1. Introduction
Adverse events (AEs) resulting from repeated COVID-19 vaccine booster doses have become a significant area of research [
1,
2,
3,
4]. While vaccines remain one of the most effective means to prevent diseases, vaccine-related adverse events have always been a concern for the public [
5,
6,
7]. Recent studies from large pharmacovigilance databases indicate substantial variability in adverse event incidence rates, emphasizing the need for context-specific analysis in vaccine safety surveillance to maintain public trust and inform future vaccination strategies [
8,
9,
10,
11,
12]. There are numerous reports of side events following COVID-19 vaccination, with particular emphasis on specific groups such as the elderly [
13], pregnant women [
2,
14,
15], and those with pre-existing medical conditions. Furthermore, retrospective reports have highlighted vaccine-related neurological adverse events [
1,
7,
16] and cardiovascular complications [
3,
17,
18]. These reports significantly impact public willingness to receive vaccinations, consequently affecting vaccination rates [
7,
19,
20]. Of particular concern are the severe adverse events associated with booster vaccines, such as vaccine-induced myocarditis and pericarditis, multisystem inflammatory syndrome in children (MIS-C), and multisystem inflammatory syndrome in adults (MIS-A)[
21,
22,
23,
24,
25,
26,
27]. The question of whether the incidence and severity of side effects increase with additional doses is of utmost importance and urgently requires detailed clinical reports to understand the current situation [
28].
Repeated administration of booster doses is crucial for enhancing protective immunity against COVID-19. However, there is a growing need for large-scale studies to support public concerns about the adverse events of these repeated doses. The risk of AE could be repeated, upon subsequent administrations of the same vaccine. Given the variety of COVID-19 vaccines available, each based on different technological platforms such as mRNA and non-replicating viral vectors, potential mechanisms for this could involve immunological responses such as hypersensitivity or immune sensitization, especially relevant in the context of repeated exposures to vaccine antigens [
3,
13,
29]. Moreover, the risk of AE could be cumulative potentially due to cumulative immunological effects or other underlying biological factors. This cumulative aspect is paramount in assessing the long-term safety and sustainability of ongoing COVID-19 vaccination strategies, particularly in the context of administering booster doses [
30]. A comprehensive analysis of these mechanisms, in terms of pharmacovigilance, is vital for enhancing the efficacy and safety of current and future vaccination strategies against COVID-19 and other diseases.
The primary objective of this study is to investigate the incidence rates of adverse events (AEs) following the administration of the second booster dose (fourth dose) of the COVID-19 vaccine. Taiwan boasts high vaccination rates globally (first dose coverage at 93.8%, second at 89.06%, third at 76.9%, and fourth at 25%) and encourages booster vaccinations for the entire population in contrast to the strategies of other countries that focus primarily on high-risk groups.[
31,
32]. This research utilizes data from the Vaccine Adverse Event Reporting System (VAERS) at Taipei Veterans General Hospital to analyze the types and incidence rates of AEs, and the correlation between the occurrence of AEs after the first and second boosters. Additionally, we evaluate whether the occurrence of AEs is a repeated phenomenon or indicative of a cumulative risk of AEs. Furthermore, we explore the relationship between the choice of vaccine brand and the booster-booster combination in relation to the occurrence of AEs. This comprehensive investigation aims to provide critical insights into the safety profile of repeated booster doses, enhancing the decision-making process for vaccine administration and public health policy.
2. Materials and Methods
2.1. Taiwan’s vaccination program for booster vaccines.
Taiwan's booster vaccine program, distinguished by its high vaccination rates (first dose coverage at 93.8%, second at 89.06%, third at 76.9%, and fourth at 25% on December 25, 2023), adopts a comprehensive and inclusive approach. The government's policy encourages staggered booster vaccinations for the entire population, a contrast to the strategies of other countries that focus primarily on high-risk groups. Integral to this program is a mix-and-match strategy, especially for booster doses, recommending mRNA or subunit vaccines following initial viral vector vaccines to enhance immune response. This strategy adapts to the availability of vaccines, exemplified by the predominant use of the Moderna BA.1 variant vaccine during the fourth dose rollout, due to its availability and the depletion of other brands. The decision for administering the second booster is informed by immunological evidence, allowing for its administration three months after the first booster. A significant focus of the research is the prevalent use of the mRNA1273 (Moderna COVID-19 vaccine, Spikevax) vaccine in the booster program, particularly notable during the administration of the second booster that included the bivalent version of Moderna COVID-19 vaccine (vaccine against original strain and BA.4/5 variant, mRNA-1273.214 in Taiwan). This scenario offers a unique opportunity providing insights into the occurrence of adverse events in a real-world context.
2.2. Vaccine Adverse Event Reporting System (VAERS) in Taipei Veterans General Hospital
In addition to a centralized Taiwan's Vaccine Adverse Event Reporting System (VAERS) maintained by Taiwan Centers for Disease Control, Taipei Veterans General Hospital (TVGH) was requested to operate its own VAERS. Individuals who receive their vaccination at TVGH are sent a reminder message seven days post-vaccination, directing them to an online, anonymous questionnaire. They are encouraged to fill out this survey regardless of whether they experienced symptoms or not. This digital survey collects data on the vaccine brand, the dates of both the first and second booster shots, and any post-vaccination symptoms. It includes a section for recipients to describe in their own words the nature of the symptoms (such as fever, fatigue, pain at the injection site, headache, severe allergies, etc.), the timing of their onset after the vaccination, and any follow-up actions they undertook, whether it was self-care at home or seeking outpatient or emergency medical treatment.
A key aspect of this approach is the mandatory reporting of any symptoms by all vaccine recipients, regardless of whether they experience any. This inclusive reporting system guarantees a detailed accumulation of data regarding post-vaccination effects, thereby enhancing the precision and dependability of the study.
2.3. Study design
This cross-sectional study investigates the vaccination status and associated adverse events reported over a three-month period at the end of 2022 in the vaccination site of Taipei Veterans General Hospital. The adverse events are categorized into severe and non-severe by the researchers. The study data includes basic demographic information of the participants, vaccination details, and descriptions of any adverse event. The analysis aims to explore the correlation between vaccine recipients who experienced adverse events and those who did not, examining potential influencing factors.
2.4. Data sources
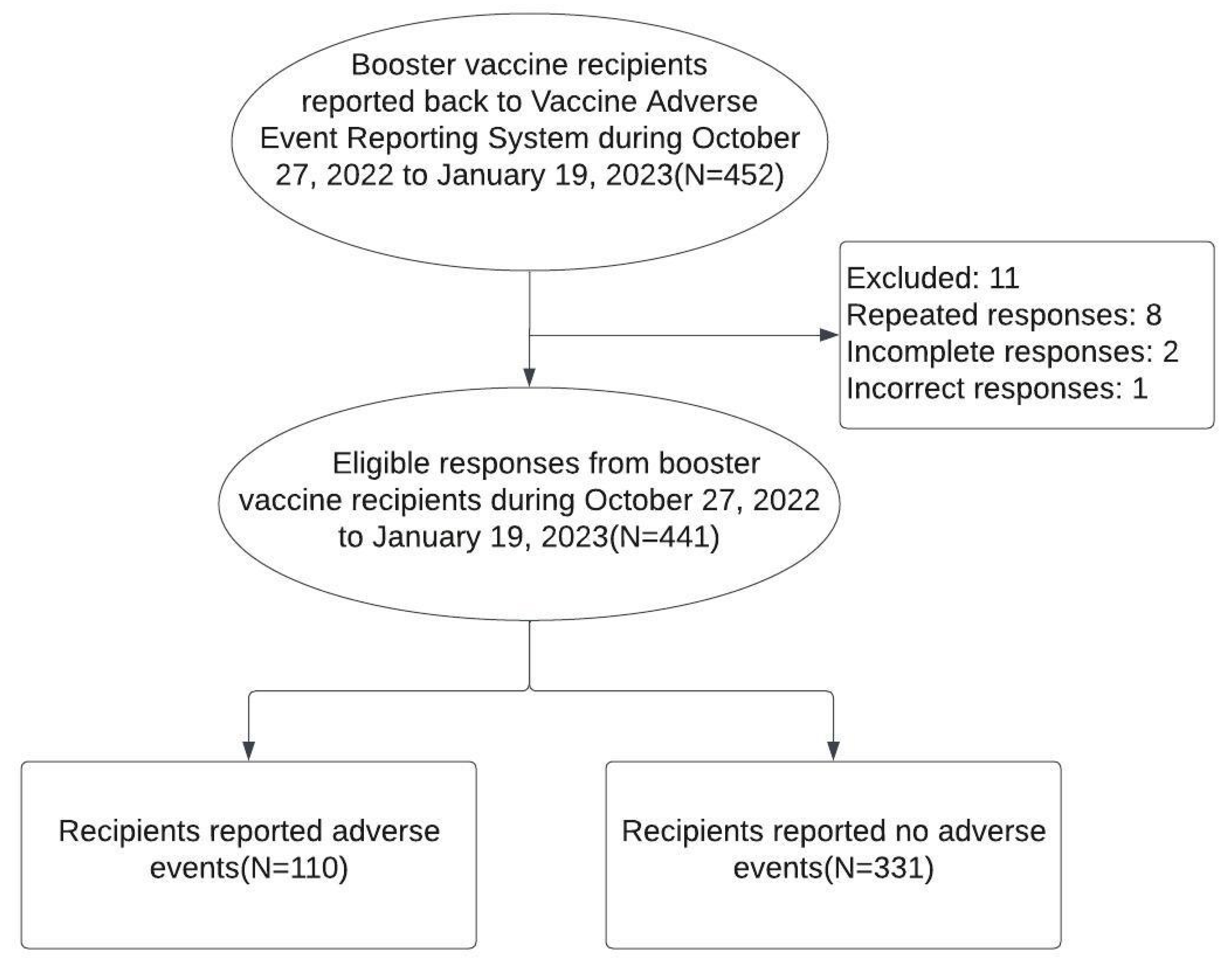
Data collection spanned from the announcement of the second booster (fourth dose) availability to citizens and foreign residents over 18 years old on October 27, 2022, until January 19, 2023. Patients receiving vaccines underwent thorough medical examinations, including checks for allergic reactions, acute infection symptoms, and major vaccine-related adverse events. Post-vaccination, patients were provided with a Chinese-language Google Form questionnaire, accessible via a QR code, to report any adverse events within seven days of vaccination. The questionnaire included demographics, vaccine brand choice for the second booster, and any adverse event experienced. To ensure privacy, gender was not included in the questionnaire. The information was anonymized upon submission, with each respondent limited to one submission. This study, approved by IRB of Taipei Veterans General Hospital (IRB 2022-12-005AC#1), ensures comprehensive data collection while maintaining participant confidentiality.
2.5. Study population
The study population comprised individuals who sent their response after received vaccinations at the vaccination site of Taipei Veterans General Hospital between October 27, 2022, and January 19, 2023. Responder who with duplicate submissions, incomplete responses, and incorrect response were excluded (
Figure 1).
2.6. Basic demographic information and vaccination history
Basic Information including respondents’ age and vaccination history including brand and time of each dose of vaccine were extracted from the VAERS. First booster and second booster with the same brand of boosters were considered as homologous otherwise were classified as heterologous. Vaccines were further divided into two groups of “mRNA1273 (Moderna)” and “others” because of high share of mRNA1273 vaccines.
2.7. Classification of AE, Serious Adverse Events (SAE) and Non-Serious Adverse Events (NSAE)
Adverse events reports were self-submitted by participants with settled options in the questionnaire and also their own descriptions. Regardless of primary doses, only AE after first booster were considered. The responses were categorized by authors Lin, C.H. and Chen, Y.C. based on the questionnaire's design. Symptoms potentially related to the cardiovascular system (like chest tightness or difficulty breathing) were classified as Serious Adverse Events (SAE). Other symptoms, including localized reactions at the injection site, flu-like symptoms, rapid heartbeat, gastrointestinal issues, and muscle aches, were categorized as Non-Serious Adverse Events (NSAE). This classification was utilized to analyze the correlation and potential influencing factors between vaccine recipients who experienced adverse events (both SAE and NSAE) and those who did not report any adverse event.
The first author (Lin, C.H.) classified the multiple descriptions into 6 domains: “cardiac symptoms”, “local reactions”, “flu-like symptoms”, “gastroin-testinal symptoms”, “muscle/joint pain”, and “others” as described in another previous research [
33]. AEs reported after the first and second boosters were referred to as first and second AEs, respectively.
2.8. Definition of repeated adverse events
Repeated adverse events (repeated AE) refers to the occurrence of adverse events following each subsequent dose of the COVID-19 vaccine. This category is particularly focused on analyzing whether individuals who experienced AEs after one dose are more likely to experience similar events following subsequent doses. Cumulative risk of AE (cumulative AE) assesses whether the likelihood or severity of adverse events increases with each subsequent dose of the vaccine. This concept is crucial for evaluating the long-term safety of the vaccination, especially in a regime involving multiple booster doses. In the current study, we investigate incidence-dependent cumulative risk by evaluating whether the incidence of AEs increases with additional doses.
2.9. Statistical analysis
To estimate the incidence rates of AEs, we employed point estimation using a binomial distribution to calculate the incidence rates (IR) and 95% confidence interval (95% CI) of AEs for each category. We used the Fisher exact test to compare the effects of various factors and identify factors associated with the occurrence of AEs after the second booster. Furthermore, Poisson regression analysis was used to identify factors associated with AEs following the second booster. Incidence rate ratios (IRR) were used to evaluate the effect of association. To test repeated occurrence of AE, McNemar test was used to explore the association between first AE and second AE. We examined IR of first AE and second AE to evaluate the cumulative risk of AE. All statistics R (R-4.3.2 for Windows), a p-value < 0.05 was considered statistically significant.
3. Results
3.1. Population characteristics and AE reports from VAERS.
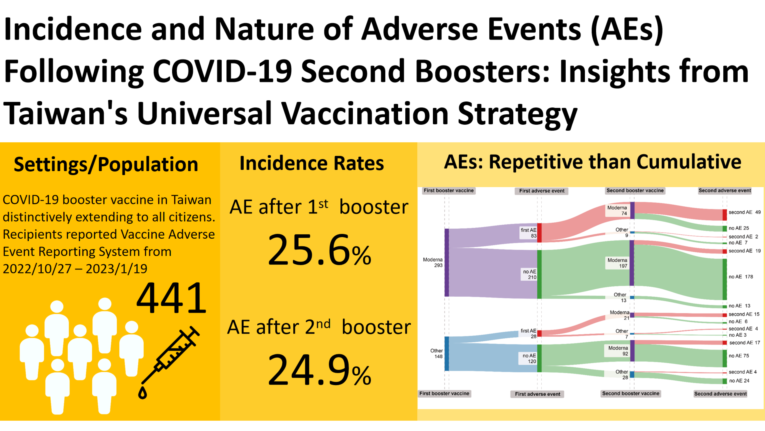
Over a three-month period, Taipei Veterans General Hospital administered COVID-19 vaccinations to 1,711 individuals. From this group, 452 reported their post-vaccination outcomes to VAERS between October 27, 2022, and January 19, 2023. A total of 441 respondents were ultimately included in this analysis. Of these, 113 reported adverse events (first AE) following their first booster and 110 reported adverse events (second AE) after the second booster. This resulted in incidence rates (IR) of 25.6% (95% CI: 21.1-30.8) after the first booster and 24.9% (95% CI: 20.5-30.0) after the second. Notably, the AE occurred repeatedly for individuals with first AE, a McNemar test showed similar IR of either first AE or second AE (difference -0.68%, 95% CI, -4.63% to 3.27%, P-value = 0.8221) (
Table 1).
The incidence of second AE appears to be influenced by age, with younger recipients (ages 18-39: 27.8%, 40-64: 24.7%) experiencing a higher rate of AEs compared to those aged 65 and above (14.6%). This trend suggests a greater occurrence of AEs among the younger age groups. Additionally, the IR of second AEs showed variation based on the vaccine brand, with mRNA-1273 having 100 cases and an IR of 26% (95% CI: 21.7-30.4). However, it is important to note that no statistically significant differences were found concerning age and vaccine brand, indicating that these factors did not significantly affect the incidence of AEs in this study (
Table 1).
3.3. Analysis of second AEs reported by recipients of the COVID-19 second booster vaccines.
The majority of adverse events (AEs) reported following the second COVID-19 booster vaccination were classified as Non-Serious Adverse Events (NSAEs). The incidence rate (IR) of Serious Adverse Events (SAEs) for the second booster was relatively low, at 1.3% (6 cases, 95% CI: 0.5-3.0). Among the NSAEs, the most common were pain at the injection site (97 cases, IR: 22%), fatigue (68 cases, IR: 15.4%), headache (60 cases, IR: 13.6%), and fever (41 cases, IR: 9.3%). The concurrent occurrence of pain at the injection site, fatigue, and headache was notably frequent, observed in 18 cases (IR: 16%). Additionally, the data suggest a lower IR of second AEs for brands other than mRNA1273, with an IR of 17.5% compared to 26.0% for mRNA1273 across all types of AEs (
Table 2).
3.4. Influential factors of second AE
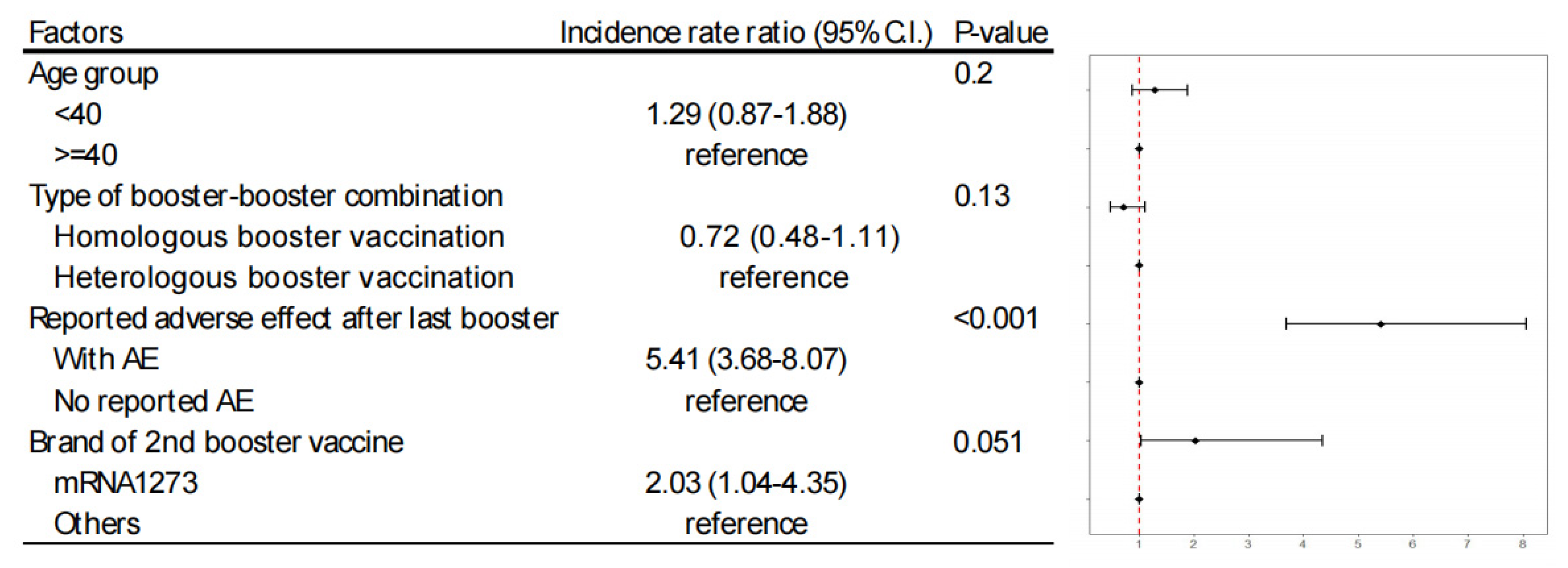
Using Poisson regression analysis, we evaluated factors such as age, type of booster-booster combination, and the occurrence of fist adverse events (AE). The analysis identified that occurrence of first AE was the most significant predictor (Incidence rate ratio, 5.41, P<0.001) for second AE. Individuals who had adverse events with an mRNA vaccine as their first booster were more prone to similar reactions with the same vaccine type for their second booster. Although the influence of vaccine brand choice did not reach statistical significance (Incidence rate ratio, 2.03, P=0.051), there was a trend indicating that participants receiving mRNA1273 had a higher incidence rate of adverse events (
Figure 2).
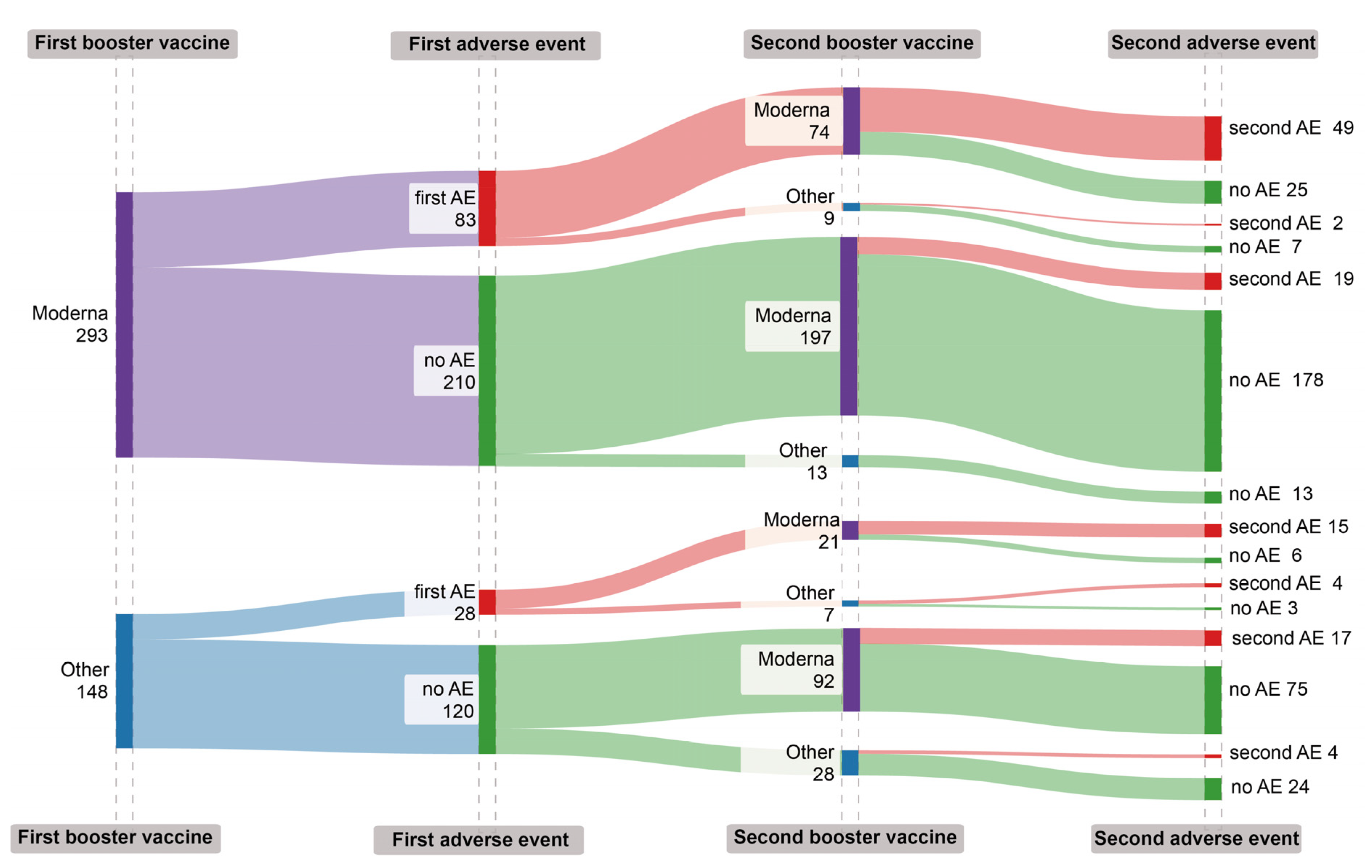
The incidence of adverse events (AEs) following COVID-19 booster vaccinations appears to be repetitive, yet the cumulative risk associated with these AEs were complex (Table3,
Figure 3). Individuals who received mRNA1273 for both doses had a stable AE rate, with 26.0% after the first booster (95% CI: 21.2 - 31.7) and a minor decrease to 25.1% post-second booster (95% CI: 19.5 - 31.8). Those who had mRNA1273 followed by a different brand observed a notable reduction in AEs to 9.1% after the second booster (95% CI: 1.1 - 32.8). In contrast, participants initially given a different brand, then mRNA1273, experienced an increase in AE incidence from 17.5% after the first booster (95% CI: 8.4 - 32.3) to a higher rate after the second mRNA1273 booster compared to those who continued with a non-mRNA1273 brand (28.3% vs. 21.6%, respectively). This suggests that for those who experienced adverse events with an mRNA1273 vaccine during their first booster, choosing a different vaccine brand for the second booster might lower the risk of subsequent adverse events (
Table 3).
4. Discussion
To address widespread public concerns about adverse events (AEs) due to repeated vaccinations, this study analyzed VAERS data from Taipei Veterans General Hospital to explore the AEs following the administration of the second COVID-19 booster dose. The incidence rates of adverse events (AEs) were relatively high, at 25.6% after the first booster and 24.9% after the second. Most notably, these AEs were predominantly non-serious, with symptoms such as injection site pain and fatigue being the most common. We observed the pattern of adverse events (AEs) following COVID-19 booster vaccinations be repetitive rather than cumulative, where individuals who experienced an AE following the first booster were more likely to report AEs after the second booster. This finding underscores the importance of considering previous AEs, the brand of the second booster, and the booster combination in devising personalized vaccination strategies. These results are vital for health providers and enhancing the understanding of vaccine safety, particularly in the context of administering booster doses.
This study offers crucial insights into the occurrence of adverse events (AEs) following COVID-19 booster vaccinations, particularly in a real-world setting in Taiwan where vaccinations are not limited to priority groups. In our study, we observed a considerable incidence rate of adverse events (AEs) post COVID-19 booster vaccinations. It's essential to recognize that the majority of these AEs were minor, frequently manifesting as injection site pain, fatigue, or low-grade fever. The temporal association of these AEs following vaccination does not necessarily imply causation, and it's important to consider the possibility of temporal bias. Additionally, the reliance on self-reported data may lead to an inflated incidence rate due to the underreporting of non-events. There is notable variability in AE incidence rates across large pharmacovigilance databases, as seen in recent studies [
8,
9,
10,
11,
12]. Our findings contribute further insights into common but non-fatal AEs, consistent with the general profile of vaccine-related side events that are typically transient and resolve without serious consequences [
2,
8,
9,
10,
11,
34,
35]. Therefore, the high incidence rate observed should be interpreted within the larger framework of vaccine safety, emphasizing that the benefits of protection against COVID-19 far outweigh the minor discomforts associated with these non-serious AEs, a fact that should be clearly communicated to address public concerns.
Our observations indicate that individuals who experienced side events after their first COVID-19 booster often faced similar issues with subsequent doses. Conversely, those who did not encounter side effects initially tended to remain symptom-free after additional doses. The finding is compatible with findings in a study focusing on AEs after primary series in Japan [
36]. This trend may be linked to the body developing a heightened sensitivity or allergic response, particularly in those already predisposed to such reactions. Ingredients in the vaccines, such as polyethylene glycol (PEG) and lipid nanoparticles (LNPs), could be responsible for triggering these responses in sensitive individuals [
30]. The presence of vaccine adjuvants, which boost the body's response to the vaccine, could also play a role. Understanding why these reactions happen is important for finding ways to prevent side effects in people who are more likely to have them, especially when considering their past experiences with allergies and sensitivities during booster vaccinations. Additionally, it's important to consider the possibility of reporting bias, where individuals more conscious of their adverse events (AEs) might be more likely to report them, potentially influencing our findings.
Our study found no evidence indicating that adverse events (AEs) increase in frequency with successive COVID-19 booster doses within the same demographic group. It suggests that although an effective immune response is crucial for vaccine effectiveness, and repeated vaccinations may activate the immune system, this does not necessarily lead to a higher frequency of adverse events (AEs) with each subsequent booster dose. This insight is particularly relevant considering the World Health Organization's (WHO) updated guidelines in 2023, which now recommend booster vaccinations mainly for priority groups, following a comprehensive assessment of the risks and benefits associated with additional doses. [
37,
38]. Our results provide reassuring evidence regarding the safety of administering second booster doses in public COVID-19 vaccination programs. This information is essential for public health communication, offering reassurance about the safety of receiving multiple booster doses during ongoing efforts to combat COVID-19.
Cautions are needed for interpretation the current result. The reliance on self-reported data in our research might have led to an overestimation of the incidence rates of adverse events. This is particularly relevant as self-reported data can vary in accuracy and completeness, which may skew the true incidence of these events. It is important to note that our findings predominantly apply to common and minor adverse events, given that the number of cases reported was relatively small. Therefore, these results may not be generalizable to rare and severe adverse events. This limitation underscores the need for further studies with more robust data collection methods to accurately assess the incidence of both common and rare adverse events following COVID-19 booster vaccinations.
Moreover, there are still some limitations to the current study. First, a significant limitation of this study is reporting bias. The reliance on self-reported data means patients without adverse events may be less inclined to report their status, potentially leading to an overestimation of the incidence of adverse events. This bias can skew the results towards those who experienced more noticeable or bothersome symptoms. Secondly, the study's methodology is susceptible to recall bias. Since data collection is based on participants’ recollections of their symptoms and the timeline of these symptoms post-vaccination, participants may forget minor symptoms or misremember the severity and timing of their symptoms. Third, the intensity of discomfort or severity of adverse events in this study is subjectively evaluated by the participants themselves, rather than through objective assessment by medical experts. This subjective evaluation can lead to a variation in the reported intensity of symptoms. Fourth, the study's findings may not be generalizable to the broader population due to the limited sample size and the specific demographic characteristics of the study participants. Larger and more diverse sample sizes are needed to validate these findings across different populations. Fifth. the study does not account for all possible confounding factors, such as underlying health conditions, concurrent medication use, or previous exposure to COVID-19, which could influence the occurrence and reporting of adverse events. Sixth, the reliance on online questionnaires for data collection may introduce a selection bias, as it excludes individuals without internet access or those who are less technologically inclined. Seventh, the study establishes a temporal association between vaccination and the occurrence of adverse events but does not prove causality. Further investigation is needed to establish a direct causal relationship.
5. Conclusions
Our study indicates that approximately one-quarter of individuals receiving COVID-19 booster vaccines reported adverse events (AEs), predominantly of a non-serious nature. Notably, these AEs tend to recur in individuals with previous adverse reactions rather than accumulate over successive doses. This repetitive nature of AEs highlights the importance of considering individual medical histories in vaccination plans. These findings are crucial for shaping personalized vaccination strategies, particularly for those with a history of AEs. The insights gained may aid healthcare providers in informing patients about potential AEs, enhancing vaccine compliance and public trust.
Funding
This research received no external funding. The article processing charge was supported by an intramural grant of Taipei Veterans General Hospital (V111E-002-1, V112E-001-1, V113E-002-1). The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Data Availability Statement
The original data is unavailable due to privacy or ethical restrictions.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Institutional Review Board of Taipei Veterans General Hospital (IRB 2022-12-005AC#1).
Informed Consent Statement
Patient consents were all well preserved. The data we analyzed were de-identified. No identifiable private information or human biospecimens was involved.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Boruah, A.P.; Heydari, K.; Wapniarski, A.E.; Caldwell, M.; Thakur, K.T. Neurological Considerations with COVID-19 Vaccinations. Semin. Neurol. 2023, 43, 297–311. [Google Scholar] [CrossRef]
- Hameed, I.; Khan, M.O.; Nusrat, K.; Mahmood, S.; Nashit, M.; Malik, S.; Siddiqui, O.M.; Samad, S.A.; Marsia, S.; Shariq, M.; Siddiqi, T.J. Is it safe and effective to administer COVID-19 vaccines during pregnancy? A systematic review and meta-analysis. Am. J. Infect. Control 2023, 51, 582–593. [Google Scholar] [CrossRef]
- Yasmin, F.; Najeeb, H.; Naeem, U.; Moeed, A.; Atif, A.R.; Asghar, M.S.; Nimri, N.; Saleem, M.; Bandyopadhyay, D.; Krittanawong, C.; et al. Adverse events following COVID-19 mRNA vaccines: A systematic review of cardiovascular complication, thrombosis, and thrombocytopenia. Immun. Inflamm. Dis. 2023, 11, 10. [Google Scholar] [CrossRef]
- Zhang, X.S.; Moreau, A.; Cruz-Santiago, D.; Langevin, S.; Nguyen, Q.D. Safety and Adverse Events Among Long-term Care Residents Receiving a Third COVID-19 mRNA Vaccine Booster Dose in Quebec. JAMA Netw. Open 2022, 5, 4. [Google Scholar] [CrossRef]
- George, G.; Strauss, M.; Lansdell, E.; Nadesan-Reddy, N.; Moroe, N.; Reddy, T.; Eshun-Wilsonova, I.; Moshabela, M. South African University Staff and Students' Perspectives, Preferences, and Drivers of Hesitancy Regarding COVID-19 Vaccines: A Multi-Methods Study. Vaccines 2022, 10, 13. [Google Scholar] [CrossRef]
- Mayfield, H.J.; Lau, C.L.; Sinclair, J.E.; Brown, S.J.; Baird, A.; Litt, J.; Vuorinen, A.; Short, K.R.; Waller, M.; Mengersen, K. Designing an evidence-based Bayesian network for estimating the risk versus benefits of AstraZeneca COVID-19 vaccine. Vaccine 2022, 40, 3072–3084. [Google Scholar] [CrossRef] [PubMed]
- Rakusa, M.; Ozturk, S.; Moro, E.; Helbok, R.; Bassetti, C.L.; Beghi, E.; Bereczki, D.; Bodini, B.; Di Liberto, G.; Jenkins, T.M.; et al. COVID-19 vaccination hesitancy among people with chronic neurological disorders: A position paper. Eur. J. Neurol. 2022, 29, 2163–2172. [Google Scholar] [CrossRef] [PubMed]
- Pillsbury, A.; Phillips, A.; Deng, L.; Quinn, H.; Macartney, K.; Gidding, H. Background incidence rates of selected adverse events of special interest (AESI) to monitor the safety of COVID-19 vaccines. Vaccine 2023, 41, 3422–3428. [Google Scholar] [CrossRef] [PubMed]
- Phillips, A.; Jiang, Y.; Walsh, D.; Andrews, N.; Artama, M.; Clothier, H.; Cullen, L.; Deng, L.; Escolano, S.; Gentile, A.; et al. Background rates of adverse events of special interest for COVID-19 vaccines: A multinational Global Vaccine Data Network (GVDN) analysis. Vaccine 2023, 41, 6227–6238. [Google Scholar] [CrossRef] [PubMed]
- Nasreen, S.; Calzavara, A.; Buchan, S.A.; Thampi, N.; Johnson, C.; Wilson, S.E.; Kwong, J.C.; Canadian Immunization Research Network Provincial Collaborative Network Ontario, i. Background incidence rates of adverse events of special interest related to COVID-19 vaccines in Ontario, Canada, 2015 to 2020, to inform COVID-19 vaccine safety surveillance. Vaccine 2022, 40, 3305–3312. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Ostropolets, A.; Makadia, R.; Shoaibi, A.; Rao, G.; Sena, A.G.; Martinez-Hernandez, E.; Delmestri, A.; Verhamme, K.; Rijnbeek, P.R.; et al. Characterising the background incidence rates of adverse events of special interest for covid-19 vaccines in eight countries: multinational network cohort study. BMJ 2021, 373, n1435. [Google Scholar] [CrossRef] [PubMed]
- Dhamanti, I.; Suwantika, A.A.; Adlia, A.; Yamani, L.N.; Yakub, F. Adverse Reactions of COVID-19 Vaccines: A Scoping Review of Observational Studies. Int J Gen Med 2023, 16, 609–618. [Google Scholar] [CrossRef]
- Xu, K.; Wang, Z.H.; Qin, M.R.; Gao, Y.Y.; Luo, N.; Xie, W.T.; Zou, Y.H.; Wang, J.; Ma, X.M. A systematic review and meta-analysis of the effectiveness and safety of COVID-19 vaccination in older adults. Front. Immunol. 2023, 14, 16. [Google Scholar] [CrossRef]
- Moro, P.L.; Olson, C.K.; Zhang, B.C.; Marquez, P.; Strid, P. Safety of Booster Doses of Coronavirus Disease 2019 (COVID-19) Vaccine in Pregnancy in the Vaccine Adverse Event Reporting System. Obstet. Gynecol. 2022, 140, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Toussia-Cohen, S.; Yinon, Y.; Peretz-Machluf, R.; Segal, O.; Regev, N.; Asraf, K.; Doolman, R.; Kubani, Y.; Gonen, T.; Regev-Yochay, G.; Peretz, S.B. Early Adverse Events and Immune Response Following Second and Third COVID-19 Vaccination in Pregnancy. J. Clin. Med. 2022, 11, 7. [Google Scholar] [CrossRef]
- Hosseini, R.; Askari, N. A review of neurological side effects of COVID-19 vaccination. Eur. J. Med. Res. 2023, 28, 8. [Google Scholar] [CrossRef] [PubMed]
- Andersson, N.W.; Thiesson, E.M.; Laursen, M.V.; Mogensen, S.H.; Kjaer, J.; Hviid, A. Safety of heterologous primary and booster schedules with ChAdOx1-S and BNT162b2 or mRNA-1273 vaccines: nationwide cohort study. BMJ-British Medical Journal 2022, 378, 10. [Google Scholar] [CrossRef]
- Simone, A.; Herald, J.; Chen, A.Y.; Nayak, R.; Shen, Y.J.A.; Lee, M.S. Acute myocarditis following a third dose of COVID-19 mRNA vaccination in adults. Int. J. Cardiol. 2022, 365, 41–43. [Google Scholar] [CrossRef]
- Al-Qerem, W.; Jarab, A.; Hammad, A.; Alsajri, A.H.; Al-Hishma, S.W.; Ling, J.; Alabdullah, A.S.; Salama, A.; Mosleh, R. Knowledge, Attitudes, and Practices of Adult Iraqi Population Towards COVID-19 Booster Dose: A Cross-Sectional Study. Patient Prefer. Adherence 2022, 16, 1525–1537. [Google Scholar] [CrossRef]
- Fazal, Z.Z.; Sen, P.; Joshi, M.; Ravichandran, N.; Lilleker, J.B.; Agarwal, V.; Kardes, S.; Kim, M.; Day, J.; Makol, A.; et al. COVAD survey 2 long-term outcomes: unmet need and protocol. Rheumatol. Int. 2022, 42, 2151–2158. [Google Scholar] [CrossRef]
- Anastassopoulou, C.; Hatziantoniou, S.; Vlachopoulos, C.; Spanakis, N.; Tsioufis, C.; Tsakris, A.; Lazaros, G. Temporal relationship of myocarditis and pericarditis following COVID-19 vaccination: A pragmatic approach. Int. J. Cardiol. 2022, 358, 136–139. [Google Scholar] [CrossRef] [PubMed]
- Constantin, T.; Pék, T.; Horváth, Z.; Garan, D.; Szabó, A.J. Multisystem inflammatory syndrome in children (MIS-C): Implications for long COVID. Inflammopharmacology 2023, 31, 2221–2236. [Google Scholar] [CrossRef]
- Diaz, G.A.; Parsons, G.T.; Gering, S.K.; Meier, A.R.; Hutchinson, I.V.; Robicsek, A. Myocarditis and Pericarditis After Vaccination for COVID-19. JAMA-J. Am. Med. Assoc. 2021, 326, 1210–1212. [Google Scholar] [CrossRef]
- Mahasing, C.; Doungngern, P.; Jaipong, R.; Nonmuti, P.; Chimmanee, J.; Wongsawat, J.; Boonyasirinant, T.; Wanlapakorn, C.; Leelapatana, P.; Yingchoncharoen, T.; et al. Myocarditis and Pericarditis following COVID-19 Vaccination in Thailand. Vaccines 2023, 11, 14. [Google Scholar] [CrossRef] [PubMed]
- Pepe, S.; Gregory, T.A.; Denniss, A.R. Myocarditis, Pericarditis and Cardiomyopathy After COVID-19 Vaccination. Heart Lung Circ. 2021, 30, 1425–1429. [Google Scholar] [CrossRef]
- Saied, M.H.; van der Griend, L.; van Straalen, J.W.; Wulffraat, N.M.; Vastert, S.; Jansen, M.H.A. The protective effect of COVID-19 vaccines on developing multisystem inflammatory syndrome in children (MIS-C): a systematic literature review and meta-analysis. Pediatr. Rheumatol. 2023, 21, 10. [Google Scholar] [CrossRef]
- Zwiers, L.C.; Ong, D.S.Y.; Grobbee, D.E. COVID-19 Vaccine-Induced Myocarditis and Pericarditis: Towards Identification of Risk Factors. Glob. Heart 2023, 18, 6. [Google Scholar] [CrossRef]
- Chen, P.Y.; Wu, B.J.; Su, M.C.; Lin, Y.H.; Chiang, S.C.; Wu, J.C.; Chen, T.J.; Chen, Y.C. Risk Factors and Incidence Rates of Self-Reported Short-Term Adverse Events of COVID-19 Vaccine Booster Dose. Vaccines 2022, 10, 12. [Google Scholar] [CrossRef]
- Parés-Badell, O.; Zules-Oña, R.; Armadans, L.; Pinós, L.; Borrás-Bermejo, B.; Otero, S.; Rodrigo-Pendás, J.Á.; Vivet-Escalé, M.; Cossio-Gil, Y.; Agustí, A.; et al. Reactogenicity to the mRNA-1273 Booster According to Previous mRNA COVID-19 Vaccination. Vaccines 2022, 10, 1217. [Google Scholar] [CrossRef]
- Lamprinou, M.; Sachinidis, A.; Stamoula, E.; Vavilis, T.; Papazisis, G. COVID-19 vaccines adverse events: potential molecular mechanisms. Immunol. Res. 2023, 71, 356–372. [Google Scholar] [CrossRef]
- Taiwan Centers for Disease Control. Statistics report of COVID-19 vaccine. Available online: https://www.cdc.gov.tw/Category/Page/9jFXNbCe-sFK9EImRRi2Og (accessed on Dec 30, 2023).
- Our World in Data. Coronavirus (COVID-19) Vaccinations. Available online: https://ourworldindata.org/covid-vaccinations (accessed on Dec 30, 2023).
- Omeish, H.; Najadat, A.A.A.; Al-Azzam, S.I.; Tarabin, N.; Abu Hameed, A.; Al-Gallab, N.; Abbas, H.G.; Rababah, L.K.; Rabadi, M.; Karasneh, R.A.; Aldeyab, M.A. Reported COVID-19 vaccines side effects among Jordanian population: a cross sectional study. Human Vaccines Immunother. 2021, 18. [Google Scholar] [CrossRef]
- Willame, C.; Dodd, C.; Duran, C.E.; Elbers, R.; Gini, R.; Bartolini, C.; Paoletti, O.; Wang, L.; Ehrenstein, V.; Kahlert, J.; et al. Background rates of 41 adverse events of special interest for COVID-19 vaccines in 10 European healthcare databases - an ACCESS cohort study. Vaccine 2023, 41, 251–262. [Google Scholar] [CrossRef]
- Lee, C.W.; Sa, S.; Hong, M.; Kim, J.; Shim, S.R.; Han, H.W. Adverse Events and Safety Profile of the COVID-19 Vaccines in Adolescents: Safety Monitoring for Adverse Events Using Real-World Data. Vaccines 2022, 10, 744. [Google Scholar] [CrossRef]
- Goda, K.; Kenzaka, T.; Yahata, S.; Okayama, M.; Nishisaki, H. Association between Adverse Reactions to the First and Second Doses of COVID-19 Vaccine. Vaccines 2022, 10, 1232. [Google Scholar] [CrossRef] [PubMed]
- Madhi, S.A.; Izu, A. Safety of COVID-19 booster dose: is the juice worth the squeeze? Lancet Infect Dis 2023, 23, 1097–1099. [Google Scholar] [CrossRef] [PubMed]
- Yamin, D.; Yechezkel, M.; Arbel, R.; Beckenstein, T.; Sergienko, R.; Duskin-Bitan, H.; Yaron, S.; Peretz, A.; Netzer, D.; Shmueli, E. Safety of monovalent and bivalent BNT162b2 mRNA COVID-19 vaccine boosters in at-risk populations in Israel: a large-scale, retrospective, self-controlled case series study. Lancet Infect Dis 2023, 23, 1130–1142. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).