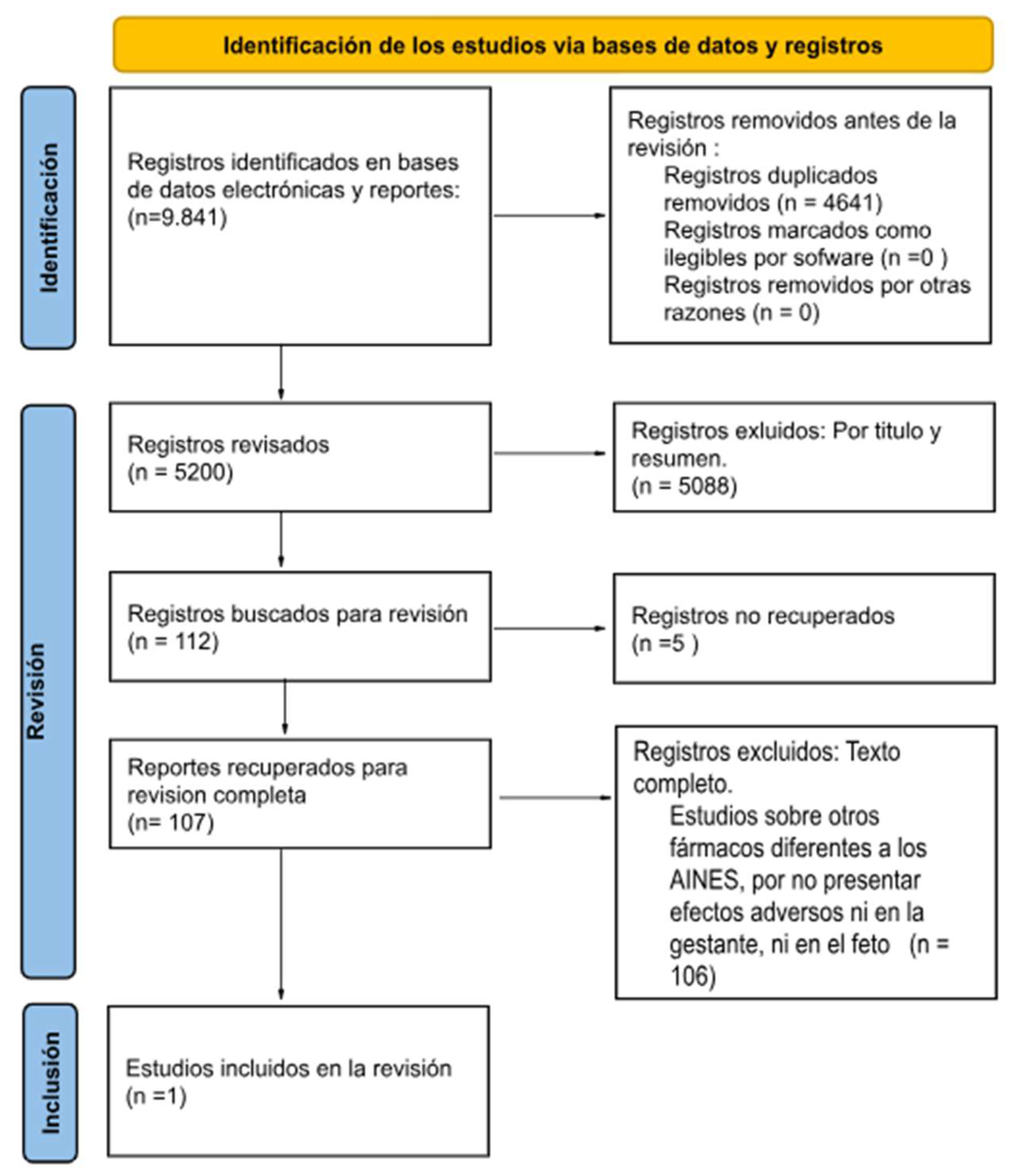
Discussion
Pregnancy is a biological process in which women are responsible for the development of a human being and starts at the time of gestation and childbirth. During this period, the woman's body experiences a series of drastic physiological and hormonal changes that allow it to provide the necessary environment for the growth and development of the fetus, generating the experience of pain or inflammation, which is often also associated with the preexisting medical conditions of the pregnant woman.
In this context, a high population of pregnant mothers has shown that they are looking for pharmacological options that allow them to reduce the symptoms generated by pregnancy without truly understanding the components and implications that it can have on their body or the fetus. Among these options are nonsteroidal anti-inflammatory drugs (NSAIDs), drugs that are frequently used during pregnancy due to the symptoms that can develop during this stage. However, the information available on its effects during pregnancy is limited and, for the most part, inconclusive. An examination of the potential risks for pregnant women and fetuses derived from self-medication with NSAIDs through a review of the literature revealed that the use of NSAIDs in the first trimester does not appear to be a significant risk factor. However, in subsequent trimesters, teratogenic effects on the fetus have been reported (4).
Consequently, these findings support the Food and Drug Administration (FDA) recommendation to avoid the use of NSAID medications during pregnancy, especially after 20 weeks, due to the risk of placental hypoperfusion and fetal kidney damage. This warning highlights the prevailing need to perform constant pharmacological surveillance on nonsteroidal anti-inflammatory drugs (NSAIDs), which, as mentioned above, are widely consumed due to their over-the-counter availability and easy access to the market. (5)
The remarkable ability of NSAIDs to relieve pain and reduce inflammation by inhibiting prostaglandin synthesis at the peripheral level exposes the paradox of their popularity and the need for caution in their use, especially during pregnancy. Additional research evidence suggests that the use of these drugs during pregnancy may be related to a slight increase in the frequency of cardiac and orofacial malformations in fetuses. These findings reinforce the importance of using these medications both moderately and under medical supervision, highlighting the complexity of balancing the need for pain relief with the precaution necessary to safeguard the health of the fetus and the pregnant woman. In this context, informed decision-making and appropriate medical advice are essential to ensure the safety of both the mother and the future baby during the gestational period (6).
On the other hand, the wide availability of NSAIDs and their attractive analgesic and anti-inflammatory action make their unrestricted use common, but the potential risk for fetal development highlights the need for careful and controlled administration of these medications, especially in pregnant women. Constant care and pharmacological monitoring are essential to ensure the safety of both expectant mothers and their babies during this crucial stage of life. Therefore, in this study, an exhaustive analysis of adverse effects during the gestation period was performed through a systematic review of the literature. The central purpose is to enrich the scientific evidence available in this field.
Abramovici's study revealed significant findings. Among the 2985 women without serious illnesses, with a gestation period between 13 and 25 weeks, the impact of the administration of analgesic drugs was observed. The initial results indicated premature birth, defined as birth before 37 weeks of gestation. This event results in a greater risk of hemorrhage, ovarian damage, and systemic or intestinal effects at the time of delivery. It should be noted that these aforementioned risks are not produced by NSAIDs but by premature birth, which is an effect of them. low birth weight <2,500 gr. In addition, an increase in the admission of newborns to neonatal intensive care units (NICUs) was observed. These results highlight the importance of carefully considering the administration of analgesics during pregnancy since it is related not only to neonatal risks but also to adverse consequences for maternal health (3).
These results, derived from specific research, underline the importance of understanding the possible adverse effects that may arise during pregnancy. Preterm birth has significant implications for maternal and fetal health. The increase in NICU admissions highlights the need for more rigorous medical care and monitoring during pregnancy to reduce the risks associated with these unfavorable outcomes. Thus, pregnant women may not be fully aware of the risks that certain medications may represent for them and their babies since they often rely on knowledge from popular culture to administer drugs since the suggestion is given by acquaintances who speak from their own experience without recognizing the effects that could be caused. Additionally, one of the main problems that is evident is that many pregnant women avoid medical advice and normalize pain treatment with the medications they normally consume but are unaware of the adverse effects that could develop during pregnancy. body or in the formation of the fetus, the body has changed, and with this change, the body processes what it consumes. Therefore, pregnant women should be regularly monitored by health professionals to guarantee a healthy pregnancy, in which the risks for the mother and her fetus are minimized.
Likewise, it is interesting to note that some of the public policies that regulate the supply of medications and care for pregnant mothers have not had adequate systematization that shows the potential impact of the use of NSAIDs and that therefore allows regulation to be carried out. through pedagogical and legislative means that restrict the risk of pregnancy for mothers. For example, through Statutory Law 1751 of 2015, which determines the regulation of the fundamental right to health, pregnant mothers were considered subjects of special constitutional protection, prioritizing the fundamental principles for them (availability, acceptability, accessibility and quality). professional:
Therefore, in the case of pregnant women, measures will be adopted to guarantee access to the health services they require during pregnancy and after pregnancy and to guarantee that they can exercise their fundamental rights within the framework of access to health services. (Congress of Colombia, 2015, Law 1751, Article 11) (7). Law 1751 addresses the prioritization that should be given to pregnant mothers and the care that should be implemented from health services, but it does not regulate public policies regarding pharmacological regulation for pregnant women and, therefore, pedagogical processes for understanding possible risks from their supply. It is interesting to see greater pharmacological regulation in contraception and safe abortion processes than in what is linked to pregnancy (7).
Another implication of this process is the easy access of pregnant women to NSAID drugs. This is related to the widespread availability of NSAIDs on the market, their sale without a prescription, and the common perception that they are low-risk medications. Easy access to these drugs may lead to greater self-medication by pregnant women, as they may consider them quick and convenient options for relieving pain or inflammation during pregnancy. However, such accessibility raises important concerns since NSAIDs can have the previously mentioned adverse effects. Furthermore, in populations with health difficulties such as arthritis, spontaneous abortions could occur, although the findings of Rojas and Cabrera are inconclusive. (8).
Ramírez et al. noted that there is a significant propensity for the use of NSAIDs for possible congenital diseases in children due to problems during pregnancy resulting from placental abruption or other difficulties during pregnancy. (8) A study by Bohórquez et al. revealed the prevalence of self-medication in pregnant women in Colombia over a period of 10 years and revealed that analgesics and nonsteroidal anti-inflammatory drugs (NSAIDs) are the drugs most commonly self-administered by these women, although they are not recommended for use in the last trimester of pregnancy (9). Arellano et al. focused on the pharmacological management of common disorders during pregnancy in primary care and noted that some NSAIDs are classified as risk category B during the first or second trimester but become category D in the last trimester, which indicates a significant risk for the fetus. The importance of avoiding its use after the third trimester is emphasized due to potential adverse effects on maternal and fetal health (10).
Although all the studies suggest the use of NSAIDs in pregnant women is alarming, the lack of definitive conclusions is due to the difficulty in generalizing the results. Different studies can yield different conclusions, especially when considering populations with particular conditions. The variability in findings highlights the complexity of the impact of nonsteroidal anti-inflammatory drugs (NSAIDs) on pregnancy. However, although adverse effects were identified in the present study, continuing to investigate the effects of NSAIDs on pregnancy is crucial. Therefore, persistent research is vital to guarantee the safety of pregnancy and fetal development, as this research could lead to the establishment of pharmacological control measures from a legislative basis. It is essential to specifically address the reasons underlying the lack of conclusive approaches in studies, considering the variables that may influence the results. Additionally, understanding the risks and benefits of these medications can play a critical role in medical decision-making and prenatal care. By taking into account the complexity of factors affecting the relationship between NSAIDs and pregnancy, the ability to guide informed decisions can be improved, thereby contributing to the health and well-being of both expectant mothers and their babies. This approach may help reduce the risks associated with the use of NSAIDs during pregnancy.
Understanding the risks associated with the use of nonsteroidal anti-inflammatory drugs (NSAIDs) is vital for a pregnant mother, as this understanding can reveal the difference between a healthy pregnancy and one with unnecessary complications. First, a pregnant mother who understands the risks of NSAIDs is therefore more prepared to make informed decisions about her health and that of her baby, resorting to a much more careful medical protocol. Knowing that some NSAIDs, such as ibuprofen, are linked to an increased risk of complications, such as premature closure of the ductus arteriosus or pulmonary hypertension in the baby, allows the mother to take preventative measures by avoiding these medications (9).
Furthermore, understanding the risks of NSAIDs can encourage open and effective communication with health professionals who supervise pregnancy and who reject self-diagnoses or those given by people in the community since ignorance often puts people at risk. the well-being of mothers and fetuses. This communication is essential for proper medical care, as it allows the doctor and mother to make decisions together based on a complete evaluation of the risks and benefits of any necessary treatment.
On the other hand, knowledge of the risks of NSAIDs may motivate pregnant women to seek safer alternatives to relieve pain or inflammation; nonpharmacological therapies, such as physical therapy, rest, physical therapy, and other approaches, may be helpful. adequate options, mainly supported by health entities that privilege the mother and fetus as subjects of special protection. By helping mothers make decisions that minimize exposure to NSAIDs, reduce unnecessary risks and protect the health of both mothers and fetuses, this knowledge empowers future mothers and contributes to healthier pregnancies without unnecessary complications.
As health professionals, we must have extensive knowledge of medical studies and the possible implications that can develop in different populations, observing patient variables in each context. In the case of pregnant mothers, the particularities of each patient must be recognized, and aggravations that may develop during the period of gestation until delivery must be understood. The pregnancy process and the transformation of the body must be recognized, and the possible implications that could affect the baby based on the mother's health particularities must be understood. In the case of NSAIDs, the effects of the gestation period must be recognized since medical treatment varies significantly from the first trimester to the last (which becomes more risky for the fetus).
The study of the effects of nonsteroidal anti-inflammatory drugs (NSAIDs) on pregnancy in young and adult women, as well as on the fetus, is a field of research with significant challenges facing the medical sector. Many of these limitations are related to medical and moral ethics, data availability, and variability of results. In the case of ethical implications, there is a confrontation with carrying out controlled clinical studies in pregnant women because many sectors do not agree with exposing pregnant women and fetuses to possible risks since the systematization of data from the observation that takes a human being as an object of testing. As a result, most studies in this area are based on retrospective observations and analyses of existing data rather than controlled experiments. The present work collected literature on the effects of nonsteroidal inflammatory agents on pregnancy in women.
The variability of the results from different studies is a common limitation in research into the effects of NSAIDs on pregnancy. This is because the effects may depend on the dose supplied, the duration of exposure during pregnancy, the presence of congenital diseases, and the context in which the mother is exposed, among other factors, which makes it difficult to draw definitive conclusions. Some repetitive patterns can be rectified, but due to the lack of further experimentation in this field, it is not possible to determine precepts.
It is relevant to note that, unlike other studies where the data depend on objective measurements such as physiological and clinical indicators, in the study of the adverse effects of NSAIDs during pregnancy, the information is often based on the truthfulness of the patients. This dependence on self-reports can introduce biases and errors in the information since there is no complete monitoring process of the mother, in part due to the ethical considerations previously stated. The lack of objective measures may affect the accuracy of the results, highlighting the need to address these limitations in future research to improve the reliability of the data and strengthen the knowledge base in this field.
Gaps in the data on population groups and diseases were noted, as was the need for specific information for children, adolescents, and pregnant and lactating women, according to the WHO. Including detailed data for these groups is essential for ensuring that they receive the necessary interventions. Although pregnancy is exclusively associated with the female sex, the importance of comprehensively addressing the different dimensions of health that can vary between genders is highlighted beyond the biological issues associated with pregnancy (11).
Due to ethical and implementation limitations, the lack of controlled clinical trials in this field leaves gaps in solid scientific evidence. This means that much of the available information is derived from observational studies and retrospective analyses, which prevents detailed implementation of the care and pedagogy of pregnant mothers. The World Health Organization (11) has sought to expand the field of application of strategies that enable clinical trials in prioritized populations and that allow more conclusive results to be determined.
Recognizing that “well-designed and well-executed clinical trials are indispensable for evaluating the safety and effectiveness of health interventions” and recognizing “the importance of promoting equity in clinical trial capacity”, the Health Assembly set out to improve the quality of the evidence generated in clinical trials and its coordination. [...] "Clinical trials of new health interventions are likely to produce a clearer result when carried out in diverse settings, especially in all the main population groups that the intervention is intended to benefit, with particular attention given to underrepresented populations"; These populations may include women, especially pregnant and breastfeeding women, children, and other groups underrepresented in such trials, such as vulnerable and marginalized people.
To advance research on the effects of nonsteroidal anti-inflammatory drugs (NSAIDs) in pregnancy, both in young and adult women and in fetuses, and to address the previously mentioned limitations, different recommendations have been derived from the present discussion.
It is essential to develop production processes for large-scale observational studies, which should be encouraged through comprehensive policies that allow the execution of the study with the protection of the health and well-being of the mother and fetus. Therefore, conducting larger-scale studies would provide more solid and generalizable data on the effects of NSAIDs during pregnancy, allowing them to be compared at local and international levels. Likewise, long-term follow-up of pregnant women exposed to NSAIDs and their babies should be performed. This approach allows us to evaluate the impact of these changes during gestation and the possible variants that may arise during a child's growth, providing crucial information on the possible long-term effects on the development of the child and the mother.
On the other hand, investigating the relationship between the dose and duration of NSAID use during pregnancy and the resulting effects on the mother and fetus is essential since understanding the stage of development of both drugs and the effects generated would help determine safer use guidelines, promoting more rigorous regulation of the sale of these drugs to pregnant women. In addition, it is necessary to encourage research into safer alternatives for the treatment of pain and inflammation in pregnant women, which could include nonpharmacological therapies, such as physiotherapy, homeopathy and analgesics, which are considered safer during pregnancy. Likewise, promoting collaboration between researchers and health professionals at an international level is crucial, as this not only seeks to compile findings but also inform the affected public.
Another important measure is to develop educational programs and awareness campaigns aimed at both health professionals and pregnant women, with a social approach based on responsibility toward others, where these initiatives must address the risks of NSAIDs and promote safe practices during pregnancy for pain management. Therefore, medical and regulatory organizations should regularly review and update clinical guidelines related to the use of NSAIDs during pregnancy. This should be based on the most recent evidence available, as well as seeking to establish the basis for general legislation.
Finally, given their importance in maternal and fetal health, state and international research funding agencies should prioritize the allocation of resources for studies related to the use of NSAIDs during pregnancy. These joint steps will significantly contribute to advancing the understanding and safe management of NSAIDs during pregnancy.