Submitted:
01 January 2024
Posted:
03 January 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results and Discussion
2.1. Synthesis

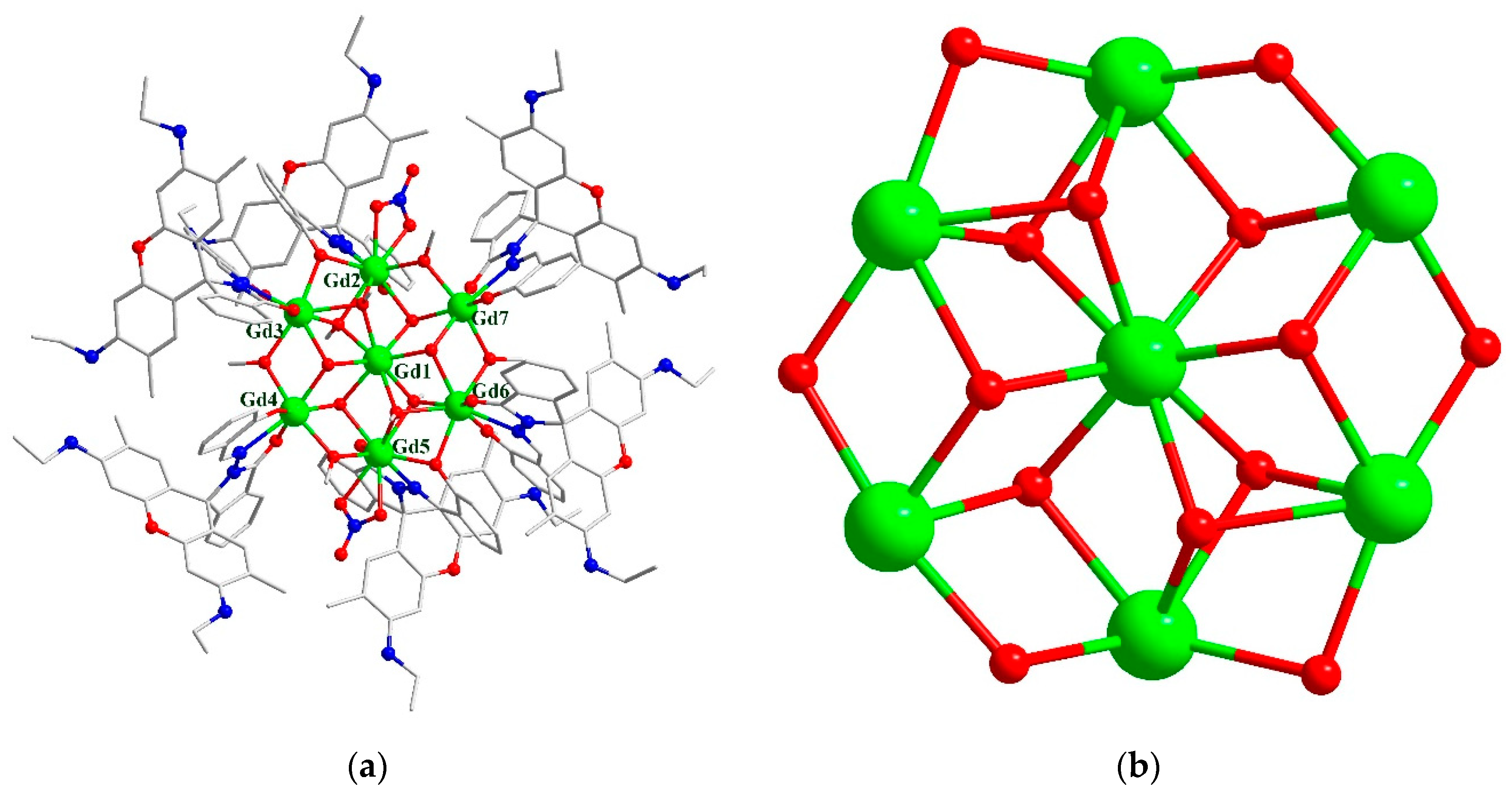
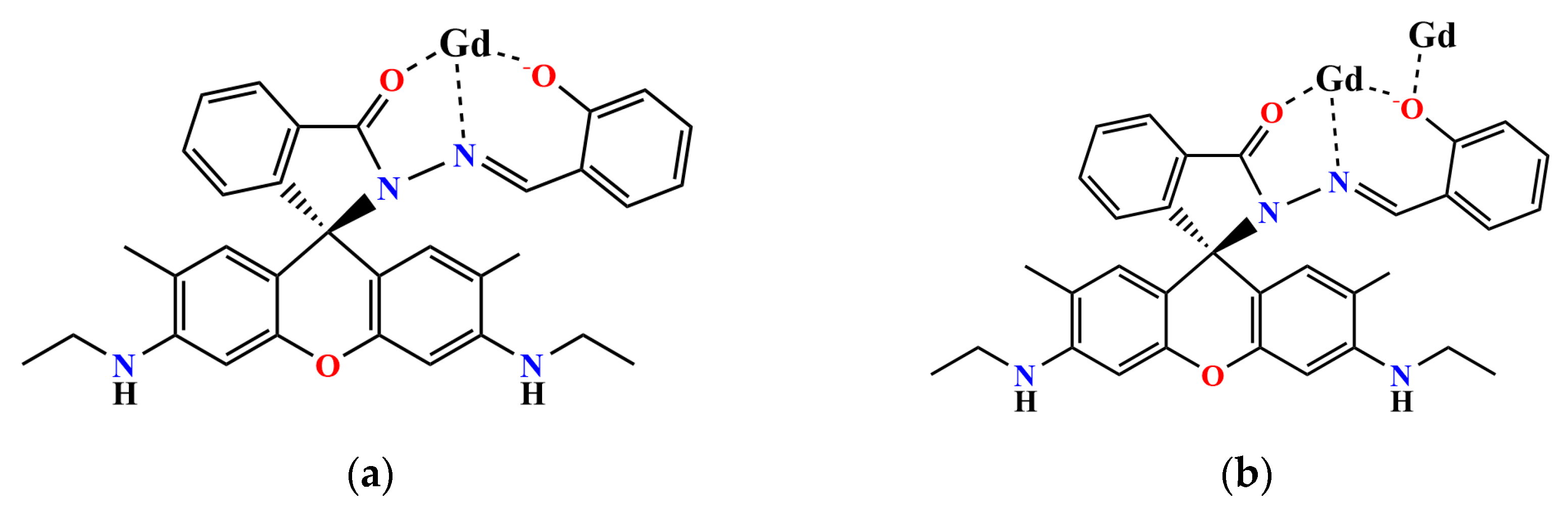
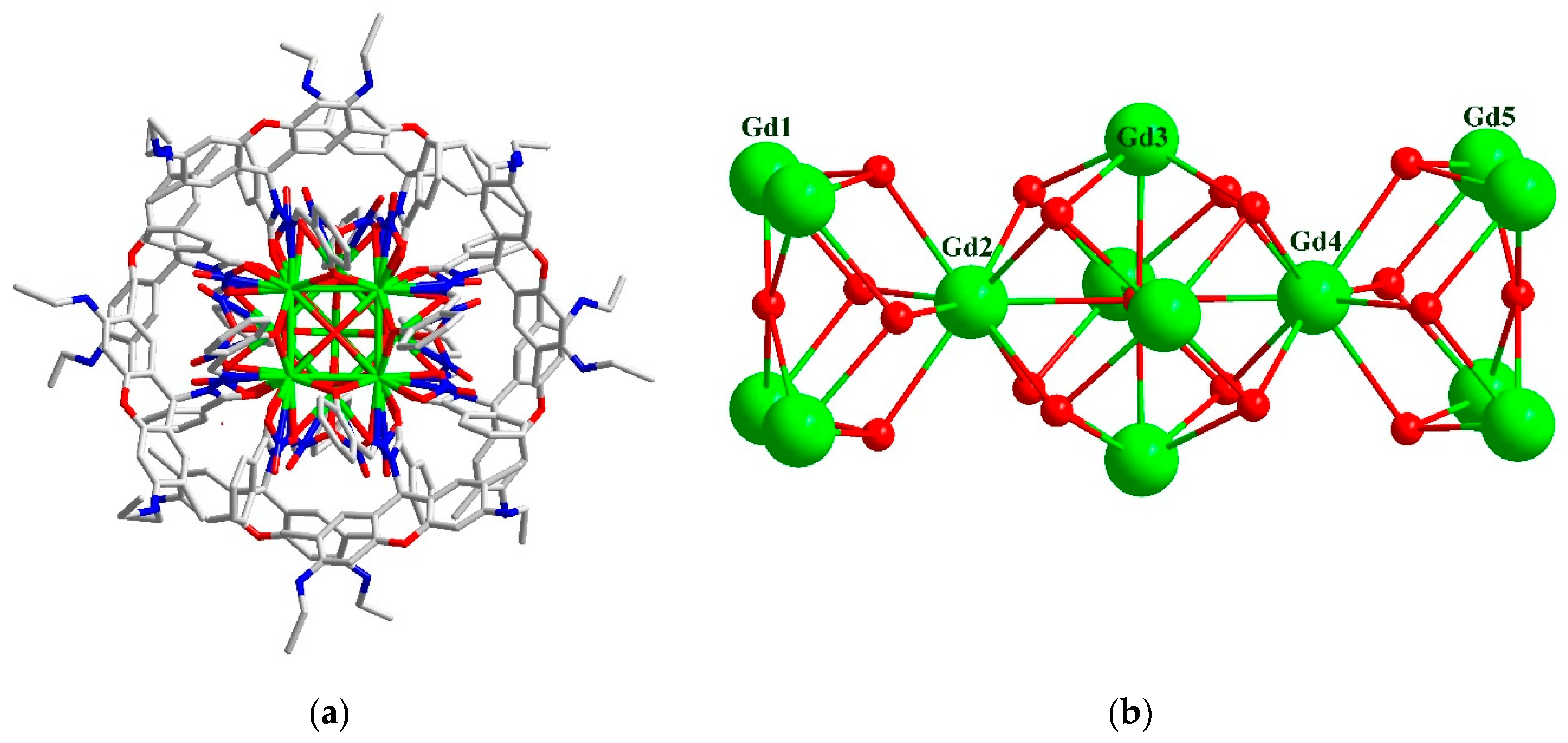
2.2. Structure
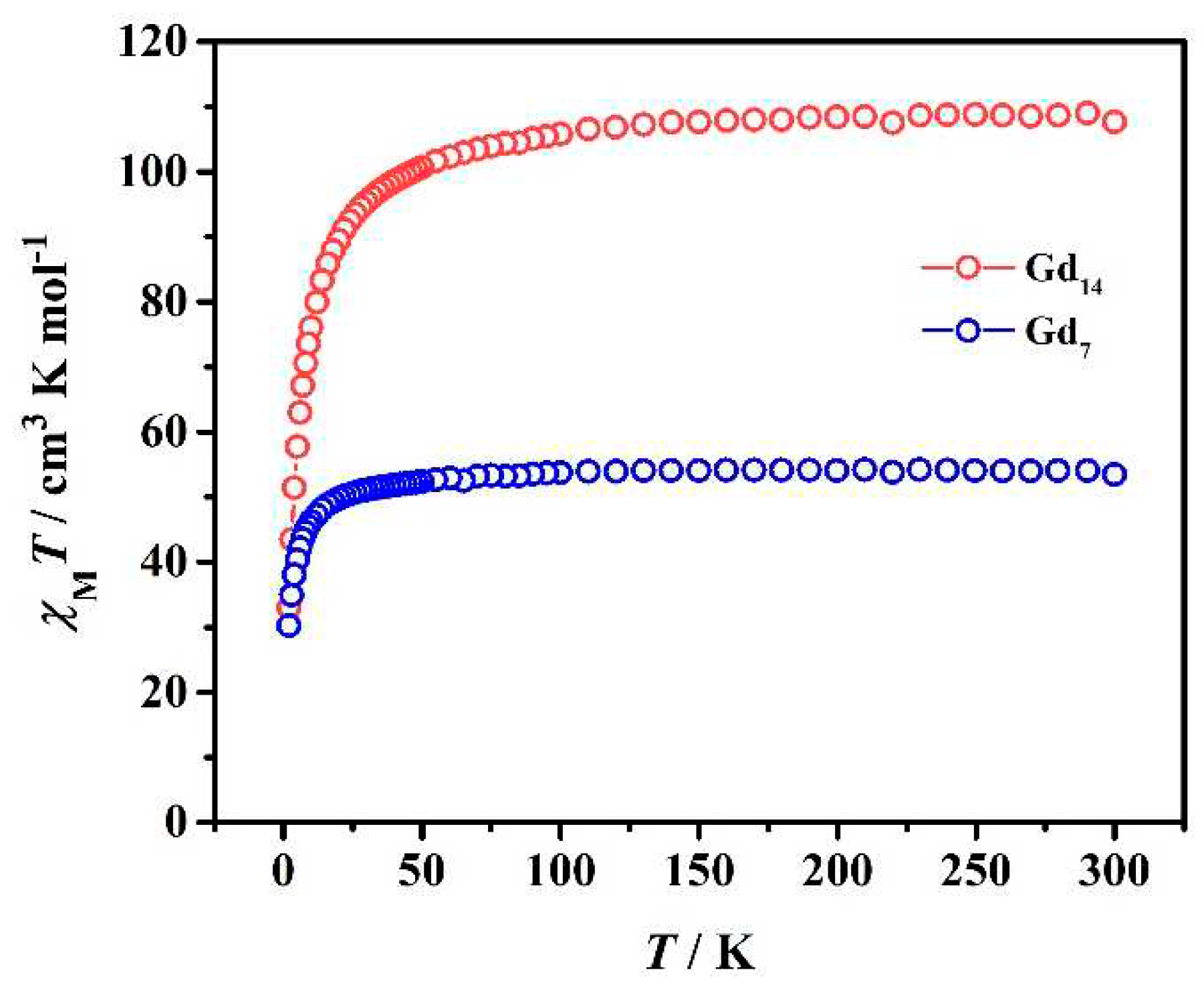
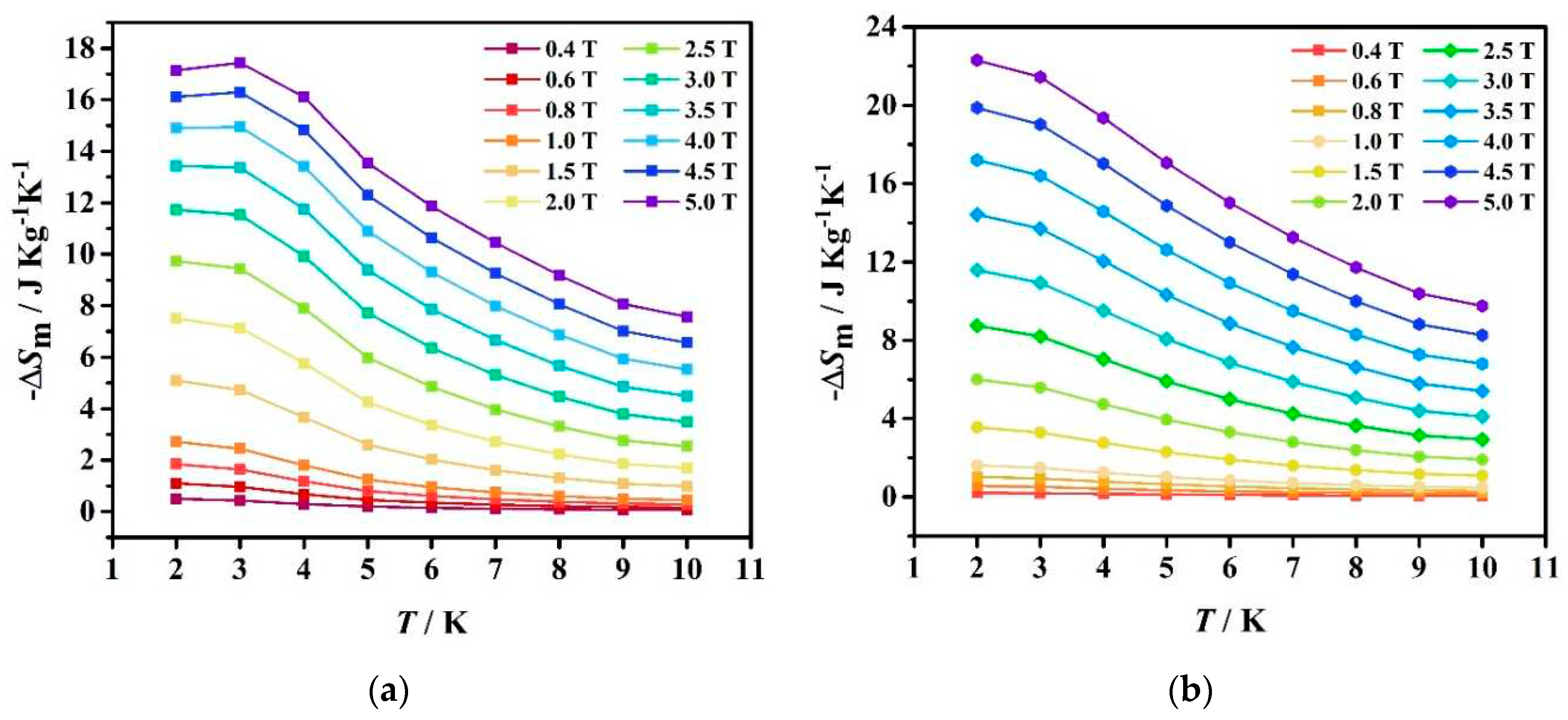
2.3. Magnetic Measurements
3. Materials and Methods
3.1. Synthesis and Preparations
3.1.1. Synthesis of [Gd7(L)6(μ2-CH3O)4(μ3-CH3O)4(μ3-OH)4(NO3)2]NO3·10CH3CN·10CH3OH·2H2O (1).
3.1.2. Synthesis of Gd14(H0.5L)8(μ6-O)(μ4-O)2(μ3-OH)16(NO3)16·9.5CH3CN·2CH3OH·11H2O (2).
3.2. Physical measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Calvez, G.; Le Natur, F.; Daiguebonne, C.; Bernot, K.; Suffren, Y.; Guillou, O. Lanthanide-based hexa-nuclear complexes and their use as molecular precursors. Coord. Chem. Rev. 2017, 340, 134–153. [Google Scholar] [CrossRef]
- Yang, X. P.; Jones, R. A.; Huang, S. M. Luminescent 4f and d-4f polynuclear complexes and coordination polymers with flexible salen-type ligands. Coord. Chem. Rev. 2014, 273, 63–75. [Google Scholar] [CrossRef]
- Ferrando-Soria, J.; Vallejo, J.; Castellano, M.; Martínez-Lillo, J.; Pardo, E.; Cano, J.; Castro, I.; Lloret, F.; Ruiz-García, R.; Julve, M. Molecular magnetism, quo vadis? A historical perspective from a coordination chemist viewpoint. Coord. Chem. Rev. 2017, 339, 17–103. [Google Scholar] [CrossRef]
- Georgopoulou, A. N.; Pissas, M.; Psycharis, V.; Sanakis, Y.; Raptopoulou, C. P. Trinuclear NiII-LnIII-NiII Complexes with Schiff Base Ligands: Synthesis, Structure, and Magnetic Properties. Molecules 2020, 25(10), 2280. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, J. A.; Jena, H. S.; Konar, S. Co3Gd4 Cage as Magnetic Refrigerant and Co3Dy4 Cage Showing Slow Relaxation of Magnetisation. Molecules 2022, 27, 1130. [Google Scholar] [CrossRef]
- Chen, W.-P.; Liao, P.-Q.; Yu, Y.; Zheng, Z.; Chen, X.-M.; Zheng, Y.-Z. A mixed-ligand approach for a gigantic and hollow heterometallic cage {Ni64RE96} for gas separation and magnetic cooling applications. Angew. Chem. Int. Ed. 2016, 55, 9375–9379. [Google Scholar] [CrossRef]
- Li, N. F.; Luo, X. M.; Wang, J.; Wang, J. L.; Mei, H.; Song, Y.; Xu, Y. Largest 3d-4f 196-nuclear Gd158Co38 clusters with excellent magnetic cooling. Sci. China Chem. 2022, 65, 1577–1583. [Google Scholar] [CrossRef]
- Miao, L.; Liu, M. J.; Zeng, M.; Kou, H. Z. Chiral Zn3Ln3 Hexanuclear Clusters of an Achiral Flexible Ligand. Inorg. Chem. 2023, 62, 12814–12821. [Google Scholar] [CrossRef]
- Zeng, M.; Hu, K. Q.; Liu, C. M.; Kou, H. Z. Heterotrimetallic Ni2Ln2Fe3 chain complexes based on [Fe(1-CH3im)(CN)5]2-. Dalton Trans. 2021, 50, 6427–6431. [Google Scholar] [CrossRef]
- Jin, Y. S.; Wang, X.; Zhang, N.; Liu, C. M.; Kou, H. Z. Assembly of Hydrazine-Bridged Cyclic FeIII4LnIII4 Octanuclear Complexes. Cryst. Growth Des. 2022, 22, 1263–1269. [Google Scholar] [CrossRef]
- Tian, H. Q.; Bao, S. S.; Zheng, L. M. Cyclic Single-Molecule Magnets: From Even-Numbered Hexanuclear to Odd-Numbered Heptanuclear Dysprosium Clusters. Eur. J. Inorg. Chem. 2016, 19, 3184–3190. [Google Scholar] [CrossRef]
- Tian, H. Q.; Bao, S. S.; Zheng, L. M. Cyclic single-molecule magnets: from the odd-numbered heptanuclear to a dimer of heptanuclear dysprosium clusters. Chem. Commun. 2016, 52, 2314–2317. [Google Scholar] [CrossRef]
- Goura, J.; Walsh, J. P. S.; Tuna, F.; Chandrasekhar, V. Synthesis, structure, and magnetism of non-planar heptanuclear lanthanide(III) complexes. Dalton Trans. 2015, 44, 1142–1149. [Google Scholar] [CrossRef]
- Mazarakioti, E. C.; Cunha-Silva, L.; Bekiari, V.; Escuer, A.; Stamatatos, T. C. New structural topologies in 4f-metal cluster chemistry from vertex-sharing butterfly units: {LnIII7} complexes exhibiting slow magnetization relaxation and ligand-centred emissions. Rsc Adv. 2015, 5, 92526–92530. [Google Scholar] [CrossRef]
- Pantelis, K. N.; Perlepe, P. S.; Grammatikopoulos, S.; Lampropoulos, C.; Tang, J. K.; Stamatatos, T. C. 4f-Metal Clusters Exhibiting Slow Relaxation of Magnetization: A {Dy7} Complex with An Hourglass-like Metal Topology. Molecules 2020, 25. [Google Scholar] [CrossRef] [PubMed]
- Peng, J. M.; Wang, H. L.; Zhu, Z. H.; Bai, J.; Liang, F. P.; Zou, H. H. Series of the Largest Dish-Shaped Dysprosium Nanoclusters Formed by in situ Reactions. Inorg. Chem. 2022, 61, 6094–6100. [Google Scholar] [CrossRef]
- Lu, T. Q.; Yin, J. J.; Chen, C.; Shi, H. Y.; Zheng, J.; Liu, Z. J.; Fang, X. L.; Zheng, X. Y. Two pairs of chiral lanthanide-oxo clusters Ln14 induced by amino acid derivatives. CrystEngComm 2021, 23, 6923–6929. [Google Scholar] [CrossRef]
- Zhu, Z. H.; Peng, J. M.; Wang, H. L.; Zou, H. H.; Liang, F. P. Assembly Mechanism and Heavy Metal Ion Sensing of Cage-Shaped Lanthanide Nanoclusters. Cell Rep. Phys. Sci. 2020, 1, 100165. [Google Scholar] [CrossRef]
- Tian, H. Q.; Bao, S. S.; Zheng, L. M. Cyclic single-molecule magnets: from the odd-numbered heptanuclear to a dimer of heptanuclear dysprosium clusters. Chem. Commun. 2016, 52, 2314–2317. [Google Scholar] [CrossRef]
- Chesman, A. S. R.; Turner, D. R.; Moubaraki, B.; Murray, K. S.; Deacon, G. B.; Batten, S. R. Tetradecanuclear polycarbonatolanthanoid clusters: Diverse coordination modes of carbonate providing access to novel core geometries. Dalton Trans. 2012, 41, 10903–10909. [Google Scholar] [CrossRef]
- Sun, P.-F.; Zhang, X.-N.; Fan, C.-H.; Chen, W.-P.; Zheng, Y.-Z. Tricine-supported polyoxo(alkoxo)lanthanide cluster {Ln15} (Ln = Eu, Gd, Tb) with magnetic refrigerant and fluorescent properties. Polyoxometalates 2023, 2, 9140026. [Google Scholar] [CrossRef]
- Du, M. H.; Chen, L. Q.; Jiang, L. P.; Liu, W. D.; Long, L. S.; Zheng, L. S.; Kong, X. J. Counterintuitive Lanthanide Hydrolysis-Induced Assembly Mechanism. J. Am. Chem. Soc. 2022, 144, 5653–5660. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Yu, Y. T.; Wang, J. L.; Li, J. N.; Li, N. F.; Fan, X. R.; Xu, Y. Two Windmill-Shaped Ln18 Nanoclusters Exhibiting High Magnetocaloric Effect and Luminescence. Inorg. Chem. 2023, 62, 3162–3169. [Google Scholar] [CrossRef]
- Wang, Q.; Lu, S. H.; Xu, L. X.; Wang, J. L.; Yu, Y. T.; Bai, X.; Mei, H.; Xu, Y. C2O42--templated cage-shaped Ln28(Ln = Gd, Eu) nanoclusters with magnetocaloric effect and luminescence. Inorg. Chem. Front. 2023, 10, 4109–4116. [Google Scholar] [CrossRef]
- Li, Y. L.; Wang, H. L.; Zhu, Z. H.; Liang, F. P.; Zou, H. H. Giant Crown-Shaped Dy34 Nanocluster with High Acid–Base Stability Assembled by an out-to-in Growth Mechanism. Inorg. Chem. 2022, 61, 10101–10107. [Google Scholar] [CrossRef]
- Wu, M.; Jiang, F.; Kong, X.; Yuan, D.; Long, L.; Al-Thabaiti, S. A.; Hong, M. Two polymeric 36-metal pure lanthanide nanosize clusters. Chem. Sci. 2013, 4, 3104–3108. [Google Scholar] [CrossRef]
- Zhou, Y.; Zheng, X. Y.; Cai, J.; Hong, Z. F.; Yan, Z. H.; Kong, X. J.; Ren, Y. P.; Long, L. S.; Zheng, L. S. Three Giant Lanthanide Clusters Ln37 (Ln = Gd, Tb, and Eu) Featuring A Double-Cage Structure. Inorg. Chem. 2017, 56, 2037–2041. [Google Scholar] [CrossRef]
- Guo, F. S.; Chen, Y. C.; Mao, L. L.; Lin, W. Q.; Leng, J. D.; Tarasenko, R.; Orendac, M.; Prokleska, J.; Sechovsky, V.; Tong, M. L. Anion-Templated Assembly and Magnetocaloric Properties of a Nanoscale {Gd38} Cage versus a {Gd48} Barrel. Chem. - Eur. J. 2013, 19, 14876–14885. [Google Scholar] [CrossRef]
- Luo, X. M.; Hu, Z. B.; Lin, Q. F.; Cheng, W.; Cao, J. P.; Cui, C. H.; Mei, H.; Song, Y.; Xu, Y. Exploring the Performance Improvement of Magnetocaloric Effect Based Gd-Exclusive Cluster Gd60. J. Am. Chem. Soc. 2018, 140, 11219–11222. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.; Yu, Y.-Z.; Liao, P.-Q.; Xue, W.; Zheng, Z.; Chen, X.-M.; Zheng, Y.-Z. A “Molecular Water Pipe”: A Giant Tubular Cluster {Dy72} Exhibits Fast Proton Transport and Slow Magnetic Relaxation. Adv. Mater. 2016, 28, 10772–10779. [Google Scholar] [CrossRef]
- Peng, J. B.; Kong, X. J.; Zhang, Q. C.; Orendac, M.; Prokleska, J.; Ren, Y. P.; Long, L. S.; Zheng, Z.; Zheng, L. S. Beauty, symmetry, and magnetocaloric effect-four-shell keplerates with 104 lanthanide atoms. J. Am. Chem. Soc. 2014, 136, 17938–17941. [Google Scholar] [CrossRef]
- Zheng, X.-Y.; Jiang, Y.-H.; Zhuang, G.-L.; Liu, D.-P.; Liao, H.-G.; Kong, X.-J.; Long, L.-S.; Zheng, L.-S. A gigantic molecular wheel of {Gd140}: a new member of the molecular wheel family. J. Am. Chem. Soc. 2017, 139, 18178–18181. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.-L.; Li, X.-X.; Qi, Y.-J.; Yu, H.; Jin, L.; Zheng, S.-T. {Nb288O768(OH)48(CO3)12}: A macromolecular polyoxometalate with close to 300 niobium atoms. Angew. Chem., Int. Ed. 2018, 57, 8572–8576. [Google Scholar] [CrossRef]
- Liu, C.-M.; Sun, R.; Hao, X.; Wang, B.-W. Two Pairs of Homochiral Parallelogram-like Dy4 Cluster Complexes with Strong Magneto-Optical Properties. Inorg. Chem. 2023, 62, 20184–20193. [Google Scholar] [CrossRef] [PubMed]
- Hao, J.; Geng, L.; Zheng, J. Y.; Wei, J. H.; Zhang, L. L.; Feng, R.; Zhao, J. X.; Li, Q. W.; Pang, J. D.; Bu, X. H. Ligand Induced Double-Chair Conformation Ln12 Nanoclusters Showing Multifunctional Magnetic and Proton Conductive Properties. Inorg. Chem. 2022, 61, 3690–3696. [Google Scholar] [CrossRef]
- Gschneidner, K. A.; Pecharsky, V. K. Thirty years of near room temperature magnetic cooling: Where we are today and future prospects. Int. J. Refrig. 2008, 31, 945–961. [Google Scholar] [CrossRef]
- Evangelisti, M.; Brechin, E. K. Recipes for enhanced molecular cooling. Dalton Trans. 2010, 39, 4672–4676. [Google Scholar] [CrossRef] [PubMed]
- Koskelo, E. C.; Liu, C.; Mukherjee, P.; Kelly, N. D.; Dutton, S. E. Free-Spin Dominated Magnetocaloric Effect in Dense Gd3+ Double Perovskites. Chem. Mater. 2022, 34, 3440–3450. [Google Scholar] [CrossRef]
- Lorusso, G.; Sharples, J. W.; Palacios, E.; Roubeau, O.; Brechin, E. K.; Sessoli, R.; Rossin, A.; Tuna, F.; McInnes, E. J. L.; Collison, D.; Evangelisti, M. A Dense Metal-Organic Framework for Enhanced Magnetic Refrigeration. Adv. Mater. 2013, 25, 4653–4656. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhang, Q.-C.; Pan, Y.-Y.; Long, L.-S.; Zheng, L.-S. Magnetocaloric effect and thermal conductivity of Gd(OH)3 and Gd2O(OH)4(H2O)2. Chem. Commun. 2015, 51, 7317–7320. [Google Scholar] [CrossRef]
- Palacios, E.; Rodríguez-Velamazán, J. A.; Evangelisti, M.; McIntyre, G. J.; Lorusso, G.; Visser, D.; de Jongh, L. J.; Boatner, L. A. Magnetic structure and magnetocalorics of GdPO4. Phys. Rev. B: Condens. Matter Mater. Phys. 2014, 90, 214423. [Google Scholar] [CrossRef]
- Chen, Y.-C.; Qin, L.; Meng, Z.-S.; Yang, D.-F.; Wu, C.; Fu, Z.; Zheng, Y.-Z.; Liu, J.-L.; Tarasenko, R.; Orendáč, M.; Prokleška, J.; Sechovský, V.; Tong, M.-L. Study of a magnetic-cooling material Gd(OH)CO3. J. Mater. Chem. A 2014, 2, 9851–9858. [Google Scholar] [CrossRef]
- Chen, Y.-C.; Prokleška, J.; Xu, W.-J.; Liu, J.-L.; Liu, J.; Zhang, W.-X.; Jia, J.-H.; Sechovský, V.; Tong, M.-L. A brilliant cryogenic magnetic coolant: magnetic and magnetocaloric study of ferromagnetically coupled GdF3. J. Mater. Chem. C 2015, 3, 12206–12211. [Google Scholar] [CrossRef]
- Xu, Q. F.; Liu, B. L.; Ye, M. Y.; Zhuang, G. L.; Long, L. S.; Zheng, L. S. Gd(OH)F2: A Promising Cryogenic Magnetic Refrigerant. J. Am. Chem. Soc. 2022, 144, 13787–13793. [Google Scholar] [CrossRef]
- Chen, Y. W.; Gong, P. F.; Guo, R. X.; Fan, F. D.; Shen, J.; Zhang, G. C.; Tu, H. Improvement on Magnetocaloric Effect through Structural Evolution in Gadolinium Borate Halides Ba2Gd(BO3)2X (X = F, Cl). Inorg. Chem. 2023, 62, 15584–15592. [Google Scholar] [CrossRef]
- Liu, M.-J.; Yuan, J.; Tao, J.; Zhang, Y.-Q.; Liu, C.-M.; Kou, H.-Z. , Rhodamine Salicylaldehyde Hydrazone Dy(III) Complexes: Fluorescence and Magnetism. Inorg. Chem. 2018, 57, 4061–4069. [Google Scholar] [CrossRef]
- Liu, M.-J.; Wu, S.-Q.; Li, J.-X.; Zhang, Y.-Q.; Sato, O.; Kou, H.-Z. , Structural Modulation of Fluorescent Rhodamine-Based Dysprosium(III) Single-Molecule Magnets. Inorg. Chem. 2020, 59, 2308–2315. [Google Scholar] [CrossRef]
- Liu, M.-J.; Fu, Z.-Y.; Sun, R.; Yuan, J.; Liu, C.-M.; Zou, B.; Wang, B.-W.; Kou, H.-Z. , Mechanochromic and Single-Molecule Magnetic Properties of a Rhodamine 6G Dy(III) Complex. ACS Appl. Electron. Mater. 2021, 3, 1368–1374. [Google Scholar] [CrossRef]
- Miao, L.; Liu, M. J.; Ding, M. M.; Zhang, Y. Q.; Kou, H. Z. A Dy(III) Fluorescent Single-Molecule Magnet Based on a Rhodamine 6G Ligand. Inorganics 2021, 9, 51. [Google Scholar] [CrossRef]
- Yuan, J.; Wu, S. Q.; Liu, M. J.; Sato, O.; Kou, H. Z. Rhodamine 6G-Labeled Pyridyl Aroylhydrazone Fe(II) Complex Exhibiting Synergetic Spin Crossover and Fluorescence. J. Am. Chem. Soc. 2018, 140, 9426–9433. [Google Scholar] [CrossRef]
- Yuan, J.; Liu, M.-J.; Wu, S.-Q.; Zhu, X.; Zhang, N.; Sato, O.; Kou, H.-Z. , Substituent effects on the fluorescent spin-crossover Fe(ii) complexes of rhodamine 6G hydrazones. Inorg. Chem. Front. 2019, 6, 1170–1176. [Google Scholar] [CrossRef]
- Li, Y.; Huang, W.; Yong, J.; Huang, S. D.; Li, Y. J.; Liu, Y.; Wu, D. Y. Aggregation-induced ratiometric emission and mechanochromic luminescence in a pyrene-benzohydrazonate conjugate. New J. Chem. 2018, 42, 12644–12648. [Google Scholar] [CrossRef]
- Sharples, J. W.; Zheng, Y. Z.; Tuna, F.; McInnes, E. J. L.; Collison, D. Lanthanide discs chill well and relax slowly. Chem. Commun. 2011, 47, 7650–7652. [Google Scholar] [CrossRef]
- Xu, C. Y.; Wu, Z. L.; Fan, C. J.; Yan, L. L.; Wang, W. M.; Ji, B. M. Synthesis of two lanthanide clusters LnIII4 (Gd4 and Dy4) with [2 x 2] square grid shape: Magnetocaloric effect and slow magnetic relaxation behaviors. J. Rare. Earth. 2021, 39, 1082–1088. [Google Scholar] [CrossRef]
- Wang, W. M.; Li, X. Z.; Zhang, L.; Chen, J. L.; Wang, J. H.; Wu, Z. L.; Cui, J. Z. A series of [2 x 2] square grid LnIII4 clusters: a large magnetocaloric effect and single-molecule-magnet behavior. New. J. Chem. 2019, 43, 7419–7426. [Google Scholar] [CrossRef]
- Baril-Robert, F.; Petit, S.; Pilet, G.; Chastanet, G.; Reber, C.; Luneau, D. Site-Selective Lanthanide Doping in a Nonanuclear Yttrium(III) Cluster Revealed by Crystal Structures and Luminescence Spectra. Inorg. Chem. 2010, 49, 10970–10976. [Google Scholar] [CrossRef] [PubMed]
- Petit, S.; Baril-Robert, F.; Pilet, G.; Reber, C.; Luneau, D. Luminescence spectroscopy of europium(III) and terbium(III) penta-, octa- and nonanuclear clusters with β-diketonate ligands. Dalton Trans. 2009, 6809–6815. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Song, D.; Wang, S. Toward constructing nanoscale hydroxo–lanthanide clusters: syntheses and characterizations of novel tetradecanuclear hydroxo–lanthanide clusters. Chem. Commun. 2002, 368–369. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-L.; He, L.-F.; Feng, X.-L.; Song, Y.; Hu, M.; Han, L.-F.; Zheng, X.-J.; Zhang, Z.-H.; Fang, S.-M. Two chiral tetradecanuclear hydroxo-lanthanide clusters with luminescent and magnetic properties. CrystEngComm 2011, 13, 3643–3645. [Google Scholar] [CrossRef]
- Bürgstein, M. R.; Gamer, M. T.; Roesky, P. W. Nitrophenolate as a building block for lanthanide chains, layers, and clusters. J. Am. Chem. Soc. 2004, 126, 5213–5218. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




