1. Introduction
In modern times of global climate change challenging society and conservation science, there are cumulative alarms on increasing human population and concerns on finding ways to enhance crop production to meet such demand [
1,
2]. Fertilizers are in authority for increasing crop yield by providing the essential nutrients for plants. On the other hand, the volatilization and leakage procedures have an impact on 30 to 50% loss of the applied fertilizers, causing severe pollution problems in rivers, lakes, waters, seawaters, and soil. Furthermore, to overcome such losses, the quantity of applied fertilizers is always greater than needed for crop uptake. New combinations of fertilizing materials have been planned to overcome these problems, namely Enhanced Efficiency Fertilizers (EEFs). The EEFs can regulate the accessibility of nutrients in the soil, decreasing nitrogen losses and pollution, so increasing crop yield for sustained food security without compromising human health and the environment [
3,
4].
Modern research in agriculture has developed distinctive groups of materials with innovative properties to improve agribusiness and increase crop production. Over the last 20 years, polysaccharide-based fertilizers have gathered significant consideration in the frame of EEFs. By being biodegradable, non-toxic, water-soluble, swellable, easily chemically modified and by increasing the disposal of nutrients, polysaccharide-based fertilizers are gaining a title role in agricultural practices [
5]. Polysaccharides have further been used in several agricultural practices, such as in plant protection against biotic and abiotic stresses and in recovery of contaminated soil and water [
6], in plant seed priming [
7] and in improvement of plant product quality and bioactivity [
8].
The INM fertilization usually employs liquid polysaccharide-based fertilizers, further consisting of commercial chemicals, systemic copper, biostimulants, organic calcium and urea as a nitrogen source. Plant biostimulants applied to plants comprise any material with the capacity to enhance nutrient use or stress toleration or to ameliorate quality features regardless their composition. The polysaccharides usually include compounds with iron, aluminum, zinc, and copper, which make them more easily available for plants. Amongst nutrients, copper is a critical micronutrient and its application together with polysaccharides has been well-established as beneficial for cultivation [
9]. The INM is mainly based on organic edible raw materials and plant extracts, resulting in a full supplement for plants with amino acids, vitamins, sugars, and macro-microelements. A characteristic of INM is that, beyond its role as a simple plant nutrient provider, it also feeds the soil biota due to its content in polysaccharides. Energetic microbial flora, in turn, closely associates with plant health, increased biomass, decreased nutrient leaking and higher nutrient availability. The microorganisms also mineralize organic materials, releasing elements such as nitrogen, phosphorus, sulphur, potassium, iron, and may enrich the soil with vitamins and antibiotics that are absorbed by crops. Furthermore, INM polysaccharides discharge and attach to clay particles, hence, together with microorganisms, contribute to the formation of stable soil aggregates. Such combinations may often lead to an early-harvesting period, achieving the highest yield and quality of products; these are able to control the biological nitrogen transformations in soils and additionally may contribute to an exceptional strengthening of plants, which can become more resistant to cold and UV-sunburns [
10]. Numerous recent publications on polysaccharide-based INM fertilization schemes have shown to date that the paybacks of EEFs as a result of evaded social costs of nitrogen losses, may substantially overshadow their costs, and thus, they are highly recommended for integration in fertilizer strategies (e.g., [
9,
10,
11,
12,
13]).
The fertilization formula sets not only the expected yield, but also defines product quality [
14]. Quality is a broad term, encompassing several features of varying importance depending on the intended use [
15,
16]. In medicinal plants, the content of antioxidant compounds is traditionally a critical index of quality [
17,
18]. These secondary metabolite compounds are referred to as health promoters, holding both preventative and therapeutic action against a variety of diseases and disorders [
19,
20]. Carotenoids, flavonoids, and phenolics are common and major antioxidant compounds exhibiting a strong antioxidant ability [
17,
18]. Notably, organic fertilizers and INM strategies have been associated with increased antioxidant compound accumulation [
19,
21,
22,
23,
24,
25,
26,
27,
28]. Organic fertilizers and INM strategies have also been suggested to act as a tool in promoting leaf chlorophyll concertation [
29] that is tightly related to green coloration intensity, which in an alternative and widely used quality index of herbal material [
15].
Plant products have been traditionally employed for healing and curing a variety of diseases and disorders in large parts of the world [
30,
31,
32] and the use of medicinal-aromatic plants (MAPs) and concomitant natural products is also bursting in Western societies to date [
33,
34]. For instance, 400 MAPs are commercially traded for therapeutic purposes in India, while over 1500 ones in Germany; in both countries and against expectations, over 93% of these volumes are still sourced from wild populations [
34]. Due to this growing demand, genetic resources are inevitably depleted in many cases, however, this situation not only raises the risk of species extinction due to overexploitation but can also disturb the ecological balance at ecosystem level [
30,
35]. Provided that alternative options are rather limited, the introduction of focal wild-growing species of commercial interest in agricultural settings is a sustainable remedy to overexploitation of wild genetic resources. To make effective such an endeavor, a well-documented initial plant material should be employed coupled with extant propagation protocols and cultivation guidelines applied in well-designed and well-managed cultivation systems.
Species-wise, the present investigation has focused on
Origanum dictamnus L. (Lamiaceae). The focal plant species is a perennial local endemic chasmophyte of Crete (Greece) threatened with extinction, i.e., assessed as Endangered according to [
36], Vulnerable according to [
37], or Near Threatened according to Flora of Greece web (
https://portal.cybertaxonomy.org/flora-greece/cdm_dataportal/taxon/11dfbc28-3e28-4187-bce3-ac6178bee770, accessed 30 November 2023).
O. dictamnus L. is traditionally cultivated at local scale in Crete as a medicinal-aromatic plant, strongly associated since ancient times with the Cretan cultural heritage [
38,
39] and as a contemporary yet traditional herbal medicinal product widely used for tea preparation, medicinal purposes natural food flavoring and/or food preservative [
39,
40]; in addition, it is associated with therapeutic indications approved by the European Medicines Agency (
https://www.ema.europa.eu/en/medicines/herbal/origani-dictamni-herba, accessed 30 November 2023). Furthermore,
O. dictamnus is known to produce polysaccharide-containing subcuticular compartments and possesses biological activity against phytopathogenic fungi, significantly affecting among others, the fungal cell wall polysaccharides [
41].
Context-wise, the present study comparatively investigated the effects of different fertilization treatments and application techniques in a pilot field cultivation of
O. dictamnus in Crete, contrasting a polysaccharide-based INM fertilization protocol with other schemes. The developed protocol responds to the extant information gap regarding the fertilization requirements of
O. dictamnus and was specifically designed to improve its yield as well as critical quality aspects (i.e., colour intensity, antioxidant element concentration). In addition, this investigation explored nutrients’ accumulation and partitioning in different plant organs of
O. dictamnus, for which only little is regrettably known [
42]. Such a knowledge would be essential to induce growth and biomass yield potential of
O. dictamnus by regulating nutrients supply according to species-specific needs, further elucidating how the latter correlates with its physiological characteristics, oil production and antioxidant activity [
43]. This research complements similar investigations [
44] or other research lines related to the herein focal species (e.g., [
45,
46]), and is scoped to contribute to bridging the way towards the effective sustainable exploitation of such valuable Mediterranean genetic resources.
2. Materials and Methods
2.1. Focal Species and Origin of Plant Material
Dittany of Crete is a mat-forming and white-woolly chasmophyte 20–30 cm in height, with rounded leaves of velvety texture and lilac flowers bordered by purple-pink bracts in summer [
47].
O. dictamnus is a seasonally dimorphic plant having different appearance in winter and summer [
48]. Shoots of late winter plants are almost leaf-naked except for their apical region which bears a cluster of small leaves covered with a thick indumentum of non-glandular hairs (
Figure 1). Shoots of summer plants are vigorous with large green leaves. Non-glandular hairs are dendroid with a five-celled vertical stub and several lateral branches. Glandular hairs are of two types, large peltate hairs and small capitate hairs. Peltate hairs are numerous and consist of a 12-celled head, a unicellular stalk, and a basal epidermal cell. Peltate hairs secrete an essential oil high in carvacrol content. Peltate glandular hairs of
O. dictamnus create at the stage of secretion two subcuticular chambers, one large and bladder-like at the apex of the head (containing essential oil), and a small and ring-like one at the bottom of the head (containing polysaccharides). In the apical chamber containing the essential oil, a small lateral compartment is present comprising polysaccharides. The secretory product occurs in the form of glucosides that pass through the plasmalemma to enter the apical chamber which are hydrolysed to the aglycone fraction (essential oil) and the sugar fraction (polysaccharides) [
49].
Botanical expeditions were performed to explore different areas for wild-growing populations of
O. dictamnus along gorges and mountain plateaus of Crete, Greece (
Figure 1) in the frame of the research project “Conservation and sustainable utilization of rare-threatened endemic plants of Crete for the development of new products with innovative precision fertilization” (acronym: PRECISE-M, Τ1ΕΔΚ-05380). Seed collections were performed using an authorized special permit of the Institute of Plant Breeding and Genetic Resources, Hellenic Agricultural Organization Demeter (Permit 82336/879 of 18 May 2019 and 26895/1527, 21 April 2021) which is issued yearly by the Greek Ministry of Environment and Energy.
The collected seeds and voucher samples were taxonomically identified, and unique IPEN (International Plant Exchange Network) accession numbers were assigned to each independent collection (GR-1-BBGK-03,2108; GR-1-BBGK-19,330; GR-1-BBGK-21,91) by the Balkan Botanic Garden of Kroussia, Institute of Plant Breeding and Genetic Resources (IPBGR) of the Hellenic Agricultural Organization, Demeter (ELGO Dimitra). New plants were initially raised ex situ for further field experimentation [
44]. The plants used in the experimental procedure were transplanted in 2 L plastic pots by the company of AFI GLAVAKI KE SIA OE Tree & Plant Nurseries, Aridea, PELLAS, GR-58400, Greece.
2.2. Field Experiment
The field experiment was performed at the farm of the Hellenic Mediterranean University, Heraklion, Crete (Greece) in a research field of 20 × 25 m (35° 19' N, 25° 6' E, 60 m). The plants were established on the ground on the 1st of March, 2021. The distance between plants was 40 cm, and between rows was 80 cm. The rows were 20 m long (
Figure 1). These rows had east-west direction and included as “guard plants” other local Cretan endemics, i.e., individuals of
Sideritis syriaca L. subsp.
syriaca (Malotira or Cretan Mountain tea),
Origanum microphyllum (Benth.)
Vogel (Cretan marjoram, [
28]),
Carlina diae (Rech.f.)
Meusel & A.Kástner (Carline of Dia), and
Verbascum arcturus L. (Cretan mullein, [
27]), all investigated in the frame of the research project PRECISE-M.
The experimental design was performed in completely randomized blocks of 10 plants of
O. dictamnus per block, three blocks per treatment (see below), which were randomly located in different rows (
Figure 1. C). To achieve the same starting plant material, all plants were trimmed at 5 cm above ground level at the end of April. At the end of May (i.e., 30 d following trimming), six fertilization treatments were initiated which were weekly performed till the final harvest. An automatic irrigation system was constructed with 2 L h-1 adjustable drippers to supply water to the plants three times per week. Pest and disease control was not necessary, but removal of weeds was regularly required, and it was manually performed. The final harvest was carried out on the 30th of June, 2021.
The soil properties of the research field are reported in previous publications [
27,
28].
2.3. Fertilization Regimes
The pilot cultivation of
O. dictamnus followed weekly fertilization treatments using water (control), chemical, biostimulant and INM in liquid or soluble granule fertilizers administered with foliar and soil applications. The foliar applications were performed using a 5 L plastic handheld sprayer (low pressure) until apparent wetness, and the soil applications were manually performed (100 mL nutrient solution per plant). The INM and biostimulants were supplied with four special fertilizers from THEOFRASTOS company, Industrial area of Korinthos, GR-20100 Korinthos, Greece. These polysaccharide-based semi-organic fertilizers are made from high quality organic edible raw materials and plant extracts, resulting in a full supplement of plants with amino acids, vitamins, sugars, and macro-microelements. The INM polysaccharides are aimed to contribute to the formation of stable soil aggregates formed in connection with active soil microorganisms; INM polysaccharides when foliar-sprayed, they are expected to be rapidly absorbed by the plants. More specifically: THEORUN is a nitrogen source liquid fertilizer with N 17 % (w/w) P
2O
5 0 % (w/w) K
2O 1.5 % (w/w), organic matter 3.2 % (w/w), C/N 0.09, pH 9.1, diurea 0.26%, electric conductivity 86 mS/cm [liquid extract (1‰)]. THEOCAL is an organic calcium powder fertilizer with raw materials based on calcium formate molecules, pH 7.1, Ca 30% (w/w) and organic matter 30 %. THEOFAST is a liquid biostimulant made from plant extracts of edible raw materials (not algae) with organic matter 4.4 % (w/w) (plant extracts), pH 9.5 and electric conductivity 78 mS/cm [liquid extract (1‰)]. THEOMASS is a liquid biostimulant including plant extracts of edible raw materials (not algae) with organic matter 5.4 % (w/w) (plant extracts), pH 9.4 and electric conductivity 85 mS/cm [liquid extract (1‰)]. The chemical fertilizers were all in soluble powder or granule form, except the liquid fertilizer for micronutrients (Plex Mix, AGRI.FE.M. LTD Fertilizers, Greece). Two foliar fertilization treatments (INM and chemical), three soil application treatments (INM, chemical and a biostimulator) and a control for the pilot cultivation of
O. dictamnus in the field were employed in the experimentation (
Appendix A.1. Methods).
2.4. Measurements of Plant Features
Several plant and leaf level measurements were regularly conducted. Among replicate plant individuals, sampling was randomly conducted. Leaf coloration and chlorophyll fluorescence were assessed at vegetative, early flowering, and full flowering stages, whereas the remaining measurements were targeted only to the full flowering stage. For the full flowering stage, non-invasive measurements were conducted 2–5 d prior to the destructive harvest (i.e., 30th of June 2021). For leaf level measurements, fully expanded leaves were selected from the upper (towards the apex) one third leaf-bearing nodes of the stem which developed under direct natural light. In all instances, the time between sampling and the initiation of the evaluation was shorter than 15 min. When was necessary, leaf samples were placed in vials, flash-frozen in liquid nitrogen and were transferred to a freezer (-80oC) for storage. Replicate leaves were sampled from separate plant individuals.
2.4.1. Non-Destructive Evaluation of Leaf Coloration at Three Growth Stages
Leaf coloration depends on the fertilization regime, and affects both photosynthetic capacity and visually perceived quality [
50]. In this study, it was assessed by three different methods as follows:
(i) Leaf SPAD value as a proxy of chlorophyll content was determined by using a SPAD-502 (Konica Minolta Corp., Solna, Sweden).
(ii) The index of absorbance difference (IAD) accurately evaluating fruit ripeness, since it is closely associated with outer mesocarp chlorophyll content and IAD was computed as the difference between the absorbance values at 670 and 720 nm, near the chlorophyll absorbance peak [
27]. The potential of IAD in reflecting respective differences in leaves has not been previously evaluated. In this investigation, IAD was determined in leaves by using the DA meter (tr DA Meter, T.R. Turoni, Italy).
(iii) Leaf colour was quantified by using a Chroma Meter (Model CR-400, Minolta Corp., Japan). CIE L*a*b* coordinates were recorded using D65 illuminants and a 10° Standard Observer as a reference system. L* [a measure of lightness, ranging from 0 (black) to 100 (white)], a* (a measure of intensity in the green to red range, where negative values refer to green and positive to red), and b* (a measure of representing intensity in the blue to yellow range, where negative values refer to blue and positive to yellow) were obtained.
These measurements were in situ conducted in attached leaves of intact plant individuals during vegetative, early flowering, and full flowering stages. Three points were recorded per replicate leaf and were further averaged. Three replicate leaves were assessed per treatment.
2.4.2. Non-Destructive Evaluation of Photosynthetic Performance at Three Growth Stages
The ratio of variable to maximum chlorophyll fluorescence (Fv/Fm) was assessed as a valid indicator of leaf photosynthetic performance [
51]. Measurements were performed by using a chlorophyll fluorometer (OS-30P, Opti Sciences, Hudson, NH, USA). Prior to evaluation, sampled leaves were dark adapted (≥ 20 min) by employing leaf clips. Fv/Fm was assessed by applying a saturated photosynthetic photon flux density of 3000 µmol m
−2 s
−1.
These measurements were in situ conducted in attached leaves of intact plant individuals at vegetative, early flowering, and full flowering stages. Three points were recorded per replicate leaf and were further averaged. Three replicate leaves were assessed per treatment.
2.4.3. Leaf Shape Indicators
A morphometric analysis was performed by analyzing leaf form. Leaf shape traits were derived from images acquired by a digital camera employing a copy stand (Sony DSC-W830, Sony Corporation, Tokyo, Japan) that was adjusted to 0.5 m away from the subject under non-reflective glass. Using specialized software (ImageJ; Wayne Rasband/NIH, Bethesda, MD, USA), leaf lamina outlines were processed to estimate the following four (dimensionless) metrics of leaf form: (a) aspect ratio [(major axis) / (minor axis); axes of the best-fitted ellipse], (b) circularity [(4π × area) / (perimeter)
2], (c) roundness [(4 × area) / [4π × (major axis)2]], and (d) solidity [(area) / (convex area)] [
52]. Each metric quantifies a distinct feature of leaf shape. Aspect ratio and roundness are affected by the length to width ratio, while circularity and solidity are sensitive to serration and lobing. Aspect ratio ranges from 1 (circle) to value without upper bound (infinitely narrow). Roundness ranges from 0 (infinitely narrow) to 1 (circle). Circularity ranges from 0 (infinitely narrow) to 1 (circle). Solidity ranges from 0 to 1, being inversely related to boundary irregularities. Solidity is sensitive to leaves with deep lobes or a distinct petiole and can be employed to detect leaves lacking such structures. Solidity, unlike circularity, is not greatly influenced by serrations and minor lobing [
53]. Thirty leaves (5 per plant × 6 plant individuals) were analyzed per treatment.
2.4.4. Plant Growth and Biomass Partitioning to Generative Organs
Plant growth and biomass partitioning to generative organs were determined. Above-ground plant and inflorescence (fresh and dry) masses were determined (± 0.01 g; MXX-412; Denver Instruments, Bohemia, NY, USA). For measuring dry weight, the samples were placed in a forced-air drying oven for 72 h at 80°C. Six replicate plants were evaluated per treatment.
2.4.5. Leaf Chlorophyll and Carotenoid Contents
The effect of growth conditions on chlorophyll and carotenoid contents was assessed. Following fine chopping, portions (0.5 g) were homogenized with the addition of 10 mL of 80% acetone. This primary acetone extract was then filtered, and the filtered extract was diluted by adding 2 mL of 80% acetone per mL of extract. Since chlorophyll is light sensitive, extraction took place in a dark room [
54]. The obtained extract was subjected to reading on a spectrophotometer (Mapada UV-1800, Mapada Instruments Co., Ltd., Shanghai, China). Total chlorophyll and carotenoid contents were calculated [
54]. Three leaves were assessed per treatment and for each replicate, four samples (collected from different plant individuals) were pooled, and the assay was performed twice.
2.4.6. Leaf Total Phenolic and Total Flavonoid Contents
Leaf samples (0.1 g) were extracted with 1 mL of 80% aqueous methanol in an ultrasonic bath (10 min) and were then centrifuged (15000 g for 10 min). The contents of total phenolic and total flavonoid were determined by using the Folin-Ciocalteu assay and aluminum chloride colorimetric assay, respectively. The absorbance against prepared reagent blank was determined using a microplate reader (Infinite 200 PRO, TECAN, Switzerland). For total phenolic content, gallic acid was used as the standard reference and gallic acid equivalent (GAE) was expressed as mg per g of fresh mass. For total flavonoid content, rutin was used as the standard reference and rutin equivalent (RUE) was expressed as mg per g of fresh mass. Three leaves were assessed per treatment. For each replicate, four samples (collected from different plant individuals) were pooled, and the assay was performed twice.
2.4.7. Leaf Soluble Sugar Content
To assess the effect of fertilization scheme on carbohydrate status, leaf samples (0.1 g) were incubated with 1 mL deionized water in a water bath (100oC for 30 min). The homogenate was centrifuged (15000g for 15 min) at room temperature (25oC). Then, 0.1 mL of the solution was mixed with anthranone ethyl acetate and sulphuric acid. Soluble sugar content was assayed in the supernatant by measuring the absorbance at 630 nm, using a spectrophotometer (Mapada UV-1800, Mapada Instruments Co., Ltd., Shanghai, China). Soluble sugar content was expressed on a per fresh weight basis (mg g−1). These measurements were conducted on three replicates per treatment. For each replicate, four samples (collected from different plant individuals) were pooled, and the assay was performed twice.
2.4.8. Leaf and Inflorescence Mineral Analysis
To assess the role of fertilization schemes on mineral uptake, leaf and inflorescence mineral analysis was conducted. Samples were washed with distilled water and then dried. The biomass was dried at 70°C, weighed, ground, and then analyzed for total N by the Kjeldahl method. In addition, sub-samples were ash-burned at 500°C for at least 4 h; the ash was then dissolved using a 2 M HCl solution, filtered, and P, K, Ca, Mg, Cu, Zn, Fe, Mn and B contents were determined in the filtrate, employing flame photometry, atomic absorption spectrometry and UV-Vis spectrophotometry, depending on the element. Mineral content was expressed on a dry weight basis. Three replicates were evaluated per treatment. For each replicate, four samples (collected from different plant individuals) were pooled, and the assay was performed twice.
2.5. Statistical Analysis
Data analysis was carried out using the SAS statistical software (SAS Institute, Cary, NC, USA). Data were tested for homogeneity of variances (Duncan's test) and subsequently the least significant differences (LSD) of treatment effects were determined (P = 0.05).
3. Results
3.1. The Fertilization Scheme Exerted Limited Effects on Leaf Colour
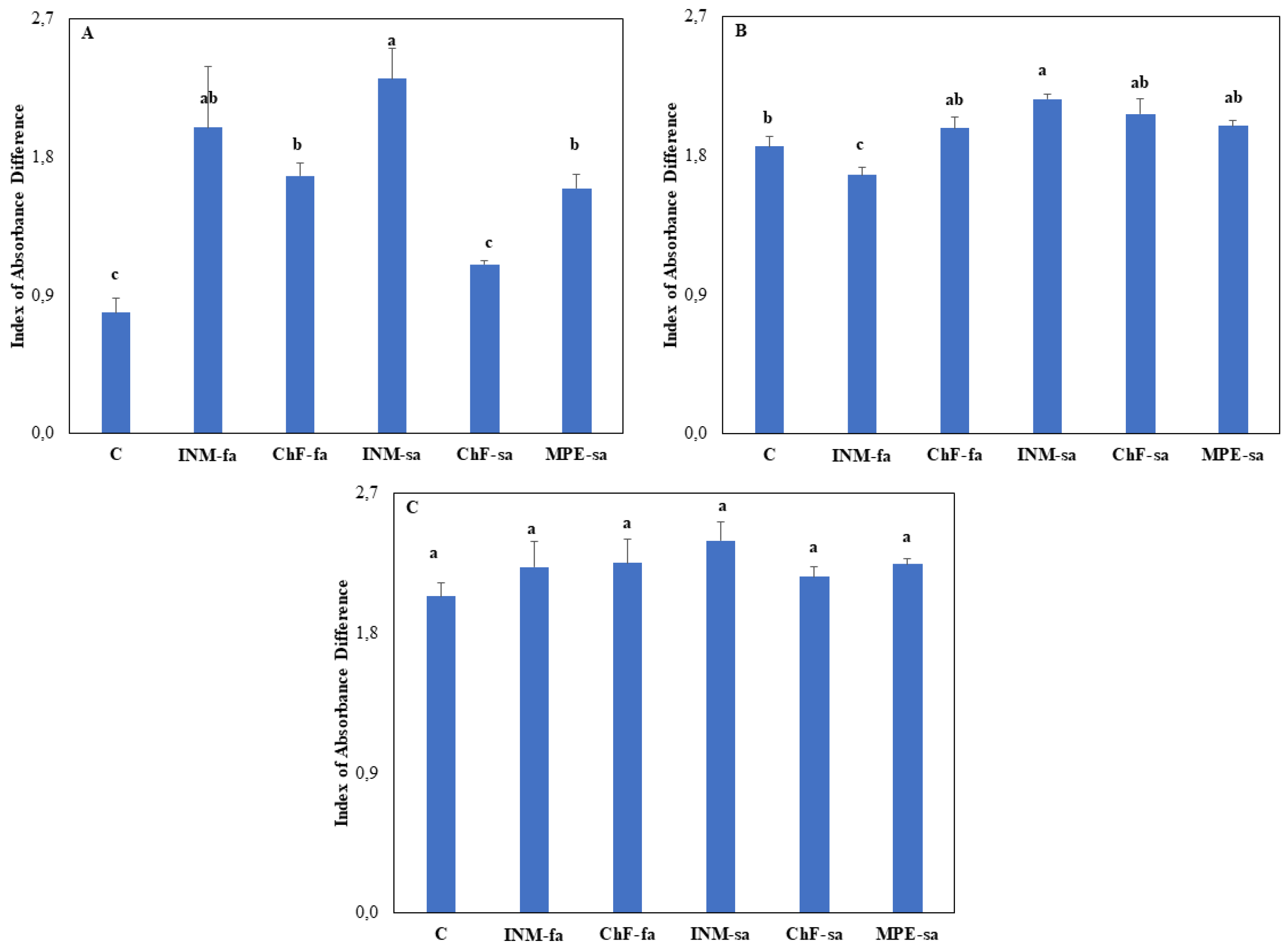
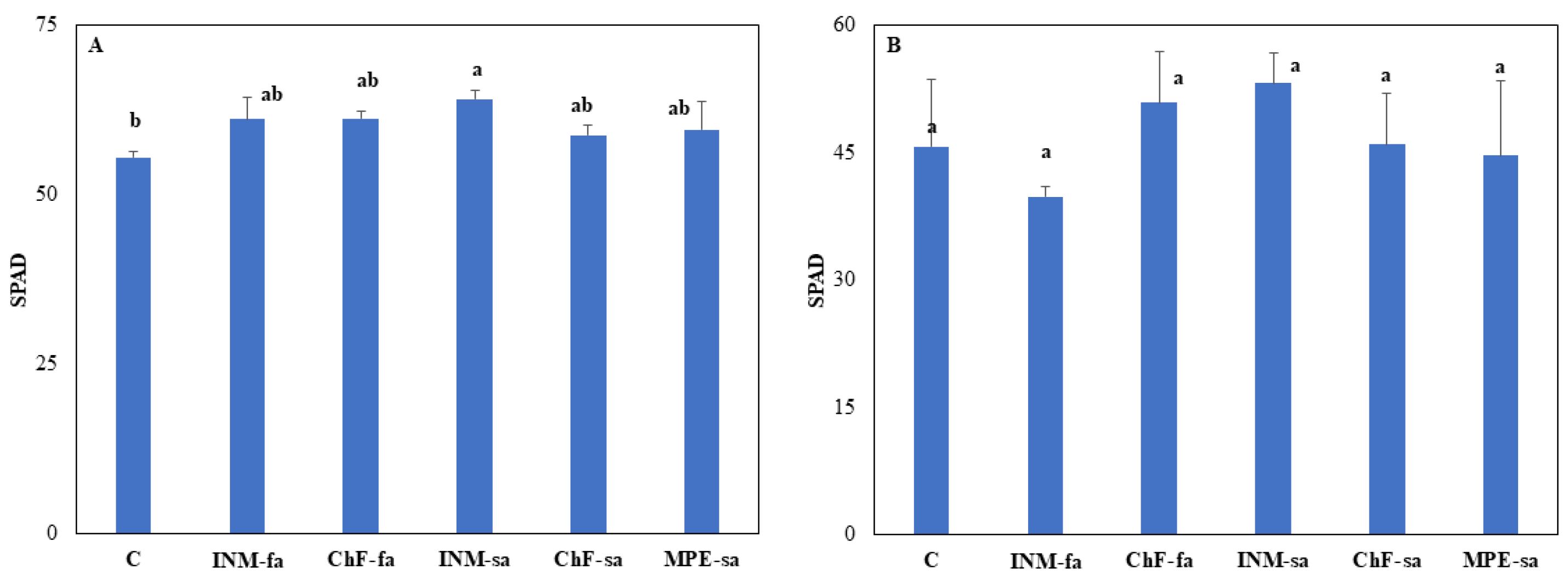
Leaf color is an important aspect of herbal quality, and therefore it was evaluated at three growth stages (i.e., vegetative, early flowering, and full flowering) by employing three methodologies (SPAD meter, DA meter, Chroma Meter). At the vegetative stage, plants receiving the INM-sa fertilization scheme presented higher leaf SPAD value as compared to control plants (
Figure 2A). At the early and full flowering stages, the leaf SPAD value was not affected by the fertilization treatment (
Figure 2B, C). At vegetative stage, control plants and plants under ChF-sa presented the lowest IAD value (i.e., being greener) as compared to the rest of the treatments (
Figure A1A). At vegetative stage, plants under INM-sa showed the highest IAD value from all treatments besides INM-fa (
Figure A1A). At early flowering stage, plants receiving INM-sa showed higher IAD value than control plants, whereas plants receiving INM-fa exhibited lower IAD value than controls (
Figure A1B). At full flowering stage, leaf IAD value was not affected by the fertilization scheme (
Figure A1C).
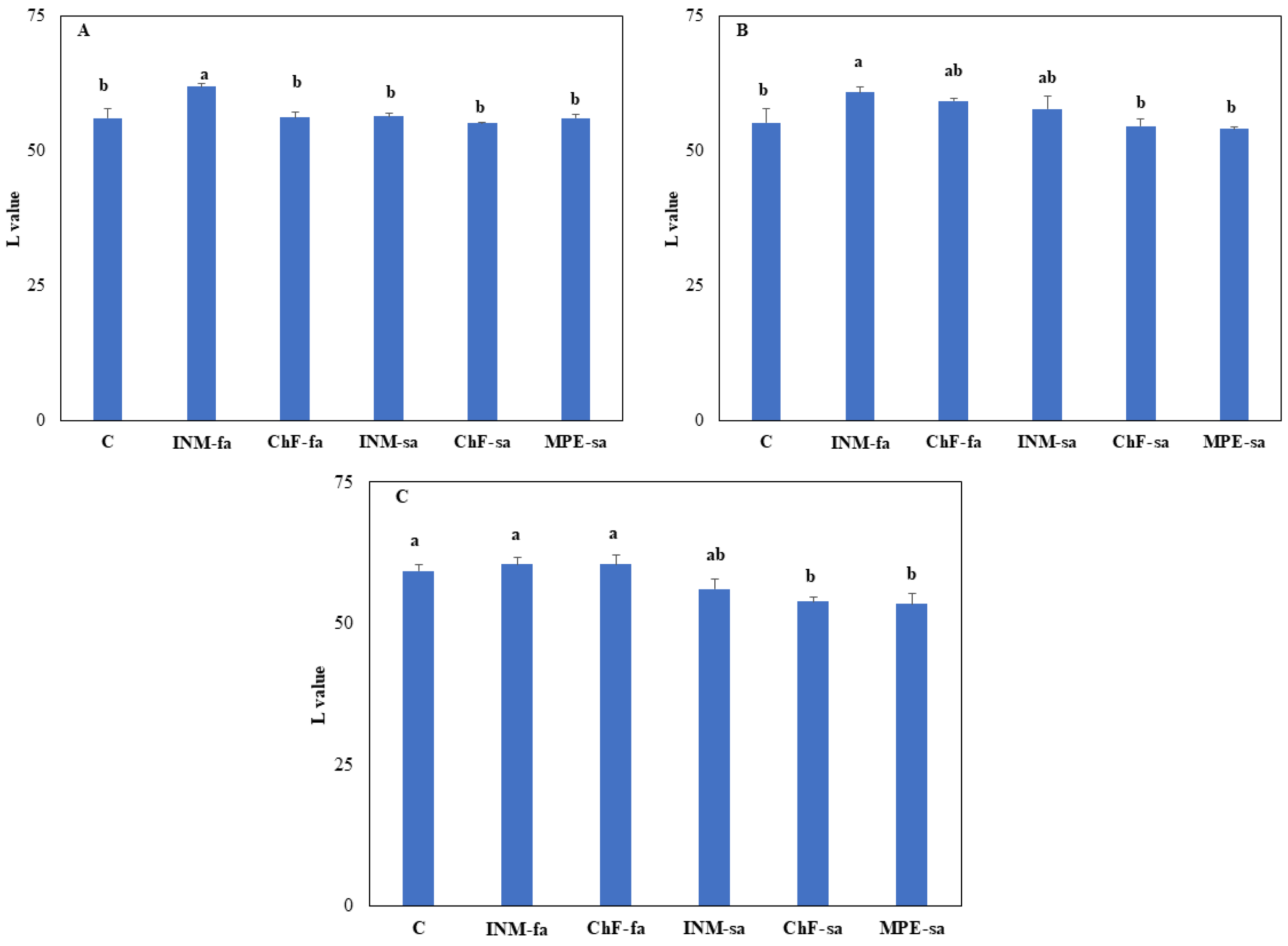
At vegetative stage, plants receiving INM-fa had higher leaf L value as compared to the remaining treatments (
Figure A2A). At early flowering stage, plants receiving INM-fa presented higher leaf L value as compared to control plants and plants receiving ChF-sa or MPE-sa (
Figure A2B). At full flowering stage, control and plants receiving both foliar fertilization schemes (INM-fa or ChF-fa) exhibited higher leaf L value as compared to those under ChF-sa or MPE-sa (
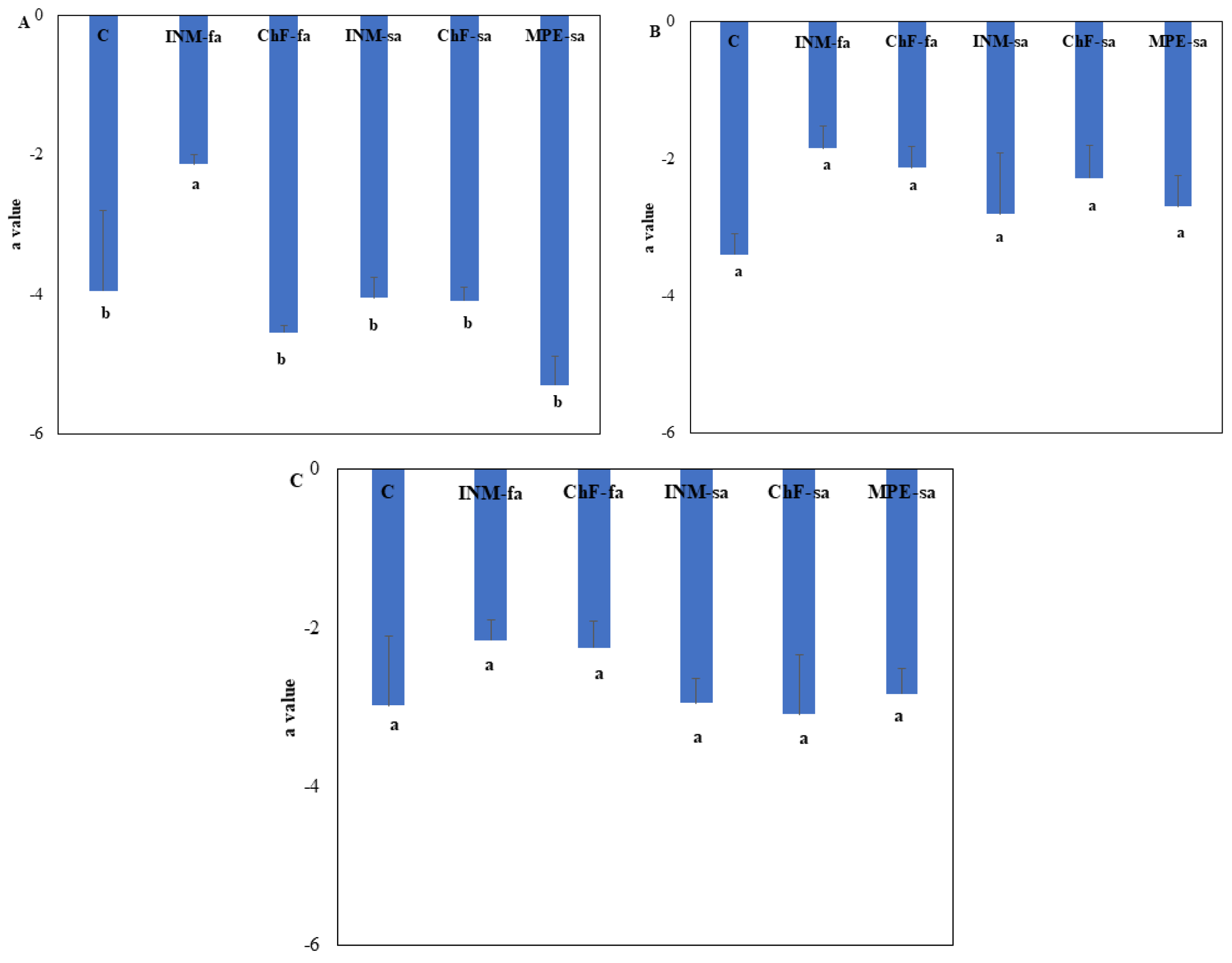
Figure A2C). At vegetative stage, plants receiving INM-fa showed higher leaf a* value as compared to the remaining treatments (
Figure A3A). At early and full flowering stages, leaf a* value was not affected by the fertilization treatment (
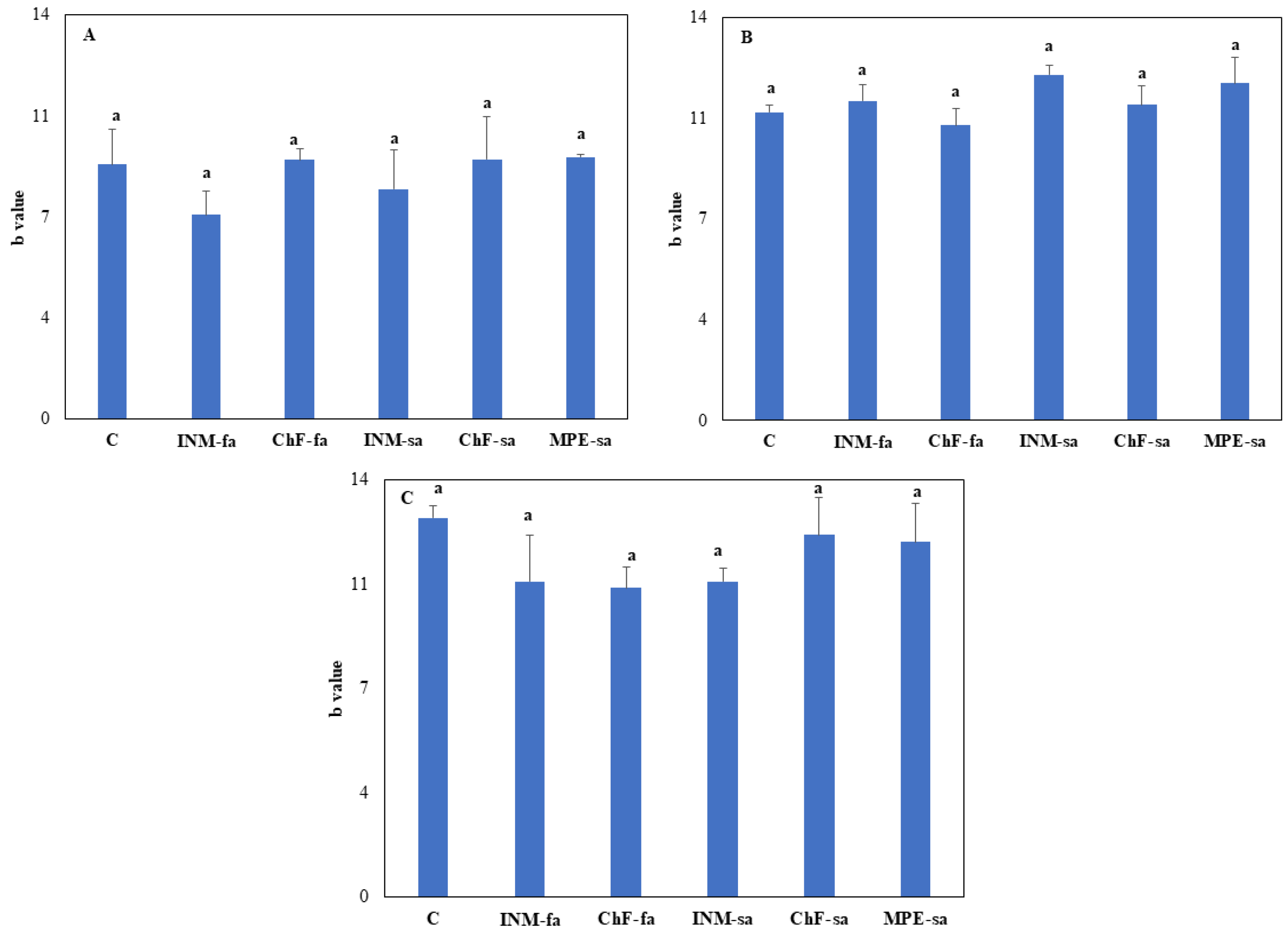
Figure A3B, C). At all three growth stages, leaf b* value was not affected by the fertilization scheme (
Figure A4).
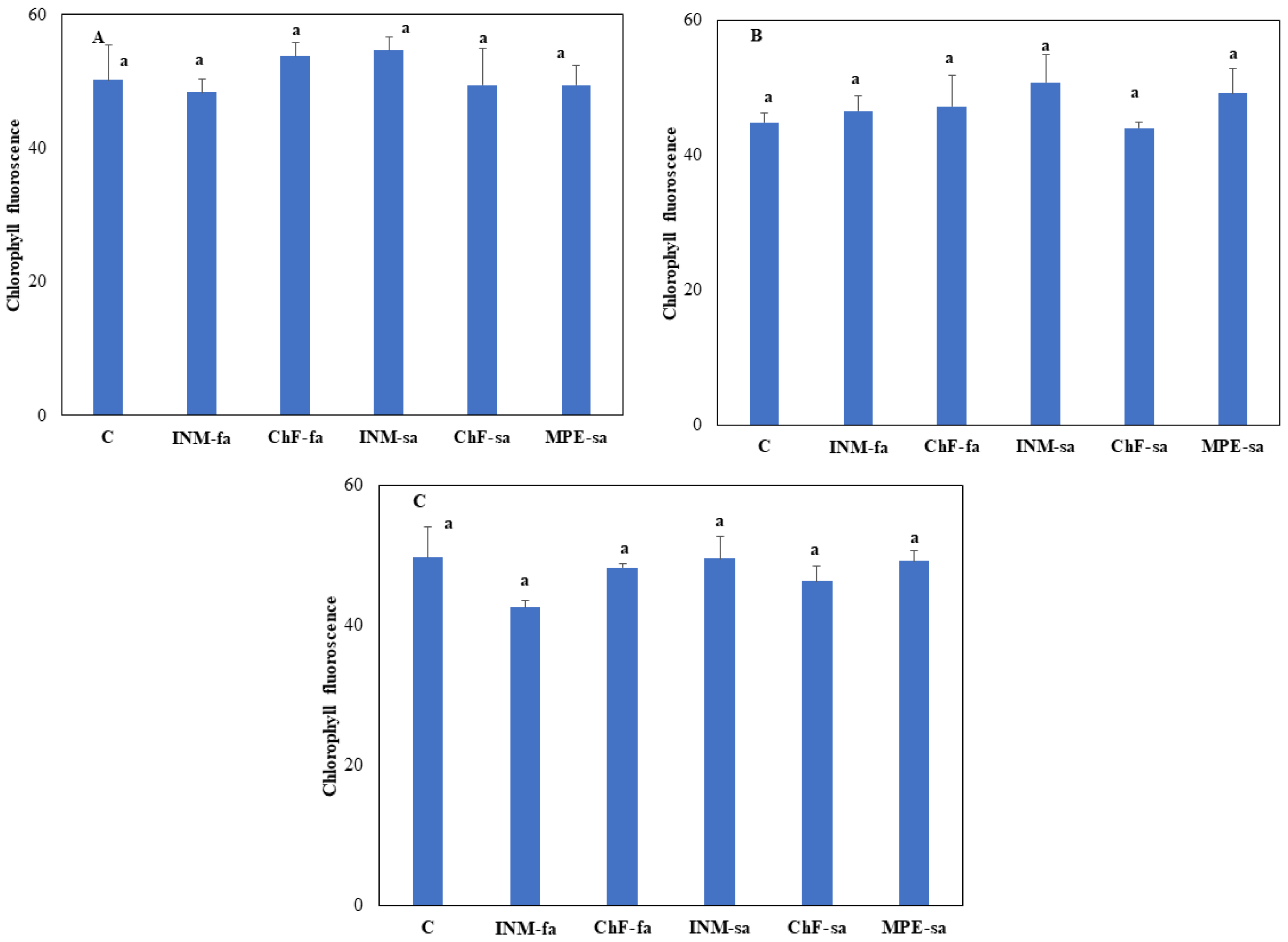
3.2. The Fertilization Scheme Did not Affect Leaf Photosynthetic Performance
At all three growth stages, leaf Fv/Fm value was not affected by the fertilization scheme (
Figure 3).
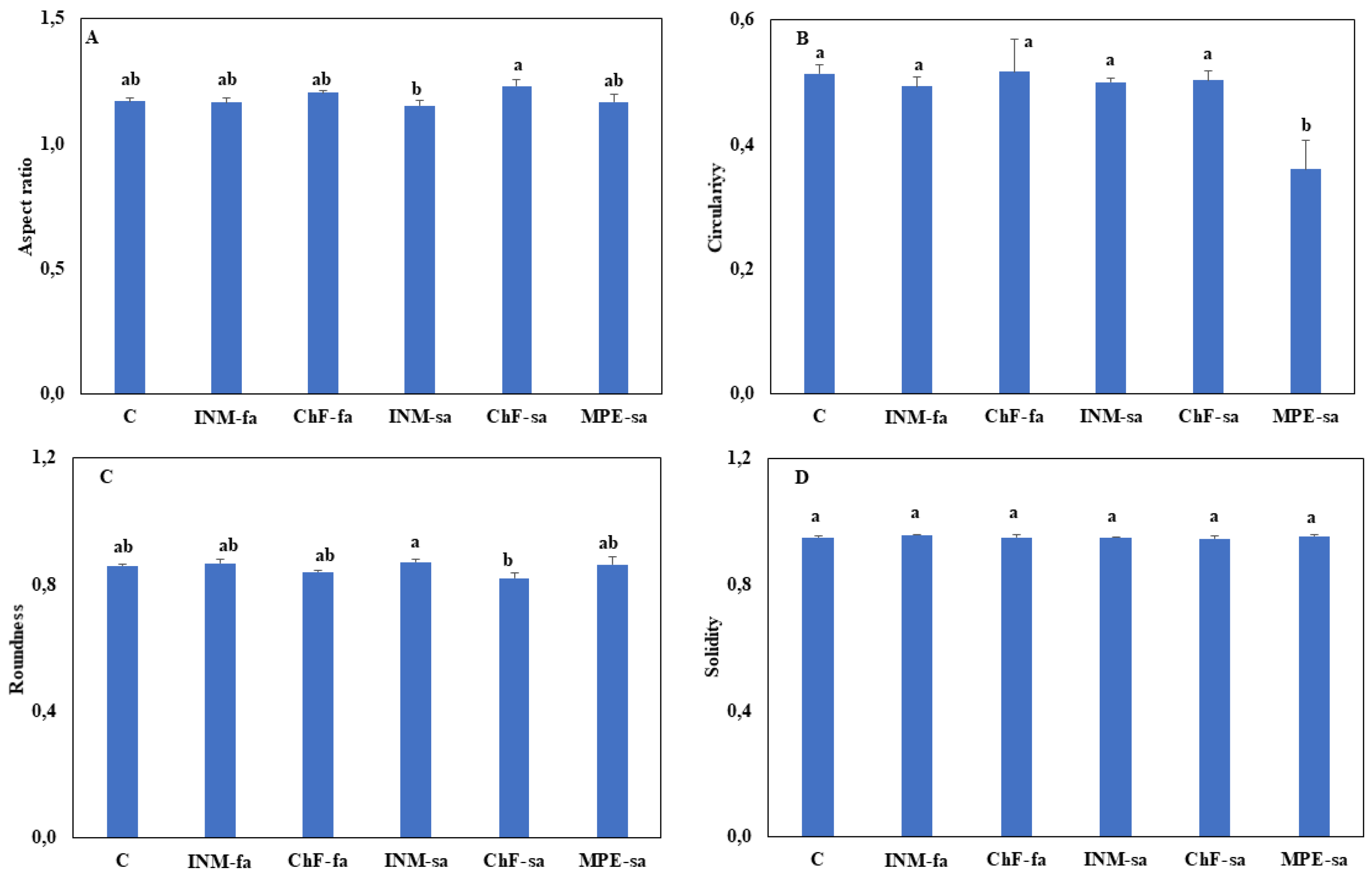
3.3. Fertilization Induced Minor Effects on Leaf Shape
To evaluate the effect of fertilization scheme on leaf form, four shape indicators (aspect ratio, circularity, roundness, solidity) were analyzed. INM-sa and ChF-sa fertilization treatments induced different leaf shapes as expressed by variation in aspect ratio (
Figure 4A) and roundness (
Figure 4C). Plants under MPE-sa showed lower circularity as compared to the remaining of the treatments (
Figure 4B). Solidity was not affected by the fertilization treatment (
Figure 4D).
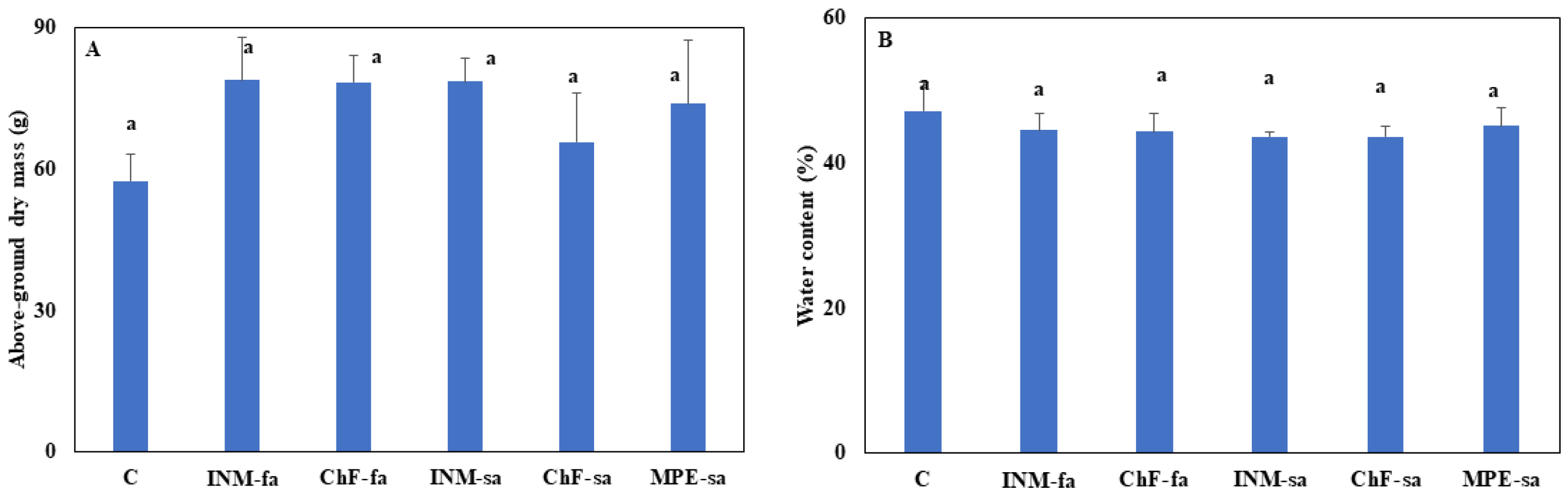
3.4. Fertilization Did not Stimulate Plant Growth but Affected Biomass Allocation
Biomass accumulation (
Figure 5A) and tissue water content (
Figure 5B) were not affected by the fertilization scheme. Plants receiving INM-sa presented the lowest partitioning to generative organs as compared to the remaining treatments including control plants (
Figure 5C). Plants receiving MPE-sa or INM-sa showed lower partitioning to generative organs as compared to the ones receiving ChF-fa (
Figure 5C).
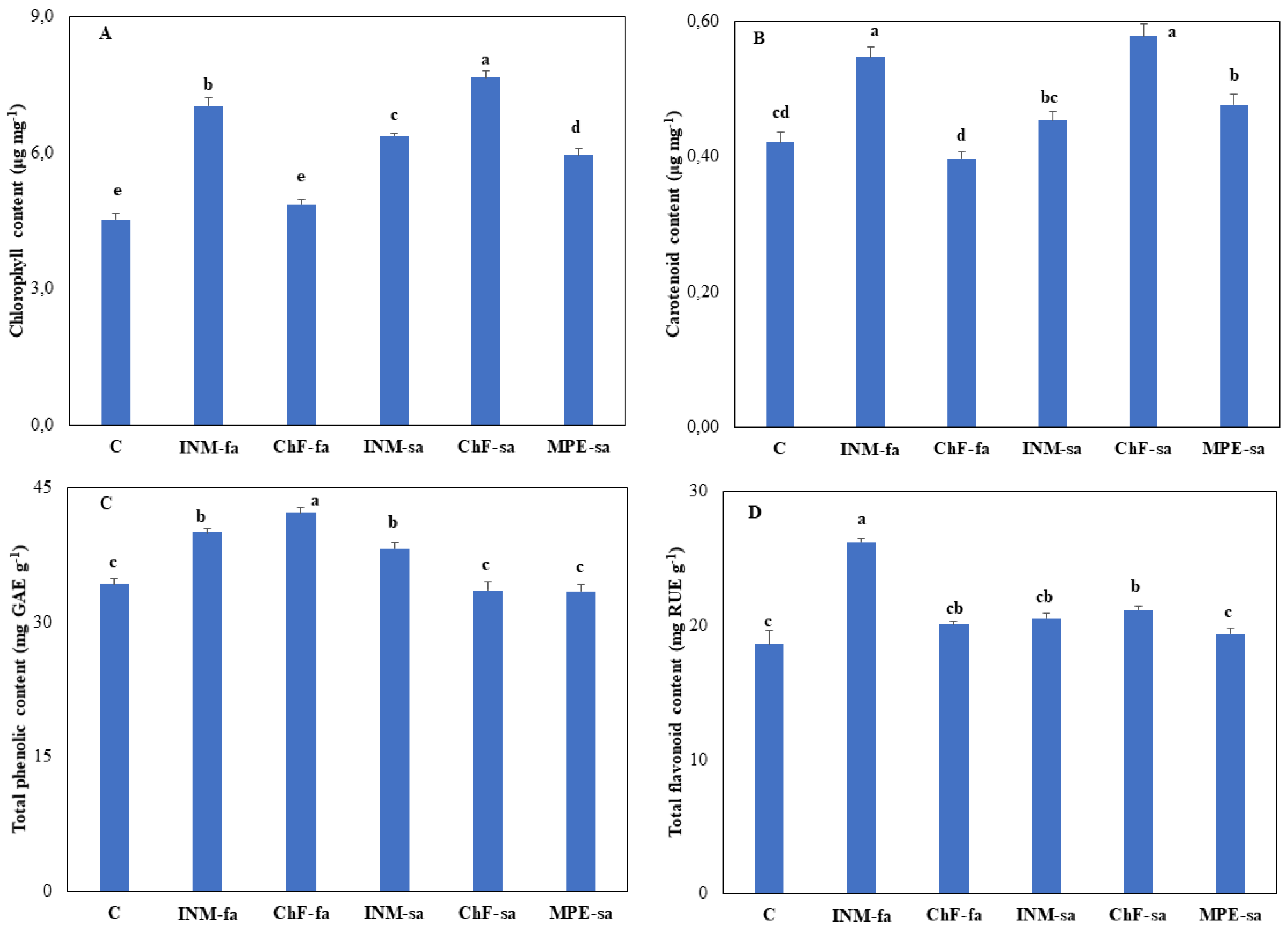
3.5. Fertilization Generally Stimulated Leaf Chlorophyll Content
As compared to control plants, fertilization stimulated leaf chlorophyll content in all cases, besides ChF-fa (
Figure 6A). The highest leaf chlorophyll content was noted in plants treated with ChF-sa, followed by the ones that received INM-fa (
Figure 6A).
3.6. Fertilization Scheme Affected Leaf Antioxidant Compound Content
Plants receiving INM-fa, ChF-sa or MPE-sa showed higher leaf carotenoid content as compared to controls (
Figure 6B). Plants receiving INM-fa or ChF-sa exhibited the highest leaf carotenoid content as compared to the remaining treatments (
Figure 6B). Plants receiving INM-fa, ChF-fa or INM-sa showed higher leaf total phenolic content as compared to controls (
Figure 6C). Plants receiving ChF-fa had the highest leaf total phenolic content as compared to the remaining treatments (
Figure 6C). Plants receiving INM-fa or ChF-sa had higher leaf total flavonoid content as compared to controls (
Figure 6D). Plants receiving INM-fa had the highest leaf total flavonoid content as compared to the remaining treatments (
Figure 6D).
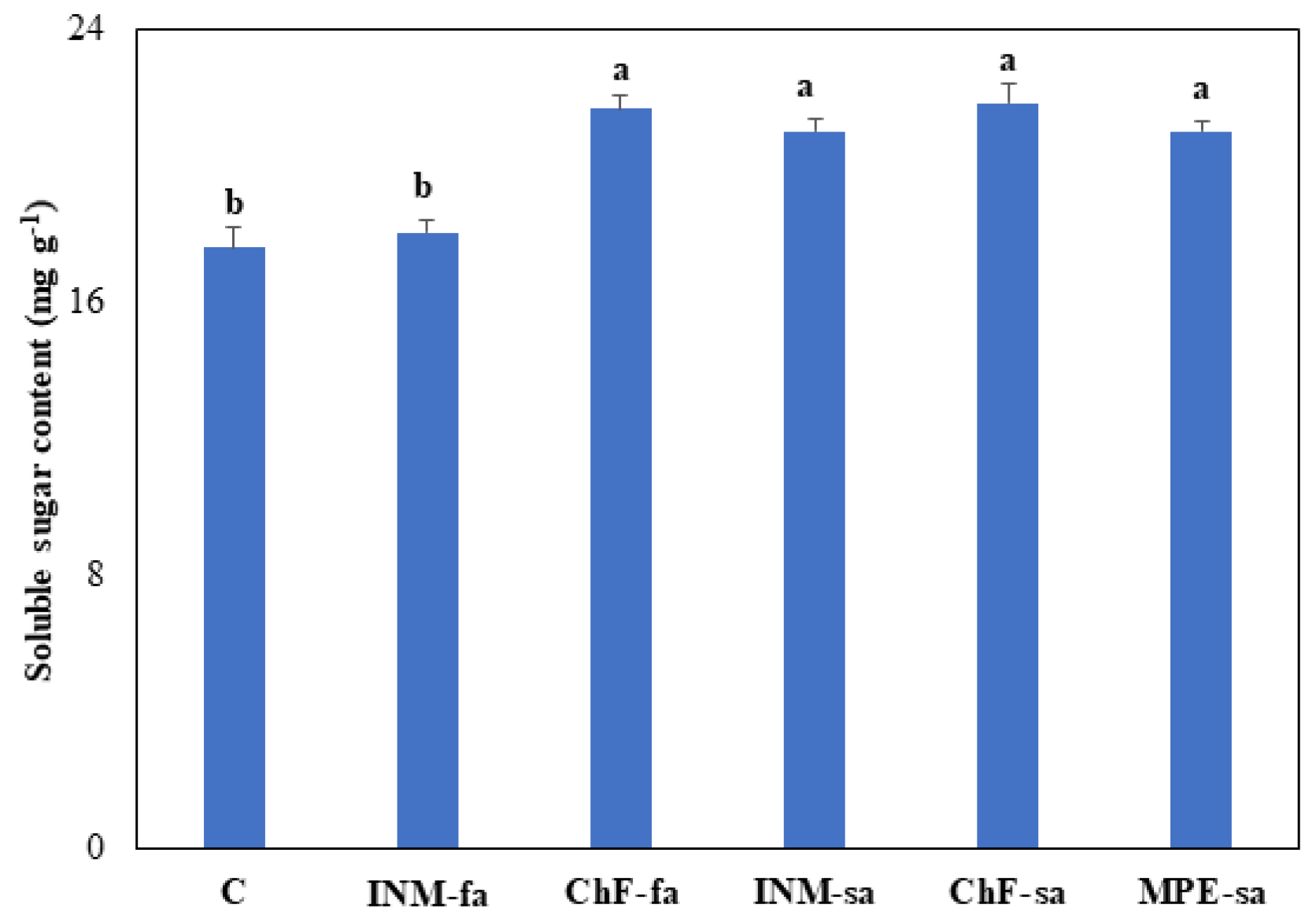
3.7. Fertilization Treatments Generally Stimulated Leaf Soluble Sugar Content
As compared to control plants, fertilization stimulated leaf soluble sugar content in all cases, besides INM-fa (
Figure 7).
3.8. Leaf and Inflorescence Mineral Analysis
Leaf macro- and micro-nutrient content analysis of
O. dictamnus showed variations depending on treatment and individual nutrients (
Table 1 and
Table 2). Chemical fertilization applied either on leaves or soil along with the INM-fa showed the highest values with respect to N and K. Also, the latter with the ChF-fa presented the highest macro- and micro-nutrients content values (except Ca and Mg) (
Table 1 and
Table 2). By contrast, ChF-fa exhibited the lowest Ca content as compared to other treatments. Both ChF treatments showed the highest Mg contents and the MPE-sa the lowest one (
Table 1). Furthermore, MPE-sa showed the lowest nutrient contents (except Mg) compared to other treatments (
Table 2). Finally, an interesting finding is that most of treatments exceeded that of control regarding the leaf nutrient content (especially in P and with exception of Ca and Mg).
Inflorescence mineral analysis showed differences among treatments (
Table 3 and
Table 4). The highest N content was observed in ChF-fa INM-sa and the lowest in MPE-sa. ChF-fa also showed the highest P and Mg contents. Regarding the K content ChF-sa showed the highest values and C the lowest (
Table 3). No differences in Ca content were observed among treatments. Regarding the inflorescences’ micro-nutrient content in O. dictamnus (
Table 4), foliar applications (“fa”) and control (C) treatments showed higher Zn contents than soil application (“sa”). INM-fa showed the highest Fe content and the ChF-sa the lowest one. No significant differences were observed among treatments regarding B content (
Table 4).
4. Discussion
The ongoing global interest on medicinal-aromatic plants adds increasing pressure on the wild-growing genetic resources and the majority of the traded herbal material is still directly collected from nature [
30,
35,
55]. In this way, wild plant resources deplete at an alarming rate [
56], threatening a wide range of species with extinction [
57,
58]. These changes may disturb the niche structure and balance, with adverse effects on the ecosystems and eventually human well-being [
56,
57,
58].
Plant species conservation necessitates the introduction of commercially valuable taxa into agricultural settings and their sustainable utilization to alleviate the over-harvesting pressure to wild populations [
48]. In response to this need, cultivation practices and farming operations ought to be developed and established, predominantly employing an optimized fertilization program. The field investigation herein presents the first comprehensive steps of introducing the threatened Cretan endemic
O. dictamnus into sustainable cultivation and evaluates a fertilization scheme which is best for stimulating crop yield as well as two key features of herbal material quality. Under this background, polysaccharide-based INM fertilizers were also tested owing not only to the reduced environmental footprint, but also to a powerful fertilization scheme leading to improved quality and yield [
59].
The fertilization treatments affected neither the above-ground plant growth (
Figure 5A) nor the tissue water content (
Figure 5B). Therefore, the fertilization scheme did not also affect the efficiency of processing (i.e., decrease in moisture content via drying; [
15,
16]). As compared to controls, the fertilization scheme did not affect biomass partitioning to generative organs, besides INM-sa which reduced it (
Figure 5C). Among fertilization treatments, plants receiving MPE-sa or INM-sa had lower partitioning to generative organs as compared to the ones receiving ChF-fa (
Figure 5C). Considering inflorescences as harvestable organs, the current research suggests that INM-sa treatment to be avoided in
O. dictamnus cultivation.
The intensity of greenness of the leaves is a critical parameter of visually perceived quality throughout production, processing, and market outlets [
15]. The intensity of leaf green color was here non-invasively determined by employing three methods (SPAD meter, DA meter, Chroma Meter). At full flowering stage, the fertilization scheme did not affect leaf SPAD value (
Figure 2C), IAD value (
Figure A1C), a* value (an index of green to red range intensity;
Figure A3C), and b value (an index of blue to yellow range intensity;
Figure A4C). An exception to this general trend was the lower L value (an index of lightness;
Figure A2C) of plants receiving fertilization through the soil (ChF-sa or MPE-sa) as compared to the remaining treatments (
Figure A2C). When taken altogether, these results indicate that the fertilization scheme did not influence leaf colour aspects in
O. dictamnus.
Another feature of visual inspection in herbal material grading is leaf outline (shape). The results of the present study denoted that different soil fertilization schemes affected leaf appearance. As compared to control plants, INM-sa and ChF-sa fertilization treatments altered both aspect ratio and roundness (
Figure 4A, C), while MPE-sa changed circularity (
Figure 4B). The former two shape indicators (aspect ratio, roundness) were affected by the length to width ratio, while the latter (circularity) were affected by serration and lobing [
53]. On this basis and given that colour variation was either lacking or limited, it is argued that instructed staff or computerized identification methods [
60] could be employed in visually distinguishing
O. dictamnus herbal material that has received soil fertilization (INM-sa, ChF-sa or MPE-sa) based on the shape indices examined herein (aspect ratio, roundness, circularity).
Foliar and inflorescence nutrient contents are largely lacking in literature regarding
O. dictamnus; only a recent pot study was found dealing with the effect of different substrates and sites on nutrient content [
42]. Herein, field data have been obtained for the first time, showing variation in the effect of different fertilization schemes depending on the element examined. Thus, regarding the leaf nutrient contents, it is remarkable that most of the treatments exceeded than the control; exception was the Ca and Mg nutrients. Also, the INM-fa and the ChF-fa showed the highest values in most cases (except for Ca and Mg) indicating the importance of the foliar applications on
O. dictamnus nutrition status. Compared to the recent pot study [
42], similar N, P and Zn but lower content values for K were obtained in the present study, while regarding other micronutrients, Cu, Fe and Mn were higher in the current study. Regarding to inflorescence nutrient content and in specific concerning N P, K, and Zn, higher values have been reported by the recent study [
42] compared to our study, showing however lower values for Fe. In the latter study, no differences were observed among tested substrates, but the opposite occurred between sites, thus suggesting that environmental conditions might be an important factor. Another finding of the current study was the slight or negligible difference of the inflorescence nutrient contents for control plants, indicating a minor effect of fertilization on inflorescence nutrient content. On the other hand, and similarly to the foliar chemical fertilization, the results herein showed that the polysaccharide-based IΝΜ treatment applied either on leaves or on soil leaded to a tendency for higher inflorescence content values considering both macro- and micro-nutrients. Taken altogether, further work is apparently needed for an extended period to validate the results prior to drawing essential conclusions regarding the accumulation and partitioning of nutrients in plant biomass.
From a phytochemical perspective, medicinal-aromatic plants are generally rich in antioxidant compounds, while their content is an important measure of herbal material quality [
17,
18]. In this study, three key non-enzymatic antioxidant metabolites (carotenoids, flavonoids, phenols) were determined. As compared to controls, no negative effect of fertilization on antioxidant metabolite content was apparent (
Figure 6B, C, D). Considering all three antioxidants together, the INM-fa was the most stimulatory treatment in phytochemical terms, due to the highest (carotenoids, total flavonoids) or the second highest (total phenolics) content as compared to the rest of the treatments (
Figure 6B, C, D). Polysaccharides have highly been connected to antioxidant enhancement using both in vitro chemical and biological models [
61]. The polysaccharide antioxidant action is greatly reliant on their solubility, sugar ring assembly, molecular weight, amount of positively or negatively charged clusters, protein moieties and covalently linked phenolic substances [
62,
63]. The polysaccharide-based INM used in this study proved to be significantly competent in order to enhance the antioxidant machinery in the glandular-haired
O. dictamnus. The increased antioxidant machinery, in turn, could be beneficial for both plant resistance to stress (plant health) and product consumer (human health). In accordance with these findings, INM has been previously related to increased antioxidant compound content in other species as well [
25,
64].
The second-best stimulatory scheme was ChF-sa, which enhanced the highest (carotenoids) or the second highest (total flavonoids) content as compared to the rest of the treatments (
Figure 6B, D). Considering all three antioxidants under study together, the current results indicated that INM-fa should be employed in the commercial cultivation of
O. dictamnus. This field investigation presents for the first time baseline information for some critical herbal quality aspects in
O. dictamnus. When inflorescences are the primary target, INM-sa ought to be avoided. When antioxidant compound content is of key commercial interest, INM-fa stands out as the optimal fertilization scheme in
O. dictamnus cultivation.
5. Conclusions and Prospects
In this field study, the fertilization scheme optimally driving plant growth and herbal material quality was investigated in O. dictamnus, a threatened local endemic of Crete (Greece) of high medicinal value and commercial interest. Different treatments encompassing chemical fertilizer (foliar/soil application), INM (foliar/soil application), and INM with biostimulant (soil application) were comparatively examined. The fertilization scheme when applied via soil, altered leaf appearance, and influenced the visually perceived quality of O. dictamnus, thus allowing potentially for standardized visual discrimination of the herbal material by instructed staff or computerized identification methods. Beyond its role as a plant nutrient provider, INM is known to feed the soil biota due to its rich polysaccharide content. Soil-applied INM fertilizers are not only absorbed by roots, but foliar-sprayed INM polysaccharides may also be directly absorbed by leaves. Considering all three antioxidants together, INM by foliar application was found to be the optimal scheme. A polysaccharide stock in the plant is vital during the whole growth period, as polysaccharides act as a direct energy source helping plants to cope and survive stress conditions. The positive outcome of INM on the enhancement of the antioxidant mechanism in O. dictamnus could be further ascribed to the activation of various subsequent functions inside plant tissues, such as the elevated hydrolysis of the unique species-specific endogenous subcuticular-located polysaccharides and/or the hydrolysis of the exogenously added polysaccharides provided by INM foliar application. As suggested by other studies, the latter, in turn, may eventually lead to the increase in the contents of physiologically active sugars, thus providing adequate biological resources for the synthesis of complex antioxidants. The data herein provided insights to the polysaccharide-based INM foliar spray system as an EEF system significantly enhancing the antioxidant machinery in O. dictamnus, thus, opening the way towards its commercial use in material sciences as well as in the food and pharmaceutical sectors.
Author Contributions
Conceptualization, G.T. (Georgios Tsoktouridis), and N.K.; Methodology, K.P., D.F., V.T., G.T. (Georgios Tsaniklidis), V.A.T., F.B., E.S., K.K., F.J.D., I.I., G.T. (Georgios Tsoktouridis), K.G., and T.M.; Software, K.P., D.F., V.T., G.T. (Georgios Tsaniklidis), V.A.T., I.I., and T.M.; Validation, D.F., V.A.T., F.B., E.S., K.K., F.J.D., I.I., T.M., K.G., and N.K.; Formal Analysis, K.P., D.F., V.T., S.V., I.K., G.T. (Georgios Tsaniklidis), V.A.T., F.B., E.S., I.I, and T.M.; Investigation, K.P., D.F., V.T., G.T. (Georgios Tsoktouridis), V.A.T., F.B., E.S., K.K., F.J.D., I.I., K.G., G.T. (Georgios Tsaniklidis), T.M.; Resources, K.P., T.M., I.I., and G.T. (Georgios Tsoktouridis); Data Curation, K.P., D.F., G.T. (Georgios Tsaniklidis), V.A.T., F.B., E.S., K.K., F.J.D., I.I., G.T. (Georgios Tsoktouridis), and T.M.; Writing—Original Draft Preparation, K.P., D.F., G.T. (Georgios Tsoktouridis), V.A.T., and N.K.; Writing—Review & Editing, K.P., D.F., V.T., G.T. (Georgios Tsoktouridis), V.A.T., F.B., E.S., K.K., F.J.D., I.I., G.T. (Georgios Tsaniklidis), T.M., and N.K.; Visualization, V.A.T., D.F., and N.K.; Supervision, T.M., D.F., G.T. (Georgios Tsoktouridis); Project Administration, G.T. (Georgios Tsoktouridis); Funding Acquisition, K.P., K.G., N.K., T.M., and G.T. (Georgios Tsoktouridis). All authors have read and agreed to the published version of the manuscript.
Funding
This research has been co-financed by the European Union and Greek National funds through the Operational Program Competitiveness, Entrepreneurship and Innovation, under the call RESEARCH—CREATE—INNOVATE (project code: T1EDK-05380), entitled “Conservation and sustainable utilization of rare threatened endemic plants of Crete for the development of new products with innovative precision fertilization”.
Institutional Review Board Statement
Non applicable.
Informed Consent Statement
Non applicable.
Data Availability Statement
All data supporting the results of this study are included in the manuscript and datasets are available upon request.
Acknowledgments
We are grateful to the laboratory staff and the undergraduate students Eleutheria Liapaki, Sofia Vouraki, Menel Ben Saleh Jouini, Mihail Kokotakis, Konstantinos Dermitzakis, Haralampos Liapakis, Emmanouella Vasilaki, Ioannis Papadakis, Emmanouil Haralampakis and Georgios Stylianakis of the Hellenic Mediterranean University for their contributions, continued diligence, and dedication to their craft.
Abbreviations
a*, green to red range intensity; b*, blue to yellow range intensity; CEC, cation exchange capacity; ECse, saturation extract electrical conductivity; EEFs, Enhanced Efficiency Fertilizers; Fv/Fm, ratio of variable to maximum chlorophyll fluorescence; GAE, gallic acid equivalent; IAD, index of absorbance difference; INM, integrated nutrient management; L*, lightness; MAPs, medicinal-aromatic plants; RUE, rutin equivalent; SAR, sodium absorption ratio
Appendix A
Appendix A.1. Methods
Fertilization treatments applied in the pilot cultivation of
O. dictamnus with other Cretan endemic plants [
27,
28] at the premises of the Hellenic Mediterranean University.
Scheme A. Integrated nutrient management (INM) by foliar application (INM-fa): The nutrient solution consisted of THEORUN at 7 ml L-1, THEOCAL at 1.5 g L-1, THEOFAST at 5 ml L-1, 10-47-10 (AGRI.FE.M. LTD Fertilizers, Greece) at 3.2 g L-1, K2SO4 (0-0-52, AGRI.FE.M. LTD Fertilizers, Greece) at 2.07 g L-1, micronutrients (Plex Mix, AGRI.FE.M. LTD Fertilizers, Greece) at 1.5 ml L-1 and MgSO4 (Mg 25.6%, AGRI.FE.M. LTD Fertilizers, Greece) at 0.6 g L-1.
Scheme Β. Chemical fertilization by foliar application (ChF-fa): The nutrient solution consisted of NH4NO3 (34,4-0-0, Neofert®, Neochim PLC, Bulgaria) at 2.7 g L-1, Ca(NO3)2 (NITROCAL, Agrohimiki, Greece) at 1.7 g L-1, 10-47-10 at 3.2 g L-1, K2SO4 (0-0-52) at 2.27 g L-1, micronutrients Plex Mix at 1.5 ml L-1 and MgSO4 (Mg 25.6 %) at 0.6 g L-1.
Scheme C. Control, with foliar and soil applications with tap water.
Scheme D. INM by soil application (INM-sa): The nutrient solution consisted of THEORUN at 7 ml L-1, THEOCAL at 1.5 g L-1, THEOMASS at 10 ml L-1, 10-47-10 at 3.2 g L-1, K2SO4 (0-0-52) at 2.1 g L-1, micronutrients Plex Mix at 1.5 ml L-1 and MgSO4 (Mg 25.6 %) at 0.3 g L-1.
Scheme E. Chemical fertilization by soil application (ChF-sa): The nutrient solution was consisted of NH4NO3 (34,4-0-0) at 2.7 g L-1, Ca(NO3)2 (NITROCAL) at 1.7 g L-1, 10-47-10 at 3.2 g L-1, K2SO4 (0-0-52) at 2.3 g L-1, micronutrients, Plex Mix at 1.5 ml L-1 and MgSO4 (Mg 25.6 %) at 0.3 g L-1.
Scheme F. Mixture of plant extracts as biostimulant by soil application (MPE-sa): The nutrient solution was consisted of THEOMASS at 10 ml L-1.
Figure A1.
Effect of fertilization scheme through different application methods (foliar/soil) on leaf index of absorbance difference of O. dictamnus at vegetative (A), early flowering (B), and full flowering (C) stage. C: Control (water); INM-fa: Integrated nutrient management (INM) by foliar application; ChF-fa: Chemical fertilization by foliar application; INM-sa: INM by soil application; ChF-sa: Chemical fertilization by soil application; MPE-sa: biostimulant by soil application (THEOMASS). Values represent the mean of three replicates ± SEM. Within each plot, different letters indicate significant differences.
Figure A1.
Effect of fertilization scheme through different application methods (foliar/soil) on leaf index of absorbance difference of O. dictamnus at vegetative (A), early flowering (B), and full flowering (C) stage. C: Control (water); INM-fa: Integrated nutrient management (INM) by foliar application; ChF-fa: Chemical fertilization by foliar application; INM-sa: INM by soil application; ChF-sa: Chemical fertilization by soil application; MPE-sa: biostimulant by soil application (THEOMASS). Values represent the mean of three replicates ± SEM. Within each plot, different letters indicate significant differences.
Figure A2.
Effect of fertilization treatment through different application methods (foliar/soil) on leaf L value of O. dictamnus at vegetative (A), early flowering (B), and full flowering (C) stage. C: Control (water); INM-fa: Integrated nutrient management (INM) by foliar application; ChF-fa: Chemical fertilization by foliar application; INM-sa: INM by soil application; ChF-sa: Chemical fertilization by soil application; MPE-sa: biostimulant by soil application (THEOMASS). Values represent the mean of three replicates ± SEM. Within each plot, different letters indicate significant differences.
Figure A2.
Effect of fertilization treatment through different application methods (foliar/soil) on leaf L value of O. dictamnus at vegetative (A), early flowering (B), and full flowering (C) stage. C: Control (water); INM-fa: Integrated nutrient management (INM) by foliar application; ChF-fa: Chemical fertilization by foliar application; INM-sa: INM by soil application; ChF-sa: Chemical fertilization by soil application; MPE-sa: biostimulant by soil application (THEOMASS). Values represent the mean of three replicates ± SEM. Within each plot, different letters indicate significant differences.
Figure A3.
Effect of fertilization treatment through different application methods (foliar/soil) on leaf a* value of O. dictamnus at vegetative (A), early flowering (B), and full flowering (C) stage. C: Control (water); INM-fa: Integrated nutrient management (INM) by foliar application; ChF-fa: Chemical fertilization by foliar application; INM-sa: INM by soil application; ChF-sa: Chemical fertilization by soil application; MPE-sa: biostimulant by soil application (THEOMASS). Values represent the mean of three replicates ± SEM. Within each plot, different letters indicate significant differences.
Figure A3.
Effect of fertilization treatment through different application methods (foliar/soil) on leaf a* value of O. dictamnus at vegetative (A), early flowering (B), and full flowering (C) stage. C: Control (water); INM-fa: Integrated nutrient management (INM) by foliar application; ChF-fa: Chemical fertilization by foliar application; INM-sa: INM by soil application; ChF-sa: Chemical fertilization by soil application; MPE-sa: biostimulant by soil application (THEOMASS). Values represent the mean of three replicates ± SEM. Within each plot, different letters indicate significant differences.
Figure A4.
Effect of fertilization scheme through different application methods (foliar/soil) on leaf b* value of O. dictamnus at vegetative (A), early flowering (B), and full flowering (C) stage. C: Control (water); INM-fa: Integrated nutrient management (INM) by foliar application; ChF-fa: Chemical fertilization by foliar application; INM-sa: INM by soil application; ChF-sa: Chemical fertilization by soil application; MPE-sa: biostimulant by soil application (THEOMASS). Values represent the mean of three replicates ± SEM. Within each plot, different letters indicate significant differences.
Figure A4.
Effect of fertilization scheme through different application methods (foliar/soil) on leaf b* value of O. dictamnus at vegetative (A), early flowering (B), and full flowering (C) stage. C: Control (water); INM-fa: Integrated nutrient management (INM) by foliar application; ChF-fa: Chemical fertilization by foliar application; INM-sa: INM by soil application; ChF-sa: Chemical fertilization by soil application; MPE-sa: biostimulant by soil application (THEOMASS). Values represent the mean of three replicates ± SEM. Within each plot, different letters indicate significant differences.
References
- Nations, U. World population prospects 2019. Retrieved from Department of Economic and Social Affairs 2019.
- Dhima, K.; Vasilakoglou, I.; Paschalidis, K.; Karagiannidis, N.; Ilias, I. Salinity tolerance evaluation of barley germplasm for marginal soil utilization. Italian J. Agronomy 2021, 16 (3), 1830. [CrossRef]
- Lam, S.K.; Wille, U.; Hu, H.; Caruso, F.; Mumford, K.; Liang, X.; Pan, B.; Malcolm, B.; Roessner, U.; Suter, H., et al. Next-generation enhanced-efficiency fertilizers for sustained food security. Nat. Food 2022, 3, 575–580 . [CrossRef]
- Ninou, E.G.; Paschalidis, K.A.; Mylonas, I.G.; Vasilikiotis, C.; A.G., M. The effect of genetic variation and nitrogen fertilization on productive characters of greek oregano. Acta Agric. Scandinavica, Soil Plant Sci. 2017 67 372-379. [CrossRef]
- Chiaregato, C.G.; Franca, D.; Messa, L.L.; Dos Santos Pereira, T.; Faez, R. A review of advances over 20 years on polysaccharide-based polymers applied as enhanced efficiency fertilizers. Carbohydr. Polym. 2022, 279, 119014. [CrossRef]
- Malerba, M.; Cerana, R. Chitin- and chitosan-based derivatives in plant protection against biotic and abiotic stresses and in recovery of contaminated soil and water. Polysaccharides 2020, 1, 21-30. [CrossRef]
- Soni, A.T.; Rookes, J.E.; Arya, S.S. Chitosan nanoparticles as seed priming agents to alleviate salinity stress in rice (oryza sativa l.) seedlings. Polysaccharides 2023, 4, 129-141. [CrossRef]
- Cruz-Monterrosa, R.G.; Rayas-Amor, A.A.; González-Reza, R.M.; Zambrano-Zaragoza, M.L.; Aguilar-Toalá, J.E.; Liceaga, A.M. Application of polysaccharide-based edible coatings on fruits and vegetables: Improvement of food quality and bioactivities. Polysaccharides 2023, 4, 99-115. [CrossRef]
- Gemin, L.G.; Bocchetti de Lara, G.; Mógor, Á.F.; Mazaro, S.M.; Sant‘Anna-Santos, B.F.; Mógor, G.; Oliveira Amatussi, J.; Negrelli Cordeiro, E.C.; Costa Marques, H.M. Polysaccharides combined to copper and magnesium improve tomato growth, yield, anti-oxidant and plant defense enzymes. Sci. Hortic. 2023, 310, 111758. [CrossRef]
- Hatzilazarou, S.; Pipinis, E.; Kostas, S.; Stagiopoulou, R.; Gitsa, K.; Dariotis, E.; Avramakis, M.; Samartza, I.; Plastiras, I.; Kriemadi, E., et al. Influence of temperature on seed germination of five wild-growing tulipa species of greece associated with their ecological profiles: Implications for conservation and cultivation. Plants (Basel) 2023, 12, 1575. [CrossRef]
- Anestis, I.; Pipinis, E.; Kostas, S.; Papaioannou, E.; Karapatzak, E.; Dariotis, E.; Tsoulpha, P.; Koundourakis, E.; Chatzileontari, E.; Tsoktouridis, G., et al. Gis-facilitated germination of stored seeds from five wild-growing populations of campanula pelviformis lam. And fertilization effects on growth, nutrients, phenol content and antioxidant potential. Horticulturae 2023, 9, 877.
- Bilias, F.; Ipsilantis, I.; Samara, E.; Tsoktouridis, G.; Glavakis, E.; Grigoriadou, K.; Krigas, N.; Matsi, T. From the wild to the field: Effect of foliar or soil application of inorganic or semi-organic fertilizers on various parameters of four local endemic plant species of crete (greece). Revista Brasileira de Botânica 2023, 46, 319 -336. [CrossRef]
- Papagrigoriou, T.; Iliadi, P.; Mitic, M.N.; Mrmosanin, J.M.; Papanastasi, K.; Karapatzak, E.; Maloupa, E.; Gkourogianni, A.V.; Badeka, A.V.; Krigas, N., et al. Wild-growing and conventionally or organically cultivated sambucus nigra germplasm: Fruit phytochemical profile, total phenolic content, antioxidant activity, and leaf elements. Plants (Basel) 2023, 12.
- Kakar, K.; Xuan, T.D.; Noori, Z.; Aryan, S.; Gulab, G. Effects of organic and inorganic fertilizer application on growth, yield, and grain quality of rice. Agriculture 2020, 10, 544. [CrossRef]
- Thamkaew, G.; Sjöholm, I.; Galindo, F. A review of drying methods for improving the quality of dried herbs. Crit. Rev. Food Sci. Nutr. 2020, 19, 1-24. [CrossRef]
- Taheri-Garavand, A.; Mumivand, H.; Fanourakis, D.; Fatahi, S.; Taghipour, S. An artificial neural network approach for non-invasive estimation of essential oil content and composition through considering drying processing factors: A case study in mentha aquatica. Ind. Crops Prod. 2021, 171, 113985. [CrossRef]
- Ibrahim, M.H.; Jaafar, H.Z.; Karimi, E.; Ghasemzadeh, A. Impact of organic and inorganic fertilizers application on the phytochemical and antioxidant activity of kacip fatimah (labisia pumila benth). Molecules (Basel, Switzerland) 2013, 18(9), 10973–10988. [CrossRef]
- Chen, Y.; Fanourakis, D.; Tsaniklidis, G.; Aliniaeifard, S.; Yang, Q.; Li, T. Low uva intensity during cultivation improves the lettuce shelf-life, an effect that is not sustained at higher intensity. Postharvest Biol. Technol. 2021, 172, 111376. [CrossRef]
- Asami D.K.; Hong Y.J.; Barrett D.M.; A.E., M. Comparison of the total phenolic and ascorbic acid content of freeze-dried and air-dried marionberry, strawberry, and corn using conventional, organic, and sustainable agricultural practices. J. Agric. Food Chem. 2003, 51, 1237–1241.
- Zhang, Y.; Ntagkas, N.; Fanourakis, D.; Tsaniklidis, G.; Zhang, Y.; Zou, J.; Cheng, R.; Yang, Q.; Li, T. The role of light intensity in mediating ascorbate content during postharvest tomato ripening: A transcriptomic analysis. Postharvest Biol. Technol. 2021, 180, 111622. [CrossRef]
- Kazimierczak, R.; Hallmann, E.; Rusaczonek, A.; Rembiałkowska, E. Antioxidant content in black currants from organic and conventional cultivation. Food Sci. Technol. Res. 2008, 2(11), 57–61.
- Wang, S.Y.; Chen, C.T.; Sciarappa, W.; C.Y.;, W.; Camp, M.J. Fruit quality, antioxidant capacity, and flavonoid content of organically and conventionally grown blueberries. J. Agric. Food Chem. 2008, 56, 5788–5794. [CrossRef]
- Tõnutare, T.; Moor, U.; Mölder, K.; Põldma, P. Fruit composition of organically and conventionally cultivated strawberry ‘polka’. Agronomy Res. 2009, 7, 755–760.
- Serri, F.; Souri, M.K.; Rezapanah, M. Growth, biochemical quality and antioxidant capacity of coriander leaves under organic and inorganic fertilization programs. Chem. Biol. Technol. Agric. 2021, 8, 33. [CrossRef]
- Mrid, R.B.; Benmrid, B.; Hafsa, J.; Boukcim, H.; Sobeh, M.; Yasri, A. Secondary metabolites as biostimulant and bioprotectant agents: A review. Sci. Total Environ. 2021, 146204. [CrossRef]
- Hatzilazarou, S.; Pipinis, E.; Kostas, S.; Stagiopoulou, R.; Gitsa, K.; Dariotis, E.; Avramakis, M.; Samartza, I.; Plastiras, I.; Kriemadi, E., et al. Influence of temperature on seed germination of five wild-growing tulipa species of greece associated with their ecological profiles: Implications for conservation and cultivation. Plants (Basel) 2023, 12. [CrossRef]
- Paschalidis, K.; Fanourakis, D.; Tsaniklidis, G.; Tzanakakis, V.A.; Bilias, F.; Samara, E.; Kalogiannakis, K.; Debouba, F.J.; Ipsilantis, I.; Tsoktouridis, G., et al. Pilot cultivation of the vulnerable cretan endemic verbascum arcturus l. (scrophulariaceae): Effect of fertilization on growth and quality features. Sustainability 2021, 13, 14030. [CrossRef]
- Fanourakis, D.; Paschalidis, K.; Tsaniklidis, G.; Tzanakakis, V.A.; Bilias, F.; Samara, E.; Liapaki, E.; Jouini, M.; Ipsilantis, I.; Maloupa, E., et al. Pilot cultivation of the local endemic cretan marjoram origanum microphyllum (benth.) vogel (lamiaceae): Effect of fertilizers on growth and herbal quality features. Agronomy 2022, 12, 94.
- Amujoyegbe, B.J.; Opabode, J.T.; Olayinka, A.; Moench. Effect of organic and inorganic fertilizer on yield and chlorophyll content of maize (zea mays l.) and sorghum (sorghum bicolour l.) Afr. J. Biotechnol. 2007, 6, 1869–1873.
- Hoareau, L.; Da Silva, E.J. Medicinal plants: A re-emerging health aid. Electron J. Biotechnol. 1999, 2, 3-4.
- Bodeker, G.; Burford, G.; Volkov, A. Integrative, traditional and complementary medicine. . In S. R. Quah, eds. 325 International Encyclopedia of Public Health, 2nd ed., Elsevier Academic Press, Amsterdam. 2017, 288–295.
- Jain, D.; Chaudhary, P.; Kotnala, A.; Hossain, R.; Bisht, K.; Hossain, M.N. Hepatoprotective activity of medicinal plants: A mini review. J. Med. Plants Stud. 2020, 8(5), 183-188. [CrossRef]
- Zuzarte, M.; Girao, H.; Salgueiro, L. Aromatic plant-based functional foods: A natural approach to manage cardiovascular diseases. Molecules 2023, 28. [CrossRef]
- Sattayakhom, A.; Wichit, S.; Koomhin, P. The effects of essential oils on the nervous system: A scoping review. Molecules 2023, 28. [CrossRef]
- Cheminal, A.; Kokkoris, I.P.; Strid, A.; Dimopoulos, P. Medicinal and aromatic lamiaceae plants in greece: Linking diversity and distribution patterns with ecosystem services. Forests 2020, 11, 661. [CrossRef]
- Kougioumoutzis, K.; Kotsakiozi, P.; Stathi, E.; Trigas, P.; Parmakelis, A. Conservation genetics of four critically endangered greek endemic plants: A preliminary assessment. Diversity 2021, 13, 152. [CrossRef]
- Turland, N.J. Origanum dictamnus l. (lamiaceae), vulnerable (vu). In: D. Phitos, A. Strid, S. Snogerup, W. Greuter (Eds.) 1995, The Red Data Book of Rare and Threatened Plants of Greece, World Wide Fund for Nature (WWF), Athens, Greece, 394-395.
- Krigas, N.; Lazari, D.; Maloupa, E.; Stikoudi, M. Introducing dittany of crete (origanum dictamnus l.) to gastronomy: A new culinary concept for a traditionally used medicinal plant. Int. J. Gastron. Food Sci. 2015, 2, 112-118. [CrossRef]
- Bourgou, S.; Ben Haj Jilani, I.; Karous, O.; Megdiche-Ksouri, W.; Ghrabi-Gammar, Z.; Libiad, M.; Khabbach, A.; El Haissoufi, M.; Lamchouri, F.; Greveniotis, V., et al. Medicinal-cosmetic potential of the local endemic plants of crete (greece), northern morocco and tunisia: Priorities for conservation and sustainable exploitation of neglected and underutilized phytogenetic resources. Biology (Basel) 2021, 10, 1344. [CrossRef]
- EMA/HMPC. Final assessment report on origanum dictamnus l., herba (200431/2012). . European Medicines Agency/Committee on Herbal Medicinal Products, London 2013.
- Skotti, E.; Kountouri, S.; Bouchagier, P.; Tsitsigiannis, D.I.; Polissiou, M.; Tarantilis, P.A. Ftir spectroscopic evaluation of changes in the cellular biochemical composition of the phytopathogenic fungus alternaria alternata induced by extracts of some greek medicinal and aromatic plants. Spectroch. Acta Mol. Biomol. Spectroscopy 2014, 127, 463-472. [CrossRef]
- Martini, A.N.; Papafotiou, M.; Massas, I.; Chorianopoulou, N. Growing of the cretan therapeutic herb origanum dictamnus in the urban fabric: The effect of substrate and cultivation site on plant growth and potential toxic element accumulation. Plants 2023, 12, 336. [CrossRef]
- Economakis, C.; Skaltsa, H.; Demetzos, C.; Soković, M.; Thanos, C.A. Effect of phosphorus concentration of the nutrient solution on the volatile constituents of leaves and bracts of origanum dictamnus. J. Agric. Food Chem. 2002, 50, 6276-6280. [CrossRef]
- Bilias, F.; Ipsilantis, I.; Samara, E.; Tsoktouridis, G.; Glavakis, E.; Grigoriadou, K.; Krigas, N.; Matsi, T. From the wild to the field: Effect of foliar or soil application of inorganic or semi-organic fertilizers on various parameters of four local endemic plant species of crete (greece). Braz. J. Bot. 2023, 46, 319–336. [CrossRef]
- Sarropoulou, V.; Maloupa, E.; Grigoriadou, K. Cretan dittany (origanum dictamnus l.), a valuable local endemic plant: In vitro regeneration potential of different type of explants for conservation and sustainable exploitation. Plants (Basel) 2023, 12(1), 182. [CrossRef]
- Lionis, C.; Petelos, E.; Linardakis, M.; Diamantakis, A.; Symvoulakis, E.; Karkana, M.-N.; Kampa, M.; Pirintsos, S.A.; Sourvinos, G.; Castanas, E. A mixture of essential oils from three cretan aromatic plants inhibits sars-cov-2 proliferation: A proof-of-concept intervention study in ambulatory patients. Diseases 2023, 11, 105.
- Strid, A. Atlas of the aegean flora. Part 1: Text & plates. Part 2: Maps; . Englera 33 (1 & 2); Botanic Garden and Botanical Museum Berlin, Freie Universität Berlin: Berlin, Germany 2016.
- Krigas, N.; Tsoktouridis, G.; Anestis, I.; Khabbach, A.; Libiad, M.; Megdiche-Ksouri, W.; Ghrabi-Gammar, Z.; Lamchouri, F.; Tsiripidis, I.; Tsiafouli, M.A., et al. Exploring the potential of neglected local endemic plants of three mediterranean regions in the ornamental sector: Value chain feasibility and readiness timescale for their sustainable exploitation. Sustainability 2021, 13, 2539. [CrossRef]
- Bosabalidis, A.M. Ultrastructure, development and histochemistry of the polysaccharide-containing subcuticular compartments in origanum dictamnus l. Peltate glandular hairs. Flavour Fragr. J. 2010 25(4), 202–205.
- Asayesh, E.J.; Aliniaeifard, S.; Askari, N.; Roozban, M.R.; Sobhani, M.; Tsaniklidis , G.; Woltering, E.J.; Fanourakis, D. Supplementary light with increased blue fraction accelerates emergence and improves development of the inflorescence in aechmea, guzmania and vriesea. Horticulturae 2021, 7, 485.
- Sørensen, H.K.; Fanourakis, D.; Tsaniklidis, G.; Bouranis, D.; Rezaei Nejad, A.; Ottosen, C.-O. Using artificial lighting based on electricity price without a negative impact on growth, visual quality or stomatal closing response in passiflora. Sci. Hortic. 2020, 267, 109354. [CrossRef]
- Koubouris, G.; Bouranis, D.; Vogiatzis, E.; Nejad, A.R.; Giday, H.; Tsaniklidis, G.; Ligoxigakis, E.K.; Blazakis, K.; Kalaitzis, P.; Fanourakis, D. Leaf area estimation by considering leaf dimensions in olive tree. Sci. Hortic. 2018, 240, 440–445. [CrossRef]
- Gupta, S.; Rosenthal, D.M.; Stinchcombe, J.R.; Baucom, R.S. The remarkable morphological diversity of leaf shape in sweet potato (ipomoea batatas): The influence of genetics, environment, and g9e. New Phytol. 2020, 225, 2183–2195.
- Ahmadi-Majd, M.; Rezaei Nejad, A.; Mousavi-Fard, S.; Fanourakis, D. Postharvest application of single, multi-walled carbon nanotubes and graphene oxide stimulates rose keeping quality. J. Hortic. Sci. Biotechnol. 2021, 97, 346-360.
- Bourgou, S.; Ben Haj Jilani, I.; Karous, O.; Megdiche-Ksouri, W.; Ghrabi-Gammar, Z.; Libiad, M.; Khabbach, A.; El Haissoufi, M.; Lamchouri, F.; Greveniotis, V., et al. Medicinal-cosmetic potential of the local endemic plants of crete (greece), northern morocco and tunisia: Priorities for conservation and sustainable exploitation of neglected and underutilized phytogenetic resources. Biology (Basel) 2021, 10. [CrossRef]
- Ross, I.A.e. Medicinal plants of the world (volume 3): Chemical constituents, traditional and modern medicinal uses. New Jersey: Humana Press Inc. 2005, 110–132.
- Bentley, R.e. Medicinal plants. London: Domville-Fife Press; 2010, 23–46.
- Pimm, S.; Russell, G.; Gittleman, J.; Brooks, T. The future of biodiversity. Science 1995, 269, 347.
- Laird, S.A.; Pierce, A.R. Promoting sustainable and ethical botanicals: Strategies to improve commercial raw material sourcing, results from the sustainable botanicals pilot project industry surveys, case studies and standards collection. Rainforest Alliance 2002, New York.
- Taheri-Garavand, A.; Nasiri, A.; Fanourakis, D.; Fatahi, S.; Omid, M.; Nikoloudakis, N. Automated in situ seed variety identification via deep learning: A case study in chickpea. . Plants (Basel) 2021, 10, 1406. [CrossRef]
- Paschalidis, K.A.; Moschou, P.N.; Aziz, A.; Toumi, T.; Roubelakis-Angelakis, K.A. Polyamines in grapevine: An update. In: Roubelakis-Angelakis, K.A. (eds) Grapevine Molecular Physiology & Biotechnology 2009 Springer, Dordrecht, 207-228.
- Fernandes, P.A.R.; Coimbra, M.A. The antioxidant activity of polysaccharides: A structure-function relationship overview. Carbohydrate Polymers 2023, 314, 120965. [CrossRef]
- Zeng, F.-S.; Yao, Y.-F.; Wang, L.-F.; Li, W.-J. Polysaccharides as antioxidants and prooxidants in managing the double-edged sword of reactive oxygen species. Biomed. Pharmacother. 2023, 159, 114221. [CrossRef]
- Serri, F.; Souri, M.K.; Rezapanah, M. Growth, biochemical quality and antioxidant capacity of coriander leaves under organic and inorganic fertilization programs. Chem. Biol. Technol. Agric. 2021, 8, 33. [CrossRef]
Figure 1.
(A) O. dictamnus wild-growing individual at the end of its flowering on rocky crevices of Petrodolakia, Mt. Psiloritis in Crete (Greece) during mid-July 2019; (B) Densely arachnoid leaf indumentum of Cretan dittany with glandular and non-glandular hair; (C) Pilot field cultivation of dittany of Crete at the farm of the Hellenic Mediterranean University, Heraklion, Crete (Greece).
Figure 1.
(A) O. dictamnus wild-growing individual at the end of its flowering on rocky crevices of Petrodolakia, Mt. Psiloritis in Crete (Greece) during mid-July 2019; (B) Densely arachnoid leaf indumentum of Cretan dittany with glandular and non-glandular hair; (C) Pilot field cultivation of dittany of Crete at the farm of the Hellenic Mediterranean University, Heraklion, Crete (Greece).
Figure 2.
Effect of fertilization scheme through different (root/foliar) application methods on leaf SPAD value of O. dictamnus at vegetative (A), early flowering (B), and full flowering (C) stage. C: Control (water); INM-fa: Integrated nutrient management (INM) by foliar application; ChF-fa: Chemical fertilization by foliar application; INM-sa: INM by soil application; ChF-sa: Chemical fertilization by soil application; MPE-sa: biostimulant by soil application (THEOMASS). Values represent the mean of three replicates ± SEM. Within each plot, different letters indicate significant differences.
Figure 2.
Effect of fertilization scheme through different (root/foliar) application methods on leaf SPAD value of O. dictamnus at vegetative (A), early flowering (B), and full flowering (C) stage. C: Control (water); INM-fa: Integrated nutrient management (INM) by foliar application; ChF-fa: Chemical fertilization by foliar application; INM-sa: INM by soil application; ChF-sa: Chemical fertilization by soil application; MPE-sa: biostimulant by soil application (THEOMASS). Values represent the mean of three replicates ± SEM. Within each plot, different letters indicate significant differences.
Figure 3.
Effect of fertilization scheme through different (root/foliar) application methods on chlorophyll fluorescence value of O. dictamnus at vegetative (A), early flowering (B), and full flowering (C) stage. C: Control (water); INM-fa: Integrated nutrient management (INM) by foliar application; ChF-fa: Chemical fertilization by foliar application; INM-sa: INM by soil application; ChF-sa: Chemical fertilization by soil application; MPE-sa: biostimulant by soil application (THEOMASS). Values represent the mean of three replicates ± SEM. Within each plot, different letters indicate significant differences.
Figure 3.
Effect of fertilization scheme through different (root/foliar) application methods on chlorophyll fluorescence value of O. dictamnus at vegetative (A), early flowering (B), and full flowering (C) stage. C: Control (water); INM-fa: Integrated nutrient management (INM) by foliar application; ChF-fa: Chemical fertilization by foliar application; INM-sa: INM by soil application; ChF-sa: Chemical fertilization by soil application; MPE-sa: biostimulant by soil application (THEOMASS). Values represent the mean of three replicates ± SEM. Within each plot, different letters indicate significant differences.
Figure 4.
Effect of fertilization scheme through different (root/foliar) application methods on four leaf shape factors of O. dictamnus. C: Control (water); INM-fa: Integrated nutrient management (INM) by foliar application; ChF-fa: Chemical fertilization by foliar application; INM-sa: INM by soil application; ChF-sa: Chemical fertilization by soil application; MPE-sa: biostimulant by soil application (THEOMASS). Values represent the mean of six replicates ± SEM. Within each plot, different letters indicate significant differences.
Figure 4.
Effect of fertilization scheme through different (root/foliar) application methods on four leaf shape factors of O. dictamnus. C: Control (water); INM-fa: Integrated nutrient management (INM) by foliar application; ChF-fa: Chemical fertilization by foliar application; INM-sa: INM by soil application; ChF-sa: Chemical fertilization by soil application; MPE-sa: biostimulant by soil application (THEOMASS). Values represent the mean of six replicates ± SEM. Within each plot, different letters indicate significant differences.
Figure 5.
Effect of fertilization sceme through different (root/foliar) application methods on above-ground dry weight (A), water content (B) and dry weight partitioning to inflorescences (C) of O. dictamnus. C: Control (water); INM-fa: Integrated nutrient management (INM) by foliar application; ChF-fa: Chemical fertilization by foliar application; INM-sa: INM by soil application; ChF-sa: Chemical fertilization by soil application; MPE-sa: biostimulant by soil application (THEOMASS). Values represent the mean of six replicates ± SEM. Within each plot, different letters indicate significant differences.
Figure 5.
Effect of fertilization sceme through different (root/foliar) application methods on above-ground dry weight (A), water content (B) and dry weight partitioning to inflorescences (C) of O. dictamnus. C: Control (water); INM-fa: Integrated nutrient management (INM) by foliar application; ChF-fa: Chemical fertilization by foliar application; INM-sa: INM by soil application; ChF-sa: Chemical fertilization by soil application; MPE-sa: biostimulant by soil application (THEOMASS). Values represent the mean of six replicates ± SEM. Within each plot, different letters indicate significant differences.
Figure 6.
Effect of fertilization scheme through different (root/foliar) application methods on leaf chlorophyll (A), carotenoid (B), total phenol (C) and total flavonoid (D) content of O. dictamnus. C: Control (water); INM-fa: Integrated nutrient management (INM) by foliar application; ChF-fa: Chemical fertilization by foliar application; INM-sa: INM by soil application; ChF-sa: Chemical fertilization by soil application; MPE-sa: s biostimulant by soil application (THEOMASS). Values represent the mean of three replicates ± SEM. Within each plot, different letters indicate significant differences.
Figure 6.
Effect of fertilization scheme through different (root/foliar) application methods on leaf chlorophyll (A), carotenoid (B), total phenol (C) and total flavonoid (D) content of O. dictamnus. C: Control (water); INM-fa: Integrated nutrient management (INM) by foliar application; ChF-fa: Chemical fertilization by foliar application; INM-sa: INM by soil application; ChF-sa: Chemical fertilization by soil application; MPE-sa: s biostimulant by soil application (THEOMASS). Values represent the mean of three replicates ± SEM. Within each plot, different letters indicate significant differences.
Figure 7.
Effect of fertilization scheme through different (root/foliar) application methods on leaf soluble sugar content of O. dictamnus. C: Control (water); INM-fa: Integrated nutrient management (INM) by foliar application; ChF-fa: Chemical fertilization by foliar application; INM-sa: INM by soil application; ChF-sa: Chemical fertilization by soil application; MPE-sa: biostimulant by soil application (THEOMASS). Soluble protein content was expressed per fresh weight basis. Values represent the mean of three replicates ± SEM. Within each plot, different letters indicate significant differences.
Figure 7.
Effect of fertilization scheme through different (root/foliar) application methods on leaf soluble sugar content of O. dictamnus. C: Control (water); INM-fa: Integrated nutrient management (INM) by foliar application; ChF-fa: Chemical fertilization by foliar application; INM-sa: INM by soil application; ChF-sa: Chemical fertilization by soil application; MPE-sa: biostimulant by soil application (THEOMASS). Soluble protein content was expressed per fresh weight basis. Values represent the mean of three replicates ± SEM. Within each plot, different letters indicate significant differences.
Table 1.
Effect of fertilization scheme through different (root/foliar) application methods on leaf essential macronutrient content of O. dictamnus. C: Control (water); INM-fa: Integrated nutrient management (INM) by foliar application; ChF-fa: Chemical fertilization by foliar application; INM-sa: INM by soil application; ChF-sa: Chemical fertilization by soil application; MPE-sa: biostimulant by soil application (THEOMASS). Mineral content was expressed on a dry weight basis. Values represent the mean of three replicates ± SEM.
Table 1.
Effect of fertilization scheme through different (root/foliar) application methods on leaf essential macronutrient content of O. dictamnus. C: Control (water); INM-fa: Integrated nutrient management (INM) by foliar application; ChF-fa: Chemical fertilization by foliar application; INM-sa: INM by soil application; ChF-sa: Chemical fertilization by soil application; MPE-sa: biostimulant by soil application (THEOMASS). Mineral content was expressed on a dry weight basis. Values represent the mean of three replicates ± SEM.
| Treatment |
N |
P |
K |
Ca |
Mg |
| |
g kg–1
|
|
| C |
15.7± 0.4 bc* |
1.7 ± 0.1c |
15.9 ± 1.1bc |
38.9 ± 0.5ab |
3.4 ± 0.0a |
| INM-fa |
18.7 ± 0.1 ab |
4.9 ± 0.2a |
17.7 ± 0.4abc |
32.8 ± 1.1bc |
2.9 ± 0.0bc |
| ChF-fa |
18.8 ± 0.4ab |
4.9 ± 0.1a |
20.2 ± 0.8ab |
29.6 ± 1.2c |
3.4 ± 0.1a |
| INM-sa |
14.7 ± 0.7c |
3.2 ± 0.1b |
15.4 ± 0.3cd |
41.1 ± 1.0a |
3.3 ± 0.0ab |
| ChF-sa |
20.8 ± 0.5 a |
3.9 ± 0.1b |
20.3 ± 0.5a |
37.0 ± 0.8ab |
3.4 ± 0.1a |
| MPE-sa |
14.0 ± 0.3 c |
1.6 ± 0.0c |
11.1 ± 0.3d |
36.7 ± 1.0ab |
2.7 ± 0.1c |
|
pF-test
|
< 0.001 |
< 0.001 |
0.001 |
0.020 |
0.013 |
Table 2.
Effect of fertilization scheme through different (root/foliar) application methods on leaf essential micronutrient content of O. dictamnus. C: Control (water); INM-fa: Integrated nutrient management (INM) by foliar application; ChF-fa: Chemical fertilization by foliar application; INM-sa: INM by soil application; ChF-sa: Chemical fertilization by soil application; MPE-sa: biostimulant by soil application (THEOMASS). Mineral content was expressed on a dry weight basis. Values represent the mean of three replicates ± SEM.
Table 2.
Effect of fertilization scheme through different (root/foliar) application methods on leaf essential micronutrient content of O. dictamnus. C: Control (water); INM-fa: Integrated nutrient management (INM) by foliar application; ChF-fa: Chemical fertilization by foliar application; INM-sa: INM by soil application; ChF-sa: Chemical fertilization by soil application; MPE-sa: biostimulant by soil application (THEOMASS). Mineral content was expressed on a dry weight basis. Values represent the mean of three replicates ± SEM.
| Treatment |
Cu |
Zn |
Fe |
Mn |
B |
| |
mg kg–1
|
| C |
14.5 ± 0.5cd* |
33.5 ± 0.6cd |
1732 ± 48b |
79 ± 5de |
36.3 ± 0.4c |
| INM-fa |
20.4 ± 0.4a |
53.1 ± 2.2a |
1916 ± 73a |
210 ± 12a |
51.4 ± 1.0a |
| ChF-fa |
18.7 ± 0.5ab |
47.6 ± 1.6ab |
2009 ± 94a |
174 ± 7ab |
42.6 ± 0.8b |
| INM-sa |
15.6 ± 0.3 bcd |
33.0 ± 0.3d |
1691 ± 49b |
118 ± 4cd |
38.6 ±0.7bc |
| ChF-sa |
17.7 ± 0.6abc |
42.4 ± 1.4bc |
1528 ± 44c |
132 ± 6bc |
42.5 ± 0.7b |
| MPE-sa |
12.5 ± 0.5d |
33.1 ± 0.7d |
1236 ± 62d |
60 ± 2e |
28.8 ±0.6d |
|
pF-test
|
< 0.001 |
< 0.001 |
0.022 |
< 0.001 |
< 0.001 |
Table 3.
Effect of fertilization scheme through different (root/foliar) application methods on floral essential macronutrient content of O. dictamnus. C: Control (water); INM-fa: Integrated nutrient management (INM) by foliar application; ChF-fa: Chemical fertilization by foliar application; INM-sa: INM by soil application; ChF-sa: Chemical fertilization by soil application; MPE-sa: biostimulant by soil application (THEOMASS). Mineral content was expressed on a dry weight basis. Values represent the mean of three replicates ± SEM.
Table 3.
Effect of fertilization scheme through different (root/foliar) application methods on floral essential macronutrient content of O. dictamnus. C: Control (water); INM-fa: Integrated nutrient management (INM) by foliar application; ChF-fa: Chemical fertilization by foliar application; INM-sa: INM by soil application; ChF-sa: Chemical fertilization by soil application; MPE-sa: biostimulant by soil application (THEOMASS). Mineral content was expressed on a dry weight basis. Values represent the mean of three replicates ± SEM.
| Treatment |
N |
P |
K |
Ca |
Mg |
| |
g kg–1
|
| C |
10.54±0.4ab* |
1.70±0.1b |
13.74±0.6b |
16.31±0.4 |
1.92±0.1a |
| INM-fa |
11.85±0.7ab |
1.83±0.1b |
15.49±0.5ab |
17.00±0.9 |
1.70±0.2ab |
| ChF-fa |
12.84±0.3a |
2.24±0.2a |
15.45±1.5ab |
15.91±0.1 |
1.90±0.1a |
| INM-sa |
12.58±0. 8a |
1.35±0.1c |
13.07±0.9b |
16.39±1.6 |
1.50±0.1ab |
| ChF-sa |
10.53±1. 6ab |
1.69±0.2b |
17.31±1.5a |
15.98±1.5 |
1.46±0.2ab |
| MPE-sa |
9.78±0.6 b |
1.48±0.1b |
13.38±1.6b |
16.00±0.7 |
1.40±0.1b |
|
pF-test
|
#NS (0.111) |
0.003 |
NS (0.15) |
NS (0.971) |
NS (0.109) |
Table 4.
Effect of fertilization scheme through different (root/foliar) application methods on floral essential micronutrient content of O. dictamnus. C: Control (water); INM-fa: Integrated nutrient management (INM) by foliar application; ChF-fa: Chemical fertilization by foliar application; INM-sa: INM by soil application; ChF-sa: Chemical fertilization by soil application; MPE-sa: biostimulant by soil application (THEOMASS). Mineral content was expressed on a dry weight basis. Values represent the mean of three replicates ± SEM.
Table 4.
Effect of fertilization scheme through different (root/foliar) application methods on floral essential micronutrient content of O. dictamnus. C: Control (water); INM-fa: Integrated nutrient management (INM) by foliar application; ChF-fa: Chemical fertilization by foliar application; INM-sa: INM by soil application; ChF-sa: Chemical fertilization by soil application; MPE-sa: biostimulant by soil application (THEOMASS). Mineral content was expressed on a dry weight basis. Values represent the mean of three replicates ± SEM.
| Treatment |
Zn |
Fe |
B |
| |
|
mg kg-1
|
|
| C |
12.97±1.4 |
21.88±1.4a |
222.79±5.1b |
| INM-fa |
14.68±2.8 |
20.25±1.1a |
322.93±18.8a |
| ChF-fa |
15.57±0.5 |
21.95±0.6a |
270.11±19.9ab |
| INM-sa |
14.31±0.4 |
12.61±1.2b |
241.95±40.8ab |
| ChF-sa |
15.42±1.0 |
15.03±2.2b |
204.19±35.3b |
| MPE-sa |
15.73±0.4 |
14.49±0.6b |
225.95±37.4ab |
|
pF-test
|
#NS (0820) |
(0.001) |
NS (0.197) |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).