1. Introduction
A common pattern is often observed in ecological communities: a few common species make up the majority of individuals, while the majority of species are rare [
1,
2,
3]. Rare species are those with low relative abundance within the communities, restricted habitat selection and/or limited distributional range [
4]. Despite their low abundance, the occurrence and distribution of rare species can play a fundamental role in shaping ecosystem dynamics by influencing community diversity, structure, and stability. In the current context of global environmental change and escalating biodiversity loss, rare species may face increased vulnerability and risk of extinction [
5] or compensate the impact on other species with their numerical abundance increase. Concerns related to the rapid loss of biodiversity due to climatic regulations and anthropogenic activities have made it important to comprehend the role of rare species in maintaining the structure, functioning and services of ecosystems [
6]. Consequently, rare species have received increased attention from conservation biologists and ecologists [
7,
8] and rarity studies within ecological communities have encompassed a broad spectrum of organisms to provide a more comprehensive understanding of biodiversity dynamics [
9]. Certain taxonomic groups have received more attention than others; in particular, plants [
10], butterflies [
11], birds [
12] and mammals [
13] in terrestrial realm and invertebrates [
14] in marine ones. On the other hand, the roles of rare species in phytoplankton communities has still received limited attention. Phytoplankton, comprise diverse photosynthetic microorganisms which form the base of aquatic food webs and contribute substantially to global biogeochemical cycles [
15]. Many studies show that phytoplankton guilds are commonly characterised by dominance effects, while the main contribution to biological diversity is given by rare species [
16,
17], but very few addressed rare species ecological role, which remains unclear.
This investigation focuses on the distribution of different species within phytoplankton communities from 24 transitional water ecosystems, ecotones between marine and freshwater environments [
18], exploring how less common species coexist with dominant ones. Through the use of an integrated individual-level, trait-based, phytoplankton dataset [
19], size-abundance relationships at global and regional scales have been analysed in phytoplankton guilds from five biogeographical regions: Northern Atlantic Ocean (Scotland), South-Western Atlantic Ocean (Brazil), South-Western Pacific Ocean (Australia), Indo-Pacific Ocean (Maldives) and Mediterranean Sea (Greece and Turkey).
Size-abundance relationships tend to show common patterns of variation in numerical populations or species abundance in relation to average body size at the individual level [
20,
21] across different animal and plant guilds, including phytoplankton [
22,
23,
24], macroinvertebrates and fish [
25,
26,
27] in aquatic ecosystems. Theoretically, a rule of -3/4 is expected for the scaling coefficient for both global and local size-abundance relationships [
28,
29,
30]. Rare species often manifest as tails or outliers in size-abundance distribution and are typically treated as artefacts or noise in the data and consequently excluded from data analysis [
31,
32].
Here we focus on the role of rare species in shaping phytoplankton size-abundance relationships and size structure addressing the patterns of change in a large phytoplankton, individual-based dataset at the removal of rare and occasional species. The paper is aimed at investigating the role of taxonomic diversity component, represented by the most rare species, on the morpho-functional diversity in phytoplankton guilds, where a hierarchical organization has already been observed [
33] with the morpho-functional diversity at the highest level of the hierarchy.
2. Materials and Methods
Sampling and data collection
This study was performed using an integrated, individual-level, trait-based phytoplankton dataset open accessible from thein the LifeWatch Italy data portal (
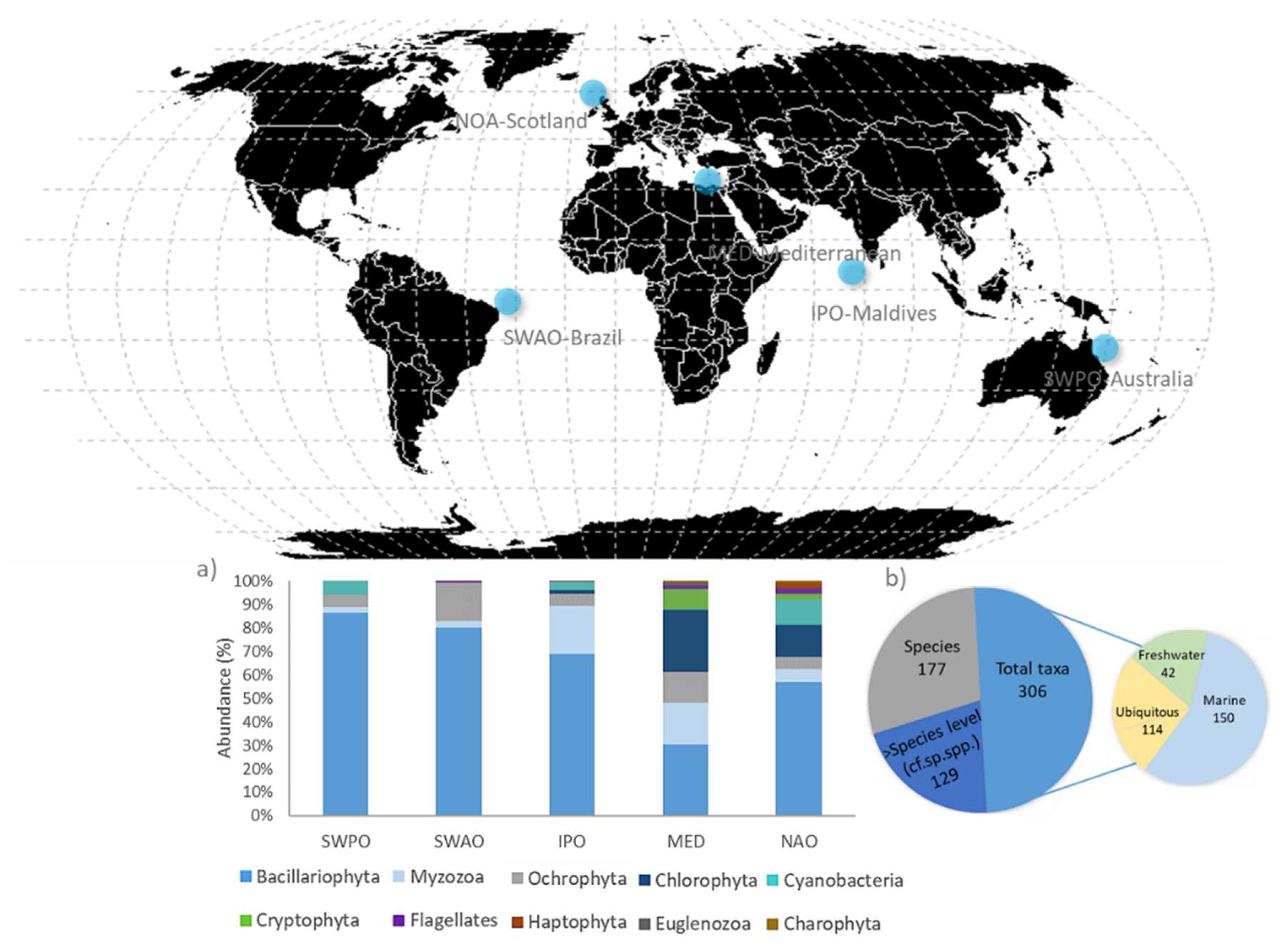
https://dataportal.lifewatchitaly.eu/data). The integrated dataset contains 6 datasets from phytoplankton data collected in 24 transitional water ecosystems, located across five biogeographical regions: Northern Atlantic Ocean (Scotland), South-Western Atlantic Ocean (Brazil), South-Western Pacific Ocean (Australia), Indo-Pacific Ocean (Maldives) and Mediterranean Sea (Greece and Turkey) (
Figure 1). The study sites in this study were mostly tidal and non-tidal lagoons that exhibit heterogeneity in hydro-morphological and physio-chemical characteristics with low anthropogenic pressure and relatively pristine nature [
34,
35,
36]. Phytoplankton samples were collected following a hierarchical sampling design: three ecosystems were selected for each biogeographical area, and within each ecosystem a maximum of three habitat types were selected. Three experimental stations were sampled per habitat type, each with three replicates, resulting in a total count of 116 sites. Samples were collected with horizontal tows from a net of 6 µm mesh at a subsurface depth of 0.5 m and preserved with Lugol’s solution (15mL/L). Phytoplankton identification and analysis were carried out using a Nikon T300E inverted microscope connected to a video-interactive image analysis system (L.U.C.I.A Version 4.8, Laboratory Imaging) following the Utermöhl's method [
37]. For each sample around 400 cells were counted, measured and identified to the lowest taxonomic level possible and the entire bottom of the sedimentation chamber was screened to ensure the possible contribution of all rare species. The qualifiers “cf.”,”sp.” and “spp.” were used to indicate the cell to its nearest nominated species. Phytoplankton cell volume (expressed in μm3) was determined by measuring the specific cell dimensions, on the basis of associated cell shape, and using geometric equations [
38,
39]. Cellular biovolume was converted to carbon content (pg C) following Menden-Deuer and Lessard [
40].
Statistical Analyses
To identify the role of rare and occasional taxa in setting phytoplankton size structure and community distribution, the integrated dataset and the individual six regional datasets were filtered by choosing a threshold value of 1%, 5%, 10% and 25% in cumulative contribution of both taxa abundance and biomass. By progressively subtracting taxa that contributed to the lower 1%, 5%, 10% and 25% of both numerical abundance and biomass, it means that only taxa occurring in both last 1% of numerical abundance and last 1% of overall biomass were actually removed, and the similar method has been used for all other thresholds. Therefore, at the threshold 1% the actual numerical abundance and biomass removed were less than 1% (0.5% abundance removed, and 0.8 % biomass removed). Similarly, for threshold 5% (3.8% abundance, 4% biomass were removed), threshold 10% (7.1% abundance, 8.4% biomass) and for threshold 25% (20% abundance, 21% biomass). From the global and regional datasets, size-abundance relationship were investigated. Global size-abundance relationship (GSAR) and local size-abundance relationship (LSAR) were shown as the linear regressions between average body size and overall taxa abundance, on logarithmic scale. Extremely rare or occasional taxa accounted for less than 1%, 5%, 10% and 25% in cumulative contribution to the overall abundance and biomass, were also investigated in terms of taxonomy and size class distribution. Size class distribution was done using the average individual cellular carbon content of phytoplankton taxa on the logarithmic (log2) scale [
41] forming 16 size classes with a size class width of 1 and clustering these classes into six ordered size classes (i.e., cluster of 2,3,3,3,3,2 classes). Linear analysis of covariance (ANCOVA) was used to test pairwise comparisons of slopes, obtained before and after filtering the datasets using 1%, 5%, 10% and 25% threshold value, from the theoretical expectation of slope values from Damut’s law [
42]. All statistical analyses were done by using R Studio; statistical and programming software (package: dplyr, version 4.2.1) [
43].
3. Results
3.1. Global and local phytoplankton size-abundance relationships
The overall phytoplankton sampled guilds, comprising 127311 cells, consisted of 306 taxa. Approximately 58% of the taxa had been identified at the species level (177 species), while the remaining taxa were classified at the genus or family level, pertaining in global to ten phyla. Among these, Bacillariophyta and Myzozoa represented most of the taxa, having high abundance and highest frequency of occurrence. Chlorophyta and Cryptophyta were also relevant at specific ecoregions (i.e., in the Northern Atlantic Ocean and in the Mediterranean Sea;
Figure 1a). At the species level, most species have a marine origin and few species were freshwater or ubiquitous (
Figure 1b).
Figure 1.
Spatial distribution of phytoplankton sampling in five biogeographic regions: South-Western Pacific Ocean (SWPO) Australia, South-Western Atlantic Ocean (SWAO) Brazil, Indo-Pacific Ocean (IPO) Maldives, Mediterranean Sea (MED) Greece and Turkey, and Northern Atlantic Ocean (NAO) Scotland. a) Stacked bar plot showing phytoplankton composition, in phyla, across ecoregions. b) Phytoplankton species composition based on their origin.
Figure 1.
Spatial distribution of phytoplankton sampling in five biogeographic regions: South-Western Pacific Ocean (SWPO) Australia, South-Western Atlantic Ocean (SWAO) Brazil, Indo-Pacific Ocean (IPO) Maldives, Mediterranean Sea (MED) Greece and Turkey, and Northern Atlantic Ocean (NAO) Scotland. a) Stacked bar plot showing phytoplankton composition, in phyla, across ecoregions. b) Phytoplankton species composition based on their origin.
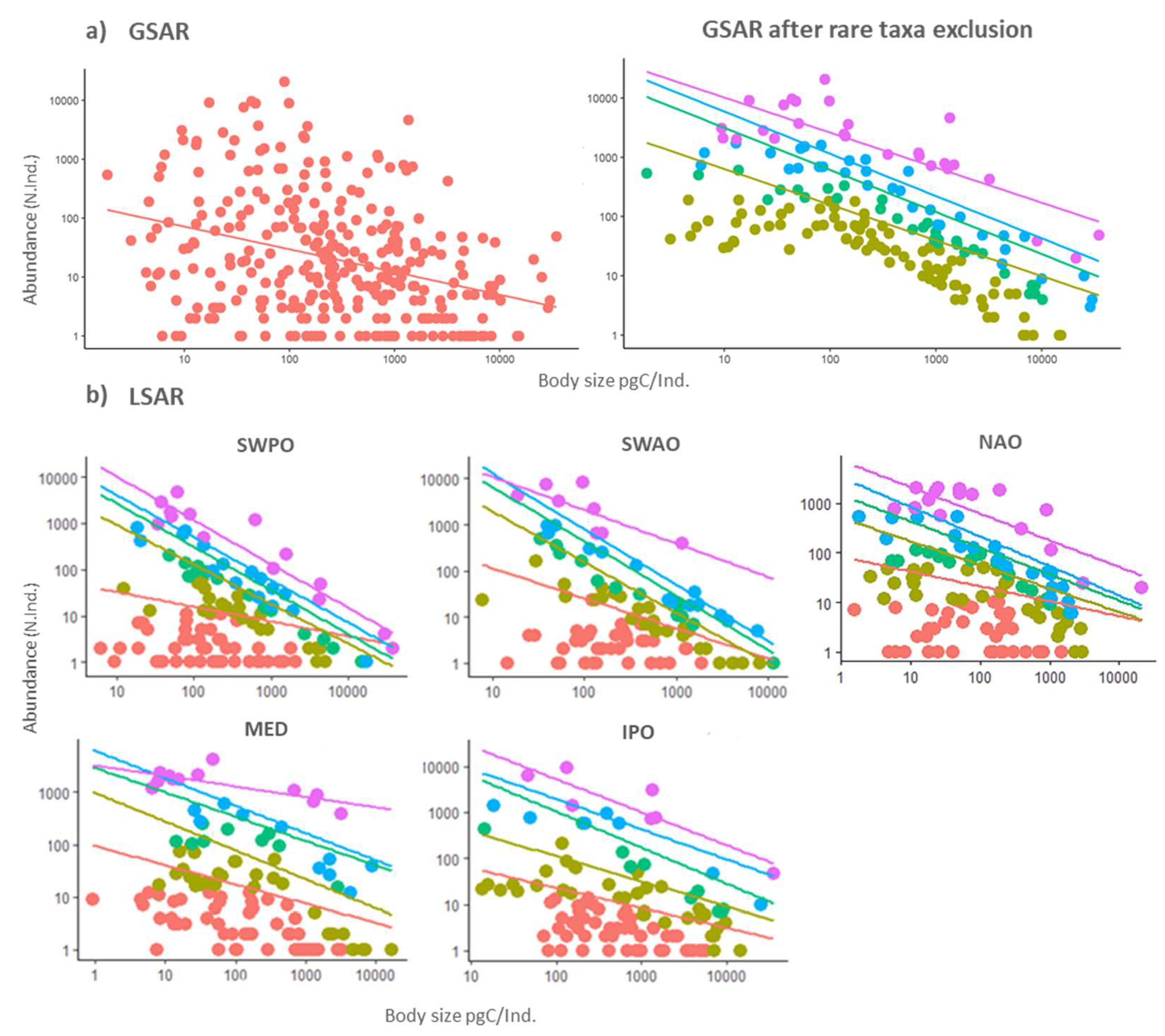
Global size-abundance relationships were all highly significant, irrespective of the percent removal of rare species, from 0% to 25%, but the scaling coefficient was lower at 0% removal than at all other rare species removal thresholds (i.e., from 1% to 25%) (
Figure 2a). A significant difference was observed in the scaling coefficient of the size-abundance relationships only when 1% most rare species had been removed (
Figure 2a), and the scaling coefficient decreased from -0.38, at 0% removal, to -0.60 at 1% removal. All parameters of the global size-abundance relationships were reported in
Table 1. When we applied 1% threshold, 131 taxa were removed, in an exclusion of approximately 42% of overall taxa richness, corresponding to 732 individuals.
In local size-abundance relationships at the biogeographical level, a significant difference in the scaling coefficient of the size-abundance relationships has been observed only when 1% rare species were removed. This was observed specifically only in the South-Western Atlantic Ocean and in South-Western Pacific Ocean (
Figure 2b;
Table 1) where the scaling coefficient decreased from -0.64, at 0% removal to-1.09, at 1% removal, in the South-Western Atlantic Ocean and from -0.31, at 0% removal, to -0.85 at 1% removal, in the South-Western Pacific Ocean. Scaling coefficients followed the same patterns in the other three biogeographical areas but the differences in the scaling coefficients were only qualitative p > 0.05 for all comparisons, with a single exception (Mediterranean Sea; comparison between 10% and 25% thresholds,
Table 1).
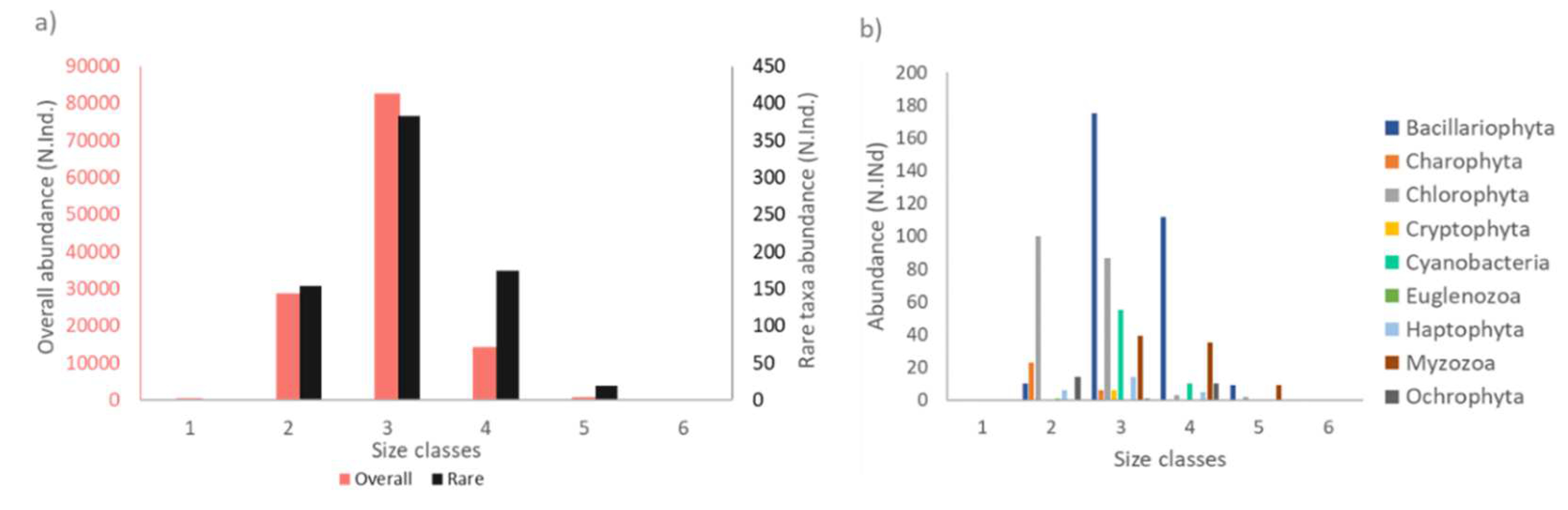
3.2. Taxonomic diversity and size class distribution of rare taxa
The phytoplankton body size spectra consisted of 16 size classes, which were grouped into 6 body size classes, as detailed in the Materials and Methods section. Globally the overall abundance was taken as the total abundance of all six datasets, and regionally it corresponded to the respective region separated. At the taxa level, the average individual cellular carbon content ranged between 1.84 pg C for the smallest taxa
Monoraphidium to 34693.89 pg C for the largest taxa
Proboscia alata and most of rare taxa were belong to intermediate size classes. Around 80% of rare taxa abundance belonged to Bacillariophyta, Chlorophyta and Myzozoa, and 20% of rare taxa abundance were belong to mostly contributed Cyanobacteria, and other phyla (
Figure 3a, b).
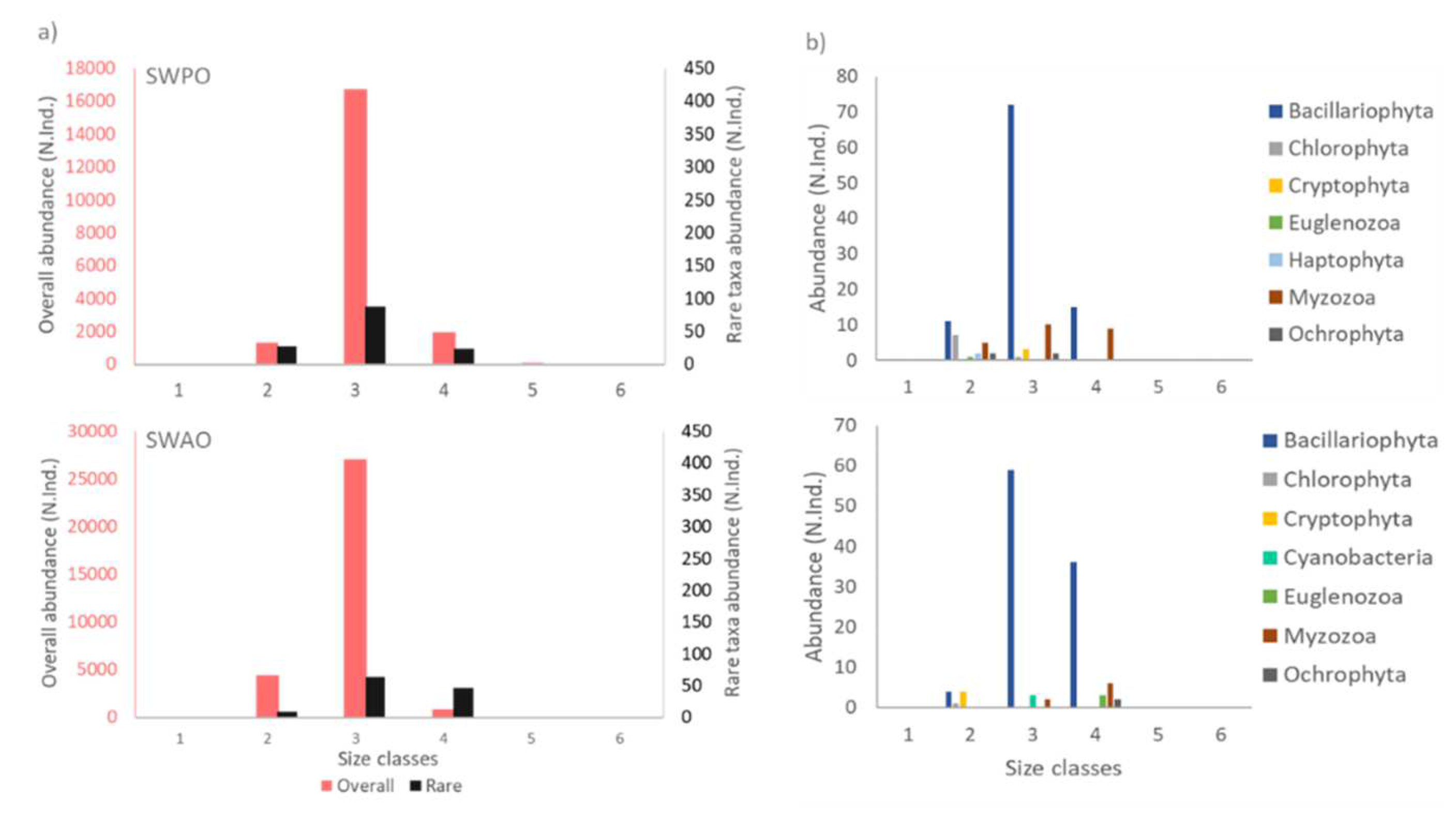
At the regional level, similarities were observed in the distribution of rare taxa only for two ecoregions: South-Western Pacific Ocean and South-Western Atlantic Ocean, where the results were found significant and coherent to the global trends after 1% rare taxa removal (
Figure 4a, b). Size class and taxonomic distributions of the phytoplankton community and 1% rare taxa present in Indo-Pacific Ocean, Mediterranean Sea and Northern Atlantic Ocean are shown as supplementary material (
Figure S1). Generally, rare taxa exhibit heterogeneous distribution across all the regions, with none found to be rare in all regions. Only few taxa occurred as rare in 2 or 3 ecoregions, such as
Thalassionema nitzschioides in the South-Western Pacific Ocean and South-Western Atlantic Ocean,
Syracosphaera pulchra, Pseudopediastrum boryanum and
Oxytoxum longiceps in the Indo-Pacific Ocean and Mediterranean Sea and
Snowella lacustris in the Mediterranean and Northern Atlantic Ocean. Moreover, 44% of the rare taxa occurring in the considered lagoon ecosystems were of marine origin, 19% freshwater and the remaining 37% were ubiquitous, adapted to occur in both marine and freshwater conditions (
Supplementary Table S1).
4. Discussion
In recent years, rarity of species has become a topic of interest in many ecological and biodiversity studies [
44]. The recent advancement in phytoplankton research has highlighted the importance of the studying rare species in phytoplankton assemblages. Available information indicates that phytoplankton communities are made up of common species with high abundance, although there is a large pool of rare species with low abundance and frequency of occurrence [
45,
46]. There are various possible ways to define and investigate rarity [
47]. In this study the cumulative contribution of taxa abundance and biomass were considered as a common denominator to categorize a species as rare [
48,
49], incorporating also the probability of occurrence. This approach determines the cut-off points for abundances and body sizes, below which species were regarded as rare. These cut-off points of rarity are inevitably somewhat arbitrary, although the cut-off/threshold of 25% has been already used in other studies [
50,
51]. One of the difficulties in assessing the importance of removing rare species is the lack of context from which to judge the impact of such a decision. However, it is challenging to find appropriate criteria for quantifying rare phytoplankton species because it is not an easy group to study in terms of rarity [
52,
53].
Our aim in this study was to verify the effect of rarity on the slope of the size scaling of phytoplankton abundance. Firstly, we observed that the removal of the most rare species, accounting for only 1% of the overall phytoplankton numerical abundance and biomass, at both global and regional scale, significantly affected the scaling rate of the size-abundance relationship. Secondly, the removal of a progressive additional fraction of rare species, globally accounting for up to the 25% of numerical abundance and biomass, did not further affect the scaling rate of the size-abundance relationships. The slope of the size-abundance relationship is a general indicator of the relative importance of cells of different sizes in terms of their contribution to total biomass. Size-abundance spectra at the global level, obtained by excluding 1% rarity, showed a trend towards steeper and more negative slopes, indicating an increased importance of smaller phytoplankton cells to biomass contribution and an exponent close to -3/4, known as Damuth’s Rule. Locally, in the South-Western Atlantic Ocean and in South-Western Pacific Ocean the removal of 1% rare species shaped the slope of the size spectra even more negatively than the expected -3/4. Local scale relationships in the Indo-Pacific Ocean, Mediterranean Sea and Northern Atlantic Ocean showed less steep slopes of the size-abundance spectrum, reflecting the increasing importance of larger cells and power-law relationships with exponents that were always shallower than expectations, not affected by the exclusion of rare taxa (at any percentage from 1- 25%). This is in accordance with other studies, probably due to size differences in competition or differences in resource availability for species of different sizes [
54,
55,
56]. Several mechanisms may contribute to biomass distribution such as the resource supply driven by changes in nutrient availability and irradiance. Another point of consideration is the assembly rules of species based on functional groups, which describe how certain groups of species assemblages tend to co-exist based on their ecological roles. Our findings are consistent with the pattern discovered by Fox for terrestrial community: “Species entering a community will tend to be drawn from a different (functional) group until each (functional) group is represented, and then the rule repeats” [
57]. The conservative patterns of individual distribution into body size classes after the removal of 1% most rare species is, in fact, analogous to the conservative pattern of guild filling in with species emphasised for the terrestrial communities [
58]. By using size-dependent functional groups, the assembly rule reflects the use of available resources and each species in an assemblage will be drawn from a different functional group until each group is represented in the community. These patterns have been further analysed and decoded, in terrestrial community, into underlying mechanisms of consumer-resource competition [
59]. In this study we do not have direct evidence of competition for nutrients in the phytoplankton or related phytoplankton-nutrient dynamics as underlying mechanisms of the observed size distribution patterns, but competition for nutrients is a mechanism commonly observed in phytoplankton communities [
60,
61] and the large-scale biogeography of phytoplankton size structure is largely determined by resource supply. Several studies have investigated the patterns and mechanisms of phytoplankton diversity in terms of functional diversity compared to taxonomic diversity. Differences in functionality have been distinguished from morphological traits [
62,
63], the majority of the study on phytoplankton functional diversity has been based on the presence or absence of identities in terms of species richness [
64,
65] and very few have done on functional groups defined by body size and the basis of abundance or biovolume based functional diversity [
66,
67,
68].
Our study is one of the first focusing on the role of rarity on the structural component of both taxonomic and functional diversity of phytoplankton guild. In this respect, our study shows that extremely rare species, accounting for less than 1% on numerical abundance and biomass, contribute consistently to lagoon phytoplankton taxonomic diversity and significantly affect its morpho-functional diversity. Therefore, the huge reserve of biological diversity, at the taxonomic and morpho-functional level, hidden in extremely rare phytoplankton species, seems to have an important role in maintaining long stability, as resilience component, of primary producers and primary productivity in transitional water ecosystems.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org. Figure S1; Table S1.
Author Contributions
A.B., M.L. and J.T. conceived the study, M.L. performed the data analysis, M.L., J.T., A.G., S.R. and A.B. discussed the results, wrote, commented and edited the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by “POR PUGLIA Progetto Strategico 2009-2012”, “LifeWatchPLUS”-CIR01_00028 and the project ITINERIS—Italian Integrated Environmental Research Infrastructure System CUP B53C22002150006 (PNRR for Mission4, Component 2, Notice 3264/2021, IR0000032).
Institutional Review Board Statement
Not applicable
Data Availability Statement
Acknowledgments
The authors thank Ilaria Rosati and Elena Stanca for their support in data collection, harmonization and compilation and the anonymous reviewers for their time and their helpful comments.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Säterberg, T.; Jonsson, T.; Yearsley, J.; Berg, S.; Ebenman, B. A potential role for rare species in ecosystem dynamics. Sci. Rep. 2019, 9, 11107. [Google Scholar] [CrossRef] [PubMed]
- Umaña, M.N.; Mi, X.; Cao, M.; Enquist, B.J.; Hao, Z.; Howe, R.; Iida, Y.; Johnson, D.; Lin, L.; Liu, X. The role of functional uniqueness and spatial aggregation in explaining rarity in trees. Glob. Ecol. Biogeogr. 2017, 26, 777–786. [Google Scholar] [CrossRef]
- Van Schalkwyk, J.; Pryke, J.S.; Samways, M.J. Contribution of common vs. rare species to species diversity patterns in conservation corridors. Ecol. Indic. 2019, 104, 279–288. [Google Scholar] [CrossRef]
- Gaston, K.J.; Lawton, J.H. The population ecology of rare species. J. Fish Biol. 1990, 37, 97–104. [Google Scholar] [CrossRef]
- Chapman, A.S.A.; Tunnicliffe, V.; Bates, A.E. Both rare and common species make unique contributions to functional diversity in an ecosystem unaffected by human activities. Divers. Distrib. 2018, 24, 568–578. [Google Scholar] [CrossRef]
- Ohlemuller, R.; Anderson, B.J.; Araujo, M.B.; Butchart, S.H.M.; Kudrna, O.; Ridgely, R.S.; Thomas, C.D. The coincidence of climatic and species rarity: High risk to small-range species from climate change. Biol. Lett. 2008, 4, 568–572. [Google Scholar] [CrossRef] [PubMed]
- Leitão, R.P.; Zuanon, J.; Villéger, S.; Williams, S.E.; Baraloto, C.; Fortunel, C.; Mendonça, F.P.; Mouillot, D. Rare species contribute disproportionately to the functional structure of species assemblages. Proc. Royal Soc. B. 2016, 283, 20160084. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Lu, Y.; Wei, G.; Jiao, S. Rare species-driven diversity–ecosystem multifunctionality relationships are promoted by stochastic community assembly. MBio 2022, 13, e00449–00422. [Google Scholar] [CrossRef]
- Kondratyeva, A.; Grandcolas, P.; Pavoine, S. Reconciling the concepts and measures of diversity, rarity and originality in ecology and evolution. Biol. Rev. 2019, 94, 1317–1337. [Google Scholar] [CrossRef]
- Wiegand, T.P.; Gentry, B.; McCoy, Z.; Tanis, C.; Klug, H.; Bonsall, M.B.; Boyd, J.N. Visualizing connectivity of ecological and evolutionary concepts-an exploration of research on plant species rarity. Ecol. Evol. 2020, 10, 9037–9047. [Google Scholar] [CrossRef]
- Clark, P.J.; Reed, J.M.; Chew, F.S. Effects of urbanization on butterfly species richness, guild structure, and rarity. Urban Ecosyst. 2007, 10, 321–337. [Google Scholar] [CrossRef]
- Booth, J.E.; Gaston, K.J.; Evans, K.L.; Armsworth, P.R. The value of species rarity in biodiversity recreation: A birdwatching example. Biol. Conserv. 2011, 144, 2728–2732. [Google Scholar] [CrossRef]
- Yu, J.; Dobson, F.S. Seven forms of rarity in mammals. J. Biogeogr. 2000, 27, 131–139. [Google Scholar] [CrossRef]
- Chapman, M.G. Are there adequate data to assess how well theories of rarity apply to marine invertebrates? Biodivers. Conserv. 1999, 8, 1295–1318. [Google Scholar] [CrossRef]
- Falkowski, P.G. The role of phytoplankton photosynthesis in global biogeochemical cycles. Photosynth. Res. 1994, 39, 235–258. [Google Scholar] [CrossRef] [PubMed]
- Hallegraeff, G.M.; Ringelbelg, J. Characterization of species diversity of phytoplankton assemblages by dominance-diversity curves: With 4 figures and 1 table in the text. Int. Ver. Theor. Angew. Limnol. Verh. 1978, 20, 939–949. [Google Scholar] [CrossRef]
- Ignatiades, L.; Gotsis-Skretas, O. The contribution of rare species to coastal phytoplankton assemblages. Mar. Ecol. 2013, 35, 132–145. [Google Scholar] [CrossRef]
- Basset, A.; Barbone, E.; Elliott, M.; Li, B.L.; Jorgensen, S.E.; Lucena-Moya, P.; Pardo, I.; Mouillot, D. A unifying approach to understanding transitional waters: Fundamental properties emerging from ecotone ecosystems. Estuar. Coast. Shelf Sci. 2013, 132, 5–16. [Google Scholar] [CrossRef]
- Laraib, M.; Titocci, J.; Rosati, I.; Basset, A. An integrated individual-level trait-based phytoplankton dataset from transitional waters. Sci. Data. 2023, 10, 897. [Google Scholar] [CrossRef]
- Brown, J.H.; Maurer, B.A. Evolution of species assemblages: Effects of energetic constraints and species dynamics on the diversification of the North American avifauna. The Am. Nat. 1987, 130, 1–17. [Google Scholar] [CrossRef]
- Gaston, K.J.; Blackburn, T.M. Global scale macroecology: Interactions between population size, geographic range size and body size in the Anseriformes. J. Anim. Ecol. 1996, 701–714. [Google Scholar] [CrossRef]
- Gjoni, V.; Glazier, D.S. A perspective on body size and abundance relationships across ecological communities. Biology 2020, 9, 42. [Google Scholar] [CrossRef]
- Belgrano, A.; Allen, A.P.; Enquist, B.J.; Gillooly, J.F. Allometric scaling of maximum population density: A common rule for marine phytoplankton and terrestrial plants. Ecol. Lett. 2002, 5, 611–613. [Google Scholar] [CrossRef]
- Huete-Ortega, M.; Cermeno, P.; Calvo-Diaz, A.; Maranon, E. Isometric size-scaling of metabolic rate and the size abundance distribution of phytoplankton. Proc. Biol. Sci. 2012, 279, 1815–1823. [Google Scholar] [CrossRef]
- Basset, A.; Sangiorgio, F.; Pinna, M. Monitoring with benthic macroinvertebrates: Advantages and disadvantages of body size descriptors. Aquat. Conserv.: Mar. Freshw. Ecosyst. 2004, 14, S43–S58. [Google Scholar] [CrossRef]
- Gjoni, V.; Basset, A. A cross-community approach to energy pathways across lagoon macroinvertebrate guilds. Estuaries Coasts. 2018, 41, 2433–2446. [Google Scholar] [CrossRef]
- Gjoni, V.; Ghinis, S.; Pinna, M.; Mazzotta, L.; Marini, G.; Ciotti, M.; Rosati, I.; Vignes, F.; Arima, S.; Basset, A. Patterns of functional diversity of macroinvertebrates across three aquatic ecosystem types, NE Mediterranean. Mediterr. Mar. Sci. 2019, 20, 703–717. [Google Scholar] [CrossRef]
- Damuth, J. Population density and body size in mammals. Nature 1981, 290, 699–700. [Google Scholar] [CrossRef]
- West, G.B.; Brown, J.H.; Enquist, B.J. A general model for the origin of allometric scaling laws in biology. Science 1997, 276, 122–126. [Google Scholar] [CrossRef]
- Cermeño, P.; Marañón, E.; Harbour, D.; Harris, R.P. Invariant scaling of phytoplankton abundance and cell size in contrasting marine environments. Ecol. Lett. 2006, 9, 1210–1215. [Google Scholar] [CrossRef] [PubMed]
- Gjoni, V.; Cozzoli, F.; Rosati, I.; Basset, A. Size–density relationships: A cross-community approach to benthic macroinvertebrates in Mediterranean and Black Sea lagoons. Estuaries Coasts 2016, 40, 1142–1158. [Google Scholar] [CrossRef]
- Blackburn, T.M.; Gaston, K.J. Who is rare? Artefacts and complexities of rarity determination. In The Biology of Rarity: Causes and Consequences of Rare—Common Differences; 1997; pp. 48–60. [Google Scholar]
- Roselli, L.; Litchman, E.; Stanca, E.; Cozzoli, F.; Basset, A. Individual trait variation in phytoplankton communities across multiple spatial scales. J. Plankton Res. 2017, 39, 577–588. [Google Scholar] [CrossRef]
- Durante, G.; Stanca, E.; Roselli, L.; Basset, A. Phytoplankton composition in six Northern Scotland lagoons (Orkney Islands). Transit. Water. Bull. 2013, 7, 159–174. [Google Scholar]
- Roselli, L.; Stanca, E.; Ludovisi, A.; Durante, G.; Souza, J.S.D.; Dural, M.; Alp, T.; Bulent, S.; Gjoni, V.; Ghinis, S. Multi-scale biodiverity patterns in phytoplankton from coastal lagoons: The Eastern Mediterranean. Transit. Water. Bull. 2013, 7, 202–219. [Google Scholar]
- Stanca, E.; Roselli, L.; Cellamare, M.; Basset, A. Phytoplankton composition in the coastal magnetic island lagoon, western pacific ocean (Australia). Transit. Water. Bull. 2013, 7, 145–158. [Google Scholar]
- Utermöhl, H. Zur vervollkommnung der quantitativen phytoplankton-methodik: Mit 1 Tabelle und 15 abbildungen im text und auf 1 tafel. Verh.-Int. Ver. Theor. Angew. Limnol.: Mitte. 1958, 9, 1–38. [Google Scholar] [CrossRef]
- Phytoplankton Virtual Research Environment VRE. Available online: https://www.phytovre.lifewatchitaly.eu/vre/shapes-groups/.
- Vadrucci, M.R.; Cabrini, M.; Basset, A. Biovolume determination of phytoplankton guilds in transitional water ecosystems of Mediterranean Ecoregion. Trans. Water. Bull. 2007, 1, 83–102. [Google Scholar] [CrossRef]
- Menden-Deuer, S.; Lessard, E.J. Carbon to volume relationships for dinoflagellates, diatoms, and other protist plankton. Limnol. Oceanogr. 2000, 45, 569–579. [Google Scholar] [CrossRef]
- Blanco, J.M.; Echeverria, F.; García, C.M. Dealing with size-spectra: Some conceptual and mathematical problems. Sci. Mar. 1994, 58, 17–29. [Google Scholar]
- Brown, J.H.; Gillooly, J.F.; Allen, A.P.; Savage, V.M.; West, G.B. Toward a metabolic theory of ecology. Ecology 2004, 85, 1771–1789. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, 2022. Available online: https://www.R-project.org/.
- Irwin, A.J.; Finkel, Z.V.; Schofield, O.M.; Falkowski, P.G. Scaling-up from nutrient physiology to the size-structure of phytoplankton communities. J. Plankton Res. 2006, 28, 459–471. [Google Scholar] [CrossRef]
- Cermeno, P.; de Vargas, C.; Abrantes, F.; Falkowski, P.G. Phytoplankton biogeography and community stability in the ocean. PLoS ONE 2010, 5, e10037. [Google Scholar] [CrossRef] [PubMed]
- Ignatiades, L.; Gotsis-Skretas, O.; Pagou, K.; Krasakopoulou, E. Diversification of phytoplankton community structure and related parameters along a large-scale longitudinal east–west transect of the Mediterranean Sea. J. Plankton Res. 2009, 31, 411–428. [Google Scholar] [CrossRef]
- Gaston, K.J. Rarity; Chapman & Hall: London, Uk, 1994. [Google Scholar]
- Rabinowitz, D.; Cairns, S.; Dillon, T. Seven forms of rarity and their frequency in the flora of the British Isles. In Conservation Biology: The Science of Scarcity and Diversity; Soule, M.E., Ed.; Sinauer Associates: Sunderland, 1986. [Google Scholar]
- Reed, J.M. A system for ranking conservation priorities for neotropical migrant birds based on relative susceptibility to extinction. In Ecology and Conservation of Neotropical Migrant Landbirds; Hangan, J.M., Johnston, D.W., Eds.; Smithsonian Institution Press: Washington, USA, 1992. [Google Scholar]
- Kunin, W.E.; Gaston, K.J. The Biology of Rarity: Causes and Consequences of Rare—Common Differences; Springer Science & Business Media, 2012. [Google Scholar]
- Ricotta, C.; Godefroid, S.; Celesti-Grapow, L. Common species have lower taxonomic diversity evidence from the urban floras of Brussels and Rome. Divers. Distrib. 2008, 14, 530–537. [Google Scholar] [CrossRef]
- Padisák, J.; Hajnal, É.; Krienitz, L.; Lakner, J.; Üveges, V. Rarity, ecological memory, rate of floral change in phytoplankton—And the mystery of the Red Cock. Hydrobiologia 2010, 653, 45–64. [Google Scholar] [CrossRef]
- Cao, Y.; Williams, D.D.; Williams, N.E. How important are rare species in aquatic community ecology and bioassessment? Limnol. Oceanogr. 1998, 43, 1403–1409. [Google Scholar] [CrossRef]
- White, E.P.; Ernest, S.K.M.; Kerkhoff, A.J.; Enquist, B.J. Relationships between body size and abundance in ecology. Trends Ecol. Evol. 2007, 22, 323–330. [Google Scholar] [CrossRef]
- Gjoni, V.; Glazier, D.S.; Ibelings, B.W.; Thomas, M.K. Temperature and resources interact to shape phytoplankton size-abundance relationships at a continental scale. bioRxiv 2022, 2022–04. [Google Scholar]
- Sabetta, L.; Fiocca, A.; Margheriti, L.; Vignes, F.; Basset, A.; Mangoni, O.; Carrada, G.C.; Ruggieri, N.; Ianni, C. Body size–abundance distributions of nano-and micro-phytoplankton guilds in coastal marine ecosystems. Estuar. Coast. Shelf Sci. 2005, 63, 645–663. [Google Scholar] [CrossRef]
- Fox, B.J.; Brown, J.H. Assembly rules for functional groups in North American desert rodent communities. Oikos 1993, 358–370. [Google Scholar] [CrossRef]
- Fox, B.J.; Kirkland Jr, G.L. An assembly rule for functional groups applied to North American soricid communities. J. Mammal. 1992, 73, 491–503. [Google Scholar] [CrossRef]
- Morris, D.W.; Knight, T.W. Can consumer-resource dynamics explain patterns of guild assembly? The Am. Nat. 1996, 147, 558–575. [Google Scholar] [CrossRef]
- Zhu, W.; Wan, L.; Zhao, L. Effect of nutrient level on phytoplankton community structure in different water bodies. J. Environ. Sci. 2010, 22, 32–39. [Google Scholar] [CrossRef]
- Litchman, E. Resource competition and the ecological success of phytoplankton. In Evolution of Primary Producers in the Sea; Falkowski, P.G., Knoll, A.H., Eds.; Academic Press: Burlington, 2007. [Google Scholar]
- Kruk, C.; Huszar, V.L.; Peeters, E.T.; Bonilla, S.; Costa, L.; Lürling, M.; Reynolds, C.S.; Scheffer, M. A morphological classification capturing functional variation in phytoplankton. Freshw. Biol. 2010, 55, 614–627. [Google Scholar] [CrossRef]
- Weithoff, G. The concepts of plant functional types and functional diversity in lake phytoplankton: A new understanding of phytoplankton ecology. Freshw. Biol. 2003, 48, 1669–1675. [Google Scholar] [CrossRef]
- Bergkemper, V.; Stadler, P.; Weisse, T. Moderate weather extremes alter phytoplankton diversity-a microcosm study. Freshw. Biol. 2018, 63, 1211–1224. [Google Scholar] [CrossRef]
- Santos, J.B.O.; Brasil, J.; Huszar, V.L.M. Responses of functional and taxonomic phytoplankton diversity to environmental gradients in subtropical and tropical reservoirs. Front. Environ. Sci. 2022, 10. [Google Scholar] [CrossRef]
- Santos, A.M.C.; Carneiro, F.M.; Cianciaruso, M.V. Predicting productivity in tropical reservoirs: The roles of phytoplankton taxonomic and functional diversity. Ecol. Indic. 2015, 48, 428–435. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, D.; Chen, L.; Giesy, J.P.; Zhang, W.; Yuan, C.; Ni, L.; Shen, H.; Xie, P. Light, but not nutrients, drives seasonal congruence of taxonomic and functional diversity of phytoplankton in a eutrophic highland lake in China. Front. Plant Sci. 2020, 11, 179. [Google Scholar] [CrossRef]
- Graco-Roza, C.; Soininen, J.; Correa, G.; Pacheco, F.S.; Miranda, M.; Domingos, P.; Marinho, M.M. Functional rather than taxonomic diversity reveals changes in the phytoplankton community of a large dammed river. Ecol. Indic. 2021, 121, 107048. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).