1. Introduction
Pain is a global healthcare concern and the most common reason for people seeking medical intervention. Chronic pain is defined as a continued painful sensation lasting for > 3 months in the clinic and can deteriorate the quality of life. The International Association for the Study of Pain (IASP) categorized chronic pain as primary and secondary pain (1). Primary pain includes fibromyalgia and nonspecific lower back pain (2). Neuropathic pain is one of chronic pain secondary to an underlying syndrome that can arise from nociceptive input even without clear damage or dysfunction (1). Chronic pain is associated with pain signal processing, central sensitization, and neural circuits. Spontaneous hyperalgesia was frequently detected with unsatisfactory therapy for neuropathic pain. Recent studies show that the central nervous system can suffer alterations in central sensitization in mice (3, 4). Pain continues due to central potentiation, a type of neuronal plasticity, and amplified neural activity after the initiation. Central sensitization also results from inflammation in either peripheral or central nervous system (5).
Neuropathic pain is demarcated as uncomfortable sensation initiated by a malunction of the somatosensory cortex (SSC) induced by central nerve system dysfunction in humans (6). In addition, strokes in the SSC region can also induce neuropathic pain (7). Maladaptive neuroinflammation beyond neuronal damage is the dominant cause of chronic pain, even after stroke resolution. Recently, the microglia has been involved in neuropathic pain as neuroinflammation triggers microglial activation (8). Neuropathic pain is a debilitating condition resulting from central sensitization with increased excitatory inputs from several brain areas, including the SSC, to anterior cingulate cortex (ACC) in response to sensory-discriminative pain features (4). Spared nerve injury (SNI) at sciatica trunk: common peroneal, tibia, or sural nerves, are commonly used to develop animal models for drug developments (9-12)
Transient receptor potential V1 (TRPV1) is a Ca2+ permeable channel associated with pain sensation and inflammation detection (13). TRPV1 is triggered by mechanical force, capsaicin, low pH, or temperatures over 43 °C. TRPV1 was indicated participating in inflammatory, fibromyalgia, and neuropathic pain (14, 15), and is highly expressed in small C-fibers. TRPV1 is significantly increased after both peripheral and central levels such as inflammatory, fibromyalgia, and neuropathic pain models. Trpv1 deletion induced desensitization to thermal stimulation and the inflammatory mediators released from glial cells (4). Moreover, injection of the TRPV1 antagonist reduces thermal pain in mice inflammatory pain. Protease-activated receptor 2 is demonstrated to activate TRPV1 through protein kinase A or C signals (14). HMGB1 was indicated as a nuclear factor enhancing transcription associated with tissue damage, bacterial infection, and inflammation. HMGB1 was known as inflammatory mediators from necrotic or glial cells activated by interleukins (ILs), tumor necrosis factor α (TNF-α), interferon-γ (IFN-γ) (16). HMGB1 can bind to receptor for advanced glycation end-products (RAGE) in regulation of DNA transcription by nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB). The S100B was a calcium- wrapping proteins, which can bind to RAGE receptor, categorized by the regulation of procedures involved in tissue damage (17).
Acupuncture has been long performed as an alternative cure for pain healing with rare side effects. Recently, electroacupuncture (EA), which provides electric stimulation along with acupuncture for better results and standardization, has been shown to relieve inflammatory, fibromyalgia, and neuropathic pain (18) in several mice models. Moreover, it can alleviate various pain conditions by increasing endogenous opiates (19), and adenosine (20). Moreover, EA was reported to alleviate fibromyalgia pain by attenuating IL-1

, TNFα, and IFNγ in mice plasma. In addition, we recently showed that EA reliably attenuate either mechanical or thermal hyperalgesia in fibromyalgia mouse models via inhibiting the TRPV1 pathway (4).
In the current research, we hypothesized that neuropathic pain is allied with amplified inflammation and neural hyperactivity from the peripheral dorsal root ganglion (DRG), spinal cord dorsal horn (SCDH), SSC, and ACC. We mimicked neuropathic pain using the SNI mice model and evaluated EA efficacy. Consistent with our hypothesis, EA treatment reduced SNI-induced neuropathic pain which also decreased in Trpv1-/- mice. TRPV1 and related factors were significantly amplified in the mice DRG, SCDH, SSC, and ACC regions. Conversely, either EA or Trpv1 gene deletion significantly attenuated this increase. Thus, EA had an anti-nociceptive effect by downregulating TRPV1. Furthermore, we used a novel chemogenetic technique to precisely attenuate hyperactivity in SSC mice. After SSC inhibition, neuropathic pain and TRPV1 signaling were attenuated both in the SSC and ACC. Thus, we provided indication that aforementioned mediators can modify TRPV1 pathway and indicated novel and prospective healing goals for treating neuropathic pain.
2. Materials and Methods
2.1. Animals
All mice conducted here were agreed by the Institute of Animal Care and Use Committee of China Medical University (Permit no. CMUIACUC-2022-408), Taiwan, next the conductor for the use of mice (National Academy Press). Our investigation was designed to include n = 9 (n = 6 for Western blot and n = 3 for immunofluorescence), This study required 45 male C57BL/6 mice including wild type and
Trpv1-/- mice (Jackson Lab, Bar Harbor, ME) 8–12 weeks’ age with body weightiness of 20–25 g. Mice had been kept at a 12-hour dark and light sequence with food and water ad libitum. A model size of 9 mice in each group was essential for

value of 0.05 with power of 80%. The laboratory worker blindly to treatment allocation during analysis and experiment. We alienated the mice into 5 individuals: Normal group (Group 1: Normal) Mice were suffered a skin incision and muscle dissection without nerve incision; Spared nerve incision group (Group 2: SNI): Mice were performed with spared nerve incision; EA group (Group 3: SNI + EA): SNI mice with 2 Hz EA treatment; Sham EA group (Group 4: Sham EA): SNI mice with sham-treatment;
Trpv1-/- group (Group 5:
Trpv1-/-):
Trpv1 knockout mice with spared nerve incision.
2.2. Neuropathic Pain Model
We used an SNI model to mimic neuropathic pain in animals (15). Mice were anesthetized with a mixture of Zoletil 50 (50 mg/mL) and Xylazine (10 mg/mL). After shaving the left side thigh and using the femur as a landmark, a linear incision was made along the femoral bone to perform a blunt dissection through the biceps femoris muscle to disclose the three branches of sciatic nerve. The sural nerve, so-called spared nerve, is the thinnest among these branches and needs to be carefully protected. The tibia and common peroneal nerves were split over 2 mm and was removed to induce neural damage and inflammation. In the normal group, only femoris muscle dissection was performed and exposure sciatic nerve and its branches without dissection.
2.3. Electroacupuncture
The 2 Hz EA group received acupuncture on the ST36 of the bilateral leg and electrical stimulation with Trio 300 stimulator (Ito, Japan). The parameters were intensity of 1 mA for 20 min at 2 Hz with a rhythm length of 100 μs, while the sham group only received needle insertion on ST36 without electrical stimulation. ST36 acupoint was selected since it was frequently conducted in traditional Chinese medicine for the handling of numerous categories of pain. Acupuncture needles (1 inch, 36G; YU KUANG, Taiwan) were consensually implanted at a depth of 3-4 mm into the murine ST36 acupoint. Mice ST36 acupoint is positioned roughly 3-4 mm lower and 1-2 mm adjacent to the center of the knee (4). The EA stimulation lasted for two weeks between the third week after SNI surgery and until the end of the fourth week.
2.4. Nociceptive Behavioral Tests
The hyperalgesic activities were evaluated double per week for four weeks after SNI initiation. The first test was three days after SNI surgery. Animals were transferred to the behavior examination area at room temperature and adjusted to the surroundings for ≥30 min. The animals were stimulated after they calmed down and not sleeping or grooming. In von Frey filament assessment, mice had been positioned at a steel net (75 × 25 × 45 cm) sheltered with a iglass cage (10 × 6 × 11 cm). We used an automated von Frey tester (IITC Life Science Inc., USA) to stimulate each mouse three times with the fiber at hind paw (lesion side) and recorded the force after paw withdrawal. After the mechanical tests, we allowed mice to calm down for ≥ 30 minutes before the Hargreaves’ test, for which we used an IITC algesimeter (IITC Life Sciences, SERIES8, Model 390G) to examine thermal hyperalgesia by counting the latency to heat challenge. The mice stood on a panes sheet in a cage under which there was a heater; the emphasis of the prediction bulb was expected exactly at left hind paw. In this test, the thermal hyperalgesic latency was judged by the time of paw removal under heat stimulation (4).
2.5. Western Blot Analysis
Mice were euthanized with isoflurane and next undergo cervical dislocation. DRG, SCDH, SSC, and ACC proteins had been used to pluck proteins. Proteins were first kept at 4 °C and future stored at –80°C. For protein mining, all contains were standardized in radioimmunoprecipitation (RIPA) lysis solution having 50 mM Tris-HCl, 1 % NP-40, 250 mM NaCl, 50 mM NaF, 5 mM EDTA, 1 mM Na3VO4,0.02% NaN3, and 1× protease inhibitor cocktail (AMRESCO). The mined proteins were run with 8% SDS-Tris glycine gel electrophoresis and relocated to a polyvinylidene difluoride (PVDF) membrane. The membrane was incubated in bovine serum albumin (BSA) in TBS-T buffer (10 mM Tris pH 7.5, 100 mM NaCl, 0.1% Tween 20), followed by incubation with 1st antibody in TBS-T with 1% BSA over 2 hours at refrigerator. Peroxidase-conjugated antibody (1: 5000) was used as suitable secondary antibody. Significant bands were imaginary using an enhanced chemiluminescent tests (PIERCE) via LAS-3000 Fujifilm (Fuji Photo Film Co., Ltd.). The concentration of specific bands in the image was measured with NIH ImageJ (Bethesda, MD, USA). α-tubulin or β-actin were used as an interior control (4).
2.6. Immunofluorescence
After all behavioral tests, mice were euthanized for sample collection by isoflurane inhalants and perfused with 4% paraformaldehyde. The DRG, SC, SSC, and ACC regions were instantly removed and post-fixed with paraformaldehyde at refrigerator for few days. Then, such samples had been transferred to a 30% sucrose solution for cold protection at 4 ºC refrigerator overnight. These fixed tissues were placed in an optimal cutting temperature (OCT) compound and fast freezing through liquid nitrogen and stored at -80ºC. Stored sections were next cut with 20-μm thickness and rapidly engaged on glass slides. The slides were then incubated with paraformaldehyde and nurtured with liquid, consisting of 2% BSA, 0.2% Triton X-100, and 0.03% sodium azide, over 1 h at apartment temperature. Later blocking, the slices were then nurtured with 1st antibody (1:200, Alomone) in a 1% BSA solution at 4 ºC refrigerator. Next, slides were further nurtured with 2nd antibody (1:500) for 2 h at room temperature before fixing with coverslips for imagining under an fluorescent microscope (Olympus, BX-51, Japan).
2.7. Chemogenetic Operation
All rodents were anesthetized with isoflurane and next fixed their heads in a stereotaxic device, a 23-gauge, 2 mm stainless cannula was implanted into SSC, 0.5 mm posterior and 1.5 mm lateral of bregma at 175 μm below the cortical superficial and fixed to the skull with dental glue. The injection cannula was implanted and linked with the Hamilton needle via a PE duct to inoculate 0.3 μL of viral liquid more than 3 min through the pump (KD Scientific). Subsequently injection, the shot cannula was preserved at SSC for extra 2 min to permit liquid to diffuse. Next, 0.3 μL of hM4D DREADD (designer receptors exclusively activated by designer drugs: AAV8-hSyn-hM4D(Gi)-mCherry; Addgene Plasmid #50477) were injected into the SSC over two weeks. Clozapine N-oxide (CNO; Sigma C0832) was injected to stimulate the DREADD. CNO was thawed in 5% dimethyl sulfoxide (DMSO; Sigma D2650) and diluted with normal saline before intraperitoneal injection of 1 mg/kg at day 15.
2.8. Statistical Analyses
Arithmetical analysis was achieved via SPSS software. All value results were offered as mean ± standard error (SEM). Shapiro-Wilk examination was accomplished to check the normality of obtained results. Statistical consequence among every groups was verified by one-way ANOVA test, surveyed by post hoc Tukey’s test. Values of P < 0.05 were reflected statistically noteworthy.
4. Discussion
When acute nerve damage progresses to a chronic stage, neuropathic pain develops, which gradually deteriorates from nociception to co-morbid emotional and cognitive discomfort (7, 21). Central nervous sensitization, induced by peripheral nerve injury, contributes to chronic neuropathic pain, indicating frequency-dependent increase in hyperexcitability of spinal and brain neuron due to peripheral nerves or tissue injury (21). This sensitization persists via continuous input of a peripheral noxious stimulation signal. Recently, neuroinflammation and associated molecules have been studied to understand neuropathic pain. When the peripheral nerve is damaged, it produces inflammatory stimuli that end up causing neuronal cell death due to oxidative stress. Microglia can secrete MCP-1, IL-1β, IL-6, IL-8, IL-17A, IL-17F, IFN-γ, and TNF-α that interact with each other and with immune T cells (22). Kiguchi et al. reported that IL-1β mRNA in macrophages and Schwann cells were increased in the damaged sciatic nerve by partial sciatic nerve ligation (PSL). Neuropathic pain can be reduced by injection of IL-1β antibody (23). Recent paper indicated that injection of IL-1β and TNF-α at sciatic nerve significantly induced neuropathic pain. Kim and Taylor determined that IL-17 can moderate microglial activation after nerve injury-induced neuropathic pain (24). This combined with immune responses induce unusual synaptic trimming, demyelination, axonal disintegration, regulation of BBB permeability, and immune cell recruitment (5). These mechanisms ultimately result in the suppression of neurogenesis and neuron apoptosis, even neuronal death.
The IASP recommends as effective drugs for neuropathic pain are tricyclic antidepressants, gabapentin, pregabalin, and Duloxetine, Venlafaxine) (25). These oral drugs influence whole -body synaptic conduction and hence, result in systemic side effects including drowsiness, dizziness, and mouth dryness. A Topical patch with capsaicin is also recommended. For clinical usage, topical capsaicin (8%) patches, applied for 60 min, can inhibit the symptoms for 3 months (26). The mechanism behind capsaicin-based relief from neuropathic pain is associated with the TRPV1 receptor, found on neuronal cell membranes, as capsaicin is a TRPV1 agonist. Theoretically, a high concentration of capsaicin binds to TRPV1 and activates it, opening calcium ion channels leading to a massive influx of calcium ions (27). Then, the neuron degenerate and thus relieve the pain. However, after three months, the neuron regenerate and the pain comes back. However, said treatment methods have many side effects, causing frequent relapses. In comparison, acupuncture is ideal for treating neuropathic pain as it has fewer side effects (28).
In neuropathic pain, neuron and microglia interactions are critical for central sensitization and further developed into chronic pain. However, the association among neurons, microglial, and chronic neuropathic pain remains uncharacterized. Yi et al. reported that microglial inhibition attenuated spinal nerve damage convinced microglial activation and chronic pain. Microglial inhibition also reduced the transcription factor interferon regulatory factor 8 (IRF8) and interleukin 1 beta (IL-1β). They also indicated that reduced microglial function alleviated synaptic transmission following SNT through in vivo spinal cord recording (29). In addition, chemogenetic blockade of the connection of locus coeruleus neuron to the basolateral amygdala decreased chronic pain, anxiety, and enhanced fear learning, whereas its activation dramatically initiated anxiety-aversive learning and memory index (30). Furthermore, CNO administration to mice that had a Gi-DREADD in sensory neuron showed increased latency of paw withdrawal for hot stimuli and indicating an analgesic effect (31). We hence validated that chemogenetic inhibition of the SSC significantly reduced the overexpression of elements in the TRPV1 pathway and down regulated these phenomena in downstream ACC areas. The main limitation of the current research indicated that microglial and TRPV1 signaling pathway were merely perceived in SNI-induced neuropathic pain mice model. Clinical trials are necessary to confirm our present data. We only determine TRPV1 receptor in neuronal level, further study may be focus on other receptors in this model such as TLR2, TLR4, or RAGE receptors (
Figure 8). Our data offers evidences of how TRPV1 and associated factors contribute to peripheral and central levels of this neuropathic pain model.
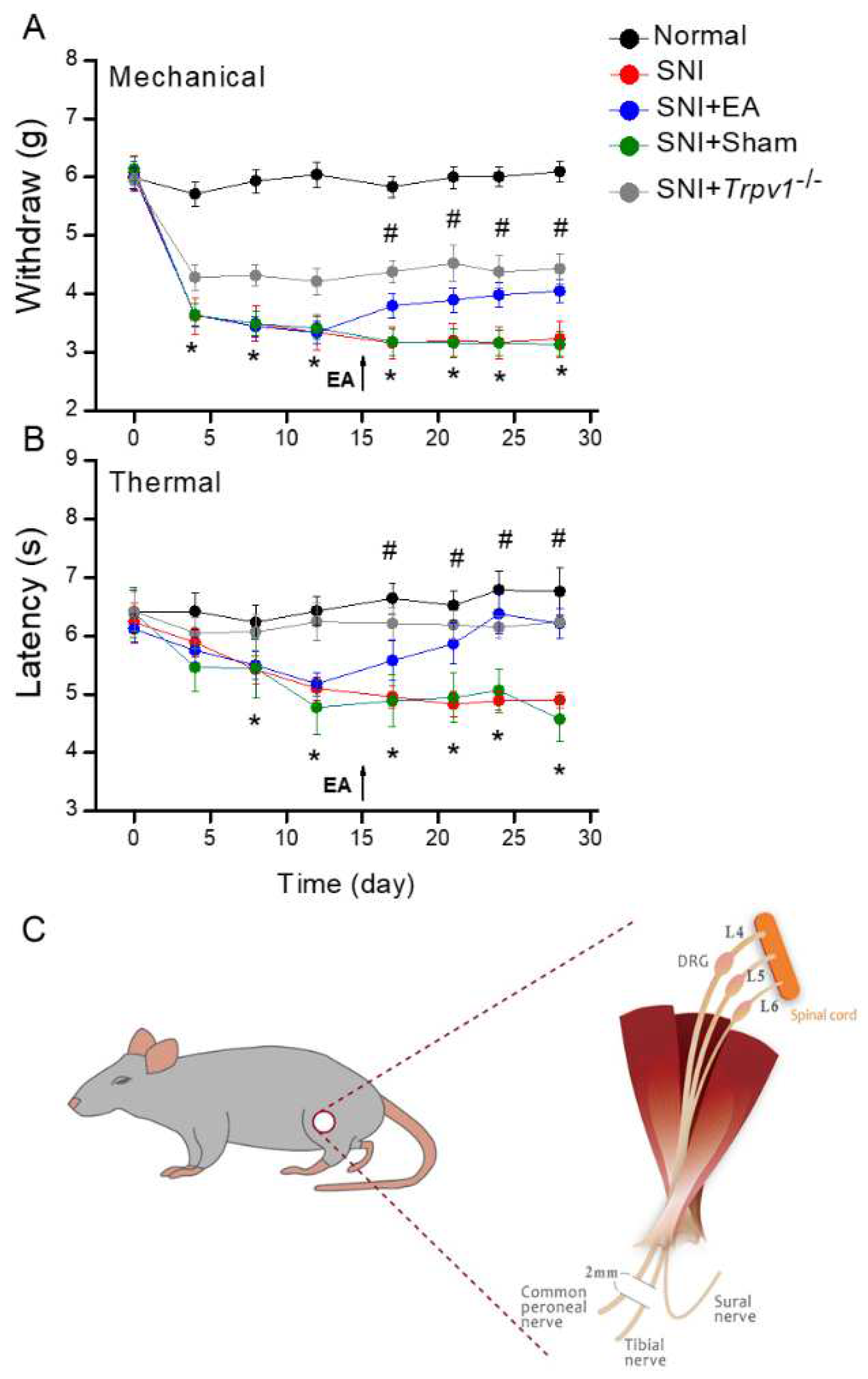
Figure 1.
Nociceptor behavior trends. Black: Normal group, red: SNI group, blue: SNI with 2Hz electroacupuncture (SNI + EA) group, green: SNI with Sham group (SNI + Sham EA), and orange: Transient receptor vanilloid member 1 deletion (SNI + Trpv1-/-) group. From day 0, each node represents post-surgery day 4, 8, 12, 17, 21, 24, 28. *P < 0.05 when compared with the normal group. #P < 0.05 when compared with the SNI group. (A) Mechanical hyperalgesia (von Frey test). (B) Thermal hyperalgesia (Hargreaves’ test). (C) Schematic showing the SNI procedure. First, dissecting the muscle and exposing the sciatica nerve with its three branches: common peroneal nerve, tibial nerve, and sural nerve. Carefully protect and preserve the sural nerve, i.e., spared nerve and cutting another two branches 2 mm at the distal end.
Figure 1.
Nociceptor behavior trends. Black: Normal group, red: SNI group, blue: SNI with 2Hz electroacupuncture (SNI + EA) group, green: SNI with Sham group (SNI + Sham EA), and orange: Transient receptor vanilloid member 1 deletion (SNI + Trpv1-/-) group. From day 0, each node represents post-surgery day 4, 8, 12, 17, 21, 24, 28. *P < 0.05 when compared with the normal group. #P < 0.05 when compared with the SNI group. (A) Mechanical hyperalgesia (von Frey test). (B) Thermal hyperalgesia (Hargreaves’ test). (C) Schematic showing the SNI procedure. First, dissecting the muscle and exposing the sciatica nerve with its three branches: common peroneal nerve, tibial nerve, and sural nerve. Carefully protect and preserve the sural nerve, i.e., spared nerve and cutting another two branches 2 mm at the distal end.
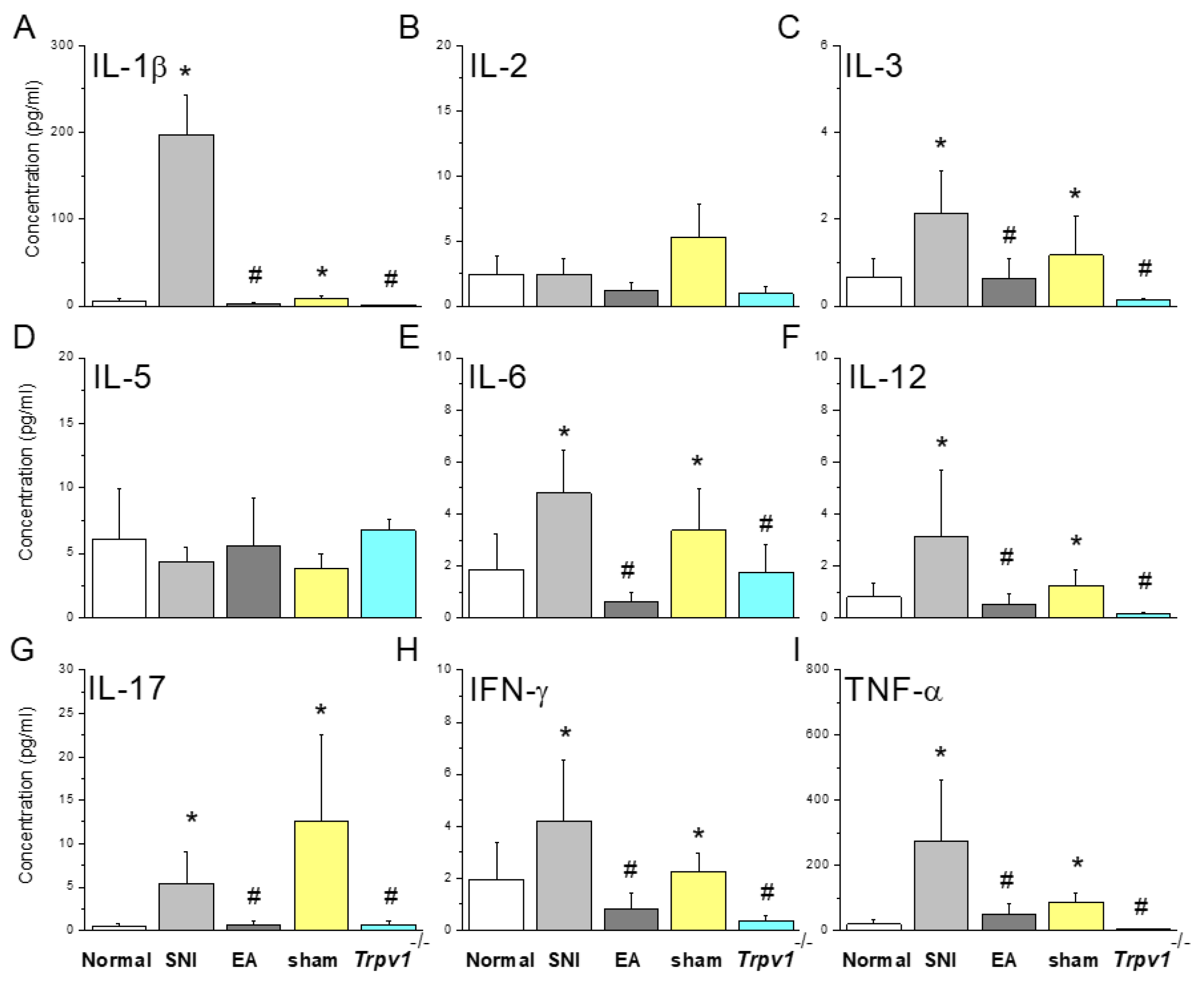
Figure 2.
Inflammatory mediators in mice plasma detected by multiplex ELISA. Inflammatory mediators including (A) IL-1β, (B) IL-2, (C) IL-3, (D) IL-5, (E) IL-6, (F) IL-12, (G) IL-17, (H) IFN-γ, and (I) TNF-α. *Indicates statistical significance when compared with the normal group. #indicates statistical significance when compared with the SNI group. IL: Interleukin, IFN: Interferon, TNF: Tumor necrosis factor. n = 9 in all groups.
Figure 2.
Inflammatory mediators in mice plasma detected by multiplex ELISA. Inflammatory mediators including (A) IL-1β, (B) IL-2, (C) IL-3, (D) IL-5, (E) IL-6, (F) IL-12, (G) IL-17, (H) IFN-γ, and (I) TNF-α. *Indicates statistical significance when compared with the normal group. #indicates statistical significance when compared with the SNI group. IL: Interleukin, IFN: Interferon, TNF: Tumor necrosis factor. n = 9 in all groups.
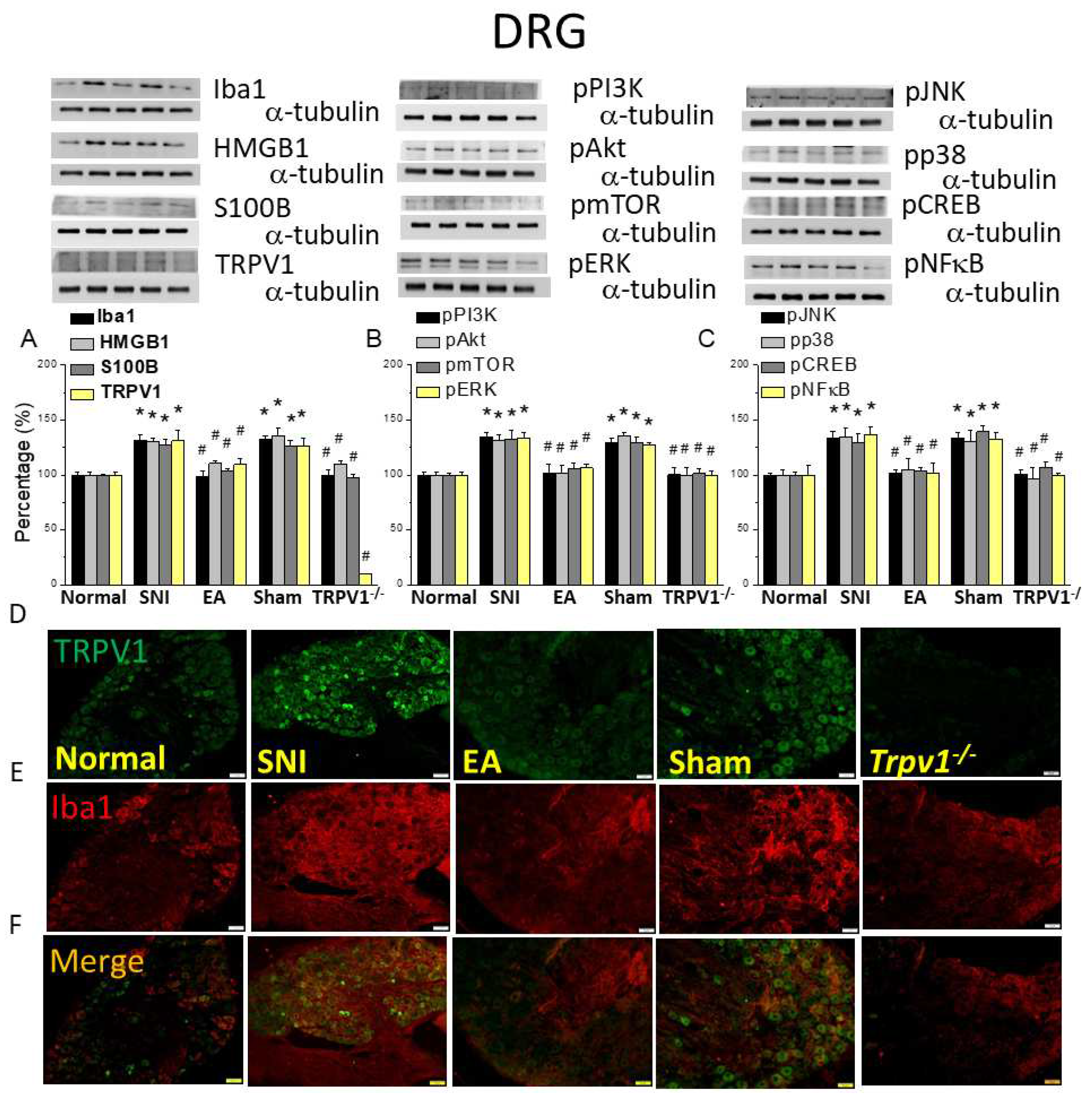
Figure 3.
Levels of signaling molecules involved in TRPV1 signaling in the mice DRG. Western blot showing protein expression in the normal, SNI, EA, sham, and Trpv1-/- groups. Protein levels of (A) Iba1, HMGB1, S100B, and TRPV1; (B) pPI3K, pAkt, pmTOR pERK; and (C) pJNK, pp38, pCREB, and pNFkB. *p <0.05 compared with the normal group. #p < 0.05 compared with the SNI group. n = 6 in all groups. Immunofluorescence staining of Iba1, TRPV1, and double staining in the mice DRG. (D) Iba1, (E) TRPV1, and (F) Iba1/ TRPV1 double staining, immuno-positive (green, red, or yellow) signals in the mouse DRG. Scale bar: 100 μm. n = 3 in all groups.
Figure 3.
Levels of signaling molecules involved in TRPV1 signaling in the mice DRG. Western blot showing protein expression in the normal, SNI, EA, sham, and Trpv1-/- groups. Protein levels of (A) Iba1, HMGB1, S100B, and TRPV1; (B) pPI3K, pAkt, pmTOR pERK; and (C) pJNK, pp38, pCREB, and pNFkB. *p <0.05 compared with the normal group. #p < 0.05 compared with the SNI group. n = 6 in all groups. Immunofluorescence staining of Iba1, TRPV1, and double staining in the mice DRG. (D) Iba1, (E) TRPV1, and (F) Iba1/ TRPV1 double staining, immuno-positive (green, red, or yellow) signals in the mouse DRG. Scale bar: 100 μm. n = 3 in all groups.
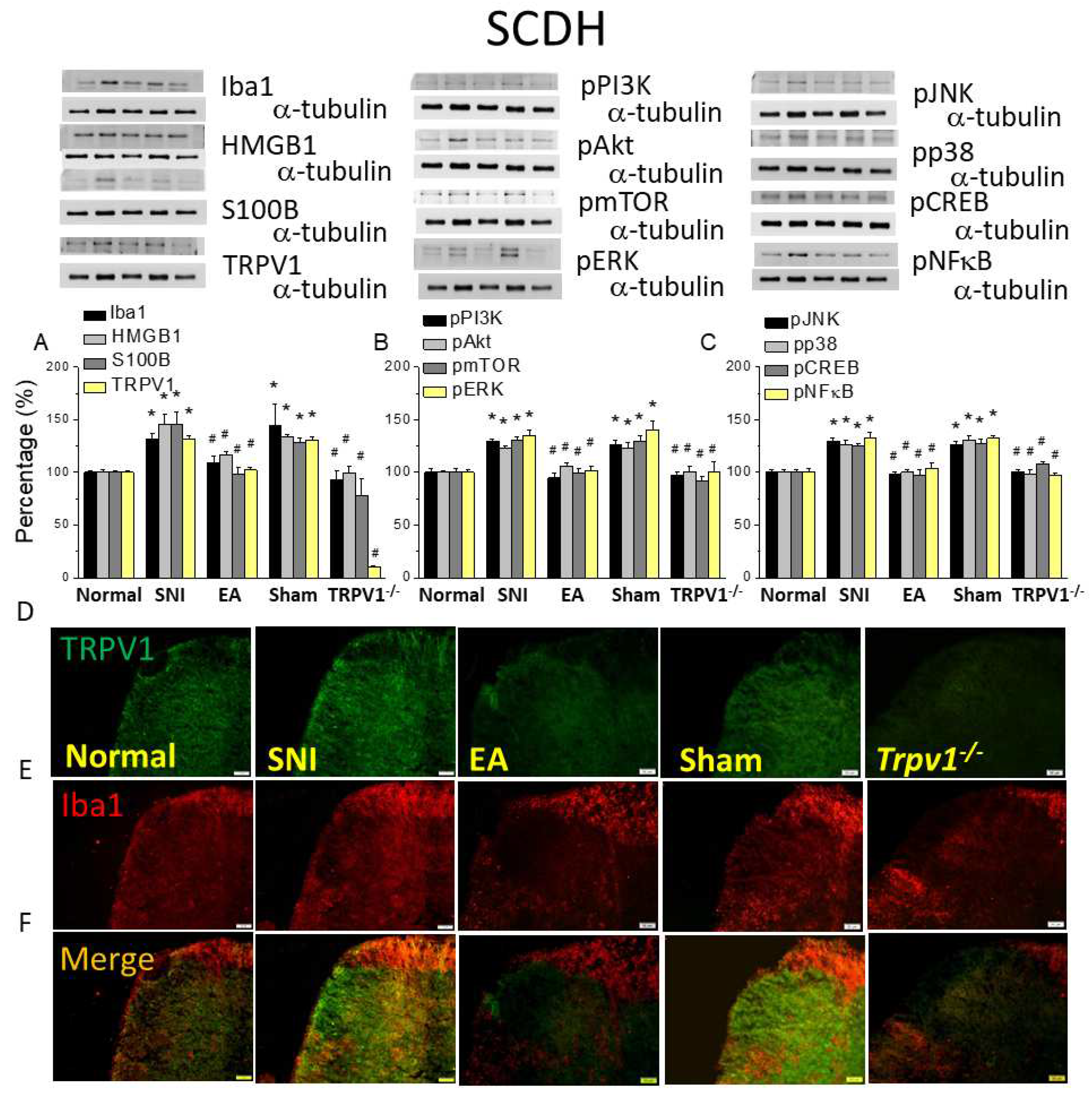
Figure 4.
Levels of signaling molecules involved in TRPV1 signaling in the mice SCDH. Western blot protein expression from normal, SNI, 2Hz EA, sham, and Trpv1-/- groups. Protein levels of (A) Iba1, HMGB1, S100B, and TRPV1; (B) pPI3K, pAkt, pmTOR pERK; and (C) pJNK, pp38, pCREB, and pNFkB. *p <0.05 compared with the normal group. #p< 0.05 compared with the SNI group. n = 6 in all groups. Immunofluorescence staining of Iba1, TRPV1, and double staining in the mice SCDH. (D) Iba1, (E) TRPV1, and (F) Iba1/ TRPV1 double staining, immuno-positive (green, red, or yellow) signals in the mice SCDH. Scale bar: 100 μm. n = 3 in all groups.
Figure 4.
Levels of signaling molecules involved in TRPV1 signaling in the mice SCDH. Western blot protein expression from normal, SNI, 2Hz EA, sham, and Trpv1-/- groups. Protein levels of (A) Iba1, HMGB1, S100B, and TRPV1; (B) pPI3K, pAkt, pmTOR pERK; and (C) pJNK, pp38, pCREB, and pNFkB. *p <0.05 compared with the normal group. #p< 0.05 compared with the SNI group. n = 6 in all groups. Immunofluorescence staining of Iba1, TRPV1, and double staining in the mice SCDH. (D) Iba1, (E) TRPV1, and (F) Iba1/ TRPV1 double staining, immuno-positive (green, red, or yellow) signals in the mice SCDH. Scale bar: 100 μm. n = 3 in all groups.
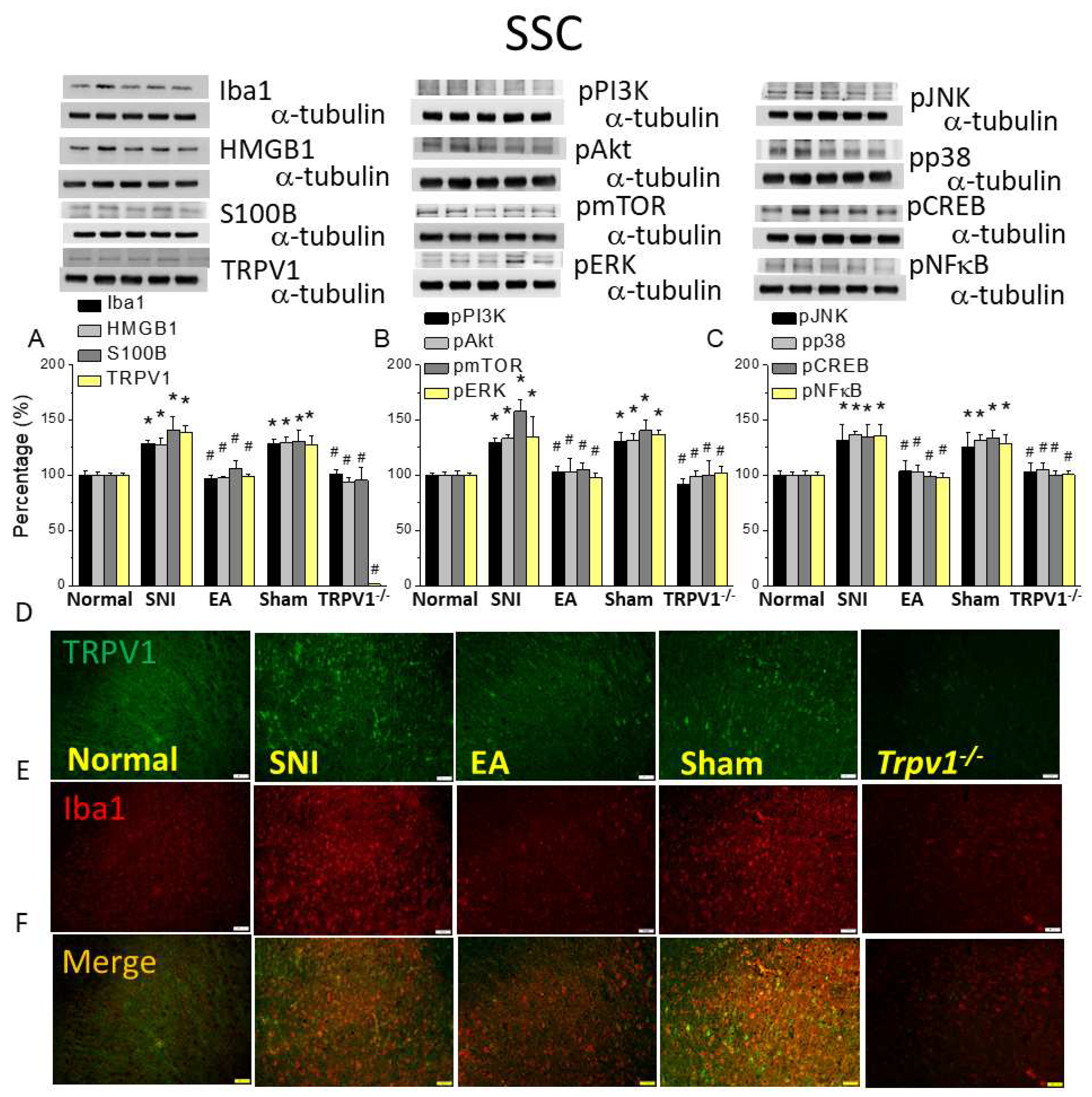
Figure 5.
Levels of signaling molecules involved in TRPV1 signaling in the somatosensory cortex (SSC) of the mice. Western blot showing protein expression from normal, SNI, 2Hz EA, sham, and Trpv1-/- groups. Protein levels of (A) Iba1, HMGB1, S100B, and TRPV1; (B) pPI3K, pAkt, pmTOR pERK; and (C) pJNK, pp38, pCREB, and pNFkB. *p <0.05 compared with the normal group. #p< 0.05 compared with the SNI group. n = 6 in all groups. Immunofluorescence staining of Iba1, TRPV1, and double staining in the mouse SCC. (D) Iba1, (E) TRPV1, and (F) Iba1/ TRPV1 double staining, immuno-positive (green, red, or yellow) signals in the mice DRG. Scale bar: 100 μm. n = 3 in all groups.
Figure 5.
Levels of signaling molecules involved in TRPV1 signaling in the somatosensory cortex (SSC) of the mice. Western blot showing protein expression from normal, SNI, 2Hz EA, sham, and Trpv1-/- groups. Protein levels of (A) Iba1, HMGB1, S100B, and TRPV1; (B) pPI3K, pAkt, pmTOR pERK; and (C) pJNK, pp38, pCREB, and pNFkB. *p <0.05 compared with the normal group. #p< 0.05 compared with the SNI group. n = 6 in all groups. Immunofluorescence staining of Iba1, TRPV1, and double staining in the mouse SCC. (D) Iba1, (E) TRPV1, and (F) Iba1/ TRPV1 double staining, immuno-positive (green, red, or yellow) signals in the mice DRG. Scale bar: 100 μm. n = 3 in all groups.
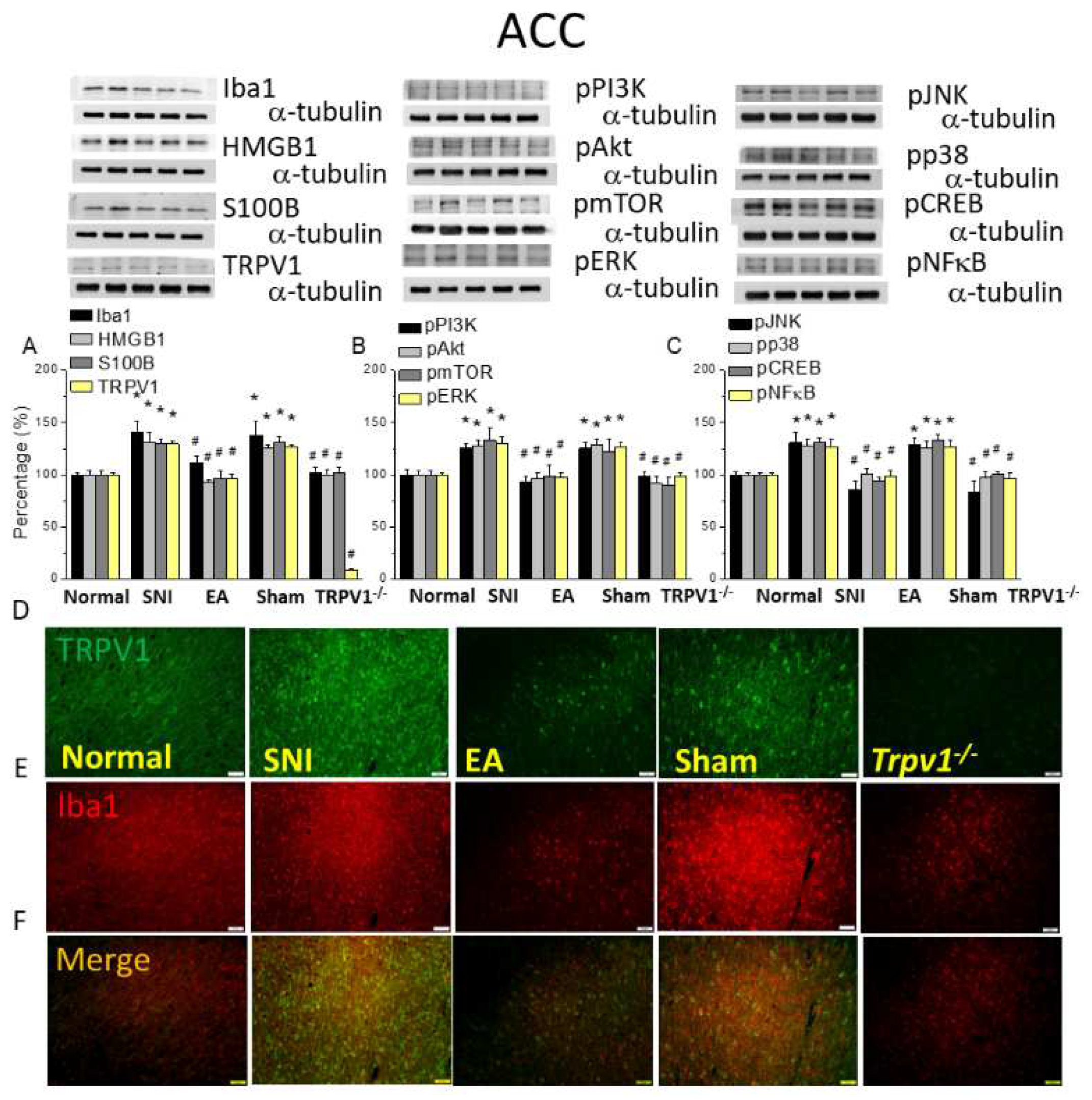
Figure 6.
Levels of signaling molecules involved in TRPV1 signaling in the mice ACC. Western blot showing protein expression from normal, SNI, 2Hz EA, sham, and Trpv1-/- groups. Protein levels of (A) Iba1, HMGB1, S100B, and TRPV1; (B) pPI3K, pAkt, pmTOR pERK; and (C) pJNK, pp38, pCREB, and pNFkB. *p <0.05 compared with the normal group. #p < 0.05 compared with the SNI group. n = 6 in all groups. Immunofluorescence staining of Iba1, TRPV1, and double staining in the mice DRG. (D) Iba1, (E) TRPV1, and (F) Iba1/ TRPV1 double staining, immuno-positive (green, red, or yellow) signals in the mice DRG. Scale bar: 100 μm. N = 3 in all groups.
Figure 6.
Levels of signaling molecules involved in TRPV1 signaling in the mice ACC. Western blot showing protein expression from normal, SNI, 2Hz EA, sham, and Trpv1-/- groups. Protein levels of (A) Iba1, HMGB1, S100B, and TRPV1; (B) pPI3K, pAkt, pmTOR pERK; and (C) pJNK, pp38, pCREB, and pNFkB. *p <0.05 compared with the normal group. #p < 0.05 compared with the SNI group. n = 6 in all groups. Immunofluorescence staining of Iba1, TRPV1, and double staining in the mice DRG. (D) Iba1, (E) TRPV1, and (F) Iba1/ TRPV1 double staining, immuno-positive (green, red, or yellow) signals in the mice DRG. Scale bar: 100 μm. N = 3 in all groups.
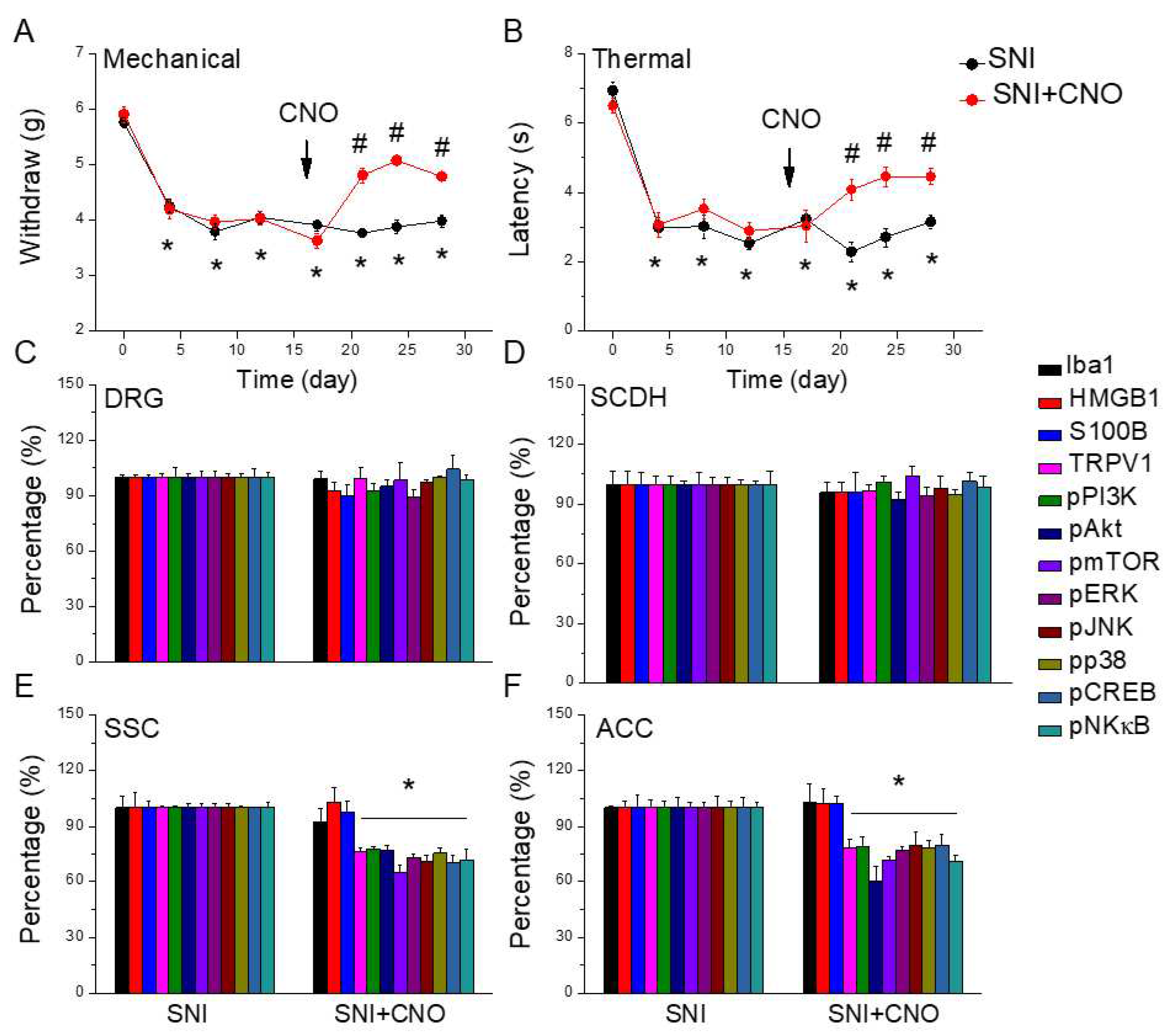
Figure 7.
Nociceptor behavior of SNI and SNI animals treated with the chemogenetic technique. Black: SNI group, red: SNI treated with chemogenetics. From day 0, each circle represents post-surgery day 4, 8, 12, 17, 21, 24, 28. * P < 0.05 compared with the normal group. # P < 0.05 compared with the SNI group. (A) Mechanical hyperalgesia (von Frey test). (B) Thermal hyperalgesia (Hargreaves’ test). Protein levels of Iba1, HMGB1, S100B, TRPV1, pPI3K, pAkt, pmTOR, pERK, pJNK, pp38, pCREB, and pNFkB were measured in mice (C) DRG, (D) SCDH, (E) SSC, and (F) ACC.
Figure 7.
Nociceptor behavior of SNI and SNI animals treated with the chemogenetic technique. Black: SNI group, red: SNI treated with chemogenetics. From day 0, each circle represents post-surgery day 4, 8, 12, 17, 21, 24, 28. * P < 0.05 compared with the normal group. # P < 0.05 compared with the SNI group. (A) Mechanical hyperalgesia (von Frey test). (B) Thermal hyperalgesia (Hargreaves’ test). Protein levels of Iba1, HMGB1, S100B, TRPV1, pPI3K, pAkt, pmTOR, pERK, pJNK, pp38, pCREB, and pNFkB were measured in mice (C) DRG, (D) SCDH, (E) SSC, and (F) ACC.
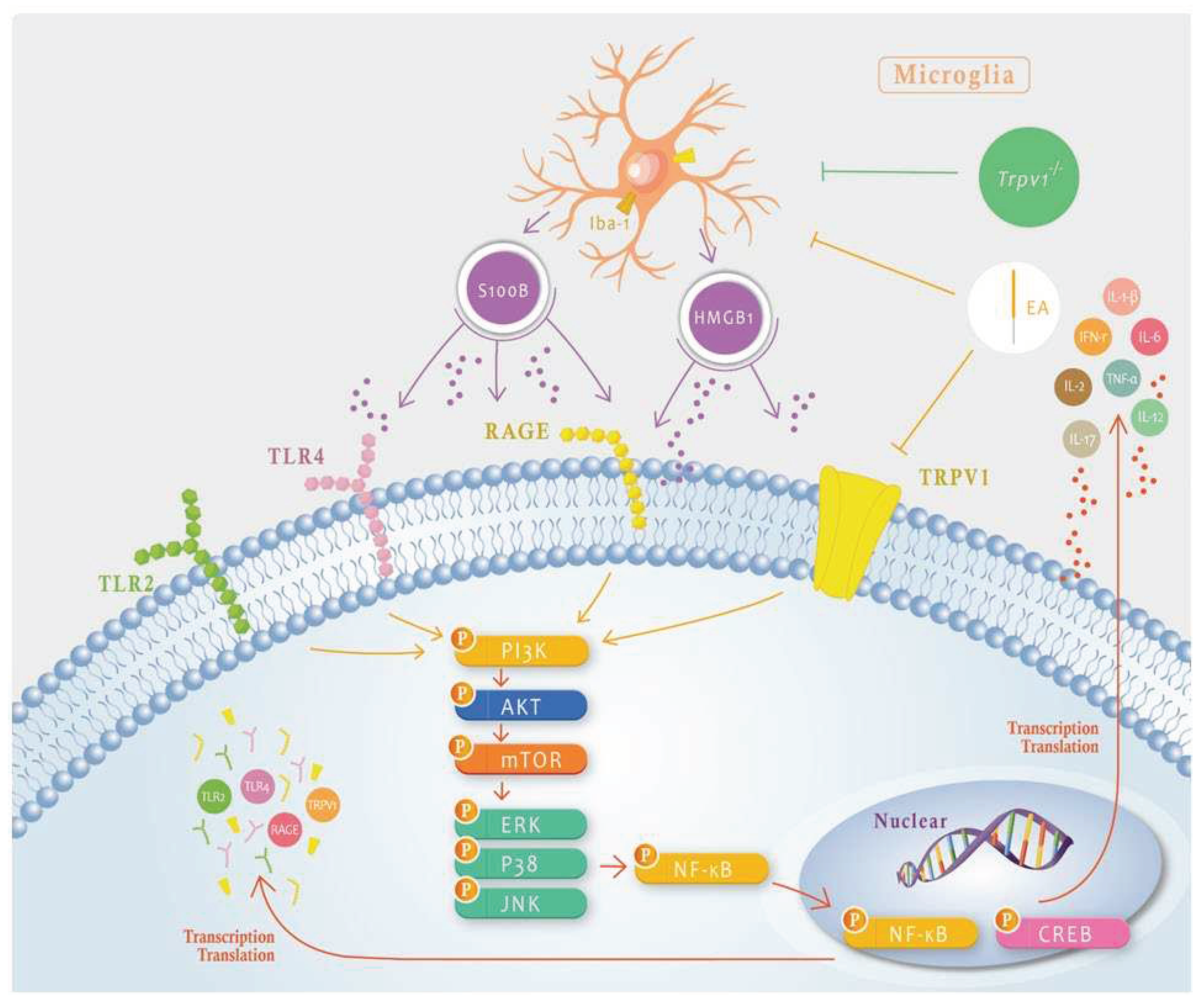
Figure 8.
Schematic illustration of the neuron-microglia interaction mechanisms underlying the 2 Hz EA-mediated analgesic effect on SNI-induced neuropathic pain. The summary diagram shows the importance of and mechanisms involving microglia and TRPV1 in neuropathic pain. EA can directly inhibit microglial activity, i.e., Iba1 presentation that will result in HMGB1 and S100B release or directly inhibit TRPV1 on the membrane. Trpv1-/- mice have the same phenotype as mice treated with EA.
Figure 8.
Schematic illustration of the neuron-microglia interaction mechanisms underlying the 2 Hz EA-mediated analgesic effect on SNI-induced neuropathic pain. The summary diagram shows the importance of and mechanisms involving microglia and TRPV1 in neuropathic pain. EA can directly inhibit microglial activity, i.e., Iba1 presentation that will result in HMGB1 and S100B release or directly inhibit TRPV1 on the membrane. Trpv1-/- mice have the same phenotype as mice treated with EA.
 , TNFα, and IFNγ in mice plasma. In addition, we recently showed that EA reliably attenuate either mechanical or thermal hyperalgesia in fibromyalgia mouse models via inhibiting the TRPV1 pathway (4).
, TNFα, and IFNγ in mice plasma. In addition, we recently showed that EA reliably attenuate either mechanical or thermal hyperalgesia in fibromyalgia mouse models via inhibiting the TRPV1 pathway (4). value of 0.05 with power of 80%. The laboratory worker blindly to treatment allocation during analysis and experiment. We alienated the mice into 5 individuals: Normal group (Group 1: Normal) Mice were suffered a skin incision and muscle dissection without nerve incision; Spared nerve incision group (Group 2: SNI): Mice were performed with spared nerve incision; EA group (Group 3: SNI + EA): SNI mice with 2 Hz EA treatment; Sham EA group (Group 4: Sham EA): SNI mice with sham-treatment; Trpv1-/- group (Group 5: Trpv1-/-): Trpv1 knockout mice with spared nerve incision.
value of 0.05 with power of 80%. The laboratory worker blindly to treatment allocation during analysis and experiment. We alienated the mice into 5 individuals: Normal group (Group 1: Normal) Mice were suffered a skin incision and muscle dissection without nerve incision; Spared nerve incision group (Group 2: SNI): Mice were performed with spared nerve incision; EA group (Group 3: SNI + EA): SNI mice with 2 Hz EA treatment; Sham EA group (Group 4: Sham EA): SNI mice with sham-treatment; Trpv1-/- group (Group 5: Trpv1-/-): Trpv1 knockout mice with spared nerve incision.