1. Introduction
Drought stress poses a persistent challenge in agriculture [
1], resulting in diminished crop yields and substantial economic losses globally [
2]. In response to the escalating aridity, plants undergo a cascade of morphological, physiological, and molecular transformations [
3,
4]. Notably, diverse plant species exhibit distinct adaptations to drought stress, involving intricate mechanisms such as abscisic acid biosynthesis regulation [
5,
6], adjustments in stomatal density [
7], and protein modifications [
8]. Signal pathways specific to different plants responding to drought stress encompass characteristic features, including phenylalanine and flavonoid metabolic pathways [
9,
10], as well as ABA metabolism and signaling pathways [
11,
12,
13]. Understanding how plants respond to drought stress is pivotal for the development of drought-tolerant varieties.
Basil (
Ocimum basilicum L.), an annual herb plant belonging to the Lamiaceae family, has gained prominence due to its versatility as a widely cultivated aromatic plant. Basil leaves are integral to Western cuisine [
14], also boast pharmaceutical properties [
15,
16]. Abundant in volatile oils such as linalool, eucalyptol, and eugenol [
17], basil essential oil exhibits calming and soothing effects on the nervous system, along with anti-inflammatory, antimicrobial, and insect-repelling activities [
18,
19,
20].
Originating from tropical Asia, bail thrives in warm and arid environments, with temperature and water stress posing significant challenges to its growth [
21]. While plants respond to drought stress by modulating gene expression and metabolite content [
22,
23], comprehensive omics studies on basil under drought conditions are limited. Although de novo transcriptome sequencing of basil leaves under various stresses has been conducted [
24], elucidating the molecular mechanisms of drought tolerance remains elusive.
To gain deeper insights into the impact of drought stress on sweet basil and unravel the associated molecular mechanisms, we conducted an experiment using sweet basil as the experimental material. Potted seedlings subjected to drought stress by withholding water, and their leaves were subsequently analyzed both transcriptomically and metabolomically. The outcomes of this study are anticipated to unveil drought-responsive genes and metabolites, providing theoretical support for molecular breeding and cultivation practices aimed at enhancing basil’s drought tolerance.
2. Materials and Methods
2.1. Plants and Drought Treatment
The experimental material comprised sweet basil. The cultivation process involved planting seeds into 32-hole trays, and two weeks after germination, the seedings were transferred to 12 cm diameter pots. The nutrient-rich soil used for cultivation included a mixture of clay at a 3:2 ratio. The seedlings were nurtured in an artificial climate chamber, maintaining a light intensity of 12000 lux at 25 °C from 7:00 to 19:00 daily, followed by darkness at 23 °C for the remaining hours. Pinching out the tops of the plants occurred when six true leaves appeared. To initiate the drought treatment, regular watering of the soil continued until the plants reached approximately 11 cm in length. Soil water content (SWC) was monitored using a moisture detector (Horde Electronic Soil Moisture Detector, China). The maximum SWC observed equated to fully watered pots (FW). Based on the relative SWC, the degree of drought stress was categorized as follows: control (CK) with 70–75% of fully watered content, mild drought (LD) with 50–55% of fully watered content, moderate drought (MD) with 30–35% of fully watered content, and severe drought (SD) with 10%–15% of fully watered content. Each SWC treatment group comprised ten pots.
2.2. Volatile Metabolic profiling analysis
Basil leaves were ground into a powder using liquid nitrogen, and three replicates of each assay were conducted. The volatile compounds were analyzed by Wuhan Metware Biotechnology Co., Ltd. (Wuhan, China) on the Agilent 8890-7000D platform. For this analysis, 500 mg of each sample was weighed in a 20 mL headspace bottle with saturated NaCl solution. The samples were subjected to shaking at 60 °C for 5 minutes, and a 120 μm divinylbenzene/carboxen/polydimethylsiloxane fiber filter was exposed to the headspace to absorb volatiles for 15 minutes. Subsequently, GC-MS separation and identification were performed after analysis at 250 °C for 5 minutes.
Volatile organic compounds (VOCs) were identified and quantified using an 8890 GC and 7000 DMS system (Agilent, USA) equipped with a 30m × 0.25mm × 0.25μm DB-5MS capillary column. Helium gas at 1.2 mL/minute served as the carrier gas and was programmed to begin at 40 °C (3.5 minutes), increase by 10 °C/minute to 100 °C, 7 °C/minute to 180 °C, 25 °C/minute to 280 °C, and maintain 280 °C for 5 minutes. Mass spectrometry (MS) employed a 70 eV electron impact ionization mode. Temperatures for the quadrupole mass detector, ion source, and transfer line were set at 150 °C, 230 °C, and 280 °C, respectively. To identify volatile compounds, mass spectra were compared with NIST data and linear retention index.
2.3. Transcriptome sequencing
Total RNA from basil leaves was isolated using Plant RNA isolation kits (Thermo Fisher, MA). The RNA libraries for CK, LD, MD, and SD samples were prepared and sequenced, with three biological replicates conducted for each variety. The cDNA library ws constructed and sequenced by Wuhan Metware Biotechnology Co., Ltd. (Wuhan, China).
Following the removal of poly-N and low-quality raw reads, the clean reads were assembled into expressed sequence tag clusters (contigs). De novo transcripts were constructed, and Q30 and GC contents were calculated using Trinity [
24]. Htseq counts were employed to determine read counts for each gene, and Cufflinks software was used to calculate fragments per kilobase million (FPKM).
2.4. Bioinformatics Analysis
To identify significantly regulated VOCs between each group, criteria of variable importance in projection (VIP) ≥ 1 and absolute Log2FC(fold change) ≥ 1 were applied. The screening process was facilitated by the MetaboAnalystR package, extracting VIP values from Orthogonal Partial Least Squares Discriminant Analysis (OPLS-DA) results, which also encompassed score plots and permutation plots. Prior to OPLS-DA, the data underwent log transformation (log2) and mean centering. A total of 200 permutation tests were conducted to prevent overfitting.
Differentially expressed VOCs and DEGs were defined as described previously [
25]. Unigenes underwent annotation with GO terms using the (
http://www.geneontology.org) platform and were further analyzed with Blast2GO. The KEGG database was employed to annotate KEGG pathways, utilizing Blastall software for this purpose.
2.5. Physiological measurements
The Alpha-linolenic acid content in basil leaves was assessed through GC-MS [
26]. Quantification of flavonoid content in the samples was conducted using HPLC [
27]. The endogenous lignin content was quantified employing a thioglycolic acid lignin method [
28].
All data were analyzed and compiled using Excel 2010. Data variance was calculated utilizing SPSS27 software, and statistical significance was assessed through Duncan's multiple comparison method (p < 0.05).
3. Results
3.1. Volatile metabolome analysis of sweet basil under four different gradient drought stresses
To explore the impact of drought stress on the VOCs in sweet basil, volatile metabolic profiling of sweet basil leaves was conducted under four gradient drought stress conditions. Physiological changes in sweet basil under these stress conditions are depicted in
Figure 1. A total of 830 VOCs were identified (
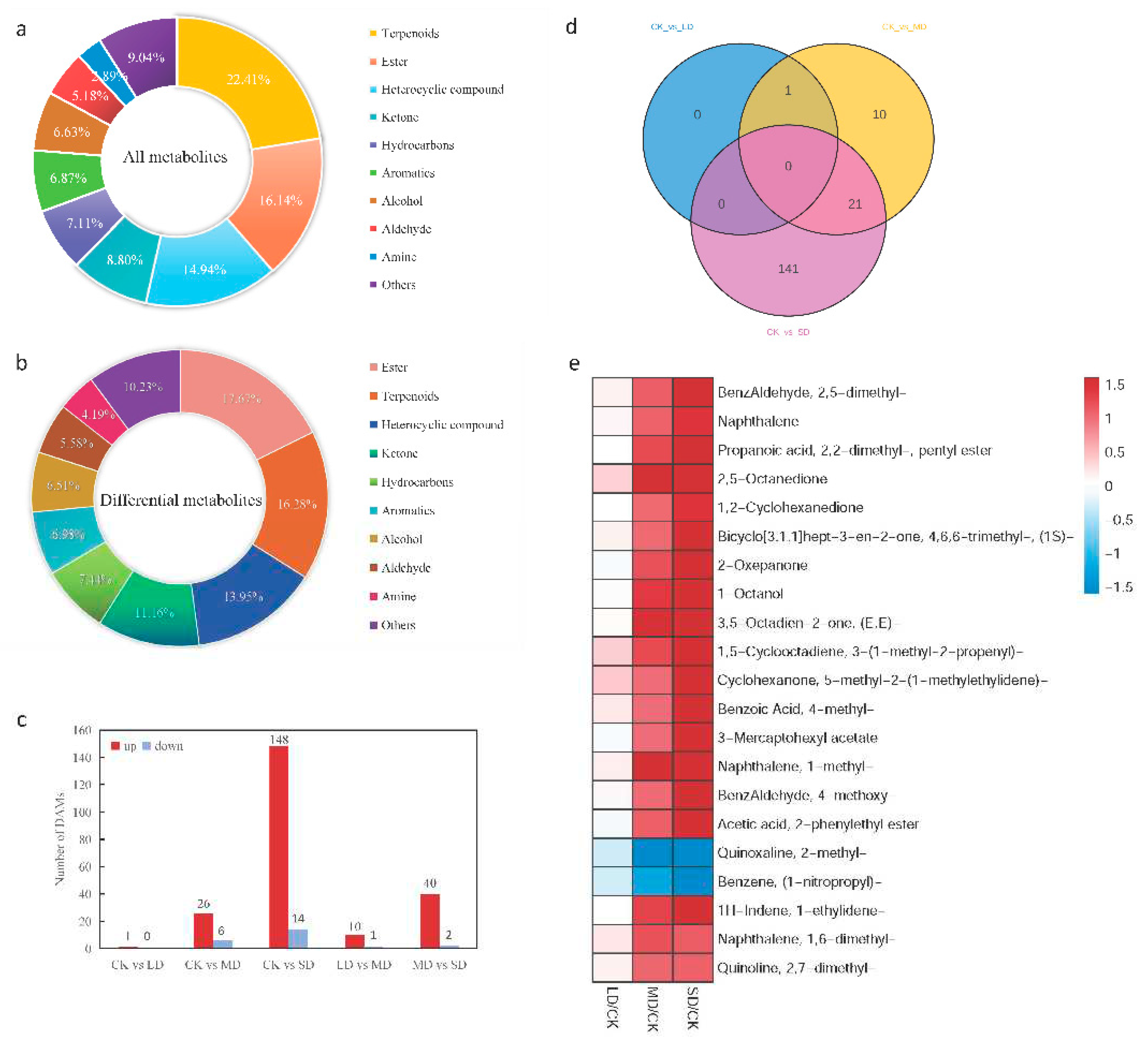
Supplementary Table S1), with linalool, eucalyptol, and eugenol representing a substantial proportion. The categorized VOCs included terpenoids (22.41%), esters (16.14%), heterocyclic compounds (14.94%), ketones (8.8%), hydrocarbons (7.11%), aromatics (6.87%), alcohols (6.63%), aldehydes (5.18%), amines (2.89%), and other compounds (9,04%), where terpedoids and esters collectively constituted 38.55% of the VOCs (
Figure 2a).
To analyze the dynamic changes of basil under drought stress, Principal Component Analysis (PCA) was performed. As shown in
Supplementary Figure S1, some basil samples under CK and LD treatments clustered together, while the separation of other samples indicated significant changes in metabolites.
OPLS-DA was employed to identify differential metabolites by extracting components of the independent variable X and dependent variable Y and calculating correlations between them. The results revealed R
2X values higher than 0.93, R
2Y scores higher than 0.99, and Q2 values higher than 0.52 in the CK vs. LD, LD vs. MD, and MD vs. SD samples, respectively. This confirmed the presence of differential metabolites responsive to drought (
Supplementary Figure S2).
For further elucidation of VOCs accumulating differentially in response to drought stress, LC-MS was utilized for volatile metabolic analysis. Metabolites with VIP values > 1 and p < 0.05 were considered significantly differentially accumulated. A total of 215 differentially accumulated metabolites were identified (
Supplementary Table S2), categorized as esters (17.64%), terpenoids (16.28%), heterocyclic compounds (13.95%), ketones (11.16%), hydrocarbons (7.44%), aromatics (6.98%), alcohols (6.51%), aldehydes (5.58%), amines (4.19%), and other compounds (10.23%) (Figure 2b). Results indicated one upregulated differential metabolite at CK vs. LD, 32 differential metabolites (26 up- and 6 downregulated) at CK vs. MD, 162 differential metabolites (148 up- and 14 downregulated) at CK vs. SD, 11 differential metabolites (10 up- and 1 downregulated) at LD vs. MD, and 42 differential metabolites (40 up- and 2 downregulated) at MD vs. SD (
Figure 2c).
Exploring the common differential metabolites in CK vs. LD, CK vs. MD, and CK vs. SD, a total of 173 differential metabolites were identified (
Figure 2d). Focusing on CK vs. MD and CK vs. SD due to the minimal differences in CK vs. LD, 21 common metabolites were found in these two comparison groups (
Supplementary Table S3).
To pinpoint drought stress-related metabolites in sweet basil leaves and discern their increasing/decreasing trends, 21 differentially accumulated metabolites common to drought stress and water sufficiency treatments were analyzed. Under drought stress, 19 metabolites exhibited an increase, while two metabolites displayed a decrease in both comparison groups. Notably, the contents of 2-methyl-quinoxaline and (1-nitropropyl)-benzene decreased gradually, while the rest metabolites increased continuously under drought stress. Minimal changes in metabolites were observed in the LD stage, but as it progressed to the MD and SD stages, the differences became stark (
Figure 2e).
3.2. Transcriptome analysis of sweet basil under four different gradient drought stresses
The sequencing analysis was conducted on the same materials used in the metabolomic analysis, yielding 86.63 Gb of clean data. Each sample reached 5 Gb, and the percentage of Q30 bases exceeded 93%. A total of 108,807 unigenes were assembled, with an N50 length of 2017bp and an average GC ratio of 50.01% (
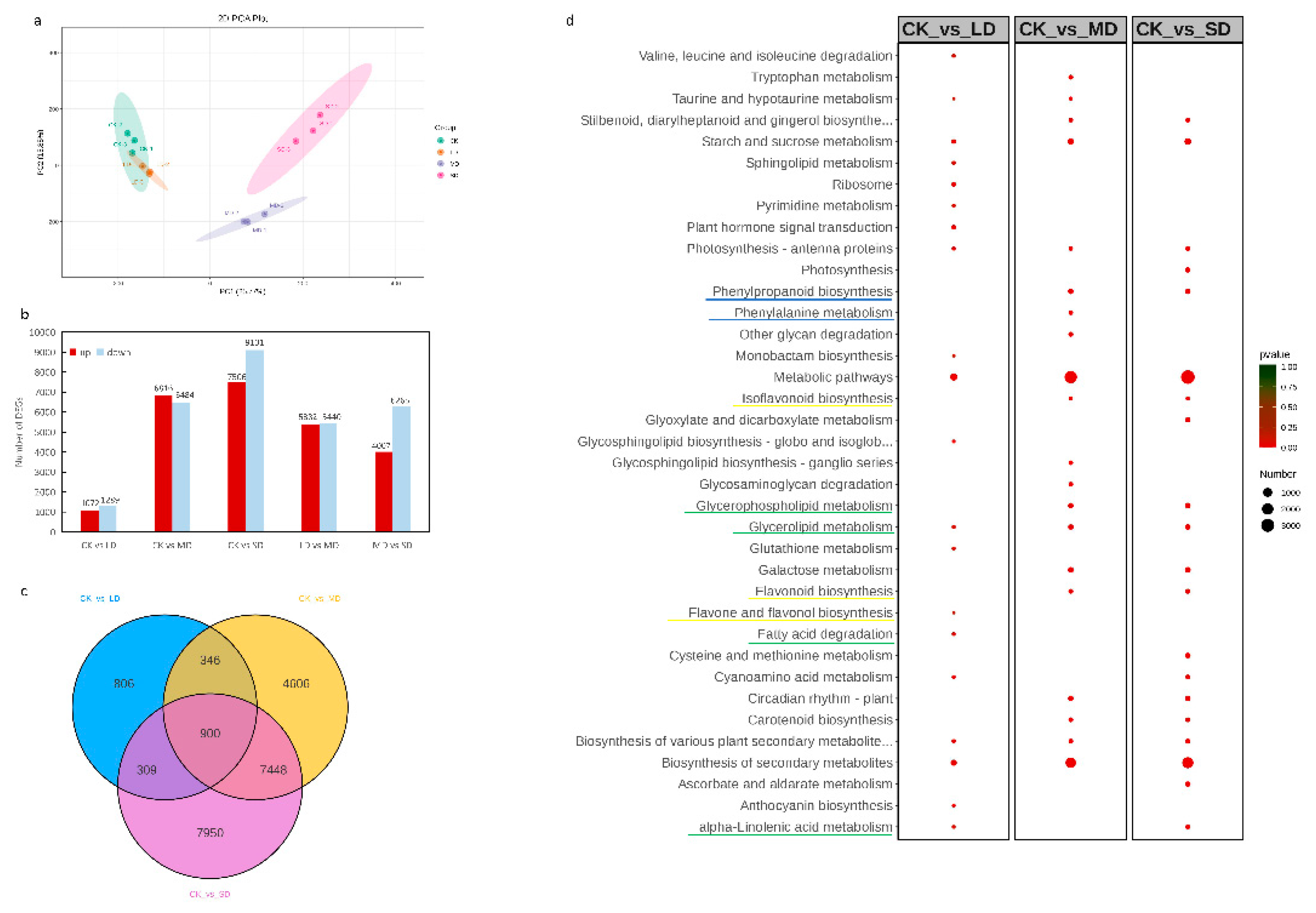
Supplementary Table S4). The sequencing data exhibited high quality, making it suitable for further analysis. A PCA analysis of 12 samples revealed strong correlations between replicates within each group and weaker correlations between treatments (
Figure 3a). The CK and MD treatment groups displayed the weakest correlation, while a good correlation was observed between CK and LD.
A total of 27,181 differentially expressed DEGs were identified in this study. A comparison of upregulated and downregulated DEGs in different drought treatments is illustrated in
Figure 3b. For LD/CK, there were 2,361 DEGs (1,072 upregulated and 1,289 downregulated); for MD/CK, 13,300 DEGs (6,816 upregulated and 6,484 downregulated); for SD/CK, 16,607 DEGs (7,506 upregulated and 9,101 downregulated); for MD/LD, 10,822DEGs (5,382 upregulated and 5,440 downregulated); for SD/MD, 10,272 DEGs (4,007 upregulated and 6,265 downregulated). The Venn diagram demonstrates that among all three treatment groups, 900 common DEGs were differentially expressed (
Figure 3c), suggesting their involvement in the response to drought stress.
To unravel the biological functions of DEGs, the top 20 enriched KEGG pathways among the groups LD/CK, MD/CK, and SD/CK were screened (
Figure 3d,
Supplementary Table S5). Metabolic pathways emerged as the most significant co-enrichment pathways. Glucose metabolism, such as starch and sucrose metabolism, and lipid metabolism, like glycerolipid metabolism, contained a substantial number of DEGs enriched in all three comparison groups.
3.3. Combined analysis of GC–MS and RNA-Seq profile
To elucidate the relationship between metabolites and DEGs under drought stress, GC–MS and RNA-Seq data were further analyzed. The findings revealed active responses in the alpha-linolenic acid and phenylpropanoid pathways to drought stress. As depicted in
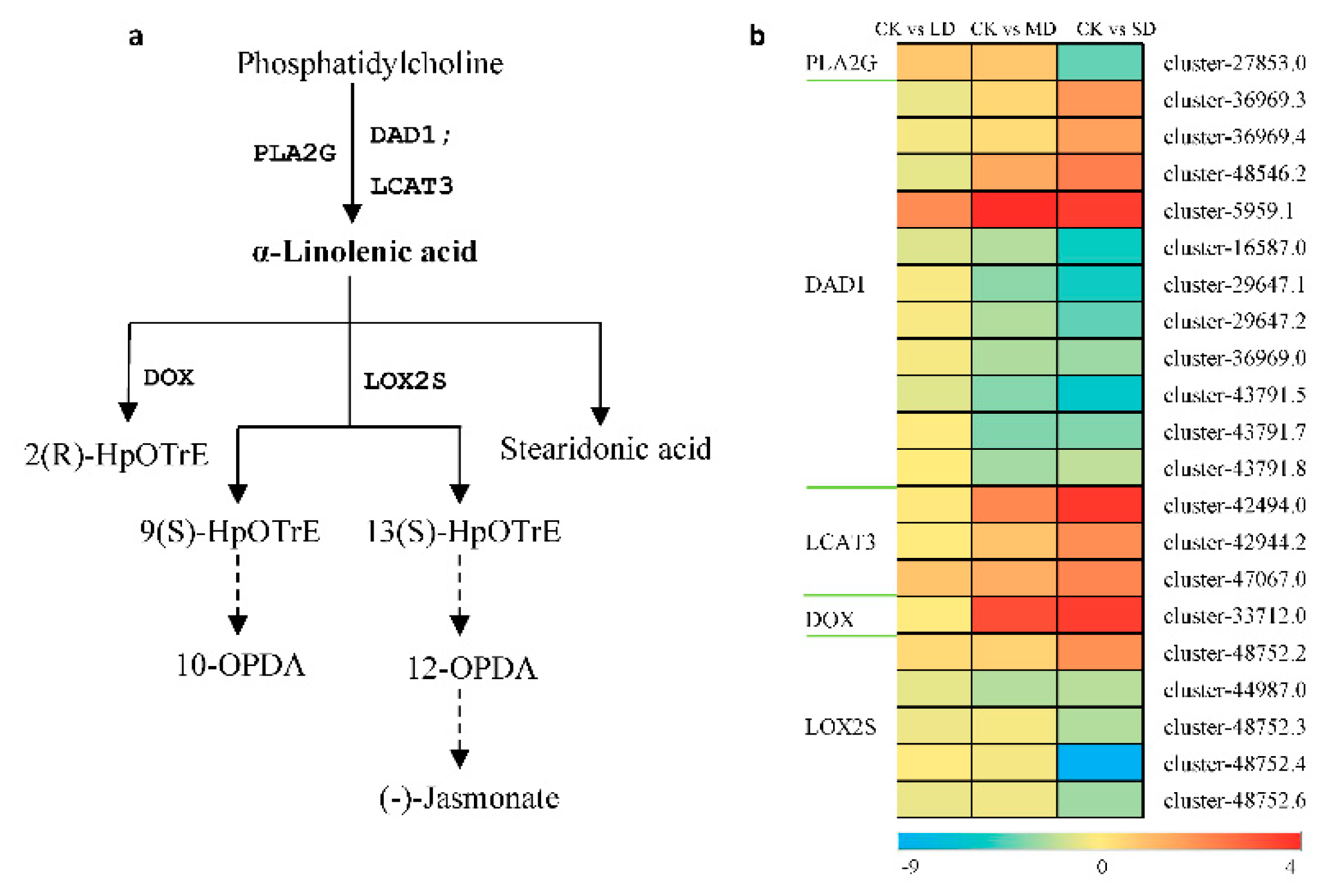
Figure 4, 21 unigenes were identified in the alpha-linolenic acid metabolic pathway.
PLA2G, DAD1, and
LCAT3 were identified as the three key genes synthesizing alpha-linolenic acid. As drought intensity increased, the expression of
PLA2G and most of the
DAD1 unigenes decreased, while all
LCAT3 unigenes were upregulated. Downstream of alpha-linolenic acid, the expression of
DOX was upregulated, while most
LOX2S were downregulated.
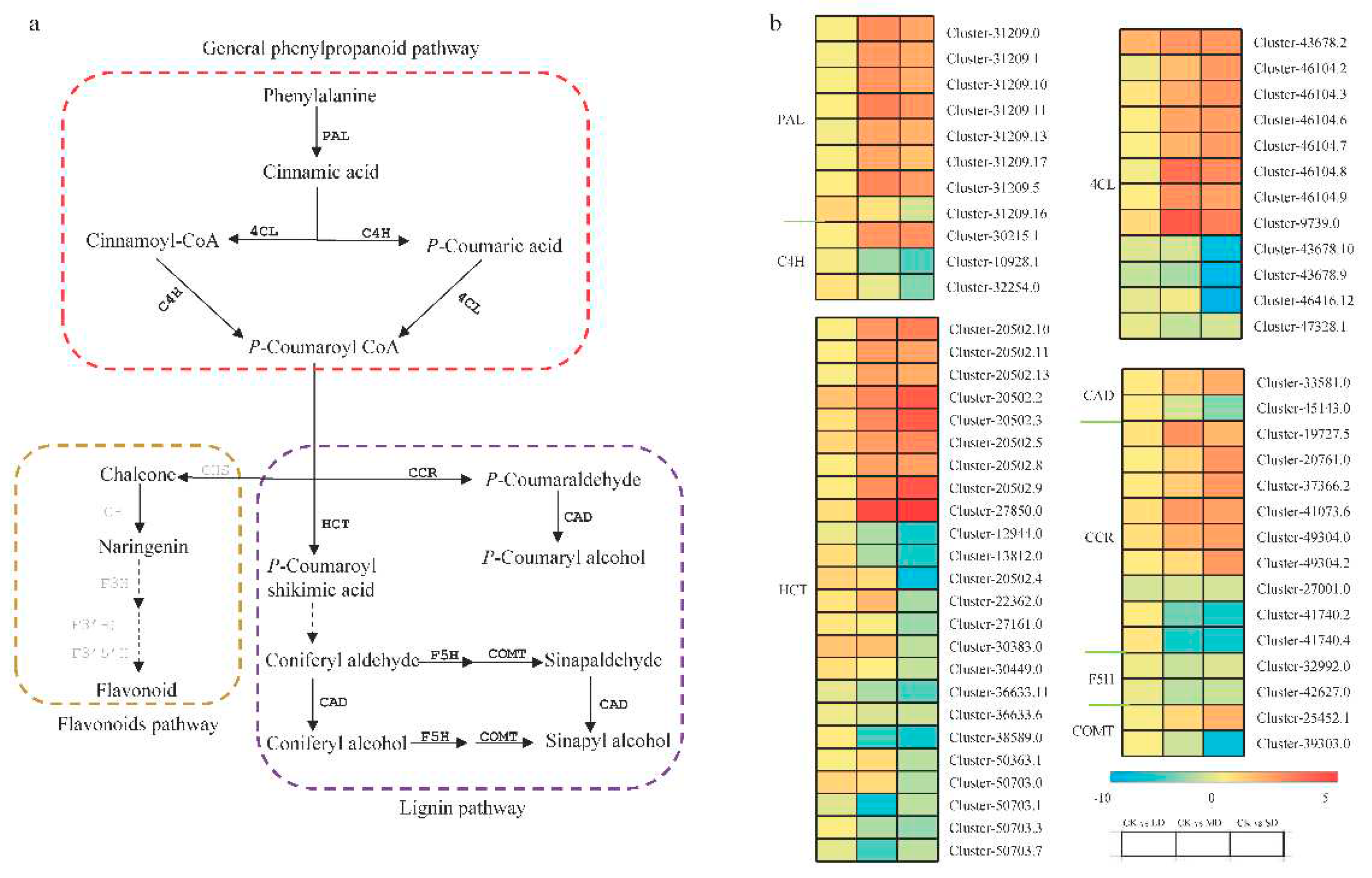
Regarding the phenylpropanoid pathway, which is associated with the flavonoid and lignin pathways (
Figure 5), it encompasses 62 differentially expressed unigenes. The majority of
PAL and
4CL unigenes were upregulated, while two-thirds of
C4H unigenes were downregulated.
HCT, a large family containing 24 differentially expressed unigenes, showed nine upregulated, six downregulated, and nine upregulated in MD/CK and downregulated in SD/CK.
CCR, containing nine differentially expressed unigenes, displayed six upregulated and three downregulated.
CAD, F5H, and
COMT, essential genes for lignin synthesis, had two downregulated unigenes for
F5H, while
CAD and
COMT had two differentially expressed unigenes, with one upregulated and one downregulated.
3.4. Verification of metabolite content
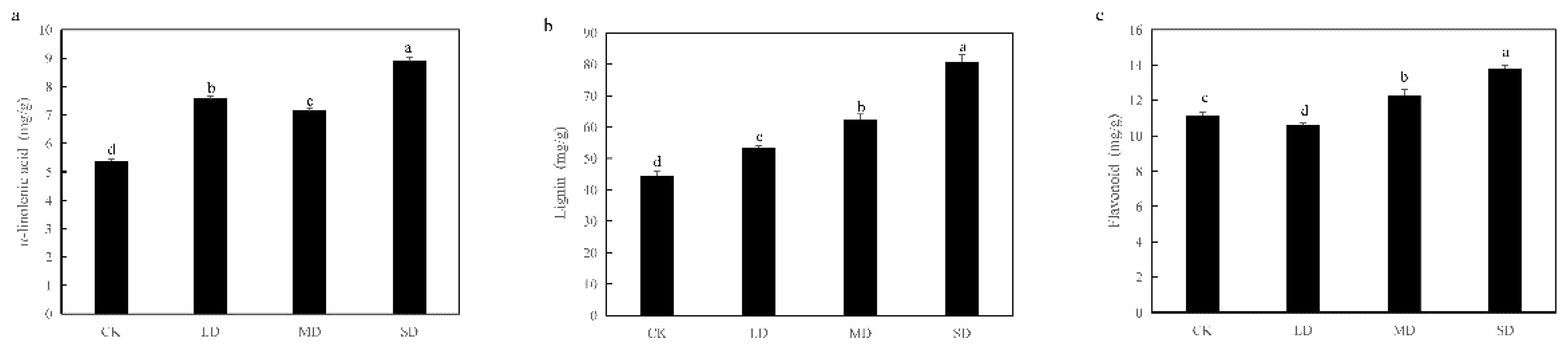
Through the integrated analysis of omics, it was discerned that alpha-linolenic acid and phenylpropanoid metabolism played pivotal roles in regulating the drought stress resistance of sweet basil. Subsequently, the variations in alpha-linolenic acid, flavonoid, and lignin contents under different degrees of drought stress were further investigated. In comparison to CK, alpha-linolenic acid and lignin contents demonstrated a significant increase under all three levels of drought stress. The flavonoid content exhibited a significant decrease in LD and a significant increase in MD and SD (
Figure 6).
4. Discussion
Ocimum basilicum L. is an annual medicinal aromatic plant renowned for its essential oil, which holds significant economic value. While basil exhibits good drought tolerance, prolonged drought stress can result in diminished plant yield, reduced quality, and even plant mortality. Therefore, investigating the drought response mechanism of basil is of paramount importance. In this study, an analysis of the morphological characteristics, volatile metabolome, and transcriptome data of sweet basil under varying degrees of drought stress was conducted. The results revealed pronounced differences in the accumulation of volatile metabolites, signal transmission, and gene expression in sweet basil as drought severity increased.
Sweet basil leaves were found to contain 830 volatile metabolites using an SPME-GC-MS method. The primary components of VOCs included terpenoids, esters, heterocyclic compounds, hydrocarbons, aromatics, alcohols, aldehydes, ketones, and other compounds. Notably, linalool, eucalyptol, and eugenol were the main components (
Supplementary Table S1). These findings align with reports on the essential oil composition in different basil species [
17]. A total of 173 differentially expressed VOCs were identified under drought stress. However, with the exception of benzaldehyde, the content of major components such as linalool, cineole, and others exhibited no significant changes, indicating that the primary volatiles of basil remain relatively stable under drought stress. This stability has been corroborated by postharvest drying experiments with basil [
29]. Twenty-one common metabolites were identified in CK vs. MD and CK vs. SD comparison groups, among which 19 metabolites were upregulated, and only two substances were downregulated. With the increasing severity of drought stress, the content of benzene and quinoxaline decreased, suggesting their potential participation in the synthesis of other metabolites as substrates.
In this study, the top 20 enriched KEGG pathways of DEGs demonstrated that both primary and secondary metabolism exhibited positive responses to drought stress (
Figure 3d). The enriched pathways encompassed glucose metabolism, lipid metabolism, photosynthesis, secondary metabolite synthesis, and others. This pattern mirrors the KEGG enrichment pathways observed in other plants under drought stress, such as maize [
30], millet [
31], soybean [
32], and rice [
33]. Intriguingly, the different DEGs were mainly enriched in phenylpropyl and flavonoid biosynthesis, as well as lipid metabolism. These pathways likely play a crucial role in the response of basil plants to drought stress.
A plant's metabolism plays a pivotal role in its resistance to stress. The accumulation of specialized metabolites, including flavonoids, terpenes, and phenols, is known to be significant across various plant species [
34,
35,
36,
37]. The combined analysis of transcriptome and volatile metabolomic data identified differences in the alpha-linolenic acid and phenylalanine metabolic pathways (
Figure 4 and
Figure 5). Previous studies have highlighted the importance of alpha-linolenic acid metabolism and the phenylalanine metabolism pathway in drought stress responses [
38,
39,
40,
41]. In this study, the content of alpha-linolenic acid increased under drought stress, which differs from a previous report indicating a decrease in linolenic acid content under drought stress [
38]. This discrepancy may be attributed to differences in plant species and the degree of drought.
The phenylpropanoid metabolic pathway serves as a crucial route from which various secondary metabolites, such as flavonoids and lignins, originate. In the present study, with the increasing severity of drought stress, the content of flavonoids decreased and then increased, while the content of lignins continued to rise. These findings suggest that the phenylpropanoid metabolic pathway may be a key regulatory pathway in the response of sweet basil to drought stress.
5. Conclusions
In this study, the volatile metabolome and transcriptome of sweet basil were systematically analyzed under various degrees of drought stress. The results revealed that the primary components of sweet basil volatiles remained stable under the influence of drought stress. Significant enrichment was observed in the DEGs associated with phenylpropanoid and flavonoid biosynthesis, as well as lipid metabolism. The resistance of sweet basil to drought stress was found to be predominantly influenced by the metabolic pathways of alpha-linolenic acid, flavonoid, and lignin. These findings provide valuable insights for screening drought-tolerant basil varieties and advancing the molecular breeding of basil with enhanced drought tolerance.
Supplementary Materials
Figure S1: Principal component analysis (PCA) between different drought treatment and quality control (QC) ; Figure S2: Orthogonal partial least squares-discriminant analysis (OPLS–DA) scores. Table S1: Information of all metabolites detected in the samples; Table S2: Information of differentially expressed metabolites; Table S3: Information of 21 differentially accumulated metabolites common to drought stress; Table S4: Summary of RNA sequencing and assembly; Table S5: KEGG pathway analysis data.
Author Contributions
Y.Z. was responsible for the experimental design, experimental performance, data analysis and writing of the manuscript; G.M. and L.X. participated in analysis of data; S.Z., X.Y. and Z.Z. involved in plant conservation, drought treatment and data collection; D.T. contributed to the reviewing and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This work received support from the Zhejiang Science and Technology Cooperation Plan (2022SNJF053).
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, (Danqing Tian), upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Grayson, M. Agriculture and drought. Nature 2013, 501, S1. [CrossRef]
- Dietz, K.J.; Zorb, C.; Geilfus, C.M. Drought and crop yield. Plant Biol. 2021, 23, 881-893. [CrossRef]
- Gonzalez, E.M. Drought Stress Tolerance in Plants. Int. J. Mol. Sci. 2023, 24. [CrossRef]
- Hura, T.; Hura, K.; Ostrowska, A. Drought-Stress Induced Physiological and Molecular Changes in Plants. Int. J. Mol. Sci. 2022, 23. [CrossRef]
- Chen, K.; Du K; Shi, Y.; Yin, L.; Shen, W.H.; Yu, Y.; Liu, B.; Dong, A. H3K36 methyltransferase SDG708 enhances drought tolerance by promoting abscisic acid biosynthesis in rice. New Phytol. 2021, 230, 1967-1984. [CrossRef]
- Yan, J.; Ninkuu, V.; Fu, Z.; Yang, T.; Ren, J.; Li, G.; Yang, X.; Zeng, H. OsOLP1 contributes to drought tolerance in rice by regulating ABA biosynthesis and lignin accumulation. Front. Plant Sci. 2023, 14, 1163939. [CrossRef]
- Caine, R.S.; Harrison, E.L.; Sloan, J.; Flis, P.M.; Fischer, S.; Khan, M.S.; Nguyen, P.T.; Nguyen, L.T.; Gray, J.E.; Croft, H. The influences of stomatal size and density on rice abiotic stress resilience. New Phytol. 2023, 237, 2180-2195. [CrossRef]
- Giri, J.; Parida, S.K.; Raghuvanshi, S.; Tyagi, A.K. Emerging Molecular Strategies for Improving Rice Drought Tolerance. Curr. Genomics 2021, 22, 16-25. [CrossRef]
- Li, W.; Wang, Y.; Ren, H.; Guo, Z.; Li, N.; Zhao, C.; Xie, Z. Transcriptomic and physiological analyses identifying Lanzhou lily (Lilium davidii var. unicolor) drought adaptation strategies. Hortic. Plant J. 2023, 9, 145-157. [CrossRef]
- Hu, H.; Fei, X.; He, B.; Luo, Y.; Qi, Y.; Wei, A. Integrated Analysis of Metabolome and Transcriptome Data for Uncovering Flavonoid Components of Zanthoxylum bungeanum Maxim. Leaves Under Drought Stress. Front. Nutr. 2022, 8. [CrossRef]
- You, J.; Zhang, Y.; Liu, A.; Li, D.; Wang, X.; Dossa, K.; Zhou, R.; Yu, J.; Zhang, Y.; Wang, L. et al. Transcriptomic and metabolomic profiling of drought-tolerant and susceptible sesame genotypes in response to drought stress. BMC Plant Biol. 2019, 19. [CrossRef]
- Wu, R.; Xu, B.; Shi, F. Leaf transcriptome analysis of Medicago ruthenica revealed its response and adaptive strategy to drought and drought recovery. BMC Plant Biol. 2022, 22. [CrossRef]
- Li, M.; Li, H.; Sun, A.; Wang, L.; Ren, C.; Liu, J.; Gao, X. Transcriptome analysis reveals key drought-stress-responsive genes in soybean. Front. Genet. 2022, 13. [CrossRef]
- Carocho, M.; Barros, L.; Barreira, J.C.; Calhelha, R.C.; Sokovic, M.; Fernandez-Ruiz, V.; Buelga, C.S.; Morales, P.; Ferreira, I.C. Basil as functional and preserving ingredient in "Serra da Estrela" cheese. Food Chem. 2016, 207, 51-59. [CrossRef]
- Romano, R.; De Luca, L.; Aiello, A.; Pagano, R.; Di Pierro, P.; Pizzolongo, F.; Masi, P. Basil (Ocimum basilicum L.) Leaves as a Source of Bioactive Compounds. Foods 2022, 11, 3212. [CrossRef]
- Zhan, Y.; An, X.; Wang, S.; Sun, M.; Zhou, H. Basil polysaccharides: A review on extraction, bioactivities and pharmacological applications. Bioorg. Med. Chem. 2020, 28, 115179. [CrossRef]
- Muráriková, A.; Ťažký, A.; Neugebauerová, J.; Planková, A.; Jampílek, J.; Mučaji, P.; Mikuš, P. Characterization of Essential Oil Composition in Different Basil Species and Pot Cultures by a GC-MS Method. Molecules 2017, 22, 1221. [CrossRef]
- Kacaniova, M.; Galovicova, L.; Borotova, P.; Vukovic, N.L.; Vukic, M.; Kunova, S.; Hanus, P.; Bakay, L.; Zagrobelna, E.; Kluz, M. et al. Assessment of Ocimum basilicum Essential Oil Anti-Insect Activity and Antimicrobial Protection in Fruit and Vegetable Quality. Plants 2022, 11. [CrossRef]
- Sakkas, H.; Papadopoulou, C. Antimicrobial Activity of Basil, Oregano, and Thyme Essential Oils. J. Microbiol. Biotechnol. 2017, 27, 429-438. [CrossRef]
- Sentari, M.; Harahap, U.; Sapiie, T.; Ritarwan, K. Blood Cortisol Level and Blood Serotonin Level in Depression Mice with Basil Leaf Essential Oil Treatment. Open Access Maced J Med Sci 2019, 7, 2652-2655. [CrossRef]
- Al-Huqail, A.; El-Dakak, R.M.; Sanad, M.N.; Badr, R.H.; Ibrahim, M.M.; Soliman, D.; Khan, F. Effects of Climate Temperature and Water Stress on Plant Growth and Accumulation of Antioxidant Compounds in Sweet Basil (Ocimum basilicum L.) Leafy Vegetable. Scientifica 2020, 2020, 1-12. [CrossRef]
- Zhao, X.; Huang, L.; Sun, X.; Zhao, L.; Wang, P. Transcriptomic and Metabolomic Analyses Reveal Key Metabolites, Pathways and Candidate Genes in Sophora davidii (Franch.) Skeels Seedlings Under Drought Stress. Front. Plant Sci. 2022, 13, doi:785702.
- Zhao, M.; Ren, Y.; Wei, W.; Yang, J.; Zhong, Q.; Li, Z. Metabolite Analysis of Jerusalem Artichoke (Helianthus tuberosus L.) Seedlings in Response to Polyethylene Glycol-Simulated Drought Stress. International Journal of Molecular Sciences 2021, 22, 3294. [CrossRef]
- Grabherr, M.G.; Haas, B.J.; Yassour, M.; Levin, J.Z.; Thompson, D.A.; Amit, I.; Adiconis, X.; Fan, L.; Raychowdhury, R.; Zeng, Q. et al. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat. Biotechnol. 2011, 29, 644-652. [CrossRef]
- Li, C.; Xin, M.; Li, L.; He, X.; Yi, P.; Tang, Y.; Li, J.; Zheng, F.; Liu, G.; Sheng, J. et al. Characterization of the aromatic profile of purple passion fruit (Passiflora edulis Sims) during ripening by HS-SPME-GC/MS and RNA sequencing. Food Chem. 2021, 355, 129685. [CrossRef]
- Zhang, Q.Y.; Yu, R.; Xie, L.H.; Rahman, M.M.; Kilaru, A.; Niu, L.X.; Zhang, Y.L. Fatty Acid and Associated Gene Expression Analyses of Three Tree Peony Species Reveal Key Genes for alpha-Linolenic Acid Synthesis in Seeds. Front. Plant Sci. 2018, 9, 106. [CrossRef]
- Andarwulan, N.; Batari, R.; Sandrasari, D.A.; Bolling, B.; Wijaya, H. Flavonoid content and antioxidant activity of vegetables from Indonesia. Food Chem. 2010, 121, 1231-1235. [CrossRef]
- Suzuki, S.; Suzuki, Y.; Yamamoto, N.; Hattori, T.; Sakamoto, M.; Umezawa, T. High-throughput determination of thioglycolic acid lignin from rice. Plant Biotechnol. 2009, 26, 337-340. [CrossRef]
- De Martino, L.; Caputo, L.; Amato, G.; Iannone, M.; Barba, A.A.; De Feo, V. Postharvest Microwave Drying of Basil (Ocimum basilicum L.): The Influence of Treatments on the Quality of Dried Products. Foods 2022, 11, 1029. [CrossRef]
- Zi, X.; Zhou, S.; Wu, B. Alpha-Linolenic Acid Mediates Diverse Drought Responses in Maize (Zea mays L.) at Seedling and Flowering Stages. Molecules 2022, 27, 771. [CrossRef]
- Cao, X.; Hu, Y.; Song, J.; Feng, H.; Wang, J.; Chen, L.; Wang, L.; Diao, X.; Wan, Y.; Liu, S. et al. Transcriptome Sequencing and Metabolome Analysis Reveals the Molecular Mechanism of Drought Stress in Millet. International Journal of Molecular Sciences 2022, 23, 10792. [CrossRef]
- Wang, X.; Song, S.; Wang, X.; Liu, J.; Dong, S. Transcriptomic and Metabolomic Analysis of Seedling-Stage Soybean Responses to PEG-Simulated Drought Stress. International Journal of Molecular Sciences 2022, 23, 6869. [CrossRef]
- Kaur, S.; Seem, K.; Duhan, N.; Kumar, S.; Kaundal, R.; Mohapatra, T. Transcriptome and Physio-Biochemical Profiling Reveals Differential Responses of Rice Cultivars at Reproductive-Stage Drought Stress. International Journal of Molecular Sciences 2023, 24, 1002. [CrossRef]
- Qin, X.; Yin, Y.; Zhao, J.; An, W.; Fan, Y.; Liang, X.; Cao, Y. Metabolomic and transcriptomic analysis of Lycium chinese and L. ruthenicum under salinity stress. BMC Plant Biol. 2022, 22. [CrossRef]
- Pichersky, E.; Raguso, R.A. Why do plants produce so many terpenoid compounds? New Phytol. 2018, 220, 692-702. [CrossRef]
- Silva, R.F.D.; Carneiro, C.N.; Sousa, C.B.D.C.; Gomez, F.J.V.; Espino, M.; Boiteux, J.; de Los Fernández, M.; Silva, M.F.; Dias, F.D.S. Sustainable extraction bioactive compounds procedures in medicinal plants based on the principles of green analytical chemistry: A review. Microchem J. 2022, 175, 107184.
- Bistgani, Z.E.; Hashemi, M.; Dacosta, M.; Craker, L.; Morshedloo, M.R. Effect of salinity stress on the physiological characteristics, phenolic compounds and antioxidant activity of Thymus vulgaris L. and Thymus daenensis Celak. Ind. Crop. Prod. 2019, 135, 311-320.
- Ullah, S.; Khan, M.N.; Lodhi, S.S.; Ahmed, I.; Tayyab, M.; Mehmood, T.; Din, I.U.; Khan, M.; Sohail, Q.; Akram, M. Targeted metabolomics reveals fatty acid abundance adjustments as playing a crucial role in drought-stress response and post-drought recovery in wheat. Front. Genet. 2022, 13. [CrossRef]
- C, X.L.A.B.; C, D.M.A.B.; D, Z.Z.; C, S.W.A.; C, S.D.A.; C, X.D.A.; C, L.Y.A. Plant lipid remodeling in response to abiotic stresses. Environ. Exp. Bot. 2019, 165, 174-184.
- Feng, H.; Lin, C.; Liu, W.; Xiao, L.; Zhao, X.; Kang, L.; Liu, X.; Sang, T.; Yi, Z.; Yan, J. et al. Transcriptomic Characterization of Miscanthus sacchariflorus × M. lutarioriparius and Its Implications for Energy Crop Development in the Semiarid Mine Area. Plants 2022, 11, 1568. [CrossRef]
- García-Calderón, M.; Pérez-Delgado, C.M.; Palove-Balang, P.; Betti, M.; Márquez, A.J. Flavonoids and Isoflavonoids Biosynthesis in the Model Legume Lotus japonicus; Connections to Nitrogen Metabolism and Photorespiration. Plants 2020, 9, 774. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).