Submitted:
28 December 2023
Posted:
28 December 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and methods
2.1. Sample collection and DNA extraction
2.2. Genome annotation
2.3. Comparative genome analysis
2.4. Repeat sequence analysis
2.5. Phylogenetic analysis
3. Results
3.1. Chloroplast genome assembly and genome features
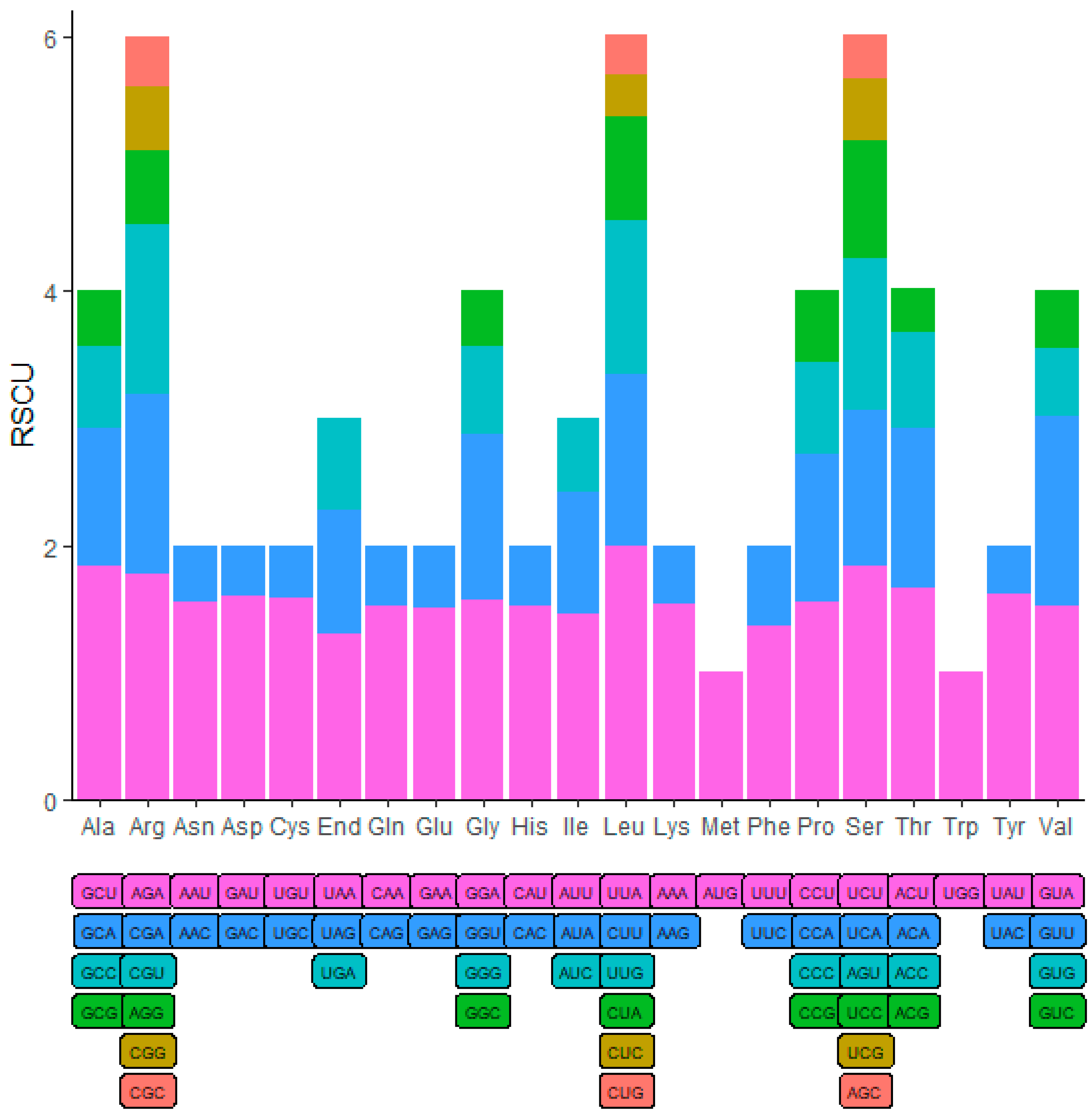
3.2. Codon usage bias
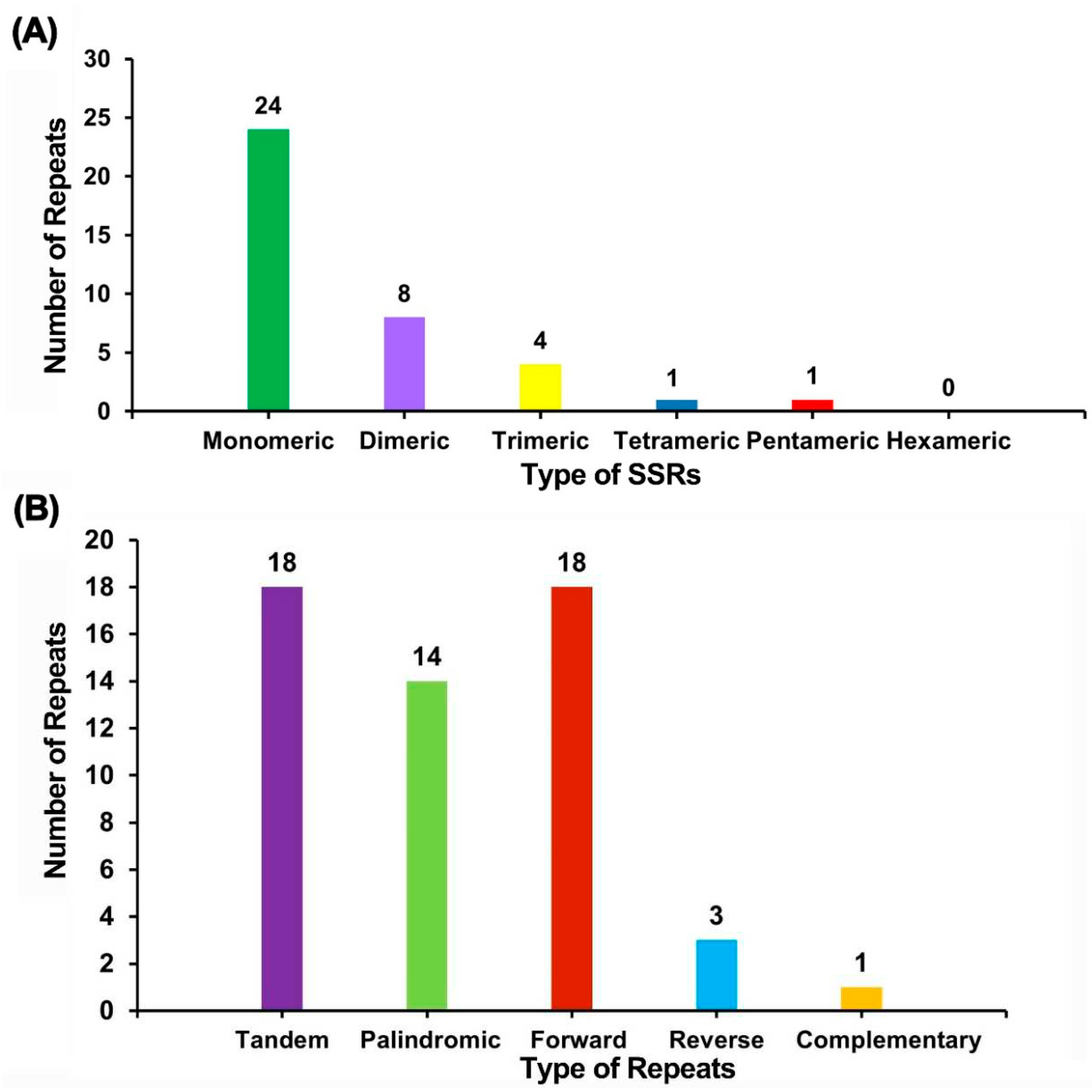
3.3. Repeat sequences and SSR analysis
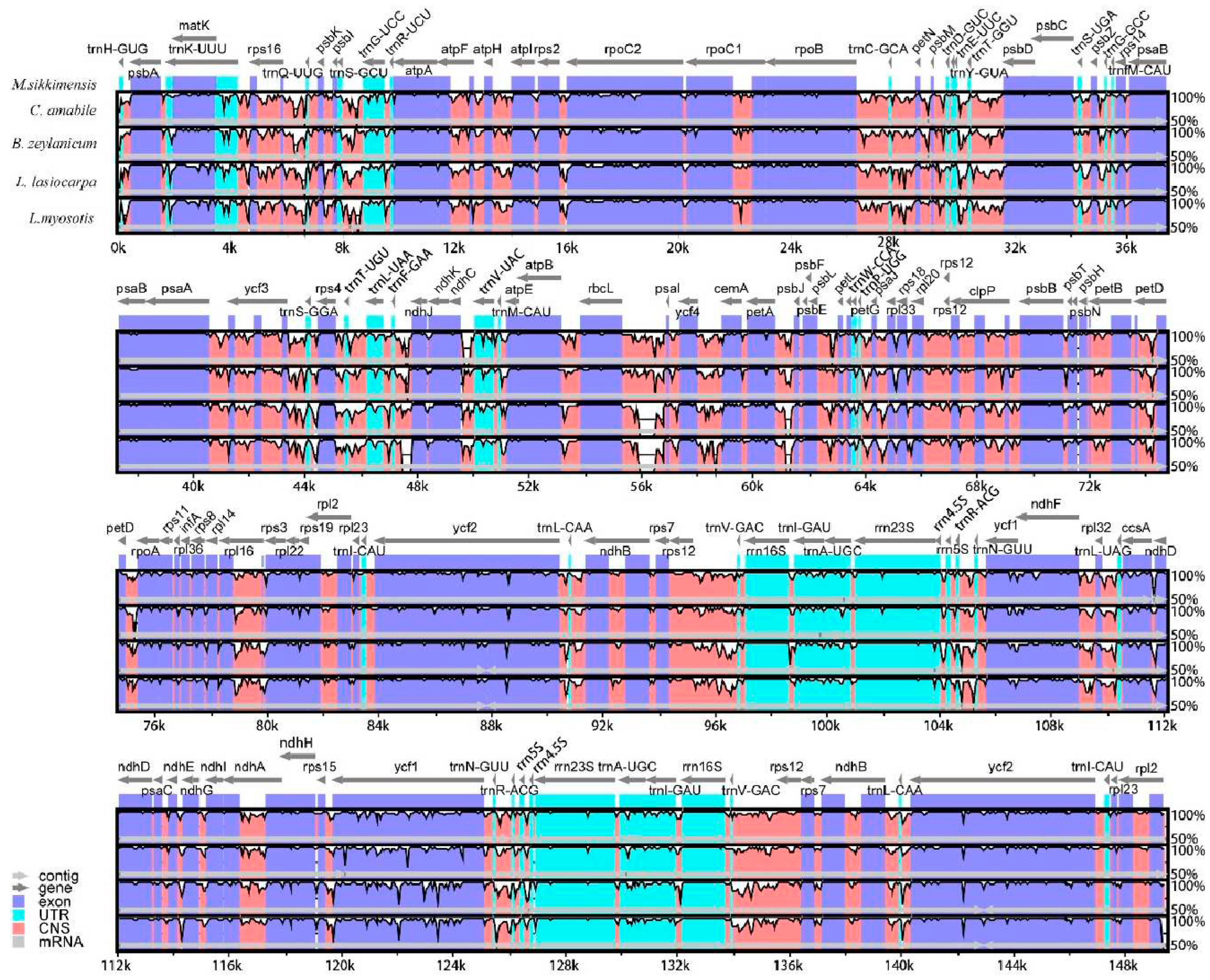
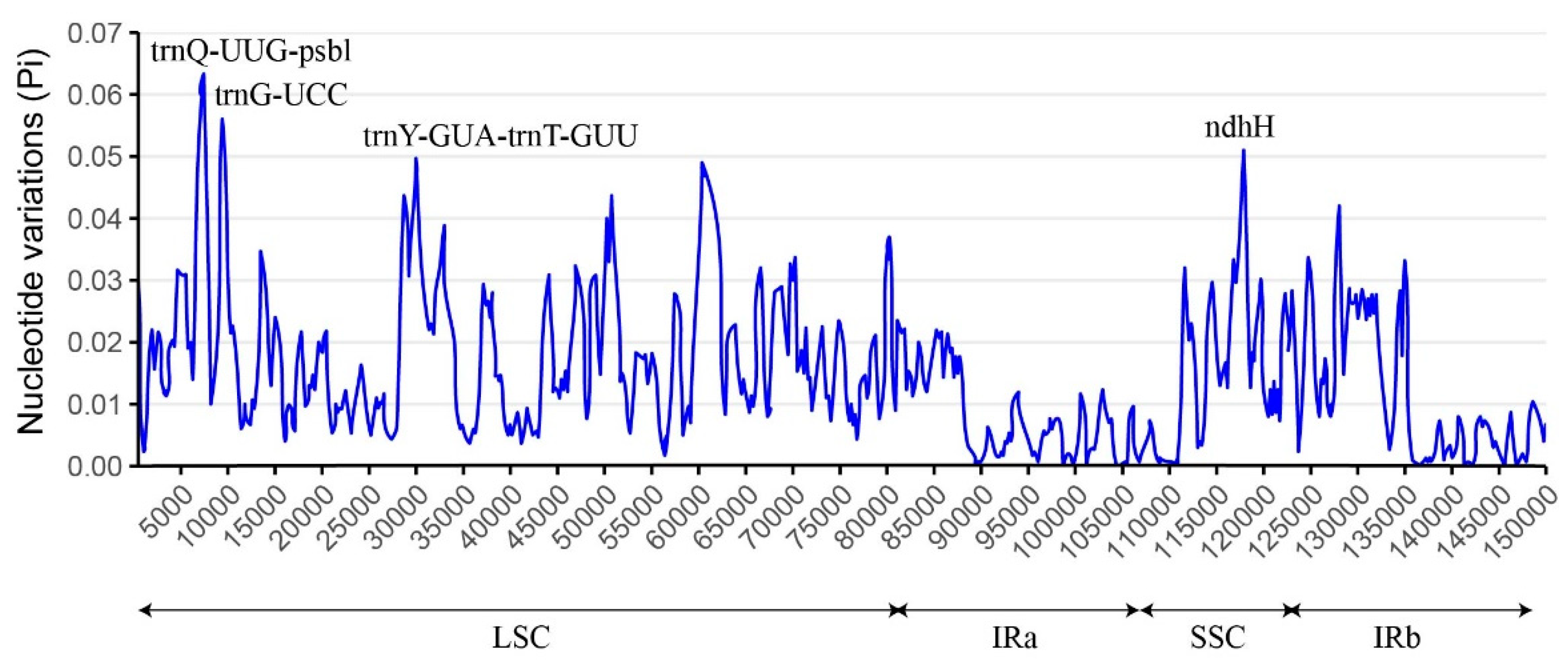
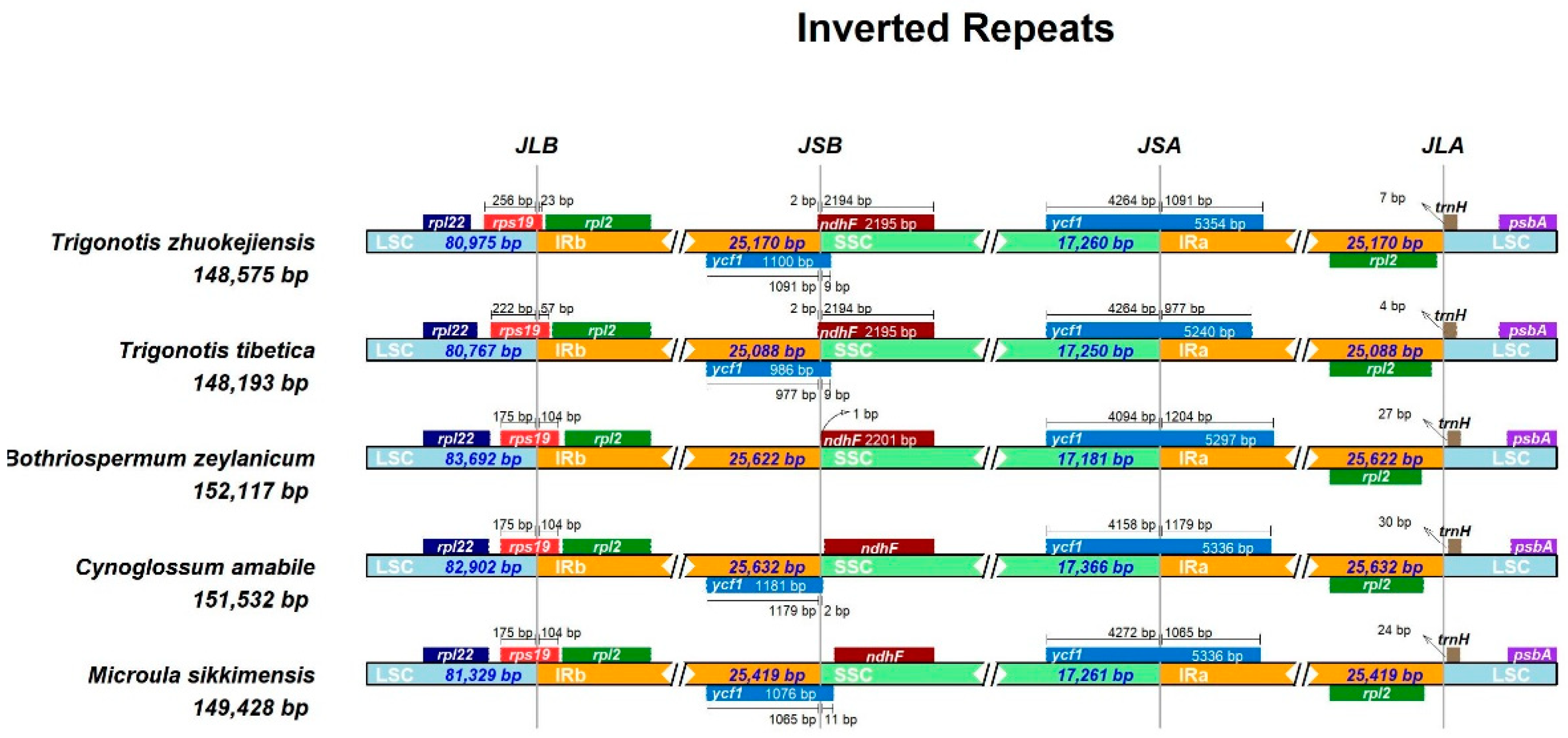
3.4. Comparative genome analysis
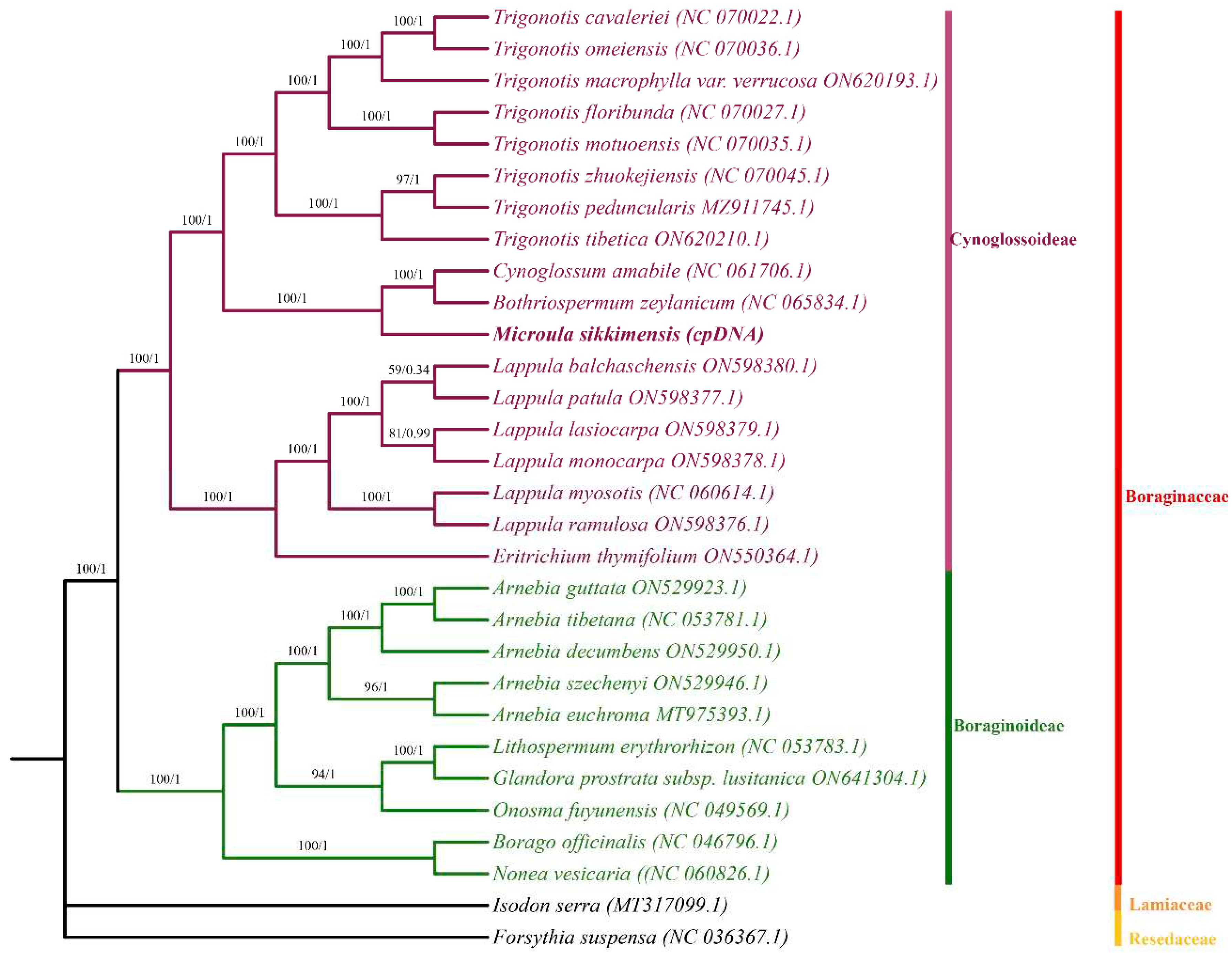
3.5. Phylogenetic analysis
4. Discussion
5. Conclusion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Han, F; Cheng, D.Z; Shi, S.B; Ran, F; Li, Y.K; Bao, S.K; Ren, F; Shi, L.N; Han, Q. The research process of a high - quality wild resource - Microula sikkimensis (in Chinese). Chinese wild plant resources 2007, 5-9.
- Zheng, S.Z; Yang, H.P; Meng, J.C; Ma, X.M; Shen, X.W. Studies on the constituents from the seeds of M.sikkimensis (in Chinese). Journal of northwest normal university (natural science) 2003, 54-57. [CrossRef]
- Wu, L.P. Exploitation and research of Microula sikkimensis resources (in Chinese). China oils and fats 1994, 41-42.
- Luo, G.R; Deng, Y.C. Experiment on feeding Microula sikkimensis straw to Tibetan sheep. Journal of grassland and forage science 2001, 56-57.
- Szabò, I.; Spetea, C. Impact of the ion transportome of chloroplasts on the optimization of photosynthesis. J Exp Bot 2017, 68, 3115-3128. [CrossRef]
- Mullineaux, P.M.; Exposito-Rodriguez, M.; Laissue, P.P.; Smirnoff, N. ROS-dependent signalling pathways in plants and algae exposed to high light: Comparisons with other eukaryotes. Free radical biology & medicine 2018, 122, 52-64. [CrossRef]
- Pollari, M.; Ruotsalainen, V.; Rantamäki, S.; Tyystjärvi, E.; Tyystjärvi, T. Simultaneous inactivation of sigma factors B and D interferes with light acclimation of the cyanobacterium Synechocystis sp. strain PCC 6803. Journal of bacteriology 2009, 191, 3992-4001. [CrossRef]
- Yang, X.; Xie, D.F.; Chen, J.P.; Zhou, S.D.; Yu, Y.; He, X.J. Comparative Analysis of the Complete Chloroplast Genomes in Allium Subgenus Cyathophora (Amaryllidaceae): Phylogenetic Relationship and Adaptive Evolution. BioMed research international 2020, 2020, 1732586. [CrossRef]
- Yang, J.B.; Tang, M.; Li, H.T.; Zhang, Z.R.; Li, D.Z. Complete chloroplast genome of the genus Cymbidium: lights into the species identification, phylogenetic implications and population genetic analyses. BMC evolutionary biology 2013, 13, 84. [CrossRef]
- Daniell, H.; Lin, C.S.; Yu, M.; Chang, W.J. Chloroplast genomes: diversity, evolution, and applications in genetic engineering. Genome biology 2016, 17, 134. [CrossRef]
- Kim, G.B.; Lim, C.E.; Kim, J.S.; Kim, K.; Lee, J.H.; Yu, H.J.; Mun, J.H. Comparative chloroplast genome analysis of Artemisia (Asteraceae) in East Asia: insights into evolutionary divergence and phylogenomic implications. BMC genomics 2020, 21, 415. [CrossRef]
- Olmstead, R.G.; Palmer, J.D. Chloroplast DNA systematics: a review of methods and data analysis. American Journal of Botany 1994, 81, 1205-1224.
- Wang, A.; Wu, H.; Zhu, X.; Lin, J. Species Identification of Conyza bonariensis Assisted by Chloroplast Genome Sequencing. Front Genet 2018, 9, 374. [CrossRef]
- Kelchner, S. The Evolution of Non-Coding Chloroplast DNA and Its Application in Plant Systematics. Annals of the Missouri Botanical Garden 2000, 87, 482-498. [CrossRef]
- Tang, J.; Xia, H.; Cao, M.; Zhang, X.; Zeng, W.; Hu, S.; Tong, W.; Wang, J.; Wang, J.; Yu, J.; et al. A comparison of rice chloroplast genomes. Plant Physiol 2004, 135, 412-420. [CrossRef]
- Li, J.; Tang, J.; Zeng, S.; Han, F.; Yuan, J.; Yu, J. Comparative plastid genomics of four Pilea (Urticaceae) species: insight into interspecific plastid genome diversity in Pilea. BMC Plant Biol 2021, 21, 25. [CrossRef]
- Saski, C.; Lee, S.B.; Daniell, H.; Wood, T.C.; Tomkins, J.; Kim, H.G.; Jansen, R.K. Complete chloroplast genome sequence of Gycine max and comparative analyses with other legume genomes. Plant Mol Biol 2005, 59, 309-322. [CrossRef]
- Du, Q.; Li, J.; Wang, L.; Chen, H.; Jiang, M.; Chen, Z.; Jiang, C.; Gao, H.; Wang, B.; Liu, C. Complete chloroplast genomes of two medicinal Swertia species: the comparative evolutionary analysis of Swertia genus in the Gentianaceae family. Planta 2022, 256, 73. [CrossRef]
- Daniell, H.; Lee, S.-B.; Grevich, J.; Saski, C.; Quesada-Vargas, T.; Guda, C.; Tomkins, J.; Jansen, R.K. Complete chloroplast genome sequences of Solanum bulbocastanum, Solanum lycopersicum and comparative analyses with other Solanaceae genomes. Theoretical and Applied Genetics 2006, 112, 1503-1518. [CrossRef]
- Shi, L.; Chen, H.; Jiang, M.; Wang, L.; Wu, X.; Huang, L.; Liu, C. CPGAVAS2, an integrated plastome sequence annotator and analyzer. Nucleic acids research 2019, 47, W65-w73. [CrossRef]
- Greiner, S.; Lehwark, P.; Bock, R. OrganellarGenomeDRAW (OGDRAW) version 1.3.1: expanded toolkit for the graphical visualization of organellar genomes. Nucleic acids research 2019, 47, W59-w64. [CrossRef]
- Lowe, T.M.; Eddy, S.R. tRNAscan-SE: a program for improved detection of transfer RNA genes in genomic sequence. Nucleic acids research 1997, 25, 955-964. [CrossRef]
- Chen, Y.; Ye, W.; Zhang, Y.; Xu, Y. High speed BLASTN: an accelerated MegaBLAST search tool. Nucleic acids research 2015, 43, 7762-7768. [CrossRef]
- Liu, S.; Ni, Y.; Li, J.; Zhang, X.; Yang, H.; Chen, H.; Liu, C. CPGView: A package for visualizing detailed chloroplast genome structures. Molecular ecology resources 2023, 23, 694-704. [CrossRef]
- Lewis, S.E.; Searle, S.M.; Harris, N.; Gibson, M.; Lyer, V.; Richter, J.; Wiel, C.; Bayraktaroglu, L.; Birney, E.; Crosby, M.A.; et al. Apollo: a sequence annotation editor. Genome biology 2002, 3, Research0082. [CrossRef]
- Frazer, K.A.; Pachter, L.; Poliakov, A.; Rubin, E.M.; Dubchak, I. VISTA: computational tools for comparative genomics. Nucleic acids research 2004, 32, W273-279. [CrossRef]
- Amiryousefi, A.; Hyvönen, J.; Poczai, P. IRscope: an online program to visualize the junction sites of chloroplast genomes. Bioinformatics (Oxford, England) 2018, 34, 3030-3031. [CrossRef]
- Katoh, K.; Standley, D.M. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Molecular biology and evolution 2013, 30, 772-780. [CrossRef]
- Rozas, J.; Ferrer-Mata, A.; Sánchez-DelBarrio, J.C.; Guirao-Rico, S.; Librado, P.; Ramos-Onsins, S.E.; Sánchez-Gracia, A. DnaSP 6: DNA Sequence Polymorphism Analysis of Large Data Sets. Molecular biology and evolution 2017, 34, 3299-3302. [CrossRef]
- Zhang, D.; Gao, F.; Jakovlić, I.; Zou, H.; Zhang, J.; Li, W.X.; Wang, G.T. PhyloSuite: An integrated and scalable desktop platform for streamlined molecular sequence data management and evolutionary phylogenetics studies. Molecular ecology resources 2020, 20, 348-355. [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Molecular biology and evolution 2016, 33, 1870-1874. [CrossRef]
- Beier, S.; Thiel, T.; Münch, T.; Scholz, U.; Mascher, M. MISA-web: a web server for microsatellite prediction. Bioinformatics (Oxford, England) 2017, 33, 2583-2585. [CrossRef]
- Benson, G. Tandem repeats finder: a program to analyze DNA sequences. Nucleic acids research 1999, 27, 573-580. [CrossRef]
- Kurtz, S.; Choudhuri, J.V.; Ohlebusch, E.; Schleiermacher, C.; Stoye, J.; Giegerich, R. REPuter: the manifold applications of repeat analysis on a genomic scale. Nucleic acids research 2001, 29, 4633-4642. [CrossRef]
- Talavera, G.; Castresana, J. Improvement of phylogenies after removing divergent and ambiguously aligned blocks from protein sequence alignments. Systematic biology 2007, 56, 564-577. [CrossRef]
- Nguyen, L.T.; Schmidt, H.A.; von Haeseler, A.; Minh, B.Q. IQ-TREE: a fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Molecular biology and evolution 2015, 32, 268-274. [CrossRef]
- Ronquist, F.; Teslenko, M.; van der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Systematic biology 2012, 61, 539-542. [CrossRef]
- Letunic, I.; Bork, P. Interactive Tree Of Life (iTOL) v4: recent updates and new developments. Nucleic acids research 2019, 47, W256-w259. [CrossRef]
- Yi, D.K.; Kim, K.J. Two complete chloroplast genome sequences of genus Paulownia (Paulowniaceae): Paulownia coreana and P. tomentosa. Mitochondrial DNA. Part B, Resources 2016, 1, 627-629. [CrossRef]
- Zheng, L.P.; Li, L.J. Characterization of the complete chloroplast genome of Centranthera grandiflora Benth (Orobanchaceae), an important species of medicinal herb. Mitochondrial DNA. Part B, Resources 2021, 6, 1784-1785. [CrossRef]
- Shen, X.; Wu, M.; Liao, B.; Liu, Z.; Bai, R.; Xiao, S.; Li, X.; Zhang, B.; Xu, J.; Chen, S. Complete Chloroplast Genome Sequence and Phylogenetic Analysis of the Medicinal Plant Artemisia annua. Molecules (Basel, Switzerland) 2017, 22. [CrossRef]
- Zhang, F.; Chen, H.; Zhou, Y.; Li, N.; Chong, X.; Li, Y.; Lu, X.; Wang, C. The complete chloroplast genome sequence and phylogenetic analysis of Ilex 'Beryl', a hybrid of Ilex cornuta × Ilex latifolia (Aquifoliaceae). Mitochondrial DNA. Part B, Resources 2021, 6, 227-228. [CrossRef]
- Zeng, S.; Zhou, T.; Han, K.; Yang, Y.; Zhao, J.; Liu, Z.L. The Complete Chloroplast Genome Sequences of Six Rehmannia Species. Genes (Basel) 2017, 8. [CrossRef]
- Nie, L.; Cui, Y.; Wu, L.; Zhou, J.; Xu, Z.; Li, Y.; Li, X.; Wang, Y.; Yao, H. Gene Losses and Variations in Chloroplast Genome of Parasitic Plant Macrosolen and Phylogenetic Relationships within Santalales. Int J Mol Sci 2019, 20. [CrossRef]
- Xue, S.; Shi, T.; Luo, W.; Ni, X.; Iqbal, S.; Ni, Z.; Huang, X.; Yao, D.; Shen, Z.; Gao, Z. Comparative analysis of the complete chloroplast genome among Prunus mume, P. armeniaca, and P. salicina. Horticulture research 2019, 6, 89. [CrossRef]
- Sun, J.; Wang, S.; Wang, Y.; Wang, R.; Liu, K.; Li, E.; Qiao, P.; Shi, L.; Dong, W.; Huang, L.; et al. Phylogenomics and Genetic Diversity of Arnebiae Radix and Its Allies (Arnebia, Boraginaceae) in China. Front Plant Sci 2022, 13, 920826. [CrossRef]
- Zhao, F.; Peng, H. The complete chloroplast genome of Caryopteris incana (Lamiaceae) and phylogenetic analysis. Mitochondrial DNA Part B 2020, 5, 1399-1400. [CrossRef]
- Duan, H.C.; Zheng, X.H.; Li, Y.Y.; Li, S.M.; Ye, L.; Jing, H.Z.; Dong, Q. The complete chloroplast genome of Fraxinus malacophylla (Oleaceae, Oleoideae). Mitochondrial DNA. Part B, Resources 2020, 5, 3588-3589. [CrossRef]
- He, Y.; Xu, X.; Liu, Q. The complete chloroplast genome of Onosma fuyunensis Y. He & Q.R. Liu and its phylogenetic analysis. Mitochondrial DNA. Part B, Resources 2021, 6, 3142-3143. [CrossRef]
- Wu, J.H.; Li, H.M.; Lei, J.M.; Liang, Z.R. The complete chloroplast genome sequence of Trigonotis peduncularis (Boraginaceae). Mitochondrial DNA. Part B, Resources 2022, 7, 456-457. [CrossRef]
- Wu, L.; Fan, P.; Zhou, J.; Li, Y.; Xu, Z.; Lin, Y.; Wang, Y.; Song, J.; Yao, H. Gene Losses and Homology of the Chloroplast Genomes of Taxillus and Phacellaria Species. Genes (Basel) 2023, 14. [CrossRef]
- Ebert, D.; Peakall, R. Chloroplast simple sequence repeats (cpSSRs): technical resources and recommendations for expanding cpSSR discovery and applications to a wide array of plant species. Molecular ecology resources 2009, 9, 673-690. [CrossRef]
- George, B.; Bhatt, B.S.; Awasthi, M.; George, B.; Singh, A.K. Comparative analysis of microsatellites in chloroplast genomes of lower and higher plants. Current genetics 2015, 61, 665-677. [CrossRef]
- Du, X.; Zeng, T.; Feng, Q.; Hu, L.; Luo, X.; Weng, Q.; He, J.; Zhu, B. The complete chloroplast genome sequence of yellow mustard (Sinapis alba L.) and its phylogenetic relationship to other Brassicaceae species. Gene 2020, 731, 144340. [CrossRef]
- Mwanzia, V.M.; Nzei, J.M.; Yan, D.Y.; Kamau, P.W.; Chen, J.M.; Li, Z.Z. The complete chloroplast genomes of two species in threatened monocot genus Caldesia in China. Genetica 2019, 147, 381-390. [CrossRef]
- Dong, S.; Ying, Z.; Yu, S.; Wang, Q.; Liao, G.; Ge, Y.; Cheng, R. Complete chloroplast genome of Stephania tetrandra (Menispermaceae) from Zhejiang Province: insights into molecular structures, comparative genome analysis, mutational hotspots and phylogenetic relationships. BMC genomics 2021, 22, 880. [CrossRef]
- Qin, Z.; Cai, Z.; Xia, G.; Wang, M. Synonymous codon usage bias is correlative to intron number and shows disequilibrium among exons in plants. BMC genomics 2013, 14, 56. [CrossRef]
- Zhang, Z.; Dai, W.; Wang, Y.; Lu, C.; Fan, H. Analysis of synonymous codon usage patterns in torque teno sus virus 1 (TTSuV1). Archives of Virology 2013, 158, 145-154. [CrossRef]
- Wang, X.; Zhou, T.; Bai, G.; Zhao, Y. Complete chloroplast genome sequence of Fagopyrum dibotrys: genome features, comparative analysis and phylogenetic relationships. Sci Rep 2018, 8, 12379. [CrossRef]
- Chen, Y.; Hu, N.; Wu, H. Analyzing and Characterizing the Chloroplast Genome of Salix wilsonii. BioMed research international 2019, 2019, 5190425. [CrossRef]
- Zhang, H.; Huang, T.; Zhou, Q.; Sheng, Q.; Zhu, Z. Complete Chloroplast Genomes and Phylogenetic Relationships of Bougainvillea spectabilis and Bougainvillea glabra (Nyctaginaceae). International Journal of Molecular Sciences 2023, 24, 13044.
- Jiao, L.; Lu, Y.; He, T.; Li, J.; Yin, Y. A strategy for developing high-resolution DNA barcodes for species discrimination of wood specimens using the complete chloroplast genome of three Pterocarpus species. Planta 2019, 250, 95-104. [CrossRef]
- Bi, Y.; Zhang, M.F.; Xue, J.; Dong, R.; Du, Y.P.; Zhang, X.H. Chloroplast genomic resources for phylogeny and DNA barcoding: a case study on Fritillaria. Sci Rep 2018, 8, 1184. [CrossRef]
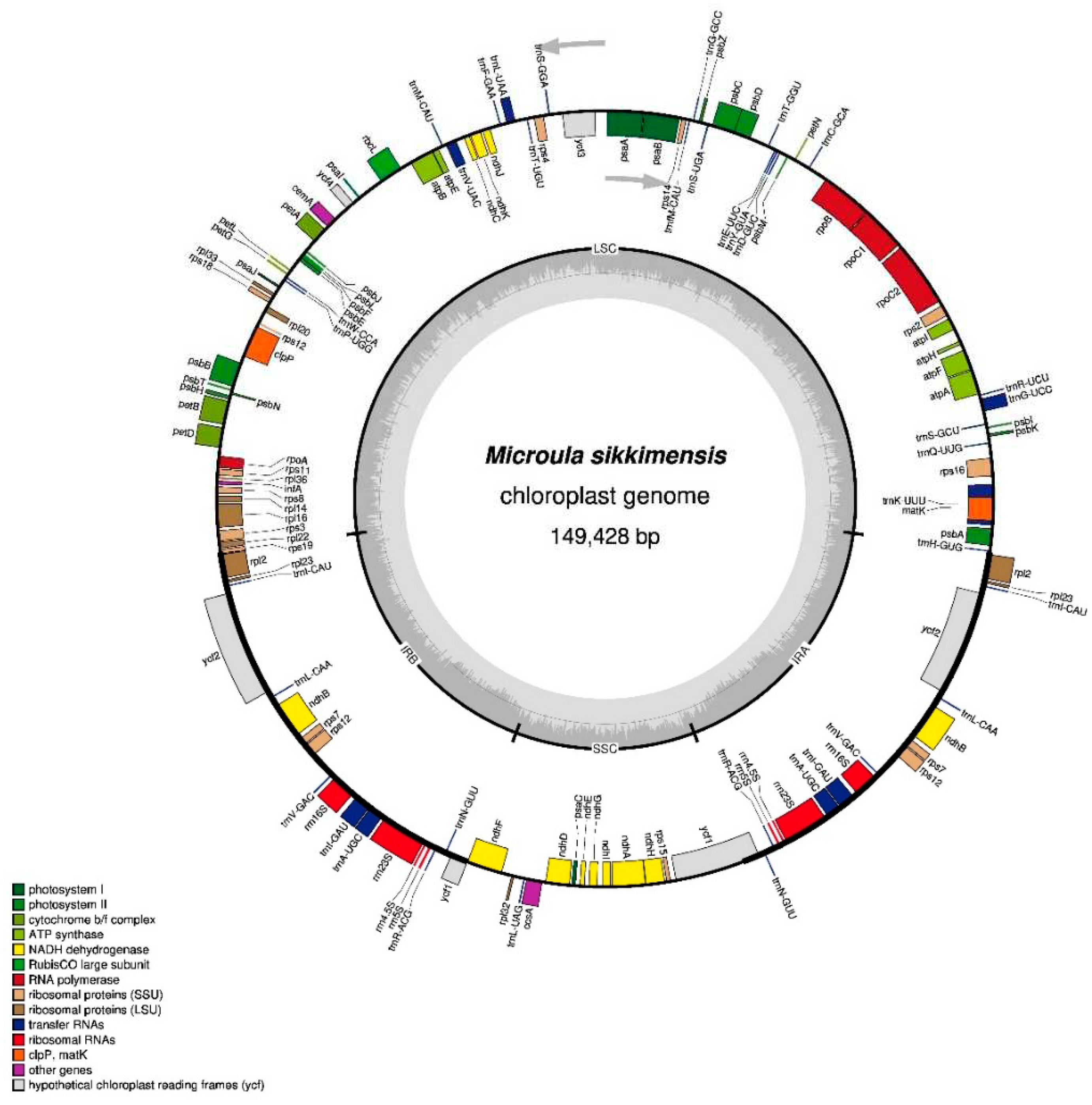
| Group of genes | Name of genes | number of genes |
| NADH-dehydrogenase | ndhA, ndhB (×2), ndhC, ndhD, ndhE, ndhF, ndhG, ndhH, ndhI, ndhJ, ndhK | 11 |
| photosystem I | psaA, psaB, psaC, psaI, psaJ | 5 |
| photosystem II | psbA, psbB, psbC, psbD, psbE, psbF, psbH, psbI, psbJ, psbK, psbL, psbM, psbN, psbT, psbZ, ycf3 | 16 |
| cytochrome b/f complex | petA, petB, petD, petG, petL, petN | 6 |
| ATP synthase | atpA, atpB, atpE, atpF, atpH, atpI | 6 |
| Large subunit of rubisco | rbcL | 1 |
| Small subunit of ribosome | rps2, rps3, rps4, rps7 (×2), rps8, rps11, rps12 (×2), rps14, rps15, rps16, rps18, rps19 | 12 |
| Large subunit of ribosome | rpl2 (×2), rpl14, rpl16, rpl20, rpl22, rpl23 (×2), rpl32, rpl33, rpl36 | 9 |
| DNA dependent RNA polymerase | rpoA, rpoB, rpoC1, rpoC2 | 4 |
| rRNA genes | rrn4.5S (×2), rrn5S (×2), rrn16S (×2), rrn23S (×2) | 4 |
| tRNA genes | 30tRNAs | 30 |
| Maturase | matK | 1 |
| Envelope membrane protein | cemA | 1 |
| Protease | clpP | 1 |
| c-type cytochrom synthesis gene | ccsA | 1 |
| Translation al initiation factor | infA | 1 |
| Genes of unknown functions Open Reading | ycf1 (×2), ycf2 (×2), ycf4 | 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





