Submitted:
31 December 2023
Posted:
02 January 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Exosomes are small, specialized extravesicular vesicles (EVs)
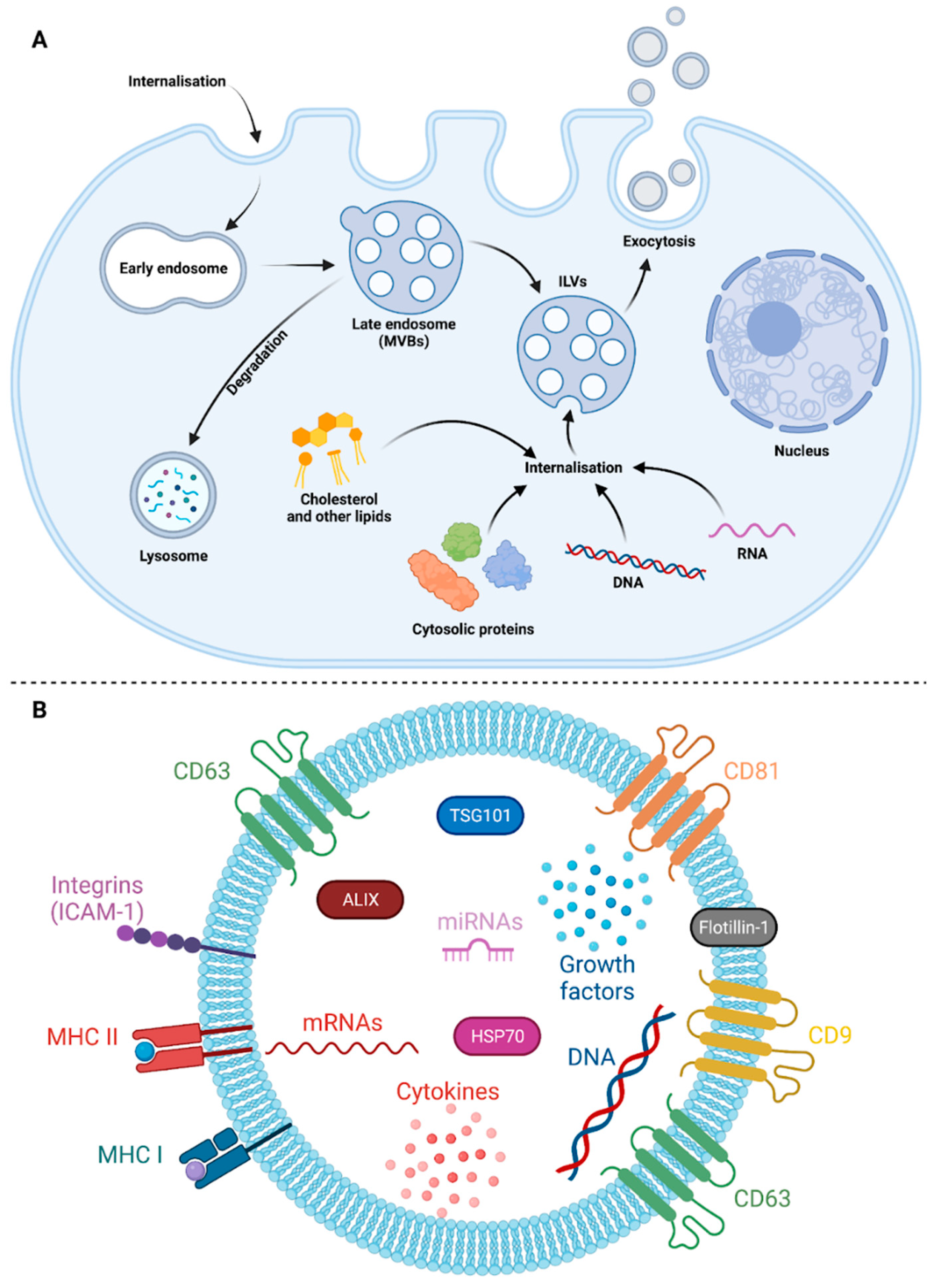
2.1. Exosomes biogenesis
2.2. Exosomes composition
2.3. Exosomes biological activities according to their mode of interaction with recipient cell
3. Exosomes as biomarkers of pathologies in human medicine
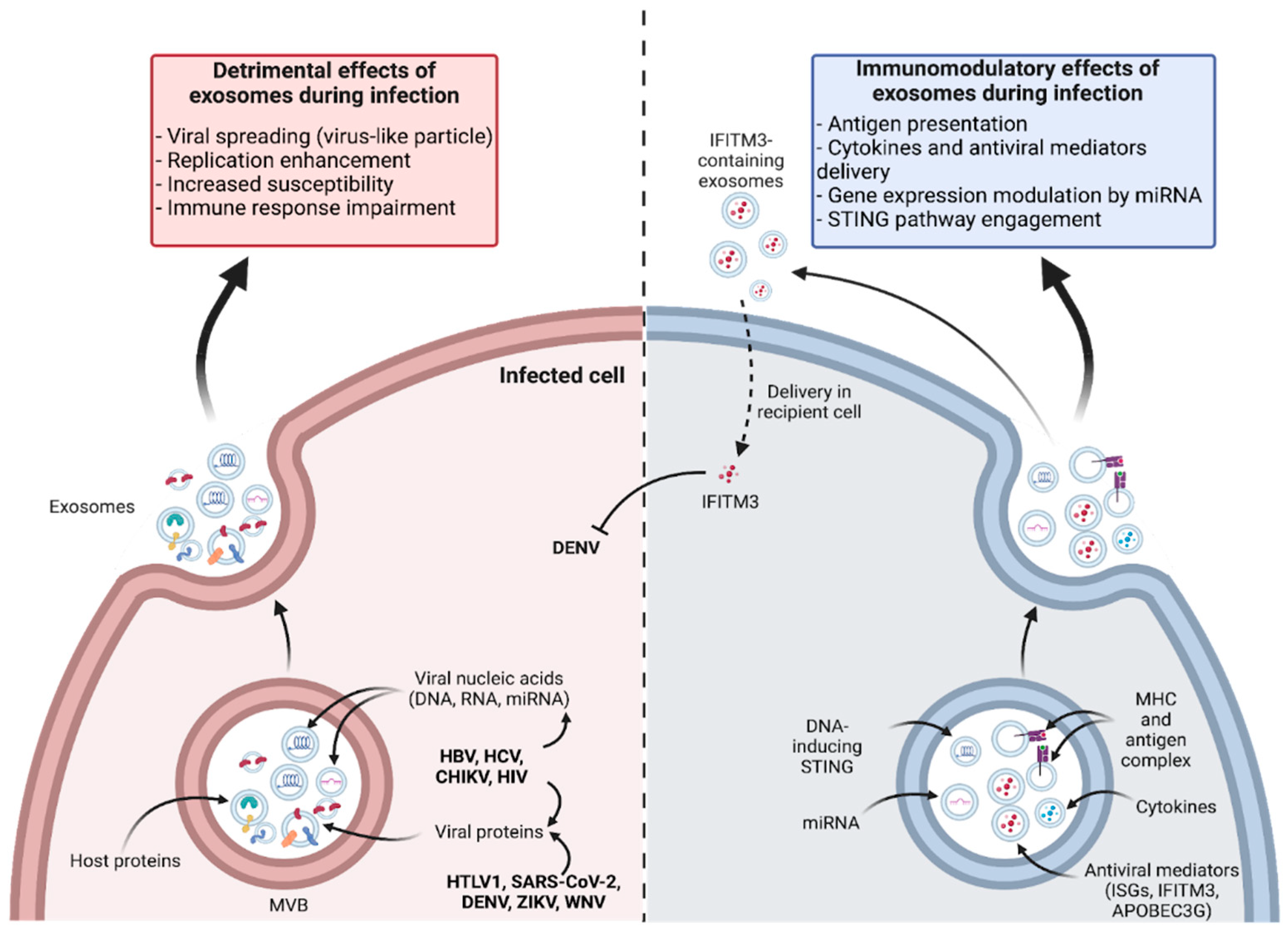
4. Exosomes and biology of infection
4.1. Exosomes are small, specialized extravesicular vesicles (EVs)
4.2. Exosomes contribute to infection resolution
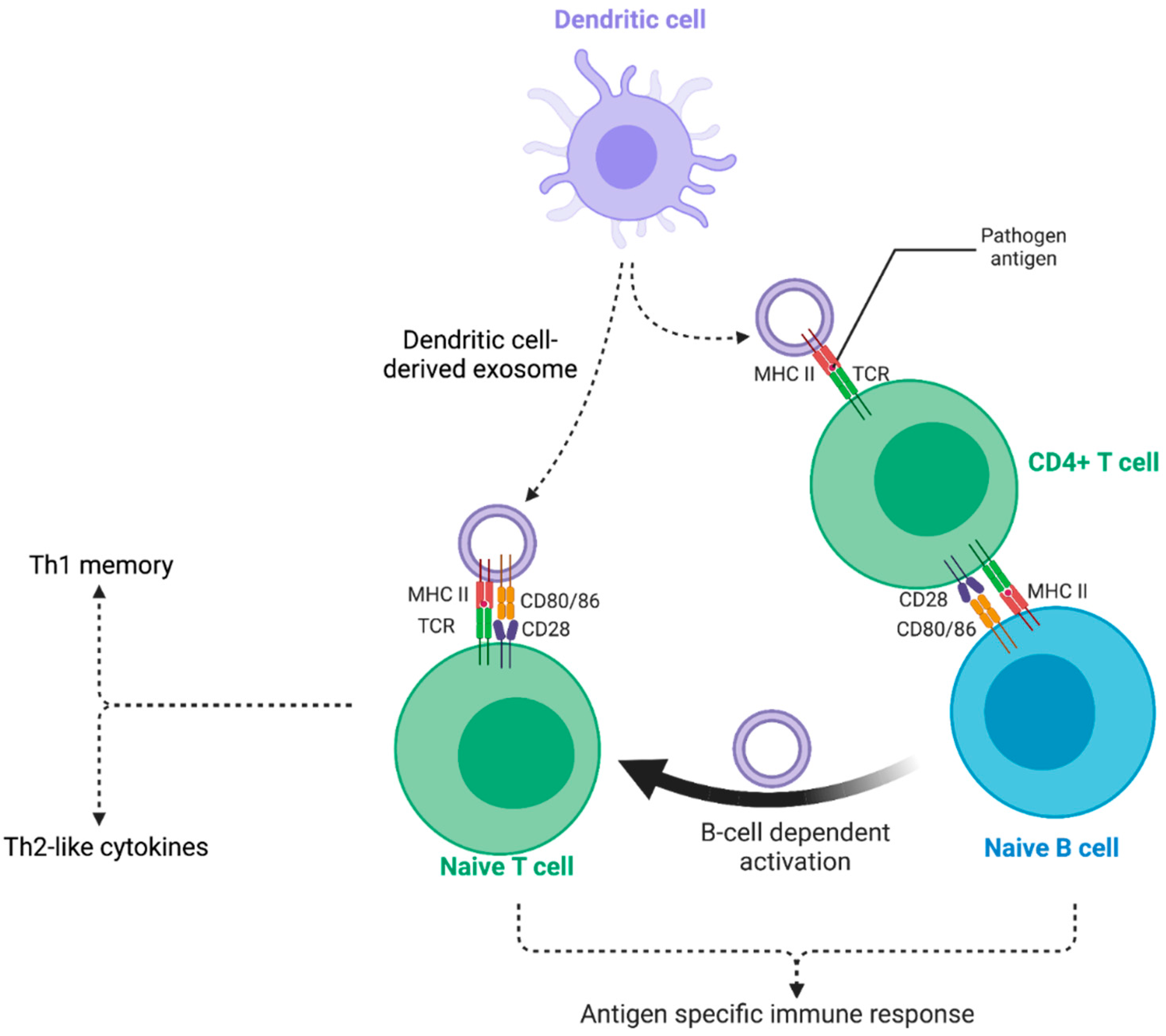
5. Exosomes and antigen presentation, perspectives in vaccinology
5.1. Exosomes and antigen presentation
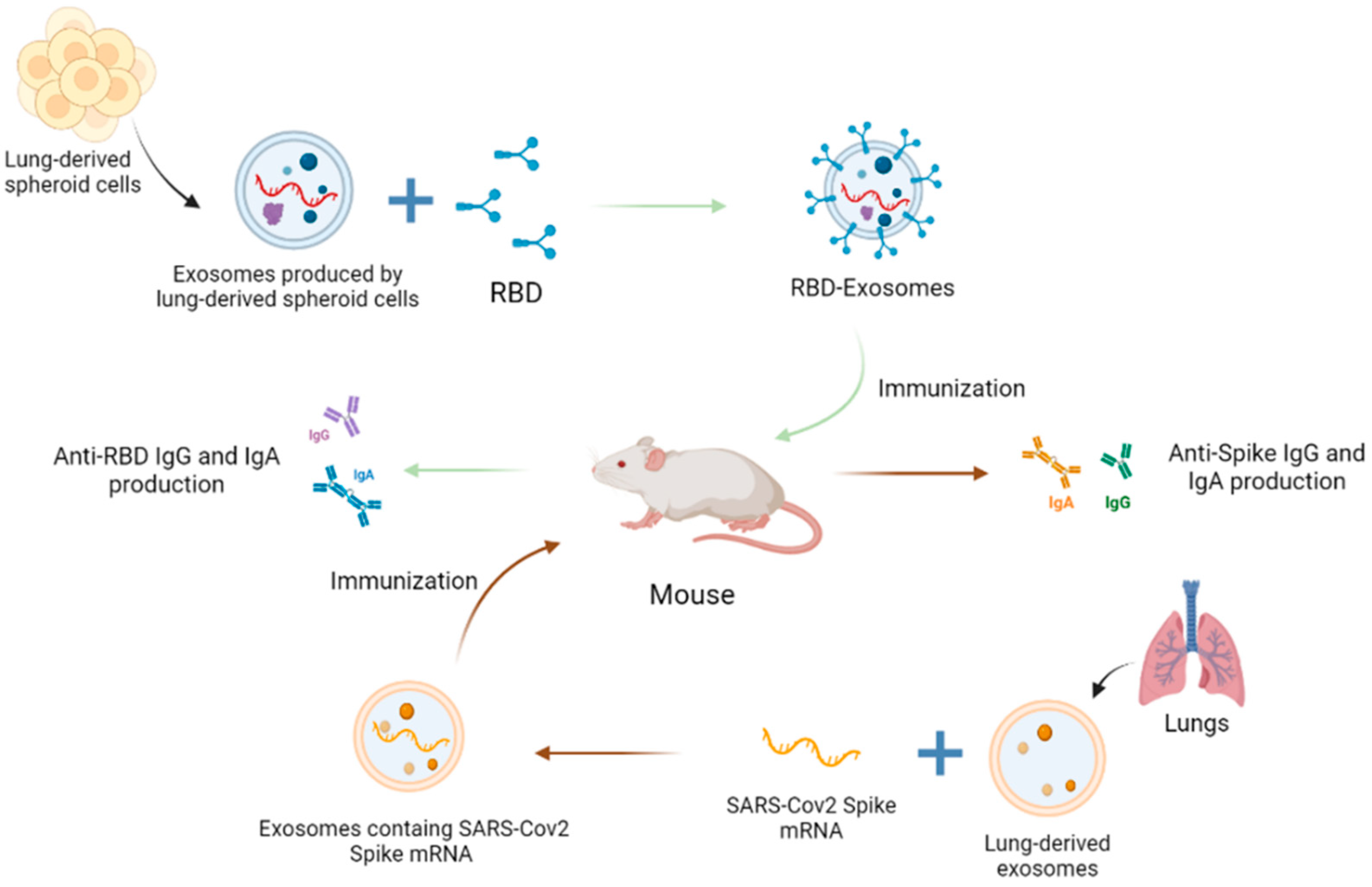
5.2. Exosomes in vaccinology
6. Challenges and issues
7. Concluding remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ALIX | ALG-2 Interacting protein X |
| APCs | Antigen-Presenting Cells |
| APOBEC3G | Apolipoprotein B mRNA editing enzyme catalytic polypeptide-like 3G |
| BMDC | Bone marrow derived dendritic cells |
| CCL2 | C-C motif chemokine ligand 2 |
| cGAS-STING | cyclic GMP-AMP synthase stimulator of interferon genes |
| CHIKV | Chikungunya virus |
| CHMP4 | Charged multivesicular body protein 4a |
| circRNAs | circular RNAs |
| CMV | Cytomegalovirus |
| CTL | Cytotoxic Lymphocyte T |
| DC | Dendritic cell |
| DENV | Dengue virus |
| DUBs | de-ubiquitylating enzymes |
| EBV | Epstein-Barr virus |
| EMT | Epithelial-mesenchymal transition |
| ESCRT | Endosomal Sorting Complex Required for Transport |
| EV | Extracellular vesicles |
| HBsAg | Hepatitis B antigen |
| HBV | Hepatitis B virus |
| HCV | Hepatitis C virus |
| HGF | hepatocyte growth factor |
| HIV | Human Immunodeficiency virus |
| HLA-DR | Human Leukocyte Antigen DR isotype |
| HNRNPs | Heterogeneous Nuclear Ribonucleoproteins |
| HNSCC | Head and Neck Squamous Cell Carcinoma |
| HSP70 | Heat Shock Protein 70 |
| ICAM-1 | Intercellular Adhesion Molecule 1 |
| IC50 | half maximal Inhibitory Concentration |
| IFITM3 | Interferon-induced transmembrane protein 3 |
| IFNα | Interferon alpha |
| IFNγ | Interferon gamma |
| IgG | immunoglobulin G |
| IL-1β | Interleukin-1β |
| ILVs | Intraluminal vesicles |
| IP-10 | Interferon gamma-induced protein |
| ISGs | Interferon-stimulated genes |
| JEV | Japanese Encephalitis virus |
| L1 | non-LTR retrotransposons LINE-1 |
| LPS | Lipopolysaccharides |
| MCP-1 | Monocyte chemoattractant protein-1 |
| MEVs | Multi-epitopic vaccines |
| MHC | Major Histocompatibility Complex |
| mo-DCs | monocyte-derived dendritic cells |
| MVBs | Multivesicular bodies |
| NS1 | Non-Structural protein-1 |
| PRRs | Pattern Recognition Receptors |
| RANTES | Regulated upon activation normal T cell expressed and secreted |
| RBD | Receptor-binding domain |
| RLRs | RIG-I like receptors |
| RSV | Respiratory syncytial virus |
| SIgA | Secretory IgA |
| SNARE | Soluble NSF attachment protein receptor |
| SARS-Cov2 | Severe Acute Respiratory Syndrome Coronavirus 2 |
| STING | Stimulator of interferon genes |
| TAR | Trans-activation response element |
| TBEV | Tick-borne encephalitis virus |
| TGFβ | Tumor Growth Factor β |
| TIM4 | T-cell immunoglobulin- and mucin-domain-containing molecule 4 |
| TNFα | Tumor Necrosis Factor α |
| TSG101 | Tumor susceptibility gene 101 |
| VLP | Virus-like particles |
| VSV-G | vesicular stomatitis virus glycoprotein |
| WNV | West Nile virus |
| ZIKV | Zika virus |
References
- C. Théry, L. Zitvogel, et S. Amigorena, « Exosomes: composition, biogenesis and function », Nat. Rev. Immunol., vol. 2, no 8, p. 569-579, août 2002. [CrossRef]
- M. F. S. Lindenbergh et W. Stoorvogel, « Antigen Presentation by Extracellular Vesicles from Professional Antigen-Presenting Cells », Annu. Rev. Immunol., vol. 36, p. 435-459, avr. 2018. [CrossRef]
- A. A. Danilushkina, C. C. Emene, N. A. Barlev, et M. O. Gomzikova, « Strategies for Engineering of Extracellular Vesicles », Int. J. Mol. Sci., vol. 24, no 17, p. 13247, août 2023. [CrossRef]
- A. Hendrix et al., « Extracellular vesicle analysis », Nat. Rev. Methods Primer, vol. 3, no 1, p. 56, juill. 2023. [CrossRef]
- G. van Niel, G. D’Angelo, et G. Raposo, « Shedding light on the cell biology of extracellular vesicles », Nat. Rev. Mol. Cell Biol., vol. 19, no 4, p. 213-228, avr. 2018. [CrossRef]
- L. M. Doyle et M. Z. Wang, « Overview of Extracellular Vesicles, Their Origin, Composition, Purpose, and Methods for Exosome Isolation and Analysis », Cells, vol. 8, no 7, p. 727, juill. 2019. [CrossRef]
- R. Kalluri et V. S. LeBleu, « The biology, function, and biomedical applications of exosomes », Science, vol. 367, no 6478, p. eaau6977, févr. 2020. [CrossRef]
- B. Cappe, P. Vandenabeele, et F. B. Riquet, « A guide to the expanding field of extracellular vesicles and their release in regulated cell death programs », FEBS J., oct. 2023. [CrossRef]
- B. L. Deatherage et B. T. Cookson, « Membrane vesicle release in bacteria, eukaryotes, and archaea: a conserved yet underappreciated aspect of microbial life », Infect. Immun., vol. 80, no 6, p. 1948-1957, juin 2012. [CrossRef]
- C. de la Torre Gomez, R. V. Goreham, J. J. Bech Serra, T. Nann, et M. Kussmann, « “Exosomics”-A Review of Biophysics, Biology and Biochemistry of Exosomes With a Focus on Human Breast Milk », Front. Genet., vol. 9, p. 92, 2018. [CrossRef]
- A. Yokoi et T. Ochiya, « Exosomes and extracellular vesicles: Rethinking the essential values in cancer biology », Semin. Cancer Biol., vol. 74, p. 79-91, sept. 2021. [CrossRef]
- M. Izadpanah, A. Seddigh, S. Ebrahimi Barough, S. A. S. Fazeli, et J. Ai, « Potential of Extracellular Vesicles in Neurodegenerative Diseases: Diagnostic and Therapeutic Indications », J. Mol. Neurosci. MN, vol. 66, no 2, p. 172-179, oct. 2018. [CrossRef]
- D. Petroni, C. Fabbri, S. Babboni, L. Menichetti, G. Basta, et S. Del Turco, « Extracellular Vesicles and Intercellular Communication: Challenges for In Vivo Molecular Imaging and Tracking », Pharmaceutics, vol. 15, no 6, p. 1639, juin 2023. [CrossRef]
- C. Théry, « Exosomes: secreted vesicles and intercellular communications », F1000 Biol. Rep., vol. 3, juill. 2011. [CrossRef]
- J. Meldolesi, « Unconventional Protein Secretion Dependent on Two Extracellular Vesicles: Exosomes and Ectosomes », Front. Cell Dev. Biol., vol. 10, p. 877344, juin 2022. [CrossRef]
- G. van Niel, D. R. F. Carter, A. Clayton, D. W. Lambert, G. Raposo, et P. Vader, « Challenges and directions in studying cell–cell communication by extracellular vesicles », Nat. Rev. Mol. Cell Biol., vol. 23, no 5, p. 369-382, mai 2022. [CrossRef]
- T. Mori, L. Giovannelli, A. R. Bilia, et F. Margheri, « Exosomes: Potential Next-Generation Nanocarriers for the Therapy of Inflammatory Diseases », Pharmaceutics, vol. 15, no 9, p. 2276, sept. 2023. [CrossRef]
- S. Li, Z. Lin, X. Jiang, et X. Yu, « Exosomal cargo-loading and synthetic exosome-mimics as potential therapeutic tools », Acta Pharmacol. Sin., vol. 39, no 4, p. 542-551, avr. 2018. [CrossRef]
- S. V. Krylova et D. Feng, « The Machinery of Exosomes: Biogenesis, Release, and Uptake », Int. J. Mol. Sci., vol. 24, no 2, p. 1337, janv. 2023. [CrossRef]
- J. Gruenberg, « Life in the lumen: The multivesicular endosome », Traffic, vol. 21, no 1, p. 76-93, janv. 2020. [CrossRef]
- N. Naslavsky et S. Caplan, « The enigmatic endosome – sorting the ins and outs of endocytic trafficking », J. Cell Sci., vol. 131, no 13, p. jcs216499, juill. 2018. [CrossRef]
- A. Norris et B. D. Grant, « Endosomal microdomains: Formation and function », Curr. Opin. Cell Biol., vol. 65, p. 86-95, août 2020. [CrossRef]
- J. A. Solinger et A. Spang, « Sorting of cargo in the tubular endosomal network », BioEssays, vol. 44, no 12, p. 2200158, déc. 2022. [CrossRef]
- S. Sigismund, L. Lanzetti, G. Scita, et P. P. Di Fiore, « Endocytosis in the context-dependent regulation of individual and collective cell properties », Nat. Rev. Mol. Cell Biol., vol. 22, no 9, p. 625-643, sept. 2021. [CrossRef]
- A.-K. Pfitzner et al., « Vps60 initiates alternative ESCRT-III filaments », J. Cell Biol., vol. 222, no 11, p. e202206028, nov. 2023. [CrossRef]
- M. Colombo, G. Raposo, et C. Théry, « Biogenesis, Secretion, and Intercellular Interactions of Exosomes and Other Extracellular Vesicles », Annu. Rev. Cell Dev. Biol., vol. 30, no 1, p. 255-289, oct. 2014. [CrossRef]
- Y. Kuchitsu et T. Taguchi, « Lysosomal microautophagy: an emerging dimension in mammalian autophagy », Trends Cell Biol., p. S0962892423002386, déc. 2023. [CrossRef]
- J. Huotari et A. Helenius, « Endosome maturation: Endosome maturation », EMBO J., vol. 30, no 17, p. 3481-3500, août 2011. [CrossRef]
- J. G. Carlton et B. Baum, « Roles of ESCRT-III polymers in cell division across the tree of life », Curr. Opin. Cell Biol., vol. 85, p. 102274, déc. 2023. [CrossRef]
- J. Schöneberg, I.-H. Lee, J. H. Iwasa, et J. H. Hurley, « Reverse-topology membrane scission by the ESCRT proteins », Nat. Rev. Mol. Cell Biol., vol. 18, no 1, p. 5-17, janv. 2017. [CrossRef]
- Y. Rivera-Cuevas et V. B. Carruthers, « The multifaceted interactions between pathogens and host ESCRT machinery », PLOS Pathog., vol. 19, no 5, p. e1011344, mai 2023. [CrossRef]
- S. L. N. Maas, X. O. Breakefield, et A. M. Weaver, « Extracellular Vesicles: Unique Intercellular Delivery Vehicles », Trends Cell Biol., vol. 27, no 3, p. 172-188, mars 2017. [CrossRef]
- M. A. Karim, D. R. Samyn, S. Mattie, et C. L. Brett, « Distinct features of multivesicular body-lysosome fusion revealed by a new cell-free content-mixing assay », Traffic Cph. Den., vol. 19, no 2, p. 138-149, févr. 2018. [CrossRef]
- N. P. Hessvik et A. Llorente, « Current knowledge on exosome biogenesis and release », Cell. Mol. Life Sci., vol. 75, no 2, p. 193-208, janv. 2018. [CrossRef]
- M. Mathieu, L. Martin-Jaular, G. Lavieu, et C. Théry, « Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication », Nat. Cell Biol., vol. 21, no 1, p. 9-17, janv. 2019. [CrossRef]
- F. Ghorbaninezhad et al., « Dendritic cell-derived exosomes: A new horizon in personalized cancer immunotherapy? », Cancer Lett., vol. 562, p. 216168, mai 2023. [CrossRef]
- X. Jin, T. Xia, S. Luo, Y. Zhang, Y. Xia, et H. Yin, « Exosomal lipid PI4P regulates small extracellular vesicle secretion by modulating intraluminal vesicle formation », J. Extracell. Vesicles, vol. 12, no 4, p. 12319, avr. 2023. [CrossRef]
- S. M. Crivelli et al., « Function of ceramide transfer protein for biogenesis and sphingolipid composition of extracellular vesicles », J. Extracell. Vesicles, vol. 11, no 6, p. e12233, juin 2022. [CrossRef]
- D. Wei et al., « RAB31 marks and controls an ESCRT-independent exosome pathway », Cell Res., vol. 31, no 2, p. 157-177, févr. 2021. [CrossRef]
- R. Horbay, A. Hamraghani, L. Ermini, S. Holcik, S. T. Beug, et B. Yeganeh, « Role of Ceramides and Lysosomes in Extracellular Vesicle Biogenesis, Cargo Sorting and Release », Int. J. Mol. Sci., vol. 23, no 23, p. 15317, déc. 2022. [CrossRef]
- B. Tancini et al., « Lysosomal Exocytosis: The Extracellular Role of an Intracellular Organelle », Membranes, vol. 10, no 12, p. 406, déc. 2020. [CrossRef]
- A. C. Dixson, T. R. Dawson, D. Di Vizio, et A. M. Weaver, « Context-specific regulation of extracellular vesicle biogenesis and cargo selection », Nat. Rev. Mol. Cell Biol., vol. 24, no 7, p. 454-476, juill. 2023. [CrossRef]
- D. Perez-Hernandez et al., « The Intracellular Interactome of Tetraspanin-enriched Microdomains Reveals Their Function as Sorting Machineries toward Exosomes », J. Biol. Chem., vol. 288, no 17, p. 11649-11661, avr. 2013. [CrossRef]
- T. Zheng, S. Xu, et J. Xu, « A Review of the Roles of Specialized Extracellular Vesicles, Migrasomes, and Exosomes in Normal Cell Physiology and Disease », Med. Sci. Monit., vol. 29, avr. 2023. [CrossRef]
- J.-M. Escola, M. J. Kleijmeer, W. Stoorvogel, J. M. Griffith, O. Yoshie, et H. J. Geuze, « Selective Enrichment of Tetraspan Proteins on the Internal Vesicles of Multivesicular Endosomes and on Exosomes Secreted by Human B-lymphocytes », J. Biol. Chem., vol. 273, no 32, p. 20121-20127, août 1998. [CrossRef]
- H. Wei et al., « Regulation of exosome production and cargo sorting », Int. J. Biol. Sci., vol. 17, no 1, p. 163-177, 2021. [CrossRef]
- W. Suwakulsiri et al., « Comparative proteomic analysis of three major extracellular vesicle classes secreted from human primary and metastatic colorectal cancer cells: Exosomes, microparticles, and shed midbody remnants », PROTEOMICS, p. 2300057, juill. 2023. [CrossRef]
- M. Mathieu et al., « Specificities of exosome versus small ectosome secretion revealed by live intracellular tracking of CD63 and CD9 », Nat. Commun., vol. 12, no 1, p. 4389, juill. 2021. [CrossRef]
- J. Kowal et al., « Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes », Proc. Natl. Acad. Sci., vol. 113, no 8, févr. 2016. [CrossRef]
- S. Gurung, D. Perocheau, L. Touramanidou, et J. Baruteau, « The exosome journey: from biogenesis to uptake and intracellular signalling », Cell Commun. Signal., vol. 19, no 1, p. 47, avr. 2021. [CrossRef]
- R. J. Simpson, S. S. Jensen, et J. W. E. Lim, « Proteomic profiling of exosomes: Current perspectives », PROTEOMICS, vol. 8, no 19, p. 4083-4099, oct. 2008. [CrossRef]
- M. P. Zaborowski, L. Balaj, X. O. Breakefield, et C. P. Lai, « Extracellular Vesicles: Composition, Biological Relevance, and Methods of Study », BioScience, vol. 65, no 8, p. 783-797, août 2015. [CrossRef]
- D. K. Jeppesen et al., « Reassessment of Exosome Composition », Cell, vol. 177, no 2, p. 428-445.e18, avr. 2019. [CrossRef]
- F. G. Kugeratski et al., « Quantitative proteomics identifies the core proteome of exosomes with syntenin-1 as the highest abundant protein and a putative universal biomarker », Nat. Cell Biol., vol. 23, no 6, p. 631-641, juin 2021. [CrossRef]
- Z. Andreu et M. Yáñez-MÃ3, « Tetraspanins in Extracellular Vesicle Formation and Function », Front. Immunol., vol. 5, sept. 2014. [CrossRef]
- I. Casari, J. A. Howard, E. E. Robless, et M. Falasca, « Exosomal integrins and their influence on pancreatic cancer progression and metastasis », Cancer Lett., vol. 507, p. 124-134, juin 2021. [CrossRef]
- A. Hoshino et al., « Tumour exosome integrins determine organotropic metastasis », Nature, vol. 527, no 7578, p. 329-335, nov. 2015. [CrossRef]
- E. Segura et al., « ICAM-1 on exosomes from mature dendritic cells is critical for efficient naive T-cell priming », Blood, vol. 106, no 1, p. 216-223, juill. 2005. [CrossRef]
- G. Raposo et al., « B lymphocytes secrete antigen-presenting vesicles. », J. Exp. Med., vol. 183, no 3, p. 1161-1172, mars 1996. [CrossRef]
- J. E. A. Braun, « Extracellular chaperone networks and the export of J-domain proteins », J. Biol. Chem., vol. 299, no 2, p. 102840, févr. 2023. [CrossRef]
- M. Linder et E. Pogge von Strandmann, « The Role of Extracellular HSP70 in the Function of Tumor-Associated Immune Cells », Cancers, vol. 13, no 18, p. 4721, sept. 2021. [CrossRef]
- Y. Qu, L. Franchi, G. Nunez, et G. R. Dubyak, « Nonclassical IL-1β Secretion Stimulated by P2X7 Receptors Is Dependent on Inflammasome Activation and Correlated with Exosome Release in Murine Macrophages », J. Immunol., vol. 179, no 3, p. 1913-1925, août 2007. [CrossRef]
- Y. Liao et al., « The Ras GTPASE -activating-like protein IQGAP1 bridges Gasdermin D to the ESCRTsystem to promote IL -1β release via exosomes », EMBO J., vol. 42, no 1, p. e110780, janv. 2023. [CrossRef]
- B. Weber et al., « Diagnostic and Prognostic Potential of Exosomal Cytokines IL-6 and IL-10 in Polytrauma Patients », Int. J. Mol. Sci., vol. 24, no 14, p. 11830, juill. 2023. [CrossRef]
- M. P. Bebelman, P. Bun, S. Huveneers, G. van Niel, D. M. Pegtel, et F. J. Verweij, « Real-time imaging of multivesicular body–plasma membrane fusion to quantify exosome release from single cells », Nat. Protoc., vol. 15, no 1, p. 102-121, janv. 2020. [CrossRef]
- T. Skotland, K. Sandvig, et A. Llorente, « Lipids in exosomes: Current knowledge and the way forward », Prog. Lipid Res., vol. 66, p. 30-41, avr. 2017. [CrossRef]
- R. C. Piper et D. J. Katzmann, « Biogenesis and Function of Multivesicular Bodies », Annu. Rev. Cell Dev. Biol., vol. 23, no 1, p. 519-547, nov. 2007. [CrossRef]
- K. O’Brien, K. Breyne, S. Ughetto, L. C. Laurent, et X. O. Breakefield, « RNA delivery by extracellular vesicles in mammalian cells and its applications », Nat. Rev. Mol. Cell Biol., vol. 21, no 10, p. 585-606, oct. 2020. [CrossRef]
- L. Geis-Asteggiante et al., « Differential Content of Proteins, mRNAs, and miRNAs Suggests that MDSC and Their Exosomes May Mediate Distinct Immune Suppressive Functions », J. Proteome Res., vol. 17, no 1, p. 486-498, janv. 2018. [CrossRef]
- C. Corrado, M. M. Barreca, C. Zichittella, R. Alessandro, et A. Conigliaro, « Molecular Mediators of RNA Loading into Extracellular Vesicles », Cells, vol. 10, no 12, p. 3355, nov. 2021. [CrossRef]
- M. Omrani et al., « Global trend in exosome isolation and application: an update concept in management of diseases », Mol. Cell. Biochem., mai 2023. [CrossRef]
- R. Heydari et al., « Exosomes as Rheumatoid Arthritis Diagnostic Biomarkers and Therapeutic Agents », Vaccines, vol. 11, no 3, p. 687, mars 2023. [CrossRef]
- F. Tavasolian et al., « Exosomes: Effectual players in rheumatoid arthritis », Autoimmun. Rev., vol. 19, no 6, p. 102511, juin 2020. [CrossRef]
- J. Silva et al., « Vesicle-related microRNAs in plasma of nonsmall cell lung cancer patients and correlation with survival », Eur. Respir. J., vol. 37, no 3, p. 617-623, mars 2011. [CrossRef]
- M. Yáñez-Mó et al., « Biological properties of extracellular vesicles and their physiological functions », J. Extracell. Vesicles, vol. 4, p. 27066, 2015. [CrossRef]
- Y. Takahashi et al., « Visualization and in vivo tracking of the exosomes of murine melanoma B16-BL6 cells in mice after intravenous injection », J. Biotechnol., vol. 165, no 2, p. 77-84, mai 2013. [CrossRef]
- H. M. Ramos-Zaldívar et al., « Extracellular vesicles through the blood–brain barrier: a review », Fluids Barriers CNS, vol. 19, no 1, p. 60, juill. 2022. [CrossRef]
- N. García-Romero et al., « DNA sequences within glioma-derived extracellular vesicles can cross the intact blood-brain barrier and be detected in peripheral blood of patients », Oncotarget, vol. 8, no 1, p. 1416-1428, janv. 2017. [CrossRef]
- M. Abdelsalam, M. Ahmed, Z. Osaid, R. Hamoudi, et R. Harati, « Insights into Exosome Transport through the Blood–Brain Barrier and the Potential Therapeutical Applications in Brain Diseases », Pharmaceuticals, vol. 16, no 4, p. 571, avr. 2023. [CrossRef]
- G. Andreola et al., « Induction of Lymphocyte Apoptosis by Tumor Cell Secretion of FasL-bearing Microvesicles », J. Exp. Med., vol. 195, no 10, p. 1303-1316, mai 2002. [CrossRef]
- M. Somiya et S. Kuroda, « Reporter gene assay for membrane fusion of extracellular vesicles », J. Extracell. Vesicles, vol. 10, no 13, p. e12171, nov. 2021. [CrossRef]
- D. Levy, M. A. Do, J. Zhang, A. Brown, et B. Lu, « Orchestrating Extracellular Vesicle With Dual Reporters for Imaging and Capturing in Mammalian Cell Culture », Front. Mol. Biosci., vol. 8, p. 680580, juin 2021. [CrossRef]
- L. A. Mulcahy, R. C. Pink, et D. R. F. Carter, « Routes and mechanisms of extracellular vesicle uptake », J. Extracell. Vesicles, vol. 3, no 1, p. 24641, janv. 2014. [CrossRef]
- L. Ginini, S. Billan, E. Fridman, et Z. Gil, « Insight into Extracellular Vesicle-Cell Communication: From Cell Recognition to Intracellular Fate », Cells, vol. 11, no 9, p. 1375, avr. 2022. [CrossRef]
- A. Gonda, J. Kabagwira, G. N. Senthil, et N. R. Wall, « Internalization of Exosomes through Receptor-Mediated Endocytosis », Mol. Cancer Res., vol. 17, no 2, p. 337-347, févr. 2019. [CrossRef]
- M. Miyanishi, K. Tada, M. Koike, Y. Uchiyama, T. Kitamura, et S. Nagata, « Identification of Tim4 as a phosphatidylserine receptor », Nature, vol. 450, no 7168, p. 435-439, nov. 2007. [CrossRef]
- B. Sims et al., « Role of TIM-4 in exosome-dependent entry of HIV-1 into human immune cells », Int. J. Nanomedicine, vol. Volume 12, p. 4823-4833, juill. 2017. [CrossRef]
- H. C. Christianson, K. J. Svensson, T. H. van Kuppevelt, J.-P. Li, et M. Belting, « Cancer cell exosomes depend on cell-surface heparan sulfate proteoglycans for their internalization and functional activity », Proc. Natl. Acad. Sci., vol. 110, no 43, p. 17380-17385, oct. 2013. [CrossRef]
- J. Ochieng et al., « Extracellular histones are the ligands for the uptake of exosomes and hydroxyapatite-nanoparticles by tumor cells via syndecan-4 », FEBS Lett., vol. 592, no 19, p. 3274-3285, oct. 2018. [CrossRef]
- E. Bonsergent, E. Grisard, J. Buchrieser, O. Schwartz, C. Théry, et G. Lavieu, « Quantitative characterization of extracellular vesicle uptake and content delivery within mammalian cells », Nat. Commun., vol. 12, no 1, p. 1864, mars 2021. [CrossRef]
- A. Zomer et al., « In Vivo Imaging Reveals Extracellular Vesicle-Mediated Phenocopying of Metastatic Behavior », Cell, vol. 161, no 5, p. 1046-1057, mai 2015. [CrossRef]
- B. Costa-Silva et al., « Pancreatic cancer exosomes initiate pre-metastatic niche formation in the liver », Nat. Cell Biol., vol. 17, no 6, p. 816-826, juin 2015. [CrossRef]
- H. Peinado et al., « Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET », Nat. Med., vol. 18, no 6, p. 883-891, juin 2012. [CrossRef]
- L. G. Lima et al., « Tumor microenvironmental cytokines bound to cancer exosomes determine uptake by cytokine receptor-expressing cells and biodistribution », Nat. Commun., vol. 12, no 1, p. 3543, juin 2021. [CrossRef]
- H. Valadi, K. Ekström, A. Bossios, M. Sjöstrand, J. J. Lee, et J. O. Lötvall, « Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells », Nat. Cell Biol., vol. 9, no 6, p. 654-659, juin 2007. [CrossRef]
- X. You et al., « Exosomal miR-663b exposed to TGF-β1 promotes cervical cancer metastasis and epithelial-mesenchymal transition by targeting MGAT3 », Oncol. Rep., vol. 45, no 4, p. 12, avr. 2021. [CrossRef]
- V. Vignard et al., « MicroRNAs in Tumor Exosomes Drive Immune Escape in Melanoma », Cancer Immunol. Res., vol. 8, no 2, p. 255-267, févr. 2020. [CrossRef]
- N. Milman, L. Ginini, et Z. Gil, « Exosomes and their role in tumorigenesis and anticancer drug resistance », Drug Resist. Updat., vol. 45, p. 1-12, juill. 2019. [CrossRef]
- G. K. Patel et al., « Exosomes confer chemoresistance to pancreatic cancer cells by promoting ROS detoxification and miR-155-mediated suppression of key gemcitabine-metabolising enzyme, DCK », Br. J. Cancer, vol. 116, no 5, p. 609-619, févr. 2017. [CrossRef]
- S. A. A. Kooijmans, R. M. Schiffelers, N. Zarovni, et R. Vago, « Modulation of tissue tropism and biological activity of exosomes and other extracellular vesicles: New nanotools for cancer treatment », Pharmacol. Res., vol. 111, p. 487-500, sept. 2016. [CrossRef]
- K. Qian, W. Fu, T. Li, J. Zhao, C. Lei, et S. Hu, « The roles of small extracellular vesicles in cancer and immune regulation and translational potential in cancer therapy », J. Exp. Clin. Cancer Res., vol. 41, no 1, p. 286, sept. 2022. [CrossRef]
- A. F. Teixeira, Y. Wang, J. Iaria, P. ten Dijke, et H.-J. Zhu, « Simultaneously targeting extracellular vesicle trafficking and TGF-β receptor kinase activity blocks signaling hyperactivation and metastasis », Signal Transduct. Target. Ther., vol. 8, no 1, p. 456, déc. 2023. [CrossRef]
- T. Briolay, T. Petithomme, M. Fouet, N. Nguyen-Pham, C. Blanquart, et N. Boisgerault, « Delivery of cancer therapies by synthetic and bio-inspired nanovectors », Mol. Cancer, vol. 20, no 1, p. 55, mars 2021. [CrossRef]
- A. Pritchard et al., « Lung Tumor Cell-Derived Exosomes Promote M2 Macrophage Polarization », Cells, vol. 9, no 5, p. 1303, mai 2020. [CrossRef]
- Z. Zhang et al., « Exosomes derived from human adipose mesenchymal stem cells ameliorate hepatic fibrosis by inhibiting PI3K/Akt/mTOR pathway and remodeling choline metabolism », J. Nanobiotechnology, vol. 21, no 1, p. 29, janv. 2023. [CrossRef]
- J. Jiang, J. Li, X. Zhou, X. Zhao, B. Huang, et Y. Qin, « Exosomes Regulate the Epithelial–Mesenchymal Transition in Cancer », Front. Oncol., vol. 12, p. 864980, mars 2022. [CrossRef]
- Y. Yao, R. Chen, G. Wang, Y. Zhang, et F. Liu, « Exosomes derived from mesenchymal stem cells reverse EMT via TGF-β1/Smad pathway and promote repair of damaged endometrium », Stem Cell Res. Ther., vol. 10, no 1, p. 225, déc. 2019. [CrossRef]
- Z. Lang et al., « Hepatocyte-derived exosomal miR-146a-5p inhibits hepatic stellate cell EMT process: a crosstalk between hepatocytes and hepatic stellate cells », Cell Death Discov., vol. 9, no 1, p. 304, août 2023. [CrossRef]
- J. Lin et al., « Exosomes: Novel Biomarkers for Clinical Diagnosis », Sci. World J., vol. 2015, p. 1-8, 2015. [CrossRef]
- S. Keller, J. Ridinger, A.-K. Rupp, J. W. Janssen, et P. Altevogt, « Body fluid derived exosomes as a novel template for clinical diagnostics », J. Transl. Med., vol. 9, no 1, p. 86, déc. 2011. [CrossRef]
- N. Ludwig, T. L. Whiteside, et T. E. Reichert, « Challenges in Exosome Isolation and Analysis in Health and Disease », Int. J. Mol. Sci., vol. 20, no 19, p. 4684, sept. 2019. [CrossRef]
- S. R. Douglas, K. T. Yeung, J. Yang, S. L. Blair, O. Cohen, et B. P. Eliceiri, « Identification of CD105+ Extracellular Vesicles as a Candidate Biomarker for Metastatic Breast Cancer », J. Surg. Res., vol. 268, p. 168-173, déc. 2021. [CrossRef]
- D. Osti et al., « Clinical Significance of Extracellular Vesicles in Plasma from Glioblastoma Patients », Clin. Cancer Res., vol. 25, no 1, p. 266-276, janv. 2019. [CrossRef]
- Q. Sabbagh et al., « The von Willebrand factor stamps plasmatic extracellular vesicles from glioblastoma patients », Sci. Rep., vol. 11, no 1, p. 22792, nov. 2021. [CrossRef]
- B. K. Thakur et al., « Double-stranded DNA in exosomes: a novel biomarker in cancer detection », Cell Res., vol. 24, no 6, p. 766-769, juin 2014. [CrossRef]
- F. Fu, W. Jiang, L. Zhou, et Z. Chen, « Circulating Exosomal miR-17-5p and miR-92a-3p Predict Pathologic Stage and Grade of Colorectal Cancer », Transl. Oncol., vol. 11, no 2, p. 221-232, avr. 2018. [CrossRef]
- J. C. Akers et al., « miR-21 in the Extracellular Vesicles (EVs) of Cerebrospinal Fluid (CSF): A Platform for Glioblastoma Biomarker Development », PLoS ONE, vol. 8, no 10, p. e78115, oct. 2013. [CrossRef]
- Y. Wang et al., « Exosomal circRNAs: biogenesis, effect and application in human diseases », Mol. Cancer, vol. 18, no 1, p. 116, déc. 2019. [CrossRef]
- J. Li et al., « Circular RNA IARS (circ-IARS) secreted by pancreatic cancer cells and located within exosomes regulates endothelial monolayer permeability to promote tumor metastasis », J. Exp. Clin. Cancer Res., vol. 37, no 1, p. 177, déc. 2018. [CrossRef]
- Z. Zhou et al., « Urinary exosomes: a promising biomarker of drug-induced nephrotoxicity », Front. Med., vol. 10, p. 1251839, sept. 2023. [CrossRef]
- C. He, S. Zheng, Y. Luo, et B. Wang, « Exosome Theranostics: Biology and Translational Medicine », Theranostics, vol. 8, no 1, p. 237-255, 2018. [CrossRef]
- A. Makler et W. Asghar, « Exosomal biomarkers for cancer diagnosis and patient monitoring », Expert Rev. Mol. Diagn., vol. 20, no 4, p. 387-400, avr. 2020. [CrossRef]
- R. Tutrone et al., « Clinical utility of the exosome based ExoDx Prostate(IntelliScore) EPI test in men presenting for initial Biopsy with a PSA 2–10 ng/mL », Prostate Cancer Prostatic Dis., vol. 23, no 4, p. 607-614, déc. 2020. [CrossRef]
- H. S. Chahar, T. Corsello, A. S. Kudlicki, N. Komaravelli, et A. Casola, « Respiratory Syncytial Virus Infection Changes Cargo Composition of Exosome Released from Airway Epithelial Cells », Sci. Rep., vol. 8, no 1, p. 387, janv. 2018. [CrossRef]
- Y. Peng, Y. Yang, Y. Li, T. Shi, Y. Luan, et C. Yin, « Exosome and virus infection », Front. Immunol., vol. 14, p. 1154217, mars 2023. [CrossRef]
- Z. Li et al., « Porcine Hemagglutinating Encephalomyelitis Virus Co-Opts Multivesicular-Derived Exosomes for Transmission », mBio, vol. 14, no 1, p. e03054-22, févr. 2023. [CrossRef]
- Y. Sato et al., « Epstein–Barr virus tegument protein BGLF2 in exosomes released from virus-producing cells facilitates de novo infection », Cell Commun. Signal., vol. 20, no 1, p. 95, déc. 2022. [CrossRef]
- C. Zhou et al., « Exosomes Carry microRNAs into Neighboring Cells to Promote Diffusive Infection of Newcastle Disease Virus », Viruses, vol. 11, no 6, p. 527, juin 2019. [CrossRef]
- S. Kopcho, M. McDew-White, W. Naushad, M. Mohan, et C. M. Okeoma, « SIV Infection Regulates Compartmentalization of Circulating Blood Plasma miRNAs within Extracellular Vesicles (EVs) and Extracellular Condensates (ECs) and Decreases EV-Associated miRNA-128 », Viruses, vol. 15, no 3, p. 622, févr. 2023. [CrossRef]
- D. E. Safadi et al., « Extracellular Vesicles Are Conveyors of the NS1 Toxin during Dengue Virus and Zika Virus Infection », Viruses, vol. 15, no 2, p. 364, janv. 2023. [CrossRef]
- Y. Ju, H. Bai, L. Ren, et L. Zhang, « The Role of Exosome and the ESCRT Pathway on Enveloped Virus Infection », Int. J. Mol. Sci., vol. 22, no 16, p. 9060, août 2021. [CrossRef]
- C. Martin, G. Ligat, et C. E. Malnou, « The Yin and the Yang of extracellular vesicles during viral infections », Biomed. J., p. 100659, sept. 2023. [CrossRef]
- C. Thepparit, S. Khongwichit, K. Ketsuwan, S. Libsittikul, P. Auewarakul, et D. R. Smith, « Dengue virus requires apoptosis linked gene-2-interacting protein X (ALIX) for viral propagation », Virus Res., vol. 261, p. 65-71, févr. 2019. [CrossRef]
- P.-T.-H. Tran, A. I. Chiramel, M. Johansson, et W. Melik, « Roles of ESCRT Proteins ALIX and CHMP4A and Their Interplay with Interferon-Stimulated Gene 15 during Tick-Borne Flavivirus Infection », J. Virol., vol. 96, no 3, p. e01624-21, févr. 2022. [CrossRef]
- L. N. Carpp, R. Galler, et M. C. Bonaldo, « Interaction between the yellow fever virus nonstructural protein NS3 and the host protein Alix contributes to the release of infectious particles », Microbes Infect., vol. 13, no 1, p. 85-95, janv. 2011. [CrossRef]
- Q. Wu et al., « Presence of Intact Hepatitis B Virions in Exosomes », Cell. Mol. Gastroenterol. Hepatol., vol. 15, no 1, p. 237-259, 2023. [CrossRef]
- N. Raab-Traub et D. P. Dittmer, « Viral effects on the content and function of extracellular vesicles », Nat. Rev. Microbiol., vol. 15, no 9, p. 559-572, sept. 2017. [CrossRef]
- F. Rey-Cadilhac, F. Rachenne, D. Missé, et J. Pompon, « Viral Components Trafficking with(in) Extracellular Vesicles », Viruses, vol. 15, no 12, p. 2333, nov. 2023. [CrossRef]
- S. J. Gould, A. M. Booth, et J. E. K. Hildreth, « The Trojan exosome hypothesis », Proc. Natl. Acad. Sci., vol. 100, no 19, p. 10592-10597, sept. 2003. [CrossRef]
- A. M. Nour, Y. Li, J. Wolenski, et Y. Modis, « Viral Membrane Fusion and Nucleocapsid Delivery into the Cytoplasm are Distinct Events in Some Flaviviruses », PLoS Pathog., vol. 9, no 9, p. e1003585, sept. 2013. [CrossRef]
- A. Longatti, B. Boyd, et F. V. Chisari, « Virion-Independent Transfer of Replication-Competent Hepatitis C Virus RNA between Permissive Cells », J. Virol., vol. 89, no 5, p. 2956-2961, mars 2015. [CrossRef]
- V. Ramakrishnaiah et al., « Exosome-mediated transmission of hepatitis C virus between human hepatoma Huh7.5 cells », Proc. Natl. Acad. Sci., vol. 110, no 32, p. 13109-13113, août 2013. [CrossRef]
- B. C. T. Le et al., « Characterization and Involvement of Exosomes Originating from Chikungunya Virus-Infected Epithelial Cells in the Transmission of Infectious Viral Elements », Int. J. Mol. Sci., vol. 23, no 20, p. 12117, oct. 2022. [CrossRef]
- D. Cortes-Galvez, J. A. Dangerfield, et C. Metzner, « Extracellular Vesicles and Their Membranes: Exosomes vs. Virus-Related Particles », Membranes, vol. 13, no 4, p. 397, mars 2023. [CrossRef]
- A. Narayanan et al., « Exosomes Derived from HIV-1-infected Cells Contain Trans-activation Response Element RNA », J. Biol. Chem., vol. 288, no 27, p. 20014-20033, juill. 2013. [CrossRef]
- L. Chen et al., « Exosomes derived from HIV-1-infected cells promote growth and progression of cancer via HIV TAR RNA », Nat. Commun., vol. 9, no 1, p. 4585, nov. 2018. [CrossRef]
- C. Fan et al., « The emerging role of Epstein-Barr virus encoded microRNAs in nasopharyngeal carcinoma », J. Cancer, vol. 9, no 16, p. 2852-2864, 2018. [CrossRef]
- S. Mukherjee, I. Akbar, B. Kumari, S. Vrati, A. Basu, et A. Banerjee, « Japanese Encephalitis Virus-induced let-7a/b interacted with the NOTCH - TLR 7 pathway in microglia and facilitated neuronal death via caspase activation », J. Neurochem., vol. 149, no 4, p. 518-534, mai 2019. [CrossRef]
- E. Barberis et al., « Circulating Exosomes Are Strongly Involved in SARS-CoV-2 Infection », Front. Mol. Biosci., vol. 8, p. 632290, févr. 2021. [CrossRef]
- Y. Fang, N. Wu, X. Gan, W. Yan, J. C. Morrell, et S. J. Gould, « Higher-Order Oligomerization Targets Plasma Membrane Proteins and HIV Gag to Exosomes », PLoS Biol., vol. 5, no 6, p. e158, juin 2007. [CrossRef]
- T. D. Campbell, M. Khan, M.-B. Huang, V. C. Bond, et M. D. Powell, « HIV-1 Nef protein is secreted into vesicles that can fuse with target cells and virions », Ethn. Dis., vol. 18, no 2 Suppl 2, p. S2-14-9, 2008.
- M. Lenassi et al., « HIV Nef is Secreted in Exosomes and Triggers Apoptosis in Bystander CD4 + T Cells », Traffic, vol. 11, no 1, p. 110-122, janv. 2010. [CrossRef]
- M. Aqil, A. R. Naqvi, A. S. Bano, et S. Jameel, « The HIV-1 Nef Protein Binds Argonaute-2 and Functions as a Viral Suppressor of RNA Interference », PLoS ONE, vol. 8, no 9, p. e74472, sept. 2013. [CrossRef]
- N. Plazolles, J.-M. Humbert, L. Vachot, B. Verrier, C. Hocke, et F. Halary, « Pivotal Advance: The promotion of soluble DC-SIGN release by inflammatory signals and its enhancement of cytomegalovirus-mediated cis -infection of myeloid dendritic cells », J. Leukoc. Biol., vol. 89, no 3, p. 329-342, oct. 2010. [CrossRef]
- R. Mishra, S. Lata, A. Ali, et A. C. Banerjea, « Dengue haemorrhagic fever: a job done via exosomes? », Emerg. Microbes Infect., vol. 8, no 1, p. 1626-1635, janv. 2019. [CrossRef]
- A. Clayton, C. L. Harris, J. Court, M. D. Mason, et B. P. Morgan, « Antigen-presenting cell exosomes are protected from complement-mediated lysis by expression of CD55 and CD59 », Eur. J. Immunol., vol. 33, no 2, p. 522-531, févr. 2003. [CrossRef]
- E. Karasu, S. U. Eisenhardt, J. Harant, et M. Huber-Lang, « Extracellular Vesicles: Packages Sent With Complement », Front. Immunol., vol. 9, p. 721, avr. 2018. [CrossRef]
- M. W. A. Hussain et al., « Exosomes for Regulation of Immune Responses and Immunotherapy », J. Nanotheranostics, vol. 3, no 1, p. 55-85, mars 2022. [CrossRef]
- R. Kulkarni et A. Prasad, « Exosomes Derived from HIV-1 Infected DCs Mediate Viral trans-Infection via Fibronectin and Galectin-3 », Sci. Rep., vol. 7, no 1, p. 14787, nov. 2017. [CrossRef]
- J. Klibi et al., « Blood diffusion and Th1-suppressive effects of galectin-9–containing exosomes released by Epstein-Barr virus–infected nasopharyngeal carcinoma cells », Blood, vol. 113, no 9, p. 1957-1966, févr. 2009. [CrossRef]
- C. Zhang et al., « Galectin-9 promotes a suppressive microenvironment in human cancer by enhancing STING degradation », Oncogenesis, vol. 9, no 7, p. 65, juill. 2020. [CrossRef]
- S. Temme, A. M. Eis-Hübinger, A. D. McLellan, et N. Koch, « The Herpes Simplex Virus-1 Encoded Glycoprotein B Diverts HLA-DR into the Exosome Pathway », J. Immunol., vol. 184, no 1, p. 236-243, janv. 2010. [CrossRef]
- H. Saari et al., « Extracellular vesicles provide a capsid-free vector for oncolytic adenoviral DNA delivery », J. Extracell. Vesicles, vol. 9, no 1, p. 1747206, sept. 2020. [CrossRef]
- Y. Kakiuchi et al., « Local oncolytic adenovirotherapy produces an abscopal effect via tumor-derived extracellular vesicles », Mol. Ther., vol. 29, no 10, p. 2920-2930, oct. 2021. [CrossRef]
- J. Dai et al., « Exosomes: key players in cancer and potential therapeutic strategy », Signal Transduct. Target. Ther., vol. 5, no 1, p. 145, août 2020. [CrossRef]
- W. Zhang, X. Jiang, J. Bao, Y. Wang, H. Liu, et L. Tang, « Exosomes in Pathogen Infections: A Bridge to Deliver Molecules and Link Functions », Front. Immunol., vol. 9, p. 90, févr. 2018. [CrossRef]
- Y. Kitai et al., « DNA-Containing Exosomes Derived from Cancer Cells Treated with Topotecan Activate a STING-Dependent Pathway and Reinforce Antitumor Immunity », J. Immunol., vol. 198, no 4, p. 1649-1659, févr. 2017. [CrossRef]
- A. Ablasser et S. Hur, « Regulation of cGAS- and RLR-mediated immunity to nucleic acids », Nat. Immunol., vol. 21, no 1, p. 17-29, janv. 2020. [CrossRef]
- A. Longatti, « The Dual Role of Exosomes in Hepatitis A and C Virus Transmission and Viral Immune Activation », Viruses, vol. 7, no 12, p. 6707-6715, déc. 2015. [CrossRef]
- B. J. Barnes et C. C. Somerville, « Modulating Cytokine Production via Select Packaging and Secretion From Extracellular Vesicles », Front. Immunol., vol. 11, p. 1040, mai 2020. [CrossRef]
- A. G. Souza et L. M. Colli, « Extracellular Vesicles and Interleukins: Novel Frontiers in Diagnostic and Therapeutic for Cancer », Front. Immunol., vol. 13, p. 836922, mars 2022. [CrossRef]
- J. Li et al., « Exosomes mediate the cell-to-cell transmission of IFN-α-induced antiviral activity », Nat. Immunol., vol. 14, no 8, p. 793-803, août 2013. [CrossRef]
- A. K. Khatua, H. E. Taylor, J. E. K. Hildreth, et W. Popik, « Exosomes Packaging APOBEC3G Confer Human Immunodeficiency Virus Resistance to Recipient Cells », J. Virol., vol. 83, no 2, p. 512-521, janv. 2009. [CrossRef]
- X. Zhu et al., « IFITM3-containing exosome as a novel mediator for anti-viral response in dengue virus infection: Antiviral effect of IFITM3 mediated by exosome », Cell. Microbiol., vol. 17, no 1, p. 105-118, janv. 2015. [CrossRef]
- A. A. Rana et al., « Poly(I:C) induces controlled release of IL-36γ from keratinocytes in the absence of cell death », Immunol. Res., vol. 63, no 1-3, p. 228-235, déc. 2015. [CrossRef]
- L. Sun, X. Wang, Y. Zhou, R.-H. Zhou, W.-Z. Ho, et J.-L. Li, « Exosomes contribute to the transmission of anti-HIV activity from TLR3-activated brain microvascular endothelial cells to macrophages », Antiviral Res., vol. 134, p. 167-171, oct. 2016. [CrossRef]
- M. L. Velandia-Romero et al., « Extracellular vesicles of U937 macrophage cell line infected with DENV-2 induce activation in endothelial cells EA.hy926 », PloS One, vol. 15, no 1, p. e0227030, 2020. [CrossRef]
- J. R. Edgar, P. T. Manna, S. Nishimura, G. Banting, et M. S. Robinson, « Tetherin is an exosomal tether », eLife, vol. 5, p. e17180, sept. 2016. [CrossRef]
- C. Villarroya-Beltri et al., « ISGylation controls exosome secretion by promoting lysosomal degradation of MVB proteins », Nat. Commun., vol. 7, no 1, p. 13588, nov. 2016. [CrossRef]
- E. Delorme-Axford et al., « Human placental trophoblasts confer viral resistance to recipient cells », Proc. Natl. Acad. Sci., vol. 110, no 29, p. 12048-12053, juill. 2013. [CrossRef]
- T. Maemura, S. Fukuyama, et Y. Kawaoka, « High Levels of miR-483-3p Are Present in Serum Exosomes Upon Infection of Mice With Highly Pathogenic Avian Influenza Virus », Front. Microbiol., vol. 11, p. 144, févr. 2020. [CrossRef]
- A. L. Hodge, A. A. Baxter, et I. K. H. Poon, « Gift bags from the sentinel cells of the immune system: The diverse role of dendritic cell-derived extracellular vesicles », J. Leukoc. Biol., vol. 111, no 4, p. 903-920, mars 2022. [CrossRef]
- E. Segura, S. Amigorena, et C. Théry, « Mature dendritic cells secrete exosomes with strong ability to induce antigen-specific effector immune responses », Blood Cells. Mol. Dis., vol. 35, no 2, p. 89-93, sept. 2005. [CrossRef]
- M. F. S. Lindenbergh et al., « Bystander T-Cells Support Clonal T-Cell Activation by Controlling the Release of Dendritic Cell-Derived Immune-Stimulatory Extracellular Vesicles », Front. Immunol., vol. 10, p. 448, mars 2019. [CrossRef]
- M. F. S. Lindenbergh, R. Wubbolts, E. G. F. Borg, E. M. Van ’T Veld, M. Boes, et W. Stoorvogel, « Dendritic cells release exosomes together with phagocytosed pathogen; potential implications for the role of exosomes in antigen presentation », J. Extracell. Vesicles, vol. 9, no 1, p. 1798606, sept. 2020. [CrossRef]
- A. Montecalvo et al., « Exosomes As a Short-Range Mechanism to Spread Alloantigen between Dendritic Cells during T Cell Allorecognition », J. Immunol., vol. 180, no 5, p. 3081-3090, mars 2008. [CrossRef]
- Y. Matsuzaka et R. Yashiro, « Regulation of Extracellular Vesicle-Mediated Immune Responses against Antigen-Specific Presentation », Vaccines, vol. 10, no 10, p. 1691, oct. 2022. [CrossRef]
- M. R. Anderson et al., « Viral antigens detectable in CSF exosomes from patients with retrovirus associated neurologic disease: functional role of exosomes », Clin. Transl. Med., vol. 7, no 1, p. e24, déc. 2018. [CrossRef]
- E. Pesce et al., « Exosomes Recovered From the Plasma of COVID-19 Patients Expose SARS-CoV-2 Spike-Derived Fragments and Contribute to the Adaptive Immune Response », Front. Immunol., vol. 12, p. 785941, janv. 2022. [CrossRef]
- J. M. Reyes-Ruiz et al., « The Regulation of Flavivirus Infection by Hijacking Exosome-Mediated Cell–Cell Communication: New Insights on Virus–Host Interactions », Viruses, vol. 12, no 7, p. 765, juill. 2020. [CrossRef]
- A. De Gassart et al., « Exosomal sorting of the cytoplasmic domain of bovine leukemia virus TM Env protein », Cell Biol. Int., vol. 33, no 1, p. 36-48, janv. 2009. [CrossRef]
- J. L. Hood, « Post isolation modification of exosomes for nanomedicine applications », Nanomed., vol. 11, no 13, p. 1745-1756, juill. 2016. [CrossRef]
- V. Sengupta, S. Sengupta, A. Lazo, P. Woods, A. Nolan, et N. Bremer, « Exosomes Derived from Bone Marrow Mesenchymal Stem Cells as Treatment for Severe COVID-19 », Stem Cells Dev., vol. 29, no 12, p. 747-754, juin 2020. [CrossRef]
- C. Yang, Y. Xue, Y. Duan, C. Mao, et M. Wan, « Exosomes and their engineering strategies, delivery systems, and biomedical applications », J. Control. Release Off. J. Control. Release Soc., p. S0168-3659(23)00782-4, déc. 2023. [CrossRef]
- P. Gangadaran et al., « The emerging role of exosomes in innate immunity, diagnosis and therapy », Front. Immunol., vol. 13, p. 1085057, janv. 2023. [CrossRef]
- J. Rädler, D. Gupta, A. Zickler, et S. E. Andaloussi, « Exploiting the biogenesis of extracellular vesicles for bioengineering and therapeutic cargo loading », Mol. Ther., vol. 31, no 5, p. 1231-1250, mai 2023. [CrossRef]
- D. Jafari et al., « Designer Exosomes: A New Platform for Biotechnology Therapeutics », BioDrugs, vol. 34, no 5, p. 567-586, oct. 2020. [CrossRef]
- M. N. Huda et M. Nurunnabi, « Potential Application of Exosomes in Vaccine Development and Delivery », Pharm. Res., vol. 39, no 11, p. 2635-2671, nov. 2022. [CrossRef]
- J. S. Schorey, Y. Cheng, P. P. Singh, et V. L. Smith, « Exosomes and other extracellular vesicles in host–pathogen interactions », EMBO Rep., vol. 16, no 1, p. 24-43, janv. 2015. [CrossRef]
- J. Davod, D. N. Fatemeh, H. Honari, et R. Hosseini, « Constructing and transient expression of a gene cassette containing edible vaccine elements and shigellosis, anthrax and cholera recombinant antigens in tomato », Mol. Biol. Rep., vol. 45, no 6, p. 2237-2246, déc. 2018. [CrossRef]
- H. Vallhov et al., « Exosomes Containing Glycoprotein 350 Released by EBV-Transformed B Cells Selectively Target B Cells through CD21 and Block EBV Infection In Vitro », J. Immunol., vol. 186, no 1, p. 73-82, janv. 2011. [CrossRef]
- S. Anticoli, E. Falcone, A. Ruggieri, et M. Federico, « Engineered exosomes boost the HCV NS3-specific CD8+ T lymphocyte immunity in humans », Trials Vaccinol., vol. 5, p. 105-110, 2016. [CrossRef]
- S. Anticoli et al., « An Exosome-Based Vaccine Platform Imparts Cytotoxic T Lymphocyte Immunity Against Viral Antigens », Biotechnol. J., vol. 13, no 4, p. 1700443, avr. 2018. [CrossRef]
- S. Jesus, E. Soares, M. T. Cruz, et O. Borges, « Exosomes as adjuvants for the recombinant hepatitis B antigen: First report », Eur. J. Pharm. Biopharm., vol. 133, p. 1-11, déc. 2018. [CrossRef]
- J. M. Pitt et al., « Dendritic Cell–Derived Exosomes as Immunotherapies in the Fight against Cancer », J. Immunol., vol. 193, no 3, p. 1006-1011, août 2014. [CrossRef]
- R. Wang, Y. Xie, T. Zhao, X. Tan, J. Xu, et J. Xiang, « HIV-1 Gag-specific exosome-targeted T cell-based vaccine stimulates effector CTL responses leading to therapeutic and long-term immunity against Gag/HLA-A2-expressing B16 melanoma in transgenic HLA-A2 mice », Trials Vaccinol., vol. 3, p. 19-25, 2014. [CrossRef]
- Y. Matsuzaka et R. Yashiro, « Extracellular Vesicle-Based SARS-CoV-2 Vaccine », Vaccines, vol. 11, no 3, p. 539, févr. 2023. [CrossRef]
- M. Cacciottolo et al., « Exosome-Based Multivalent Vaccine: Achieving Potent Immunization, Broadened Reactivity, and Strong T-Cell Responses with Nanograms of Proteins », Microbiol. Spectr., vol. 11, no 3, p. e00503-23, juin 2023. [CrossRef]
- S. J. Tsai et al., « Exosome-mediated mRNA delivery in vivo is safe and can be used to induce SARS-CoV-2 immunity », J. Biol. Chem., vol. 297, no 5, p. 101266, nov. 2021. [CrossRef]
- K. D. Popowski et al., « Inhalable dry powder mRNA vaccines based on extracellular vesicles », Matter, vol. 5, no 9, p. 2960-2974, sept. 2022. [CrossRef]
- Q. Zhang et al., « Intraduodenal Delivery of Exosome-Loaded SARS-CoV-2 RBD mRNA Induces a Neutralizing Antibody Response in Mice », Vaccines, vol. 11, no 3, p. 673, mars 2023. [CrossRef]
- S. Kuate, J. Cinatl, H. W. Doerr, et K. Überla, « Exosomal vaccines containing the S protein of the SARS coronavirus induce high levels of neutralizing antibodies », Virology, vol. 362, no 1, p. 26-37, mai 2007. [CrossRef]
- V. Karn et al., « Extracellular Vesicle-Based Therapy for COVID-19: Promises, Challenges and Future Prospects », Biomedicines, vol. 9, no 10, p. 1373, oct. 2021. [CrossRef]
- Z. Wang et al., « Exosomes decorated with a recombinant SARS-CoV-2 receptor-binding domain as an inhalable COVID-19 vaccine », Nat. Biomed. Eng., vol. 6, no 7, p. 791-805, juill. 2022. [CrossRef]
- A. Schwab et al., « Extracellular vesicles from infected cells: potential for direct pathogenesis », Front. Microbiol., vol. 6, oct. 2015. [CrossRef]
- R. C. Lai, R. W. Y. Yeo, K. H. Tan, et S. K. Lim, « Exosomes for drug delivery — a novel application for the mesenchymal stem cell », Biotechnol. Adv., vol. 31, no 5, p. 543-551, sept. 2013. [CrossRef]
- M. Marcus et J. Leonard, « FedExosomes: Engineering Therapeutic Biological Nanoparticles that Truly Deliver », Pharmaceuticals, vol. 6, no 5, p. 659-680, avr. 2013. [CrossRef]
- L. Luketic et al., « Antigen Presentation by Exosomes Released from Peptide-Pulsed Dendritic Cells Is not Suppressed by the Presence of Active CTL », J. Immunol., vol. 179, no 8, p. 5024-5032, oct. 2007. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




