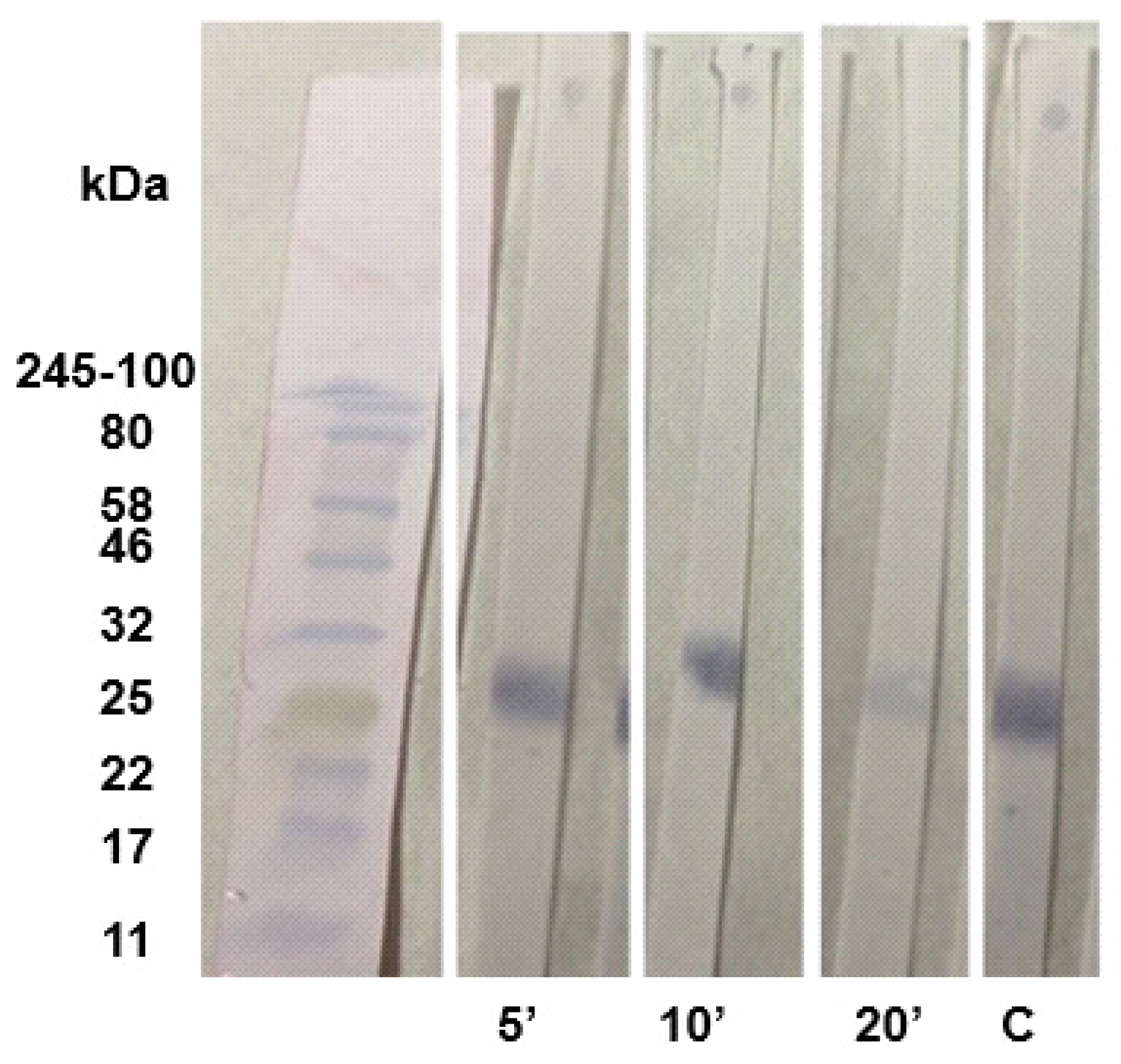
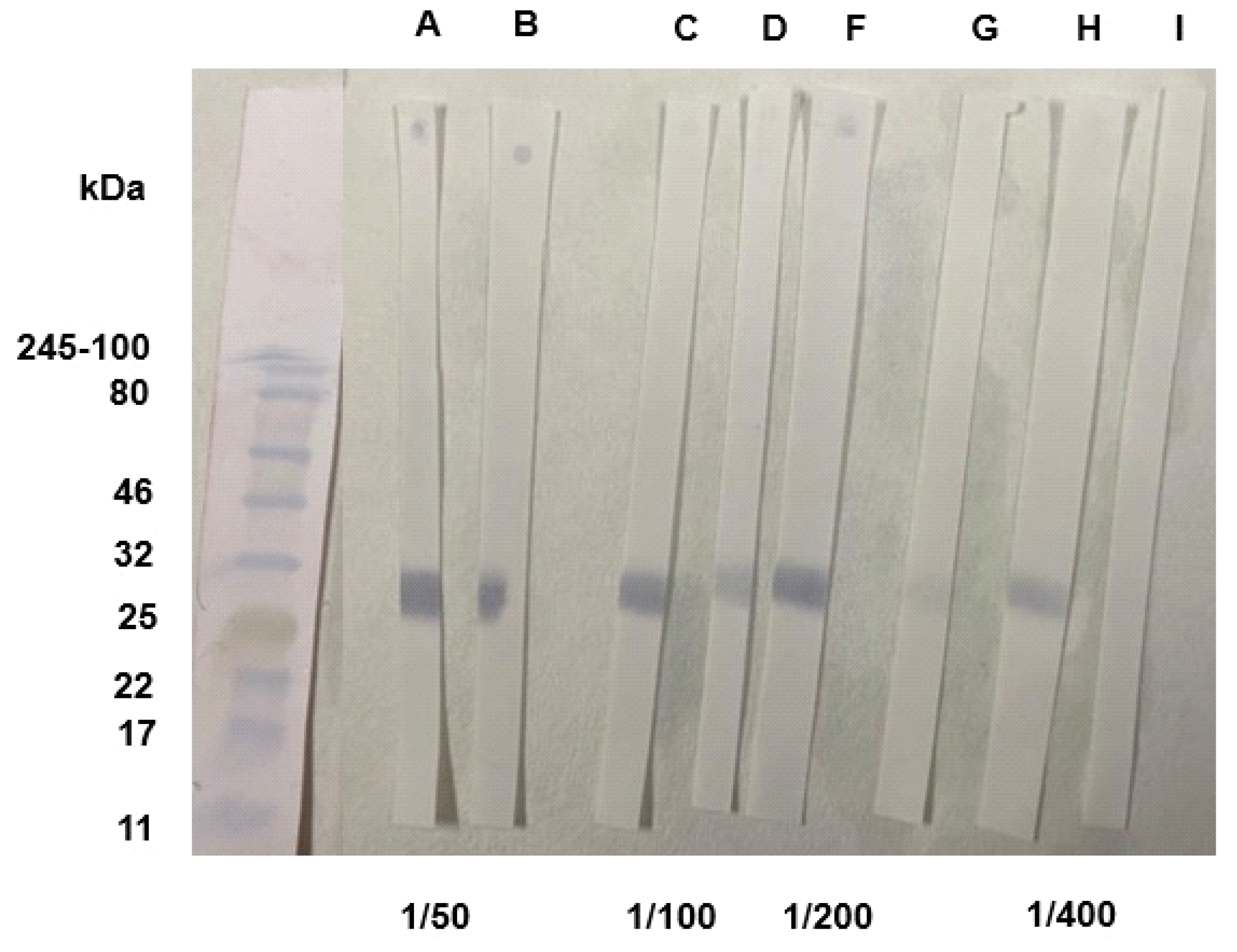
3.2. Standardization of SARS-CoV-2 Immunoblot and Dot-Blot for RBD
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgements
Conflicts of Interest
References
- Bauer, G. et al. The challenge of avidity determination in SARS-CoV-2 serology. J. Med. Virol., 2021, 93, 3092–3104. [CrossRef] [PubMed]
- Bekkat-Berkani et al. Public health perspective of a pentavalent meningococcal vaccine combining antigens of MenACWY-CRM and 4CMenB. J. Infect., 2022, 85, 481–491. [CrossRef] [PubMed]
- Belo, E.F.T.; Farhat, C.K.; De Gaspari, E.N. Comparision of dot-ELISA and standard ELISA for detection of Neisseria meningitidis outer membrane complex-specific antibodies. Braz. J. Infect. Dis., 2010, 14, 35–40. [Google Scholar] [PubMed]
- Benner, S.E. et al. SARS-CoV-2 antibody avidity responses in COVID-19 patients and convalescent plasma donors. J. Infect. Dis., 2020, 222, 1974–1984. [CrossRef]
- Brady, A. M.; Unger, E. R.; Panicker, G. Description of a novel multiplex avidity assay for evaluating HPV antibodies. J. Immunol. Methods, 2017, 447, 31–26. [Google Scholar] [CrossRef]
- Brito, L.T. et al. Study of different routes of immunization using outer membrane vesicles of Neisseria meningitidis B and comparison of two adjuvants. Vaccine, 2020, 38, 7674–7682. [CrossRef]
- Comanducci, M. et al. NadA, a novel vaccine candidate of Neisseria meningitidis. J. Exp. Med., 2002, 195, 1445–1454. [CrossRef]
- Correa, V.A.; Rodrigues, T.S.; Portilho, A.I.; Trzewikoswki de Lima, G.; De Gaspari, E. Modified ELISA for antibody avidity evaluation: The need for standardization. Biomed. J., 2021, 44, 433–348. [Google Scholar] [CrossRef]
- Da Costa, H.H.M. et al. RBD and Spike DNA-Based Immunization in Rabbits Elicited IgG Avidity Maturation and High Neutralizing Antibody Responses against SARS-CoV-2. Viruses, 2023, 15, 555. [CrossRef]
- Da Porto, F. et al. Antibody avidity and neutralizing response against SARS-CoV-2 omicron variant after infection or vaccination. J. Immunol. Res., 2022, 2022, 1–9. [CrossRef]
- Eickhoff, T.C.; Myers, M. Workshop summary. Aluminum in vaccines. Vaccine, 2002, 20, S1–S4. [Google Scholar] [CrossRef] [PubMed]
- Eisen, H. N. Affinity enhancement of antibodies: how low-affinity antibodies produced early in immune responses are followed by high-affinity antibodies later and in memory B-cell responses. Cancer Immunol. Res., 2014, 2, 381–392. [Google Scholar] [CrossRef] [PubMed]
- Estabrook, M.M.; Zhou,D.; Apicella, M.A.A. Nonopsonic phagocytosis of group C Neisseria meningitidis by human neutrophils. Infect. and Immun., 1998, 66, 1028–1036. [CrossRef] [PubMed]
- Ferraz, A. S. et al. Storage and stability of IgG and IgM monoclonal antibodies dried on filter paper and utility in Neisseria meningitidis serotyping by Dot-blot ELISA. BMC Infect. Dis., 2008, 8, 1–8. [CrossRef]
- Gaspar, E.B.; De Gaspari, E. Avidity assay to test the functionality of anti-SARS-Cov-2 antibodies. Vaccine, 2021, 39, 1473. [Google Scholar] [CrossRef]
- Granoff, D.M., et al. A modified enzyme-linked immunosorbent assay for measurement of antibody responses to meningococcal C polysaccharide that correlate with bactericidal responses. Clin. Diagn. Lab. Immunol., 1998, 5, 479–485. [CrossRef]
- Heyderman, R. S. et al. Neutrophil response to Neisseria meningitidis: inhibition of adhesion molecule expression and phagocytosis by recombinant bactericidal/permeability-increasing protein (rBPI21). J. Infect. Dis., 1999, 179, 1288–1292. [CrossRef]
- Humphries, H.E. et al. Multivalent liposome-based vaccines containing different serosubtypes of PorA protein induce cross-protective bactericidal immune responses against Neisseria meningitidis. Vaccine, 2006, 24, 36–44. [CrossRef]
- Ise, W.; Kurosaki, T. Regulation of Plasma Cell Differentiation. B Cells in Immun. Tol., 2020, 1254, 63–74. [Google Scholar] [CrossRef]
- Lima, G.G.; Portilho, A.I.; De Gaspari, E. Adjuvants to increase immunogenicity of SARS-CoV-2 RBD and support maternal–fetal transference of antibodies in mice. Pathog. Dis., 2022, 80, ftac038. [Google Scholar] [CrossRef]
- Luczkowiak, J. et al. Cross neutralization of SARS-CoV-2 omicron subvariants after repeated doses of COVID-19 mRNA vaccines. J. Med.Virol., 2023, 95, e28268. [CrossRef]
- Marrack, P.; Mckee, A.S.; Munks, M. W. Towards an understanding of the adjuvant action of aluminum. Nat. Rev. Immunol., 2009, 9, 287–293. [CrossRef]
- McNeil, G.; Virji, M. Phenotypic variants of meningococci and their potential in phagocytic interactions: the influence of opacity proteins, pili, PilC and surface sialic acids. Microb. Pathog., 1997, 22, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Nompari, L. et al. Quality by design approach in the development of an ultra-high-performance liquid chromatography method for the Bexsero meningococcal group B vaccine. Talanta, 2018, 178, 552–562. [CrossRef] [PubMed]
- Pollard, A. J.; Frasch, C. Development of natural immunity to Neisseria meningitidis. Vaccine, 2001, 19, 1327–1346. [Google Scholar] [CrossRef] [PubMed]
- Portilho, A.I.; Santos, J.S.; Trzewikoswki de Lima, G.; Lima, G.G. ; De Gaspari,, E. Study of avidity-ELISA: Comparison of chaotropic agents, incubation temperature and affinity maturation after meningococcal immunization. J. Immunol. Methods, 2023, 512, 11337–2023. [Google Scholar] [CrossRef]
- Principi, N.; Esposito, S. Aluminum in vaccines: Does it create a safety problem? . Vaccine, 2018, 36, 5825–5831. [Google Scholar] [CrossRef] [PubMed]
- Pullen, G. R.; Fitzgerald, M. G.; Hosking, C. S. Antibody avidity determination by ELISA using thiocyanate elution. J. Immunol. Methods, 1986, 86, 83–87. [Google Scholar] [CrossRef]
- Reddy, S.B et al. Affinity antibodies to Plasmodium falciparum merozoite antigens are associated with protection from malaria. PLoS One, 2012, 7, 232242. [CrossRef]
- Smolander, H. et al. A novel antibody avidity methodology for rapid point-of-care serological diagnosis. J. Virol. Methods, 2010, 166, 86–91. [CrossRef]
- Standlbauer, D. et al. SARS-CoV-2 seroconversion in humans: a detailed protocol for a serological assay, antigen production, and test setup. Curr. Protoc. Microbiol., 2020, 57, e100. [CrossRef]
- Steward, M.W; Stanley, C.M.; Dimarchi, R.; Mulcahy, G.; Doel, T.R. High-affinity antibody induced by immunization with a synthetic peptide is associated with protection of cattle against foot-and-mouth disease. Immunology, 1991, 72, 79. [Google Scholar]
- Trzewikoswki de Lima, G.; Rodrigues, T.S.; Portilho, A. I.; Correa, V.A.; Gaspar, E.B.; De Gaspari, E. Immune responses of meningococcal B outer membrane vesicles in middle-aged mice. Pathog. Dis., 2020, 78, ftaa028. [Google Scholar] [CrossRef]
- Vermont, C.L.; Van Dijken, H.H.; Van Limpt, C.J.; De Groot, R.; Van Alphen, L.; Van Den Dobbelsteen, G.P. Antibody avidity and immunoglobulin G isotype distribution following immunization with a monovalent meningococcal B outer membrane vesicle vaccine. Infect. Immun., 2002, 70, 584–590. [Google Scholar] [CrossRef] [PubMed]
- Victora, G.D.; Nussenzweig, M.C. Germinal centers. Annu. Revi. Immunol., 2022, 40, 413–442. [Google Scholar] [CrossRef] [PubMed]
- Virji, M. et al. The N-domain of the human CD66a adhesion molecule is a target for Opa proteins of Neisseria meningitidis and Neisseria gonorrhoeae. Mol. Microbiol., 1996, 22, 929–939. [CrossRef]
- Watson, P.S.; Turner, D.P.J. Clinical experience with the meningococcal B vaccine, Bexsero®: Prospects for reducing the burden of meningococcal serogroup B disease. Vaccine, 2015, 34, 875–880. [Google Scholar] [CrossRef]
- Weynants, V. E. et al. Additive and synergistic bactericidal activity of antibodies directed against minor outer membrane proteins of Neisseria meningitidis. Infect. and Immun., 2007, 75, 5434–5442. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).




