1. Introduction
The color industry creates dyes and pigments that in turn are the raw materials for other manufacturing fields, such as textile, food, cosmetic and paint companies. Demand for dyes and pigments by these huge industries and also by artisans and artists stimulate constantly development of new colorful materials [
1,
2,
3]. Materials acting as pigments are solid materials providing color to the object on which they are incorporated. It is suitable that pigments are compatible with substrate on they are used but insoluble with the medium. Depending on the final application of pigment, the requirements are more or less severe. For example, if they are proposed to provide color to food or cosmetics, the toxicity, thermal stability, dispersion in a specific medium [
4,
5,
6] will be crucial characteristics to keep in mind. Actually, the most used pigments in cosmetic industry are organic and they are used in the form of lakes and toners [
7]. However, sometimes the pure organic or inorganic pigments are insufficient to reach a final purpose. Fortunately, the hybrid materials can be designed from various components in order to get original properties in a single material [
8,
9,
10]. In this context, it is documented that hybrid pigments can be prepared from several sources of color, such as anthocyanins, betalains, azoic dyes, among others [
11,
12]. These dyes are stabilized onto surface of an inorganic matrix and then pigments with unique properties emerge [
13,
14]. Earlier it was pointed out that layered double hydroxide act as host of azoic dyes to produce stable hybrid pigments [
15]. After that, several works confirmed the versatility of LDHs to produce original pigments [
16,
17,
18,
19]. Some additional works proposed other inorganic matrices and also micelle media in order to stabilize organic, both natural and synthetic, chromophores [
20]. Emphasizing in the LDH as a host to stabilize azoic dyes, the matrix is particularly interesting as its physical-chemical properties are easily modulated. Thus, we started this work with the goal to find evidence that modification of LDH, and even if this modification is minor, changes significantly its properties to host chromophores molecules. We show at end the use of pigment hybrid based on LDH as the source of color of stable creams that can be potentially used in the cosmetic or food industry.
2. Materials and Methods
2.1. Materials
Carbonate-containing Mg–Al LDHs with a Mg/Al atomic ratio close to 3 have been synthesized by the sol-gel technique from magnesium ethoxide and aluminum tri-sec-butoxide. Aluminum tri-sec-butoxide (ATB, Aldrich 99.9 %) dissolved in ethanol was refluxed and stirred for 1 h. Subsequently the temperature was lowered to 0 °C and HNO3 was added. The mixture was stirred for 1 h. After that, magnesium methoxide (Aldrich, 99%) dissolved in butanol, and water were dropped into the solution until a gel was formed, which was dried at 70 °C. Molar amounts of reagents and solvents were as follows: EtOH=1.54x10-1, ATB=2.7x10-3, HNO3=7.725x10-5, MetMg=5.76x10-2.
Fluorinated LDH was obtained when during the synthesis [
21] a part of ATB was replaced by sodium hexa-fluoroaluminate, Na
3AlF
6 (1.018x10
-3 molar). The nominal ratio of Mg/Al was always equal to 3.
Both LDH and fluorinated LDH were used as hosts of two chromophores (carminic acid or hydroxy naphthol blue) in order to produce hybrid pigments. The chromophore was incorporated to LDH either during the LDH synthesis or using the memory effect of LDH. In the first case, during the synthesis, just before formation of gel a solution 2.11x10-3 M of carminic aid or hydroxyl naphthol blue was dropped. A modification was also experimented, after 5 hours added the chromophore magnesium methoxide was dropped in order to have a pigment covered with Mg(OH)2.
For production of pigments through memory effect LDH was thermal treated at 400 °C and then the layered structured recovered by contact thermal treated samples with a chromophore solution.
All the colored samples were rinsed with distilled water. The amount of dye in the washes was quantified by UV vis spectroscopy in order to estimate the amount of dye contained in the pigment.
Table 1 summarizes the pigments under study. All the selected pigments in this work contained close to 4% wt of dye (CA or HNB). Other pigments with higher or lower amount of dye were discarded in this manuscript.
2.2. Characterization
X-ray powder diffraction (XRD) patterns were obtained using a Bruker D8 advance diffractometer with a copper anode tube. The CuKα radiation (λ = 1.54186 Å) was selected with a beam monochromator.
27Al solid state nuclear magnetic resonance (MAS NMR) spectra were obtained operating a Bruker Avance II spectrometer at a Larmor frequency of 78.21 MHz. Spectra were acquired with short single pulses (π/12) and delay times of 0.5 s. The samples were spun at 10 kHz and the chemical shifts were referenced to a 1M AlCl3 solution.
The 19F MAS NMR spectra were measured by operating the spectrometer at 376.3 MHz, using π/2 pulses of 6 μs with a recycle delay of 1 s; 19F chemical shifts were referenced to those of CFCl3 at 0 ppm.
Table 1.
Summary of pigments, including their components, prepared in this work.
Table 1.
Summary of pigments, including their components, prepared in this work.
| Code sample |
Chromophore |
Add of chromophore |
Add of Mg(OH)2
|
| LDH-CA |
Carminic acid |
During sol-gel synthesis |
Non |
| LDH-HNB |
Hydroxynaphthol blue |
During sol-gel synthesis |
Non |
| FLDH-CA |
Carminic acid |
During sol-gel synthesis |
Non |
| FLDH-HNB |
Hydroxynaphthol blue |
During sol-gel synthesis |
Non |
| FLDH-CA+MgO |
Carminic acid |
During sol-gel synthesis |
Yes |
| FLDH-HNB+MgO |
Hydroxynaphthol blue |
During sol-gel synthesis |
Yes |
| ME-FLDH-CA |
Carminic acid |
Memory effect |
Non |
| ME-FLDH-HNB |
Hydroxynaphthol blue |
Memory effect |
Non |
| ME-FLDH-CA+MgO |
Carminic acid |
Memory effect |
Yes |
| ME-FLDH-HNB+MgO |
Hydroxynaphthol blue |
Memory effect |
Yes |
13C CP MAS NMR spectra were collected at a frequency of 100.58 MHz using a Bruker Avance II HD 400 spectrometer. The spectra were acquired using a 4 mm cross-polarization (CP) MAS probe spinning at a rate of 5 kHz. Typical 13C CP MAS NMR conditions for 1H–13C polarization experiment used a π/2 pulse of 4 μs, contact time of 1 ms and delay time of 5 s. Chemical shifts were referenced to a chemical shift at 38.2 ppm of CH signal of adamantane, relative to TMS
2.3. Formulation of the pigmented cream
An emollient cream was prepared by the emulsification method [
22,
23] starting from stearic acid (14 g), almond oil (1 ml), NaOH (1g), triethanolamine (1 ml), ethylenediamine tetra acetic acid (0.2 g), glycerin (6 ml) and methyl paraben (0.1 g). Briefly: The oil soluble components and the emulsifier were melted in a water bath at 75°C. Separately, water-soluble components were melted at 75°C. After heating, the oil phase was taken in a mortar and pestle and slowly the water phase was added. When the temperature cools down the pigment was dispersed in the cream.
3. Results
3.1. Pigments
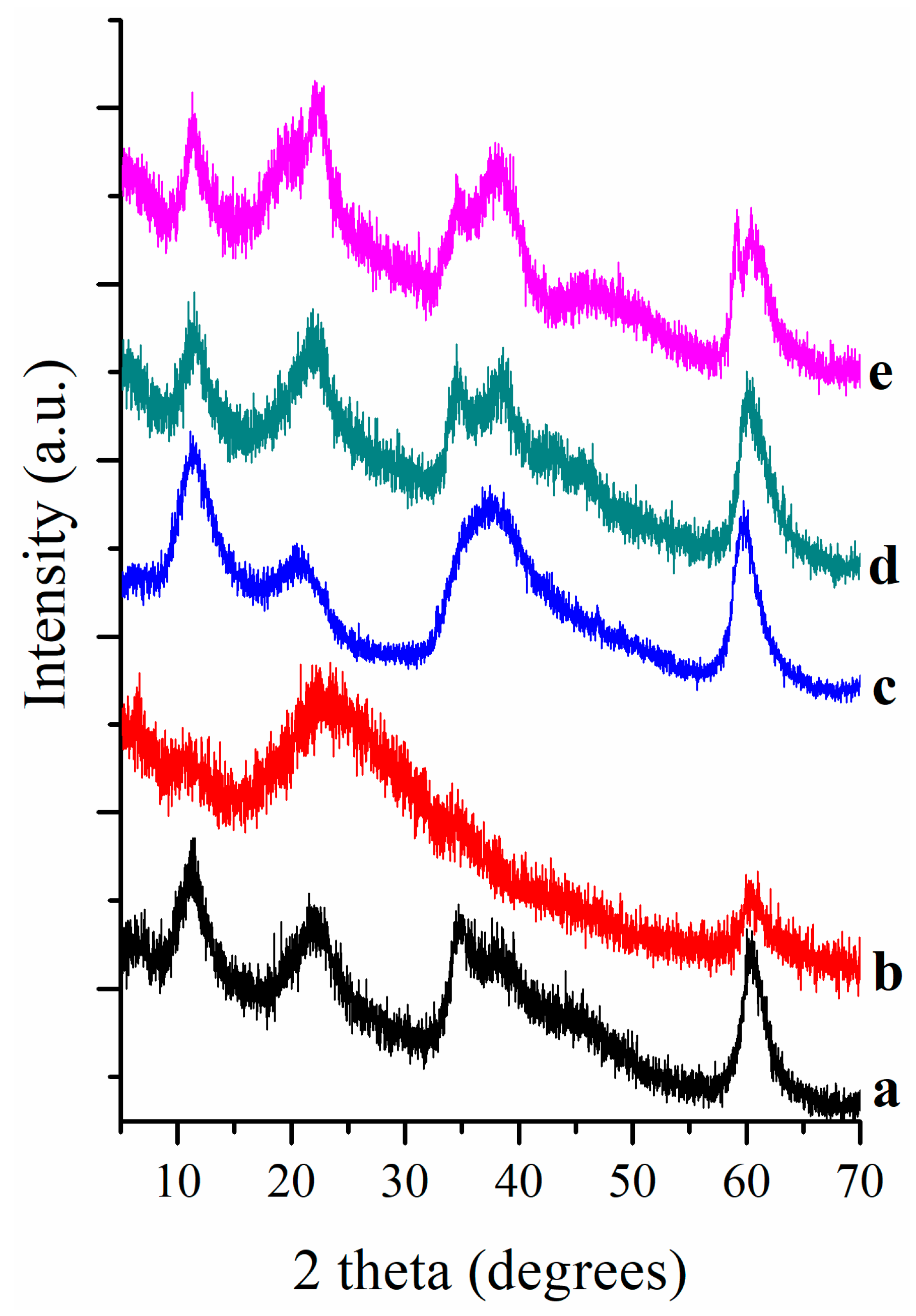
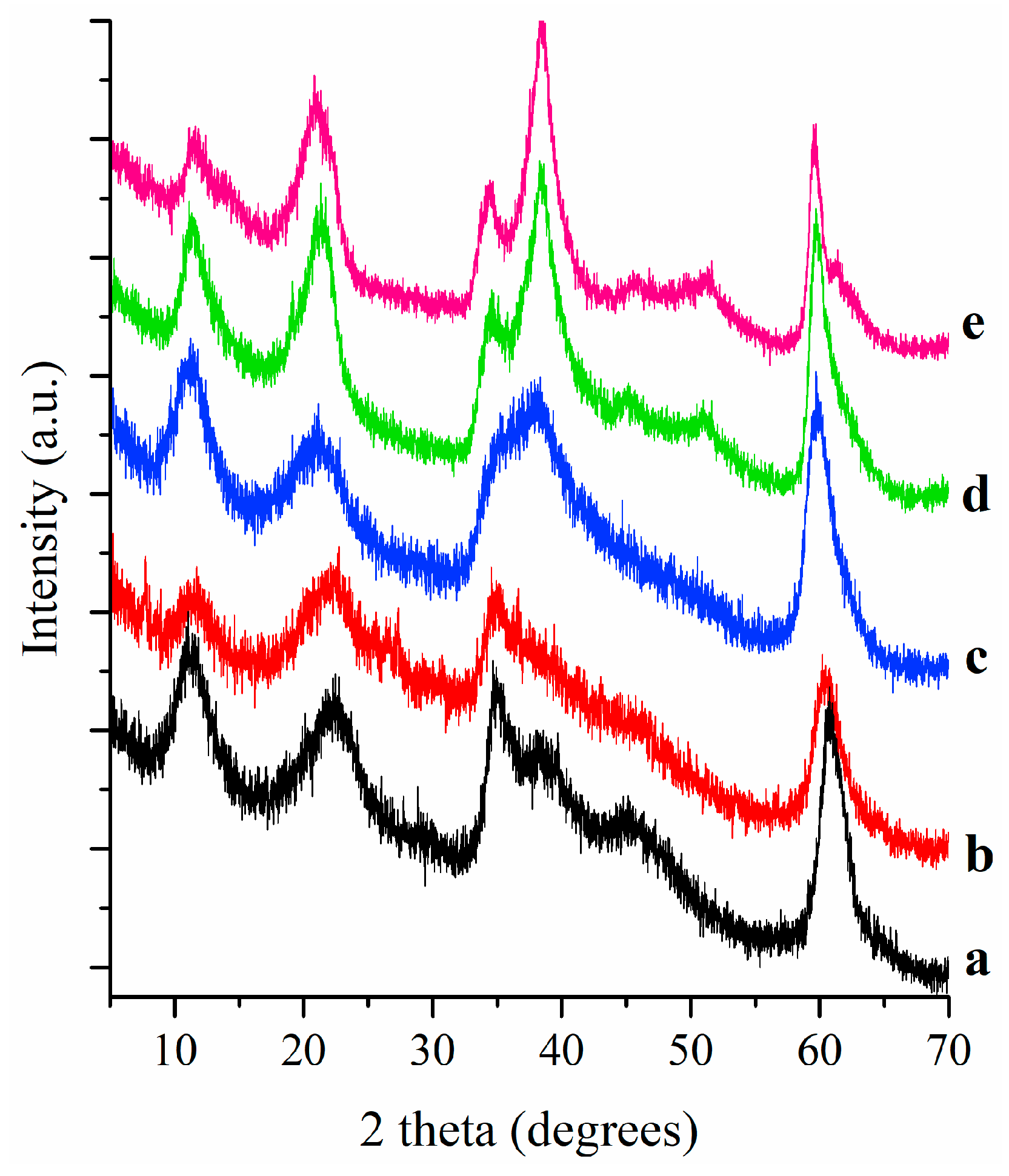
3.1.1. Effect of fluorination in colored LDH
Figure 1 displays XRD patterns of pigments where clearly is observed that LDH differ structurally depending their chemical composition. XRD pattern of LDH-CA sample is a one typical of an LDH synthesized by a sol-gel method; the peaks are broad and correspond to samples with a small size, which is expected for LDH samples synthesized by a sol-gel procedure. On the contrary, the analogue fluorinated pigment, sample FLDH-CA, shows an XRD pattern with very low intense peaks matching to an LDH phase, suggesting that fluorine and carminic acid disordered the layered structure. A most relevant result is that found for sample MEFLDH-CA that has an XRD pattern matching with an LDH more ordered, however the peak at 38 degrees suggests that a periclase mixed oxide phase is present; in this context, it should remember that this pigment was prepared by treatment of fluorinated LDH and rehydration in carminic acid. Thus, it seems that the presence of carminic acid in rehydration inhibits the rehydration. Lastly, the XRD patterns of samples synthesized with Mg(OH)
2 adding exhibited the distinctive peaks of MgO phase at 38.1 and 62 degrees.
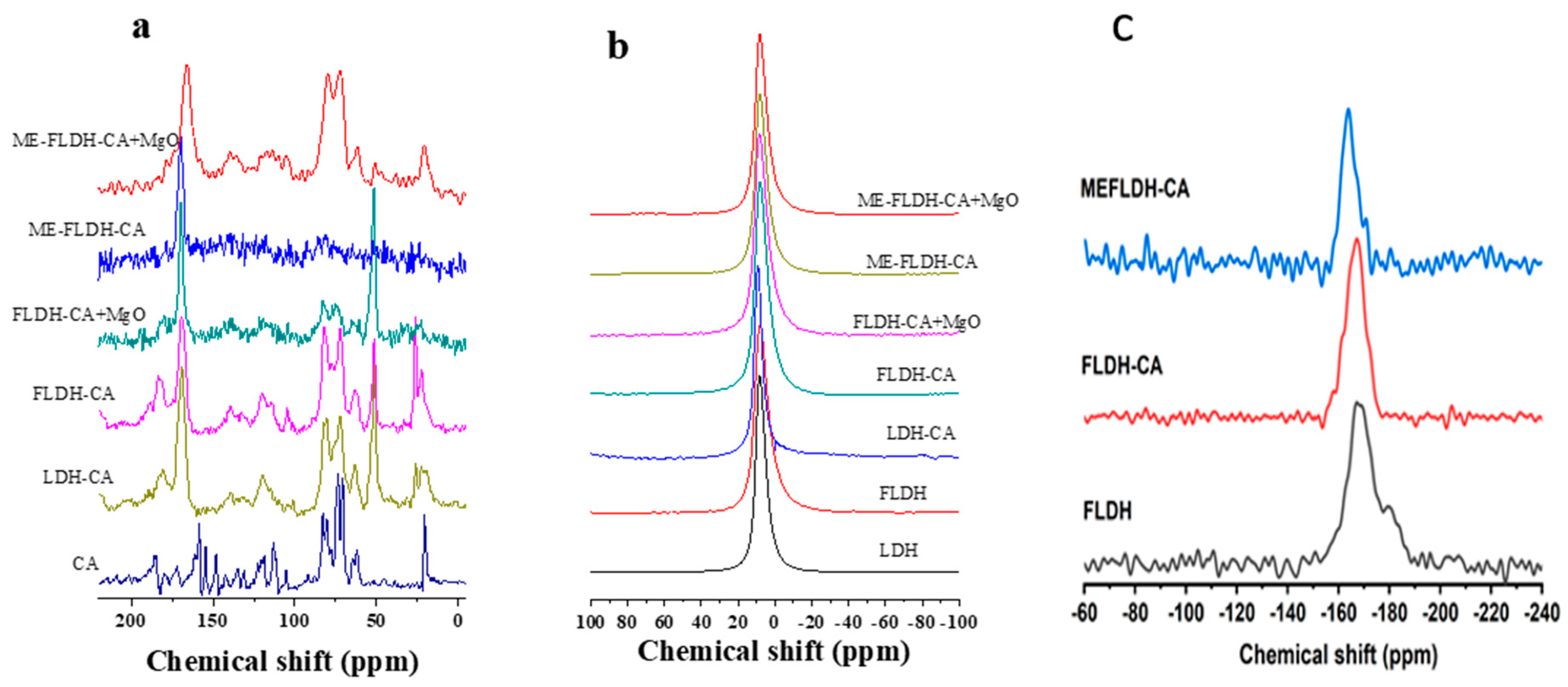
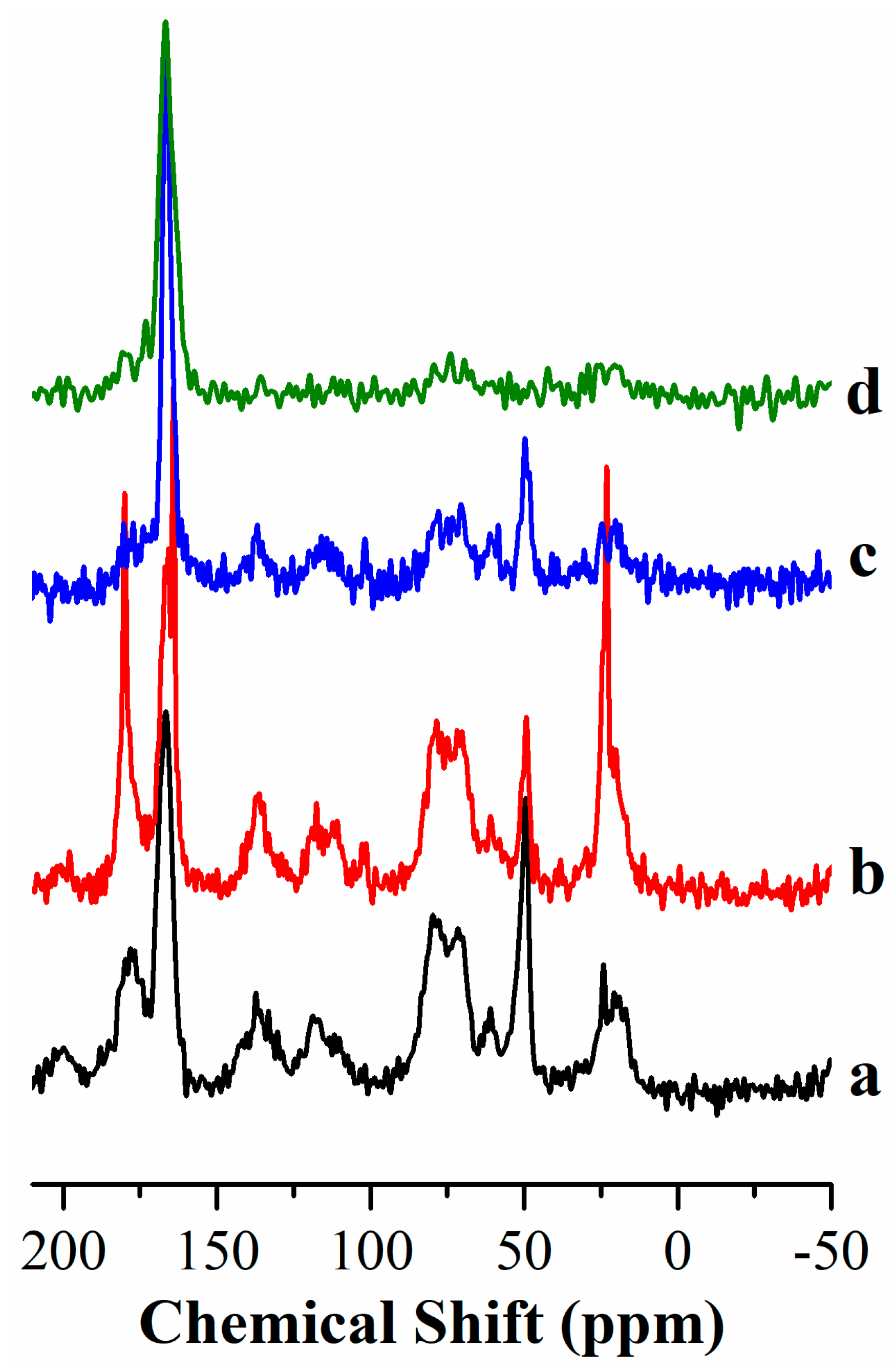
Figure 2a shows that
13C CP MAS NMR spectrum of CA as a part of hybrid pigments change significantly if compared with the spectrum of CA pure. The peaks due to resonances of aromatic carbons (168-188 ppm) in pigments are significantly broader and less intense than in CA pure. In the range 62-87 ppm the signals due to glucose unit [
24] also are a little broader in pigments. These results suggest that CA molecule is partially immobilized in the pigment, in other words an interaction between aromatic part of CA and layers of LDH should take place. The
27Al MAS NMR (
Figure 2b) spectra of all colored and white samples are composed of a single peak centered around 9 ppm, which is due to aluminum six-fold coordinated [
25] that is in line with a pure hydrotalcite structure. No significant differences are observed in spectra as a function of the composition of pigments.
19F NMR spectra included in
Figure 2c confirm that fluorine does not change its chemical environment when the white sample (FLDH) becomes the colored one (FLDH-CA), in both samples only one single peak is observed at -168.1 ppm, assigned to AlF6-xOx, with x taking values from 1 to 6 [
26]. In contrast, when the colored sample is coated by magnesium hydroxide, the resonance peak shifts to high fields, to -163.8 ppm, supporting that fluorine is redistributed leading to fluorinated and oxygenated magnesium species, which is an expected result as the number of oxygen atoms is higher in FLDH-CA than in FLDH-CA+MgO.
3.1.2. Loading chromophore: during sol-gel synthesis versus memory effect
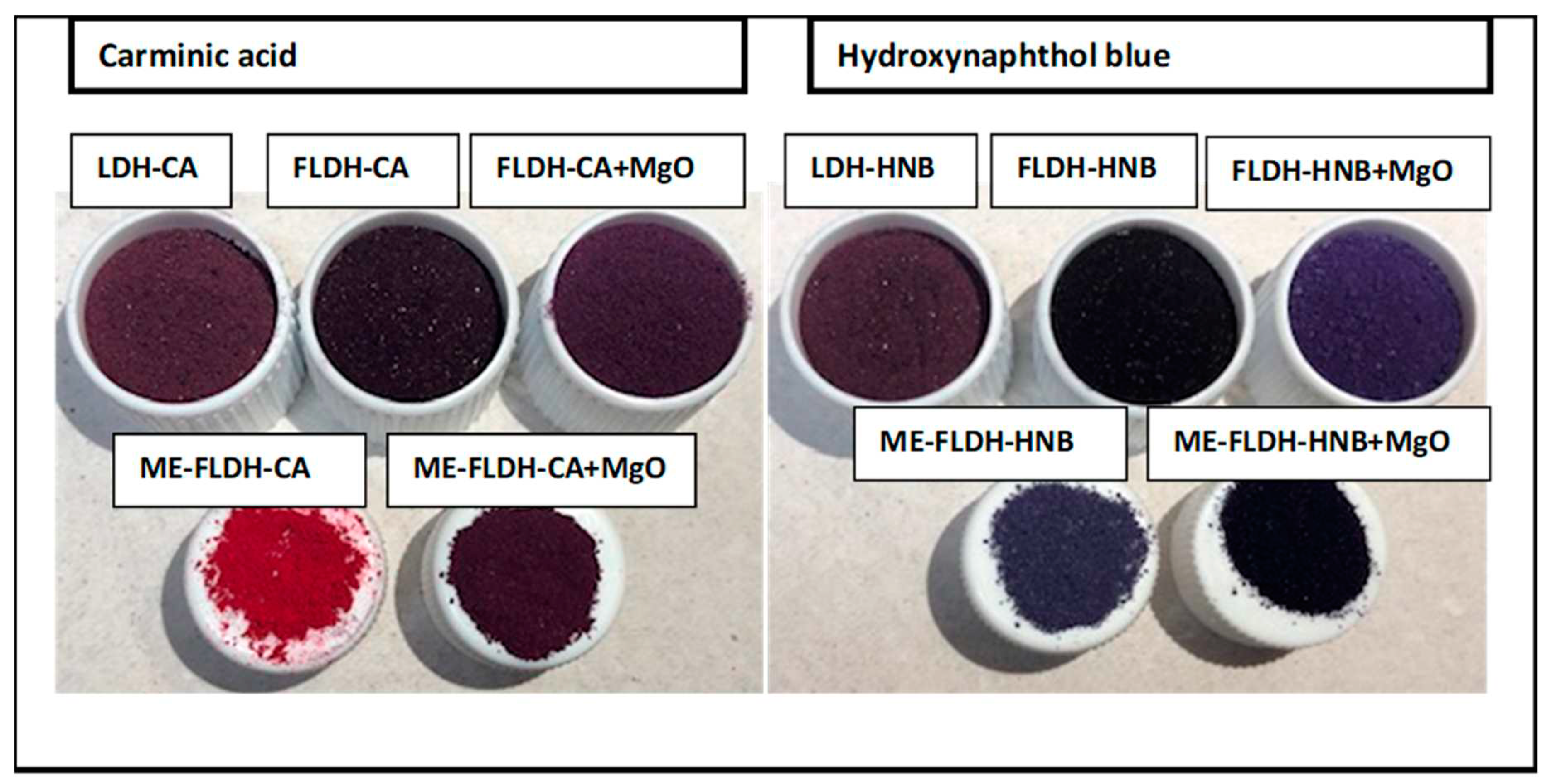
As described in experimental section, the pigments were obtained by incorporating CA or HNB into LDH. Two ways for this loading were tested: on the one hand, the chromophore was loaded during sol-gel synthesis and on the other hand the LDH was thermal treated and rehydrated, the chromophore was added during rehydration step.
Figure 3 displays the picture of fresh pigments obtained. Note that method used to load chromophore influences the final color of pigment. The pigments FLDH-CA and ME-FLDH-CA differ significantly in color appreciable to human eye. Loading chromophore during sol-gel synthesis leads to a purple pigment and loading chromophore in rehydration step during memory effect of LDH drives to a red pigment. After the coating with magnesium hydroxide both pigments have similar purple color. When the chromophore was HNB, also the color differed between the FLDH-HNB and ME-FLDH-HNB pigments and became similar after recovery with the magnesium phase.
Figure 2.
Multinuclear solid-state NMR spectra of pigments. (a) 1H→13C CP/MAS NMR, (b) 27Al MAS NMR and (c) 19F MAS NMR.
Figure 2.
Multinuclear solid-state NMR spectra of pigments. (a) 1H→13C CP/MAS NMR, (b) 27Al MAS NMR and (c) 19F MAS NMR.
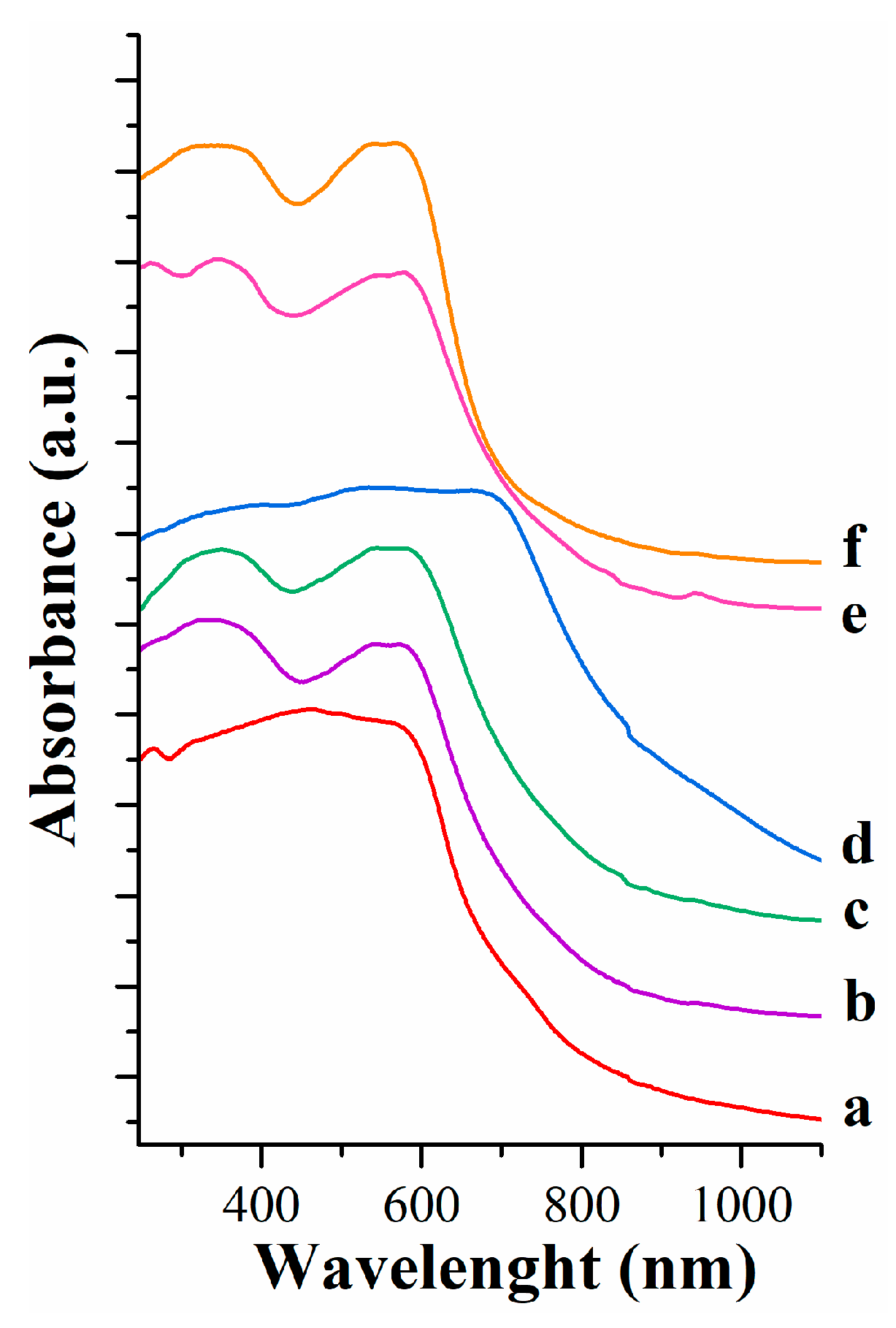
UV-vis spectra of pigments containing carminic acid are included in
Figure 4. A broad band centered at 450 nm composes the spectrum of reference, that of pure carminic acid. In pigments LDH-CA and FLDH-CA two bands are observed at 340 and 555 nm. These two bands are often observed when carminic acid is treated with mordents based in aluminum. The presence of fluorine in LDH shifted slightly to red the UV-vis band. It was earlier [
21,
27] pointed out that a consequence of the fluorination of a magnesium-aluminum hydrotalcite is the formation of MgO
6-xF
x species which in turn induces the creation of strong basic sites but also aluminum with low coordination numbers. These sites should act as the adsorption sites for CA. However, this interaction is not enough strong as the absorption wavelength is easily modified by the presence of Mg(OH)
2. The presence of Mg(OH)
2 in these two pigments leads to a shifting of band to red. More interesting, the spectra of pigments prepared by memory effect shows also two broad absorption bands centered at 355 and 562 nm. They are shifted to red if compared with bands observed in pigments prepared with chromophore loaded during sol-gel synthesis.
3.1.3. Aging of pigments
Fresh pigments were stored at room temperature for ten years and then characterized again in order to verify any change regarding structure and color. The XRD patterns of the aged pigments were close to that of the fresh pigments as the
Figure 5 shows. Only one pigment evolved within XRD limit detection: the XRD of aged pigment seems to be closer to hydrotalcite structure in contrast of fresh one that was close to the MgO periclase structure. The
13C CP MAS NMR spectra of the aged pigments are displayed in
Figure 6. When comparing the spectra of LDH-CA and FLDH-CA+MgO (
Figure 2a) with their respective aged samples, no important changes are observed, only the signal that appears near 170 ppm shifts 3 ppm to higher field with aging, indicating that group C=O fades as time goes on, in other words the interaction of CA with LDH has changed slightly.
The spectrum of the FLDH-CA sample also changed once it was aged, in the interval of 62-87 ppm, where the glucose unit signals appear, a decrease in intensity is observed and the peaks are no longer well defined once the sample ages. This is probably because the glucose unit interacts with LDA, via the –OH groups, resulting in some rigidity to the glucose unit.
In general, the sample that changed the least as a result of aging was the sample with Mg(OH)2 coating, while the FLDH-AC sample presented the most changes. Therefore, the coated hybrid pigment is the most stable under moderate changes in temperature, humidity and radiation.
The more significant differences between aged and fresh pigments were observed in color. Color was measured according to the CIEL*a*b* parameters [
30]. In order to calculate a fade of color, was calculated as follows.
Where L is the brightness parameter and a and b the color coordinates.
3.2. Pigmented cream
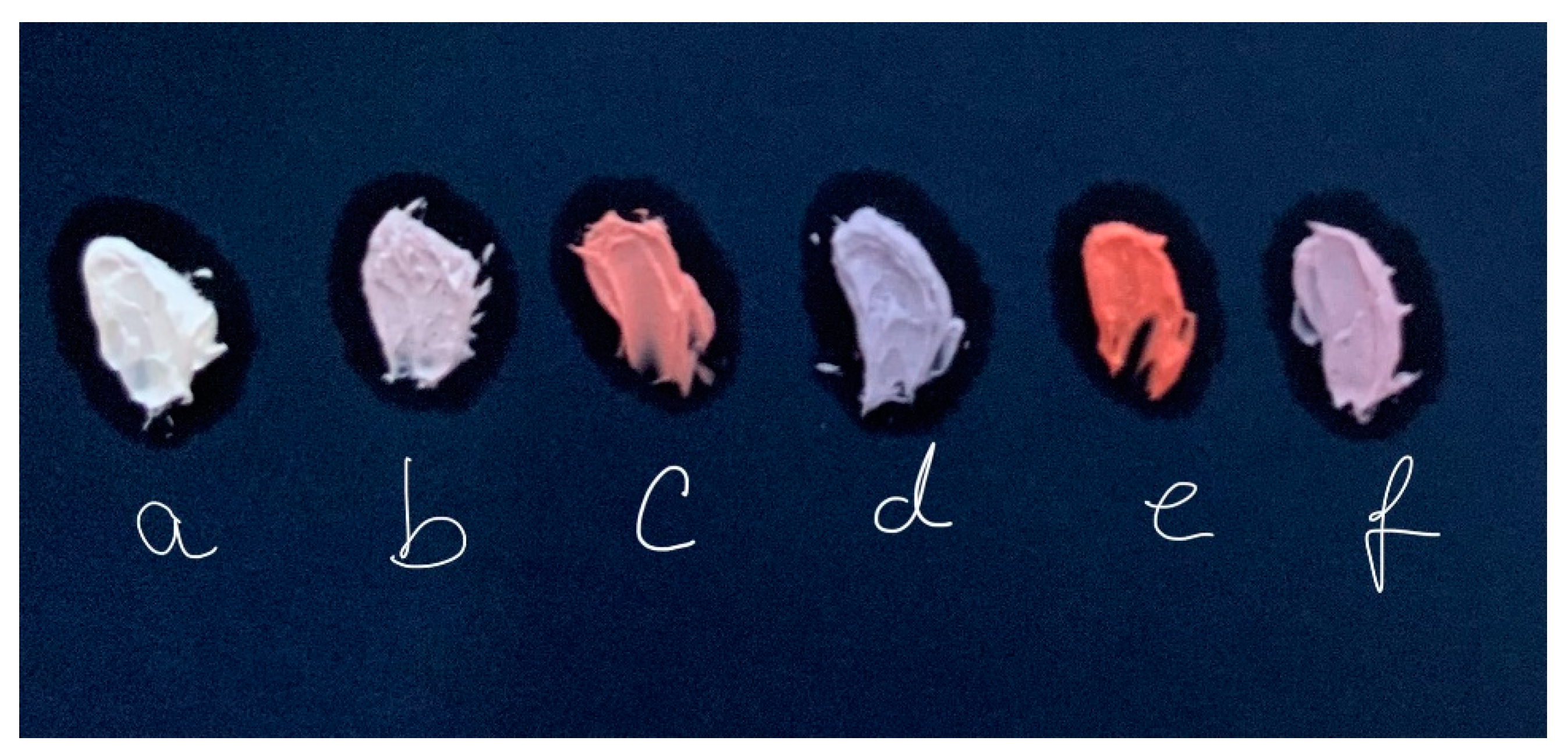
Figure 7 includes photographs of emollient cream pigmented with several of the pigments prepared in this work. The image was updated as time passed.
Fresh colored creams prepared with hybrid pigments preserve the same consistency that the white cream. The color acquired by cream was, at beginning, the same that the pigment used in coloring. However, as time passes, the LDH-CA pigmented cream fades slightly and small red aqueous droplets are secreted (aqueous phase confirmed by FTIR spectroscopy).
4. Discussion
The presence of carminic acid in pigments does not collapse the layered structure of hydrotalcite acting of a host of chromophores and the main interaction between CA and LDH is through the aromatic rings of CA and hydroxylated layers of LDH. When fluorine is present in LDH it can play an important role stabilizing the magnesium phase coating as discussed below.
The results in pigments containing hydroxy naphthol blue where similar to that found for the carminic acid series, the interaction with the aromatic part of HNB was also confirmed and the role of fluorine with magnesium phase in the coated pigments also observed. For the shake of brevity, the corresponding NMR spectra and XRD patterns of HNB series are presented in the supplementary information (
Figures S1 and S2) section.
Regarding the textural properties, all the pigments were non-porous, with a specific surface area (SSA) between 1 and 7 m
2/g (
Table S1, supplementary information). Considering that the white samples of LDH and FLDH have SSA of up to 310 and 318 m
2/g, respectively, it should be concluded that CA and HNB molecules are sufficient to block the pores of LDH.
The
27Al NMR MAS and UV-Vis results suggest a stronger interaction CA-aluminum in pigments prepared by memory effect than that observed in pigments LDH-CA and FLDH-CA. It was earlier [
21,
27] pointed out that a consequence of the fluorination of a magnesium-aluminum hydrotalcite is the formation of MgO
6-xF
x species which in turn induces the creation of strong basic sites but also aluminum with low coordination numbers. These sites should act as the adsorption sites for CA. However, this interaction is not enough strong as the absorption wavelength is easily modified by the presence of Mg(OH)
2. The presence of Mg(OH)
2 in these two pigments leads to a shifting of band to red. More interesting, the spectra of pigments prepared by memory effect shows also two broad absorption bands centered at 355 and 562 nm. They are shifted to red if compared with bands observed in pigments prepared with chromophore loaded during sol-gel synthesis.It should be emphasized that memory effect includes the thermal treatment of LDH, creating then centers of unsaturated aluminum sites, such as 4-fold coordinated Al
3+ [
28,
29].
In
Table 2 is reported
. The higher
the higher the loss of color. Overall, the pigments were stable, the
values where < 20. Small changes in color where observed: For example, sample with major loss of color was LDH-CA but the fluorinated pigment diminishes significantly the fade of color, the presence of recovery magnesium phase also inhibits the loss of color. All pigments containing the magnesium phase coating decreased
as compared to the uncoated pigment. Interestingly, the pigments synthesized using the memory effect were the pigments where the recovery with Mg(OH)
2 was less significant. Actually the color faded similar with or without Mg(OH)
2, in line with the explanation that pigments prepared by memory effect contain immobilized the chromophore in coordinated unsaturated aluminum sites. Note, however, that the pigments discolor significantly, as hydration leads to a layered hydrotalcite-like structure, as shown by the XRD results.
Changes observed in the cream pigmented with LDH-CA can be interpreted as a release of the chromophore from the pigment and dissolution in the water contained in cream. The cream recovered its original appearance when it was mixed for 15 seconds. All other creams were unchanging for periods as long as 4 years. This result is in line with the most stable pigments are that prepared with fluorinated LDH and that improved with Mg(OH)2 phase.
5. Conclusions
Carminic acid and hydroxynaphthol dyes are adsorbed on hydrotalcite support trough weak interactions, emerging then hybrid pigments. However, dye is slightly removed from hydrotalcite when it comes into contact with water, but when Mg(OH)2 is incorporated as a coating of hybrid pigments, it interacts with the surface of the dye, functioning as a kind of shell to prevent the dye chromophore from release.
The adsorption of the dyes drastically decreases the surface area of the hydrotalcite, evidencing that the dye occupies most of available specific area, however the presence of fluorine or magnesium hydroxide does not help to develop large surface areas.
The hybrid pigments showed slight structural and textural changes, however the changes are not drastic.
The pigments obtained by memory effect are the ones that suffer the most changes in their surface properties and porosity.
The results obtained in colorimetry reveal that the hybrid pigments obtained by sol-gel do not show a great difference in color before and after subjecting it to the aging, and especially the coated samples. However, the pigments obtained by memory effect are the most discolored. This means that the pigments obtained by sol-gel are the most stable under air atmosphere for periods as long as ten years, since the color remains almost unaltered.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org, Figure S1: XRD patterns of pigments prepared from combination of layered double hydroxides and hydroxinaphtol blue. Figure S2: 13CP/MAS NMR and 19F MAS NMR spectra of pigments prepared from combination of layered double hydroxides and hydroxinaphtol blue.; Table S1: Textural properties of white layered double hydroxides and their respective pigments.
Author Contributions
Formal analysis, M. Hernández; methodology, C. Felipe; writing—original draft preparation, M. Hernández, and A. Guzmán; supervision, J.L. Rivera; conceptualization, supervision and project administration, E. Lima.
References
- Lu, Y.; Wu, Y.; Yang, J.; Zhu, X.; Sun, F.; Li, L.; Shen, Z.; Pang, Y.; Wu, Q.; Chen, H. Gentle fabrication of colorful superhydrophobic bamboo based on metal-organic framework. J. Colloid Interface Sci. 2021, 593, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, H.; Shao, L.-M.; He, P.-J. Preparation of a metal-phosphate/chromium oxide nanocomposite from Cr(III) containing electroplating sludge and its optical properties as a nanopigment. Process Saf. Environ. Prot. 2015, 98, 261–267. [Google Scholar] [CrossRef]
- Kong, M. , Meng, F.; Zhang, S.; Tang B. Self-supporting structural color films with excellent stability and flexibility through hot-press assisted assembly. Dyes and Pigments, 2021; 195, 109742. [Google Scholar] [CrossRef]
- Lan, Y.-F.; Lin, J.-J. Clay-assisted dispersion of organic pigments in water. Dyes and Pigments 2011, 90, 21–27. [Google Scholar] [CrossRef]
- R. Lambourne (ed.). Paint and Surface Coatings: Theory and Practice; Ellis Horwood, Chichester. 1987; pp. 58–61.
- Lee, P. T.C.; Chiu, C.-W.; Chang, L.-Y.; Chou, P.-Y.; Lee, T.-M.; Chang, T.-Y.; Wu, M.-T.; Cheng, W.-Y.; Kuo, S.-W.; Lin, J.-J. Tailoring Pigment Dispersants with Polyisobutylene Twin-Tail Structures for Electrowetting Display Application. ACS Appl. Mater. Interfaces 2014, 6, 14345–14352. [Google Scholar] [CrossRef]
- Sivamani, R. K; Jagdeo, J. R.; Elsner, P.; Maibach, H. I. (Eds.) Cosmeceuticals and Active Cosmetics. 3rd Edition; CRC Press, 2015; p. 472. ISBN 9781482214161. [Google Scholar]
- Kojima, Y.; Usuki, A.; Kawasumi, M.; Okada, A.; Fukushima, Y.; Kurauchi, T.; Kamigaito, O. Mechanical properties of nylon 6-clay hybrid. J. Mater. Res. 1993, 8, 1185–1189. [Google Scholar] [CrossRef]
- Jeon, N.J.; Noh, J.H.; Kim, Y.C.; Yang, W.S.; Ryu, S.; Seok, S.I. Solvent engineering for high-performance inorganic-organic hybrid perovskite solar cells. Nat. Mater. 2014, 13, 897–903. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, C.; Julián, B.; Belleville, P.; Popall, M. Applications of hybrid organic-inorganic nanocomposites. J. Mater. Chem. 2005, 15, 3559–3592. [Google Scholar] [CrossRef]
- Koczorowski, T.; Cerbin-Koczorowska, M.; Rębiś, T. Azaporphyrins embedded on carbon-based nanomaterials for potential use in electrochemical sensing—A review. Nanomaterials 2021, 11, 2861. [Google Scholar] [CrossRef]
- Pérez, E.; Ibarra, I. A.; Guzmán, A.; Lima, E. Hybrid pigments resulting from several guest dyes onto γ-alumina host: A spectroscopic analysis. Spectrochim. Acta - Part A: Mol. Biomol. Spectr. 2017, 172, 174–181. [Google Scholar] [CrossRef]
- Cavalcanti, G. R. S.; Rodrigues, F.; Zhuang, G.; Balme, S.; Janot, J.-M.; Fonseca, M.G.; Jaber, M. Inorganic-organic hybrid pigments based on carminic acid and clay minerals. Dyes and Pigments. 2021, 190, 109306. [Google Scholar] [CrossRef]
- Lima, E.; Guzmán, A.; Vera, M.; Rivera, J. L.; Fraissard, J. Aged natural and synthetic Maya Blue-like pigments: What difference does it make? J Phys Chem C 2012, 116, 4556–4563. [Google Scholar] [CrossRef]
- Laguna, H.; Loera, S.; Ibarra, I.A.; Lima, E.; Vera, M.A.; Lara, V. Azoic dyes hosted on hydrotalcite-like compounds: Non-toxic hybrid pigments. Micr. Mes. Mater. 2007, 98, 234–2415. [Google Scholar] [CrossRef]
- Tang, P.; Xu, X.; Lin, Y.; Li, D. Enhancement of the thermo- and photostability of an anionic dye by intercalation in a zinc-aluminum layered double hydroxide host, Ind. Eng. Chem. Res. 2008, 47, 2478–2483. [Google Scholar] [CrossRef]
- Marangoni, R.; Ramos, L. P.; Wypych, F. New multifunctional materials obtained by the intercalation of anionic dyes into layered zinc hydroxide nitrate followed by dispersion into poly(vinyl alcohol) (PVA). J. Col. Interface Sc. 2009, 330, 303–309. [Google Scholar] [CrossRef]
- Mandal, S.; Tichit, D.; Lerner, D. A.; Marcotte, N. Azoic dye hosted in layered double hydroxide: Physicochemical characterization of the intercalated materials. Langmuir, 2009; 25, 10980–10986. [Google Scholar] [CrossRef]
- Marzec, A.; Szadkowski, B.; Rogowski, J.; Maniukiewicz, W.; Rybiński, P.; Prochoń, M. New organic/inorganic pigments based on azo dye and aluminum-magnesium hydroxycarbonates with various Mg/Al ratios. Materials 2019, 12, 134910. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Shi, J.; Wei, C.; Jiang, P.; Huang, W.; Zhuang, D. , Azo chromophore monomerically bonded mesostructured silica films with large third-order nonlinearity but negligible nonlinear absorption. J. Phys. Chem. C 2008, 112, 13754–1376. [Google Scholar] [CrossRef]
- Lima, E.; Martínez-Ortiz, M. J.; Gutiérrez Reyes, R. I.; Vera, M. Fluorinated Hydrotalcites: The Addition of Highly Electronegative Species in Layered Double Hydroxides To Tune Basicity. Inorg. Chem. 2012, 51, 7774–7781. [Google Scholar] [CrossRef] [PubMed]
- Park, C.I.; Cho, W.-G.; Lee, S.J. Emulsion stability of cosmetic creams based on water-in-oil high internal phase emulsions. Korea-Australia Rheol J. 2003, 15, 125–130. [Google Scholar]
- Liu, Y.; Lee, W. J.; Tan, C. P.; Lai, O. M.; Wang, Y.; Qiu, C. W/O high internal phase emulsion featuring by interfacial crystallization of diacylglycerol and different internal compositions. Food Chem. 2022, 372, 131305. [Google Scholar] [CrossRef]
- Alexandersson, E.; Nestor, G. Complete 1H and 13C NMR spectral assignment of d-glucofuranose. Carbohydrate Res. 2022, 511, 108477. [Google Scholar] [CrossRef]
- Coster, D.; Fripiat, J. J. Memory Effects in Gel-Solid Transformations: Coordinately Unsaturated Al Sites in Nanosized Aluminas. Chem. Mater. 1993, 5, 1204–1210. [Google Scholar] [CrossRef]
- Scholz, G.; Stosiek, C.; Noack, J.; Kemnitz, E. Local fluorine environments in nanoscopic magnesium hydr(oxide) fluorides studied by 19F MAS NMR. J. Fluorine Chem. 2011, 132, 1079–1085. [Google Scholar] [CrossRef]
- Lima, E.; Pfeiffer, H.; Flores, J. Some consequences of the fluorination of brucite-like layers in layered double hydroxides: Adsorption. Appl. Clay Sc, 2014; 88–89, 26–32. [Google Scholar]
- Valente, J. S.; López-Salinas, E.; Bokhimi, X.; Flores, J.; Maubert, A. M.; Lima, E. Sulfated Nanocapsular Aluminas: Controlling their Brönsted and Lewis Acidity. J. Phys. Chem. C 2009, 113, 16476–16484. [Google Scholar] [CrossRef]
- Valente, J. S.; Falcón, S.; Lima, E.; Vera, M. A.; Bosch, P.; López-Salinas, E. Phosphating alumina: A way to tailor its surface properties. Micr. Mes. Mater. 2006, 94, 277–282. [Google Scholar] [CrossRef]
- Brainard, D.H.; Stockman, A. Colorimetry. In the OSA handbook of optics, 3rd ed.; McGraw-Hill: New York, 2010. [Google Scholar]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).