1. Introduction
Optimal treatment strategy for acute ischemic stroke in patients presenting with so-called tandem lesions, i.e. comprising both occlusion or high grade stenosis of the extracranial segment of the internal carotid artery (ICA) coexisting with occlusion of the intracranial cerebral arteries (intracranial segments of the ICA, the middle or the anterior cerebral artery), still remains controversial [
1,
2,
3]. Data that come from clinical trials are scarce, since such patients typically represent only 10-20% of the cohorts studied. Quite often patients presenting with tandem lesions are excluded from randomized controlled trials [
4,
5,
6,
7]. However, it is known that intravenous trombolysis in these patients is able to recanalyze occlusions in about 5% of the cases only, while the risk of hemorrhagic complications is high. Moreover, good clinical outcomes after standard intravenous trombolysis in stroke patients with tandem lesions are at the level of 17%, with mortality rate at the level of 50% [
8,
9]. On the other hand, data coming from observational studies suggest that reperfusion rates and clinical outcomes are better if extracranial lesions in these patients are managed with angioplasty and stenting [
9,
10,
11,
12].
Antiplatelet therapy is another controversial issue associated with the treatment of strokes in patients presenting with tandem lesions [
11,
12,
13,
14,
15]. Recommended pharmacological treatment accompanying elective carotid stent placement include dual-antiplatelet therapy and intraprocedural anticoagulation. Unfortunately, such a strategy cannot be used in the setting of acute stroke, because of the risk of intracranial hemorrhage, which is especially high if heparin is used. The risk of intracranial hemorrhage in stroke patients particularly concerns tandem occlusions. An increased risk of intracranial bleeding is also seen when antiplatelet agents are used in these patients. Therefore, usually these pharmacological agents are not used at the beginning of treatment. Moreover, bleeding associated with long half-life antiplatelet agents, such as aspirin or clopidogrel, cannot be easily controlled and almost always is associated with neurologic deterioration and poor clinical outcome [
10,
11,
14,
15,
16,
17,
18].
This problem could be overcome if a short half-life drug was used. Theoretically, early administration of such an antiplatelet agent in patients managed with angioplasty and stenting should decrease the risk of intraprocedural cerebral embolic events, as well as the risk of stent thrombosis. For this purpose GP IIB/IIIA receptor antagonists have been used, the most common agent being eptifibatide. Yet, data regarding safety and efficacy of early administration of eptifibatide in this particular group of stroke patients are scarce and inconclusive [
17,
18,
19,
20,
21]. Moreover, according to the producer, this pharmacological agent is contraindicated in stroke patients; thus all published reports on eptifibatide in stroke patients concern its off-label administration. Meta-analysis by Liu et al. revealed that the treatment of stroke patients with eptifibatide neither had an effect on favorable neurological outcomes, nor did it increase the mortality [
22]. On the other hand, several studies demonstrated that the treatment with eptifibatide in stroke patients can be
Relatively safe and can improve stroke outcomes. It is possible that these dissimilar observations of this agent resulted from its different dosing of. Accompanying coronary stent implantation, eptifibatide is typically administered as intravenous infusion at the rate of 2 μg/kg/min. It has already been suggested that perhaps such a dosing in stroke patients is too high, and indeed in several trials it has been lowered to 0.75 μg/kg/min or 1 μg/kg/min.
The aim of this single- centre retrospective analysis was to assess safety and efficacy of the administration of the modified low dosage of eptifibatide (Integrelin, GlaxoSmithKline, UK) accompanying endovascular angioplasty and stenting of the ICA in the patients presenting with tandem lesions in acute phase of ischemic stroke. This drug was administered in the standard initial intravenous bolus of 180 μg/kg, but then, instead of usually recommended infusion at the rate of 2 μg/kg/min, the dose 1 μg/kg/min was used. This modification was primarily aimed at the reduction of potential hemorrhagic complications.
2. Materials and Methods
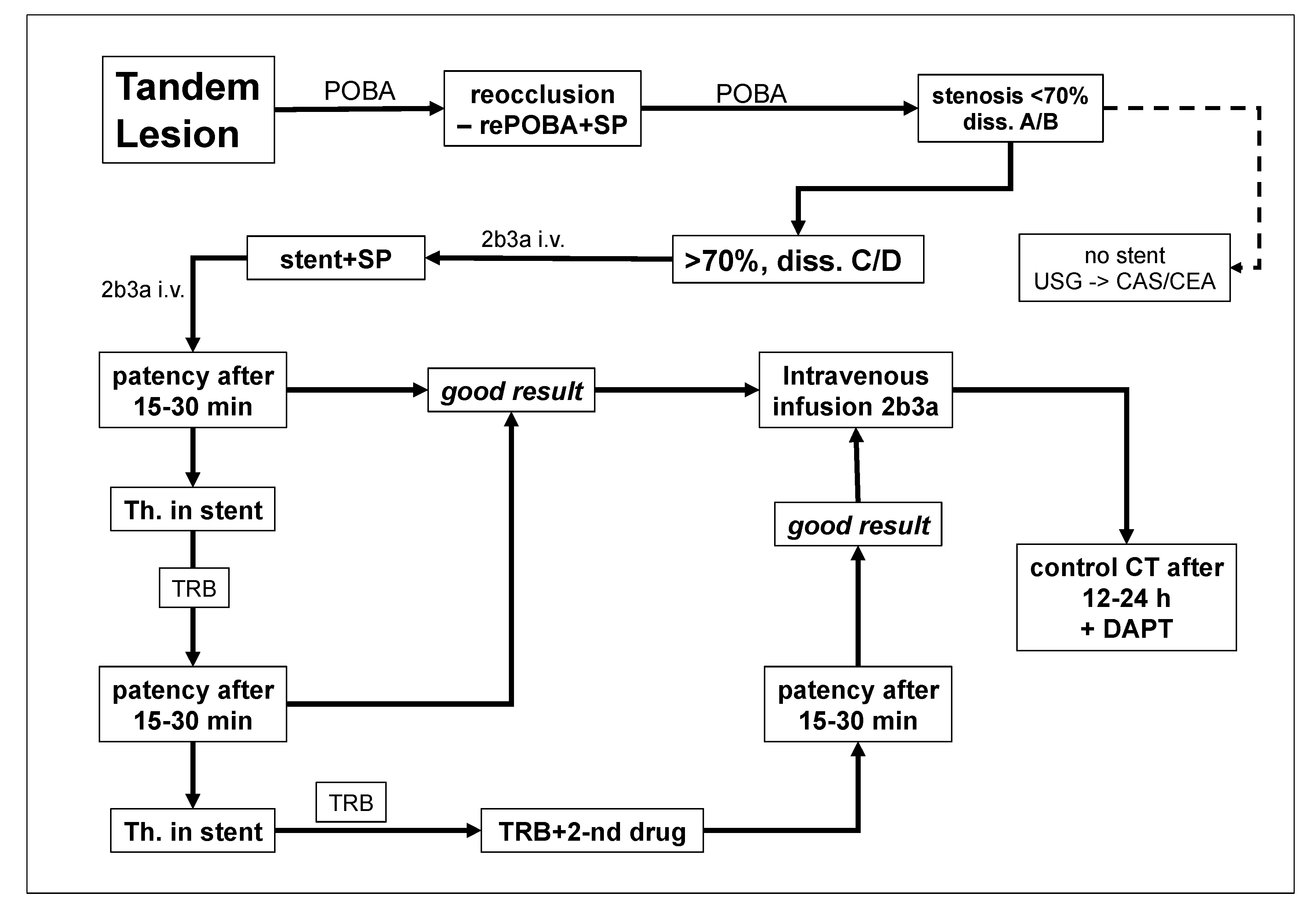
This study is a post-hoc analysis, which focused at the safety of early administration of eptifibatide in stroke patients with tandem lesions. We retrospectively evaluated results of the endovascular treatment in 148 consecutive patients (114 men and 34 women) presenting with acute ischemic stroke due to tandem lesions, who were managed from 2016 to 2023. All of them were treated following our standard hospital protocol for the management of ischemic stroke caused by tandem lesions (
Figure 1).
Patients were aged 23-98 years (mean: 65±12.8). One-hundred-eleven patients (75%) presented with total occlusion of the ICA, while the remaining 37 patients (25%) had 80-99% stenosis of this artery. All of them had also occlusions in the intracranial arteries supplying the brain – details are given in (
Table 1).
The primary endpoint of this study was the proportion of patients who had 30-days mortality of any cause, any intracranial bleeding or any periprocedural complications comprising target artery dissection, perforation and/or new embolic cerebral infarction. Symptomatic intracranial hemorrhage, according to the European Cooperative Acute Stroke Study II, was defined as any intraparenchymal, subarachnoid or intraventricular bleeding, which was associated with clinical worsening of at least 4 points in the National Institutes of Health Stroke Scale (NIHSS). Stent thrombosis and/or hemorrhagic complications were diagnosed either intraprocedurally, or during routine CT or MR imaging, which was performed 24 hours after the procedure, or during diagnostics that was started immediately in a case of clinical worsening or recurrence of neurologic symptoms. For the latter purpose, Doppler sonography together with CT or MR angiography were utilized. The secondary endpoint of this study was neurological status measured with the NIHSS at 30-day follow-up.
For the purpose of this survey, all patients were divided into two groups: i. receiving a short half-life GP IIB/IIIA receptor antagonist (eptifibatide) intra- and postprocedurally, and ii. patients in whom antiplatelet treatment was postponed until the second postprocedural day, when—after exclusion of intracranial bleeding—all patients were allowed to receive standard antiplatelet therapy if such a therapy was clinically justified. No patients received heparin during endovascular procedure.
Decision upon administration of eptifibatide was primarily associated with early stent implantation. If endovascular angioplasty of extracranial occlusion/stenosis of the ICA, using a long inflation of the balloon, resulted in satisfactory dilatation of the artery and good flow, the decision upon stent implantation was postponed until 2-7 postprocedural day. Otherwise, if angioplasty failed or there was an immediate reocclusion, stent was implanted and eptifibatide was administered. This agent was given in an initial intravenous bolus of 180 μg/kg (total dose of such a bolus in our patients: 11-16 mg), and then as intravenous infusion 1 μg/kg/min for 24 hours. Eptifibatide was also administered in the same doses if there were the above-described indications for stent implantation, yet the stent was not implanted due to technical problems; in this case series there were two such patients. Given these indications, patients receiving eptifibatide presented with an unfavorable morphology of tandem lesions, potentially with a high risk of thrombotic re-occlusion, which justified antiplatelet treatment. Of note, the reduced dose of eptifibatide - 1 μg/kg/min instead of standard 2 μg/kg/min, was primarily aimed at the reduction of hemorrhagic complications.
Out of the total of 148 patients assessed in this survey, 115 individuals (78%) received eptifibatide. In this group, 111 patients had stent implantation, including 7 patients in whom two stents were implanted. The remaining 33 patients (22%) did not receive early antiplatelet treatment and had no stents implanted.
Clinical and radiologic characteristics of strokes in both groups (i.e. receiving or not receiving early antiplatelet therapy) were similar. Median scores of the NIHSS at the baseline were 15 and 16, respectively, and this difference was statistically insignificant (see:
Table 1). Average ASPECT score (The Alberta stroke program early CT score) in patients receiving eptifibatide was 7.7, while in those without antiplatelet treatment it was 7.3. Similarly, postprocedural recanalization rates of the middle cerebral artery in both groups were comparable. The Thrombolysis in Cerebral Infarction (TICI) score in patients receiving eptifibatide was TICI2b in 20% of them, TICI2c – in 8%, and TICI3 – in 72%. In patients without antiplatelet treatment these scores were: TICI2b – in 27% of the patients, TICI2c – in 12%, and TICI3 – in 61%. Mean volumes of the necrosis and penumbra in patients receiving eptifibatide were 16.2 mL and 114.3 mL, respectively, while in those without antiplatelet treatment – 28.6 mL and 135.8 mL, respectively. Difference between the groups regarding necrosis volume was statistically significant (p=0.01; assessed using the two-sample t-test), while the difference in therms of penumbra volume was statistically insignificant. Preprocedural clinical characteristics of the patients are given in
Table 1.
Extracranial occlusions or stenoses of the ICA were managed with balloon angioplasty, with or without stent implantation. In addition, all patients received standard intravenous thrombolysis with alteplase. Decision upon stent implantation, as well as upon the administration of antiplatelet agent was left to the discretion of doctor performing the endovascular procedure. In all patients it was tried to perform thrombectomy of the intracranial occlusion first, and to address extracranial lesion thereafter. If introduction of the reperfusion catheter into the distal segments of the ICA was not possible, the proximal critical lesion was managed firstly, either with angioplasty alone, or with angioplasty and stenting.
Protection systems were used in 44 patients. Proximal protection devices used comprised the Mo.Ma Ultra 8F (Medtronic, Minneapolis, MN, USA) and the Cello (Covidien, ev3 Endovascular, Inc., Plymouth, MN, USA); the SpiderFX™ Embolic Protection Device (Medtronic, Minneapolis, MN, USA) was used as a distal protection system. Decision upon distal or proximal protection depended on morphology of occluding lesions, as well as on the angioarchitecture of the carotid arteries. Stents were implanted if balloon angioplasty alone failed to reopen the extracranial occlusion or if the flow improvement in this segment lasted only a few minutes and control intraprocedural angiography revealed re-occlusion of the artery. We used several types of stents: the Carotid WallstentTM (Boston Scientific, Natick, MA, USA), the RoadSaver™ stent (Terumo, Tokio, Japan), and the XACT stent (Abbott Vascular, Abbott Park, IL, USA). Type, length and diameter of the stents were tailored to the angioarchitecture of the target lesion.
In order to reveal potential reocclusion and/or bleeding control intraprocedural angiography was performed in each patient 5, 10 and 15 minutes after the stent implantation. Angiographic success of the procedure was evaluated using the modified Treatment in Cerebral Infarction score (mTICI), with the assessment of previously occluded part of cerebral circulation at the end of endovascular repair.
In all patients, in order to assess efficacy of the treatment, patency of the stent and/or target artery, and to reveal potential early intracranial bleeding, CT or MR of the head was performed 24 hours after the procedure. Then, if there were no hemorrhagic complications, patients were recommended to take dual antiplatelet therapy (aspirin + clopidogrel or ticagrelor) for 1-3 months. The algorithm of patients’ management is presented in
Figure 1.
Statistical analysis
In order to compare preprocedural clinical characteristics of the patients, as well as the rates of severe complications in both groups, such as bleeding, thrombosis or death, the Fisher exact test was used. To compare parametric pre-interventional neurologic status (e.g., NIHSS scores) and the duration of hospital stay we used the two-sample t-test; in the later case patients who died were excluded. To compare neurologic status at 30-day follow-up we categorized the patients into six ordinal groups: without neurologic symptoms (NIHSS score: 0), with mild stroke symptoms (NIHSS score: 1-4), moderate stroke symptoms (NIHSS score: 5-14), severe stroke symptoms (NIHSS score: 15-24), very severe stroke symptoms (NIHSS score: 25 and more), and those who died for any reason. Significance of the differences regarding neurologic status at the 30-day follow-up was calculated using the Mann-Whitney U test. In order to assess the correlation between the preprocedural necrosis volume and the NIHSS score at the 30-day follow-up, the Pearson’s r coefficient was calculated. The significance of p values of all statistical tests used was set at p < 0.05. Statistical analysis was performed using the PAST data analysis package (version 3.0; University of Oslo, Norway).
3. Results
Clinical assessment at 30-day follow-up was possible in all patients in this series, except for those who died. There were 12 (8.1%) fatalities, 9 (7.8%) in the group of patients receiving eptifibatide and 3 death (9.1%) in the group of patients who were not managed with this drug. This difference was statistically insignificant. Of note, the frequencies of thrombotic and hemorrhagic complications were higher in patients not receiving eptifibatide, yet these differences were not statistically significant. Duration of hospital stay was similar in both groups of patients. Baseline neurological status measured with the NIHSS was almost the same in both groups (15 vs. 16 points). Yet, at 30-day follow-up, clinical status of patients who received eptifibatide was better (median NIHSS: 3 vs. 7 points). Details are given in
Table 2.
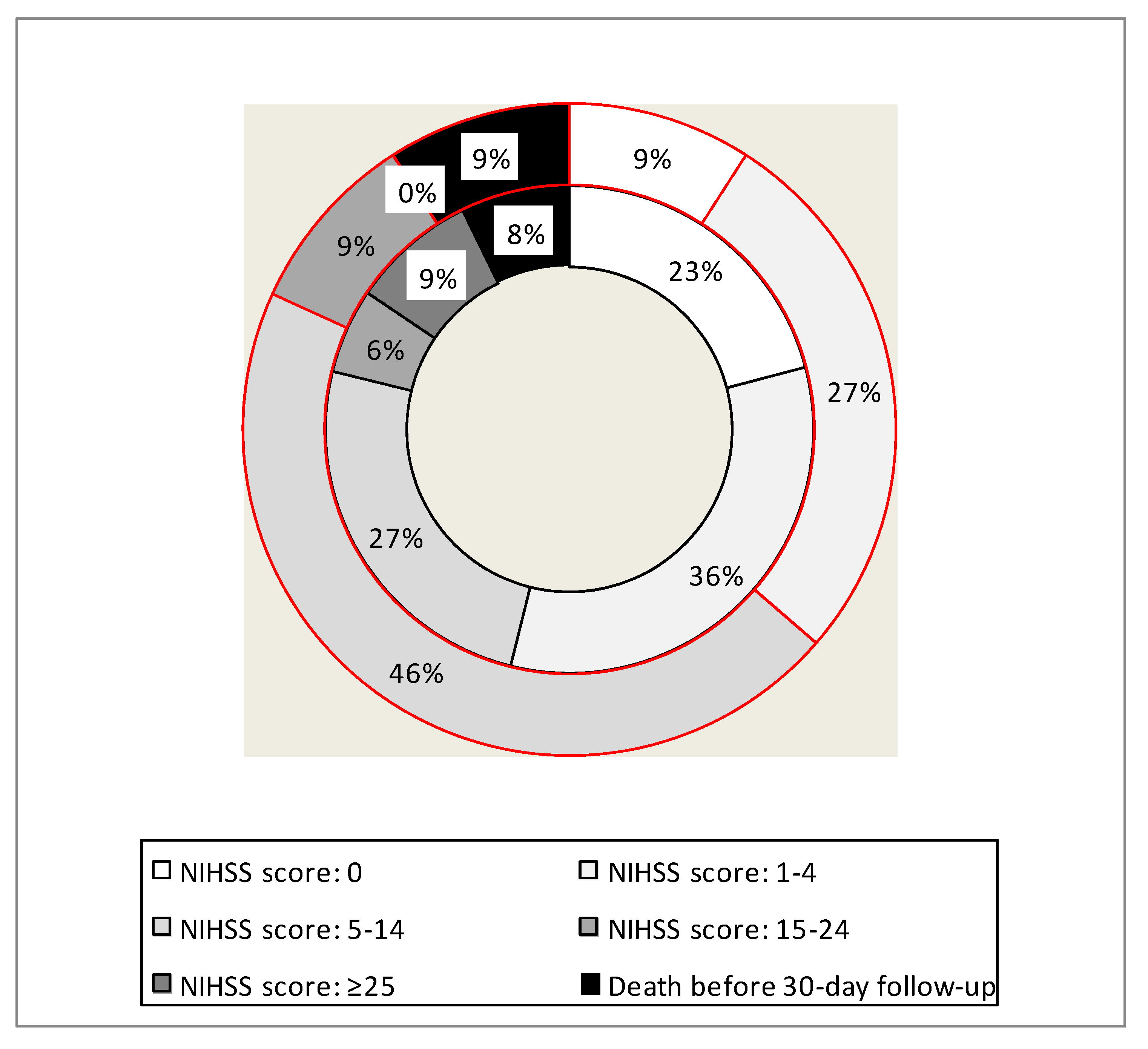
Detailed analysis of neurological status at the 30-day follow-up has revealed that early administration of this short half-life antiplatelet agent in a modified lower dose was associated with a higher rate of either no neurological symptoms, or symptoms of a mild stroke (maximally 4 NIHSS points). This beneficial effect of the modified early administration of eptifibatide was statistically significant, while the rates of severe adverse events, including fatalities, were not significantly different from those in the group of patients not receiving such a medication. Details of this analysis are given in
Table 3 and also shown in
Figure 2. Since there was statistically significant difference between the groups regarding baseline necrosis volume, we have checked whether this parameter was correlated with the clinical outcome. Still, the Pearson’s r coefficient was 0.21, revealing a weak correlation only.
4. Discussion
In this retrospective analysis we have fund that administration of eptifibatide in stroke patients presenting with tandem lesions was relatively safe. The rates of thromboses of the target artery, as well as the rates of intracranial hemorrhages were not statistically different from those in the group of patients not receiving this antiplatelet agent. Neither the mortality rate was significantly higher after administration of eptifibatide. Besides, frequencies of these adverse events were similar to those reported by other studies [
10,
12,
16,
17,
18,
19].
A quite unexpected finding from our survey was significantly better neurological outcome at the 30-day follow-up in patients who were managed with eptifibatide. Theoretically, since this antiplatelet agent was given only to patients with unsuccessful first balloon angioplasty, thus generally with unfavorable status of the target artery, a worse clinical outcome should be expected. Perhaps, these better clinical outcomes in the eptifibatide group resulted from lower rates of thrombotic and hemorrhagic complications in comparison with patients not receiving this drug (even if these differences in our material were not statistically significant). Theoretically these differences could be associated with fewer thrombotic occlusions in the cerebral microvasculature [
16,
20,
21,
22]. Of note, re-occlusion of the ICA or suboptimal thrombectomy are independent predictors of the intracranial bleeding in the settings of ischemic stroke [
16,
23]. In the study by
Renu et al. it was found that the highest rate of bleeding complications occurred in the group in which stent implantation was used in combination with antiplatelet drug but there was either a suboptimal intracranial recanalization (TICI ≤2a) or there was stent thrombosis [
23]. Probably, incomplete recanalization and damage to the blood-brain barrier, in combination with the antiplatelet drugs, increase the risk of intracranial bleeding [
23].
Although, considering retrospective nature of this study, our results should be interpreted with caution; perhaps all stroke patients with tandem lesions should be managed with low dose eptifibatide. Indeed, such an approach was reported by
Jost et al. They were giving this antiplatelet agent in all tandem lesion patients with stroke, irrespective if they were treated with stent placement or angioplasty [
19]. These authors also recommend the dose 1 μg/kg/min of eptifibatide, since they have found that the dosing of 0.5 μg/kg/min was associated with high reocclusion rates, while other studies suggested that 2 μg/kg/min can result in an increased risk of potentially fatal intracranial bleeding.
Our study showed that stenting of extracranial ICA lesions and intracranial thrombectomy augmented with low-dose eptifibatide can be a good strategy for the management of difficult to recanalyze tandem lesions. Perhaps, such a medication should be given to all and to only to selected tandem lesion patients. A similar beneficial effect of low dose low-dose of eptifibatide in tandem lesion patients has been recently demonstrated in the retrospective analysis by Waters et al. [25]. Still, prospective trials should unequivocally demonstrate safety and efficacy of eptifibatide or other short half-life antiplatelet agents in these challenging stroke patients. For the time being, no such data are available.
We acknowledge that there are important limitations of our survey, comprising its retrospective character, relatively small number of patients assessed and a high heterogeneity of the cohort studied.
5. Conclusions
Administration of modified low-dose of eptifibatide in stroke patients presenting with tandem lesions is relatively safe. Moreover, treatment with this drug can possibly improve clinical outcomes in these patients.
Author Contributions
Conceptualization, P.L. and M.S.; methodology, P.L., T.P.; validation, M.S., P.L.; formal analysis, M.S.; investigation, P.L., T.P., P.B., B.L., K.K.; resources, X.X.; data curation, M.S.; writing—original draft preparation, P.L.; writing—review and editing, M.S.; supervision, P.L.; project administration, P.L., T.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Linfante, I.; Llinas, R.H.; Selim, M.; et al. Clinical and vascular outcome in internal carotid artery versus middle cerebral artery occlusions after intravenous tissue plasminogen activator. Stroke 2002, 33, 2066–2071. [Google Scholar] [CrossRef] [PubMed]
- Pechlaner, R.; Knoflach, M.; Matosevic, B.; et al. Recanalization of extracranial internal carotid artery occlusion after i.v. thrombolysis for acute ischemic stroke. PLoS One 2013, 8, e55318. [Google Scholar] [CrossRef] [PubMed]
- Rubiera, M.; Ribo. M.; Delgado-Mederos, R.; et al. Tandem internal carotid artery/middle cerebral artery occlusion: An independent predictor of poor outcome after systemic thrombolysis. Stroke 2006, 37, 2301–2305. [CrossRef] [PubMed]
- Berkhemer, O.A.; Fransen, P.S.; Beumer, D.; et al. A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med 2015, 372, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Campbell, B.C.; Mitchell, P.J.; Kleinig, T.J.; et al. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med 2015, 372, 1009–1018. [Google Scholar] [CrossRef] [PubMed]
- Goyal, M,; Demchuk, A.M.; Menon, B.K.; et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med 2015, 372, 1019–1030. [CrossRef]
- Veunac, L.; Saliou, G.; Knebel, J.F.; et al. Revascularization of carotid artery occlusion using stenting versus non stenting in endovascular management of tandem occlusion stroke. J ClinNeurosci 2022, 98, 15–20. [Google Scholar] [CrossRef]
- Jovin, T.G.; Chamorro, A.; Cobo, E.; et al. Thrombectomy within 8 hours after symptom onsetin ischemic stroke. N Engl J Med 2015, 372: 2296-2306. [CrossRef]
- Jadhav, A.P.; Zaidat, O.O.; Liebeskind, D.S.; et al. Emergent management of tandem lesions inacute ischemic stroke: analysis of the STRATIS registry. Stroke 2019, 50, 428–433. [Google Scholar] [CrossRef]
- Papanagiotou, P.; Haussen, D.C.; Turjman, F.; et al. (TITAN Investigators), Carotid stenting with antithrombotic agents and intracranial thrombectomy leads to the highest recanalization rate in patients with acute stroke with tandem lesions. ACC Cardiovasc Interv 2018, 11, 1290–1299. [Google Scholar] [CrossRef] [PubMed]
- Wallocha, M.; Chapot, R.; Nordmeyer, H.; et al. Treatment methods and early neurologic improvement after endovascular treatment of tandem occlusions in acute ischemic stroke. Front Neurol 2019, 10, 127. [Google Scholar] [CrossRef] [PubMed]
- Gory, B.; Haussen, D.C.; Piotin, M.; et al. Impact of intravenous thrombolysis and emergent carotid stenting on reperfusion and clinical outcomes in patients with acute stroke with tandem lesion treated with thrombectomy: a collaborative pooled analysis. Eur J Neurol 2018, 25, 1115–1120. [CrossRef]
- Neuberger, U.; Moteva, K.; Vollherbst, D.F.; et al. Tandem occlusions in acute ischemic stroke: impact of antithrombotic medication and complementary heparin on clinical outcome and stent patency. J Neurointerv Surg 2020, 12, 1088–1093. [Google Scholar] [CrossRef]
- Heck, D.; Brown, M. Carotid stenting and intracranial thrombectomy for treatment of acute stroke due to tandem occlusions with aggressive antiplatelet therapy may be associated with a high incidence of intracranial hemorrhage. J Neurointerv Surg 2015, 7, 170–175. [Google Scholar] [CrossRef] [PubMed]
- Pop, R.; Zinchenko, I.; Quenardelle, V.; et al. Predictors and clinical impact of delayed stent thrombosis after thrombectomy for acute stroke with tandem lesions. Am J Neuroradiol 2019, 40, 533–539. [Google Scholar] [CrossRef] [PubMed]
- Poppe, A.Y.; Jacquin, G.; Roy, D.; Stapf, C.; Derex, L. Tandem carotid lesions in acute ischemic stroke: mechanisms, therapeutic challenges, and future directions. AJNR Am J Neuroradiol 2020, 41, 1142–1148. [Google Scholar] [CrossRef] [PubMed]
- Goyal, M.; Yoshimura, S.; Milot, G.; et al. Considerations for antiplatelet management of carotid stenting in the setting of mechanical thrombectomy: A Delphi consensus statement. AJNR Am J Neuroradiol 2020, 41, 2274–2279. [Google Scholar] [CrossRef]
- Osteraas, N.D.; Crowley, R.W.; Panos, N.; Dafer, R.M. Eptifibatide use following emergent carotid stenting in acute anterior circulation ischemic stroke with tandem occlusion. J Stroke Cerebrovasc Dis 2020, 29, 105021. [Google Scholar] [CrossRef]
- Jost, A.; Roels, C.; Brown, M.; Janjua, R.; Heck, D. Low-dose eptifibatide for tandem occlusion in stroke: safety and carotid artery patency. AJNR Am J Neuroradiol 2021, 42, 738–742. [Google Scholar] [CrossRef] [PubMed]
- Gaoting, M.; Sun, X.; Cheng, H.; et al. Combined approach to eptifibatide and thrombectomy in acute ischemic stroke because of large vessel occlusion: a matched-control analysis. Stroke 2022, 53, 1580–1588. [Google Scholar] [CrossRef]
- Luo, L.; Lin, J.; Deng, Y.; Li, Z.; Yuan, Y.; Zhang, W. Treatment of progressive ischemic stroke with low dose eptifibatide: A retrospective case control study. Exp Ther Med 2023, 25, 22. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Yang, Y.; Liu, H. Efficacy outcomes and safety measures of intravenous tirofiban or eptifibatide for patients with acute ischemic stroke: a systematic review and meta-analysis of prospective studies. J Thromb Thrombolysis 2022, 53, 898–910. [Google Scholar] [CrossRef]
- Renu, A.; Blasco, J.; Laredo, C.; et al. Carotid stent occlusion after emergent stenting in acute ischemic stroke: Incidence, predictors and clinical relevance. Atherosclerosis 2020, 313, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Marnat, G.; Mourand, I.; Eker, O.; et al. Endovascular management of tandem occlusion stroke related to internal carotid artery dissection using a distal to proximal approach: Insight from the RECOST Study. AJNR Am J Neuroradiol 2016, 37, 1281–1288. [Google Scholar] [CrossRef] [PubMed]
- Waters, M.J.; Vargas, J.; Turk, A.; Chaudry, I.; Turner, R.D. Safety and efficacy of eptifibatide in acute ischemic stroke requiring extracranial carotid artery stenting. Interventional Neuroradiology 2023. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).