Submitted:
01 January 2024
Posted:
03 January 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
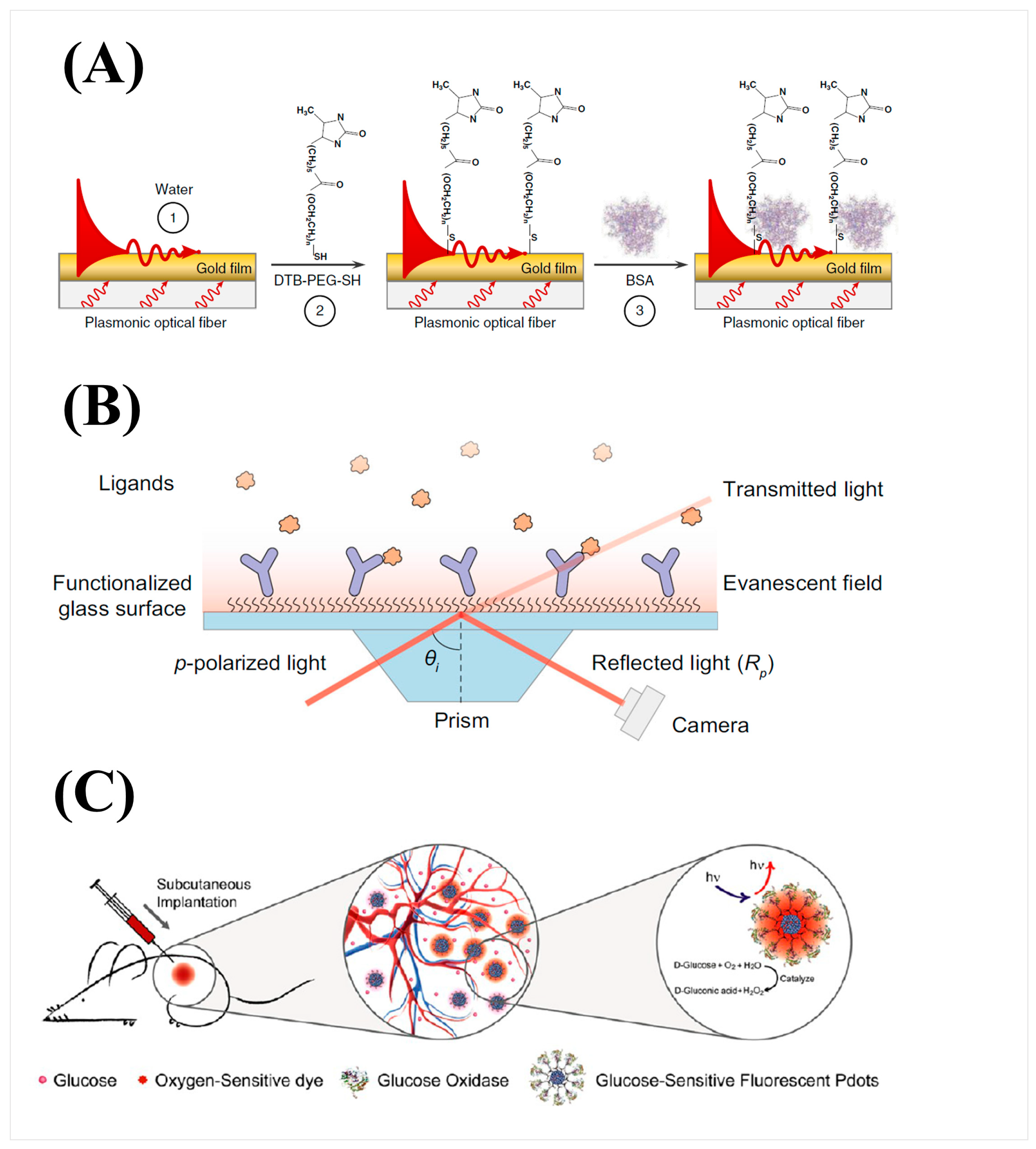
2. Optical Transduction
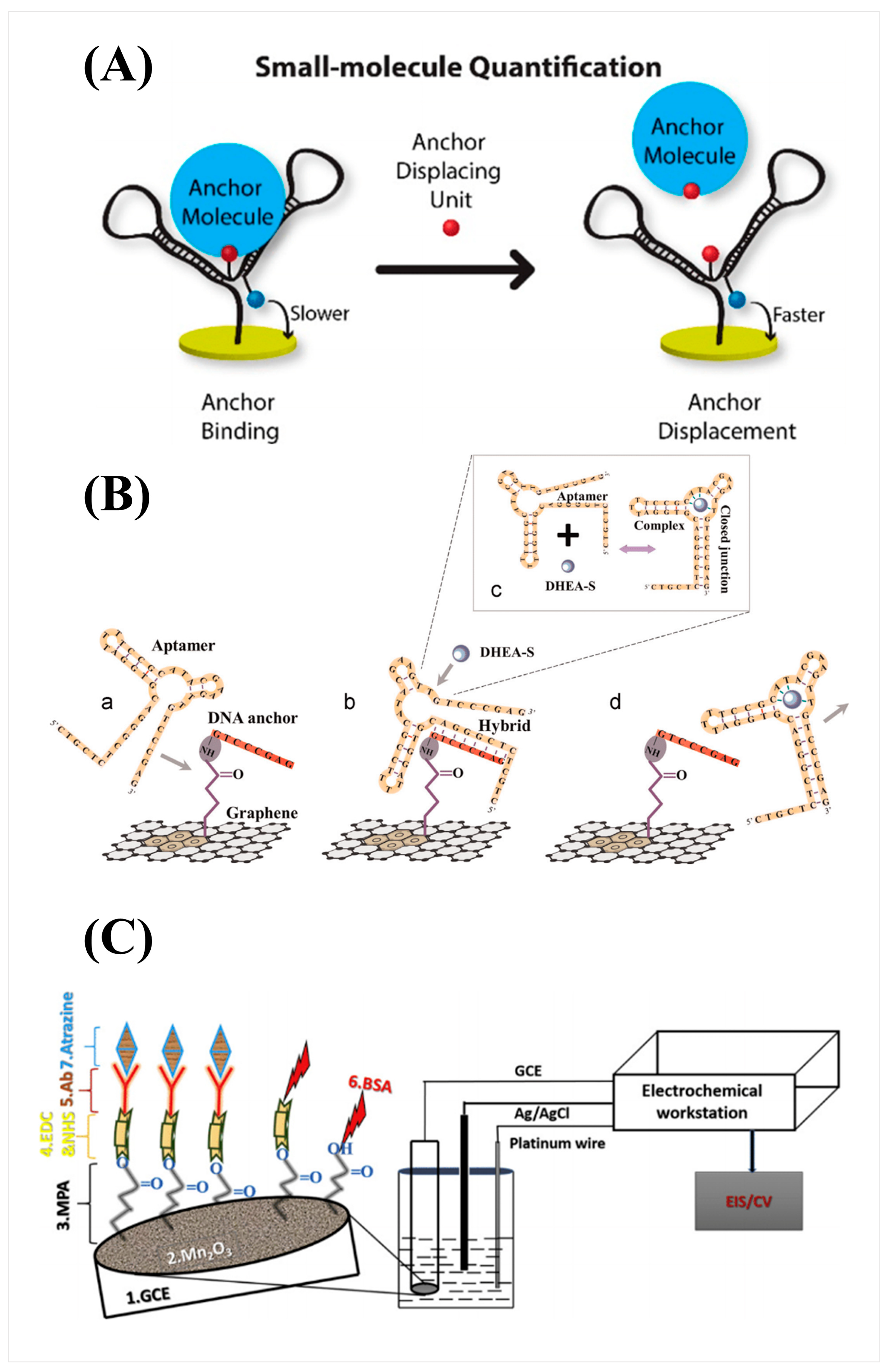
3. Electrochemical Transduction
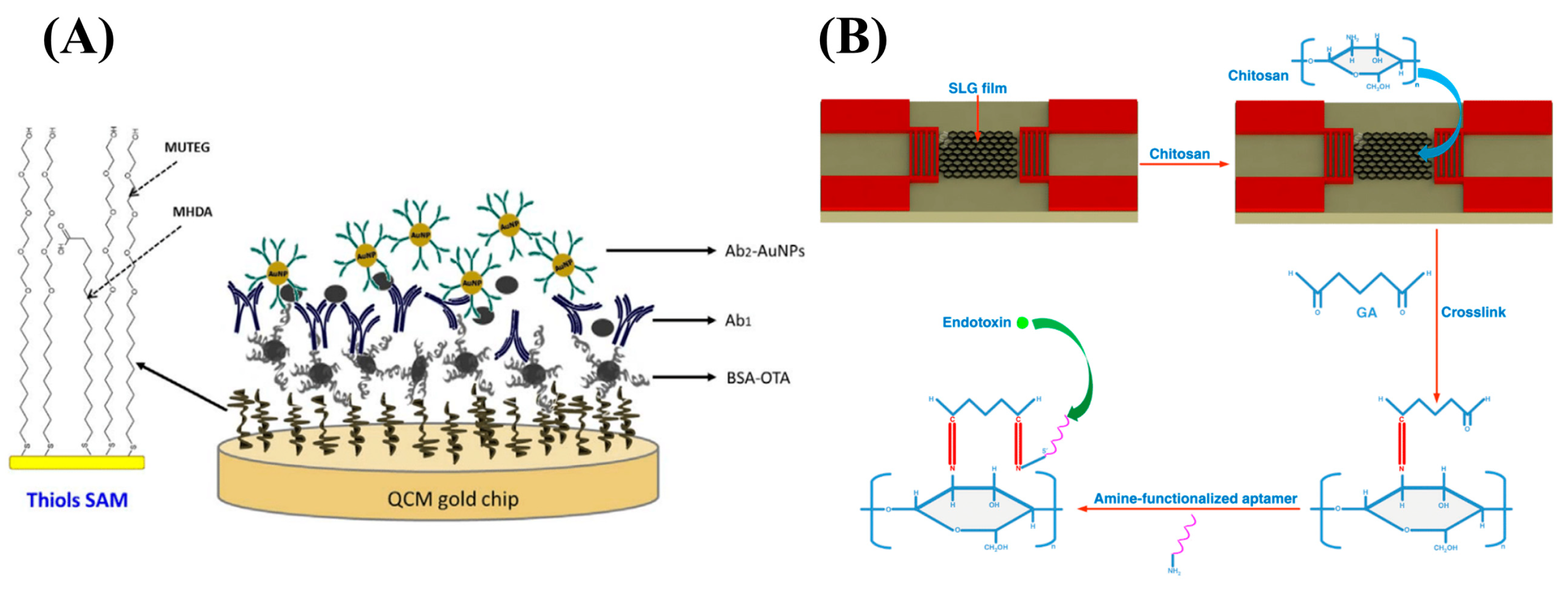
4. Piezoelectric Transduction
5. Conclusions
Funding
Conflicts of Interest
References
- Fechner, P.; Bleher, O.; Ewald, M.; Freudenberger, K.; Furin, D.; Hilbig, U.; Kolarov, F.; Krieg, K.; Leidner, L.; Markovic, G.; et al. Size Does Matter! Label-Free Detection of Small Molecule-Protein Interaction. Analytical and Bioanalytical Chemistry 2014, 406, 4033–4051. [Google Scholar] [CrossRef]
- Peltomaa, R.; Glahn-Martínez, B.; Benito-Peña, E.; Moreno-Bondi, M.C. Optical Biosensors for Label-Free Detection of Small Molecules. Sensors (Basel, Switzerland) 2018, 18. [Google Scholar] [CrossRef]
- Tiz, D.B.; Bagnoli, L.; Rosati, O.; Marini, F.; Santi, C.; Sancineto, L. FDA-Approved Small Molecules in 2022: Clinical Uses and Their Synthesis. Pharmaceutics 2022, 14. [Google Scholar] [CrossRef]
- Wu, X.; Zhang, Q.; Guo, Y.; Zhang, H.; Guo, X.; You, Q.; Wang, L. Methods for the Discovery and Identification of Small Molecules Targeting Oxidative Stress-Related Protein–Protein Interactions: An Update. Antioxidants 2022, 11. [Google Scholar] [CrossRef]
- Singh, S.; Srivastava, A.; Oh, H.M.; Ahn, C.Y.; Choi, G.G.; Asthana, R.K. Recent Trends in Development of Biosensors for Detection of Microcystin. Toxicon 2012, 60, 878–894. [Google Scholar] [CrossRef] [PubMed]
- Vogiazi, V.; De La Cruz, A.; Mishra, S.; Shanov, V.; Heineman, W.R.; Dionysiou, D.D. A Comprehensive Review: Development of Electrochemical Biosensors for Detection of Cyanotoxins in Freshwater. ACS Sensors 2019, 4, 1151–1173. [Google Scholar] [CrossRef] [PubMed]
- Moulahoum, H.; Ghorbanizamani, F.; Guler Celik, E.; Zihnioglu, F.; Timur, S. Nanoconjugated Materials as Sensors in Point-of-Care Diagnostic Tools: Detection of Small Molecules and Viruses; Elsevier B.V., 2023; Vol. 102; ISBN 978-0-443-13199-8.
- Sayago, I.; Matatagui, D.; Fernández, M.J.; Fontecha, J.L.; Jurewicz, I.; Garriga, R.; Muñoz, E. Graphene Oxide as Sensitive Layer in Love-Wave Surface Acoustic Wave Sensors for the Detection of Chemical Warfare Agent Simulants. Talanta 2016, 148, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Crivat, G.; Taraska, J.W. Imaging Proteins inside Cells with Fluorescent Tags. Trends in Biotechnology 2012, 30, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Jensen, E.C. Use of Fluorescent Probes: Their Effect on Cell Biology and Limitations. Anatomical Record 2012, 295, 2031–2036. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Finney, N.S. Small-Molecule Fluorescent Probes and Their Design. RSC Advances 2018, 8, 29051–29061. [Google Scholar] [CrossRef]
- Wysocki, L.M.; Lavis, L.D. Advances in the Chemistry of Small Molecule Fluorescent Probes. Current Opinion in Chemical Biology 2011, 15, 752–759. [Google Scholar] [CrossRef]
- Powers, J.L.; Rippe, K.D.; Imarhia, K.; Swift, A.; Scholten, M.; Islam, N. A Direct, Competitive Enzyme-Linked Immunosorbent Assay (ELISA) as a Quantitative Technique for Small Molecules. Journal of Chemical Education 2012, 89, 1587–1590. [Google Scholar] [CrossRef]
- Hämäläinen, M.D.; Markgren, P.-O.; Schaal, W.; Karlén, A.; Classon, B.; Vrang, L.; Samuelsson, B.; Hallberg, A.; Danielson, U.H. Characterization of a Set of HIV-1 Protease Inhibitors Using Binding Kinetics Data from a Biosensor-Based Screen. J Biomol Screen 2000, 5, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Myszka, D.G. Analysis of Small-Molecule Interactions Using Biacore S51 Technology. Analytical Biochemistry 2004, 329, 316–323. [Google Scholar] [CrossRef]
- Wadhera, T.; Kakkar, D.; Wadhwa, G.; Raj, B. Recent Advances and Progress in Development of the Field Effect Transistor Biosensor: A Review. Journal of Electronic Materials 2019, 48, 7635–7646. [Google Scholar] [CrossRef]
- Vu, C.A.; Chen, W.Y. Predicting Future Prospects of Aptamers in Field-Effect Transistor Biosensors. Molecules 2020, 25. [Google Scholar] [CrossRef] [PubMed]
- Shoorideh, K.; Chui, C.O. Optimization of the Sensitivity of FET-Based Biosensors via Biasing and Surface Charge Engineering. IEEE Transactions on Electron Devices 2012, 59, 3104–3110. [Google Scholar] [CrossRef]
- Pohanka, M. Overview of Piezoelectric Biosensors, Immunosensors and DNA Sensors and Their Applications. Materials 2018, 11. [Google Scholar] [CrossRef]
- García-Martinez, G.; Bustabad, E.A.; Perrot, H.; Gabrielli, C.; Bucur, B.; Lazerges, M.; Rose, D.; Rodriguez-Pardo, L.; Fariña, J.; Compère, C.; et al. Development of a Mass Sensitive Quartz Crystal Microbalance (QCM)-Based DNA Biosensor Using a 50 MHz Electronic Oscillator Circuit. Sensors 2011, 11, 7656–7664. [Google Scholar] [CrossRef]
- Guo, X. Surface Plasmon Resonance Based Biosensor Technique: A Review. Journal of Biophotonics 2012, 5, 483–501. [Google Scholar] [CrossRef]
- Singh, P. SPR Biosensors: Historical Perspectives and Current Challenges. Sensors and Actuators, B: Chemical 2016, 229, 110–130. [Google Scholar] [CrossRef]
- Wang, Q.; Ren, Z.H.; Zhao, W.M.; Wang, L.; Yan, X.; Zhu, A.S.; Qiu, F.M.; Zhang, K.K. Research Advances on Surface Plasmon Resonance Biosensors. Nanoscale 2022, 14, 564–591. [Google Scholar] [CrossRef] [PubMed]
- Gosu, R.; Zaheer, S.M. Principle of Surface Plasmon Resonance (OneStep) BT - Methods for Fragments Screening Using Surface Plasmon Resonance. In; Zaheer, S.M., Gosu, R., Eds.; Springer Singapore: Singapore, 2021; pp. 5–7 ISBN 978-981-16-1536-8.
- Pockrand, I.; Swalen, J.D.; Gordon, J.G.; Philpott, M.R. Surface Plasmon Spectroscopy of Organic Monolayer Assemblies. Surface Science 1978, 74, 237–244. [Google Scholar] [CrossRef]
- Zhao, Y.; Tong, R. jie; Xia, F.; Peng, Y. Current Status of Optical Fiber Biosensor Based on Surface Plasmon Resonance. Biosensors and Bioelectronics 2019, 142, 111505. [Google Scholar] [CrossRef]
- Jorgenson, R.C.; Yee, S.S. A Fiber-Optic Chemical Sensor Based on Surface Plasmon Resonance. Sensors and Actuators B: Chemical 1993, 12, 213–220. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, X.; Zhu, Q.; Li, K.; Lu, Y.; Zhou, X.; Guo, T. Ultrasensitive Detection of Endocrine Disruptors via Superfine Plasmonic Spectral Combs. Light: Science and Applications 2021, 10. [Google Scholar] [CrossRef]
- Ma, G.; Syu, G.-D.; Shan, X.; Henson, B.; Wang, S.; Desai, P.J.; Zhu, H.; Tao, N. Measuring Ligand Binding Kinetics to Membrane Proteins Using Virion Nano-Oscillators. J Am Chem Soc 2018, 140, 11495–11501. [Google Scholar] [CrossRef]
- Ma, G.; Shan, X.; Wang, S.; Tao, N. Quantifying Ligand–Protein Binding Kinetics with Self-Assembled Nano-Oscillators. Anal. Chem. 2019, 91, 14149–14156. [Google Scholar] [CrossRef]
- Ma, G.; Liang, R.; Wan, Z.; Wang, S. Critical Angle Reflection Imaging for Quantification of Molecular Interactions on Glass Surface. Nature Communications 2021, 12, 1–9. [Google Scholar] [CrossRef]
- Sun, K.; Tang, Y.; Li, Q.; Yin, S.; Qin, W.; Yu, J.; Chiu, D.T.; Liu, Y.; Yuan, Z.; Zhang, X.; et al. In Vivo Dynamic Monitoring of Small Molecules with Implantable Polymer-Dot Transducer. ACS Nano 2016, 10, 6769–6781. [Google Scholar] [CrossRef]
- Kozma, P.; Hamori, A.; Cottier, K.; Kurunczi, S.; Horvath, R. Grating Coupled Interferometry for Optical Sensing. Applied Physics B 2009, 97, 5–8. [Google Scholar] [CrossRef]
- Kozma, P.; Hámori, A.; Kurunczi, S.; Cottier, K.; Horvath, R. Grating Coupled Optical Waveguide Interferometer for Label-Free Biosensing. Sensors and Actuators B: Chemical 2011, 155, 446–450. [Google Scholar] [CrossRef]
- Orgovan, N.; Peter, B.; Bősze, S.; Ramsden, J.J.; Szabó, B.; Horvath, R. Dependence of Cancer Cell Adhesion Kinetics on Integrin Ligand Surface Density Measured by a High-Throughput Label-Free Resonant Waveguide Grating Biosensor. Scientific Reports 2014, 4, 4034. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.C.; Hsieh, W.H.; Chau, L.K.; Chang, G.E. Intensity-Detection-Based Guided-Mode-Resonance Optofluidic Biosensing System for Rapid, Low-Cost, Label-Free Detection. Sensors and Actuators, B: Chemical 2017, 250, 659–666. [Google Scholar] [CrossRef]
- Zhang, F.; Jing, W.; Hunt, A.; Yu, H.; Yang, Y.; Wang, S.; Chen, H.Y.; Tao, N. Label-Free Quantification of Small-Molecule Binding to Membrane Proteins on Single Cells by Tracking Nanometer-Scale Cellular Membrane Deformation. ACS Nano 2018, 12, 2056–2064. [Google Scholar] [CrossRef]
- Guan, Y.; Shan, X.; Wang, S.; Zhang, P.; Tao, N. Detection of Molecular Binding via Charge-Induced Mechanical Response of Optical Fibers. Chemical Science 2014, 5, 4375–4381. [Google Scholar] [CrossRef]
- Ma, G.; Guan, Y.; Wang, S.; Xu, H.; Tao, N. Study of Small-Molecule-Membrane Protein Binding Kinetics with Nanodisc and Charge-Sensitive Optical Detection. Analytical Chemistry 2016, 88, 2375–2379. [Google Scholar] [CrossRef]
- Liang, R.; Ma, G.; Jing, W.; Wang, Y.; Yang, Y.; Tao, N.; Wang, S. Charge-Sensitive Optical Detection of Small Molecule Binding Kinetics in Normal Ionic Strength Buffer. ACS Sensors 2021, 6, 364–370. [Google Scholar] [CrossRef]
- Lu, L.M.; Zhang, X.B.; Kong, R.M.; Yang, B.; Tan, W. A Ligation-Triggered DNAzyme Cascade for Amplified Fluorescence Detection of Biological Small Molecules with Zero-Background Signal. Journal of the American Chemical Society 2011, 133, 11686–11691. [Google Scholar] [CrossRef]
- Rong, M.; Lin, L.; Song, X.; Zhao, T.; Zhong, Y.; Yan, J.; Wang, Y.; Chen, X. A Label-Free Fluorescence Sensing Approach for Selective and Sensitive Detection of 2,4,6-Trinitrophenol (TNP) in Aqueous Solution Using Graphitic Carbon Nitride Nanosheets. Analytical Chemistry 2015, 87, 1288–1296. [Google Scholar] [CrossRef]
- Liang, M.; Li, Z.; Wang, W.; Liu, J.; Liu, L.; Zhu, G.; Karthik, L.; Wang, M.; Wang, K.F.; Wang, Z.; et al. A CRISPR-Cas12a-Derived Biosensing Platform for the Highly Sensitive Detection of Diverse Small Molecules. Nature Communications 2019, 10. [Google Scholar] [CrossRef]
- Wang, J. Electrochemical Glucose Biosensors. Electrochemical Sensors, Biosensors and their Biomedical Applications 2008, 57,I-69,I. [CrossRef]
- Liao, S.; Ding, H.; Wu, Y.; Wu, Z.; Shen, G.; Yu, R. Label-Free Liquid Crystal Biosensor for L-Histidine: A DNAzyme-Based Platform for Small Molecule Assay. Biosensors and Bioelectronics 2016, 79, 650–655. [Google Scholar] [CrossRef]
- Xiao, F.; Wang, L.; Duan, H. Nanomaterial Based Electrochemical Sensors for in Vitro Detection of Small Molecule Metabolites. Biotechnology Advances 2016, 34, 234–249. [Google Scholar] [CrossRef]
- Chen, R.J.; Choi, H.C.; Bangsaruntip, S.; Yenilmez, E.; Tang, X.; Wang, Q.; Chang, Y.L.; Dai, H. An Investigation of the Mechanisms of Electronic Sensing of Protein Adsorption on Carbon Nanotube Devices. Journal of the American Chemical Society 2004, 126, 1563–1568. [Google Scholar] [CrossRef]
- Bogomolova, A.; Komarova, E.; Reber, K.; Gerasimov, T.; Yavuz, O.; Bhatt, S.; Aldissi, M. Challenges of Electrochemical Impedance Spectroscopy in Protein Biosensing. Analytical Chemistry 2009, 81, 3944–3949. [Google Scholar] [CrossRef]
- Wiegand, C.; Pflugmacher, S. Ecotoxicological Effects of Selected Cyanobacterial Secondary Metabolites a Short Review. Toxicology and Applied Pharmacology 2005, 203, 201–218. [Google Scholar] [CrossRef]
- Regenthal, R.; Krueger, M.; Koeppel, C.; Preiss, R. Special Article DRUG LEVELS: THERAPEUTIC AND TOXIC SERUM/ PLASMA CONCENTRATIONS OF COMMON DRUGS; KluwerAcademic Publishers, 1999; Vol. 15.
- Somasundaram, S.; Easley, C.J. A Nucleic Acid Nanostructure Built through On-Electrode Ligation for Electrochemical Detection of a Broad Range of Analytes. Journal of the American Chemical Society 2019, 141, 11721–11726. [Google Scholar] [CrossRef]
- Qi, X.; Yan, X.; Zhao, L.; Huang, Y.; Wang, S.; Liang, X. A Facile Label-Free Electrochemical Aptasensor Constructed with Nanotetrahedron and Aptamer-Triplex for Sensitive Detection of Small Molecule: Saxitoxin. Journal of Electroanalytical Chemistry 2020, 858. [Google Scholar] [CrossRef]
- Wang, C.; Kim, J.; Zhu, Y.; Yang, J.; Lee, G.H.; Lee, S.; Yu, J.; Pei, R.; Liu, G.; Nuckolls, C.; et al. An Aptameric Graphene Nanosensor for Label-Free Detection of Small-Molecule Biomarkers. Biosensors and Bioelectronics 2015, 71, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Supraja, P.; Tripathy, S.; Krishna Vanjari, S.R.; Singh, V.; Singh, S.G. Label Free, Electrochemical Detection of Atrazine Using Electrospun Mn2O3 Nanofibers: Towards Ultrasensitive Small Molecule Detection. Sensors and Actuators, B: Chemical 2019, 285, 317–325. [Google Scholar] [CrossRef]
- Nakatsuka, N.; Yang, K.A.; Abendroth, J.M.; Cheung, K.M.; Xu, X.; Yang, H.; Zhao, C.; Zhu, B.; Rim, Y.S.; Yang, Y.; et al. Aptamer-Field-Effect Transistors Overcome Debye Length Limitations for Small-Molecule Sensing. Science 2018, 362, 319–324. [Google Scholar] [CrossRef]
- Wang, L.; Wang, X.; Wu, Y.; Guo, M.; Gu, C.; Dai, C.; Kong, D.; Wang, Y.; Zhang, C.; Qu, D.; et al. Rapid and Ultrasensitive Electromechanical Detection of Ions, Biomolecules and SARS-CoV-2 RNA in Unamplified Samples. Nature Biomedical Engineering 2022, 6, 276–285. [Google Scholar] [CrossRef]
- Lee, D.H.; Lee, W.Y.; Kim, J. Introducing Nanoscale Electrochemistry in Small-Molecule Detection for Tackling Existing Limitations of Affinity-Based Label-Free Biosensing Applications. Journal of the American Chemical Society 2023, 145, 17767–17778. [Google Scholar] [CrossRef]
- He, Y.; Zhou, L.; Deng, L.; Feng, Z.; Cao, Z.; Yin, Y. An Electrochemical Impedimetric Sensing Platform Based on a Peptide Aptamer Identified by High-Throughput Molecular Docking for Sensitive L-Arginine Detection. Bioelectrochemistry 2021, 137, 107634. [Google Scholar] [CrossRef]
- Kim, N.Y.; Adhikari, K.K.; Dhakal, R.; Chuluunbaatar, Z.; Wang, C.; Kim, E.S. Rapid, Sensitive, and Reusable Detection of Glucose by a Robust Radiofrequency Integrated Passive Device Biosensor Chip. Scientific Reports 2015, 5, 1–9. [Google Scholar] [CrossRef]
- Munro, N.B.; Talmage, S.S.; Griffin, G.D.; Waters, L.C.; Watson, A.P.; King, J.F.; Hauschild4, V. RESEA The Sources, Fate, and Toxicity of Chemical Warfare Agent Degradation Products; 1999; Vol. 107, pp. 933–974.
- Cui, H.; Cheng, C.; Lin, X.; Wu, J.; Chen, J.; Eda, S.; Yuan, Q. Rapid and Sensitive Detection of Small Biomolecule by Capacitive Sensing and Low Field AC Electrothermal Effect. Sensors and Actuators, B: Chemical 2016, 226, 245–253. [Google Scholar] [CrossRef]
- Skládal, P. Piezoelectric Biosensors. TrAC - Trends in Analytical Chemistry 2016, 79, 127–133. [Google Scholar] [CrossRef]
- P J, J.; Prabakaran, K.; Luo, J.; M G, D.H. Effective Utilization of Quartz Crystal Microbalance as a Tool for Biosensing Applications. Sensors and Actuators A: Physical 2021, 331, 113020. [Google Scholar] [CrossRef]
- Karczmarczyk, A.; Haupt, K.; Feller, K.H. Development of a QCM-D Biosensor for Ochratoxin A Detection in Red Wine. Talanta 2017, 166, 193–197. [Google Scholar] [CrossRef]
- Neves, M.A.D.; Blaszykowski, C.; Bokhari, S.; Thompson, M. Ultra-High Frequency Piezoelectric Aptasensor for the Label-Free Detection of Cocaine. Biosensors and Bioelectronics 2015, 72, 383–392. [Google Scholar] [CrossRef]
- Koutsoumpeli, E.; Tiede, C.; Murray, J.; Tang, A.; Bon, R.S.; Tomlinson, D.C.; Johnson, S. Antibody Mimetics for the Detection of Small Organic Compounds Using a Quartz Crystal Microbalance. Analytical Chemistry 2017, 89, 3051–3058. [Google Scholar] [CrossRef]
- Gronewold, T.M.A. Surface Acoustic Wave Sensors in the Bioanalytical Field: Recent Trends and Challenges. Analytica Chimica Acta 2007, 603, 119–128. [Google Scholar] [CrossRef]
- Ji, J.; Pang, Y.; Li, D.; Huang, Z.; Zhang, Z.; Xue, N.; Xu, Y.; Mu, X. An Aptamer-Based Shear Horizontal Surface Acoustic Wave Biosensor with a CVD-Grown Single-Layered Graphene Film for High-Sensitivity Detection of a Label-Free Endotoxin. Microsystems and Nanoengineering 2020, 6. [Google Scholar] [CrossRef]
- Yen, Y.K.; Chiu, C.Y. A CMOS MEMS-Based Membrane-Bridge Nanomechanical Sensor for Small Molecule Detection. Scientific Reports 2020, 10, 1–8. [Google Scholar] [CrossRef]
| Transducer | Technique | Analyte | LOD1 | Ref. |
|---|---|---|---|---|
| Optical | SPR | Estradiol | 1.5 pg/mL | [28] |
| SPR-based Oscillator | EMPPP | 4.0 fg/mm2 | [30] | |
| GMR | DNP | 75 ng/mL | [36] | |
| CAR | Furosemide | 1.5 pg/mm2 | [31] | |
| CSOD | Imatinib | 0.14 e-/µm2 | [38] | |
| Fluorescence | Picric acid | 1.9 ng/mL | [41] | |
| Fluorescence | Uric acid | 1.68 ng/mL | [43] | |
| Fluorescence | Glucose | 8 mg/mL | [32] | |
| Liquid Crystal | L-histidine | 7.8 mg/mL | [45] | |
| Electrochemical | Amperometric | Biotin | 0.9 µg/mL | [51] |
| Voltammetry | Saxitoxin | 0.28 ng/mL | [52] | |
| FET | Dehydroepiandosterone sulfate | 16.5 ng/mL | [53] | |
| Quantum EIS | Cortisol | 57.6 ng/mL | [57] | |
| EIS | Atrazine | 0.22 zg/mL2 | [54] | |
| EIS | L-arginine | 19.2 fg/mL | [58] | |
| RF Resonator | Glucose | 0.11 ng/mL | [59] | |
| Capitative Affinity | Bisphenol A | 2 fg/mL | [61] | |
| Piezoelectric | QCM | Ochratoxin | 0.4 pg/mL | [64] |
| QCM-based | Cocaine | 27 µg/mL | [65] | |
| SAW | Dipropylene glycol monomethyl ether | 20 µg/mL | [8] | |
| SAW | Endotoxin | 2.53 ng/mL | [68] | |
| MEMS-based | Phenytoin | 4.06 µg/mL | [69] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





