1. Introduction
Humans are continuously exposed to various toxic elements, which can appear in increased quantities in the atmosphere, water, soil, plants, and animals. These differences may be due to natural causes (volcanic activity, soils, and waters with locally increased metal content) and anthropogenic causes (intensive agriculture, animal husbandry, mining, chemical metallurgy, transport, and households). Humans interact in a variety of environmental matrices (changes in the composition of inorganic and organic compounds) and biological matrices (changes in the abundance and diversity of fungi, bacteria, and other living forms) that can alter their degree of risk from toxic elements (both in the environment and their organism)[
1]. Therefore, the human chemical environment is very complex and variable over time, leading to diseases with difficult-to-detect causes. Often, chronic and/or acute poisoning by a toxic element through ingestion, inhalation, or dermal contact cannot be ruled out as one of the causes. The result is most often an accumulation of metals in the body and changes in the functioning of organs and glands, such as the heart, brain, kidneys, bones, and liver [
2]. This situation is of particular concern in developing countries, which have increased their industrial activities, especially in an era of increased fossil resource exploitation or urban agglomerations [
3,
4]. It is not surprising, therefore, that a positive association between metal levels in patients with diseases such as Alzheimer's disease, breast cancer, endocrine disorders, hypertension, rheumatoid arthritis, and childhood neurocognitive impacts has been demonstrated from studies [
2,
5,
6]. For example, lead, cadmium, cobalt, chromium and copper cause significant environmental problems due to their persistent and toxic character. Their accumulation in living organisms causes damage to the nervous system, kidneys, liver, and reproductive system and can even cause death [
2,
7,
8]. Therefore, there is an increasing demand for cheap and easily accessible diagnostic methods to prevent and control the patient's condition during therapy.
It is essential to determine the levels of heavy metals to which humans are exposed, but analysis of air, soil, water, and food samples can be inconclusive in assessing health risks from toxic elements [
2]. For these reasons, biological monitoring of contaminants has been identified as the optimal choice for assessing the impact of contaminant exposure on human health [
2,
8]. The primary biological samples used for these analyses are urine, blood, hair, nails, and tissue samples taken when implants are replaced [
9,
10]. Metals in biological materials are present in trace amounts and, therefore, require sensitive instrumental techniques. Among the most commonly used are atomic absorption spectroscopy (ASA) with atomization in a graphite cuvette (in a few cases in a flame) [
11,
12,
13,
14], optical emission spectrometry [
15,
16], and mass spectrometry [
4,
5,
6,
17,
18] with plasma as a source of excitation or ionization of elements (ICP-OES, ICP-MS), respectively and total reflection X-ray fluorescence spectrometry (TXRF) [
19,
20]. These techniques have high operational costs and additionally require sample mineralization [
5,
6,
16,
21] or extraction combined with preconcentration [
13,
16,
18,
22]. Therefore, many methods for determining metal ions are being developed using electrochemical [
23] or spectrophotometric techniques [
24], the selectivity of which depends on the leaching, enrichment, and complexation method of metal ions.
It should be noted that the content of an element in biological material does not have total diagnostic value if it is not presented concerning other elements, especially chemically similar ones. This way, the degree of advancement of metal homeostasis changes is described, which may better reflect the patient's condition. For this reason, techniques such as ICP-OES/MS that allow the simultaneous determination of many elements offer high sensitivity, selectivity, and even atomic/isotopic specificity are most often used [
6,
21,
25]. In the case of urine and blood, metals can be determined with ICP-MS also in dissolved/diluted, not mineralized samples [
26,
27]. Hair, fingernails, and tissue samples are usually mineralized to decrease interferences.
Another exciting solution is the application of energy or wavelength dispersive X-ray fluorescence spectrometry (WDX or EDX), which allows for the simultaneous determination of metals in a solid material [
28]. This technique requires that the bulk material be homogeneous, representative of the sample, and dense enough to increase the efficiency of the emitted radiation relative to the reflected and diffuse ones [
29]. These features are achieved by adequately grinding the material and packing it under high-pressure conditions (up to 20 bar) supported, if required, by adding a suitable binder [
19]. This technique is cheap and easy to use and, therefore, creates the possibility of creating a cheap and ecological method for metal determination in easily collected samples of hair and nails. It should be noted, however, that the grinding process carries serious challenges related to losses of the tested material and electrostatic interactions leading to its granulation. Lowering the temperature and moistening the material by adding an organic solvent (acetone, methanol, isopropanol) increases the abrasion process's efficiency and material losses [
30].
The purpose of the work was to develop a method for the determination of elements using ED-XRF in non-mineralized hair and nails. The method was supposed to be simple and allow for quick screening tests. Many problems related to the amount of material taken for analysis, its homogenization, and the preparation of a representative pellet were solved during its development. The developed sample preparation method can be implemented for metal analysis using other ED/WD/T-XRF systems.
2. Results and discussion
Since preparing samples and standard pellets for metal determination using ED-XRF is a procedure not yet described in the literature, it will be the subject of a thorough discussion. However, the procedure and most results obtained using ICP-MS/MS have been placed in the support information (
Tables S1–S5).
2.1. Parameters of the ED-XRF influencing the sensitivity of the method
Due to the lack of available certified pellets for the material of biological origin, at the initial stage, it was decided to use certified pellets made of polyethylene containing known contents of Br, Cr, Cd, Hg, and Pb (Calibration Set for PE Analysis, VHG-ROHS-PE-SET1D, VHG Labs, Menchester, NH, USA). In this way, the impact of the pellet's homogeneity and width on the quality of the results was excluded.
Three parameters were detected that significantly affect the sensitivity and precision of the technique: the pressure, the collimator, and the measurement time.
The air pressure in the measuring chamber significantly influenced sensitivity, but this is not a controllable parameter. The level of vacuum depends on the model of the instrument used. Initial work was carried out using the model 7000, which allowed operation in low vacuum conditions (below 50 Pa). When the model 7000 apparatus was used, detecting light elements at contents above 200 ppm was only possible. Applying the 8100 model, in which the pressure in the measurement chamber reaches 1 - 5 Pa, improved sensitivity up to 50 times for the determination of light elements (e.g., Na, Mg, Ca, and K).
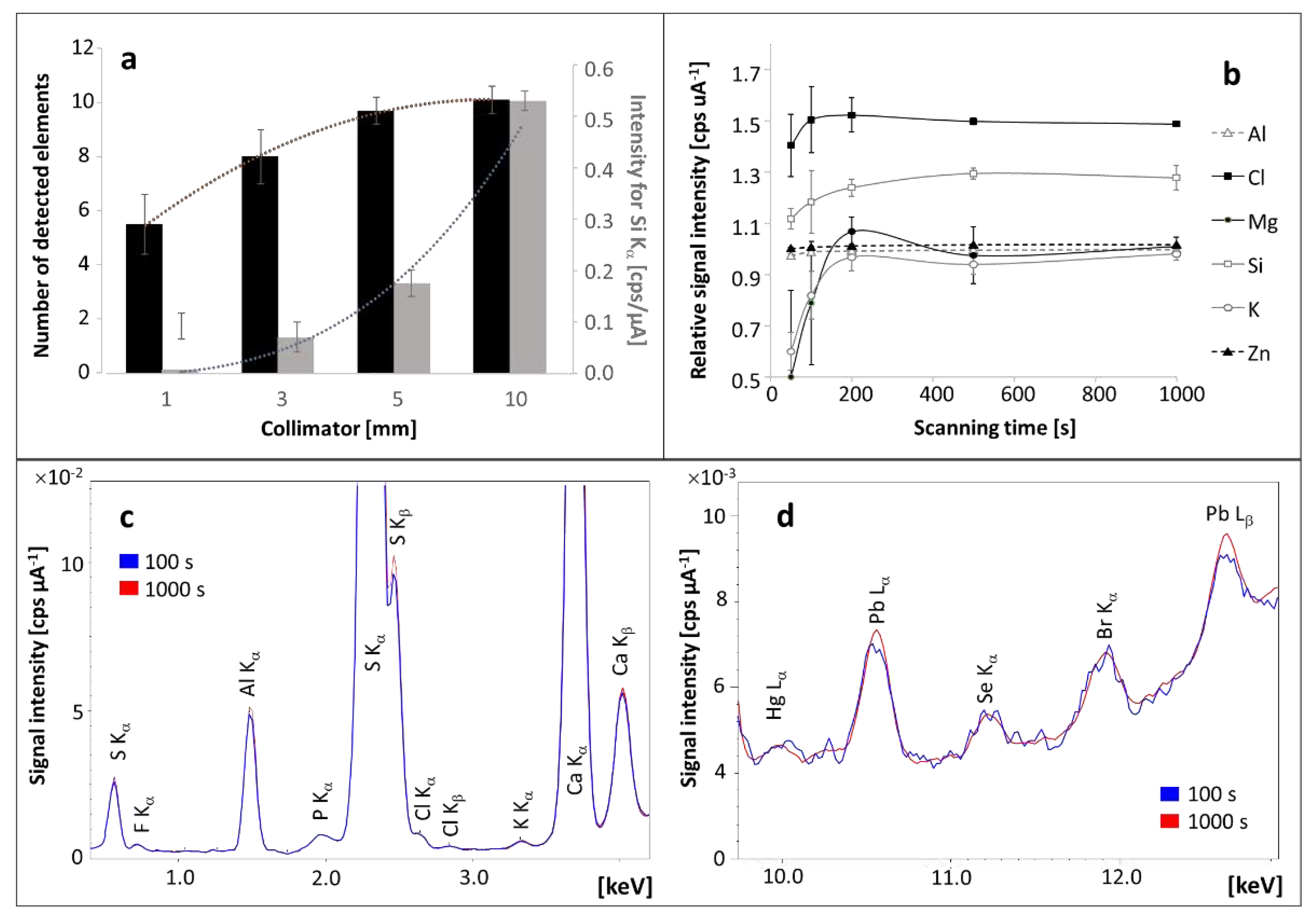
Increasing the diameter of the radiation beam (collimator) significantly increased the height of the signals from the elements and thus decreased the standard deviation (
Figure 1a). Moreover, more elements were detected when the diameter of the collimator was increased. It was due to the improvement of signal-to-noise ratio. It was decided to carry out measurements for the largest permissible diameter of the radiation beam - 10 mm.
An important parameter influencing the signal-to-noise ratio (S/N) was the scanning time. It was found that analysis carried out between 5 and 18 minutes offers the highest S/N (
Figure 1b). It was achieved through improved repeatability of the method obtained with a more accurately defined spectrum with reduced noise (
Figure 1 c,d). Lower noise in the spectrum was responsible for three different effects: (1) slightly increasing signals offering better repeatability for elements in amounts higher than LOQ (such as Cl, Cr, Hg, Mn, Na, Si, Tl), (2) improved repeatability with scanning time for stable signal– observed for elements in an amount close to the upper limit of quantitation (such as Al, Br, Ca, Cu, Fe, Ni, Pb, S, Se, Sr, Y, Zn), (3) random changes of signal intensity and slight improvement of the precision for elements with content close to LOD (such as As, K, Mg) (
Figure 1b).
It was also noted that in the case of a material with a complex matrix such as a biological one, the principle of obtaining better sensitivity for lower X-ray energy when determining light elements (smaller atomic radius allowing more straightforward electron knockout) and higher energy for heavier elements is still fulfilled.
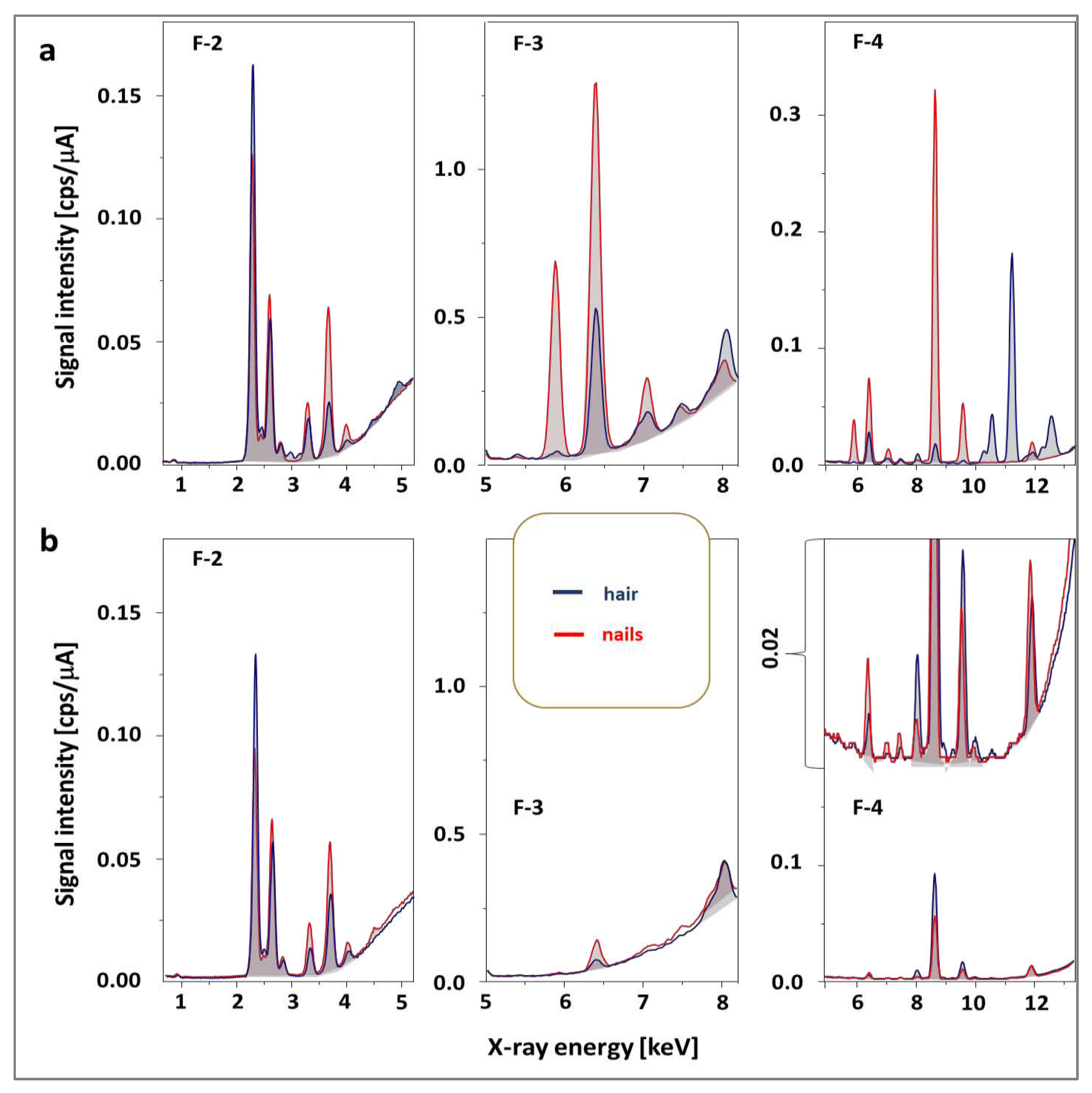
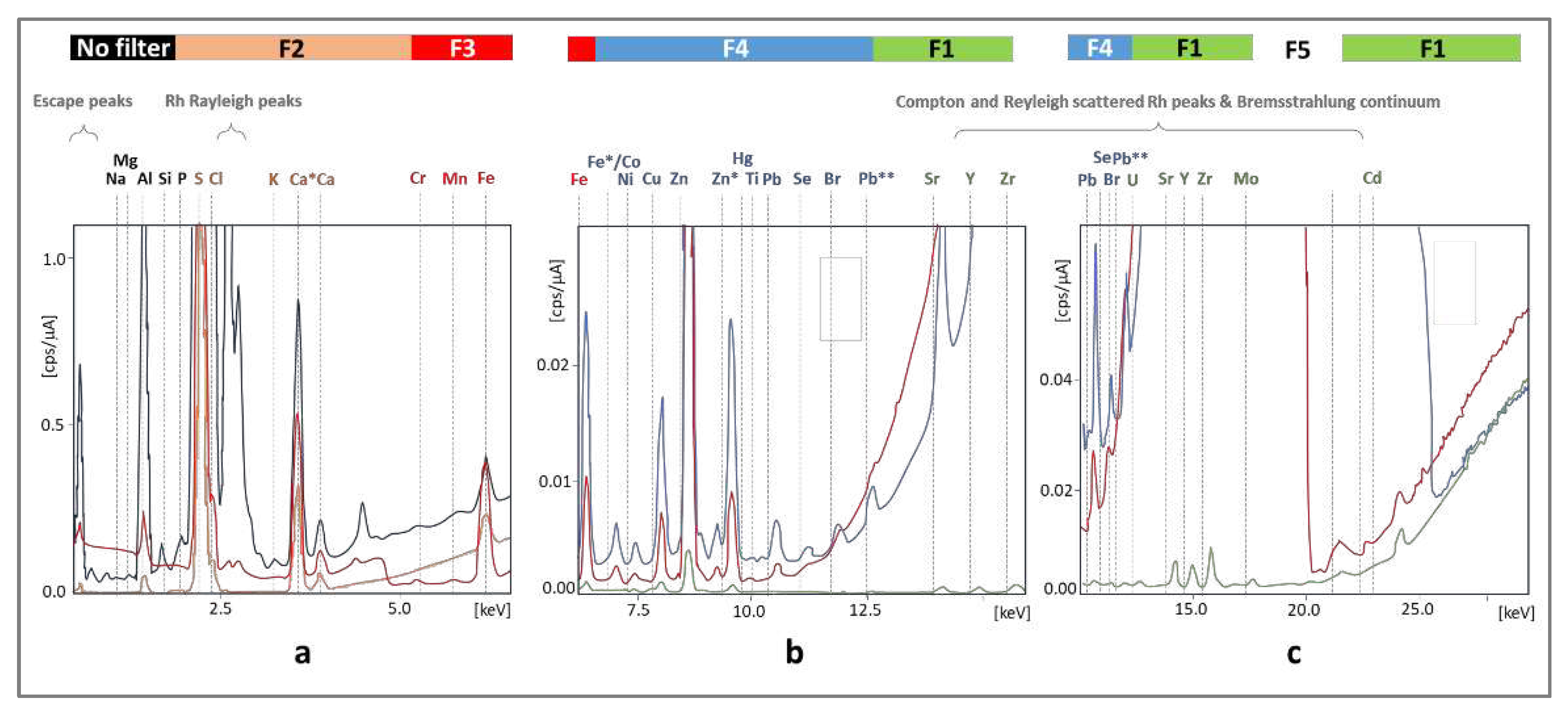
Another problem is interference, the basis of which may be as follows: (1) the incident radiation emitted from the source to the sample leading to the high background and the presence of several signals, (2) reflected x-ray from the atoms of the sample without losing any energy (Rayleigh/elastic scattering,
Figure 2a) corresponding to the characteristic energies of the x-ray tube target element (Rh), (3) scattered x-ray without loss of energy due to the excitement of an inner-shell electron (Compton scattering), (4) other incidents related to Rayleigh and Compton scattering leading to the presence of Bremsstrahlung continuum (high background,
Figure 2b), (5) fluorescence in the detector if x-ray entering the detector in the case when the energy is greater than the absorption edge of an element in the detector (escape peaks) and (6) matrix effect related to reabsorption of characteristic x-rays of other elements and fluorescence obtained through secondary excitation of elements. However, it is possible to use a filter between the radiation source and the sample to attenuate radiation in specific energy ranges.
The best results were obtained for filters 1-4 made of aluminum, titanium, and copper with different relative thicknesses of the layers of these metals. For low energies in the case of the determination of light elements (Ca, K, S), the best results were obtained for filter F2; for medium energies applied to determine Cr, Fe, and Mn - filter F3 and filter F4 made it possible to reduce the background to the greatest extent without losing signals in the case of Br and Pb atoms (
Figure 2b), while for the highest energies, the most elements could be detected after suppressing the radiation emitted by rhodium with filter F1. The average intensity of the generator was 100 A due to the need to limit the influence of the organic components of the matrix on the signal height (in the case of metal alloys, the intensity was 3-10 times lower). In this way, the measurement conditions that were used during the analyses of the tested materials of biological origin were determined (
Table 1).
2.2. Modification of autosampler's wells
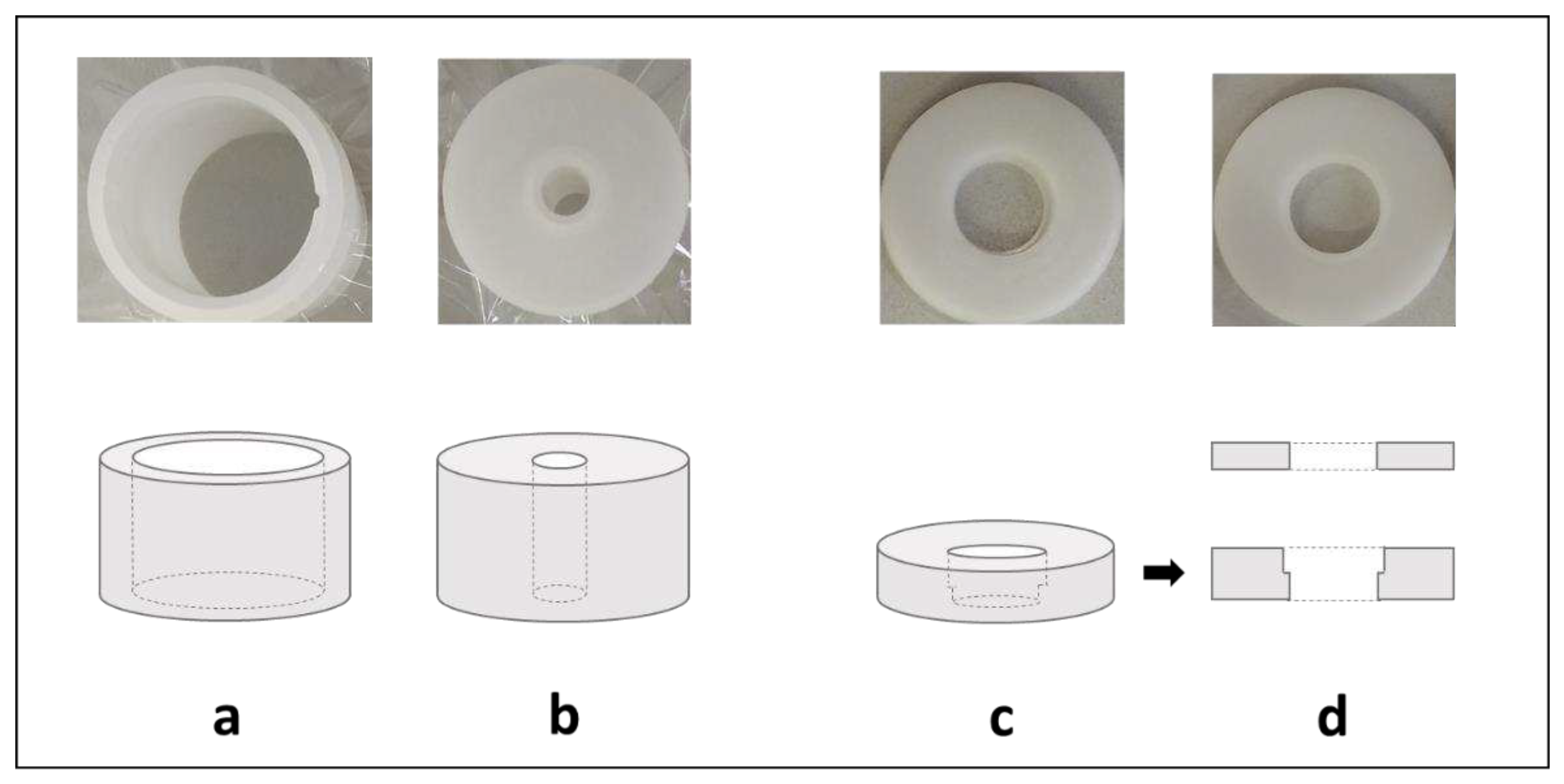
In the case of material of biological origin, the amount of sample available for analysis is limited. Hair and nails are a perfect example because a large volume of material has a low mass. To prepare a standard-size EDX pellet (30 mm diameter x 5 mm thickness), 0.5 g of hair or nails is needed. Meanwhile, an average of 0.1-0.2 g of material from one nail's cut can be expected. The apparatus manufacturer offers vessels that reduce the amount of the necessary sample for measurement. However, they force a change in the diameter of the collimator, which reduces the sensitivity of the method (
Figure 3a). It was decided to make an insert reducing the diameter of the sample well in the EDX detection chamber, allowing for the placement of pellets with a diameter of 13 mm (
Figure 3b). The second part of the reducer (cover) was used to block the position of the pellet, which was of particular concern when the appropriate vacuum was established.
Sample-well reducers were initially made of steel, but it turned out that the elements present in steel (Fe, Cu, Ni) were visible in the spectrum regardless of the width of the X-ray beam. Once the MOP insert was made, a 10 mm diameter beam could be used, and no signals from the insert were observed. The minimum thickness of the pellet was also checked to ensure adequate X-ray absorption efficiency and to obtain the highest possible fluorescence. It turned out that the fluorescence increased with the thickness of the pellet up to 3 mm and then reached the plateau (
Figure S1). It should be noted that it was established for hair, and value may change depending on the sample type, binder, and determined elements.
This way, the amount of sample necessary to obtain a 13 mm pellet was reduced to 0.2 g when no binder was used. MOP-made reducers were placed in all 12 wells of autosamplers, and their cohesion was checked by establishing the precision and trueness by measuring the metals in certified PE powder pelleted and placed in all 12 wells with inserted well-reducer (RSD for Br, Cd, Cr, Hg, Pb was lower than 2%).
2.3. Formation of pellets for calibration of EDX
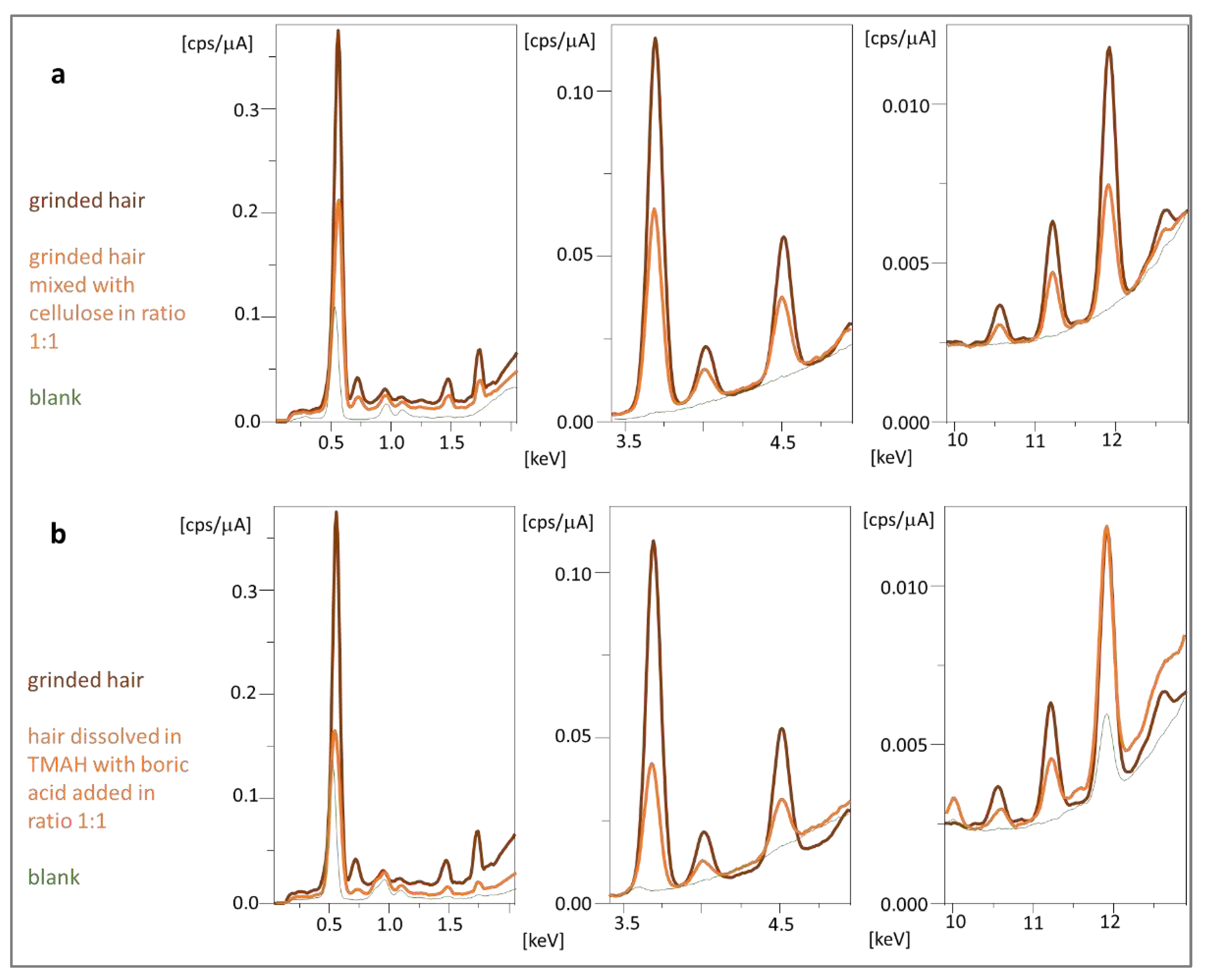
Pellets made from ground hair without adding a binder were quite unstable. After 24 hours, they began to crumble due to moisture absorption from the air. The addition of a binder in the form of cellulose solved this problem. Wax as a binder melted during pelleting, which increased the variance of the method. In turn, using boric acid increases the friability of the tablet. Cellulose (as the best additive) can be used in ratios of 1:1 and 2:1 to the tested material (added in mortar and mixed accurately), which reduces the required amount of tested material to 50 mg. The stable cellulose-hair pellets for up to two weeks provided good precision for 10 tested elements (RSD 3.97% for n = 12), but they were stored in vacuum bags or a desiccator. The spectrum of the hair pellet obtained with the cellulose binder is shown in figure 4a. In addition, the spectrum derived from cellulose is shown, based on which it can be concluded that cellulose as a binder is not a source of interference and can be used to determine metals in biological material.
Hair and nails are a significant challenge when grinding (electrostatic effects reduced by adding a small volume of polar organic solvent; need to use grinders with a small chamber to reduce waste, avoiding contamination of the material by metals from elements made of steel). Using agate instead of steel significantly extends the time of the grinding process (from 3 min to 1 h), which leads to an increase in the temperature of the material. Therefore, it is necessary to cool the grinding bowl properly. It can be achieved with liquid nitrogen (incompatible with agate) or by discontinuous grinding (for example, 10 min of grinding interrupted with 5 min pauses). Still, it can be problematic when a higher number of samples has to be prepared. Therefore, a second method of preparing pellets from a material that is not ground but is dissolved has been developed so that after evaporation of the solvent reaches an appropriate/expected homogeneity.
Samples were dissolved using a 25% TMAH (m/v) solution in methanol. Methanol shortens the sample drying time before pelletization, making sample preconcentration more efficient. The obtained biomass was viscous and made it possible to obtain more durable pellets with boric acid as a binder (RSD 2.97% for n = 12 measurements carried out within 10 days). As a result, three calibration methods were obtained: based on the ground (G), dissolved hair, and based on gelatin spiked with metal ions standard solutions (TH, TM). In each case, the slope coefficients of standard curves for the tested elements differed significantly (usually by fold of 3-5,
Table S5). The difference is caused by matrix components (cellulose vs boric acid, gelatin), changes in metal speciation, structure of protein, and pellet density. It means that matrix influence on signal intensity is significant, and the instrument calibration method should consider it. Despite a similar background, pellets based on gelatin offered higher sensitivity (slope coefficients) and the addition of boric acid instead of cellulose enabled the detection and determination of the largest number of elements.
It should be noted that sample preparation via dissolution with TMAH is more manageable to automate and less time-consuming than grinding. Moreover, samples offer better homogeneity and stability than ground hair or nail pellets. Also, detection limits established for blank were lower for the majority of elements when a procedure based on TMAH was used.
The proposed TMAH-based method for sample preparation does not require grinding of hair. It can also be used for metal determination by ICP-MS. This way, microwave-assisted mineralization can be avoided, requiring high amounts (from 5 to 7 mL per sample) of concentrated nitric acid and hydrogen peroxide. Moreover, samples in the form of pellets can be mineralized after analysis with EDX for further investigation via ICP-MS. However, the background will be increased by boric acid and other chemicals added during sample preparation and pellet formation. For example, Pb, which signal was observed in a blank sample at 12.6 keV (Lβ), is used in the mathematical correction of Pb determination.
2.4. Validation of EDX method for determination of selected elements in hair
It was decided that metal determinations would be performed in hair dissolved with TMAH, increasing the method's throughput and significantly reducing its cost. Pellets were made from mixed hair in various proportions (including two types of CRMs), in which ICP-MS determined the metal content. The metal content in the CRM hair samples is higher than normally detected; therefore, to determine the LOD and enlarge the linear range, the hair sample (waste from the hairdressing salon) was subjected to a demetallization by cyclic washing in hot solutions of 10% nitric acid, 3.6% hydrochloric acid, ammonium acetate solution with 50 mM EDTA and 50 mM thiourea. The hair obtained this way still contained metal ions, detected by ICP-MS in the majority but not by EDX. Such a hair sample was used to determine the LOD (blank) and as a matrix component to mix with other metal-rich hair to obtain samples of different metals. Obtained calibration curves with statistical descriptions are presented in
Table 1. It should be noted that the linear response range does not depend on the detector's dynamic range but on keratin's ability to adsorb metal ions.
Instrument stability for 2 months was tested using reference metal alloy (stable in time regardless of storage conditions) and was analyzed (n = 15) using calibration obtained for the determination of metals in hair and nails. Relative standard deviation was below 2% for most elements except chromium (4.3%), sulfur (6.8%), magnesium (6.1%), and lead (4.1%). Higher variability for the sulfur and magnesium amounts was related to their quantity below LOQ. It can be concluded that the EDX instrument is not only able to work stably but can also return to the expected conditions after a week of non-use without recalibration of the instrument. Meanwhile, the ICP-MS instrument needs to be recalibrated each day before sample analysis due to plasma fluctuation and changes in the dimensions of the orifice in the interface section exposed to corrosive solutions with acids and salts.
Four pellets were made to check the repeatability for manufacturing the pellets for one type of hair, each on a different day. Measurements were made three times for each pellet to determine the coefficient of variation for the sample preparation process vs instrumental precision. Based on the obtained results (
Table 2), it can be concluded that the variation coefficient is higher for the sample preparation step than for repeated measurements obtained for one pellet. The exception is the coefficient of variation for silver, which is below LOQ.
The method's accuracy was tested by determining elements in certified reference materials and ICP-MS/MS (
Table 3,
Figure S2). It can be noted that the method's accuracy was positively verified for the two materials certified for 13 elements whose content was, in some cases, less than 1 ppm, and these elements were selected for further tests. For four elements (Ag, Co, Cd, Mo, V), their content in at least one of the materials was less than 0.1 ppm, which is problematic due to the instrument's limited sensitivity. Light metals such as Na and K also proved to be troublesome to determine due to the negligible energy dispersion characteristic of atoms with a small number of orbitals. In no case was it possible to correctly determine V due to interference from Cr.
2.5. Application of EDX method for determination of selected elements in nails
Given that keratin is the basic microfibre from which hair, nails, hooves, and bird beaks are made up, it was decided to see if the method developed for hair determination could be applied to determine elements in nails. Waste nails were collected and ground (with the addition of isopropanol as an anti-granulation agent) to produce material with a grain size of less than 20 m (verified using optical microscopy) and divided into two representative portions, prepared in the same way as hair samples and subjected to determination by ICP-MS/MS and by EDX. A comparison of the ED-XRF spectrum obtained for the hair with that obtained for the nails confirms the assumption that the composition of the sample matrix is significantly similar spectrally (
Figure 6). It can be noticed that nails have a greater ability to accumulate selected elements than hair. These are: Ca (K
α 3.7 keV), Mn (K
α 5.9 keV), Fe (K
α 6.4 keV, K
β 7.1 keV), Ni (K
β 8.3 keV), Zn (K
β 9.6 keV). In such cases, the sample was diluted using binder and recalculated according to the nail:binder ratio. It is worth noticing that spectral background and height of signals with increased additions of boric acid were changing the same way as for hair samples, leading to the conclusion that the same calibration can be applied for both hair and nail samples.
Figure 5.
ED-XRF spectra obtained for hair and nails containing the highest (a) and the lowest (b) amounts of metals in examined samples.
Figure 5.
ED-XRF spectra obtained for hair and nails containing the highest (a) and the lowest (b) amounts of metals in examined samples.
A comparison of the results obtained by ICP-MS/MS and ED-XRF methods shows that in both methods, in the case of 15 elements, the determined amounts agree within the range of the expanded uncertainty of the method (k=2,
Table 4). However, It should be noted that in chromium and nickel determination by ICP-MS/MS, it was necessary to use platinized cones instead of the standard nickel-plated ones to avoid positive error.
The levels of metal content in the nails are similar to those obtained for the hair used to calibrate the method and mostly were within the linear response range of the method established using hair certified reference materials. If the amounts of metals exceeded the upper limit of the linear range of the method, the pellet was ground in mortar with the addition of boric acid and pressed again prior to re-analysis. The determined metal content recalculated considering the degree of dilution.
The validity of the EDX method (by comparing the results of determinations using EDX and ICP-MS/MS) was confirmed in the case of 15 elements. However, it should be noted that the bromine, chlorine, mercury, and silicon determination was performed using ICP-MS/MS only in a pooled sample (combined material of various biological origins). As amount of nail material is limited, there is only one way to dissolve the material. The nitric acid method was chosen because it allowed the determination of more elements than the method using an alkalic medium. Reconstituted samples had to be analyzed within 6 hours. After 18 hours, the recovery of many elements (Ag, Al, Ba, Cd, Cl, Cu, Fe, Zn) decreased by up to 60%, and the precipitate was observed at the bottom of the flask. The EDX method was more accurate for determining bromine, silicon, sulfur, chlorine, mercury, and selenium. This was due to the different chemical nature of the elements, which required a different method of digestion or stabilization of the elements. The method using ICP-MS/MS was more accurate for determining cadmium, cobalt, manganese, molybdenum, and silver.
Moreover, it should be noted that MQ-water of the resistance 18 MΩ obtained by the Milipore system used in our laboratory was still contaminated by Sr. To obtain detection limits comparable with the EDX method, two additional purification systems were tested, and one offered appropriate water purity as a dilution medium. It is of particular importance as the sample needed to be diluted by factor of 500, 1000, and 10000 to determine different elements in the mineralized sample. Such problems were avoided in the the EDX-based method, as the sample was usually diluted twice or a maximum of four times prior to pellet formation.
2.6. Applicability of the EDX method considering biological and environmental diversity of samples
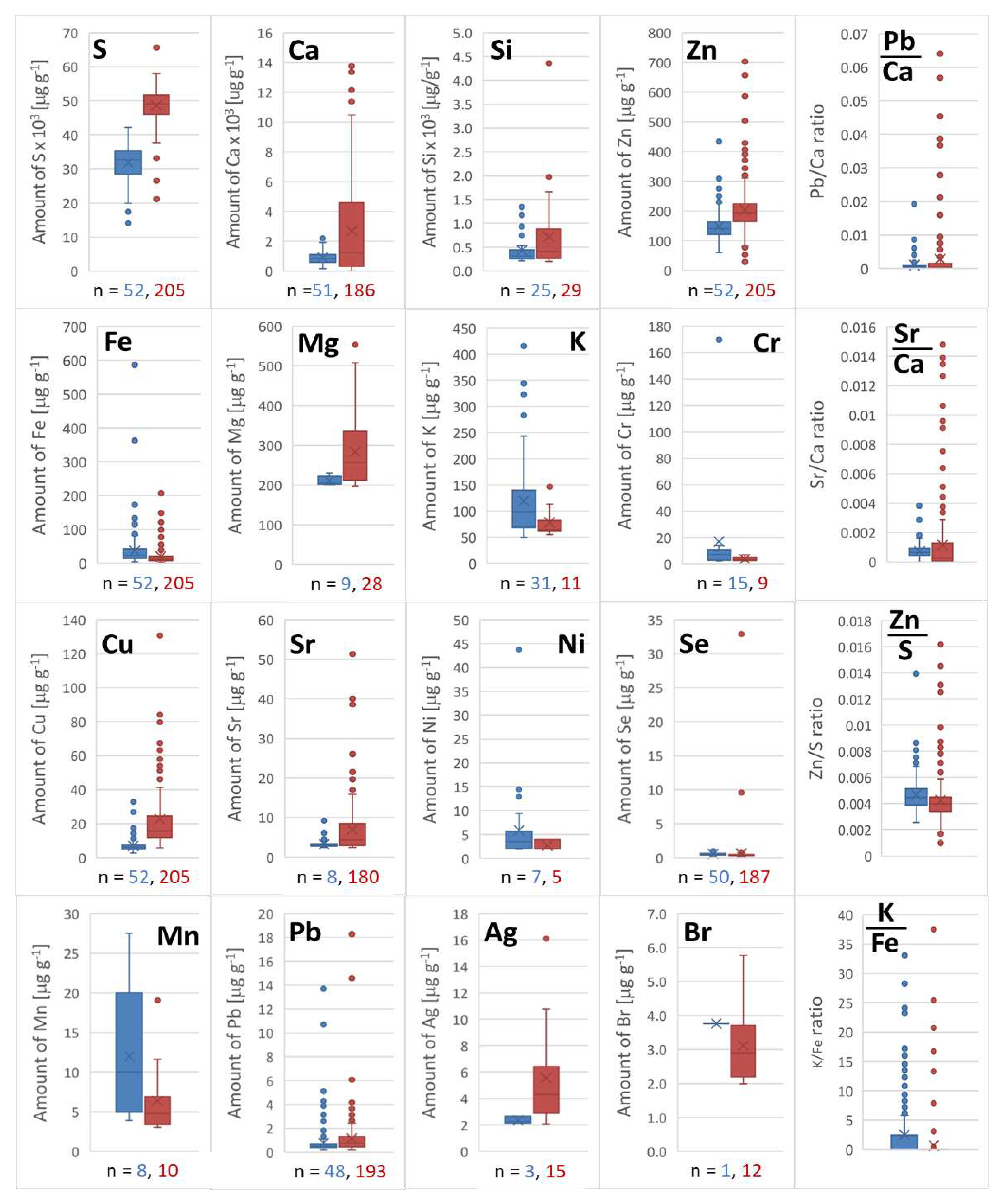
Waste hair and nail samples were collected from the beauty departments in Warsaw. Hair of different colors was fractionated and classified as the sample obtained from one person. The nail samples were mixed material collected at one station. Obtained results for 208 samples of hair and 52 of nails are presented in the form of box charts to show data distribution as an interquartile range, median, and statistical dispersion to indicate outliers (
Figure 6). The number n in the chart is the number of the results above LOQ of the EDX-based method.
It can be easily noticed in figure 6 that for elements such as Ag, Br, Ca, Cu, Mg, Mn, S, Si, and Sr, the interquartile range was the widest. In the case of Ag, Br, and Mn, the number of the results is very low, and statistical dispersion is very high. It is the result of high noise typical for sample amounts close to the lower limit of quantitation. Distribution of Ca, Cu, Mg, S, Si, Sr, and additionally Fe with Zn elements can be an effect of different grooming treatments such as hair cleansing, conditioning, coloring, and styling in daily life different among people [
31]. It can influence the hair fiber's condition and increase its ability to adsorb metal ions. All these elements are essential for humans except strontium, which can cause a risk to health in the case of prolonged exposure, resulting in the replacement of calcium ions.
The ratio of Ca/Sr and Ca/Pb in the hair and nail sample should be additionally monitored and above 700 and 1100 in hair and nails, respectively. Meanwhile, for three samples of hair containing outlier amounts of Sr and Pb, the ratio was below 60 and 33, respectively [
32]. It can indicate alarming exposure to toxic elements, which significantly stand out from typical amounts of strontium and lead in hair for citizens of Warsaw exposed to similar water and air quality. Similar effect was observed in the case of nail samples as excess of Ca to Pb was below 100 in the case of two samples.
In the case of nail samples, most diverse results were obtained only for K and Mn. Potassium can be monitored additionally as a ratio K/Fe. The dominating ratios were up to 2.5, but in four samples the ratio was above 20 indicating that the deviating content results from a more complex disturbance of metal homeostasis which may be the result of incorrect supplementation, a side effect of medications or severe deficiency of iron [
33]. This relationship should, of course, be verified in relation to the metal content in the blood.
Detected Ag by EDX was quite surprising, but probable, because it can be an effect of application of different cosmetic products containing antibacterial silver applied on the skin in the form of aerosol. The random presence of silver in hair and nail samples is therefore probable.
3. Materials and Methods
3.1. Chemicals
Acetone and methanol of HPLC grade, nitric acid, hydrochloric acid, and 25% (v/v) hydrogen peroxide of purity for trace analysis were purchased from VWR (Germany) and Sigma-Aldrich (France). A 25% (m/v) tetramethylammonium hydroxide (TMAH) solution in methanol and the second in water were purchased from Alfa Aesar (Kandel, Germany) and JKchemical (Pforzheim, Germany), respectively. Boric acid of analytical purity (SO-press001) was purchased from PD Instruments (Kleve, Germany). Environmental Calibration Standard (5183-4688) containing metal ions at two concentration levels was purchased from Agilent Technologies (Stara Zagora, Bulgaria). Three certified reference materials (CRM) prepared from human hair (NCS DC73347a and NCS ZC 8100 2b, CISRI, Beijing, China, and ERM-DB001, ERM, Geel, Belgium) were used toward calibration of ED-XRF and methods' validation. Milli-Q system (US) was used to prepare ultrapure water (18.2 MΩ/cm).
3.2. Instrumentation
Hair and fingernails were homogenized using a planetary ball mill (Fritsch, Oberstein, Germany) with a single grinding station made of agate to avoid contamination with metals. 13 mm pellets from powdered hair and nails were obtained with pressure (up to 15 US tons) applied by hydraulic press (PIKE technologies, Madison, WI, USA). Metals in pellets were determined using an energy dispersion X-ray fluorescence spectrometer (ED-XRF, Model EDX-8100, Shimadzu, Japan) equipped with a vacuum pump. Special plastic reduction inserts (matching the wells of the autosampler) were made of polyoxymethylene (MOP) to place and lock smaller 13 mm pellets in the center of the autosampler's well.
Metals in hair and nails mineralized using nitric acid and dissolved in TMAH water solution were determined using inductively coupled plasma mass spectrometry (ICP-MS/MS, Model 8900, Agilent Technologies, Japan) equipped with SPS4 autosampler and MicroMist nebulizer (0.4 mL min-1) with ratchet gas fitting.
3.3. Hair/nail washing and homogenization
Hair and nail discards were collected from several beauty salons (Warsaw, Poland). Hair was then fractionated according to color and texture, originating from a single source, while nails were collected together as one sample at each workstation.
Samples were placed in clean 30 mL (hair) and 10 mL (nails) glass containers. The material was subjected to sequential washing with acetone, deionized water at 40 °C, again acetone enhanced with shaking at 320 rpm for 20 minutes (water bath Memmert WN 22, Buechenbach, Germany) [
34]. Once the washing was complete, the samples were filtered and left to dry at room temperature on a filter in a Petri dish.
Samples were pre-cut with ceramic scissors and placed in the bowl of the mill. To prevent granulation of the material due to electrostatic effects, the acetone was added to the material at a ratio of 1 µL per 3 mg for hair and 3 µL per 1 mg for nails. The samples were ground for more than two hours in cycles (3 x 30 min grinding, 2 x 30 min cooling). The obtained powder was filtered and left to dry at room temperature on a filter in a Petri dish; next, it was inspected with a microscope for homogeneity and particle size (
Figure S1).
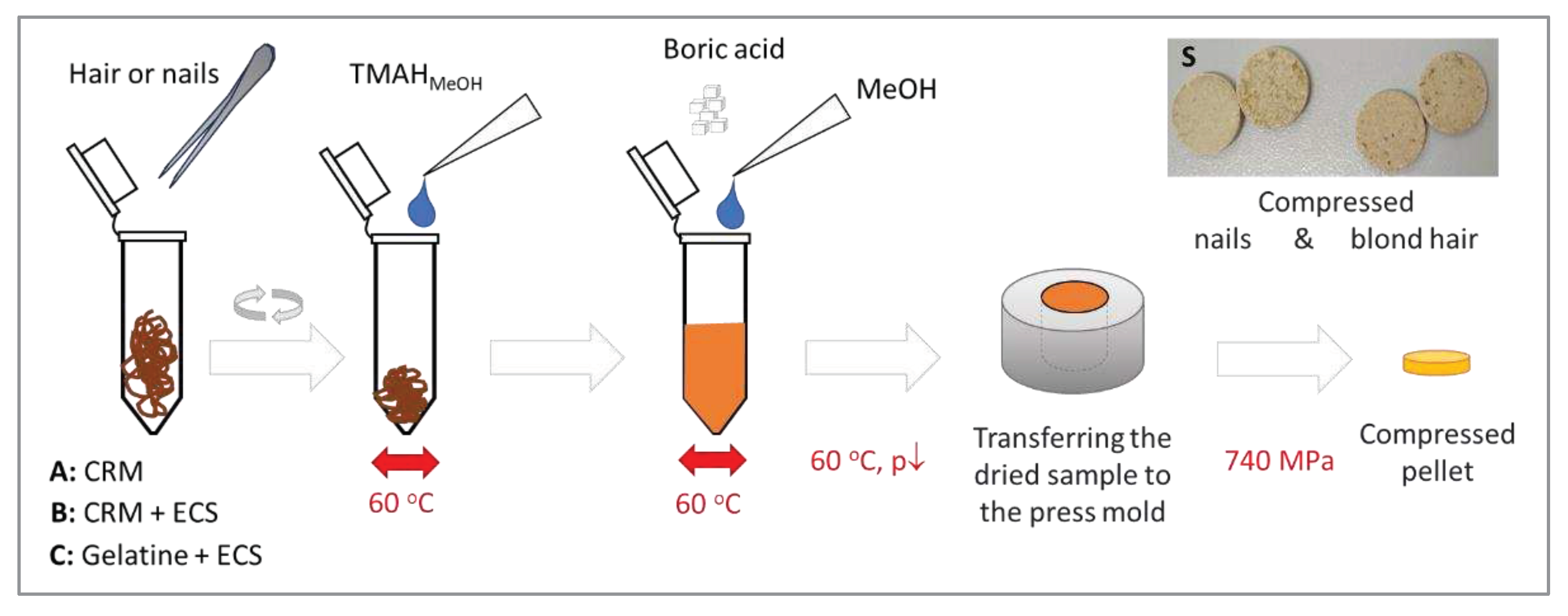
3.4. Hair/nail washing and homogenization
To 100 mg of test material (hair/nails) was weighed into the Eppendorf tube, and 250 µL of TMAH solution was added. Solubilization was enhanced by vortexing the sample at 4200 rpm for 1 min at 60
oC. After 2 h, samples were centrifuged at 10,000 rpm for 6 min and incubated at 60
oC for 1 h. Next, 50 µL of methanol and 250 mg of boric acid were added to the resulting suspension and mixed thoroughly at 4200 rpm for 1 min (
Figure 7).
The mixed sample was dried in a desiccator under reduced pressure (680 mmHg) for 1 h and placed on a heating plate for 2 h at 60 oC. The dried sample was transferred to a hydraulic press, and the pellet was formed within 2 minutes at a pressure of 10 tons. Prepared samples were analyzed within 24 hours. Otherwise, they were stored in vacuum bags or containers to protect against moisture. Samples were prepared in a set of 12 (number of wells in the autosampler).
3.5. Calibration of EDX
Pellets for calibration of EDX were prepared following the procedure described in section 3.4 but with two types of modifications: (A) different amounts of CRM's powder were weighted (
Table 5) to obtain different dilution factors against a fixed amount of binder and (B) different amounts of metal ions were added using Environmental Calibration Solution (ECS,
Table 1) to obtain different levels of dissolved CRM. Suspensions were prepared in Eppendorf tubes, shaken, dried, and pressed as previously described.
Amounts of metals in calibration pellets (C
cal) were calculated according to the following equations:
x is the weight of CRM according to table 1 [mg], 100 – serves as the correction factor representing fixed weight for investigated samples [mg], CCRM – element content in CRM [µg g-1].
0.05 corresponds to the fixed weight of the CRM powder [g], CCRM – represents element content in CRM [µg g-1], Cst and Vst – represents element concentration in the standard solution (ECS) [µgmL-1] added in appropriate volume to CRM [µL], 1000 – is a conversion factor for volume unit from µL to mL, 0.1 – serves as correction factor representing fixed weight for investigated samples [g].
3.6. Mineralization and solubilization of hair and nails toward metal determination by ICP-MS/MS.
3.5 ml of concentrated nitric acid was added to about 0.15 g of hair or nails or in a PTFE vessel. After 1 hour, 1.5 ml of hydrogen peroxide was added. After waiting a minimum 2 hours, microwave-assisted mineralization was performed using Speed Wave Microwave (Berghof, Eningen, Germany). The program (
Table S1) was established by comparing the obtained metal recovery (to obtain as high as possible) and method variance (as low as possible) against a certified reference hair material. After mineralization, the sample was quantitatively transferred to a 20 ml volumetric flask and analyzed on the same day because the stability tests of the samples showed that for some elements, the signals decreased to 20% after 18 h and to 45% after 48 h.
The second sample preparation method was the solubilization of hair and nails. Weights of hair (0.2 g) or nails (0.1 g) were placed in a polypropylene falcon. The appropriate amount (5 and 2.5 mL, respectively) of concentrated TMAH solution (25%, v/v in water) was added and sealed. After ~12 hours of incubation at 30
oC, the other reagents were added to the samples (thiourea, triton, and ethylenediaminetetraacetic acid) in the amounts shown in
Table S2 and mixed thoroughly. Next, samples were quantitatively transferred to 20 mL volumetric flasks and analyzed on the same day.
3.7. EDX and ICP-MS/MS detection conditions
Optimal conditions for determining elements by EDX-8100 were established using pellets prepared from human hair certified reference material NCS DC73347a to detect the highest number of elements and S/N ratio. Optimal detection parameters ICP-MS/MS were established using a tune mixture containing 1 ng mL-1 metal ions (Li, Co, Y, Ce, Tl) in 5% nitric acid (RF power, nebulization parameters), next environmental standard solution diluted to obtain concentration of trace metals 5 ng mL
-1 was used to establish optimal conditions for collision/reaction cell. Final integration times and isotopes were established after analysis of human hair and nail samples according to their amount in the samples. Optimal parameters for both techniques are presented in
Table 6.
3.8. Calibration of ICP-MS/MS.
ICP-MS was calibrated using standard curves obtained for solutions prepared in a 2% (v/v) nitric acid solution (for samples after mineralization) and 1% (v/v) TMAH solution containing 0.25% EDTA and 0.025% Thiourea and Triton.
The following standard solutions were used to prepare the calibration for (A) selected non-metals (S at concentration 10 μg·mL-1), P, I, Br (at concentration 0.1 μg·mL-1) prepared in water and for (B) metals/metaloids Fe, K, Ca, Na, Mg at a concentration of 10 μg mL-1 and Ag, Al, As, Be, Cd, Co, C, Cu, Mn, Mo, Ni, Pb, Sb, Se Tl, V, Zn, Th, U, Sn, Hg, Sr at a concentration of 0.1 μg·mL-1 in an aqueous solution of 2% HNO3. Precision and recovery were compensated by a signal obtained for internals standards (Ge, Y, Rh) with a concentration of 10 ng mL-1 in each solution.
The linear response range for micronutrients was 0 - 80 ng mL
-1, while for macronutrients, it was 100 - 8000 ng mL
-1. The sequential dilution of the sample enlarged the applicability of the linear range of the method. Statistical description of calibration graphs is presented in
Tables S3 and S4.
3.9. Data registration and analysis.
MassHunter Software registered data obtained by ICP-MS/MS including calibration curves and their statistical analysis. Data obtained by ED-XRF were collected using Shimadzu EDX software. The part of software applied to create reports containing quantitative analysis data was modified to transfer data without mathematical rounding of numbers (it led to the loss of significant changes of signals for elements present at trace levels as software was prepared with the aim to determine major components in samples) and collect it combined in a spreadsheet (See section 3 of Supplementary Material). Obtained data for screening tests were analyzed using STATISTICA 13.1 and Excel software.
4. Conclusions
Energy dispersive x-ray fluorescence (EDXRF) can be applied to determine essential and toxic elements in human hair and nails. However, laboratory or certified reference materials are necessary for correct instrument calibration. An entire spectrum is acquired practically simultaneously so that 16 elements (S, Si, Ca, Br, Fe, Cu, Cr, Mg, Si, K, Mn, Ni, Zn, Se, Sr, Pb) can be determined within 20 min. Filters improve detection limits and can be used to analyze trace elements.
Alternative sample preparation of hair and nails without using a mill but based on solubilization by a strong alkalic medium (tetramethylammonium hydroxide, TMAH) reduces losses during the hair/nail homogenization and enables its automation. Adding boric acid as a binder allows diluting samples in different ratios and increases the method's applicability.
The developed method of producing smaller pellets and modifying the wells in the autosampler allowed for reducing the amount of material required to perform analyses. The obtained pellets are more stable than solutions prepared for analysis using ICP-MS. They enable repeating the analyses, dissolving the pellet, and analyzing its contents using ICP-MS or ICP-MS/MS.
EDX is not as sensitive as ICP-MS, but it allows the determination of many elements that cause problems to ICP-MS due to high ionization energy of element (Hg), numerous interferences (S, Si, Cr), or the need to repeatedly dilute the sample increasing risk for contamination of sample (for example with Sr).
To sum up, EDX enables the performance of complementary analyzes to ICP-MS of metal content in materials of biological origin.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org. Figure S1: The comparison of the homogeneity of milled hair offered by the European Research Institute (JRC) (a), the Chinese Research Institute (b), and those produced within the project (c), Figure S2: Effect of pellet thickness on changes in signal intensity and relative standard deviation (RSD); Table S1: Mineralization program for hair and nail samples toward ICP-MS/MS analysis, Table S2: The volumes of reagents used for the alkaline extraction of metals from a sample of hair and nails toward ICP-MS/MS analysis, Table S3: Statistical description of calibration curves obtained by ICP-MS/MS in 2% HNO3, Table S4: Statistical description of calibration curves obtained by ICP-MS/MS in 1% TMAH, Table S5: Comparison of the metals' amounts established by ICP-MS/MS with certified values obtained for CRM (NCS ZC 81OO2b human hair). (unit μg/g), Table S6: Statistical description of calibration curves obtained by EDX using different methods for pellets' preparation (unit μg/g), Section S3: Modification of ED-XRF software.
Author Contributions
Conceptualization, K.P.; methodology, Z.M. and K.P.; software, K.H.; validation, Z.M., M.N. and K.Z.; formal analysis, K.P.; investigation, Z.M.; resources, T.B.; data curation, K.P., Z.M., P.Ś. and K.H.; writing—original draft preparation, K.P.; writing—review and editing, K.P.; visualization, K.P.; supervision, K.P.; project administration, T.B.; funding acquisition, K.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by National Centre for Research and Development (NCBiR), grant number POIR.01.01.01-00-0120/18.
Institutional Review Board Statement
Not applicable. Hair and nails were obtained from communal waste collected at the beauty salons. As the hair and nails were waste materials, it is unnecessary to submit to the biological ethics committee appreciation at the Regional Medical Chambers in Warsaw.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.
Acknowledgments
The authors would like to thank Shim-Pol company for providing the EDX device, model 7000, for measurements in the initial phase of the project.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Tchounwou, P.B.; Yedjou, C.G.; Patlolla, A.K.; Sutton, D.J. Heavy Metal Toxicity and the Environment BT - Molecular, Clinical and Environmental Toxicology: Volume 3: Environmental Toxicology. In; Luch, A., Ed.; Springer Basel: Basel, 2012; pp. 133–164 ISBN 978-3-7643-8340-4.
- Briffa, J.; Sinagra, E.; Blundell, R. Heliyon Heavy metal pollution in the environment and their toxicological effects on humans. Heliyon 2020, 6, e04691. [CrossRef]
- Anyanwu, B.O.; Ezejiofor, A.N.; Igweze, Z.N.; Orisakwe, O.E. Heavy Metal Mixture Exposure and Effects in Developing Nations: An Update. Toxics 2018, 6, 65–97. [CrossRef]
- Tokumaru, T.; Ozaki, H.; Onwona-Agyeman, S.; Ofosu-Anim, J.; Watanabe, I. Determination of the Extent of Trace Metals Pollution in Soils, Sediments and Human Hair at e-Waste Recycling Site in Ghana. Arch. Environ. Contam. Toxicol. 2017, 73, 377–390. [CrossRef]
- Luo, J.; Meng, J.; Ye, Y.; Wang, Y.; Bai, L. Population health risk via dietary exposure to trace elements (Cu, Zn, Pb, Cd, Hg, and As) in Qiqihar, Northeastern China. Environ. Geochem. Health 2018, 40, 217–227. [CrossRef]
- Tippairote, T.; Temviriyanukul, P.; Benjapong, W.; Trachootham, D. Hair Zinc and Severity of Symptoms Are Increased in Children with Attention Deficit and Hyperactivity Disorder: a Hair Multi-Element Profile Study. Biol. Trace Elem. Res. 2017, 179, 185–194. [CrossRef]
- Hair, I.N.; Autistic, O.F.; Karczewski, J.; Żendzian-piotrowska, M. Analysis of trace element content in hair of autistic children. J. Elem. 2017, 22, 1285–1293. [CrossRef]
- Jaishankar, M.; Tseten, T.; Anbalagan, N.; Mathew, B.B.; Beeregowda, K.N. Toxicity, mechanism and health effects of some heavy metals. Interdiscip. Toxicol. 2014, 7, 60–72. [CrossRef]
- Griffin, R.M. Biological Monitoring for Heavy Metals: Practical Concerns. J. Occup. Med. 1986, 28, 615–618.
- Tehrani, M.W.; Yang, K.X.; Parsons, P.J. Development and characterization of reference materials for trace element analysis of keratinized matrices. Anal. Bioanal. Chem. 2020, 412, 1847–1861. [CrossRef]
- Pengping, S.; Kungwankunakorn, S. Determination of Some Heavy Metals in Human Hair by Ultrasonic Acid Digestion and Atomic Absorption Spectrophotometry. Chiang Mai J. Sci. 2014, 41, 148–155.
- Panhwar, A.H.; Kazi, T.G.; Afridi, H.I.; Arain, S.A.; Arain, S.S.; Ullah, N.; Brahman, K.D.; Khan, A.R. Preconcentration of Cadmium in Water and Hair by Supramolecular Solvent-Based Dispersive Liquid – Liquid Microextraction. Anal. Lett. 2016, 49, 2436–2445. [CrossRef]
- Rajabi, M.; Arghavani-beydokhti, S.; Bar, B.; Asghari, A. Dissolvable layered double hydroxide as an ef fi cient nanosorbent for centrifugeless air-agitated dispersive solid-phase extraction of potentially toxic metal ions from biofluid samples. Anal. Chim. Acta 2017, 957, 1–9. [CrossRef]
- Forero-Mendieta, J.R.; David, J.; Angelica, D.; Riaño-herrera, D.A.; Alexander, J. Validation of an Analytical Method for the Determination of Manganese and Lead in Human Hair and Nails Using Graphite Furnace Atomic Absorption Spectrometry. Seperations 2022, 9, 158–173. [CrossRef]
- Brummer-holder, M.; Cassill, B.D.; Hayes, S.H. Interrelationships Between Age and Trace Element Concentration in Horse Mane Hair and Whole Blood. J. Equine Vet. Sci. 2020, 87, 102922. [CrossRef]
- Ghorbani, A.; Mohit, A.; Kuhi, H.D. Effects of Dietary Mineral Intake on Hair and Serum Mineral Contents of Horses. J. Equine Vet. Sci. 2015, 35, 295–300. [CrossRef]
- Domanico, F.; Forte, G.; Majorani, C.; Senofonte, O.; Petrucci, F.; Pezzi, V.; Alimonti, A. Determination of mercury in hair: Comparison between gold amalgamation-atomic absorption spectrometry and mass spectrometry. J. Trace Elem. Med. Biol. 2017, 43, 3–8. [CrossRef]
- He, M.; Huang, L.; Zhao, B.; Chen, B.; Hu, B. Advanced functional materials in solid phase extraction for ICP-MS determination of trace elements and their species - A review. Anal. Chim. Acta 2017, 973, 1–24. [CrossRef]
- Baranowska, I.; Barchański, L.; Bąk, M.; Smolec, B.; Mzyk, Z. X-Ray Fluorescence Spectrometry in Multielemental Analysis of Hair and Teeth. Polish J. Environ. Stud. 2004, 13, 639–646.
- Gruber, A.; Müller, R.; Wagner, A.; Colucci, S.; Spasić, M.V.; Leopold, K. Total reflection X-ray fluorescence spectrometry for trace determination of iron and some additional elements in biological samples. Anal. Bioanal. Chem. 2020, 412, 6419–6429. [CrossRef]
- Lokvencová, L.; Zvěřina, O.; Kuta, J. Different trends of Cr, Fe and Zn contents in hair between obese, overweight and normal-weight men. Cent. Eur. J. Public Health 2021, 29, 301–304. [CrossRef]
- Damokhi, A.; Yousefinejad, S.; Yarmohammadi, R.; Jafari, S. Ionic liquids in biological monitoring for exposure assessments. J. Mol. Liq. 2021, 344, 117732. [CrossRef]
- Almeida, L. De; Oliveira, Í.; Lucena, D.; Oliveira, J. De; Grandis, F.; Luna, D. De; Silva, F. New strategies for the simultaneous voltammetric quanti fi cation of Pb and Zn in hair cosmetics samples employing chemically modi fi ed composite electrodes. Measurement 2018, 125, 651–658. [CrossRef]
- Khan ab, M.; Soylak, M. Switchable solvent based liquid phase microextraction of mercury from environmental samples: a green aspect. 2016. [CrossRef]
- Benes, B.; Sladká, J.; Spevácková, V.; Smid, J. Determination of normal concentration levels of Cd, Cr, Cu, Hg, Pb, Se and Zn in hair of the child population in the Czech Republic. Cent. Eur. J. Public Health 2003, 11, 184–186.
- Ullucci, P.A. Metals in Blood and Urine: Biological Monitoring for Worker Exposure. In Encyclopedia of Analytical Chemistry; John Wiley & Sons, Ltd., 2006; pp. 1–21 ISBN 9780470027318.
- Gajek, R. Analytical Methods Determination of essential and toxic metals in blood by ICP-MS with calibration in synthetic matrix. Anal. Methods 2015, 5(9), 2193–2202. [CrossRef]
- Byers, H.L.; Mchenry, L.J.; Grundl, T.J. Food Chemistry: X XRF techniques to quantify heavy metals in vegetables at low detection limits. Food Chem. X 2019, 1, 100001. [CrossRef]
- Buhrke, V.E.; Jenkins, R.; Smith, D.K. A Practical Guide for the Preparation of Specimens for X-Ray Fluorescence and X-Ray Diffraction Analysis; Buhrke, V.E., Jenkins, R., Smith, D.K., Eds.; WILEY-VCH, 1997; ISBN 9780471194583.
- Haraguchi, K.; Sakamoto, M.; Matsuyama, A.; Yamamoto, M.; Hung, D.T.; Nagasaka, H.; Uchida, K.; Ito, Y.; Kodamatani, H.; Horvat, M.; et al. Development of Human Hair Reference Material Supporting the Biomonitoring of Methylmercury. Anal. Sci. 2020, 36, 561–565. [CrossRef]
- Mostafaii, G.; Karamali, F.; AbooSaedi, Z.; Atoof, F.; Hesami Arani, M.; Miranzadeh, M.B. Determination of Heavy Metals in Hair Dye Sale in Iranian Market: Dermal Sensitivity and Carcinogenicity Assessment. Biol. Trace Elem. Res. 2022, 200, 1464–1472. [CrossRef]
- McPherson, C.A.; Lawrence, G.S.; Elphick, J.R.; Chapman, P.M. Development of a strontium chronic effects benchmark for aquatic life in freshwater. Environ. Toxicol. Chem. 2014, 33, 2472–2478. [CrossRef]
- Salsbury, G.; Cambridge, E.L.; McIntyre, Z.; Arends, M.J.; Karp, N.A.; Isherwood, C.; Shannon, C.; Hooks, Y.; Ramirez-Solis, R.; Adams, D.J.; et al. Disruption of the potassium channel regulatory subunit KCNE2 causes iron-deficient anemia. Exp. Hematol. 2014, 42, 1053–8.e1. [CrossRef]
- Gellein, K.; Lierhagen, S.; Brevik, P.S.; Teigen, M.; Kaur, P.; Singh, T.; Flaten, T.P.; Syversen, T. Trace element profiles in single strands of human hair determined by HR-ICP-MS. Biol. Trace Elem. Res. 2008, 123, 250–260. [CrossRef]
Figure 1.
The effect of the radiation beam diameter on the height of the signals and number of detected elements in a pellet made of hair (a). The influence of scanning time on signal intensity and repeatability is presented in chart (b) and on spectra obtained for primary (c) and trace elements (d).
Figure 1.
The effect of the radiation beam diameter on the height of the signals and number of detected elements in a pellet made of hair (a). The influence of scanning time on signal intensity and repeatability is presented in chart (b) and on spectra obtained for primary (c) and trace elements (d).
Figure 2.
The influence of the type of filter on the attenuation of signals coming from the detection elements on the quality of the spectrum obtained for a hair sample for different X-ray energy ranges (a-c). The color of the spectral line corresponds to the background with the specified filter type above the spectra. Filter 5 was excluded from further studies.
Figure 2.
The influence of the type of filter on the attenuation of signals coming from the detection elements on the quality of the spectrum obtained for a hair sample for different X-ray energy ranges (a-c). The color of the spectral line corresponds to the background with the specified filter type above the spectra. Filter 5 was excluded from further studies.
Figure 3.
Simplified schemes and pictures of commercially available vials for typical samples (a) and low amounts of samples (b) with mylar or polypropylene film to secure the sample. Simplified schemes and pictures of the manufactured sample-well reducer used to place 13 mm pellets in the EDX detector chamber (c) and cover (d) to stabilize the position of the pellet immersed in the well-reducer (c).
Figure 3.
Simplified schemes and pictures of commercially available vials for typical samples (a) and low amounts of samples (b) with mylar or polypropylene film to secure the sample. Simplified schemes and pictures of the manufactured sample-well reducer used to place 13 mm pellets in the EDX detector chamber (c) and cover (d) to stabilize the position of the pellet immersed in the well-reducer (c).
Figure 4.
EDX spectra obtained for pellet obtained from ground hair compared to (a) ground hair mixed with cellulose used as a binder and (b) hair dissolved in TMAH mixed with boric acid as binder.
Figure 4.
EDX spectra obtained for pellet obtained from ground hair compared to (a) ground hair mixed with cellulose used as a binder and (b) hair dissolved in TMAH mixed with boric acid as binder.
Figure 6.
Box charts illustrating the distribution of obtained results for selected elements determined in nails (blue) and hair (red) and ratios for selected elements.
Figure 6.
Box charts illustrating the distribution of obtained results for selected elements determined in nails (blue) and hair (red) and ratios for selected elements.
Figure 7.
The scheme of preparing hair and nails (A) for measurement using ED-XRF and reference pellets (B, C) for calibration. CRM – hair certified reference materials, ECS - environmental calibration solution containing metal ions, S – photographs of pellets prepared for measurements.
Figure 7.
The scheme of preparing hair and nails (A) for measurement using ED-XRF and reference pellets (B, C) for calibration. CRM – hair certified reference materials, ECS - environmental calibration solution containing metal ions, S – photographs of pellets prepared for measurements.
Table 1.
Statistical characteristics of calibration graphs obtained for selected elements by EDX in pellets obtained for hair dissolved by TMAH and containing boric acid as a binder.
Table 1.
Statistical characteristics of calibration graphs obtained for selected elements by EDX in pellets obtained for hair dissolved by TMAH and containing boric acid as a binder.
| El. |
y = ax + b |
r2 |
Linear range [ug/g] |
n |
SDa
|
SDb
|
LOD |
LOQ |
| Ag |
y = 0.000352x + 0.000206 |
0.950 |
0.5-5.0 |
15 |
3.3 ⋅ 10-5
|
4.8 ⋅ 10-5
|
0.15 |
0.45 |
| Al |
y = 0.0071x + 0.0538 |
0.995 |
39 - 2000 |
6 |
2.4 ⋅ 10-5
|
2.8 ⋅ 10-2
|
13 |
39 |
| Br |
y = 0.00949x + 0.0377 |
0.924 |
0.9-5.7 |
11 |
9.8 ⋅ 10-4
|
2.7 ⋅ 10-3
|
0.31 |
0.93 |
| Ca |
y = 0.000301x + 0.141 |
0.934 |
217-1740 |
12 |
1.7⋅ 10-5
|
2.0 ⋅ 10-2
|
72 |
217 |
| Cd |
y = 0.0098x + 0.141 |
0.794 |
0.07 – 0.12 |
4 |
5.5 ⋅ 10-3
|
2.6 ⋅ 10-2
|
0.02 |
0.07 |
| Co |
y = 0.0086x + 0.0014 |
0.864 |
0.3 – 0.6 |
4 |
3.1 ⋅ 10-3
|
3.4 ⋅ 10-4
|
0.10 |
0.30 |
| Cu |
y = 0.001813x + 0.00452 |
0.978 |
2.3-39.6 |
13 |
5.6 ⋅ 10-6
|
1.3 ⋅ 10-3
|
2.3 |
2.3 |
| Cr |
y = 0.00452x + 0.00779 |
0.913 |
0.7-8.8 |
15 |
2.6 ⋅ 10-4
|
9.9 ⋅ 10-4
|
0.24 |
0.72 |
| Fe |
y = 0.008972x + 0.0414 |
0.999 |
1.8-160.0 |
8 |
8.0 ⋅ 10-5
|
5.0 ⋅10-3
|
0.6 |
1.8 |
| K |
y = 0.000333x + 0.1199 |
0.969 |
24-510 |
10 |
1.4 ⋅ 10-5
|
2.5 ⋅10-3
|
8.4 |
25 |
| Mg |
y = 0.000053x + 0.0034 |
0.824 |
101-570 |
15 |
0.8 ⋅ 10-5
|
1.6 ⋅ 10-3
|
61 |
201 |
| Mn |
y = 0.00587x + 0.0036 |
0.969 |
1.3-6.0 |
9 |
8.1 ⋅ 10-4
|
2.2 ⋅ 10-3
|
0.4 |
1.3 |
| Mo |
y = 0.00091x + 0.000066 |
0.884 |
0.21 – 1.5 |
8 |
2.2 ⋅10-4
|
1.4 ⋅ 10-5
|
0.07 |
0.21 |
| Na |
y = 0.00091x + 0.000066 |
0.890 |
96 - 1599 |
5 |
7.6 ⋅ 10-5
|
5.2 ⋅ 10-3
|
137 |
417 |
| Ni |
y = 0.001135x + 0.00370 |
0.993 |
0.4-5.8 |
28 |
6.0 ⋅ 10-5
|
1.5 ⋅ 10-4
|
0.13 |
0.40 |
| Pb |
y = 0.002727x – 0.00035 |
0.984 |
0.4-6.8 |
10 |
8.3 ⋅ 10-5
|
3.1 ⋅ 10-4
|
0.13 |
0.38 |
| S |
y = 0.000035x – 0.263 |
0.983 |
7300-69800 |
5 |
0.2 ⋅ 10-5
|
7.6 ⋅ 10-2
|
2431 |
7294 |
| Se |
y = 0.005864x – 0.00034 |
0.995 |
0.1-5.3 |
12 |
8.7 ⋅ 10-5
|
1.8 ⋅ 10-4
|
0.03 |
0.10 |
| Si |
y = 0.000069x – 0.0058 |
0.984 |
371-720 |
3 |
1.3 ⋅ 10-5
|
7.8 ⋅ 10-3
|
124 |
371 |
| Sr |
y = 0.000226x + 0.000325 |
0.970 |
1.1-9.2 |
7 |
1.1 ⋅ 10-5
|
7.6 ⋅ 10-5
|
0.37 |
1.11 |
| V |
y = 0.0072x + 0.016 |
0.999 |
4.5 - 43 |
12 |
1.0 ⋅ 10-4
|
2.8 ⋅ 10-3
|
1.5 |
4.5 |
| Zn |
y = 0.002505x + 0.035 |
0.974 |
16-251 |
13 |
8.4 ⋅ 10-5
|
1.2 ⋅ 10-2
|
5.3 |
16 |
Table 2.
Comparison of measurement repeatability vs. preparative repeatability (pellet formation) for selected elements (*value below LOQ).
Table 2.
Comparison of measurement repeatability vs. preparative repeatability (pellet formation) for selected elements (*value below LOQ).
| Element |
Average amount [µg g-1] |
RSD for one pellet (n = 3) [%] |
RSD for three pellets [%] |
| S |
49423 |
0.26 |
3.6 |
| Ca |
1190 |
0.63 |
4.4 |
| Ag |
0.217* |
9.4 |
9.8 |
| Fe |
25.9 |
0.33 |
5.0 |
| Cu |
11.04 |
1.6 |
2.6 |
| Si |
531 |
3.9 |
5.5 |
| Zn |
100.8 |
0.34 |
4.9 |
| Se |
0.587 |
2.28 |
4.3 |
| Sr |
6.190 |
2.6 |
2.8 |
| Pb |
4.116 |
1.1 |
1.6 |
Table 3.
Comparison of elements amounts established by EDX with certified amounts for analyzed reference material made of hair (DC/NC – the type of reference material with indicated (○-dis and, ●-agreement). Recovery (RE) and coefficient of variation (CV) are presented for the EDX method.
Table 3.
Comparison of elements amounts established by EDX with certified amounts for analyzed reference material made of hair (DC/NC – the type of reference material with indicated (○-dis and, ●-agreement). Recovery (RE) and coefficient of variation (CV) are presented for the EDX method.
| Element |
CDC-CRM ± U |
CDC-EDX ±U |
REDC [%] |
CVDC [%] |
DC/NC agreed |
| Ag |
0.050 ± 0.005 |
<LOD |
N/A |
N/A |
○/○ |
| Al |
20000 ±N/A |
19710 ±190 |
99 |
1.9 |
●/● |
| Br |
1.10± N/A |
0.989 ± 0.014 |
90 |
2.9 |
●/○ |
| Ca |
1450 ±200 |
1326 ±35 |
91 |
5.3 |
●/● |
| Cd |
0.070 ±0.010 |
0.0857 ±0.0031 |
122 |
7.2 |
●/○ |
| Cl |
180 ± 21 |
163.9 ± 3.7 |
91 |
4.5 |
●/○ |
| Co |
0.045 ± 0.009 |
<LOD |
N/A |
N/A |
○/● |
| Cr |
0.41 ±0.12 |
0.329 ±0.079 |
80 |
4.8 |
●/● |
| Cu |
14.3 ± 1.6 |
16.2 ± 1.9 |
113 |
2.4 |
●/● |
| Fe |
36.0 ±5.0 |
35.10 ± 0.93 |
98 |
5.3 |
●/● |
| K |
20.0 ± 1.8 |
17.93 ±0.20 |
90 |
2.2 |
●/○ |
| Mg |
140 ± 14 |
134.5 ± 6.9 |
96 |
10 |
●/● |
| Mn |
2.0 ±0.3 |
1.84 ±0.25 |
92 |
13 |
●/● |
| Mo |
0.17 ±0.03 |
<LOD |
N/A |
N/A |
○/● |
| Na |
89 ±12 |
<LOD |
N/A |
N/A |
○/● |
| Ni |
0.43 ±0.12 |
0.53 ±0.14 |
123 |
26 |
●/● |
| Pb |
5.7 ±0.5 |
5.58 ±0.22 |
98 |
7.9 |
●/● |
| S |
41900 ±1100 |
41 760 ± 293 |
100 |
1.4 |
●/● |
| Se |
0.58 ±0.12 |
0.600 ±0.036 |
103 |
12 |
●/● |
| Si |
600 ± N/A |
613 ± 14 |
102 |
4.5 |
●/● |
| Sr |
7.70 ±0.40 |
7.81 ± 0.14 |
101 |
3.6 |
●/● |
| V |
0.50 ± 0.18 |
<LOD |
N/A |
N/A |
○/○ |
| Zn |
137 ±9 |
138.8 ± 1.9 |
101 |
2.7 |
●/● |
Table 4.
Comparison of amounts of elements [μg g-1] established by ICP-MS/MS and EDX nail pulled sample. Uncertainty of the method was calculated for k = 2. Values are in agreement when |CICP-MS – CEDX|≤∑U.
Table 4.
Comparison of amounts of elements [μg g-1] established by ICP-MS/MS and EDX nail pulled sample. Uncertainty of the method was calculated for k = 2. Values are in agreement when |CICP-MS – CEDX|≤∑U.
| Element |
CICP-MS ± U |
CEDX ±U |
|CICP-MS – CEDX| |
∑U |
Agreement |
| Ag |
1.45 ± 0.94 |
2.52 ± 0.98 |
0.07 |
0.14 |
√ |
| Al |
29.2 ± 5.9 |
N/A |
N/A |
N/A |
N/A |
| Ca |
1596 ± 359 |
1891 ±267 |
295 |
447 |
√ |
| Cd |
0.022 ±0.009 |
0.011 ±0.005 |
0.012 |
0.011 |
- |
| Cr |
2.18 ±0.79 |
2.34 ±0.38 |
0.16 |
0.88 |
√ |
| Cu |
4.07 ± 1.37 |
3.77 ± 0.68 |
0.30 |
1.53 |
√ |
| Fe |
15.3 ±3.7 |
10.8 ± 3.0 |
4.5 |
4.8 |
√ |
| K |
154 ± 39 |
133 ±81 |
21 |
21 |
√ |
| Mg |
246 ± 68 |
224 ± 55 |
42 |
87 |
√ |
| Mn |
5.08 ±0.30 |
4.84 ±0.25 |
0.24 |
0.39 |
√ |
| Mo |
2.98 ±0.58 |
N/A |
N/A |
N/A |
N/A |
| Na |
139 ±62 |
N/A |
N/A |
N/A |
N/A |
| Ni |
3.89 ±0.22 |
3.63 ±0.32 |
0.26 |
0.38 |
√ |
| Pb |
0.26 ±0.11 |
0.21 ±0.17 |
0.06 |
0.21 |
√ |
| S |
33 247 ±1 829 |
31 638 ± 1265 |
1609 |
2223 |
√ |
| Se |
0.51 ±0.14 |
0.44 ±0.10 |
0.07 |
0.17 |
√ |
| Si |
306 ± 88 |
263 ± 34 |
43 |
94 |
√ |
| Sr |
3.33 ±0.98 |
3.11 ± 0.91 |
0.22 |
1.3 |
√ |
| Zn |
125 ±50 |
111 ± 18 |
14 |
53 |
√ |
Table 5.
Composition of calibration pellets for EDX.
Table 5.
Composition of calibration pellets for EDX.
| |
Method A: CRM diluted with binder |
Method B: CRM fortified with ECS |
| Used CRMs |
NCS DC73347a/NCS ZC 8100 2b/ERM-DB001 |
NCS DC73347a |
| Amount of CRM [mg] - x |
120, 100, 80, 60 |
50 |
| Volume of ECS solution [µL] |
N/A |
5, 10, 20, 50 |
| Volume of TMAHMeOH [µL] |
250 |
250 |
| VMeOH [µL] |
50 |
50 |
| Amount of boric acid [mg] |
250 |
250 |
Table 6.
Optimal conditions for metal determination by EDX and ICP-MS/MS. See
Table S3 for more detailed information.
Table 6.
Optimal conditions for metal determination by EDX and ICP-MS/MS. See
Table S3 for more detailed information.
| Parameter |
Value |
| X-ray fluorescence spectrometry |
| Instrument model and mode |
Model EDX-8100, Vaccum (< 5 Pa) |
| X-ray lamp |
Rh |
| Collimator |
10 mm |
| Generator current XRF |
Medium: 100 μA (automatic, filter dependent) |
| Generator voltage XRF, Scanning time, Dead Time (DT) for two groups of elements |
15 kV, 400 s, DT 30% - Al, Ca, K, Na, Mg, P, S
50kV, 600 s, DT 30%, - As, Ag, Cd, Fe, Mn Se, Zn, Cu, Pb, Br, Sr, Y, Tl, U |
| Filters assigned for determination of appropriate elements |
No filter – Al*, Na*, Mg*, P*, Si*
1-Ag*, Cd*, Mo*, Rb*, Sb*, Sr*, Y*, Tl***, U***
2 –Ba***, Ca*, Cl*, K*, S*, Ti*, V*
3 –Cr*, Fe*, Mn*
4 –As**, Br*, Co*, Cu*, Hg***, Ni*, Pb***, Se*, Zn*,
*Kα, **Kβ, ***Lα, |
| Inductively coupled plasma mass spectrometry, ICP-MS/MS |
| Model |
8900, QqQ |
| Generator RF power |
1550 W |
| Nebulizer gas flow |
1.05 L∙min-1
|
| Collision gas flow |
[No gas], [He] 5.5 mL∙min-1, [O2] 0.45 mL∙min-1
|
| Gas flow for high matrix dilution |
0,9 L∙min-1
|
| Sampling program |
Sample injection: 50 s, stabilization: 40 s, acquisition: 30 s |
| Integration times for monitored isotopes |
5 ms: 56Fe, 10 ms: 24Mg, 27Al
50 ms: 31P, 39K, 44Ca, 45Sc
100 ms: 7Li, 9Be, 47Ti, 51V, 52Cr, 55Mn, 59Co, 60Ni, 63Cu, 66Zn, 71Ga, 72GeIS, 88Sr, 89YIS, 95Mo, 103RhIS, 107Ag, 115In, 118Sn, 121Sb, 125Te, 127I, 137Ba, 201Hg, 205Tl, 206Pb, 209Bi, 232Th, 238U
300 ms: 75As, 78Se, 79Br, 23Na
1000 ms: 111Cd |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).