Introduction
The COVID-19 pandemic, brought about by the emergence of the novel SARS-CoV-2 virus, quickly escalated into a global health emergency of unprecedented proportions. The introduction of vaccines emerged as a beacon of hope [
1], offering much-needed relief to regions like Uganda, where limited healthcare resources had intensified the pandemic's impact. The single-dose Janssen Ad26.COV2.S COVID-19 vaccine, strategically prioritised in Uganda for key demographic groups such as teachers and hard-to-reach populations, including mobile and remote communities, due to its logistical practicality, offered a valuable opportunity to examine the immune responses induced by this vaccine within the African context. This study informs local public health strategies and contributes to the global discourse on vaccine efficacy.
Emerging research has underscored the critical role of spike (S)-directed [
2,
3] immune responses in conferring protection against SARS-CoV-2 [
4]. Prevailing literature suggests that vaccine-induced immunity against SARS-CoV-2 can significantly vary across different vaccines and populations [
5,
6,
7,
8]. While most studies have concentrated on spike (S) protein-directed immune responses [
7,
9], few study has explored the context of nucleoprotein (N)-directed responses, especially in the setting of the spike protein-based Janssen Ad26.COV2.S COVID-19 vaccine [
10]. Here, we monitored both spike (S) and nucleocapsid (N) protein-directed antibody responses, recognising that N-directed responses, which are unexpected in spike-focused vaccines, could signal post-vaccination infections. This dual-tracking approach provided critical insights into the real-world effectiveness of the vaccine, shedding light not only its ability to provoke an immune response but also on its potential to prevent subsequent infections.
We hypothesised that the single-dose Janssen Ad26.COV2.S COVID-19 vaccine would elicit a robust immune response, and examined this through longitudinal analysis of 319 specimens from 60 individuals over 12 months. In our study, we meticulously measured both Spike (S-IgG) and Nucleocapsid (N-IgG) antibody responses. This approach enabled us to comprehensively delineate the patterns of seroconversion, assess the longevity of immune protection, and track the incidence of breakthrough infections. The significance of this study was amplified by the evolving landscape of the SARS-CoV-2 virus, especially with the concurrent emergence of new variants [
11] that continually challenged the efficacy of existing vaccines [
12,
13]. We sought to fill a vital gap in understanding vaccine-induced immunity, particularly within an African context. By delving into the immune responses elicited by the Janssen Ad26.COV2.S COVID-19 vaccine in Uganda, our research not only enriches global insights into its immunogenicity within the African context but also sets a precedent for similar investigations in other areas where Janssen Ad26.COV2.S COVID-19 vaccine has been pivotal [
15,
16]. This insight is crucial for informing vaccine-related public health strategies and policies, especially in regions where logistical challenges make single-dose vaccine regimens a more feasible option.
Materials and Methods
Study Population.
This study analysed 319 specimens collected over 12 months from 60 individuals who received a single dose of the Janssen Ad26.COV2.S COVID-19 vaccine. Samples were collected between November 15, 2021, and June 2, 2023, from individuals aged 18 to 64 years, with a median age of 22 years (IQR: 19-25 years). The cohort comprised 13 females (21.7%) and 47 males (78.3%). In this study, baseline blood samples were obtained from 58 of 60 participants. These individuals were subsequently classified based on their baseline S-IgG responses measured at day 0. Subjects with S-IgG levels above the established cut-off were classified as baseline S-IgG positive (S-IgG+), while those below this threshold were considered baseline S-IgG negative (S-IgG-). Among the 58 subjects, 39 (67%) were identified as S-IgG +, contributing 218 samples, while the remaining 19 (33%) were S-IgG-, providing 95 samples. This categorisation at baseline provided a foundational reference for analysing S-IgG responses in our cohort.
Binding antibody ELISA to detect SARS-CoV-2-specific IgG, IgM, and IgA levels.
We used a validated ELISA [
14,
15], to detect the prescence of SARS-CoV-2-specific IgG, IgM, and IgA antibodies against the spike (S) and nucleocapsid (N) proteins. ELISA plates were coated with an antigen at a concentration of 3 μg/ml, which had been verified to have the highest possible specificity and sensitivity. Optical densities (OD) were measured at 450 nm to quantify the antibody concentrations in nanograms per millilitre (ng/ml). Seropositivity was determined using previously established cut-off optical density (OD) values specific to this population, as described before [
15]. The OD thresholds were 0.432 for IgG, 0.459 for IgM, 0.226 for IgA for spike-specific antibodies, 0.454 for IgG, 0.229 for IgM, and 0.225 for IgA for nucleoprotein-specific antibodies. These values were determined from an extensive analysis of a large sample population.
Statistical analysis
Seroconversion percentages at each follow-up time point were visualized using diverging bar graphs, and boxplots were utilised to evaluate and contrast medians (represented by horizontal lines), means (indicated by black dots), and quartile ranges (denoted by the top and bottom edges of the box). The Wilcoxon test, with Hochberg correction for multiple testing adjustments, was conducted to determine differences in antibody responses between pairwise comparisons at different time points. Unpaired tests were selected due to missing data at various time points, and a significance threshold of p > 0.05 indicated non-significance (ns). Statistical significance was denoted as follows: * for p ≤ 0.05, ** for p < 0.01, *** for p < 0.001, and **** for p < 0.0001.
Results
Dynamic Patterns of Seroconversion and Long-Lasting Immunity Post-Vaccination with the Janssen Ad26.COV2.S COVID-19 vaccine
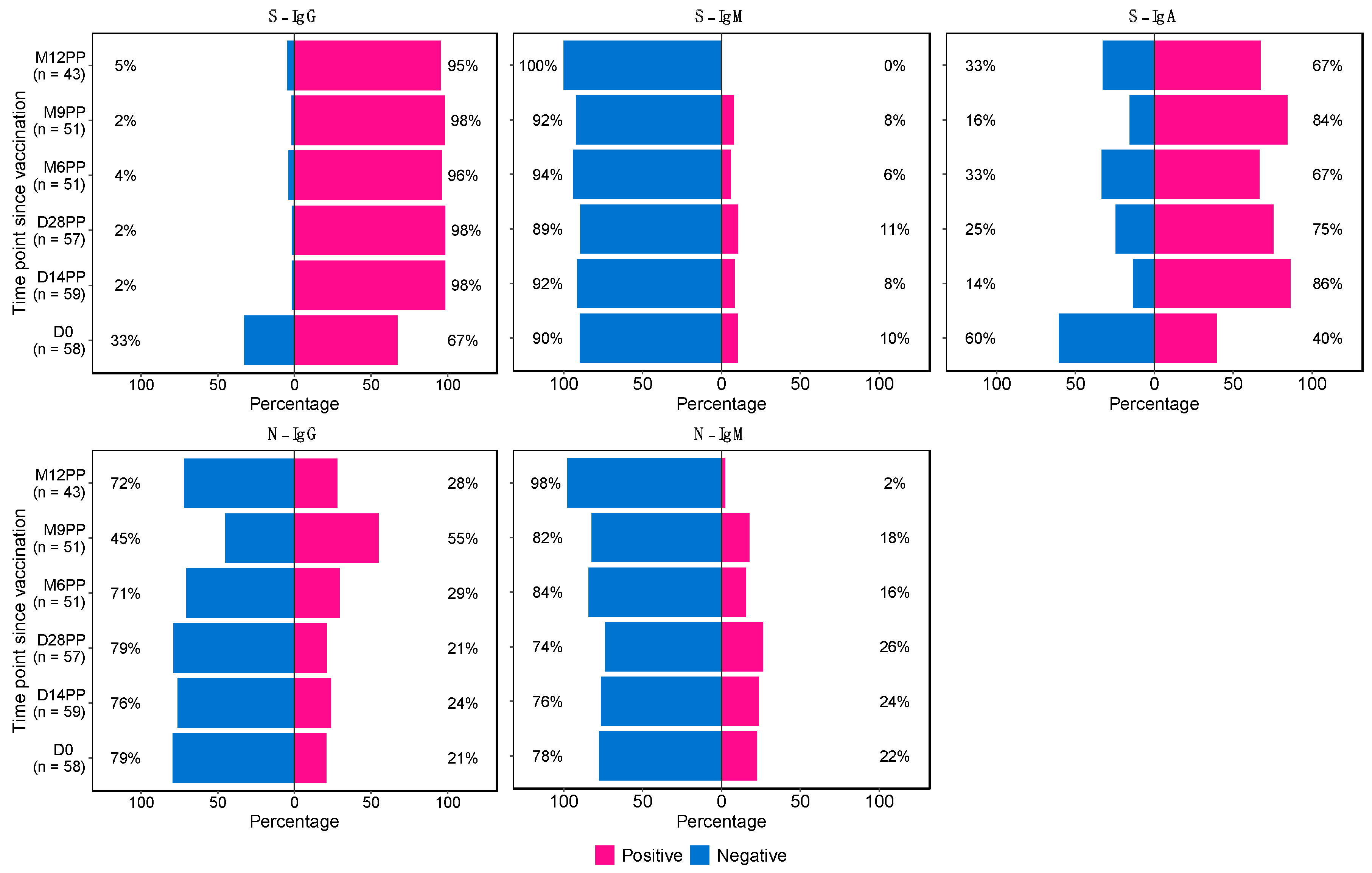
This longitudinal study presents evidence of the seroconversion patterns in response to vaccination with the single dose Janssen Ad26.COV2.S COVID-19 vaccine in Uganda. The data, captured over 12 months since initial vaccination, show the temporal changes in various immunoglobulin responses following a priming dose of the vaccine, illustrated in
Figure 1. The study demonstrated a marked and sustained enhancement in S-IgG positivity, increasing from 67% at baseline to 98% by day 14 post-priming (D14PP). This elevated seropositivity was maintained till the 12-month follow-up mark, highlighting the vaccine's effectiveness in eliciting a durable and robust hybrid immune response.
In contrast, initial S-IgM responses were minimal, with only 10% seropositivity at day 0, maiataining these levels over an extended period, until 12 months (M12PP) when the seropositivity rate eventually reducing to 0%. This decline in S-IgM seroprevalence highlights the typical switch to a predominantly IgG-mediated immune responses [
16]. Meanwhile, S-IgA seropositivity substantially increased, rising from 40% baseline seropositivity to 86% by day 14 post-prime (D14PP). This seropositivity was sustained over the 12-month follow-up, with proportions at 75%, 67%, and 84% at subsequent intervals, concluding with 67% at the 12-month mark. Overall, approximately 70% of subjects demonstrated consistent S-IgA seropositivity throughout the study period after the first vaccine dose, indicating durable mucosal immunity. N-IgG responses were initially detected in 21% of subjects, with a notable rise to 55% nine months after the primary dose (M9PP), that dropped to 28% at 12 months, indicating a delayed yet significant serological response. Correspondingly, N-IgM seropositivity initially rose modestly from a baseline of 22% to 26% by 28 days after the primary dose before decreasing to 16% at six months, culminating in a 2% level by 12 months post-vaccination, consistent with the expected serological trends. The sustained levels of S-IgG and S-IgA seropositivity, coupled with the modulated S-IgM and N-IgM frequencies, affirm the vaccine's effectiveness in inducing a robust and lasting immune response. These results reflect the longevity of Janssen Ad26.COV2.S COVID-19 vaccine -induced immunity in a landscape of a continuing epidemic, pivotal for shaping future vaccination strategies and informing public health policies.
A Temporal Analysis of the Evolving Antibody Response Dynamics Following Vaccination
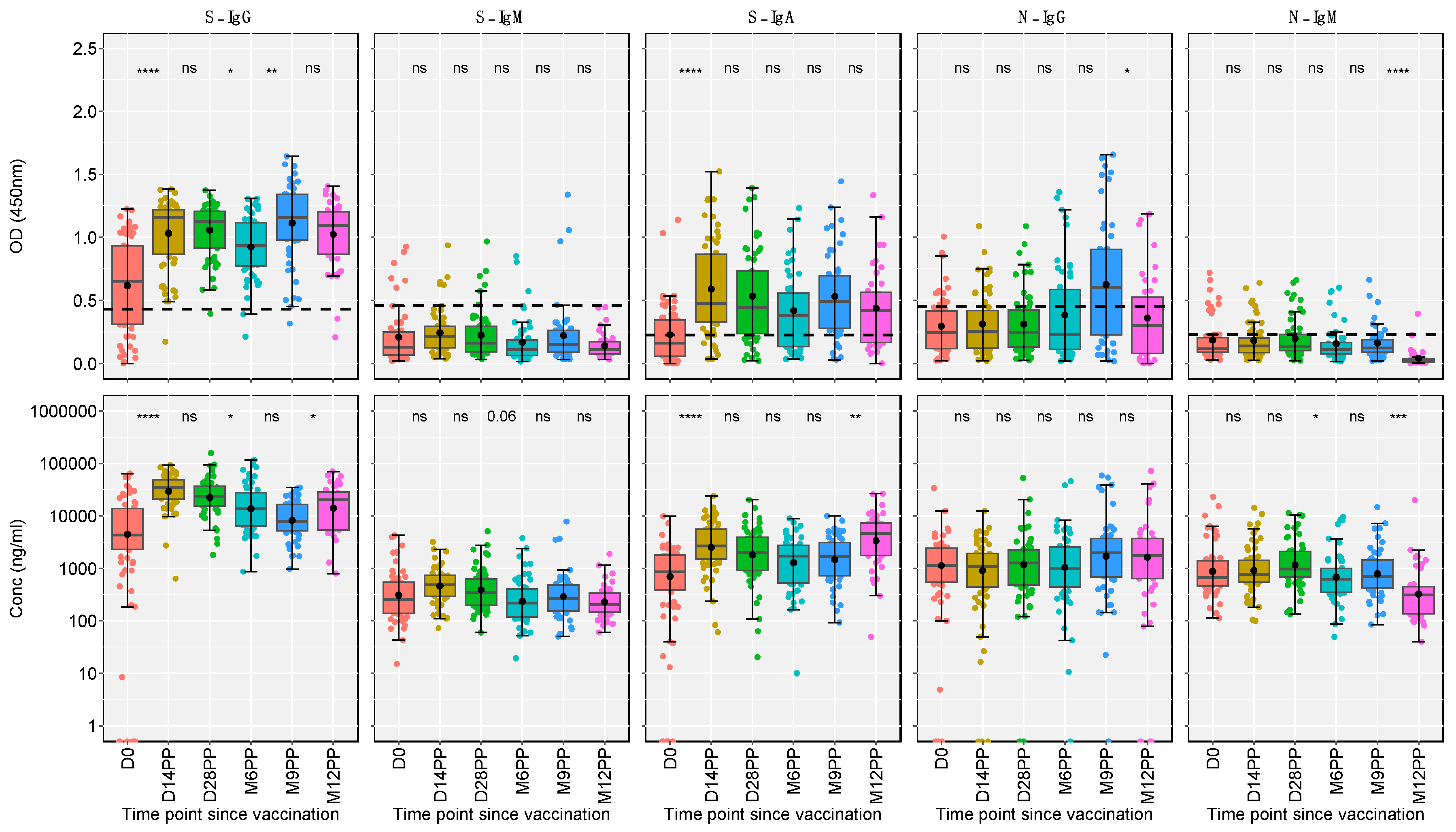
Our analysis delineated the temporal dynamics of antibody responses post-vaccination (
Figure 2). Applying unpaired Wilcoxon tests with Hochberg corrections for multiple comparisons, we observed a significant elevation in S-IgG optical density (OD) and antibody concentrations at 14 days post-vaccination. This increase plateaued by day 28 post-vaccination (D28PP). However, by month six post-vaccination (M6PP), a marked decline in S-IgG concentrations was noted, suggesting a time-dependent waning of these antibodies (
Table 1). Significantly elevated S-IgG antibody levels observed at the 12-month mark may indicate breakthrough infections, as shown in
Table 1. In contrast, S-IgM antibody optical density (OD) levels and The S-IgA response dynamics were significant, showing an immediate post-vaccination increase, a gradual decline, and then a notable resurgence at 9 months post-vaccination, possibly indicative of re-infection. N-IgG responses throughout the study period were predominantly low and below optimal thresholds, with only a marginal, non-significant increase observed at the 9-month mark, indicating an overall subdued N-IgG response. In parallel, N-IgM levels maintained a consistently low profile, culminating in a significant decrease at the 12-month follow-up. These findings show the diverse antibody response dynamics following Janssen Ad26.COV2.S COVID-19 vaccine vaccination advancing our understanding of post-vaccination immunological processes.
Antibody Fold Changes and Breakthrough Infections Following SARS-CoV-2 Vaccination
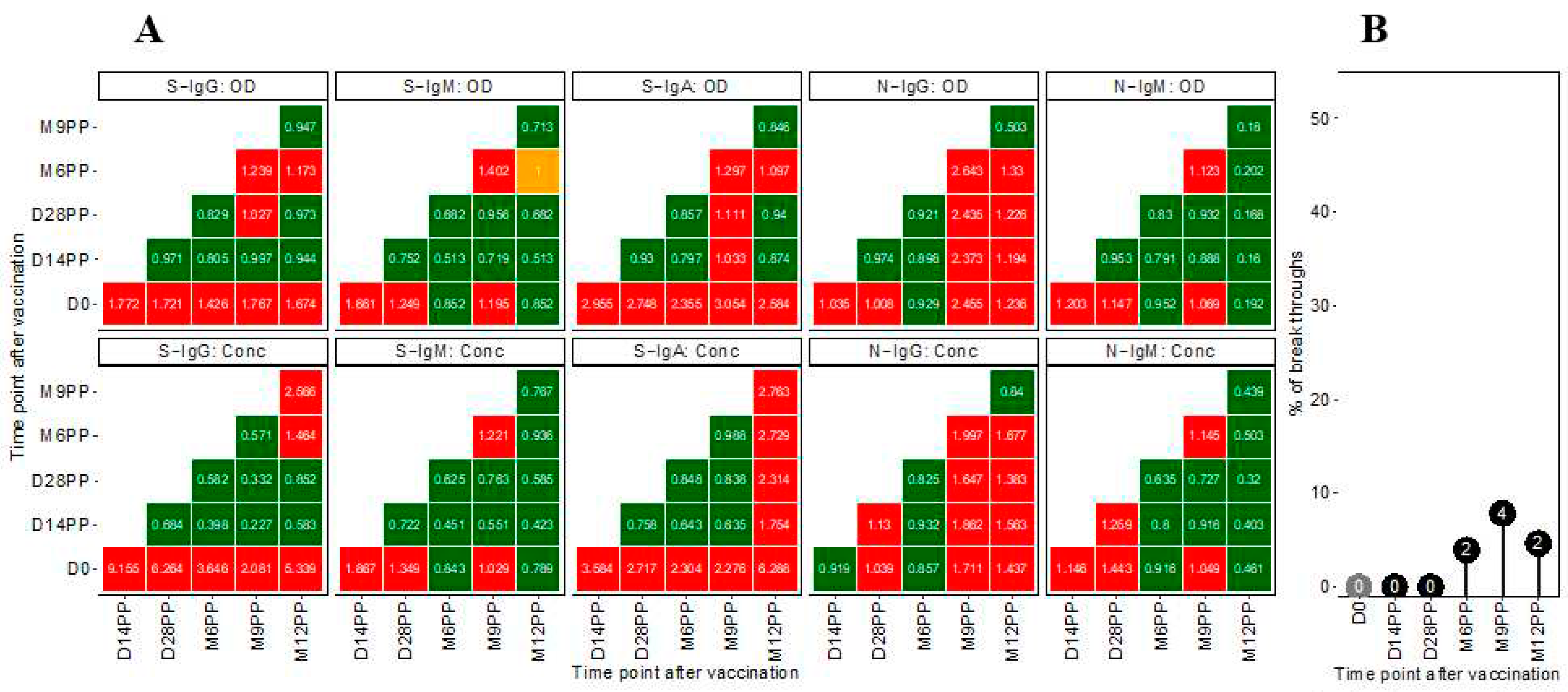
Fold change analyses demonstrated noticeable increments in antibody responses post-vaccination, with S-IgG and S-IgA OD levels increasing by 2-fold and 3-fold elevations respectively, within 14 days post-prime (
Figure 3). This initial upsurge stabilised, as indicated by minimal OD level alterations at later time points. A modest 1.2-fold rise in S-IgM OD levels was noted two weeks post-prime, alongside minimal changes in N-IgG and N-IgM, reflecting a comparatively subdued response. More pronounced changes were observed in antibody concentrations. S-IgG concentrations surged, registering over 9-fold and 6-fold increases at 14 and 28 days post-prime, respectively. S-IgA concentrations also rose significantly, showing 3.5-fold and 3-fold increments at the same time points. However, N-directed IgG and IgM concentrations remained relatively unchanged throughout the study, as summarised in
Figure 3A.Subjects were categorised as breakthrough cases if they exhibited an 11-fold or more significant increase in N-IgG concentration, presumed to indicate infection, occurring 14 days or more after completing the vaccination regimen. Analysis revealed a total of eight breakthrough COVID-19 cases following primary vaccination with the Janssen Ad26.COV2.S COVID-19 vaccine. These cases were temporally distributed as follows: two cases at six months post-primary vaccination (M6PP), four cases at nine months (M9PP), and two cases at twelve months (M12PP). Among these, three cases occurred in subjects who were baseline S-IgG negative, while five cases were in baseline S-IgG positive individuals, summarised in
Figure 3B.
Impact of Baseline Cross-Reactivity on Spike-Directed Antibody Responses Post-Vaccination
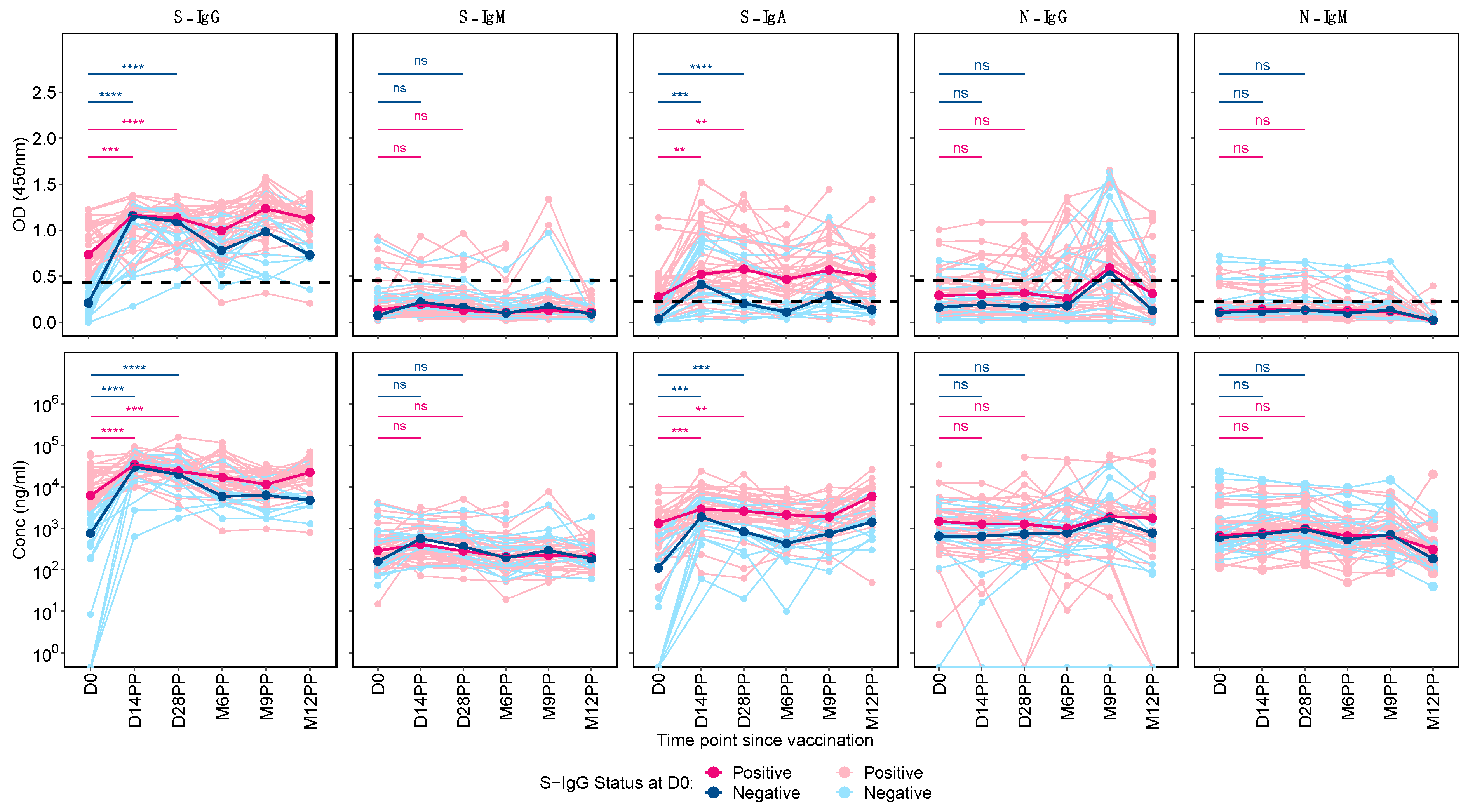
Finally, we identified distinct patterns in Spike-directed antibody responses based on S-IgG serostatus at baseline. Individuals with baseline (day 0) S-IgG antibody OD levels at or above the cutoff were categorised as S-IgG positive (S-IgG+), whereas those below the threshold were considered S-IgG negative (S-IgG-). Significant increases in S-IgG responses from day 0 to both day 14 and day 28 post-prime were observed in both groups, as indicated by unpaired Wilcoxon tests, illustrated in
Figure 4. We observed a plateau in Spike-protein-specific Immunoglobulin G (S-IgG) antibody OD levels and concentrations in both baseline S-IgG positive and negative groups from Day 14 to Day 28 after the first vaccination. Following Day 28, a marked decline in S-IgG levels was noted in the baseline S-IgG negative group, with these levels remaining consistently lower than those in the baseline positive group for the duration of the study period. S-IgM antibody responses were consistently low in both groups, with most participants showing optical density (OD) levels below the threshold throughout the follow-up period. S-IgM antibody responses remained consistently low in both groups, with most participants showing optical density (OD) levels below the threshold throughout the follow-up. In this study, we observed distinct differences in S-IgA antibody responses between subjects with pre-existing S-IgG (S-IgG+) and those without (S-IgG-). Initially, baseline S-IgG+ individuals exhibited S-IgA levels above the established cutoff, whereas S-IgG- subjects presented lower S-IgA responses. Following prime vaccination, both cohorts showed a significant increase in S-IgA levels by day 14 and day 28 relative to day 0. Notably, the S-IgG+ group maintained higher S-IgA responses compared to the S-IgG- group throughout the study.
Both S-IgG+ and S-IgG- groups demonstrated consistently low median N-IgG and N-IgM antibody levels, with negligible differences between the two groups. Notably, a modest increase in N-IgG levels was observed between 6 and 9 months, subsequently followed by a reduction to levels below the established threshold.
Discussion
In this study, we evaluated the immunogenicity of the single-dose Janssen Ad26.COV2.S COVID-19 vaccine in a Ugandan cohort. This vaccination was strategically prioritised for selected key demographic populations, such as teachers, highly mobile populations, and residents in remote hard-to-reach areas. This approach provided crucial data on the vaccine's effectiveness in diverse, and in logistically challenging communities, often underserved in healthcare. In our study, a pronounced and sustained increase in Spike-directed Immunoglobulin G (S-IgG) responses was observed following the administration of the Janssen Ad26.COV2.S COVID-19 vaccine. S-IgG seropositivity rapidly escalated from 67% at baseline to 98% within 14 days of the first dose, consistently maintaining these elevated levels over the subsequent observed period. This result corroborates previous studies demonstrating the vaccine's effectiveness in generating robust and long-lasting spike-specific antibodies [
8,
17,
18]. This is particularly significant in scenarios where administering subsequent doses poses logistical hurdles.
In our study, we observed a notable rise in nucleoprotein-directed IgG (N-IgG) and IgM (N-IgM) antibodies among eight individuals fully vaccinated with the Janssen Ad26.COV2.S COVID-19 vaccine. The rise in N-IgG and N-IgM levels, given the vaccine's focus on the spike protein, suggests potential breakthrough infections rather than a direct response to the vaccine [
3]. These findings are critical for understanding post-vaccination infection rates and underscore the importance of continuous sero-surveillance and booster vaccinations in response to evolving viral strains. The observed breakthrough rate of 13% (8 of 60) following the Janssen vaccine administration aligns closely with the rates of 10% (6 of 60) reported in comparative studies in this population using Coronavac COVID-19 vaccine (Sinovac) administered during the same period [
1].Similar trends were noted in just concluded studies for Pfizer-BioNTech's BNT162b2 and Moderna's mRNA-1273 vaccines, with rates of 23% (11 of 48) and 16% (3 of 19), respectively (manuscripts under review). However, these comparisons should be interpreted cautiously due to the small sample sizes involved. Initial S-IgM responses were minimal, decreasing to zero by 12 months post-prime, a trend that is consistent with the expected serological progression towards an IgG-dominant response [
19,
20,
21]. Our study demonstrates a marked increase in S-IgA responses following vaccination with the Janssen Ad26.COV2.S COVID-19 vaccine, indicative of a rapid and sustained mucosal immunity, an aspect often underrepresented in vaccine research [
22,
23,
24]. This pattern of prolonged immunity is consistent with the natural infection responses previously observed within this population [
3]. This study corroborates earlier South African research demonstrating sustained, robust spike-specific immune responses for up to six months post-administration of the Ad26.COV2.S vaccine, independent of prior infection history [
25]. It also aligns with responses elicited by other COVID-19 vaccines used in this demographic, such as Sinovac Biotech's CoronaVac COVID-19 vaccine [
26] and the Oxford/AstraZeneca ChadOx1-S COVID-19 vaccine [
27], collectively supporting the vaccine’s effectiveness in this landscape. These finding align with literature that shows the importance of mucosal immunity in respiratory infections [
28,
29,
30] and could have implications for future vaccine designs and public health strategies[
28,
29,
30].
Our study, conducted in a Ugandan cohort, demonstrates the elicitation of sustained immune responses to the Janssen Ad26.COV2.S COVID-19 vaccine, offering valuable insights for vaccine strategies in similar settings, complementing global data that often overlook regional variations in immune response due to demographic, genetic, and epidemiological factors [
31,
32]. The findings highlightthe persistence of antibody responses for up to a year, despite observed breakthrough infections primarily occurring after six months, thus contributing to a broader understanding of vaccine-induced immunity against SARS-CoV-2.
Our methodology, though robust, needed to be improved in tracking breakthrough infections. The reliance on N-IgG as a post-vaccination infection marker may not fully represent the immune response spectrum of reinfections, particularly in cases with subdued secondary responses after boosting [
33]. Future studies should integrate viral sequencing and epidemiological insights to determine breakthrough infections and vaccine efficacy against diverse strains more accurately. The study's analysis of antibody responses, while informative, could have been enriched by incorporating cellular immunity assessments for a fuller evaluation of vaccine efficacy. Constraints such as high baseline exposure and missing data at various points, necessitating the use of unpaired tests, may have impacted the robustness of our findings. Additionally, the unique demographic and epidemiological context of Uganda's equatorial positioning suggests the need for further studies in diverse settings to enhance the generalizability of our results.
In conclusion, the single-dose Janssen Ad26.COV2.S COVID_19 vaccine demonstrated potent and lasting immune responses, which is crucial for remote and hard-to-reach populations. The rise in N-directed antibodies post-vaccination indicates possible breakthrough infections, underscoring the need for vigilant surveillance and adaptive vaccination strategies. These results contribute significantly to the global understanding of COVID-19 vaccine effectiveness, informing public health policy and vaccination strategies.
Author Contributions
Conceptualization, Jennifer Serwanga; Data curation, Jennifer Serwanga, Violet Ankunda, Claire Baine, Gerald Oluka and Peter Ejou; Formal analysis, Jennifer Serwanga and Violet Ankunda; Funding acquisition, Jennifer Serwanga; Investigation, Jennifer Serwanga, Laban Kato, Isaac Kitabye, Angella Namuyanja, Solomon Opio, Jackson Sembera, Claire Baine, Joseph Katende, Gerald Oluka, Peter Ejou and The COVID-19 Immunoprofiling Team; Methodology, Jennifer Serwanga, Laban Kato, Violet Ankunda, Claire Baine and Pontiano Kaleebu; Supervision, Jennifer Serwanga; Validation, Jennifer Serwanga, Violet Ankunda, Isaac Kitabye, Solomon Opio, Jackson Sembera, Claire Baine, Joseph Katende, Gerald Oluka and The COVID-19 Immunoprofiling Team; Visualization, Jennifer Serwanga and Violet Ankunda; Writing – original draft, Jennifer Serwanga, Violet Ankunda and Angella Namuyanja; Writing – review & editing, Jennifer Serwanga, Laban Kato, Violet Ankunda, Isaac Kitabye, Solomon Opio, Jackson Sembera, Claire Baine, Gerald Oluka, Peter Ejou and The COVID-19 Immunoprofiling Team.
Funding
This research was made possible through funding from several sources. The Science, Technology, and Innovation Secretariat at the Office of the President in Uganda (STI-OP) played a pivotal role by providing cohort development financial support via the MOSTI-PRESIDE-COVID-19-2020/15 grant. Additionally, the European & Developing Countries Clinical Trials Partnership (EDCTP2) program, supported by the European Union, contributed through grant RIA2020EF-3008-COVAB, aiding specific objectives of the study. The research activities were conducted at the MRC/UVRI and LSHTM Uganda Research Unit. This unit is a collaborative venture involving the UK Medical Research Council, which is part of UK Research and Innovation, and the UK Foreign, Commonwealth, and Development Office, operating under the MRC/FCDO Concordat agreement and affiliated with the EDCTP2 programme backed by the EU. The Bill & Melinda Gates Foundation also extended support through the GIISER Uganda Grant (Investment ID INV-036306). The insights and conclusions drawn in this study are those of the authors and do not necessarily reflect the views of the funding bodies.
Ethics approval and consent to participate
The study obtained clearance from the Research and Ethics Committee (GC/127/833) of the Uganda Virus Research Institute and the Uganda National Council for Science and Technology (HS637ES). All procedures were conducted in accordance with the relevant ethical norms. All participants provided written informed consent.
Data availability statement
This study contains all of the original data that was generated. For further information or access to the raw data supporting the article's results, please contact the relevant author. The authors will supply this information without hesitation.
Acknowledgments
We express our gratitude to the individuals who generously provided samples for this comprehensive longitudinal study. We used the Monoclonal Anti-SARS Coronavirus Recombinant Human Antibody (Clone CR3022, HEK293 Cell production) obtained from BEI Resources, supported by NIAID, NIH, under contract HHSN272201400008C. Additionally, the Monoclonal Anti-SARS Coronavirus Recombinant Human IgG1 (Clone CR3022), produced in Nicotiana benthamiana, was acquired from BEI Resources, NIAID, NIH, catalogued as NR-52392. The Nucleoprotein mAb CR3009 (Product No. 101011), crucial as a positive control in our experiments, was procured from the Centre for AIDS Reagents, NIBSC, UK.
Conflict of Interest
The authors declare that there are no commercial or financial interests that could be construed as potential conflicts of interest in relation to this research.
Figure Legends
Figure 1: Twelve-Month Longitudinal Study of Seroconversion Dynamics Using S- and N-Protein-Directed Antibody Detection in Individuals Vaccinated with Janssen Ad26.COV2.S COVID-19 Vaccine
This figure displays the percentage of subjects seroconverting against S (spike) and N (nucleocapsid) proteins, segmented by the detection of S-IgG, S-IgM, and S-IgA antibodies. Data is stratified based on baseline S-IgG seropositivity: baseline positives are indicated in pink, and negatives in blue.
Figure 2: Longitudinal Analysis of Antibody Responses Over 12 months Following Administration of the Single-Dose Janssen Ad26.COV2.S COVID-19 Vaccine
This figure illustrates the antibody response levels, measured in optical density (OD) and concentration (ng/ml), throughout the study period. Each boxplot displays the interquartile range, with the mean represented by a black solid circle and the median by a horizontal line. Statistical analysis of the antibody response variation over time was conducted using an unpaired Wilcoxon test, with a Hochberg correction for multiple comparisons. Significance thresholds are indicated as: 'ns' for p > 0.05 (non-significant), '' for p ≤ 0.05, '' for p < 0.01, '' for p < 0.001, and '****' for p < 0.0001."Median
Figure 3: Temporal Dynamics of Median Antibody Response and Incidence of Breakthrough Cases Post-Vaccination
Figure 3A illustrates the median fold changes in antibody responses between sequential time points. Fold changes are quantified as ratios, with a value of one indicating no change, values greater than 1 denoting an increase, and values less than one signifying a decrease. Increases in antibody responses are highlighted in red, decreases in green, and instances with no change are marked in orange.
Figure 3B delineates the prevalence of presumed infection and breakthrough cases in the study cohort, measured by the change in N-IgG antibody levels, before and after completion of the COVID-19 vaccination regimen. Grey circles indicate the percentage of subjects presumed infected at each time point before completing the vaccination regimen, while black circles represent the percentage of breakthrough cases post-full vaccination. The y-axis quantifies these percentages. Breakthrough cases, defined as subjects with an 11-fold increase in N-IgG levels indicative of infection occurring 14 days or more after the complete vaccination, amounted to three individuals, all of whom were identified six months post-vaccination.
Figure 4: Comparative Profiling of Median Spike-Directed Antibody Responses Post-Janssen Janssen Ad26.COV2.S COVID-19 Vaccination Stratified by Baseline S-IgG Seropositivity
This figure depicts the individual trends in Spike-directed antibody responses (light-shaded lines) and the median responses (dark, thicker lines), categorized based on baseline S-IgG antibody levels. Subjects are classified as S-IgG positive (shown in red) if their baseline S-IgG levels are at or above the established cutoff value and S-IgG negative (illustrated in blue) if below this threshold. The data tracks these antibody responses over 12 months following the initial vaccination, providing a detailed temporal view of the immune response elicited by the Ad26.COV2.S COVID-19 Janssen vaccine.
References
- Turley CB, Tables L, Fuller T, Sanders LJ, Scott H, Moodley A, Woodward Davis A, Leav B, Miller J, Schoemaker K et al: Modifiers of COVID-19 vaccine efficacy: Results from four COVID-19 prevention network efficacy trials. Vaccine 2023, 41(33):4899-4906. [CrossRef]
- Ssali I, Mugaba S, Watelo AK, Bemanzi J, Katende JS, Oluka GK, Ankunda V, Baine C, Kato L, Onyachi N et al: Spike protein is a key target for stronger and more persistent T-cell responses-a study of mild and asymptomatic SARS-CoV-2 infection. Int J Infect Dis 2023, 136:49-56. [CrossRef]
- Serwanga J, Ankunda V, Sembera J, Kato L, Oluka GK, Baine C, Odoch G, Kayiwa J, Auma BO, Jjuuko M et al: Rapid, early, and potent Spike-directed IgG, IgM, and IgA distinguish asymptomatic from mildly symptomatic COVID-19 in Uganda, with IgG persisting for 28 months. Front Immunol 2023, 14:1152522. [CrossRef]
- Yiska Weisblum FS, Fengwen Zhang1, Justin DaSilva1, Daniel Poston1, Julio C. C. Lorenzi2, Frauke Muecksch1, Magdalena Rutkowska1,Hans-Heinrich Hoffmann3 Eleftherios Michailidis3, Christian Gaebler2, Marianna Agudelo2, Alice Cho2, Zijun Wang2, Anna Gazumyan2, Melissa Cipolla2, Larry Luchsinger4, Christopher D. Hillyer4, Marina Caskey2 Davide F. Robbiani2,5, Charles M. Rice3, Michel C. Nussenzweig2,6, Theodora Hatziioannou1 and Paul D. Bieniasz: Escape from neutralizing antibodies by SARS-CoV-2 spike protein variants. 2020.
- Zhang Z, Mateus J, Coelho CH, Dan JM, Moderbacher CR, Gálvez RI, Cortes FH, Grifoni A, Tarke A, Chang J et al: Humoral and cellular immune memory to four COVID-19 vaccines. Cell 2022, 185(14):2434-2451.e2417. [CrossRef]
- Fedele G, Trentini F, Schiavoni I, Abrignani S, Antonelli G, Baldo V, Baldovin T, Bandera A, Bonura F, Clerici P et al: Evaluation of humoral and cellular response to four vaccines against COVID-19 in different age groups: A longitudinal study. Front Immunol 2022, 13:1021396. [CrossRef]
- Cho A, Muecksch F, Wang Z, Ben Tanfous T, DaSilva J, Raspe R, Johnson B, Bednarski E, Ramos V, Schaefer-Babajew D et al: Antibody evolution to SARS-CoV-2 after single-dose Ad26.COV2.S vaccine in humans. J Exp Med 2022, 219(8). [CrossRef]
- El-Shesheny R, El Taweel A, Gomaa MR, Roshdy WH, Kandeil A, Webby RJ, Kayali G, Ali MA: Induced humoral immunity of different types of vaccines against most common variants of SARS-CoV-2 in Egypt prior to Omicron outbreak. Vaccine 2022, 40(32):4303-4306. [CrossRef]
- Samanovic MI, Oom AL, Cornelius AR, Gray-Gaillard SL, Karmacharya T, Tuen M, Wilson JP, Tasissa MF, Goins S, Herati RS et al: Vaccine-Acquired SARS-CoV-2 Immunity versus Infection-Acquired Immunity: A Comparison of Three COVID-19 Vaccines. Vaccines (Basel) 2022, 10(12). [CrossRef]
- Batchi-Bouyou AL, Djontu JC, Vouvoungui JC, Mfoutou Mapanguy CC, Lobaloba Ingoba L, Mougany JS, Boumpoutou KR, Diafouka-Kietela S, Ampa R, Ntoumi F: Assessment of neutralizing antibody responses after natural SARS-CoV-2 infection and vaccination in congolese individuals. BMC Infect Dis 2022, 22(1):610. [CrossRef]
- Tegally H, San JE, Cotten M, Moir M, Tegomoh B, Mboowa G, Martin DP, Baxter C, Lambisia AW, Diallo A et al: The evolving SARS-CoV-2 epidemic in Africa: Insights from rapidly expanding genomic surveillance. Science 2022, 378(6615):eabq5358. [CrossRef]
- Mistry P, Barmania F, Mellet J, Peta K, Strydom A, Viljoen IM, James W, Gordon S, Pepper MS: SARS-CoV-2 Variants, Vaccines, and Host Immunity. Front Immunol 2021, 12:809244. [CrossRef]
- Chakraborty C, Sharma AR, Bhattacharya M, Lee SS: A Detailed Overview of Immune Escape, Antibody Escape, Partial Vaccine Escape of SARS-CoV-2 and Their Emerging Variants With Escape Mutations. Front Immunol 2022, 13:801522. [CrossRef]
- Baine C, Sembera, J., Oluka, GK., Katende JS., Ankunda, V., Serwanga,. J.: An Optimised Indirect ELISA Protocol for Detecting and Quantifying Anti-Viral Antibodies in Human Plasma or Serum: A Case Study using SARS-CoV-2; in press. Bioprotocol, Manuscript ID: 2305159 2023.
- Oluka GK, Namubiru P, Kato L, Ankunda V, Gombe B, Cotten M, Team C-I, Musenero M, Kaleebu P, Fox J et al: Optimisation and Validation of a conventional ELISA and cut-offs for detecting and quantifying anti-SARS-CoV-2 Spike, RBD, and Nucleoprotein IgG, IgM, and IgA antibodies in Uganda. Front Immunol 2023, 14:1113194. [CrossRef]
- Stavnezer J, Schrader CE: IgH chain class switch recombination: mechanism and regulation. J Immunol 2014, 193(11):5370-5378. [CrossRef]
- Cerqueira-Silva T, Andrews JR, Boaventura VS, Ranzani OT, de Araújo Oliveira V, Paixão ES, Júnior JB, Machado TM, Hitchings MDT, Dorion M et al: Effectiveness of CoronaVac, ChAdOx1 nCoV-19, BNT162b2, and Ad26.COV2.S among individuals with previous SARS-CoV-2 infection in Brazil: a test-negative, case-control study. Lancet Infect Dis 2022, 22(6):791-801. [CrossRef]
- Sadoff J, Le Gars M, Brandenburg B, Cárdenas V, Shukarev G, Vaissiere N, Heerwegh D, Truyers C, de Groot AM, Jongeneelen M et al: Durable antibody responses elicited by 1 dose of Ad26.COV2.S and substantial increase after boosting: 2 randomized clinical trials. Vaccine 2022, 40(32):4403-4411. [CrossRef]
- Jeffery-Smith A, Burton AR, Lens S, Rees-Spear C, Davies J, Patel M, Gopal R, Muir L, Aiano F, Doores KJ et al: SARS-CoV-2-specific memory B cells can persist in the elderly who have lost detectable neutralizing antibodies. J Clin Invest 2022, 132(2). [CrossRef]
- Goh YS, Chavatte JM, Lim Jieling A, Lee B, Hor PX, Amrun SN, Lee CY, Chee RS, Wang B, Lee CY et al: Sensitive detection of total anti-Spike antibodies and isotype switching in asymptomatic and symptomatic individuals with COVID-19. Cell Rep Med 2021, 2(2):100193. [CrossRef]
- Brynjolfsson SF, Sigurgrimsdottir H, Einarsdottir ED, Bjornsdottir GA, Armannsdottir B, Baldvinsdottir GE, Bjarnason A, Gudlaugsson O, Gudmundsson S, Sigurdardottir ST et al: Detailed Multiplex Analysis of SARS-CoV-2 Specific Antibodies in COVID-19 Disease. Front Immunol 2021, 12:695230. [CrossRef]
- Wang Z, Lorenzi JCC, Muecksch F, Finkin S, Viant C, Gaebler C, Cipolla M, Hoffmann H-H, Oliveira TY, Oren DA et al: Enhanced SARS-CoV-2 neutralization by dimeric IgA. Science Translational Medicine 2021, 13(577):eabf1555. [CrossRef]
- Puhach O, Bellon M, Adea K, Bekliz M, Hosszu-Fellous K, Sattonnet P, Hulo N, Kaiser L, Eckerle I, Meyer B: SARS-CoV-2 convalescence and hybrid immunity elicits mucosal immune responses. EBioMedicine 2023, 98:104893. [CrossRef]
- Zhu F, Zhuang C, Chu K, Zhang L, Zhao H, Huang S, Su Y, Lin H, Yang C, Jiang H et al: Safety and immunogenicity of a live-attenuated influenza virus vector-based intranasal SARS-CoV-2 vaccine in adults: randomised, double-blind, placebo-controlled, phase 1 and 2 trials. Lancet Respir Med 2022, 10(8):749-760. [CrossRef]
- Moyo-Gwete T, Richardson SI, Keeton R, Hermanus T, Spencer H, Manamela NP, Ayres F, Makhado Z, Motlou T, Tincho MB et al: Homologous Ad26.COV2.S vaccination results in reduced boosting of humoral responses in hybrid immunity, but elicits antibodies of similar magnitude regardless of prior infection. PLoS Pathog 2023, 19(11):e1011772. [CrossRef]
- Sembera J, Baine C, Ankunda V, Katende JS, Oluka GK, Akoli CH, Kato L, Odoch G, Ejou P, Opio S et al: Sustained spike-specific IgG antibodies following CoronaVac (Sinovac) vaccination in sub-Saharan Africa, but increased breakthrough infections in baseline spike-naive individuals. Frontiers in Immunology 2023, 14. [CrossRef]
- Serwanga J, Baine C, Mugaba S, Ankunda V, Auma BO, Oluka GK, Kato L, Kitabye I, Sembera J, Odoch G et al: Seroprevalence and durability of antibody responses to AstraZeneca vaccination in Ugandans with prior mild or asymptomatic COVID-19: implications for vaccine policy. Front Immunol 2023, 14:1183983. [CrossRef]
- Sterlin D, Mathian A, Miyara M, Mohr A, Anna F, Claer L, Quentric P, Fadlallah J, Devilliers H, Ghillani P et al: IgA dominates the early neutralizing antibody response to SARS-CoV-2. Sci Transl Med 2021, 13(577). [CrossRef]
- Nyagwange J, Kutima B, Mwai K, Karanja HK, Gitonga JN, Mugo D, Sein Y, Wright D, Omuoyo DO, Nyiro JU et al: Serum immunoglobulin G and mucosal immunoglobulin A antibodies from prepandemic samples collected in Kilifi, Kenya, neutralize SARS-CoV-2 in vitro. Int J Infect Dis 2023, 127:11-16. [CrossRef]
- Wang X, Zhang J, Wu Y, Xu Y, Zheng J: SIgA in various pulmonary diseases. Eur J Med Res 2023, 28(1):299. [CrossRef]
- Muyanja E, Ssemaganda A, Ngauv P, Cubas R, Perrin H, Srinivasan D, Canderan G, Lawson B, Kopycinski J, Graham AS et al: Immune activation alters cellular and humoral responses to yellow fever 17D vaccine. J Clin Invest 2014, 124(7):3147-3158. [CrossRef]
- Muir R, Metcalf T, Fourati S, Bartsch Y, Kyosiimire-Lugemwa J, Canderan G, Alter G, Muyanja E, Okech B, Namatovu T et al: Schistosoma mansoni infection alters the host pre-vaccination environment resulting in blunted Hepatitis B vaccination immune responses. PLoS Negl Trop Dis 2023, 17(7):e0011089. [CrossRef]
- Möhlendick B, Čiučiulkaitė I, Elsner C, Anastasiou OE, Trilling M, Wagner B, Zwanziger D, Jöckel KH, Dittmer U, Siffert W: Individuals With Weaker Antibody Responses After Booster Immunization Are Prone to Omicron Breakthrough Infections. Front Immunol 2022, 13:907343. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).