Submitted:
31 December 2023
Posted:
03 January 2024
Read the latest preprint version here
Abstract
Keywords:
1. Introduction
2. Chemistry
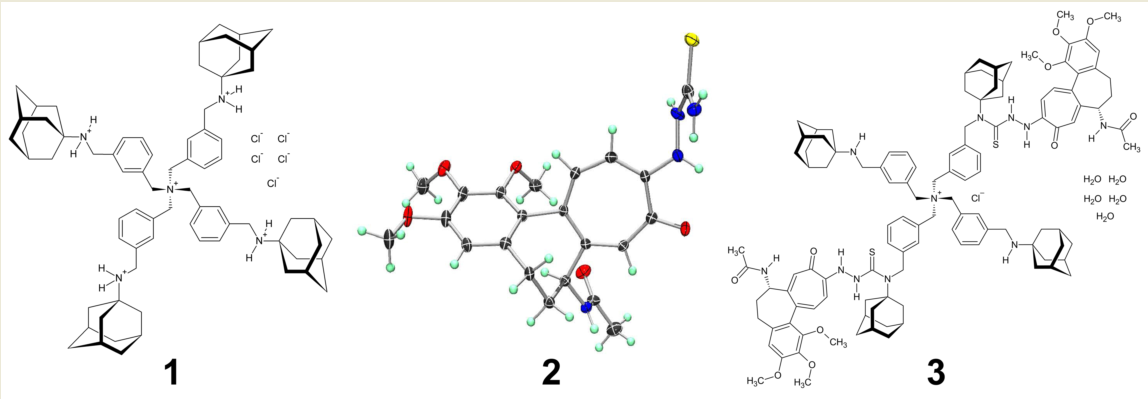
2.1. Compound 1
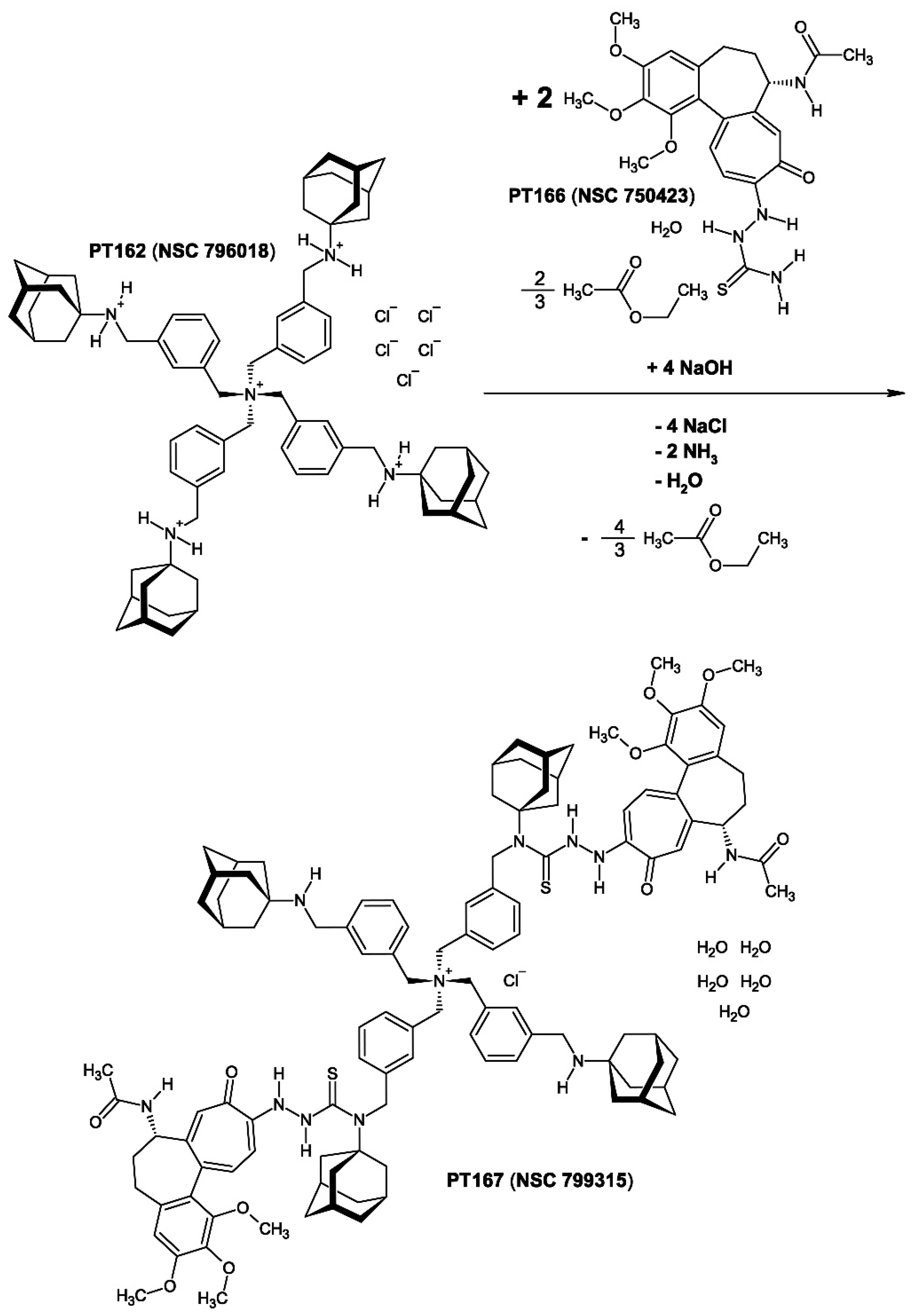
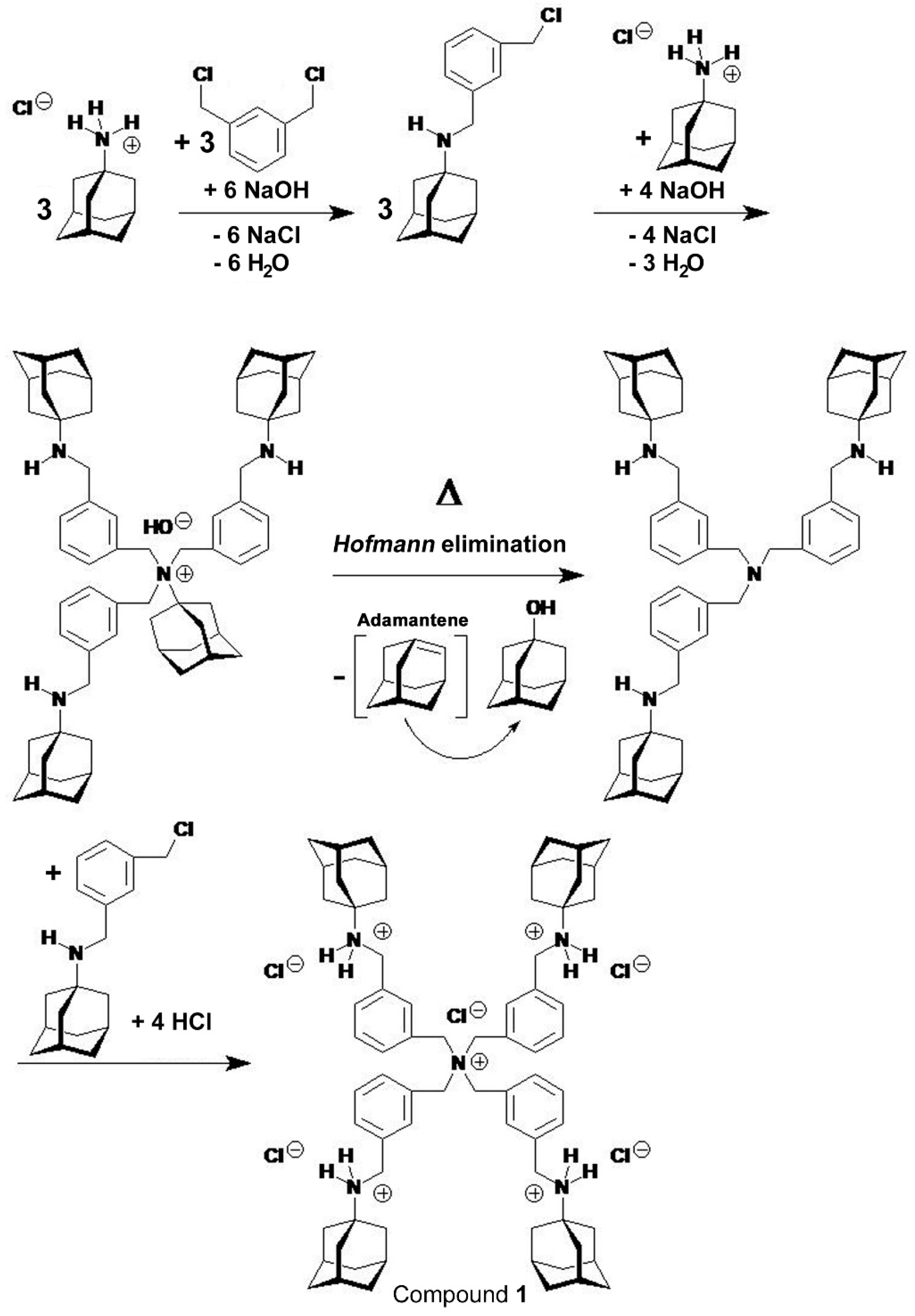
2.1.1. The Synthesis of Salt-Containing Compound 1 (PENTA)
2.1.2. The Synthesis of Pure Compound 1 [PT162 (NSC 796018)]
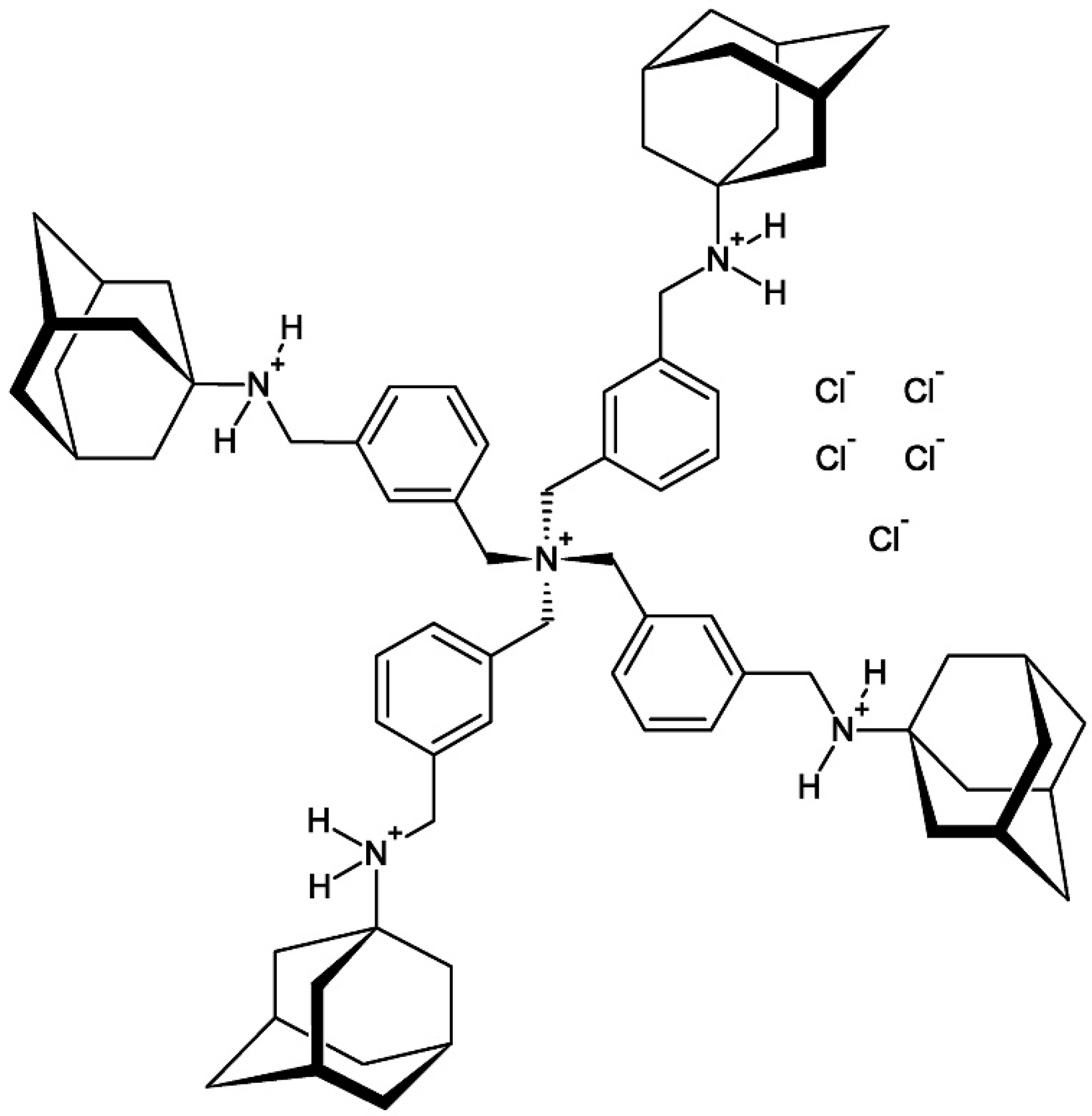
2.1.3. Structure Elucidation of Compound 1 [PT162 (NSC 796018)]
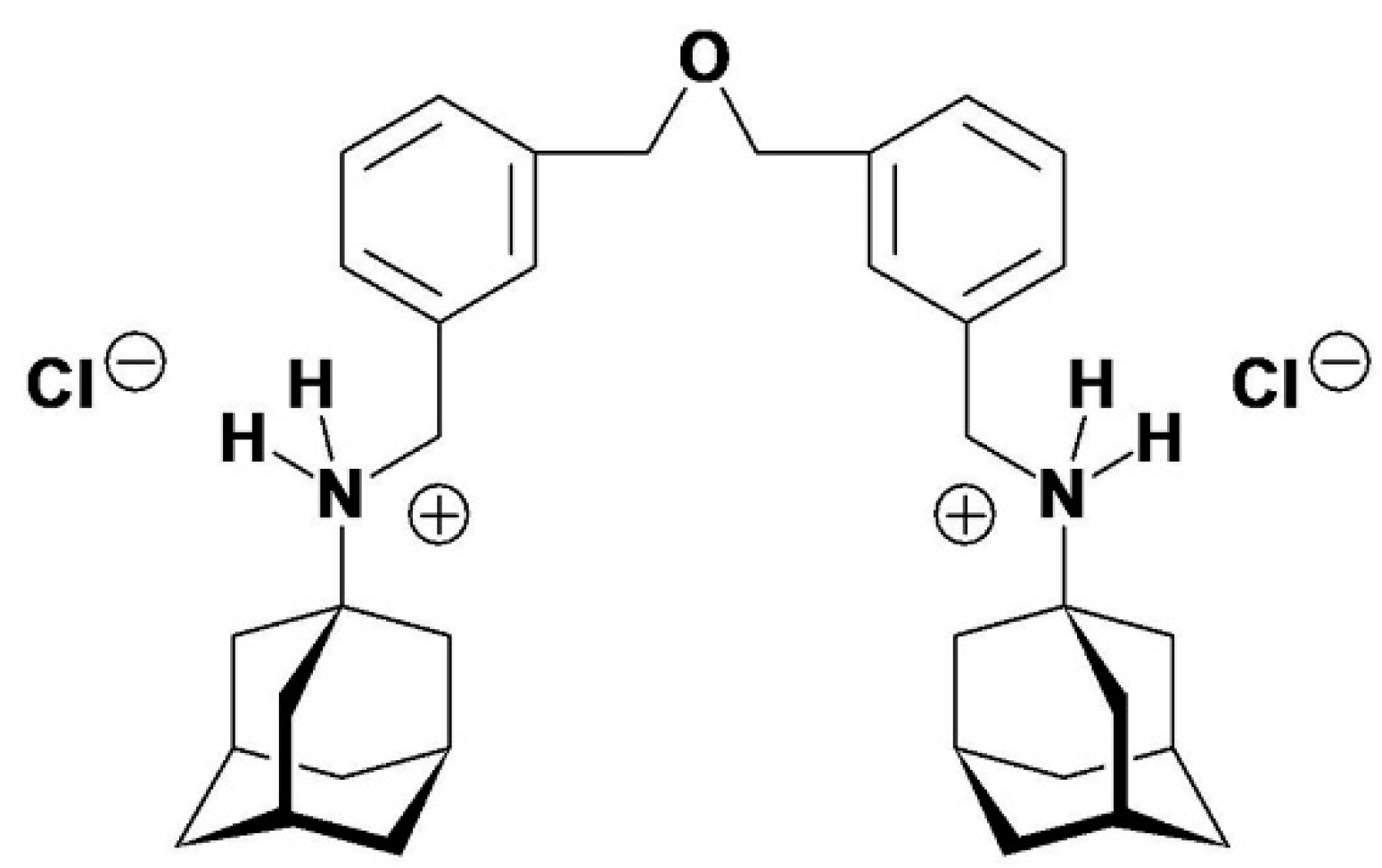
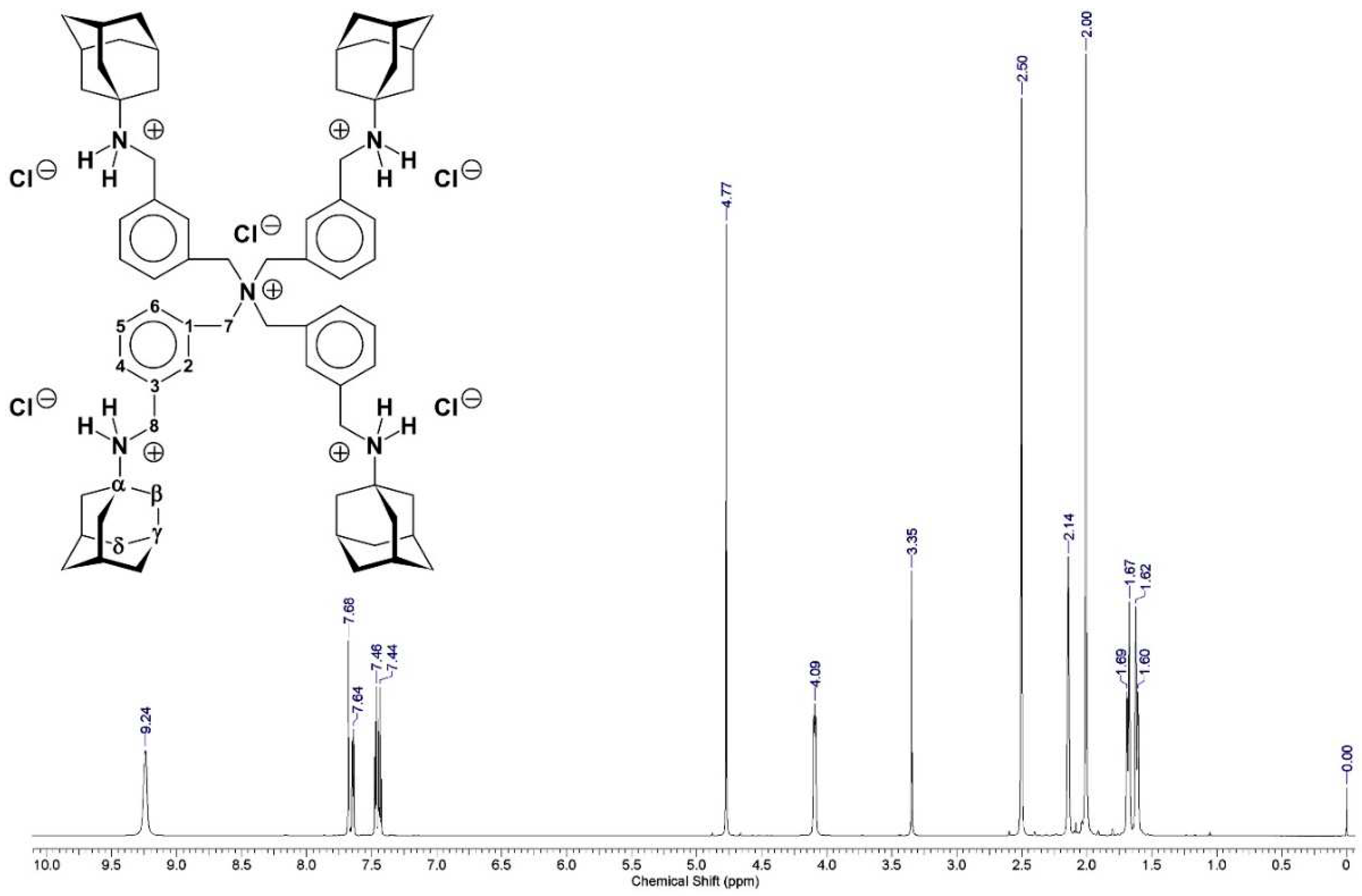
2.2. Compound 2
2.2.1. The Synthesis of Colchic(in)oid Compound 2 [PT166 (NSC 750423)]

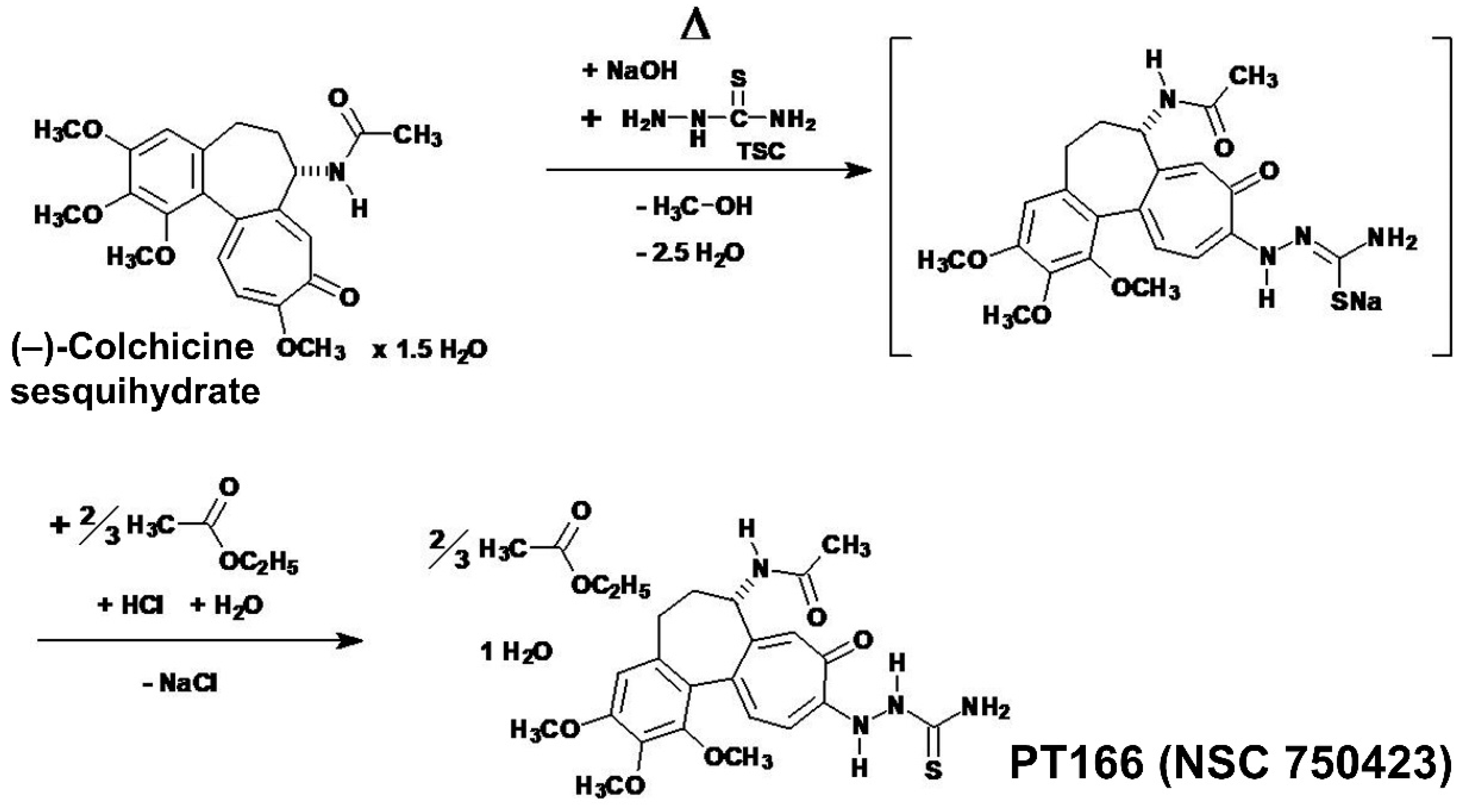
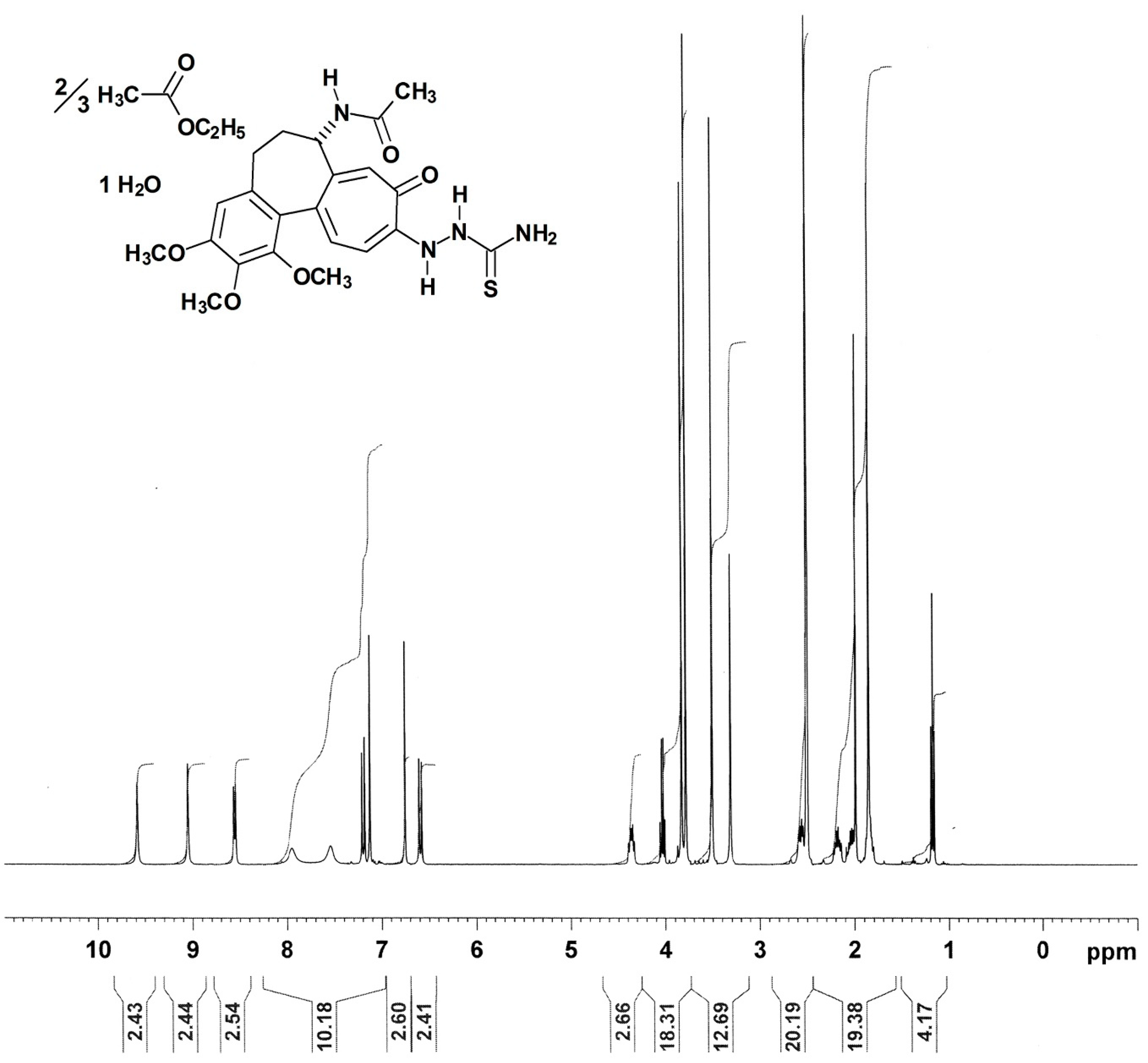
2.2.2. The Nuclear Magnetic Resonance Spectra of Compound 2
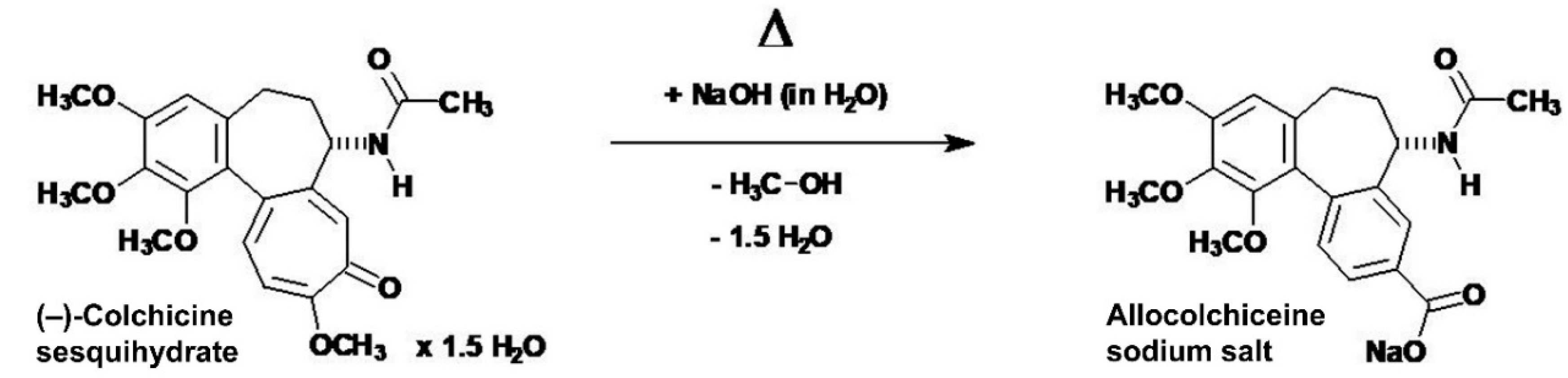
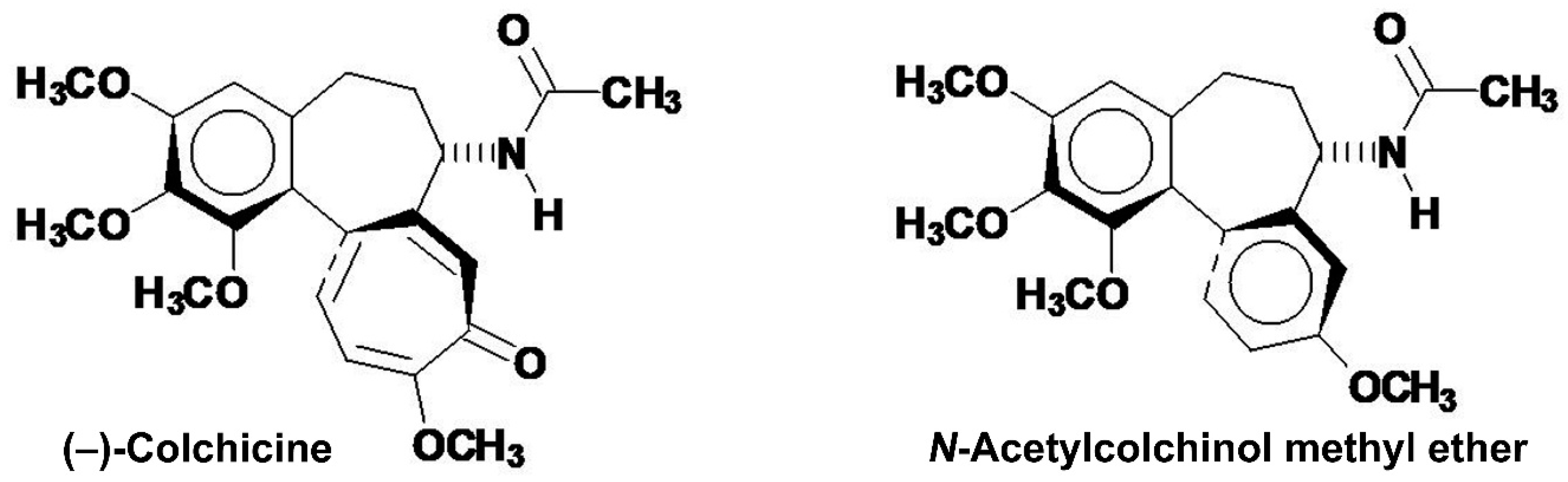
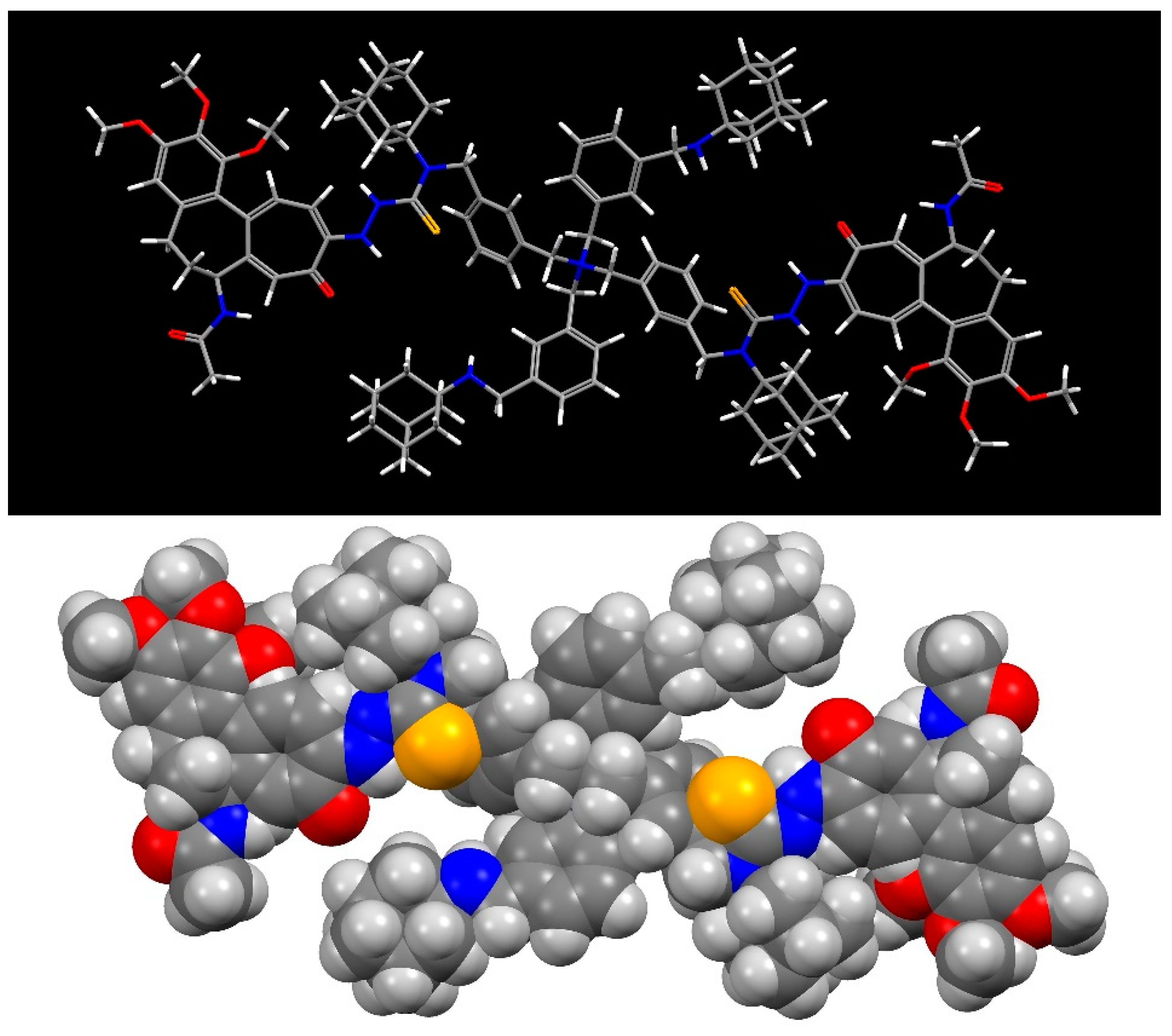
2.2.3. The X-Ray Crystallographic Crystal and Molecular Structure Determination of Compound 2
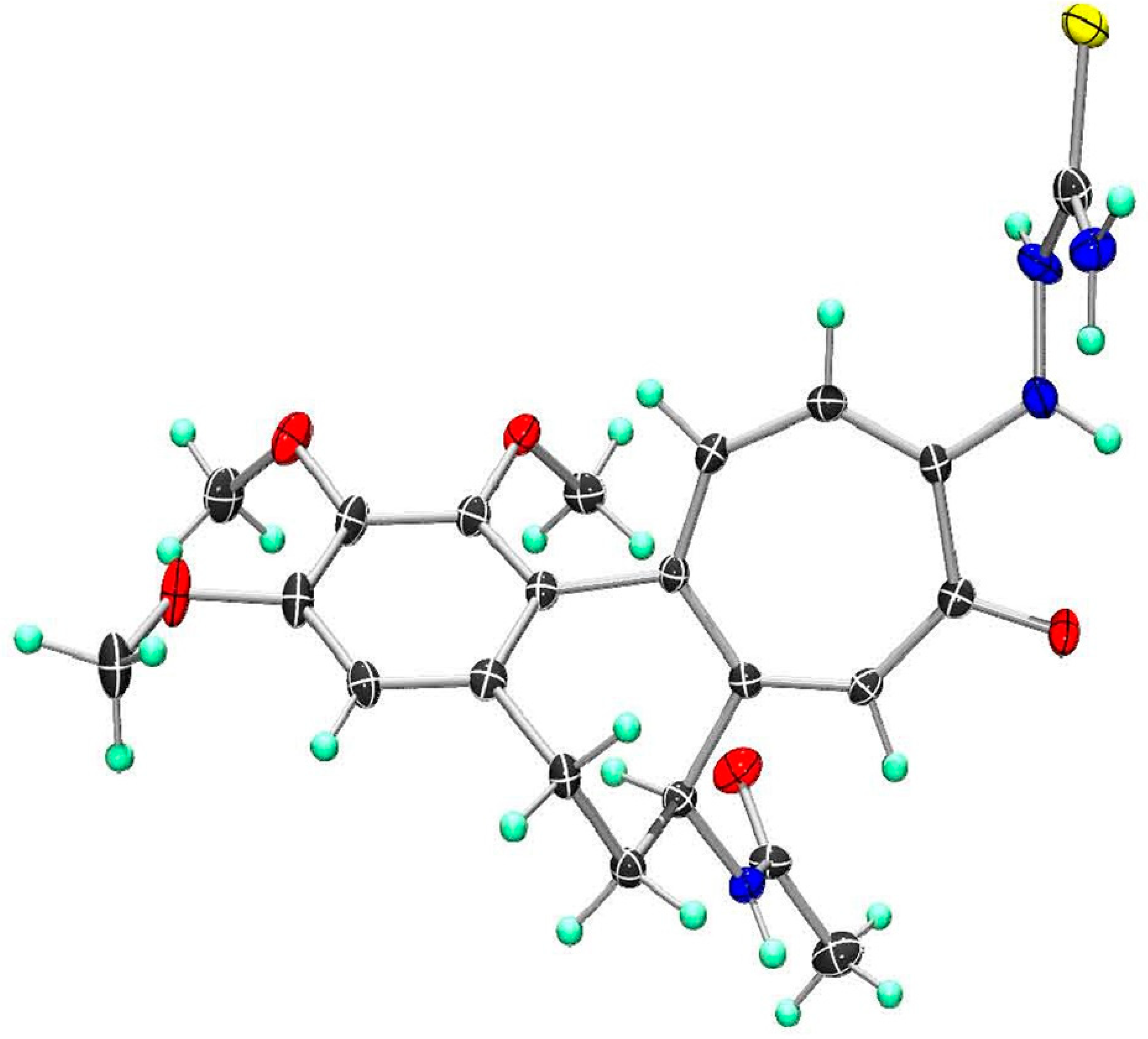
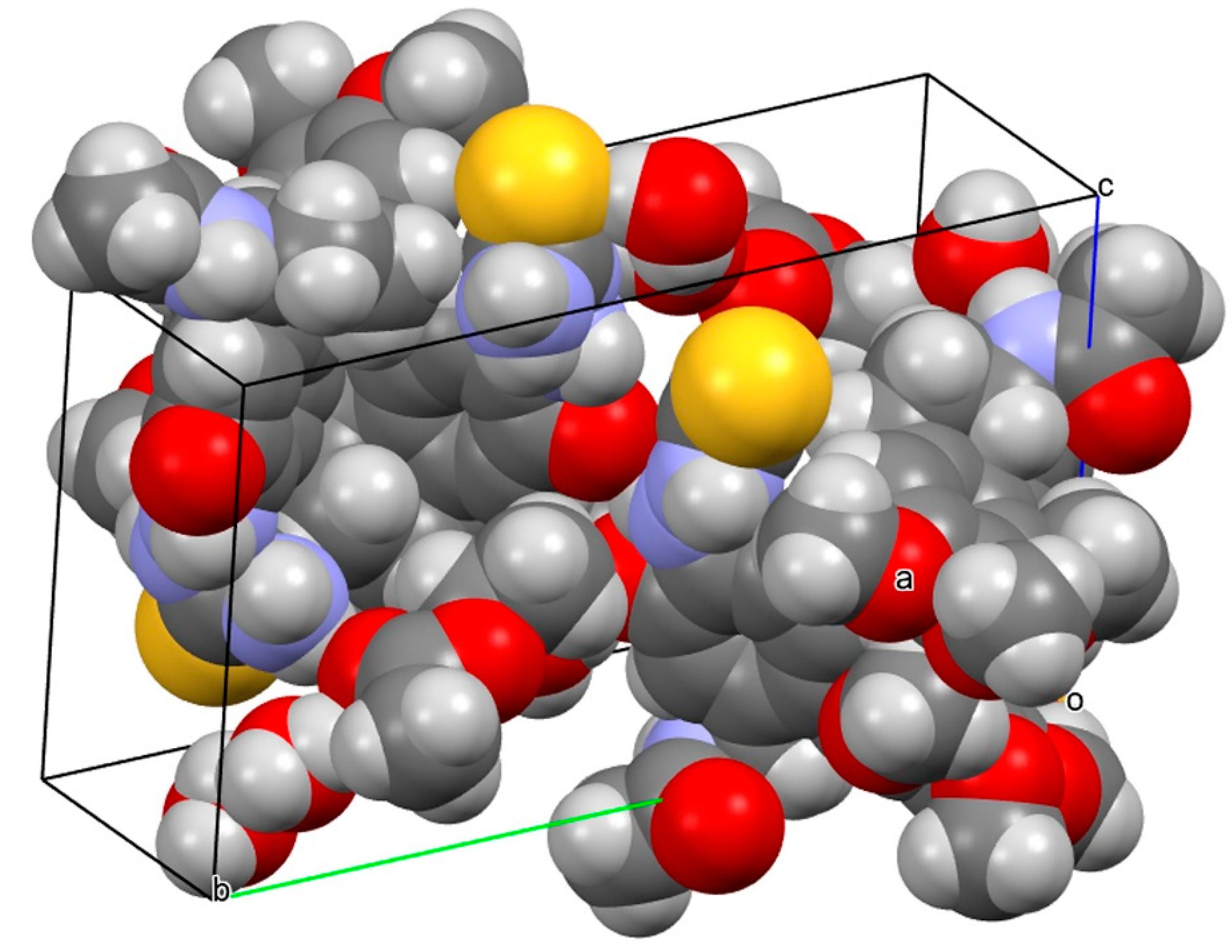
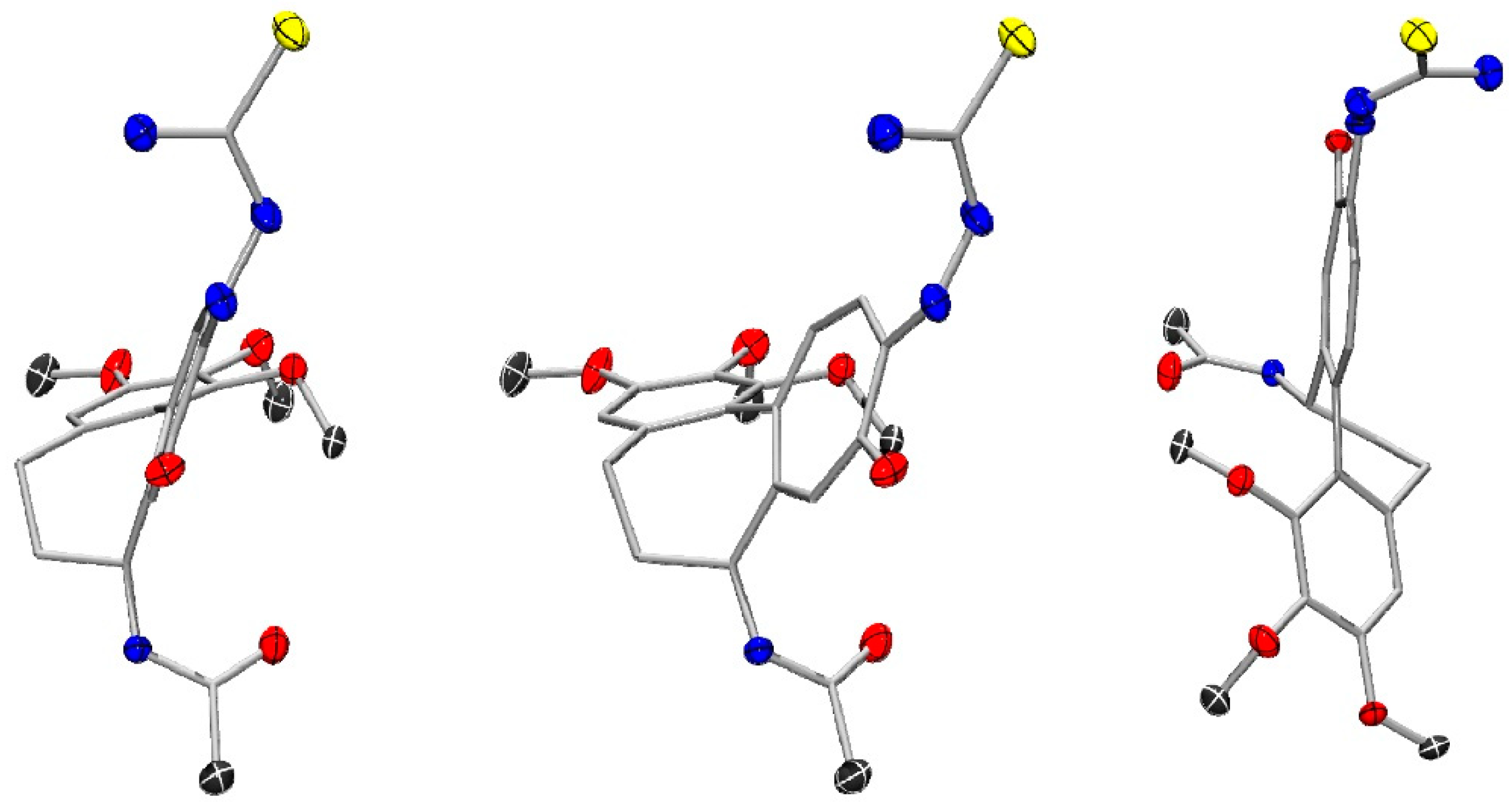
| Empirical formula | C48H66N8O15S2 |
| Formula weight, Mr (g·mol–1) | 1059.21 |
| Temperature, T (K) | 100(2) |
| Radiation, λ (Å) | MoK 0.71073 |
| Crystal system | Monoclinic |
| Space group | P21 |
| Unit cell dimensions | |
| a (Å) | 9.1886(5) |
| b (Å) | 20.9047(10) |
| c (Å) | 13.9841(7) |
| α (°) | 90.00 |
| β (°) | 106.153(2) |
| γ (°) | 90.00 |
| Unit cell volume, V (Å3) | 2580.1(2) |
| Formula units per unit cell, Z | 2 |
| Calculated density, ρcalc (Mg·m–3) | 1.363 |
| Absorption coefficient, μ (mm–1) | 0.178 |
| F(000) | 1124 |
| Theta (ϑ) range for collection | 1.80 to 26.05° |
| Reflections collected | 49598 |
| Independent reflections | 9686 |
| Minimum / maximum transmission, Tmin / Tmax | 0.9193 / 0.9929 |
| Absorption correction | Multi-scan, SADABS 2008/1 (G.M. Sheldrick, 2008) |
| Refinement method | Full-matrix least-squares on F2 |
| Data / parameters / restraints | 9686 / 693 / 5 |
| Goodness−of−fit on F2, S | 1.021 |
| Flack parameter, x | 0.09(6) |
| Final R indices [I > 2σ(I)] | R1 = 0.0506, wR2 = 0.1269 |
| R indices (all data) | R1 = 0.0594, wR2 = 0.1325 |
| Maximum / minimum residual electron density, Δρmax / Δρmin (e·Å–3) | 0.747 / –0.496 |
2.3. Compound 3
2.3.1. The Synthesis of Compound 3 [PT167 (NSC 799315)]
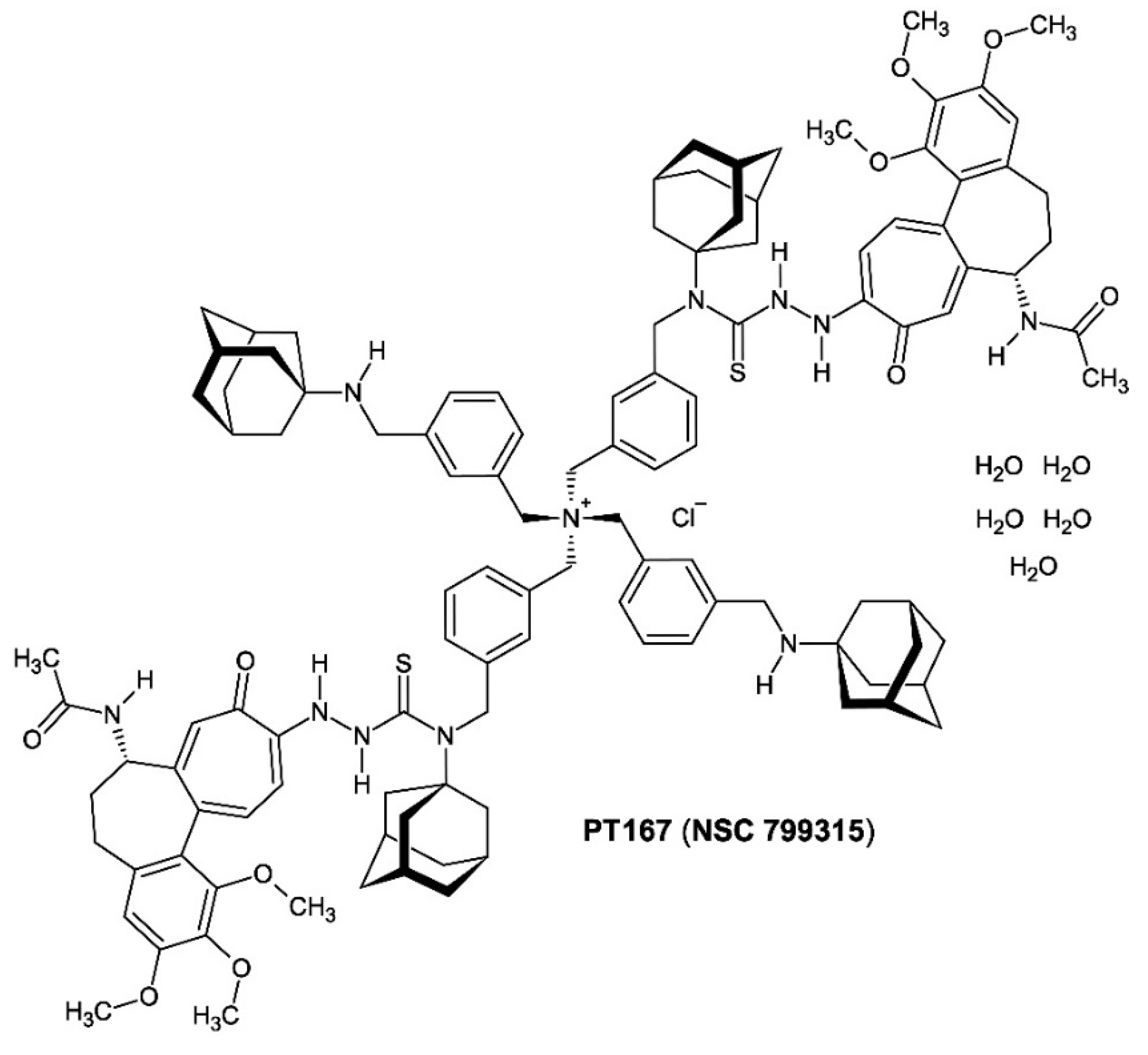
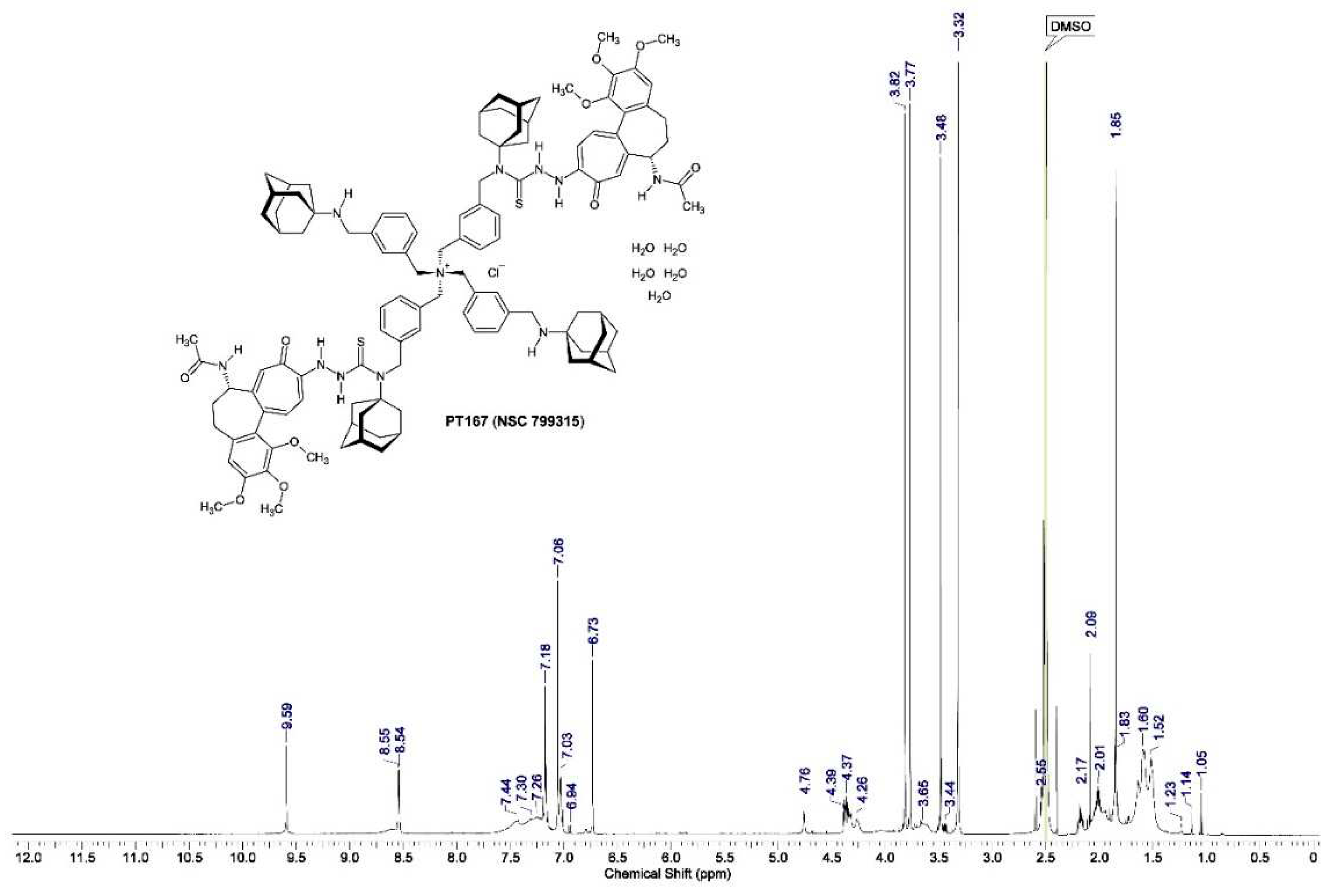
2.3.2. The Proton Nuclear Magnetic Resonance Spectrum (1H-NMR) of Freshly Synthesized Compound 3
2.3.3. The Proton Nuclear Magnetic Resonance Spectrum (1H-NMR) of Compound 3 after Six Year Storage at +0−4 °C in the Refrigerator
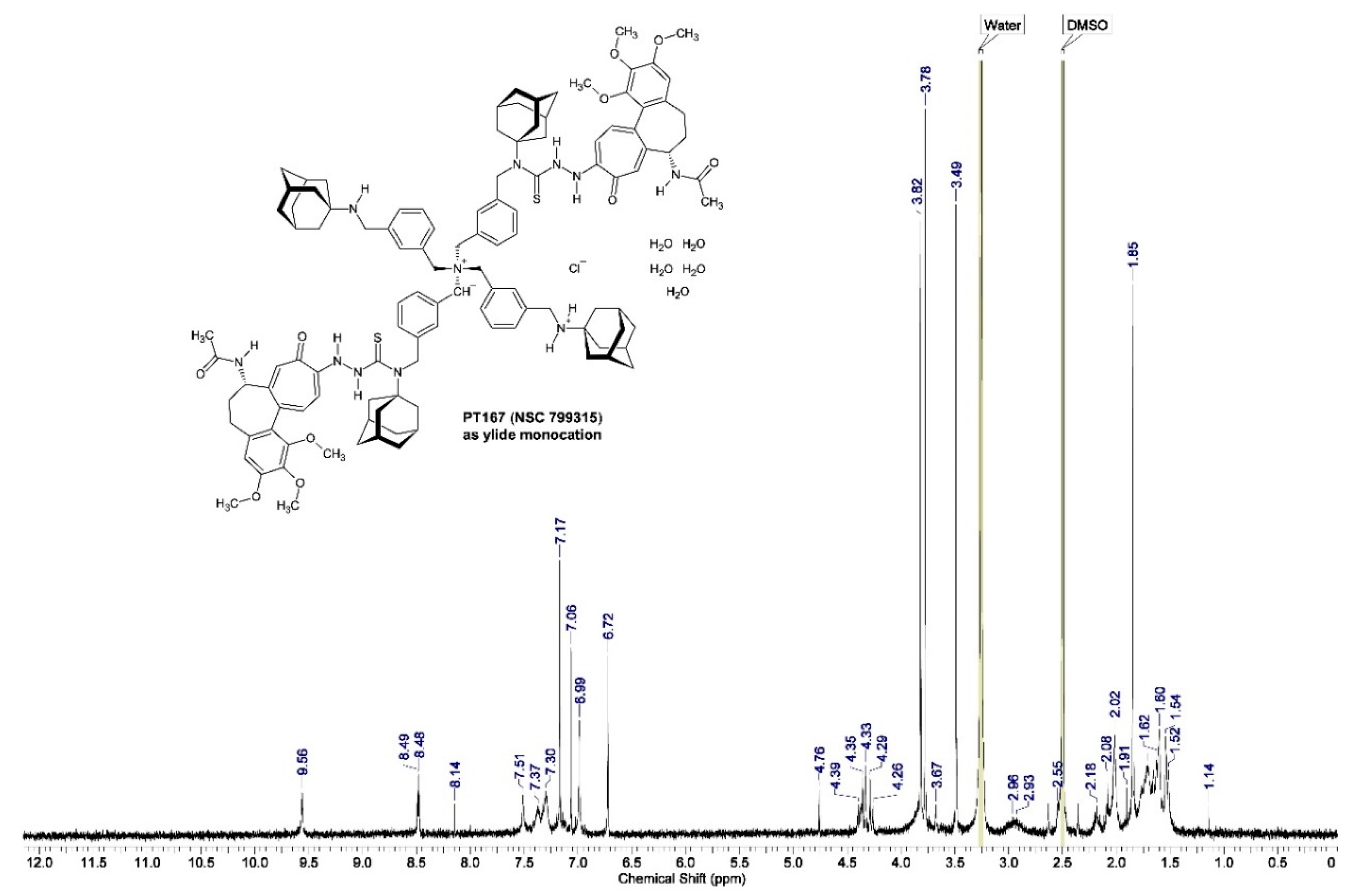
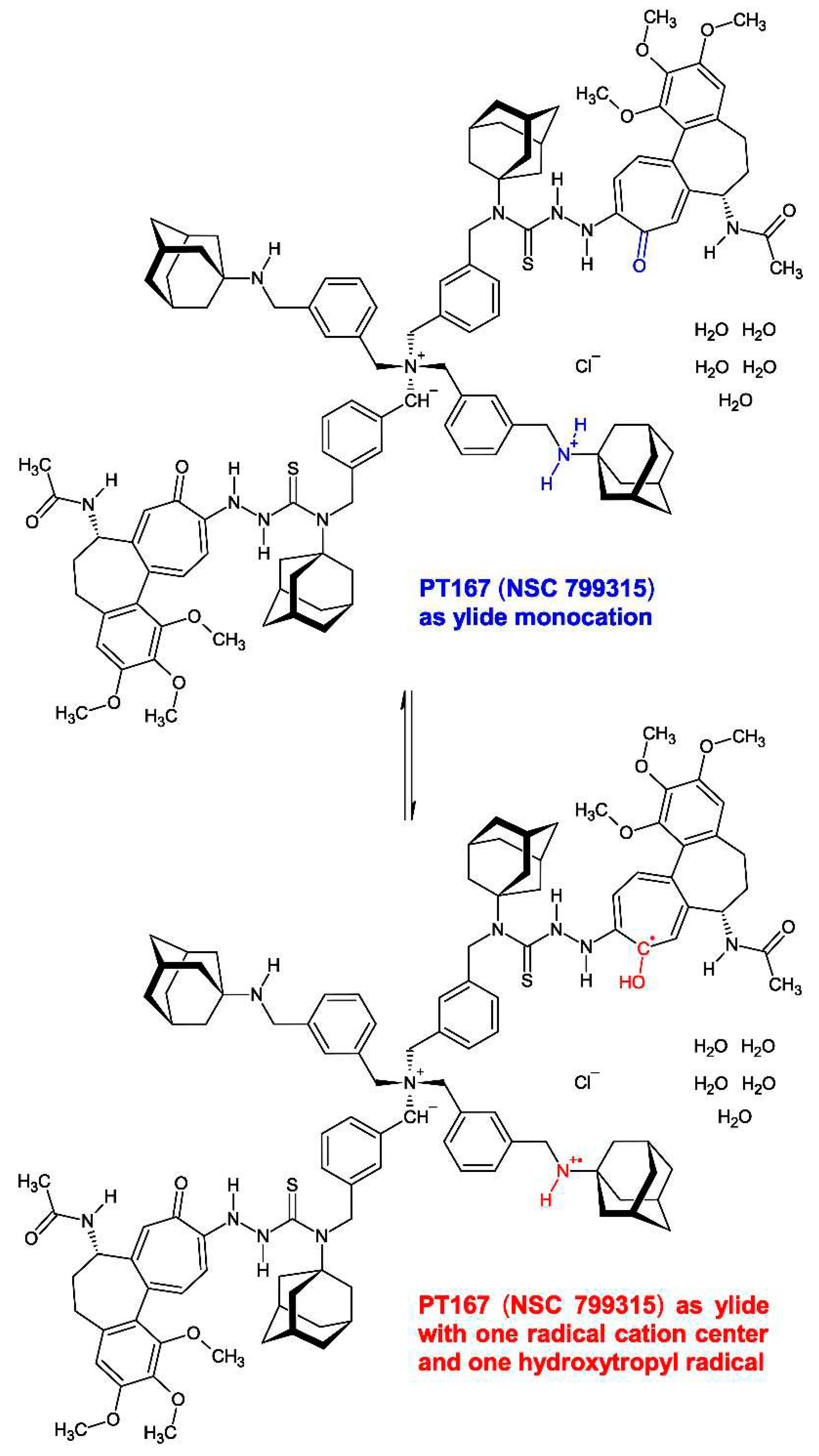
2.3.4. The Liquid Chromatographic (HPLC) Investigation of Compound 3
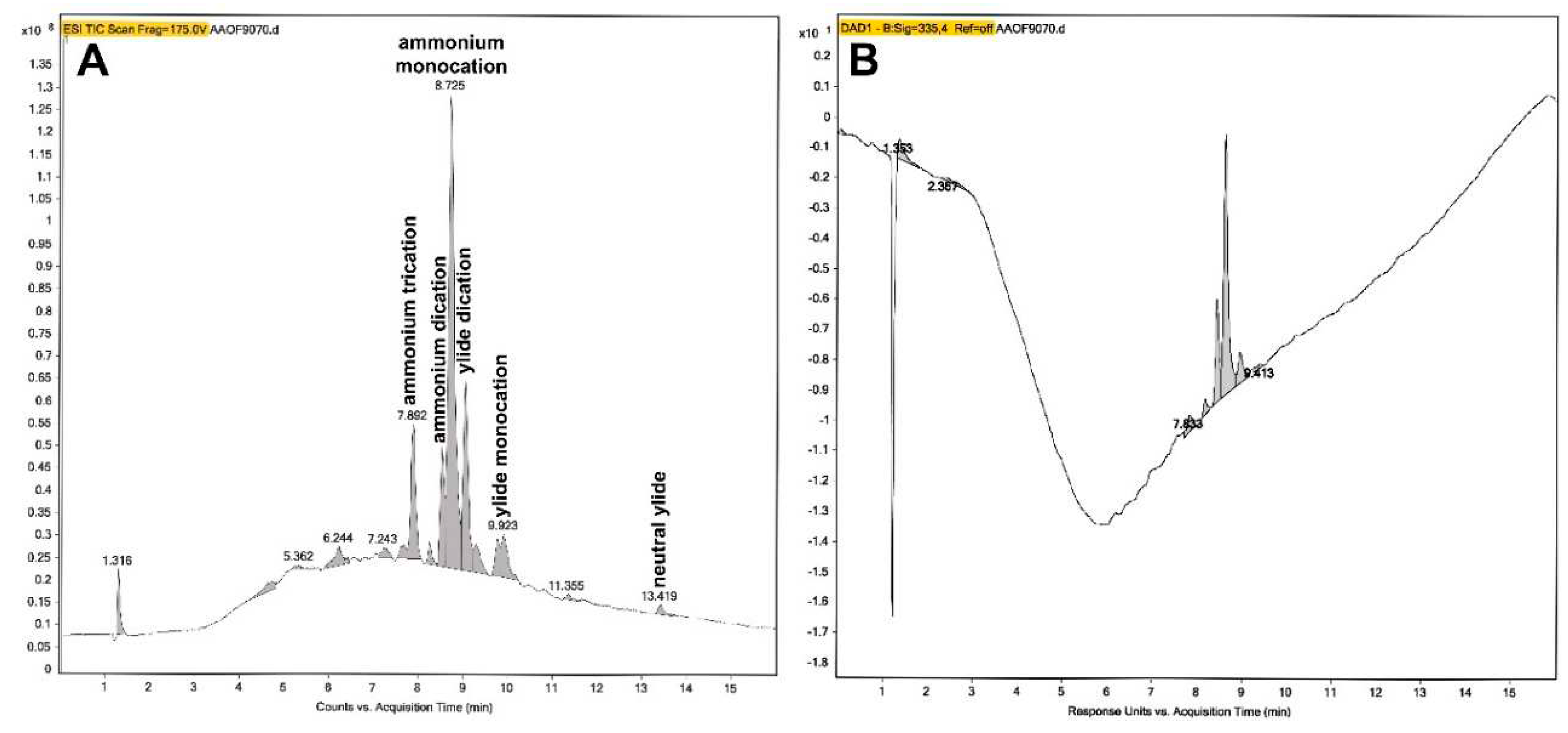
| HPLC peak (min) | Peak identity | ESI−MS fragment peaks (m/z) (relative peak intensity of the 100% base peak) |
|---|---|---|
| 7.842−7.925 | ammonium trication | 290.1670 (100%), 678.3662 (12.5%), 135.1157 (9.6%) |
| 8.508−8.558 | ammonium dication | 712.3545 (100%), 123.0807 (16.5%), 356.6799 (2.1%), 483.2713 (1.3%) |
| 8.608−8.658 | ammonium monocation | 712.3550 (100%), 543.3511 (27.9%), 356.6804 (18.1%), 123.0806 (14.2%), 653.3701 (5.4%), 483.2714 (2.5%), 409.2404 (1.7%) |
| 8.691−8.741 | ammonium monocation | 712.3558 (100%), 356.6814 (19.1%), 578.2448 (6.3%), 135.1165 (5.8%) |
| 9.024−9.074 | ylide dication | 483.2727 (100%), 965.5391 (80.9%), 712.3534 (19.5%), 123.0798 (14.1%), 356.6793 (5.0%), 831.4260 (4.3%), 577.2110 (3.4%), 213.1784 (2.3%) |
| 9.740−9.973 | ylide monocation | 227.2000 (100%), 123.0804 (47.2%), 609.8644 (31.0%), 712.3522 (6.6%), 398.7700 (5.8%), 1218.7191 (5.0%), 796.5327 (4.0%), 483.2712 (2.5%) |
| 13.386−13.453 | neutral ylide | 123.0805 (100%), 637.3055 (45.6%), 227.1988 (7.1%), 439.2038 (5.8%), 712.3521 (3.2%) |
2.3.5. The Electrospray Ionization (ESI) Mass Spectrometric Investigation of Compound 3 after HPLC Separation

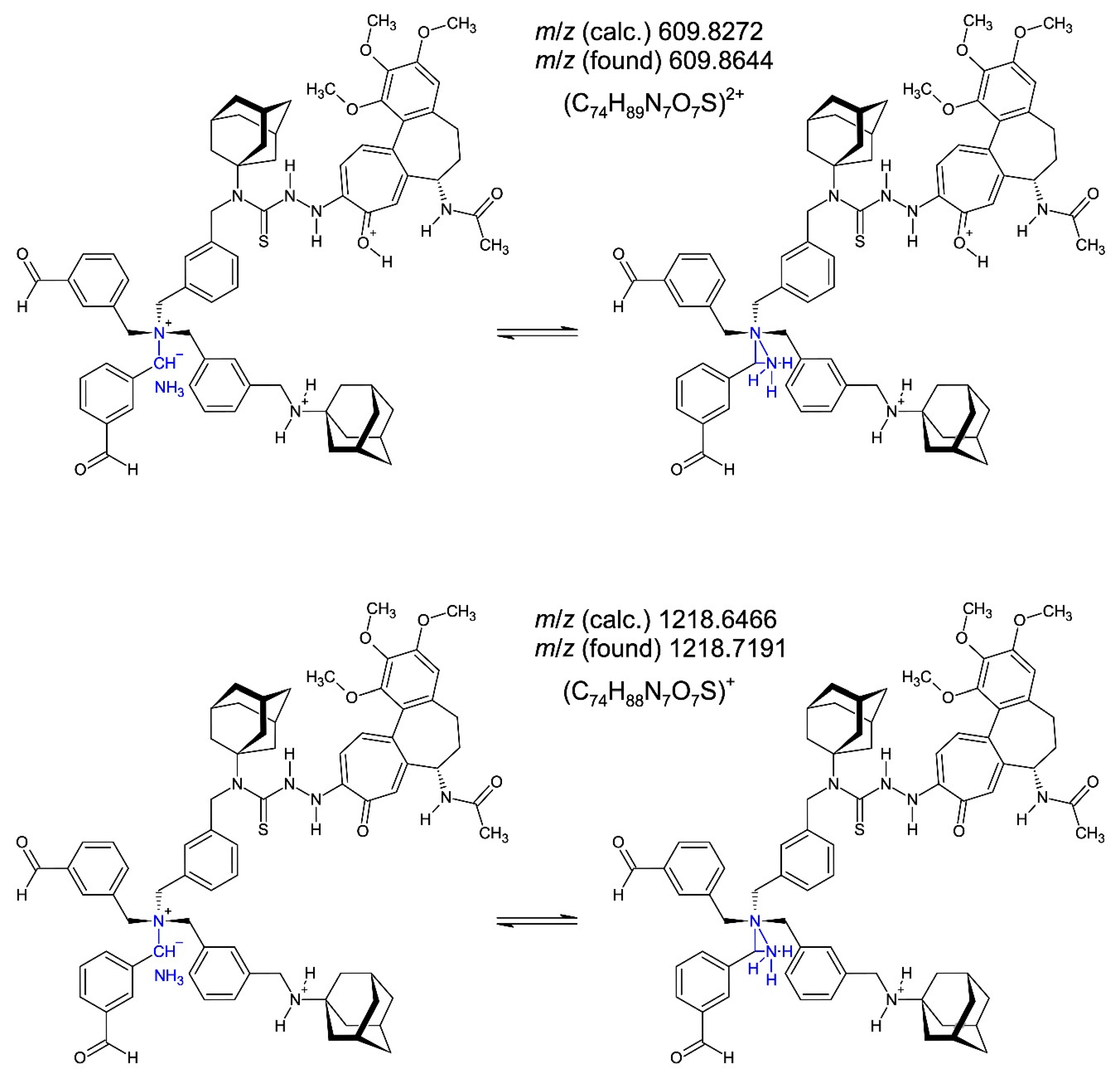
3. Biological Activities
3.1. NCI 60 Cell Five-Dose Screen with the Drugs Compound 1, Compound 2 and Compound 3
3.1.1. National Cancer Institute (NCI) Developmental Therapeutics Program (DTP) 60-Cancer Cell 5-Dose Testing
3.1.2. Overall NCI 60 Cell Five-Dose Screen Results with the Drugs Compound 1, Compound 2 and Compound 3
3.1.3. National Cancer Institute (NCI) Developmental Therapeutics Program (DTP) 60-Cancer Cell 5-Dose Testing Results with Compound 1
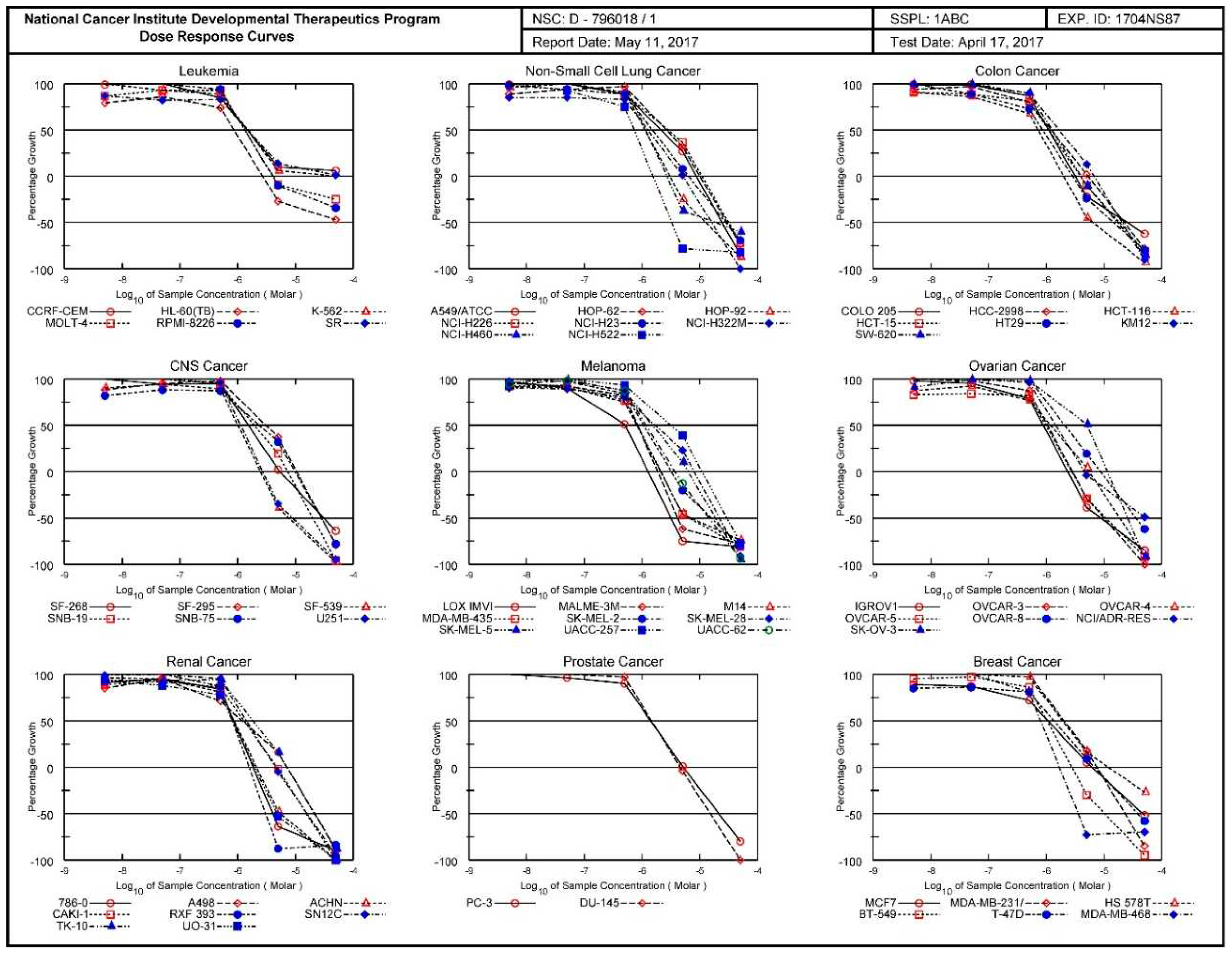
3.1.4. National Cancer Institute (NCI) Developmental Therapeutics Program (DTP) 60-Cancer Cell 5-Dose Testing Results with Compound 2

3.1.4. National Cancer Institute (NCI) Developmental Therapeutics Program (DTP) 60-Cancer Cell 5-Dose Testing Results with Compound 3
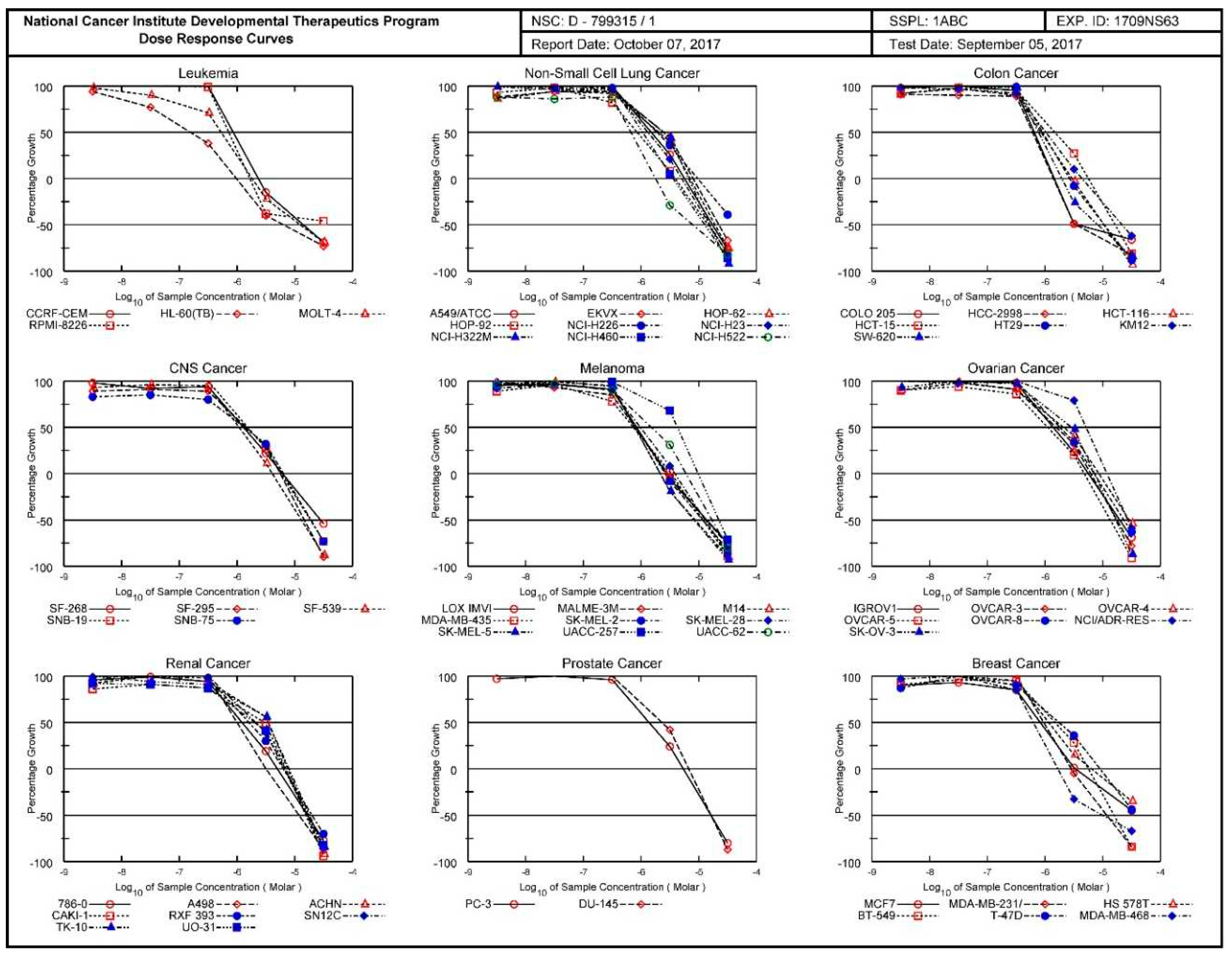
3.1.5. Cytochrome c Assay (Mitochondrial and Cytosolic) with the Drugs Compound 1, Compound 2 and Compound 3 in the Human Prostate Cancer Cell Lines PC-3 and DU-145
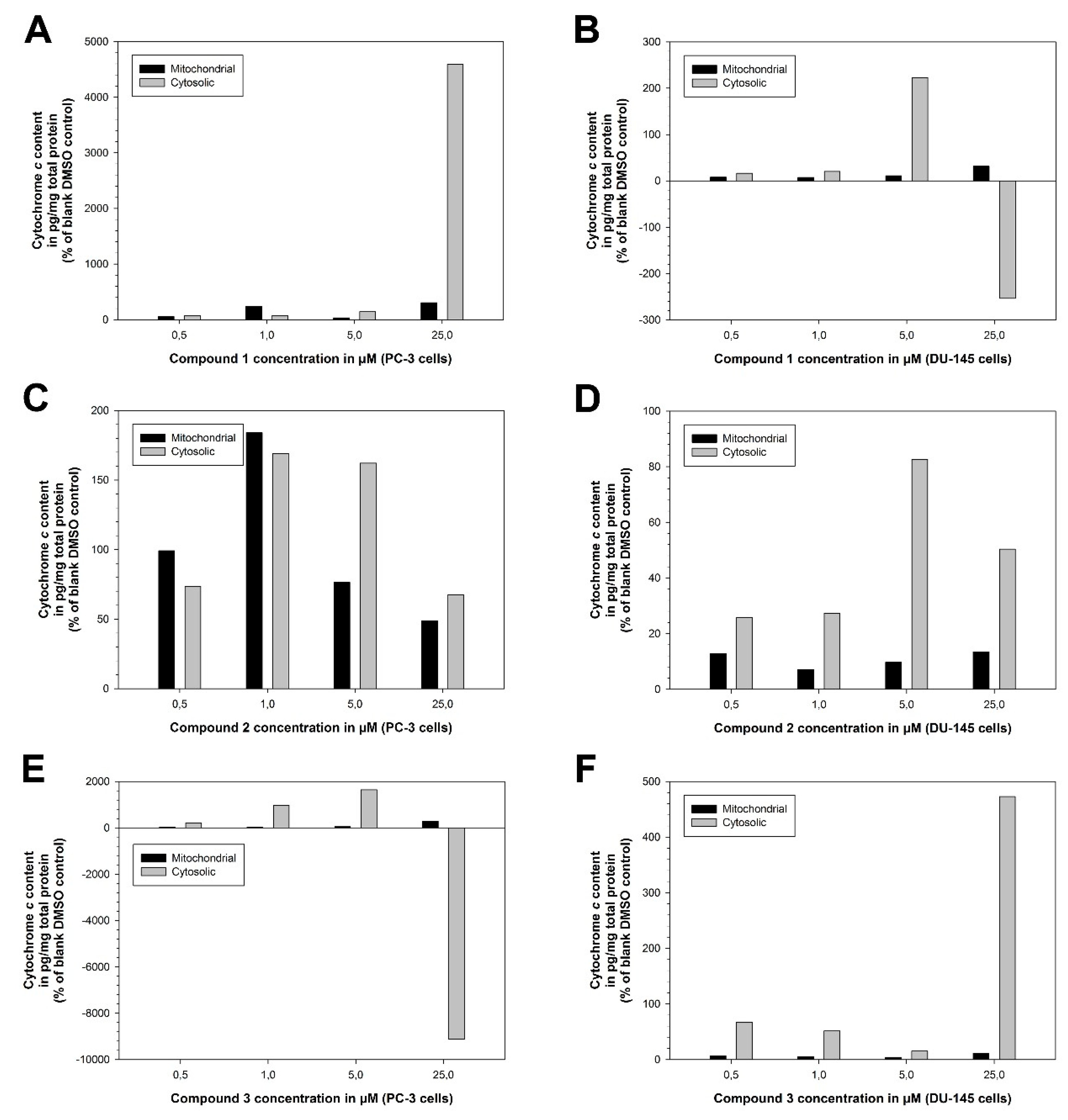
3.1.6. HIV-1LAI Replication Reverse Transcriptase Assay with the Drug Compound 1 in Primary PBL Cells
| Drug | Cytotoxicity CC50 (µM) and CCRF–CEM growth inhibition at fixed 1 µM concentration (%, in parentheses) | Anti-HIV-1LAI activity EC50 (µM)/EC90 (µM) in PBM cells | |||||
| PBM cells | CCRF −CEM |
Vero | EC50 | EC90 | SI50 | r2 | |
| Compound 1 (PT162, NSC 796018) |
2.2 | < 1 (60.0) |
1.8 | 0.56 | 4.3 | 3.9 | 0.93 |
| AZT* | > 100 | 14.3 | 56.0 | 0.0044 ± 0.0018 | 0.031 ± 0.015 | > 22,727 | 0.99 |
4. Experimental Section
4.1. Materials and Methods
4.1.1. Chemicals
4.1.2. Cell Lines
4.1.3. Analytical Methods
4.1.4. Software
4.2. Biological Testing
4.2.1. National Cancer Institute (NCI) Developmental Therapeutics Program (DTP) 60-Cancer Cell 5-Dose Testing
4.2.2. Cytochrome c Assay (Mitochondrial and Cytosolic)
4.2.3. Cytotoxicity and HIV-1LAI Replication Reverse Transcriptase Assays with Compound 1
4.3. X-Ray Crystallographic Determination of the Crystal and Molecular Structure of (M)-10-(2-Carbamothioylhydrazinyl)-10-demethoxycolchicine Sesquihydrate × ½ (Ethyl Acetate) = N-[(aS,7S)-10-(2-Carbamothioylhydrazinyl)-1,2,3-trimethoxy-9-oxo-5,6,7,9-tetrahydrobenzo[a]heptalen-7-yl]acetamide Sesquihydrate × ½ (Ethyl Acetate) (Crystalline Compound 2)
4.3.1. Crystallization of Compound 2 Single Crystals
4.3.2. Crystal Data of Compound 2 Single Crystals
4.3.3. Deposition of the X-Ray Crystallographic Structure Determination of Compound 2
4.4. Chemical Synthesis
4.4.1. Salt-Containing Tetrakis{3-[(tricyclo[3.3.1.13,7]decan-1-ammonio)methyl]benzyl}ammonium Pentachloride (Compound 1 × 1.5 NaCl, PT162 × 1.5 NaCl)
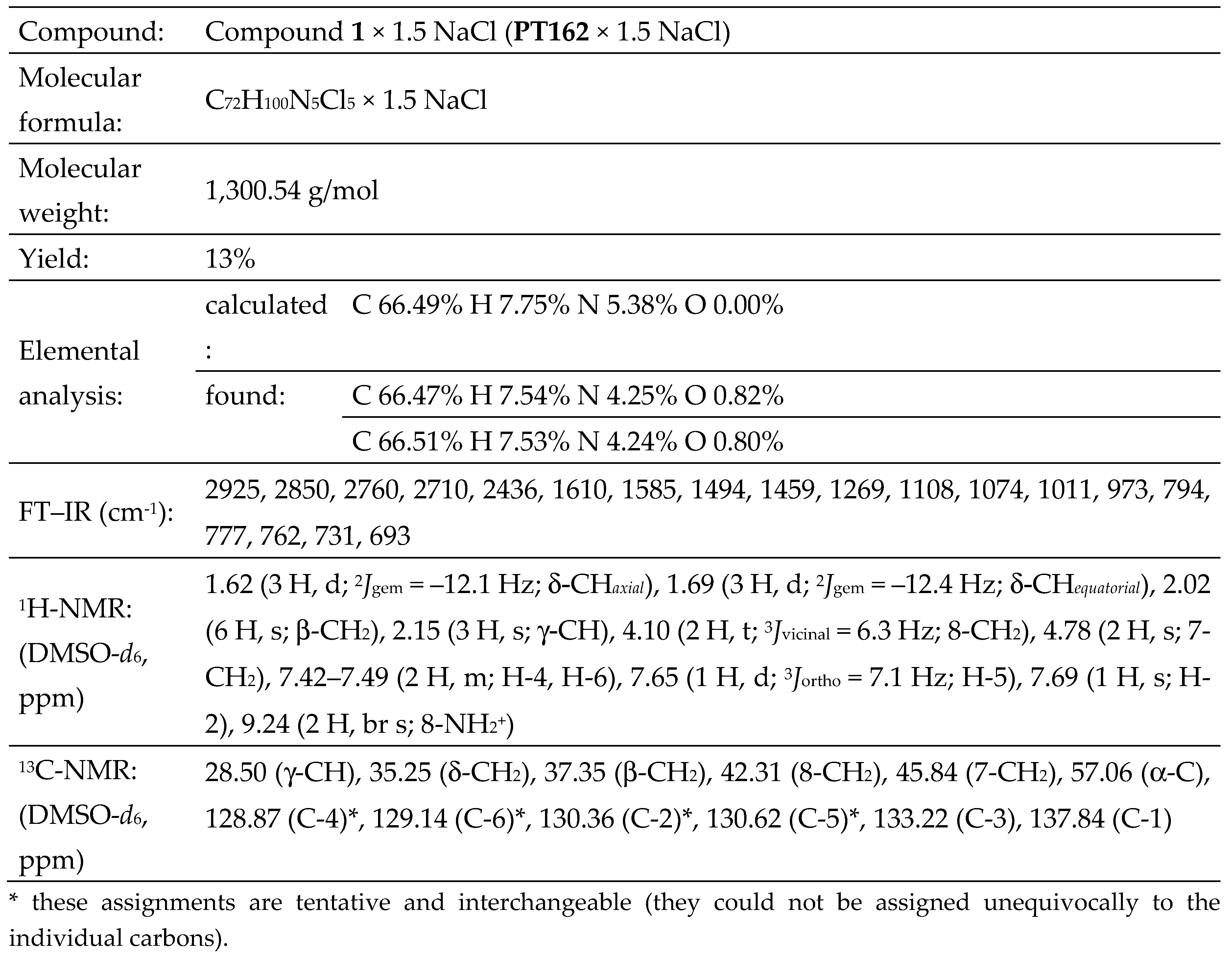
4.4.2. Pure (Salt-Free) Tetrakis{3-[(tricyclo[3.3.1.13,7]decan-1-ammonio)methyl]benzyl}ammonium Pentachloride (Compound 1, PT162, NSC 796018)

- (Compound 1, PT162, NSC 796018) = tetrakis{3-[(tricyclo[3.3.1.13,7]decan-1-ammonio)methyl]benzyl}ammonium pentachloride (C72H100Cl5N5) (M = 1,212.86 g/mol) [w (n/n) ≥ 99% (1H-NMR and elemental analysis)], synthesized at Friday, December 30th, 2016.
- (Compound 2, PT166, NSC 750423) = N-[(7S)-10-(2-carbamothioylhydrazinyl)-1,2,3-trimethoxy-9-oxo-5,6,7,9-tetrahydrobenzo[a]heptalen-7-yl]acetamide monohydrate × ⅔ (ethyl acetate) [C22H26N4O5S × H2O × ⅔ (C4H8O2)] (M = 535.28 g/mol) [w (n/n) ≥ 98% (1H-NMR and elemental analysis)], synthesized at Thursday, January 29th, 2009.

5. Conclusions
- p53 can activate DNA repair proteins when DNA has suffered sustained damage. Thus, it may be an important factor in aging.
- p53 can arrest growth by holding the cell cycle at the G1/S boundary on DNA damage recognition — if it stops the cell cycle here for long enough time, the DNA repair machinery will have time to fix the damage and the cells will be allowed to continue the cell cycle [104].
- p53 can initiate apoptosis (genetically programmed cell death) if DNA damage proves to be irreparable [104].
- p53 is essential for the senescence response to shortened telomeres.
- p53 acts as an inhibitor of angiogenesis generally mediated by vascular endothelial growth factor A (VEGF-A).
- p53 plays an important role in the differentiation and maintenance of human stem cells throughout embryonal development and adult human life.
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Warburg, O. The Metabolism of Tumours (translated by Dickens, F.); Constable: London, 1930. [Google Scholar]
- Warburg, O.; Posener, K.; Negelein, E. Über den Stoffwechsel der Carcinomzelle. Biochem. Z. (Berl.) 1924, 152, 309–344. [Google Scholar] [CrossRef]
- Warburg, O. Über den Stoffwechsel der Carcinomzelle. Naturwissenschaften 1924, 12, 1131–1137. [Google Scholar] [CrossRef]
- Warburg, O.; Wind, F.; Negelein, E. The metabolism of tumors in the body. J. Gen. Physiol. 1927, 8, 519–530. [Google Scholar] [CrossRef]
- Warburg, O. On the origin of cancer cells. Science 1956, 123, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Koppenol, W.H.; Bounds, P.L.; Dang, C.V. Otto Warburg’s contributions to current concepts of cancer metabolism. Nat. Rev. Cancer 2011, 11, 325–337. [Google Scholar] [CrossRef] [PubMed]
- Cuezva, J.M.; Krajewska, M.; López de Heredia, M.; Krajewski, S.; Santamaría, G.; Kim, H.; Zapata, J.M.; Marusawa, H.; Chamorro, M.; Reed, J.C. The bioenergetic signature of cancer: A marker of tumor progression. Cancer Res. 2002, 62, 6674–6681. [Google Scholar]
- Kim, J.-w.; Dang, C.V. Cancer’s molecular sweet tooth and the Warburg effect. Cancer Res. 2006, 66, 8927–8930. [Google Scholar] [CrossRef]
- Kiebish, M.A.; Han, X.; Cheng, H.; Chuang, J.H.; Seyfried, T.N. Cardiolipin and electron transport chain abnormalities in mouse brain tumor mitochondria: Lipidomic evidence supporting the Warburg theory of cancer. J. Lipid Res. 2008, 49, 2545–2556. [Google Scholar] [CrossRef]
- Jahnke, V.E.; Sabido, O.; Defour, A.; Castells, J.; Lefai, E.; Roussel, D.; Freyssenet, D. Evidence for mitochondrial respiratory deficiency in rat rhabdomyosarcoma cells. PLoS One 2010, 5, e8637. [Google Scholar] [CrossRef]
- Cori, C.F.; Cori, G.T. The carbohydrate metabolism of tumors. II. Changes in the sugar, lactic acid, and CO2-combining power of blood passing through a tumor. J. Biol. Chem. 1925, 65, 397–405. [Google Scholar] [CrossRef]
- Weinhouse, S. On respiratory impairment in cancer cells. Science 1956, 124, 267–269. [Google Scholar] [CrossRef]
- Warburg, O. Reply to the Editor. Science 1956, 124, 269–270. [Google Scholar] [CrossRef] [PubMed]
- Burk, D.; Schade, A.L. Letter to the Editor. Science 1956, 124, 270–272. [Google Scholar] [CrossRef] [PubMed]
- Quinn, P.J.; Dawson, R.M.C. Interactions of cytochrome c and [14C]carboxymethylated cytochrome c with monolayers of phosphatidylcholine, phosphatidic acid and cardiolipin. Biochem. J. 1969, 115, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Vik, S.B.; Georgevich, G.; Capaldi, R.A. Diphosphatidylglycerol is required for optimal activity of beef heart cytochrome c oxidase. Proc. Natl. Acad. Sci. U. S. A. 1981, 78, 1456–1460. [Google Scholar] [CrossRef] [PubMed]
- Speck, S.H.; Neu, C.A.; Swanson, M.S.; Margoliash, E. Role of phospholipid in the low affinity reactions between cytochrome c and cytochrome oxidase. FEBS Lett. 1983, 164, 379–382. [Google Scholar] [CrossRef]
- Rytömaa, M.; Mustonen, P.; Kinnunen, P.K.J. Reversible, nonionic, and pH-dependent association of cytochrome c with cardiolipin-phosphatidylcholine liposomes. J. Biol. Chem. 1992, 267, 22243–22248. [Google Scholar] [CrossRef] [PubMed]
- Hengartner, M.O. The biochemistry of apoptosis. Nature 2000, 407, 770–776. [Google Scholar] [CrossRef] [PubMed]
- Indran, I.R.; Tufo, G.; Pervaiz, S.; Brenner, C. Recent advances in apoptosis, mitochondria and drug resistance in cancer cells. Biochim. Biophys. Acta 2011, 1807, 735–745. [Google Scholar] [CrossRef]
- Danysz, W.; Parsons, C.G.; Kornhuber, J.; Schmidt, W.J.; Quack, G. Aminoadamantanes as NMDA receptor antagonists and antiparkinsonian agents – Preclinical studies. Neurosci. Biobehav. Rev. 1997, 21, 455–468. [Google Scholar] [CrossRef]
- Davies, W.L.; Grunert, R.R.; Haff, R.F.; McGahen, J.W.; Neumayer, E.M.; Paulshock, M.; Watts, J.C.; Wood, T.R.; Hermann, E.C.; Hoffmann, C.E. Antiviral activity of 1-adamantanamine (amantadine). Science 1964, 144, 862–863. [Google Scholar] [CrossRef] [PubMed]
- Kamada, R.; Nomura, T.; Anderson, C.W.; Sakaguchi, K. Cancer-associated p53 tetramerization domain mutants. Quantitative analysis reveals a low threshold for tumor suppressor inactivation. J. Biol. Chem. 2011, 286, 252–258. [Google Scholar] [CrossRef]
- Kesel, A.J. Broad-spectrum antiviral activity including human immunodeficiency and hepatitis C viruses mediated by a novel retinoid thiosemicarbazone derivative. Eur. J. Med. Chem. 2011, 46, 1656–1664. [Google Scholar] [CrossRef] [PubMed]
- Kesel, A.J.; Huang, Z.; Murray, M.G.; Prichard, M.N.; Caboni, L.; Nevin, D.K.; Fayne, D.; Lloyd, D.G.; Detorio, M.A.; Schinazi, R.F. Retinazone inhibits certain blood-borne human viruses including Ebola virus Zaire. Antivir. Chem. Chemother. 2014, 23, 197–215. [Google Scholar] [CrossRef]
- Landa, S.; Macháček, V. Sur l’adamantane, nouvel hydrocarbure extrait du naphte. Coll. Czech. Chem. Commun. 1933, 5, 1–5. [Google Scholar] [CrossRef]
- Hofmann, A.W. Einwirkung der Wärme auf die Ammoniumbasen. Ber. dtsch. chem. Ges. 1881, 14, 659–669. [Google Scholar] [CrossRef]
- Gano, J.E.; Eizenberg, L. Short-lived intermediates. IV. Adamantene. J. Am. Chem. Soc. 1973, 95, 972–974. [Google Scholar] [CrossRef]
- Bian, N.; Jones, M., Jr. More on adamantene. J. Am. Chem. Soc. 1995, 117, 8957–8961. [Google Scholar] [CrossRef]
- Tae, E.L.; Zhu, Z.; Platz, M.S. A matrix isolation, laser flash photolysis, and computational study of adamantene. J. Phys. Chem. A 2001, 105, 3803–3807. [Google Scholar] [CrossRef]
- Baek, J.Y.; Lee, S.J.; Han, B.H. Direct synthesis of symmetric ethers from carbonyl compounds using SbI3/PhSiH3. J. Korean Chem. Soc. 2004, 48, 220–224. [Google Scholar] [CrossRef]
- Nishiyama, T.; Kameyama, H.; Maekawa, H.; Watanuki, K. Ether synthesis using trifluoromethanesulfonic anhydride or triflates under mild reaction conditions. Can. J. Chem. 1999, 77, 258–262. [Google Scholar] [CrossRef]
- Burger, R.; Bigler, P. DEPTQ: Distorsionless enhancement by polarization transfer including the detection of quaternary nuclei. J. Magn. Res. 1998, 135, 529–534. [Google Scholar] [CrossRef] [PubMed]
- Berger, S.; Braun, S. 200 and More NMR Experiments. A Practical Course; 1st ed., Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, 2004. [Google Scholar]
- Kozłowski, E.; Biziuk, M. A new method for simultaneous determination of nitrogen, hydrogen and halogen in organic compounds. Mikrochim. Acta (Wien) 1979, 72, 1–17. [Google Scholar] [CrossRef]
- Sharifi, N.; Hamel, E.; Lill, M.A.; Risbood, P.; Kane, C.T., Jr.; Hossain, M.T.; Jones, A.; Dalton, J.T.; Farrar, W.L. A bifunctional colchicinoid that binds to the androgen receptor. Mol. Cancer Ther. 2007, 6, 2328–2336. [Google Scholar] [CrossRef] [PubMed]
- Mackay, M.F.; Morrison, J.D.; Gulbis, J.M. Crystal structure of triclinic colchiceine hemihydrate. Aust. J. Phys. 1985, 38, 413–420. [Google Scholar] [CrossRef]
- Silverton, J.V. The structure of colchiceine ethyl acetate–water solvate. Acta Crystallogr. B 1979, 35, 2800–2803. [Google Scholar] [CrossRef]
- Zeisel, S. Über Colchicin und Colchiceïn. Monatsh. Chem. 1883, 4, 162–164. [Google Scholar] [CrossRef]
- Zeisel, S. Über das Colchicin. I. Abhandlung. Monatsh. Chem. 1886, 7, 557–596. [Google Scholar] [CrossRef]
- Cook, J.W.; Loudon, J.D. Colchicine; In: Manske, R.H.F.; Holmes, H.L. (Eds.) The Alkaloids: Chemistry and Physiology; Academic Press Inc.: New York, 1952; Volume II, p. 265. [Google Scholar]
- King, M.V.; de Vries, J.L.; Pepinsky, R. An X-ray diffraction determination of the chemical structure of colchicine. Acta Crystallogr. 1952, 5, 437–440. [Google Scholar] [CrossRef]
- Lessinger, L.; Margulis, T.N. The crystal structure of colchicine. A new application of magic integers to multiple-solution direct methods. Acta Crystallogr. B 1978, 34, 578–584. [Google Scholar] [CrossRef]
- Clewer, H.W.B.; Green, S.J.; Tutin, F. The constituents of Gloriosa superba. J. Chem. Soc., Trans. 1915, 107, 835–846. [Google Scholar] [CrossRef]
- Clerici, F.; Mottadelli, S.; Rossi, L.M. 1H- and 13C-NMR spectra of thiocolchicine and derivatives: A complete analysis. J. Nat. Prod. 1995, 58, 259–263. [Google Scholar] [CrossRef]
- Meksuriyen, D.; Lin, L.-J.; Cordell, G.A.; Mukhopadhyay, S.; Banerjee, S.K. NMR studies of colchicine and its photoisomers, - and -lumicolchicines. J. Nat. Prod. 1988, 51, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Iorio, M.A.; Brossi, A.; Silverton, J.V. 7-Oxo-deacetamidocolchiceine and 7-benzylimino-deacetamido-colchiceine: Two novel products from the base catalysed reaction of (–)-N-benzylidene-deacetylcolchiceine. Helv. Chim. Acta 1978, 61, 1213–1220. [Google Scholar] [CrossRef]
- Hufford, C.D.; Capraro, H.-G.; Brossi, A. 13C- and 1H-NMR. Assignments for colchicine derivatives. Helv. Chim. Acta 1980, 63, 50–56. [Google Scholar] [CrossRef]
- Horowitz, R.M.; Ullyot, G.E. Colchicine. Some reactions of ring C. J. Am. Chem. Soc. 1952, 74, 587–592. [Google Scholar] [CrossRef]
- Šantavý, Fr. Préparation de l’acide colchicique à partir de la colchicine. Helv. Chim. Acta 1948, 31, 821–826. [Google Scholar] [CrossRef]
- Fernholz, H. Über die Umlagerung des Colchicins mit Natriumalkoholat und die Struktur des Ringes C. Justus Liebigs Ann. Chem. 1950, 568, 63–72. [Google Scholar] [CrossRef]
- Hufford, C.D.; Collins, C.C.; Clark, A.M. Microbial transformations and 13C-NMR analysis of colchicine. J. Pharm. Sci. 1979, 68, 1239–1243. [Google Scholar] [CrossRef]
- Nozoe, T.; Ikemi, T.; Itô, S. Some derivatives from colchicine. Proc. Japan Acad. (Nippon-gakushiin-kiyô, Tokyo) 1954, 30, 609–613. [Google Scholar] [CrossRef]
- Nozoe, T.; Ikemi, T.; Itô, S. Derivatives of colchicine. Sci. Rep. Tôhoku Univ. Ser. 1 (Tôhoku-daigaku-rika-hôkoku, dai 1-shû, kagaku, Sendai) 1954, 38, 117–129. [Google Scholar]
- Windaus, A.; Schiele, H. Die Konstitution des Colchicins. Nachr. Ges. Wiss. Göttingen Math.-Phys. 1924. [Google Scholar]
- Cook, J.W.; Loudon, J.D. Colchicine; In: Manske, R.H.F.; Holmes, H.L. (Eds.) The Alkaloids: Chemistry and Physiology; Academic Press Inc.: New York, 1952; Volume II, p. 273. [Google Scholar]
- Cook, J.W.; Loudon, J.D. The tropolones. Quart. Rev. Chem. Soc. (London) 1951, 5, 99–130. [Google Scholar] [CrossRef]
- Pauson, P.L. Tropones and tropolones. Chem. Rev. 1955, 55, 9–136. [Google Scholar] [CrossRef]
- Dauben, H.J., Jr.; Ringold, H.J. Synthesis of tropone. J. Am. Chem. Soc. 1951, 73, 876–876. [Google Scholar] [CrossRef]
- Doering, W. von E.; Detert, F.L. Cycloheptatrienylium oxide. J. Am. Chem. Soc. 1951, 73, 876–877. [Google Scholar] [CrossRef]
- Redington, R.L.; Bock, C.W. MO study of singlets, triplets, and tunneling in tropolone. 1. Geometries, tunneling, and vibrations in the ground electronic state. J. Phys. Chem. 1991, 95, 10284–10294. [Google Scholar] [CrossRef]
- Brossi, A.; Yeh, H.J.C.; Chrzanowska, M.; Wolff, J.; Hamel, E.; Lin, C.M.; Quin, F.; Suffness, M.; Silverton, J. Colchicine and its analogues: Recent findings. Med. Res. Rev. 1988, 8, 77–94. [Google Scholar] [CrossRef]
- Brossi, A.; Boyé, O.; Muzaffar, A.; Yeh, H.J.C.; Toome, V.; Wegrzynski, B.; George, C. aS,7S-absolute configuration of natural (–)-colchicine and allo-congeners. FEBS Lett. 1990, 262, 5–7. [Google Scholar] [CrossRef]
- Cahn, R.S.; Ingold, C.; Prelog, V. Spezifikation der molekularen Chiralität. Angew. Chem. 1966, 78, 413–447. [Google Scholar] [CrossRef]
- Cahn, R.S.; Ingold, C.; Prelog, V. Specification of Molecular Chirality. Angew. Chem. Int. Ed. Engl. 1966, 5, 385–415. [Google Scholar] [CrossRef]
- Detrich, H.W., III; Williams, R.C., Jr.; Macdonald, T.L.; Wilson, L.; Puett, D. Changes in the circular dichroic spectrum of colchicine associated with its binding to tubulin. Biochemistry 1981, 20, 5999–6005. [Google Scholar] [CrossRef] [PubMed]
- Berg, U.; Bladh, H. The absolute configuration of colchicine by correct application of the CIP rules. Helv. Chim. Acta 1999, 82, 323–325. [Google Scholar] [CrossRef]
- Corrodi, H.; Hardegger, E. Die Konfiguration des Colchicins und verwandter Verbindungen. Helv. Chim. Acta 1955, 38, 2030–2033. [Google Scholar] [CrossRef]
- Bagnato, J.D.; Eilers, A.L.; Horton, R.A.; Grissom, C.B. Synthesis and characterization of a cobalamin–colchicine conjugate as a novel tumor-targeted cytotoxin. J. Org. Chem. 2004, 69, 8987–8996. [Google Scholar] [CrossRef]
- Bombuwala, K.; Kinstle, T.; Popik, V.; Uppal, S.O.; Olesen, J.B.; Viña, J.; Heckman, C.A. Colchitaxel, a coupled compound made from microtubule inhibitors colchicine and paclitaxel. Beilstein J. Org. Chem. 2006, 2, 13. [Google Scholar] [CrossRef] [PubMed]
- Sustmann, R.; Brandes, D.; Lange, F.; Tashtoush, H.I. Substituent effects in cycloheptatrienyl radicals: An ESR spectroscopic study. Magn. Reson. Chem. 1989, 27, 340–343. [Google Scholar] [CrossRef]
- Fischer, K.H.; Hemberger, P.; Bodi, A.; Fischer, I. Photoionisation of the tropyl radical. Beilstein J. Org. Chem. 2013, 9, 681–688. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.W. Organic Chemistry; Volume 7, Ylid Chemistry, Ed.; 1st ed., Academic Press: New York, London, 1966; p. 251283. [Google Scholar]
- Musker, W.K.; Stevens, R.R. Nitrogen ylides. V. Coordination chemistry of trimethylammonium methylide. Inorg. Chem. 1969, 8, 255–264. [Google Scholar] [CrossRef]
- Wittig, G.; Wetterling, M.-H. Darstellung und Eigenschaften des Trimethyl-ammonium-methylids. Justus Liebigs Ann. Chem. 1947, 557, 193–201. [Google Scholar] [CrossRef]
- Nekrasov, B.V. Pentavalent nitrogen. Bull. Acad. Sci. U. S. S. R., Div. Chem. Sci. (Russ. Chem. Bull.) 1979, 28, 1789–1790. [Google Scholar] [CrossRef]
- Kurzydłowski, D.; Zaleski-Ejgierd, P. Hexacoordinated nitrogen(V) stabilized by high pressure. Sci. Rep. 2016, 6, 36049. [Google Scholar] [CrossRef]
- Yan, C.; Takeshita, M.; Nakatsuji, J.-y.; Kurosaki, A.; Sato, K.; Shang, R.; Nakamoto, M.; Yamamoto, Y.; Adachi, Y.; Furukawa, K.; Kishi, R.; Nakano, M. Synthesis and properties of hypervalent electron-rich pentacoordinate nitrogen compounds. Chem. Sci. 2020, 11, 5082–5088. [Google Scholar] [CrossRef]
- Ikeda, E.; Tranter, R.S.; Kiefer, J.H.; Kern, R.D.; Singh, H.J.; Zhang, Q. The pyrolysis of methylcyclopentadiene: Isomerization and formation of aromatics. Proc. Combust. Inst. 2000, 28, 1725–1732. [Google Scholar] [CrossRef]
- Hanamirian, B.; Della Libera, A.; Pratali Maffei, L.; Cavallotti, C. Investigation of methylcyclopentadiene reactivity: Abstraction reactions and methylcyclopentadienyl radical unimolecular decomposition. J. Phys. Chem. A 2023, 127, 1314–1328. [Google Scholar] [CrossRef] [PubMed]
- Berglind, H.; Pawitan, Y.; Kato, S.; Ishioka, C.; Soussi, T. Analysis of p53 mutation status in human cancer cell lines. A paradigm for cell line cross-contamination. Cancer Biol. Ther. 2008, 7, 699–708. [Google Scholar] [CrossRef] [PubMed]
- Muller, P.A.J.; Vousden, K.H. Mutant p53 in cancer: New functions and therapeutic opportunities. Cancer Cell 2014, 25, 304–317. [Google Scholar] [CrossRef]
- Foley, G.E.; Lazarus, H.; Farber, S.; Uzman, B.G.; Boone, B.A.; McCarthy, R.E. Continuous culture of human lymphoblasts from peripheral blood of a child with acute leukemia. Cancer 1965, 18, 522–529. [Google Scholar] [CrossRef]
- Subler, M.A.; Martin, D.W.; Deb, S. Inhibition of viral and cellular promoters by human wild-type p53. J. Virol. 1992, 66, 4757–4762. [Google Scholar] [CrossRef] [PubMed]
- Subler, M.A.; Martin, D.W.; Deb, S. Activation of the human immunodeficiency virus type 1 long terminal repeat by transforming mutants of human p53. J. Virol. 1994, 68, 103–110. [Google Scholar] [CrossRef]
- Duan, L.; Ozaki, I.; Oakes, J.W.; Taylor, J.P.; Khalili, K.; Pomerantz, R.J. The tumor suppressor protein p53 strongly alters human immunodeficiency virus type 1 replication. J. Virol. 1994, 68, 4302–4313. [Google Scholar] [CrossRef]
- El-Deiry, W.S.; Tokino, T.; Velculescu, V.E.; Levy, D.B.; Parsons, R.; Trent, J.M.; Lin, D.; Mercer, W.E.; Kinzler, K.W.; Vogelstein, B. WAF1, a potential mediator of p53 tumor suppression. Cell 1993, 75, 817–825. [Google Scholar] [CrossRef] [PubMed]
- Shi, B.; Sharifi, H.J.; DiGrigoli, S.; Kinnetz, M.; Mellon, K.; Hu, W.; de Noronha, C.M.C. Inhibition of HIV early replication by the p53 and its downstream gene p21. Virol. J. 2018, 15, 53. [Google Scholar] [CrossRef]
- Chowdhury, I.H.; Wang, X.-F.; Landau, N.R.; Robb, M.L.; Polonis, V.R.; Birx, D.L.; Kim, J.H. HIV-1 Vpr activates cell cycle inhibitor p21/Waf1/Cip1: A potential mechanism of G2/M cell cycle arrest. Virology 2003, 305, 371–377. [Google Scholar] [CrossRef] [PubMed]
- Li, C.J.; Wang, C.; Friedman, D.J.; Pardee, A.B. Reciprocal modulations between p53 and Tat of human immunodeficiency virus type 1. Proc. Natl. Acad. Sci. U. S. A. 1995, 92, 5461–5464. [Google Scholar] [CrossRef] [PubMed]
- Longo, F.; Marchetti, M.A.; Castagnoli, L.; Battaglia, P.A.; Gigliani, F. A novel approach to protein‒protein interaction: Complex formation between the p53 tumor suppressor and the HIV Tat proteins. Biochem. Biophys. Res. Commun. 1995, 206, 326–334. [Google Scholar] [CrossRef] [PubMed]
- Ariumi, Y.; Kaida, A.; Hatanaka, M.; Shimotohno, K. Functional cross-talk of HIV-1 Tat with p53 through its C-terminal domain. Biochem. Biophys. Res. Commun. 2001, 287, 556–561. [Google Scholar] [CrossRef] [PubMed]
- Greenway, A.L.; McPhee, D.A.; Allen, K.; Johnstone, R.; Holloway, G.; Mills, J.; Azad, A.; Sankovich, S.; Lambert, P. Human immunodeficiency virus type 1 Nef binds to tumor suppressor p53 and protects cells against p53-mediated apoptosis. J. Virol. 2002, 76, 2692–2702. [Google Scholar] [CrossRef]
- Sawaya, B.E.; Khalili, K.; Mercer, W.E.; Denisova, L.; Amini, S. Cooperative actions of HIV-1 Vpr and p53 modulate viral gene transcription. J. Biol. Chem. 1998, 273, 20052–20057. [Google Scholar] [CrossRef] [PubMed]
- Vichai, V.; Kirtikara, K. Sulforhodamine B colorimetric assay for cytotoxicity screening. Nat. Protoc. 2006, 1, 1112–1116. [Google Scholar] [CrossRef]
- Schinazi, R.F.; Sommadossi, J.-P.; Saalmann, V.; Cannon, D.L.; Xie, M.-Y.; Hart, G.C.; Smith, G.A.; Hahn, E.F. Activities of 3′-azido-3′-deoxythymidine nucleotide dimers in primary lymphocytes infected with human immunodeficiency virus type 1. Antimicrob. Agents Chemother. 1990, 34, 1061–1067. [Google Scholar] [CrossRef]
- Spira, T.J.; Bozeman, L.H.; Holman, R.C.; Warfield, D.T.; Phillips, S.K.; Feorino, P.M. Micromethod for assaying reverse transcriptase of human T-cell lymphotropic virus type III/lymphadenopathy-associated virus. J. Clin. Microbiol. 1987, 25, 97–99. [Google Scholar] [CrossRef]
- Stuyver, L.J.; Lostia, S.; Adams, M.; Mathew, J.S.; Pai, B.S.; Grier, J.; Tharnish, P.M.; Choi, Y.; Chong, Y.; Choo, H.; Chu, C.K.; Otto, M.J.; Schinazi, R.F. Antiviral activities and cellular toxicities of modified 2′,3′-dideoxy-2′,3′-didehydrocytidine analogues. Antimicrob. Agents Chemother. 2002, 46, 3854–3860. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. A 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Flack, H.D. On enantiomorph-polarity estimation. Acta Crystallogr. A 1983, 39, 876–881. [Google Scholar] [CrossRef]
- Lane, D.P.; Crawford, L.V. T antigen is bound to a host protein in SV40-transformed cells. Nature 1979, 278, 261–263. [Google Scholar] [CrossRef] [PubMed]
- Linzer, D.I.H.; Levine, A.J. Characterization of a 54K dalton cellular SV40 tumor antigen present in SV40-transformed cells and uninfected embryonal carcinoma cells. Cell 1979, 17, 43–52. [Google Scholar] [CrossRef] [PubMed]
- DeLeo, A.B.; Jay, G.; Appella, E.; Dubois, G.C.; Law, L.W.; Old, L.J. Detection of a transformation-related antigen in chemically induced sarcomas and other transformed cells of the mouse. Proc. Natl. Acad. Sci. U. S. A. 1979, 76, 2420–2424. [Google Scholar] [CrossRef]
- Levine, A.J.; Lane, D.P. (Eds.) The p53 Family, Cold Spring Harbor Perspectives in Biology; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 2010. [Google Scholar]
- Musgrove, E.A.; Caldon, C.E.; Barraclough, J.; Stone, A.; Sutherland, R.L. Cyclin D as a therapeutic target in cancer. Nat. Rev. Cancer 2011, 11, 558–572. [Google Scholar] [CrossRef]
- Tashiro, E.; Tsuchiya, A.; Imoto, M. Functions of cyclin D1 as an oncogene and regulation of cyclin D1 expression. Cancer Sci. 2007, 98, 629–635. [Google Scholar] [CrossRef]
- Diehl, J.A. Cycling to cancer with cyclin D1. Cancer Biol. Ther. 2002, 1, 226–231. [Google Scholar] [CrossRef]
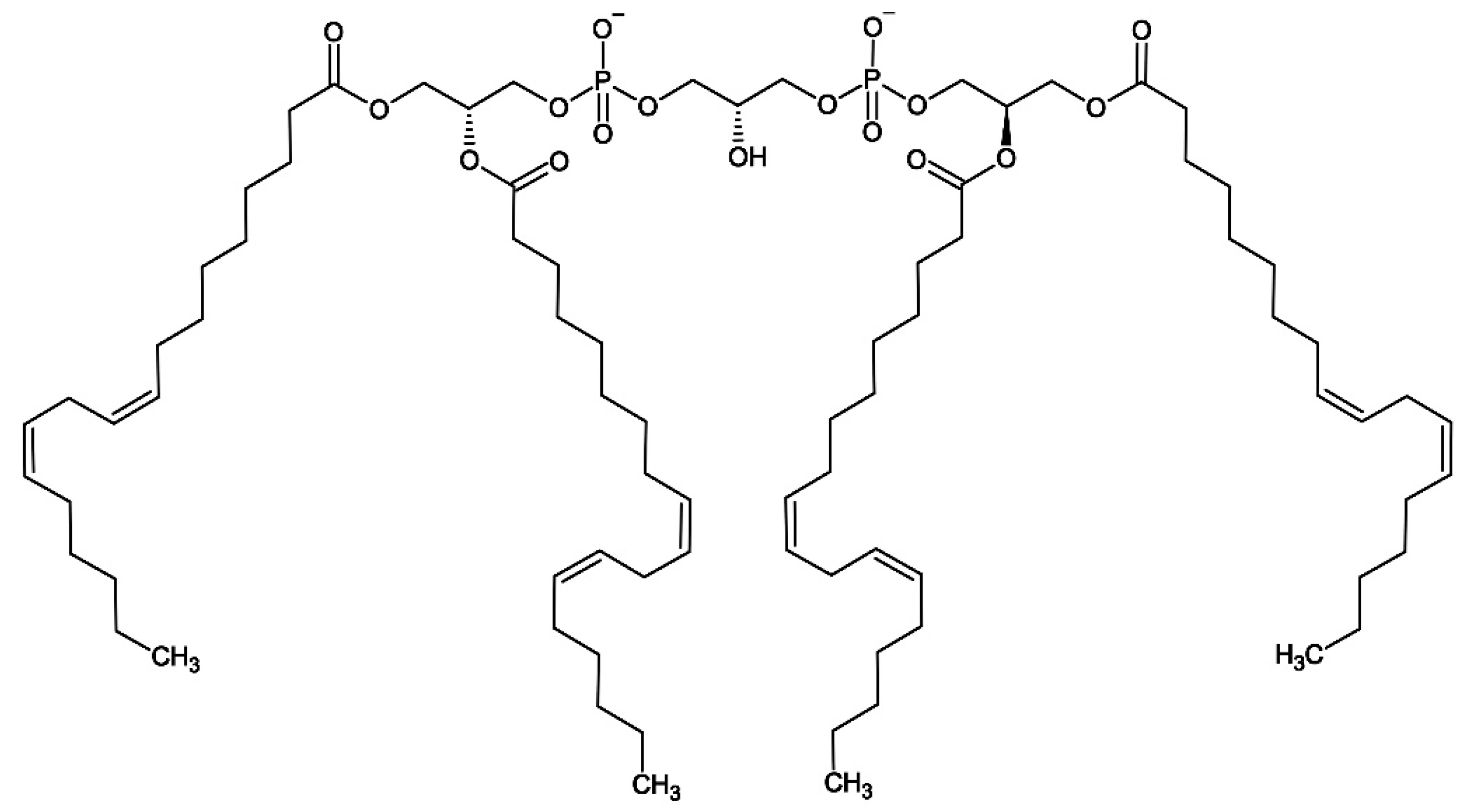
- Schlame, M.; Brody, S.; Hostetler, K.Y. Mitochondrial cardiolipin in diverse eukaryotes. Comparison of biosynthetic reactions and molecular acyl species. Eur. J. Biochem. 1993, 212, 727–735. [Google Scholar] [CrossRef] [PubMed]
- Uppuluri, S.; Knipling, L.; Sackett, D.L.; Wolf, J. Localization of the colchicine-binding site of tubulin. Proc. Natl. Acad. Sci. U. S. A. 1993, 90, 11598–11602. [Google Scholar] [CrossRef] [PubMed]
- Andreu, J.M.; Perez-Ramirez, B.; Gorbunoff, M.J.; Ayala, D.; Timasheff, S.N. Role of the colchicine ring A and its methoxy groups in the binding to tubulin and microtubule inhibition. Biochemistry 1998, 37, 8356–8368. [Google Scholar] [CrossRef] [PubMed]
- Bai, R.; Covell, D.G.; Pei, X.-F.; Ewell, J.B.; Nguyen, N.Y.; Brossi, A.; Hamel, E. Mapping the binding site of colchicinoids on -tubulin. 2-Chloroacetyl-2-demethylthiocolchicine covalently reacts predominantly with cysteine 239 and secondarily with cysteine 354. J. Biol. Chem. 2000, 275, 40443–40452. [Google Scholar] [CrossRef] [PubMed]
- Ravelli, R.B.G.; Gigant, B.; Curmi, P.A.; Jourdain, I.; Lachkar, S.; Sobel, A.; Knossow, M. Insight into tubulin regulation from a complex with colchicine and a stathmin-like domain. Nature 2004, 428, 198–202. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, H.; Gigant, B.; Yu, Y.; Wu, Y.; Chen, X.; Lai, Q.; Yang, Z.; Chen, Q.; Yang, J. Structures of a diverse set of colchicine binding site inhibitors in complex with tubulin provide a rationale for drug discovery. FEBS J. 2016, 283, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Pascale, R.M.; Calvisi, D.F.; Simile, M.M.; Feo, C.F.; Feo, F. The Warburg effect 97 years after its discovery. Cancers (Basel) 2020, 12, 2819. [Google Scholar] [CrossRef]
- Liu, C.; Jin, Y.; Fan, Z. Mechanism of Warburg effect-induced chemoresistance in cancer. Front. Oncol. 2021, 11, 698023. [Google Scholar] [CrossRef] [PubMed]
- Sapandowski, A.; Stope, M.; Evert, K.; Evert, M.; Zimmermann, U.; Peter, D.; Päge, I.; Burchardt, M.; Schild, L. Cardiolipin composition correlates with prostate cancer cell proliferation. Mol. Cell. Biochem. 2015, 410, 175–185. [Google Scholar] [CrossRef]
- Ahmadpour, S.T.; Mahéo, K.; Servais, S.; Brisson, L.; Dumas, J.-F. Cardiolipin, the mitochondrial signature lipid: Implication in cancer. Int. J. Mol. Sci. 2020, 21, 8031. [Google Scholar] [CrossRef]
- Di Carlo, C.; Sousa, B.C.; Manfredi, M.; Brandi, J.; Dalla Pozza, E.; Marengo, E.; Palmieri, M.; Dando, I.; Wakelam, M.J.O.; Lopez-Clavijo, A.F.; Cecconi, D. Integrated lipidomics and proteomics reveal cardiolipin alterations, upregulation of HADHA and long chain fatty acids in pancreatic cancer stem cells. Sci. Rep. 2021, 11, 13297. [Google Scholar] [CrossRef] [PubMed]
- Krieger, A.C.; Macias, L.A.; Goodman, J.C.; Brodbelt, J.S.; Eberlin, L.S. Mass spectrometry imaging reveals abnormalities in cardiolipin composition and distribution in astrocytoma tumor tissues. Cancers (Basel) 2023, 15, 2842. [Google Scholar] [CrossRef] [PubMed]
- Kerr, J.F.R.; Wyllie, A.H.; Currie, A.R. Apoptosis: A basic biological phenomenon with wide-ranging implications in tissue kinetics. Br. J. Cancer 1972, 26, 239–257. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z. The chemical biology of apoptosis: Exploring protein-protein interactions and the life and death of cells with small molecules. Chem. Biol. (Cell Chem. Biol.) 2002, 9, 1059–1072. [Google Scholar]
- Wong, R.S.Y. Apoptosis in cancer: From pathogenesis to treatment. J. Exp. Clin. Cancer Res. 2011, 30, 87. [Google Scholar] [CrossRef] [PubMed]
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef]
- Cavalcante, G.C.; Schaan, A.P.; Cabral, G.F.; Santana-da-Silva, M.N.; Pinto, P.; Vidal, A.F.; Ribeiro-Dos-Santos, Â. A cell’s fate: An overview of the molecular biology and genetics of apoptosis. Int. J. Mol. Sci. 2019, 20, 4133. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).