Submitted:
01 January 2024
Posted:
03 January 2024
You are already at the latest version
Abstract
Keywords:
Introduction
Clinical Findings in Visceral Pain Sexual Dimorphism
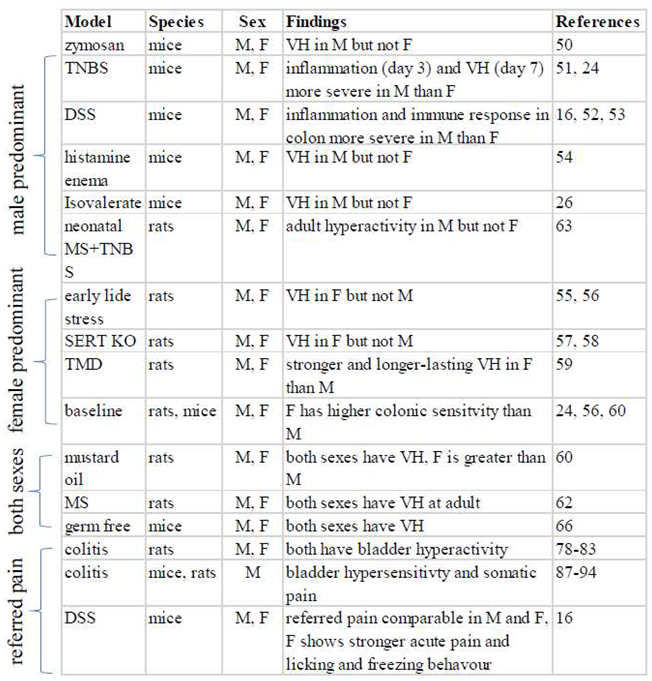
Visceral Pain Preclinical Models with Sexual Dimorphism
Sex Differences in Visceral Pain Comorbidity
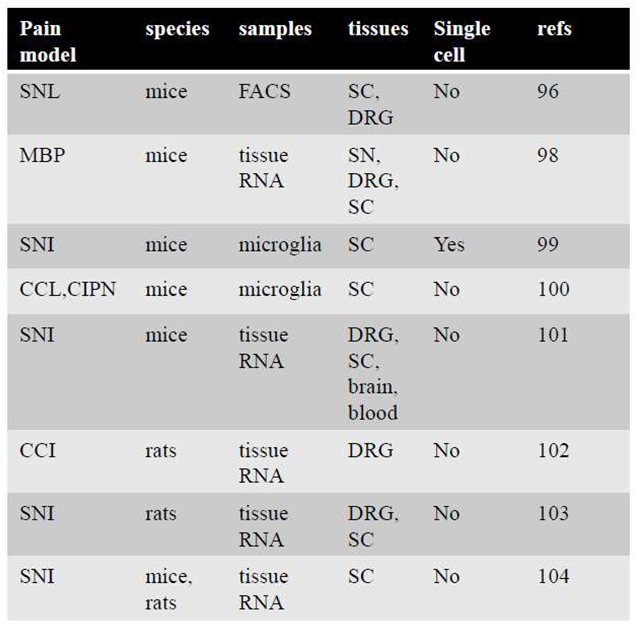
Cellular and Molecular Mechanisms in Pain Sexual Dimorphism
Nociceptors:
Nonneuronal cells:
Sexual Dimorphisms of Brain Circuits and Emotional Effects on Visceral Pain and Pain at Large
Concluding Remarks:
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kennedy, R.; Abd-Elsayed, A. The International Association for the Study of Pain (IASP) Classification of Chronic Pain Syndromes. Abd-Elsayed, A. (eds) Pain 2019. [CrossRef]
- Mayer, E.A.; Ryu, H.J.; Bhatt, R.R. The neurobiology of irritable bowel syndrome. Mol Psychiatry 2023, 28, 1451–1465. [Google Scholar] [CrossRef] [PubMed]
- Engel, F.; Stadnitski, T.; Stroe-Kunold, E.; Berens, S.; Schaefert, R.; Wild, B. Pain and psyche in a patient with irritable bowel syndrome: chicken or egg? A time series case report. BMC Gastroenterol 2021, 21, 309. [Google Scholar] [CrossRef]
- Riedl, A.; Schmidtmann, M.; Stengel, A.; Goebel, M.; Wisser, A.S.; Klapp, B.F.; Monnikes, H. Somatic comorbidities of irritable bowel syndrome: a systematic analysis. J Psychosom Res 2008, 64, 573–582. [Google Scholar] [CrossRef]
- Chang, L.; Mayer, E.A.; Labus, J.S.; Schmulson, M.; Lee, O.Y.; Olivas, T.I.; Stains, J.; Naliboff, B.D. Effect of sex on perception of rectosigmoid stimuli in irritable bowel syndrome. Am J Physiol Regul Integr Comp Physiol 2006, 291, R277–R284. [Google Scholar] [CrossRef] [PubMed]
- Simren, M.; Abrahamsson, H.; Bjornsson, E.S. Lipid-induced colonic hypersensitivity in the irritable bowel syndrome: the role of bowel habit, sex, and psychologic factors. Clin Gastroenterol Hepatol 2007, 5, 201–208. [Google Scholar] [CrossRef]
- Chang, L.; Heitkemper, M.M. Gender differences in irritable bowel syndrome. Gastroenterology 2002, 123, 1686–1701. [Google Scholar] [CrossRef]
- Choghakhori, R.; Abbasnezhad, A.; Amani, R.; Alipour, M. Sex-Related Differences in Clinical Symptoms, Quality of Life, and Biochemical Factors in Irritable Bowel Syndrome. Digest Dis Sci 2017, 62, 1550–1560. [Google Scholar] [CrossRef]
- Hong, J.Y.; Labus, J.S.; Kilpatrick, L.A.; Stains, J.; Heendeniya, N.; Smith, S.R.; Katibian, D.; Tillisch, K.; Mayer, E.A. Patients With Irritable Bowel Syndrome Show Sex Related Differences in Resting-State Functional Connectivity. Gastroenterology 2014, 146, S847. [Google Scholar] [CrossRef]
- Goodman, W.A.; Erkkila, I.P.; Pizarro, T.T. Sex matters: impact on pathogenesis, presentation and treatment of inflammatory bowel disease. Nat Rev Gastroenterol Hepatol 2020, 17, 740–754. [Google Scholar] [CrossRef]
- Camilleri, M. Sex as a biological variable in irritable bowel syndrome. Neurogastroent Motil 2020, 32, e13802. [Google Scholar] [CrossRef]
- Mogil, J.S. Qualitative sex differences in pain processing: emerging evidence of a biased literature. Nat Rev Neurosci 2020, 21, 353–365. [Google Scholar] [CrossRef] [PubMed]
- Stephens, K.E.; Zhou, W.; Ji, Z.; Chen, Z.; He, S.; Ji, H.; Guan, Y.; Taverna, S.D. Sex differences in gene regulation in the dorsal root ganglion after nerve injury. BMC Genomics 2019, 20, 147. [Google Scholar] [CrossRef]
- Presto, P.; Mazzitelli, M.; Junell, R.; Griffin, Z.; Neugebauer, V. Sex differences in pain along the neuraxis. Neuropharmacology 2022, 210, 109030. [Google Scholar] [CrossRef] [PubMed]
- Bartley, E.J.; Fillingim, R.B. Sex differences in pain: a brief review of clinical and experimental findings. Br J Anaesth 2013, 111, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Francis-Malave, A.M.; Martinez Gonzalez, S.; Pichardo, C.; Wilson, T.D.; Rivera-Garcia, L.G.; Brinster, L.R.; Carrasquillo, Y. Sex differences in pain-related behaviors and clinical progression of disease in mouse models of colonic pain. Pain 2023, 164, 197–215. [Google Scholar] [CrossRef] [PubMed]
- Classification of chronic pain. Descriptions of chronic pain syndromes and definitions of pain terms. Prepared by the International Association for the Study of Pain, Subcommittee on Taxonomy. Pain Suppl 1986, 3, S1–S226. [Google Scholar]
- Mazure, C.M.; Jones, D.P. Twenty years and still counting: including women as participants and studying sex and gender in biomedical research. BMC Womens Health 2015, 15, 94. [Google Scholar] [CrossRef] [PubMed]
- Legato, M.J. The International Society for Gender Medicine History and Highlights PREFACE. International Society for Gender Medicine: History and Highlights 2017, Xiii-Xvi.
- Samulowitz, A.; Gremyr, I.; Eriksson, E.; Hensing, G. "Brave Men" and "Emotional Women": A Theory-Guided Literature Review on Gender Bias in Health Care and Gendered Norms towards Patients with Chronic Pain. Pain Res Manag 2018, 2018, 6358624. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Kim, N. Sex-Gender Differences in Irritable Bowel Syndrome. J Neurogastroenterol Motil 2018, 24, 544–558. [Google Scholar] [CrossRef]
- Gregus, A.M.; Levine, I.S.; Eddinger, K.A.; Yaksh, T.L.; Buczynski, M.W. Sex differences in neuroimmune and glial mechanisms of pain. Pain 2021, 162, 2186–2200. [Google Scholar] [CrossRef]
- Beery, A.K. Inclusion of females does not increase variability in rodent research studies. Curr Opin Behav Sci 2018, 23, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Madar, J.; Tiwari, N.; Smith, C.; Sharma, D.; Shen, S.; Elmahdi, A.; Qiao, L.Y. Piezo2 regulates colonic mechanical sensitivity in a sex specific manner in mice. Nat Commun 2023, 14, 2158. [Google Scholar] [CrossRef] [PubMed]
- Obeidat, A.M.; Wood, M.J.; Adamczyk, N.S.; Ishihara, S.; Li, J.; Wang, L.; Ren, D.; Bennett, D.A.; Miller, R.J.; Malfait, A.M.; et al. Piezo2 expressing nociceptors mediate mechanical sensitization in experimental osteoarthritis. Nat Commun 2023, 14, 2479. [Google Scholar] [CrossRef]
- Bayrer, J.R.; Castro, J.; Venkataraman, A.; Touhara, K.K.; Rossen, N.D.; Morrie, R.D.; Maddern, J.; Hendry, A.; Braverman, K.N.; Garcia-Caraballo, S.; et al. Gut enterochromaffin cells drive visceral pain and anxiety. Nature 2023, 616, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Icenhour, A.; Labrenz, F.; Roderigo, T.; Siebert, C.; Elsenbruch, S.; Benson, S. Are there sex differences in visceral sensitivity in young healthy men and women? Neurogastroenterol Motil 2019, 31, e13664. [Google Scholar] [CrossRef]
- Posserud, I.; Syrous, A.; Lindström, L.; Tack, J.; Abrahamsson, H.; Simrén, M. Altered rectal perception in irritable bowel syndrome is associated with symptom severity. Gastroenterology 2007, 133, 1113–1123. [Google Scholar] [CrossRef]
- Almario, C.V.; Sharabi, E.; Chey, W.D.; Lauzon, M.; Higgins, C.S.; Spiegel, B.M.R. Prevalence and Burden of Illness of Rome IV Irritable Bowel Syndrome in the United States: Results From a Nationwide Cross-Sectional Study. Gastroenterology 2023, 165, 1475–1487. [Google Scholar] [CrossRef]
- Adeyemo, M.A.; Spiegel, B.M.; Chang, L. Meta-analysis: do irritable bowel syndrome symptoms vary between men and women? Aliment Pharmacol Ther 2010, 32, 738–755. [Google Scholar] [CrossRef]
- Lungaro, L.; Costanzini, A.; Manza, F.; Barbalinardo, M.; Gentili, D.; Guarino, M.; Caputo, F.; Zoli, G.; De Giorgio, R.; Caio, G. Impact of Female Gender in Inflammatory Bowel Diseases: A Narrative Review. J Pers Med 2023, 13. [Google Scholar] [CrossRef]
- Barbara, G.; Grover, M.; Bercik, P.; Corsetti, M.; Ghoshal, U.C.; Ohman, L.; Rajilic-Stojanovic, M. Rome Foundation Working Team Report on Post-Infection Irritable Bowel Syndrome. Gastroenterology 2019, 156, 46–58. [Google Scholar] [CrossRef]
- Park, J.H.; Kang, S.H.; Kim, J.S.; Moon, H.S.; Sung, J.K.; Jeong, H.Y. Contribution of sex and gender roles to the incidence of post-infectious irritable bowel syndrome in a prospective study. Sci Rep 2023, 13, 19467. [Google Scholar] [CrossRef]
- Grinsvall, C.; Tornblom, H.; Tack, J.; Van Oudenhove, L.; Simren, M. Relationships between psychological state, abuse, somatization and visceral pain sensitivity in irritable bowel syndrome. United European Gastroenterol J 2018, 6, 300–309. [Google Scholar] [CrossRef] [PubMed]
- Oka, P.; Parr, H.; Barberio, B.; Black, C.J.; Savarino, E.V.; Ford, A.C. Global prevalence of irritable bowel syndrome according to Rome III or IV criteria: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol 2020, 5, 908–917. [Google Scholar] [CrossRef] [PubMed]
- Greuter, T.; Manser, C.; Pittet, V.; Vavricka, S.R.; Biedermann, L.; on behalf of Swiss Ibdnet. Gender Differences in Inflammatory Bowel Disease. Digestion 2020, 101 Suppl 1, 98–104. [Google Scholar] [CrossRef]
- Smith, Y.R.; Stohler, C.S.; Nichols, T.E.; Bueller, J.A.; Koeppe, R.A.; Zubieta, J.K. Pronociceptive and antinociceptive effects of estradiol through endogenous opioid neurotransmission in women. J Neurosci 2006, 26, 5777–5785. [Google Scholar] [CrossRef] [PubMed]
- Aranda, G.; Fernandez-Rebollo, E.; Pradas-Juni, M.; Hanzu, F.A.; Kalko, S.G.; Halperin, I.; Mora, M. Effects of sex steroids on the pattern of methylation and expression of the promoter region of estrogen and androgen receptors in people with gender dysphoria under cross-sex hormone treatment. J Steroid Biochem Mol Biol 2017, 172, 20–28. [Google Scholar] [CrossRef]
- Mulak, A.; Tache, Y.; Larauche, M. Sex hormones in the modulation of irritable bowel syndrome. World J Gastroenterol 2014, 20, 2433–2448. [Google Scholar] [CrossRef]
- Heitkemper, M.M.; Jarrett, M. Gender differences and hormonal modulation in visceral pain. Curr Pain Headache Rep 2001, 5, 35–43. [Google Scholar] [CrossRef]
- Athnaiel, O.; Cantillo, S.; Paredes, S.; Knezevic, N.N. The Role of Sex Hormones in Pain-Related Conditions. Int J Mol Sci 2023, 24. [Google Scholar] [CrossRef]
- Lacy, B.E.; Rosemore, J.; Robertson, D.; Corbin, D.A.; Grau, M.; Crowell, M.D. Physicians' attitudes and practices in the evaluation and treatment of irritable bowel syndrome. Scand J Gastroenterol 2006, 41, 892–902. [Google Scholar] [CrossRef]
- Moon, E.C.; Chambers, C.T.; Larochette, A.C.; Hayton, K.; Craig, K.D.; McGrath, P.J. Sex differences in parent and child pain ratings during an experimental child pain task. Pain Res Manag 2008, 13, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Chaloner, A.; Greenwood-Van Meerveld, B. Early life adversity as a risk factor for visceral pain in later life: importance of sex differences. Front Neurosci 2013, 7, 13. [Google Scholar] [CrossRef] [PubMed]
- Blankenburg, M.; Boekens, H.; Hechler, T.; Maier, C.; Krumova, E.; Scherens, A.; Magerl, W.; Aksu, F.; Zernikow, B. Reference values for quantitative sensory testing in children and adolescents: developmental and gender differences of somatosensory perception. Pain 2010, 149, 76–88. [Google Scholar] [CrossRef] [PubMed]
- Boerner, K.E.; Birnie, K.A.; Caes, L.; Schinkel, M.; Chambers, C.T. Sex differences in experimental pain among healthy children: A systematic review and meta-analysis. Pain 2014, 155, 983–993. [Google Scholar] [CrossRef] [PubMed]
- Martin, A.L.; McGrath, P.A.; Brown, S.C.; Katz, J. Children with chronic pain: impact of sex and age on long-term outcomes. Pain 2007, 128, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Ray, P.R.; Shiers, S.; Caruso, J.P.; Tavares-Ferreira, D.; Sankaranarayanan, I.; Uhelski, M.L.; Li, Y.; North, R.Y.; Tatsui, C.; Dussor, G.; et al. RNA profiling of human dorsal root ganglia reveals sex differences in mechanisms promoting neuropathic pain. Brain 2023, 146, 749–766. [Google Scholar] [CrossRef] [PubMed]
- LaCroix-Fralish, M.L.; Austin, J.S.; Zheng, F.Y.; Levitin, D.J.; Mogil, J.S. Patterns of pain: meta-analysis of microarray studies of pain. Pain 2011, 152, 1888–1898. [Google Scholar] [CrossRef]
- Guo, T.; Liu, J.; Chen, L.; Bian, Z.; Zheng, G.; Feng, B. Sex differences in zymosan-induced behavioral visceral hypersensitivity and colorectal afferent sensitization. Am J Physiol Gastrointest Liver Physiol 2023. [Google Scholar] [CrossRef]
- Kozik, A.J.; Nakatsu, C.H.; Chun, H.; Jones-Hall, Y.L. Age, sex, and TNF associated differences in the gut microbiota of mice and their impact on acute TNBS colitis. Exp Mol Pathol 2017, 103, 311–319. [Google Scholar] [CrossRef]
- Babickova, J.; Tothova, L.; Lengyelova, E.; Bartonova, A.; Hodosy, J.; Gardlik, R.; Celec, P. Sex Differences in Experimentally Induced Colitis in Mice: a Role for Estrogens. Inflammation 2015, 38, 1996–2006. [Google Scholar] [CrossRef]
- Hases, L.; Birgersson, M.; Indukuri, R.; Archer, A.; Williams, C. Colitis Induces Sex-Specific Intestinal Transcriptomic Responses in Mice. Int J Mol Sci 2022, 23. [Google Scholar] [CrossRef]
- McClain, J.L.; Morales-Soto, W.; Gonzales, J.; Parmar, V.; Demireva, E.Y.; Gulbransen, B.D. Sexually Dimorphic Effects of Histamine Degradation by Enteric Glial Histamine N-Methyltransferase (HNMT) on Visceral Hypersensitivity. Biomolecules 2023, 13. [Google Scholar] [CrossRef] [PubMed]
- Louwies, T.; Greenwood-Van Meerveld, B. Sex differences in the epigenetic regulation of chronic visceral pain following unpredictable early life stress. Neurogastroenterol Motil 2020, 32, e13751. [Google Scholar] [CrossRef]
- Chen, J.H.; Sun, Y.; Ju, P.J.; Wei, J.B.; Li, Q.J.; Winston, J.H. Estrogen augmented visceral pain and colonic neuron modulation in a double-hit model of prenatal and adult stress. World J Gastroenterol 2021, 27, 5060–5075. [Google Scholar] [CrossRef]
- Galligan, J.J.; Patel, B.A.; Schneider, S.P.; Wang, H.; Zhao, H.; Novotny, M.; Bian, X.; Kabeer, R.; Fried, D.; Swain, G.M. Visceral hypersensitivity in female but not in male serotonin transporter knockout rats. Neurogastroenterol Motil 2013, 25, e373–e381. [Google Scholar] [CrossRef] [PubMed]
- El-Ayache, N.; Galligan, J.J. 5-HT(3) receptor signaling in serotonin transporter-knockout rats: a female sex-specific animal model of visceral hypersensitivity. Am J Physiol Gastrointest Liver Physiol 2019, 316, G132–G143. [Google Scholar] [CrossRef]
- Da Silva, J.T.; Hernandez-Rojas, L.G.; Mekonen, H.K.; Hanson, S.; Melemedjian, O.; Scott, A.J.; Ernst, R.K.; Seminowicz, D.A.; Traub, R.J. Sex differences in visceral sensitivity and brain activity in a rat model of comorbid pain: a longitudinal study. Pain 1097. [Google Scholar] [CrossRef]
- Ji, Y.; Tang, B.; Cao, D.Y.; Wang, G.; Traub, R.J. Sex differences in spinal processing of transient and inflammatory colorectal stimuli in the rat. Pain 2012, 153, 1965–1973. [Google Scholar] [CrossRef]
- Qiao, L.Y.; Gulick, M.A. Region-specific changes in the phosphorylation of ERK1/2 and ERK5 in rat micturition pathways following cyclophosphamide-induced cystitis. Am J Physiol Regul Integr Comp Physiol 2007, 292, R1368–R1375. [Google Scholar] [CrossRef] [PubMed]
- Yi, L.; Zhang, H.; Sun, H.; Zhou, L.; Chen, Y.; Xuan, L.; Jiang, Y.; Xu, S. Maternal Separation Induced Visceral Hypersensitivity from Childhood to Adulthood. J Neurogastroenterol Motil 2017, 23, 306–315. [Google Scholar] [CrossRef]
- Hasegawa, R.; Saito-Nakaya, K.; Gu, L.; Kanazawa, M.; Fukudo, S. Maternal separation and TNBS-induced gut inflammation synergistically alter the sexually differentiated stress response in rats. Biopsychosoc Med 2023, 17, 7. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Woo, S.Y.; Raza, S.; Ho, D.; Jeon, S.W.; Chang, Y.; Ryu, S.; Kim, H.L.; Kim, H.N. Association between gut microbiota and anxiety symptoms: A large population-based study examining sex differences. J Affect Disord 2023, 333, 21–29. [Google Scholar] [CrossRef] [PubMed]
- McGee, J.S.; Huttenhower, C. Of mice and men and women: Sexual dimorphism of the gut microbiome. Int J Womens Dermatol 2021, 7, 533–538. [Google Scholar] [CrossRef] [PubMed]
- Pujo, J.; De Palma, G.; Lu, J.; Galipeau, H.J.; Surette, M.G.; Collins, S.M.; Bercik, P. Gut microbiota modulates visceral sensitivity through calcitonin gene-related peptide (CGRP) production. Gut Microbes 2023, 15, 2188874. [Google Scholar] [CrossRef] [PubMed]
- Tao, E.; Long, G.; Yang, T.; Chen, B.; Guo, R.; Ye, D.; Fang, M.; Jiang, M. Maternal Separation Induced Visceral Hypersensitivity Evaluated via Novel and Small Size Distention Balloon in Post-weaning Mice. Front Neurosci 2021, 15, 803957. [Google Scholar] [CrossRef] [PubMed]
- Wyndaele, M.; De Winter, B.Y.; Pelckmans, P.A.; De Wachter, S.; Van Outryve, M.; Wyndaele, J.J. Exploring associations between lower urinary tract symptoms (LUTS) and gastrointestinal (GI) problems in women: a study in women with urological and GI problems vs a control population. BJU Int 2015, 115, 958–967. [Google Scholar] [CrossRef] [PubMed]
- Wyndaele, M.; De Winter, B.Y.; Pelckmans, P.; Wyndaele, J.J. Lower bowel function in urinary incontinent women, urinary continent women and in controls. Neurourol Urodyn 2011, 30, 138–143. [Google Scholar] [CrossRef]
- Carter, D.; Beer-Gabel, M. Lower urinary tract symptoms in chronically constipated women. Int Urogynecol J 2012, 23, 1785–1789. [Google Scholar] [CrossRef] [PubMed]
- Thurmon, K.L.; Breyer, B.N.; Erickson, B.A. Association of bowel habits with lower urinary tract symptoms in men: findings from the 2005-2006 and 2007-2008 National Health and Nutrition Examination Survey. J Urol 2013, 189, 1409–1414. [Google Scholar] [CrossRef]
- Coyne, K.S.; Cash, B.; Kopp, Z.; Gelhorn, H.; Milsom, I.; Berriman, S.; Vats, V.; Khullar, V. The prevalence of chronic constipation and faecal incontinence among men and women with symptoms of overactive bladder. BJU Int 2011, 107, 254–261. [Google Scholar] [CrossRef]
- Wolfe-Christensen, C.; Manolis, A.; Guy, W.C.; Kovacevic, N.; Zoubi, N.; El-Baba, M.; Kovacevic, L.G.; Lakshmanan, Y. Bladder and bowel dysfunction: evidence for multidisciplinary care. J Urol 2013, 190, 1864–1868. [Google Scholar] [CrossRef] [PubMed]
- Sampaio, C.; Sousa, A.S.; Fraga, L.G.; Veiga, M.L.; Bastos Netto, J.M.; Barroso, U., Jr. Constipation and Lower Urinary Tract Dysfunction in Children and Adolescents: A Population-Based Study. Front Pediatr 2016, 4, 101. [Google Scholar] [CrossRef] [PubMed]
- Corre, C.S.; Grant, N.; Sadjadi, R.; Hayden, D.; Becker, C.; Gomery, P.; Eichler, F.S. Beyond gait and balance: urinary and bowel dysfunction in X-linked adrenoleukodystrophy. Orphanet J Rare Dis 2021, 16, 14. [Google Scholar] [CrossRef]
- Stabell, N.; Stubhaug, A.; Flaegstad, T.; Nielsen, C.S. Increased pain sensitivity among adults reporting irritable bowel syndrome symptoms in a large population-based study. Pain 2013, 154, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Stabell, N.; Stubhaug, A.; Flaegstad, T.; Mayer, E.; Naliboff, B.D.; Nielsen, C.S. Widespread hyperalgesia in adolescents with symptoms of irritable bowel syndrome: results from a large population-based study. J Pain 2014, 15, 898–906. [Google Scholar] [CrossRef] [PubMed]
- Pezzone, M.A.; Liang, R.; Fraser, M.O. A model of neural cross-talk and irritation in the pelvis: implications for the overlap of chronic pelvic pain disorders. Gastroenterology 2005, 128, 1953–1964. [Google Scholar] [CrossRef] [PubMed]
- Xia, C.M.; Gulick, M.A.; Yu, S.J.; Grider, J.R.; Murthy, K.S.; Kuemmerle, J.F.; Akbarali, H.I.; Qiao, L.Y. Up-regulation of brain-derived neurotrophic factor in primary afferent pathway regulates colon-to-bladder cross-sensitization in rat. J Neuroinflammation 2012, 9, 30. [Google Scholar] [CrossRef]
- Yoshikawa, S.; Kawamorita, N.; Oguchi, T.; Funahashi, Y.; Tyagi, P.; Chancellor, M.B.; Yoshimura, N. Pelvic organ cross-sensitization to enhance bladder and urethral pain behaviors in rats with experimental colitis. Neuroscience 2015, 284, 422–429. [Google Scholar] [CrossRef]
- Noor-Mohammadi, E.; Ligon, C.O.; Mackenzie, K.D.; Stratton, J.; Shnider, S.J.; Greenwood-Van Meerveld, B. Antinociceptive Effects of an Anti-CGRP Antibody in Rat Models of Colon-Bladder Cross-Organ Sensitization. J Pharmacol Exp Ther 2023, 387, 4–14. [Google Scholar] [CrossRef]
- Majima, T.; Funahashi, Y.; Kawamorita, N.; Takai, S.; Matsukawa, Y.; Yamamoto, T.; Yoshimura, N.; Gotoh, M. Role of microglia in the spinal cord in colon-to-bladder neural crosstalk in a rat model of colitis. Neurourol Urodyn 2018, 37, 1320–1328. [Google Scholar] [CrossRef]
- Malykhina, A.P.; Qin, C.; Greenwood-van Meerveld, B.; Foreman, R.D.; Lupu, F.; Akbarali, H.I. Hyperexcitability of convergent colon and bladder dorsal root ganglion neurons after colonic inflammation: mechanism for pelvic organ cross-talk. Neurogastroenterol Motil 2006, 18, 936–948. [Google Scholar] [CrossRef] [PubMed]
- Funahashi, Y.; Takahashi, R.; Mizoguchi, S.; Suzuki, T.; Takaoka, E.; Ni, J.; Wang, Z.; DeFranco, D.B.; de Groat, W.C.; Tyagi, P.; et al. Bladder overactivity and afferent hyperexcitability induced by prostate-to-bladder cross-sensitization in rats with prostatic inflammation. J Physiol 2019, 597, 2063–2078. [Google Scholar] [CrossRef] [PubMed]
- Grundy, L.; Harrington, A.M.; Castro, J.; Garcia-Caraballo, S.; Deiteren, A.; Maddern, J.; Rychkov, G.Y.; Ge, P.; Peters, S.; Feil, R.; et al. Chronic linaclotide treatment reduces colitis-induced neuroplasticity and reverses persistent bladder dysfunction. JCI Insight 2018, 3. [Google Scholar] [CrossRef] [PubMed]
- Lei, Q.; Pan, X.Q.; Villamor, A.N.; Asfaw, T.S.; Chang, S.; Zderic, S.A.; Malykhina, A.P. Lack of transient receptor potential vanilloid 1 channel modulates the development of neurogenic bladder dysfunction induced by cross-sensitization in afferent pathways. J Neuroinflammation 2013, 10, 3. [Google Scholar] [CrossRef] [PubMed]
- Lamb, K.; Zhong, F.; Gebhart, G.F.; Bielefeldt, K. Experimental colitis in mice and sensitization of converging visceral and somatic afferent pathways. Am J Physiol Gastrointest Liver Physiol 2006, 290, G451–G457. [Google Scholar] [CrossRef] [PubMed]
- Atmani, K.; Wuestenberghs, F.; Baron, M.; Boulete, I.; Guerin, C.; Bahlouli, W.; Vaudry, D.; do Rego, J.C.; Cornu, J.N.; Leroi, A.M.; et al. Bladder-colon chronic cross-sensitization involves neuro-glial pathways in male mice. World J Gastroenterol 2022, 28, 6935–6949. [Google Scholar] [CrossRef] [PubMed]
- Jain, P.; Hassan, A.M.; Koyani, C.N.; Mayerhofer, R.; Reichmann, F.; Farzi, A.; Schuligoi, R.; Malle, E.; Holzer, P. Behavioral and molecular processing of visceral pain in the brain of mice: impact of colitis and psychological stress. Front Behav Neurosci 2015, 9, 177. [Google Scholar] [CrossRef] [PubMed]
- Amoroso, M.; Kempter, E.; Eleslambouly, T.; Lowry, C.A.; Langgartner, D.; Reber, S.O. Intranasal Mycobacterium vaccae administration prevents stress-induced aggravation of dextran sulfate sodium (DSS) colitis. Brain Behav Immun 2019, 80, 595–604. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Price, D.D.; Caudle, R.M.; Verne, G.N. Visceral and somatic hypersensitivity in TNBS-induced colitis in rats. Dig Dis Sci 2008, 53, 429–435. [Google Scholar] [CrossRef]
- Zhou, Q.; Price, D.D.; Caudle, R.M.; Verne, G.N. Visceral and somatic hypersensitivity in a subset of rats following TNBS-induced colitis. Pain 2008, 134, 9–15. [Google Scholar] [CrossRef]
- Jain, P.; Materazzi, S.; De Logu, F.; Rossi Degl'Innocenti, D.; Fusi, C.; Li Puma, S.; Marone, I.M.; Coppi, E.; Holzer, P.; Geppetti, P.; et al. Transient receptor potential ankyrin 1 contributes to somatic pain hypersensitivity in experimental colitis. Sci Rep 2020, 10, 8632. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Han, S.; Huang, Q.; He, S.Q.; Ford, N.C.; Zheng, Q.; Chen, Z.; Yu, S.; Dong, X.; Guan, Y. Calcium imaging in population of dorsal root ganglion neurons unravels novel mechanisms of visceral pain sensitization and referred somatic hypersensitivity. Pain 2021, 162, 1068–1081. [Google Scholar] [CrossRef]
- Mecklenburg, J.; Zou, Y.; Wangzhou, A.; Garcia, D.; Lai, Z.; Tumanov, A.V.; Dussor, G.; Price, T.J.; Akopian, A.N. Transcriptomic sex differences in sensory neuronal populations of mice. Sci Rep 2020, 10, 15278. [Google Scholar] [CrossRef] [PubMed]
- Lopes, D.M.; Malek, N.; Edye, M.; Jager, S.B.; McMurray, S.; McMahon, S.B.; Denk, F. Sex differences in peripheral not central immune responses to pain-inducing injury. Sci Rep 2017, 7, 16460. [Google Scholar] [CrossRef]
- Vacca, V.; Marinelli, S.; Pieroni, L.; Urbani, A.; Luvisetto, S.; Pavone, F. 17beta-estradiol counteracts neuropathic pain: a behavioural, immunohistochemical, and proteomic investigation on sex-related differences in mice. Sci Rep 2016, 6, 18980. [Google Scholar] [CrossRef] [PubMed]
- Chernov, A.V.; Hullugundi, S.K.; Eddinger, K.A.; Dolkas, J.; Remacle, A.G.; Angert, M.; James, B.P.; Yaksh, T.L.; Strongin, A.Y.; Shubayev, V.I. A myelin basic protein fragment induces sexually dimorphic transcriptome signatures of neuropathic pain in mice. J Biol Chem 2020, 295, 10807–10821. [Google Scholar] [CrossRef]
- Tansley, S.; Uttam, S.; Urena Guzman, A.; Yaqubi, M.; Pacis, A.; Parisien, M.; Deamond, H.; Wong, C.; Rabau, O.; Brown, N.; et al. Single-cell RNA sequencing reveals time- and sex-specific responses of mouse spinal cord microglia to peripheral nerve injury and links ApoE to chronic pain. Nat Commun 2022, 13, 843. [Google Scholar] [CrossRef]
- Fiore, N.T.; Yin, Z.R.; Guneykaya, D.; Gauthier, C.D.; Hayes, J.; D'Hary, A.; Butovsky, O.; Moalem-Taylor, G. Sex-specific transcriptome of spinal microglia in neuropathic pain due to peripheral nerve injury. Glia 2022, 70, 675–696. [Google Scholar] [CrossRef]
- Parisien, M.; Samoshkin, A.; Tansley, S.N.; Piltonen, M.H.; Martin, L.J.; El-Hachem, N.; Dagostino, C.; Allegri, M.; Mogil, J.S.; Khoutorsky, A.; et al. Genetic pathway analysis reveals a major role for extracellular matrix organization in inflammatory and neuropathic pain. Pain 2019, 160, 932–944. [Google Scholar] [CrossRef]
- Stephens, K.E.; Chen, Z.Y.; Sivanesan, E.; Raja, S.N.; Linderoth, B.; Taverna, S.D.; Guan, Y. RNA-seq of spinal cord from nerve-injured rats after spinal cord stimulation. Mol Pain 2018, 14. [Google Scholar] [CrossRef]
- Ahlström, F.H.G.; Mätlik, K.; Viisanen, H.; Blomqvist, K.J.; Liu, X.; Lilius, T.O.; Sidorova, Y.; Kalso, E.A.; Rauhala, P.V. Spared Nerve Injury Causes Sexually Dimorphic Mechanical Allodynia and Differential Gene Expression in Spinal Cords and Dorsal Root Ganglia in Rats. Mol Neurobiol 2021, 58, 5396–5419. [Google Scholar] [CrossRef] [PubMed]
- Ghazisaeidi, S.; Muley, M.M.; Tu, Y.S.; Finn, D.P.; Kolahdouzan, M.; Pitcher, G.M.; Kim, D.; Sengar, A.S.; Ramani, A.K.; Brudno, M.; et al. Conserved transcriptional programming across sex and species after peripheral nerve injury predicts treatments for neuropathic pain. Brit J Pharmacol 2023, 180, 2822–2836. [Google Scholar] [CrossRef] [PubMed]
- Usoskin, D.; Furlan, A.; Islam, S.; Abdo, H.; Lonnerberg, P.; Lou, D.; Hjerling-Leffler, J.; Haeggstrom, J.; Kharchenko, O.; Kharchenko, P.V.; et al. Unbiased classification of sensory neuron types by large-scale single-cell RNA sequencing. Nat Neurosci 2015, 18, 145–153. [Google Scholar] [CrossRef]
- Chiu, I.M.; Heesters, B.A.; Ghasemlou, N.; Von Hehn, C.A.; Zhao, F.; Tran, J.; Wainger, B.; Strominger, A.; Muralidharan, S.; Horswill, A.R.; et al. Bacteria activate sensory neurons that modulate pain and inflammation. Nature 2013, 501, 52. [Google Scholar] [CrossRef] [PubMed]
- Qiao, L.Y.; Grider, J.R. Colitis induces calcitonin gene-related peptide expression and Akt activation in rat primary afferent pathways. Exp Neurol 2009, 219, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.; Tiwari, N.; Madar, J.; Mehta, P.; Qiao, L.Y. Beta 2-adrenergic receptor mediates noradrenergic action to induce cyclic adenosine monophosphate response element-binding protein phosphorylation in satellite glial cells of dorsal root ganglia to regulate visceral hypersensitivity. Pain 2022, 163, 180–192. [Google Scholar] [CrossRef] [PubMed]
- Delafoy, L.; Gelot, A.; Ardid, D.; Eschalier, A.; Bertrand, C.; Doherty, A.M.; Diop, L. Interactive involvement of brain derived neurotrophic factor, nerve growth factor, and calcitonin gene related peptide in colonic hypersensitivity in the rat. Gut 2006, 55, 940–945. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, N.; Smith, C.; Sharma, D.; Shen, S.; Mehta, P.; Qiao, L.Y. Satellite glial cells drive colonic and somatic mechanical pain via upregulating Piezo2 in DRG neurons Cell Reports 2023, SSRN.
- Han, J.S.; Li, W.; Neugebauer, V. Critical role of calcitonin gene-related peptide 1 receptors in the amygdala in synaptic plasticity and pain behavior. J Neurosci 2005, 25, 10717–10728. [Google Scholar] [CrossRef] [PubMed]
- Kay, J.C.; Xia, C.M.; Liu, M.; Shen, S.; Yu, S.J.; Chung, C.; Qiao, L.Y. Endogenous PI3K/Akt and NMDAR act independently in the regulation of CREB activity in lumbosacral spinal cord in cystitis. Exp Neurol 2013, 250, 366–375. [Google Scholar] [CrossRef]
- Presto, P.; Neugebauer, V. Sex Differences in CGRP Regulation and Function in the Amygdala in a Rat Model of Neuropathic Pain. Front Mol Neurosci 2022, 15, 928587. [Google Scholar] [CrossRef]
- Paige, C.; Plasencia-Fernandez, I.; Kume, M.; Papalampropoulou-Tsiridou, M.; Lorenzo, L.E.; David, E.T.; He, L.; Mejia, G.L.; Driskill, C.; Ferrini, F.; et al. A Female-Specific Role for Calcitonin Gene-Related Peptide (CGRP) in Rodent Pain Models. J Neurosci 2022, 42, 1930–1944. [Google Scholar] [CrossRef] [PubMed]
- Szabo-Pardi, T.A.; Barron, L.R.; Lenert, M.E.; Burton, M.D. Sensory Neuron TLR4 mediates the development of nerve-injury induced mechanical hypersensitivity in female mice. Brain Behav Immun 2021, 97, 42–60. [Google Scholar] [CrossRef] [PubMed]
- Inyang, K.E.; Szabo-Pardi, T.; Wentworth, E.; McDougal, T.A.; Dussor, G.; Burton, M.D.; Price, T.J. The antidiabetic drug metformin prevents and reverses neuropathic pain and spinal cord microglial activation in male but not female mice. Pharmacol Res 2019, 139, 1–16. [Google Scholar] [CrossRef]
- Saika, F.; Matsuzaki, S.; Kobayashi, D.; Ideguchi, Y.; Nakamura, T.Y.; Kishioka, S.; Kiguchi, N. Chemogenetic Regulation of CX3CR1-Expressing Microglia Using Gi-DREADD Exerts Sex-Dependent Anti-Allodynic Effects in Mouse Models of Neuropathic Pain. Front Pharmacol 2020, 11, 925. [Google Scholar] [CrossRef] [PubMed]
- Sorge, R.E.; Mapplebeck, J.C.; Rosen, S.; Beggs, S.; Taves, S.; Alexander, J.K.; Martin, L.J.; Austin, J.S.; Sotocinal, S.G.; Chen, D.; et al. Different immune cells mediate mechanical pain hypersensitivity in male and female mice. Nat Neurosci 2015, 18, 1081–1083. [Google Scholar] [CrossRef] [PubMed]
- Tsujikawa, S.; DeMeulenaere, K.E.; Centeno, M.V.; Ghazisaeidi, S.; Martin, M.E.; Tapies, M.R.; Maneshi, M.M.; Yamashita, M.; Stauderman, K.A.; Apkarian, A.V.; et al. Regulation of neuropathic pain by microglial Orai1 channels. Sci Adv 2023, 9, eade7002. [Google Scholar] [CrossRef] [PubMed]
- Saika, F.; Matsuzaki, S.; Kishioka, S.; Kiguchi, N. Chemogenetic Activation of CX3CR1-Expressing Spinal Microglia Using Gq-DREADD Elicits Mechanical Allodynia in Male Mice. Cells 2021, 10. [Google Scholar] [CrossRef] [PubMed]
- Mapplebeck, J.C.S.; Dalgarno, R.; Tu, Y.; Moriarty, O.; Beggs, S.; Kwok, C.H.T.; Halievski, K.; Assi, S.; Mogil, J.S.; Trang, T.; et al. Microglial P2X4R-evoked pain hypersensitivity is sexually dimorphic in rats. Pain 2018, 159, 1752–1763. [Google Scholar] [CrossRef] [PubMed]
- Doyle, H.H.; Eidson, L.N.; Sinkiewicz, D.M.; Murphy, A.Z. Sex Differences in Microglia Activity within the Periaqueductal Gray of the Rat: A Potential Mechanism Driving the Dimorphic Effects of Morphine. J Neurosci 2017, 37, 3202–3214. [Google Scholar] [CrossRef]
- Hanamsagar, R.; Alter, M.D.; Block, C.S.; Sullivan, H.; Bolton, J.L.; Bilbo, S.D. Generation of a microglial developmental index in mice and in humans reveals a sex difference in maturation and immune reactivity. Glia 2018, 66, 460. [Google Scholar] [CrossRef]
- Chen, G.; Luo, X.; Qadri, M.Y.; Berta, T.; Ji, R.R. Sex-Dependent Glial Signaling in Pathological Pain: Distinct Roles of Spinal Microglia and Astrocytes. Neurosci Bull 2018, 34, 98–108. [Google Scholar] [CrossRef] [PubMed]
- Xie, A.X.; Madayag, A.; Minton, S.K.; McCarthy, K.D.; Malykhina, A.P. Sensory satellite glial Gq-GPCR activation alleviates inflammatory pain via peripheral adenosine 1 receptor activation. Sci Rep 2020, 10, 14181. [Google Scholar] [CrossRef]
- Labus, J.S.; Gupta, A.; Coveleskie, K.; Tillisch, K.; Kilpatrick, L.; Jarcho, J.; Feier, N.; Bueller, J.; Stains, J.; Smith, S.; et al. Sex differences in emotion-related cognitive processes in irritable bowel syndrome and healthy control subjects. Pain 2013, 154, 2088–2099. [Google Scholar] [CrossRef]
- Hong, J.Y.; Kilpatrick, L.A.; Labus, J.; Gupta, A.; Jiang, Z.G.; Ashe-McNalley, C.; Stains, J.; Heendeniya, N.; Ebrat, B.; Smith, S.; et al. Patients with Chronic Visceral Pain Show Sex-Related Alterations in Intrinsic Oscillations of the Resting Brain. Journal of Neuroscience 2013, 33, 11994–12002. [Google Scholar] [CrossRef] [PubMed]
- Bushnell, M.C.; Ceko, M.; Low, L.A. Cognitive and emotional control of pain and its disruption in chronic pain. Nature Reviews Neuroscience 2013, 14, 502–511. [Google Scholar] [CrossRef] [PubMed]
- Han, G.S.; Domaille, D.W. Connecting the dynamics and reactivity of arylboronic acids to emergent and stimuli-responsive material properties. J Mater Chem B 2022, 10, 6263–6278. [Google Scholar] [CrossRef]
- Li, X.H.; Matsuura, T.; Xue, M.; Chen, Q.Y.; Liu, R.H.; Lu, J.S.; Shi, W.; Fan, K.; Zhou, Z.; Miao, Z.; et al. Oxytocin in the anterior cingulate cortex attenuates neuropathic pain and emotional anxiety by inhibiting presynaptic long-term potentiation. Cell Rep 2021, 36, 109411. [Google Scholar] [CrossRef]
- Monroe, T.B.; Fillingim, R.B.; Bruehl, S.P.; Rogers, B.P.; Dietrich, M.S.; Gore, J.C.; Atalla, S.W.; Cowan, R.L. Sex Differences in Brain Regions Modulating Pain Among Older Adults: A Cross-Sectional Resting State Functional Connectivity Study. Pain Med 2018, 19, 1737–1747. [Google Scholar] [CrossRef]
- Wang, G.; Erpelding, N.; Davis, K.D. Sex differences in connectivity of the subgenual anterior cingulate cortex. Pain 2014, 155, 755–763. [Google Scholar] [CrossRef]
- Osborne, N.R.; Cheng, J.C.; Rogachov, A.; Kim, J.A.; Hemington, K.S.; Bosma, R.L.; Inman, R.D.; Davis, K.D. Abnormal subgenual anterior cingulate circuitry is unique to women but not men with chronic pain. Pain 2021, 162, 97–108. [Google Scholar] [CrossRef]
- Liu, R.H.; Xue, M.; Li, X.H.; Zhuo, M. Sex difference in synaptic plasticity in the anterior cingulate cortex of adult mice. Mol Brain 2020, 13, 41. [Google Scholar] [CrossRef] [PubMed]
- Bristow, G.C.; Bostrom, J.A.; Haroutunian, V.; Sodhi, M.S. Sex differences in GABAergic gene expression occur in the anterior cingulate cortex in schizophrenia. Schizophr Res 2015, 167, 57–63. [Google Scholar] [CrossRef] [PubMed]
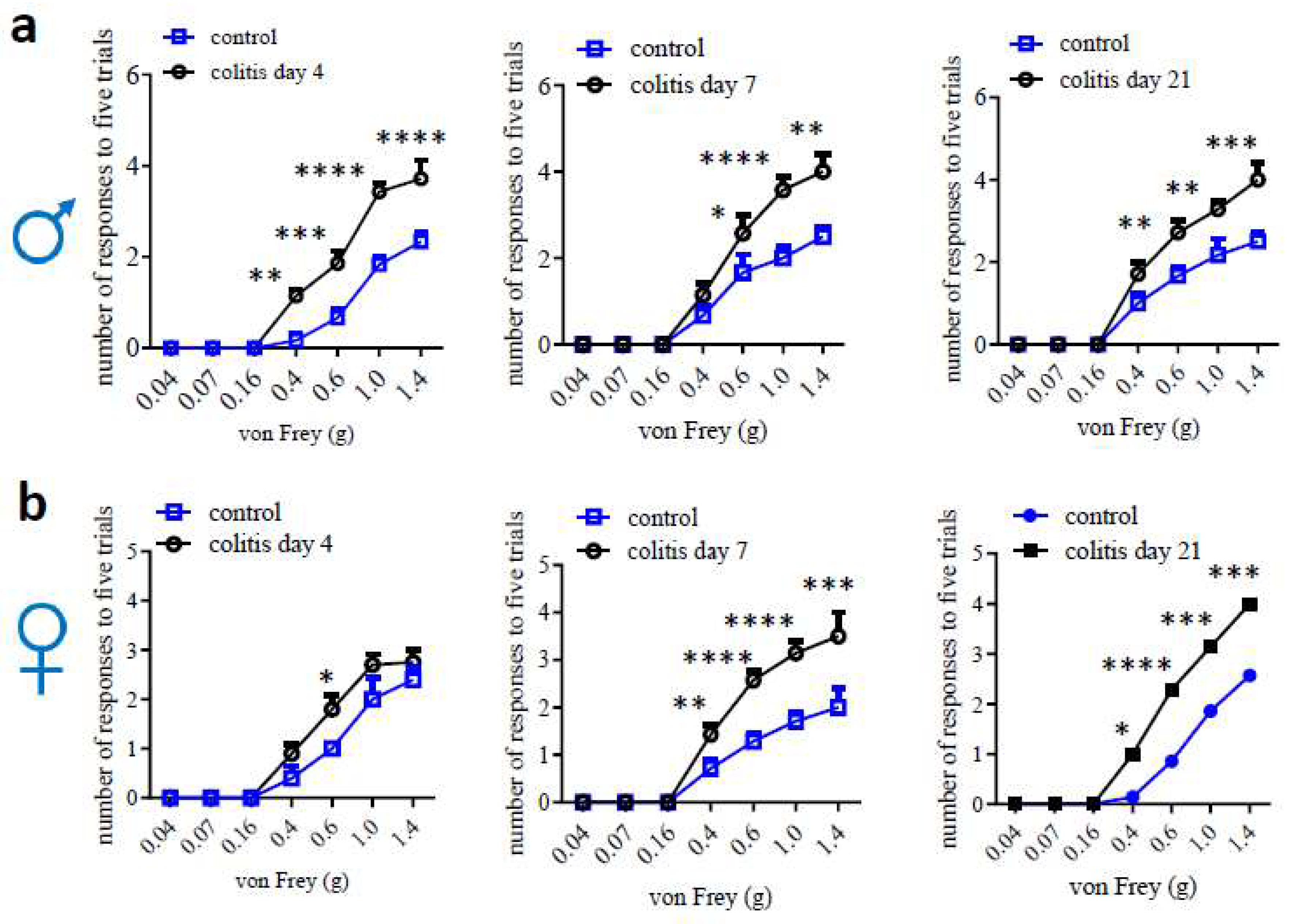
 |
 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





