1. Introduction
Approximately 30% of primary CNS tumors in children and 6.4% of primary CNS tumors in adults are low-grade gliomas (LGGs), a diverse group of neuroepithelial tumors originating from CNS astrocytes. LGGs are classified as Grade1 or Grade 2 gliomas by the World Health Organization (WHO). While they are benign and grow slowly, many of them rarely improve and frequently develop into higher-grade tumors. Survival times ranging from less than two years to more than ten years are indicative of the diverse clinical effects of LGGs. This diversity is influenced by molecular changes, treatment regimens, tumor features, and patient demographics. Forty percent of childhood cancer survivors experience cognitive impairment between 5 and 10 years after diagnosis [
1,
2,
3,
4,
5,
6].
There is little consensus on a neuro-oncological treatment that improves cognitive functioning, which involves knowing the state of functioning linked to the diagnosis and treatment process. However, 196 case reports were found in PubMed as of 7 November 2023, none of which assessed executive performance in the patient. We evaluated executive function in a patient who presented with a supratentorial tumor of oligodendroglioma grade and type in a longitudinal mannerthrough the treatment process from the preoperative phase to the first phase of complementary treatment with concomitant chemotherapy and radiation therapy.
The case was addressed from a neurological, neuropsychological and biopsychosocial perspective. A longitudinal survey was administered from 2020 to the present day, taking into account the understanding of the immediate context of the case. The tools used during the preparation of this case report were selected within the framework of neuroimaging techniques (MRI - iRM with T1, T2 and FLAIR sequences; Spectroscopy and Electroencephalography - EEG); and the Wisconsin Card Classification Test - WCST.
2. Case Presentation
A clinical picture of a male patient aged 59 years was consulted in February 2020 with repeated convulsive events of a partial complex type and subsequent amnesia of the facts with complete recovery; as a pathological background, chronic hypertension was diagnosed, and the patient was autonomous about activities of daily living. The physical examination showed no neurological deficits.
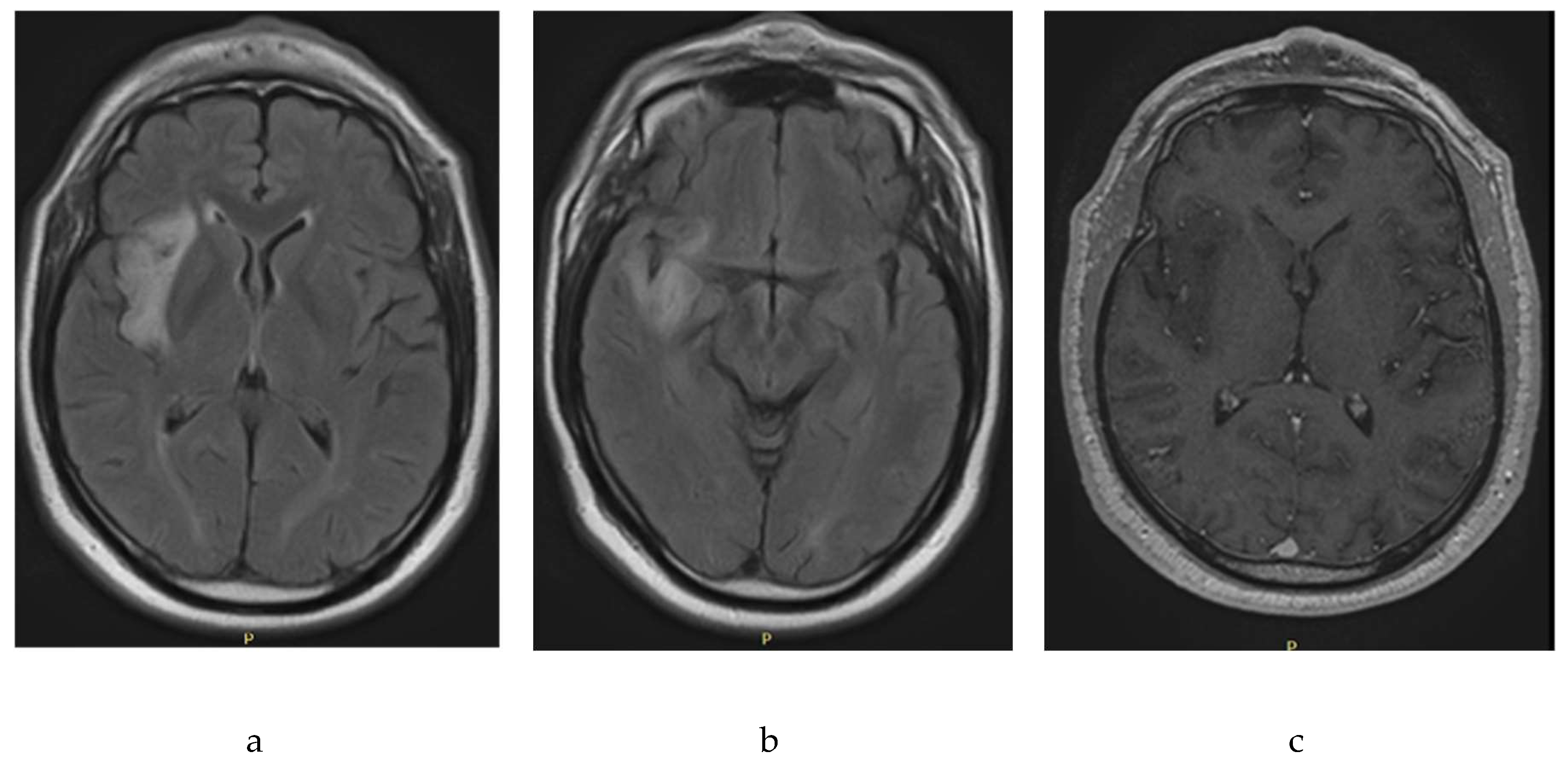
The initial paraclinical studies of the type of magnetic resonance imaging of the brain that were carried out in February 2020 revealed abnormalities (
Figure 1). Images with hyperintensity, oval, well-defined, and hippocaptant signals were located in the right periinsular territory with extension to the right temporal lobe. The injury had a diameter of 4.4 × 1.5 cm and discrete perilesional edema with a compressive effect on the lenticular nucleus. White substances with nonspecific signal hyperintensities associated with gliosis foci. No abnormal contrast absorption was observed. As a probable diagnosis, nodular appearance injury in the Perissilvian territory and right temporal area compatible with low-grade glioma was noted.
An extension study with magnetic resonance spectroscopy carried out sequences of multivoxel and univoxel spectroscopy findings in the area of interest, revealing an obvious decrease in the peak N acetylpartate with increased peaks of choline and creatine, as well as Cho/Cr indices of 2.94 and a Cho/Cr of 0.52, associated with probable astrocytoma-type gliomas of low degree. The supplementary studies carried out to exclude other pathologies were: Normal lumbar punctuation (February 2020) with gram without microorganisms, glucose 62.5 mg/dl, proteins 29.2 mg, pH 8, Chinese ink negative, nonreactive serology; Measured tumor markers, which were normal, were: testosterone 593, alphafetoprotein 1.85, carcinoembrionary antigen 1.87, ca. 19-9 27.45, psa 2.02. Electroencephalogram recording was performed (February 2020), which was normal.
The treatment plan was open surgery with the aim of extensive resection and obtaining tissue for pathological study, as anticonvulsant treatment included levetiracetam 1000 mg daily and valproic acid valcote ER 1000 mg per day. The patient and his relatives did not accept the surgical procedure and discontinued and continued the observation process during the external consultation for 18 months without changes in health conditions or controlled epilepsy.
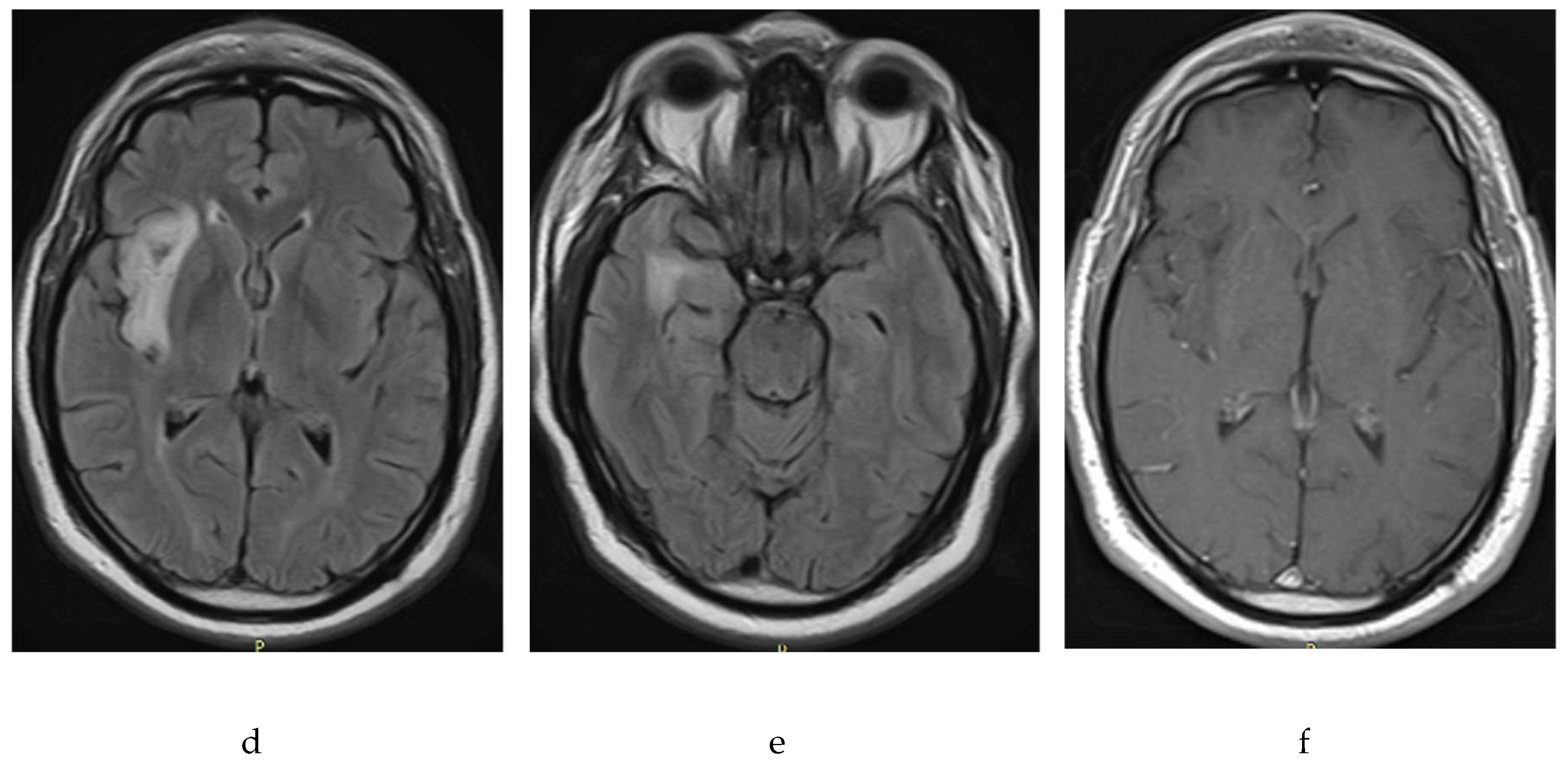
For the control of September 2021, brings brain resonance (
Figure 2) that showed changes in the volume of the tumor and it was described that in the right island region there is cortical and subcortical compromise by an area of alteration of the intensity that is hypointensive at t1, hyperintensive in t2 and flair, without enhancement with the gadolinium and has restriction to the diffusion of peripheral predominance, measured 33.5 x 50 x 21 mm, compatible with a low-grade glioma. The patient and family decided to authorize the surgery, which was carried out in October 2021. Surgical intervention was carried out through partial resection of the injury without subsequent neurological deficits or postoperative complications.
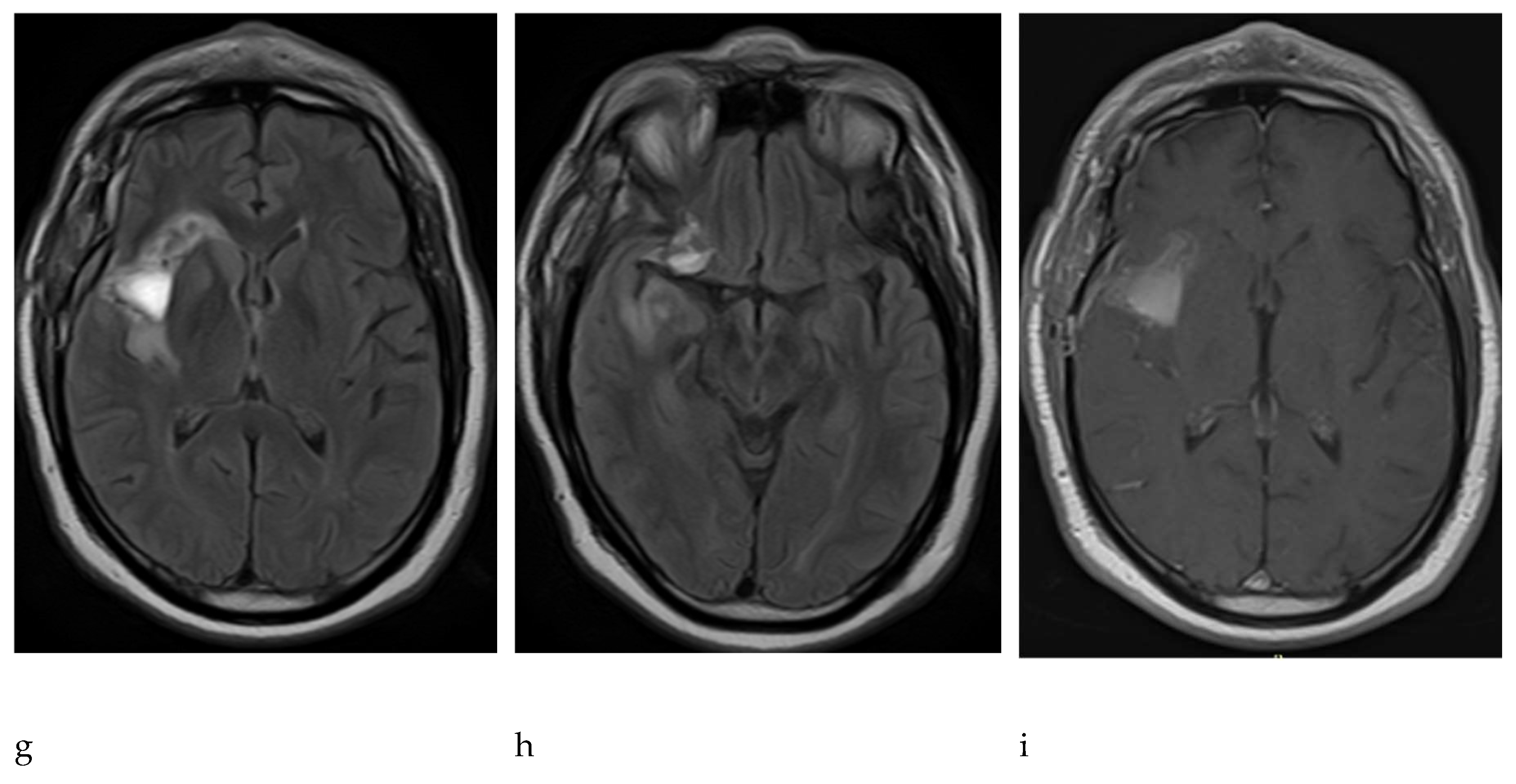
The control resonance in November 2021 described postoperative changes in the right island region with the presence of hematoma and persistence of residual tumor lesions prior to the area of surgical intervention. The pathology of the tumor revealed a glial neoplasm concordant with oligodendroglioma, olig2+, idh 1+, and ki67 less than 3%; p53-, no necrosis; no significant mitotic activity; and no endothelial proliferation. In consensus, the treating physicians considered that the patient had evidence of risk factors such as age group, persistence of tumor lesion by the last resonance performed and increase in the volume of the lesion over the period of approximately 18 months in observation; therefore, the pathology showed a low-grade glial neoplasm. The patient was treated as a high-grade tumor, with the stupp protocol: chemotherapy with temozolomide and radiation therapy in combination and subsequent adjuvant therapy with temozolomide. The patient is stable with periodic checks and receives the protocol of treatment at the institution on an outpatient basis.
The clinical psychological interview process did not reveal a history of neurological, oncological or psychopathological pathologies in the family. The caregiver had a history of partner therapy due to the preexistence of relationship difficulties between both individuals prior to the patient’s clinical condition. She came from a family with parents who died at an early age and whose loss at the age of ten had a significant impact in terms of the deficit of instrumental and affective support on the part of her parent figures. His wife described the patient's father as authoritarian, given the small sample of people affected during childhood.
The patient's literacy includes up to the third grade of primary school, with adequate reading and writing and remarkable skills for calculation according to what was shown by his wife. This indicates deterioration in the patient's postsurgical visual health and deterioration of calculation skills. He worked from childhood in agriculture and mining and developed his early life in precarious economic conditions due to the loss of his parent figures. He was characterized by having a broad social circle throughout his life and was a charismatic and receptive individual. These changes occurred as the treatment of glioma progressed.
The symptomatology of the tumor was demonstrated through nightly seizures in 2018, with a duration of approximately ten seconds per episode and increasing frequency over time with daytime and nightly episodes. The diagnosis was formalized in 2019, and treatment began with resection of the tumor in November 2021, accompanied by oral chemotherapy and radiation therapy in January 2022. The caregiver exhibited noticeable behavioral changes beginning at the onset of the tumor. Increased isolation and disconnection from the environment, constant sadness and emotional shutdown, poor communication and reserved temperament were observed. The inhibition of attention to environmental stimuli, such as conversations at social gatherings, is well known. Thus, relevant defects were identified in attention, short-term memory and possibly working memory from the beginning of treatment and in accordance with the impressions narrated by the caregiver.
Prior to the onset of the tumor, he highlighted the outstanding performance of the patient's memory in general terms and in contrast with other components of the family core, as well as remarkable abilities with computation. Some signs of anterograde amnesia were observed when he began to develop difficulties in evoking episodic memories of conversations and people. In terms of orientation, the patients showed a tendency toward spatial disorientation, although their orientation both in person and in time was still preserved.
With regard to executive functioning, failure results are presented for attitude maintenance, learning to learn and persistence. Cognitive flexibility is expressed in learning and perseveration. However, operating memory fails to maintain the attitude. The findings are shown as pre- and postsurgical sequences of tumor resection in the patient (
Table 1).
At the perseverative level, prior to resection of the tumor, the borderline was located with a typical score of 79. This indicates that the patient population exceeded eight percent (8%) of the normative group. She completed three of the six WCST categories, with thirty-five (35) attempts to complete the first, resulting in a learn-to-learn performance, reflecting a performance that exceeded more than 16% of the standard population. Despite the percentile in which the individual is situated, the number of attempts required to complete the first category and the number of categories completed allow us to see a cognitive flexibility to improve compared to the normative group. The completed categories were placed between the sixth and tenth percentiles, exceeding between six and ten percent of the population; moreover, there was a percentile location between two and five percent relative to the standard population around the number of attempts to complete the first category.
Notably, cognitive flexibility deteriorated seven days after resection of the tumor. The postsurgical WCST showed a moderate deterioration in performance, which translates to a typical score of 68 on the Perseverations subscale. This implies a considerable deficit in the performance of the presurgical test. The deficit in cognitive flexibility was notable in the retest, as it failed to complete any WCST category. This places it in a percentile that does not exceed the performance of one percent of the standard population (<1%). It is highlighted that the patient required the 128 reagents to try to complete the first category of the test unsuccessfully; this situation places him or her between percentiles two and five in contrast with the normative group (2% - 5%).
There was no possibility of calculating learning to learn due to the performance of the subject, so due to the absence of calculations in this domain and the increase in perseverations, it is evident that the cognitive flexibility of the patient hasdeteriorated. It was therefore impossible to consider and structure alternative strategies to solve the problem of the task. The role of cognitive flexibility may lead to possible deficits in operational memory given the difficulties in accessinginformation in working memory. Similarly, the inventory of the frontal lobe seeks to objectivize the behavior of the patient by interrogating with the caregiver; in this respect, 13 points out of 40 possible points are obtained, which places it close to the minimum overall functional compromise.
The results concerning perseverations allow consideration of possible cognitive deficits in the patient in the pretest, which were more evident in the posttest. The performance during the first test was 47.5% closer than was the performance in theWCST clinical group. The increase in the deficit was most evident in the posttest, in which the proximity of the deficit to the total clinical group increased to 73.7%.
Similarity of patient performance to clinical groups according to WCST score. Percentage compared to the frontal lobe (42.2%, 67.9%). Compared to the frontal lobe, 41.5% and 71.7% more patients were affected, respectively. Compared to the diffuse group, 46.3% and 73.9% of the patients in the two groups, respectively. Compared to the percentage of patients in the nonfrontal group, 62.9% and 81.5%. Compared to the clinical group, the total percentage was 47.5%±73.7%. The increased percentage of similarity between the pretest and posttest scores in the clinical group of patients with brain injury is highlighted.
3. Discussion
Executive functioning, evidenced by perseverations, learning to learn and failure to maintain an attitude, was more deteriorated than was observed in neurological or psychological interviews. The use of complementary instruments forneuropsychological evaluation allows us to report the extent to which executive functions are affected by the tumor, treatment or surgery.
Executive functioning is crucial for the patient to observe and control his/her behavior and adapt it to the contextual demands arising from the evolution of diagnosis, treatment, family, work and personal changes, which together are key factors in adherence to treatment [
7]. Moreover, when evaluating the cognitive state of neuro-oncological patients, other risk factors involved in the deterioration observed, such as age, extent of surgery, use of antiepileptic drugs, tumor variants associated with different survival outcomes (benign astrocytoma type or malignant forms such as glioblastoma) and early progression of the tumor, should be considered [
8].
Tumors are generated where both local damage and disorganization of cognitive networks occur, and the areas most affected in patients are involved in attention, memory and executive functions [
3,
4]. Oncological treatment applieddirectly to the CNS in the early stages of life compromises the development of the brain by reducing the volume of the cerebral cortex and altering connectivity; additionally, biological changes can lead to vascular brain damage, a reduction in the population of pluripotential cells or stem cells, oxidative stress and inflammation. Ionizing radiation causes cellular senescence, epigenetic changes, and alterations in DNA repair. Chemotherapy drugs such as methotrexate generate epigenetic changes and inhibit the reduction of free radicals. Cyclophosphamide induces ovarian insufficiency early in menopause, and a reduction in estrogen affects the protection of patients from these conditions in the cardiovascular system [
1].
Studies comparing temozolomide-type chemotherapy with radiation therapy have shown no improvement in aspects such as quality of life or cognitive improvement or in survival without progression. Studies on cognitive impairment include radiation therapy; proton therapy schemes reduce doses of brain tissue exposed to radiation compared to photon therapy, which seeks to reduce structural damage and preserve cognosctive capabilities. Studies that compared both therapies and the application of neurocognitive testing have shown no differences in results at the start of engagement [
8,
9,
10].
Radiation therapy currently prevents irradiation of structures such as the hippocampus, and in metastatic patients, stereotaxic radiosurgery has been used as a treatment option with the aim of reducing the risk of deterioration. According to the reviewed expert consensus, no well-developed studies have been found to support the use of a drug that significantly improves cognitive performance in the neuro-oncological population [
10].
Therapeutic interventions focused on the preservation and improvement of cognitive capacity involve both cognitiverehabilitation therapy and pharmacological treatment [
3]. Currently, vorasidenib is a promising drug for improving survival and decreasing the duration of subsequent intervention in patients with low-grade gliomas, enabling patients and their relatives to find effective intervention options for observed cognitive and executive decline [
11].
Neuropsychological tests applied to neuro-oncological patients have evaluated executive functions, memory, attention, and processing speed [
12,
13,
14,
15]. There are different tests to be applied to patients, for example, the European Organization for Research and Treatment of Brain Cancer. In the papers consulted, the revised Hopkins Verbal Learning Test (HVLT),which measures domains of memory and evocation of achieved learning; the Trail Making Test (TMT), which values attention, speed and mental flexibility; and the Controlled Oral Words (COWA) test, which evaluates the spontaneous production of words in conditions of restricted search; other tests, such as the Montreal Cognitive Assessment (MoCA) [
14].
From this perspective, the usefulness of the Wisconsin Card Classification Test (WCST), as one of the most widely used tools at the research level, is highlighted due to its degree of sensitivity in discriminating between clinical and nonclinicalsamples of executive functions; therefore, low function suggests pathologies of the frontal lobe. This test provides an explanatory framework for why subjects with frontal pathologies, as well as in areas peripheral to the frontal lobe, obtain unfavorable results in the test [
15].
5. Conclusions
The Wisconsin Card Classification Test (WCST) was used to assess executive function, which is difficult to quantify during neurological or psychological interviews in relation to neurotypic functioning. We emphasize that visualizing executive functions enables patients to gain metacognitive and emotional knowledge about changes related to diagnosis and treatment, which are elements that help physicians understand adherence to treatment.
Author Contributions
Conceptualization, AMJ. and ABC.; methodology, AMJ.; validation, MG., FG. and AMJ.; formal analysis, ABC.; data curation, MG.; writing—original draft preparation, all authors.; writing—review and editing, all authors.; supervision, AMJ.; project administration, AMJ.; funding acquisition, ABC. All authors have read and agreed to the published version of the manuscript.
Funding
This research has been funded by Dirección General de Investigaciones of Universidad Santiago de Cali under call No. 02-2023
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board (or Ethics Committee) of Clinica de occidente.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data is available for solicitation to AMJ with valid reasons.
Acknowledgments
To the personnel of Clinica de Occidente and the families of patients.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Phillips NS, Stratton K, Williams A, et al.: Late-onset Cognitive Impairment and Modifiable Risk Factors in Adult Childhood Cancer Survivors. JAMA Network Open. 2023, 6:2316077. [CrossRef]
- Haldbo-Classen L, Ali Amidi L, Wu SL, et al.: Long-term cognitive dysfunction after radiation therapy for primary brain tumors. Acta Oncol. 2019, 58(5):745-752. [CrossRef]
- Marijke B, Coomansa SD, Van der L, et al.: Treatment of cognitive effects in brain tumor patients: current status and future directions. Curr Opin Oncol. 2019, 31:540-547. [CrossRef]
- Zhang J, Jie C, Boni D, et al.: Probiotic supplement attenuates chemotherapy-related cognitive impairment in patients with breast cancer: a randomized, double-blind, and placebo-controlled trial. European Journal of Cancer. Volume 161, P10-22, January 01. 2022, 10:1016. [CrossRef]
- van Lingen MR, Breedt LC, Geurts JJG, et al.: The longitudinal relationship between executive functioning and multilayer network topology in glioma patients. Brain Imaging Behav. 2023, 17:425-435. [CrossRef]
- Cao J, Yan W, Zhan Z, et al.: Epidemiology and risk stratification of low-grade gliomas in the United States, 2004-2019: A competing-risk regression model for survival analysis. Front Oncol. 2023, 13:1079597-2023. [CrossRef]
- Zhang R, Wang DM, Liu YL, et al.: Symptom management in adult brain tumors: A literature review. Nurs Open. 2023, 10:4892-4906. [CrossRef]
- Brown PD, Gondi V, Pugh S, et al.: Hippocampal avoidance during whole-brain radiotherapy plus memantine for patients with brain metastases: phase III trial NRG Oncology CC001. J Clin Oncol. 2020, 38:1019-1029. [CrossRef]
- Klein M, Drijver AJ, van den Bent MJ, et al.: Memory in low-grade glioma patients treated with radiotherapy or temozolomide: a correlative analysis of EORTC study 22033-26033. Neuro Oncol. 20201101093252. [CrossRef]
- Koekkoek JAF, van der Meer PB, Pace A, et al.: Palliative care and end-of-life care in adults with malignant brain tumors [published correction appears in. Neuro Oncol. 2023, 5:212-2023. [CrossRef]
- Mellinghoff IK, van den Bent MJ, Blumenthal DT, et al.: Vorasidenib in IDH1- or IDH2-Mutant Low-Grade Glioma. N Engl J Med. 2023, 389:589-601. [CrossRef]
- K Leemans, M De Ridder: Cognition: development of a cognitive testing battery on the iPad for the evaluation of patients with brain Mets. Acta Neurol Belg. 2022, 122:145-152. [CrossRef]
- Blommaert J, Schroyen G, Vandenbulcke M, et al.: Age-dependent brain volume and neuropsychological changes after chemotherapy in breast cancer patients. Hum Brain Mapp. 2019, 40:4994-5010. [CrossRef]
- Caramanna I, Bottomley A, Drijver J, et al.: Jaap C Reijneveld. Objective neurocognitive functioning and neurocognitive complaints in patients with high-grade glioma: Evidence of cognitive awareness from the European Organization for Research and Treatment of Cancer brain tumor clinical trials. Eur J Cancer. 2021, 144:162-168. [CrossRef]
- Stuss DT, Levine B, Alexander MP, et al.: Wisconsin Card Sorting Test performance in patients with focal frontal and posterior brain damage: effects of lesion location and test structure on separable cognitive processes. Neuropsychologia. 2000, 38(4):388-402. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).