Submitted:
27 December 2023
Posted:
28 December 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
- ≥18 years old
- Breast cancer or hormone-sensitive prostate cancer diagnosed by biopsy
- Overweight or obesity defined as BMI ≥ 25 kg/m2 and 30 kg/m2 respectively
- Indication of radiotherapy with a curative intent.
- BMI < 25 kg/m2
- Patients undergoing nutritional treatment or malnutrition
- Patients with diabetes mellitus using insulin
- Diagnosis of vascular ischemia, uncontrolled thyroid disease, mental illness without medical supervision, liver disease or malabsorption syndromes (inflammatory bowel disease, celiac disease)
- Patients with gastric by-pass or gastric sleeve
- Use of corticosteroids or anti-depressants
- Moderate or high level of physical activity.
- A.
- Nutritional evaluation: baseline measurement of weight, BMI, waist circumference, along with serum levels of glucose, basal insulin and lipid were performed.
- B.
- Quality of life (QoL): QoL was assessed through the EORTC-c30 questionnaire with all patients, and additionally the EORTC QLQ - BR23 questionnaire in breast cancer and the Expanded Prostate Cancer Index Composite (EPIC 2.0) in prostate cancer, if appropriate, which were taken at the same time as the EORTC-30.
- C.
- Physical activity: international physical activity questionnaire (IPAQ) from the World Health Organization (WHO).
- D.
- Eating habits: a 24-hour food survey and consumption trend was used to determine the patient’s eating habits (Supplementary Material, “Supplementary Spreadsheet 1”).
- A.
- The TRF group had a sugar and saturated fat free diet with caloric intake according to the patient's total energy expenditure, distributed in 8 hours of food intake and 16-hour daily fast.
- B.
- The CR group was based on the Obesity Society guidelines, with a total caloric intake 25% less than total energy expenditure. This was distributed in 4 meals and 1 snack according to the following proportions: 55% for carbohydrates, 15% protein and 30% fat, without sugar or saturated fat.
- C.
- Every patient, independent of their group, was tasked with a dairy registry of what they ate.
- A.
- Treatment adherence: patients were called once a week and their dairy diet registry was evaluated according to their corresponding group, where important concepts related to their intervention were reinforced.
- B.
- Anthropometric changes: every 4 weeks every patient’s weight and waist circumference were measured.
- C.
- Toxicity: once a week during their consultation with radiation oncology, acute toxicity was assessed using the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE).
- A.
- Acute Toxicity
- B.
- Weight and waist circumference
- C.
- Fasting glucose, basal insulin and lipid profile.
- D.
- QoL with EORTC-c30, EORTC QLQ - BR23 (for breast cancer) and the EPIC 2.0 (for prostate cancer)
3. Hypothesis
4. Primary objectives
5. Secondary Objectives
- A.
- Assess adherence, defined as a reduction in body weight of at least 5% compared to baseline and in at least 50% of the recruited patients.
- B.
- Establish which intervention (CR or TRF) has better adherence.
- C.
- Assess the impact on quality of life in patients under nutritional interventions
- D.
- Assess differences in weight and waist circumference in patients under nutritional interventions
- E.
- Assess differences in serum levels of fasting glucose, basal insulin and lipid profile in patients under nutritional interventions
- F.
- Assess acute toxicity grade ≥2 in patients under nutritional interventions
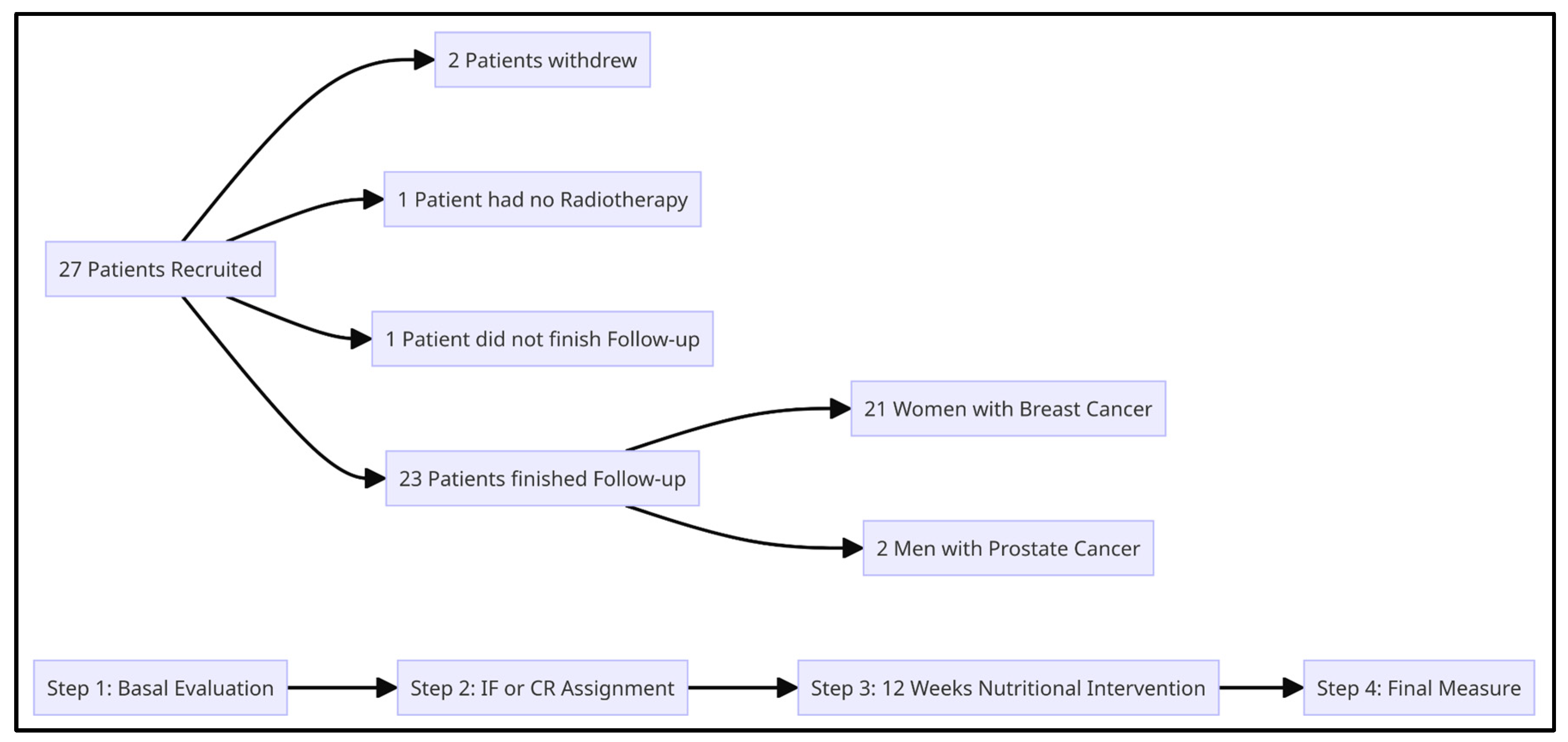
6. Results
- A.
- There was a reported loss of 5% or more of the initial weight in 52,1% of patients (12/23).
- B.
- Within the patients with breast cancer, 100% of those who chose TRF showed true adherence (5 out of 5), whereas only 43.75% (7 out of 16) showed true adherence in the CR group.
- C.
- Within the patients with prostate cancer, 0% showed true adherence in the TRF group (0 out of 2).
- D.
- There was a mean reduction in weight of 3.6 kg and waist circumference of 4.9 cm.
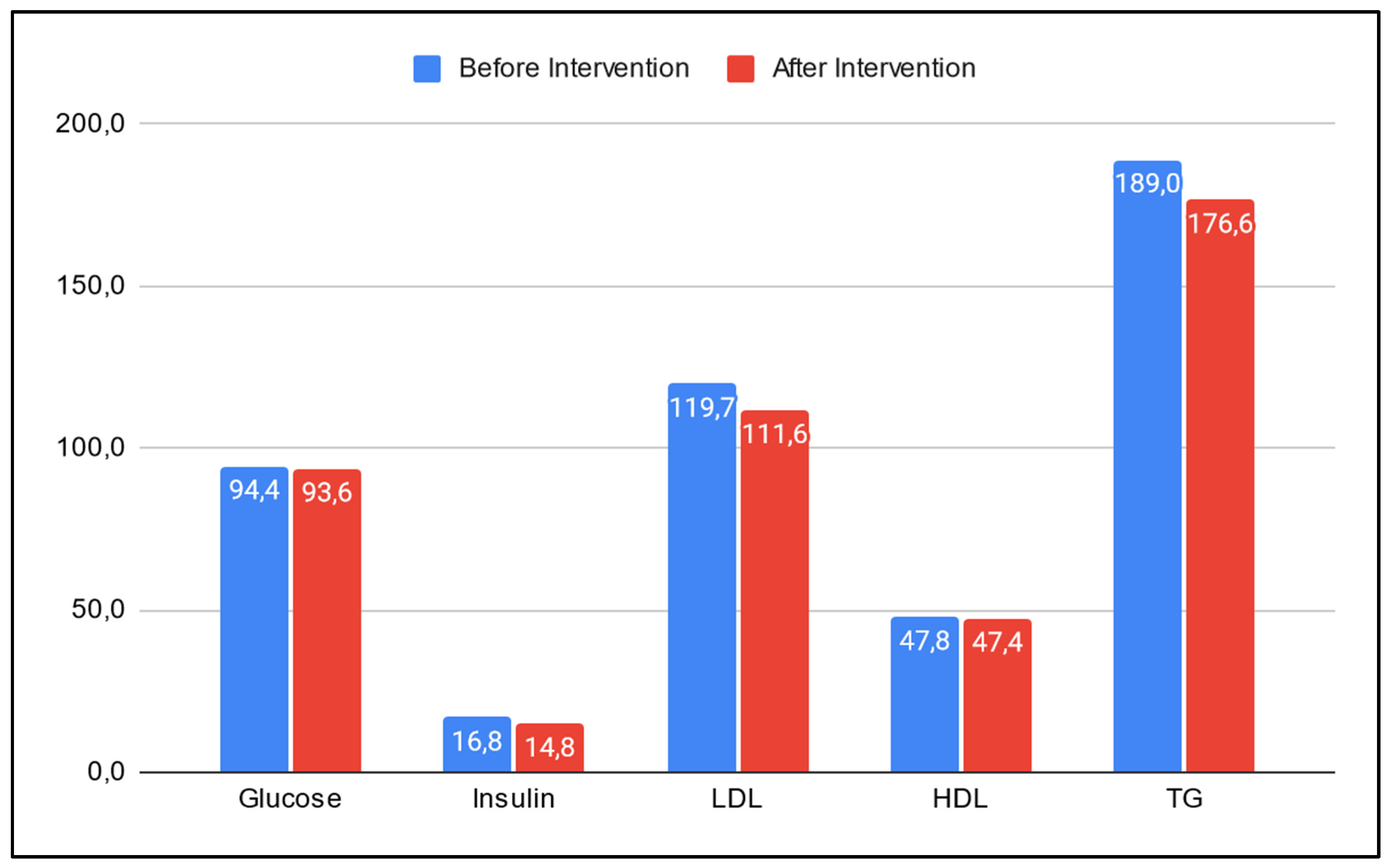
- E.
- Serum levels of glucose, insulin, LDL, HDL and TG showed a mean reduction of 0.6 mg/dL, 2.0 uU/mL, 8.1 mg/dL, 0.4 mg/dL and 12.4 mg/dL respectively (Figure 3).
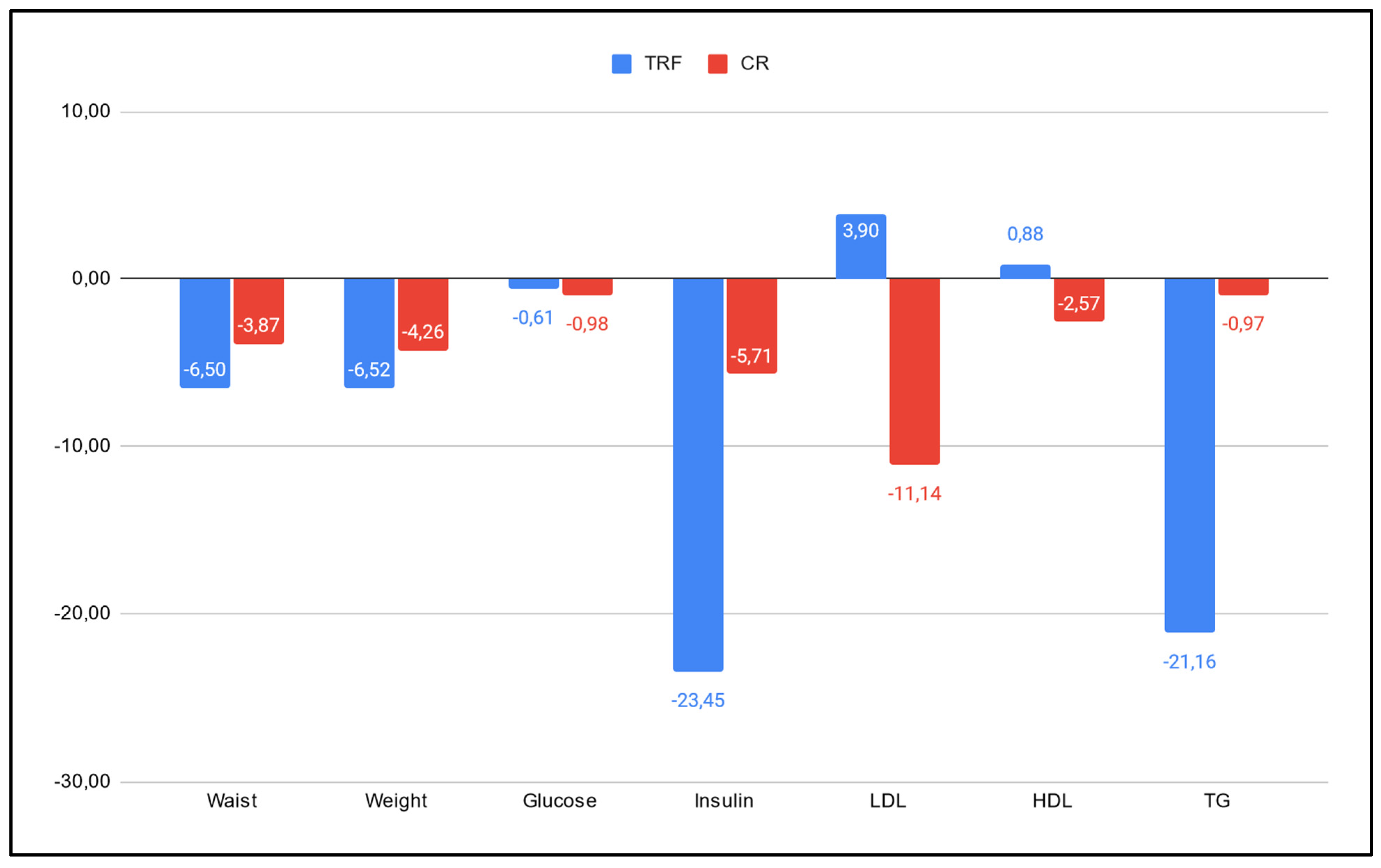
- A.
- Anthropometric measures: patients in the TRF group lost 61.8 % more weight and reduced their waist circumference by 49.5% more in comparison to those in the CR group (6.50% and 6.52% vs 3.87% and 4.26%, respectively).
- B.
- Glucose and insulin: TRF showed a reduction 38% smaller than the CR group in mean serum glucose levels (0.57 vs 0.92), but a 270% bigger decrease in their mean serum insulin level, in comparison to the CR group (3.65 vs 0.99).
- C.
- LDL and HDL: in mean serum levels of LDL, the TRF group showed an increase of 3.9%, whereas the CR group showed a decrease of 11.14%. On the other hand, the TRF group showed an increase of 0.88% in their mean serum levels of HDL, whereas the CR group showed a reduction of 2.57%.
- D.
- TG: the TRF group showed a decrease in their TG mean serum levels 1900% higher than those in the CR group (21.16% vs 0.97% reduction, respectively).
- E.
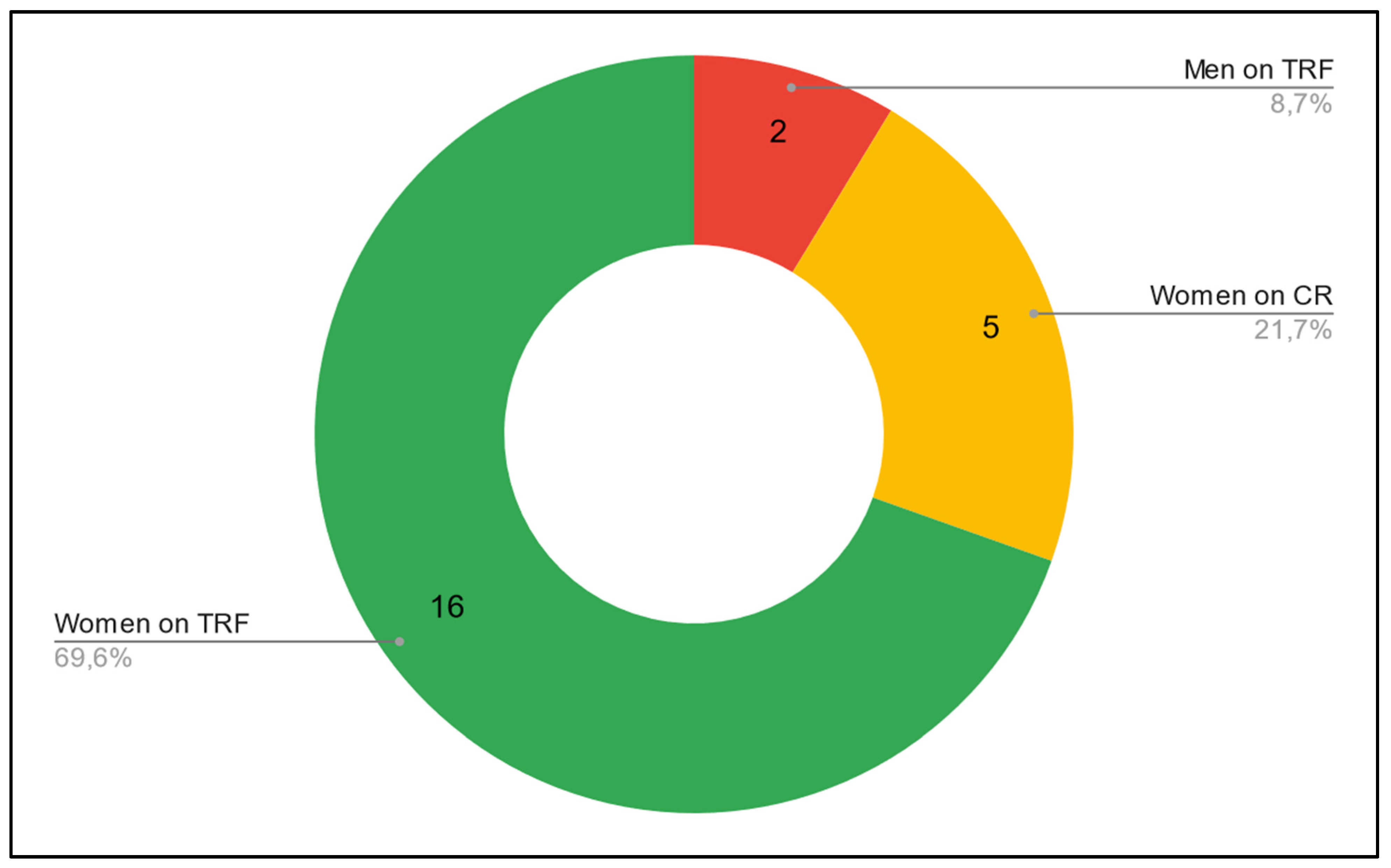
- Adherence: of the 12 patients who showed true adherence, all 12 were women. 5 were on the TRF group (5 out of 7 patients in the TRF group) and 7 were on the CR group (7 out of 16 patients in the CR group). None of the men showed true adherence.
7. Discussion
8. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- The International Agency for Research on Cancer (IARC). Global Cancer Observatory. Retrieved August 10, 2023, from https://gco.iarc.fr/today/home.
- World Health Organization. (2020, December 9). The top 10 causes of death. Retrieved August 10, 2023, from https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death.
- Margozzini, P.; Passi, A. Encuesta Nacional de Salud, ENS 2016-2017: Un aporte a la planificación sanitaria y políticas públicas en Chile.
- World Cancer Research Fund/American Institute For Cancer Research, «Continuous Update Project Report Summary. Food, Nutrition, Physical Activity, And The Prevention Of Colorectal Cancer». 2011.
- Rock, C.L.; Thomson, C.; Gansler, T.; Gapstur, S.M.; McCullough, M.L.; Patel, A.V.; Bandrews, K.S.; Bandera, E.V.; Spees, C.K.; Robien, K.; et al. American Cancer Society guideline for diet and physical activity for cancer prevention. CA A Cancer J. Clin. 2020, 70, 245–271. [Google Scholar] [CrossRef] [PubMed]
- Protani, M.; Coory, M.; Martin, J.H. Effect of obesity on survival of women with breast cancer: Systematic review and meta-analysis. Breast Cancer Res. Treat. 2010, 123, 627–635. [Google Scholar] [CrossRef] [PubMed]
- De Cabo, R.; Mattson, M.P. Effects of intermittent fasting on health, aging, and disease. N. Engl. J. Med. 2019, 381, 2541–2551. [Google Scholar] [CrossRef] [PubMed]
- Nebeling, L.C.; Miraldi, F.; Shurin, S.B. Effects of a ketogenic diet on tumor metabolism and nutritional status in pediatric oncology patients: Two case reports. J. Am. Coll. Nutr. 1995, 14, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Tagde, P.; Tagde, S.; Bhattacharya, T.; Tagde, P.; Akter, R.; Rahman, M.H. Multifaceted effects of intermittent fasting on the treatment and prevention of diabetes, cancer, obesity or other chronic diseases. Curr. Diabetes Rev. 2022, 18, 33–49. [Google Scholar] [CrossRef] [PubMed]
- International Food Information Council. 2023 Food & Health Survey. 23 May 2023. [https://foodinsight.org/2023-food-health-survey/].
- Vasim, I.; Majeed, C.N.; DeBoer, M.D. Intermittent Fasting and Metabolic Health. Nutrients 2022, 14, 631. [Google Scholar] [CrossRef]
- Most, J.; Tosti, V.; Redman, L.M.; Fontana, L. Calorie restriction in humans: An update. Ageing Res. Rev. 2017, 39, 36–45. [Google Scholar] [CrossRef]
- Flanagan, E.W.; Most, J.; Mey, J.T.; Redman, L.M. Calorie Restriction and Aging in Humans. Annu. Rev. Nutr. 2020, 40, 105–133. [Google Scholar] [CrossRef] [PubMed]
- Simone, B. The design and implementation of caloric restriction for oncology research (CaReFOR): Is starving cancer cells in the clinical realm feasible? Hematol. Med. Oncol. 2017, 2, 1–6. [Google Scholar] [CrossRef]
- Masood W, Annamaraju P, Khan Suheb MZ; et al. Ketogenic Diet. [Updated 2023 Jun 16]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK499830/.
- Madeo, F.; Carmona-Gutierrez, D.; Hofer, S.J.; Kroemer, G. Caloric Restriction Mimetics against Age-Associated Disease: Targets, Mechanisms, and Therapeutic Potential. Cell Metab. 2019, 29, 592–610. [Google Scholar] [CrossRef]
- Alidadi, M.; Banach, M.; Guest, P.C.; Bo, S.; Jamialahmadi, T.; Sahebkar, A. The effect of caloric restriction and fasting on cancer. Semin. Cancer Biol. 2020, 73, 30–44. [Google Scholar] [CrossRef] [PubMed]
- Klement, R.J.; Champ, C.E. Calories, carbohydrates, and cancer therapy with radiation: Exploiting the five R’s through dietary manipulation. Cancer Metastasis Rev. 2014, 33, 217–229. [Google Scholar] [CrossRef] [PubMed]
- Trepanowski, J.F.; Kroeger, C.M.; Barnosky, A.; Klempel, M.; Bhutani, S.; Hoddy, K.K.; Rood, J.; Ravussin, E.; Varady, K.A. Effects of alternate-day fasting or daily calorie restriction on body composition, fat distribution, and circulating adipokines: Secondary analysis of a randomized controlled trial. Clin. Nutr. 2017, 37, 1871–1878. [Google Scholar] [CrossRef] [PubMed]
- Kharazi, A.; James, S.; Taylor, J.; Lubinski, J.; Nakamura, L.; Makinodan, T. Combined chronic low dose radiation-caloric restriction: A model for regression of spontaneous mammary tumor. Endocrine 1994, 28, 641–647. [Google Scholar] [CrossRef] [PubMed]
- Safdie, F.; Brandhorst, S.; Wei, M.; Wang, W.; Lee, C.; Hwang, S.; Conti, P.S.; Chen, T.C.; Longo, V.D. Fasting Enhances the Response of Glioma to Chemo- and Radiotherapy. PLoS ONE 2012, 7, e44603. [Google Scholar] [CrossRef] [PubMed]
- Saleh, A.; Simone, B.; Palazzo, J.; Savage, J.E.; Sano, Y.; Dan, T.; Jin, L.; Champ, C.; Zhao, S.; Lim, M.; et al. Caloric restriction augments radiation efficacy in breast cancer. Cell Cycle 2013, 12, 1955–1963. [Google Scholar] [CrossRef] [PubMed]
- Simone, B.A.; Dan, T.; Palagani, A.; Jin, L.; Han, S.Y.; Wright, C.; Savage, J.E.; Gitman, R.; Lim, M.K.; Palazzo, J.; et al. Caloric restriction coupled with radiation decreases metastatic burden in triple negative breast cancer. Cell Cycle 2016, 15, 2265–2274. [Google Scholar] [CrossRef] [PubMed]
- Mercier, B.D.; Tizpa, E.; Philip, E.J.; Feng, Q.; Huang, Z.; Thomas, R.M.; Pal, S.K.; Dorff, T.B.; Li, Y.R. Dietary Interventions in Cancer Treatment and Response: A Comprehensive Review. Cancers 2022, 14, 5149. [Google Scholar] [CrossRef]
- Rock, C.L.; Thomson, C.A.; Sullivan, K.R.; Howe, C.L.; Kushi, L.H.; Caan, B.J.; Neuhouser, M.L.; Bandera, E.V.; Wang, Y.; Robien, K.; et al. American Cancer Society nutrition and physical activity guideline for cancer survivors. CA A Cancer J. Clin. 2022, 72, 230–262. [Google Scholar] [CrossRef]
- Sanft, T.; Day, A.; Peterson, L.; Rodriguez, M.A.; Ansbaugh, S.; Armenian, S.; Baker, K.S.; Ballinger, T.; Broderick, G.; Demark-Wahnefried, W.; et al. NCCN Guidelines® Insights: Survivorship, Version 1.2022. J. Natl. Compr. Cancer Netw. 2022, 20, 1080–1090. [Google Scholar] [CrossRef]
- Muscaritoli, M.; Arends, J.; Bachmann, P.; Baracos, V.; Barthelemy, N.; Bertz, H.; Bozzetti, F.; Hütterer, E.; Isenring, E.; Kaasa, S.; et al. ESPEN practical guideline: Clinical Nutrition in cancer. Clin. Nutr. 2021, 40, 2898–2913. [Google Scholar] [CrossRef] [PubMed]
- Ligibel, J.A.; Bohlke, K.; May, A.M.; Clinton, S.K.; Demark-Wahnefried, W.; Gilchrist, S.C.; Irwin, M.L.; Late, M.; Mansfield, S.; Marshall, T.F.; et al. Exercise, Diet, and Weight Management During Cancer Treatment: ASCO Guideline. J. Clin. Oncol. 2022, 40, 2491–2507. [Google Scholar] [CrossRef] [PubMed]
- Icard, P.; Ollivier, L.; Forgez, P.; Otz, J.; Alifano, M.; Fournel, L.; Loi, M.; Thariat, J. Perspective: Do Fasting, Caloric Restriction, and Diets Increase Sensitivity to Radiotherapy? A Literature Review. Adv. Nutr. Int. Rev. J. 2020, 11, 1089–1101. [Google Scholar] [CrossRef] [PubMed]
- Kalam, F.; James, D.L.; Li, Y.R.; Coleman, M.F.; A Kiesel, V.; Feliciano, E.M.C.; Hursting, S.D.; Sears, D.D.; Kleckner, A.S. Intermittent fasting interventions to leverage metabolic and circadian mechanisms for cancer treatment and supportive care outcomes. JNCI Monogr. 2023, 2023, 84–103. [Google Scholar] [CrossRef] [PubMed]
- Anemoulis, M.; Vlastos, A.; Kachtsidis, V.; Karras, S.N. Intermittent Fasting in Breast Cancer: A Systematic Review and Critical Update of Available Studies. Nutrients 2023, 15, 532. [Google Scholar] [CrossRef] [PubMed]
- Vega, C.; Merino, T. Radiotherapy and Oncology, 2022-05-01, Volume 170, Pages S1028–S1029.
- Leung, A.W.Y.; Chan, R.S.M.; Sea, M.M.M.; Woo, J. An Overview of Factors Associated with Adherence to Lifestyle Modification Programs for Weight Management in Adults. Int. J. Environ. Res. Public Health 2017, 14, 922. [Google Scholar] [CrossRef] [PubMed]
- Aycinena, A.C.; Valdovinos, C.; Crew, K.D.; Tsai, W.Y.; Mata, J.M.; Sandoval, R.; Hershman, D.; Greenlee, H. Barriers to Recruitment and Adherence in a Randomized Controlled Diet and Exercise Weight Loss Intervention Among Minority Breast Cancer Survivors. J. Immigr. Minor. Health 2017, 19, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Dent, R.; McPherson, R.; Harper, M.-E. Factors affecting weight loss variability in obesity. Metabolism 2020, 113, 154388. [Google Scholar] [CrossRef]
- Harvey, B.I.; Youngblood, S.M.; Kleckner, A.S. Barriers and Facilitators to Adherence to a Mediterranean Diet Intervention during Chemotherapy Treatment: A Qualitative Analysis. Nutr. Cancer 2023, 75, 1349–1360. [Google Scholar] [CrossRef]
- Harvie, M.; Pegington, M.; Howell, S.J.; Bundred, N.; Foden, P.; Adams, J.; Graves, L.; Greystoke, A.; Mattson, M.P.; Cutler, R.G.; et al. Randomised controlled trial of intermittent vs continuous energy restriction during chemotherapy for early breast cancer. Br. J. Cancer 2022, 126, 1157–1167. [Google Scholar] [CrossRef]
- Cortellino, S.; Quagliariello, V.; Delfanti, G.; Blaževitš, O.; Chiodoni, C.; Maurea, N.; Di Mauro, A.; Tatangelo, F.; Pisati, F.; Shmahala, A.; et al. Fasting mimicking diet in mice delays cancer growth and reduces immunotherapy-associated cardiovascular and systemic side effects. Nat. Commun. 2023, 14, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Bonilla, M.d.l.C.; Stemler, K.M.; Jeter-Jones, S.; Fujimoto, T.N.; Molkentine, J.; Torres, G.M.A.; Zhang, X.; Broaddus, R.R.; Taniguchi, C.M.; Piwnica-Worms, H. Fasting Reduces Intestinal Radiotoxicity, Enabling Dose-Escalated Radiation Therapy for Pancreatic Cancer. Endocrine 2019, 105, 537–547. [Google Scholar] [CrossRef]
| Intervention | Approach | Definition |
|---|---|---|
| Intermittent Fasting | Periods of voluntary abstinence from food and drink [11]. There are various ways to approach IF. Most of them have no caloric restriction associated, or have caloric restriction for short periods of time. | |
| TRF | Fasting that requires limiting the consumption of calories to a window of time, typically between 4 and 12 h daily | |
| 5:2 | Two days per week, 24 hours each day, a very low calorie diet is applied. | |
| B2 | Two large meals are eaten per day: breakfast between 06:00 a.m. and 10:00 a.m., and lunch between 12:00 p.m. and 04:00 p.m. No dinner. | |
| Calorie Restriction | Nutritional intervention where the focus is in reducing energy intake, but keeping adequate nutrition [12]. In general, 10 - 30% of restriction is accepted as having beneficial effects and being tolerable [13,14]. | |
| Ketogenic Diet | Diet primarily consists of high fat intake, moderate protein consumption, and low carbohydrate intake. The macronutrient distribution typically ranges from approximately 55% to 60% fat, 30% to 35% protein, and 5% to 10% carbohydrates [15]. |
| ACS | Published in 2022. Focuses mostly on BMI, dietary patterns, specific food avoidance and physical activity. |
| NCCN | Published in 2022. Similar to ACS, it focuses mostly on BMI, dietary patterns, specific food avoidance and physical activity. No mention of intermittent fasting or caloric restriction at all in their guidelines. |
| ESPEN | Published in 2021. Does not recommend fasting unless there is evidence of a benefit. |
| ASCO | Published in 2022. Mentions intermittent fasting as an intervention, but refrains from recommending it as there is insufficient evidence and points to 5 systematic reviews. These reviews revised 177 articles, where only 7 talk about any kind of weight loss intervention and of these, 5 of them investigated short term fasting finding it to be well tolerated and having beneficial effects. |
| General | |||
| Male patients | 2 | ||
| Female patients | 21 | ||
| Mean Age (y) | 53,1 | ||
| Mean Height (m) | 1,57 | ||
| Cancer | |||
| Breast | 21 | ||
| Prostate | 2 | ||
| Stage | |||
| Breast Cancer | 0 | 1 | |
| I | 13 | ||
| II | 2 | ||
| III | 2 | ||
| N/A | 3 | ||
| Prostate Cancer | Recurrence | 2 | |
| Intervention | |||
| TRF | 7 | ||
| CR | 16 | ||
| Mean serum levels | Before | After | |
| Glucose | 94,4 | 93,6 | |
| Insulin | 16,8 | 14,8 | |
| LDL | 119,7 | 111,6 | |
| HDL | 47,8 | 47,4 | |
| TG | 189,0 | 176,6 | |
| Mean Anthropometry | Before | After | |
| Weight (kg) | 77,2 | 73,6 | |
| Waist Circ. (cm) | 95,8 | 90,9 |
| General | ||||
| Male | 2 | |||
| TRF | 2 | |||
| CR | 0 | |||
| Female | 21 | |||
| TRF | 5 | |||
| CR | 16 | |||
| Type | ||||
| Breast | ||||
| DCIS | 1 | |||
| CDI | 18 | |||
| CLI | 3 | |||
| Poorly Differentiated | 1 | |||
| Prostate | ||||
| Adenocarcinoma | Gleason 3 + 3 | 1 | ||
| Gleason 3 + 4 | 1 | |||
| Molecular Subtypes | ||||
| Breast | Luminal | 18 | ||
| Triple Negative | 3 | |||
| Mean Serum Levels by Intervention | Before | After | Difference (%) | |
| TRF | ||||
| Glucose | 94,43 | 93,86 | -0,61 | |
| Insulin | 15,58 | 11,93 | -23,45 | |
| LDL | 122,23 | 127,00 | 3,90 | |
| HDL | 48,43 | 48,86 | 0,88 | |
| TG | 180,29 | 142,14 | -21,16 | |
| CR | ||||
| Glucose | 94,38 | 93,45 | -0,98 | |
| Insulin | 17,26 | 16,28 | -5,71 | |
| LDL | 116,93 | 103,90 | -11,14 | |
| HDL | 47,88 | 46,64 | -2,57 | |
| TG | 195,75 | 193,86 | -0,97 | |
| Mean Anthropometry by Intervention | Before | After | Difference (%) | |
| TRF | ||||
| Weight (kg) | 75,11 | 70,23 | -6,50 | |
| Waist Circ. (cm) | 94,21 | 88,07 | -6,52 | |
| CR | ||||
| Weight (kg) | 78,09 | 75,07 | -3,87 | |
| Waist Circ. (cm) | 96,47 | 92,36 | -4,26 | |
| Loss of 5% or more of initial weight (True adherence) | Yes | No | ||
| Breast Cancer | ||||
| TRF (n = 5) | 5 | 0 | ||
| CR (n = 16) | 7 | 9 | ||
| Prostate Cancer | ||||
| TRF (n = 2) | 0 | 2 | ||
| CR (n = 0) | 0 | 0 |
| TRF | CR | ||||
| QD | PD (%) | QD | PD (%) | TRF:CR | |
| Glucose | -0,57 | -0,61 | -0,92 | -0,98 | - 38,0 |
| Insulin | -3,65 | -23,45 | -0,99 | -5,71 | 270,8 |
| LDL | 4,77 | 3,90 | -13,03 | -11,14 | - |
| HDL | 0,43 | 0,88 | -1,23 | -2,57 | - |
| TG | -38,14 | -21,16 | -1,89 | -0,97 | 1915,1 |
| Weight (kg) | -4,89 | -6,50 | -3,02 | -3,87 | 61,8 |
| Waist Circ. (cm) | -6,14 | -6,52 | -4,11 | -4,26 | 49,5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





