Submitted:
27 December 2023
Posted:
29 December 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Structure and cellular localization of FADD
2.1. The structural organization of FADD
2.2. Expression regulation and localization of FADD
3. FADD: cell intrinsic molecular intraction
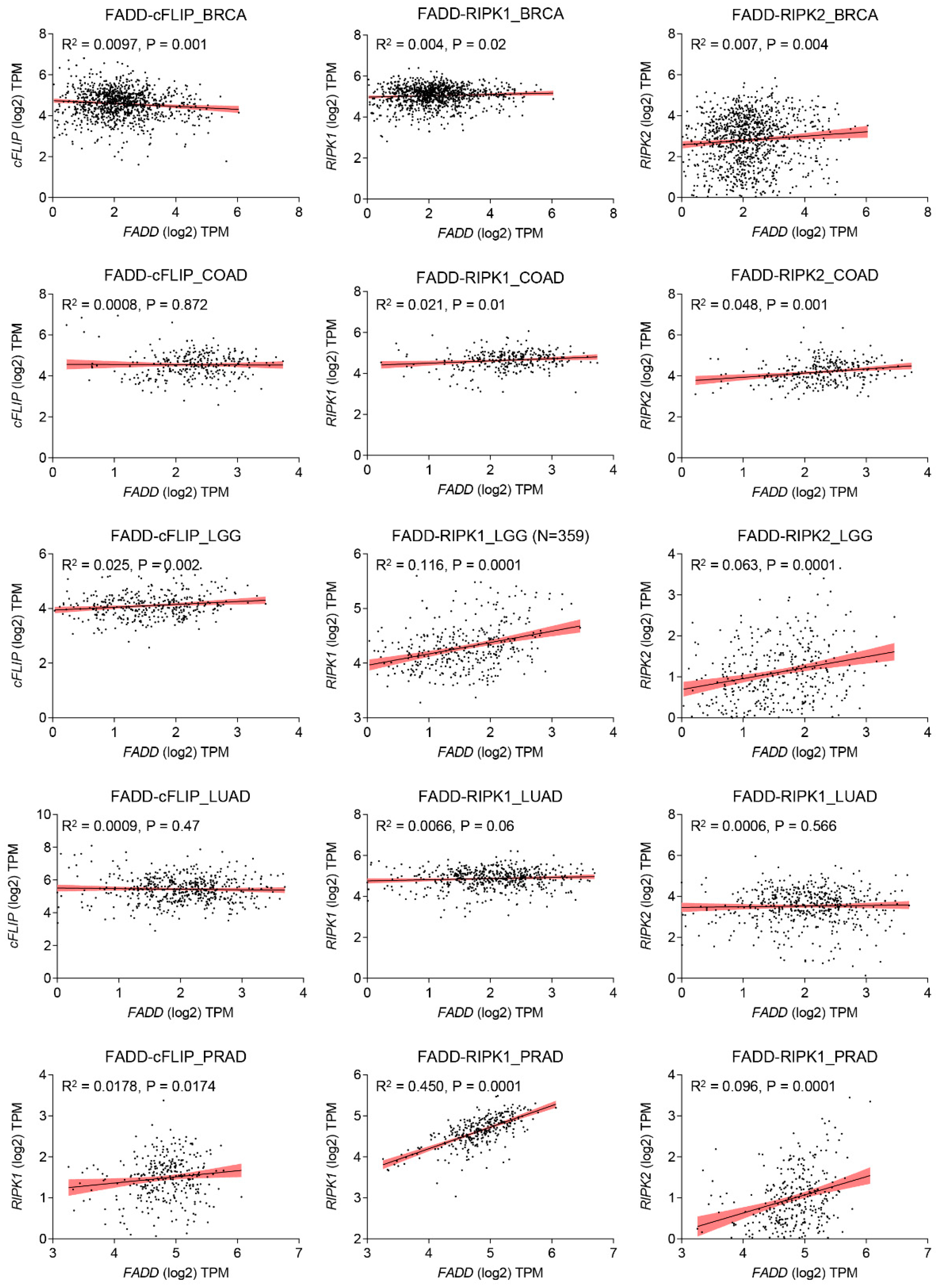
3.1. FADD and cFLIP interactions
3.2. FADD and RIP1 interaction
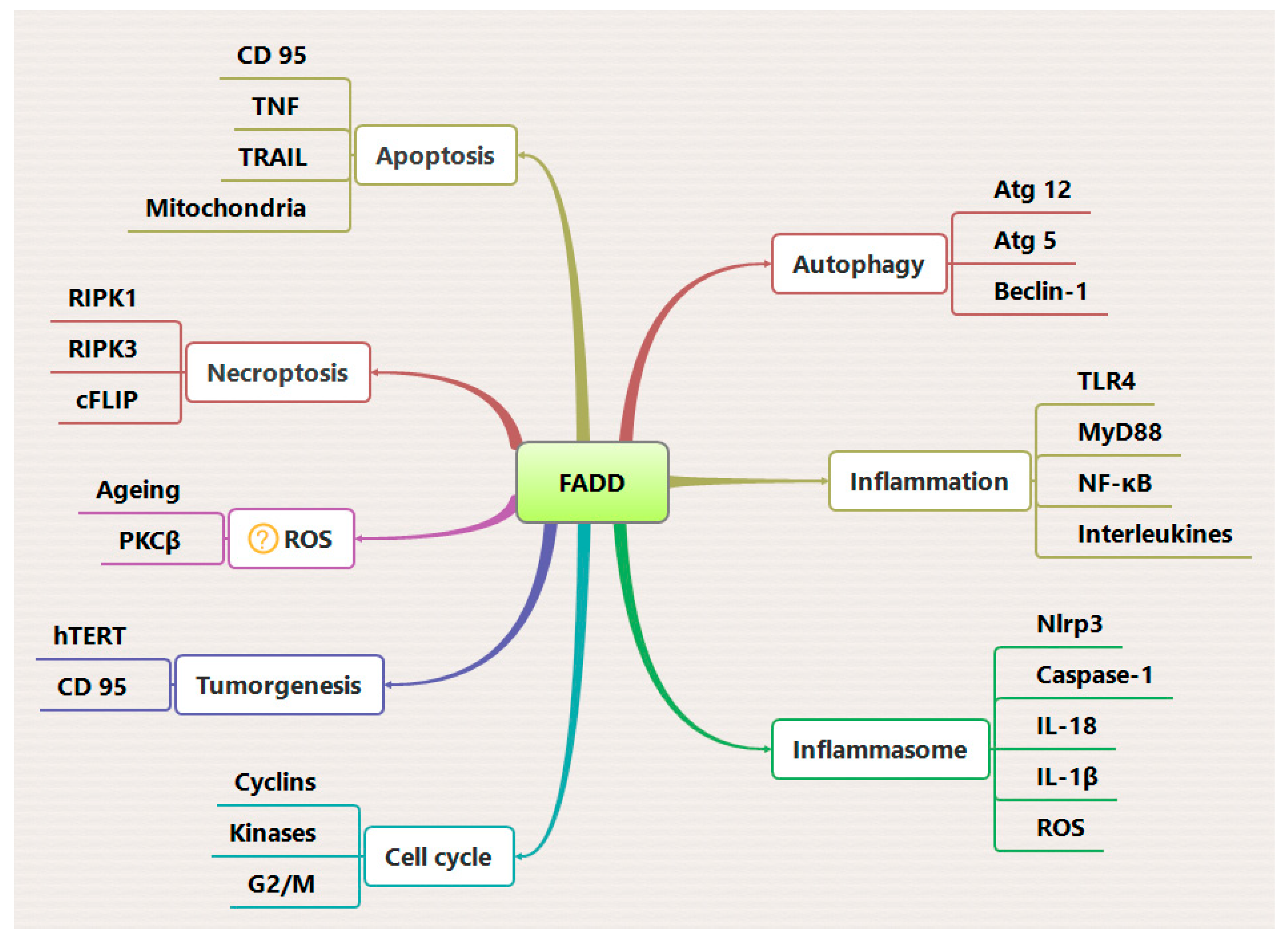
4. Role of FADD in cell death and Inflammatory signaling
4.1. FADD in the regulation of the TNFα-NF-κB signaling axis
4.2. Role of FADD in Necroptosis
4.3. Role of FADD in Inflammation
4.4. Role of FADD in Autophagy
5. FADD in cancer therapeutic
6. Future perspective
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Tourneur, L.; Chiocchia, G. FADD: a regulator of life and death. Trends in immunology 2010, 31, 260-269. [CrossRef]
- Scaffidi, C.; Fulda, S.; Srinivasan, A.; Friesen, C.; Li, F.; Tomaselli, K.J.; Debatin, K.M.; Krammer, P.H.; Peter, M.E. Two CD95 (APO-1/Fas) signaling pathways. EMBO J 1998, 17, 1675-1687. [CrossRef]
- Imtiyaz, H.Z.; Zhou, X.; Zhang, H.; Chen, D.; Hu, T.; Zhang, J. The death domain of FADD is essential for embryogenesis, lymphocyte development, and proliferation. The Journal of biological chemistry 2009, 284, 9917-9926. [CrossRef]
- Marin-Rubio, J.L.; Vela-Martin, L.; Fernandez-Piqueras, J.; Villa-Morales, M. FADD in Cancer: Mechanisms of Altered Expression and Function, and Clinical Implications. Cancers (Basel) 2019, 11. [CrossRef]
- Algeciras-Schimnich, A.; Shen, L.; Barnhart, B.C.; Murmann, A.E.; Burkhardt, J.K.; Peter, M.E. Molecular ordering of the initial signaling events of CD95. Molecular and cellular biology 2002, 22, 207-220.
- Jeong, E.J.; Bang, S.; Lee, T.H.; Park, Y.I.; Sim, W.S.; Kim, K.S. The solution structure of FADD death domain. Structural basis of death domain interactions of Fas and FADD. The Journal of biological chemistry 1999, 274, 16337-16342.
- Scott, F.L.; Stec, B.; Pop, C.; Dobaczewska, M.K.; Lee, J.J.; Monosov, E.; Robinson, H.; Salvesen, G.S.; Schwarzenbacher, R.; Riedl, S.J. The Fas-FADD death domain complex structure unravels signalling by receptor clustering. Nature 2009, 457, 1019-1022. [CrossRef]
- Gomez-Angelats, M.; Cidlowski, J.A. Molecular evidence for the nuclear localization of FADD. Cell death and differentiation 2003, 10, 791-797. [CrossRef]
- O'Reilly, L.A.; Divisekera, U.; Newton, K.; Scalzo, K.; Kataoka, T.; Puthalakath, H.; Ito, M.; Huang, D.C.; Strasser, A. Modifications and intracellular trafficking of FADD/MORT1 and caspase-8 after stimulation of T lymphocytes. Cell death and differentiation 2004, 11, 724-736. [CrossRef]
- Screaton, R.A.; Kiessling, S.; Sansom, O.J.; Millar, C.B.; Maddison, K.; Bird, A.; Clarke, A.R.; Frisch, S.M. Fas-associated death domain protein interacts with methyl-CpG binding domain protein 4: a potential link between genome surveillance and apoptosis. Proceedings of the National Academy of Sciences of the United States of America 2003, 100, 5211-5216. [CrossRef]
- Tourneur, L.; Mistou, S.; Michiels, F.M.; Devauchelle, V.; Renia, L.; Feunteun, J.; Chiocchia, G. Loss of FADD protein expression results in a biased Fas-signaling pathway and correlates with the development of tumoral status in thyroid follicular cells. Oncogene 2003, 22, 2795-2804. [CrossRef]
- Park, S.M.; Schickel, R.; Peter, M.E. Nonapoptotic functions of FADD-binding death receptors and their signaling molecules. Current opinion in cell biology 2005, 17, 610-616. [CrossRef]
- Werner, M.H.; Wu, C.; Walsh, C.M. Emerging roles for the death adaptor FADD in death receptor avidity and cell cycle regulation. Cell cycle 2006, 5, 2332-2338.
- Cheng, W.; Zhang, R.; Yao, C.; He, L.; Jia, K.; Yang, B.; Du, P.; Zhuang, H.; Chen, J.; Liu, Z.; et al. A critical role of Fas-associated protein with death domain phosphorylation in intracellular reactive oxygen species homeostasis and aging. Antioxidants & redox signaling 2014, 21, 33-45. [CrossRef]
- Zhou, K.; Bai, L.; Nan, X.; Zhao, K.; Song, Y.; Li, W.; Wang, Q. FADD regulates antibacterial immune responses via the immune deficiency signaling pathway in the Chinese mitten crab. Dev Comp Immunol 2022, 128, 104326. [CrossRef]
- Pyo, J.O.; Jang, M.H.; Kwon, Y.K.; Lee, H.J.; Jun, J.I.; Woo, H.N.; Cho, D.H.; Choi, B.; Lee, H.; Kim, J.H.; et al. Essential roles of Atg5 and FADD in autophagic cell death: dissection of autophagic cell death into vacuole formation and cell death. The Journal of biological chemistry 2005, 280, 20722-20729. [CrossRef]
- Bell, B.D.; Leverrier, S.; Weist, B.M.; Newton, R.H.; Arechiga, A.F.; Luhrs, K.A.; Morrissette, N.S.; Walsh, C.M. FADD and caspase-8 control the outcome of autophagic signaling in proliferating T cells. Proceedings of the National Academy of Sciences of the United States of America 2008, 105, 16677-16682. [CrossRef]
- Welz, P.S.; Wullaert, A.; Vlantis, K.; Kondylis, V.; Fernandez-Majada, V.; Ermolaeva, M.; Kirsch, P.; Sterner-Kock, A.; van Loo, G.; Pasparakis, M. FADD prevents RIP3-mediated epithelial cell necrosis and chronic intestinal inflammation. Nature 2011, 477, 330-334. [CrossRef]
- Han, J.; Zhong, C.Q.; Zhang, D.W. Programmed necrosis: backup to and competitor with apoptosis in the immune system. Nature immunology 2011, 12, 1143-1149. [CrossRef]
- Holler, N.; Zaru, R.; Micheau, O.; Thome, M.; Attinger, A.; Valitutti, S.; Bodmer, J.L.; Schneider, P.; Seed, B.; Tschopp, J. Fas triggers an alternative, caspase-8-independent cell death pathway using the kinase RIP as effector molecule. Nature immunology 2000, 1, 489-495. [CrossRef]
- Feng, S.; Yang, Y.; Mei, Y.; Ma, L.; Zhu, D.E.; Hoti, N.; Castanares, M.; Wu, M. Cleavage of RIP3 inactivates its caspase-independent apoptosis pathway by removal of kinase domain. Cellular signalling 2007, 19, 2056-2067. [CrossRef]
- Salaun, B.; Romero, P.; Lebecque, S. Toll-like receptors' two-edged sword: when immunity meets apoptosis. European journal of immunology 2007, 37, 3311-3318. [CrossRef]
- Mouasni, S.; Tourneur, L. FADD at the Crossroads between Cancer and Inflammation. Trends Immunol 2018, 39, 1036-1053. [CrossRef]
- Alappat, E.C.; Feig, C.; Boyerinas, B.; Volkland, J.; Samuels, M.; Murmann, A.E.; Thorburn, A.; Kidd, V.J.; Slaughter, C.A.; Osborn, S.L.; et al. Phosphorylation of FADD at serine 194 by CKIalpha regulates its nonapoptotic activities. Molecular cell 2005, 19, 321-332. [CrossRef]
- Bhojani, M.S.; Chen, G.; Ross, B.D.; Beer, D.G.; Rehemtulla, A. Nuclear localized phosphorylated FADD induces cell proliferation and is associated with aggressive lung cancer. Cell cycle 2005, 4, 1478-1481.
- Lee, E.W.; Kim, J.H.; Ahn, Y.H.; Seo, J.; Ko, A.; Jeong, M.; Kim, S.J.; Ro, J.Y.; Park, K.M.; Lee, H.W.; et al. Ubiquitination and degradation of the FADD adaptor protein regulate death receptor-mediated apoptosis and necroptosis. Nature communications 2012, 3, 978. [CrossRef]
- Goto, E.; Tokunaga, F. Decreased linear ubiquitination of NEMO and FADD on apoptosis with caspase-mediated cleavage of HOIP. Biochemical and biophysical research communications 2017, 485, 152-159. [CrossRef]
- Ranjan, K.; Pathak, C. FADD regulates NF-kappaB activation and promotes ubiquitination of cFLIPL to induce apoptosis. Sci Rep 2016, 6, 22787. [CrossRef]
- Ranjan, K.; Pathak, C. Expression of FADD and cFLIP(L) balances mitochondrial integrity and redox signaling to substantiate apoptotic cell death. Mol Cell Biochem 2016, 422, 135-150. [CrossRef]
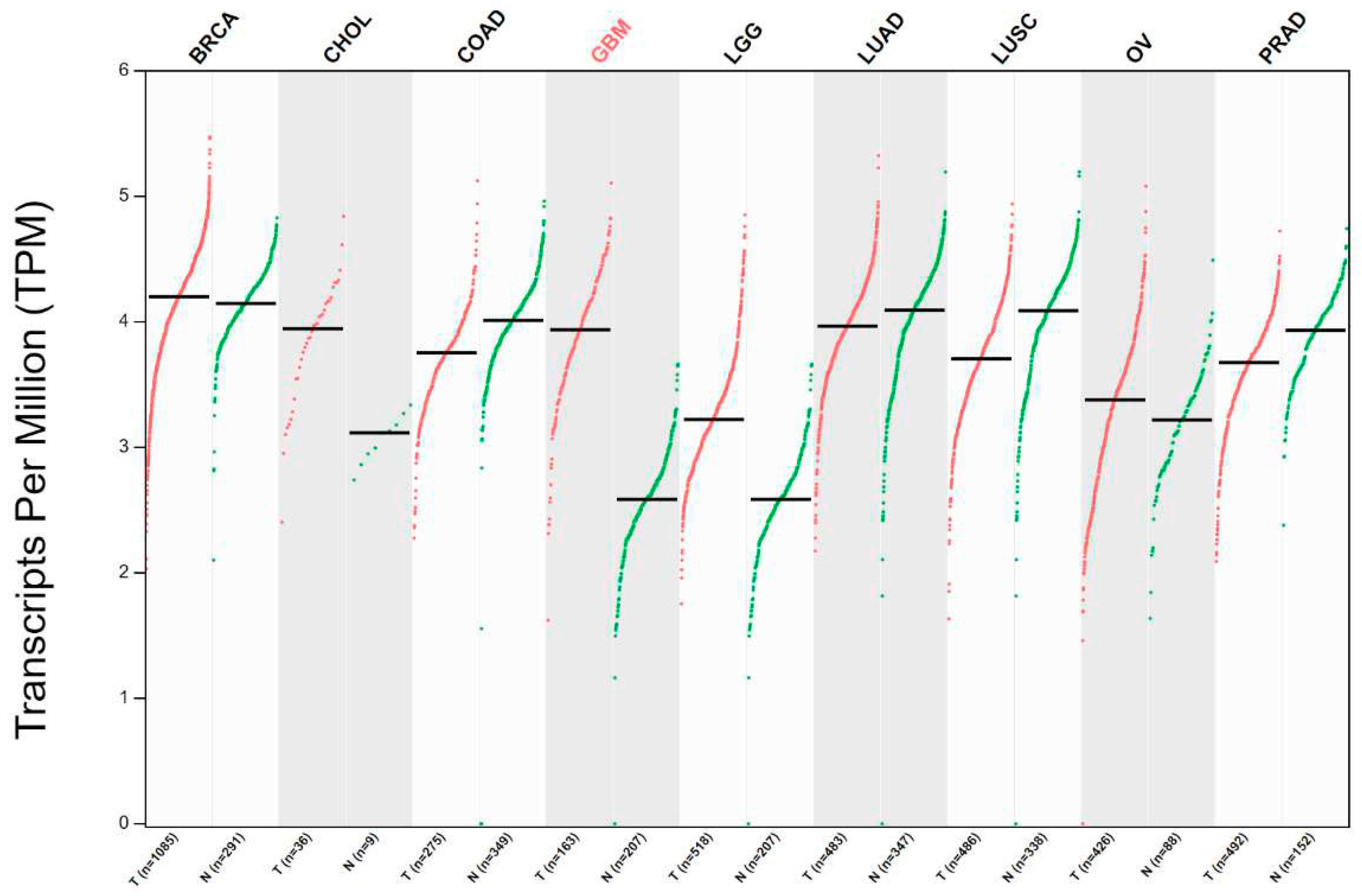
- Tang, Z.; Li, C.; Kang, B.; Gao, G.; Li, C.; Zhang, Z. GEPIA: a web server for cancer and normal gene expression profiling and interactive analyses. Nucleic Acids Res 2017, 45, W98-W102. [CrossRef]
- Kim, P.K.; Dutra, A.S.; Chandrasekharappa, S.C.; Puck, J.M. Genomic structure and mapping of human FADD, an intracellular mediator of lymphocyte apoptosis. Journal of immunology 1996, 157, 5461-5466.
- Chinnaiyan, A.M.; O'Rourke, K.; Tewari, M.; Dixit, V.M. FADD, a novel death domain-containing protein, interacts with the death domain of Fas and initiates apoptosis. Cell 1995, 81, 505-512.
- Hsu, H.; Shu, H.B.; Pan, M.G.; Goeddel, D.V. TRADD-TRAF2 and TRADD-FADD interactions define two distinct TNF receptor 1 signal transduction pathways. Cell 1996, 84, 299-308.
- Barnhart, B.C.; Lee, J.C.; Alappat, E.C.; Peter, M.E. The death effector domain protein family. Oncogene 2003, 22, 8634-8644. [CrossRef]
- Boldin, M.P.; Goncharov, T.M.; Goltsev, Y.V.; Wallach, D. Involvement of MACH, a novel MORT1/FADD-interacting protease, in Fas/APO-1- and TNF receptor-induced cell death. Cell 1996, 85, 803-815.
- Muzio, M.; Chinnaiyan, A.M.; Kischkel, F.C.; O'Rourke, K.; Shevchenko, A.; Ni, J.; Scaffidi, C.; Bretz, J.D.; Zhang, M.; Gentz, R.; et al. FLICE, a novel FADD-homologous ICE/CED-3-like protease, is recruited to the CD95 (Fas/APO-1) death--inducing signaling complex. Cell 1996, 85, 817-827.
- Carrington, P.E.; Sandu, C.; Wei, Y.; Hill, J.M.; Morisawa, G.; Huang, T.; Gavathiotis, E.; Wei, Y.; Werner, M.H. The structure of FADD and its mode of interaction with procaspase-8. Molecular cell 2006, 22, 599-610. [CrossRef]
- Hill, J.M.; Morisawa, G.; Kim, T.; Huang, T.; Wei, Y.; Wei, Y.; Werner, M.H. Identification of an expanded binding surface on the FADD death domain responsible for interaction with CD95/Fas. The Journal of biological chemistry 2004, 279, 1474-1481. [CrossRef]
- Yang, J.K. Death effecter domain for the assembly of death-inducing signaling complex. Apoptosis : an international journal on programmed cell death 2015, 20, 235-239. [CrossRef]
- Park, Y.H.; Jeong, M.S.; Park, H.H.; Jang, S.B. Formation of the death domain complex between FADD and RIP1 proteins in vitro. Biochimica et biophysica acta 2013, 1834, 292-300. [CrossRef]
- Eberstadt, M.; Huang, B.; Chen, Z.; Meadows, R.P.; Ng, S.C.; Zheng, L.; Lenardo, M.J.; Fesik, S.W. NMR structure and mutagenesis of the FADD (Mort1) death-effector domain. Nature 1998, 392, 941-945. [CrossRef]
- Tibbetts, M.D.; Zheng, L.; Lenardo, M.J. The death effector domain protein family: regulators of cellular homeostasis. Nature immunology 2003, 4, 404-409. [CrossRef]
- Siegel, R.M.; Martin, D.A.; Zheng, L.; Ng, S.Y.; Bertin, J.; Cohen, J.; Lenardo, M.J. Death-effector filaments: novel cytoplasmic structures that recruit caspases and trigger apoptosis. The Journal of cell biology 1998, 141, 1243-1253.
- Zhang, J.; Winoto, A. A mouse Fas-associated protein with homology to the human Mort1/FADD protein is essential for Fas-induced apoptosis. Molecular and cellular biology 1996, 16, 2756-2763.
- Sandu, C.; Morisawa, G.; Wegorzewska, I.; Huang, T.; Arechiga, A.F.; Hill, J.M.; Kim, T.; Walsh, C.M.; Werner, M.H. FADD self-association is required for stable interaction with an activated death receptor. Cell death and differentiation 2006, 13, 2052-2061. [CrossRef]
- Chinnaiyan, A.M.; Tepper, C.G.; Seldin, M.F.; O'Rourke, K.; Kischkel, F.C.; Hellbardt, S.; Krammer, P.H.; Peter, M.E.; Dixit, V.M. FADD/MORT1 is a common mediator of CD95 (Fas/APO-1) and tumor necrosis factor receptor-induced apoptosis. The Journal of biological chemistry 1996, 271, 4961-4965.
- Zornig, M.; Hueber, A.O.; Evan, G. p53-dependent impairment of T-cell proliferation in FADD dominant-negative transgenic mice. Current biology : CB 1998, 8, 467-470.
- Matsuyoshi, S.; Shimada, K.; Nakamura, M.; Ishida, E.; Konishi, N. FADD phosphorylation is critical for cell cycle regulation in breast cancer cells. British journal of cancer 2006, 94, 532-539. [CrossRef]
- Scaffidi, C.; Volkland, J.; Blomberg, I.; Hoffmann, I.; Krammer, P.H.; Peter, M.E. Phosphorylation of FADD/ MORT1 at serine 194 and association with a 70-kDa cell cycle-regulated protein kinase. Journal of immunology 2000, 164, 1236-1242.
- Sakamaki, K.; Takagi, C.; Kominami, K.; Sakata, S.; Yaoita, Y.; Kubota, H.Y.; Nozaki, M.; Yonehara, S.; Ueno, N. The adaptor molecule FADD from Xenopus laevis demonstrates evolutionary conservation of its pro-apoptotic activity. Genes to cells : devoted to molecular & cellular mechanisms 2004, 9, 1249-1264. [CrossRef]
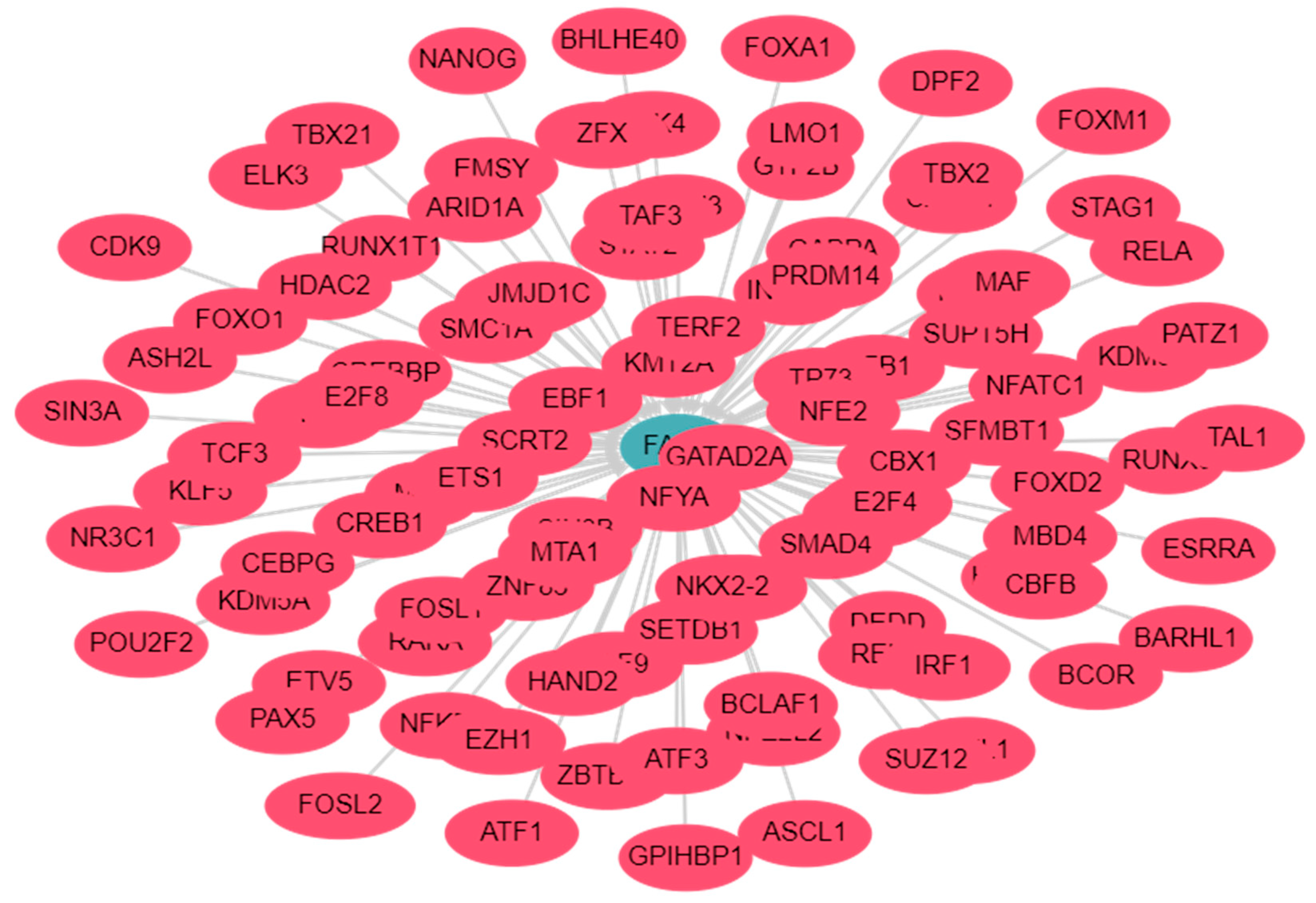
- Liska, O.; Bohar, B.; Hidas, A.; Korcsmaros, T.; Papp, B.; Fazekas, D.; Ari, E. TFLink: an integrated gateway to access transcription factor-target gene interactions for multiple species. Database (Oxford) 2022, 2022. [CrossRef]
- Hindryckx, P.; De Vos, M.; Jacques, P.; Ferdinande, L.; Peeters, H.; Olievier, K.; Bogaert, S.; Brinkman, B.; Vandenabeele, P.; Elewaut, D.; et al. Hydroxylase inhibition abrogates TNF-alpha-induced intestinal epithelial damage by hypoxia-inducible factor-1-dependent repression of FADD. Journal of immunology 2010, 185, 6306-6316. [CrossRef]
- Nguyen, D.D.; Lee, D.G.; Kim, S.; Kang, K.; Rhee, J.K.; Chang, S. Integrative Bioinformatics and Functional Analyses of GEO, ENCODE, and TCGA Reveal FADD as a Direct Target of the Tumor Suppressor BRCA1. International journal of molecular sciences 2018, 19. [CrossRef]
- Vilmont, V.; Filhol, O.; Hesse, A.M.; Coute, Y.; Hue, C.; Remy-Tourneur, L.; Mistou, S.; Cochet, C.; Chiocchia, G. Modulatory role of the anti-apoptotic protein kinase CK2 in the sub-cellular localization of Fas associated death domain protein (FADD). Biochimica et biophysica acta 2015, 1853, 2885-2896. [CrossRef]
- Zhang, J.; Zhang, D.; Hua, Z. FADD and its phosphorylation. IUBMB life 2004, 56, 395-401. [CrossRef]
- Cimino, Y.; Costes, A.; Damotte, D.; Validire, P.; Mistou, S.; Cagnard, N.; Alifano, M.; Regnard, J.F.; Chiocchia, G.; Sautes-Fridman, C.; et al. FADD protein release mirrors the development and aggressiveness of human non-small cell lung cancer. British journal of cancer 2012, 106, 1989-1996. [CrossRef]
- Ozturk, S.; Schleich, K.; Lavrik, I.N. Cellular FLICE-like inhibitory proteins (c-FLIPs): fine-tuners of life and death decisions. Experimental cell research 2012, 318, 1324-1331. [CrossRef]
- Safa, A.R. c-FLIP, a master anti-apoptotic regulator. Exp Oncol 2012, 34, 176-184.
- Tsuchiya, Y.; Nakabayashi, O.; Nakano, H. FLIP the Switch: Regulation of Apoptosis and Necroptosis by cFLIP. International journal of molecular sciences 2015, 16, 30321-30341. [CrossRef]
- Irmler, M.; Thome, M.; Hahne, M.; Schneider, P.; Hofmann, K.; Steiner, V.; Bodmer, J.L.; Schroter, M.; Burns, K.; Mattmann, C.; et al. Inhibition of death receptor signals by cellular FLIP. Nature 1997, 388, 190-195. [CrossRef]
- Longley, D.B.; Wilson, T.R.; McEwan, M.; Allen, W.L.; McDermott, U.; Galligan, L.; Johnston, P.G. c-FLIP inhibits chemotherapy-induced colorectal cancer cell death. Oncogene 2006, 25, 838-848. [CrossRef]
- Bagnoli, M.; Ambrogi, F.; Pilotti, S.; Alberti, P.; Ditto, A.; Barbareschi, M.; Galligioni, E.; Biganzoli, E.; Canevari, S.; Mezzanzanica, D. c-FLIPL expression defines two ovarian cancer patient subsets and is a prognostic factor of adverse outcome. Endocrine-related cancer 2009, 16, 443-453. [CrossRef]
- Yu, J.W.; Jeffrey, P.D.; Shi, Y. Mechanism of procaspase-8 activation by c-FLIPL. Proceedings of the National Academy of Sciences of the United States of America 2009, 106, 8169-8174. [CrossRef]
- Panaitiu, A.E.; Basiashvili, T.; Mierke, D.F.; Pellegrini, M. An engineered construct of cFLIP provides insight into DED1 structure and interactions. Structure 2022, 30, 229-239 e225. [CrossRef]
- Majkut, J.; Sgobba, M.; Holohan, C.; Crawford, N.; Logan, A.E.; Kerr, E.; Higgins, C.A.; Redmond, K.L.; Riley, J.S.; Stasik, I.; et al. Differential affinity of FLIP and procaspase 8 for FADD's DED binding surfaces regulates DISC assembly. Nature communications 2014, 5, 3350. [CrossRef]
- Chang, D.W.; Xing, Z.; Pan, Y.; Algeciras-Schimnich, A.; Barnhart, B.C.; Yaish-Ohad, S.; Peter, M.E.; Yang, X. c-FLIP(L) is a dual function regulator for caspase-8 activation and CD95-mediated apoptosis. The EMBO journal 2002, 21, 3704-3714. [CrossRef]
- Dempsey, P.W.; Doyle, S.E.; He, J.Q.; Cheng, G. The signaling adaptors and pathways activated by TNF superfamily. Cytokine & growth factor reviews 2003, 14, 193-209.
- Waters, J.P.; Pober, J.S.; Bradley, J.R. Tumour necrosis factor and cancer. The Journal of pathology 2013, 230, 241-248. [CrossRef]
- Karin, M.; Lin, A. NF-kappaB at the crossroads of life and death. Nature immunology 2002, 3, 221-227. [CrossRef]
- Marques-Fernandez, F.; Planells-Ferrer, L.; Gozzelino, R.; Galenkamp, K.M.; Reix, S.; Llecha-Cano, N.; Lopez-Soriano, J.; Yuste, V.J.; Moubarak, R.S.; Comella, J.X. TNFalpha induces survival through the FLIP-L-dependent activation of the MAPK/ERK pathway. Cell death & disease 2013, 4, e493. [CrossRef]
- Kreuz, S.; Siegmund, D.; Rumpf, J.J.; Samel, D.; Leverkus, M.; Janssen, O.; Hacker, G.; Dittrich-Breiholz, O.; Kracht, M.; Scheurich, P.; et al. NFkappaB activation by Fas is mediated through FADD, caspase-8, and RIP and is inhibited by FLIP. The Journal of cell biology 2004, 166, 369-380. [CrossRef]
- Fulda, S.; Kufer, M.U.; Meyer, E.; van Valen, F.; Dockhorn-Dworniczak, B.; Debatin, K.M. Sensitization for death receptor- or drug-induced apoptosis by re-expression of caspase-8 through demethylation or gene transfer. Oncogene 2001, 20, 5865-5877. [CrossRef]
- Micheau, O.; Lens, S.; Gaide, O.; Alevizopoulos, K.; Tschopp, J. NF-kappaB signals induce the expression of c-FLIP. Molecular and cellular biology 2001, 21, 5299-5305. [CrossRef]
- Higuchi, H.; Yoon, J.H.; Grambihler, A.; Werneburg, N.; Bronk, S.F.; Gores, G.J. Bile acids stimulate cFLIP phosphorylation enhancing TRAIL-mediated apoptosis. The Journal of biological chemistry 2003, 278, 454-461. [CrossRef]
- Fukazawa, T.; Fujiwara, T.; Uno, F.; Teraishi, F.; Kadowaki, Y.; Itoshima, T.; Takata, Y.; Kagawa, S.; Roth, J.A.; Tschopp, J.; et al. Accelerated degradation of cellular FLIP protein through the ubiquitin-proteasome pathway in p53-mediated apoptosis of human cancer cells. Oncogene 2001, 20, 5225-5231. [CrossRef]
- Roberts, J.Z.; Crawford, N.; Longley, D.B. The role of Ubiquitination in Apoptosis and Necroptosis. Cell death and differentiation 2022, 29, 272-284. [CrossRef]
- Panner, A.; Crane, C.A.; Weng, C.; Feletti, A.; Parsa, A.T.; Pieper, R.O. A novel PTEN-dependent link to ubiquitination controls FLIPS stability and TRAIL sensitivity in glioblastoma multiforme. Cancer Res 2009, 69, 7911-7916. [CrossRef]
- Wilkie-Grantham, R.P.; Matsuzawa, S.; Reed, J.C. Novel phosphorylation and ubiquitination sites regulate reactive oxygen species-dependent degradation of anti-apoptotic c-FLIP protein. The Journal of biological chemistry 2013, 288, 12777-12790. [CrossRef]
- Kerr, E.; Holohan, C.; McLaughlin, K.M.; Majkut, J.; Dolan, S.; Redmond, K.; Riley, J.; McLaughlin, K.; Stasik, I.; Crudden, M.; et al. Identification of an acetylation-dependant Ku70/FLIP complex that regulates FLIP expression and HDAC inhibitor-induced apoptosis. Cell death and differentiation 2012, 19, 1317-1327. [CrossRef]
- Tang, G.; Cho, M.; Wang, X. OncoDB: an interactive online database for analysis of gene expression and viral infection in cancer. Nucleic Acids Res 2022, 50, D1334-D1339. [CrossRef]
- Sakurai, H.; Suzuki, S.; Kawasaki, N.; Nakano, H.; Okazaki, T.; Chino, A.; Doi, T.; Saiki, I. Tumor necrosis factor-alpha-induced IKK phosphorylation of NF-kappaB p65 on serine 536 is mediated through the TRAF2, TRAF5, and TAK1 signaling pathway. The Journal of biological chemistry 2003, 278, 36916-36923. [CrossRef]
- Kelliher, M.A.; Grimm, S.; Ishida, Y.; Kuo, F.; Stanger, B.Z.; Leder, P. The death domain kinase RIP mediates the TNF-induced NF-kappaB signal. Immunity 1998, 8, 297-303.
- Zhang, H.; Zhou, X.; McQuade, T.; Li, J.; Chan, F.K.; Zhang, J. Functional complementation between FADD and RIP1 in embryos and lymphocytes. Nature 2011, 471, 373-376. [CrossRef]
- Liu, Y.; Fan, C.; Zhang, Y.; Yu, X.; Wu, X.; Zhang, X.; Zhao, Q.; Zhang, H.; Xie, Q.; Li, M.; et al. RIP1 kinase activity-dependent roles in embryonic development of Fadd-deficient mice. Cell death and differentiation 2017, 24, 1459-1469. [CrossRef]
- Grunert, M.; Gottschalk, K.; Kapahnke, J.; Gundisch, S.; Kieser, A.; Jeremias, I. The adaptor protein FADD and the initiator caspase-8 mediate activation of NF-kappaB by TRAIL. Cell death & disease 2012, 3, e414. [CrossRef]
- Wang, L.; Du, F.; Wang, X. TNF-alpha induces two distinct caspase-8 activation pathways. Cell 2008, 133, 693-703. [CrossRef]
- Jang, T.H.; Zheng, C.; Li, J.; Richards, C.; Hsiao, Y.S.; Walz, T.; Wu, H.; Park, H.H. Structural study of the RIPoptosome core reveals a helical assembly for kinase recruitment. Biochemistry 2014, 53, 5424-5431. [CrossRef]
- Tenev, T.; Bianchi, K.; Darding, M.; Broemer, M.; Langlais, C.; Wallberg, F.; Zachariou, A.; Lopez, J.; MacFarlane, M.; Cain, K.; et al. The Ripoptosome, a signaling platform that assembles in response to genotoxic stress and loss of IAPs. Molecular cell 2011, 43, 432-448. [CrossRef]
- Lin, Y.; Devin, A.; Rodriguez, Y.; Liu, Z.G. Cleavage of the death domain kinase RIP by caspase-8 prompts TNF-induced apoptosis. Genes & development 1999, 13, 2514-2526.
- Ea, C.K.; Deng, L.; Xia, Z.P.; Pineda, G.; Chen, Z.J. Activation of IKK by TNFalpha requires site-specific ubiquitination of RIP1 and polyubiquitin binding by NEMO. Molecular cell 2006, 22, 245-257. [CrossRef]
- Wertz, I.E.; Dixit, V.M. Regulation of death receptor signaling by the ubiquitin system. Cell death and differentiation 2010, 17, 14-24. [CrossRef]
- Chen, Z.J. Ubiquitin signalling in the NF-kappaB pathway. Nature cell biology 2005, 7, 758-765. [CrossRef]
- Sun, J.; Yu, X.; Wang, C.; Yu, C.; Li, Z.; Nie, W.; Xu, X.; Miao, X.; Jin, X. RIP-1/c-FLIPL Induce Hepatic Cancer Cell Apoptosis Through Regulating Tumor Necrosis Factor-Related Apoptosis-Inducing Ligand (TRAIL). Med Sci Monit 2017, 23, 1190-1199. [CrossRef]
- Duckett, C.S. Apoptosis and NF-kappa B: the FADD connection. The Journal of clinical investigation 2002, 109, 579-580. [CrossRef]
- Kang, J.W.; Yan, J.; Ranjan, K.; Zhang, X.; Turner, J.R.; Abraham, C. Myeloid Cell Expression of LACC1 Is Required for Bacterial Clearance and Control of Intestinal Inflammation. Gastroenterology 2020, 159, 1051-1067. [CrossRef]
- Ranjan, K. Intestinal Immune Homeostasis and Inflammatory Bowel Disease: A Perspective on Intracellular Response Mechanisms. Gastrointestinal Disorders 2020, 2, 246-266.
- Kalliolias, G.D.; Ivashkiv, L.B. TNF biology, pathogenic mechanisms and emerging therapeutic strategies. Nat Rev Rheumatol 2016, 12, 49-62. [CrossRef]
- Liu, D.; Zhong, Z.; Karin, M. NF-kappaB: A Double-Edged Sword Controlling Inflammation. Biomedicines 2022, 10. [CrossRef]
- Baldwin, A.S. Control of oncogenesis and cancer therapy resistance by the transcription factor NF-kappaB. The Journal of clinical investigation 2001, 107, 241-246. [CrossRef]
- Ranjan, K.; Sharma, A.; Surolia, A.; Pathak, C. Regulation of HA14-1 mediated oxidative stress, toxic response, and autophagy by curcumin to enhance apoptotic activity in human embryonic kidney cells. Biofactors 2014, 40, 157-169. [CrossRef]
- Hayden, M.S.; Ghosh, S. Regulation of NF-kappaB by TNF family cytokines. Seminars in immunology 2014, 26, 253-266. [CrossRef]
- Blonska, M.; Shambharkar, P.B.; Kobayashi, M.; Zhang, D.; Sakurai, H.; Su, B.; Lin, X. TAK1 is recruited to the tumor necrosis factor-alpha (TNF-alpha) receptor 1 complex in a receptor-interacting protein (RIP)-dependent manner and cooperates with MEKK3 leading to NF-kappaB activation. The Journal of biological chemistry 2005, 280, 43056-43063. [CrossRef]
- Broglie, P.; Matsumoto, K.; Akira, S.; Brautigan, D.L.; Ninomiya-Tsuji, J. Transforming growth factor beta-activated kinase 1 (TAK1) kinase adaptor, TAK1-binding protein 2, plays dual roles in TAK1 signaling by recruiting both an activator and an inhibitor of TAK1 kinase in tumor necrosis factor signaling pathway. The Journal of biological chemistry 2010, 285, 2333-2339. [CrossRef]
- Zhou, L.; Zhang, Y.; Meads, M.B.; Dai, Y.; Ning, Y.; Hu, X.; Li, L.; Sharma, K.; Nkwocha, J.; Parker, R.; et al. IAP and HDAC inhibitors interact synergistically in myeloma cells through noncanonical NF-kappaB- and caspase-8-dependent mechanisms. Blood Adv 2021, 5, 3776-3788. [CrossRef]
- Chaudhary, P.M.; Eby, M.T.; Jasmin, A.; Kumar, A.; Liu, L.; Hood, L. Activation of the NF-kappaB pathway by caspase 8 and its homologs. Oncogene 2000, 19, 4451-4460. [CrossRef]
- Budd, R.C. Death receptors couple to both cell proliferation and apoptosis. The Journal of clinical investigation 2002, 109, 437-441. [CrossRef]
- Vanden Berghe, T.; Linkermann, A.; Jouan-Lanhouet, S.; Walczak, H.; Vandenabeele, P. Regulated necrosis: the expanding network of non-apoptotic cell death pathways. Nature reviews. Molecular cell biology 2014, 15, 135-147. [CrossRef]
- Vandenabeele, P.; Galluzzi, L.; Vanden Berghe, T.; Kroemer, G. Molecular mechanisms of necroptosis: an ordered cellular explosion. Nature reviews. Molecular cell biology 2010, 11, 700-714. [CrossRef]
- Christofferson, D.E.; Yuan, J. Necroptosis as an alternative form of programmed cell death. Current opinion in cell biology 2010, 22, 263-268. [CrossRef]
- Degterev, A.; Huang, Z.; Boyce, M.; Li, Y.; Jagtap, P.; Mizushima, N.; Cuny, G.D.; Mitchison, T.J.; Moskowitz, M.A.; Yuan, J. Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nature chemical biology 2005, 1, 112-119. [CrossRef]
- Kalai, M.; Van Loo, G.; Vanden Berghe, T.; Meeus, A.; Burm, W.; Saelens, X.; Vandenabeele, P. Tipping the balance between necrosis and apoptosis in human and murine cells treated with interferon and dsRNA. Cell death and differentiation 2002, 9, 981-994. [CrossRef]
- Upton, J.W.; Kaiser, W.J.; Mocarski, E.S. Virus inhibition of RIP3-dependent necrosis. Cell host & microbe 2010, 7, 302-313. [CrossRef]
- Davis, C.W.; Hawkins, B.J.; Ramasamy, S.; Irrinki, K.M.; Cameron, B.A.; Islam, K.; Daswani, V.P.; Doonan, P.J.; Manevich, Y.; Madesh, M. Nitration of the mitochondrial complex I subunit NDUFB8 elicits RIP1- and RIP3-mediated necrosis. Free radical biology & medicine 2010, 48, 306-317. [CrossRef]
- Vandenabeele, P.; Declercq, W.; Van Herreweghe, F.; Vanden Berghe, T. The role of the kinases RIP1 and RIP3 in TNF-induced necrosis. Science signaling 2010, 3, re4. [CrossRef]
- Cho, Y.S.; Challa, S.; Moquin, D.; Genga, R.; Ray, T.D.; Guildford, M.; Chan, F.K. Phosphorylation-driven assembly of the RIP1-RIP3 complex regulates programmed necrosis and virus-induced inflammation. Cell 2009, 137, 1112-1123. [CrossRef]
- Ch'en, I.L.; Tsau, J.S.; Molkentin, J.D.; Komatsu, M.; Hedrick, S.M. Mechanisms of necroptosis in T cells. The Journal of experimental medicine 2011, 208, 633-641. [CrossRef]
- Lu, J.V.; Weist, B.M.; van Raam, B.J.; Marro, B.S.; Nguyen, L.V.; Srinivas, P.; Bell, B.D.; Luhrs, K.A.; Lane, T.E.; Salvesen, G.S.; et al. Complementary roles of Fas-associated death domain (FADD) and receptor interacting protein kinase-3 (RIPK3) in T-cell homeostasis and antiviral immunity. Proceedings of the National Academy of Sciences of the United States of America 2011, 108, 15312-15317. [CrossRef]
- Osborn, S.L.; Diehl, G.; Han, S.J.; Xue, L.; Kurd, N.; Hsieh, K.; Cado, D.; Robey, E.A.; Winoto, A. Fas-associated death domain (FADD) is a negative regulator of T-cell receptor-mediated necroptosis. Proceedings of the National Academy of Sciences of the United States of America 2010, 107, 13034-13039. [CrossRef]
- Lin, Y.; Choksi, S.; Shen, H.M.; Yang, Q.F.; Hur, G.M.; Kim, Y.S.; Tran, J.H.; Nedospasov, S.A.; Liu, Z.G. Tumor necrosis factor-induced nonapoptotic cell death requires receptor-interacting protein-mediated cellular reactive oxygen species accumulation. The Journal of biological chemistry 2004, 279, 10822-10828. [CrossRef]
- Irrinki, K.M.; Mallilankaraman, K.; Thapa, R.J.; Chandramoorthy, H.C.; Smith, F.J.; Jog, N.R.; Gandhirajan, R.K.; Kelsen, S.G.; Houser, S.R.; May, M.J.; et al. Requirement of FADD, NEMO, and BAX/BAK for aberrant mitochondrial function in tumor necrosis factor alpha-induced necrosis. Molecular and cellular biology 2011, 31, 3745-3758. [CrossRef]
- Alvarez-Diaz, S.; Dillon, C.P.; Lalaoui, N.; Tanzer, M.C.; Rodriguez, D.A.; Lin, A.; Lebois, M.; Hakem, R.; Josefsson, E.C.; O'Reilly, L.A.; et al. The Pseudokinase MLKL and the Kinase RIPK3 Have Distinct Roles in Autoimmune Disease Caused by Loss of Death-Receptor-Induced Apoptosis. Immunity 2016, 45, 513-526. [CrossRef]
- Dillon, C.P.; Weinlich, R.; Rodriguez, D.A.; Cripps, J.G.; Quarato, G.; Gurung, P.; Verbist, K.C.; Brewer, T.L.; Llambi, F.; Gong, Y.N.; et al. RIPK1 blocks early postnatal lethality mediated by caspase-8 and RIPK3. Cell 2014, 157, 1189-1202. [CrossRef]
- Newton, K.; Wickliffe, K.E.; Maltzman, A.; Dugger, D.L.; Strasser, A.; Pham, V.C.; Lill, J.R.; Roose-Girma, M.; Warming, S.; Solon, M.; et al. RIPK1 inhibits ZBP1-driven necroptosis during development. Nature 2016, 540, 129-133. [CrossRef]
- Young, J.A.; Sermwittayawong, D.; Kim, H.J.; Nandu, S.; An, N.; Erdjument-Bromage, H.; Tempst, P.; Coscoy, L.; Winoto, A. Fas-associated death domain (FADD) and the E3 ubiquitin-protein ligase TRIM21 interact to negatively regulate virus-induced interferon production. The Journal of biological chemistry 2011, 286, 6521-6531. [CrossRef]
- Schock, S.N.; Young, J.A.; He, T.H.; Sun, Y.; Winoto, A. Deletion of FADD in macrophages and granulocytes results in RIP3- and MyD88-dependent systemic inflammation. PloS one 2015, 10, e0124391. [CrossRef]
- Ma, Y.; Liu, H.; Tu-Rapp, H.; Thiesen, H.J.; Ibrahim, S.M.; Cole, S.M.; Pope, R.M. Fas ligation on macrophages enhances IL-1R1-Toll-like receptor 4 signaling and promotes chronic inflammation. Nature immunology 2004, 5, 380-387. [CrossRef]
- Henry, C.M.; Martin, S.J. Caspase-8 Acts in a Non-enzymatic Role as a Scaffold for Assembly of a Pro-inflammatory "FADDosome" Complex upon TRAIL Stimulation. Molecular cell 2017, 65, 715-729 e715. [CrossRef]
- Hartwig, T.; Montinaro, A.; von Karstedt, S.; Sevko, A.; Surinova, S.; Chakravarthy, A.; Taraborrelli, L.; Draber, P.; Lafont, E.; Arce Vargas, F.; et al. The TRAIL-Induced Cancer Secretome Promotes a Tumor-Supportive Immune Microenvironment via CCR2. Molecular cell 2017, 65, 730-742 e735. [CrossRef]
- Kawai, T.; Akira, S. TLR signaling. Seminars in immunology 2007, 19, 24-32. [CrossRef]
- Waghela, B.N.; Vaidya, F.U.; Ranjan, K.; Chhipa, A.S.; Tiwari, B.S.; Pathak, C. AGE-RAGE synergy influences programmed cell death signaling to promote cancer. Mol Cell Biochem 2021, 476, 585-598. [CrossRef]
- Bannerman, D.D.; Tupper, J.C.; Kelly, J.D.; Winn, R.K.; Harlan, J.M. The Fas-associated death domain protein suppresses activation of NF-kappa B by LPS and IL-1 beta. The Journal of clinical investigation 2002, 109, 419-425. [CrossRef]
- Aliprantis, A.O.; Yang, R.B.; Weiss, D.S.; Godowski, P.; Zychlinsky, A. The apoptotic signaling pathway activated by Toll-like receptor-2. The EMBO journal 2000, 19, 3325-3336. [CrossRef]
- Zhande, R.; Dauphinee, S.M.; Thomas, J.A.; Yamamoto, M.; Akira, S.; Karsan, A. FADD negatively regulates lipopolysaccharide signaling by impairing interleukin-1 receptor-associated kinase 1-MyD88 interaction. Molecular and cellular biology 2007, 27, 7394-7404. [CrossRef]
- Bossaller, L.; Chiang, P.I.; Schmidt-Lauber, C.; Ganesan, S.; Kaiser, W.J.; Rathinam, V.A.; Mocarski, E.S.; Subramanian, D.; Green, D.R.; Silverman, N.; et al. Cutting edge: FAS (CD95) mediates noncanonical IL-1beta and IL-18 maturation via caspase-8 in an RIP3-independent manner. Journal of immunology 2012, 189, 5508-5512. [CrossRef]
- Kelley, N.; Jeltema, D.; Duan, Y.; He, Y. The NLRP3 Inflammasome: An Overview of Mechanisms of Activation and Regulation. International journal of molecular sciences 2019, 20. [CrossRef]
- Latz, E.; Xiao, T.S.; Stutz, A. Activation and regulation of the inflammasomes. Nature reviews. Immunology 2013, 13, 397-411. [CrossRef]
- Moriwaki, K.; Bertin, J.; Gough, P.J.; Chan, F.K. A RIPK3-caspase 8 complex mediates atypical pro-IL-1beta processing. Journal of immunology 2015, 194, 1938-1944. [CrossRef]
- Gurung, P.; Anand, P.K.; Malireddi, R.K.; Vande Walle, L.; Van Opdenbosch, N.; Dillon, C.P.; Weinlich, R.; Green, D.R.; Lamkanfi, M.; Kanneganti, T.D. FADD and caspase-8 mediate priming and activation of the canonical and noncanonical Nlrp3 inflammasomes. Journal of immunology 2014, 192, 1835-1846. [CrossRef]
- Xue, Y.; Enosi Tuipulotu, D.; Tan, W.H.; Kay, C.; Man, S.M. Emerging Activators and Regulators of Inflammasomes and Pyroptosis. Trends Immunol 2019, 40, 1035-1052. [CrossRef]
- Sefik, E.; Qu, R.; Junqueira, C.; Kaffe, E.; Mirza, H.; Zhao, J.; Brewer, J.R.; Han, A.; Steach, H.R.; Israelow, B.; et al. Inflammasome activation in infected macrophages drives COVID-19 pathology. Nature 2022, 606, 585-593. [CrossRef]
- Karki, R.; Sharma, B.R.; Tuladhar, S.; Williams, E.P.; Zalduondo, L.; Samir, P.; Zheng, M.; Sundaram, B.; Banoth, B.; Malireddi, R.K.S.; et al. Synergism of TNF-alpha and IFN-gamma Triggers Inflammatory Cell Death, Tissue Damage, and Mortality in SARS-CoV-2 Infection and Cytokine Shock Syndromes. Cell 2021, 184, 149-168 e117. [CrossRef]
- Raman, R.; Patel, K.J.; Ranjan, K. COVID-19: Unmasking Emerging SARS-CoV-2 Variants, Vaccines and Therapeutic Strategies. Biomolecules 2021, 11. [CrossRef]
- Raman, R.; Patel, K.J.; Ranjan, K. SARS-CoV-2 Variants: Impact of Spike Mutations on Vaccine and Therapeutic Strategies. In Frontiers of COVID-19: Scientific and Clinical Aspects of the Novel Coronavirus 2019, Adibi, S., Griffin, P., Sanicas, M., Rashidi, M., Lanfranchi, F., Eds.; Springer International Publishing: Cham, 2022; pp. 143-160.
- Singh, S.; Kishore, D.; Singh, R.K.; Pathak, C.; Ranjan, K. Higher BCG-induced trained immunity prevalence predicts protection from COVID-19: Implications for ongoing BCG trials. Clin Transl Discov 2022, 2, e60. [CrossRef]
- Kaser, A.; Zeissig, S.; Blumberg, R.S. Inflammatory bowel disease. Annu Rev Immunol 2010, 28, 573-621. [CrossRef]
- Ranjan, K.; Hedl, M.; Abraham, C. The E3 ubiquitin ligase RNF186 and RNF186 risk variants regulate innate receptor-induced outcomes. Proceedings of the National Academy of Sciences of the United States of America 2021, 118. [CrossRef]
- Huang, C.; Hedl, M.; Ranjan, K.; Abraham, C. LACC1 Required for NOD2-Induced, ER Stress-Mediated Innate Immune Outcomes in Human Macrophages and LACC1 Risk Variants Modulate These Outcomes. Cell Rep 2019, 29, 4525-4539 e4524. [CrossRef]
- Ranjan, K.; Hedl, M.; Sinha, S.; Zhang, X.; Abraham, C. Ubiquitination of ATF6 by disease-associated RNF186 promotes the innate receptor-induced unfolded protein response. The Journal of clinical investigation 2021, 131. [CrossRef]
- Gunther, C.; Martini, E.; Wittkopf, N.; Amann, K.; Weigmann, B.; Neumann, H.; Waldner, M.J.; Hedrick, S.M.; Tenzer, S.; Neurath, M.F.; et al. Caspase-8 regulates TNF-alpha-induced epithelial necroptosis and terminal ileitis. Nature 2011, 477, 335-339. [CrossRef]
- Stolzer, I.; Kaden-Volynets, V.; Ruder, B.; Letizia, M.; Bittel, M.; Rausch, P.; Basic, M.; Bleich, A.; Baines, J.F.; Neurath, M.F.; et al. Environmental Microbial Factors Determine the Pattern of Inflammatory Lesions in a Murine Model of Crohn's Disease-Like Inflammation. Inflamm Bowel Dis 2020, 26, 66-79. [CrossRef]
- Levine, B.; Yuan, J. Autophagy in cell death: an innocent convict? The Journal of clinical investigation 2005, 115, 2679-2688. [CrossRef]
- Klionsky, D.J. The molecular machinery of autophagy: unanswered questions. Journal of cell science 2005, 118, 7-18. [CrossRef]
- Mukhopadhyay, S.; Panda, P.K.; Sinha, N.; Das, D.N.; Bhutia, S.K. Autophagy and apoptosis: where do they meet? Apoptosis : an international journal on programmed cell death 2014, 19, 555-566. [CrossRef]
- Pua, H.H.; Dzhagalov, I.; Chuck, M.; Mizushima, N.; He, Y.W. A critical role for the autophagy gene Atg5 in T cell survival and proliferation. The Journal of experimental medicine 2007, 204, 25-31. [CrossRef]
- Li, C.; Capan, E.; Zhao, Y.; Zhao, J.; Stolz, D.; Watkins, S.C.; Jin, S.; Lu, B. Autophagy is induced in CD4+ T cells and important for the growth factor-withdrawal cell death. Journal of immunology 2006, 177, 5163-5168.
- Walsh, C.M.; Edinger, A.L. The complex interplay between autophagy, apoptosis, and necrotic signals promotes T-cell homeostasis. Immunological reviews 2010, 236, 95-109. [CrossRef]
- Tait, S.W.; Ichim, G.; Green, D.R. Die another way--non-apoptotic mechanisms of cell death. Journal of cell science 2014, 127, 2135-2144. [CrossRef]
- Newton, K.; Harris, A.W.; Bath, M.L.; Smith, K.G.; Strasser, A. A dominant interfering mutant of FADD/MORT1 enhances deletion of autoreactive thymocytes and inhibits proliferation of mature T lymphocytes. The EMBO journal 1998, 17, 706-718. [CrossRef]
- Newton, K.; Harris, A.W.; Strasser, A. FADD/MORT1 regulates the pre-TCR checkpoint and can function as a tumour suppressor. The EMBO journal 2000, 19, 931-941. [CrossRef]
- Ranjan, K.; Pathak, C. Expression of cFLIPL Determines the Basal Interaction of Bcl-2 With Beclin-1 and Regulates p53 Dependent Ubiquitination of Beclin-1 During Autophagic Stress. J Cell Biochem 2016, 117, 1757-1768. [CrossRef]
- Ranjan, K.; Surolia, A.; Pathak, C. Apoptotic potential of Fas-associated death domain on regulation of cell death regulatory protein cFLIP and death receptor mediated apoptosis in HEK 293T cells. J Cell Commun Signal 2012, 6, 155-168. [CrossRef]
- Komata, T.; Koga, S.; Hirohata, S.; Takakura, M.; Germano, I.M.; Inoue, M.; Kyo, S.; Kondo, S.; Kondo, Y. A novel treatment of human malignant gliomas in vitro and in vivo: FADD gene transfer under the control of the human telomerase reverse transcriptase gene promoter. International journal of oncology 2001, 19, 1015-1020.
- Pope, R.M. Apoptosis as a therapeutic tool in rheumatoid arthritis. Nature reviews. Immunology 2002, 2, 527-535. [CrossRef]
- Kondo, S.; Ishizaka, Y.; Okada, T.; Kondo, Y.; Hitomi, M.; Tanaka, Y.; Haqqi, T.; Barnett, G.H.; Barna, B.P. FADD gene therapy for malignant gliomas in vitro and in vivo. Human gene therapy 1998, 9, 1599-1608. [CrossRef]
- Shinoura, N.; Yoshida, Y.; Sadata, A.; Hanada, K.I.; Yamamoto, S.; Kirino, T.; Asai, A.; Hamada, H. Apoptosis by retrovirus- and adenovirus-mediated gene transfer of Fas ligand to glioma cells: implications for gene therapy. Human gene therapy 1998, 9, 1983-1993. [CrossRef]
- Kobayashi, T.; Okamoto, K.; Kobata, T.; Hasunuma, T.; Kato, T.; Hamada, H.; Nishioka, K. Novel gene therapy for rheumatoid arthritis by FADD gene transfer: induction of apoptosis of rheumatoid synoviocytes but not chondrocytes. Gene therapy 2000, 7, 527-533. [CrossRef]
- Ho, I.A.; Ng, W.H.; Lam, P.Y. FasL and FADD delivery by a glioma-specific and cell cycle-dependent HSV-1 amplicon virus enhanced apoptosis in primary human brain tumors. Mol Cancer 2010, 9, 270. [CrossRef]
- Zhang, Y.; Roise, J.J.; Lee, K.; Li, J.; Murthy, N. Recent developments in intracellular protein delivery. Curr Opin Biotechnol 2018, 52, 25-31. [CrossRef]
- Kumar, M.; Ranjan, K.; Singh, V.; Pathak, C.; Pappachan, A.; Singh, D.D. Hydrophilic Acylated Surface Protein A (HASPA) of Leishmania donovani: Expression, Purification and Biophysico-Chemical Characterization. Protein J 2017, 36, 343-351. [CrossRef]
- Pathak, C.; Vaidya, F.U.; Waghela, B.N.; Jaiswara, P.K.; Gupta, V.K.; Kumar, A.; Rajendran, B.K.; Ranjan, K. Insights of Endocytosis Signaling in Health and Disease. International journal of molecular sciences 2023, 24. [CrossRef]
- Ranjan, K.; Waghela, B.N.; Vaidya, F.U.; Pathak, C. Cell-Penetrable Peptide-Conjugated FADD Induces Apoptosis and Regulates Inflammatory Signaling in Cancer Cells. International journal of molecular sciences 2020, 21. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




