Submitted:
28 December 2023
Posted:
29 December 2023
You are already at the latest version
Abstract
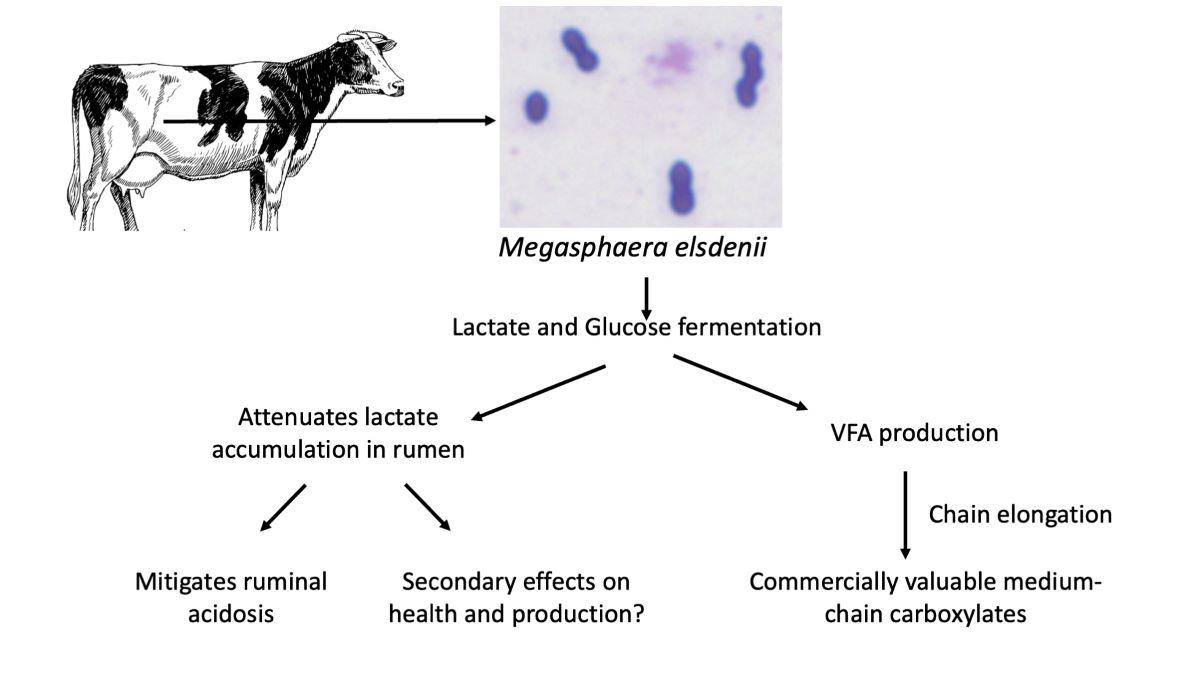
Keywords:
1. Introduction
2. Classification and morphology
3. Abundance in the rumen
4. Isolation and cultivation
5. Nutrient metabolism and growth
6. Resistance to environmental stress
7. Relationships with others ruminal microorganisms
8. Nutritional Importance
9. VFA production
10. Final considerations
Author Contributions
Conflicts of Interest
References
- Van Soest, P.J. Nutritional Ecology of the Ruminant. 2nd ed. Cornell University Press: New York, 1994.
- Hungate, R.E. The Rumen and Its Microbes. Academic Press: New York, 1966.
- Joblin, K.N. Isolation, enumeration and maintenance of rumen anaerobic fungi in roll tubes. Appl. Environ. Microbiol. 1981, 42, 1119–1122. [Google Scholar] [CrossRef] [PubMed]
- Kay, R. Digestion of protein in the intestines of adult ruminants. Proc. Nutrition Soc. 1969, 28, 140–151. [Google Scholar] [CrossRef]
- Bergman, E.N. Energy contributions of volatile fatty acids from the gastrointestinal tract in various species. Physiol. Rev. 1990, 70, 567–590. [Google Scholar] [CrossRef] [PubMed]
- Won, M.Y.; Oyama, L.B.; Courtney, S.J.; Creevey, C.J.; Huws, S.A. Can rumen bacteria communicate to each other? Microbiome 2020, 8, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Huhtanen, C.N.; Gall, L.S. Rumen organisms II: two lactate utilizers and six miscellaneous types. J. Bacteriol. 1953, 65, 554–559. [Google Scholar] [CrossRef]
- Agler, M.; Wrenn, B.A.; Zinder, S.H.; Angenent, L.T. Waste to bioproduct conversion with undefined mixed cultures: the carboxylate platform. Trends Biotechnol. 2011, 29, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Sugihara, P.T.; Sutter, V.L.; Attebery, H.R.; Bricknell, K.S.; Finegold, S.M. Isolation of Acidaminococcus fermentans and Megasphaera elsdenii from normal human feces. Appl. Microbiol. 1974, 27, 274–275. [Google Scholar] [CrossRef] [PubMed]
- Giesecke, D.; Wiesmayr, S.; Ledinek, M. Peptostreptococcus elsdenii from the caecum of pigs. J. Gen. Microbiol. 1970, 64, 123–126. [Google Scholar] [CrossRef]
- Yutin, N.; Galperin, M.Y. A genomic update on clostridial phylogeny: Gram-negative spore formers and other misplaced clostridia. Environ. Microbiol. 2013, 15, 2631–2641. [Google Scholar] [CrossRef]
- Sarmikasoglou, E.; Faciola, A.P. Ruminal bacteria lipopolysaccharides: an immunological and microbial outlook. J. Animal Sci. Biotechnol. 2022, 13, 41. [Google Scholar] [CrossRef]
- Shetty, S.A.; Marathe, N.P.; Laniekar, V.; Ranade, D.; Shouche, Y.S. Comparative genome analysis of Megasphaera sp. reveals niche specialization and its potential role in the human gut. Plos One. 2013, 8, 1–13. [Google Scholar] [CrossRef]
- Rogosa, M. Transfer of Peptostreptococcus elsdenii Gutierrez et al. to a new genus, Megasphaera [M. elsdenii (Gutierrez et al.) comb. nov.]. Int. J. Syst. Bacteriol 1972, 21, 187–189. [Google Scholar] [CrossRef]
- Bucci, M. Cell motility: Bigger and faster. Nat. Chem. Biol. 2015, 11, 381. [Google Scholar] [CrossRef]
- Levin, P.A.; Angert, E.R. Small but mighty: cell size and bacteria. Cold Spring Harbor Perspect. Biol. 2015, 7, a019216. [Google Scholar] [CrossRef] [PubMed]
- Elsden, S.R.; Gilchrist, F.M.C.; Lewis, D.; Volcani, B.E. Properties of a fatty acid forming organism isolated from the rumen of sheep. J. Bacteriol. 1956, 72, 681–689. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, J.; Davis, R.E.; Lindahl, I.L.; Warwick, E.J. Bacterial changes in the rumen during the onset of feed-lot bloat of cattle and characteristics of Peptostreptococcus elsdenii n. sp. Appl. Microbiol. 1959, 7, 16–22. [Google Scholar] [CrossRef]
- Elsden, S.R; Lewis, D. The production of fatty acids by a gram-negative coccus. Biochem. J. 1953, 55, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Kluyver, A.J.; Van Niel, C.B. Prospects for a natural system of classification of bacteria. Zentralbl. Bakteriol. Parisitenk. Abt. 1935, 94, 369–403. [Google Scholar]
- Piknova, M.; Bires, O.; Javorsky, P.; Pristas, P. Limited genetic variability in Megasphaera elsdenii strains. Folia Microbiol. 2006, 51, 299–302. [Google Scholar] [CrossRef]
- Marx, H.; Graf, A.B.; Tatto, N.E.; Thallinger, G.G.; Mattanovich, D; Saueri, M. Genome sequence of the ruminal bacterium Megasphaera elsdenii. J. Bacteriol. 2011, 193, 5578–5579. [Google Scholar] [CrossRef]
- Hatmaker, E.A.; Klingeman, D.M.; O’Dell, K.B.; Riley, L.A.; Papanek, B.; Guss, A.M. Complete genome sequences of two Megasphaera elsdenii strains, NCIMB 702410 and ATCC 25940. Microbiol. Res. Announc. 2019, 8, e01430–18. [Google Scholar] [CrossRef] [PubMed]
- Bag, S.; Ghosh, T.S.; Das, B. Whole-genome sequence of a Megasphaera elsdenii strain isolated from the gut of a healthy Indian adult subject. Genome Announc. 2017, 5, e01033–17. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Gutierrez, C.A.; Aylward, F.O. Genome size distributions in bacteria and archaea are strongly linked to evolutionary history at broad phylogenetic scales. PLoS Genet. 2022, 18, e1010220. [Google Scholar] [CrossRef] [PubMed]
- Suen, G.; Weimer, P.J.; Stevenson, D.M.; Aylward, F.O.; Boyum, J. The complete genome sequence of Fibrobacter succinogenes S85 reveals a cellulolytic and metabolic specialist. PLoS ONE, 2011, 6, e18814. [Google Scholar] [CrossRef] [PubMed]
- Hobson, P.N.; Mann, S.O.; Oxford, A.E. Some studies on the occurrence and properties of a large Gram-negative coccus from the rumen. J. Gen. Microbiol. 1958, 19, 462–472. [Google Scholar] [CrossRef] [PubMed]
- Stewart, C.S.; Bryant, M.P. The rumen bacteria. In The Rumen Microbial Ecosystem; Hobson, P.N., Stewart, C.S., Springer Dordrecht, United Kingdom, 1988; pp.10-72.
- Stewart, C.S.; Fonty, G.; Gouet, P.H. The establishment of rumen microbial communities. Anim. Feed Sci. Technol. 1988, 21, 69–97. [Google Scholar] [CrossRef]
- Mackie, R.I.; Gilchrist, F.M.C. Changes in lactate-producing and lactate-utilizing bacteria in relation to pH in the rumen of sheep during stepwise adaptation to a high-concentrate diet. Appl. Environ. Microbiol. 1979, 38, 422–430. [Google Scholar] [CrossRef]
- Yang, H.E.; Zotti, C.A.; McKinnon, J.J.; McAllister, T.A. Lactobacilli are prominent members of the microbiota involved in the ruminal digestion of barley and corn. Front. Microbiol. 2018, 9, 718. [Google Scholar] [CrossRef] [PubMed]
- Ouwerkerk, D.; Klieve, A.V.; Forster, R.J. Enumeration of Megasphaera elsdenii in rumen contents by real-time Taq nuclease assay. J. Appl. Microbiol. 2022, 97, 753–758. [Google Scholar] [CrossRef]
- Klieve, A.V.; Hennessy, D.; Ouwerkerk, D.; Forster, R.J.; Mackie, R.I.; Attwood, G.T. Establishing populations of Megasphaera elsdenii YE 34 and Butyrivibrio fibrisolvens YE 44 in the rumen of cattle fed high grain diets. J. Appl. Microbiol. 2003, 95, 621–630. [Google Scholar] [CrossRef]
- Větrovský, T.; Baldrian, P. The variability of the 16S rRNA gene in bacterial genomes and its consequences for bacterial community analyses. PLoS One. 2013, 8, e57923. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, D.M.; Weimer, P.J. Dominance of Prevotella and low abundance of classical ruminal bacterial species in the bovine rumen revealed by relative quantification real-time PCR. Appl. Microbiol. Biotechnol. 2007, 75, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Weimer, P.J.; Stevenson, D.M.; Mertens, D.R. Shifts in bacterial community composition in the rumen of lactating dairy cows under milk-fat depression conditions. J. Dairy Sci. 2010, 93, 265–278. [Google Scholar] [CrossRef] [PubMed]
- Palmonari, A.; Stevenson, D.M.; Mertens, D.R.; Cruywagen, C.W.; Weimer, P.J. pH dynamics and bacterial community composition in the rumen of lactating dairy cows. J. Dairy Sci. 2010, 93, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Jami, E.; Mizrahi, I. Similarity of the ruminal bacteria across individual lactating cows. Anaerobe. 2012, 2012 18, 338e343. [Google Scholar] [CrossRef]
- Jami, E.; Israel, A.; Kotser, A.; Mizrahi, I. Exploring the bovine rumen bacterial community from birth to adulthood. ISME J. 2013, 7, 1069–1079. [Google Scholar] [CrossRef] [PubMed]
- Rico, D.; Preston, S.; Risser, J.; Harvatine, K. Rapid changes in key ruminal microbial populations during the induction of and recovery from diet-induced milk fat depression in dairy cows. Br. J. Nutr. 2015, 114, 358–367. [Google Scholar] [CrossRef]
- Yohe, T.T.; Enger, B.D.; Wang, L.; Tucker, H.L.M.; Ceh, C.A.; Parsons, C.L.M.; Yu, Z.; Daniels, K.M. Does early-life administration of a Megasphaera elsdenii probiotic affect long-term establishment of the organism in the rumen and alter rumen metabolism in the dairy calf? J. Dairy Sci. 2018, 101, 1747–1751. [Google Scholar] [CrossRef]
- de Melo, H.S.A.; Ítavo, L.C.V.; de Castro, A.P.; Ítavo, C.C.B.F.; de Araújo Caldas, R.; Mateus, R.G.; Niwa, M.V.G.; de Moraes, G.J.; da Silva Zornitta, C.; Gurgel, A.L.C.; Benchaar, C. Bacterial species in the ruminal content of steers fed oilseeds in the diet. Trop. Anim. Health. Prod. 2022, 54, 396. [Google Scholar] [CrossRef]
- Park, T.; Cersosimo, L.M.; Radloff, W.; Zanton, G.I.; Li, W. The rumen liquid metatranscriptome of post-weaned dairy calves differed by pre-weaning ruminal administration of differentially-enriched, rumen-derived inocula. Anim. Microbiome, 2022, 4, 4. [Google Scholar] [CrossRef]
- Fernando, S.C.; Purvis, H.T. II,, Najar, F.Z.; Sukharnikov, L.O.; Krehbiel, C.R.; Nagaraja, T.G.; Roe, B.A.; DeSilva, U. Rumen microbial population dynamics during adaptation to a high-grain diet. Appl. Environ. Microbiol., 2010, 76, 7482–7490. [Google Scholar] [CrossRef] [PubMed]
- Khafipour, E.; Li, S.; Plaizier, J.C.; Krause, D.O. Rumen microbiome composition determined using two nutritional models of subacute ruminal acidosis. Appl. Environ. Microbiol. 2009, 75, 7115–7124. [Google Scholar] [CrossRef] [PubMed]
- McCann, J.C.; Luan, S.; Cardoso, F.C.; Derakhshani, H.; Khafipour, E.; Loor, J.J. Induction of subacute ruminal acidosis affects the ruminal microbiome and epithelium. Front Microbiol. 2016, 7, 701. [Google Scholar] [CrossRef] [PubMed]
- He, B.; Fan, Y.; Wang, H. Lactate uptake in the rumen and its contributions to subacute rumen acidosis of goats induced by high-grain diets. Front. Vet. Sci. 2022, 9, 964027. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.; Yang, Y.; Lin, C.; Zhang, J.; Pan, J.; Luo, L.; Wu, G.; Awasthi, M.K.; Yan, B. Caproic acid production from anaerobic fermentation of organic waste – Pathways and microbial perspective. Renew. Sust. Energy Revs., 2023, 175, 113181. [Google Scholar] [CrossRef]
- Weimer, P.J.; Moen, G.N. Quantitative analysis of growth and volatile fatty acid production by the anaerobic ruminal bacterium Megasphaera elsdenii T81. Appl. Microbiol. Biotechnol. 2013, 97, 4075–4081. [Google Scholar] [CrossRef]
- Weimer, P.J.; da Silva Cabral, L.; Cacite, F. Effects of ruminal dosing of Holstein cows with Megasphaera elsdenii on milk fat production, ruminal chemistry, and bacterial strain persistence. J. Dairy Sci. 2015, 98, 8078–8092. [Google Scholar] [CrossRef] [PubMed]
- Bryant, M.P.; Robinson, I.M. Some nutritional characteristics of predominant culturable ruminal bacteria. J. Bacteriol. 1962, 84, 605–614. [Google Scholar] [CrossRef]
- Forsberg, C.W. Nutritional characteristics of Megasphaera elsdenii. Can. J. Microbiol. 1978, 24, 981–985. [Google Scholar] [CrossRef]
- Lee, N.R.; Lee, C.H.; Lee, D.Y.; Park, J.B. Genome-scale metabolic network reconstruction and in silico analysis of hexanoic acid producing Megasphaera elsdenii. Microorganisms 2020, 8, 539. [Google Scholar] [CrossRef]
- Wallace, R.J. Catabolism of amino acids by Megasphaera elsdenii LC1. Appl. Environ. Microbiol., 1986, 51, 1141–1143. [Google Scholar] [CrossRef] [PubMed]
- Rychlik, J.R.; LaVera, R.; Russell, J.B. Amino acid deamination by ruminal Megasphaera elsdenii strains. Curr. Microbiol. 2002, 45, 340–345. [Google Scholar] [CrossRef] [PubMed]
- Russell, J.B.; Baldwin, R.L. Comparison of maintenance energy expenditures and growth yields among several rumen bacteria grown on continuous culture. Appl. Environ. Microbiol. 1979, 37, 537–543. [Google Scholar] [CrossRef] [PubMed]
- Russell, J.B.; Dombrowski, D.B. Effect of pH on the efficiency of growth by pure cultures of rumen bacteria in continuous culture. Appl. Environ. Microbiol. 1980, 39, 604–610. [Google Scholar] [CrossRef]
- Panikov, N.S. True and illusory benefits of modeling: comment on “Genome-scale metabolic network reconstruction and in silico analysis of hexanoic acid producing Megasphaera elsdenii. Microorganisms 2020, 8, 539”. Microorganisms 2020, 8, 1742. [Google Scholar] [CrossRef] [PubMed]
- Marounek, M.; Fliegrova, K.; Bartos, S. Metabolism and some characteristics of ruminal strains of Megasphaera elsdenii. Appl. Environ. Microbiol. 1989, 55, 1570–1573. [Google Scholar] [CrossRef]
- Russell, J.B.; Baldwin, R.L. Substrate preferences in rumen bacteria: evidence of catabolite regulatory mechanisms. Appl. Environ. Microbiol. 1978, 36, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Russell, J.B.; Baldwin, R.L. Comparison of substrate affinities among several rumen bacteria: a possible determinant of rumen bacterial competition. Appl. Environ. Microbiol. 1979, 37, 531–536. [Google Scholar] [CrossRef] [PubMed]
- Hino, T.; Shimada, K.; Maruyama, T. Substrate preference in a strain of Megasphaera elsdenii, a ruminal bacterium, and its implications in propionate production and growth competition. Appl. Environ. Microbiol. 1994, 60, 1827–1831. [Google Scholar] [CrossRef]
- Hino, T.; Miyazaki, K.; Kuroda, S. Role of extracellular acetate in the fermentation of glucose by a ruminal bacterium, Megasphaera elsdenii. J. Gen. Appl. Microbiol. 1991, 37, 121–129. [Google Scholar] [CrossRef]
- Thauer, R.K.; Jungermann, K.; Decker, K. Energy conservation in chemotrophic anaerobic bacteria. Bacteriol Rev. 1977, 31, 100–180. [Google Scholar] [CrossRef] [PubMed]
- Hino, T.; Kuroda, S. Presence of lactate dehydrogenase and lactate racemase in Megasphaera elsdenii grown on glucose or lactate. Appl. Environ. Microbiol. 1993, 59, 255–259. [Google Scholar] [CrossRef] [PubMed]
- Prabhu, R.; Altman, E.; Eiteman, M.A. Lactate and acrylate metabolism by Megasphaera elsdenii under batch and steady-state conditions. Appl. Environ. Microbiol. 2012, 78, 8564–8570. [Google Scholar] [CrossRef]
- Counotte, G.H.; Prins, R.A.; Janssen, R.H.A.M.; DeBie, M.J.A. Role of Megasphaera elsdenii in the fermentation of DL-[2-13C]-lactate in the rumen of dairy cattle. Appl. Environ. Microbiol. 1981, 42, 649–655. [Google Scholar] [CrossRef]
- Madigan, M.T.; Martinko, J.M. Brock - Biology of Microorganisms, 11th ed.; Prentice-Hall International: Upper Saddle River, NJ, USA, 2006; p. 157. [Google Scholar]
- Moat, A.G.; Foster, J.W. Microbial Physiology. 3rd ed. John Wiley & Sons: New York, 1995, p.26.
- Jin, Q.; Kirk, M.F. pH as a primary control in environmental microbiology: 1. Thermodynamic perspective. Front. Environ. Sci. 2018, 6, 21. [Google Scholar] [CrossRef]
- Tortora, G.; Funke, B.; Case, C. Microbiology: An Introduction. 12thed. Benjamin-Cummings: Boston. 2015, p.152.
- Russell, J.B. Rumen Microbiology and Its Role in Ruminant Nutrition. James B. Russell Publications, Ithaca, USA, 2002. Available at https://www.ars.usda.gov/research/software/download/?softwareid=409.
- Nagaraja, T.G.; Titgemeyer, E.C. Ruminal acidosis in beef cattle: the current microbiological and nutritional outlook. J. Dairy Sci. 2007, 90, E17–E38. [Google Scholar] [CrossRef] [PubMed]
- Nocek, J.E. Bovine acidosis: implications on laminitis. J. Dairy Sci. 1997, 80, 1005–1028. [Google Scholar] [CrossRef] [PubMed]
- Russell, J.B.; Sharp, W.M.; Baldwin, R.L. The effect of pH on maximum bacterial growth rate and its possible role as a determinant of bacterial competition in the rumen. J. Anim. Sci. 1979, 48, 251–255. [Google Scholar] [CrossRef]
- Therion, J.T.; Kistner, A.; Kornelius, J.H. Effect of pH on growth rates of rumen amylolytic and lactilytic bacteria. Appl. Environ. Microbiol. 1982, 44, 428–434. [Google Scholar] [CrossRef]
- Waldrip, H.M.; Martin, S.A. Effects of an Aspegillus oryzae fermentation extract and other factors on lactate utilization by the ruminal bacterium Megasphaera elsdenii. J. Anim. Sci. 1993, 71, 2770–2776. [Google Scholar] [CrossRef]
- Noyes, N.R.; Yang, X.; Linke, L.M.; Magnuson, R.J.; Cook, S.; Zaheer, R.; Yang, H.; Woerner, D.R.; Geornaras, I.; Mcart, J.A.; et al. Characterization of the resistome in manure, soil and wastewater from dairy and beef production systems. Sci. Reports 2016, 6, 24645. [Google Scholar] [CrossRef] [PubMed]
- Cameron, A.; McAllister, T.A. Antimicrobial usage and resistance in beef cattle production. J. Anim. Sci. Biotechnol. 2016, 7, 1–22. [Google Scholar] [CrossRef] [PubMed]
- El Akkad, I.; Hobson, P. Effect of antibiotics on some rumen and intestinal bacteria. Nature. 1966, 209, 1046–1047. [Google Scholar] [CrossRef]
- Fulghum, R.S.; Baldwin, B.B.; Williams, P.P. Antibiotic susceptibility of anaerobic ruminal bacteria. Appl. Microbiol. 1968, 16, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Flint, H.J.; Duncan, S.H.; Bisset, J.; Stewart, C.S. The isolation of tetracycline-resistant strains of strictly anaerobic bacteria from the rumen. Lett. Appl. Microbiol. 1988, 5, 113–115. [Google Scholar] [CrossRef]
- Nagaraja, T.G.; Taylor, M.B. Susceptibility and resistance of ruminal bacteria to antimicrobial feed additives. Appl. Environ. Microbiol. 1987, 53, 1620–1625. [Google Scholar] [CrossRef] [PubMed]
- Wang, C. L.; Baldwin, B.B.; Fulghum, R.S.; Williams, P.P. Quantitative antibiotic sensitivities of ruminal bacteria. Appl. Microbiol. 1969, 18, 677–679. [Google Scholar] [CrossRef]
- Nelson, R.S.; Peterson, D.J.; Karp, E.M.; Beckham, G.T.; Salvachua, D. Mixed carboxylic acid production by Megasphaera elsdenii from glucose and lignocellulosic hydrolysate. Fermentation 2017, 3, 10. [Google Scholar] [CrossRef]
- Russell, J.B. Another explanation for the toxicity of fermentation acids at low pH: anion accumulation versus uncoupling. J. Appl. Microbiol. 1992, 73, 363–370. [Google Scholar] [CrossRef]
- Wolin, M.J. The rumen fermentation: a model for microbial interactions in anaerobic ecosystems. Adv. Microbial Physiol., 1979, 3, 49–77. [Google Scholar] [CrossRef]
- Weimer, P.J. Redundancy, resilience, and host specificity of the ruminal microbiota: implications for engineering improved ruminal fermentations. Front. Microbiol. 2015, 6, 296. [Google Scholar] [CrossRef] [PubMed]
- Russell, J.B.; Cotta, M.A.; Dombrowski, D.B. Rumen bacteria competition in continuous culture: Streptococcus bovis versus Megasphaera elsdenii. Appl. Environ. Microbiol. 1981, 41, 1394–1399. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Shen, Y.; Wang, C.; Ding, L.; Zhao, F.; Wang, M.; Fu, J.; Wang, H. Megasphaera elsdenii lactate degradation pattern shifts in rumen acidosis models. Front. Microbiol. 2019, 10, 162. [Google Scholar] [CrossRef]
- Kung, L.J.; Hessian, A.O. Preventing in vitro lactate accumulation in ruminal fermentations by inoculation with Megasphaera elsdenii. J. Anim. Sci. 1995, 73, 250–256. [Google Scholar] [CrossRef]
- Ricke, S.C.; Martin, S.A.; Nisbet, D.J. Ecology, metabolism, and genetics of ruminal selenomonads. Crit. Rev. Microbiol. 1996, 22, 27–65. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, K.; Yamada-Narita, S.; Abe, N.; Onodera, T.; Eiichiro Kan, E.; Kojima, S.; Miyazaki, T.; Yamamoto, Y.; Oguchi, A.; Ankai, A.; et al. Complete genome sequence of Selenomonas ruminantium subsp. lactilytica will accelerate further understanding of the nature of the class Negativicutes. FEMS Microbiology Letters 2015, 362, fnv050. [Google Scholar] [CrossRef]
- Fan, Y.; Xia, G.; Jin, Y.; Wang, H. Ambient pH regulates lactate catabolism pathway of the ruminal Megasphaera elsdenii BE2-2083 and Selenomonas ruminantium HD4. J. Appl. Microbiol. 2022, 132, 2661–2672. [Google Scholar] [CrossRef]
- Baldwin, R.L.; Wood, W.A.; Emery, R.S. Conversion of lactate-C14 to propionate by the rumen microbiota. J. Bacteriol. 1962, 83, 907–913. [Google Scholar] [CrossRef]
- Baldwin, R.L.; Wood, W.A.; Emery, R.S. Conversion of glucose-C14 to propionate by the rumen microbiota. J. Bacteriol. 1963, 85, 1346–1349. [Google Scholar] [CrossRef]
- Sarmikasoglou, E.; Ferrell, J.; Vinyard, J.R.; Flythe, M.D.; Tuanyok, A.; Faciola, A.P. Effects of ruminal lipopolysaccharides on growth and fermentation end products of pure cultured bacteria. Sci. Reports 2023, 12, 15932. [Google Scholar] [CrossRef]
- Bandarupalli, V.V.K.; St-Pierre, B. Metagenomics-based analysis of candidate lactate utilizers from the rumen of beef cattle. Microorganisms 2023, 11, 658. [Google Scholar] [CrossRef] [PubMed]
- Miura, H.; Horguchi, M.; Matsumoto, T. Nutritional interdependence among rumen bacteria, Bacteroides amylophilus, Megasphaera elsdenii, and Ruminococcus albus. Appl. Environ. Microbiol. 1980, 40, 294–300. [Google Scholar] [CrossRef] [PubMed]
- Shabat, S.K.B.; Sasson, G.; Doron-Faigenboim, A.; Durman, T.; Yaacoby, S.; Miller, M.E.B.; White, B.A.; Shterzer, N.; Mizrahi, I. Specific microbiome-dependent mechanisms underlie the energy harvest efficiency of ruminants. ISME J. 2016, 10, 2958–2972. [Google Scholar] [CrossRef] [PubMed]
- Betancour-Murillo, C.L.; Aguilar-Marin, S.B.; Jovel, J. Prevotella: A key player in ruminal metabolism. Microorganisms. 2022, 11, 18. [Google Scholar] [CrossRef] [PubMed]
- Jewell, K.A.; McCormick, C.; Odt, C.L.; Weimer, P.J.; Suen, G. Ruminal bacterial community composition in dairy cows is dynamic over the course of multiple lactations and correlates with feed efficiency. Appl. Environ. Microbiol. 2015, 81, 4697–4710. [Google Scholar] [CrossRef] [PubMed]
- Weimer, P.J.; Cox, M.S.; Vieira de Paula, T.; Lin, L.; Hall, M.B.; Suen, G. Transient changes in milk production efficiency and bacterial community composition resulting from near-total exchange of ruminal contents between high- and low-efficiency Holstein cows. J. Dairy Sci. 2017, 100, 7165–7182. [Google Scholar] [CrossRef] [PubMed]
- Bauman, D.E.; Griinari, J.M. Nutritional regulation of milk fat synthesis. Annu. Rev. Nutr. 2003, 23, 203–27. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, T.C.; Wallace, R.J.; Moate, P.J.; Mosley, E.E. Board-invited review: Recent advances in biohydrogenation of unsaturated fatty acids within the rumen microbial ecosystem. J Anim. Sci. 2008, 86, 397–412. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Liu, R.H.; Bond, D.; Russell, J.B. Effect of linoleic acid concentration on the conjugated linoleic acid (CLA) production of Butyrivibrio fibrisolvens A38. Appl. Environ. Microbiol. 2000, 66, 5226–5230. [Google Scholar] [CrossRef]
- Kim, Y.J.; Liu, R.H.; Rychlik, J.L.; Russell, J.B. Enrichment of a ruminal bacterium (Megasphaera elsdenii YJ-4) that produces the trans-10, cis-12 isomer of conjugated linoleic acid (CLA). J. Appl. Microbiol. 2002, 92, 976–982. [Google Scholar] [CrossRef]
- Kim, T.W.; Choi, N.J.; Hwangbo, J.; Hsu, J.T.; Lee, S.S.; Song, M.K.; Seo, L.J.; Kim, Y.J. Production of trans-10, cis-12 conjugated linoleic acid by Megasphaera elsdenii YJ-4: physiological roles in the rumen. Asian-Austr. J. Anim. Sci 2005, 18, 1425–1429. [Google Scholar] [CrossRef]
- Muya, M.C.; Nherera, F.V.; Miller, K.A.; Aperce, C.C.; Moshidi, P.M.; Erasmus, L.J. Effect of Megasphaera elsdenii NCIMB 41125 dosing on rumen development, volatile fatty acid production and blood β-hydroxybutyrate in neonatal dairy calves. J. Anim. Physiol. Anim. Nut. 2015, 99, 913–918. [Google Scholar] [CrossRef] [PubMed]
- Susanto, I.; Wiryawab, K.G.; Suharti, S.; Retnani, Y.; Zahera, R. Jayanegara. Evaluation of Megasphaera elsdenii supplementation on rumen fermentation, production performance, carcass traits and health of ruminants: a meta-analysis. Anim. Biosci. 2023, 36, 879–890. [Google Scholar] [CrossRef] [PubMed]
- Robinson, J.A.; Smolenski, W.J.; Greening, R.C.; Ogilvie, M.L.; Bell, R.L.; Barsuhn, K.; Peters, J.P. Prevention of acute acidosis and enhancement of feed intake in the bovine by Megasphaera elsdenii 407A. J. Anim. Sci 1992, 70, 310 (Abstract). [Google Scholar]
- McDaniel, M.R. The effects of dosing feedlot cattle with Megasphaera elsdenii strain NCIMB 41125 prior to the introduction of a grain rich diet. M.S. Thesis, Kansas State University, 2009. https://krex.k-state.edu/bitstream/handle/2097/1666/MichaelMcDaniel2009.pdf;sequence=1.
- Leeuw, K.J.; Siebrits, F.K.; Henning, P.H.; Meissner, H.H. Effect of Megasphaera elsdenii NCIMB 41125 drenching on health and performance of steers fed high and low roughage diets in the feedlot. S. Afr. J. Anim. Sci. 2009, 39, 337–348. [Google Scholar] [CrossRef]
- Henning, P.H.; Horn, C.H.; Steyn, D.G.; Meissner, H.H.; Hagg, F.M. The potential of Megasphaera elsdenii isolates to control ruminal acidosis. Anim. Feed Sci. Technol. 2010, 157, 13–19. [Google Scholar] [CrossRef]
- Henning, P.H.; Horn, C.H.; Leeuw, K.J.; Meissner, H.H.; Hagg, F.M. Effect of ruminal administration of the lactate-utilizing strain Megasphaera elsdenii (Me) NCIMB 41125 on abrupt or gradual transition from forage to concentrate diets. Anim. Feed Sci. Technol. 2010, 157, 20–29. [Google Scholar] [CrossRef]
- Aikman, P.C.; Henning, P.H.; Humphries, D.J.; Hom, C.H. Rumen pH and fermentation characteristics in dairy cows supplemented with Megasphaera elsdenii NCIMB 41125 in early lactation. J. Dairy Sci. 2011, 94, 2840–2849. [Google Scholar] [CrossRef]
- Zebeli, Q.; Terrill, S.J.; Mazzolari, A.; Dunn, S.M.; Yang, W.Z.; Ametaj, B.N. Intraruminal administration of Megasphaera elsdenii modulated rumen fermentation profile in mid-lactation dairy cows. J. Dairy Res. 2012, 79, 16–25. [Google Scholar] [CrossRef]
- Klieve, A.V.; McLennan, S.R.; Ouwerkerk, D. Persistence of orally administered Megasphaera elsdenii and Ruminococcus bromii in the rumen of beef cattle fed a high grain (barley) diet. Anim. Prod. Sci. 2012, 52, 297–304. [Google Scholar] [CrossRef]
- Drouillard, J.S.; Henning, P.H.; Meissner, H.H.; Leeuw, K.J. Short communication: Megasphaera elsdenii on the performance of steers adapting to a high-concentrate diet, using three or five transition diets. S. Afr. J. Anim. Sci. 2012, 42, 195–199. [Google Scholar] [CrossRef]
- Ye, D.; Eastridge, M.L. Oral administration of Megasphaera elsdenii to Jersey cows during early lactation. Profess. Anim. Sci. 2018, 34, 67–74. [Google Scholar] [CrossRef]
- Arik, H.D.; Gulsen, N.; Hayirli, A.; Alatas, M.S. Efficacy of Megasphaera elsdenii inoculation in subacute ruminal acidosis in cattle. J. Anim. Physiol. Anim. Nutr. 2018, 103, 416–426. [Google Scholar] [CrossRef] [PubMed]
- Sedighi, R.; Alipour, D. Assessment of probiotic effects of isolated Megasphaera elsdenii strains in Mehraban sheep and Holstein lactating cows. Anim. Feed Sci. Technol. 2019, 248, 126–131. [Google Scholar] [CrossRef]
- DeClerck, J.C.; Reeves, N.R.; Miller, M.F.; Johnson, B.J.; Ducharme, G.A.; Rathmann, R.J. Influence of dietary roughage level and Megasphaera elsdenii on feedlot performance and carcass composition of thin cull beef cows fed for a lean market. Transl. Anim. Sci. 2020, 29, 159–169. [Google Scholar] [CrossRef] [PubMed]
- DeClerck, J.C.; Wade, Z.E.; Reeves, N.R.; Miller, M.F.; Johnson, B.J.; Ducharme, G.A.; Rathmann, R.J. Influence of Megasphaera elsdenii and feeding strategies on feedlot performance, compositional growth, and carcass parameters of early weaned, beef calves. Transl. Ani. Sci. 2020, 4, 863–875. [Google Scholar] [CrossRef]
- Mazon, G.; Campler, M.R.; Holcomb, C.; Bewley, J.M.; Costa, J.H.C. Effects of a Megasphaera elsdenii oral drench on reticulorumen pH dynamics in lactating dairy cows under subacute ruminal acidosis challenge. Anim. Feed Sci. Technol. 2020, 261, 1–9. [Google Scholar] [CrossRef]
- Lopes, A.L.; Santos, F.A.P.; Meschiatti, M.; Oliveira, M.O.; Fernandes, J.J.R.; Drouillard, J.S.; Cappellozza, B.I. Effects of Megasphaera elsdenii administration on performance and carcass traits of finishing Bos indicus feedlot cattle. Transl. Anim. Sci. 2021, 28, 1–9. [Google Scholar] [CrossRef]
- Diez-Gonzalez, F.; Callaway, T.R.; Kizoulis, M.G.; Russell, J.B. Grain feeding and the dissemination of acid-resistant Escherichia coli from cattle. Science. 1998, 281, 1666–1668. [Google Scholar] [CrossRef]
- Maher, J.M.; Drouillard, J.S.; Baker, A.N.; Veloso, V. d.A.; Kang, Q.; Kasstner, J.J.; Gregg, S.E. Impact of the probiotic organism Megasphaera elsdenii on Escherichia coli O157:H7 prevalence in finishing cattle. J. Food Protection 2023, 86, 100133. [Google Scholar] [CrossRef]
- Jeon, B.S.; Kim, S.; Sang, B.I. Megasphaera hexanoica sp. nov., a medium-chain carboxylic acid-producing bacterium isolated from a cow rumen. Int. J. Syst. Evol. Microb. 2017, 67, 2114–2120. [Google Scholar] [CrossRef] [PubMed]
- Lanjekar, V.B.; Marathe, N.P.; Ramana, V.V.; Shouche, Y.S.; Ranade, D.R. Megasphaera indica sp. nov., an obligate anaerobic bacteria isolated from human faeces. Int. J. Syst. Evol. Microbiol. 2014, 64, 2250–2256. [Google Scholar] [CrossRef] [PubMed]
- Holtzapple, M.T.; Lonkar, S.; Granda, C. Producing biofuels via the carboxylate platform. Chem. Eng. Progr. 2015, 111, 52–57. [Google Scholar]
- Weimer, P.J.; Digman, M.F. Fermentation of alfalfa wet-fractionation liquids to volatile fatty acids by Streptococcus bovis and Megasphaera elsdenii. Biores. Technol. 2013, 142, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, S.; Araoka, R.; Kajihara, Y.; Ito, T.; Miyamoto, H.; Kodama, H. Valerate production from Megasphaera elsdenii isolated from pig feces. J. Biosci. Bioeng. 2018, 125, 519–534. [Google Scholar] [CrossRef] [PubMed]
- Roddick, F.A.; Britz, F.A. Production of hexanoic acid by free and immobilised cells of Megasphaera elsdenii: influence of in-situ product removal using ion exchange resin. J. Chem. Technol. Biotechnol. 1997, 69, 383–391. [Google Scholar] [CrossRef]
- Choi, K.; Jeon, BS.; Kim, B.C.; Oh, M.K.; Um, Y.; Sang, B.I. In situ biphasic extractive fermentation for hexanoic acid production from sucrose by Megasphaera elsdenii NCIMB 702410. Appl. Biochem. Biotechnol. 2013, 171, 1094–1107. [Google Scholar] [CrossRef] [PubMed]
- Jeon, B.S.; Choi, O.; Um, Y.; Sang, B.I. Production of medium-chain carboxylic acids by Megasphaera sp. MH with supplemental electron acceptors. Biotechnol. Biofuels 2016, 9, 129. [Google Scholar] [CrossRef]
- Fernández-Blanco, C.; Veiga, M.C.; Kennes, C. Effect of pH and medium composition on chain elongation with Megasphaera hexanoica producing C4-C8 fatty acids. Front. Microbiol. 2023, 14, 1281103. [Google Scholar] [CrossRef]
- Lange, J.P.; Price, R.; Ayoub, P.M.; Louis, J.; Petrus, L.; Clarke, L.; Gosselink, H. Valeric biofuels: a platform of cellulosic transportation fuels. Angew. Chem. Int. Ed. 2010, 49, 4479–4483. [Google Scholar] [CrossRef]
- Urban, C.; Xu, J.; Strauber, H.; Dantas, T.R.S.; Muhlenberg, J.; Hartig, C.; Angenent, L.T.; Harnisch, F. Production of drop-in fuels from biomass at high selectivity by combined microbial and electrochemical conversion. Energy Environ. Sci. 2017, 10, 2231–2244. [Google Scholar] [CrossRef]
- Scarborough, M.J.; Lynch, G.; Dickson, M.; McGee, M.; Donohue, T.J.; Noguera, D.R. Increasing the economic value of lignocellulosic stillage through medium-chain fatty acid production. Biotech. Biofuels. 2018, 11, 200. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Yan, B.; Xu, S.; Zhou, J.; Liang, J.; Zhao, J., Tyangi; Wong, J.W.C. Regulation of acidogenic fermentation through exogenous additives for promoting carbon conversion of food waste in two-phase anaerobic system. Biores. Technol. 2023, 368, 128368. [Google Scholar] [CrossRef] [PubMed]
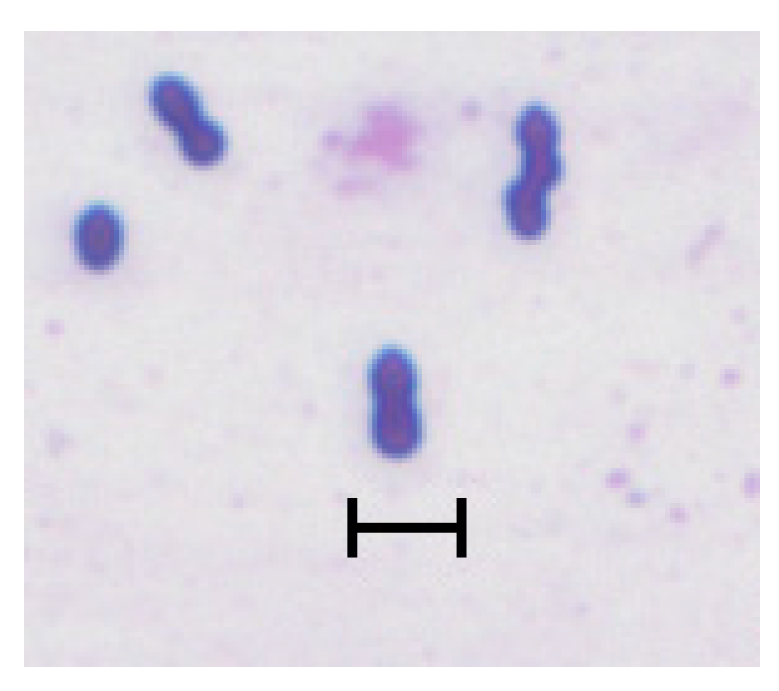
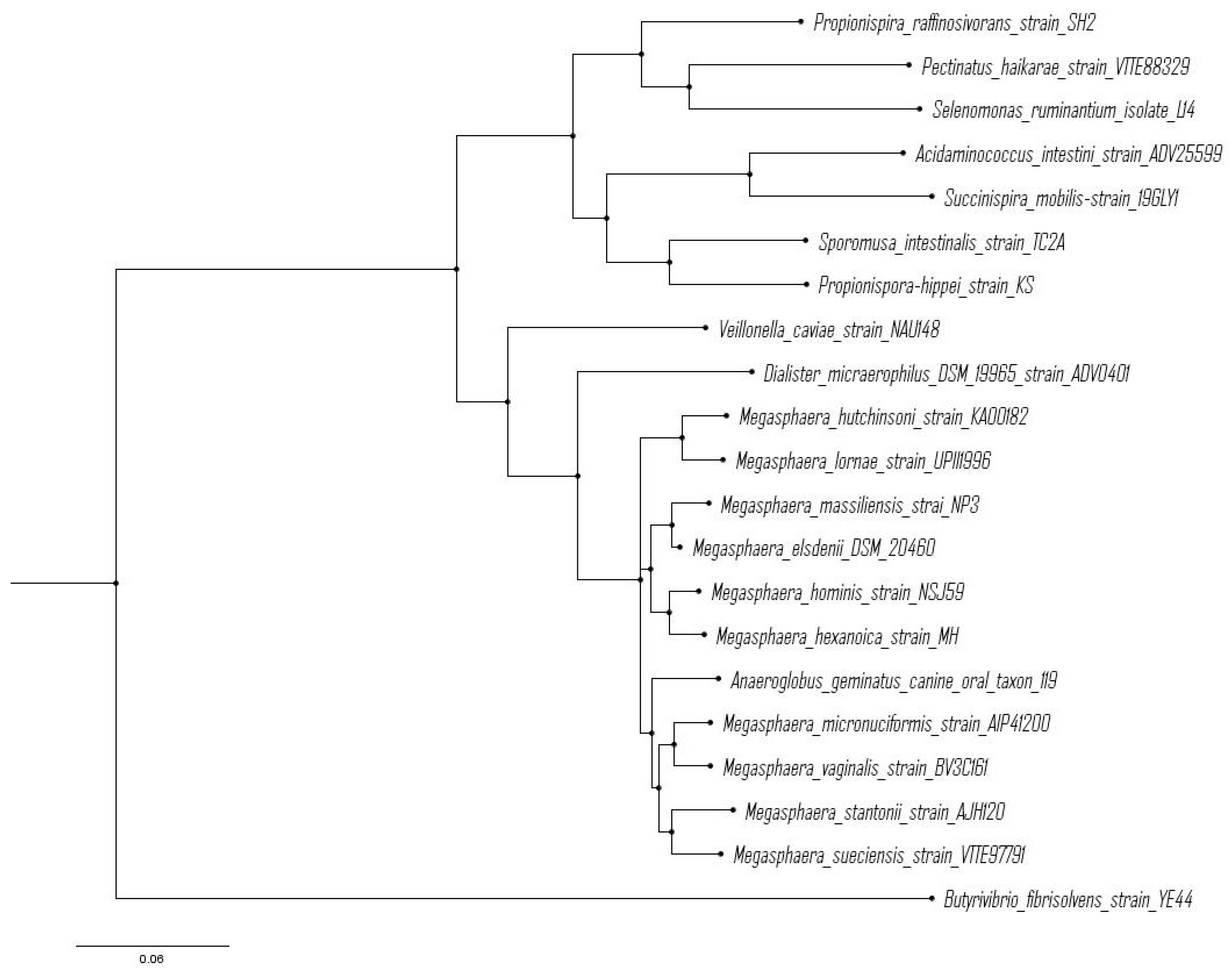

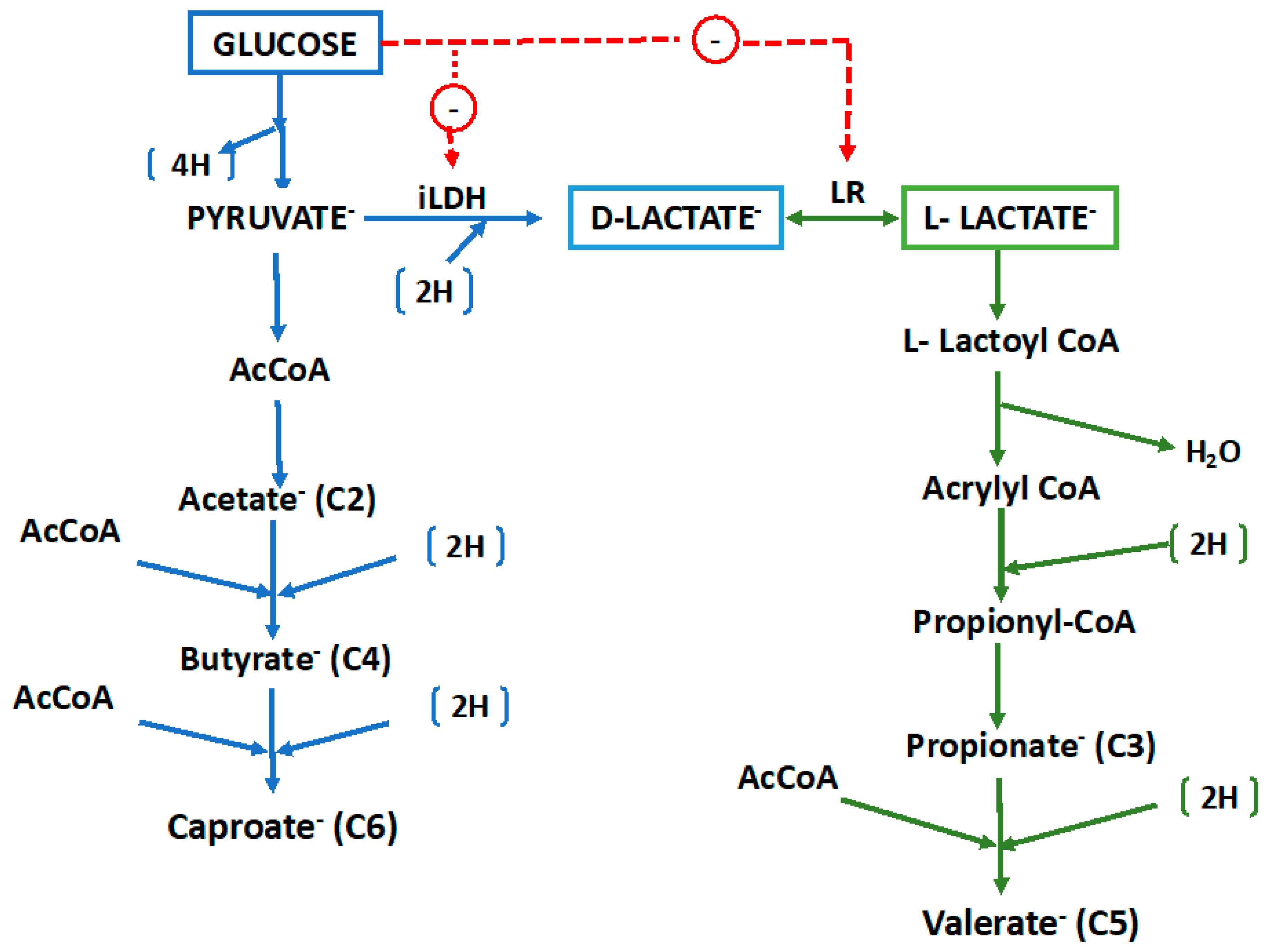
| Strain | Source | Genome size (bp) | Putative number of genes | Average G+C content | Reference |
|---|---|---|---|---|---|
| DSM 20640T a | Rumen | 2,474,718 | 2220 | 54 | [22] |
| ATCC 25940 a | Rumen | 2,478,842 | 2194 | 52.8 | [23] |
| NCIMB 702410 | Rumen | 2,566,193 | 2280 | 52.7 | [23] |
| indica | Human feces | 2,429,033 | 2184 | 53.2 | [24] |
| Organism designation | Comparison | Counts/mL | Reference |
|---|---|---|---|
| Large coccus | Young calves (3-9weeks) Adult cattle and sheep |
109 to 109 absent to 105 |
[27] |
| Large coccus | Before bloat Moderate bloat |
< 1 x104 to 2 x107 2 x 106 to 2 x108 |
[18] |
| Total lactate fermenting bacteria | Forage diet High grain diets |
4.8 x 106 1.89 x 1011 |
[30] |
| Animal | n | % RA a | Method b | Notes | Reference |
|---|---|---|---|---|---|
| Holstein cows | 2 | 0.0001 - 0.0011 | qPCR | TMR, 27.5% NDF, 18% CP | [35] |
| Holstein cows | 2 | 0.002 - 0.40 | qPCR | High concentrate diet. RA declined upon monensin feeding and withdrawal | [36] |
| Holstein cows | 2 2 |
0.018 - 0.032 0.320 - 1.946 |
qPCR | Cows not fat-depressed Fat-depressed cows |
[37] |
| Holstein cows | 16 | 0.03 - 0.01 | qPCR | 30% forage/70% concentrate diet | [38] |
| Holstein calf to adult | 5 | 0.0003 - 0.10 | 16S 454 | Calf development study, diet varied over 5 time points from 1 d to 730 d age | [39] |
| Holstein cows | 8 | 0.0001- 0.0005 0.0025 - 0.0038 |
qPCR | Control or after recovery from MFD After induction of MFD |
[40] |
| Holstein heifer calves | 6 | 0 to 0.70 | 16S Illumina | Dosed with M. elsdenii probiotic at d14; RA declined to below uninoculated controls by d84 | [41] |
| Crossbred steers | 6 | 0.02 to 0.10 | 16S Illumina | 6 diets varying in oilseed source, sampled 14 d after each dietary switch | [42] |
| Holstein bull calves (post-weaning) | 5 5 |
0.379 0.790 |
RNA-seq | Inoculated with protozoal enrichment culture Uninoculated controls |
[43] |
| Strain | Time (h) | From Glucose | From Lactate | Reference | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Si d (mM) | Mol % product e | Si d (mM) | Mol % product e | |||||||||||||
| For (C1) |
Ace (C2) | Pro (C3) | But (C4) | Val (C5) |
Cap (C6) | For (C1) |
Ace (C2) | Pro (C3) | But (C4) | Val (C5) |
Cap (C6) | |||||
| LC | nra | nr | 26.7 | 22.4 | 22.4 | 28.6 | [18] | |||||||||
| LC-3 | 168 | 27.8 | 4.0 | 7.2 | 1.8 | 14.3 | 11.1 | 61.6 | 62 | 0 | 18.7 | 32.0 | 21.6 | 27.7 | 0 | [17] |
| LC-S | nra | 12.9 | 22.1 | 26.3 | 22.1 | 27.9 | 1.6 | [17] | ||||||||
| nra | nra | 55.5 | + | 33.9 | 1.3 | 52.1 | 6.7 | 5.9 | 111 | 35.5 | 38.2 | 13.7 | 12.6 | - | [27] | |
| B159 b | 72 | 27.8 | 20.4 | - | 77.5 | 2.0 | + | 17.7 | 31.3 | 37.5 | 12.5 | 18.8 | - | [52] | ||
| L8 | 20 | 10 | 27.9 | - | - | 48.6 | 2.7 | 20.7 | 20 | - | 29.7 | 30.6 | 18.0 | 21.6 | - | [59] |
| LC-1 | 4.2 | - | - | 59.4 | 19.8 | 16.7 | - | 51.3 | 38.0 | 6.8 | 3.8 | - | ||||
| J1 | 19.4 | - | - | 37.4 | 21.6 | 21.6 | - | 24.3 | 27.4 | 39.4 | - | 8.8 | ||||
| AW106 | 0.7 | 29.2 | - | 47.4 | 11.7 | 10.9 | 9.9 | 40.1 | 29.2 | 7.0 | 4.7 | - | ||||
| NIAH102 | 10 | 11.1 | 32.0 | - | 61.5 | 0.6 | 6.0 | [63] | ||||||||
| T81 c | nr | 35 | 43.1 | 50.4 | 3.4 | [66] | ||||||||||
| T81 c | 48 | 41 | - | 16.4 | - | 45.5 | 0.7 | 37.3 | 100 | 36.8 | 37.4 | 13.1 | 10.7 | 0.1 | [49] | |
| Property | M. elsdenii | S. ruminantium lactilytica |
| Phylum | Firmicutes | Firmicutes |
| Class | Clostridia | Negativicutes |
| Gram reaction | Negative | Positive |
| Motility | Nonmotile | Motile |
| Genome | Single chromosome, ~2.5 Mbp | Single chromosome 3,003,680 bp, 9 plasmids 2619 -285,449 bp. Total genome size 3,631,933 bp |
| Preferred growth substrate | D- or L-Lactate | D-lactate |
| Other growth substrates | Glucose, Fructose, Maltose, Amino acids | Glucose, Cellobiose, Mannose, Xylose, Arabinose, Mannitol, Glycerol |
| Lactate fermentation pathway | Acrylate | Succinate |
| Lactate utilization enzymes | NAD-independent L-lactate dehydrogenase, Lactate racemase | NAD-independent D-lactate dehydrogenase. No L-LDH, No Lactate racemase |
| VFA fermentation productsa | From lactate, C2, C3, C4, C5 From glucose: C2, C4, C6 |
From lactate: C2, C3 From glucose: Lactate, Acetate |
| Response to monensin | Relatively resistant | Sensitive |
| mb on glucose | 0.187 | 0.021 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





