1. Introduction
The coronavirus disease 2019 (COVID-19) pandemic has become a serious challenge of the 21
st century. Clinical and laboratory signs of the hyperinflammatory syndrome that develops with COVID-19 and related factors continue to be widely studied [
1,
2,
3].
Evidence regarding the relationship between factors of innate and adaptive immunity in acute COVID-19 is still far from clear. At different stages of infection, antibodies to viral antigens are produced, which are first immunoglobulin M (IgM) and then immunoglobulin G (IgG) [
4]. Of the entire pool of immunoglobulins in normal blood serum, 80% of all immunoglobulins are IgG, the main protective immunoglobulins only a small part of which are pathogen-specific [
5]. Some types of antibodies are used for serological diagnosis of past infections. For COVID-19, аntibodies to spike (S) protein, nucleocapsid (N) protein and RBD (receptor-binding domain) are the best characterized. Antibodies to RBD and the N-terminal domain of the S-protein exhibit neutralizing properties by interfering with the S-protein interaction with the human angiotensin-converting enzyme 2 receptor (ACE2) [
6].
Antibody detection provides important clinical information about the infectious process during COVID-19. The different characteristics of IgG isotypes and subclasses in severe and non-severe group of COVID-19 will contribute to the development of future therapeutic measures such as the use of convalescent plasma [
7]. Besides, the increase in antibody titers to SARS-Cov-2 proteins in paired sera of COVID-19 patients can serve as an additional diagnosis in the absence of a positive PCR test [
8].
A number of studies suggests that virus-specific IgG in acute COVID-19 have been identified in hospitalized patients within 2 weeks after the onset of symptoms [
8,
9,
10,
11]. However, as with other systemic viral infections, during COVID-19, the most noticeable increase in the level of immunoglobulins, especially IgG, is observed during the recovery stage [
10]. The predominant virus-specific antibody responses to SARS-Cov-2, as with other viral infections, are represented by the IgG1 and IgG3 subclasses [
11]. The increase of IgG1 and IgG3 in respiratory viral infections are associated with immune functions such as virus neutralization, opsonization and complement activation [
12]. The study of virus-specific IgG subclasses in combination with age, medical history and severity of primary infection can serve to predict the likelihood of developing the currently widely studied post-acute COVID-19 syndrome [
13]. In addition, studying the IgG antibody subclasses can provide insight into the strength and durability of immune memory following natural infection or vaccination, which is important for predicting the level of protection. For example, the production of RBD-specific IgG1 corresponds to the activation of Th1 lymphocytes [
14].
There is some evidence that there is an increase in virus-specific IgG in severe COVID-19 compared to mild COVID-19 infections 19 [
15,
16,
17]. At the same time, the absence of IgM and IgG antibodies to SARS-Cov-2 at the time of admission was negatively correlated with in-hospital mortality [
18]. Antibody-dependent enhancement (ADE) theory has been proposed to play a role in the pathogenesis of viral infections, including COVID-19. Antibodies that bind to Fc receptors can activate antibody-dependent complement deposition, antibody-dependent cell-mediated phagocytosis, and antibody-dependent cell-mediated cytotoxicity. It was previously shown that patients with pre-existing high levels of pathogen-specific antibodies appear to have a more severe clinical course [
19]. There is an assumption about the relationship between systemic antibodies and macrophage or mast cell activation syndrome in coronavirus infection [
20]. Antibodies that bind to Fc receptors can activate antibody-dependent complement deposition, antibody-dependent cell-mediated phagocytosis, and antibody-dependent cell-mediated cytotoxicity. These reactions of the innate immune system limit viral replication, also contribute to increased inflammatory reactions [
21].
Thus, in order to effectively combat COVID-19, it is necessary to study all the factors of immunity to better understand their participation in pathogenesis. The aim of this work was to search the relationship between factors of adaptive immunity, such as virus-specific IgG subclasses, and the severity of the disease, accompanied by an increase in inflammatory markers.
2. Materials and Methods
2.1. Ethics statement
A retrospective study was conducted using serum/plasma samples remaining from clinical examinations of COVID-19 patients hospitalized at the beginning of the first wave of the pandemic caused by SARS-Cov-2. The protocol of the study was approved by the local Ethics Committee of the FSBSI "IEM" (protocol 3/20 from 06/05/2020). After obtaining Ethics Committee approval, the sera were released to the investigators, none of whom had access to the patients' personal data. When analyzing clinical data, the primary patient data was anonymized and the employees did not have access to the patients’ personal data. Because this was a retrospective study, informed consent was not required, although all patients signed an informed consent upon admission to the hospital.
2.2. Study participants and specimens
A total of 45 blood samples were collected from COVID-19 patients during the first three days of their hospital stay. Peripheral blood was taken into collecting tubes with heparin or ethylenediaminetetraacetic acid (EDTA) upon admission to the hospital and after 6-7 days. Serum samples were provided for serological testing in aliquots, which were stored at -20°C until testing, so that they were thawed only once.
The study cohort was divided into 3 groups, depending on the severity of
the course of the disease at the time of hospitalization
admission to the hospital. In February-April 2020, patients were hospitalized at the Vsevolozhsk Clinical Interdistrict Hospital of the Leningrad Region of the Russian Federation. The COVID-19 severity was estimated according to “Interim guidelines for the prevention, diagnosis and treatment of coronavirus disease 2019 (COVID-19), Version 8” (
https://static-0.minzdrav.gov.ru/system/attachments/attaches/000/064/610/original/%D0%92%D0%9C%D0%A0_COVID-19_V18.pdf, accessed on the 3
rd of September, 2020). Mild course of disease was characterized by body temperature <38° C, cough, weakness, sore throat with lack of criteria for moderate and severe course. The moderate course was characterized by body temperature >38° C, respiration rate > 22 /min, dyspnea on exertion, changes on X-ray typical of a viral lesion (the extent of damage is minimal or moderate), oxygen saturation <95%, serum C-reactive protein (CRP) >10 mg/L. Severe course of infection was characterized by a respiratory rate >30/min, oxygen saturation <93%, unstable hemodynamics (systolic blood pressure less than 90 mm Hg or diastolic blood pressure less than 60 mm Hg, urine output less than 20 ml/hour), changes in the lungs on radiographs typical of a viral lesion (the volume of damage >70%), arterial blood lactate >2 mmol/L. The severe course of the infection was characterized by the addition of acute respiratory distress syndrome (ARD) to the previous symptoms. Nasopharyngeal and pharyngeal swabs were tested by real-time polymerase chain reaction at hospitalization.
2.3. PCR test
PCR analysis of nasal swabs and the level of viremia in blood serum were determined using RealBest RNA SARS-CoV-2 kits (Vector best, Novosibirsk, Russia) accordingly to provider’s manual.
2.4. Laboratory data
The concentration of C-reactive protein (CRP) and fibrinogen in blood serum was studied by the turbidimetric method with BioSystems reagents (BioSystems, Barcelona, Spain). Serum histamine levels were detected using Histamine ELISA kit (Cloud-Clone Corp., Wuhan, Hubei) according to the manufacturer's instructions. All blood tests were done with unheated blood samples. The neutrophil/lymphocyte ratio (NLR) was calculated based on clinical blood test data using the following formula: absolute neutrophil count/absolute lymphocyte count. The reference values for laboratory markers were: 0.0-5.0 mg/L for CRP; 1.13-3.79 conventional units for NLR; 2.0-4.0 mg/L for fibrinogen and 0.0-9.3 nm/L for histamine.
2.5. Detection of antibodies to SARS-CoV-2 S- and N- proteins using enzyme-linked immunosorbent assay (ELISA)
Blood samples were tested using an in-house developed ELISA for the detection of IgG or IgG subclasses specific for proteins of SARS-CoV-2. The principle of the test was based on an indirect ELISA method make use of microwells coated with a certain quantity of the SARS-CoV-2 virus antigens [
8]. For this purpose, 96-well Nunc MaxiSorp plates (Thermo Fisher Scientific, Waltham, United States) were sensitized with recombinant S-protein at a concentration of 2 µg/ml containing 496-646 amino acids (GenBank: OL447006.1) obtained in
E. Coli as described previously [
22] or 2 µg/ml of commercial SARS-CoV-2 N-protein (AtaGenix, Wuhan, China). After washing 3 times , serial dilutions of sera were added to the well of sensitized plates in of 1:4 were introduced into the wells of sensitized plates. All tests were done in duplicates. After 1.5 hours of incubation at 37°C, a washing decision was used to excisionunbound antibodies. The HRP-linked goat anti-human IgG antibody (Sigma, St. Louis, USA) was used to detect serum IgG as described earlier [
8]. To identify IgG subclasses, a specially developed technique was used using rabbit anti-human IgG1, IgG2, IgG3, IgG4 (‘Polygnost’, Saint Petersburg, Russia) as conjugates. ELISA end-point titers were expressed as the highest dilution that gave an optical density at 450 nm (OD450) that was greater than the mean OD450 plus 3 standard deviations (SD) of control wells. Control well means were determined for each 4x to 6x dilution of negative blood sera obtained before the emergence of SARS-CoV-2.
2.6. Statistical analysis
Statistical analysis of the data was performed using the Statistica 12.0 software package (StatSoft, Inc. Tulsa, Oklahoma, USA) and graphics data were generated using Prism 8 (GraphPad software, San Diego, USA). The data was normalized using the mean normalization method (Z-normalization). Medians (Me) and lower and upper quartiles (Q1; Q3) were calculated and used to represent the antibody response and blood tests levels. To compare two independent samples with a normal distribution, the Student's t-test was used for unpaired samples with a preliminary analysis of the equality of variances using the D'Agostino & Pearson omnibus normality test. If the data were not normally distributed, comparisons of two independent groups were made with nonparametric test, to wit the Mann-Whitney test. For nominal data, Fisher exact 2-tailed tests, or McNemar's chi-square tests were used for the same purposes. The presence of a statistical relationship between the variables was assessed using correlation analysis in Python 3 applying the Pandas library, ‘Corr’ function by Spearmen's method. The p-value < 0.05 was considered to be statistically significant.
3. Results
3.1. Patient’s characteristics
In this retrospective cohort research, we assessed serum/plasma samples obtained from 45 patients with mild, moderate and severe forms of COVID-19 who were hospitalized in February-April 2020 at the Vsevolozhsk Clinical Interdistrict Hospital of the Leningrad Region
Table 1 presents the patient characteristics among a group of 45 COVID-19 patients at the time of hospitalization. The exclusion criteria were oncological diseases or immunodeficiency states. The cohort included 22 men and 23 women aged 27 to 92 years old, the median age was 62 years old. There were no significant differences between the main indicators between men and women (
Suppl. Table S1). It was shown that 65% of the examined patients over 60 years of age had concomitant diseases compared to younger people (
P = 0.016), and the average level of CRP was 1.5 times higher than in younger subjects (
P=0.03), (
Suppl. Table S2). The other markers considered in the study did not differ between these age groups (
Suppl. Table S2). The most common comorbidities in hospitalized patients with COVID-19 were arterial hypertension and ischemic heart disease (55.5%), diabetes (13.3%), and chronic pulmonary disorders such as bronchial asthma and chronic obstructive bronchitis (4.4%). It was shown that there were no differences in age or time of admission to the hospital from the beginning of the onset of symptoms among the studied groups. A positive PCR test for SARS-Cov-2 was observed most frequently in mild COVID-19 patients and to a lesser extent in severe cases.
Bacterial complications have been identified in 26.6% of examined patients. Bacteriological analysis revealed opportunistic pathogenic microorganisms, such as Staphylococcus aureus, Klebsiella pneumoniae, Enterobacter aerogenes, Candida albicans. For mild COVID-19, all patients were recovered; for medium severe COVID-19, four patients died (25.0%); in severe course of the disease the lethal outcome was in 11 cases (73.3%).
3.2. The results of blood tests
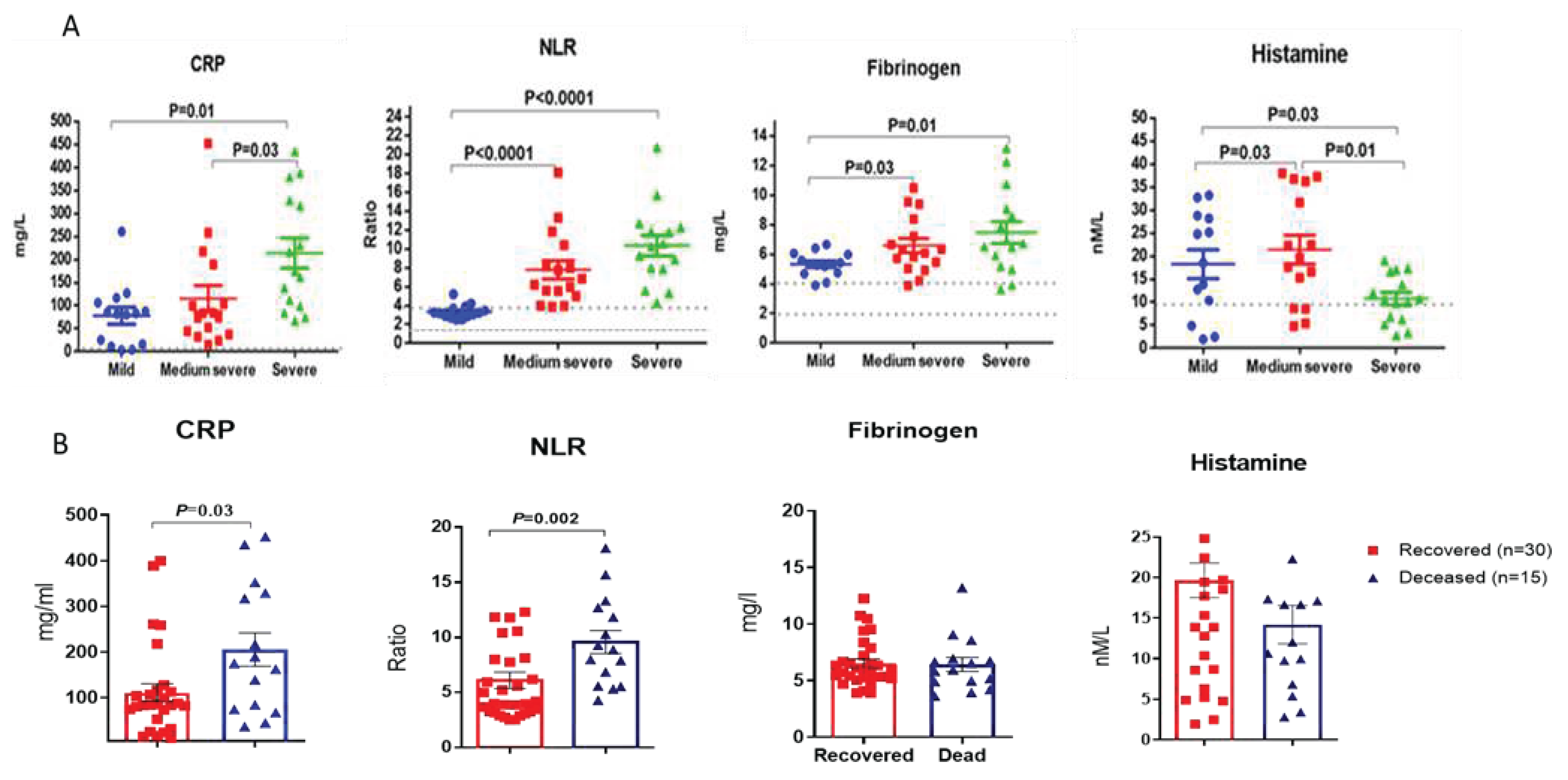
Figure 1 shows a comparative analysis of individual blood samples of COVID-19 patients with various forms of COVID-19 severity. Blood sampling for testing in the first days of hospitalization was performed in 60% of cases before the appointment of any specific therapy, so the effect of drugs was minimal. The level of CRP was higher than normal in 96% of those examined COVID-19 patients with an average of 136.7 mg/L. CRP levels were apparently different in mild and medium severe forms of COVID-19 patients (
Figure 1, a). Thus, in patients with mild COVID, the average level of CRP was 77.8 mg/l; with a medium-severe COVID-19 it was 117.95 mg/l, and with a severe disease course of the disease it reached 214.2 mg/l.
For such laboratory markers as NLR, fibrinogen and histamine, statistically significant differences between COVID-19 patients with mild and moderate or severe infection were revealed (
Figure 1, A). In contrast to other parameters, histamine levels in severe COVID-19 patients were statistically significantly lower than in patients with mild to moderate disease course (
Figure 1, d).
It should be noted that out of 30 recovered patients from the observed cohort, the mean CRP levels were 101.5 and were significantly lower than those of the 15 patients who died during hospitalization, in whom this indicator was on average 207.0 (P = 0.03) (
Figure 2, a). Mean levels of NLR were also significantly higher among patients with poor outcomes. (
Figure 2, b). Among 11 patients with NLR level not exceeding the reference values (1.13-3.79), all patients recovered, while among 34 patients with increased NLR levels, 15 people died (44.1%), (P = 0.0054; Fisher's exact test). It turned out that mean fibrinogen levels among survivors were slightly lower than those of non-survivors, although the differences were not statistically significant (
Figure 2, c). Histamine levels were higher among surviving patients, although the differences were not statistically significant.
3.3. Serum IgG sybtypes in COVID-19 patients
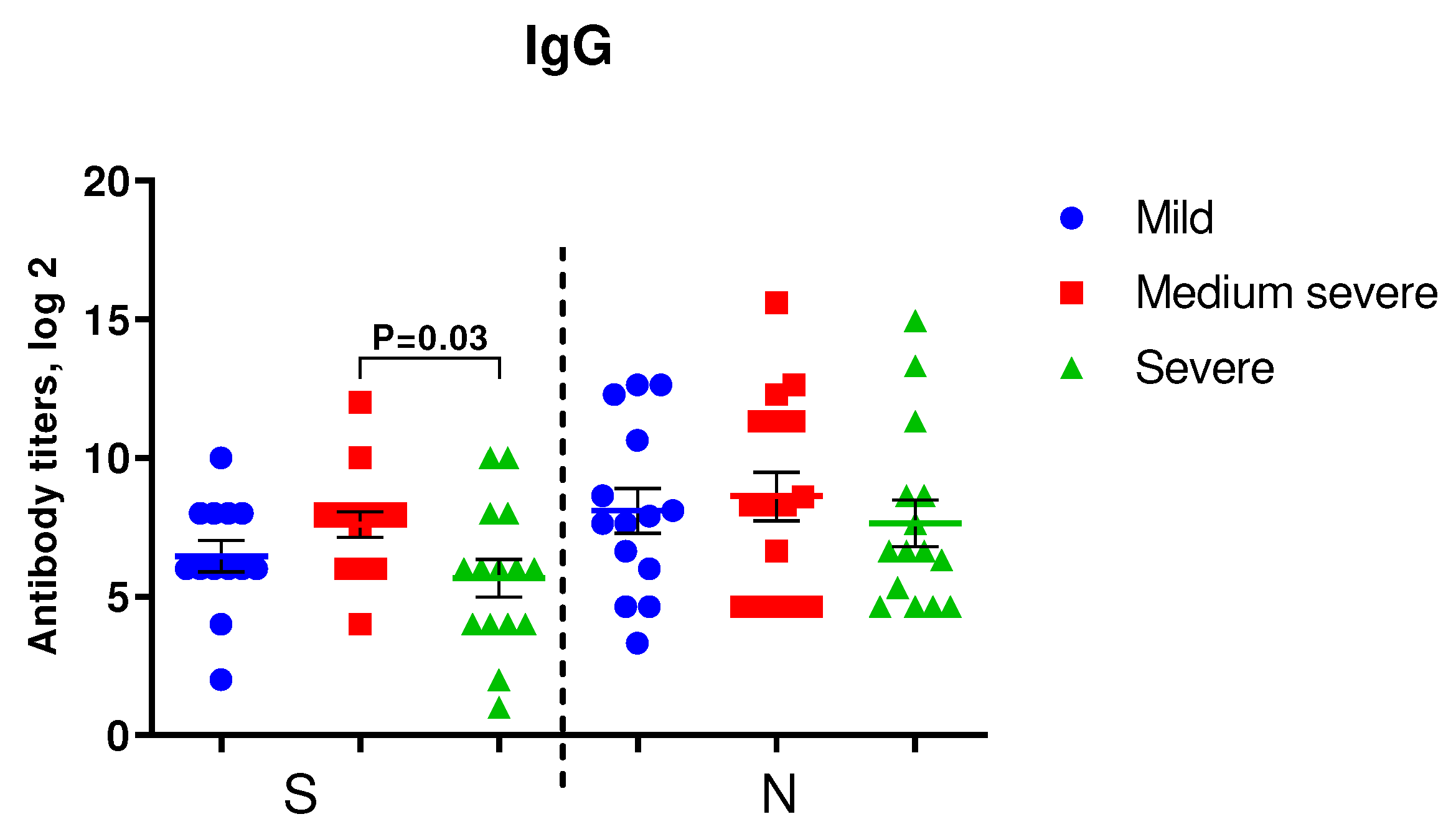
Figure 2 shows the results of IgG to S- and N-protein of SARS-CoV-2 among COVID-19 patients of varying severity. In patients with medium severe COVID-19, levels of IgG to SARS-CoV-2 were higher than in severe COVID-19 patients, although the differences were statistically significant only for IgG to S-protein.
Figure 2.
Detection of IgG in the blood sera of the examined patients. A. IgG to S- and N-proteins of SARS-CoV-2 in COVID-19 patients with mild (n=14), moderate (n=16) and severe (n=15) infections upon admission to the hospital. We used Student's test to determine differences in anti-spike IgG and Mann-Whitney U-test was used for anti-N IgG analysis.
Figure 2.
Detection of IgG in the blood sera of the examined patients. A. IgG to S- and N-proteins of SARS-CoV-2 in COVID-19 patients with mild (n=14), moderate (n=16) and severe (n=15) infections upon admission to the hospital. We used Student's test to determine differences in anti-spike IgG and Mann-Whitney U-test was used for anti-N IgG analysis.
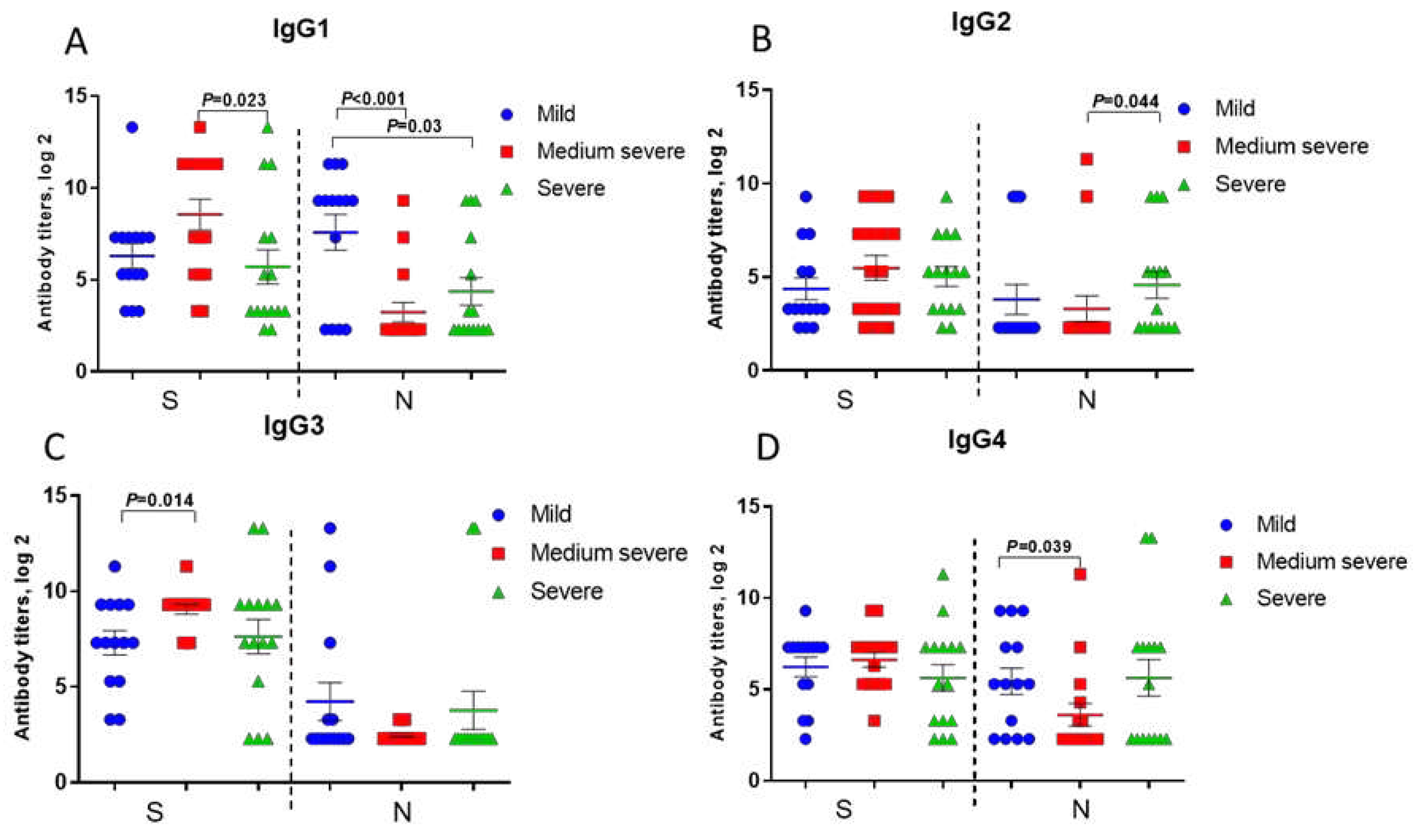
Anti-spike IgG1, IgG2, IgG3, IgG4 levels in patients with severe COVID-19 were even lower compared to those in patients with moderate COVID-19, although statistically significant differences were noted only for IgG1 (
Figure 3, a-d).
Anti-N IgG2 and IgG4 antibodies in patients with severe COVID-19 were slightly higher compared to moderate disease (
Figure 3, b, d). Besides, IgG and IgG1 to SARS-CoV-2 N-protein were significantly lower in subsequent poor outcome compared to those in recovered patients (
Supplementary Figure S1).
3.4. Correlation analysis
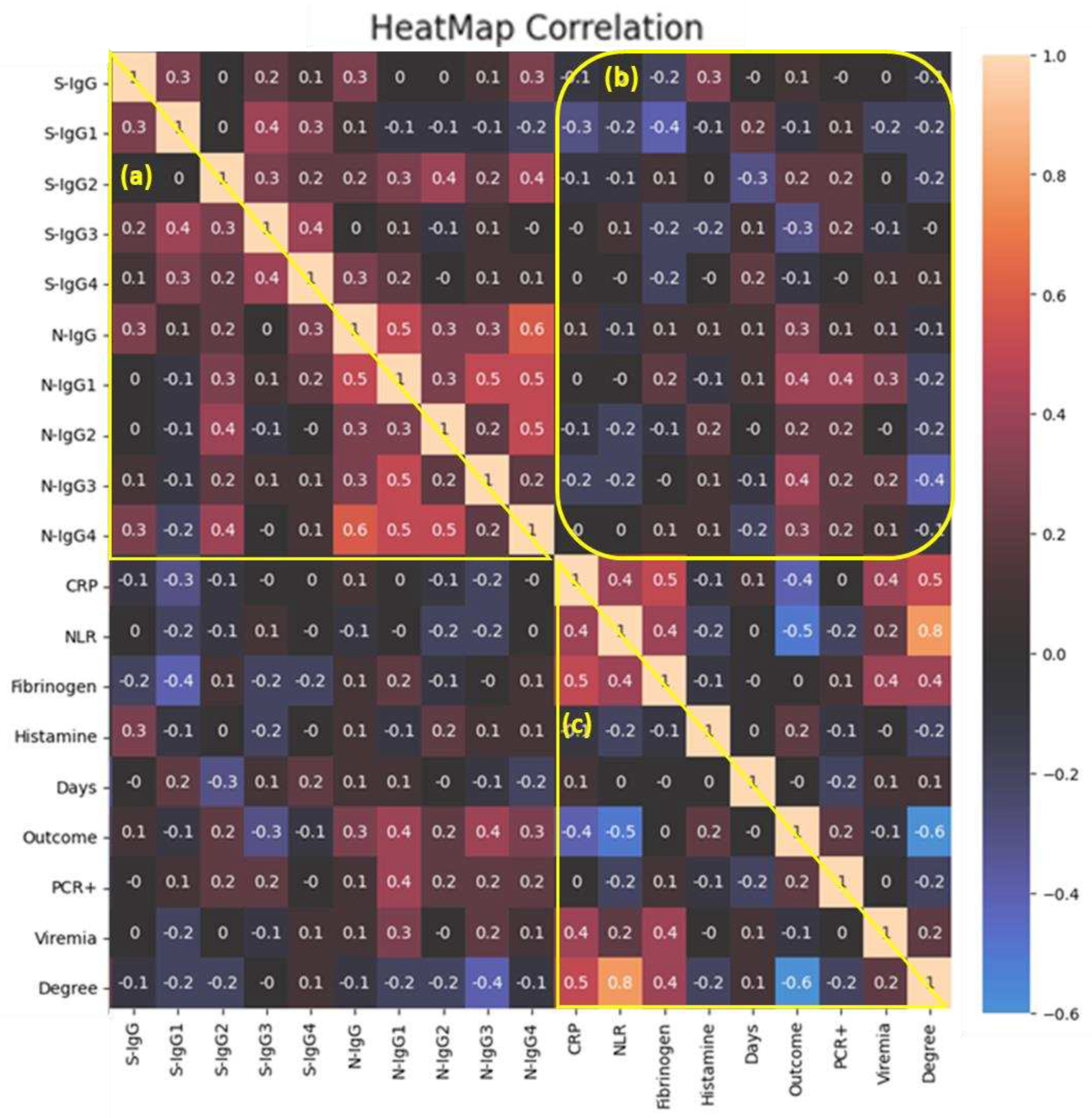
Figure 4 shows the results of a correlation analysis of clinical features, markers of systemic inflammation and IgG titers to SARS-CoV-2 proteins.
A medium-strength relationship between the levels of anti-spike IgG1 and IgG3 in the same blood sera (r
s=0.4) (
Figure 4, a) was demonstrated. A similar pattern was observed in relation to anti-N IgG1 and IgG3 (r
s=0.5), at the same time, anti-N IgG1 also positively correlated with IgG4 (r
s=0.5). Аnti-N IgG antibody titers were medium positively correlated with anti-N IgG1 (r
s=0.5) and anti-N IgG4 (r
s=0.6); anti-N IgG2 was medium positively correlated with IgG4 (r
s=0.5) (
Figure 4, а).
A medium negative relationship was shown between anti-spike IgG-1 with CRP and fibrinogen (r
s=-0.3, r
s=-0.4 respectively) (
Figure 4, b). There was a weak positive relationship between anti-spike IgG levels and histamine levels (r
s=0.3).
A medium-strength relationship was found between the favorable disease outcome and anti-N IgG1 or anti-N IgG3 levels (r
s=0.4) (
Figure 4, b). And other subtypes of anti-N IgG were weakly positively correlated with a favorable outcome of the disease, with the exception of anti-spike IgG3, which was the opposite weakly negatively correlated with subsequent recovery (
Figure 4, b).
Quite expectedly, severity of the disease correlated positively with inflammatory markers, but most of all with NLR levels (r
s=0.8) (
Figure 4, c). Detection of viremia medium positively correlated with CRP and fibrinogen levels (r
s=0.4). The favorable outcome of the disease negatively correlated with the severity (r
s=-0.6) as well as the content of CRP and NLR (r
s=-0.4, r
s=-0.5 respectively) but not depended from fibrinogen content (
Figure 4, c).
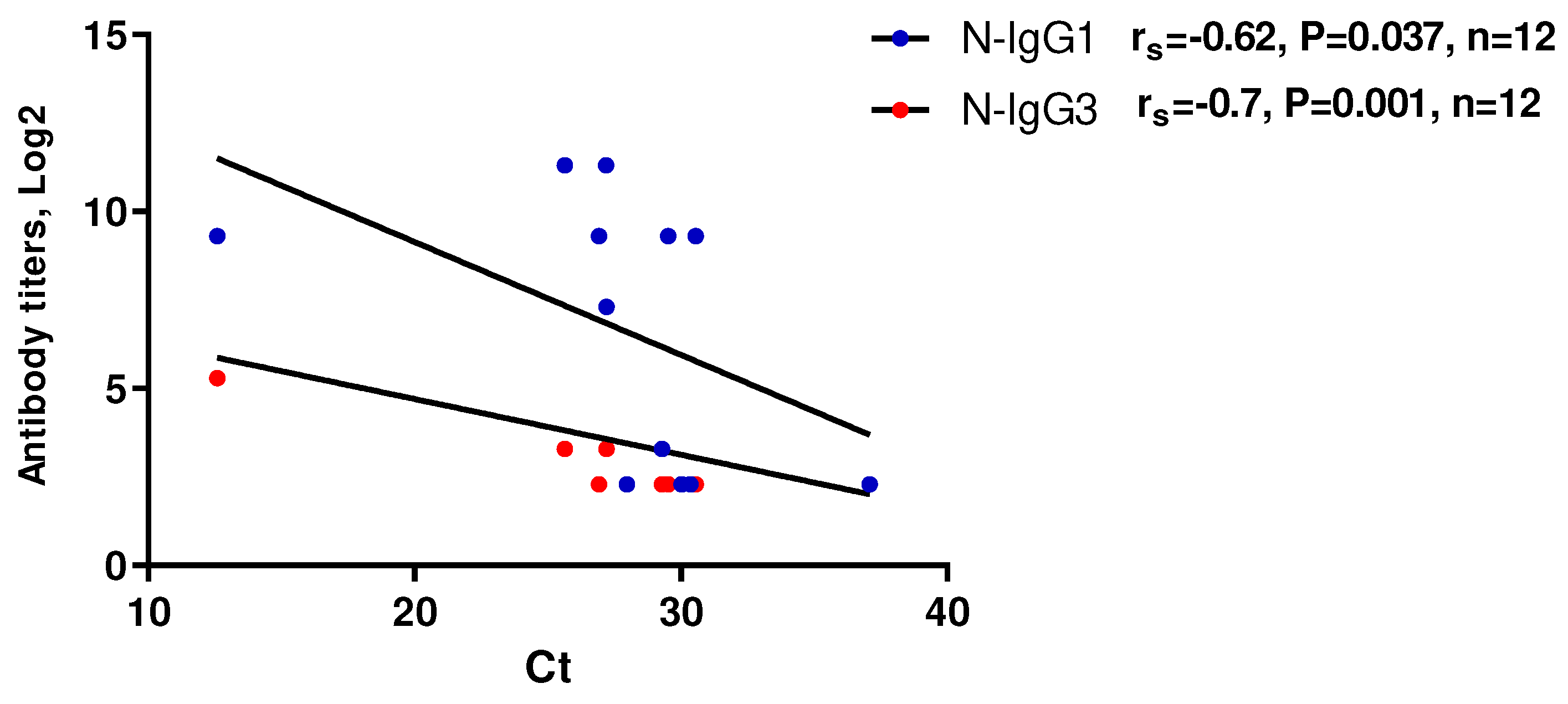
A moderate to strong negative correlation was obtained between anti-N IgG1 and IgG3 titers and threshold cycles (Ct) when detecting SARS-CoV-2 RNA in the studied blood samples using RT-PCR (Figure 5). This may suggest a potential relationship between the levels of these antibodies and the levels of viremia (Figure 5, b).
Figure 6.
Scatterplot of RT-PCR Ct values as a function of anti-N protein IgG subclasses levels (n=12). RT-PCR analysis of serum samples was performed using RealBest RNA SARS-CoV-2 kits (Vector best, Novosibirsk, Russia) according to the manufacturer’s instructions.
Figure 6.
Scatterplot of RT-PCR Ct values as a function of anti-N protein IgG subclasses levels (n=12). RT-PCR analysis of serum samples was performed using RealBest RNA SARS-CoV-2 kits (Vector best, Novosibirsk, Russia) according to the manufacturer’s instructions.
Thus, some degree of relationship between clinical indicators and antibodies to SARS-CoV-2 has been identified. To summarize, we can conclude that the presence of anti-spike IgG showed a medium negative correlation with laboratory markers indicating an increase in the severity of the infection. Conversely, IgG subclasses to the N-protein were positively correlated with both inflammatory markers and viremia.
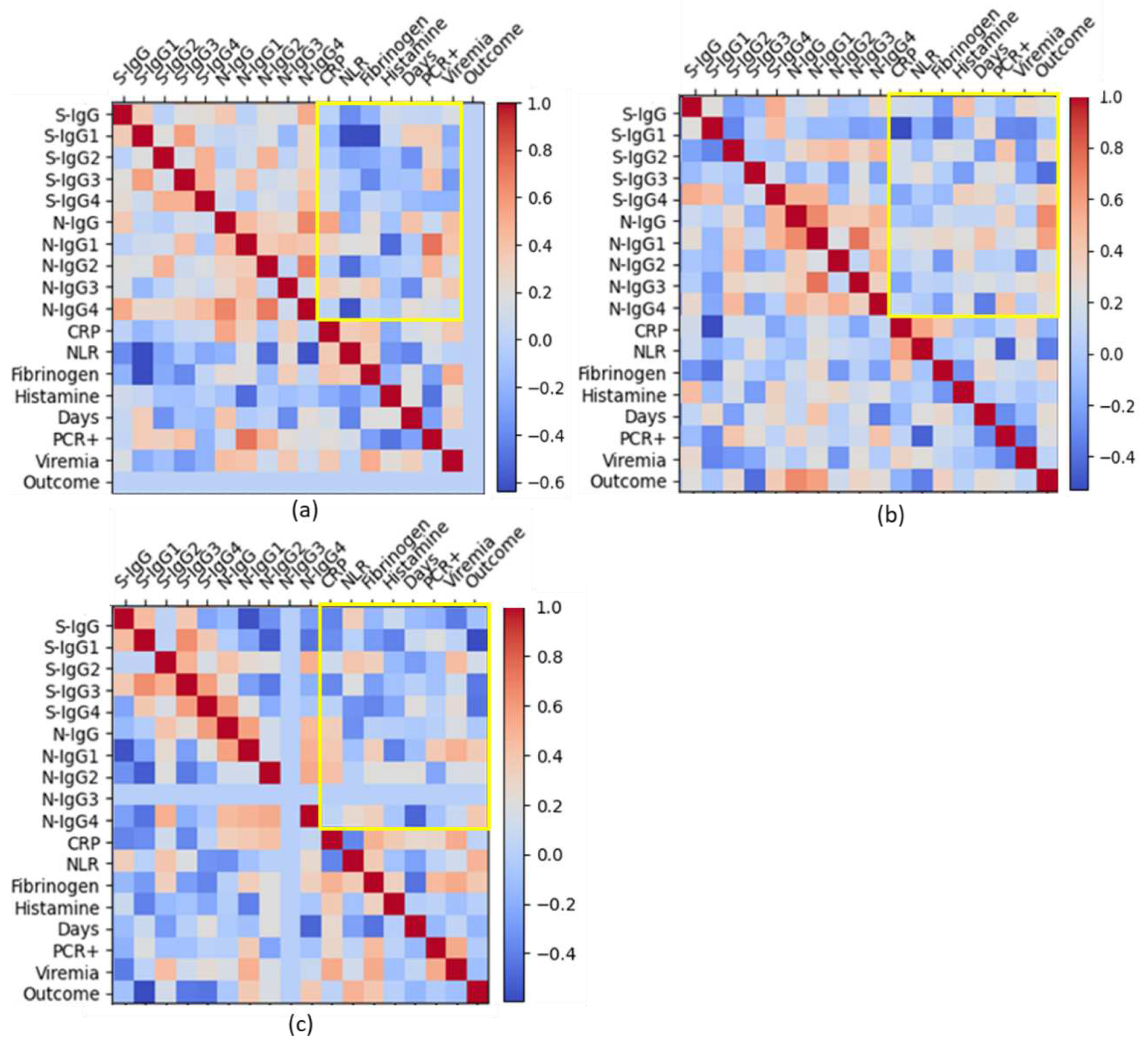
Next, we tried to assess the relationship between these same laboratory markers in different groups patients with varying severity of COVID-19.
With a mild course of infection, anti-spike IgG and IgG1 titers negatively correlated with NLR levels (r
s=-0.4, rs =-0.6 respectively); and anti-spike IgG1 and IgG3 titers negatively correlated with fibrinogen levels (r
s=-0.6, r
s=-0.4 respectively). At the same time, anti-N IgG1 titers correlated well with positive PCR test (r
s=0.7) and moderately positive correlated with viremia (r
s=0.4) (
Figure 7, a;
Suppl. Table S3). Anti-N IgG2 correlated with positive PCR test (rs=0.4).
In medium severe cases, anti-spike IgG positively correlated with histamine levels (r
s=0.5), and negatively correlated with CRP levels (r
s=-0.5); anti-spike IgG4 was found to be moderately positively correlated with histamine levels and recovery (r
s=0.4); a strong connection was found between anti-N IgG and a favorable outcome of the disease (r
s=0.7). And also, for moderate disease course, there is a rather good correlation of CRP and NLR (rs=0.6) (
Figure 7, b;
Suppl. Table S4).
In severe cases, anti-spike antibodies negatively correlated with subsequent recovery, and anti-N IgG1 and IgG 4 positively correlated with a good outcome of the disease (
Figure 7, c). In severe COVID-19, NLR levels showed a medium-positive correlation with antibody titers to S-protein and, on the contrary a medium negative correlation with IgG to N-protein (
Figure 7, c,
Suppl. Table S5).
And quite unexpectedly, in severe COVID-19, levels of NLR and fibrinogen showed a moderately positive correlation with a favorable outcome of the disease (r
s=0.5, r
s =0.4 respectively) (
Figure 7, c;
Suppl. Table S5). And even in severe COVID-19, CRP levels mild negatively correlated with NLR levels (r
s =-0.4) and positively correlated with fibrinogen (
Figure 7, c;
Suppl. Table S5).
4. Discussion
Sera from patients in this study were collected early in the pandemic, when the pathogenicity of the virus was much higher than in the later stages of the pandemic [
23], and severe forms of COVID-19 with high mortality were observed. This accompanied by a ‘cytokine storm’, acute respiratory distress syndrome (ARDS) and coagulopathy [
24].
The features and kinetics of the formation of types of immune response during COVID-19 are still insufficiently studied. [
25]. Previous extensive analysis of the antibody response, showed that SARS-CoV-2 induces virus-specific antibodies, mediated by all immunoglobulin isotypes including IgM, IgG, and IgA; with IgG1 and IgG3 subclasses being the most dominant [
26]. Our study like many other studies showed that in acute COVID-19, among other subclasses of virus-specific antibodies, IgG1 and IgG3 prevailed.
The immune presentation of S and N proteins during coronavirus infection can differ in terms of their roles and the nature of the immune response they elicit. As shown in this study, seroconversion of both anti-spike and anti-N IgG may occur later in patients with more severe pneumonia. The delayed seroconversion to intracellular pathogens like SARS-CoV-2 may be a result of the intricate and time-consuming processes involved in the activation and maturation of the adaptive immune response [
27]. Antigen-presenting cells, such as dendritic cells, need time to process and present viral antigens to T cells, which, in turn, stimulate B cells to produce antibodies [
28]. This antigen presentation process is an essential step in the immune response and contributes to the delay in seroconversion. Besides, in severe cases of COVID-19, there is severe impairment of helping T cells and, as a result, fewer antigen-specific antibodies and a longer symptomatic period of the disease [
29].
Previous studies examining the dynamics of seroconversion in COVID-19 have shown that neutralizing antibody responses developed within two weeks of symptom onset correlate with recovery, whereas antibodies generated after this time point do not contribute to viral clearance and recovery patients [
30]. It has been suggested that in this case, the coronavirus at certain stages of infection becomes inaccessible to antibodies when located in infected tissues, and in addition, in the later stages of infection, the development of antibody-mediated immunopathology may occur. For instance, antibodies from patients with severe COVID-19 show pro-inflammatory Fc modification signatures, including high levels of afucosylated IgG1, which could potentially drive pathologic responses [
31]. These findings may explain to some extent the prognostic value of early antibody response in COVID-19.
Our study showed that the presence of anti-N IgG at the time of hospitalization correlated with subsequent recovery. The fact that anti-N IgG1 and IgG3 correlated with viral load in the blood suggests that these antibodies are markers of viral reproduction. The N protein is involved in packaging the viral RNA, forming the nucleocapsid. It plays a crucial role in the replication and assembly of the virus [
32]. While antibodies against the N protein can be detected during infection, they may not necessarily confer strong neutralizing activity. The immune response to the N protein is often more associated with cellular immunity, involving T cells [
33]. Thus, the immune presentation of S and N proteins during coronavirus infection can differ in terms of their roles and the nature of the immune response they elicit. The S protein is a primary target for neutralizing antibodies, which are essential for preventing viral entry, while the N protein is more associated with cellular immunity. Understanding the immune response to both proteins is important for vaccine development, diagnostic testing, and gaining insights into the dynamics of the immune response against coronaviruses. Along with this, it should be noted that the dynamics of the immune response can vary between individuals, and the factors influencing seroconversion are complex. Additionally, the severity of the infection may not be the only determinant of the timing of seroconversion; individual variability, genetics, and other factors also play a role.
Interestingly, serum histamine concentrations were significantly higher in moderate COVID-19 than in mild or severe COVID-19, with a trend toward higher concentrations in recovered patients compared with deceased patients. Histamine is the main product of mast cells (MCs), which belong to the innate immune system [
34]. There is evidence that MCs may play a positive role in the body's defenses in the early stages of infection. Recent studies have shown that macrophages can also produce histamine and are involved in histamine-mediated pathogenesis [
35]. The increase in histamine in moderate versus mild COVID-19 may be due to increased antibody-dependent Fc-mediated phagocytosis by monocytes and macrophages, which, although stopping viral replication, also leads to increased inflammation [
36]. The role of histamine pathway in the pathogenesis of the COVID-19 cytokine storm is discussed in the literature [
37]. Some hypotheses suggest that histamine may play a protective role in COVID-19 by modulating the immune response or inhibiting viral entry into cells. However, these hypotheses are not generally accepted and the available evidence is limited. Understanding of the pathology of COVID-19 is still evolving, and a number of studies continue to explore the interaction between the virus and the immune system [
38].
In conclusion, it should be noted that COVID-19 is a complex disease, and multiple factors contribute to its progression and severity. Research is ongoing to understand the complexities of the immune response to SARS-CoV-2 and its implications for disease severity and vaccine development.
Study limitations
This study has several limitations. The serological results of this study were obtained from small subjects. Because this was a retrospective study, the researchers did not have access to the inpatients' personal information. We were able to associate each of the samples analyzed with varying degrees of severity of the disease according to specific signs determined by the recommendations of the Ministry of Health of the Russian Federation specified in in the Materials and Methods. From the previous anamnesis, it is known that none of the examined patients were vaccinated with the Sputnik V vaccine, since study was conducted using blood sera obtained in March-April 2020 when vaccines against COVID-19 were not used.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on
Preprints.org. Covid-19 patient characteristics depending on the gender of the participants; Table S2: Covid-19 patient characteristics depending on the age of the participants; Table S3: Correlation analysis of ant-spike and anti-N IgG subclasses, blood test and clinical data of medium severe COVID-19 patients; Table S4: Correlation analysis of ant-spike and anti-N IgG subclasses, blood test and clinical data of severe COVID-19 patients. Table S5. Correlation analysis of ant-spike and anti-N IgG subclasses. blood test and clinical data of severe COVID-19 patients; Figure S1: Bacterial complications in COVID-19 patients. (a) Correlation analysis of the relationship between disease outcome and the presence of bacterial complications. Class variables have been converted to numeric as follows: Outcome - Died - 0, Survived - 1; bacterial coinfections: Yes - 0, No – 1. (b) Proportion of bacterial complications in recovered patients. (c) Proportion of bacterial complications in deceased patients.
Author Contributions
сonceptualization, Y.D. and A.L.; methodology, G.L.; validation, G.L.., and I.C.; formal analysis, D.P.; investigation, P.C., O.K.; data curation, T.Sh. and S.P.; writing—original draft preparation, Y.D.; writing—review and editing, Y.D. and G.L.; supervision, A.S.; project administration, Y.D.; funding acquisition, A.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by budgetary funds of the Federal State Budgetary Institution "IEM", topic: FGWG-2023-0002.
Institutional Review Board Statement
The study was approved by the Local Ethics committee of the FSBSI "IEM" (protocol 3/20 from 06/05/2020).
Informed Consent Statement
Written informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Acknowledgments
The authors acknowledge Dr. Valentina Smelova for her excellent support in statistical processing and graphical representation of the data obtained.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Jiang, Y.; Rubin, L.; Peng, T.; Liu, L.; Xing, X.; Lazarovici, P.; Zheng, W. Cytokine storm in COVID-19: From viral infection to immune responses, diagnosis and therapy. International journal of biological sciences 2022, 18, 459. [Google Scholar] [CrossRef] [PubMed]
- Abbas, A.K.; Lichtman, A.H.; Pillai, S. Innate immunity, p 73. Cellular and molecular immunology, 10th ed.; Elsevier Saunders: Philadelphia, 2021. [Google Scholar]
- Jimeno, S.; Ventura, P.S.; Castellano, J.M.; García-Adasme, S.I.; Miranda, M.; Touza, P.; López-Escobar, A. Prognostic implications of neutrophil-lymphocyte ratio in COVID-19. European journal of clinical investigation 2021, 51, e13404. [Google Scholar] [CrossRef] [PubMed]
- Eisen, H.N. Affinity enhancement of antibodies: How low-affinity antibodies produced early in immune responses are followed by high-affinity antibodies later and in memory B-cell responses. Cancer immunology research 2014, 2, 381–392. [Google Scholar] [CrossRef] [PubMed]
- Mix, E.; Goertsches, R.; Zett, U.K. Immunoglobulins—Basic considerations. Journal of neurology 2006, 253, v9–v17. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Zheng, J.; Tai, W.; Verma, A.K.; Zhang, X.; Geng, Q.; Du, L. A glycosylated RBD protein induces enhanced neutralizing antibodies against Omicron and other variants with improved protection against SARS-CoV-2 infection. Journal of virology 2022, 96, e00118–22. [Google Scholar] [CrossRef]
- Wang H, Yan D, Li Y, Gong Y, Mai Y, Li B, Zhu X, Wan X, Xie L, Jiang H, Zhang M, Sun, M.; Yao, Y.; Zhu, Y. Clinical and antibody characteristics reveal diverse signatures of severe and non-severe SARS-CoV-2 patients. Infect Dis Poverty. 2022, 11, 15. [CrossRef]
- Desheva, Y.; Lerner, A.; Shvedova, T.; Kopteva, O.; Kudar, P.; Koroleva, I.; Suvorov, A. Pilot Study Results on Antibodies to the S-and N-Proteins of SARS-CoV-2 in Paired Sera from COVID-19 Patients with Varying Severity. Antibodies 2023, 12, 19. [Google Scholar] [CrossRef] [PubMed]
- Qi, H.; Liu, B.; Wang, X.; Zhang, L. The humoral response and antibodies against SARS-CoV-2 infection. Nature Immunology 2022, 23, 1008–1020. [Google Scholar] [CrossRef] [PubMed]
- Chvatal-Medina, M.; Mendez-Cortina, Y.; Patino, P.J.; Velilla, P.A.; Rugeles, M.T. Antibody responses in COVID-19: A review. Frontiers in immunology 2021, 12, 633184. [Google Scholar] [CrossRef] [PubMed]
- Korobova, Z. R., Zueva, E. V., Arsentieva, N. A., Batsunov, O. K., Liubimova, N. E., Khamitova, I. V., ... & Totolian, A. A. (2022). Changes in anti-SARS-CoV-2 IgG subclasses over time and in association with disease severity. Viruses, 14(5), 941, Frasca, D., Diaz, A., Romero, M., Mendez, N. V., Landin, A. M., &Blomberg, B. B. (2013). Effects of age on H1N1-specific serum IgG1 and IgG3 levels evaluated during the 2011–2012 influenza vaccine season. Immunity & ageing, 10, 1-9.
- Suthar, M.S.; Zimmerman, M.G.; Kauffman, R.C.; Mantus, G.; Linderman, S.L.; Hudson, W.H.; Wrammert, J. Rapid generation of neutralizing antibody responses in COVID-19 patients. Cell Reports Medicine 2020, 1. [Google Scholar] [CrossRef]
- Cervia, C.; Zurbuchen, Y.; Taeschler, P.; Ballouz, T.; Menges, D.; Hasler, S.; Boyman, O. Immunoglobulin signature predicts risk of post-acute COVID-19 syndrome. Nature Communications 2022, 13, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Kawano, Y.; Noma, T.; Yata, J. Regulation of human IgG subclass production by cytokines. IFN-gamma and IL-6 act antagonistically in the induction of human IgG1 but additively in the induction of IgG2. J. Immunol. 1994, 153, 4948–4958. [Google Scholar] [CrossRef]
- Luo, H.; Jia, T.; Chen, J.; Zeng, S.; Qiu, Z.; Wu, S.; Shu, Y. The characterization of disease severity associated IgG subclasses response in COVID-19 patients. Frontiers in immunology 2021, 12. [Google Scholar] [CrossRef]
- Salgado, B.B.; Jordão, M.F.; de Morais, T.B.D.N.; da Silva, D.S.S.; Pereira Filho, I.V.; Salgado Sobrinho, W.B.; Lalwani, P. Antigen-Specific Antibody Signature Is Associated with COVID-19 Outcome. Viruses 2023, 15. [Google Scholar] [CrossRef] [PubMed]
- Cervia, C.; Nilsson, J.; Zurbuchen, Y.; Valaperti, A.; Schreiner, J.; Wolfensberger, A.; Boyman, O. Systemic and mucosal antibody responses specific to SARS-CoV-2 during mild versus severe COVID-19. Journal of Allergy and Clinical Immunology 2021, 147, 545–557. [Google Scholar] [CrossRef] [PubMed]
- Jurenka, J.; Nagyová, A.; Dababseh, M.; Mihalov, P.; Stankovič, I.; Boža, V.; Sabaka, P. Anti-SARS-CoV-2 antibody status at the time of hospital admission and the prognosis of patients with COVID-19: A prospective observational study. Infectious Disease Reports 2022, 14, 1004–1016. [Google Scholar] [CrossRef]
- Ricke, D.O. Two different antibody-dependent enhancement (ADE) risks for SARS-CoV-2 antibodies. Frontiers in immunology 2021, 443. [Google Scholar] [CrossRef] [PubMed]
- McGonagle, D.; Ramanan, A.V.; Bridgewood, C. Immune cartography of macrophage activation syndrome in the COVID-19 era. Nature Reviews Rheumatology 2021, 17, 145–157. [Google Scholar] [CrossRef] [PubMed]
- Adeniji, O.S.; Giron, L.B.; Purwar, M.; Zilberstein, N.F.; Kulkarni, A.J.; Shaikh, M.W.; Abdel-Mohsen, M. COVID-19 severity is associated with differential antibody Fc-mediated innate immune functions. MBio 2021, 12, 10–1128. [Google Scholar] [CrossRef]
- Suvorov, A.; Gupalova, T.; Desheva, Y.; Kramskaya, T.; Bormotova, E.; Koroleva, I.; Leontieva, G. Construction of the enterococcal strain expressing immunogenic fragment of SARS-Cov-2 virus. Frontiers in pharmacology 2022, 12. [Google Scholar] [CrossRef]
- Mattiuzzi, C.; Lippi, G. Timeline analysis of clinical severity of COVID-19 in the general population. European Journal of Internal Medicine 2023, 110, 97–98. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.R.; Cao, Q.D.; Hong, Z.S.; Tan, Y.Y.; Chen, S.D.; Jin, H.J.; Yan, Y. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak–an update on the status. Military medical research 2020, 7, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Perevaryukha, A.Y. A continuous model of three scenarios of the infection process with delayed immune response factors. Biophysics 2021, 66, 327–348. [Google Scholar] [CrossRef] [PubMed]
- Wasiluk, T.; Sredzinska, M.; Rogowska, A.; Zebrowska, A.; Boczkowska-Radziwon, B.; Stasiak-Barmuta, A.; Radziwon, P. Analysis of the IgG subclass profile and IgG sum-total discrepancy in COVID-19 convalescent plasma donors: A single-centre prospective cohort study. Transfusion and Apheresis Science 2023, 62. [Google Scholar] [CrossRef] [PubMed]
- Prompetchara, E.; Ketloy, C.; Palaga, T. Immune responses in COVID-19 and potential vaccines: Lessons learned from SARS and MERS epidemic. Asian Pac J Allergy Immunol 2020, 38, 1–9. [Google Scholar]
- Newell, K.L.; Clemmer, D.C.; Cox, J.B.; et al. Switched and unswitched memory B cells detected during SARS-CoV-2 convalescence correlate with limited symptom duration. PLoS ONE 2021, 16, e0244855. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, N.; Kuo, H.-H.; Boucau, J.; et al. Loss of Bcl-6-expressing T follicular helper cells and germinal centers in COVID-19. Cell 2020, 183, 143–57. [Google Scholar] [CrossRef] [PubMed]
- Lucas, C.; Klein, J.; Sundaram, M.E.; Liu, F.; Wong, P.; Silva, J.; Iwasaki, A. Delayed production of neutralizing antibodies correlates with fatal COVID-19. Nature medicine 2021, 27, 1178–1186. [Google Scholar] [CrossRef] [PubMed]
- Abry, P.; Pustelnik, N.; Roux, S.; Jensen, P.; Flandrin, P.; Gribonval, R.; Garnier, N. Spatial and temporal regularization to estimate COVID-19 reproduction number R (t): Promoting piecewise smoothness via convex optimization. PLoS ONE 2020, 15, e0237901. [Google Scholar] [CrossRef]
- Seim, I.; Roden, C.A.; Gladfelter, A.S. Role of spatial patterning of N-protein interactions in SARS-CoV-2 genome packaging. Biophysical journal 2021, 120, 2771–2784. [Google Scholar] [CrossRef]
- Federico, M. Virus-induced CD8+ T-cell immunity and its exploitation to contain the SARS-CoV-2 pandemic. Vaccines 2021, 9, 922. [Google Scholar] [CrossRef] [PubMed]
- Malone, R.W.; Tisdall, P.; Fremont-Smith, P.; Liu, Y.; Huang, X.P.; White, K.M.; Ricke, D.O. COVID-19: Famotidine, histamine, mast cells, and mechanisms. Frontiers in Pharmacology 2021, 12, 633680. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, N.; Terawaki, S.; Shimizu, K.; Oikawa, D.; Sakamoto, H.; Sunami, K.; Tokunaga, F. Th2 cells and macrophages cooperatively induce allergic inflammation through histamine signaling. PLoS ONE 2021, 16. [Google Scholar] [CrossRef] [PubMed]
- Junqueira, C.; Crespo, Â.; Ranjbar, S.; De Lacerda, L.B.; Lewandrowski, M.; Ingber, J.; Lieberman, J. FcγR-mediated SARS-CoV-2 infection of monocytes activates inflammation. Nature 2022, 606, 576–584. [Google Scholar] [CrossRef]
- Mamontov, A.; Polevshchikov, A.; Desheva, Y. Mast cells in severe respiratory virus infections: Insights for treatment and vaccine administration. AIMS Allergy and Immunology 2023, 7, 1–23. [Google Scholar] [CrossRef]
- Kakavas, S.; Karayiannis, D.; Mastora, Z. The complex interplay between immunonutrition, mast cells, and histamine signaling in COVID-19. Nutrients 2021, 13, 3458. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).