You are currently viewing a beta version of our website. If you spot anything unusual, kindly let us know.
Preprint
Review
Peptide-Based Biomaterials for Osteochondral Tissue Regeneration
Altmetrics
Downloads
186
Views
124
Comments
0
A peer-reviewed article of this preprint also exists.
This version is not peer-reviewed
Abstract
The healing of osteochondral defects (OCD) resulting from injury, osteochondritis, or osteoarthritis in middle- and old-age individuals, bearing lesions in the cartilage and bone, pain, and loss of joint function, presents challenges to the clinical practitioners because of non-regenerative cartilage and the limitations of current therapies. Bioactive peptide-based osteochondral (OC) tissue regeneration is becoming more popular because it does not have the immunogenicity, misfolding, or denaturation problems associated with original proteins. Periodically, reviews are published on the regeneration of bone and cartilage; however, none of them address the simultaneous healing of these tissues in the complicated heterogeneous environment of the OC interface. As regulators of cell adhesion, proliferation, differentiation, angiogenesis, immunomodulation, and antibacterial activity, potential therapeutic strategies for OC defects utilizing bone and cartilage-specific peptides should be examined and investigated. Therefore, the main goal of this review is to study how they contribute to the healing of OC defects, either alone or in conjunction with other peptides and biomaterials.
Keywords:
Subject: Chemistry and Materials Science - Biomaterials
1. Introduction
An articulating joint's osteochondral (OC) unit comprises the vascularized, mineralized subchondral bone and the acellular, avascular hyaline cartilage connected by a seamless interface.[1] The structural heterogenicity of the OC tissue arises from its diverse organic (cells, aggrecan, collagen) and inorganic (hydroxyapatite) components, along with their spatial orientation, forming gradients from the superficial cartilage to the subchondral bone via middle, deeper, and calcified layers (Figure 1). Cartilage exerts cushioning effects on joint bones and prevents damage from the physiological load; however, an injured cartilage cannot spontaneously regenerate. OC defects (OCD) are created by a variety of biological (aging, osteochondritis, osteoarthritis) or mechanical (accidental trauma, sports injury, prolonged wear) factors, characterized by cartilage and bone lesions, severe joint pain, and loss of joint function. They are more common in the middle-aged and older population. The World Health Organization estimates that 595 million people worldwide or 7.6 percent of the world's population suffered from osteoarthritis alone in 2020. This number has increased by 132.2 percent since 1990. [2]
When cartilage is damaged, the body's natural healing process is triggered by an inflammatory response, which leads to excessive production of reactive oxygen species (ROS) and matrix metalloproteinases (MMPs), further deteriorating the cartilage matrix and eventually inducing osteoarthritis. The treatment of OCD can be palliative (e.g., chondroplasty or microfracture), reparative (fixation), or restorative (autologous chondrocyte implantation (ACI), autologous matrix induced-chondrogenesis (AMIC), osteochondral autograft transplantation (OAT)) with their advantages and limitations.[3,4,5,6] Unfortunately, none of these techniques can fully repair the damaged tissue or form hyaline cartilage instead of fibrocartilage. [7]
OC tissue engineering (TE), which combines cells, scaffolds, and biomolecules to mimic extracellular microenvironment, seems promising for regenerating a functional OC tissue. The cells can be used for this purpose are bone marrow- or adipose-derived mesenchymal stem cells (MSCs), osteoblasts and chondroblasts derived through differentiation of MSCs, autologous chondrocytes, or MSCs cocultured with chondrocytes.[8] Scaffolds can be fabricated from various extracellular matrix (ECM)-mimetic biopolymers and synthetic polymers using solvent casting, freeze-drying, electrospinning, microfluidic-based methods, and three-dimensional (3D) printing. Considering heterogenicity of the OC tissue, a bi-layer or multilayer design is usually preferred over injectable hydrogels or single-layered scaffolds, since they possess osteoblasts-seeded porous bone layer forming continuous gradient with nonporous cartilage layer seeded with chondrocytes. Bioactive molecules, such as drugs (e.g., alendronate, dexamethasone), drug-like molecules (e.g., kartogenin, berberine), growth factors or peptides derived from them, are often incorporated into the scaffolds forming smart materials with enhanced regenerative capacity, facilitating cell migration, osteogenic or chondrogenic activity.[9,10] The transforming growth factor-β (TGF-β), bone morphogenetic proteins (BMPs) (especially BMP-2, BMP-4, and BMP-7), stromal cell-derived factor-1α (SDF-1α), insulin-like growth factor (IGF) (IGF-1 and IGF-2), fibroblast growth factor (FGF), platelet-derived growth factor (PDGF), and vascular endothelial growth factor (VEGF) are the major growth factors which promote MSCs, osteoblasts or chondrocytes recruitment, proliferation and differentiation, vascularization and maintenance of cartilage homeostasis. However, regulating their target selectivity, dose selection, release kinetics, and spatial distribution is quite challenging.
However, bioactive peptides are short-chain amino acid sequences that mimic signaling or binding domains of larger proteins and create a biomimetic environment for recruiting host cells, while controlling their activity and differentiation. They are either delivered alone or combined with biomaterials for tissue regeneration [9]. For instance, peptides either derived from ECM proteins or growth factors, or mimic their function, exhibit selective cells affinity, enhance cell penetration, or form hydrogels through self-assembly were investigated for OC regeneration. They are more effective, stable, scalable, and affordable than large proteins and also, they do not experience issues like immunogenicity, protein folding, and denaturation.[11]
Several reviews have been published highlighting the last five years’ clinical and experimental data on bioactive peptides for bone and cartilage regeneration as well as usage of peptide-enhanced bone graft substitutes. [11,12,13,14] However, none of them has focused specifically on the recovery of OC defects. Given that peptides could promote cell adhesion, motility, membrane penetration, immunomodulation, and antibacterial activities, besides inducing chondrogenic, osteogenic, and angiogenic differentiation, the primary focus of this review is to highlight their role in healing of OC defects when used alone or in combination with other peptides or biomaterials. Peptides belonging to different subcategories for bone and cartilage regeneration have been discussed after a brief review of the heterogeneity of the OC unit and the prerequisites for OC regeneration. Peptides that aid in osteo-, angio-, or chondrogenic activity, such as adhesion and migration of reparative cells, the creation of a 3D matrix, or an in vivo environment free from infection or inflammation, are covered in the following section. The review concludes with a summary and outlook after summarizing the results of several in vitro and in vivo studies using peptide-conjugated biomaterials.
2. Requirements for Osteochondral Tissue Regeneration
Successful recovery of OC defect faces significant challenge in reproducing the heterogeneous structure of the OC tissue. The present goal of OC regeneration is reviving the basic tissue structure and function under clinical settings, particularly in growth plate defects, cartilage defects in load-bearing sites, or full-thickness OC defects. The prerequisites are native tissue mimetic scaffolds, host tissue mimetic regenerated tissue and a well-controlled inflammatory state to counter MMPs-mediated irreversible destruction of cartilage matrix. The existing platelet-rich plasma (PRP) and exosomes-based therapies greatly passivated MMPs activity. Stem cell therapy maintained cytokine levels by expressing growth factors and limiting production of inflammatory cytokine, such as tumor necrosis factor alpha (TNF-α), interleukin-6 (IL-6).[15] Treating with the recombinant IL-1 receptor antagonists and monoclonal antibodies against TNF-α can generate a favorable cartilage microenvironment, which suggests MSCs and drug therapy may complement each other. Genetic engineering aims state reversal by transfecting seed cells with bone/cartilage-specific genes and arthritis inhibitory genes, thus ensuring necessary cytokines production.
Although tissue engineering holds promise for simultaneous bone and cartilage regeneration, injectable hydrogels and monolayer scaffolds fail to replicate the intricate spatial distribution of biochemical cues, particularly at the bone and cartilage interface.[16,17] Advanced techniques such as 3D printing or 3D bioprinting enable customized scaffold fabrication in terms of structure, anisotropy, and cell distribution; however, they involve high cost and intricate setup.[18]
Two transcription factors, Sox9 and Runx2, are essential for converting MSCs into chondrocytes and subsequent transformation into the hypertrophic phenotype, [19,20] while FGFs and BMPs are necessary for triggering bone formation.[21] Despite osteogenic and chondrogenic activity, BMPs have less control over chondrocyte hypertrophy and mineralization.[15,22] Localized delivery of bioactive peptides inducing target protein expression could be an ideal choice for biomimetic bone and cartilage regeneration, avoiding risks associated with growth factor or cell delivery. Adhesion and affinity peptides can be used to promote specific cell attachment and motility, respectively, while chondrogenic and osteogenic peptides can be delivered through multilayered scaffolds to accomplish site-specific differentiation. An antimicrobial peptide can potentially resist bacterial colonization at the defect site. On the other hand, inflammation or immunogenicity can be reduced using penetration peptides which enable selective cargo delivery to the cells and induce macrophage polarization.
3. Peptides for Bone Regeneration
The natural bone healing occurs in two different pathways: intramembranous ossification and endochondral ossification [23]. The former involves direct differentiation of MSC into osteoblasts, which then deposits mineralized matrix in flat bone. The latter is a complicated multi-step process of long bone formation that starts with inflammation and continues with hematoma development, platelet-mediated cytokine release, stem cell and fibroblast migration, chondrogenic differentiation, formation of fibrocartilaginous matrix (soft callus) and then mineralization into woven bones following chondrocyte hypertrophy. The woven bones develop into mature lamellar bone through remodeling and neovascularization. During this process, a variety of cytokines with distinct roles are released, including TGF-β1, PDGF-1, BMPs, VEGF-1, FGF, SDF-1α, TNF-α, Chemokine (CC-motif) ligand 2 (CCL2), IL-1b, IL-6, and Wnt proteins.[24] Among these, BMPs, TGF-β2, and -β3 play a crucial role in stem cell differentiation by causing hypertrophy and mineralization, while VEGF and angiopoietins promote neoangiogenesis.[25,26] BMP-2, 3, 4, 7, TNF-α, interferon-γ (IFN-γ), and certain hormones regulate the remodeling phase.[27] Since bioactive peptides follow similar cell signaling pathways with these proteins (Figure 2a), and may be beneficial for both in vitro and in vivo bone formation, they are grouped into two categories based on their role: osteo-inducers and angiogenic.
3.1. Osteo-Inducers
3.1.1. Collagen-Mimetic/Derived Peptides
Collagens are a superfamily of proteins consisting of 46 distinct polypeptide chains that supramolecularly assemble into 28 different types of fibrils, fibril-associated with interrupted triple helices (FACIT) and nonfibrillar forms that provide structural support and encourage cell adhesion via integrins. Peptides derived from integrin-binding motifs of collagen type I, the most significant ECM protein, are GFOGER, P15, KOD, DGEA, and BCSP1, which can promote osteogenic activity and in vivo bone formation.
Collagen-mimetic GFOGER (Gly-Phe-Hyp-Gly-Glu-Arg) peptide, derived from collagen α1 chain, selectively promotes α2β1 integrin binding required for osteoblastic differentiation. [28] Besides improving cell attachment, GFOGER successfully induced in vitro osteogenic differentiation and in vivo bone healing. [28,29,30,31,32] GFOGER coating on synthetic PCL scaffolds remarkably enhanced bone formation in critically sized segmental defects in rats by stimulating osteoblast adhesion and differentiation (Figure 2b). [31] P15, a 15-mer peptide (Gly-Thr-Pro-Gly-Pro-Gln-Gly-Ile-Ala-Gly-Gln-Arg-Gly-Val-Val), derived from collagen type I α1 chain, has a strong affinity for the cell surface α2β1 integrin receptors. By releasing growth factors and cytokines, the peptide dramatically enhanced the osteogenic differentiation of MSCs.[33] The commercially available P15 formulations significantly enhanced regeneration of alveolar bone and tibial defects in osteoporotic dogs. [34] [35] The collagen-mimetic KOD peptide, made of three units: ((PKG)4-(POG)4-(DOG)4), forms hydrogel through self-assembly inducing platelet activation and blood clotting associated with hematoma formation. [36] POG-based poly-amphiphilic hydrogels allowed faster recovery (within two weeks) of intervertebral disc defects in rabbits due to a significant increase in ECM deposition.[37] DGEA (Asp-Gly-Glu-Ala) derived from collagen type I adhesive motif serves as a crucial ligand for osteoblast differentiation. [38] DGEA-containing PA hydrogels seeded with hMSCs substantially upregulated osteogenic markers (OCN, RUNX2, and ALP). [39]
Using bone and cartilage stimulating peptide (BCSP™-1 or NGLPGPIGP) present in human collagen type-I, proliferation of rat bone marrow derived osteoblasts and human or bovine chondrocytes was drastically improved with enhanced bone mineral density (BMD) and bone mineral content (BMC) in male Wistar rats.[40] Three highly osteogenic peptides (GPAGPHGPVG, APDPFRMY, and TPERYY) derived from tilapia scale collagen hydrolysate notably increased the MC3T3-E1 cell proliferation, and mineralization activity (ALP synthesis, osteogenic-related gene expression) at concentrations of 50 μg/mL.[41]
3.1.2. BMP-Mimetic/Derived Peptides
As members of the TGF-β superfamily, BMPs are primarily produced by endothelial cells, osteoblasts, and hypertrophic chondrocytes and can recruit MSCs to the site of injury, differentiate into osteoblasts, while inducing ectopic bone formation.
KIPKASSVPTELSAISTLYL peptide, derived from the knuckle epitope of BMP2, increased ALP activity of osteoprogenitor cells.[42] P24, a BMP-2 mimetic peptide, with 24-mer peptide (Ser-Lys-Ile-Pro-Lys-Ala-Ser-Ser-Val-Pro-Thr-Gly-Leu-Ser-Ala-Ile-Ser-Thr-Leu-Tyr-Leu-Asp-Asp-Asp), bearing the knuckle epitope of the protein that facilitate binding with BMP receptors. P24 successfully induced ectopic bone formation in rodents.[43,44,45] PEP7 peptide (CKIPKPSSVP-TELSAISMLYL) derived from BMP-2, promoted adhesion, proliferation, and differentiation of MG-63 cells, besides new bone formation in supra-alveolar peri-implant defect model in micropig mandible[46]. BMP peptide (KIPKASSVPTELSAISTLYL), derived from BMP2, increased ALP activity, an early marker for bone formation, in murine osteoprogenitor cells [47] and other cell types, [48,49,50,51,52] besides dose-dependent healing of rabbit radial bone-defects. [53] The other osteoinductive or osteogenic peptides derived from BMP-2 (NSVNSKIPKACCVPTELSAI, KIPKASSVPTELSAISTLYL, DWIVA) produced differential effects on in vitro osteogenic differentiation as well as ectopic or orthotopic bone formation in vivo.[12] Bone-forming peptide (BFP-2) with VEHDKEFFHPRYHH sequence, isolated from the immature BMP-7 precursor, triggered osteogenic differentiation of BMSCs, while induced ectopic bone formation after subcutaneous implantation of BFP-2-treated BMSCs in mice (Figure 2c).[54] Similarly, effects of various osteoinductive peptides derived from BMP-4 (RKKNPNCRRH), BMP-7 (TVPKPSSAPTQLNAISTLYF, GQGFSYPYKAVFSTQ, ETLDGQSINPKLAGL), BMP-9 (KVGKACCVPTKLSPISVLY) have been reviewed. [55] Casein kinase 2 (CK2) related peptide has great influence on cell proliferation, and apoptosis, it facilitates in vivo bone formation by interacting with BMP receptor type Ia (BMPRIa).[56] Three BMP-2 mimetic peptides, CK2.1, CK2.2, and CK2.3, triggered the BMP signaling pathways by inhibiting CK2 binding to BMPRIa. [57] C2C12 cells, treated with CK2.3 peptide resulted in osteogenesis, while CK2.2 led to both osteogenesis and adipogenesis. [56,58]
3.1.3. Hormone-Derived Peptides
Parathyroid hormone (PTH) is a major regulator of mineral homeostasis. Parathyroid hormone (PTH)-related peptides, called Teriparatide, which is 1–34 peptide domains of PTH (PTH1–34), stimulated osteoblast activity and increased bone density at the fracture site, leading to healing of non-unions. [59,60,61,62,63] On the other hand, endogenous PTH-related protein (PTHrP) analogues, PTHrP1–34, PTHrP1–36, and PTHrP107–111, increased osteoblast activity and local bone formation.[64,65,66] Calcitonin gene-related peptide (CGRP) is a 37-mer neuropeptide, having two isoforms: α and β-CGRP. They have been found to stimulate the proliferation and differentiation of osteoprogenitor cells, [67,68,69,70] production of osteogenic molecules like IGF-I, BMP-2, [71,72] and reparative bone formation. [73]
3.1.4. Circulating Peptides
Osteogenic growth peptide (OGP), a 14-mer peptide occurring in mammalian blood, increases bone formation through anabolic effects on bone cells [74,75] and differentiation of osteoprogenitor cells leading to upregulated osteogenic markers, including mineralization [76,77,78] Thrombin peptide 508 (TP508) or Chrysalin is a 23-amino acid peptide, receptor binding domain of thrombin, which enhanced the proliferation, differentiation, and chemotaxis of human osteoblasts [79,80] and VEGF-stimulated angiogenesis [81]. TP508 injected into the fracture gap promoted fracture healing and increased blood vessel formation [82,83,84].
3.1.5. Other ECM-Derived Peptides
Signaling domains on ECM protein chains, capable of interacting with cell membrane receptors. Various peptides (e.g., FN III9-10/12-14) derived from fibronectin (FN), have shown to promote osteoblast activity and mineralization, [85] rabbit calvarial defects healing, [86] and augmented BMP-2 and PDGF-BB activities for bone regeneration in vivo. [87]
Collagen-binding motif (CBM) is a cleavage product of osteopontin (OPN) that can specifically bind to collagen [88] and promote migration and osteogenic differentiation [89], bone formation in rabbit calvarial defect model.[90] SVVYGLR (Ser-Val-Val-Tyr-Gly-Leu-Arg) peptide adjacent to the RGD sequence in OPN significantly enhanced the adhesion and proliferation of MSCs, neovascularization, upregulation of osteogenesis and angiogenesis when delivered through a collagen sponge. [91,92,93] FHRRIKA (Phe-His-Arg-Arg-Ile-Lys-Ala), a cell-binding and heparin-binding domain of bone sialoprotein (BSP) exerts favorable effect on osteoblast adhesion, spreading and mineralization [94] Higher cell proliferation and viability was observed on rat calvarial osteoblasts seeded scaffolds containing the RGD and FHRRIKA sequences.[95]
3.2. Angiogenic
Vascularization is a crucial process during natural bone formation. Many peptides are derived from angiogenic growth factors (e.g., VEGF, FGF-2, PDGF, etc.), ECM (e.g., OPN, ON) and other proteins that have crucial roles in blood vessel formation [96].
VEGF-mimicking QK or KLT peptide (KLTWQELQLKYKGIGGG), derived from the VEGF receptors binding domain 17–35, not only induce endothelial cell migration and proliferation, but also to trigger other complex processes like chemotaxis, capillary sprouting and organization similar to VEGF [97]. PDGF-BB derived PBA2-1c peptide interacts with α- and β-PDGF receptors. Though it’s in vivo proangiogenic activity is still unclear, it functions similarly to PDGF in establishing mature blood vessels that are created by VEGF [98]. Exendin-4, a glucagon-like peptide 1 (Glp-1) analogue, stimulates human umbilical vein endothelial cells (HUVECs) motility, sprouting, and tube formation in vitro in addition to in vivo sprout outgrowth.[99] While OPN is widely distributed in bone matrix helping in bone metabolism, OPN-derived peptide (OPD) does not induce endothelial cell proliferation in vitro. However, like VEGF, it facilitated endothelial cell migration and tube formation using 3D collagen gels [100], suppressed osteoclastogenesis,[91] promoted adhesion and proliferation of MSCs, besides neovascularization in a rat tibial defect model [101]. SPARC113 and SPARC118, two OPN-derived peptides, which exhibit potent angiogenic activity [102], stimulated in vivo angiogenesis when delivered through MMPs degradable hydrogel [103]. TP508 enhanced neoangiogenesis in femoral defects produced in rats [84] and mice [82] following one hour of local administration. The synthetic 12-mer peptides, known as RoY peptides, which were created via the phage-display technology, may also promote in vitro endothelial cell proliferation, tube formation, and sprouting, besides inducing in vivo angiogenesis via a distinct mechanism from VEGF [104].
4. Peptides for Cartilage Regeneration
4.1. Chondroinductive/Chondrogenic Peptides
Numerous peptides have been identified to imitate the functions of ECM components, cell-cell junction molecules, and chondroinductive/chondrogenic ligands triggering specific cell signaling pathways. Motifs derived fibronectin like RGD, decorin, collagen, MMPs have displayed chondrogenic properties. These peptides are often used to functionalize scaffolds encouraging chondrocyte adhesion, migration, and proliferation in addition to MSC differentiation into the chondrogenic lineage.
4.1.1. TGF-β Mimetic Peptides
TGF-β improves cell differentiation, collagen synthesis, and matrix deposition in cartilage tissue engineering.[105] Therefore, peptides mimicking TGF-β activity were used in OC TE for cartilage tissue regeneration. TGF-β mimetic peptides, i.e., cytomodulins (CMs), are oligopeptides containing 4-6 amino acids [106]. CMs immobilized to a solid surface can potentially induce the chondrogenic differentiation compared to its soluble form (Figure 2d). [107,108]
4.1.2. BMP2-Derived/Mimetic Peptides
BMP-2, a member of the TGF-β super-family, is one of the main chondrogenic growth factors that induce in vitro chondrogenic differentiation and cartilage regeneration in vivo. Human MSCs (hMSCs) cultured with ≥100 mg/mL of the BMP peptide resulted in glycosaminoglycan (GAGs) production, increased in levels of collagen production and matrix accumulation without extensive upregulation of hypertrophic markers.[107,108,109,110,111] Injection of BMP-2 mimetic CK2.1 peptide into the mice tail vein enhanced chondrogenesis and enhanced articular cartilage formation, without any effects on osteogenesis or BMD.[112] BMP peptide stimulated chondrogenic differentiation of hMSCs without additional growth factors. At a 100 μg/mL concentration, BMP peptide enhanced proteoglycan production and chondrogenic gene expression without causing hypertrophy, as occurs with BMP-2. [113]
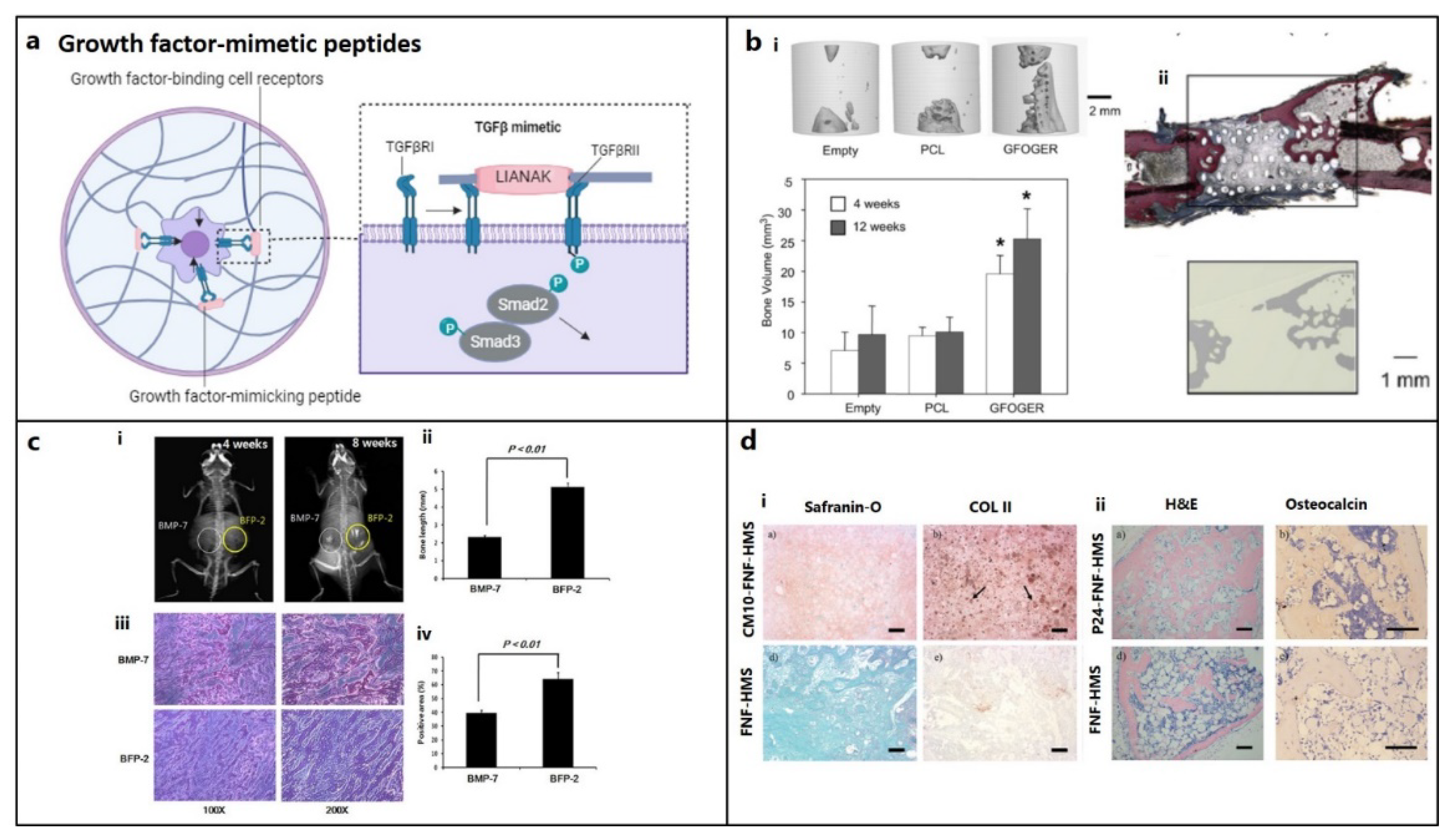
Figure 2.
Growth-factor mimetic peptides for bone and cartilage regeneration in osteochondral defects. (a) Growth factor-mimetic peptide epitopes inducing direct signaling, e.g., mimicking transforming growth factor-β (TGF-β) pathway for TGFβ receptors (TGFβR) binding. (figure generated via biorender.com) (b) (i) Critical bone defects treated with GFOGER-coated scaffolds shows significantly higher bone formation compared to uncoated PCL scaffolds and empty defect (control), as revealed by micro-CT imaging after 12 weeks of implantation and the plot also depicts a similar pattern at 4 weeks. (ii) Histology confirms that areas of high attenuation in GFOGER-coated sample, as revealed by 2D micro-CT image (inset) are bone tissue (red/pink) (soft tissue appearing blue/green in Sanderson’s rapid bone stain).[31] Copyright© 2009 Elsevier Ltd. (c) Bone formation in mice treated with BFP-2 for 4 or 8 weeks in comparison to BMP-7 was evaluated by (i) radiography, (ii) bone length estimation, (iii) histological assessment after hematoxylin and eosin staining and (iv) measurement of the osteogenic area from histology samples using the Image-Pro Plus 6.0 software (n=4).[54] Copyright© 2017, Springer Nature. (d) Histological analysis (i) CM10 conjugated FNF-HMS (CM10-FNF-HMS) with rabbit BMSCs demonstrates positive in SO staining (GAGs) and collagen type II (COL II) staining indicating hyaline cartilage formation after 2 weeks and (ii) P24 conjugated FNF-HMS (P24- FNF-HMS) with rabbit BMSCs reveals significantly higher bone formation in H&E and osteocalcin staining after 5 weeks of subcutaneous implantation, respectively compared to FNF-HMS (D–F) (Scale bars: 100 μm).[108] Copyright© 2014 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figure 2.
Growth-factor mimetic peptides for bone and cartilage regeneration in osteochondral defects. (a) Growth factor-mimetic peptide epitopes inducing direct signaling, e.g., mimicking transforming growth factor-β (TGF-β) pathway for TGFβ receptors (TGFβR) binding. (figure generated via biorender.com) (b) (i) Critical bone defects treated with GFOGER-coated scaffolds shows significantly higher bone formation compared to uncoated PCL scaffolds and empty defect (control), as revealed by micro-CT imaging after 12 weeks of implantation and the plot also depicts a similar pattern at 4 weeks. (ii) Histology confirms that areas of high attenuation in GFOGER-coated sample, as revealed by 2D micro-CT image (inset) are bone tissue (red/pink) (soft tissue appearing blue/green in Sanderson’s rapid bone stain).[31] Copyright© 2009 Elsevier Ltd. (c) Bone formation in mice treated with BFP-2 for 4 or 8 weeks in comparison to BMP-7 was evaluated by (i) radiography, (ii) bone length estimation, (iii) histological assessment after hematoxylin and eosin staining and (iv) measurement of the osteogenic area from histology samples using the Image-Pro Plus 6.0 software (n=4).[54] Copyright© 2017, Springer Nature. (d) Histological analysis (i) CM10 conjugated FNF-HMS (CM10-FNF-HMS) with rabbit BMSCs demonstrates positive in SO staining (GAGs) and collagen type II (COL II) staining indicating hyaline cartilage formation after 2 weeks and (ii) P24 conjugated FNF-HMS (P24- FNF-HMS) with rabbit BMSCs reveals significantly higher bone formation in H&E and osteocalcin staining after 5 weeks of subcutaneous implantation, respectively compared to FNF-HMS (D–F) (Scale bars: 100 μm).[108] Copyright© 2014 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
5. Other Supporting Peptides
5.1. Adhesion, Binding, or Affinity Peptides
RGD Peptide: RGD (Arg-Gly-Asp) motif is an essential cell adhesion peptide found in collagen, FN, vitronectin (VN), etc., promotes cell adhesion and/or differentiation through binding with transmembrane integrin receptors (Figure 3a). RGD sequences, such as GRGDS, RGDS, YRGDS and c(RGDfk), are widely used in the field of cartilage and bone repair. Bone regeneration using cyclic RGD in sheep spinal fusion model was comparable to that of rhBMP-2, due to its increased receptor affinity.[114] RGD, particularly the longer chain RGD (GPenGRGDSPCA), was not always effective in increasing cell adhesion and spreading. This could be because pre-absorbed proteins with stronger binding sites (like FN and VN) compete with weekly adsorbed RGD for cell binding, necessitating covalent attachment. [115,116] RGD is usually capable of increasing cellular adhesion of MSCs, but unable to induce osteogenic differentiation without osteogenic supplements or BMP-2 (Figure 3b). [29,48,49]
PHSRN Peptide: PHSRN (Pro-His-Ser-Arg-Asn) is a FN-derived peptide synergistically enhance cellular activity with RGD and other adhesion peptides through α5β1 integrin binding, though ineffective alone.[30,117] Due to competitive binding with α5β1 integrin sites, RGD and PHSRN did not improve the spreading of rat bone MSC on titanium surfaces[118]. However, a dimeric platform consisting of RGD and PHSRN, with a spacing between them resembling natural fibronectin, significantly improved the spreading and proliferation of SAOS-2 osteoblast-like cells when compared to RGD alone or their combination.[117] Further improvements in attachment, spreading, and cell differentiation were made possible by a longer chain RGD that allowed for wider spacings between the peptides like natural fibronectin; nevertheless, such modifications did not result in any osteogenic activity. [119]
FHRRIKA and KRSR Peptides: FHRRIKA (Phe-His-Arg-Arg-Ile-Lys-Ala) peptide, isolated from heparin binding domain of BSP, significantly enhanced cell proliferation, spreading, as well as matrix mineralization [116,120]. KRSR (Lys-Arg-Ser-Arg) is another peptide isolated from heparin-binding sites of BSP, along with FN, VN, OPN, thrombospondin, etc. [121], increased osteoblast adhesion and osteogenic gene expression. [122,123,124,125] A combination of FHRRIKA and longer chain RGD (CGGNGEPRGDTYRAY) stimulated rat osteoblast matrix mineralization, which was absent when they were employed alone.[94] Similarly, KRSR peptides delivered with RGD promoted cellular attachment on glass surface due to the due to this dual binding mechanism with proteoglycans and integrins, respectively.[121]
N-Cadherin is a calcium-dependent adhesion molecule consisting of five extracellular domains, in which, EC-1 domain having His-Ala-Val (HAV) motif, plays substantial role in cell adhesion. [126] hMSC-seeded nanofibrous hydrogels formed by a cadherin-mimic self-assembling peptide significantly upregulated the expression of chondrogenic markers such Col-II, Sox-9, and aggrecan.[127] hMSCs seeded hydrogels prepared from self-assembling KLD-12 peptide couples with N-cadherin mimetic peptide (HAVDI), remarkably increased chondrogenesis, upregulating cartilage-specific genes expression (collagen II, Aggrecan, and Sox9).[128] hMSC-seeded hyaluronic acid hydrogels functionalized with RGD and N-cadherin mimetic peptide improved osteogenic gene expression, besides new bone formation after 12 weeks of rat MSCs seeded hydrogel implantation into the rat calvarial defects.[129] On the other hand, NEMO-binding domain (NBD) peptide has shown to promote osteoblast differentiation and inhibit bone resorption,[130] osteoclastogenesis [131]. The other integrin binding peptides derived from collagen IV (NYYSNS), fibronectin (KQAGDV, PRARI), laminin (IKLLI, LRGDN and SINNNR), fibrinogen (GWTVFQKRLDGS, YSMKKTTMKIIPFNRLTIG and GWTVIQNRQ), netrin-1 (QWRDTWARRLRKFQREKKGKCRKA), CAM-1 (QIDS, LDT, DELPQLVTLPHPNLHGPEILDVPST), thrombospondin (CSVTCG), nidogen-1 (FRGDGQ) have been reported.[132]
Affinity peptides can bind with specific cells, scaffolds, and cytokines associated with cartilage regeneration. Using anti-CD44 antibody, biotin-avidin binding system [133], chondrocyte affinity peptides, [134] E7 peptide, [135], etc., cell adhesion was greatly improved. A TGF-β affinity peptide which can recruit TGF-β to the impaired region [136,137] was ligated to the PA nanofibers, promoted in vitro chondrogenic differentiation of hMSCs and cartilage repair in rabbits. [137] ECM affinity peptides can help mimic the native environment of chondrocytes thus integrated with scaffolds [138,139]. Hydrogels modified with HA - and chondroitin sulfate-binding peptides, significantly promoted BMSCs differentiation into chondrogenic lineage (Figure 3c). [140]
While RGD peptides immobilized at a low density effectively promoted cell adhesion and chondrogenenesis [141,142,143], a higher density of RGD induced hypertrophic transformation of chondrocytes due to the activation of integrin-mediated pathway.[144,145,146] However, compared to simple RGD adsorption, chemical grafting of RGD peptides on to the biomaterial surface offered improved coating stability and osteoconductivity (Figure 3d). [147] Incorporation of N-cadherin mimetic peptide, HAVDIGGGC, into HA hydrogels increased chondrogenic differentiation at an early stage followed by deposition of cartilaginous matrix [148,149]. Similarly, laminin-related peptide, CDPGYIGSR, significantly promoted cell adhesion with increased ECM deposition by bovine knee chondrocytes seeded on to the polyethylene oxide PEO/chitosan scaffolds.[150]
5.2. Cell Penetrating Peptides (CPPs)
CPPs are peptides derived from bacteria, viruses or synthetic method transverse the cellular membrane to transport their 'cargo' (proteins, siRNA, nanoparticles, oligonucleotides and other peptides), into the cytoplasm. [111]
hMSCs delivered with CPP-conjugated co-activator-associated arginine methyltransferase 1 (CARM1) proteins, transcriptional factor fusion proteins, or recombinant adenovirus expressing BMP-2, promoted in vitro differentiation, and highly mineralized bone formation in vivo. [151,152,153] NLS-TAT peptides were employed to introduce the hTGF-β3 plasmid into a self-assembled peptide scaffold or precartilaginous stem cells (PCSCs), and to facilitate chondrogenic differentiation. [154,155]
5.3. Peptides Promoting Cell Migration
For the healing of osteochondral defects, reparative cells, such as fibroblasts, MSCs, osteoblasts, chondrocytes, and endothelial cells, should migrate towards the defect site for participating in various stages of the healing process. ECM proteins and GFs (EGF, PDGF) facilitate cell migration by acting as mitogens or chemotactic factors.[156] Peptides derived from them often exhibit superior cell migration than the parent protein. They work via modulating adhesion proteins (integrins), enzymes (MMPs), kinase-linked receptors, or formyl peptide receptors. SDF-1 is known recruiter of endothelial progenitor cells that differentiate into mature vascular endothelium in the subchondral bone environment, thus promote osteochondral repair.[157] SDF-1 elastin-like peptide (SDF1-ELP) derived from SDF-1 showed comparable in vitro endothelial cell migration and vascularization to SDF, however it was more effective than SDF1 during in vivo healing of full-thickness wounds in diabetic mice. [158] Histatin-1, a 38-mer peptide with antimicrobial properties found in saliva, is a proangiogenic agent that stimulates the adhesion, migration, and angiogenesis of endothelial cells. [159] Other examples are the endogenous histatins, the annexin A1-derived peptide Ac2-26, [160] or the frog-skin derived esculentin 1-21 peptide,[161] induced better wound-healing driven by cell migration. Following administration of the endogenous antimicrobial peptide LL-37, vascularization increased significantly because of enhanced endothelial cell migration. [162] Like FGF-2 (bFGF), FGF-2-mimicking peptide, YRSRKYSSWYVALKR, derived from the 106–120 peptide domain in FGF-2, a partial agonist of FGF receptors, could successfully promote in vitro HUVEC cells proliferation and migration.[163]
5.4. Self-Assembly (SA) Peptides
SA peptides are composed of either alternating hydrophilic and hydrophobic amino acids or peptide amphiphiles (PA), which can self-assemble into β-sheet structures and interwoven nanofibrous hydrogel matrices under physiological conditions [164,165,166] SA peptides, isolated from various growth factors and bone-related proteins (RANKL-binding peptide, AC-100, B2A) have been studied for osteoblast differentiation and bone regeneration. [53,167,168] RADA16-I (AcN-RADARADARADARADA-CONH2) peptide immobilized on to the BMP-2 loaded hydrogel promoted osteogenic differentiation of MSCs leading to higher expression of osteogenic-related genes (ALP, OCN, Runx2) and in vivo bone regeneration [169]. PuraMatrixTM, a commercially available RADA16-1 peptide containing hydrogel, strongly supported cartilage formation [170,171]. Repeating units of KLD and RAD in a peptide hydrogel enhanced cartilage formation compared to agarose gel [172].
5.5. Degradable Peptides
Degradable peptides accelerate degradation of scaffolds to facilitate cell penetration and migration, ECM synthesis and deposition, consequently tissue ingrowth. MMPs are the best examples of ECM-degrading proteins. Inclusion of a MMPs-derived peptide (KCGPQGIWGQCK) remarkably promoted GAGs and collagen deposition on to the crosslinked poly (ethylene glycol) norbornene hydrogels.[140,173]
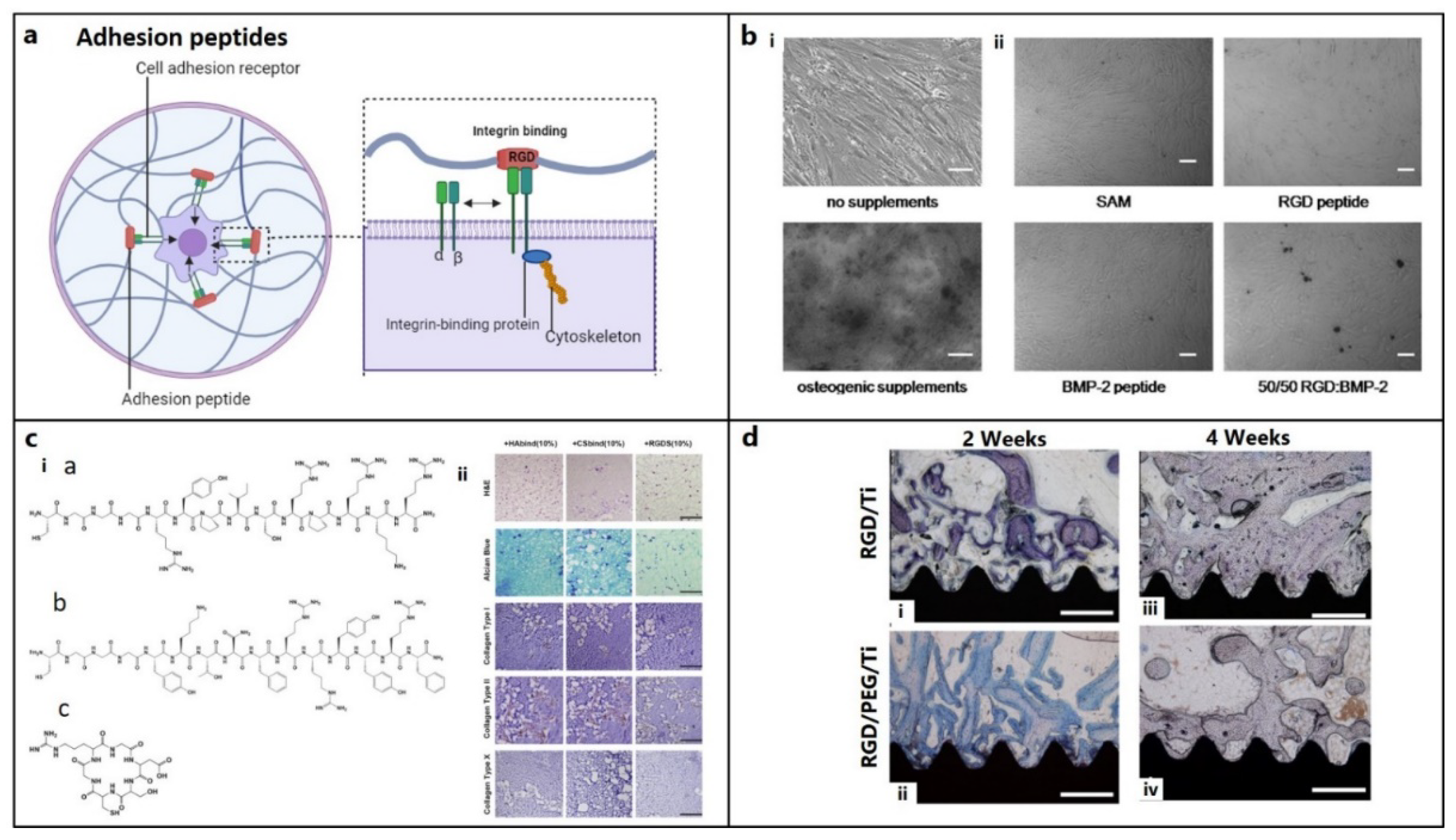
Figure 3.
Role of other supporting peptides for osteochondral regeneration. (a) Signaling of the adhesion peptides, e.g., integrin binding. (figure generated via biorender.com) (b) Evaluation of mineralization activity at 3 weeks of hBMSC culture on (i) tissue culture plate with or without osteogenic supplements (ii) self-assembled monolayer (SAM), bone morphogenic protein (BMP) and RGD peptide gradients on glass cover slips supplemented with osteogenic medium. Scale bars 100 μm.[48] Copyright© 2011 Elsevier Ltd. (i) (c) Structures of acrylated streptoccocal collagen-like 2 (Scl2) protein conjugated with hyaluronic acid (HA) binding CGGGRYPISRPRKR peptide (HAbind), chondroitin sulfate (CS) binding CGGGYKTNFRRYYRF peptide (CSbind) and cell adhesive GRGDSC peptide (RGDS) (ii) HAbind, CSbind and RGDS peptides based hydrogels cultured with hMSCs for 4 weeks, showed uniform cell and ECM distribution (H&E staining, in panel 1), extensive sGAG accumulation (Alcian Blue staining, in panel 2), high, low, and negative expressions of collagen type I (cartilage marker, in panel 3), type II (bone marker, in panel 4) and type X (hypertrophic marker, in panel 5), respectively (IHC staining), in all hydrogels, indicating chondrogenic differentiation without any hypertrophic transformation. Scale bars: 200 μm. [140] Copyright© 2015 Elsevier Ltd. (d) Histological analysis showing higher bone formation on RGD/PEG/Ti implants compared to RGD/Ti implants after 2 and 4 weeks of implantation in rabbit femoral condyles. (Mag: 40x; scale bar = 0.5 mm).[147] Copyright© 2011 Elsevier Ltd.
Figure 3.
Role of other supporting peptides for osteochondral regeneration. (a) Signaling of the adhesion peptides, e.g., integrin binding. (figure generated via biorender.com) (b) Evaluation of mineralization activity at 3 weeks of hBMSC culture on (i) tissue culture plate with or without osteogenic supplements (ii) self-assembled monolayer (SAM), bone morphogenic protein (BMP) and RGD peptide gradients on glass cover slips supplemented with osteogenic medium. Scale bars 100 μm.[48] Copyright© 2011 Elsevier Ltd. (i) (c) Structures of acrylated streptoccocal collagen-like 2 (Scl2) protein conjugated with hyaluronic acid (HA) binding CGGGRYPISRPRKR peptide (HAbind), chondroitin sulfate (CS) binding CGGGYKTNFRRYYRF peptide (CSbind) and cell adhesive GRGDSC peptide (RGDS) (ii) HAbind, CSbind and RGDS peptides based hydrogels cultured with hMSCs for 4 weeks, showed uniform cell and ECM distribution (H&E staining, in panel 1), extensive sGAG accumulation (Alcian Blue staining, in panel 2), high, low, and negative expressions of collagen type I (cartilage marker, in panel 3), type II (bone marker, in panel 4) and type X (hypertrophic marker, in panel 5), respectively (IHC staining), in all hydrogels, indicating chondrogenic differentiation without any hypertrophic transformation. Scale bars: 200 μm. [140] Copyright© 2015 Elsevier Ltd. (d) Histological analysis showing higher bone formation on RGD/PEG/Ti implants compared to RGD/Ti implants after 2 and 4 weeks of implantation in rabbit femoral condyles. (Mag: 40x; scale bar = 0.5 mm).[147] Copyright© 2011 Elsevier Ltd.
5.6. Antimicrobial and Immunomodulatory Peptides
AMPs or cationic host defense peptides (CHDPs) are oligopeptides (5–100 amino acids), cationic (net charge: +2 to +13), hydrophobic (50% hydrophobic residues), or amphipathic, derived from bacteriophages, bacteria, fungus, plants, and animals, possessing broad-spectrum antibacterial properties without causing antibiotic resistance. [174] Few also have angiogenic, cell migratory, and immunomodulatory properties facilitating tissue regeneration.
A wide range of AMPs have been reported.[175] They include helix-based (LL-37, magainin, melittin), sheet-based (protegrins, bactenecin, defensins) exhibiting β-hairpin like conformation, coil-based (indolicidin, omiganan) which are devoid of both α-helical and β-sheet structures, and composite AMPs, such as melimine (TLISWIKNKRKQRPRVSRRRRRRGGRRRR), which amalgamates functional regions of mellitin (α-helix) and protamine (random coil with a partially ordered α-helix). LL-37 (LLGDFFRKSKEKIGKEFKRIVQRIKDFLRNLVPRTES) is a 37-mer peptide α-helical AMP, derived from human cathelicidin antimicrobial protein, possessing antibacterial, antibiofilm, immunomodulation, and angiogenesis properties. Pexiganan (GIGKFLKKAKKFGKAFVKILKK), a synthetic magainin (GIGKFLHSAGKFGKAFVGEIMKS) analogue, PLG0206 (RRWVRRVRRWVRRVVRVVRRWVRR) rich in arginine, valine, and tryptophan residues, reduce biofilm formation. Protegrin-1 (RGGRLCYCRRRFCVCVGR), bactenecin (RLCRIVVIRVCR) and its derivatives (RKWRIVVIRVRR and RWRRIVVIRVRR), arginine-rich PEP8R (VRVRVRVRVDPPTRVRVRVRV) peptide, show broad-spectrum antimicrobial activities and reduced cytotoxicity. Indolicidin (ILPWKWPWWPWRR), rich in tryptophan and proline, is high potent against bacteria, fungi, and viruses, whereas omiganan (ILRWPWWPWRRK) shows improved bioactivity with reduced cytotoxicity.
AMPs work by disrupting bacterial cell wall or cytoplasmic membrane (Figure 4a), as well as interfering with the other cellular processes like cell wall synthesis. Bactericidal properties of keratin-derived anti-inflammatory KAMP-19 (RAIGGGLSSVGGGSSTIKY) peptide originates from the destruction of bacterial cell membrane via pore formation.[176] α-defensin, known as human Neutrophil Peptide 1 (HNP-1), exhibits high affinity for lipid II, an important component for cell wall synthesis, thereby destabilizes the cell wall integrity [177]. Likewise, RWRWRW-NH2 delocalizes peripheral membrane proteins in Gram-positive bacteria,[178] LL-37 affects cell wall synthesis by regulating Sfp1 gene, buforin II interrupts cellular functions by interacting with nucleic acids [179], and TO17 degrades DNA and RNA. [180]
Biofilms associated with chronic bone infection are difficult to treat because of bacterial resistance.[181] By interacting with mannan, a key component of cell walls, L-37 decreases cell adhesion before it causes biofilms, therefore lowering Candida infection (Figure 4b).[182] AMP 1037, a 9-mer peptide, decreases swimming and motility of bacteria [183]. Piscidin and sculentin (1–21) degrades pre-formed biofilm matrix by activating nuclease activity and disturbing membrane function, respectively.[184,185]
Anti-inflammatory cytokines (AMPs) can suppress pro-inflammatory cytokines and their associated signaling pathways, or stimulate the synthesis of anti-inflammatory cytokines, which can initiate both an innate and adaptive immune response to eliminate infection and inflammation (Figure 4c). Immunomodulatory metalloproteinases (AMPs) such as cathelicidin-WA stimulate macrophage polarisation from the M1 phenotype (caused by E. Coli) to the M2 phenotype (facilitating bone repair) (Figure 4d).[186] Macrophage polarization plays a significant role in mediating chondrogenesis. Anti-inflammatory M2 macrophages significantly contribute to tissue remodeling and repair, [187] while failure to transform M1 to the M2 subtype induces the progression of cartilage injury.[188]
Regenerative AMPs are beneficial for the regeneration of bone tissue because they can inhibit osteoclastogenesis and stimulate angiogenesis. Histatin-1, 38-mer peptide occurring in saliva, induces angiogenesis and tube formation.[159] On the other hand, human β defensin 3 stimulates MSC osteogenesis,[189] GL13K (GKIIKLKASLKLL) suppresses osteoclastogenesis,[190] and LL-37 supports the proliferation, migration, and differentiation of MSCs to osteogenic lineage [191].
6. Peptide-Conjugated Biomaterials
Natural ECM-mimetic biomaterials like collagen, gelatin, chitosan, alginate, and silk, etc., are often combined with synthetic materials, such as polyethylene glycol (PEG), polylactic acid (PLA), and polycaprolactone (PCL), to fabricate an OC construct possessing tailorable strength, bioactivity, biocompatibility, and biodegradation characteristics, with reduced hydrophobicity, immunogenicity, and inflammatory properties. Bioactive materials, such as hydroxyapatite, calcium phosphate, and biological ceramics, are also combined to encourage biomineralization.[22]
6.1. Osteo-Inductive Scaffold
Delivery of BMP2-derived KIPKASSVPTELSAISTLYL peptide through TCP scaffolds promoted bone healing rabbit radial bone-defect.[192] BMP-2 mimetic peptides attached to a substrate enhanced BMSC attachment and differentiation without affecting mineralization. [49] However, when these peptides were combined with RGD, the rate of mineralization increased because RGD induced higher interaction cells in absence of osteogenic supplementation. [48] Incorporation of BMP-2-mimetic peptide conjugated to a heptaglutamate moiety (E7-BMP-2) into the mineralized PLGA-collagen-gelatin nanofiber promoted bone formation in critical-sized alveolar bone defects in rats after 4 weeks of implantation.[193] Electrospun porous cellulose acetate nanofibrous mat modified with adhesive peptides KRSR, RGD, and growth factor BMP-2 enhanced adhesion and proliferation of pre-osteoblastic cells.[194] PCL nanofibrous scaffolds containing different concentrations of the bioactive with KIPKASSVPTELSAISTLYL peptide derived from the BMP-2 developed by Lukasova et al. Scaffolds seeded with porcine MSCs showed increased expression of OCN and collagen I significantly osteogenic differentiation.[195] KRSR, BMP mimetic peptides, and FHRIKKA were successfully integrated into the outer, middle, and inner layers of PLGA electrospun membranes through layer-by-layer assembly, and such modification demonstrated synergistic effects on bone healing by enhancing cell attachment, differentiation, and mineralization. [196] FHRRIKA and KRSR peptides covalently linked with the surface of polymeric nanoparticles significantly improved MSC attachment and ALP activity due to an osteogenic differentiation, which was not very profound in case of KRSR.[197,198] Immobilization of KRSR on a silane functionalized borosilicate glass,[121] or micropatterning with KRSR on borosilicate glass,[199] and the other substrates.[116,124,125,200] selectively enhanced osteoblasts adhesion through αvβ5 integrin receptors binding.
On the other hand, GFOGER functionalized PEG-BMP-2 hydrogels increased in vitro cell spreading and differentiation of BMSCs into osteogenic lineage, besides improved bone healing in mice radial defect model even in absence of BMP-2.[29] Implants coated with GFOGER were found to improve peri-implant bone regeneration and osseointegration[201]. However, hydroxyapatite disks adsorbed with GFOGER failed to attach cells with a decrease in cell spreading,[115] possibly due to a lack in nature-mimicking conformation of GFOGER.[31] P15-coated bovine bone grafts allowed faster recovery of periodontal defects in humans. [202] P-15-containing bone graft substitutes could facilitate the process of early bone formation[203,204], bone healing and regeneration[205]. P24 delivery to the defect site was achieved by encapsulating in chitosan microspheres,[50] covalent binding with polymeric backbone, [43] or simple adsorption on hydroxyapatite,[43] greatly enhancing the bioactive properties of the scaffolds.
DGEA immobilized on HA was found to enhance the adhesion and osteoblastic differentiation of MSCs, and new bone formation[206]. BCSP™-1, when delivered through HA and tricalcium phosphate (TCP) grafts, stimulated ALP activity in murine calvarial osteoprogenitor cells[207]. OGP rich PLGA scaffold accelerated bone healing in 1.5-cm rabbit segmental defects.[208] PEG hydrogel containing PTH1–34 promoted in situ bone augmentation in rabbits, [209] whereas TP508 loaded PPF composite and microsphere scaffolds showed enhanced bone formation in rabbit segmental bone defects. [210] RGD coated implants were found to have an increased peri-implant bone formation and enhanced direct bone apposition even in areas of poor surrounding bone. [147,211,212,213] An increased osteoblast density was observed, when anodized nanotubular titanium surface was coated with RGD or RGDS (Arg-Gly-Asp-Ser).[214,215]
6.2. Chondro-Inductive Scaffolds
Collagen type II-derived peptide conjugated zwitterionic carbon nano-dots (pCD) induced chondrogenic differentiation of ADMSCs. Pluronic F-127 hydrogel mediated delivery of pCD could successfully promote rabbit auricular cartilage defect healing after 60 days.[179] Self-assembled KLD12 and KLD12-CMP7 peptide hydrogels loaded into poly (L-lactide-co-caprolactone) scaffolds could successfully create a chondrogenic microenvironment to rat BMSCs seeded scaffolds after subcutaneous implantation in nude mice.[216] Self-assembling PA scaffolds loaded with a TGF-binding domain, HSNGLPLGGGSEEEAAAVVV(K)-CO(CH2)10CH3 could support viability and chondrogenic differentiation of hMSC, besides accelerated healing of articular cartilage defects in rabbit.[137] Similarly, HSNGLPL peptide coated porous chitosan scaffolds or gelatin methacryloyl (GelMA) hydrogel successfully induced chondrogenic differentiation of MSCs and recovery of osteochondral defects in mice and rabbit model.[217,218] RGD coating on the synthetic bioinert materials surface significantly improved properties like cell adhesion, viability, and differentiation. Composite scaffolds were prepared from RGD functionalized hydroxyapatite/methoxy poly (ethylene glycol)-block-poly(ε-caprolactone) (1:2 ratio) scaffold followed by infiltration of TGF-β1 functionalized glycidyl methacrylate-hyaluronic acid hydrogel. The scaffolds significantly upregulated the expression of cartilage-specific genes (aggrecan, Col2a1, Sox9) with higher accumulation of sulphated glycosaminoglycan (sGAG), whereas successful OC defect healing was also achieved after 12 weeks of implantation in rabbit knee defect model.[219]
6.3. Multifunctional Scaffolds
Subcutaneous injection of nanofibrous hollow microspheres conjugated with TGF-β1 mimetic CM10 combined with rabbit BMSCs successfully induced ectopic cartilage formation in nude mice via chondrogenic differentiation of MSCs; in contrast, BMP-2 mimetic P24 peptide-loaded microspheres demonstrated osteogenic activity leading to bone formation.[108] Implantation of 3D printed porous bi-layered scaffolds infiltrated with TGF-β1 binding HSNGLPLGG(MA) peptides-rich GelMA hydrogels in the top layer and hydroxyapatite in the bottom layer successfully repaired the osteochondral of defects in SD rats through simultaneous cartilage and bone regeneration, respectively.[220] Hyaluronic-acid-based hydrogel particles (HGPs) functionalized with cysteine-tagged CK2.1 peptide showed promising results for cartilage repair in a mouse model, without inducing chondrocyte hypertrophy, unlike BMP-2. [57] Whereas, using same model, CK2.2 and CK2.3 peptides induced osteoblast differentiation and mineralization, similar to BMP-2 [112]. RGD functionalized poly (ethylene glycol)-diacrylate (PEGDA) hydrogel encapsulating rat osteoblasts facilitated the adhesion and spreading of rat osteoblasts leading to matrix mineralization.[221] RGD immobilized on hydroxyapatite improved healing of femoral condylar defects in rabbits.[222] Though a longer chain RGD, was unable to do the same without an TGF-β supplementation.[223] RGD peptide attached to glass coverslips through silanization, did not show osteoblast adhesion, since attachment happens through integrin binding.[121]
Wang et al developed cryogenic 3D printed bilayer β-tricalcium phosphate/PLGA osteochondral scaffold, enriched with osteogenic peptide sequence, KIPKA SSVPT ELSAI STLYL SGGC, and TGF-β1 loaded collagen I hydrogel for cartilage repair. The osteochondral scaffold showed improved chondrogenic differentiation of rBMSCs, by up-regulated expression of chondrogenic markers and glycosaminoglycan (GAG) production, while enhanced osteogenic differentiation of rBMSCs at the subchondral layer by up-regulated expression RUNX2, osteocalcin and alkaline phosphatase (ALP), and calcium deposition.[224]
Due to the bactericidal or anti-biofilm, immunomodulatory, and regenerative capacity, AMPs with adequate cytotocompatibility have been explored for bone and cartilage tissue regeneration. Titanium surface immobilized with Mel4 peptides demonstrated significant antimicrobial efficacy after implantation into the rabbit femoral defects.[225] While GL13K peptide (GKIIKLKASLKLL-NH2) coated titanium surface could effectively inhibit peri-implantitis through immunomodulatory function[226,227] Titanium plate modified with antimicrobial LF1-11-coupled RGD peptide demonstrated good antibacterial properties with improved osteoblast adhesion, proliferation and mineralization.[228] Inclusion of HHC-36 (KRWWKWWRR), a broad-spectrum cationic AMP, and osteoconductive Laponite nanosilicates into GelMA hydrogel, which was used for coating of titanium implant surface significantly promoted expression of osteogenic-related genes and mineralization of hMSCs, besides effective inhibition of Gram-positive bacteria (S. aureus and S. epidermidis) and Gram-negative bacteria (P. aeruginosa and E. coli).[229] On the other hand, HA bone scaffolds coated with PSI10 (RRWPWWPWRR) and BMP2-MP [230] or a nano-hydroxyapatite coated titanium surface containing antimicrobial human β-defensin 3 (HBD-3) peptide and BMP-2,[231] significantly promoted antibacterial and osteogenic activity of the scaffolds.
Multifunctional PEEK coated with lithium-ion and mussel-inspired antimicrobial peptide enhanced osteogenesis-associated genes/proteins expression, and osseointegration at bone-implant interface, besides inhibition of E. coli and S. aureus.[232] Coated with mouse beta-defensin-14 (MBD-14) also improved antimicrobial and osseointegration properties of PEEK.[233] KR-12 analogue immobilized on the surface of titanium or PEEK bone implants via a polydopamine coating showed remarkable antibacterial activity, osteogenic differentiation of MSCs and peri-implant bone formation.[234,235]
7. Summary and Outlook
The goal of bioengineering is tissue emulation, including mimicking ECM and facilitating cell-material interaction, as well as tissue shape, function, and the in vivo milieu. Peptides, as the cell-instructive molecular building blocks, can bind or co-assemble with ECM or synthetic biomaterials, that not only offer structural and functional complexity, but also additively or synergistically work for better cellular activity, immune response, or tissue remodeling. A significant number of peptides were investigated as potential candidates for the promoting bone and cartilage healing, either directly by promoting cell adhesion, migration, angiogenesis, and differentiation into osteo/chondrogenic lineage, or indirectly by helping cargo penetration into the cells. Considering the structural and functional heterogenicity of an osteochondral unit, a strategy combining multiple peptides with biomaterials for simultaneous bone and cartilage tissue regeneration has been proposed. For example, a collagen-mimetic GFOGER or P15 peptide, or a BMP-2 mimetic P24 or BFP-2 peptide can be combined with VEGF mimetic QK peptides or TP508 or synthetic RoY peptides for osteo-inductive effect. On the other hand, a TGF-β or BMP-2 mimetic peptide can be used to induce chondrogenic activity in conjunction with RGD or PHSRN conjugated hydrogel for improved adhesion and proliferation, or SDF1-ELP or MMP-cleavable peptide-based hydrogels for cell migration into the hydrogel matrix. Hydrogel nanocomposites with improved mechanical strength and biological properties may be created by co-assembling peptides with structural proteins or inorganic fillers or by short SA peptides with macromolecules. Peptides that prevent microbial infection or help pro-inflammatory M1 to anti-inflammatory M2 macrophage polarization can be added to overcome post-implantation issues. The advent of electrospinning, inkjet printing, bioprinting, and other advanced manufacturing strategies enable fabrication of peptide hydrogel constructs with complex geometry and multi-scale hierarchy resembling native tissue. More focus should be given on investigating phage display or artificial intelligence (AI) and machine learning (ML) tools, which can provide a pattern-based search to find novel sequences or bioactive motifs from full-length proteins by establishing rules for protein interactions and signaling.
Acknowledgments
The work was supported by NIPER Kolkata and the Department of Pharmaceuticals, Ministry of Chemicals and Fertilizers, Government of India.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Yildirim, N.; Amanzhanova, A.; Kulzhanova, G.; Mukasheva, F.; Erisken, C. Osteochondral interface: regenerative engineering and challenges. ACS Biomaterials Science & Engineering 2023, 9, 1205–1223. [Google Scholar]
- Steinmetz, J.D.; Culbreth, G.T.; Haile, L.M.; Rafferty, Q.; Lo, J.; Fukutaki, K.G.; Cruz, J.A.; Smith, A.E.; Vollset, S.E.; Brooks, P.M. Global, regional, and national burden of osteoarthritis, 1990–2020 and projections to 2050: a systematic analysis for the Global Burden of Disease Study 2021. The Lancet Rheumatology 2023, 5, e508–e522. [Google Scholar] [CrossRef] [PubMed]
- Howell, M.; Liao, Q.; Gee, C.W. Surgical management of osteochondral defects of the knee: an educational review. Current Reviews in Musculoskeletal Medicine 2021, 14, 60–66. [Google Scholar] [CrossRef]
- Zhu, M.; Zhong, W.; Cao, W.; Zhang, Q.; Wu, G. Chondroinductive/chondroconductive peptides and their-functionalized biomaterials for cartilage tissue engineering. Bioactive materials 2022, 9, 221–238. [Google Scholar] [CrossRef] [PubMed]
- Madry, H. Surgical therapy in osteoarthritis. Osteoarthritis and cartilage 2022, 30, 1019–1034. [Google Scholar] [CrossRef]
- Wei, W.; Dai, H. Articular cartilage and osteochondral tissue engineering techniques: Recent advances and challenges. Bioactive materials 2021, 6, 4830–4855. [Google Scholar] [CrossRef]
- Makris, E.A.; Gomoll, A.H.; Malizos, K.N.; Hu, J.C.; Athanasiou, K.A. Repair and tissue engineering techniques for articular cartilage. Nature Reviews Rheumatology 2015, 11, 21–34. [Google Scholar] [CrossRef]
- Chen, L.; Wei, L.; Su, X.; Qin, L.; Xu, Z.; Huang, X.; Chen, H.; Hu, N. Preparation and Characterization of Biomimetic Functional Scaffold with Gradient Structure for Osteochondral Defect Repair. Bioengineering 2023, 10, 213. [Google Scholar] [CrossRef]
- Rizzo, M.G.; Palermo, N.; D’Amora, U.; Oddo, S.; Guglielmino, S.P.P.; Conoci, S.; Szychlinska, M.A.; Calabrese, G. Multipotential Role of Growth Factor Mimetic Peptides for Osteochondral Tissue Engineering. International Journal of Molecular Sciences 2022, 23, 7388. [Google Scholar] [CrossRef]
- Gonçalves, A.M.; Moreira, A.; Weber, A.; Williams, G.R.; Costa, P.F. Osteochondral tissue engineering: The potential of electrospinning and additive manufacturing. Pharmaceutics 2021, 13, 983. [Google Scholar] [CrossRef]
- Bullock, G.; Atkinson, J.; Gentile, P.; Hatton, P.; Miller, C. Osteogenic peptides and attachment methods determine tissue regeneration in modified bone graft substitutes. Journal of Functional Biomaterials 2021, 12, 22. [Google Scholar] [CrossRef] [PubMed]
- Visser, R.; Rico-Llanos, G.A.; Pulkkinen, H.; Becerra, J. Peptides for bone tissue engineering. Journal of controlled release 2016, 244, 122–135. [Google Scholar] [CrossRef] [PubMed]
- Pountos, I.; Panteli, M.; Lampropoulos, A.; Jones, E.; Calori, G.M.; Giannoudis, P.V. The role of peptides in bone healing and regeneration: a systematic review. BMC medicine 2016, 14, 1–15. [Google Scholar] [CrossRef]
- Ai, C.; Lee, Y.H.D.; Tan, X.H.; Tan, S.H.S.; Hui, J.H.P.; Goh, J.C.-H. Osteochondral tissue engineering: perspectives for clinical application and preclinical development. Journal of Orthopaedic Translation 2021, 30, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Le, H.; Wang, Y.; Liu, H.; Li, Z.; Yang, X.; Wang, C.; Ding, J.; Chen, X. Instructive cartilage regeneration modalities with advanced therapeutic implantations under abnormal conditions. Bioactive Materials 2022, 11, 317–338. [Google Scholar] [CrossRef] [PubMed]
- Bittner, S.M.; Guo, J.L.; Melchiorri, A.; Mikos, A.G. Three-dimensional printing of multilayered tissue engineering scaffolds. Materials Today 2018, 21, 861–874. [Google Scholar] [CrossRef] [PubMed]
- Kapat, K.; Rameshbabu, A.P.; Maity, P.P.; Mandal, A.; Bankoti, K.; Dutta, J.; Das, D.K.; Dey, G.; Mandal, M.; Dhara, S. Osteochondral defects healing using extracellular matrix mimetic phosphate/sulfate decorated GAGs-agarose gel and quantitative micro-CT evaluation. ACS Biomaterials Science & Engineering 2018, 5, 149–164. [Google Scholar]
- Bittner, S.M.; Guo, J.L.; Mikos, A.G. Spatiotemporal control of growth factors in three-dimensional printed scaffolds. Bioprinting 2018, 12, e00032. [Google Scholar] [CrossRef]
- Gibson, J.D.; O’Sullivan, M.B.; Alaee, F.; Paglia, D.N.; Yoshida, R.; Guzzo, R.M.; Drissi, H. Regeneration of articular cartilage by human ESC-derived mesenchymal progenitors treated sequentially with BMP-2 and Wnt5a. Stem cells translational medicine 2017, 6, 40–50. [Google Scholar] [CrossRef]
- McGee-Lawrence, M.E.; Carpio, L.R.; Bradley, E.W.; Dudakovic, A.; Lian, J.B.; van Wijnen, A.J.; Kakar, S.; Hsu, W.; Westendorf, J.J. Runx2 is required for early stages of endochondral bone formation but delays final stages of bone repair in Axin2-deficient mice. Bone 2014, 66, 277–286. [Google Scholar] [CrossRef]
- Du, X.; Xie, Y.; Xian, C.J.; Chen, L. Role of FGFs/FGFRs in skeletal development and bone regeneration. Journal of cellular physiology 2012, 227, 3731–3743. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.L.; Diaz-Gomez, L.; Xie, V.Y.; Bittner, S.M.; Jiang, E.Y.; Wang, B.; Mikos, A.G. Three-dimensional printing of click functionalized, peptide patterned scaffolds for osteochondral tissue engineering. Bioprinting 2021, 22, e00136. [Google Scholar] [CrossRef] [PubMed]
- Einhorn, T.A.; Gerstenfeld, L.C. Fracture healing: mechanisms and interventions. Nature Reviews Rheumatology 2015, 11, 45–54. [Google Scholar] [CrossRef]
- Pozos-Guillén, A.; Molina, G.; Soviero, V.; Arthur, R.A.; Chavarria-Bolaños, D.; Acevedo, A.M. Management of dental caries lesions in Latin American and Caribbean countries. Brazilian Oral Research 2021, 35, e055. [Google Scholar] [CrossRef]
- Mehta, M.; Schmidt-Bleek, K.; Duda, G.N.; Mooney, D.J. Biomaterial delivery of morphogens to mimic the natural healing cascade in bone. Advanced drug delivery reviews 2012, 64, 1257–1276. [Google Scholar] [CrossRef]
- Gerber, H.-P.; Vu, T.H.; Ryan, A.M.; Kowalski, J.; Werb, Z.; Ferrara, N. VEGF couples hypertrophic cartilage remodeling, ossification and angiogenesis during endochondral bone formation. Nature medicine 1999, 5, 623–628. [Google Scholar] [CrossRef] [PubMed]
- Calori, G.; Albisetti, W.; Agus, A.; Iori, S.; Tagliabue, L. Risk factors contributing to fracture non-unions. Injury 2007, 38, S11–S18. [Google Scholar] [CrossRef] [PubMed]
- Reyes, C.D.; Petrie, T.A.; Burns, K.L.; Schwartz, Z.; García, A.J. Biomolecular surface coating to enhance orthopaedic tissue healing and integration. Biomaterials 2007, 28, 3228–3235. [Google Scholar] [CrossRef]
- Shekaran, A.; García, J.R.; Clark, A.Y.; Kavanaugh, T.E.; Lin, A.S.; Guldberg, R.E.; García, A.J. Bone regeneration using an alpha 2 beta 1 integrin-specific hydrogel as a BMP-2 delivery vehicle. Biomaterials 2014, 35, 5453–5461. [Google Scholar] [CrossRef]
- Connelly, J.; Petrie, T.; García, A.; Levenston, M. Fibronectin-and Collagen-Mimetic Ligands Regulate BMSC Chondrogenesis in 3D Hydrogels. European cells & materials 2011, 22, 168. [Google Scholar]
- Wojtowicz, A.M.; Shekaran, A.; Oest, M.E.; Dupont, K.M.; Templeman, K.L.; Hutmacher, D.W.; Guldberg, R.E.; García, A.J. Coating of biomaterial scaffolds with the collagen-mimetic peptide GFOGER for bone defect repair. Biomaterials 2010, 31, 2574–2582. [Google Scholar] [CrossRef] [PubMed]
- Kolambkar, Y.M.; Bajin, M.; Wojtowicz, A.; Hutmacher, D.W.; García, A.J.; Guldberg, R.E. Nanofiber orientation and surface functionalization modulate human mesenchymal stem cell behavior in vitro. Tissue Engineering Part A 2014, 20, 398–409. [Google Scholar] [CrossRef] [PubMed]
- Bhatnagar, R.S.; Qian, J.J.; Wedrychowska, A.; Sadeghi, M.; Wu, Y.M.; Smith, N. Design of biomimetic habitats for tissue engineering with P-15, a synthetic peptide analogue of collagen. Tissue Engineering 1999, 5, 53–65. [Google Scholar] [CrossRef] [PubMed]
- Hestehave Pedersen, R.; Rasmussen, M.; Overgaard, S.; Ding, M. Effects of P-15 peptide coated hydroxyapatite on Tibial defect repair in vivo in Normal and osteoporotic rats. BioMed Research International 2015, 2015. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, C.M.; Koepple, M.; Moest, T.; Neumann, K.; Weisel, T.; Schlegel, K.A. In vivo evaluation of biofunctionalized implant surfaces with a synthetic peptide (P-15) and its impact on osseointegration. A preclinical animal study. Clinical Oral Implants Research 2016, 27, 1339–1348. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.A.; Taylor, N.L.; Jalan, A.A.; Hwang, L.K.; Wang, B.K.; Hartgerink, J.D. A nanostructured synthetic collagen mimic for hemostasis. Biomacromolecules 2014, 15, 1484–1490. [Google Scholar] [PubMed]
- Uysal, O.; Arslan, E.; Gulseren, G.; Kilinc, M.C.; Dogan, I.; Ozalp, H.; Caglar, Y.S.; Guler, M.O.; Tekinay, A.B. Collagen peptide presenting nanofibrous scaffold for intervertebral disc regeneration. ACS Applied Bio Materials 2019, 2, 1686–1695. [Google Scholar] [PubMed]
- Harbers, G.M.; Healy, K.E. The effect of ligand type and density on osteoblast adhesion, proliferation, and matrix mineralization. Journal of Biomedical Materials Research Part A: An Official Journal of The Society for Biomaterials, The Japanese Society for Biomaterials, and The Australian Society for Biomaterials and the Korean Society for Biomaterials 2005, 75, 855–869. [Google Scholar] [CrossRef]
- Anderson, J.M.; Vines, J.B.; Patterson, J.L.; Chen, H.; Javed, A.; Jun, H.-W. Osteogenic differentiation of human mesenchymal stem cells synergistically enhanced by biomimetic peptide amphiphiles combined with conditioned medium. Acta biomaterialia 2011, 7, 675–682. [Google Scholar]
- Sindrey, D.; Pugh, S.; Smith, T. Connective Tissue Stimulating Peptides. US20050288229A1, US Patent 2005/02888229 A1. 2005. [Google Scholar]
- Huang, W.; Yu, K.; Kang, M.; Wang, Q.; Liao, W.; Liang, P.; Liu, G.; Cao, Y.; Miao, J. Identification and functional analysis of three novel osteogenic peptides isolated from tilapia scale collagen hydrolysate. Food Research International 2022, 162, 111993. [Google Scholar] [CrossRef]
- Lam, H.; Li, S.; Lou, N.; Chu, J.; Bhatnagar, R. Synthetic peptides cytomodulin-1 (CM-1) and cytomodulin-2 (CM-2) promote collagen synthesis and wound healing in vitro. In Proceedings of the The 26th Annual International Conference of the IEEE Engineering in Medicine and Biology Society; 2004; pp. 5028–5030. [Google Scholar]
- Lin, Z.-Y.; Duan, Z.-X.; Guo, X.-D.; Li, J.-F.; Lu, H.-W.; Zheng, Q.-X.; Quan, D.-P.; Yang, S.-H. Bone induction by biomimetic PLGA-(PEG-ASP) n copolymer loaded with a novel synthetic BMP-2-related peptide in vitro and in vivo. Journal of Controlled Release 2010, 144, 190–195. [Google Scholar] [CrossRef]
- Duan, Z.; Zheng, Q.; Guo, X.; Yuan, Q.; Chen, S. Experimental research on ectopic osteogenesis of BMP2-derived peptide P24 combined with PLGA copolymers. Journal of Huazhong University of Science and Technology 2007, 27, 179–182. [Google Scholar] [CrossRef]
- Saito, A.; Suzuki, Y.; Ogata, S.I.; Ohtsuki, C.; Tanihara, M. Prolonged ectopic calcification induced by BMP-2–derived synthetic peptide. Journal of Biomedical Materials Research Part A: An Official Journal of The Society for Biomaterials, The Japanese Society for Biomaterials, and The Australian Society for Biomaterials and the Korean Society for Biomaterials 2004, 70, 115–121. [Google Scholar] [CrossRef]
- Kang, E.-J.; Kim, S.-K.; Eom, T.-G.; Choi, K.-O.; Lee, T.-H. Evaluation of the osteogenic activity of the BMP-2 mimetic peptide, PEP7, in vitro and in vivo. International Journal of Oral & Maxillofacial Implants 2013, 28. [Google Scholar]
- Saito, A.; Suzuki, Y.; Ogata, S.-i.; Ohtsuki, C.; Tanihara, M. Activation of osteo-progenitor cells by a novel synthetic peptide derived from the bone morphogenetic protein-2 knuckle epitope. Biochimica et Biophysica Acta (BBA)-Proteins and Proteomics 2003, 1651, 60–67. [Google Scholar] [CrossRef]
- Moore, N.M.; Lin, N.J.; Gallant, N.D.; Becker, M.L. Synergistic enhancement of human bone marrow stromal cell proliferation and osteogenic differentiation on BMP-2-derived and RGD peptide concentration gradients. Acta biomaterialia 2011, 7, 2091–2100. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Ma, J.; Jabbari, E. Effect of grafting RGD and BMP-2 protein-derived peptides to a hydrogel substrate on osteogenic differentiation of marrow stromal cells. Langmuir 2008, 24, 12508–12516. [Google Scholar] [CrossRef]
- Niu, X.; Feng, Q.; Wang, M.; Guo, X.; Zheng, Q. Porous nano-HA/collagen/PLLA scaffold containing chitosan microspheres for controlled delivery of synthetic peptide derived from BMP-2. Journal of Controlled Release 2009, 134, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.-J.; Yeo, S.-I.; Park, J.-W.; Shin, H.-I.; Bae, Y.-C.; Suh, J.-Y. The effects of synthetic peptide derived from hBMP-2 on bone formation in rabbit calvarial defect. Tissue Eng Regen Me 2008, 5, 488–497. [Google Scholar]
- Lee, J.S.; Lee, J.S.; Murphy, W.L. Modular peptides promote human mesenchymal stem cell differentiation on biomaterial surfaces. Acta biomaterialia 2010, 6, 21–28. [Google Scholar] [CrossRef]
- Saito, A.; Suzuki, Y.; Kitamura, M.; Ogata, S.I.; Yoshihara, Y.; Masuda, S.; Ohtsuki, C.; Tanihara, M. Repair of 20-mm long rabbit radial bone defects using BMP-derived peptide combined with an α-tricalcium phosphate scaffold. Journal of Biomedical Materials Research Part A: An Official Journal of The Society for Biomaterials, The Japanese Society for Biomaterials, and The Australian Society for Biomaterials and the Korean Society for Biomaterials 2006, 77, 700–706. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.K.; Lee, J.S.; Kim, J.H.; Seon, J.K.; Park, K.S.; Jeong, M.H.; Yoon, T.R. Bone-forming peptide-2 derived from BMP-7 enhances osteoblast differentiation from multipotent bone marrow stromal cells and bone formation. Experimental & molecular medicine 2017, 49, e328–e328. [Google Scholar]
- Patterson, J. Peptide-functionalized Biomaterials with Osteoinductive or Anti-biofilm Activity. Racing for the Surface: Antimicrobial and Interface Tissue Engineering, 2020; 129–168. [Google Scholar]
- Bragdon, B.; Thinakaran, S.; Moseychuk, O.; Gurski, L.; Bonor, J.; Price, C.; Wang, L.; Beamer, W.G.; Nohe, A. Casein kinase 2 regulates in vivo bone formation through its interaction with bone morphogenetic protein receptor type Ia. Bone 2011, 49, 944–954. [Google Scholar] [CrossRef] [PubMed]
- Akkiraju, H.; Bonor, J.; Nohe, A. CK2. 1, a novel peptide, induces articular cartilage formation in vivo. Journal of Orthopaedic Research 2017, 35, 876–885. [Google Scholar] [CrossRef] [PubMed]
- Akkiraju, H.; Bonor, J.; Olli, K.; Bowen, C.; Bragdon, B.; Coombs, H.; Donahue, L.R.; Duncan, R.; Nohe, A. Systemic injection of CK2. 3, a novel peptide acting downstream of bone morphogenetic protein receptor BMPRIa, leads to increased trabecular bone mass. Journal of Orthopaedic Research 2015, 33, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Alkhiary, Y.M.; Gerstenfeld, L.C.; Krall, E.; Westmore, M.; Sato, M.; Mitlak, B.H.; Einhorn, T.A. Enhancement of experimental fracture-healing by systemic administration of recombinant human parathyroid hormone (PTH 1-34). JBJS 2005, 87, 731–741. [Google Scholar]
- Komrakova, M.; Stuermer, E.K.; Werner, C.; Wicke, M.; Kolios, L.; Sehmisch, S.; Tezval, M.; Daub, F.; Martens, T.; Witzenhausen, P. Effect of human parathyroid hormone hPTH (1–34) applied at different regimes on fracture healing and muscle in ovariectomized and healthy rats. Bone 2010, 47, 480–492. [Google Scholar] [CrossRef]
- Mognetti, B.; Marino, S.; Barberis, A.; Bravo Martin, A.-S.; Bala, Y.; Di Carlo, F.; Boivin, G.; Portigliatti Barbos, M. Experimental stimulation of bone healing with teriparatide: histomorphometric and microhardness analysis in a mouse model of closed fracture. Calcified tissue international 2011, 89, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Chintamaneni, S.; Finzel, K.; Gruber, B. Successful treatment of sternal fracture nonunion with teriparatide. Osteoporosis international 2010, 21, 1059–1063. [Google Scholar] [CrossRef]
- Whitfield, J.F.; Motley, P.; Willick, G.E. Parathyroid hormone, its fragments and their analogs for the treatment of osteoporosis. Treatments in Endocrinology 2002, 1, 175–190. [Google Scholar] [CrossRef]
- Peggion, E.; Mammi, S.; Schievano, E.; Silvestri, L.; Schiebler, L.; Bisello, A.; Rosenblatt, M.; Chorev, M. Structure− Function Studies of Analogues of Parathyroid Hormone (PTH)-1− 34 Containing β-Amino Acid Residues in Positions 11− 13. Biochemistry 2002, 41, 8162–8175. [Google Scholar] [CrossRef] [PubMed]
- de Castro, L.F.; Lozano, D.; Portal-Núñez, S.; Maycas, M.; De la Fuente, M.; Caeiro, J.R.; Esbrit, P. Comparison of the skeletal effects induced by daily administration of PTHrP (1–36) and PTHrP (107–139) to ovariectomized mice. Journal of cellular physiology 2012, 227, 1752–1760. [Google Scholar] [CrossRef]
- Trejo, C.G.; Lozano, D.; Manzano, M.; Doadrio, J.C.; Salinas, A.J.; Dapía, S.; Gómez-Barrena, E.; Vallet-Regí, M.; García-Honduvilla, N.; Buján, J. The osteoinductive properties of mesoporous silicate coated with osteostatin in a rabbit femur cavity defect model. Biomaterials 2010, 31, 8564–8573. [Google Scholar] [CrossRef] [PubMed]
- Mrak, E.; Guidobono, F.; Moro, G.; Fraschini, G.; Rubinacci, A.; Villa, I. Calcitonin gene-related peptide (CGRP) inhibits apoptosis in human osteoblasts by β-catenin stabilization. Journal of cellular physiology 2010, 225, 701–708. [Google Scholar] [CrossRef] [PubMed]
- Villa, I.; Melzi, R.; Pagani, F.; Ravasi, F.; Rubinacci, A.; Guidobono, F. Effects of calcitonin gene-related peptide and amylin on human osteoblast-like cells proliferation. European journal of pharmacology 2000, 409, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Shi, X.; Zhao, R.; Halloran, B.P.; Clark, D.J.; Jacobs, C.R.; Kingery, W.S. Calcitonin-gene-related peptide stimulates stromal cell osteogenic differentiation and inhibits RANKL induced NF-κB activation, osteoclastogenesis and bone resorption. Bone 2010, 46, 1369–1379. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Jiang, D. The role and mechanism of exogenous calcitonin gene-related peptide on mesenchymal stem cell proliferation and osteogenetic formation. Cell biochemistry and biophysics 2014, 69, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Calland, J.W.; Harris, S.E.; Carnes Jr, D.L. Human pulp cells respond to calcitonin gene-related peptide in vitro. Journal of endodontics 1997, 23, 485–489. [Google Scholar] [CrossRef] [PubMed]
- Vignery, A.; McCarthy, T. The neuropeptide calcitonin gene-related peptide stimulates insulin-like growth factor I production by primary fetal rat osteoblasts. Bone 1996, 18, 331–335. [Google Scholar] [CrossRef]
- Ballica, R.; Valentijn, K.; Khachatryan, A.; Guerder, S.; Kapadia, S.; Gundberg, C.; Gilligan, J.; Flavell, R.A.; Vignery, A. Targeted expression of calcitonin gene-related peptide to osteoblasts increases bone density in mice. Journal of bone and mineral research 1999, 14, 1067–1074. [Google Scholar] [CrossRef]
- Bab, I.; Gazit, D.; Chorev, M.; Muhlrad, A.; Shteyer, A.; Greenberg, Z.; Namdar, M.; Kahn, A. Histone H4-related osteogenic growth peptide (OGP): a novel circulating stimulator of osteoblastic activity. The EMBO journal 1992, 11, 1867–1873. [Google Scholar] [CrossRef] [PubMed]
- Gabet, Y.; Müller, R.; Regev, E.; Sela, J.; Shteyer, A.; Salisbury, K.; Chorev, M.; Bab, I. Osteogenic growth peptide modulates fracture callus structural and mechanical properties. Bone 2004, 35, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Brager, M.A.; Patterson, M.J.; Connolly, J.F.; Nevo, Z. Osteogenic growth peptide normally stimulated by blood loss and marrow ablation has local and systemic effects on fracture healing in rats. Journal of Orthopaedic Research 2000, 18, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Fei, Q.; Guo, C.; Xu, X.; Gao, J.; Zhang, J.; Chen, T.; Cui, D. Osteogenic growth peptide enhances the proliferation of bone marrow mesenchymal stem cells from osteoprotegerin-deficient mice by CDK2/cyclin A. Acta Biochim Biophys Sin 2010, 42, 801–806. [Google Scholar] [CrossRef]
- An, G.; Xue, Z.; Zhang, B.; Deng, Q.; Wang, Y.; Lv, S. Expressing osteogenic growth peptide in the rabbit bone mesenchymal stem cells increased alkaline phosphatase activity and enhanced the collagen accumulation. Eur Rev Med Pharmacol Sci 2014, 18, 1618–1624. [Google Scholar] [PubMed]
- Li, G.; Cui, Y.; McILmurray, L.; Allen, W.E.; Wang, H. rhBMP-2, rhVEGF165, rhPTN and thrombin-related peptide, TP508 induce chemotaxis of human osteoblasts and microvascular endothelial cells. Journal of orthopaedic research 2005, 23, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Vordemvenne, T.; Paletta, J.R.; Hartensuer, R.; Pap, T.; Raschke, M.J.; Ochman, S. Cooperative effects in differentiation and proliferation between PDGF-BB and matrix derived synthetic peptides in human osteoblasts. BMC musculoskeletal disorders 2011, 12, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Olszewska-Pazdrak, B.; Carney, D.H. Systemic administration of thrombin peptide TP508 enhances VEGF-stimulated angiogenesis and attenuates effects of chronic hypoxia. Journal of vascular research 2013, 50, 186–196. [Google Scholar] [CrossRef]
- Hanratty, B.M.; Ryaby, J.T.; Pan, X.-H.; Li, G. Thrombin related peptide TP508 promoted fracture repair in a mouse high energy fracture model. Journal of Orthopaedic Surgery and Research 2009, 4, 1–10. [Google Scholar] [CrossRef]
- Li, X.; Wang, H.; Touma, E.; Qi, Y.; Rousseau, E.; Quigg, R.J.; Ryaby, J.T. TP508 accelerates fracture repair by promoting cell growth over cell death. Biochemical and biophysical research communications 2007, 364, 187–193. [Google Scholar] [CrossRef]
- Wang, H.; Li, X.; Tomin, E.; Doty, S.B.; Lane, J.M.; Carney, D.H.; Ryaby, J.T. Thrombin peptide (TP508) promotes fracture repair by up-regulating inflammatory mediators, early growth factors, and increasing angiogenesis. Journal of orthopaedic research 2005, 23, 671–679. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-J.; Park, Y.-J.; Lee, Y.-M.; Rhyu, I.-C.; Ku, Y. The biological effects of fibrin-binding synthetic oligopeptides derived from fibronectin on osteoblast-like cells. Journal of Periodontal & Implant Science 2012, 42, 113–118. [Google Scholar]
- Lee, J.-A.; Ku, Y.; Rhyu, I.-C.; Chung, C.-P.; Park, Y.-J. Effects of fibrin-binding oligopeptide on osteopromotion in rabbit calvarial defects. Journal of periodontal & implant science 2010, 40, 211–219. [Google Scholar]
- Martino, M.M.; Tortelli, F.; Mochizuki, M.; Traub, S.; Ben-David, D.; Kuhn, G.A.; Müller, R.; Livne, E.; Eming, S.A.; Hubbell, J.A. Engineering the growth factor microenvironment with fibronectin domains to promote wound and bone tissue healing. Science translational medicine 2011, 3, 100ra189. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-Y.; Choo, J.-E.; Park, H.-J.; Park, J.-B.; Lee, S.-C.; Jo, I.; Lee, S.-J.; Chung, C.-P.; Park, Y.-J. Injectable gel with synthetic collagen-binding peptide for enhanced osteogenesis in vitro and in vivo. Biochemical and biophysical research communications 2007, 357, 68–74. [Google Scholar] [CrossRef]
- Shin, M.K.; Kim, M.-K.; Bae, Y.-S.; Jo, I.; Lee, S.-J.; Chung, C.-P.; Park, Y.-J. A novel collagen-binding peptide promotes osteogenic differentiation via Ca2+/calmodulin-dependent protein kinase II/ERK/AP-1 signaling pathway in human bone marrow-derived mesenchymal stem cells. Cellular signalling 2008, 20, 613–624. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-Y.; Choo, J.-E.; Choi, Y.-S.; Park, J.-B.; Min, D.-S.; Lee, S.-J.; Rhyu, H.K.; Jo, I.-H.; Chung, C.-P.; Park, Y.-J. Assembly of collagen-binding peptide with collagen as a bioactive scaffold for osteogenesis in vitro and in vivo. Biomaterials 2007, 28, 4257–4267. [Google Scholar] [CrossRef]
- Egusa, H.; Kaneda, Y.; Akashi, Y.; Hamada, Y.; Matsumoto, T.; Saeki, M.; Thakor, D.K.; Tabata, Y.; Matsuura, N.; Yatani, H. Enhanced bone regeneration via multimodal actions of synthetic peptide SVVYGLR on osteoprogenitors and osteoclasts. Biomaterials 2009, 30, 4676–4686. [Google Scholar] [CrossRef] [PubMed]
- Hamada, Y.; Yuki, K.; Okazaki, M.; Fujitani, W.; Matsumoto, T.; Hashida, M.K.; Harutsugu, K.; Nokihara, K.; Daito, M.; Matsuura, N. Osteopontin-derived peptide SVVYGLR induces angiogenesis in vivo. Dental materials journal 2004, 23, 650–655. [Google Scholar] [CrossRef]
- Park, K.M.; Lee, Y.; Son, J.Y.; Bae, J.W.; Park, K.D. In situ SVVYGLR peptide conjugation into injectable gelatin-poly (ethylene glycol)-tyramine hydrogel via enzyme-mediated reaction for enhancement of endothelial cell activity and neo-vascularization. Bioconjugate chemistry 2012, 23, 2042–2050. [Google Scholar] [CrossRef]
- Rezania, A.; Healy, K.E. Biomimetic peptide surfaces that regulate adhesion, spreading, cytoskeletal organization, and mineralization of the matrix deposited by osteoblast-like cells. Biotechnology progress 1999, 15, 19–32. [Google Scholar] [CrossRef] [PubMed]
- Stile, R.A.; Healy, K.E. Thermo-responsive peptide-modified hydrogels for tissue regeneration. Biomacromolecules 2001, 2, 185–194. [Google Scholar] [CrossRef]
- Van Hove, A.H.; Benoit, D.S. Depot-based delivery systems for pro-angiogenic peptides: a review. Frontiers in bioengineering and biotechnology 2015, 3, 102. [Google Scholar] [CrossRef] [PubMed]
- Finetti, F.; Basile, A.; Capasso, D.; Di Gaetano, S.; Di Stasi, R.; Pascale, M.; Turco, C.M.; Ziche, M.; Morbidelli, L.; D’Andrea, L.D. Functional and pharmacological characterization of a VEGF mimetic peptide on reparative angiogenesis. Biochemical pharmacology 2012, 84, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.R.; Silva, E.A.; Yuen, W.W.; Mooney, D.J. Spatio–temporal VEGF and PDGF delivery patterns blood vessel formation and maturation. Pharmaceutical research 2007, 24, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.-M.; Kang, Y.; Chun, H.J.; Jeong, J.-W.; Park, C. Evaluation of the in vitro and in vivo angiogenic effects of exendin-4. Biochemical and biophysical research communications 2013, 434, 150–154. [Google Scholar] [CrossRef] [PubMed]
- Hamada, Y.; Nokihara, K.; Okazaki, M.; Fujitani, W.; Matsumoto, T.; Matsuo, M.; Umakoshi, Y.; Takahashi, J.; Matsuura, N. Angiogenic activity of osteopontin-derived peptide SVVYGLR. Biochemical and biophysical research communications 2003, 310, 153–157. [Google Scholar] [CrossRef] [PubMed]
- Hamada, Y.; Egusa, H.; Kaneda, Y.; Hirata, I.; Kawaguchi, N.; Hirao, T.; Matsumoto, T.; Yao, M.; Daito, K.; Suzuki, M. Synthetic osteopontin-derived peptide SVVYGLR can induce neovascularization in artificial bone marrow scaffold biomaterials. Dental materials journal 2007, 26, 487–492. [Google Scholar] [CrossRef] [PubMed]
- Lane, T.F.; Iruela-Arispe, M.L.; Johnson, R.S.; Sage, E.H. SPARC is a source of copper-binding peptides that stimulate angiogenesis. The Journal of cell biology 1994, 125, 929–943. [Google Scholar] [CrossRef]
- Van Hove, A.H.; Burke, K.; Antonienko, E.; Brown III, E.; Benoit, D.S. Enzymatically-responsive pro-angiogenic peptide-releasing poly (ethylene glycol) hydrogels promote vascularization in vivo. Journal of Controlled Release 2015, 217, 191–201. [Google Scholar] [CrossRef]
- Hardy, B.; Raiter, A.; Weiss, C.; Kaplan, B.; Tenenbaum, A.; Battler, A. Angiogenesis induced by novel peptides selected from a phage display library by screening human vascular endothelial cells under different physiological conditions. Peptides 2007, 28, 691–701. [Google Scholar] [CrossRef]
- Wang, W.; Rigueur, D.; Lyons, K.M. TGFβ signaling in cartilage development and maintenance. Birth Defects Research Part C: Embryo Today: Reviews 2014, 102, 37–51. [Google Scholar] [CrossRef] [PubMed]
- Bhatnagar, R.S.; Qian, J.J. Peptide compositions with growth factor-like activity. 1997.
- Renner, J.N.; Liu, J.C. Investigating the effect of peptide agonists on the chondrogenic differentiation of human mesenchymal stem cells using design of experiments. Biotechnology Progress 2013, 29, 1550–1557. [Google Scholar] [CrossRef]
- Zhang, Z.; Gupte, M.J.; Jin, X.; Ma, P.X. Injectable peptide decorated functional nanofibrous hollow microspheres to direct stem cell differentiation and tissue regeneration. Advanced functional materials 2015, 25, 350–360. [Google Scholar] [CrossRef] [PubMed]
- Mittal, A.; Kumar, R.; Parsad, D.; Kumar, N. Cytomodulin-functionalized porous PLGA particulate scaffolds respond better to cell migration, actin production and wound healing in rodent model. Journal of tissue engineering and regenerative medicine 2014, 8, 351–363. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Yang, D.; Wong, J.H.; Wang, J.; Yin, C.; Zhu, Y.; Fan, S.; Ng, T.B.; Xia, J.; Li, Z. A Thioether-Stabilized d-Proline–l-Proline-Induced β-Hairpin Peptide of Defensin Segment Increases Its Anti-Candida albicans Ability. ChemBioChem 2016, 17, 1416–1420. [Google Scholar] [CrossRef] [PubMed]
- Ruczynski, J.; Wierzbicki, P.M.; Kogut-Wierzbicka, M.; Mucha, P.; Siedlecka-Kroplewska, K.; Rekowski, P. Cell-penetrating peptides as a promising tool for delivery of various molecules into the cells. Folia histochemica et cytobiologica 2014, 52, 257–269. [Google Scholar] [CrossRef] [PubMed]
- Akkiraju, H.; Srinivasan, P.P.; Xu, X.; Jia, X.; Safran, C.B.K.; Nohe, A. CK2. 1, a bone morphogenetic protein receptor type Ia mimetic peptide, repairs cartilage in mice with destabilized medial meniscus. Stem Cell Research & Therapy 2017, 8, 1–11. [Google Scholar]
- Renner, J.N.; Kim, Y.; Liu, J.C. Bone morphogenetic protein-derived peptide promotes chondrogenic differentiation of human mesenchymal stem cells. Tissue Engineering Part A 2012, 18, 2581–2589. [Google Scholar] [CrossRef]
- Scholz, M.; Schleicher, P.; Sewing, A.; Gelinsky, M.; Kandziora, F. Cyclic-RGD is as effective as rhBMP-2 in anterior interbody fusion of the sheep cervical spine. Spine 2013, 38, E59–E65. [Google Scholar] [CrossRef]
- Hennessy, K.M.; Pollot, B.E.; Clem, W.C.; Phipps, M.C.; Sawyer, A.A.; Culpepper, B.K.; Bellis, S.L. The effect of collagen I mimetic peptides on mesenchymal stem cell adhesion and differentiation, and on bone formation at hydroxyapatite surfaces. Biomaterials 2009, 30, 1898–1909. [Google Scholar] [CrossRef]
- Sawyer, A.A.; Hennessy, K.M.; Bellis, S.L. The effect of adsorbed serum proteins, RGD and proteoglycan-binding peptides on the adhesion of mesenchymal stem cells to hydroxyapatite. Biomaterials 2007, 28, 383–392. [Google Scholar] [CrossRef]
- Mas-Moruno, C.; Fraioli, R.; Albericio, F.; Manero, J.M.; Gil, F.J. Novel peptide-based platform for the dual presentation of biologically active peptide motifs on biomaterials. ACS applied materials & interfaces 2014, 6, 6525–6536. [Google Scholar]
- Paredes, V.; Salvagni, E.; Rodríguez-Castellon, E.; Gil, F.; Manero, J. Study on the use of 3-aminopropyltriethoxysilane and 3-chloropropyltriethoxysilane to surface biochemical modification of a novel low elastic modulus Ti–Nb–Hf alloy. Journal of Biomedical Materials Research Part B: Applied Biomaterials 2015, 103, 495–502. [Google Scholar] [CrossRef]
- Benoit, D.S.; Anseth, K.S. The effect on osteoblast function of colocalized RGD and PHSRN epitopes on PEG surfaces. Biomaterials 2005, 26, 5209–5220. [Google Scholar] [CrossRef]
- Schuler, M.; Hamilton, D.W.; Kunzler, T.P.; Sprecher, C.M.; de Wild, M.; Brunette, D.M.; Textor, M.; Tosatti, S.G. Comparison of the response of cultured osteoblasts and osteoblasts outgrown from rat calvarial bone chips to nonfouling KRSR and FHRRIKA-peptide modified rough titanium surfaces. Journal of Biomedical Materials Research Part B: Applied Biomaterials: An Official Journal of The Society for Biomaterials, The Japanese Society for Biomaterials, and The Australian Society for Biomaterials and the Korean Society for Biomaterials 2009, 91, 517–527. [Google Scholar] [CrossRef]
- Dee, K.C.; Andersen, T.T.; Bizios, R. Design and function of novel osteoblast-adhesive peptides for chemical modification of biomaterials. Journal of biomedical materials research 1998, 40, 371–377. [Google Scholar] [CrossRef]
- Palchesko, R.N.; Romeo, J.D.; McGowan, K.A.; Gawalt, E.S. Increased osteoblast adhesion on physically optimized KRSR modified calcium aluminate. Journal of Biomedical Materials Research Part A 2012, 100, 1229–1238. [Google Scholar] [CrossRef]
- Sun, S.; Yu, W.; Zhang, Y.; Zhang, F. Increased preosteoblast adhesion and osteogenic gene expression on TiO 2 nanotubes modified with KRSR. Journal of Materials Science: Materials in Medicine 2013, 24, 1079–1091. [Google Scholar]
- Nelson, M.; Balasundaram, G.; Webster, T.J. Increased osteoblast adhesion on nanoparticulate crystalline hydroxyapatite functionalized with KRSR. International journal of nanomedicine 2006, 1, 339. [Google Scholar]
- Balasundaram, G.; Webster, T.J. Increased osteoblast adhesion on nanograined Ti modified with KRSR. Journal of Biomedical Materials Research Part A 2007, 80, 602–611. [Google Scholar] [CrossRef] [PubMed]
- Frith, J.E.; Mills, R.J.; Hudson, J.E.; Cooper-White, J.J. Tailored integrin–extracellular matrix interactions to direct human mesenchymal stem cell differentiation. Stem cells and development 2012, 21, 2442–2456. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, M.; Lai, T.-S.; Lin, H.-C. Substrate stiffness and sequence dependent bioactive peptide hydrogels influence the chondrogenic differentiation of human mesenchymal stem cells. Journal of Materials Chemistry B 2021, 9, 1676–1685. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Xu, J.; Wong, D.S.H.; Li, J.; Zhao, P.; Bian, L. Self-assembled N-cadherin mimetic peptide hydrogels promote the chondrogenesis of mesenchymal stem cells through inhibition of canonical Wnt/β-catenin signaling. Biomaterials 2017, 145, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Lin, S.; Sun, Y.; Feng, Q.; Li, G.; Bian, L. Hydrogels functionalized with N-cadherin mimetic peptide enhance osteogenesis of hMSCs by emulating the osteogenic niche. Biomaterials 2016, 77, 44–52. [Google Scholar] [CrossRef]
- Li, W.; Yu, B.; Li, M.; Sun, D.; Hu, Y.; Zhao, M.; Cui, C.-B.; Hou, S. NEMO-binding domain peptide promotes osteoblast differentiation impaired by tumor necrosis factor alpha. Biochemical and biophysical research communications 2010, 391, 1228–1233. [Google Scholar] [CrossRef] [PubMed]
- Dai, S.; Hirayama, T.; Abbas, S.; Abu-Amer, Y. The IκB kinase (IKK) inhibitor, NEMO-binding domain peptide, blocks osteoclastogenesis and bone erosion in inflammatory arthritis. Journal of Biological Chemistry 2004, 279, 37219–37222. [Google Scholar] [CrossRef]
- Ligorio, C.; Mata, A. Synthetic extracellular matrices with function-encoding peptides. Nature Reviews Bioengineering 2023, 1–19. [Google Scholar] [CrossRef]
- Lin, H.; Zhou, J.; Shen, L.; Ruan, Y.; Dong, J.; Guo, C.; Chen, Z. Biotin-conjugated anti-CD44 antibody–avidin binding system for the improvement of chondrocyte adhesion to scaffolds. Journal of Biomedical Materials Research Part A 2014, 102, 1140–1148. [Google Scholar] [CrossRef]
- Cheung, C.S.; Lui, J.C.; Baron, J. Identification of chondrocyte-binding peptides by phage display. Journal of Orthopaedic research 2013, 31, 1053–1058. [Google Scholar] [CrossRef]
- Shao, Z.; Zhang, X.; Pi, Y.; Wang, X.; Jia, Z.; Zhu, J.; Dai, L.; Chen, W.; Yin, L.; Chen, H. Polycaprolactone electrospun mesh conjugated with an MSC affinity peptide for MSC homing in vivo. Biomaterials 2012, 33, 3375–3387. [Google Scholar] [CrossRef] [PubMed]
- Webb, D.J.; Roadcap, D.W.; Dhakephalkar, A.; Gonias, S.L. A 16-amino acid peptide from human α2-macroglobulin binds transforming growth factor-β and platelet-derived growth factor-BB. Protein Science 2000, 9, 1986–1992. [Google Scholar] [CrossRef]
- Shah, R.N.; Shah, N.A.; Del Rosario Lim, M.M.; Hsieh, C.; Nuber, G.; Stupp, S.I. Supramolecular design of self-assembling nanofibers for cartilage regeneration. Proceedings of the National Academy of Sciences 2010, 107, 3293–3298. [Google Scholar] [CrossRef]
- Thakkar, S.; Fernandes, H.; Moroni, L. Decellularized extracellular matrix scaffolds for cartilage regeneration. Cartilage Tissue Engineering: Methods and Protocols, 2015; 133–151. [Google Scholar]
- Masters, K.S.; Shah, D.N.; Leinwand, L.A.; Anseth, K.S. Crosslinked hyaluronan scaffolds as a biologically active carrier for valvular interstitial cells. Biomaterials 2005, 26, 2517–2525. [Google Scholar] [CrossRef] [PubMed]
- Parmar, P.A.; Chow, L.W.; St-Pierre, J.-P.; Horejs, C.-M.; Peng, Y.Y.; Werkmeister, J.A.; Ramshaw, J.A.; Stevens, M.M. Collagen-mimetic peptide-modifiable hydrogels for articular cartilage regeneration. Biomaterials 2015, 54, 213–225. [Google Scholar] [CrossRef] [PubMed]
- Ruoslahti, E.; Pierschbacher, M.D. Arg-Gly-Asp: a versatile cell recognition signal. Cell 1986, 44, 517–518. [Google Scholar] [CrossRef]
- Kim, H.D.; Heo, J.; Hwang, Y.; Kwak, S.-Y.; Park, O.K.; Kim, H.; Varghese, S.; Hwang, N.S. Extracellular-matrix-based and Arg-Gly-Asp–modified photopolymerizing hydrogels for cartilage tissue engineering. Tissue Engineering Part A 2015, 21, 757–766. [Google Scholar] [CrossRef] [PubMed]
- Jeschke, B.; Meyer, J.; Jonczyk, A.; Kessler, H.; Adamietz, P.; Meenen, N.M.; Kantlehner, M.; Goepfert, C.; Nies, B. RGD-peptides for tissue engineering of articular cartilage. Biomaterials 2002, 23, 3455–3463. [Google Scholar] [CrossRef]
- Kim, I.L.; Khetan, S.; Baker, B.M.; Chen, C.S.; Burdick, J.A. Fibrous hyaluronic acid hydrogels that direct MSC chondrogenesis through mechanical and adhesive cues. Biomaterials 2013, 34, 5571–5580. [Google Scholar] [CrossRef]
- Homandberg, G.A.; Hui, F. Arg-Gly-Asp-Ser peptide analogs suppress cartilage chondrolytic activities of integrin-binding and nonbinding fibronectin fragments. Archives of biochemistry and biophysics 1994, 310, 40–48. [Google Scholar] [CrossRef]
- Zhang, T.; Wen, F.; Wu, Y.; Goh, G.S.H.; Ge, Z.; Tan, L.P.; Hui, J.H.P.; Yang, Z. Cross-talk between TGF-beta/SMAD and integrin signaling pathways in regulating hypertrophy of mesenchymal stem cell chondrogenesis under deferral dynamic compression. Biomaterials 2015, 38, 72–85. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-W.; Kurashima, K.; Tustusmi, Y.; An, C.-H.; Suh, J.-Y.; Doi, H.; Nomura, N.; Noda, K.; Hanawa, T. Bone healing of commercial oral implants with RGD immobilization through electrodeposited poly (ethylene glycol) in rabbit cancellous bone. Acta Biomaterialia 2011, 7, 3222–3229. [Google Scholar] [CrossRef] [PubMed]
- Tavella, S.; Raffo, P.; Tacchetti, C.; Cancedda, R.; Castagnola, P. N-CAM and N-cadherin expression during in vitro chondrogenesis. Experimental cell research 1994, 215, 354–362. [Google Scholar] [CrossRef] [PubMed]
- Bian, L.; Guvendiren, M.; Mauck, R.L.; Burdick, J.A. Hydrogels that mimic developmentally relevant matrix and N-cadherin interactions enhance MSC chondrogenesis. Proceedings of the National Academy of Sciences 2013, 110, 10117–10122. [Google Scholar] [CrossRef] [PubMed]
- Kuo, Y.-C.; Wang, C.-C. Surface modification with peptide for enhancing chondrocyte adhesion and cartilage regeneration in porous scaffolds. Colloids and Surfaces B: Biointerfaces 2011, 84, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Jo, J.; Song, H.; Park, S.G.; Lee, S.H.; Ko, J.J.; Park, J.H.; Jeong, J.; Cheon, Y.P.; Lee, D.R. Regulation of Differentiation Potential of Human Mesenchymal Stem Cells by Intracytoplasmic Delivery of Coactivator-Associated Arginine Methyltransferase 1 Protein Using Cell-Penetrating Peptide. Stem Cells 2012, 30, 1703–1713. [Google Scholar] [CrossRef] [PubMed]
- Suh, J.S.; Lee, J.Y.; Choi, Y.J.; You, H.K.; Hong, S.-D.; Chung, C.P.; Park, Y.J. Intracellular delivery of cell-penetrating peptide-transcriptional factor fusion protein and its role in selective osteogenesis. International journal of nanomedicine 2014, 1153–1166. [Google Scholar]
- Park, S.; Doh, J.; Park, S.; Lim, J.; Kim, S.; Youn, J.; Jin, H.; Seo, S.; Song, M.; Sung, S. Branched oligomerization of cell-permeable peptides markedly enhances the transduction efficiency of adenovirus into mesenchymal stem cells. Gene therapy 2010, 17, 1052–1061. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Chu, X.; Li, W.; Pan, Q.; You, H. Chondrogenic effect of precartilaginous stem cells following NLS-TAT cell penetrating peptide-assisted transfection of eukaryotic h TGF β3. Journal of Cellular Biochemistry 2013, 114, 2588–2594. [Google Scholar] [CrossRef]
- Pan, Q.; Li, W.; Yuan, X.; Rakhmanov, Y.; Wang, P.; Lu, R.; Mao, Z.; Shang, X.; You, H. Chondrogenic effect of cell-based scaffold of self-assembling peptides/PLGA-PLL loading the hTGFβ3 plasmid DNA. Journal of Materials Science: Materials in Medicine 2016, 27, 1–11. [Google Scholar] [CrossRef]
- Trepat, X.; Chen, Z.; Jacobson, K. Cell migration. Comprehensive Physiology 2012, 2, 2369. [Google Scholar] [PubMed]
- Shen, W.; Chen, X.; Chen, J.; Yin, Z.; Heng, B.C.; Chen, W.; Ouyang, H.-W. The effect of incorporation of exogenous stromal cell-derived factor-1 alpha within a knitted silk-collagen sponge scaffold on tendon regeneration. Biomaterials 2010, 31, 7239–7249. [Google Scholar] [CrossRef] [PubMed]
- Yeboah, A.; Cohen, R.I.; Faulknor, R.; Schloss, R.; Yarmush, M.L.; Berthiaume, F. The development and characterization of SDF1α-elastin-like-peptide nanoparticles for wound healing. Journal of controlled release 2016, 232, 238–247. [Google Scholar] [CrossRef]
- Torres, P.; Díaz, J.; Arce, M.; Silva, P.; Mendoza, P.; Lois, P.; Molina-Berríos, A.; Owen, G.I.; Palma, V.; Torres, V.A. The salivary peptide histatin-1 promotes endothelial cell adhesion, migration, and angiogenesis. The FASEB Journal 2017, 31, 4946–4958. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.J.; Xia, C.J.; Wei, Y.; Yao, Y.; Dong, M.W.; Lin, K.Z.; Yu, L.S.; Gao, Y.; Fan, Y.Y. Annexin A1-derived peptide Ac2-26 facilitates wound healing in diabetic mice. Wound Repair and Regeneration 2020, 28, 772–779. [Google Scholar] [CrossRef] [PubMed]
- Di Grazia, A.; Cappiello, F.; Imanishi, A.; Mastrofrancesco, A.; Picardo, M.; Paus, R.; Mangoni, M.L. The frog skin-derived antimicrobial peptide esculentin-1a (1-21) NH2 promotes the migration of human HaCaT keratinocytes in an EGF receptor-dependent manner: a novel promoter of human skin wound healing? PloS one 2015, 10, e0128663. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, K.; Okumura, K.; Isogai, H.; Isogai, E. The human cathelicidin antimicrobial peptide LL-37 and mimics are potential anticancer drugs. Frontiers in oncology 2015, 5, 144. [Google Scholar] [CrossRef]
- Rubert Pérez, C.M.; Álvarez, Z.; Chen, F.; Aytun, T.; Stupp, S.I. Mimicking the bioactivity of fibroblast growth factor-2 using supramolecular nanoribbons. ACS Biomaterials Science & Engineering 2017, 3, 2166–2175. [Google Scholar]
- Zhang, S. Emerging biological materials through molecular self-assembly. Biotechnology advances 2002, 20, 321–339. [Google Scholar] [CrossRef]
- Ryan, D.M.; Nilsson, B.L. Self-assembled amino acids and dipeptides as noncovalent hydrogels for tissue engineering. Polymer Chemistry 2012, 3, 18–33. [Google Scholar] [CrossRef]
- Avinash, M.; Govindaraju, T. Nanoarchitectonics of biomolecular assemblies for functional applications. Nanoscale 2014, 6, 13348–13369. [Google Scholar] [CrossRef]
- Lee, J.Y.; Choo, J.E.; Choi, Y.S.; Lee, K.Y.; Min, D.S.; Pi, S.H.; Seol, Y.J.; Lee, S.J.; Jo, I.H.; Chung, C.P. Characterization of the surface immobilized synthetic heparin binding domain derived from human fibroblast growth factor-2 and its effect on osteoblast differentiation. Journal of Biomedical Materials Research Part A: An Official Journal of The Society for Biomaterials, The Japanese Society for Biomaterials, and The Australian Society for Biomaterials and the Korean Society for Biomaterials 2007, 83, 970–979. [Google Scholar] [CrossRef] [PubMed]
- Furuya, Y.; Inagaki, A.; Khan, M.; Mori, K.; Penninger, J.M.; Nakamura, M.; Udagawa, N.; Aoki, K.; Ohya, K.; Uchida, K. Stimulation of bone formation in cortical bone of mice treated with a receptor activator of nuclear factor-κB ligand (RANKL)-binding peptide that possesses osteoclastogenesis inhibitory activity. Journal of Biological Chemistry 2013, 288, 5562–5571. [Google Scholar] [CrossRef]
- Ikeno, M.; Hibi, H.; Kinoshita, K.; Hattori, H.; Ueda, M. Effects of self-assembling peptide hydrogel scaffold on bone regeneration with recombinant human bone morphogenetic protein-2. Oral & Craniofacial Tissue Engineering 2011, 1. [Google Scholar]
- Recha-Sancho, L.; Semino, C.E. Heparin-based self-assembling peptide scaffold reestablish chondrogenic phenotype of expanded de-differentiated human chondrocytes. Journal of Biomedical Materials Research Part A 2016, 104, 1694–1706. [Google Scholar] [CrossRef]
- Yamaoka, H.; Asato, H.; Ogasawara, T.; Nishizawa, S.; Takahashi, T.; Nakatsuka, T.; Koshima, I.; Nakamura, K.; Kawaguchi, H.; Chung, U.i. Cartilage tissue engineering using human auricular chondrocytes embedded in different hydrogel materials. Journal of Biomedical Materials Research Part A: An Official Journal of The Society for Biomaterials, The Japanese Society for Biomaterials, and The Australian Society for Biomaterials and the Korean Society for Biomaterials 2006, 78, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Kisiday, J.; Jin, M.; Kurz, B.; Hung, H.; Semino, C.; Zhang, S.; Grodzinsky, A. Self-assembling peptide hydrogel fosters chondrocyte extracellular matrix production and cell division: implications for cartilage tissue repair. Proceedings of the National Academy of Sciences 2002, 99, 9996–10001. [Google Scholar] [CrossRef]
- Sridhar, B.V.; Brock, J.L.; Silver, J.S.; Leight, J.L.; Randolph, M.A.; Anseth, K.S. Development of a cellularly degradable PEG hydrogel to promote articular cartilage extracellular matrix deposition. Advanced healthcare materials 2015, 4, 702–713. [Google Scholar] [CrossRef]
- Bin Hafeez, A.; Jiang, X.; Bergen, P.J.; Zhu, Y. Antimicrobial peptides: An update on classifications and databases. International journal of molecular sciences 2021, 22, 11691. [Google Scholar] [CrossRef]
- Hao, Z.; Chen, R.; Chai, C.; Wang, Y.; Chen, T.; Li, H.; Hu, Y.; Feng, Q.; Li, J. Antimicrobial peptides for bone tissue engineering: Diversity, effects and applications. Frontiers in Bioengineering and Biotechnology 2022, 10, 1030162. [Google Scholar] [CrossRef]
- Sun, Y.; Chan, J.; Bose, K.; Tam, C. Simultaneous control of infection and inflammation with keratin-derived antibacterial peptides targeting TLRs and co-receptors. Science Translational Medicine 2023, 15, eade2909. [Google Scholar] [CrossRef] [PubMed]
- de Leeuw, E.; Li, C.; Zeng, P.; Li, C.; Diepeveen-de Buin, M.; Lu, W.-Y.; Breukink, E.; Lu, W. Functional interaction of human neutrophil peptide-1 with the cell wall precursor lipid II. FEBS letters 2010, 584, 1543–1548. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, M.; Chiriac, A.I.; Otto, A.; Zweytick, D.; May, C.; Schumacher, C.; Gust, R.; Albada, H.B.; Penkova, M.; Krämer, U. Small cationic antimicrobial peptides delocalize peripheral membrane proteins. Proceedings of the National Academy of Sciences 2014, 111, E1409–E1418. [Google Scholar] [CrossRef] [PubMed]
- Park, C.B.; Kim, H.S.; Kim, S.C. Mechanism of action of the antimicrobial peptide buforin II: buforin II kills microorganisms by penetrating the cell membrane and inhibiting cellular functions. Biochemical and biophysical research communications 1998, 244, 253–257. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Jin, Y.; Wang, X.; Yao, S.; Li, Y.; Wu, Q.; Ma, G.; Cui, F.; Liu, H. An antimicrobial peptide-loaded gelatin/chitosan nanofibrous membrane fabricated by sequential layer-by-layer electrospinning and electrospraying techniques. Nanomaterials 2018, 8, 327. [Google Scholar] [CrossRef] [PubMed]
- Vallet-Regí, M.; Lozano, D.; González, B.; Izquierdo-Barba, I. Biomaterials against bone infection. Adv. Healthc. Mater. 2020, 9. [Google Scholar] [CrossRef] [PubMed]
- Tsai, P.-W.; Yang, C.-Y.; Chang, H.-T.; Lan, C.-Y. Human antimicrobial peptide LL-37 inhibits adhesion of Candida albicans by interacting with yeast cell-wall carbohydrates. PloS one 2011, 6, e17755. [Google Scholar] [CrossRef] [PubMed]
- De La Fuente-Núñez, C.; Korolik, V.; Bains, M.; Nguyen, U.; Breidenstein, E.B.; Horsman, S.; Lewenza, S.; Burrows, L.; Hancock, R.E. Inhibition of bacterial biofilm formation and swarming motility by a small synthetic cationic peptide. Antimicrobial agents and chemotherapy 2012, 56, 2696–2704. [Google Scholar] [CrossRef] [PubMed]
- Libardo, M.D.J.; Bahar, A.A.; Ma, B.; Fu, R.; McCormick, L.E.; Zhao, J.; McCallum, S.A.; Nussinov, R.; Ren, D.; Angeles-Boza, A.M. Nuclease activity gives an edge to host-defense peptide piscidin 3 over piscidin 1, rendering it more effective against persisters and biofilms. The FEBS journal 2017, 284, 3662–3683. [Google Scholar] [CrossRef]
- Luca, V.; Stringaro, A.; Colone, M.; Pini, A.; Mangoni, M.L. Esculentin (1-21), an amphibian skin membrane-active peptide with potent activity on both planktonic and biofilm cells of the bacterial pathogen Pseudomonas aeruginosa. Cellular and molecular life sciences 2013, 70, 2773–2786. [Google Scholar] [CrossRef]
- Chen, S.; Lu, Z.; Wang, F.; Wang, Y. Cathelicidin-WA polarizes E. coli K88-induced M1 macrophage to M2-like macrophage in RAW264. 7 cells. International Immunopharmacology 2018, 54, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Dai, M.; Sui, B.; Xue, Y.; Liu, X.; Sun, J. Cartilage repair in degenerative osteoarthritis mediated by squid type II collagen via immunomodulating activation of M2 macrophages, inhibiting apoptosis and hypertrophy of chondrocytes. Biomaterials 2018, 180, 91–103. [Google Scholar] [CrossRef]
- Zhang, H.; Lin, C.; Zeng, C.; Wang, Z.; Wang, H.; Lu, J.; Liu, X.; Shao, Y.; Zhao, C.; Pan, J. Synovial macrophage M1 polarisation exacerbates experimental osteoarthritis partially through R-spondin-2. Annals of the rheumatic diseases 2018, 77, 1524–1534. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Bian, A.; Jia, F.; Lan, J.; Yang, H.; Yan, K.; Xie, L.; Qiao, H.; Chang, X.; Lin, H. “Dual-functional” strontium titanate nanotubes designed based on fusion peptides simultaneously enhancing anti-infection and osseointegration. Biomaterials Advances 2022, 133, 112650. [Google Scholar] [CrossRef]
- Zhou, L.; Han, Y.; Ding, J.; Chen, X.; Huang, S.; Xing, X.; Wu, D.; Chen, J. Regulation of an antimicrobial peptide GL13K-modified titanium surface on osteogenesis, osteoclastogenesis, and angiogenesis base on osteoimmunology. ACS Biomaterials Science & Engineering 2021, 7, 4569–4580. [Google Scholar]
- Liu, H.-W.; Wei, D.-X.; Deng, J.-Z.; Zhu, J.-J.; Xu, K.; Hu, W.-H.; Xiao, S.-H.; Zhou, Y.-G. Combined antibacterial and osteogenic in situ effects of a bifunctional titanium alloy with nanoscale hydroxyapatite coating. Artificial cells, nanomedicine, and biotechnology 2018, 46, 460–470. [Google Scholar] [CrossRef] [PubMed]
- Basu, S.; Kumar, M.; Chansuria, J.; Singh, T.B.; Bhatnagar, R.; Shukla, V.K. Effect of Cytomodulin-10 (TGF-ß1 analogue) on wound healing by primary intention in a murine model. International Journal of Surgery 2009, 7, 460–465. [Google Scholar] [CrossRef]
- Boda, S.K.; Wang, H.; John, J.V.; Reinhardt, R.A.; Xie, J. Dual delivery of alendronate and E7-BMP-2 peptide via calcium chelation to mineralized nanofiber fragments for alveolar bone regeneration. ACS biomaterials science & engineering 2020, 6, 2368–2375. [Google Scholar]
- Laboy-López, S.; Méndez Fernández, P.O.; Padilla-Zayas, J.G.; Nicolau, E. Bioactive Cellulose Acetate Electrospun Mats as Scaffolds for Bone Tissue Regeneration. International Journal of Biomaterials 2022, 2022. [Google Scholar] [CrossRef]
- Lukasova, V.; Buzgo, M.; Sovkova, V.; Dankova, J.; Rampichova, M.; Amler, E. Osteogenic differentiation of 3D cultured mesenchymal stem cells induced by bioactive peptides. Cell Proliferation 2017, 50, e12357. [Google Scholar] [CrossRef]
- Gentile, P.; Ferreira, A.M.; Callaghan, J.T.; Miller, C.A.; Atkinson, J.; Freeman, C.; Hatton, P.V. Multilayer nanoscale encapsulation of biofunctional peptides to enhance bone tissue regeneration in vivo. Advanced healthcare materials 2017, 6, 1601182. [Google Scholar] [CrossRef]
- Gentile, P.; Ghione, C.; Tonda-Turo, C.; Kalaskar, D. Peptide functionalisation of nanocomposite polymer for bone tissue engineering using plasma surface polymerisation. RSC advances 2015, 5, 80039–80047. [Google Scholar] [CrossRef]
- Shekaran, A.; García, A.J. Extracellular matrix-mimetic adhesive biomaterials for bone repair. Journal of biomedical materials research Part A 2011, 96, 261–272. [Google Scholar] [CrossRef]
- Hasenbein, M.; Andersen, T.; Bizios, R. Micropatterned surfaces modified with select peptides promote exclusive interactions with osteoblasts. Biomaterials 2002, 23, 3937–3942. [Google Scholar] [CrossRef]
- Posadowska, U.; Storm, I.; de Wolf, F.; van den Beucken, J.; Leeuwenburgh, S. Nanofibrillar hydrogel scaffolds from recombinant protein-based polymers with integrin-and proteoglycan-binding domains. Production of protein-based polymers in Pichia pastoris 1, 135.
- Reyes, C.D.; García, A.J. α2β1 integrin-specific collagen-mimetic surfaces supporting osteoblastic differentiation. Journal of Biomedical Materials Research Part A: An Official Journal of The Society for Biomaterials, The Japanese Society for Biomaterials, and The Australian Society for Biomaterials and the Korean Society for Biomaterials 2004, 69, 591–600. [Google Scholar] [CrossRef]
- Yukna, R.A.; Callan, D.P.; Krauser, J.T.; Evans, G.H.; Aichelmann-Reidy, M.E.; Moore, K.; Cruz, R.; Scott, J.B. Multi-center clinical evaluation of combination anorganic bovine-derived hydroxyapatite matrix (ABM)/cell binding peptide (P-15) as a bone replacement graft material in human periodontal osseous defects. 6-month results. Journal of periodontology 1998, 69, 655–663. [Google Scholar] [CrossRef]
- Thorwarth, M.; Schultze-Mosgau, S.; Wehrhan, F.; Srour, S.; Wiltfang, J.; Neukam, F.W.; Schlegel, K.A. Enhanced bone regeneration with a synthetic cell-binding peptide—in vivo results. Biochemical and biophysical research communications 2005, 329, 789–795. [Google Scholar] [CrossRef]
- Thorwarth, M.; Schultze-Mosgau, S.; Wehrhan, F.; Kessler, P.; Srour, S.; Wiltfang, J.; Schlegel, K.A. Bioactivation of an anorganic bone matrix by P-15 peptide for the promotion of early bone formation. Biomaterials 2005, 26, 5648–5657. [Google Scholar] [CrossRef]
- Matos, S.; Guerra, F.; Krauser, J.T.; Figueiredo, H.; Marcelino, J.P.; Sanz, M. Evaluation of an anorganic bovine-derived mineral with P-15 hydrogel bone graft: preliminary study in a rabbit cranial bone model. Clinical Oral Implants Research 2012, 23, 698–705. [Google Scholar] [CrossRef]
- Culpepper, B.K.; Phipps, M.C.; Bonvallet, P.P.; Bellis, S.L. Enhancement of peptide coupling to hydroxyapatite and implant osseointegration through collagen mimetic peptide modified with a polyglutamate domain. Biomaterials 2010, 31, 9586–9594. [Google Scholar] [CrossRef]
- Wang, V.; Misra, G.; Amsden, B. Immobilization of a bone and cartilage stimulating peptide to a synthetic bone graft. Journal of Materials Science: Materials in Medicine 2008, 19, 2145–2155. [Google Scholar] [CrossRef] [PubMed]
- Shuqiang, M.; Kunzheng, W.; Xiaoqiang, D.; Wei, W.; Mingyu, Z.; Daocheng, W. Osteogenic growth peptide incorporated into PLGA scaffolds accelerates healing of segmental long bone defects in rabbits. Journal of plastic, reconstructive & aesthetic surgery 2008, 61, 1558–1560. [Google Scholar]
- Jung, R.E.; Hämmerle, C.H.; Kokovic, V.; Weber, F.E. Bone regeneration using a synthetic matrix containing a parathyroid hormone peptide combined with a grafting material. International Journal of Oral & Maxillofacial Implants 2007, 22. [Google Scholar]
- Sheller, M.R.; Crowther, R.S.; Kinney, J.H.; Yang, J.; Di Jorio, S.; Breunig, T.; Carney, D.H.; Ryaby, J.T. Repair of rabbit segmental defects with the thrombin peptide, TP508. Journal of orthopaedic research 2004, 22, 1094–1099. [Google Scholar] [CrossRef] [PubMed]
- Rammelt, S.; Illert, T.; Bierbaum, S.; Scharnweber, D.; Zwipp, H.; Schneiders, W. Coating of titanium implants with collagen, RGD peptide and chondroitin sulfate. Biomaterials 2006, 27, 5561–5571. [Google Scholar] [CrossRef] [PubMed]
- Ferris, D.; Moodie, G.; Dimond, P.; Giorani, C.; Ehrlich, M.; Valentini, R. RGD-coated titanium implants stimulate increased bone formation in vivo. Biomaterials 1999, 20, 2323–2331. [Google Scholar] [CrossRef] [PubMed]
- Schneiders, W.; Reinstorf, A.; Pompe, W.; Grass, R.; Biewener, A.; Holch, M.; Zwipp, H.; Rammelt, S. Effect of modification of hydroxyapatite/collagen composites with sodium citrate, phosphoserine, phosphoserine/RGD-peptide and calcium carbonate on bone remodelling. Bone 2007, 40, 1048–1059. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Hemraz, U.D.; Fenniri, H.; Webster, T.J. Tuning cell adhesion on titanium with osteogenic rosette nanotubes. Journal of biomedical materials research Part A 2010, 95, 550–563. [Google Scholar] [CrossRef] [PubMed]
- Balasundaram, G.; Shimpi, T.M.; Sanow, W.R.; Storey, D.M.; Kitchell, B.S.; Webster, T.J. Molecular plasma deposited peptides on anodized nanotubular titanium: an osteoblast density study. Journal of Biomedical Materials Research Part A 2011, 98, 192–200. [Google Scholar] [CrossRef]
- Kim, J.E.; Kim, S.H.; Jung, Y. In situ chondrogenic differentiation of bone marrow stromal cells in bioactive self-assembled peptide gels. Journal of bioscience and bioengineering 2015, 120, 91–98. [Google Scholar] [CrossRef]
- Chen, J.; Li, Y.; Wang, B.; Yang, J.; Heng, B.C.; Yang, Z.; Ge, Z.; Lin, J. TGF-β1 affinity peptides incorporated within a chitosan sponge scaffold can significantly enhance cartilage regeneration. Journal of Materials Chemistry B 2018, 6, 675–687. [Google Scholar] [CrossRef]
- Ju, X.; Liu, X.; Zhang, Y.; Chen, X.; Chen, M.; Shen, H.; Feng, Y.; Liu, J.; Wang, M.; Shi, Q. A photo-crosslinked proteinogenic hydrogel enabling self-recruitment of endogenous TGF-β1 for cartilage regeneration. Smart Materials in Medicine 2022, 3, 85–93. [Google Scholar] [CrossRef]
- Hsieh, Y.-H.; Hsieh, M.-F.; Fang, C.-H.; Jiang, C.-P.; Lin, B.; Lee, H.-M. Osteochondral regeneration induced by TGF-β loaded photo cross-linked hyaluronic acid hydrogel infiltrated in fused deposition-manufactured composite scaffold of hydroxyapatite and poly (ethylene glycol)-block-poly (ε-caprolactone). Polymers 2017, 9, 182. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Gao, J.; Yu, X.; Shi, J.; Chen, J.; Yu, L.; Chen, S.; Ding, J. 3D-printed porous scaffolds of hydrogels modified with TGF-β1 binding peptides to promote in vivo cartilage regeneration and animal gait restoration. ACS applied materials & interfaces 2022, 14, 15982–15995. [Google Scholar]
- Burdick, J.A.; Anseth, K.S. Photoencapsulation of osteoblasts in injectable RGD-modified PEG hydrogels for bone tissue engineering. Biomaterials 2002, 23, 4315–4323. [Google Scholar] [CrossRef]
- Zhang, X.; Gu, J.; Zhang, Y.; Tan, Y.; Zhou, J.; Zhou, D. Immobilization of RGD peptide onto the surface of apatite-wollastonite ceramic for enhanced osteoblast adhesion and bone regeneration. Journal of Wuhan University of Technology-Mater. Sci. Ed. 2014, 29, 626–634. [Google Scholar] [CrossRef]
- Pan, H.; Zheng, Q.; Yang, S.; Guo, X.; Wu, B.; Zou, Z.; Duan, Z. A novel peptide-modified and gene-activated biomimetic bone matrix accelerating bone regeneration. Journal of Biomedical Materials Research Part A 2014, 102, 2864–2874. [Google Scholar] [CrossRef]
- Wang, C.; Yue, H.; Huang, W.; Lin, X.; Xie, X.; He, Z.; He, X.; Liu, S.; Bai, L.; Lu, B. Cryogenic 3D printing of heterogeneous scaffolds with gradient mechanical strengths and spatial delivery of osteogenic peptide/TGF-β1 for osteochondral tissue regeneration. Biofabrication 2020, 12, 025030. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhou, X.; Liu, T.; Huang, Y.; Li, J. The effects of Peptide Mel4-coated titanium plates on infection rabbits after internal fixation of open fractures. Archives of Orthopaedic and Trauma Surgery 2022, 1–6. [Google Scholar] [CrossRef]
- Chen, X.; Zhou, L.; Wu, D.; Huang, W.; Lin, Y.; Zhou, B.; Chen, J. The effects of titanium surfaces modified with an antimicrobial peptide GL13K by silanization on polarization, anti-inflammatory, and proinflammatory properties of macrophages. BioMed Research International 2020, 2020. [Google Scholar] [CrossRef]
- Fischer, N.G.; Chen, X.; Astleford-Hopper, K.; He, J.; Mullikin, A.F.; Mansky, K.C.; Aparicio, C. Antimicrobial and enzyme-responsive multi-peptide surfaces for bone-anchored devices. Materials Science and Engineering: C 2021, 125, 112108. [Google Scholar] [CrossRef] [PubMed]
- Hoyos-Nogues, M.; Velasco, F.; Ginebra, M.-P.; Manero, J.M.; Gil, F.J.; Mas-Moruno, C. Regenerating bone via multifunctional coatings: the blending of cell integration and bacterial inhibition properties on the surface of biomaterials. ACS Applied Materials & Interfaces 2017, 9, 21618–21630. [Google Scholar]
- Cheng, H.; Yue, K.; Kazemzadeh-Narbat, M.; Liu, Y.; Khalilpour, A.; Li, B.; Zhang, Y.S.; Annabi, N.; Khademhosseini, A. Mussel-inspired multifunctional hydrogel coating for prevention of infections and enhanced osteogenesis. ACS applied materials & interfaces 2017, 9, 11428–11439. [Google Scholar]
- Zhongxing, L.; Shaohong, W.; Jinlong, L.; Limin, Z.; Yuanzheng, W.; Haipeng, G.; Jian, C. Three-dimensional printed hydroxyapatite bone tissue engineering scaffold with antibacterial and osteogenic ability. Journal of Biological Engineering 2021, 15, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Yuan, X.; Liu, M.; Fernandes, G.; Zhang, Y.; Yang, S.; Ionita, C.N.; Yang, S. Antimicrobial peptide combined with BMP2-modified mesenchymal stem cells promotes calvarial repair in an osteolytic model. Molecular Therapy 2018, 26, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Bai, J.; Wang, W.; Liang, X.; Zhang, W.; Li, W.; Lu, L.; Xiao, L.; Xu, Y.; Wang, Z. Facile and versatile surface functional polyetheretherketone with enhanced bacteriostasis and osseointegrative capability for implant application. ACS Applied Materials & Interfaces 2021, 13, 59731–59746. [Google Scholar]
- Yuan, X.; Ouyang, L.; Luo, Y.; Sun, Z.; Yang, C.; Wang, J.; Liu, X.; Zhang, X. Multifunctional sulfonated polyetheretherketone coating with beta-defensin-14 for yielding durable and broad-spectrum antibacterial activity and osseointegration. Acta Biomaterialia 2019, 86, 323–337. [Google Scholar] [CrossRef]
- Trzcińska, Z.; Bruggeman, M.; Ijakipour, H.; Hodges, N.J.; Bowen, J.; Stamboulis, A. Polydopamine linking substrate for amps: Characterisation and stability on ti6al4v. Materials 2020, 13, 3714. [Google Scholar] [CrossRef]
- Meng, X.; Zhang, J.; Chen, J.; Nie, B.; Yue, B.; Zhang, W.; Lyu, Z.; Long, T.; Wang, Y. KR-12 coating of polyetheretherketone (PEEK) surface via polydopamine improves osteointegration and antibacterial activity in vivo. Journal of Materials Chemistry B 2020, 8, 10190–10204. [Google Scholar] [CrossRef]
Figure 1.
Diagram of the osteochondral unit showing the structural hierarchy, zonal arrangements, and extracellular matrix composition of the articular cartilage. (figure generated via biorender.com).
Figure 1.
Diagram of the osteochondral unit showing the structural hierarchy, zonal arrangements, and extracellular matrix composition of the articular cartilage. (figure generated via biorender.com).

Figure 4.
Role of antimicrobial and immunomodulatory peptides for osteochondral regeneration. (a) schematic representation of bacterial cell membrane damage using antimicrobial peptides. (figure generated via biorender.com) (b) Inhibition of C. albicans adhesion by different concentrations of LL-37 evaluated by (i) XTT reduction assay and whole-cell ELISA (*, p<0.05; **, p<0.01; ***, p<0.001) (ii) spot assay and (iii) optical microscopy of the floating cells (Mag. 400×).[182] Copyright© 2011 Tsai et al. (c) mechanism of immunomodulatory peptides eliciting immune-cell response. (figure generated via biorender.com) (d) E. coli K88-induced RAW264.7 cells (macrophages) treated with varying doses of cathelicidin-WA (CWA) and gentamicin shows (i) significant reduction of reactive oxygen species (ROS) (in fluorescent images) (ii) decrease in proinflammatory cytokines (IL-1β, IL-6) and (iii) increase in anti-inflammatory cytokines (IL-4, IL-10) levels.[186] Copyright© 2017 Elsevier B.V.
Figure 4.
Role of antimicrobial and immunomodulatory peptides for osteochondral regeneration. (a) schematic representation of bacterial cell membrane damage using antimicrobial peptides. (figure generated via biorender.com) (b) Inhibition of C. albicans adhesion by different concentrations of LL-37 evaluated by (i) XTT reduction assay and whole-cell ELISA (*, p<0.05; **, p<0.01; ***, p<0.001) (ii) spot assay and (iii) optical microscopy of the floating cells (Mag. 400×).[182] Copyright© 2011 Tsai et al. (c) mechanism of immunomodulatory peptides eliciting immune-cell response. (figure generated via biorender.com) (d) E. coli K88-induced RAW264.7 cells (macrophages) treated with varying doses of cathelicidin-WA (CWA) and gentamicin shows (i) significant reduction of reactive oxygen species (ROS) (in fluorescent images) (ii) decrease in proinflammatory cytokines (IL-1β, IL-6) and (iii) increase in anti-inflammatory cytokines (IL-4, IL-10) levels.[186] Copyright© 2017 Elsevier B.V.
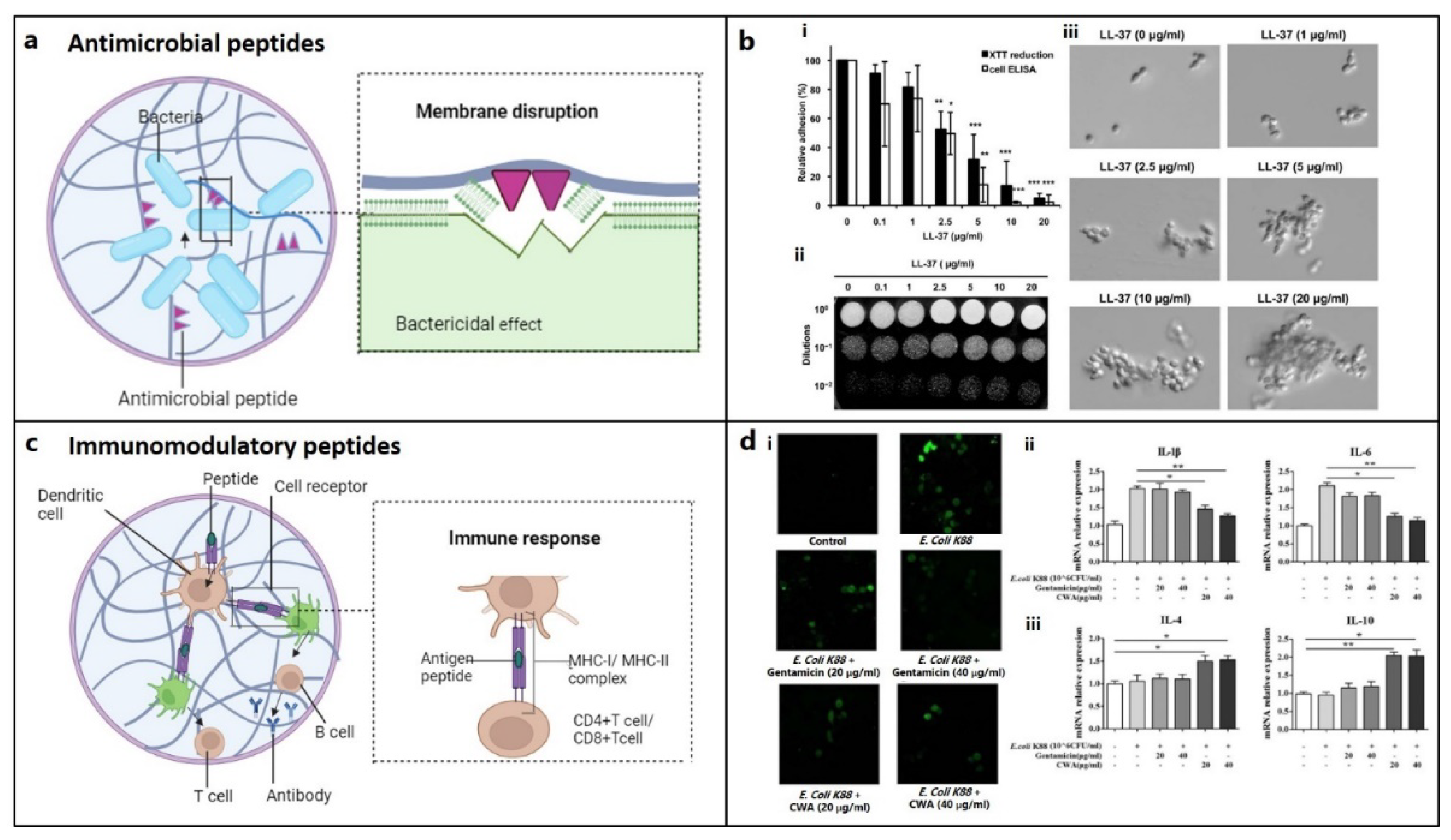
Table 1.
Bioactive peptides for osteochondral regeneration.
| Types | Source | Function | Ref |
|---|---|---|---|
| a. Osteogenic peptides | |||
| (i) Osteo-inductive peptides | |||
| GFOGER | Col | ↑ α2β1 integrin binding, osteoblastic differentiation | [28] |
| GTPGQGIAGQRGVV (P15 peptide) |
↑ osteogenic differentiation | [33] | |
| ((PKG)4-(POG)4-(DOG)4) (KOD peptide) |
↑ECM deposition | [37] | |
| DGEA | ↑expression of osteogenic markers (ALP, RUNX2, OCN). | [39] | |
| NGLPGPIGP (BCSP™-1) |
↑ mineralization | [40] | |
| GPAGPHGPVG, APDPFRMY, TPERYY | ↑ALP activity, mineralization, osteogenic gene expression | [41] | |
| KIPKASSVPTELSAISTLYL | BMP-2 | ↑ALP activity | [42] |
| SKIPKASSVPTGLSAISTLYLAAA (P24) | ↑ bone formation | [43,44,45] | |
| CKIPKPSSVP-TELSAISMLYL (PEP7) | ↑ bone formation | [46] | |
| KIPKASSVPTELSAISTLYL | ↑ALP activity, osteogenic marker | [47] | |
| NSVNSKIPKACCVPTELSAI, KIPKASSVPTELSAISTLYL, DWIVA | ↑Osteogenic differentiation, in vivo bone formation | [12] | |
| RKKNPNCRRH | BMP-4 | ↑Osteogenic differentiation | [55] |
| VEHDKEFFHPRYHH (BFP-2) | BMP-7 | ↑Osteogenic differentiation | [54] |
| TVPKPSSAPTQLNAISTLYF, GQGFSYPYKAVFSTQ, ETLDGQSINPKLAGL | ↑Osteogenic differentiation | [55] | |
| KVGKACCVPTKLSPISVLY | BMP-9 | ↑Osteogenic differentiation | [55] |
| CK2.2, CK2.3 | synthetic | ↑ BMP signaling | [57] |
| PTHrP1–34, PTHrP1–36, PTHrP107–111 | PTH | ↑ Osteoblast differentiation, bone formation | [64,65,66] |
| CGRP–α and β-CGRP | CGRP | ↑ Osteogenic differentiation, bone formation | [67,68,69,70] |
| ALKRQGRTLYGFGG (OGP) | Mammalian blood | ↑ Osteogenic differentiation, ALP, OCN and collagen secretion, mineralization | [76,77,78] |
| AGYKPDEGKRGDACEGDSGGPFV (TP508) | Thrombin | ↑ chemotaxis, proliferation, osteogenic differentiation | [79,80] |
| FN III9-10/12-14 | FN | ↑ osteoblast activity, mineralization | [85] |
| CBM | OPN | ↑ osteogenic differentiation, bone formation | [90] |
| SVVYGLR | OPN | ↑ osteogenesis, neovascularization | [91,92,93] |
| FHRRIKA | BSP | ↑ osteoblast activity, mineralization | [94] |
| (ii) Angiogenic peptides | |||
| QK | VEGF | ↑EC migration, proliferation | [97] |
| PBA2-1c | PDGF-BB | Blood vessels formation | [98] |
| Exendin-4 | Exendin-4 | ↑ tube formation of HUVECs | [99] |
| SPARC113, SPARC118 | OPN | ↑ in vivo angiogenesis | [103] |
| TP508 | Thrombin | ↑ neo-angiogenesis | [84] |
| RoY | Synthetic | ↑ EC proliferation, sprouting, in vivo angiogenesis | [104] |
| b. Chondroinductive peptides | |||
| CMs | TGF-β | ↑Chondrogenic differentiation | [107,108] |
| CK2.1 | synthetic | ↑Chondrogenesis | [112] |
| c. Other supporting peptides | |||
| (i) Adhesion, binding, affinity peptides | |||
| RGD | Col, FN, VN | ↑Cellular adhesion/ or differentiation | [114] |
| PHSRN | FN | ↑Cell adhesion | [30,117] |
| FHRRIKA | BSP | ↑Cell proliferation, spreading, mineralization | [116,120] |
| KRSR | BSP, FN, VN, OPN, thrombospondin | ↑Osteoblast adhesion, osteogenic gene expression | [122,123,124,125] |
| HAV | N-Cadherin | ↑Cell adhesion | [126] |
| NEMO-binding domain (NBD) peptide | synthetic | ↑Osteoblast diferentiation ↓bone resorption, osteoclastogenesis | [130] [131] |
| CDPGYIGSR | Laminin | ↑Cell adhesion, ECM deposition | [150] |
| (ii) Peptides supporting cell migration | |||
| SDF1-ELP | SDF-1 | EC migration, vascularization | [158] |
| Histatin-1 | Saliva | EC adhesion, migration, angiogenesis | [159] |
| Ac2-26 | Annexin A1 | Cell migration | [160] |
| Esculentin 1-21 | Frog-skin | Cell migration | [161] |
| LL-37 | Human | EC migration | [162] |
| (ii) Cell penetrating peptides (CPPs) | |||
| NLS-TAT | HIV-1 Tat protein | ↑Chondrogenic differentiation | [154,155] |
| (iii) Self-assembly (SA) peptides | |||
| AcN-RADARADARADARADA-CONH2 (RADA16-I) | synthetic | ↑ ALP, OCN, Runx2 | [169] |
| (iv) Degradable peptides | |||
| KCGPQGIWGQCK | MMP-derived | ↑GAGs, collagen deposition | [140,173] |
| Antimicrobial and immunomodulatory peptides | |||
| TLISWIKNKRKQRPRVSRRRRRRGGRRRR (Melamine) | synthetic | destabilization of cell membrane | [175] |
| LLGDFFRKSKEKIGKEFKRIVQRIKDFLRNLVPRTES (LL-37) | human cathelicidin | inhibit cell wall synthesis | [179] |
| GIGKFLKKAKKFGKAFVKILKK (Pexiganan) | synthetic analogue of magainin2 | disrupts membrane | [175] |
| GIGKFLHSAGKFGKAFVGEIMKS (Magainin-1) |
frog skin | destabilizes or disrupts bacterial membrane | [175] |
| RRWVRRVRRWVRRVVRVVRRWVRR (PLG0206) | synthetic | disrupts cell membrane | [175] |
| RGGRLCYCRRRFCVCVGR (Protegrin-1) | porcine leukocytes | disrupts cell membrane | [175] |
| RLCRIVVIRVCR (Bactenecin) | bovine neutrophils | inhibit protein synthesis | [175] |
| VRVRVRVRVDPPTRVRVRVRV (PEP8R) | synthetic | disrupts bacterial cell membrane | [175] |
| ILPWKWPWWPWRR (Indolicidin) | bovine neutrophil | Increase cell membrane permeability | [175] |
| ILRWPWWPWRRK (Omiganan) | synthetic | destabilizes cell membrane | [175] |
| RAIGGGLSSVGGGSSTIKY (KAMP-19) | keratin | pore formation in bacterial cell membra | [176] |
| HNP-1 | human neutrophil | destabilizes cell wall integrity | [177] |
| RWRWRW-NH2 | synthetic | delocalizes peripheral membrane proteins | [178] |
| AMP 1037 | synthetic | ↓swimming and motility of bacteria, gene expression | [183] |
| Piscidin and sculentin 1–21 | fish | degrades biofilm | [184,185] |
| Histatin-1, 38 | saliva | antimicrobial | [159] |
| β defensin 3 | human | immunomodulator | [189] |
| GKIIKLKASLKLL (GL13K) | salivary protein | delocalizes cell membrane lipids | [190] |
| Abbreviations: BMP: Bone morphogenic protein, ALP: Alkaline Phosphatase, OCN: Osteocalcin, Col: Collagen, CK: Calmodulin Complexes, PTH: Parathyroid hormone, CGRP: Calcitonin gene-related peptide, OGP: Osteogenic growth peptide, FN: Fibronectin, VN: Vironectin, OPN: Osteopontin, CBM: Collagen-binding motif, BSP: bone sialoprotein, VEGF: Vascular endothelial growth factor, PDGF-BB, EC: Endothelial cell; CMs: Cytomodulins, GAG: Glycosaminoglycan; SDF-1: Stromal cell-derived factor 1, SDF1-ELP: SDF1 elastin-like peptide, CK2: casein kinase II | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Copyright: This open access article is published under a Creative Commons CC BY 4.0 license, which permit the free download, distribution, and reuse, provided that the author and preprint are cited in any reuse.
Submitted:
28 December 2023
Posted:
29 December 2023
You are already at the latest version
Alerts
A peer-reviewed article of this preprint also exists.
This version is not peer-reviewed
Submitted:
28 December 2023
Posted:
29 December 2023
You are already at the latest version
Alerts
Abstract
The healing of osteochondral defects (OCD) resulting from injury, osteochondritis, or osteoarthritis in middle- and old-age individuals, bearing lesions in the cartilage and bone, pain, and loss of joint function, presents challenges to the clinical practitioners because of non-regenerative cartilage and the limitations of current therapies. Bioactive peptide-based osteochondral (OC) tissue regeneration is becoming more popular because it does not have the immunogenicity, misfolding, or denaturation problems associated with original proteins. Periodically, reviews are published on the regeneration of bone and cartilage; however, none of them address the simultaneous healing of these tissues in the complicated heterogeneous environment of the OC interface. As regulators of cell adhesion, proliferation, differentiation, angiogenesis, immunomodulation, and antibacterial activity, potential therapeutic strategies for OC defects utilizing bone and cartilage-specific peptides should be examined and investigated. Therefore, the main goal of this review is to study how they contribute to the healing of OC defects, either alone or in conjunction with other peptides and biomaterials.
Keywords:
Subject: Chemistry and Materials Science - Biomaterials
1. Introduction
An articulating joint's osteochondral (OC) unit comprises the vascularized, mineralized subchondral bone and the acellular, avascular hyaline cartilage connected by a seamless interface.[1] The structural heterogenicity of the OC tissue arises from its diverse organic (cells, aggrecan, collagen) and inorganic (hydroxyapatite) components, along with their spatial orientation, forming gradients from the superficial cartilage to the subchondral bone via middle, deeper, and calcified layers (Figure 1). Cartilage exerts cushioning effects on joint bones and prevents damage from the physiological load; however, an injured cartilage cannot spontaneously regenerate. OC defects (OCD) are created by a variety of biological (aging, osteochondritis, osteoarthritis) or mechanical (accidental trauma, sports injury, prolonged wear) factors, characterized by cartilage and bone lesions, severe joint pain, and loss of joint function. They are more common in the middle-aged and older population. The World Health Organization estimates that 595 million people worldwide or 7.6 percent of the world's population suffered from osteoarthritis alone in 2020. This number has increased by 132.2 percent since 1990. [2]
When cartilage is damaged, the body's natural healing process is triggered by an inflammatory response, which leads to excessive production of reactive oxygen species (ROS) and matrix metalloproteinases (MMPs), further deteriorating the cartilage matrix and eventually inducing osteoarthritis. The treatment of OCD can be palliative (e.g., chondroplasty or microfracture), reparative (fixation), or restorative (autologous chondrocyte implantation (ACI), autologous matrix induced-chondrogenesis (AMIC), osteochondral autograft transplantation (OAT)) with their advantages and limitations.[3,4,5,6] Unfortunately, none of these techniques can fully repair the damaged tissue or form hyaline cartilage instead of fibrocartilage. [7]
OC tissue engineering (TE), which combines cells, scaffolds, and biomolecules to mimic extracellular microenvironment, seems promising for regenerating a functional OC tissue. The cells can be used for this purpose are bone marrow- or adipose-derived mesenchymal stem cells (MSCs), osteoblasts and chondroblasts derived through differentiation of MSCs, autologous chondrocytes, or MSCs cocultured with chondrocytes.[8] Scaffolds can be fabricated from various extracellular matrix (ECM)-mimetic biopolymers and synthetic polymers using solvent casting, freeze-drying, electrospinning, microfluidic-based methods, and three-dimensional (3D) printing. Considering heterogenicity of the OC tissue, a bi-layer or multilayer design is usually preferred over injectable hydrogels or single-layered scaffolds, since they possess osteoblasts-seeded porous bone layer forming continuous gradient with nonporous cartilage layer seeded with chondrocytes. Bioactive molecules, such as drugs (e.g., alendronate, dexamethasone), drug-like molecules (e.g., kartogenin, berberine), growth factors or peptides derived from them, are often incorporated into the scaffolds forming smart materials with enhanced regenerative capacity, facilitating cell migration, osteogenic or chondrogenic activity.[9,10] The transforming growth factor-β (TGF-β), bone morphogenetic proteins (BMPs) (especially BMP-2, BMP-4, and BMP-7), stromal cell-derived factor-1α (SDF-1α), insulin-like growth factor (IGF) (IGF-1 and IGF-2), fibroblast growth factor (FGF), platelet-derived growth factor (PDGF), and vascular endothelial growth factor (VEGF) are the major growth factors which promote MSCs, osteoblasts or chondrocytes recruitment, proliferation and differentiation, vascularization and maintenance of cartilage homeostasis. However, regulating their target selectivity, dose selection, release kinetics, and spatial distribution is quite challenging.
However, bioactive peptides are short-chain amino acid sequences that mimic signaling or binding domains of larger proteins and create a biomimetic environment for recruiting host cells, while controlling their activity and differentiation. They are either delivered alone or combined with biomaterials for tissue regeneration [9]. For instance, peptides either derived from ECM proteins or growth factors, or mimic their function, exhibit selective cells affinity, enhance cell penetration, or form hydrogels through self-assembly were investigated for OC regeneration. They are more effective, stable, scalable, and affordable than large proteins and also, they do not experience issues like immunogenicity, protein folding, and denaturation.[11]
Several reviews have been published highlighting the last five years’ clinical and experimental data on bioactive peptides for bone and cartilage regeneration as well as usage of peptide-enhanced bone graft substitutes. [11,12,13,14] However, none of them has focused specifically on the recovery of OC defects. Given that peptides could promote cell adhesion, motility, membrane penetration, immunomodulation, and antibacterial activities, besides inducing chondrogenic, osteogenic, and angiogenic differentiation, the primary focus of this review is to highlight their role in healing of OC defects when used alone or in combination with other peptides or biomaterials. Peptides belonging to different subcategories for bone and cartilage regeneration have been discussed after a brief review of the heterogeneity of the OC unit and the prerequisites for OC regeneration. Peptides that aid in osteo-, angio-, or chondrogenic activity, such as adhesion and migration of reparative cells, the creation of a 3D matrix, or an in vivo environment free from infection or inflammation, are covered in the following section. The review concludes with a summary and outlook after summarizing the results of several in vitro and in vivo studies using peptide-conjugated biomaterials.
2. Requirements for Osteochondral Tissue Regeneration
Successful recovery of OC defect faces significant challenge in reproducing the heterogeneous structure of the OC tissue. The present goal of OC regeneration is reviving the basic tissue structure and function under clinical settings, particularly in growth plate defects, cartilage defects in load-bearing sites, or full-thickness OC defects. The prerequisites are native tissue mimetic scaffolds, host tissue mimetic regenerated tissue and a well-controlled inflammatory state to counter MMPs-mediated irreversible destruction of cartilage matrix. The existing platelet-rich plasma (PRP) and exosomes-based therapies greatly passivated MMPs activity. Stem cell therapy maintained cytokine levels by expressing growth factors and limiting production of inflammatory cytokine, such as tumor necrosis factor alpha (TNF-α), interleukin-6 (IL-6).[15] Treating with the recombinant IL-1 receptor antagonists and monoclonal antibodies against TNF-α can generate a favorable cartilage microenvironment, which suggests MSCs and drug therapy may complement each other. Genetic engineering aims state reversal by transfecting seed cells with bone/cartilage-specific genes and arthritis inhibitory genes, thus ensuring necessary cytokines production.
Although tissue engineering holds promise for simultaneous bone and cartilage regeneration, injectable hydrogels and monolayer scaffolds fail to replicate the intricate spatial distribution of biochemical cues, particularly at the bone and cartilage interface.[16,17] Advanced techniques such as 3D printing or 3D bioprinting enable customized scaffold fabrication in terms of structure, anisotropy, and cell distribution; however, they involve high cost and intricate setup.[18]
Two transcription factors, Sox9 and Runx2, are essential for converting MSCs into chondrocytes and subsequent transformation into the hypertrophic phenotype, [19,20] while FGFs and BMPs are necessary for triggering bone formation.[21] Despite osteogenic and chondrogenic activity, BMPs have less control over chondrocyte hypertrophy and mineralization.[15,22] Localized delivery of bioactive peptides inducing target protein expression could be an ideal choice for biomimetic bone and cartilage regeneration, avoiding risks associated with growth factor or cell delivery. Adhesion and affinity peptides can be used to promote specific cell attachment and motility, respectively, while chondrogenic and osteogenic peptides can be delivered through multilayered scaffolds to accomplish site-specific differentiation. An antimicrobial peptide can potentially resist bacterial colonization at the defect site. On the other hand, inflammation or immunogenicity can be reduced using penetration peptides which enable selective cargo delivery to the cells and induce macrophage polarization.
3. Peptides for Bone Regeneration
The natural bone healing occurs in two different pathways: intramembranous ossification and endochondral ossification [23]. The former involves direct differentiation of MSC into osteoblasts, which then deposits mineralized matrix in flat bone. The latter is a complicated multi-step process of long bone formation that starts with inflammation and continues with hematoma development, platelet-mediated cytokine release, stem cell and fibroblast migration, chondrogenic differentiation, formation of fibrocartilaginous matrix (soft callus) and then mineralization into woven bones following chondrocyte hypertrophy. The woven bones develop into mature lamellar bone through remodeling and neovascularization. During this process, a variety of cytokines with distinct roles are released, including TGF-β1, PDGF-1, BMPs, VEGF-1, FGF, SDF-1α, TNF-α, Chemokine (CC-motif) ligand 2 (CCL2), IL-1b, IL-6, and Wnt proteins.[24] Among these, BMPs, TGF-β2, and -β3 play a crucial role in stem cell differentiation by causing hypertrophy and mineralization, while VEGF and angiopoietins promote neoangiogenesis.[25,26] BMP-2, 3, 4, 7, TNF-α, interferon-γ (IFN-γ), and certain hormones regulate the remodeling phase.[27] Since bioactive peptides follow similar cell signaling pathways with these proteins (Figure 2a), and may be beneficial for both in vitro and in vivo bone formation, they are grouped into two categories based on their role: osteo-inducers and angiogenic.
3.1. Osteo-Inducers
3.1.1. Collagen-Mimetic/Derived Peptides
Collagens are a superfamily of proteins consisting of 46 distinct polypeptide chains that supramolecularly assemble into 28 different types of fibrils, fibril-associated with interrupted triple helices (FACIT) and nonfibrillar forms that provide structural support and encourage cell adhesion via integrins. Peptides derived from integrin-binding motifs of collagen type I, the most significant ECM protein, are GFOGER, P15, KOD, DGEA, and BCSP1, which can promote osteogenic activity and in vivo bone formation.
Collagen-mimetic GFOGER (Gly-Phe-Hyp-Gly-Glu-Arg) peptide, derived from collagen α1 chain, selectively promotes α2β1 integrin binding required for osteoblastic differentiation. [28] Besides improving cell attachment, GFOGER successfully induced in vitro osteogenic differentiation and in vivo bone healing. [28,29,30,31,32] GFOGER coating on synthetic PCL scaffolds remarkably enhanced bone formation in critically sized segmental defects in rats by stimulating osteoblast adhesion and differentiation (Figure 2b). [31] P15, a 15-mer peptide (Gly-Thr-Pro-Gly-Pro-Gln-Gly-Ile-Ala-Gly-Gln-Arg-Gly-Val-Val), derived from collagen type I α1 chain, has a strong affinity for the cell surface α2β1 integrin receptors. By releasing growth factors and cytokines, the peptide dramatically enhanced the osteogenic differentiation of MSCs.[33] The commercially available P15 formulations significantly enhanced regeneration of alveolar bone and tibial defects in osteoporotic dogs. [34] [35] The collagen-mimetic KOD peptide, made of three units: ((PKG)4-(POG)4-(DOG)4), forms hydrogel through self-assembly inducing platelet activation and blood clotting associated with hematoma formation. [36] POG-based poly-amphiphilic hydrogels allowed faster recovery (within two weeks) of intervertebral disc defects in rabbits due to a significant increase in ECM deposition.[37] DGEA (Asp-Gly-Glu-Ala) derived from collagen type I adhesive motif serves as a crucial ligand for osteoblast differentiation. [38] DGEA-containing PA hydrogels seeded with hMSCs substantially upregulated osteogenic markers (OCN, RUNX2, and ALP). [39]
Using bone and cartilage stimulating peptide (BCSP™-1 or NGLPGPIGP) present in human collagen type-I, proliferation of rat bone marrow derived osteoblasts and human or bovine chondrocytes was drastically improved with enhanced bone mineral density (BMD) and bone mineral content (BMC) in male Wistar rats.[40] Three highly osteogenic peptides (GPAGPHGPVG, APDPFRMY, and TPERYY) derived from tilapia scale collagen hydrolysate notably increased the MC3T3-E1 cell proliferation, and mineralization activity (ALP synthesis, osteogenic-related gene expression) at concentrations of 50 μg/mL.[41]
3.1.2. BMP-Mimetic/Derived Peptides
As members of the TGF-β superfamily, BMPs are primarily produced by endothelial cells, osteoblasts, and hypertrophic chondrocytes and can recruit MSCs to the site of injury, differentiate into osteoblasts, while inducing ectopic bone formation.
KIPKASSVPTELSAISTLYL peptide, derived from the knuckle epitope of BMP2, increased ALP activity of osteoprogenitor cells.[42] P24, a BMP-2 mimetic peptide, with 24-mer peptide (Ser-Lys-Ile-Pro-Lys-Ala-Ser-Ser-Val-Pro-Thr-Gly-Leu-Ser-Ala-Ile-Ser-Thr-Leu-Tyr-Leu-Asp-Asp-Asp), bearing the knuckle epitope of the protein that facilitate binding with BMP receptors. P24 successfully induced ectopic bone formation in rodents.[43,44,45] PEP7 peptide (CKIPKPSSVP-TELSAISMLYL) derived from BMP-2, promoted adhesion, proliferation, and differentiation of MG-63 cells, besides new bone formation in supra-alveolar peri-implant defect model in micropig mandible[46]. BMP peptide (KIPKASSVPTELSAISTLYL), derived from BMP2, increased ALP activity, an early marker for bone formation, in murine osteoprogenitor cells [47] and other cell types, [48,49,50,51,52] besides dose-dependent healing of rabbit radial bone-defects. [53] The other osteoinductive or osteogenic peptides derived from BMP-2 (NSVNSKIPKACCVPTELSAI, KIPKASSVPTELSAISTLYL, DWIVA) produced differential effects on in vitro osteogenic differentiation as well as ectopic or orthotopic bone formation in vivo.[12] Bone-forming peptide (BFP-2) with VEHDKEFFHPRYHH sequence, isolated from the immature BMP-7 precursor, triggered osteogenic differentiation of BMSCs, while induced ectopic bone formation after subcutaneous implantation of BFP-2-treated BMSCs in mice (Figure 2c).[54] Similarly, effects of various osteoinductive peptides derived from BMP-4 (RKKNPNCRRH), BMP-7 (TVPKPSSAPTQLNAISTLYF, GQGFSYPYKAVFSTQ, ETLDGQSINPKLAGL), BMP-9 (KVGKACCVPTKLSPISVLY) have been reviewed. [55] Casein kinase 2 (CK2) related peptide has great influence on cell proliferation, and apoptosis, it facilitates in vivo bone formation by interacting with BMP receptor type Ia (BMPRIa).[56] Three BMP-2 mimetic peptides, CK2.1, CK2.2, and CK2.3, triggered the BMP signaling pathways by inhibiting CK2 binding to BMPRIa. [57] C2C12 cells, treated with CK2.3 peptide resulted in osteogenesis, while CK2.2 led to both osteogenesis and adipogenesis. [56,58]
3.1.3. Hormone-Derived Peptides
Parathyroid hormone (PTH) is a major regulator of mineral homeostasis. Parathyroid hormone (PTH)-related peptides, called Teriparatide, which is 1–34 peptide domains of PTH (PTH1–34), stimulated osteoblast activity and increased bone density at the fracture site, leading to healing of non-unions. [59,60,61,62,63] On the other hand, endogenous PTH-related protein (PTHrP) analogues, PTHrP1–34, PTHrP1–36, and PTHrP107–111, increased osteoblast activity and local bone formation.[64,65,66] Calcitonin gene-related peptide (CGRP) is a 37-mer neuropeptide, having two isoforms: α and β-CGRP. They have been found to stimulate the proliferation and differentiation of osteoprogenitor cells, [67,68,69,70] production of osteogenic molecules like IGF-I, BMP-2, [71,72] and reparative bone formation. [73]
3.1.4. Circulating Peptides
Osteogenic growth peptide (OGP), a 14-mer peptide occurring in mammalian blood, increases bone formation through anabolic effects on bone cells [74,75] and differentiation of osteoprogenitor cells leading to upregulated osteogenic markers, including mineralization [76,77,78] Thrombin peptide 508 (TP508) or Chrysalin is a 23-amino acid peptide, receptor binding domain of thrombin, which enhanced the proliferation, differentiation, and chemotaxis of human osteoblasts [79,80] and VEGF-stimulated angiogenesis [81]. TP508 injected into the fracture gap promoted fracture healing and increased blood vessel formation [82,83,84].
3.1.5. Other ECM-Derived Peptides
Signaling domains on ECM protein chains, capable of interacting with cell membrane receptors. Various peptides (e.g., FN III9-10/12-14) derived from fibronectin (FN), have shown to promote osteoblast activity and mineralization, [85] rabbit calvarial defects healing, [86] and augmented BMP-2 and PDGF-BB activities for bone regeneration in vivo. [87]
Collagen-binding motif (CBM) is a cleavage product of osteopontin (OPN) that can specifically bind to collagen [88] and promote migration and osteogenic differentiation [89], bone formation in rabbit calvarial defect model.[90] SVVYGLR (Ser-Val-Val-Tyr-Gly-Leu-Arg) peptide adjacent to the RGD sequence in OPN significantly enhanced the adhesion and proliferation of MSCs, neovascularization, upregulation of osteogenesis and angiogenesis when delivered through a collagen sponge. [91,92,93] FHRRIKA (Phe-His-Arg-Arg-Ile-Lys-Ala), a cell-binding and heparin-binding domain of bone sialoprotein (BSP) exerts favorable effect on osteoblast adhesion, spreading and mineralization [94] Higher cell proliferation and viability was observed on rat calvarial osteoblasts seeded scaffolds containing the RGD and FHRRIKA sequences.[95]
3.2. Angiogenic
Vascularization is a crucial process during natural bone formation. Many peptides are derived from angiogenic growth factors (e.g., VEGF, FGF-2, PDGF, etc.), ECM (e.g., OPN, ON) and other proteins that have crucial roles in blood vessel formation [96].
VEGF-mimicking QK or KLT peptide (KLTWQELQLKYKGIGGG), derived from the VEGF receptors binding domain 17–35, not only induce endothelial cell migration and proliferation, but also to trigger other complex processes like chemotaxis, capillary sprouting and organization similar to VEGF [97]. PDGF-BB derived PBA2-1c peptide interacts with α- and β-PDGF receptors. Though it’s in vivo proangiogenic activity is still unclear, it functions similarly to PDGF in establishing mature blood vessels that are created by VEGF [98]. Exendin-4, a glucagon-like peptide 1 (Glp-1) analogue, stimulates human umbilical vein endothelial cells (HUVECs) motility, sprouting, and tube formation in vitro in addition to in vivo sprout outgrowth.[99] While OPN is widely distributed in bone matrix helping in bone metabolism, OPN-derived peptide (OPD) does not induce endothelial cell proliferation in vitro. However, like VEGF, it facilitated endothelial cell migration and tube formation using 3D collagen gels [100], suppressed osteoclastogenesis,[91] promoted adhesion and proliferation of MSCs, besides neovascularization in a rat tibial defect model [101]. SPARC113 and SPARC118, two OPN-derived peptides, which exhibit potent angiogenic activity [102], stimulated in vivo angiogenesis when delivered through MMPs degradable hydrogel [103]. TP508 enhanced neoangiogenesis in femoral defects produced in rats [84] and mice [82] following one hour of local administration. The synthetic 12-mer peptides, known as RoY peptides, which were created via the phage-display technology, may also promote in vitro endothelial cell proliferation, tube formation, and sprouting, besides inducing in vivo angiogenesis via a distinct mechanism from VEGF [104].
4. Peptides for Cartilage Regeneration
4.1. Chondroinductive/Chondrogenic Peptides
Numerous peptides have been identified to imitate the functions of ECM components, cell-cell junction molecules, and chondroinductive/chondrogenic ligands triggering specific cell signaling pathways. Motifs derived fibronectin like RGD, decorin, collagen, MMPs have displayed chondrogenic properties. These peptides are often used to functionalize scaffolds encouraging chondrocyte adhesion, migration, and proliferation in addition to MSC differentiation into the chondrogenic lineage.
4.1.1. TGF-β Mimetic Peptides
TGF-β improves cell differentiation, collagen synthesis, and matrix deposition in cartilage tissue engineering.[105] Therefore, peptides mimicking TGF-β activity were used in OC TE for cartilage tissue regeneration. TGF-β mimetic peptides, i.e., cytomodulins (CMs), are oligopeptides containing 4-6 amino acids [106]. CMs immobilized to a solid surface can potentially induce the chondrogenic differentiation compared to its soluble form (Figure 2d). [107,108]
4.1.2. BMP2-Derived/Mimetic Peptides
BMP-2, a member of the TGF-β super-family, is one of the main chondrogenic growth factors that induce in vitro chondrogenic differentiation and cartilage regeneration in vivo. Human MSCs (hMSCs) cultured with ≥100 mg/mL of the BMP peptide resulted in glycosaminoglycan (GAGs) production, increased in levels of collagen production and matrix accumulation without extensive upregulation of hypertrophic markers.[107,108,109,110,111] Injection of BMP-2 mimetic CK2.1 peptide into the mice tail vein enhanced chondrogenesis and enhanced articular cartilage formation, without any effects on osteogenesis or BMD.[112] BMP peptide stimulated chondrogenic differentiation of hMSCs without additional growth factors. At a 100 μg/mL concentration, BMP peptide enhanced proteoglycan production and chondrogenic gene expression without causing hypertrophy, as occurs with BMP-2. [113]
Figure 2.
Growth-factor mimetic peptides for bone and cartilage regeneration in osteochondral defects. (a) Growth factor-mimetic peptide epitopes inducing direct signaling, e.g., mimicking transforming growth factor-β (TGF-β) pathway for TGFβ receptors (TGFβR) binding. (figure generated via biorender.com) (b) (i) Critical bone defects treated with GFOGER-coated scaffolds shows significantly higher bone formation compared to uncoated PCL scaffolds and empty defect (control), as revealed by micro-CT imaging after 12 weeks of implantation and the plot also depicts a similar pattern at 4 weeks. (ii) Histology confirms that areas of high attenuation in GFOGER-coated sample, as revealed by 2D micro-CT image (inset) are bone tissue (red/pink) (soft tissue appearing blue/green in Sanderson’s rapid bone stain).[31] Copyright© 2009 Elsevier Ltd. (c) Bone formation in mice treated with BFP-2 for 4 or 8 weeks in comparison to BMP-7 was evaluated by (i) radiography, (ii) bone length estimation, (iii) histological assessment after hematoxylin and eosin staining and (iv) measurement of the osteogenic area from histology samples using the Image-Pro Plus 6.0 software (n=4).[54] Copyright© 2017, Springer Nature. (d) Histological analysis (i) CM10 conjugated FNF-HMS (CM10-FNF-HMS) with rabbit BMSCs demonstrates positive in SO staining (GAGs) and collagen type II (COL II) staining indicating hyaline cartilage formation after 2 weeks and (ii) P24 conjugated FNF-HMS (P24- FNF-HMS) with rabbit BMSCs reveals significantly higher bone formation in H&E and osteocalcin staining after 5 weeks of subcutaneous implantation, respectively compared to FNF-HMS (D–F) (Scale bars: 100 μm).[108] Copyright© 2014 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figure 2.
Growth-factor mimetic peptides for bone and cartilage regeneration in osteochondral defects. (a) Growth factor-mimetic peptide epitopes inducing direct signaling, e.g., mimicking transforming growth factor-β (TGF-β) pathway for TGFβ receptors (TGFβR) binding. (figure generated via biorender.com) (b) (i) Critical bone defects treated with GFOGER-coated scaffolds shows significantly higher bone formation compared to uncoated PCL scaffolds and empty defect (control), as revealed by micro-CT imaging after 12 weeks of implantation and the plot also depicts a similar pattern at 4 weeks. (ii) Histology confirms that areas of high attenuation in GFOGER-coated sample, as revealed by 2D micro-CT image (inset) are bone tissue (red/pink) (soft tissue appearing blue/green in Sanderson’s rapid bone stain).[31] Copyright© 2009 Elsevier Ltd. (c) Bone formation in mice treated with BFP-2 for 4 or 8 weeks in comparison to BMP-7 was evaluated by (i) radiography, (ii) bone length estimation, (iii) histological assessment after hematoxylin and eosin staining and (iv) measurement of the osteogenic area from histology samples using the Image-Pro Plus 6.0 software (n=4).[54] Copyright© 2017, Springer Nature. (d) Histological analysis (i) CM10 conjugated FNF-HMS (CM10-FNF-HMS) with rabbit BMSCs demonstrates positive in SO staining (GAGs) and collagen type II (COL II) staining indicating hyaline cartilage formation after 2 weeks and (ii) P24 conjugated FNF-HMS (P24- FNF-HMS) with rabbit BMSCs reveals significantly higher bone formation in H&E and osteocalcin staining after 5 weeks of subcutaneous implantation, respectively compared to FNF-HMS (D–F) (Scale bars: 100 μm).[108] Copyright© 2014 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.

5. Other Supporting Peptides
5.1. Adhesion, Binding, or Affinity Peptides
RGD Peptide: RGD (Arg-Gly-Asp) motif is an essential cell adhesion peptide found in collagen, FN, vitronectin (VN), etc., promotes cell adhesion and/or differentiation through binding with transmembrane integrin receptors (Figure 3a). RGD sequences, such as GRGDS, RGDS, YRGDS and c(RGDfk), are widely used in the field of cartilage and bone repair. Bone regeneration using cyclic RGD in sheep spinal fusion model was comparable to that of rhBMP-2, due to its increased receptor affinity.[114] RGD, particularly the longer chain RGD (GPenGRGDSPCA), was not always effective in increasing cell adhesion and spreading. This could be because pre-absorbed proteins with stronger binding sites (like FN and VN) compete with weekly adsorbed RGD for cell binding, necessitating covalent attachment. [115,116] RGD is usually capable of increasing cellular adhesion of MSCs, but unable to induce osteogenic differentiation without osteogenic supplements or BMP-2 (Figure 3b). [29,48,49]
PHSRN Peptide: PHSRN (Pro-His-Ser-Arg-Asn) is a FN-derived peptide synergistically enhance cellular activity with RGD and other adhesion peptides through α5β1 integrin binding, though ineffective alone.[30,117] Due to competitive binding with α5β1 integrin sites, RGD and PHSRN did not improve the spreading of rat bone MSC on titanium surfaces[118]. However, a dimeric platform consisting of RGD and PHSRN, with a spacing between them resembling natural fibronectin, significantly improved the spreading and proliferation of SAOS-2 osteoblast-like cells when compared to RGD alone or their combination.[117] Further improvements in attachment, spreading, and cell differentiation were made possible by a longer chain RGD that allowed for wider spacings between the peptides like natural fibronectin; nevertheless, such modifications did not result in any osteogenic activity. [119]
FHRRIKA and KRSR Peptides: FHRRIKA (Phe-His-Arg-Arg-Ile-Lys-Ala) peptide, isolated from heparin binding domain of BSP, significantly enhanced cell proliferation, spreading, as well as matrix mineralization [116,120]. KRSR (Lys-Arg-Ser-Arg) is another peptide isolated from heparin-binding sites of BSP, along with FN, VN, OPN, thrombospondin, etc. [121], increased osteoblast adhesion and osteogenic gene expression. [122,123,124,125] A combination of FHRRIKA and longer chain RGD (CGGNGEPRGDTYRAY) stimulated rat osteoblast matrix mineralization, which was absent when they were employed alone.[94] Similarly, KRSR peptides delivered with RGD promoted cellular attachment on glass surface due to the due to this dual binding mechanism with proteoglycans and integrins, respectively.[121]
N-Cadherin is a calcium-dependent adhesion molecule consisting of five extracellular domains, in which, EC-1 domain having His-Ala-Val (HAV) motif, plays substantial role in cell adhesion. [126] hMSC-seeded nanofibrous hydrogels formed by a cadherin-mimic self-assembling peptide significantly upregulated the expression of chondrogenic markers such Col-II, Sox-9, and aggrecan.[127] hMSCs seeded hydrogels prepared from self-assembling KLD-12 peptide couples with N-cadherin mimetic peptide (HAVDI), remarkably increased chondrogenesis, upregulating cartilage-specific genes expression (collagen II, Aggrecan, and Sox9).[128] hMSC-seeded hyaluronic acid hydrogels functionalized with RGD and N-cadherin mimetic peptide improved osteogenic gene expression, besides new bone formation after 12 weeks of rat MSCs seeded hydrogel implantation into the rat calvarial defects.[129] On the other hand, NEMO-binding domain (NBD) peptide has shown to promote osteoblast differentiation and inhibit bone resorption,[130] osteoclastogenesis [131]. The other integrin binding peptides derived from collagen IV (NYYSNS), fibronectin (KQAGDV, PRARI), laminin (IKLLI, LRGDN and SINNNR), fibrinogen (GWTVFQKRLDGS, YSMKKTTMKIIPFNRLTIG and GWTVIQNRQ), netrin-1 (QWRDTWARRLRKFQREKKGKCRKA), CAM-1 (QIDS, LDT, DELPQLVTLPHPNLHGPEILDVPST), thrombospondin (CSVTCG), nidogen-1 (FRGDGQ) have been reported.[132]
Affinity peptides can bind with specific cells, scaffolds, and cytokines associated with cartilage regeneration. Using anti-CD44 antibody, biotin-avidin binding system [133], chondrocyte affinity peptides, [134] E7 peptide, [135], etc., cell adhesion was greatly improved. A TGF-β affinity peptide which can recruit TGF-β to the impaired region [136,137] was ligated to the PA nanofibers, promoted in vitro chondrogenic differentiation of hMSCs and cartilage repair in rabbits. [137] ECM affinity peptides can help mimic the native environment of chondrocytes thus integrated with scaffolds [138,139]. Hydrogels modified with HA - and chondroitin sulfate-binding peptides, significantly promoted BMSCs differentiation into chondrogenic lineage (Figure 3c). [140]
While RGD peptides immobilized at a low density effectively promoted cell adhesion and chondrogenenesis [141,142,143], a higher density of RGD induced hypertrophic transformation of chondrocytes due to the activation of integrin-mediated pathway.[144,145,146] However, compared to simple RGD adsorption, chemical grafting of RGD peptides on to the biomaterial surface offered improved coating stability and osteoconductivity (Figure 3d). [147] Incorporation of N-cadherin mimetic peptide, HAVDIGGGC, into HA hydrogels increased chondrogenic differentiation at an early stage followed by deposition of cartilaginous matrix [148,149]. Similarly, laminin-related peptide, CDPGYIGSR, significantly promoted cell adhesion with increased ECM deposition by bovine knee chondrocytes seeded on to the polyethylene oxide PEO/chitosan scaffolds.[150]
5.2. Cell Penetrating Peptides (CPPs)
CPPs are peptides derived from bacteria, viruses or synthetic method transverse the cellular membrane to transport their 'cargo' (proteins, siRNA, nanoparticles, oligonucleotides and other peptides), into the cytoplasm. [111]
hMSCs delivered with CPP-conjugated co-activator-associated arginine methyltransferase 1 (CARM1) proteins, transcriptional factor fusion proteins, or recombinant adenovirus expressing BMP-2, promoted in vitro differentiation, and highly mineralized bone formation in vivo. [151,152,153] NLS-TAT peptides were employed to introduce the hTGF-β3 plasmid into a self-assembled peptide scaffold or precartilaginous stem cells (PCSCs), and to facilitate chondrogenic differentiation. [154,155]
5.3. Peptides Promoting Cell Migration
For the healing of osteochondral defects, reparative cells, such as fibroblasts, MSCs, osteoblasts, chondrocytes, and endothelial cells, should migrate towards the defect site for participating in various stages of the healing process. ECM proteins and GFs (EGF, PDGF) facilitate cell migration by acting as mitogens or chemotactic factors.[156] Peptides derived from them often exhibit superior cell migration than the parent protein. They work via modulating adhesion proteins (integrins), enzymes (MMPs), kinase-linked receptors, or formyl peptide receptors. SDF-1 is known recruiter of endothelial progenitor cells that differentiate into mature vascular endothelium in the subchondral bone environment, thus promote osteochondral repair.[157] SDF-1 elastin-like peptide (SDF1-ELP) derived from SDF-1 showed comparable in vitro endothelial cell migration and vascularization to SDF, however it was more effective than SDF1 during in vivo healing of full-thickness wounds in diabetic mice. [158] Histatin-1, a 38-mer peptide with antimicrobial properties found in saliva, is a proangiogenic agent that stimulates the adhesion, migration, and angiogenesis of endothelial cells. [159] Other examples are the endogenous histatins, the annexin A1-derived peptide Ac2-26, [160] or the frog-skin derived esculentin 1-21 peptide,[161] induced better wound-healing driven by cell migration. Following administration of the endogenous antimicrobial peptide LL-37, vascularization increased significantly because of enhanced endothelial cell migration. [162] Like FGF-2 (bFGF), FGF-2-mimicking peptide, YRSRKYSSWYVALKR, derived from the 106–120 peptide domain in FGF-2, a partial agonist of FGF receptors, could successfully promote in vitro HUVEC cells proliferation and migration.[163]
5.4. Self-Assembly (SA) Peptides
SA peptides are composed of either alternating hydrophilic and hydrophobic amino acids or peptide amphiphiles (PA), which can self-assemble into β-sheet structures and interwoven nanofibrous hydrogel matrices under physiological conditions [164,165,166] SA peptides, isolated from various growth factors and bone-related proteins (RANKL-binding peptide, AC-100, B2A) have been studied for osteoblast differentiation and bone regeneration. [53,167,168] RADA16-I (AcN-RADARADARADARADA-CONH2) peptide immobilized on to the BMP-2 loaded hydrogel promoted osteogenic differentiation of MSCs leading to higher expression of osteogenic-related genes (ALP, OCN, Runx2) and in vivo bone regeneration [169]. PuraMatrixTM, a commercially available RADA16-1 peptide containing hydrogel, strongly supported cartilage formation [170,171]. Repeating units of KLD and RAD in a peptide hydrogel enhanced cartilage formation compared to agarose gel [172].
5.5. Degradable Peptides
Degradable peptides accelerate degradation of scaffolds to facilitate cell penetration and migration, ECM synthesis and deposition, consequently tissue ingrowth. MMPs are the best examples of ECM-degrading proteins. Inclusion of a MMPs-derived peptide (KCGPQGIWGQCK) remarkably promoted GAGs and collagen deposition on to the crosslinked poly (ethylene glycol) norbornene hydrogels.[140,173]
Figure 3.
Role of other supporting peptides for osteochondral regeneration. (a) Signaling of the adhesion peptides, e.g., integrin binding. (figure generated via biorender.com) (b) Evaluation of mineralization activity at 3 weeks of hBMSC culture on (i) tissue culture plate with or without osteogenic supplements (ii) self-assembled monolayer (SAM), bone morphogenic protein (BMP) and RGD peptide gradients on glass cover slips supplemented with osteogenic medium. Scale bars 100 μm.[48] Copyright© 2011 Elsevier Ltd. (i) (c) Structures of acrylated streptoccocal collagen-like 2 (Scl2) protein conjugated with hyaluronic acid (HA) binding CGGGRYPISRPRKR peptide (HAbind), chondroitin sulfate (CS) binding CGGGYKTNFRRYYRF peptide (CSbind) and cell adhesive GRGDSC peptide (RGDS) (ii) HAbind, CSbind and RGDS peptides based hydrogels cultured with hMSCs for 4 weeks, showed uniform cell and ECM distribution (H&E staining, in panel 1), extensive sGAG accumulation (Alcian Blue staining, in panel 2), high, low, and negative expressions of collagen type I (cartilage marker, in panel 3), type II (bone marker, in panel 4) and type X (hypertrophic marker, in panel 5), respectively (IHC staining), in all hydrogels, indicating chondrogenic differentiation without any hypertrophic transformation. Scale bars: 200 μm. [140] Copyright© 2015 Elsevier Ltd. (d) Histological analysis showing higher bone formation on RGD/PEG/Ti implants compared to RGD/Ti implants after 2 and 4 weeks of implantation in rabbit femoral condyles. (Mag: 40x; scale bar = 0.5 mm).[147] Copyright© 2011 Elsevier Ltd.
Figure 3.
Role of other supporting peptides for osteochondral regeneration. (a) Signaling of the adhesion peptides, e.g., integrin binding. (figure generated via biorender.com) (b) Evaluation of mineralization activity at 3 weeks of hBMSC culture on (i) tissue culture plate with or without osteogenic supplements (ii) self-assembled monolayer (SAM), bone morphogenic protein (BMP) and RGD peptide gradients on glass cover slips supplemented with osteogenic medium. Scale bars 100 μm.[48] Copyright© 2011 Elsevier Ltd. (i) (c) Structures of acrylated streptoccocal collagen-like 2 (Scl2) protein conjugated with hyaluronic acid (HA) binding CGGGRYPISRPRKR peptide (HAbind), chondroitin sulfate (CS) binding CGGGYKTNFRRYYRF peptide (CSbind) and cell adhesive GRGDSC peptide (RGDS) (ii) HAbind, CSbind and RGDS peptides based hydrogels cultured with hMSCs for 4 weeks, showed uniform cell and ECM distribution (H&E staining, in panel 1), extensive sGAG accumulation (Alcian Blue staining, in panel 2), high, low, and negative expressions of collagen type I (cartilage marker, in panel 3), type II (bone marker, in panel 4) and type X (hypertrophic marker, in panel 5), respectively (IHC staining), in all hydrogels, indicating chondrogenic differentiation without any hypertrophic transformation. Scale bars: 200 μm. [140] Copyright© 2015 Elsevier Ltd. (d) Histological analysis showing higher bone formation on RGD/PEG/Ti implants compared to RGD/Ti implants after 2 and 4 weeks of implantation in rabbit femoral condyles. (Mag: 40x; scale bar = 0.5 mm).[147] Copyright© 2011 Elsevier Ltd.

5.6. Antimicrobial and Immunomodulatory Peptides
AMPs or cationic host defense peptides (CHDPs) are oligopeptides (5–100 amino acids), cationic (net charge: +2 to +13), hydrophobic (50% hydrophobic residues), or amphipathic, derived from bacteriophages, bacteria, fungus, plants, and animals, possessing broad-spectrum antibacterial properties without causing antibiotic resistance. [174] Few also have angiogenic, cell migratory, and immunomodulatory properties facilitating tissue regeneration.
A wide range of AMPs have been reported.[175] They include helix-based (LL-37, magainin, melittin), sheet-based (protegrins, bactenecin, defensins) exhibiting β-hairpin like conformation, coil-based (indolicidin, omiganan) which are devoid of both α-helical and β-sheet structures, and composite AMPs, such as melimine (TLISWIKNKRKQRPRVSRRRRRRGGRRRR), which amalgamates functional regions of mellitin (α-helix) and protamine (random coil with a partially ordered α-helix). LL-37 (LLGDFFRKSKEKIGKEFKRIVQRIKDFLRNLVPRTES) is a 37-mer peptide α-helical AMP, derived from human cathelicidin antimicrobial protein, possessing antibacterial, antibiofilm, immunomodulation, and angiogenesis properties. Pexiganan (GIGKFLKKAKKFGKAFVKILKK), a synthetic magainin (GIGKFLHSAGKFGKAFVGEIMKS) analogue, PLG0206 (RRWVRRVRRWVRRVVRVVRRWVRR) rich in arginine, valine, and tryptophan residues, reduce biofilm formation. Protegrin-1 (RGGRLCYCRRRFCVCVGR), bactenecin (RLCRIVVIRVCR) and its derivatives (RKWRIVVIRVRR and RWRRIVVIRVRR), arginine-rich PEP8R (VRVRVRVRVDPPTRVRVRVRV) peptide, show broad-spectrum antimicrobial activities and reduced cytotoxicity. Indolicidin (ILPWKWPWWPWRR), rich in tryptophan and proline, is high potent against bacteria, fungi, and viruses, whereas omiganan (ILRWPWWPWRRK) shows improved bioactivity with reduced cytotoxicity.
AMPs work by disrupting bacterial cell wall or cytoplasmic membrane (Figure 4a), as well as interfering with the other cellular processes like cell wall synthesis. Bactericidal properties of keratin-derived anti-inflammatory KAMP-19 (RAIGGGLSSVGGGSSTIKY) peptide originates from the destruction of bacterial cell membrane via pore formation.[176] α-defensin, known as human Neutrophil Peptide 1 (HNP-1), exhibits high affinity for lipid II, an important component for cell wall synthesis, thereby destabilizes the cell wall integrity [177]. Likewise, RWRWRW-NH2 delocalizes peripheral membrane proteins in Gram-positive bacteria,[178] LL-37 affects cell wall synthesis by regulating Sfp1 gene, buforin II interrupts cellular functions by interacting with nucleic acids [179], and TO17 degrades DNA and RNA. [180]
Biofilms associated with chronic bone infection are difficult to treat because of bacterial resistance.[181] By interacting with mannan, a key component of cell walls, L-37 decreases cell adhesion before it causes biofilms, therefore lowering Candida infection (Figure 4b).[182] AMP 1037, a 9-mer peptide, decreases swimming and motility of bacteria [183]. Piscidin and sculentin (1–21) degrades pre-formed biofilm matrix by activating nuclease activity and disturbing membrane function, respectively.[184,185]
Anti-inflammatory cytokines (AMPs) can suppress pro-inflammatory cytokines and their associated signaling pathways, or stimulate the synthesis of anti-inflammatory cytokines, which can initiate both an innate and adaptive immune response to eliminate infection and inflammation (Figure 4c). Immunomodulatory metalloproteinases (AMPs) such as cathelicidin-WA stimulate macrophage polarisation from the M1 phenotype (caused by E. Coli) to the M2 phenotype (facilitating bone repair) (Figure 4d).[186] Macrophage polarization plays a significant role in mediating chondrogenesis. Anti-inflammatory M2 macrophages significantly contribute to tissue remodeling and repair, [187] while failure to transform M1 to the M2 subtype induces the progression of cartilage injury.[188]
Regenerative AMPs are beneficial for the regeneration of bone tissue because they can inhibit osteoclastogenesis and stimulate angiogenesis. Histatin-1, 38-mer peptide occurring in saliva, induces angiogenesis and tube formation.[159] On the other hand, human β defensin 3 stimulates MSC osteogenesis,[189] GL13K (GKIIKLKASLKLL) suppresses osteoclastogenesis,[190] and LL-37 supports the proliferation, migration, and differentiation of MSCs to osteogenic lineage [191].
6. Peptide-Conjugated Biomaterials
Natural ECM-mimetic biomaterials like collagen, gelatin, chitosan, alginate, and silk, etc., are often combined with synthetic materials, such as polyethylene glycol (PEG), polylactic acid (PLA), and polycaprolactone (PCL), to fabricate an OC construct possessing tailorable strength, bioactivity, biocompatibility, and biodegradation characteristics, with reduced hydrophobicity, immunogenicity, and inflammatory properties. Bioactive materials, such as hydroxyapatite, calcium phosphate, and biological ceramics, are also combined to encourage biomineralization.[22]
6.1. Osteo-Inductive Scaffold
Delivery of BMP2-derived KIPKASSVPTELSAISTLYL peptide through TCP scaffolds promoted bone healing rabbit radial bone-defect.[192] BMP-2 mimetic peptides attached to a substrate enhanced BMSC attachment and differentiation without affecting mineralization. [49] However, when these peptides were combined with RGD, the rate of mineralization increased because RGD induced higher interaction cells in absence of osteogenic supplementation. [48] Incorporation of BMP-2-mimetic peptide conjugated to a heptaglutamate moiety (E7-BMP-2) into the mineralized PLGA-collagen-gelatin nanofiber promoted bone formation in critical-sized alveolar bone defects in rats after 4 weeks of implantation.[193] Electrospun porous cellulose acetate nanofibrous mat modified with adhesive peptides KRSR, RGD, and growth factor BMP-2 enhanced adhesion and proliferation of pre-osteoblastic cells.[194] PCL nanofibrous scaffolds containing different concentrations of the bioactive with KIPKASSVPTELSAISTLYL peptide derived from the BMP-2 developed by Lukasova et al. Scaffolds seeded with porcine MSCs showed increased expression of OCN and collagen I significantly osteogenic differentiation.[195] KRSR, BMP mimetic peptides, and FHRIKKA were successfully integrated into the outer, middle, and inner layers of PLGA electrospun membranes through layer-by-layer assembly, and such modification demonstrated synergistic effects on bone healing by enhancing cell attachment, differentiation, and mineralization. [196] FHRRIKA and KRSR peptides covalently linked with the surface of polymeric nanoparticles significantly improved MSC attachment and ALP activity due to an osteogenic differentiation, which was not very profound in case of KRSR.[197,198] Immobilization of KRSR on a silane functionalized borosilicate glass,[121] or micropatterning with KRSR on borosilicate glass,[199] and the other substrates.[116,124,125,200] selectively enhanced osteoblasts adhesion through αvβ5 integrin receptors binding.
On the other hand, GFOGER functionalized PEG-BMP-2 hydrogels increased in vitro cell spreading and differentiation of BMSCs into osteogenic lineage, besides improved bone healing in mice radial defect model even in absence of BMP-2.[29] Implants coated with GFOGER were found to improve peri-implant bone regeneration and osseointegration[201]. However, hydroxyapatite disks adsorbed with GFOGER failed to attach cells with a decrease in cell spreading,[115] possibly due to a lack in nature-mimicking conformation of GFOGER.[31] P15-coated bovine bone grafts allowed faster recovery of periodontal defects in humans. [202] P-15-containing bone graft substitutes could facilitate the process of early bone formation[203,204], bone healing and regeneration[205]. P24 delivery to the defect site was achieved by encapsulating in chitosan microspheres,[50] covalent binding with polymeric backbone, [43] or simple adsorption on hydroxyapatite,[43] greatly enhancing the bioactive properties of the scaffolds.
DGEA immobilized on HA was found to enhance the adhesion and osteoblastic differentiation of MSCs, and new bone formation[206]. BCSP™-1, when delivered through HA and tricalcium phosphate (TCP) grafts, stimulated ALP activity in murine calvarial osteoprogenitor cells[207]. OGP rich PLGA scaffold accelerated bone healing in 1.5-cm rabbit segmental defects.[208] PEG hydrogel containing PTH1–34 promoted in situ bone augmentation in rabbits, [209] whereas TP508 loaded PPF composite and microsphere scaffolds showed enhanced bone formation in rabbit segmental bone defects. [210] RGD coated implants were found to have an increased peri-implant bone formation and enhanced direct bone apposition even in areas of poor surrounding bone. [147,211,212,213] An increased osteoblast density was observed, when anodized nanotubular titanium surface was coated with RGD or RGDS (Arg-Gly-Asp-Ser).[214,215]
6.2. Chondro-Inductive Scaffolds
Collagen type II-derived peptide conjugated zwitterionic carbon nano-dots (pCD) induced chondrogenic differentiation of ADMSCs. Pluronic F-127 hydrogel mediated delivery of pCD could successfully promote rabbit auricular cartilage defect healing after 60 days.[179] Self-assembled KLD12 and KLD12-CMP7 peptide hydrogels loaded into poly (L-lactide-co-caprolactone) scaffolds could successfully create a chondrogenic microenvironment to rat BMSCs seeded scaffolds after subcutaneous implantation in nude mice.[216] Self-assembling PA scaffolds loaded with a TGF-binding domain, HSNGLPLGGGSEEEAAAVVV(K)-CO(CH2)10CH3 could support viability and chondrogenic differentiation of hMSC, besides accelerated healing of articular cartilage defects in rabbit.[137] Similarly, HSNGLPL peptide coated porous chitosan scaffolds or gelatin methacryloyl (GelMA) hydrogel successfully induced chondrogenic differentiation of MSCs and recovery of osteochondral defects in mice and rabbit model.[217,218] RGD coating on the synthetic bioinert materials surface significantly improved properties like cell adhesion, viability, and differentiation. Composite scaffolds were prepared from RGD functionalized hydroxyapatite/methoxy poly (ethylene glycol)-block-poly(ε-caprolactone) (1:2 ratio) scaffold followed by infiltration of TGF-β1 functionalized glycidyl methacrylate-hyaluronic acid hydrogel. The scaffolds significantly upregulated the expression of cartilage-specific genes (aggrecan, Col2a1, Sox9) with higher accumulation of sulphated glycosaminoglycan (sGAG), whereas successful OC defect healing was also achieved after 12 weeks of implantation in rabbit knee defect model.[219]
6.3. Multifunctional Scaffolds
Subcutaneous injection of nanofibrous hollow microspheres conjugated with TGF-β1 mimetic CM10 combined with rabbit BMSCs successfully induced ectopic cartilage formation in nude mice via chondrogenic differentiation of MSCs; in contrast, BMP-2 mimetic P24 peptide-loaded microspheres demonstrated osteogenic activity leading to bone formation.[108] Implantation of 3D printed porous bi-layered scaffolds infiltrated with TGF-β1 binding HSNGLPLGG(MA) peptides-rich GelMA hydrogels in the top layer and hydroxyapatite in the bottom layer successfully repaired the osteochondral of defects in SD rats through simultaneous cartilage and bone regeneration, respectively.[220] Hyaluronic-acid-based hydrogel particles (HGPs) functionalized with cysteine-tagged CK2.1 peptide showed promising results for cartilage repair in a mouse model, without inducing chondrocyte hypertrophy, unlike BMP-2. [57] Whereas, using same model, CK2.2 and CK2.3 peptides induced osteoblast differentiation and mineralization, similar to BMP-2 [112]. RGD functionalized poly (ethylene glycol)-diacrylate (PEGDA) hydrogel encapsulating rat osteoblasts facilitated the adhesion and spreading of rat osteoblasts leading to matrix mineralization.[221] RGD immobilized on hydroxyapatite improved healing of femoral condylar defects in rabbits.[222] Though a longer chain RGD, was unable to do the same without an TGF-β supplementation.[223] RGD peptide attached to glass coverslips through silanization, did not show osteoblast adhesion, since attachment happens through integrin binding.[121]
Wang et al developed cryogenic 3D printed bilayer β-tricalcium phosphate/PLGA osteochondral scaffold, enriched with osteogenic peptide sequence, KIPKA SSVPT ELSAI STLYL SGGC, and TGF-β1 loaded collagen I hydrogel for cartilage repair. The osteochondral scaffold showed improved chondrogenic differentiation of rBMSCs, by up-regulated expression of chondrogenic markers and glycosaminoglycan (GAG) production, while enhanced osteogenic differentiation of rBMSCs at the subchondral layer by up-regulated expression RUNX2, osteocalcin and alkaline phosphatase (ALP), and calcium deposition.[224]
Due to the bactericidal or anti-biofilm, immunomodulatory, and regenerative capacity, AMPs with adequate cytotocompatibility have been explored for bone and cartilage tissue regeneration. Titanium surface immobilized with Mel4 peptides demonstrated significant antimicrobial efficacy after implantation into the rabbit femoral defects.[225] While GL13K peptide (GKIIKLKASLKLL-NH2) coated titanium surface could effectively inhibit peri-implantitis through immunomodulatory function[226,227] Titanium plate modified with antimicrobial LF1-11-coupled RGD peptide demonstrated good antibacterial properties with improved osteoblast adhesion, proliferation and mineralization.[228] Inclusion of HHC-36 (KRWWKWWRR), a broad-spectrum cationic AMP, and osteoconductive Laponite nanosilicates into GelMA hydrogel, which was used for coating of titanium implant surface significantly promoted expression of osteogenic-related genes and mineralization of hMSCs, besides effective inhibition of Gram-positive bacteria (S. aureus and S. epidermidis) and Gram-negative bacteria (P. aeruginosa and E. coli).[229] On the other hand, HA bone scaffolds coated with PSI10 (RRWPWWPWRR) and BMP2-MP [230] or a nano-hydroxyapatite coated titanium surface containing antimicrobial human β-defensin 3 (HBD-3) peptide and BMP-2,[231] significantly promoted antibacterial and osteogenic activity of the scaffolds.
Multifunctional PEEK coated with lithium-ion and mussel-inspired antimicrobial peptide enhanced osteogenesis-associated genes/proteins expression, and osseointegration at bone-implant interface, besides inhibition of E. coli and S. aureus.[232] Coated with mouse beta-defensin-14 (MBD-14) also improved antimicrobial and osseointegration properties of PEEK.[233] KR-12 analogue immobilized on the surface of titanium or PEEK bone implants via a polydopamine coating showed remarkable antibacterial activity, osteogenic differentiation of MSCs and peri-implant bone formation.[234,235]
7. Summary and Outlook
The goal of bioengineering is tissue emulation, including mimicking ECM and facilitating cell-material interaction, as well as tissue shape, function, and the in vivo milieu. Peptides, as the cell-instructive molecular building blocks, can bind or co-assemble with ECM or synthetic biomaterials, that not only offer structural and functional complexity, but also additively or synergistically work for better cellular activity, immune response, or tissue remodeling. A significant number of peptides were investigated as potential candidates for the promoting bone and cartilage healing, either directly by promoting cell adhesion, migration, angiogenesis, and differentiation into osteo/chondrogenic lineage, or indirectly by helping cargo penetration into the cells. Considering the structural and functional heterogenicity of an osteochondral unit, a strategy combining multiple peptides with biomaterials for simultaneous bone and cartilage tissue regeneration has been proposed. For example, a collagen-mimetic GFOGER or P15 peptide, or a BMP-2 mimetic P24 or BFP-2 peptide can be combined with VEGF mimetic QK peptides or TP508 or synthetic RoY peptides for osteo-inductive effect. On the other hand, a TGF-β or BMP-2 mimetic peptide can be used to induce chondrogenic activity in conjunction with RGD or PHSRN conjugated hydrogel for improved adhesion and proliferation, or SDF1-ELP or MMP-cleavable peptide-based hydrogels for cell migration into the hydrogel matrix. Hydrogel nanocomposites with improved mechanical strength and biological properties may be created by co-assembling peptides with structural proteins or inorganic fillers or by short SA peptides with macromolecules. Peptides that prevent microbial infection or help pro-inflammatory M1 to anti-inflammatory M2 macrophage polarization can be added to overcome post-implantation issues. The advent of electrospinning, inkjet printing, bioprinting, and other advanced manufacturing strategies enable fabrication of peptide hydrogel constructs with complex geometry and multi-scale hierarchy resembling native tissue. More focus should be given on investigating phage display or artificial intelligence (AI) and machine learning (ML) tools, which can provide a pattern-based search to find novel sequences or bioactive motifs from full-length proteins by establishing rules for protein interactions and signaling.
Acknowledgments
The work was supported by NIPER Kolkata and the Department of Pharmaceuticals, Ministry of Chemicals and Fertilizers, Government of India.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Yildirim, N.; Amanzhanova, A.; Kulzhanova, G.; Mukasheva, F.; Erisken, C. Osteochondral interface: regenerative engineering and challenges. ACS Biomaterials Science & Engineering 2023, 9, 1205–1223. [Google Scholar]
- Steinmetz, J.D.; Culbreth, G.T.; Haile, L.M.; Rafferty, Q.; Lo, J.; Fukutaki, K.G.; Cruz, J.A.; Smith, A.E.; Vollset, S.E.; Brooks, P.M. Global, regional, and national burden of osteoarthritis, 1990–2020 and projections to 2050: a systematic analysis for the Global Burden of Disease Study 2021. The Lancet Rheumatology 2023, 5, e508–e522. [Google Scholar] [CrossRef] [PubMed]
- Howell, M.; Liao, Q.; Gee, C.W. Surgical management of osteochondral defects of the knee: an educational review. Current Reviews in Musculoskeletal Medicine 2021, 14, 60–66. [Google Scholar] [CrossRef]
- Zhu, M.; Zhong, W.; Cao, W.; Zhang, Q.; Wu, G. Chondroinductive/chondroconductive peptides and their-functionalized biomaterials for cartilage tissue engineering. Bioactive materials 2022, 9, 221–238. [Google Scholar] [CrossRef] [PubMed]
- Madry, H. Surgical therapy in osteoarthritis. Osteoarthritis and cartilage 2022, 30, 1019–1034. [Google Scholar] [CrossRef]
- Wei, W.; Dai, H. Articular cartilage and osteochondral tissue engineering techniques: Recent advances and challenges. Bioactive materials 2021, 6, 4830–4855. [Google Scholar] [CrossRef]
- Makris, E.A.; Gomoll, A.H.; Malizos, K.N.; Hu, J.C.; Athanasiou, K.A. Repair and tissue engineering techniques for articular cartilage. Nature Reviews Rheumatology 2015, 11, 21–34. [Google Scholar] [CrossRef]
- Chen, L.; Wei, L.; Su, X.; Qin, L.; Xu, Z.; Huang, X.; Chen, H.; Hu, N. Preparation and Characterization of Biomimetic Functional Scaffold with Gradient Structure for Osteochondral Defect Repair. Bioengineering 2023, 10, 213. [Google Scholar] [CrossRef]
- Rizzo, M.G.; Palermo, N.; D’Amora, U.; Oddo, S.; Guglielmino, S.P.P.; Conoci, S.; Szychlinska, M.A.; Calabrese, G. Multipotential Role of Growth Factor Mimetic Peptides for Osteochondral Tissue Engineering. International Journal of Molecular Sciences 2022, 23, 7388. [Google Scholar] [CrossRef]
- Gonçalves, A.M.; Moreira, A.; Weber, A.; Williams, G.R.; Costa, P.F. Osteochondral tissue engineering: The potential of electrospinning and additive manufacturing. Pharmaceutics 2021, 13, 983. [Google Scholar] [CrossRef]
- Bullock, G.; Atkinson, J.; Gentile, P.; Hatton, P.; Miller, C. Osteogenic peptides and attachment methods determine tissue regeneration in modified bone graft substitutes. Journal of Functional Biomaterials 2021, 12, 22. [Google Scholar] [CrossRef] [PubMed]
- Visser, R.; Rico-Llanos, G.A.; Pulkkinen, H.; Becerra, J. Peptides for bone tissue engineering. Journal of controlled release 2016, 244, 122–135. [Google Scholar] [CrossRef] [PubMed]
- Pountos, I.; Panteli, M.; Lampropoulos, A.; Jones, E.; Calori, G.M.; Giannoudis, P.V. The role of peptides in bone healing and regeneration: a systematic review. BMC medicine 2016, 14, 1–15. [Google Scholar] [CrossRef]
- Ai, C.; Lee, Y.H.D.; Tan, X.H.; Tan, S.H.S.; Hui, J.H.P.; Goh, J.C.-H. Osteochondral tissue engineering: perspectives for clinical application and preclinical development. Journal of Orthopaedic Translation 2021, 30, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Le, H.; Wang, Y.; Liu, H.; Li, Z.; Yang, X.; Wang, C.; Ding, J.; Chen, X. Instructive cartilage regeneration modalities with advanced therapeutic implantations under abnormal conditions. Bioactive Materials 2022, 11, 317–338. [Google Scholar] [CrossRef] [PubMed]
- Bittner, S.M.; Guo, J.L.; Melchiorri, A.; Mikos, A.G. Three-dimensional printing of multilayered tissue engineering scaffolds. Materials Today 2018, 21, 861–874. [Google Scholar] [CrossRef] [PubMed]
- Kapat, K.; Rameshbabu, A.P.; Maity, P.P.; Mandal, A.; Bankoti, K.; Dutta, J.; Das, D.K.; Dey, G.; Mandal, M.; Dhara, S. Osteochondral defects healing using extracellular matrix mimetic phosphate/sulfate decorated GAGs-agarose gel and quantitative micro-CT evaluation. ACS Biomaterials Science & Engineering 2018, 5, 149–164. [Google Scholar]
- Bittner, S.M.; Guo, J.L.; Mikos, A.G. Spatiotemporal control of growth factors in three-dimensional printed scaffolds. Bioprinting 2018, 12, e00032. [Google Scholar] [CrossRef]
- Gibson, J.D.; O’Sullivan, M.B.; Alaee, F.; Paglia, D.N.; Yoshida, R.; Guzzo, R.M.; Drissi, H. Regeneration of articular cartilage by human ESC-derived mesenchymal progenitors treated sequentially with BMP-2 and Wnt5a. Stem cells translational medicine 2017, 6, 40–50. [Google Scholar] [CrossRef]
- McGee-Lawrence, M.E.; Carpio, L.R.; Bradley, E.W.; Dudakovic, A.; Lian, J.B.; van Wijnen, A.J.; Kakar, S.; Hsu, W.; Westendorf, J.J. Runx2 is required for early stages of endochondral bone formation but delays final stages of bone repair in Axin2-deficient mice. Bone 2014, 66, 277–286. [Google Scholar] [CrossRef]
- Du, X.; Xie, Y.; Xian, C.J.; Chen, L. Role of FGFs/FGFRs in skeletal development and bone regeneration. Journal of cellular physiology 2012, 227, 3731–3743. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.L.; Diaz-Gomez, L.; Xie, V.Y.; Bittner, S.M.; Jiang, E.Y.; Wang, B.; Mikos, A.G. Three-dimensional printing of click functionalized, peptide patterned scaffolds for osteochondral tissue engineering. Bioprinting 2021, 22, e00136. [Google Scholar] [CrossRef] [PubMed]
- Einhorn, T.A.; Gerstenfeld, L.C. Fracture healing: mechanisms and interventions. Nature Reviews Rheumatology 2015, 11, 45–54. [Google Scholar] [CrossRef]
- Pozos-Guillén, A.; Molina, G.; Soviero, V.; Arthur, R.A.; Chavarria-Bolaños, D.; Acevedo, A.M. Management of dental caries lesions in Latin American and Caribbean countries. Brazilian Oral Research 2021, 35, e055. [Google Scholar] [CrossRef]
- Mehta, M.; Schmidt-Bleek, K.; Duda, G.N.; Mooney, D.J. Biomaterial delivery of morphogens to mimic the natural healing cascade in bone. Advanced drug delivery reviews 2012, 64, 1257–1276. [Google Scholar] [CrossRef]
- Gerber, H.-P.; Vu, T.H.; Ryan, A.M.; Kowalski, J.; Werb, Z.; Ferrara, N. VEGF couples hypertrophic cartilage remodeling, ossification and angiogenesis during endochondral bone formation. Nature medicine 1999, 5, 623–628. [Google Scholar] [CrossRef] [PubMed]
- Calori, G.; Albisetti, W.; Agus, A.; Iori, S.; Tagliabue, L. Risk factors contributing to fracture non-unions. Injury 2007, 38, S11–S18. [Google Scholar] [CrossRef] [PubMed]
- Reyes, C.D.; Petrie, T.A.; Burns, K.L.; Schwartz, Z.; García, A.J. Biomolecular surface coating to enhance orthopaedic tissue healing and integration. Biomaterials 2007, 28, 3228–3235. [Google Scholar] [CrossRef]
- Shekaran, A.; García, J.R.; Clark, A.Y.; Kavanaugh, T.E.; Lin, A.S.; Guldberg, R.E.; García, A.J. Bone regeneration using an alpha 2 beta 1 integrin-specific hydrogel as a BMP-2 delivery vehicle. Biomaterials 2014, 35, 5453–5461. [Google Scholar] [CrossRef]
- Connelly, J.; Petrie, T.; García, A.; Levenston, M. Fibronectin-and Collagen-Mimetic Ligands Regulate BMSC Chondrogenesis in 3D Hydrogels. European cells & materials 2011, 22, 168. [Google Scholar]
- Wojtowicz, A.M.; Shekaran, A.; Oest, M.E.; Dupont, K.M.; Templeman, K.L.; Hutmacher, D.W.; Guldberg, R.E.; García, A.J. Coating of biomaterial scaffolds with the collagen-mimetic peptide GFOGER for bone defect repair. Biomaterials 2010, 31, 2574–2582. [Google Scholar] [CrossRef] [PubMed]
- Kolambkar, Y.M.; Bajin, M.; Wojtowicz, A.; Hutmacher, D.W.; García, A.J.; Guldberg, R.E. Nanofiber orientation and surface functionalization modulate human mesenchymal stem cell behavior in vitro. Tissue Engineering Part A 2014, 20, 398–409. [Google Scholar] [CrossRef] [PubMed]
- Bhatnagar, R.S.; Qian, J.J.; Wedrychowska, A.; Sadeghi, M.; Wu, Y.M.; Smith, N. Design of biomimetic habitats for tissue engineering with P-15, a synthetic peptide analogue of collagen. Tissue Engineering 1999, 5, 53–65. [Google Scholar] [CrossRef] [PubMed]
- Hestehave Pedersen, R.; Rasmussen, M.; Overgaard, S.; Ding, M. Effects of P-15 peptide coated hydroxyapatite on Tibial defect repair in vivo in Normal and osteoporotic rats. BioMed Research International 2015, 2015. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, C.M.; Koepple, M.; Moest, T.; Neumann, K.; Weisel, T.; Schlegel, K.A. In vivo evaluation of biofunctionalized implant surfaces with a synthetic peptide (P-15) and its impact on osseointegration. A preclinical animal study. Clinical Oral Implants Research 2016, 27, 1339–1348. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.A.; Taylor, N.L.; Jalan, A.A.; Hwang, L.K.; Wang, B.K.; Hartgerink, J.D. A nanostructured synthetic collagen mimic for hemostasis. Biomacromolecules 2014, 15, 1484–1490. [Google Scholar] [PubMed]
- Uysal, O.; Arslan, E.; Gulseren, G.; Kilinc, M.C.; Dogan, I.; Ozalp, H.; Caglar, Y.S.; Guler, M.O.; Tekinay, A.B. Collagen peptide presenting nanofibrous scaffold for intervertebral disc regeneration. ACS Applied Bio Materials 2019, 2, 1686–1695. [Google Scholar] [PubMed]
- Harbers, G.M.; Healy, K.E. The effect of ligand type and density on osteoblast adhesion, proliferation, and matrix mineralization. Journal of Biomedical Materials Research Part A: An Official Journal of The Society for Biomaterials, The Japanese Society for Biomaterials, and The Australian Society for Biomaterials and the Korean Society for Biomaterials 2005, 75, 855–869. [Google Scholar] [CrossRef]
- Anderson, J.M.; Vines, J.B.; Patterson, J.L.; Chen, H.; Javed, A.; Jun, H.-W. Osteogenic differentiation of human mesenchymal stem cells synergistically enhanced by biomimetic peptide amphiphiles combined with conditioned medium. Acta biomaterialia 2011, 7, 675–682. [Google Scholar]
- Sindrey, D.; Pugh, S.; Smith, T. Connective Tissue Stimulating Peptides. US20050288229A1, US Patent 2005/02888229 A1. 2005. [Google Scholar]
- Huang, W.; Yu, K.; Kang, M.; Wang, Q.; Liao, W.; Liang, P.; Liu, G.; Cao, Y.; Miao, J. Identification and functional analysis of three novel osteogenic peptides isolated from tilapia scale collagen hydrolysate. Food Research International 2022, 162, 111993. [Google Scholar] [CrossRef]
- Lam, H.; Li, S.; Lou, N.; Chu, J.; Bhatnagar, R. Synthetic peptides cytomodulin-1 (CM-1) and cytomodulin-2 (CM-2) promote collagen synthesis and wound healing in vitro. In Proceedings of the The 26th Annual International Conference of the IEEE Engineering in Medicine and Biology Society; 2004; pp. 5028–5030. [Google Scholar]
- Lin, Z.-Y.; Duan, Z.-X.; Guo, X.-D.; Li, J.-F.; Lu, H.-W.; Zheng, Q.-X.; Quan, D.-P.; Yang, S.-H. Bone induction by biomimetic PLGA-(PEG-ASP) n copolymer loaded with a novel synthetic BMP-2-related peptide in vitro and in vivo. Journal of Controlled Release 2010, 144, 190–195. [Google Scholar] [CrossRef]
- Duan, Z.; Zheng, Q.; Guo, X.; Yuan, Q.; Chen, S. Experimental research on ectopic osteogenesis of BMP2-derived peptide P24 combined with PLGA copolymers. Journal of Huazhong University of Science and Technology 2007, 27, 179–182. [Google Scholar] [CrossRef]
- Saito, A.; Suzuki, Y.; Ogata, S.I.; Ohtsuki, C.; Tanihara, M. Prolonged ectopic calcification induced by BMP-2–derived synthetic peptide. Journal of Biomedical Materials Research Part A: An Official Journal of The Society for Biomaterials, The Japanese Society for Biomaterials, and The Australian Society for Biomaterials and the Korean Society for Biomaterials 2004, 70, 115–121. [Google Scholar] [CrossRef]
- Kang, E.-J.; Kim, S.-K.; Eom, T.-G.; Choi, K.-O.; Lee, T.-H. Evaluation of the osteogenic activity of the BMP-2 mimetic peptide, PEP7, in vitro and in vivo. International Journal of Oral & Maxillofacial Implants 2013, 28. [Google Scholar]
- Saito, A.; Suzuki, Y.; Ogata, S.-i.; Ohtsuki, C.; Tanihara, M. Activation of osteo-progenitor cells by a novel synthetic peptide derived from the bone morphogenetic protein-2 knuckle epitope. Biochimica et Biophysica Acta (BBA)-Proteins and Proteomics 2003, 1651, 60–67. [Google Scholar] [CrossRef]
- Moore, N.M.; Lin, N.J.; Gallant, N.D.; Becker, M.L. Synergistic enhancement of human bone marrow stromal cell proliferation and osteogenic differentiation on BMP-2-derived and RGD peptide concentration gradients. Acta biomaterialia 2011, 7, 2091–2100. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Ma, J.; Jabbari, E. Effect of grafting RGD and BMP-2 protein-derived peptides to a hydrogel substrate on osteogenic differentiation of marrow stromal cells. Langmuir 2008, 24, 12508–12516. [Google Scholar] [CrossRef]
- Niu, X.; Feng, Q.; Wang, M.; Guo, X.; Zheng, Q. Porous nano-HA/collagen/PLLA scaffold containing chitosan microspheres for controlled delivery of synthetic peptide derived from BMP-2. Journal of Controlled Release 2009, 134, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.-J.; Yeo, S.-I.; Park, J.-W.; Shin, H.-I.; Bae, Y.-C.; Suh, J.-Y. The effects of synthetic peptide derived from hBMP-2 on bone formation in rabbit calvarial defect. Tissue Eng Regen Me 2008, 5, 488–497. [Google Scholar]
- Lee, J.S.; Lee, J.S.; Murphy, W.L. Modular peptides promote human mesenchymal stem cell differentiation on biomaterial surfaces. Acta biomaterialia 2010, 6, 21–28. [Google Scholar] [CrossRef]
- Saito, A.; Suzuki, Y.; Kitamura, M.; Ogata, S.I.; Yoshihara, Y.; Masuda, S.; Ohtsuki, C.; Tanihara, M. Repair of 20-mm long rabbit radial bone defects using BMP-derived peptide combined with an α-tricalcium phosphate scaffold. Journal of Biomedical Materials Research Part A: An Official Journal of The Society for Biomaterials, The Japanese Society for Biomaterials, and The Australian Society for Biomaterials and the Korean Society for Biomaterials 2006, 77, 700–706. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.K.; Lee, J.S.; Kim, J.H.; Seon, J.K.; Park, K.S.; Jeong, M.H.; Yoon, T.R. Bone-forming peptide-2 derived from BMP-7 enhances osteoblast differentiation from multipotent bone marrow stromal cells and bone formation. Experimental & molecular medicine 2017, 49, e328–e328. [Google Scholar]
- Patterson, J. Peptide-functionalized Biomaterials with Osteoinductive or Anti-biofilm Activity. Racing for the Surface: Antimicrobial and Interface Tissue Engineering, 2020; 129–168. [Google Scholar]
- Bragdon, B.; Thinakaran, S.; Moseychuk, O.; Gurski, L.; Bonor, J.; Price, C.; Wang, L.; Beamer, W.G.; Nohe, A. Casein kinase 2 regulates in vivo bone formation through its interaction with bone morphogenetic protein receptor type Ia. Bone 2011, 49, 944–954. [Google Scholar] [CrossRef] [PubMed]
- Akkiraju, H.; Bonor, J.; Nohe, A. CK2. 1, a novel peptide, induces articular cartilage formation in vivo. Journal of Orthopaedic Research 2017, 35, 876–885. [Google Scholar] [CrossRef] [PubMed]
- Akkiraju, H.; Bonor, J.; Olli, K.; Bowen, C.; Bragdon, B.; Coombs, H.; Donahue, L.R.; Duncan, R.; Nohe, A. Systemic injection of CK2. 3, a novel peptide acting downstream of bone morphogenetic protein receptor BMPRIa, leads to increased trabecular bone mass. Journal of Orthopaedic Research 2015, 33, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Alkhiary, Y.M.; Gerstenfeld, L.C.; Krall, E.; Westmore, M.; Sato, M.; Mitlak, B.H.; Einhorn, T.A. Enhancement of experimental fracture-healing by systemic administration of recombinant human parathyroid hormone (PTH 1-34). JBJS 2005, 87, 731–741. [Google Scholar]
- Komrakova, M.; Stuermer, E.K.; Werner, C.; Wicke, M.; Kolios, L.; Sehmisch, S.; Tezval, M.; Daub, F.; Martens, T.; Witzenhausen, P. Effect of human parathyroid hormone hPTH (1–34) applied at different regimes on fracture healing and muscle in ovariectomized and healthy rats. Bone 2010, 47, 480–492. [Google Scholar] [CrossRef]
- Mognetti, B.; Marino, S.; Barberis, A.; Bravo Martin, A.-S.; Bala, Y.; Di Carlo, F.; Boivin, G.; Portigliatti Barbos, M. Experimental stimulation of bone healing with teriparatide: histomorphometric and microhardness analysis in a mouse model of closed fracture. Calcified tissue international 2011, 89, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Chintamaneni, S.; Finzel, K.; Gruber, B. Successful treatment of sternal fracture nonunion with teriparatide. Osteoporosis international 2010, 21, 1059–1063. [Google Scholar] [CrossRef]
- Whitfield, J.F.; Motley, P.; Willick, G.E. Parathyroid hormone, its fragments and their analogs for the treatment of osteoporosis. Treatments in Endocrinology 2002, 1, 175–190. [Google Scholar] [CrossRef]
- Peggion, E.; Mammi, S.; Schievano, E.; Silvestri, L.; Schiebler, L.; Bisello, A.; Rosenblatt, M.; Chorev, M. Structure− Function Studies of Analogues of Parathyroid Hormone (PTH)-1− 34 Containing β-Amino Acid Residues in Positions 11− 13. Biochemistry 2002, 41, 8162–8175. [Google Scholar] [CrossRef] [PubMed]
- de Castro, L.F.; Lozano, D.; Portal-Núñez, S.; Maycas, M.; De la Fuente, M.; Caeiro, J.R.; Esbrit, P. Comparison of the skeletal effects induced by daily administration of PTHrP (1–36) and PTHrP (107–139) to ovariectomized mice. Journal of cellular physiology 2012, 227, 1752–1760. [Google Scholar] [CrossRef]
- Trejo, C.G.; Lozano, D.; Manzano, M.; Doadrio, J.C.; Salinas, A.J.; Dapía, S.; Gómez-Barrena, E.; Vallet-Regí, M.; García-Honduvilla, N.; Buján, J. The osteoinductive properties of mesoporous silicate coated with osteostatin in a rabbit femur cavity defect model. Biomaterials 2010, 31, 8564–8573. [Google Scholar] [CrossRef] [PubMed]
- Mrak, E.; Guidobono, F.; Moro, G.; Fraschini, G.; Rubinacci, A.; Villa, I. Calcitonin gene-related peptide (CGRP) inhibits apoptosis in human osteoblasts by β-catenin stabilization. Journal of cellular physiology 2010, 225, 701–708. [Google Scholar] [CrossRef] [PubMed]
- Villa, I.; Melzi, R.; Pagani, F.; Ravasi, F.; Rubinacci, A.; Guidobono, F. Effects of calcitonin gene-related peptide and amylin on human osteoblast-like cells proliferation. European journal of pharmacology 2000, 409, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Shi, X.; Zhao, R.; Halloran, B.P.; Clark, D.J.; Jacobs, C.R.; Kingery, W.S. Calcitonin-gene-related peptide stimulates stromal cell osteogenic differentiation and inhibits RANKL induced NF-κB activation, osteoclastogenesis and bone resorption. Bone 2010, 46, 1369–1379. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Jiang, D. The role and mechanism of exogenous calcitonin gene-related peptide on mesenchymal stem cell proliferation and osteogenetic formation. Cell biochemistry and biophysics 2014, 69, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Calland, J.W.; Harris, S.E.; Carnes Jr, D.L. Human pulp cells respond to calcitonin gene-related peptide in vitro. Journal of endodontics 1997, 23, 485–489. [Google Scholar] [CrossRef] [PubMed]
- Vignery, A.; McCarthy, T. The neuropeptide calcitonin gene-related peptide stimulates insulin-like growth factor I production by primary fetal rat osteoblasts. Bone 1996, 18, 331–335. [Google Scholar] [CrossRef]
- Ballica, R.; Valentijn, K.; Khachatryan, A.; Guerder, S.; Kapadia, S.; Gundberg, C.; Gilligan, J.; Flavell, R.A.; Vignery, A. Targeted expression of calcitonin gene-related peptide to osteoblasts increases bone density in mice. Journal of bone and mineral research 1999, 14, 1067–1074. [Google Scholar] [CrossRef]
- Bab, I.; Gazit, D.; Chorev, M.; Muhlrad, A.; Shteyer, A.; Greenberg, Z.; Namdar, M.; Kahn, A. Histone H4-related osteogenic growth peptide (OGP): a novel circulating stimulator of osteoblastic activity. The EMBO journal 1992, 11, 1867–1873. [Google Scholar] [CrossRef] [PubMed]
- Gabet, Y.; Müller, R.; Regev, E.; Sela, J.; Shteyer, A.; Salisbury, K.; Chorev, M.; Bab, I. Osteogenic growth peptide modulates fracture callus structural and mechanical properties. Bone 2004, 35, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Brager, M.A.; Patterson, M.J.; Connolly, J.F.; Nevo, Z. Osteogenic growth peptide normally stimulated by blood loss and marrow ablation has local and systemic effects on fracture healing in rats. Journal of Orthopaedic Research 2000, 18, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Fei, Q.; Guo, C.; Xu, X.; Gao, J.; Zhang, J.; Chen, T.; Cui, D. Osteogenic growth peptide enhances the proliferation of bone marrow mesenchymal stem cells from osteoprotegerin-deficient mice by CDK2/cyclin A. Acta Biochim Biophys Sin 2010, 42, 801–806. [Google Scholar] [CrossRef]
- An, G.; Xue, Z.; Zhang, B.; Deng, Q.; Wang, Y.; Lv, S. Expressing osteogenic growth peptide in the rabbit bone mesenchymal stem cells increased alkaline phosphatase activity and enhanced the collagen accumulation. Eur Rev Med Pharmacol Sci 2014, 18, 1618–1624. [Google Scholar] [PubMed]
- Li, G.; Cui, Y.; McILmurray, L.; Allen, W.E.; Wang, H. rhBMP-2, rhVEGF165, rhPTN and thrombin-related peptide, TP508 induce chemotaxis of human osteoblasts and microvascular endothelial cells. Journal of orthopaedic research 2005, 23, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Vordemvenne, T.; Paletta, J.R.; Hartensuer, R.; Pap, T.; Raschke, M.J.; Ochman, S. Cooperative effects in differentiation and proliferation between PDGF-BB and matrix derived synthetic peptides in human osteoblasts. BMC musculoskeletal disorders 2011, 12, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Olszewska-Pazdrak, B.; Carney, D.H. Systemic administration of thrombin peptide TP508 enhances VEGF-stimulated angiogenesis and attenuates effects of chronic hypoxia. Journal of vascular research 2013, 50, 186–196. [Google Scholar] [CrossRef]
- Hanratty, B.M.; Ryaby, J.T.; Pan, X.-H.; Li, G. Thrombin related peptide TP508 promoted fracture repair in a mouse high energy fracture model. Journal of Orthopaedic Surgery and Research 2009, 4, 1–10. [Google Scholar] [CrossRef]
- Li, X.; Wang, H.; Touma, E.; Qi, Y.; Rousseau, E.; Quigg, R.J.; Ryaby, J.T. TP508 accelerates fracture repair by promoting cell growth over cell death. Biochemical and biophysical research communications 2007, 364, 187–193. [Google Scholar] [CrossRef]
- Wang, H.; Li, X.; Tomin, E.; Doty, S.B.; Lane, J.M.; Carney, D.H.; Ryaby, J.T. Thrombin peptide (TP508) promotes fracture repair by up-regulating inflammatory mediators, early growth factors, and increasing angiogenesis. Journal of orthopaedic research 2005, 23, 671–679. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-J.; Park, Y.-J.; Lee, Y.-M.; Rhyu, I.-C.; Ku, Y. The biological effects of fibrin-binding synthetic oligopeptides derived from fibronectin on osteoblast-like cells. Journal of Periodontal & Implant Science 2012, 42, 113–118. [Google Scholar]
- Lee, J.-A.; Ku, Y.; Rhyu, I.-C.; Chung, C.-P.; Park, Y.-J. Effects of fibrin-binding oligopeptide on osteopromotion in rabbit calvarial defects. Journal of periodontal & implant science 2010, 40, 211–219. [Google Scholar]
- Martino, M.M.; Tortelli, F.; Mochizuki, M.; Traub, S.; Ben-David, D.; Kuhn, G.A.; Müller, R.; Livne, E.; Eming, S.A.; Hubbell, J.A. Engineering the growth factor microenvironment with fibronectin domains to promote wound and bone tissue healing. Science translational medicine 2011, 3, 100ra189. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-Y.; Choo, J.-E.; Park, H.-J.; Park, J.-B.; Lee, S.-C.; Jo, I.; Lee, S.-J.; Chung, C.-P.; Park, Y.-J. Injectable gel with synthetic collagen-binding peptide for enhanced osteogenesis in vitro and in vivo. Biochemical and biophysical research communications 2007, 357, 68–74. [Google Scholar] [CrossRef]
- Shin, M.K.; Kim, M.-K.; Bae, Y.-S.; Jo, I.; Lee, S.-J.; Chung, C.-P.; Park, Y.-J. A novel collagen-binding peptide promotes osteogenic differentiation via Ca2+/calmodulin-dependent protein kinase II/ERK/AP-1 signaling pathway in human bone marrow-derived mesenchymal stem cells. Cellular signalling 2008, 20, 613–624. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-Y.; Choo, J.-E.; Choi, Y.-S.; Park, J.-B.; Min, D.-S.; Lee, S.-J.; Rhyu, H.K.; Jo, I.-H.; Chung, C.-P.; Park, Y.-J. Assembly of collagen-binding peptide with collagen as a bioactive scaffold for osteogenesis in vitro and in vivo. Biomaterials 2007, 28, 4257–4267. [Google Scholar] [CrossRef]
- Egusa, H.; Kaneda, Y.; Akashi, Y.; Hamada, Y.; Matsumoto, T.; Saeki, M.; Thakor, D.K.; Tabata, Y.; Matsuura, N.; Yatani, H. Enhanced bone regeneration via multimodal actions of synthetic peptide SVVYGLR on osteoprogenitors and osteoclasts. Biomaterials 2009, 30, 4676–4686. [Google Scholar] [CrossRef] [PubMed]
- Hamada, Y.; Yuki, K.; Okazaki, M.; Fujitani, W.; Matsumoto, T.; Hashida, M.K.; Harutsugu, K.; Nokihara, K.; Daito, M.; Matsuura, N. Osteopontin-derived peptide SVVYGLR induces angiogenesis in vivo. Dental materials journal 2004, 23, 650–655. [Google Scholar] [CrossRef]
- Park, K.M.; Lee, Y.; Son, J.Y.; Bae, J.W.; Park, K.D. In situ SVVYGLR peptide conjugation into injectable gelatin-poly (ethylene glycol)-tyramine hydrogel via enzyme-mediated reaction for enhancement of endothelial cell activity and neo-vascularization. Bioconjugate chemistry 2012, 23, 2042–2050. [Google Scholar] [CrossRef]
- Rezania, A.; Healy, K.E. Biomimetic peptide surfaces that regulate adhesion, spreading, cytoskeletal organization, and mineralization of the matrix deposited by osteoblast-like cells. Biotechnology progress 1999, 15, 19–32. [Google Scholar] [CrossRef] [PubMed]
- Stile, R.A.; Healy, K.E. Thermo-responsive peptide-modified hydrogels for tissue regeneration. Biomacromolecules 2001, 2, 185–194. [Google Scholar] [CrossRef]
- Van Hove, A.H.; Benoit, D.S. Depot-based delivery systems for pro-angiogenic peptides: a review. Frontiers in bioengineering and biotechnology 2015, 3, 102. [Google Scholar] [CrossRef] [PubMed]
- Finetti, F.; Basile, A.; Capasso, D.; Di Gaetano, S.; Di Stasi, R.; Pascale, M.; Turco, C.M.; Ziche, M.; Morbidelli, L.; D’Andrea, L.D. Functional and pharmacological characterization of a VEGF mimetic peptide on reparative angiogenesis. Biochemical pharmacology 2012, 84, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.R.; Silva, E.A.; Yuen, W.W.; Mooney, D.J. Spatio–temporal VEGF and PDGF delivery patterns blood vessel formation and maturation. Pharmaceutical research 2007, 24, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.-M.; Kang, Y.; Chun, H.J.; Jeong, J.-W.; Park, C. Evaluation of the in vitro and in vivo angiogenic effects of exendin-4. Biochemical and biophysical research communications 2013, 434, 150–154. [Google Scholar] [CrossRef] [PubMed]
- Hamada, Y.; Nokihara, K.; Okazaki, M.; Fujitani, W.; Matsumoto, T.; Matsuo, M.; Umakoshi, Y.; Takahashi, J.; Matsuura, N. Angiogenic activity of osteopontin-derived peptide SVVYGLR. Biochemical and biophysical research communications 2003, 310, 153–157. [Google Scholar] [CrossRef] [PubMed]
- Hamada, Y.; Egusa, H.; Kaneda, Y.; Hirata, I.; Kawaguchi, N.; Hirao, T.; Matsumoto, T.; Yao, M.; Daito, K.; Suzuki, M. Synthetic osteopontin-derived peptide SVVYGLR can induce neovascularization in artificial bone marrow scaffold biomaterials. Dental materials journal 2007, 26, 487–492. [Google Scholar] [CrossRef] [PubMed]
- Lane, T.F.; Iruela-Arispe, M.L.; Johnson, R.S.; Sage, E.H. SPARC is a source of copper-binding peptides that stimulate angiogenesis. The Journal of cell biology 1994, 125, 929–943. [Google Scholar] [CrossRef]
- Van Hove, A.H.; Burke, K.; Antonienko, E.; Brown III, E.; Benoit, D.S. Enzymatically-responsive pro-angiogenic peptide-releasing poly (ethylene glycol) hydrogels promote vascularization in vivo. Journal of Controlled Release 2015, 217, 191–201. [Google Scholar] [CrossRef]
- Hardy, B.; Raiter, A.; Weiss, C.; Kaplan, B.; Tenenbaum, A.; Battler, A. Angiogenesis induced by novel peptides selected from a phage display library by screening human vascular endothelial cells under different physiological conditions. Peptides 2007, 28, 691–701. [Google Scholar] [CrossRef]
- Wang, W.; Rigueur, D.; Lyons, K.M. TGFβ signaling in cartilage development and maintenance. Birth Defects Research Part C: Embryo Today: Reviews 2014, 102, 37–51. [Google Scholar] [CrossRef] [PubMed]
- Bhatnagar, R.S.; Qian, J.J. Peptide compositions with growth factor-like activity. 1997.
- Renner, J.N.; Liu, J.C. Investigating the effect of peptide agonists on the chondrogenic differentiation of human mesenchymal stem cells using design of experiments. Biotechnology Progress 2013, 29, 1550–1557. [Google Scholar] [CrossRef]
- Zhang, Z.; Gupte, M.J.; Jin, X.; Ma, P.X. Injectable peptide decorated functional nanofibrous hollow microspheres to direct stem cell differentiation and tissue regeneration. Advanced functional materials 2015, 25, 350–360. [Google Scholar] [CrossRef] [PubMed]
- Mittal, A.; Kumar, R.; Parsad, D.; Kumar, N. Cytomodulin-functionalized porous PLGA particulate scaffolds respond better to cell migration, actin production and wound healing in rodent model. Journal of tissue engineering and regenerative medicine 2014, 8, 351–363. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Yang, D.; Wong, J.H.; Wang, J.; Yin, C.; Zhu, Y.; Fan, S.; Ng, T.B.; Xia, J.; Li, Z. A Thioether-Stabilized d-Proline–l-Proline-Induced β-Hairpin Peptide of Defensin Segment Increases Its Anti-Candida albicans Ability. ChemBioChem 2016, 17, 1416–1420. [Google Scholar] [CrossRef] [PubMed]
- Ruczynski, J.; Wierzbicki, P.M.; Kogut-Wierzbicka, M.; Mucha, P.; Siedlecka-Kroplewska, K.; Rekowski, P. Cell-penetrating peptides as a promising tool for delivery of various molecules into the cells. Folia histochemica et cytobiologica 2014, 52, 257–269. [Google Scholar] [CrossRef] [PubMed]
- Akkiraju, H.; Srinivasan, P.P.; Xu, X.; Jia, X.; Safran, C.B.K.; Nohe, A. CK2. 1, a bone morphogenetic protein receptor type Ia mimetic peptide, repairs cartilage in mice with destabilized medial meniscus. Stem Cell Research & Therapy 2017, 8, 1–11. [Google Scholar]
- Renner, J.N.; Kim, Y.; Liu, J.C. Bone morphogenetic protein-derived peptide promotes chondrogenic differentiation of human mesenchymal stem cells. Tissue Engineering Part A 2012, 18, 2581–2589. [Google Scholar] [CrossRef]
- Scholz, M.; Schleicher, P.; Sewing, A.; Gelinsky, M.; Kandziora, F. Cyclic-RGD is as effective as rhBMP-2 in anterior interbody fusion of the sheep cervical spine. Spine 2013, 38, E59–E65. [Google Scholar] [CrossRef]
- Hennessy, K.M.; Pollot, B.E.; Clem, W.C.; Phipps, M.C.; Sawyer, A.A.; Culpepper, B.K.; Bellis, S.L. The effect of collagen I mimetic peptides on mesenchymal stem cell adhesion and differentiation, and on bone formation at hydroxyapatite surfaces. Biomaterials 2009, 30, 1898–1909. [Google Scholar] [CrossRef]
- Sawyer, A.A.; Hennessy, K.M.; Bellis, S.L. The effect of adsorbed serum proteins, RGD and proteoglycan-binding peptides on the adhesion of mesenchymal stem cells to hydroxyapatite. Biomaterials 2007, 28, 383–392. [Google Scholar] [CrossRef]
- Mas-Moruno, C.; Fraioli, R.; Albericio, F.; Manero, J.M.; Gil, F.J. Novel peptide-based platform for the dual presentation of biologically active peptide motifs on biomaterials. ACS applied materials & interfaces 2014, 6, 6525–6536. [Google Scholar]
- Paredes, V.; Salvagni, E.; Rodríguez-Castellon, E.; Gil, F.; Manero, J. Study on the use of 3-aminopropyltriethoxysilane and 3-chloropropyltriethoxysilane to surface biochemical modification of a novel low elastic modulus Ti–Nb–Hf alloy. Journal of Biomedical Materials Research Part B: Applied Biomaterials 2015, 103, 495–502. [Google Scholar] [CrossRef]
- Benoit, D.S.; Anseth, K.S. The effect on osteoblast function of colocalized RGD and PHSRN epitopes on PEG surfaces. Biomaterials 2005, 26, 5209–5220. [Google Scholar] [CrossRef]
- Schuler, M.; Hamilton, D.W.; Kunzler, T.P.; Sprecher, C.M.; de Wild, M.; Brunette, D.M.; Textor, M.; Tosatti, S.G. Comparison of the response of cultured osteoblasts and osteoblasts outgrown from rat calvarial bone chips to nonfouling KRSR and FHRRIKA-peptide modified rough titanium surfaces. Journal of Biomedical Materials Research Part B: Applied Biomaterials: An Official Journal of The Society for Biomaterials, The Japanese Society for Biomaterials, and The Australian Society for Biomaterials and the Korean Society for Biomaterials 2009, 91, 517–527. [Google Scholar] [CrossRef]
- Dee, K.C.; Andersen, T.T.; Bizios, R. Design and function of novel osteoblast-adhesive peptides for chemical modification of biomaterials. Journal of biomedical materials research 1998, 40, 371–377. [Google Scholar] [CrossRef]
- Palchesko, R.N.; Romeo, J.D.; McGowan, K.A.; Gawalt, E.S. Increased osteoblast adhesion on physically optimized KRSR modified calcium aluminate. Journal of Biomedical Materials Research Part A 2012, 100, 1229–1238. [Google Scholar] [CrossRef]
- Sun, S.; Yu, W.; Zhang, Y.; Zhang, F. Increased preosteoblast adhesion and osteogenic gene expression on TiO 2 nanotubes modified with KRSR. Journal of Materials Science: Materials in Medicine 2013, 24, 1079–1091. [Google Scholar]
- Nelson, M.; Balasundaram, G.; Webster, T.J. Increased osteoblast adhesion on nanoparticulate crystalline hydroxyapatite functionalized with KRSR. International journal of nanomedicine 2006, 1, 339. [Google Scholar]
- Balasundaram, G.; Webster, T.J. Increased osteoblast adhesion on nanograined Ti modified with KRSR. Journal of Biomedical Materials Research Part A 2007, 80, 602–611. [Google Scholar] [CrossRef] [PubMed]
- Frith, J.E.; Mills, R.J.; Hudson, J.E.; Cooper-White, J.J. Tailored integrin–extracellular matrix interactions to direct human mesenchymal stem cell differentiation. Stem cells and development 2012, 21, 2442–2456. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, M.; Lai, T.-S.; Lin, H.-C. Substrate stiffness and sequence dependent bioactive peptide hydrogels influence the chondrogenic differentiation of human mesenchymal stem cells. Journal of Materials Chemistry B 2021, 9, 1676–1685. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Xu, J.; Wong, D.S.H.; Li, J.; Zhao, P.; Bian, L. Self-assembled N-cadherin mimetic peptide hydrogels promote the chondrogenesis of mesenchymal stem cells through inhibition of canonical Wnt/β-catenin signaling. Biomaterials 2017, 145, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Lin, S.; Sun, Y.; Feng, Q.; Li, G.; Bian, L. Hydrogels functionalized with N-cadherin mimetic peptide enhance osteogenesis of hMSCs by emulating the osteogenic niche. Biomaterials 2016, 77, 44–52. [Google Scholar] [CrossRef]
- Li, W.; Yu, B.; Li, M.; Sun, D.; Hu, Y.; Zhao, M.; Cui, C.-B.; Hou, S. NEMO-binding domain peptide promotes osteoblast differentiation impaired by tumor necrosis factor alpha. Biochemical and biophysical research communications 2010, 391, 1228–1233. [Google Scholar] [CrossRef] [PubMed]
- Dai, S.; Hirayama, T.; Abbas, S.; Abu-Amer, Y. The IκB kinase (IKK) inhibitor, NEMO-binding domain peptide, blocks osteoclastogenesis and bone erosion in inflammatory arthritis. Journal of Biological Chemistry 2004, 279, 37219–37222. [Google Scholar] [CrossRef]
- Ligorio, C.; Mata, A. Synthetic extracellular matrices with function-encoding peptides. Nature Reviews Bioengineering 2023, 1–19. [Google Scholar] [CrossRef]
- Lin, H.; Zhou, J.; Shen, L.; Ruan, Y.; Dong, J.; Guo, C.; Chen, Z. Biotin-conjugated anti-CD44 antibody–avidin binding system for the improvement of chondrocyte adhesion to scaffolds. Journal of Biomedical Materials Research Part A 2014, 102, 1140–1148. [Google Scholar] [CrossRef]
- Cheung, C.S.; Lui, J.C.; Baron, J. Identification of chondrocyte-binding peptides by phage display. Journal of Orthopaedic research 2013, 31, 1053–1058. [Google Scholar] [CrossRef]
- Shao, Z.; Zhang, X.; Pi, Y.; Wang, X.; Jia, Z.; Zhu, J.; Dai, L.; Chen, W.; Yin, L.; Chen, H. Polycaprolactone electrospun mesh conjugated with an MSC affinity peptide for MSC homing in vivo. Biomaterials 2012, 33, 3375–3387. [Google Scholar] [CrossRef] [PubMed]
- Webb, D.J.; Roadcap, D.W.; Dhakephalkar, A.; Gonias, S.L. A 16-amino acid peptide from human α2-macroglobulin binds transforming growth factor-β and platelet-derived growth factor-BB. Protein Science 2000, 9, 1986–1992. [Google Scholar] [CrossRef]
- Shah, R.N.; Shah, N.A.; Del Rosario Lim, M.M.; Hsieh, C.; Nuber, G.; Stupp, S.I. Supramolecular design of self-assembling nanofibers for cartilage regeneration. Proceedings of the National Academy of Sciences 2010, 107, 3293–3298. [Google Scholar] [CrossRef]
- Thakkar, S.; Fernandes, H.; Moroni, L. Decellularized extracellular matrix scaffolds for cartilage regeneration. Cartilage Tissue Engineering: Methods and Protocols, 2015; 133–151. [Google Scholar]
- Masters, K.S.; Shah, D.N.; Leinwand, L.A.; Anseth, K.S. Crosslinked hyaluronan scaffolds as a biologically active carrier for valvular interstitial cells. Biomaterials 2005, 26, 2517–2525. [Google Scholar] [CrossRef] [PubMed]
- Parmar, P.A.; Chow, L.W.; St-Pierre, J.-P.; Horejs, C.-M.; Peng, Y.Y.; Werkmeister, J.A.; Ramshaw, J.A.; Stevens, M.M. Collagen-mimetic peptide-modifiable hydrogels for articular cartilage regeneration. Biomaterials 2015, 54, 213–225. [Google Scholar] [CrossRef] [PubMed]
- Ruoslahti, E.; Pierschbacher, M.D. Arg-Gly-Asp: a versatile cell recognition signal. Cell 1986, 44, 517–518. [Google Scholar] [CrossRef]
- Kim, H.D.; Heo, J.; Hwang, Y.; Kwak, S.-Y.; Park, O.K.; Kim, H.; Varghese, S.; Hwang, N.S. Extracellular-matrix-based and Arg-Gly-Asp–modified photopolymerizing hydrogels for cartilage tissue engineering. Tissue Engineering Part A 2015, 21, 757–766. [Google Scholar] [CrossRef] [PubMed]
- Jeschke, B.; Meyer, J.; Jonczyk, A.; Kessler, H.; Adamietz, P.; Meenen, N.M.; Kantlehner, M.; Goepfert, C.; Nies, B. RGD-peptides for tissue engineering of articular cartilage. Biomaterials 2002, 23, 3455–3463. [Google Scholar] [CrossRef]
- Kim, I.L.; Khetan, S.; Baker, B.M.; Chen, C.S.; Burdick, J.A. Fibrous hyaluronic acid hydrogels that direct MSC chondrogenesis through mechanical and adhesive cues. Biomaterials 2013, 34, 5571–5580. [Google Scholar] [CrossRef]
- Homandberg, G.A.; Hui, F. Arg-Gly-Asp-Ser peptide analogs suppress cartilage chondrolytic activities of integrin-binding and nonbinding fibronectin fragments. Archives of biochemistry and biophysics 1994, 310, 40–48. [Google Scholar] [CrossRef]
- Zhang, T.; Wen, F.; Wu, Y.; Goh, G.S.H.; Ge, Z.; Tan, L.P.; Hui, J.H.P.; Yang, Z. Cross-talk between TGF-beta/SMAD and integrin signaling pathways in regulating hypertrophy of mesenchymal stem cell chondrogenesis under deferral dynamic compression. Biomaterials 2015, 38, 72–85. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-W.; Kurashima, K.; Tustusmi, Y.; An, C.-H.; Suh, J.-Y.; Doi, H.; Nomura, N.; Noda, K.; Hanawa, T. Bone healing of commercial oral implants with RGD immobilization through electrodeposited poly (ethylene glycol) in rabbit cancellous bone. Acta Biomaterialia 2011, 7, 3222–3229. [Google Scholar] [CrossRef] [PubMed]
- Tavella, S.; Raffo, P.; Tacchetti, C.; Cancedda, R.; Castagnola, P. N-CAM and N-cadherin expression during in vitro chondrogenesis. Experimental cell research 1994, 215, 354–362. [Google Scholar] [CrossRef] [PubMed]
- Bian, L.; Guvendiren, M.; Mauck, R.L.; Burdick, J.A. Hydrogels that mimic developmentally relevant matrix and N-cadherin interactions enhance MSC chondrogenesis. Proceedings of the National Academy of Sciences 2013, 110, 10117–10122. [Google Scholar] [CrossRef] [PubMed]
- Kuo, Y.-C.; Wang, C.-C. Surface modification with peptide for enhancing chondrocyte adhesion and cartilage regeneration in porous scaffolds. Colloids and Surfaces B: Biointerfaces 2011, 84, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Jo, J.; Song, H.; Park, S.G.; Lee, S.H.; Ko, J.J.; Park, J.H.; Jeong, J.; Cheon, Y.P.; Lee, D.R. Regulation of Differentiation Potential of Human Mesenchymal Stem Cells by Intracytoplasmic Delivery of Coactivator-Associated Arginine Methyltransferase 1 Protein Using Cell-Penetrating Peptide. Stem Cells 2012, 30, 1703–1713. [Google Scholar] [CrossRef] [PubMed]
- Suh, J.S.; Lee, J.Y.; Choi, Y.J.; You, H.K.; Hong, S.-D.; Chung, C.P.; Park, Y.J. Intracellular delivery of cell-penetrating peptide-transcriptional factor fusion protein and its role in selective osteogenesis. International journal of nanomedicine 2014, 1153–1166. [Google Scholar]
- Park, S.; Doh, J.; Park, S.; Lim, J.; Kim, S.; Youn, J.; Jin, H.; Seo, S.; Song, M.; Sung, S. Branched oligomerization of cell-permeable peptides markedly enhances the transduction efficiency of adenovirus into mesenchymal stem cells. Gene therapy 2010, 17, 1052–1061. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Chu, X.; Li, W.; Pan, Q.; You, H. Chondrogenic effect of precartilaginous stem cells following NLS-TAT cell penetrating peptide-assisted transfection of eukaryotic h TGF β3. Journal of Cellular Biochemistry 2013, 114, 2588–2594. [Google Scholar] [CrossRef]
- Pan, Q.; Li, W.; Yuan, X.; Rakhmanov, Y.; Wang, P.; Lu, R.; Mao, Z.; Shang, X.; You, H. Chondrogenic effect of cell-based scaffold of self-assembling peptides/PLGA-PLL loading the hTGFβ3 plasmid DNA. Journal of Materials Science: Materials in Medicine 2016, 27, 1–11. [Google Scholar] [CrossRef]
- Trepat, X.; Chen, Z.; Jacobson, K. Cell migration. Comprehensive Physiology 2012, 2, 2369. [Google Scholar] [PubMed]
- Shen, W.; Chen, X.; Chen, J.; Yin, Z.; Heng, B.C.; Chen, W.; Ouyang, H.-W. The effect of incorporation of exogenous stromal cell-derived factor-1 alpha within a knitted silk-collagen sponge scaffold on tendon regeneration. Biomaterials 2010, 31, 7239–7249. [Google Scholar] [CrossRef] [PubMed]
- Yeboah, A.; Cohen, R.I.; Faulknor, R.; Schloss, R.; Yarmush, M.L.; Berthiaume, F. The development and characterization of SDF1α-elastin-like-peptide nanoparticles for wound healing. Journal of controlled release 2016, 232, 238–247. [Google Scholar] [CrossRef]
- Torres, P.; Díaz, J.; Arce, M.; Silva, P.; Mendoza, P.; Lois, P.; Molina-Berríos, A.; Owen, G.I.; Palma, V.; Torres, V.A. The salivary peptide histatin-1 promotes endothelial cell adhesion, migration, and angiogenesis. The FASEB Journal 2017, 31, 4946–4958. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.J.; Xia, C.J.; Wei, Y.; Yao, Y.; Dong, M.W.; Lin, K.Z.; Yu, L.S.; Gao, Y.; Fan, Y.Y. Annexin A1-derived peptide Ac2-26 facilitates wound healing in diabetic mice. Wound Repair and Regeneration 2020, 28, 772–779. [Google Scholar] [CrossRef] [PubMed]
- Di Grazia, A.; Cappiello, F.; Imanishi, A.; Mastrofrancesco, A.; Picardo, M.; Paus, R.; Mangoni, M.L. The frog skin-derived antimicrobial peptide esculentin-1a (1-21) NH2 promotes the migration of human HaCaT keratinocytes in an EGF receptor-dependent manner: a novel promoter of human skin wound healing? PloS one 2015, 10, e0128663. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, K.; Okumura, K.; Isogai, H.; Isogai, E. The human cathelicidin antimicrobial peptide LL-37 and mimics are potential anticancer drugs. Frontiers in oncology 2015, 5, 144. [Google Scholar] [CrossRef]
- Rubert Pérez, C.M.; Álvarez, Z.; Chen, F.; Aytun, T.; Stupp, S.I. Mimicking the bioactivity of fibroblast growth factor-2 using supramolecular nanoribbons. ACS Biomaterials Science & Engineering 2017, 3, 2166–2175. [Google Scholar]
- Zhang, S. Emerging biological materials through molecular self-assembly. Biotechnology advances 2002, 20, 321–339. [Google Scholar] [CrossRef]
- Ryan, D.M.; Nilsson, B.L. Self-assembled amino acids and dipeptides as noncovalent hydrogels for tissue engineering. Polymer Chemistry 2012, 3, 18–33. [Google Scholar] [CrossRef]
- Avinash, M.; Govindaraju, T. Nanoarchitectonics of biomolecular assemblies for functional applications. Nanoscale 2014, 6, 13348–13369. [Google Scholar] [CrossRef]
- Lee, J.Y.; Choo, J.E.; Choi, Y.S.; Lee, K.Y.; Min, D.S.; Pi, S.H.; Seol, Y.J.; Lee, S.J.; Jo, I.H.; Chung, C.P. Characterization of the surface immobilized synthetic heparin binding domain derived from human fibroblast growth factor-2 and its effect on osteoblast differentiation. Journal of Biomedical Materials Research Part A: An Official Journal of The Society for Biomaterials, The Japanese Society for Biomaterials, and The Australian Society for Biomaterials and the Korean Society for Biomaterials 2007, 83, 970–979. [Google Scholar] [CrossRef] [PubMed]
- Furuya, Y.; Inagaki, A.; Khan, M.; Mori, K.; Penninger, J.M.; Nakamura, M.; Udagawa, N.; Aoki, K.; Ohya, K.; Uchida, K. Stimulation of bone formation in cortical bone of mice treated with a receptor activator of nuclear factor-κB ligand (RANKL)-binding peptide that possesses osteoclastogenesis inhibitory activity. Journal of Biological Chemistry 2013, 288, 5562–5571. [Google Scholar] [CrossRef]
- Ikeno, M.; Hibi, H.; Kinoshita, K.; Hattori, H.; Ueda, M. Effects of self-assembling peptide hydrogel scaffold on bone regeneration with recombinant human bone morphogenetic protein-2. Oral & Craniofacial Tissue Engineering 2011, 1. [Google Scholar]
- Recha-Sancho, L.; Semino, C.E. Heparin-based self-assembling peptide scaffold reestablish chondrogenic phenotype of expanded de-differentiated human chondrocytes. Journal of Biomedical Materials Research Part A 2016, 104, 1694–1706. [Google Scholar] [CrossRef]
- Yamaoka, H.; Asato, H.; Ogasawara, T.; Nishizawa, S.; Takahashi, T.; Nakatsuka, T.; Koshima, I.; Nakamura, K.; Kawaguchi, H.; Chung, U.i. Cartilage tissue engineering using human auricular chondrocytes embedded in different hydrogel materials. Journal of Biomedical Materials Research Part A: An Official Journal of The Society for Biomaterials, The Japanese Society for Biomaterials, and The Australian Society for Biomaterials and the Korean Society for Biomaterials 2006, 78, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Kisiday, J.; Jin, M.; Kurz, B.; Hung, H.; Semino, C.; Zhang, S.; Grodzinsky, A. Self-assembling peptide hydrogel fosters chondrocyte extracellular matrix production and cell division: implications for cartilage tissue repair. Proceedings of the National Academy of Sciences 2002, 99, 9996–10001. [Google Scholar] [CrossRef]
- Sridhar, B.V.; Brock, J.L.; Silver, J.S.; Leight, J.L.; Randolph, M.A.; Anseth, K.S. Development of a cellularly degradable PEG hydrogel to promote articular cartilage extracellular matrix deposition. Advanced healthcare materials 2015, 4, 702–713. [Google Scholar] [CrossRef]
- Bin Hafeez, A.; Jiang, X.; Bergen, P.J.; Zhu, Y. Antimicrobial peptides: An update on classifications and databases. International journal of molecular sciences 2021, 22, 11691. [Google Scholar] [CrossRef]
- Hao, Z.; Chen, R.; Chai, C.; Wang, Y.; Chen, T.; Li, H.; Hu, Y.; Feng, Q.; Li, J. Antimicrobial peptides for bone tissue engineering: Diversity, effects and applications. Frontiers in Bioengineering and Biotechnology 2022, 10, 1030162. [Google Scholar] [CrossRef]
- Sun, Y.; Chan, J.; Bose, K.; Tam, C. Simultaneous control of infection and inflammation with keratin-derived antibacterial peptides targeting TLRs and co-receptors. Science Translational Medicine 2023, 15, eade2909. [Google Scholar] [CrossRef] [PubMed]
- de Leeuw, E.; Li, C.; Zeng, P.; Li, C.; Diepeveen-de Buin, M.; Lu, W.-Y.; Breukink, E.; Lu, W. Functional interaction of human neutrophil peptide-1 with the cell wall precursor lipid II. FEBS letters 2010, 584, 1543–1548. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, M.; Chiriac, A.I.; Otto, A.; Zweytick, D.; May, C.; Schumacher, C.; Gust, R.; Albada, H.B.; Penkova, M.; Krämer, U. Small cationic antimicrobial peptides delocalize peripheral membrane proteins. Proceedings of the National Academy of Sciences 2014, 111, E1409–E1418. [Google Scholar] [CrossRef] [PubMed]
- Park, C.B.; Kim, H.S.; Kim, S.C. Mechanism of action of the antimicrobial peptide buforin II: buforin II kills microorganisms by penetrating the cell membrane and inhibiting cellular functions. Biochemical and biophysical research communications 1998, 244, 253–257. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Jin, Y.; Wang, X.; Yao, S.; Li, Y.; Wu, Q.; Ma, G.; Cui, F.; Liu, H. An antimicrobial peptide-loaded gelatin/chitosan nanofibrous membrane fabricated by sequential layer-by-layer electrospinning and electrospraying techniques. Nanomaterials 2018, 8, 327. [Google Scholar] [CrossRef] [PubMed]
- Vallet-Regí, M.; Lozano, D.; González, B.; Izquierdo-Barba, I. Biomaterials against bone infection. Adv. Healthc. Mater. 2020, 9. [Google Scholar] [CrossRef] [PubMed]
- Tsai, P.-W.; Yang, C.-Y.; Chang, H.-T.; Lan, C.-Y. Human antimicrobial peptide LL-37 inhibits adhesion of Candida albicans by interacting with yeast cell-wall carbohydrates. PloS one 2011, 6, e17755. [Google Scholar] [CrossRef] [PubMed]
- De La Fuente-Núñez, C.; Korolik, V.; Bains, M.; Nguyen, U.; Breidenstein, E.B.; Horsman, S.; Lewenza, S.; Burrows, L.; Hancock, R.E. Inhibition of bacterial biofilm formation and swarming motility by a small synthetic cationic peptide. Antimicrobial agents and chemotherapy 2012, 56, 2696–2704. [Google Scholar] [CrossRef] [PubMed]
- Libardo, M.D.J.; Bahar, A.A.; Ma, B.; Fu, R.; McCormick, L.E.; Zhao, J.; McCallum, S.A.; Nussinov, R.; Ren, D.; Angeles-Boza, A.M. Nuclease activity gives an edge to host-defense peptide piscidin 3 over piscidin 1, rendering it more effective against persisters and biofilms. The FEBS journal 2017, 284, 3662–3683. [Google Scholar] [CrossRef]
- Luca, V.; Stringaro, A.; Colone, M.; Pini, A.; Mangoni, M.L. Esculentin (1-21), an amphibian skin membrane-active peptide with potent activity on both planktonic and biofilm cells of the bacterial pathogen Pseudomonas aeruginosa. Cellular and molecular life sciences 2013, 70, 2773–2786. [Google Scholar] [CrossRef]
- Chen, S.; Lu, Z.; Wang, F.; Wang, Y. Cathelicidin-WA polarizes E. coli K88-induced M1 macrophage to M2-like macrophage in RAW264. 7 cells. International Immunopharmacology 2018, 54, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Dai, M.; Sui, B.; Xue, Y.; Liu, X.; Sun, J. Cartilage repair in degenerative osteoarthritis mediated by squid type II collagen via immunomodulating activation of M2 macrophages, inhibiting apoptosis and hypertrophy of chondrocytes. Biomaterials 2018, 180, 91–103. [Google Scholar] [CrossRef]
- Zhang, H.; Lin, C.; Zeng, C.; Wang, Z.; Wang, H.; Lu, J.; Liu, X.; Shao, Y.; Zhao, C.; Pan, J. Synovial macrophage M1 polarisation exacerbates experimental osteoarthritis partially through R-spondin-2. Annals of the rheumatic diseases 2018, 77, 1524–1534. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Bian, A.; Jia, F.; Lan, J.; Yang, H.; Yan, K.; Xie, L.; Qiao, H.; Chang, X.; Lin, H. “Dual-functional” strontium titanate nanotubes designed based on fusion peptides simultaneously enhancing anti-infection and osseointegration. Biomaterials Advances 2022, 133, 112650. [Google Scholar] [CrossRef]
- Zhou, L.; Han, Y.; Ding, J.; Chen, X.; Huang, S.; Xing, X.; Wu, D.; Chen, J. Regulation of an antimicrobial peptide GL13K-modified titanium surface on osteogenesis, osteoclastogenesis, and angiogenesis base on osteoimmunology. ACS Biomaterials Science & Engineering 2021, 7, 4569–4580. [Google Scholar]
- Liu, H.-W.; Wei, D.-X.; Deng, J.-Z.; Zhu, J.-J.; Xu, K.; Hu, W.-H.; Xiao, S.-H.; Zhou, Y.-G. Combined antibacterial and osteogenic in situ effects of a bifunctional titanium alloy with nanoscale hydroxyapatite coating. Artificial cells, nanomedicine, and biotechnology 2018, 46, 460–470. [Google Scholar] [CrossRef] [PubMed]
- Basu, S.; Kumar, M.; Chansuria, J.; Singh, T.B.; Bhatnagar, R.; Shukla, V.K. Effect of Cytomodulin-10 (TGF-ß1 analogue) on wound healing by primary intention in a murine model. International Journal of Surgery 2009, 7, 460–465. [Google Scholar] [CrossRef]
- Boda, S.K.; Wang, H.; John, J.V.; Reinhardt, R.A.; Xie, J. Dual delivery of alendronate and E7-BMP-2 peptide via calcium chelation to mineralized nanofiber fragments for alveolar bone regeneration. ACS biomaterials science & engineering 2020, 6, 2368–2375. [Google Scholar]
- Laboy-López, S.; Méndez Fernández, P.O.; Padilla-Zayas, J.G.; Nicolau, E. Bioactive Cellulose Acetate Electrospun Mats as Scaffolds for Bone Tissue Regeneration. International Journal of Biomaterials 2022, 2022. [Google Scholar] [CrossRef]
- Lukasova, V.; Buzgo, M.; Sovkova, V.; Dankova, J.; Rampichova, M.; Amler, E. Osteogenic differentiation of 3D cultured mesenchymal stem cells induced by bioactive peptides. Cell Proliferation 2017, 50, e12357. [Google Scholar] [CrossRef]
- Gentile, P.; Ferreira, A.M.; Callaghan, J.T.; Miller, C.A.; Atkinson, J.; Freeman, C.; Hatton, P.V. Multilayer nanoscale encapsulation of biofunctional peptides to enhance bone tissue regeneration in vivo. Advanced healthcare materials 2017, 6, 1601182. [Google Scholar] [CrossRef]
- Gentile, P.; Ghione, C.; Tonda-Turo, C.; Kalaskar, D. Peptide functionalisation of nanocomposite polymer for bone tissue engineering using plasma surface polymerisation. RSC advances 2015, 5, 80039–80047. [Google Scholar] [CrossRef]
- Shekaran, A.; García, A.J. Extracellular matrix-mimetic adhesive biomaterials for bone repair. Journal of biomedical materials research Part A 2011, 96, 261–272. [Google Scholar] [CrossRef]
- Hasenbein, M.; Andersen, T.; Bizios, R. Micropatterned surfaces modified with select peptides promote exclusive interactions with osteoblasts. Biomaterials 2002, 23, 3937–3942. [Google Scholar] [CrossRef]
- Posadowska, U.; Storm, I.; de Wolf, F.; van den Beucken, J.; Leeuwenburgh, S. Nanofibrillar hydrogel scaffolds from recombinant protein-based polymers with integrin-and proteoglycan-binding domains. Production of protein-based polymers in Pichia pastoris 1, 135.
- Reyes, C.D.; García, A.J. α2β1 integrin-specific collagen-mimetic surfaces supporting osteoblastic differentiation. Journal of Biomedical Materials Research Part A: An Official Journal of The Society for Biomaterials, The Japanese Society for Biomaterials, and The Australian Society for Biomaterials and the Korean Society for Biomaterials 2004, 69, 591–600. [Google Scholar] [CrossRef]
- Yukna, R.A.; Callan, D.P.; Krauser, J.T.; Evans, G.H.; Aichelmann-Reidy, M.E.; Moore, K.; Cruz, R.; Scott, J.B. Multi-center clinical evaluation of combination anorganic bovine-derived hydroxyapatite matrix (ABM)/cell binding peptide (P-15) as a bone replacement graft material in human periodontal osseous defects. 6-month results. Journal of periodontology 1998, 69, 655–663. [Google Scholar] [CrossRef]
- Thorwarth, M.; Schultze-Mosgau, S.; Wehrhan, F.; Srour, S.; Wiltfang, J.; Neukam, F.W.; Schlegel, K.A. Enhanced bone regeneration with a synthetic cell-binding peptide—in vivo results. Biochemical and biophysical research communications 2005, 329, 789–795. [Google Scholar] [CrossRef]
- Thorwarth, M.; Schultze-Mosgau, S.; Wehrhan, F.; Kessler, P.; Srour, S.; Wiltfang, J.; Schlegel, K.A. Bioactivation of an anorganic bone matrix by P-15 peptide for the promotion of early bone formation. Biomaterials 2005, 26, 5648–5657. [Google Scholar] [CrossRef]
- Matos, S.; Guerra, F.; Krauser, J.T.; Figueiredo, H.; Marcelino, J.P.; Sanz, M. Evaluation of an anorganic bovine-derived mineral with P-15 hydrogel bone graft: preliminary study in a rabbit cranial bone model. Clinical Oral Implants Research 2012, 23, 698–705. [Google Scholar] [CrossRef]
- Culpepper, B.K.; Phipps, M.C.; Bonvallet, P.P.; Bellis, S.L. Enhancement of peptide coupling to hydroxyapatite and implant osseointegration through collagen mimetic peptide modified with a polyglutamate domain. Biomaterials 2010, 31, 9586–9594. [Google Scholar] [CrossRef]
- Wang, V.; Misra, G.; Amsden, B. Immobilization of a bone and cartilage stimulating peptide to a synthetic bone graft. Journal of Materials Science: Materials in Medicine 2008, 19, 2145–2155. [Google Scholar] [CrossRef] [PubMed]
- Shuqiang, M.; Kunzheng, W.; Xiaoqiang, D.; Wei, W.; Mingyu, Z.; Daocheng, W. Osteogenic growth peptide incorporated into PLGA scaffolds accelerates healing of segmental long bone defects in rabbits. Journal of plastic, reconstructive & aesthetic surgery 2008, 61, 1558–1560. [Google Scholar]
- Jung, R.E.; Hämmerle, C.H.; Kokovic, V.; Weber, F.E. Bone regeneration using a synthetic matrix containing a parathyroid hormone peptide combined with a grafting material. International Journal of Oral & Maxillofacial Implants 2007, 22. [Google Scholar]
- Sheller, M.R.; Crowther, R.S.; Kinney, J.H.; Yang, J.; Di Jorio, S.; Breunig, T.; Carney, D.H.; Ryaby, J.T. Repair of rabbit segmental defects with the thrombin peptide, TP508. Journal of orthopaedic research 2004, 22, 1094–1099. [Google Scholar] [CrossRef] [PubMed]
- Rammelt, S.; Illert, T.; Bierbaum, S.; Scharnweber, D.; Zwipp, H.; Schneiders, W. Coating of titanium implants with collagen, RGD peptide and chondroitin sulfate. Biomaterials 2006, 27, 5561–5571. [Google Scholar] [CrossRef] [PubMed]
- Ferris, D.; Moodie, G.; Dimond, P.; Giorani, C.; Ehrlich, M.; Valentini, R. RGD-coated titanium implants stimulate increased bone formation in vivo. Biomaterials 1999, 20, 2323–2331. [Google Scholar] [CrossRef] [PubMed]
- Schneiders, W.; Reinstorf, A.; Pompe, W.; Grass, R.; Biewener, A.; Holch, M.; Zwipp, H.; Rammelt, S. Effect of modification of hydroxyapatite/collagen composites with sodium citrate, phosphoserine, phosphoserine/RGD-peptide and calcium carbonate on bone remodelling. Bone 2007, 40, 1048–1059. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Hemraz, U.D.; Fenniri, H.; Webster, T.J. Tuning cell adhesion on titanium with osteogenic rosette nanotubes. Journal of biomedical materials research Part A 2010, 95, 550–563. [Google Scholar] [CrossRef] [PubMed]
- Balasundaram, G.; Shimpi, T.M.; Sanow, W.R.; Storey, D.M.; Kitchell, B.S.; Webster, T.J. Molecular plasma deposited peptides on anodized nanotubular titanium: an osteoblast density study. Journal of Biomedical Materials Research Part A 2011, 98, 192–200. [Google Scholar] [CrossRef]
- Kim, J.E.; Kim, S.H.; Jung, Y. In situ chondrogenic differentiation of bone marrow stromal cells in bioactive self-assembled peptide gels. Journal of bioscience and bioengineering 2015, 120, 91–98. [Google Scholar] [CrossRef]
- Chen, J.; Li, Y.; Wang, B.; Yang, J.; Heng, B.C.; Yang, Z.; Ge, Z.; Lin, J. TGF-β1 affinity peptides incorporated within a chitosan sponge scaffold can significantly enhance cartilage regeneration. Journal of Materials Chemistry B 2018, 6, 675–687. [Google Scholar] [CrossRef]
- Ju, X.; Liu, X.; Zhang, Y.; Chen, X.; Chen, M.; Shen, H.; Feng, Y.; Liu, J.; Wang, M.; Shi, Q. A photo-crosslinked proteinogenic hydrogel enabling self-recruitment of endogenous TGF-β1 for cartilage regeneration. Smart Materials in Medicine 2022, 3, 85–93. [Google Scholar] [CrossRef]
- Hsieh, Y.-H.; Hsieh, M.-F.; Fang, C.-H.; Jiang, C.-P.; Lin, B.; Lee, H.-M. Osteochondral regeneration induced by TGF-β loaded photo cross-linked hyaluronic acid hydrogel infiltrated in fused deposition-manufactured composite scaffold of hydroxyapatite and poly (ethylene glycol)-block-poly (ε-caprolactone). Polymers 2017, 9, 182. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Gao, J.; Yu, X.; Shi, J.; Chen, J.; Yu, L.; Chen, S.; Ding, J. 3D-printed porous scaffolds of hydrogels modified with TGF-β1 binding peptides to promote in vivo cartilage regeneration and animal gait restoration. ACS applied materials & interfaces 2022, 14, 15982–15995. [Google Scholar]
- Burdick, J.A.; Anseth, K.S. Photoencapsulation of osteoblasts in injectable RGD-modified PEG hydrogels for bone tissue engineering. Biomaterials 2002, 23, 4315–4323. [Google Scholar] [CrossRef]
- Zhang, X.; Gu, J.; Zhang, Y.; Tan, Y.; Zhou, J.; Zhou, D. Immobilization of RGD peptide onto the surface of apatite-wollastonite ceramic for enhanced osteoblast adhesion and bone regeneration. Journal of Wuhan University of Technology-Mater. Sci. Ed. 2014, 29, 626–634. [Google Scholar] [CrossRef]
- Pan, H.; Zheng, Q.; Yang, S.; Guo, X.; Wu, B.; Zou, Z.; Duan, Z. A novel peptide-modified and gene-activated biomimetic bone matrix accelerating bone regeneration. Journal of Biomedical Materials Research Part A 2014, 102, 2864–2874. [Google Scholar] [CrossRef]
- Wang, C.; Yue, H.; Huang, W.; Lin, X.; Xie, X.; He, Z.; He, X.; Liu, S.; Bai, L.; Lu, B. Cryogenic 3D printing of heterogeneous scaffolds with gradient mechanical strengths and spatial delivery of osteogenic peptide/TGF-β1 for osteochondral tissue regeneration. Biofabrication 2020, 12, 025030. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhou, X.; Liu, T.; Huang, Y.; Li, J. The effects of Peptide Mel4-coated titanium plates on infection rabbits after internal fixation of open fractures. Archives of Orthopaedic and Trauma Surgery 2022, 1–6. [Google Scholar] [CrossRef]
- Chen, X.; Zhou, L.; Wu, D.; Huang, W.; Lin, Y.; Zhou, B.; Chen, J. The effects of titanium surfaces modified with an antimicrobial peptide GL13K by silanization on polarization, anti-inflammatory, and proinflammatory properties of macrophages. BioMed Research International 2020, 2020. [Google Scholar] [CrossRef]
- Fischer, N.G.; Chen, X.; Astleford-Hopper, K.; He, J.; Mullikin, A.F.; Mansky, K.C.; Aparicio, C. Antimicrobial and enzyme-responsive multi-peptide surfaces for bone-anchored devices. Materials Science and Engineering: C 2021, 125, 112108. [Google Scholar] [CrossRef] [PubMed]
- Hoyos-Nogues, M.; Velasco, F.; Ginebra, M.-P.; Manero, J.M.; Gil, F.J.; Mas-Moruno, C. Regenerating bone via multifunctional coatings: the blending of cell integration and bacterial inhibition properties on the surface of biomaterials. ACS Applied Materials & Interfaces 2017, 9, 21618–21630. [Google Scholar]
- Cheng, H.; Yue, K.; Kazemzadeh-Narbat, M.; Liu, Y.; Khalilpour, A.; Li, B.; Zhang, Y.S.; Annabi, N.; Khademhosseini, A. Mussel-inspired multifunctional hydrogel coating for prevention of infections and enhanced osteogenesis. ACS applied materials & interfaces 2017, 9, 11428–11439. [Google Scholar]
- Zhongxing, L.; Shaohong, W.; Jinlong, L.; Limin, Z.; Yuanzheng, W.; Haipeng, G.; Jian, C. Three-dimensional printed hydroxyapatite bone tissue engineering scaffold with antibacterial and osteogenic ability. Journal of Biological Engineering 2021, 15, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Yuan, X.; Liu, M.; Fernandes, G.; Zhang, Y.; Yang, S.; Ionita, C.N.; Yang, S. Antimicrobial peptide combined with BMP2-modified mesenchymal stem cells promotes calvarial repair in an osteolytic model. Molecular Therapy 2018, 26, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Bai, J.; Wang, W.; Liang, X.; Zhang, W.; Li, W.; Lu, L.; Xiao, L.; Xu, Y.; Wang, Z. Facile and versatile surface functional polyetheretherketone with enhanced bacteriostasis and osseointegrative capability for implant application. ACS Applied Materials & Interfaces 2021, 13, 59731–59746. [Google Scholar]
- Yuan, X.; Ouyang, L.; Luo, Y.; Sun, Z.; Yang, C.; Wang, J.; Liu, X.; Zhang, X. Multifunctional sulfonated polyetheretherketone coating with beta-defensin-14 for yielding durable and broad-spectrum antibacterial activity and osseointegration. Acta Biomaterialia 2019, 86, 323–337. [Google Scholar] [CrossRef]
- Trzcińska, Z.; Bruggeman, M.; Ijakipour, H.; Hodges, N.J.; Bowen, J.; Stamboulis, A. Polydopamine linking substrate for amps: Characterisation and stability on ti6al4v. Materials 2020, 13, 3714. [Google Scholar] [CrossRef]
- Meng, X.; Zhang, J.; Chen, J.; Nie, B.; Yue, B.; Zhang, W.; Lyu, Z.; Long, T.; Wang, Y. KR-12 coating of polyetheretherketone (PEEK) surface via polydopamine improves osteointegration and antibacterial activity in vivo. Journal of Materials Chemistry B 2020, 8, 10190–10204. [Google Scholar] [CrossRef]
Figure 1.
Diagram of the osteochondral unit showing the structural hierarchy, zonal arrangements, and extracellular matrix composition of the articular cartilage. (figure generated via biorender.com).
Figure 1.
Diagram of the osteochondral unit showing the structural hierarchy, zonal arrangements, and extracellular matrix composition of the articular cartilage. (figure generated via biorender.com).

Figure 4.
Role of antimicrobial and immunomodulatory peptides for osteochondral regeneration. (a) schematic representation of bacterial cell membrane damage using antimicrobial peptides. (figure generated via biorender.com) (b) Inhibition of C. albicans adhesion by different concentrations of LL-37 evaluated by (i) XTT reduction assay and whole-cell ELISA (*, p<0.05; **, p<0.01; ***, p<0.001) (ii) spot assay and (iii) optical microscopy of the floating cells (Mag. 400×).[182] Copyright© 2011 Tsai et al. (c) mechanism of immunomodulatory peptides eliciting immune-cell response. (figure generated via biorender.com) (d) E. coli K88-induced RAW264.7 cells (macrophages) treated with varying doses of cathelicidin-WA (CWA) and gentamicin shows (i) significant reduction of reactive oxygen species (ROS) (in fluorescent images) (ii) decrease in proinflammatory cytokines (IL-1β, IL-6) and (iii) increase in anti-inflammatory cytokines (IL-4, IL-10) levels.[186] Copyright© 2017 Elsevier B.V.
Figure 4.
Role of antimicrobial and immunomodulatory peptides for osteochondral regeneration. (a) schematic representation of bacterial cell membrane damage using antimicrobial peptides. (figure generated via biorender.com) (b) Inhibition of C. albicans adhesion by different concentrations of LL-37 evaluated by (i) XTT reduction assay and whole-cell ELISA (*, p<0.05; **, p<0.01; ***, p<0.001) (ii) spot assay and (iii) optical microscopy of the floating cells (Mag. 400×).[182] Copyright© 2011 Tsai et al. (c) mechanism of immunomodulatory peptides eliciting immune-cell response. (figure generated via biorender.com) (d) E. coli K88-induced RAW264.7 cells (macrophages) treated with varying doses of cathelicidin-WA (CWA) and gentamicin shows (i) significant reduction of reactive oxygen species (ROS) (in fluorescent images) (ii) decrease in proinflammatory cytokines (IL-1β, IL-6) and (iii) increase in anti-inflammatory cytokines (IL-4, IL-10) levels.[186] Copyright© 2017 Elsevier B.V.

Table 1.
Bioactive peptides for osteochondral regeneration.
| Types | Source | Function | Ref |
|---|---|---|---|
| a. Osteogenic peptides | |||
| (i) Osteo-inductive peptides | |||
| GFOGER | Col | ↑ α2β1 integrin binding, osteoblastic differentiation | [28] |
| GTPGQGIAGQRGVV (P15 peptide) |
↑ osteogenic differentiation | [33] | |
| ((PKG)4-(POG)4-(DOG)4) (KOD peptide) |
↑ECM deposition | [37] | |
| DGEA | ↑expression of osteogenic markers (ALP, RUNX2, OCN). | [39] | |
| NGLPGPIGP (BCSP™-1) |
↑ mineralization | [40] | |
| GPAGPHGPVG, APDPFRMY, TPERYY | ↑ALP activity, mineralization, osteogenic gene expression | [41] | |
| KIPKASSVPTELSAISTLYL | BMP-2 | ↑ALP activity | [42] |
| SKIPKASSVPTGLSAISTLYLAAA (P24) | ↑ bone formation | [43,44,45] | |
| CKIPKPSSVP-TELSAISMLYL (PEP7) | ↑ bone formation | [46] | |
| KIPKASSVPTELSAISTLYL | ↑ALP activity, osteogenic marker | [47] | |
| NSVNSKIPKACCVPTELSAI, KIPKASSVPTELSAISTLYL, DWIVA | ↑Osteogenic differentiation, in vivo bone formation | [12] | |
| RKKNPNCRRH | BMP-4 | ↑Osteogenic differentiation | [55] |
| VEHDKEFFHPRYHH (BFP-2) | BMP-7 | ↑Osteogenic differentiation | [54] |
| TVPKPSSAPTQLNAISTLYF, GQGFSYPYKAVFSTQ, ETLDGQSINPKLAGL | ↑Osteogenic differentiation | [55] | |
| KVGKACCVPTKLSPISVLY | BMP-9 | ↑Osteogenic differentiation | [55] |
| CK2.2, CK2.3 | synthetic | ↑ BMP signaling | [57] |
| PTHrP1–34, PTHrP1–36, PTHrP107–111 | PTH | ↑ Osteoblast differentiation, bone formation | [64,65,66] |
| CGRP–α and β-CGRP | CGRP | ↑ Osteogenic differentiation, bone formation | [67,68,69,70] |
| ALKRQGRTLYGFGG (OGP) | Mammalian blood | ↑ Osteogenic differentiation, ALP, OCN and collagen secretion, mineralization | [76,77,78] |
| AGYKPDEGKRGDACEGDSGGPFV (TP508) | Thrombin | ↑ chemotaxis, proliferation, osteogenic differentiation | [79,80] |
| FN III9-10/12-14 | FN | ↑ osteoblast activity, mineralization | [85] |
| CBM | OPN | ↑ osteogenic differentiation, bone formation | [90] |
| SVVYGLR | OPN | ↑ osteogenesis, neovascularization | [91,92,93] |
| FHRRIKA | BSP | ↑ osteoblast activity, mineralization | [94] |
| (ii) Angiogenic peptides | |||
| QK | VEGF | ↑EC migration, proliferation | [97] |
| PBA2-1c | PDGF-BB | Blood vessels formation | [98] |
| Exendin-4 | Exendin-4 | ↑ tube formation of HUVECs | [99] |
| SPARC113, SPARC118 | OPN | ↑ in vivo angiogenesis | [103] |
| TP508 | Thrombin | ↑ neo-angiogenesis | [84] |
| RoY | Synthetic | ↑ EC proliferation, sprouting, in vivo angiogenesis | [104] |
| b. Chondroinductive peptides | |||
| CMs | TGF-β | ↑Chondrogenic differentiation | [107,108] |
| CK2.1 | synthetic | ↑Chondrogenesis | [112] |
| c. Other supporting peptides | |||
| (i) Adhesion, binding, affinity peptides | |||
| RGD | Col, FN, VN | ↑Cellular adhesion/ or differentiation | [114] |
| PHSRN | FN | ↑Cell adhesion | [30,117] |
| FHRRIKA | BSP | ↑Cell proliferation, spreading, mineralization | [116,120] |
| KRSR | BSP, FN, VN, OPN, thrombospondin | ↑Osteoblast adhesion, osteogenic gene expression | [122,123,124,125] |
| HAV | N-Cadherin | ↑Cell adhesion | [126] |
| NEMO-binding domain (NBD) peptide | synthetic | ↑Osteoblast diferentiation ↓bone resorption, osteoclastogenesis | [130] [131] |
| CDPGYIGSR | Laminin | ↑Cell adhesion, ECM deposition | [150] |
| (ii) Peptides supporting cell migration | |||
| SDF1-ELP | SDF-1 | EC migration, vascularization | [158] |
| Histatin-1 | Saliva | EC adhesion, migration, angiogenesis | [159] |
| Ac2-26 | Annexin A1 | Cell migration | [160] |
| Esculentin 1-21 | Frog-skin | Cell migration | [161] |
| LL-37 | Human | EC migration | [162] |
| (ii) Cell penetrating peptides (CPPs) | |||
| NLS-TAT | HIV-1 Tat protein | ↑Chondrogenic differentiation | [154,155] |
| (iii) Self-assembly (SA) peptides | |||
| AcN-RADARADARADARADA-CONH2 (RADA16-I) | synthetic | ↑ ALP, OCN, Runx2 | [169] |
| (iv) Degradable peptides | |||
| KCGPQGIWGQCK | MMP-derived | ↑GAGs, collagen deposition | [140,173] |
| Antimicrobial and immunomodulatory peptides | |||
| TLISWIKNKRKQRPRVSRRRRRRGGRRRR (Melamine) | synthetic | destabilization of cell membrane | [175] |
| LLGDFFRKSKEKIGKEFKRIVQRIKDFLRNLVPRTES (LL-37) | human cathelicidin | inhibit cell wall synthesis | [179] |
| GIGKFLKKAKKFGKAFVKILKK (Pexiganan) | synthetic analogue of magainin2 | disrupts membrane | [175] |
| GIGKFLHSAGKFGKAFVGEIMKS (Magainin-1) |
frog skin | destabilizes or disrupts bacterial membrane | [175] |
| RRWVRRVRRWVRRVVRVVRRWVRR (PLG0206) | synthetic | disrupts cell membrane | [175] |
| RGGRLCYCRRRFCVCVGR (Protegrin-1) | porcine leukocytes | disrupts cell membrane | [175] |
| RLCRIVVIRVCR (Bactenecin) | bovine neutrophils | inhibit protein synthesis | [175] |
| VRVRVRVRVDPPTRVRVRVRV (PEP8R) | synthetic | disrupts bacterial cell membrane | [175] |
| ILPWKWPWWPWRR (Indolicidin) | bovine neutrophil | Increase cell membrane permeability | [175] |
| ILRWPWWPWRRK (Omiganan) | synthetic | destabilizes cell membrane | [175] |
| RAIGGGLSSVGGGSSTIKY (KAMP-19) | keratin | pore formation in bacterial cell membra | [176] |
| HNP-1 | human neutrophil | destabilizes cell wall integrity | [177] |
| RWRWRW-NH2 | synthetic | delocalizes peripheral membrane proteins | [178] |
| AMP 1037 | synthetic | ↓swimming and motility of bacteria, gene expression | [183] |
| Piscidin and sculentin 1–21 | fish | degrades biofilm | [184,185] |
| Histatin-1, 38 | saliva | antimicrobial | [159] |
| β defensin 3 | human | immunomodulator | [189] |
| GKIIKLKASLKLL (GL13K) | salivary protein | delocalizes cell membrane lipids | [190] |
| Abbreviations: BMP: Bone morphogenic protein, ALP: Alkaline Phosphatase, OCN: Osteocalcin, Col: Collagen, CK: Calmodulin Complexes, PTH: Parathyroid hormone, CGRP: Calcitonin gene-related peptide, OGP: Osteogenic growth peptide, FN: Fibronectin, VN: Vironectin, OPN: Osteopontin, CBM: Collagen-binding motif, BSP: bone sialoprotein, VEGF: Vascular endothelial growth factor, PDGF-BB, EC: Endothelial cell; CMs: Cytomodulins, GAG: Glycosaminoglycan; SDF-1: Stromal cell-derived factor 1, SDF1-ELP: SDF1 elastin-like peptide, CK2: casein kinase II | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Copyright: This open access article is published under a Creative Commons CC BY 4.0 license, which permit the free download, distribution, and reuse, provided that the author and preprint are cited in any reuse.
The Incorporation of Etanercept into a Porous Tri-Layer Scaffold for Restoring and Repairing Cartilage Tissue
Yaima Campos
et al.
Pharmaceutics,
2022
PHB/CHIT Scaffold as a Promising Biopolymer in the Treatment of Osteochondral Defects—An Experimental Animal Study
Eva Petrovova
et al.
Polymers,
2021
Potential Implantable Nanofibrous Biomaterials Combined with Stem Cells for Subchondral Bone Regeneration
Rana Smaida
et al.
Materials,
2020
MDPI Initiatives
Important Links
© 2024 MDPI (Basel, Switzerland) unless otherwise stated






