1. Introduction
Ventricular arrhythmia is an important predictor for mortality in mitral valve prolapse (MVP) [
1]. The mechanism of ventricular arrhythmia in MVP is poorly understood. Advanced cardiac imaging has identified myocardial inflammation in MVP which may mediate its association with ventricular scarring and arrhythmia [
2]. Two easily obtainable markers of systemic inflammation, namely the neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR), have emerged as potent prognostic indicators in those with cardiovascular disease and the general population, correlating with increased risk of cardiovascular events, including myocardial infarction, major adverse cardiac events, and mortality [
3,
4,
5,
6]. NLR and PLR have been associated with MVP, but they have not yet been utilized to predict ventricular arrhythmia in MVP patients [
7]. The objective of this study is to investigate the association of NLR and PLR with ventricular arrhythmia in MVP. If such an association is demonstrated, these may allow for better identifying those at increased risk ventricular arrhythmia and sudden death from MVP, as well as identifying those who may benefit from interventions targeting inflammation to reduce risk in MVP.
2. Materials and Methods
Patients aged 18 to 90 years diagnosed with MVP on echocardiography between 2016 – 2019 at our institution were identified, and clinical and demographic data was extracted from electronic medical records. Ambulatory ECG (A-ECG) data was available in 207 of the 632 patients with MVP.
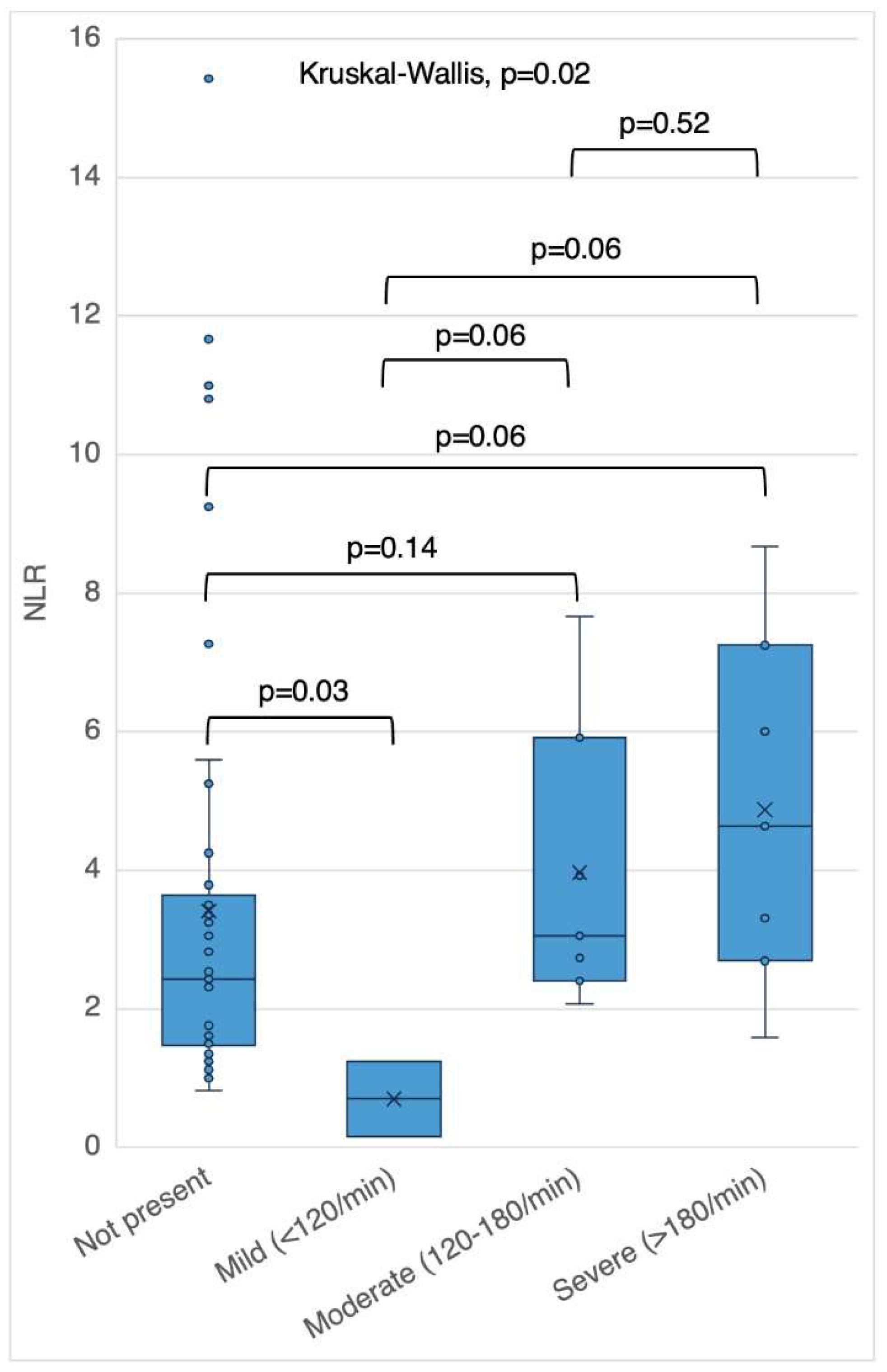
The A-ECG closest to date of the index echocardiogram was reviewed for PVC burden, and non-sustained ventricular tachycardia (NSVT). NSVT was further categorized using fastest average NSVT rate as mild (<120/minute), moderate (120-179/minute) or severe (≥180/minute) [
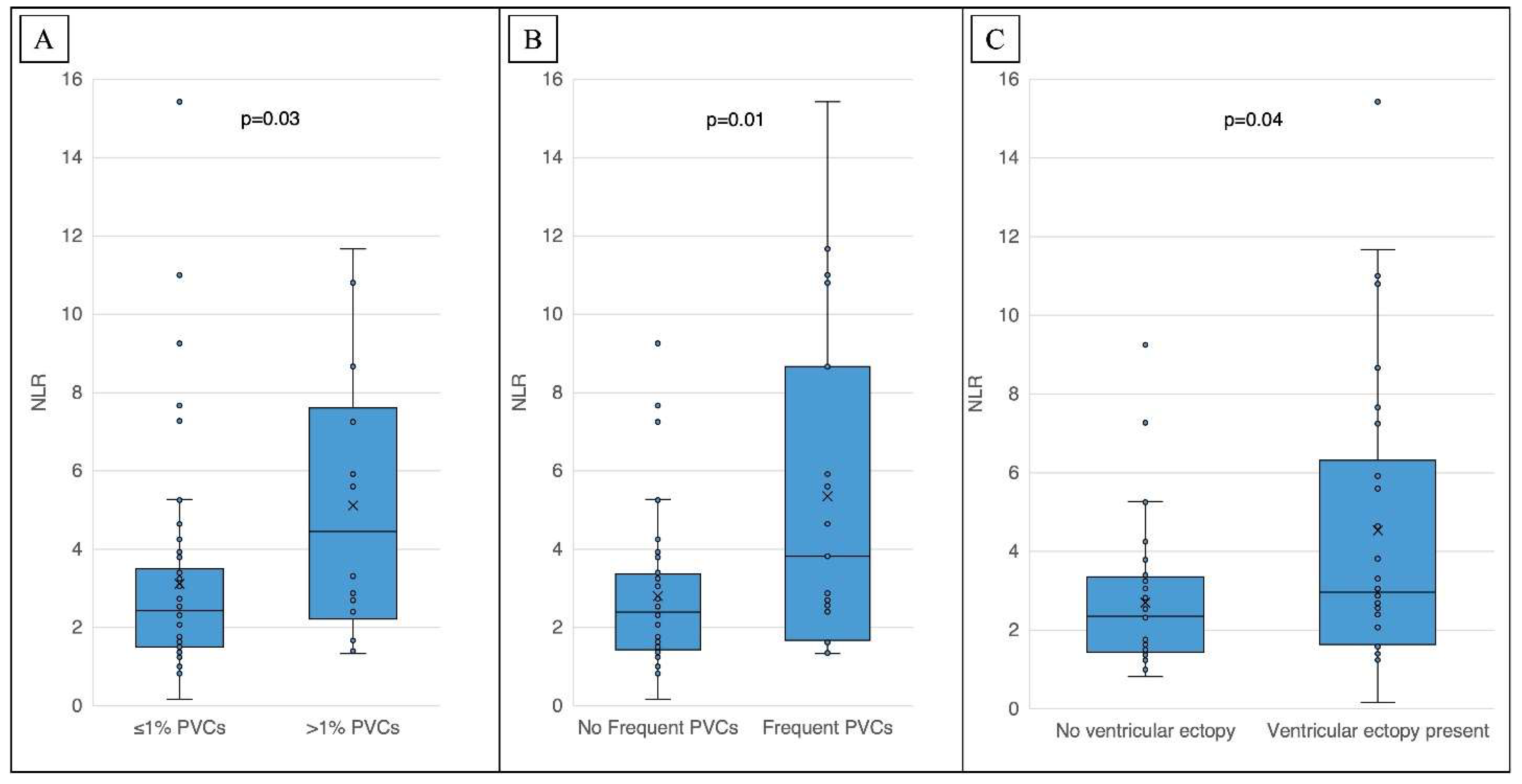
1]. The presence of complex or frequent ventricular ectopy (cfVE) was defined as the presence of two or more premature ventricular contractions (PVCs) on a 10-second ECG tracing or greater than 1% PVC burden or NSVT on ambulatory ECG monitoring.
Complete blood counts (CBC) with differential cell counts within six months prior to A- ECG monitor placement were reviewed, and the most recent of these results was used for data collection. Additionally, patients with invasive or surgical procedure, infection, cancer, or autoimmune conditions within three months of CBC collection date were excluded. NLR and PLR were calculated by dividing absolute neutrophil count and platelet count by absolute lymphocyte count, respectively. ESR and CRP were not included in data collection due to low availability within this subset of patients.
Chi-squared test, Fisher's exact test, and Spearman correlation were used to analyze clinical, ECG, and echocardiographic factors associated NLR and PLR, and correlations of NLR and PLR with PVC burden, NSVT and cfVE. Statistical significance was taken at a p-value of 0.05. Statistical analysis was performed by using JMP version 16.0 (SAS, Cary, NC, USA).
3. Results
3.1. Study population and mitral valve features
We identified 65 patients with a differential blood count within six months prior to A-ECG. There were 40 women (62%), median age (range) was 70 (61-77) years, and 8 (12%) were African American. Mitral valve morphology was myxomatous in 25 (38%) and 24 (37%) had bileaflet prolapse.
3.2. NLR and PLR
An elevated NLR (≥ 3.92) was present in 17 (26%) and an elevated PLR (≥ 239) was present in 12 (18%).7 There was a positive correlation between age and NLR (rs=0.36, p=0.003). Other features associated with higher NLR (median NLR [IQR]) were heart failure (6.0 (3.0-10.9) vs 2.4 [1.5-3.5] without heart failure, p=0.005), bileaflet prolapse (3.1 [1.9-5.8] vs 2.3 [1.4-3.4] with unileaflet prolapse, p=0.04), male sex (3.3 [1.9-6.6] vs 2.3 [1.4-3.3] among female sex, p=0.01). Clinical characteristics of those with normal and elevated NLR are shown in
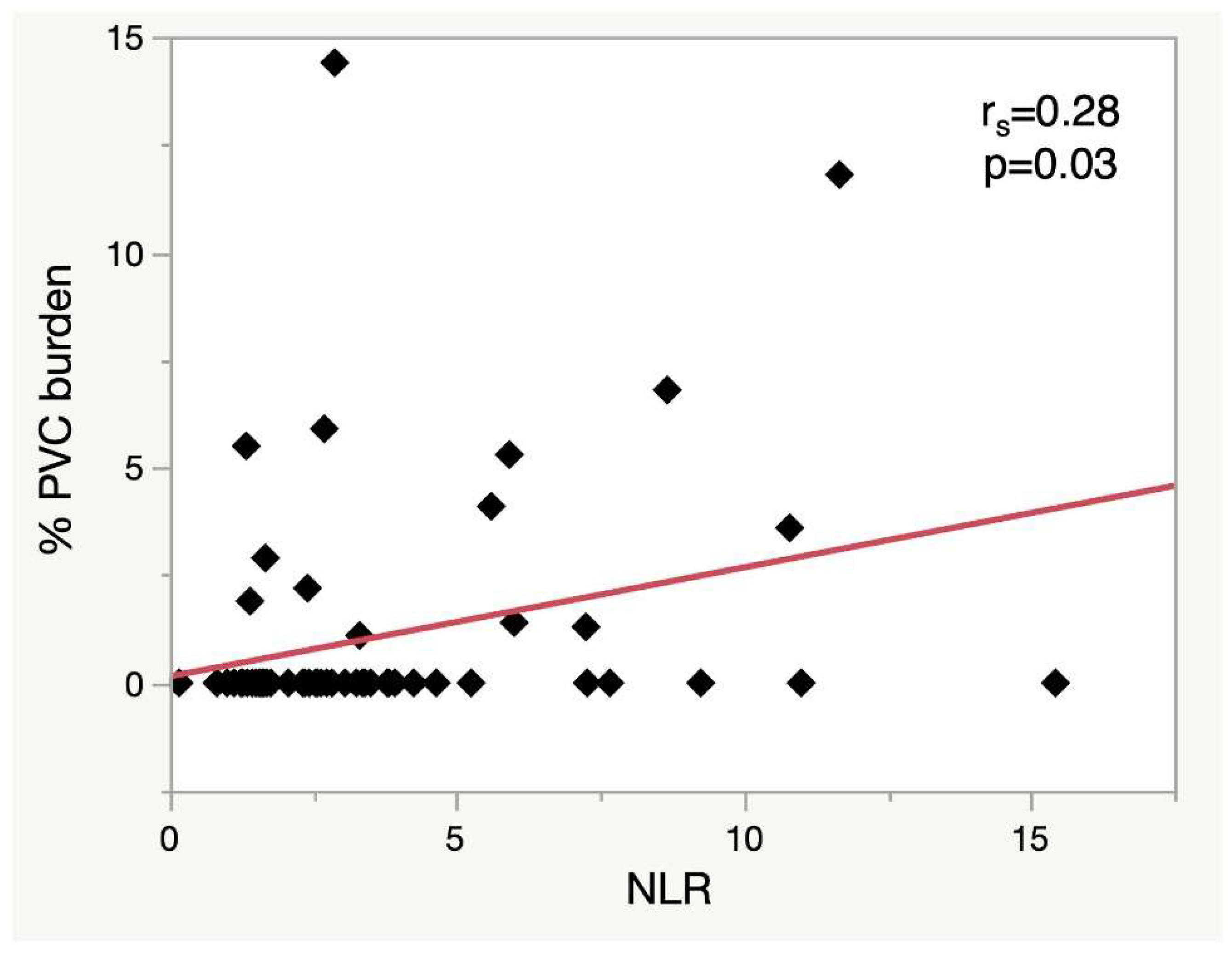
Table 1. NLR, but not PLR, was associated with PVC burden, cfVE and NSVT severity (
Figure 1,
Figure 2 and
Figure 3). An NLR of 5.6 yielded 50% sensitivity and 90% specificity for PVC burden >1% (AUC 0.69).
4. Discussion
NLR has previously been explored as an indicative inflammatory marker in MVP.[
7] In this retrospective study of patients with MVP at our institution, we observed associations of NLR with PVC burden, cfVE and severity of NSVT. Furthermore, an elevated NLR was present in those with bileaflet mitral valve prolapse and congestive heart failure. These observations suggests that inflammation is a significant factor in the pathogenesis of ventricular arrhythmia in mitral valve prolapse, and lend support to inflammation as a mechanistic link between mitral morphological features, cardiac dysfunction and ventricular arrhythmia. Frequent PVCs and NSVT in MVP have been associated with higher rates of severe ventricular arrhythmia and NSVT severity has been associated with decreased survival in MVP patients [
1,
8]. Our findings that NLR is elevated in those with higher rates of NSVT lends further support of the potential importance of inflammation as a risk factor for SCD in MVP.
The NLR is a widely studied and easily obtainable marker that reflects the balance between the innate immune response, mainly due to neutrophils, and adaptive immune response, mediated predominantly by lymphocytes. It is calculated by dividing the absolute neutrophil count by the absolute lymphocyte count, both of which can be obtained from a complete blood count (CBC) analysis. Normal NLR has been shown to be <3.53 in one study and <3.92 in another study [
9,
10]. An elevated NLR has been recognized as a useful indicator of systemic inflammation in numerous medical conditions including cardiovascular disease. The NLR can become increased within a few hours of a stressful stimulus and can increase in response to cytokines, catecholamines and corticosteroids [
11]. Neutrophilia is associated with the release of pro-inflammatory cytokines and reactive oxygen species, while lymphopenia may reflect impaired immune regulation. The rapid rise in NLR ( <6 hours) following acute physiological stress compared to CRP (12-24 hours) and ESR (24-48 hours) proves advantageous for early recognition of inflammatory changes [
11,
12].
This is the first study correlating NLR with ventricular arrhythmia. Inflammation has been proposed to be an important etiological factor in the pathogenesis of ventricular arrhythmia [
13]. Specifically in MVP, a subset of patients with MVP have increased risk of ventricular arrhythmia resulting in sudden death, bileaflet prolapse, complex ventricular ectopy including NSVT and ventricular fibrosis have been identified as conferring increased risk of sudden death in this condition [
14,
15]. A study of 20 patients demonstrated using hybrid positron emission tomography – magnetic resonance imaging that myocardial inflammation is common in patients with mitral valve prolapse and is associated with ventricular scarring and ventricular arrhythmia [
2]. NLR has also been associated with incident heart failure and worsened heart failure prognosis [
6,
16]. In keeping with these findings, we found significant associations of bileaflet prolapse and heart failure with NLR. It seems likely, therefore, that an elevated NLR in mitral valve prolapse identifies increased ventricular arrhythmia risk due to myocardial inflammation, with possible mechanisms including repetitive myocardial injury from the prolapsing mitral valve and myocardial dysfunction. In addition, the systemic inflammatory response can impact cardiac electrophysiology, leading to alterations in ion channel function and electrical remodeling, which are critical determinants of ventricular arrhythmia development [
13].
The study was inherently limited by its small numbers and retrospective design, such as selection bias and nonuniform assessment of arrhythmia. We were careful to ensure that event monitoring data and blood counts were performed within an acceptable time frame of each other. However, in doing so we excluded a significant number of patients identified initially. Owing to the small size, we did not assess outcomes including mortality. We made considerable efforts to exclude confounders of NLR elevation, but may have overlooked factors not documented or measured. NLR is higher in men and older patients, associations also observed in this study [
10]. Patients on steroids or immunosuppressants were not excluded. Patients on antibiotics were excluded from the study. We limited the analysis to ventricular arrhythmia and did not measure supraventricular arrhythmia.
5. Conclusions
In mitral valve prolapse, NLR is associated with bileaflet prolapse, heart failure and ventricular arrhythmia, suggests that inflammation may contribute to arrhythmogenicity in this population. The NLR, as a simple and easily measurable marker, shows promise as a predictive biomarker of adverse cardiovascular outcomes in MVP. Further research is needed to better understand underlying mechanisms and define the clinical utility of NLR in risk stratification and management of ventricular arrhythmias in MVP patients.
Author Contributions
Conceptualization, F.S..; methodology, F.S and S.G.; software, S.G, A.M.C and A.P.C.; validation, F.S., R.S.. and T.W.; formal analysis, A.C.; investigation, S.G., A.M.C., A.P.C.; resources, F.S..; data curation, S.G, A.M.C and A.P.C.; writing—original draft preparation, S.G and T.P..; writing—review and editing, F.S, A.P.C, R.S, T.W..; visualization, A.C.; supervision, F.S, R.S, T.W.. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board (or Ethics Committee) of The University of North Carolina at Chapel Hill (IRB 19-0069, approved January 2019.
Informed Consent Statement
Patient consent was waived in accordance with the Institutional Review Board determination of the study meeting the regulatory definition of minimal risk.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Essayagh, B.; Sabbag, A.; Antoine, C.; Benfari, G.; Yang, L.T.; Maalouf, J.; Asirvatham, S.; Michelena, H.; Enriquez-Sarano, M. Presentation and Outcome of Arrhythmic Mitral Valve Prolapse. J Am Coll Cardiol 2020, 76, 637–649. [CrossRef]
- Miller, M.A.; Adams, D.H.; Pandis, D.; Robson, P.M.; Pawale, A.; Pyzik, R.; Liao, S.L.; El-Eshmawi, A.; Boateng, P.; Garg, J.; et al. Hybrid Positron Emission Tomography/Magnetic Resonance Imaging in Arrhythmic Mitral Valve Prolapse. JAMA Cardiol 2020, 5, 1000–1005. [CrossRef]
- Silvestre-Roig, C.; Braster, Q.; Ortega-Gomez, A.; Soehnlein, O. Neutrophils as Regulators of Cardiovascular Inflammation. Nat Rev Cardiol. [CrossRef]
- Song, M.; Graubard, B.I.; Rabkin, C.S.; Engels, E.A. Neutrophil-to-Lymphocyte Ratio and Mortality in the United States General Population. Sci Rep 2021, 11. [CrossRef]
- Zhai, G.; Wang, J.; Liu, Y.; Zhou, Y. Platelet-Lymphocyte Ratio as a New Predictor of in-Hospital Mortality in Cardiac Intensive Care Unit Patients. Sci Rep 2021, 11. [CrossRef]
- Davison, B.A.; Takagi, K.; Edwards, C.; Adams, K.F.; Butler, J.; Collins, S.P.; Dorobantu, M.I.; Ezekowitz, J.A.; Filippatos, G.; Greenberg, B.H.; et al. Neutrophil-to-Lymphocyte Ratio and Outcomes in Patients Admitted for Acute Heart Failure (As Seen in the BLAST-AHF, Pre-RELAX-AHF, and RELAX-AHF Studies). Am J Cardiol 2022, 180, 72–80. [CrossRef]
- Yalim, Z.; Ersoy, İ. Evaluation of Inflammation Markers in Mitral Valve Prolapse. Arch Cardiol Mex 2022, 92, 181–188. [CrossRef]
- Aabel, E.W.; Chivulescu, M.; Lie, Ø.H.; Hopp, E.; Gjertsen, E.; Ribe, M.; Helle-Valle, T.M.; Edvardsen, T.; Hegbom, F.; Dejgaard, L.A.; et al. Ventricular Arrhythmias in Arrhythmic Mitral Valve Syndrome—a Prospective Continuous Long-Term Cardiac Monitoring Study. Europace 2023, 25, 506. [CrossRef]
- Forget, P.; Khalifa, C.; Defour, J.P.; Latinne, D.; Van Pel, M.C.; De Kock, M. What Is the Normal Value of the Neutrophil-to-Lymphocyte Ratio? BMC Res Notes 2017, 10, 1–4. [CrossRef]
- Fest, J.; Ruiter, R.; Ikram, M.A.; Voortman, T.; Van Eijck, C.H.J.; Stricker, B.H. Reference Values for White Blood-Cell-Based Inflammatory Markers in the Rotterdam Study: A Population-Based Prospective Cohort Study. Sci Rep 2018, 8. [CrossRef]
- Buonacera, A.; Stancanelli, B.; Colaci, M.; Malatino, L. Neutrophil to Lymphocyte Ratio: An Emerging Marker of the Relationships between the Immune System and Diseases. Int J Mol Sci 2022, 23. [CrossRef]
- Markanday, A. Acute Phase Reactants in Infections: Evidence-Based Review and a Guide for Clinicians. Open Forum Infect Dis 2015, 2. [CrossRef]
- Grune, J.; Yamazoe, M.; Nahrendorf, M. Electroimmunology and Cardiac Arrhythmia. Nat Rev Cardiol 2021, 18, 547–564. [CrossRef]
- Sriram, C.S.; Syed, F.F.; Ferguson, M.E.; Johnson, J.N.; Enriquez-Sarano, M.; Cetta, F.; Cannon, B.C.; Asirvatham, S.J.; Ackerman, M.J. Malignant Bileaflet Mitral Valve Prolapse Syndrome in Patients with Otherwise Idiopathic Out-of-Hospital Cardiac Arrest. J Am Coll Cardiol 2013, 62, 222–230. [CrossRef]
- Basso, C.; Perazzolo Marra, M.; Rizzo, S.; De Lazzari, M.; Giorgi, B.; Cipriani, A.; Frigo, A.C.; Rigato, I.; Migliore, F.; Pilichou, K.; et al. Arrhythmic Mitral Valve Prolapse and Sudden Cardiac Death. Circulation 2015, 132, 556–566. [CrossRef]
- Afari, M.E.; Bhat, T. Neutrophil to Lymphocyte Ratio (NLR) and Cardiovascular Diseases: An Update. Expert Rev Cardiovasc Ther 2016, 14, 573–577. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).