Submitted:
29 December 2023
Posted:
03 January 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
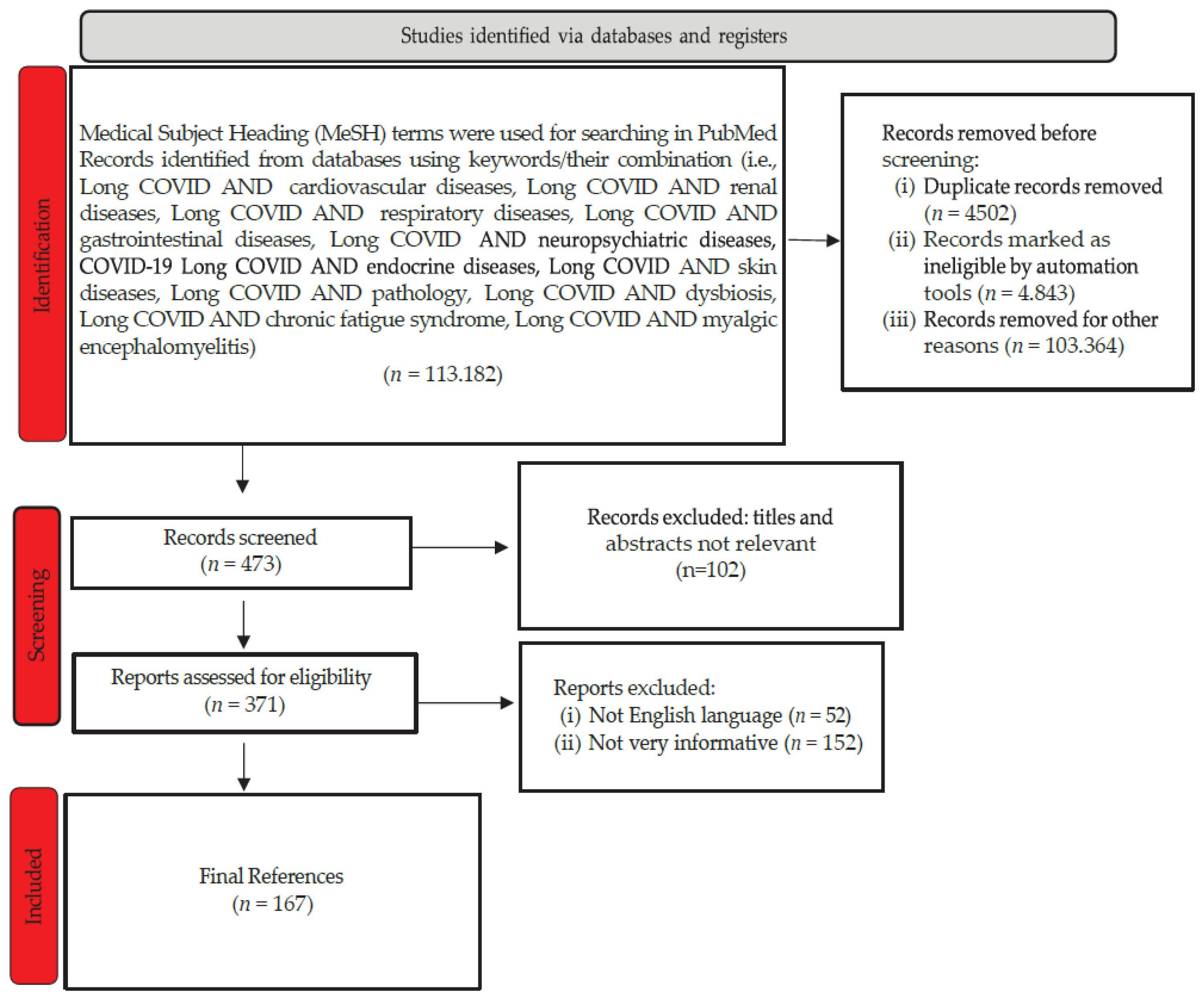
2. Materials and Methods
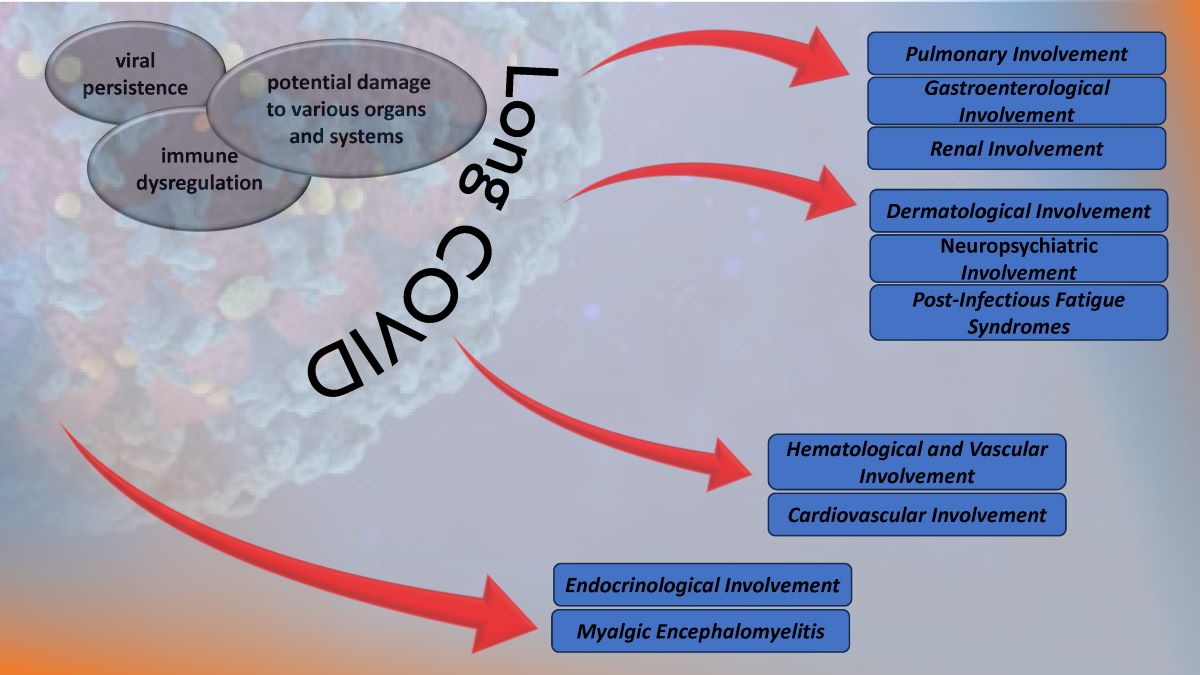
3. The Multisystem Impact of Long COVID
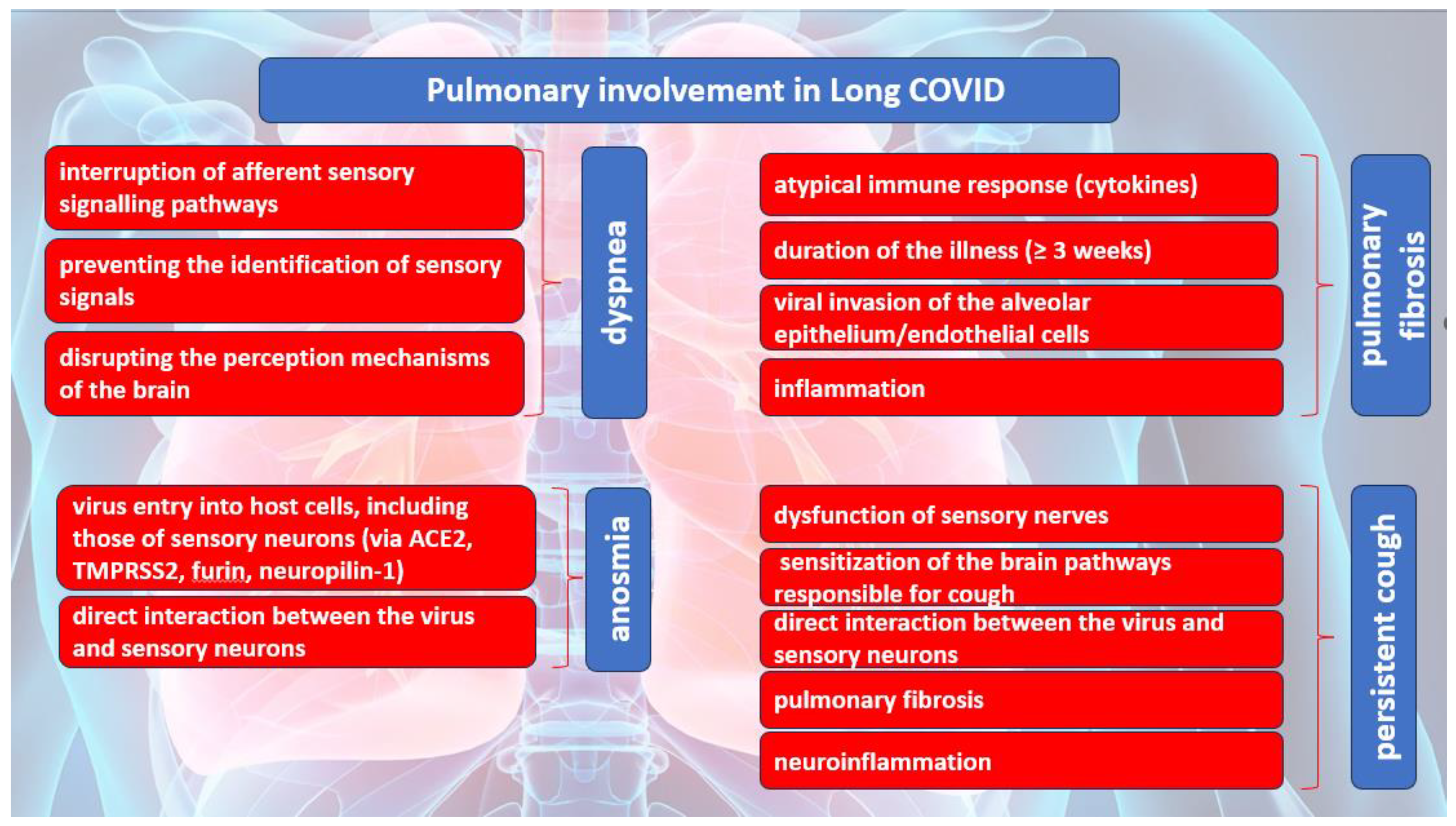
3.1. Pulmonary Involvement in Long COVID
3.2. Endocrinological Involvement in Long COVID
3.2.1. Pituitary Gland
3.2.2. Thyroid Gland
3.2.3. Pancreas
3.2.4. Adrenal Glands
3.2.5. Gonads
3.3. Hematological and Vascular Mechanism of Long COVID
3.3.1. Cell counts Alterations
3.3.2. SARS-CoV-2 Persistence in Extracellular Vesicles
3.3.3. Persistent Vascular Inflammatory State
3.3.4. Endothelial Damage and Dysfunction
3.3.5. Long Covid related Hypercoagulability
3.3.6. Microangiopathy
3.3.7. Gastroenterological Involvement in Long COVID
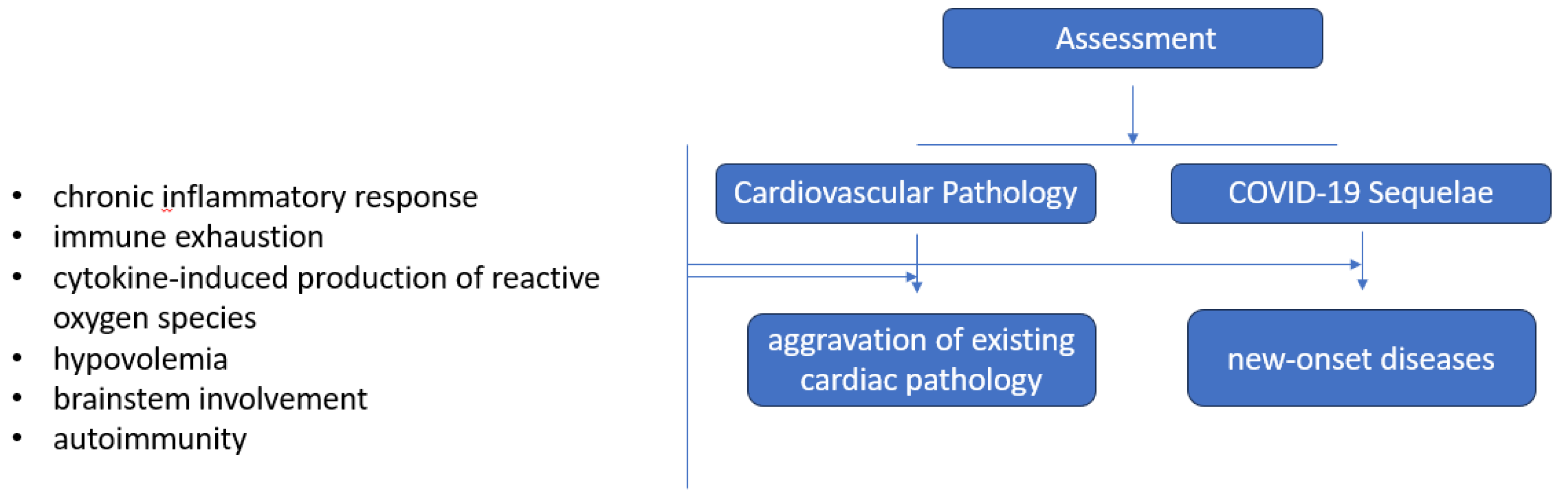
3.4. Cardiovascular Involvement in Long COVID
3.5. Renal Involvement in Long COVID
3.6. Dermatological Involvement in Long COVID
3.7. Neuropsychiatric Mechanism of Long COVID
3.7.1. SARS-CoV-2 Neurotropism
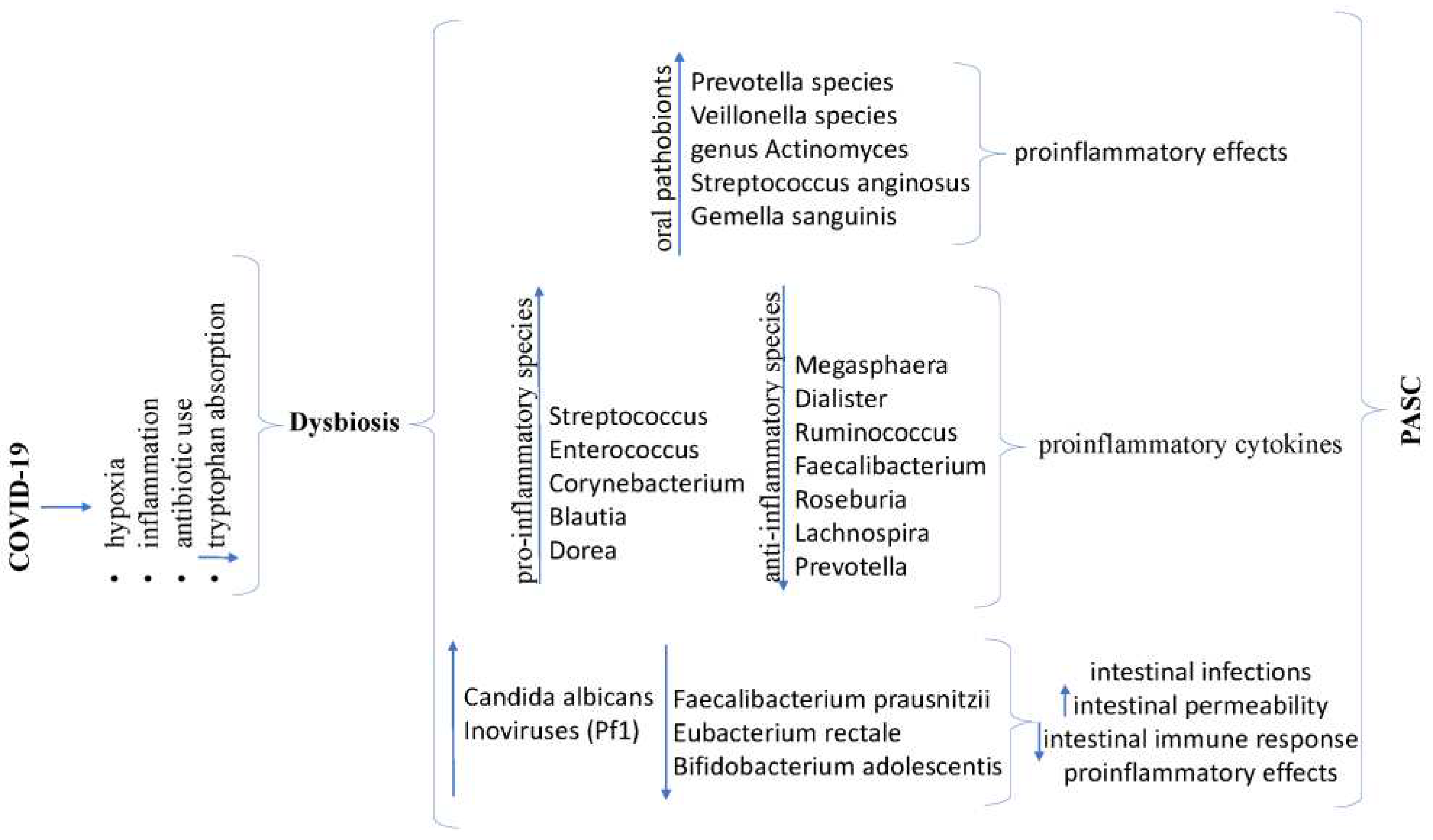
3.7.2. Brain-Gut Axis - Dysbiosis
3.7.3. Long COVID Neuroinflammation
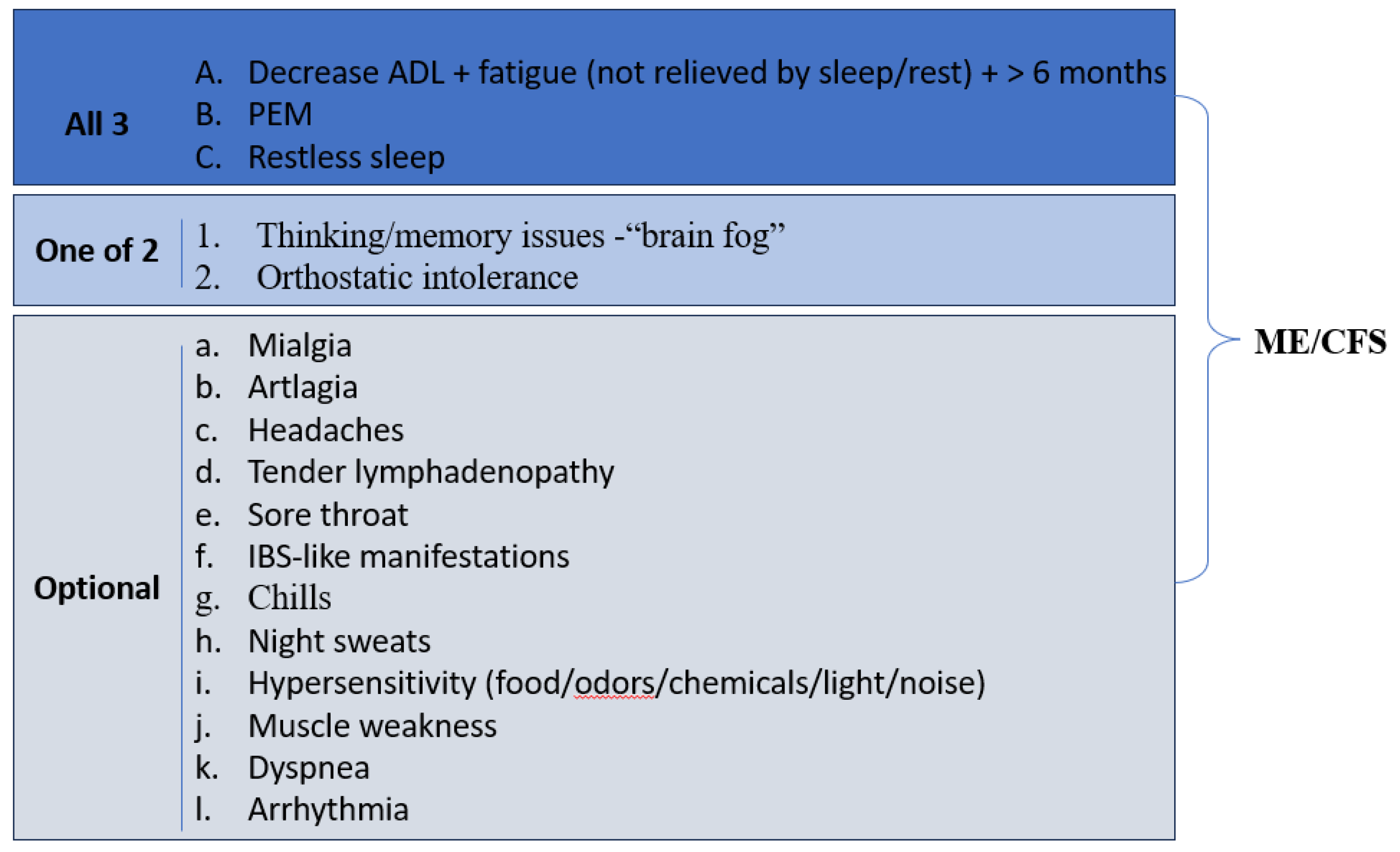
3.8. Myalgic Encephalomyelitis and Chronic Fatigue Syndrome in Long COVID-19
4. Conclusions
5. Patents
Author Contributions
Funding
Conflicts of Interest
References
- Carvalho-Schneider, C.; Laurent, E.; Lemaignen, A.; Beaufils, E.; Bourbao-Tournois, C.; Laribi, S.; Flament, T.; Ferreira-Maldent, N.; Bruyère, F.; Stefic, K.; Gaudy-Graffin, C.; Grammatico-Guillon, L.; Bernard, L. Follow-up of Adults with Non-Critical COVID-19 Two Months after Symptoms’ Onset. Clinical Microbiology and Infection 2020, 0 (0). [CrossRef]
- Sigfrid, L.; Drake, T. M.; Pauley, E.; Jesudason, E. C.; Olliaro, P.; Lim, W. S.; Gillesen, A.; Berry, C.; Lowe, D. J.; McPeake, J.; Lone, N.; Munblit, D.; Cevik, M.; Casey, A.; Bannister, P.; Russell, C. D.; Goodwin, L.; Ho, A.; Turtle, L.; O’Hara, M. E. Long Covid in Adults Discharged from UK Hospitals after Covid-19: A Prospective, Multicentre Cohort Study Using the ISARIC WHO Clinical Characterisation Protocol. The Lancet Regional Health - Europe 2021, 100186. [CrossRef]
- Desgranges, F.; Tadini, E.; Munting, A.; Regina, J.; Filippidis, P.; Viala, B.; Karachalias, E.; Suttels, V.; Haefliger, D.; Kampouri, E.; Van Singer, M.; Tschopp, J.; Rochat Stettler, L.; Schaad, S.; Brahier, T.; Hugli, O.; Mueller, Y.; Gouveia, A.; Opota, O.; Carron, P.-N. Post-COVID-19 Syndrome in Outpatients: A Cohort Study. Journal of General Internal Medicine 2022, 37 (8), 1943–1952. [CrossRef]
- Subramanian, A.; Nirantharakumar, K.; Hughes, S.; Myles, P.; Williams, T.; Gokhale, K. M.; Taverner, T.; Chandan, J. S.; Brown, K.; Simms-Williams, N.; Shah, A. D.; Singh, M.; Kidy, F.; Okoth, K.; Hotham, R.; Bashir, N.; Cockburn, N.; Lee, S. I.; Turner, G. M.; Gkoutos, G. V. Symptoms and Risk Factors for Long COVID in Non-Hospitalized Adults. Nature Medicine 2022, 28 (8), 1706–1714. [CrossRef]
- Pelà, G.; Goldoni, M.; Solinas, E.; Cavalli, C.; Tagliaferri, S.; Ranzieri, S.; Frizzelli, A.; Marchi, L.; Mori, P. A.; Majori, M.; Aiello, M.; Corradi, M.; Chetta, A. Sex-Related Differences in Long-COVID-19 Syndrome. Journal of Women’s Health (2002) 2022. [CrossRef]
- Gebhard, C. E.; Sütsch, C.; Bengs, S.; Todorov, A.; Deforth, M.; Buehler, K. P.; Meisel, A.; Schuepbach, R. A.; Zinkernagel, A. S.; Brugger, S. D.; Acevedo, C.; Patriki, D.; Wiggli, B.; Gysi, B.; Beer, J. H.; Friedl, A.; Twerenbold, R.; Kuster, G. M.; Pargger, H.; Tschudin-Sutter, S. Understanding the Impact of Sociocultural Gender on Post-Acute Sequelae of COVID-19: A Bayesian Approach. 2021. [CrossRef]
- Tleyjeh, I. M.; Saddik, B.; Ramakrishnan, R. K.; AlSwaidan, N.; AlAnazi, A.; Alhazmi, D.; Aloufi, A.; AlSumait, F.; Berbari, E. F.; Halwani, R. Long Term Predictors of Breathlessness, Exercise Intolerance, Chronic Fatigue and Well-Being in Hospitalized Patients with COVID-19: A Cohort Study with 4 Months Median Follow-Up. Journal of Infection and Public Health 2022, 15 (1), 21–28. [CrossRef]
- García-Abellán, J.; Padilla, S.; Fernández-González, M.; José Luis García; García, J. A.; María Andreo; Bibiana, S.; Galiana, A.; Félix Gutiérrez; Mar Masiá. Antibody Response to SARS-CoV-2 Is Associated with Long-Term Clinical Outcome in Patients with COVID-19: A Longitudinal Study. 2021, 41 (7), 1490–1501. [CrossRef]
- Asadi-Pooya, A. A.; Akbari, A.; Emami, A.; Lotfi, M.; Rostamihosseinkhani, M.; Nemati, H.; Barzegar, Z.; Kabiri, M.; Zeraatpisheh, Z.; Farjoud-Kouhanjani, M.; Jafari, A.; Sasannia, F.; Ashrafi, S.; Nazeri, M.; Nasiri, S.; Shahisavandi, M. Risk Factors Associated with Long COVID Syndrome: A Retrospective Study. Iranian Journal of Medical Sciences 2021, 46 (6), 428–436. [CrossRef]
- Munblit, D.; Bobkova, P.; Spiridonova, E.; Shikhaleva, A.; Gamirova, A.; Blyuss, O.; Nekliudov, N.; Bugaeva, P.; Andreeva, M.; DunnGalvin, A.; Comberiati, P.; Apfelbacher, C.; Genuneit, J.; Avdeev, S.; Kapustina, V.; Guekht, A.; Fomin, V.; Svistunov, A. A.; Timashev, P.; Subbot, V. S. Incidence and Risk Factors for Persistent Symptoms in Adults Previously Hospitalized for COVID-19. Clinical & Experimental Allergy 2021, 51 (9), 1107–1120. [CrossRef]
- Fernández-de-las-Peñas, C.; Martín-Guerrero, J. D.; Pellicer-Valero, Ó. J.; Navarro-Pardo, E.; Gómez-Mayordomo, V.; Cuadrado, M. L.; Arias-Navalón, J. A.; Cigarán-Méndez, M.; Hernández-Barrera, V.; Arendt-Nielsen, L. Female Sex Is a Risk Factor Associated with Long-Term Post-COVID Related-Symptoms but Not with COVID-19 Symptoms: The LONG-COVID-EXP-CM Multicenter Study. Journal of Clinical Medicine 2022, 11 (2), 413. [CrossRef]
- Bai, F.; Tomasoni, D.; Falcinella, C.; Barbanotti, D.; Castoldi, R.; Mulè, G.; Augello, M.; Mondatore, D.; Allegrini, M.; Cona, A.; Tesoro, D.; Tagliaferri, G.; Viganò, O.; Suardi, E.; Tincati, C.; Beringheli, T.; Varisco, B.; Battistini, C. L.; Piscopo, K.; Vegni, E. Female Gender Is Associated with “Long COVID” Syndrome: A Prospective Cohort Study. Clinical Microbiology and Infection 2021, 28 (4). [CrossRef]
- Chudzik, M.; Lewek, J.; Kapusta, J.; Banach, M.; Jankowski, P.; Bielecka-Dabrowa, A. Predictors of Long COVID in Patients without Comorbidities: Data from the Polish Long-COVID Cardiovascular (PoLoCOV-CVD) Study. Journal of Clinical Medicine 2022, 11 (17), 4980. [CrossRef]
- Chudzik, M.; Babicki, M.; Kapusta, J.; Kałuzińska-Kołat, Ż.; Kołat, D.; Jankowski, P.; Mastalerz-Migas, A. Long-COVID Clinical Features and Risk Factors: A Retrospective Analysis of Patients from the STOP-COVID Registry of the PoLoCOV Study. Viruses 2022, 14 (8), 1755. [CrossRef]
- Notarte, K. I.; de Oliveira, M. H. S.; Peligro, P. J.; Velasco, J. V.; Macaranas, I.; Ver, A. T.; Pangilinan, F. C.; Pastrana, A.; Goldrich, N.; Kavteladze, D.; Gellaco, M. M. L.; Liu, J.; Lippi, G.; Henry, B. M.; Fernández-de-las-Peñas, C. Age, Sex and Previous Comorbidities as Risk Factors Not Associated with SARS-CoV-2 Infection for Long COVID-19: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine 2022, 11 (24), 7314. [CrossRef]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; Guan, L.; Wei, Y.; Li, H.; Wu, X.; Xu, J.; Tu, S.; Zhang, Y.; Chen, H.; Cao, B. Clinical Course and Risk Factors for Mortality of Adult Inpatients with COVID-19 in Wuhan, China: A Retrospective Cohort Study. The Lancet 2020, 395 (10229), 1054–1062. [CrossRef]
- Zhang, H.; Penninger, J. M.; Li, Y.; Zhong, N.; Slutsky, A. S. Angiotensin-Converting Enzyme 2 (ACE2) as a SARS-CoV-2 Receptor: Molecular Mechanisms and Potential Therapeutic Target. Intensive Care Medicine 2020, 46 (4), 586–590. [CrossRef]
- Thorne, L. G.; Reuschl, A.; Zuliani-Alvarez, L.; Whelan, M. V. X.; Turner, J.; Noursadeghi, M.; Jolly, C.; Towers, G. J. SARS-CoV-2 Sensing by RIG-I and MDA5 Links Epithelial Infection to Macrophage Inflammation. The EMBO Journal 2021, 40 (15). [CrossRef]
- Zhao, F.; Ma, Q.; Yue, Q.; Chen, H. SARS-CoV-2 Infection and Lung Regeneration. Clinical Microbiology Reviews 2022. [CrossRef]
- Zhang, H.; Rostami, M. R.; Leopold, P. L.; Mezey, J. G.; O’Beirne, S. L.; Strulovici-Barel, Y.; Crystal, R. G. Expression of the SARS-CoV-2 ACE2 Receptor in the Human Airway Epithelium. American Journal of Respiratory and Critical Care Medicine 2020. [CrossRef]
- Duclos, G. E.; Teixeira, V. H.; Autissier, P.; Gesthalter, Y. B.; Reinders-Luinge, M. A.; Terrano, R.; Dumas, Y. M.; Liu, G.; Mazzilli, S. A.; Brandsma, C.-A.; van den Berge, M.; Janes, S. M.; Timens, W.; Lenburg, M. E.; Spira, A.; Campbell, J. D.; Beane, J. Characterizing Smoking-Induced Transcriptional Heterogeneity in the Human Bronchial Epithelium at Single-Cell Resolution. Science Advances 2019, 5 (12). [CrossRef]
- Hou, Y. J.; Okuda, K.; Edwards, C. E.; Martinez, D. R.; Asakura, T.; Dinnon, K. H.; Kato, T.; Lee, R. E.; Yount, B. L.; Mascenik, T. M.; Chen, G.; Olivier, K. N.; Ghio, A.; Tse, L. V.; Leist, S. R.; Gralinski, L. E.; Schäfer, A.; Dang, H.; Gilmore, R.; Nakano, S. SARS-CoV-2 Reverse Genetics Reveals a Variable Infection Gradient in the Respiratory Tract. Cell 2020, 182 (2). [CrossRef]
- Zou, X.; Chen, K.; Zou, J.; Han, P.; Hao, J.; Han, Z. Single-Cell RNA-Seq Data Analysis on the Receptor ACE2 Expression Reveals the Potential Risk of Different Human Organs Vulnerable to 2019-NCoV Infection. Frontiers of Medicine 2020, 14 (2), 185–192. [CrossRef]
- Ziegler, C. G. K.; Allon, S. J.; Nyquist, S. K.; Mbano, I. M.; Miao, V. N.; Tzouanas, C. N.; Cao, Y.; Yousif, A. S.; Bals, J.; Hauser, B. M.; Feldman, J.; Muus, C.; Wadsworth, M. H.; Kazer, S. W.; Hughes, T. K.; Doran, B.; Gatter, G. J.; Vukovic, M.; Taliaferro, F.; Mead, B. E. SARS-CoV-2 Receptor ACE2 Is an Interferon-Stimulated Gene in Human Airway Epithelial Cells and Is Detected in Specific Cell Subsets across Tissues. Cell 2020, 181 (5). [CrossRef]
- Lukassen, S.; Chua, R. L.; Trefzer, T.; Kahn, N. C.; Schneider, M. A.; Muley, T.; Winter, H.; Meister, M.; Veith, C.; Boots, A. W.; Hennig, B. P.; Kreuter, M.; Conrad, C.; Eils, R. SARS -CoV-2 Receptor ACE 2 and TMPRSS 2 Are Primarily Expressed in Bronchial Transient Secretory Cells. The EMBO Journal 2020, 39 (10). [CrossRef]
- Hentsch, L.; Cocetta, S.; Allali, G.; Santana, I.; Eason, R.; Adam, E.; Janssens, J.-P. Breathlessness and COVID-19: A Call for Research. Respiration 2021, 1–11. [CrossRef]
- Coccia, C. B. I.; Palkowski, G. H.; Schweitzer, B.; Motsohi, T.; Ntusi, N. Dyspnoea: Pathophysiology and a Clinical Approach. South African Medical Journal 2016, 106 (1), 32. [CrossRef]
- Liu, J.; Zheng, X.; Tong, Q.; Li, W.; Wang, B.; Sutter, K.; Trilling, M.; Lu, M.; Dittmer, U.; Yang, D. Overlapping and Discrete Aspects of the Pathology and Pathogenesis of the Emerging Human Pathogenic Coronaviruses SARS-CoV, MERS-CoV, and 2019-NCoV. Journal of Medical Virology 2020, 92 (5), 491–494. [CrossRef]
- Burnham, E. L.; Janssen, W. J.; Riches, D. W. H.; Moss, M.; Downey, G. P. The Fibroproliferative Response in Acute Respiratory Distress Syndrome: Mechanisms and Clinical Significance. European Respiratory Journal 2013, 43 (1), 276–285. [CrossRef]
- Wu, C.; Chen, X.; Cai, Y.; Xia, J.; Zhou, X.; Xu, S.; Huang, H.; Zhang, L.; Zhou, X.; Du, C.; Zhang, Y.; Song, J.; Wang, S.; Chao, Y.; Yang, Z.; Xu, J.; Zhou, X.; Chen, D.; Xiong, W.; Xu, L. Risk Factors Associated with Acute Respiratory Distress Syndrome and Death in Patients with Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Internal Medicine 2020, 180 (7). [CrossRef]
- Liu, X.; Zhou, H.; Zhou, Y.; Wu, X.; Zhao, Y.; Lu, Y.; Tan, W.; Yuan, M.; Ding, X.; Zou, J.; Li, R.; Liu, H.; Ewing, R. M.; Hu, Y.; Nie, H.; Wang, Y. Risk Factors Associated with Disease Severity and Length of Hospital Stay in COVID-19 Patients. Journal of Infection 2020, 81 (1), e95–e97. [CrossRef]
- Rai, D. K.; Sharma, P.; Kumar, R. Post Covid 19 Pulmonary Fibrosis- Is It Reversible? The Indian Journal of Tuberculosis 2020. [CrossRef]
- George, P. M.; Wells, A. U.; Jenkins, R. G. Pulmonary Fibrosis and COVID-19: The Potential Role for Antifibrotic Therapy. The Lancet Respiratory Medicine 2020, 8 (8). [CrossRef]
- Shang, J.; Wan, Y.; Luo, C.; Ye, G.; Geng, Q.; Auerbach, A.; Li, F. Cell Entry Mechanisms of SARS-CoV-2. Proceedings of the National Academy of Sciences 2020, 117 (21). [CrossRef]
- Menni, C.; Valdes, A. M.; Freidin, M. B.; Sudre, C. H.; Nguyen, L. H.; Drew, D. A.; Ganesh, S.; Varsavsky, T.; Cardoso, M. J.; El-Sayed Moustafa, J. S.; Visconti, A.; Hysi, P.; Bowyer, R. C. E.; Mangino, M.; Falchi, M.; Wolf, J.; Ourselin, S.; Chan, A. T.; Steves, C. J.; Spector, T. D. Real-Time Tracking of Self-Reported Symptoms to Predict Potential COVID-19. Nature Medicine 2020, 1–4. [CrossRef]
- Mazzone, S. B.; Tian, L.; Moe, A. A. K.; Trewella, M. W.; Ritchie, M. E.; McGovern, A. E. Transcriptional Profiling of Individual Airway Projecting Vagal Sensory Neurons. Molecular Neurobiology 2020, 57 (2), 949–963. [CrossRef]
- Davies, J.; Randeva, H.; Chatha, K.; Hall, M.; Spandidos, D.; Karteris, E.; Kyrou, I. Neuropilin-1 as a New Potential SARS-CoV-2 Infection Mediator Implicated in the Neurologic Features and Central Nervous System Involvement of COVID-19. Molecular Medicine Reports 2020. [CrossRef]
- Brann, D. H.; Tsukahara, T.; Weinreb, C.; Lipovsek, M.; Berge, K. V. den; Gong, B.; Chance, R.; Macaulay, I. C.; Chou, H.-J.; Fletcher, R. B.; Das, D.; Street, K.; Bezieux, H. R. de; Choi, Y.-G.; Risso, D.; Dudoit, S.; Purdom, E.; Mill, J.; Hachem, R. A.; Matsunami, H. Non-Neuronal Expression of SARS-CoV-2 Entry Genes in the Olfactory System Suggests Mechanisms Underlying COVID-19-Associated Anosmia. Science Advances 2020, 6 (31), eabc5801. [CrossRef]
- Chen, M.; Shen, W.; Rowan, N. R.; Kulaga, H.; Hillel, A.; Ramanathan, M.; Lane, A. P. Elevated ACE-2 Expression in the Olfactory Neuroepithelium: Implications for Anosmia and Upper Respiratory SARS-CoV-2 Entry and Replication. European Respiratory Journal 2020, 56 (3), 2001948. [CrossRef]
- Shiers, S.; Ray, P. R.; Wangzhou, A.; Sankaranarayanan, I.; Tatsui, C. E.; Rhines, L. D.; Li, Y.; Uhelski, M. L.; Dougherty, P. M.; Price, T. J. ACE2 and SCARF Expression in Human Dorsal Root Ganglion Nociceptors: Implications for SARS-CoV-2 Virus Neurological Effects. Pain 2020, 161 (11), 2494–2501. [CrossRef]
- Rhea, E. M.; Logsdon, A. F.; Hansen, K. M.; Williams, L. M.; Reed, M. J.; Baumann, K. K.; Holden, S. J.; Raber, J.; Banks, W. A.; Erickson, M. A. The S1 Protein of SARS-CoV-2 Crosses the Blood–Brain Barrier in Mice. Nature Neuroscience 2020. [CrossRef]
- Ojha, V.; Mani, A.; Pandey, N. N.; Sharma, S.; Kumar, S. CT in Coronavirus Disease 2019 (COVID-19): A Systematic Review of Chest CT Findings in 4410 Adult Patients. European Radiology 2020. [CrossRef]
- Jones, R. M.; Hilldrup, S.; Hope-Gill, B. D.; Eccles, R.; Harrison, N. K. Mechanical Induction of Cough in Idiopathic Pulmonary Fibrosis. Cough 2011, 7 (1), 2. [CrossRef]
- Ryan, N. M.; Birring, S. S.; Gibson, P. G. Gabapentin for Refractory Chronic Cough: A Randomised, Double-Blind, Placebo-Controlled Trial. The Lancet 2012, 380 (9853), 1583–1589. [CrossRef]
- Vertigan, A. E.; Kapela, S. L.; Ryan, N. M.; Birring, S. S.; McElduff, P.; Gibson, P. G. Pregabalin and Speech Pathology Combination Therapy for Refractory Chronic Cough. Chest 2016, 149 (3), 639–648. [CrossRef]
- Walls, A. C.; Park, Y.-J.; Tortorici, M. A.; Wall, A.; McGuire, A. T.; Veesler, D. Structure, Function, and Antigenicity of the Sars-Cov-2 Spike Glycoprotein. Cell 2020, 181 (2), 281–292. [CrossRef]
- Gui, M.; Song, W.; Zhou, H.; Xu, J.; Chen, S.; Xiang, Y.; Wang, X. Cryo-Electron Microscopy Structures of the SARS-CoV Spike Glycoprotein Reveal a Prerequisite Conformational State for Receptor Binding. Cell Research 2016, 27 (1), 119–129. [CrossRef]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Krüger, N.; Herrler, T.; Erichsen, S.; Schiergens, T. S.; Herrler, G.; Wu, N.-H.; Nitsche, A.; Müller, M. A.; Drosten, C.; Pöhlmann, S. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020, 181 (2), 271–280. [CrossRef]
- Lazartigues, E.; Qadir, M. M. F.; Mauvais-Jarvis, F. Endocrine Significance of SARS-CoV-2′S Reliance on ACE2. Endocrinology 2020, 161 (9). [CrossRef]
- Chan, J. L.; Gregory, K. D.; Smithson, S. S.; Naqvi, M.; Mamelak, A. N. Pituitary Apoplexy Associated with Acute COVID-19 Infection and Pregnancy. Pituitary 2020, 23 (6), 716–720. [CrossRef]
- Ghosh, R.; Roy, D.; Roy, D.; Mandal, A.; Dutta, A.; Naga, D.; Benito-León, J. A Rare Case of SARS-CoV-2 Infection Associated with Pituitary Apoplexy without Comorbidities. Journal of the Endocrine Society 2021, 5 (3). [CrossRef]
- Bordes, S. J.; Phang-Lyn, S.; Najera, E.; Borghei-Razavi, H.; Adada, B. Pituitary Apoplexy Attributed to COVID-19 Infection in the Absence of an Underlying Macroadenoma or Other Identifiable Cause. Cureus 2021, 13 (2). [CrossRef]
- Rotondi, M.; Coperchini, F.; Ricci, G.; Denegri, M.; Croce, L.; Ngnitejeu, S. T.; Villani, L.; Magri, F.; Latrofa, F.; Chiovato, L. Detection of SARS-COV-2 Receptor ACE-2 MRNA in Thyroid Cells: A Clue for COVID-19-Related Subacute Thyroiditis. Journal of Endocrinological Investigation 2020, 1–6. [CrossRef]
- Lazartigues, E.; Qadir, M. M. F.; Mauvais-Jarvis, F. Endocrine Significance of SARS-CoV-2′S Reliance on ACE2. Endocrinology 2020, 161 (9). [CrossRef]
- Croce, L.; Gangemi, D.; Ancona, G.; Liboà, F.; Bendotti, G.; Minelli, L.; Chiovato, L. The Cytokine Storm and Thyroid Hormone Changes in COVID-19. Journal of Endocrinological Investigation 2021, 44 (5), 891–904. [CrossRef]
- Kumari, K.; Chainy, G. B. N.; Subudhi, U. Prospective Role of Thyroid Disorders in Monitoring COVID-19 Pandemic. Heliyon 2020, 6 (12), e05712. [CrossRef]
- Popa, A.; Chereji, A.-I.; Dodu, M. A.; Chereji, I.; Fitero, A.; Daina, C. M.; Daina, L. G.; Badau, D.; Neculoiu, D. C.; Domnariu, C. The Impact of Changes Regarding Working Circumstances during COVID-19 Pandemic upon Patients Evaluated for Thyroid Dysfunction. International Journal of Environmental Research and Public Health 2022, 19 (16), 9856. [CrossRef]
- Fatourechi, V.; Aniszewski, J. P.; Fatourechi, G. Z. E.; Atkinson, E. J.; Jacobsen, S. J. Clinical Features and Outcome of Subacute Thyroiditis in an Incidence Cohort: Olmsted County, Minnesota, Study. The Journal of Clinical Endocrinology & Metabolism 2003, 88 (5), 2100–2105. [CrossRef]
- Correia de Sá, T.; Soares, C.; Rocha, M. Acute Pancreatitis and COVID-19: A Literature Review. World Journal of Gastrointestinal Surgery 2021, 13 (6), 574–584. [CrossRef]
- Amidifar, S.; Elahi, R.; Siahmansouri, A.; Maleki, A. J.; Moradi, A. Endocrine and Metabolic Complications of COVID-19: Lessons Learned and Future Prospects. Journal of Molecular Endocrinology 2022. [CrossRef]
- Rubino, F.; Amiel, S. A.; Zimmet, P.; Alberti, G.; Bornstein, S.; Eckel, R. H.; Mingrone, G.; Boehm, B.; Cooper, M. E.; Chai, Z.; Del Prato, S.; Ji, L.; Hopkins, D.; Herman, W. H.; Khunti, K.; Mbanya, J.-C.; Renard, E. New-Onset Diabetes in Covid-19. New England Journal of Medicine 2020. [CrossRef]
- Landstra, C. P.; de Koning, E. J. P. COVID-19 and Diabetes: Understanding the Interrelationship and Risks for a Severe Course. Frontiers in Endocrinology 2021, 12. [CrossRef]
- Rubino, F.; Amiel, S. A.; Zimmet, P.; Alberti, G.; Bornstein, S.; Eckel, R. H.; Mingrone, G.; Boehm, B.; Cooper, M. E.; Chai, Z.; Del Prato, S.; Ji, L.; Hopkins, D.; Herman, W. H.; Khunti, K.; Mbanya, J.-C.; Renard, E. New-Onset Diabetes in Covid-19. New England Journal of Medicine 2020. [CrossRef]
- Carlsson, P. O. The Renin-Angiotensin System in the Endocrine Pancreas. JOP: Journal of the pancreas 2001, 2 (1), 26–32.
- Goossens, G. H. The Renin-Angiotensin System in the Pathophysiology of Type 2 Diabetes. Obesity Facts 2012, 5 (4), 611–624. [CrossRef]
- Hayden, M. R.; Karuparthi, P. R.; Habibi, J.; Wasekar, C.; Lastra, G.; Manrique, C.; Stas, S.; Sowers, J. R. Ultrastructural Islet Study of Early Fibrosis in the Ren2 Rat Model of Hypertension. Emerging Role of the Islet Pancreatic Pericyte-Stellate Cell. JOP: Journal of the pancreas 2007, 8 (6), 725–738.
- Wang, F.; Wang, H.; Fan, J.; Zhang, Y.; Wang, H.; Zhao, Q. Pancreatic Injury Patterns in Patients with COVID-19 Pneumonia. Gastroenterology 2020. [CrossRef]
- Aloysius, M. M.; Thatti, A.; Gupta, A.; Sharma, N.; Bansal, P.; Goyal, H. COVID-19 Presenting as Acute Pancreatitis. Pancreatology 2020. [CrossRef]
- Lopez-Leon, S.; Wegman-Ostrosky, T.; Perelman, C.; Sepulveda, R.; Rebolledo, P. A.; Cuapio, A.; Villapol, S. More than 50 Long-Term Effects of COVID-19: A Systematic Review and Meta-Analysis. medRxiv: The Preprint Server for Health Sciences 2021. [CrossRef]
- Kamin, H. S.; Kertes, D. A. Cortisol and DHEA in Development and Psychopathology. Hormones and Behavior 2017, 89, 69–85. [CrossRef]
- Wheatland, R. Molecular Mimicry of ACTH in SARS – Implications for Corticosteroid Treatment and Prophylaxis. Medical Hypotheses 2004, 63 (5), 855–862. [CrossRef]
- Chu, K. Y.; Nackeeran, S.; Horodyski, L.; Masterson, T. A.; Ramasamy, R. COVID-19 Infection Is Associated with New Onset Erectile Dysfunction: Insights from a National Registry. Sexual Medicine 2022, 10 (1), 100478. [CrossRef]
- Barragan, M.; Guillén, J. J.; Martin-Palomino, N.; Rodriguez, A.; Vassena, R. Undetectable Viral RNA in Oocytes from SARS-CoV-2 Positive Women. Human Reproduction 2020, 36 (2), 390–394. [CrossRef]
- Li, K.; Chen, G.; Hou, H.; Liao, Q.; Chen, J.; Bai, H.; Lee, S.; Wang, C.; Li, H.; Cheng, L.; Ai, J. Analysis of Sex Hormones and Menstruation in COVID-19 Women of Child-Bearing Age. Reproductive BioMedicine Online 2021, 42 (1), 260–267. [CrossRef]
- Proal, A. D.; VanElzakker, M. B. Long COVID or Post-Acute Sequelae of COVID-19 (PASC): An Overview of Biological Factors That May Contribute to Persistent Symptoms. Frontiers in Microbiology 2021, 12. [CrossRef]
- Report: What Does COVID-19 Recovery Actually Look Like? Patient Led Research. https://patientresearchcovid19.com/research/report-1/.
- Erdinc, B.; Sahni, S.; Gotlieb, V. Hematological Manifestations and Complications of COVID-19. Advances in Clinical and Experimental Medicine 2021, 30 (1), 101–107. [CrossRef]
- Sava, C. N.; Bodog, T.-M.; Niulas, L. R.; Iuhas, A. R.; Marinau, C. P.; Negrut, N.; Balmos, A. B.; Pasca, B.; Roman, N. A.; Delia Nistor-Cseppento, C. Biomarker Changes in Pediatric Patients with COVID-19: A Retrospective Study from a Single Center Database. In Vivo (Athens, Greece) 2022, 36 (6), 2813–2822. [CrossRef]
- Amer Harky; Avesta Ala’Aldeen; None SundasButt; Duric, B.; Roy, S.; Zeinah, M. COVID-19 and Multiorgan Response: The Long-Term Impact. 2023, 101756–101756. [CrossRef]
- Barrett, T.J.; Bilaloglu, S.; Cornwell, M.; Burgess, H.M.; Virginio, V.W.; Drenkova, K.; Ibrahim, H.; Yuriditsky, E.; Aphinyanaphongs, Y.; Lifshitz, M.; et al. Platelets contribute to disease severity in COVID-19. J Thromb Haemost 2021, 19, 3139-3153. [CrossRef]
- Targosz-Korecka, M.; Kubisiak, A.; Kloska, D.; Kopacz, A.; Grochot-Przeczek, A.; Szymonski, M. Endothelial Glycocalyx Shields the Interaction of SARS-CoV-2 Spike Protein with ACE2 Receptors. Scientific Reports 2021, 11 (1), 12157. [CrossRef]
- Prasad, M.; Leon, M.; Lerman, L. O.; Lerman, A. Viral Endothelial Dysfunction: A Unifying Mechanism for COVID-19. Mayo Clinic Proceedings 2021. [CrossRef]
- Evangelos Oikonomou; Nektarios Souvaliotis; Stamatios Lampsas; Gerasimos Siasos; Garyphallia Poulakou; Panagiotis Theofilis; Papaioannou, T. G.; Anna-Bettina Haidich; Tsaousi, G.; Ntousopoulos Vasileios; Sakka Vissaria; Charalambous, G.; Rapti, V.; Raftopoulou, S.; Syrigos, K. N.; Costas Tsioufis; Dimitrios Tousoulis; Manolis Vavuranakis. Endothelial Dysfunction in Acute and Long Standing COVID−19: A Prospective Cohort Study. 2022, 144, 106975–106975. [CrossRef]
- Chioh, F. W.; Fong, S.-W.; Young, B. E.; Wu, K.-X.; Siau, A.; Krishnan, S.; Chan, Y.-H.; Carissimo, G.; Teo, L. L.; Gao, F.; Tan, R. S.; Zhong, L.; Koh, A. S.; Tan, S.-Y.; Tambyah, P. A.; Renia, L.; Ng, L. F.; Lye, D. C.; Cheung, C. Convalescent COVID-19 Patients Are Susceptible to Endothelial Dysfunction due to Persistent Immune Activation. eLife 2021, 10. [CrossRef]
- Fogarty, H.; Townsend, L.; Morrin, H.; Ahmad, A.; Comerford, C.; Karampini, E.; Englert, H.; Byrne, M.; Bergin, C.; O’Sullivan, J. M.; Martin-Loeches, I.; Nadarajan, P.; Bannan, C.; Mallon, P. W.; Curley, G. F.; Preston, R. J. S.; Rehill, A. M.; McGonagle, D.; Ni Cheallaigh, C.; Baker, R. I. Persistent Endotheliopathy in the Pathogenesis of Long COVID Syndrome. Journal of Thrombosis and Haemostasis 2021, 19 (10), 2546–2553. [CrossRef]
- Hernán Mejía-Rentería; Travieso, A.; A Sagir; Martínez-Gómez, E.; Carrascosa-Granada, A.; Toya, T.; Núñez-Gil, I. J.; Estrada, V.; Lerman, A.; Escaned, J. In-Vivo Evidence of Systemic Endothelial Vascular Dysfunction in COVID-19. 2021, 345, 153–155. [CrossRef]
- Manolis, A. S.; Manolis, T. A.; Manolis, A. A.; Papatheou, D.; Melita, H. COVID-19 Infection: Viral Macro- and Micro-Vascular Coagulopathy and Thromboembolism/Prophylactic and Therapeutic Management. Journal of Cardiovascular Pharmacology and Therapeutics 2020, 26 (1), 12–24. [CrossRef]
- Poor, H. D. Pulmonary Thrombosis and Thromboembolism in COVID-19. Chest 2021. [CrossRef]
- Pretorius, E.; Vlok, M.; Venter, C.; Bezuidenhout, J. A.; Laubscher, G. J.; Steenkamp, J.; Kell, D. B. Persistent Clotting Protein Pathology in Long COVID/Post-Acute Sequelae of COVID-19 (PASC) Is Accompanied by Increased Levels of Antiplasmin. Cardiovascular Diabetology 2021, 20 (1). [CrossRef]
- Pretorius, E.; Venter, C.; Laubscher, G. J.; Kotze, M. J.; Oladejo, S. O.; Watson, L. R.; Rajaratnam, K.; Watson, B. W.; Kell, D. B. Prevalence of Symptoms, Comorbidities, Fibrin Amyloid Microclots and Platelet Pathology in Individuals with Long COVID/Post-Acute Sequelae of COVID-19 (PASC). Cardiovascular Diabetology 2022, 21 (1). [CrossRef]
- Proal, A. D.; VanElzakker, M. B. Long COVID or Post-Acute Sequelae of COVID-19 (PASC): An Overview of Biological Factors That May Contribute to Persistent Symptoms. Frontiers in Microbiology 2021, 12. [CrossRef]
- Chen, W.; Pan, J. Y. Anatomical and Pathological Observation and Analysis of SARS and COVID-19: Microthrombosis Is the Main Cause of Death. Biological Procedures Online 2021, 23. [CrossRef]
- Guervilly, C.; Bonifay, A.; Burtey, S.; Sabatier, F.; Cauchois, R.; Abdili, E.; Arnaud, L.; Lano, G.; Pietri, L.; Robert, T.; Velier, M.; Papazian, L.; Albanese, J.; Kaplanski, G.; Dignat-George, F.; Lacroix, R. Dissemination of Extreme Levels of Extracellular Vesicles: Tissue Factor Activity in Patients with Severe COVID-19. Blood Advances 2021, 5 (3), 628–634. [CrossRef]
- Barberis, E.; Vanella, V. V.; Falasca, M.; Caneapero, V.; Cappellano, G.; Raineri, D.; Ghirimoldi, M.; De Giorgis, V.; Puricelli, C.; Vaschetto, R.; Sainaghi, P. P.; Bruno, S.; Sica, A.; Dianzani, U.; Rolla, R.; Chiocchetti, A.; Cantaluppi, V.; Baldanzi, G.; Marengo, E.; Manfredi, M. Circulating Exosomes Are Strongly Involved in SARS-CoV-2 Infection. Frontiers in Molecular Biosciences 2021, 8. [CrossRef]
- Eymieux, S.; Uzbekov, R.; Rouillé, Y.; Blanchard, E.; Hourioux, C.; Dubuisson, J.; Belouzard, S.; Roingeard, P. Secretory Vesicles Are the Principal Means of SARS-CoV-2 Egress. Cells 2021, 10 (8), 2047. [CrossRef]
- Fogarty, H.; Townsend, L.; Morrin, H.; Ahmad, A.; Comerford, C.; Karampini, E.; Englert, H.; Byrne, M.; Bergin, C.; O’Sullivan, J. M.; Martin-Loeches, I.; Nadarajan, P.; Bannan, C.; Mallon, P. W.; Curley, G. F.; Preston, R. J. S.; Rehill, A. M.; McGonagle, D.; Ni Cheallaigh, C.; Baker, R. I. Persistent Endotheliopathy in the Pathogenesis of Long COVID Syndrome. Journal of Thrombosis and Haemostasis 2021, 19 (10), 2546–2553. [CrossRef]
- Celestino Rodríguez; Luque, N.; Blanco, I.; Sebastian, L.; Joan Albert Barberà; Peinado, V. I.; Tura-Ceide, O. Pulmonary Endothelial Dysfunction and Thrombotic Complications in Patients with COVID-19. 2021, 64 (4), 407–415. [CrossRef]
- Wang, C.; Yu, C.; Jing, H.; Wu, X.; Novakovic, V. A.; Xie, R.; Shi, J. Long COVID: The Nature of Thrombotic Sequelae Determines the Necessity of Early Anticoagulation. Frontiers in Cellular and Infection Microbiology 2022, 12. [CrossRef]
- Sharma, P.; Behl, T.; Sharma, N.; Singh, S.; Grewal, A.S.; Albarrati, A.; Albratty, M.; Meraya, A.M.; Bungau, S. COVID-19 and diabetes: Association intensify risk factors for morbidity and mortality. Biomed Pharmacother 2022, 151, 113089. [CrossRef]
- Kabir, M.T.; Uddin, M.S.; Hossain, M.F.; Abdulhakim, J.A.; Alam, M.A.; Ashraf, G.M.; Bungau, S.G.; Bin-Jumah, M.N.; Abdel-Daim, M.M.; Aleya, L. nCOVID-19 Pandemic: From Molecular Pathogenesis to Potential Investigational Therapeutics. Front Cell Dev Biol 2020, 8, 616. [CrossRef]
- Gheorghe, G.; Ilie, M.; Bungau, S.; Stoian, A.M.P.; Bacalbasa, N.; Diaconu, C.C. Is There a Relationship between COVID-19 and Hyponatremia? Medicina (Kaunas) 2021, 57. [CrossRef]
- Tagde, P.; Tagde, S.; Tagde, P.; Bhattacharya, T.; Monzur, S.M.; Rahman, M.H.; Otrisal, P.; Behl, T.; ul Hassan, S.S.; Abdel-Daim, M.M.; et al. Nutraceuticals and Herbs in Reducing the Risk and Improving the Treatment of COVID-19 by Targeting SARS-CoV-2. Biomedicines 2021, 9, 1266. [CrossRef]
- Perumal, R.; Shunmugam, L.; Naidoo, K.; Wilkins, D.; Garzino-Demo, A.; Brechot, C.; Anders Vahlne; Janko Nikolich-Žugich. Biological Mechanisms Underpinning the Development of Long COVID. 2023, 26 (6), 106935–106935. [CrossRef]
- Mayte Suárez-Fariñas; Minami Tokuyama; Wei, G.; Huang, R.; Livanos, A. E.; Jha, D.; Anaïs Levescot; Haritz Irizar; Kosoy, R.; Cording, S.; Wang, W.-H.; Bojan Losic; Ungaro, R. C.; Di’Narzo, A.; Martinez-Delgado, G.; Suprun, M.; Corley, M. J.; Aleksandar Stojmirović; Houten, S. M.; Peters, L. A. Intestinal Inflammation Modulates the Expression of ACE2 and TMPRSS2 and Potentially Overlaps with the Pathogenesis of SARS-CoV-2–Related Disease. 2021, 160 (1), 287-301.e20. [CrossRef]
- Mehandru, S.; Merad, M. Pathological Sequelae of Long-Haul COVID. Nature Immunology 2022, 23 (2), 194–202. [CrossRef]
- Gaebler, C.; Wang, Z.; Lorenzi, J. C. C.; Muecksch, F.; Finkin, S.; Tokuyama, M.; Cho, A.; Jankovic, M.; Schaefer-Babajew, D.; Oliveira, T. Y.; Cipolla, M.; Viant, C.; Barnes, C. O.; Bram, Y.; Breton, G.; HägglöfT.; Mendoza, P.; Hurley, A.; Turroja, M.; Gordon, K. Evolution of Antibody Immunity to SARS-CoV-2. Nature 2021, 591. [CrossRef]
- Choudhury, A.; Tariq, R.; Jena, A.; Vesely, E. K.; Singh, S.; Khanna, S.; Sharma, V. Gastrointestinal Manifestations of Long COVID: A Systematic Review and Meta-Analysis. Therapeutic Advances in Gastroenterology 2022, 15, 175628482211184. [CrossRef]
- Adame, M. J.; Nada, K. M.; Seashore, J. Late Sequelae of COVID-19 Infection in Patients without Comorbidities. American Journal of Respiratory and Critical Care Medicine 2021.
- Suárez-Fariñas, M.; Tokuyama, M.; Wei, G.; Huang, R.; Livanos, A.; Jha, D.; Levescot, A.; Irizar, H.; Kosoy, R.; Cording, S.; et al. Intestinal Inflammation Modulates the Expression of ACE2 and TMPRSS2 and Potentially Overlaps With the Pathogenesis of SARS-CoV-2–related Disease. Gastroenterology 2021, 160, 287-301.e220. [CrossRef]
- Berna Akıncı Özyürek; Tuğçe Şahin Özdemirel; Esma Sevil Akkurt; Derya Yenibertiz; Zeynep Tilbe Saymaz; Sertaç Büyükyaylacı Özden; Zuhal Eroglu. What Are the Factors That Affect Post COVID 1st Month’s Continuing Symptoms? 2021, 75 (11). [CrossRef]
- Faycal, A.; Ndoadoumgue, A. L.; Sellem, B.; Blanc, C.; Dudoit, Y.; Schneider, L.; Tubiana, R.; Valantin, M.-A. .; Seang, S.; Palich, R.; Bleibtreu, A.; Monsel, G.; Godefroy, N.; Itani, O.; Paccoud, O.; Pourcher, V.; Caumes, E.; Ktorza, N.; Chermak, A.; Abdi, B. Prevalence and Factors Associated with Symptom Persistence: A Prospective Study of 429 Mild COVID-19 Outpatients. Infectious Diseases Now 2022, 52 (2), 75–81. [CrossRef]
- Fernández-de-las-Peñas, C.; Martín-Guerrero, J.; Navarro-Pardo, E.; Torres-Macho, J.; Canto-Diez, M. G.; Pellicer-Valero, O. Gastrointestinal Symptoms at the Acute COVID-19 Phase Are Risk Factors for Developing Gastrointestinal Post-COVID Symptoms: A Multicenter Study. Internal and Emergency Medicine 2021. [CrossRef]
- Kozak, R.; Armstrong, S. M.; Salvant, E.; Ritzker, C.; Feld, J.; Biondi, M. J.; Tsui, H. Recognition of Long-COVID-19 Patients in a Canadian Tertiary Hospital Setting: A Retrospective Analysis of Their Clinical and Laboratory Characteristics. Pathogens 2021, 10 (10), 1246. [CrossRef]
- Leth, S.; Gunst, J. D.; Mathiasen, V. D.; Hansen, K. S.; Søgaard, O. S.; Østergaard, L.; Jensen-Fangel, S.; Storgaard, M.; Agergaard, J. Persistent Symptoms in Hospitalized Patients Recovering from COVID-19 in Denmark. Open Forum Infectious Diseases 2021. [CrossRef]
- Lombardo, M. D. M.; Foppiani, A.; Peretti, G. M.; Mangiavini, L.; Battezzati, A.; Bertoli, S.; Martinelli Boneschi, F.; Zuccotti, G. V. Long-Term Coronavirus Disease 2019 Complications in Inpatients and Outpatients: A One-Year Follow-up Cohort Study. Open Forum Infectious Diseases 2021, 8 (8), ofab384. [CrossRef]
- Shang, Y. F.; Liu, T.; Yu, J. N.; Xu, X. R.; Zahid, K. R.; Wei, Y. C.; Wang, X. H.; Zhou, F. L. Half-Year Follow-up of Patients Recovering from Severe COVID-19: Analysis of Symptoms and Their Risk Factors. Journal of Internal Medicine 2021, 290 (2), 444–450. [CrossRef]
- Lamers, M. M.; Beumer, J.; Vaart, J. van der; Knoops, K.; Puschhof, J.; Breugem, T. I.; Ravelli, R. B. G.; Schayck, J. P. van; Mykytyn, A. Z.; Duimel, H. Q.; Donselaar, E. van; Riesebosch, S.; Kuijpers, H. J. H.; Schippers, D.; Wetering, W. J. van de; Graaf, M. de; Koopmans, M.; Cuppen, E.; Peters, P. J.; Haagmans, B. L. SARS-CoV-2 Productively Infects Human Gut Enterocytes. Science 2020, 369 (6499). [CrossRef]
- Mouchati, C.; Durieux, J. C.; Zisis, S. N.; Labbato, D.; Rodgers, M. A.; Ailstock, K.; Reinert, B. L.; Funderburg, N. T.; McComsey, G. A. Increase in Gut Permeability and Oxidized Ldl Is Associated with Post-Acute Sequelae of SARS-CoV-2. Frontiers in Immunology 2023, 14, 1182544. [CrossRef]
- Gupta, S.; Parker, J.; Smits, S.; Underwood, J.; Dolwani, S. Persistent viral shedding of SARS-CoV-2 in faeces - a rapid review. Colorectal Dis 2020, 22, 611-620. [CrossRef]
- Xing, Y.-H.; Ni, W.; Wu, Q.; Li, W.-J.; Li, G.-J.; Wang, W.-D.; Tong, J.-N.; Song, X.-F.; Wing-Kin Wong, G.; Xing, Q.-S. Prolonged Viral Shedding in Feces of Pediatric Patients with Coronavirus Disease 2019. Journal of Microbiology, Immunology and Infection 2020, 53 (3), 473–480. [CrossRef]
- Amirian, E. S. Potential Fecal Transmission of SARS-CoV-2: Current Evidence and Implications for Public Health. International Journal of Infectious Diseases 2020, 95, 363–370. [CrossRef]
- Chen, Y.; Chen, L.; Deng, Q.; Zhang, G.; Wu, K.; Ni, L.; Yang, Y.; Liu, B.; Wang, W.; Wei, C.; Yang, J.; Ye, G.; Cheng, Z. The Presence of SARS-CoV-2 RNA in the Feces of COVID-19 Patients. Journal of Medical Virology 2020, 92 (7), 833–840. [CrossRef]
- Jones, D. L.; Baluja, M. Q.; Graham, D. W.; Corbishley, A.; McDonald, J. E.; Malham, S. K.; Hillary, L. S.; Connor, T. R.; Gaze, W. H.; Moura, I. B.; Wilcox, M. H.; Farkas, K. Shedding of SARS-CoV-2 in Feces and Urine and Its Potential Role in Person-To-Person Transmission and the Environment-Based Spread of COVID-19. Science of The Total Environment 2020, 749, 141364. [CrossRef]
- Peng, L.; Liu, J.; Xu, W.; Luo, Q.; Chen, D.; Lei, Z.; Huang, Z.; Li, X.; Deng, K.; Lin, B.; Gao, Z. SARS-CoV-2 Can Be Detected in Urine, Blood, Anal Swabs, and Oropharyngeal Swabs Specimens. Journal of Medical Virology 2020. [CrossRef]
- Tian, Y.; Rong, L.; Nian, W.; He, Y. Review Article: Gastrointestinal Features in COVID-19 and the Possibility of Faecal Transmission. Alimentary Pharmacology & Therapeutics 2020, 51 (9), 843–851. [CrossRef]
- Khreefa, Z.; Barbier, M. T.; Koksal, A. R.; Love, G.; Del Valle, L. Pathogenesis and Mechanisms of SARS-CoV-2 Infection in the Intestine, Liver, and Pancreas. Cells 2023, 12 (2), 262. [CrossRef]
- Phetsouphanh, C.; Darley, D. R.; Wilson, D. B.; Howe, A.; Munier, C. M. L.; Patel, S. K.; Juno, J. A.; Burrell, L. M.; Kent, S. J.; Dore, G. J.; Kelleher, A. D.; Matthews, G. V. Immunological Dysfunction Persists for 8 Months Following Initial Mild-To-Moderate SARS-CoV-2 Infection. Nature Immunology 2022, 23 (2), 210–216. [CrossRef]
- Gaebler, C.; Wang, Z.; Lorenzi, J. C. C.; Muecksch, F.; Finkin, S.; Tokuyama, M.; Cho, A.; Jankovic, M.; Schaefer-Babajew, D.; Oliveira, T. Y.; Cipolla, M.; Viant, C.; Barnes, C. O.; Bram, Y.; Breton, G.; HägglöfT.; Mendoza, P.; Hurley, A.; Turroja, M.; Gordon, K. Evolution of Antibody Immunity to SARS-CoV-2. Nature 2021, 591. [CrossRef]
- Ståhlberg, M.; Reistam, U.; Fedorowski, A.; Villacorta, H.; Horiuchi, Y.; Bax, J.; Pitt, B.; Matskeplishvili, S.; Lüscher, T. F.; Weichert, I.; Thani, K. B.; Maisel, A. Post-Covid-19 Tachycardia Syndrome: A Distinct Phenotype of Post-Acute Covid-19 Syndrome. The American Journal of Medicine 2021. [CrossRef]
- Vernino, S.; Stiles, L. E. Autoimmunity in Postural Orthostatic Tachycardia Syndrome: Current Understanding. Autonomic Neuroscience 2018, 215, 78–82. [CrossRef]
- Bisaccia, G.; Ricci, F.; Recce, V.; Serio, A.; Iannetti, G.; Chahal, A.A.; Ståhlberg, M.; Khanji, M.Y.; Fedorowski, A.; Gallina, S. Post-Acute Sequelae of COVID-19 and Cardiovascular Autonomic Dysfunction: What Do We Know? J Cardiovasc Dev Dis 2021, 8. [CrossRef]
- Taquet, M.; Dercon, Q.; Luciano, S.; Geddes, J. R.; Husain, M.; Harrison, P. J. Incidence, Co-Occurrence, and Evolution of Long-COVID Features: A 6-Month Retrospective Cohort Study of 273,618 Survivors of COVID-19. PLOS Medicine 2021, 18 (9), e1003773. [CrossRef]
- Schiffl, H.; Lang, S. M. Long-Term Interplay between COVID-19 and Chronic Kidney Disease. International Urology and Nephrology 2023. [CrossRef]
- Bowe, B.; Xie, Y.; Xu, E.; Al-Aly, Z. Kidney Outcomes in Long COVID. Journal of the American Society of Nephrology 2021, 32 (11), 2851–2862. [CrossRef]
- Chand, S.; Kapoor, S.; Naqvi, A.; Thakkar, J.; Fazzari, M. J.; Orsi, D.; Dieiev, V.; Lewandowski, D. C.; Dicpinigaitis, P. V. Long-Term Follow up of Renal and Other Acute Organ Failure in Survivors of Critical Illness due to Covid-19. Journal of Intensive Care Medicine 2021, 37 (6), 736–742. [CrossRef]
- Su, H.; Yang, M.; Wan, C.; Yi, L.-X.; Tang, F.; Zhu, H.-Y.; Yi, F.; Yang, H.-C.; Fogo, A. B.; Nie, X.; Zhang, C. Renal Histopathological Analysis of 26 Postmortem Findings of Patients with COVID-19 in China. Kidney International 2020, 0 (0). [CrossRef]
- Wang, K.; Chen, W.; Zhang, Z.; Deng, Y.; Lian, J.-Q.; Du, P.; Wei, D.; Zhang, Y.; Sun, X.-X.; Gong, L.; Yang, X.; He, L.; Zhang, L.; Yang, Z.; Geng, J.-J.; Chen, R.; Zhang, H.; Wang, B.; Zhu, Y.-M.; Nan, G. CD147-Spike Protein Is a Novel Route for SARS-CoV-2 Infection to Host Cells. Signal Transduction and Targeted Therapy 2020, 5 (1). [CrossRef]
- Behl, T., Kaur, I., Aleya, L., Sehgal, A., Singh, S., Sharma, N., Bhatia, S., Al-Harrasi, A., Bungau, S.* CD147-spike protein interaction in COVID-19: Get the ball rolling with a novel receptor and therapeutic target. Sci. Tot. Environ. 2022, 808, 152072. [CrossRef]
- Monteil, V.; Kwon, H.; Prado, P.; Hagelkrüys, A.; Wimmer, R.A.; Stahl, M.; Leopoldi, A.; Garreta, E.; Hurtado del Pozo, C.; Prosper, F.; et al. Inhibition of SARS-CoV-2 Infections in Engineered Human Tissues Using Clinical-Grade Soluble Human ACE2. Cell 2020, 181, 905-913.e907. [CrossRef]
- Diao, B.; Wang, C.; Wang, R.; Feng, Z.; Zhang, J.; Yang, H.; Tan, Y.; Wang, H.; Wang, C.; Liu, L.; Liu, Y.; Liu, Y.; Wang, G.; Yuan, Z.; Hou, X.; Ren, L.; Wu, Y.; Chen, Y. Human Kidney Is a Target for Novel Severe Acute Respiratory Syndrome Coronavirus 2 Infection. Nature Communications 2021, 12 (1), 2506. [CrossRef]
- Magro, C.; Mulvey, J. J.; Berlin, D.; Nuovo, G.; Salvatore, S.; Harp, J.; Baxter-Stoltzfus, A.; Laurence, J. Complement Associated Microvascular Injury and Thrombosis in the Pathogenesis of Severe COVID-19 Infection: A Report of Five Cases. Translational Research 2020. [CrossRef]
- Sanchez, A.; Sohier, P.; Benghanem, S.; L’Honneur, A.-S.; Rozenberg, F.; Dupin, N.; Garel, B. Digitate Papulosquamous Eruption Associated with Severe Acute Respiratory Syndrome Coronavirus 2 Infection. JAMA Dermatology 2020, 156 (7), 819. [CrossRef]
- Gottlieb, M.; Long, B. Dermatologic Manifestations and Complications of COVID-19. The American Journal of Emergency Medicine 2020. [CrossRef]
- Galván Casas, C.; Català, A.; Carretero Hernández, G.; Rodríguez-Jiménez, P.; Fernández Nieto, D.; Rodríguez-Villa Lario, A.; Navarro Fernández, I.; Ruiz-Villaverde, R.; Falkenhain, D.; Llamas Velasco, M.; García-Gavín, J.; Baniandrés, O.; González-Cruz, C.; Morillas-Lahuerta, V.; Cubiró, X.; Figueras Nart, I.; Selda-Enriquez, G.; Romaní, J.; Fustà-Novell, X.; Melian-Olivera, A. Classification of the Cutaneous Manifestations of COVID-19: A Rapid Prospective Nationwide Consensus Study in Spain with 375 Cases. British Journal of Dermatology 2020. [CrossRef]
- Kaya, G.; Kaya, A.; Saurat, J.-H. Clinical and Histopathological Features and Potential Pathological Mechanisms of Skin Lesions in COVID-19: Review of the Literature. Dermatopathology 2020, 7 (1), 3–16. [CrossRef]
- Suchonwanit, P.; Leerunyakul, K.; Kositkuljorn, C. Cutaneous Manifestations in COVID-19: Lessons Learned from Current Evidence. Journal of the American Academy of Dermatology 2020. [CrossRef]
- Criado, P. R.; Abdalla, B. M. Z.; de Assis, I. C.; van Blarcum de Graaff Mello, C.; Caputo, G. C.; Vieira, I. C. Are the Cutaneous Manifestations during or due to SARS-CoV-2 Infection/COVID-19 Frequent or Not? Revision of Possible Pathophysiologic Mechanisms. Inflammation Research 2020, 1–12. [CrossRef]
- Goren, A.; Vaño-Galván, S.; Wambier, C. G.; McCoy, J.; Gomez-Zubiaur, A.; Moreno-Arrones, O. M.; Shapiro, J.; Sinclair, R. D.; Gold, M. H.; Kovacevic, M.; Mesinkovska, N. A.; Goldust, M.; Washenik, K. A Preliminary Observation: Male Pattern Hair Loss among Hospitalized COVID-19 Patients in Spain – a Potential Clue to the Role of Androgens in COVID-19 Severity. Journal of Cosmetic Dermatology 2020, 19 (7), 1545–1547. [CrossRef]
- A. Mahé; E. Birckel; C. Merklen; Lefebvre, P.; Cédric Hannedouche; Jost, M.; L. Droy-Dupré. Histology of Skin Lesions Establishes That the Vesicular Rash Associated with COVID-19 Is Not “Varicella-Like.” 2020, 34 (10). [CrossRef]
- Hamming, I.; Timens, W.; Bulthuis, M.; Lely, A.; Navis, G.; van Goor, H. Tissue Distribution of ACE2 Protein, the Functional Receptor for SARS Coronavirus. A First Step in Understanding SARS Pathogenesis. The Journal of Pathology 2004, 203 (2), 631–637. [CrossRef]
- Leng, A.; Shah, M.; Ahmad, S. A.; Premraj, L.; Wildi, K.; Li Bassi, G.; Pardo, C. A.; Choi, A.; Cho, S.-M. Pathogenesis Underlying Neurological Manifestations of Long COVID Syndrome and Potential Therapeutics. Cells 2023, 12 (5), 816. [CrossRef]
- Stefanou, M.-I.; Palaiodimou, L.; Bakola, E.; Smyrnis, N.; Papadopoulou, M.; Paraskevas, G. P.; Rizos, E.; Boutati, E.; Grigoriadis, N.; Krogias, C.; Giannopoulos, S.; Tsiodras, S.; Gaga, M.; Tsivgoulis, G. Neurological Manifestations of Long-COVID Syndrome: A Narrative Review. Therapeutic Advances in Chronic Disease 2022, 13, 20406223221076890. [CrossRef]
- Balcom, E. F.; Nath, A.; Power, C. Acute and Chronic Neurological Disorders in COVID-19: Potential Mechanisms of Disease. Brain 2021. [CrossRef]
- Meinhardt, J.; Radke, J.; Dittmayer, C.; Franz, J.; Thomas, C.; Mothes, R.; Laue, M.; Schneider, J.; Brünink, S.; Greuel, S.; Lehmann, M.; Hassan, O.; Aschman, T.; Schumann, E.; Chua, R. L.; Conrad, C.; Eils, R.; Stenzel, W.; Windgassen, M.; Rößler, L. Olfactory Transmucosal SARS-CoV-2 Invasion as a Port of Central Nervous System Entry in Individuals with COVID-19. Nature Neuroscience 2020, 24 (2), 1–8. [CrossRef]
- Monteil, V.; Kwon, H.; Prado, P.; Hagelkrüys, A.; Wimmer, R.A.; Stahl, M.; Leopoldi, A.; Garreta, E.; Hurtado del Pozo, C.; Prosper, F.; et al. Inhibition of SARS-CoV-2 Infections in Engineered Human Tissues Using Clinical-Grade Soluble Human ACE2. Cell 2020, 181, 905-913.e907. [CrossRef]
- Cheng, X.; Zhang, Y.; Li, Y.; Wu, Q.; Wu, J.; Park, S.K.; Guo, C.; Lu, J. Meta-analysis of 16S rRNA microbial data identified alterations of the gut microbiota in COVID-19 patients during the acute and recovery phases. BMC Microbiol 2022, 22, 274. [CrossRef]
- Wang, M.; Zhang, Y.; Li, C.; Chang, W.; Zhang, L. The relationship between gut microbiota and COVID-19 progression: new insights into immunopathogenesis and treatment. Front Immunol 2023, 14, 1180336. [CrossRef]
- Liu, Q.; Su, Q.; Zhang, F.; Tun, H.M.; Mak, J.W.Y.; Lui, G.C.; Ng, S.S.S.; Ching, J.Y.L.; Li, A.; Lu, W.; et al. Multi-kingdom gut microbiota analyses define COVID-19 severity and post-acute COVID-19 syndrome. Nat Commun 2022, 13, 6806. [CrossRef]
- Yeoh, Y.K.; Zuo, T.; Lui, G.C.; Zhang, F.; Liu, Q.; Li, A.Y.; Chung, A.C.; Cheung, C.P.; Tso, E.Y.; Fung, K.S.; et al. Gut microbiota composition reflects disease severity and dysfunctional immune responses in patients with COVID-19. Gut 2021, 70, 698-706. [CrossRef]
- Haran, J.P.; Bradley, E.; Zeamer, A.L.; Cincotta, L.; Salive, M.C.; Dutta, P.; Mutaawe, S.; Anya, O.; Meza-Segura, M.; Moormann, A.M.; et al. Inflammation-type dysbiosis of the oral microbiome associates with the duration of COVID-19 symptoms and long COVID. JCI Insight 2021, 6. [CrossRef]
- Roth, W.; Zadeh, K.; Vekariya, R.; Ge, Y.; Mohamadzadeh, M. Tryptophan Metabolism and Gut-Brain Homeostasis. Int J Mol Sci 2021, 22. [CrossRef]
- Almulla, A.F.; Supasitthumrong, T.; Tunvirachaisakul, C.; Algon, A.A.A.; Al-Hakeim, H.K.; Maes, M. The tryptophan catabolite or kynurenine pathway in COVID-19 and critical COVID-19: a systematic review and meta-analysis. BMC Infect Dis 2022, 22, 615. [CrossRef]
- Almutairi, M. M.; Sivandzade, F.; Albekairi, T. H.; Alqahtani, F.; Cucullo, L. Neuroinflammation and Its Impact on the Pathogenesis of COVID-19. Frontiers in Medicine 2021, 8. [CrossRef]
- Fitero, A.; Bungau, S. G.; Tit, D. M.; Endres, L.; Khan, S. A.; Bungau, A. F.; Romanul, I.; Vesa, C. M.; Radu, A.-F.; Tarce, A. G.; Bogdan, M. A.; Nechifor, A. C.; Negrut, N. Comorbidities, Associated Diseases, and Risk Assessment in COVID-19—a Systematic Review. International Journal of Clinical Practice 2022, 2022, 1–24. [CrossRef]
- Moghimi, N.; Di Napoli, M.; Biller, J.; Siegler, J. E.; Shekhar, R.; McCullough, L. D.; Harkins, M. S.; Hong, E.; Alaouieh, D. A.; Mansueto, G.; Divani, A. A. The Neurological Manifestations of Post-Acute Sequelae of SARS-CoV-2 Infection. Current Neurology and Neuroscience Reports 2021, 21 (9). [CrossRef]
- Moga, T. D.; Nistor-Cseppento, C. D.; Bungau, S. G.; Tit, D. M.; Sabau, A. M.; Behl, T.; Nechifor, A. C.; Bungau, A. F.; Negrut, N. The Effects of the “Catabolic Crisis” on Patients’ Prolonged Immobility after COVID-19 Infection. Medicina 2022, 58 (6), 828. [CrossRef]
- Nistor-Cseppento, C. D.; Moga, T. D.; Bungau, A. F.; Tit, D. M.; Negrut, N.; Pasca, B.; Bochis, C. F.; Ghitea, T. C.; Jurcau, A.; Purza, A. L.; Uivarosan, D. The Contribution of Diet Therapy and Probiotics in the Treatment of Sarcopenia Induced by Prolonged Immobilization Caused by the COVID-19 Pandemic. Nutrients 2022, 14 (21), 4701. [CrossRef]
| Gland | Clinical manifestation |
|---|---|
| 1. Pituitary gland | pituitary apoplexy |
| 2. Thyroid gland | euthyroid sick syndrome subacute thyroiditis Graves’ disease postpartum thyroiditis Hashimoto’s disease silent thyroiditis |
| 3. Pancreas | acute pancreatitis necrotizing pancreatitis impaired glucose tolerance insulin resistance diabetes mellitus severe metabolic complications (diabetic ketoacidosis) hyperglycemia abnormalities level of amylase/lipase |
| 4. Adrenal glands | necrosis hypocortisolism cross-reactive antibodies against endogenous ACTH |
| 5. Gonads | hypogonadism erectile dysfunction menstrual disturbances |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





