Introduction:
Since the first Autistic Spectrum Disease (ASD) patient was diagnosed 80 years ago (1), important progress was made yet many aspects remain unknown on both its diagnosis and treatment. Children with ASD present with various manifestations – repetitive gestures, irritability, anxiety, sometimes aggressive behavior - and different degrees of impairment in verbalization, focus, understanding, interaction, initiative, following commands, overall intelligence quotient (IQ), - corresponding to various areas of cortex affected (2,3). Therapeutic options in ASD are limited both in types and results, with psychotherapy being the main therapeutic intervention; besides this the antipsychotics risperidone and aripiprazole are prescribed in overly agitated kids, atomoxetine for increasing focus in kids with hyperactivity symptoms, and in some cases nootropics – piracetam, pyritinol, Cerebrolysin (porcine brain protein hydrolysate), Actovegin (deproteinized hemoderivative from calf blood), etc, with modest results.
Cellular therapies are offering a new therapeutic perspective in ASD (4-11); however their results vary greatly and this is very likely due to both the polymorphic nature of the ASD pathogenesis, and also the difference in the characteristics of the individual autologous cord blood (CB) or stem cell products, both of which we will discuss below in the respective section.
Designing a clinical study involving administration of umbilical CB is complicated by the presence of the obligatory cryopreserving agent dimethyl sulfoxide (DMSO), a special substance with unique properties. Known as a universal aprotic solvent for organic molecules including DNA, it protects cells against the ice crystals formed intracellularly while freezing - the main factor damaging intracellular architecture and organelles. DMSO is metabolized after intravenous infusion in dimethyl sulfide and dimethyl sulfone (12); both are molecules with a sulfur content and characteristic smell, which can be felt in patient’s breath beginning a few minutes after infusion and lasting 24-48 hours. This is a telltale sign which makes “blinding” a clinical study very difficult, and can be partly solved by using a crossover design for the clinical study.
Besides being a vital cryoprotectant DMSO also has a multitude of actions at cellular and molecular level: acts as antioxidant (13, 14), prevents accumulation of DNA breakage and facilitates DNA damage repair (15-17), is neuroprotective via decreasing glutamate and NMDA activation (18), has positive actions on neurons (19), modifies the blood-brain barrier permeability, decreases thrombosis with other vascular actions (20-24), induces stem cell differentiation into neuronal precursors via activation of necessary intracellular pathways (25-29); inhibits the pro-inflammatory function of leukocytes, including TNF-alpha function (30) and has other immunomodulatory actions (31-35). Because of its inhibitory action on leukocytes DMSO is “washed” from the cryopreserved grafts used for hematopoietic reconstitution (in hematology-oncology transplants), however intracellular DMSO cannot be removed and its metabolites are still felt after its administration in patients’ breath. In ASD cord blood is administered to patients who are not conditioned for transplant – where DMSO can have a possible deleterious action on white cells-, and because it has actually beneficial actions on neurons and neuronal precursors, we have chosen to not wash the DMSO out of the cord blood graft prior to infusion, and we have used the dilution technique which is potentially a better fit for the purpose of improving neuronal activity and simultaneously modulating the immune system functions. This technique also keeps manipulation of the graft to a minimum (and improves viability and vitality of cells infused), and preserves the other components of cord blood which potentially can be lost after centrifugation and removal of supernatant.
The CORDUS Clinical Study was granted ethics approval by the National Bioethics Committee of the Romanian Medicine Agency ANM: IS/4/12.02. and is registered on
www.clinicaltrials.gov with NCT04007224. Informed Consent was obtained from all the children’s parents prior to enrolling and testing (both parents needed to agree on enrollment).
Materials and Method:
Participants included in this study were children diagnosed with ASD, age 3-7 years, body weight 15-30 kg, who received prior multiple treatments including psychotherapy and had not achieved significant progress.
For the purpose of evaluating differences in efficacy and safety, exceptions were made for children age 8 or older and/or body weight more than 30 kg the body weight so that treatments were also administered to them; however they were not included in the final statistical analysis which has only considered the ASD children meeting both inclusion criteria - age and body weight.
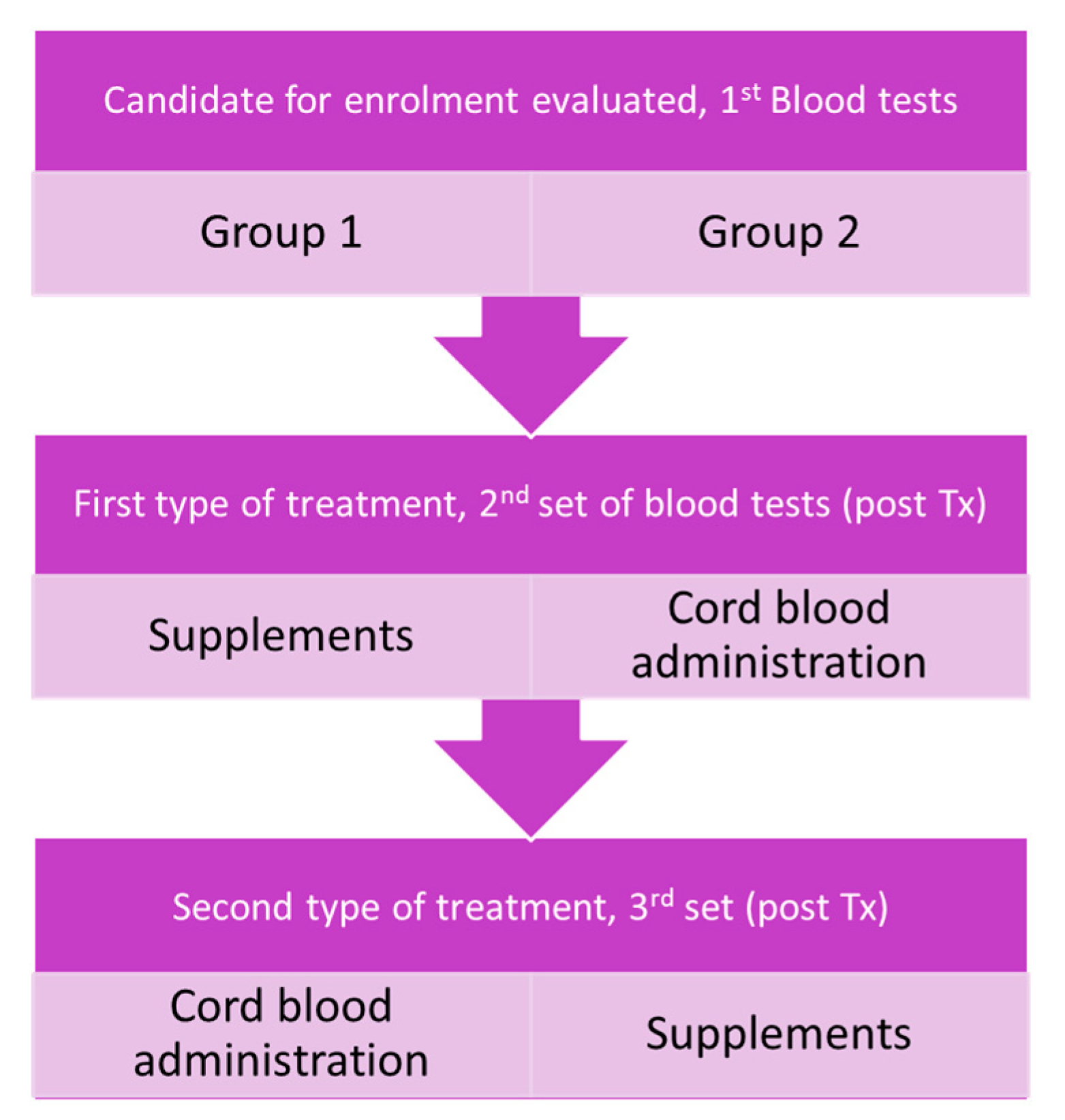
A flowchart summarizing the study design is represented in
Figure 1
CORDUS study participants had the following blood tests performed before and after the 2 treatments (supplements and CB): blood type/Rh (Rhesus factor); HLA (Human Leukocyte Antigen) – A; - B, and -DRB1 typed from both the peripheral blood and the child’s cryopreserved cord blood; complete blood count with differential; liver function tests - AST (aspartate transaminase), ALT (alanine transaminase) and/or GGT (gamma-glutamyl transferase)); renal function (serum urea, serum creatinine); electrolytes - Na (sodium), K (potassium), Ca ( calcium)); NSE (neuron-specific enolase); markers of inflammation: ESR (erythrocyte sedimentation rate), CRP (C-Reactive Protein), TNF-α (Tumor Necrosis Factor-alpha), ferritin, alpha-2 globulins on serum protein electrophoresis; anti-neuronal antibodies: Ac. Anti-amphifizin: Ac. Anti-CV2 : Ac. Anti-PNMA2; Ac. Anti-Ri : Ac. Anti-Yo: Ac. Anti-Hu: Ac. Anti-recoverin; Ac. Anti-SOX1: Ac. Anti-titin; and individually (only some kids): IL(Interleukin)-1 beta, IL-6, IL-8, neopterin, procalcitonin, cationic protein of eosinophils, homocysteine, TSH (thyroid stimulating hormone), GH (growth hormone), IGF (insulin-like growth factor)-1, etc..
Administration of supplements was done in an individualized manner in all the ASD kids, following blood tests results and using these guidelines:
- -
for immune dysfunction type I: over-activation of pro-inflammatory actions (ex type M1 macrophages) resulting in subacute inflammation (increased TNF-α or α-2 globulin fraction occurred in about 70% of the 56 ASD kids tested – (36): Uridine or Boswellia (extracted from Boswellia serrata) or curcumin (from Curcuma longa);
- -
for immune dysfunction type II: over-activation of allergic-type immune reaction (ex. activation of type M2 macrophages) resulting in eosinophilia and/or increased IgE and/or cationic protein of eosinophils (about 30% of ASD kids): blackcurrant extract (Ribes nigrum contains a natural steroid) and/or Viola tricolor extract;
- -
for high ferritin (in a few cases is associated with hemoglobinopathies); one or more antioxidants: glutathione; polyphenols from blueberry (Vaccinium mirtillus extract), ascorbate, N-acetylcysteine, etc.
- -
for high homocysteine (most likely due to suboptimal folate metabolism or receptor issue): methylcianocobalamin (vitamin B12 conjugate) and folate or leucovorin (folinic acid);
- -
for low homocysteine – antioxidants, resveratrol or antioxidants which cross the blood-brain barrier – proanthocyanidins from blueberry, luteolin (flavonoid, the main yellow pigment in Reseda luteola), astaxanthin (carotenoid red pigment from algae), zeaxanthin (carotenoid alkaloid from plants);
- -
for low ferritin - an antioxidant and a cell membrane stabilizer - fish or vegetable oil, omega 3, DHA (Docosahexaenoic acid) or EPA (Eicosapentaenoic acid), and vitamin D3;
- -
for intestinal dysbiosis – pro-biotics (especially Bifidobacterium salivarius) and pre-biotic or inulin (fructose-containing oligosaccharides) to decrease intestinal inflammation and Candida sp proliferation, to reduce formation of inflammatory cytokines as well as excess amines, dopamine and balances serotonin;
- -
for high lactate and/or LDH (lactate dehydrogenase): mitochondrial enhancers such as PQQ (Pyrroloquinoline quinone), Uridine, luteolin to improve aerobic glycolysis and the pyruvate/lactate imbalance
- -
for metabolic or liver issues (increased AST, ALT, bilirubin) – Astragalus (Astragalus lentiginosus), antioxidants
- -
for low GH, IGF-1 (Insulin-like growth factor) – L-arginine; for high TSH - spirulina (Arthrospira platensis) or Kanchanar guggul extracts;
- -
if demyelination on MRI – Bacopa (from Bacopa monnieri), citicoline (CDP-choline), plus an anti-inflammatory – Boswellia, curcuma
- -
for high NSE values (especially above 30 pg/mL) administration of supplements which stimulate and support neurogenesis, alongside natural anti-inflammatory agents and anti-oxidants
- -
for anxiety, agitation and focus deficits - supplements containing combinations of Passiflora, Humulus lupulus, Valerian (Valeriana officinalis), chamomile (Chamomilla recutita and Chamomilla nobile), trillium (extracted from Trillium species), and sometimes echinacea (Echinacea purpurea), GABA (Gamma-aminobutyric acid), theanine (extracted from Camellia sinensis), or Rhodiola rosea extracts.
Administration of autologous cord blood was performed after confirmatory blood group, Rh and HLA typing, with a minimum of 5 x 106 TNCs/kg being available in each graft; two grafts were microbiologically contaminated and they were successfully de-contaminated prior to infusion while preserving the viability of stem cells (37). Each cord blood graft was used integrally, meaning that both of the 2 contiguous compartments (20 ml and the 5 ml compartment) were processed by adding Dextran 40 and albumin to minimize the osmotic modifications of the cells post-decryogenation. The Rubinstein decryogenation protocol (38) was employed with minimal handling of the decryogenated graft – the dilution technique - in order to minimize the stress on cells and avoid cell and exosome loss. After decryogenation and while preparing the cord blood for infusion, an extemporaneous viability determination was performed with Trypan Blue 0,4% at the brightfield microscope 200x and the cord blood was administered intravenously (iv) after pre-medicating the child with antihistaminic and hydrocortisone hemi succinate (5); children also received iv sedation with midazolam and ketamine as needed. Cord blood was infused via gravitational drip; the CB graft volumes infused varied between 47 and 75 ml, the infusion time between 20 and 35 minutes, after which saline flushing of the iv line was given with a volume of 75-150 ml. A small sample (1-2 ml) from the cord blood infused was taken from the administration bag and at about 2-6 hours after the infusion, flowcytometry was performed on the sampled cord blood prepared for infusion. Flowcytometry was performed at an independent laboratory – The Biochemistry Institute from The Romanian Academy -; viability by 7-actinomycin D (7-AAD), and the presence of surface cellular markers CD34+, CD45+, CD271+, and CD133+ was analyzed; these results will be presented in a separate article.
Psychometric evaluation was done by a clinical psychologist who interviewed both the parents and also the therapist of the respective child on the behavior of the child before and after the treatments. The evaluations were done by using the ATEC, Q-CHAT and CAST questionnaires and a 16-item table of symptoms; they assessed initial behavior and the post-treatment behavior after 4 -8 weeks of supplements, and approximately at 6 months after CB infusion.
On the psychometric evaluation of the child by the clinician at his/her office with programmed office visits it is important to note that it can be biased by various factors, including unexpected modifications of the health status of the patient on that specific day (“cranky” kid with acute infections or pain), differences in the mood of the kid in various days (“our kid has good days and bad days”) or based on prior unpleasant procedures experienced by the child in a health setting, or the child mirroring the mood of the parents’ “having a bad day”; all these factors may increase anxiety and make the kid cooperate less, act differently and introduce an important bias in evaluations. This may explain why some studies report statistically significant differences while evaluating with the ABC (Autistic Behavior Checklist) and CARS (Childhood Autism Rating Scale) scales but not on VABS (Vineland Adaptive Behavior Scale) scale (7, 11).
The table used for evaluation by the psychotherapist consisted of 16 items on which the parents and the therapists were prompted to give grades from 0 - which indicates complete absence of the respective symptom/behavioral aspect) - to 10, corresponding to the highest possible intensity for the respective symptom/behavior. Parents and therapists had to grade 6 positive items (attention, understanding, verbalization, social interaction, initiative and maintaining focus), and ten negative items (irritability, hyperactivity, stereotypies, inadequate verbalization, aggressivity, withdrawal, somnolence, headaches, constipation/diarrhea) including 1 general health item (presence of specific manifestations such as food preferences in type, shape, texture, color, taste, smell; fears/phobias, nail biting, etc.). Scores were analyzed individually, comparing scores on same items before and after treatments, and the total score for the positive items and for the negative items.
All the resulting data from the study was tabulated in Microsoft Excel and statistical analysis was done with the same software by employing descriptive statistics (means, standard deviations), Pearson correlation between two datasets and the two-tailed paired t-test, which was considered statistically significant if the p <0.05.
Results:
Cord blood and supplements were administered to a total of 56 children with ASD, only 28 of whom met simultaneously the inclusion criteria of age of less than 8 years and body weight less than 30 kg and also were available for psychometric evaluations; these 28 participants were considered for the final study data analysis; psychometric data from kids not meeting the study inclusion criteria (28 kids age >8, body weight >35 kg) was not considered for the final study results. The blood test data from all the 56 kids and the respective results and analysis was published in a recent article (36) which discussed all the values obtained after the blood tests were performed. We have made exceptions on enrollment in order to see if age and body weight were important determinants in treatment efficacy – and they are indeed - and also to increase the power of the study in order to identify blood markers with a predictive value towards the efficacy of the treatments.
On the safety side there were no major adverse reactions to either the supplements or the cord blood administered. The most common side effect encountered after both treatments was temporary agitation; this was not a new symptom as it manifested only in children who previously had such episodes (about 30% of children) and it was controlled with administration of natural anxiolytic/sedatives (Passiflora, Chamomile, GABA, etc) or the risperidone/aripiprazole medication on which the child was on previously; we were also able to wean off risperidone or aripiprazole in 3 kids following cord blood administration. Agitation occurred the day after administering either new supplements or cord blood, and is likely due to the nonspecific, global stimulation of the neuronal activity; some agitation episodes lasted for hours and were intense, but they could be controlled with supplements or medication which subsequently was tapered off. Agitation was more common after CB administration (15 patients) compared to supplements (4 patients). Additionally, side effects related to cord blood administration consisted of emission of red urine - first micturition after CB infusion - which occurred in 6 children and was self-limiting and resolved spontaneously; this is likely explained by the elimination of hemoglobin released from some of the red cells infused and destroyed shortly after infusion. Another 4 children had vomiting after CB infusion, likely due to gallbladder spasm; this also was self-limiting and required no treatment. One child had symptoms of gastroesophageal reflux and administration of an antacid solved the issue; one child (history of allergies and atopic dermatitis) had facial flushing lasting a few minutes during infusion which also resolved spontaneously.
Following administration of cord blood, the improvement of ASD symptoms was present in an ample range, from truly transformative (around 10% of kids age 3-7) with respective parents stating “it was like we saw a different kid”, to no effect (also about 10% of parents). For children older than 8 years and 35kg body weight, the improvements were much more reduced both in occurrence and amplitude, so that only one in ten children (around 10%) in this group were improved after cord blood administration.
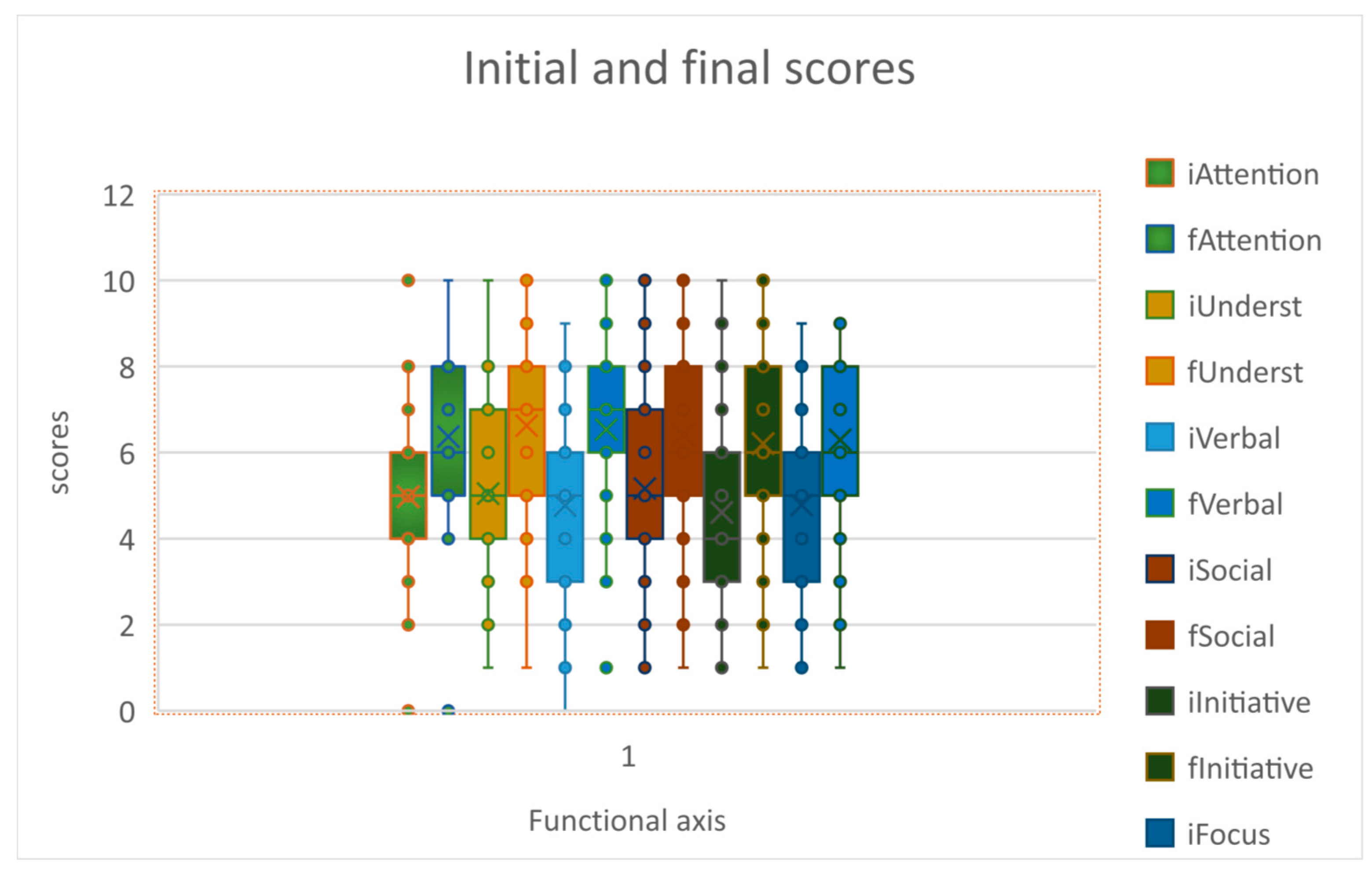
Analyzing the scores on the 16-item table, we see that the CB administration was followed by statistically significant improvements in all 6 of the “positive” areas: attention, understanding, verbalization, social interaction, initiative and maintaining focus; so that the 2-tailed T test had p<0.05 when comparing respective scores before and after CB administration
Figure 2.
Scores on 6 items before and after CB.
Figure 2.
Scores on 6 items before and after CB.
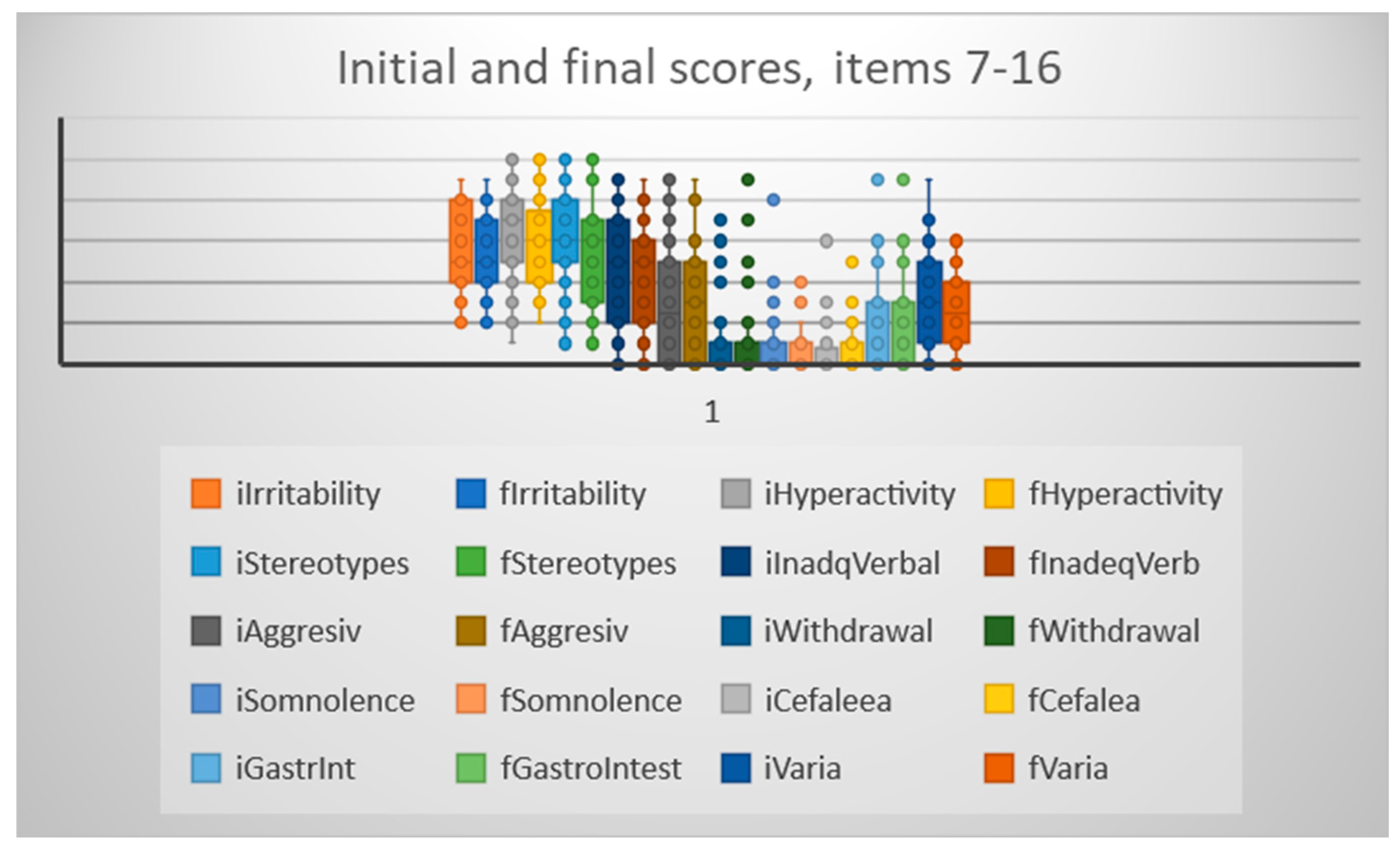
Comparing the scores on the 10 “negative” items, we have seen significant improvements in 4 areas (hyperactivity, stereotypical behavior, inadequate verbalization and varia) while on the other 6 items there was no significant improvement (irritability, aggressivity, withdrawal, somnolence, headaches, constipation/diarrhea).
Figure 3.
Scores on 10 “negative” items before and after CB infusion.
Figure 3.
Scores on 10 “negative” items before and after CB infusion.
When considering all 6 positive items, their combined scores were also significantly better after the CB infusion - average score 29.11 +/- 10.14 SD before infusion, and 38.16 +/- 9.83 after infusion, p<0.05; similarly the combined 10 negative items had significantly better total score after infusion 29.17 +/- 9.96 vs 32.62+/- 11.09 before CB infusion, p<0.05.
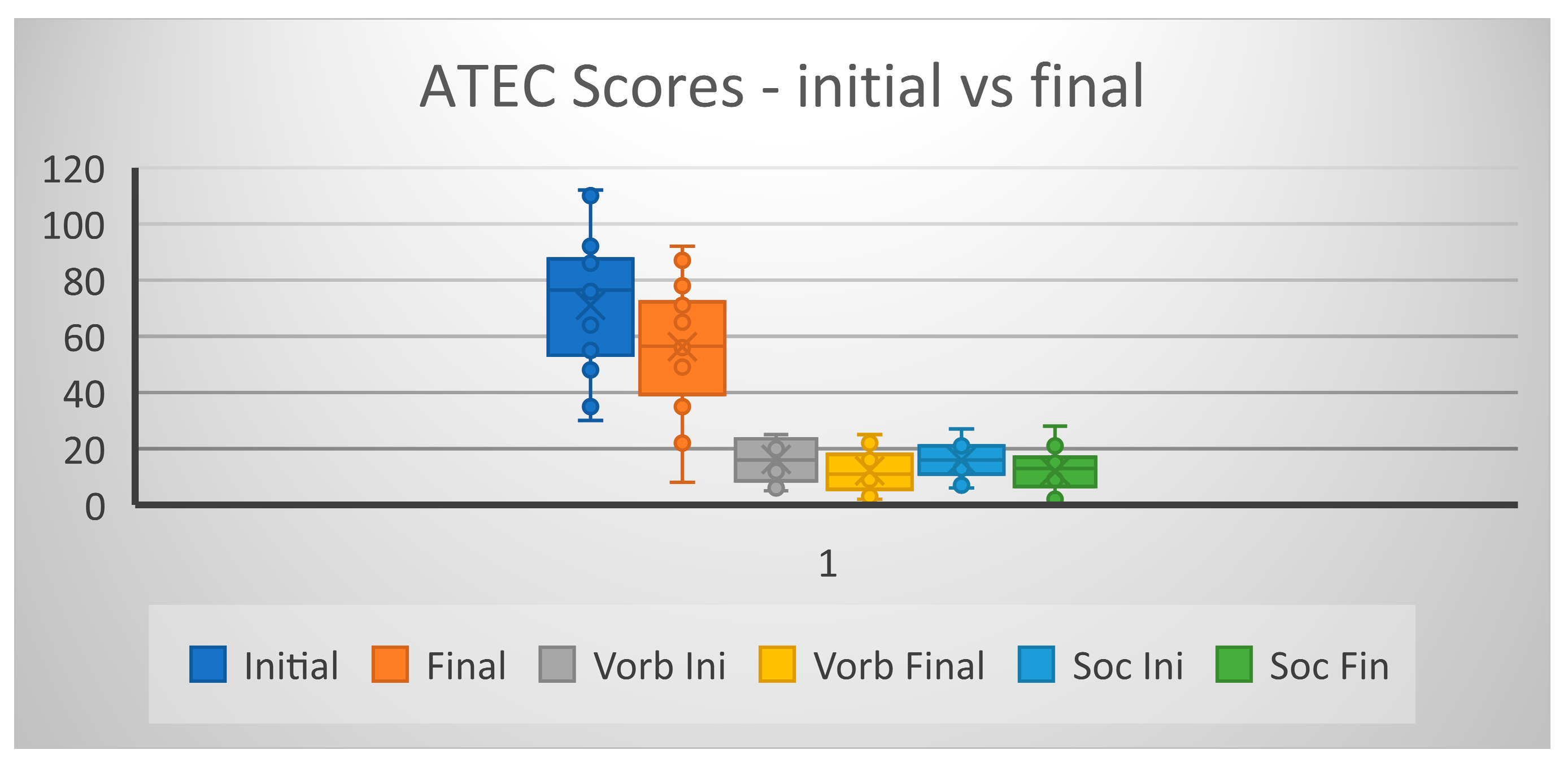
Analyzing the ATEC scores before and after the CB infusion we have also seen that three scores were significantly improved: - on subscale Speech (p = 0.0043), and Socialization (p=0.012) and Total score (p = 0.0051), but not on the subscale Sensory/Cognitive (p=0.09) and neither on subscale Overall Health/Behavior (p=0.18).
Figure 4.
ATEC scores before and after CB.
Figure 4.
ATEC scores before and after CB.
In the case of supplement administration, after analyzing the total scores on both ATEC and the 16-item table scores before and after administration of supplements, neither showed a statistically significant improvement, making the overall CB efficacy superior to that of individualized supplements. However, in 5 of 28 kids (age < 7, body weight <35 kg) the improvement obtained with supplements was bigger than that observed after CB administration.
When entering the evaluations done by the psychotherapist, which were all recorded and transcribed, we have noted in a few cases a discrepancy between the very enthusiastic statements made by some parents during the interview with the psychotherapists, and the very small scores resulting from completing ATEC and the 16-item table by the same parents. Some examples are: P1 - “The second day after transplant [….] like he was another kid” while the respective improvement on the 16-item table was of only 8 points; P2 - “the kid had an explosion for the better after both treatments with stem cells” and respectively only a 2 points improvement on the Total score on the 16-item table; P3 - “Extraordinary good evolution after cell administration. He started saying words” and the respective improvement on the 16-item table totaled 1 point. This may be due to the unidirectional progress of the child (only one aspect improved, such as verbalization) but also a possible difference in the perceptions of parents and their quantification by the questionnaires; it also underscores the subjective nature of the evaluations and the need to interview also the therapist with whom the child worked before and after the treatments. In our study, while comparing the parents’ interview evaluations and the interview of the child’s psychotherapist, there was a very strong correlation between the evaluation scores given by the parents and the respective psychotherapists – Pearson correlation r = 0.913
Given the variability of the results after CB administration we have looked for markers which can predict efficacy of CB administration, especially correlations between various blood markers and psychometric evaluations. Previously it was found that a good predictor for the efficacy of cord blood in ASD was the posterior beta power on EEG recorded prior to cord blood infusion; a higher value was associated with increased alpha and beta and decreased theta power on EEG 12 months post-infusion (39); however no subsequent study has validated this marker.
First we have analyzed all blood test values (including children older than 7 years and/or body weight more than 30 kg, n=56) for NSE, TNF-α, and α-2 albumin, and we have found that while there was a strong correlation between inflammation markers - values of TNF-α and α-2 albumin (r = 0.883), there was only a moderate correlation between the levels of NSE and TNF-α (r = 0.508) and NSE and α-2 albumin (r = 0.502), and this strongly suggests that factors other than inflammation (genetic, metabolic, oxidative stress, etc) are involved in the neuronal apoptosis observed in ASD children.
To evaluate the predictive value of the blood markers for the success of CB administration, we have included the psychometric evaluations and we have only considered the children meeting both inclusion criteria (age an body weight, n=28). There was no correlation between the levels of TNF-α and NSE (r= 0.052); there was a very small correlation (r= 0.138) between the initial TNF-α levels and the improvement on the difference in ATEC scores initial vs final (dATEC = initial ATEC – final ATEC); and a better correlation between initial NSE levels and the dATEC (r=0.401). This can be interpreted as the treatment being more efficacious in kids where neuronal destruction is more ample – children with higher initial NSE values.
It is possible that the baseline presence of both inflammation (abnormal TNF-α and/or alpha-2 globulins) and neuronal destruction (Neuron Specific Enolase – NSE > 20 pg/ml) may be good prognostic factors for the therapeutic success of stem cells in ASD, but the CORDUS study was not designed or powered to study this correlation, and we plan to investigate in a future clinical study the predictive value of these two markers taken together, as well as other possible markers (miRNA profiles in both the ASD child and the cord blood administered).
Discussion
ASD is associated with a wide variety of causative factors which can be grouped based on their levels of action in: genetic, epigenetic, and allogeneic/environmental - infections, nutrient imbalance, toxins – (40), these may act alone or in combination and makes identification and treatment of the predominant factor very difficult. So far more than 800 genes were identified as associated with the ASD pathogenesis, these genes are involved in pathways also active in cell cycle, metabolism, adhesion and signaling, inflammation and cancer (41 - 44).
Phenotypic expression of these genes results in the abnormal levels of various proteins, and more than 100 proteins were showed to be dysregulated in ASD, among which cytokines, markers of mitochondrial dysfunction, oxidative stress, and epigenetic markers indicating deficient methylation (45)
Modifications occurring at a macroscopic level in the CNS – patches of cortex with abnormal morphology observed via post-mortem studies (2) or imagistic studies (46, 47), have corresponding disturbances at a cellular and molecular level represented by genetic and epigenetic factors which affect neurons, glia and astrocytes. Modifications of single or multiple genes ranging from single nucleotide polymorphisms – SNPs- to deletions, inversions, repetitions, aneuploidy) result in groups of abnormal or non-functional cells (neurons, glia, astrocytes or combinations) - brain mosaicism - which form defective or no neuronal networks in various areas of the brain and result in functional impairments of various degrees. Such modifications could be shown only recently after single cell sequencing became possible, and were found more frequently in ASD compared to typical brains (48); however a recent study revealed that in a typical adult brain a mean of 17% of neurons (range 4-78%) had autosomal mutations and 4% of brain cells had a mean of 26 SNP variants and there is at least one somatic mutation with function-altering potential in the brain of approximately half of all adults (49). In the typical human frontal cortex up to 41% of neurons had CNVs (copy number variations) of more than one megabase, with some regions having highly aberrant genomes with multiple alterations (48); another recent study found that around 10% of neurons from the frontal cortex of a typical individual have CNVs (50), and it is estimated that about 4% of the approximately 1 trillion cells in the typical brain have chromosome 21 aneuploidy (51); to this is added another modification – DNA content variation – DCV – of up to 250 megabases between individual cells and also cell populations in different brain areas, especially in the frontal cortex (52).
It is also interesting to note that different types of mitochondrial cytochromes are more active in specific areas of CNS so that specific SNP cytochrome alterations lead to dysfunctions of specific neural paths; CYP2D is present in the neurons and astrocytes (N, A) from olfactory bulb and substantia nigra, linked to processing of adrenaline, dopamine, synephrine, deoxycorticosterone; CYP2B and CYP2C are abundant in blood-brain barrier (BBB) and linked to processing of nitric oxide, harmine, harmalol; while CYP2B6 (N, A); CYP2D6 (N), CYP2E1 (N), CYP1A1 (N, A)– are abundant in the cortex, hippocampus, cerebellum and involved with processing of serotonin, tyramine, arachidonic acid, estradiol, linoleic and oleic acid, retinol (53).
Other genetic impairments can also be found in specific areas of the brain and produce impairments of certain brain circuitry; the mitochondrial mutation mtDNA4977 was found especially in dopamine-producing neurons from substantia nigra, putamen and caudate (54) – dopaminergic networks also frequently affected in ASD.
Related to this, a question arises about the possible use of autologous stem cells when the child has a somatic mutation on whole exome sequencing (WES) or whole genome sequencing (WGS): is it logical to use the child’s own stem cells knowing this? We observe that genetic mutations arise during every cell division at a rate of 2-3 SNPs/cell with every generation (48) so it is possible that de novo mutations in somatic cells are not present in the perinatal stem cells which can thus be administered; this approach has led us to good results in a child with GABBR2 mutation – followed by a significant improvement in both the child’s NSE values and ATEC score. Additionally, because WES and WGS are performed with 20x coverage, we recommend a neurology gene panel (approximately 1000 genes associated with neurodegenerative disease) which has 100x coverage and can clarify the problem; furthermore, a genetic testing of the cord blood itself may be the most useful test in determining whether autologous cord blood also has a genetic anomaly which can preclude its administration.
Somatic mosaicism is a common occurrence in healthy individuals; CNVs of around 100 kilobases are present in many tissues analyzed in any given individual (55, 56) and it can be evaluated by sequencing DNA from both a buccal swab and blood in same individual, which analyzes both ectodermal- and mesodermal-derived cells (57).
The frequency of mosaic chromosomal alterations (mCAs) was analyzed in peripheral blood from a public bank (58) and in the autosomal chromosomes it was around ten-fold less than that of SNPs; mosaicism in the X and Y chromosomes was around 1000-fold higher than somatic mutations in adult cells and this latter observation is in line with the observed frequency of ASD, which is affecting more boys than girls, likely because of uncompensated heterozygous X/Y mutations (ex. EXT1, ASTN2, MACROD2, HDAC4) which alone or combined with other somatic mutations affect cell function.
Adding another layer of complexity to the genetic component is the epigenetic control of gene expression to the extent that phenotypic differences in monozygotic twins can be explained by differences in both histone acetylation and 5-methylcytosine DNA content (59); this also may be the differentiating factor acting in certain siblings, one with ASD while the other is typically developing.
Due to the complexity of the genetic picture in ASD, treatment-wise it is very unlikely to find a single common denominator in the form of a target molecule which could be successfully modulated by a therapeutic agent and lead to a cure for ASD – ex. NFkB or mTOR or SIRT1.
Cellular therapies and especially cord blood have inherent advantages over any other therapeutic modality, because they make possible the simultaneous administration of a wide range of molecules with various biological actions contained in extracellular vesicles – EVs or exosomes – to which are added the actions of the administered stem cells themselves.
Cord blood and other stem cell treatments can address important pathophysiologic modifications associated with ASD, such as genetic brain anomalies – brain mosaicism - by increasing neurogenesis from healthy stem cells which can migrate and populate brain areas with genetic anomalies, also by providing neuronal precursors while neuronal destruction is increased (high NSE) and finally by supporting formation of synapses integrated in functional neural networks via exosomes which improve the mitochondrial function of neurons and modulate microglia primarily by decreasing its pro-inflammatory functions (ex. increased GFAP).
Compared to adult stem cells, CB has a number of advantages: it contains eight times more colony-forming cells with high proliferative potential than bone marrow; has more primitive hematopoietic stem cells in a higher proportion, its CD34+ cells have more adhesion molecules such as CD44 proteoglycan and integrins like CD49d or CD49f, and have longer telomers – capable of more self-renewal; also a higher proportion of immature B cells - CD19+ CD5+; and a characteristic precursor of T cell CD3−/CD8−; are less sensitive to the possible toxic environmental substances; CD34+ from cord blood hone better to injury sites due to a higher affinity stromal cell derived factor (SDF-1) (60). Cord blood also has mesenchymal precursor cells which are more primitive and able to generate cells from all three germ layers (61) and CB has more proteins with different properties than adult ones; proteomic analysis showed that perinatal-derived EVs vs adult MSCs are enriched in key proteins involved in immune, metabolic, and regenerative pathways (62). Perinatal stem cells have a better overall genetic status than adult stem cells since every cell division produces an average of 2-3 genetic mutations in each generation resulting in about 80 SNPs in more than 2% of somatic cells, and there is at least one somatic mutation with function-altering potential in the brain of approximately half of all adults (49).
Given that subacute systemic inflammation is present in a majority of ASD children (63 -65) alongside neuroinflammation (66, 67); administration of naïve, non-opsonized leukocytes and their precursors from CB can both replenish the T and B lymphocytes with unaffected ones and also re-establish the balance between pro- and anti-inflammatory cytokines which is affecting microglia in ASD. It was shown that increased, sustained immune cell activation is followed by an impairment in their function associated with impaired thymic production of T cells and impaired antigen presentation and production (68), and also activation and proliferation of immune cells is dependent on mitochondrial energy production, with various roles for tricarboxylic cycle and oxidative phosphorylation; so that an extended inflammatory state and mitochondrial exhaustion may lead to alterations of the immune function, both innate and acquired (69).
As stated above, besides the various causative ASD pathogenesis, the great variation in the effect of the CB infusions is also very likely due to the qualitative differences of the cord blood administered, leading to sometimes opposing results in the clinical studies which analyzed the outcomes (70 -72). Cord blood contains both hematopoietic and mesenchymal stem cells in various proportions, and there are indications that the ratio of these two populations greatly impact the site of implantation of stem cells (lung vs brain) after intravenous administration (73). The capability of MSCs to generate new neurons in the CNS can thus be greatly influenced by the properties of the cord blood infused. In the CORDUS study we have analyzed the CD133+/CD271+ ratio, and also the numbers and ratios of CD45+ and CD34+; the results of the flowcytometry testing and correlations with the psychometric evaluations will be published in a subsequent paper.
Autologous cord blood also contains variable numbers and proportions of embryonic – like stem cells – cells with positive surface markers such as NANOG, OCT etc (74, 75). We have not tested in our study for the presence of these surface markers, but they may be important determinants of the effects of the cord blood infusion, and we plan to do this in a new clinical study.
CB of various origins contains exosomes of different quality and quantity and can also be a source of the major differences in the results seen after its administration. Using the dilution technique for decryogenation of the cord blood graft, - which we have used in our study - has the advantage of minimal manipulation (a possible increase in cell viability) and also preserves all the contents of the graft, including exosomes and very small embryonic like stem cells which otherwise can be excluded as supernatants after centrifugations. We also plan to test for the differences in the exosome content via profiling the microRNAs in both the cord blood and the ASD children who will receive the treatment, knowing that there are important differences observed after testing microRNAs in ASD children obtained by different teams (76 – 79).
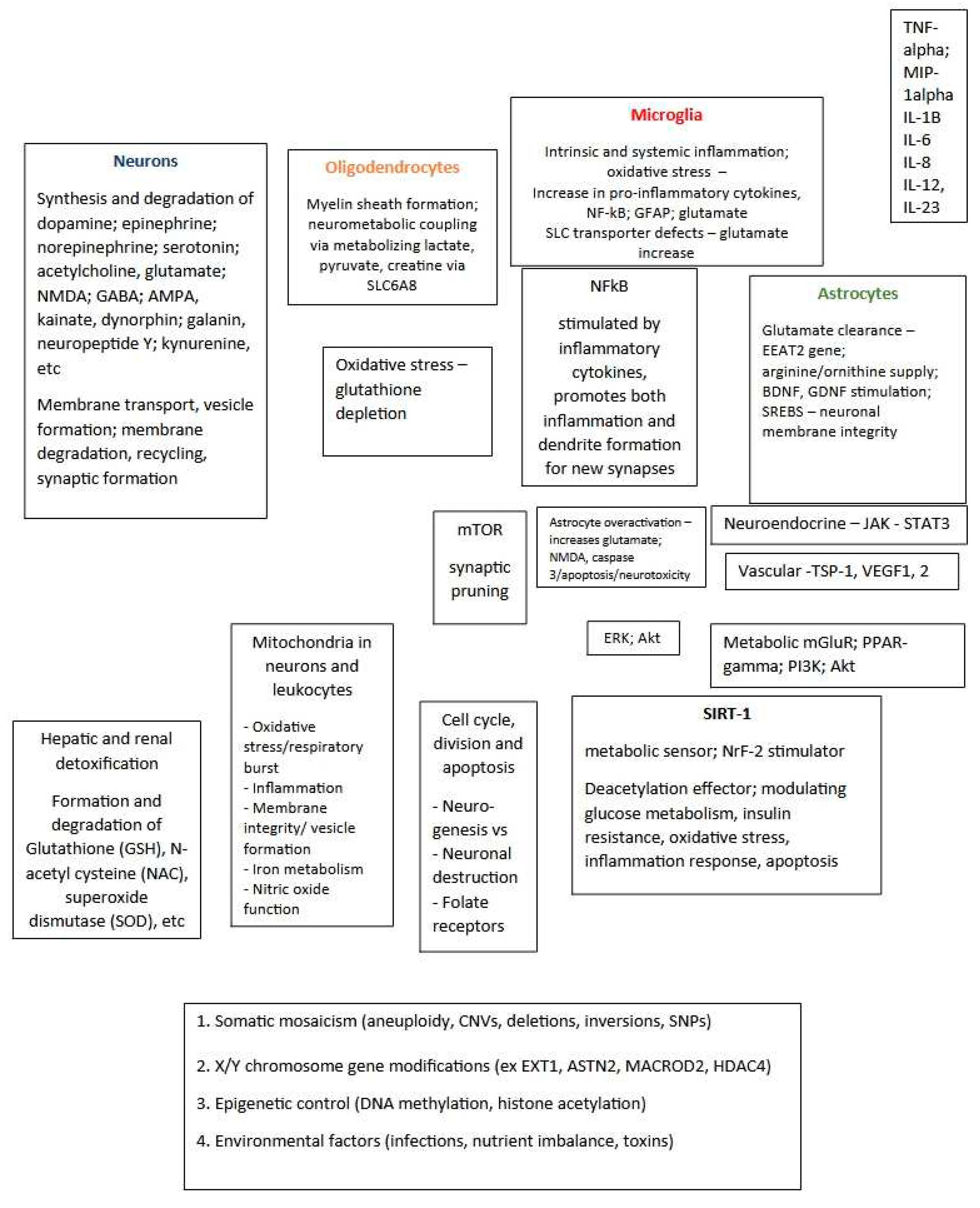
Since behavior is a consequence of activation/inhibition of various cortical areas (amygdala, hippocampus, prefrontal cortex) and a complex interplay between secretion and inactivation of various neurotransmitters and modulators such as dopamine, serotonin, epinephrine, acetylcholine, GABA, glutamate; NMDA, neuropeptide Y, galanin, etc., the activity of the respective neurons can be directly or indirectly modified or influenced by a multitude of factors, (
Figure 5)
Given the hundreds of genes and epigenetic factors linked to the pathological modifications in ASD, sometimes it is next to impossible to test for and pinpoint the main causal factor (which can be partly masked by another compensatory modification) and in these situations when modern precision molecular medicine cannot yield a clear molecular cause and effect, a combination of information from clinical exam, reaction to previous treatments and various tests can guide a more empirical treatment towards therapeutic success in a return to good, old fashioned clinical medicine.
Besides the genetic/epigenetic/environmental causality, the ASD causative factors can also be classified based on the primary organ affected – intrinsic or extrinsic to the central nervous system (CNS) – (36); for example a mutation directly affecting neurons (ex GABBR2) is considered intrinsic to CNS (primary ASD) while a metabolic or immune system dysfunction resulting in systemic inflammation (microglia activation evidentiated by increased glial fibrillary acidic protein – GFAP – , NFkB and other markers such as TNF-α, α2-albumins) or in rare cases presence of antibodies to neuronal proteins or folate receptors is extrinsic to CNS (secondary ASD) – in this secondary/extrinsic cases treatments can be given with good results.
Following blood tests in ASD children, supplements can be administered more specifically and be an effective initial treatment before considering CB or other stem cell treatment; besides the examples mentioned above we are adding:
- -
in kids with low ferritin there is a likely association with ferroptosis which occurs as a compensatory mechanism for increased lipid oxidation and cell membrane degradation; while a supplement with iron gives noticeable short-term improvement especially in sleeping, an antioxidant or a cell membrane stabilizer is more helpful as a causal treatment and for long-term administration;
- -
if marginally low serum sodium is repeatedly seen (ex. 137 mmol/L; normal above 138) while the other electrolytes are being normal, there is likely a sodium transporter defect and the child may benefit from the administration of the diuretic bumetanide or torasemide (80, 81);
- -
in kids with associated hyperactivity, viloxazine may give better results than the non-stimulant atomoxetine (82).
- -
for children with anxiety, agitation and/or focus deficits not improved with above-mentioned supplements, administration of an adaptogen – Rhodiola -, and magnesium citrate and vit B6 may help and short-term administration of low-dose aripiprazole may be needed; but epigenetic factors such as methylation or acetylation should also be considered in this situation, and administration of folate/cobalamin derivatives or SAM (S-adenosyl-methionine) can improve the underlying deficits leading to altered behavior
Even though stem cell administration in ASD so far has had no constant solid results making it difficult to recommend as standard second- or third-line therapy in ASD clinical practice (83) it is worth noting the exceptional results which followed administration of cord blood in some children (around 1 in 10 children) and the fact that a majority of children (3 out of 4) aged 3-7 and body weight less than 30 kg were presenting improvements.
Conclusions
In the CORDUS study we have administered autologous cord blood to 56 kids with ASD, and results were very good in the age range 3-7 years where about 3 out of 4 kids had clear improvements especially on verbalization, initiative, social interaction and understanding. Kids older than 8 years had much less improvement, with only 1 out of 10 kids older than 8 years showing clear improvement in behavior. Autologous cord blood administration has a very good risk/benefit ratio, especially because of the minimal risk involved (vs unknown sources, which although thoroughly tested carry an undetermined risk for future problems). The benefit of cord blood seems to reside in the immune modulation and regenerative capacity of the cord blood, both directly from the infused cells and also from the exosomes, which influence the activity of other cells in the body, especially immune cells, microglia and neurons.
Considering the excellent risk profile of administering autologous stem cells, we advocate further studying the autologous stem cell administration in ASD via new clinical studies, especially for identifying prognostic factors associated with good results which will allow us to select the patients who would very likely benefit from stem cell treatment, and also for further understanding and treating ASD.
Ethics approval was granted by the National Ethics Committee in accordance to the principles stated in The Declaration of Helsinki. Informed Consent was granted in writing by both parents of all the children prior to enrolling and administering treatments.
Conflicts of Interest: Dr Felician Stancioiu has received speaker honorariums and consultant fees from three family cord blood banks after the CORDUS study was designed and started; Dr Bogdan Ivanescu is the medical director for a private cord blood bank; Dr Raluca Bogdan and Dr Radu Dumitrescu have no conflicts of interest.
References
- Kanner, L. (1943). Autistic disturbances of affective contact. Nervous child .pdf. Nervous Child, 2(3), 217–250. Retrieved from http://mail.neurodiversity.com/library_kanner_1943.pdf.
- Stoner, R., Chow, M. L., Boyle, M. P., Sunkin, S. M., Mouton, P. R., Roy, S., … Courchesne, E. (2014). Patches of Disorganization in the Neocortex of Children with Autism. New England Journal of Medicine, 370(13). [CrossRef]
- Buch, A. M., Vértes, P. E., Seidlitz, J., Kim, S. H., Grosenick, L., & Liston, C. (2023). Molecular and network-level mechanisms explaining individual differences in autism spectrum disorder. Nature Neuroscience, 26(4). [CrossRef]
- Nabetani, M., Mukai, T., & Taguchi, A. (2023). Cell Therapies for Autism Spectrum Disorder Based on New Pathophysiology: A Review. Cell Transplantation. [CrossRef]
- Dawson, G., Sun, J. M., Davlantis, K. S., Murias, M., Franz, L., Troy, J., … Kurtzberg, J. (2017). Autologous cord blood infusions are safe and feasible in young children with autism spectrum disorder: Results of a single-center phase I open-label trial. Stem Cells Translational Medicine, 6(5). [CrossRef]
- Villarreal-Martínez, L., González-Martínez, G., Sáenz-Flores, M., Bautista-Gómez, A. J., González-Martínez, A., Ortiz-Castillo, M., … Garza-López, E. (2022). Stem Cell Therapy in the Treatment of Patients With Autism Spectrum Disorder: a Systematic Review and Meta-analysis. Stem Cell Reviews and Reports. [CrossRef]
- Qu, J., Liu, Z., Li, L., Zou, Z., He, Z., Zhou, L., … Ye, J. (2022). Efficacy and Safety of Stem Cell Therapy in Children With Autism Spectrum Disorders: A Systematic Review and Meta-Analysis. Frontiers in Pediatrics. [CrossRef]
- Carpenter, K. L. H., Major, S., Tallman, C., Chen, L. W., Franz, L., Sun, J., … Dawson, G. (2019). White Matter Tract Changes Associated with Clinical Improvement in an Open-Label Trial Assessing Autologous Umbilical Cord Blood for Treatment of Young Children with Autism. Stem Cells Translational Medicine, 8(2).
- Sharma, A. K., Gokulchandran, N., Kulkarni, P. P., Sane, H. M., Sharma, R., Jose, A., & Badhe, P. B. (2020). Cell transplantation as a novel therapeutic strategy for autism spectrum disorders: a clinical study. American Journal of Stem Cells, 9(5).
- Tamouza, R., Volt, F., Richard, J. R., Wu, C. L., Bouassida, J., Boukouaci, W., … Gluckman, E. (2022). Possible Effect of the use of Mesenchymal Stromal Cells in the Treatment of Autism Spectrum Disorders: A Review. Frontiers in Cell and Developmental Biology. [CrossRef]
- Villarreal-Martínez, L., González-Martínez, G., Sáenz-Flores, M., Bautista-Gómez, A. J., González-Martínez, A., Ortiz-Castillo, M., … Garza-López, E. (2022). Stem Cell Therapy in the Treatment of Patients With Autism Spectrum Disorder: a Systematic Review and Meta-analysis. Stem Cell Reviews and Reports.
- Egorin, M. J., Rosen, D. M., Sridhara, R., Sensenbrenner, L., & Cottler-Fox, M. (1998). Plasma concentrations and pharmacokinetics of dimethylsulfoxide and its metabolites in patients undergoing peripheral-blood stem-cell transplants. Journal of Clinical Oncology, 16(2). [CrossRef]
- Sanmartín-Suárez, C., Soto-Otero, R., Sánchez-Sellero, I., & Méndez-Álvarez, E. (2011). Antioxidant properties of dimethyl sulfoxide and its viability as a solvent in the evaluation of neuroprotective antioxidants. Journal of Pharmacological and Toxicological Methods, 63(2). [CrossRef]
- Wuputra, K., Tsai, M. H., Kato, K., Yang, Y. han, Pan, J. Bin, Ku, C. C., … Yokoyama, K. K. (2022). Dimethyl sulfoxide stimulates the AhR-Jdp2 axis to control ROS accumulation in mouse embryonic fibroblasts. Cell Biology and Toxicology, 38(2). [CrossRef]
- Huang, Z., Peng, R., Yu, H., Chen, Z., Wang, S., Wang, Z., … Li, Q. (2022). Dimethyl Sulfoxide Attenuates Radiation-Induced Testicular Injury through Facilitating DNA Double-Strand Break Repair. Oxidative Medicine and Cellular Longevity, 2022. [CrossRef]
- Yang, C., Tang, H., Wang, L., Peng, R., Bai, F., Shan, Y., … Cong, Y. (2018). Dimethyl Sulfoxide Prevents Radiation-Induced Oral Mucositis Through Facilitating DNA Double-Strand Break Repair in Epithelial Stem Cells. International Journal of Radiation Oncology Biology Physics, 102(5). [CrossRef]
- Tunçer, S., Gurbanov, R., Sheraj, I., Solel, E., Esenturk, O., & Banerjee, S. (2018). Low dose dimethyl sulfoxide driven gross molecular changes have the potential to interfere with various cellular processes. Scientific Reports, 8(1). [CrossRef]
- Jacob, S. W., & de la Torre, J. C. (2009). Pharmacology of dimethyl sulfoxide in cardiac and CNS damage. Pharmacological Reports. [CrossRef]
- Santos, N. C., Figueira-Coelho, J., Martins-Silva, J., & Saldanha, C. (2003). Multidisciplinary utilization of dimethyl sulfoxide: Pharmacological, cellular, and molecular aspects. Biochemical Pharmacology. [CrossRef]
- Bulama, I., Nasiru, S., Bello, A., Abbas, A. Y., Nasiru, J. I., Saidu, Y., … Suleman, B. L. (2022). Antioxidant-based neuroprotective effect of dimethylsulfoxide against induced traumatic brain injury in a rats model. Frontiers in Pharmacology, 13. [CrossRef]
- Di Giorgio, A. M., Hou, Y., Zhao, X., Zhang, B., Lyeth, B. G., & Russell, M. J. (2008). Dimethyl sulfoxide provides neuroprotection in a traumatic brain injury model. Restorative Neurology and Neuroscience, 26(6).
- Broadwell, R. D., Salcman, M., & Kaplan, R. S. (1982). Morphologic effect of dimethyl sulfoxide on the blood-brain barrier. Science, 217(4555). [CrossRef]
- Pardridge, W. M. (2022). A historical review of brain drug delivery. Pharmaceutics. [CrossRef]
- Camici, G. G., Steffel, J., Akhmedov, A., Schafer, N., Baldinger, J., Schulz, U., … Tanner, F. C. (2006). Dimethyl sulfoxide inhibits tissue factor expression, thrombus formation, and vascular smooth muscle cell activation: A potential treatment strategy for drug-eluting stents. Circulation, 114(14).
- Chetty, S., Pagliuca, F. W., Honore, C., Kweudjeu, A., Rezania, A., & Melton, D. A. (2013). A simple tool to improve pluripotent stem cell differentiation. Nature Methods, 10(6). [CrossRef]
- Sambo, D., Li, J., Brickler, T., & Chetty, S. (2019). Transient treatment of human pluripotent stem cells with dmso to promote differentiation. Journal of Visualized Experiments, 2019(149). [CrossRef]
- Li, J., Narayanan, C., Bian, J., Sambo, D., Brickler, T., Zhang, W., & Chetty, S. (2018). A transient DMSO treatment increases the differentiation potential of human pluripotent stem cells through the Rb family. PLoS ONE, 13(12).
- Qiu, Z., Mishra, A., Li, M., Farnsworth, S. L., Guerra, B., Lanford, R. E., & Hornsby, P. J. (2015). Marmoset induced pluripotent stem cells: Robust neural differentiation following pretreatment with dimethyl sulfoxide. Stem Cell Research, 15(1). [CrossRef]
- Slack, R. S., Skerjanc, I. S., Lach, B., Craig, J., Jardine, K., & McBurney, M. W. (1995). Cells differentiating into neuroectoderm undergo apoptosis in the absence of functional retinoblastoma family proteins. Journal of Cell Biology, 129(3). [CrossRef]
- Javaid, N., Patra, M. C., Seo, H., Yasmeen, F., & Choi, S. (2020). A rational insight into the effect of dimethyl sulfoxide on TNF-α activity. International Journal of Molecular Sciences, 21(24). [CrossRef]
- De Abreu Costa, L., Ottoni, M. H. F., Dos Santos, M. G., Meireles, A. B., De Almeida, V. G., De Fátima Pereira, W., … Brito-Melo, G. E. A. (2017). Dimethyl sulfoxide (DMSO) decreases cell proliferation and TNF-α, IFN-, and IL-2 cytokines production in cultures of peripheral blood lymphocytes. Molecules, 22(11). [CrossRef]
- Elisia, I., Nakamura, H., Lam, V., Hofs, E., Cederberg, R., Cait, J., … Krystal, G. (2016). DMSO represses inflammatory cytokine production from human blood cells and reduces autoimmune arthritis. PLoS ONE, 11(3). [CrossRef]
- Lin, G. J., Sytwu, H. K., Yu, J. C., Chen, Y. W., Kuo, Y. L., Yu, C. C., … Huang, S. H. (2015). Dimethyl sulfoxide inhibits spontaneous diabetes and autoimmune recurrence in non-obese diabetic mice by inducing differentiation of regulatory T cells. Toxicology and Applied Pharmacology, 282(2). [CrossRef]
- Huang, S. H., Wu, C. H., Chen, S. J., Sytwu, H. K., & Lin, G. J. (2020). Immunomodulatory effects and potential clinical applications of dimethyl sulfoxide. Immunobiology. [CrossRef]
- Teraoka, H., Mikoshiba, M., Takase, K., Yamamoto, K., & Tsukada, K. (1996). Reversible G1 arrest induced by dimethyl sulfoxide in human lymphoid cell lines: Dimethyl sulfoxide inhibits IL-6-induced differentiation of SKW6-CL4 into IgM-secreting plasma cells. Experimental Cell Research, 222(1). [CrossRef]
- Stancioiu, F., Bogdan, R., & Dumitrescu, R. (2023). Neuron-Specific Enolase (NSE) as a Biomarker for Autistic Spectrum Disease (ASD). Life, 13(8). [CrossRef]
- Stancioiu, F., Bogdan, R., Bulumac, B., Ivanescu, B., & Dumitrscu, R. (2022). Decontamination of Two Umbilical Cord Blood Grafts Prior to Autologous Administration. Maedica, 17(4), 885–892.
- Rubinstein, P., Dobrila, L., Rosenfield, R. E., Adamson, J. W., Migliaccio, G., Migliaccio, A. R., … Stevens, C. E. (1995). Processing and cryopreservation of placental/umbilical cord blood for unrelated bone marrow reconstitution. Proceedings of the National Academy of Sciences of the United States of America, 92(22). [CrossRef]
- Murias, M., Major, S., Compton, S., Buttinger, J., Sun, J. M., Kurtzberg, J., & Dawson, G. (2018). Electrophysiological Biomarkers Predict Clinical Improvement in an Open-Label Trial Assessing Efficacy of Autologous Umbilical Cord Blood for Treatment of Autism. Stem Cells Translational Medicine, 7(11). [CrossRef]
- Pugsley, K., Scherer, S. W., Bellgrove, M. A., & Hawi, Z. (2022). Environmental exposures associated with elevated risk for autism spectrum disorder may augment the burden of deleterious de novo mutations among probands. Molecular Psychiatry. [CrossRef]
- Autism Spectrum Disorders Working Group of the Psychiatric Genomics Consortium. Meta-analysis of GWAS of over 16,000 individuals with autism spectrum disorder highlights a novel locus at 10q24.32 and a significant overlap with schizophrenia. Mol Autism. 2017 May 22;8:21. doi: 10.1186/s13229-017-0137-9. eCollection 2017.
- Havdahl, A., Niarchou, M., Starnawska, A., Uddin, M., Van Der Merwe, C., & Warrier, V. (2021). Genetic contributions to autism spectrum disorder. Psychological Medicine. [CrossRef]
- Wiśniowiecka-Kowalnik, B., & Nowakowska, B. A. (2019). Genetics and epigenetics of autism spectrum disorder—current evidence in the field. Journal of Applied Genetics. [CrossRef]
- Rylaarsdam, L., & Guemez-Gamboa, A. (2019). Genetic Causes and Modifiers of Autism Spectrum Disorder. Frontiers in Cellular Neuroscience. [CrossRef]
- Hewitson, L., Mathews, J. A., Devlin, M., Schutte, C., Lee, J., & German, D. C. (2021). Blood biomarker discovery for autism spectrum disorder: A proteomic analysis. PLoS ONE, 16(2 February 2021). [CrossRef]
- Sussman, D., Leung, R. C., Vogan, V. M., Lee, W., Trelle, S., Lin, S., … Taylor, M. J. (2015). The autism puzzle: Diffuse but not pervasive neuroanatomical abnormalities in children with ASD. NeuroImage: Clinical, 8. [CrossRef]
- Khundrakpam, B. S., Lewis, J. D., Kostopoulos, P., Carbonell, F., & Evans, A. C. (2017). Cortical thickness abnormalities in autism spectrum disorders through late childhood, adolescence, and adulthood: A large-scale mri study. Cerebral Cortex, 27(3). [CrossRef]
- McConnell, M. J., Lindberg, M. R., Brennand, K. J., Piper, J. C., Voet, T., Cowing-Zitron, C., … Gage, F. H. (2013). Mosaic copy number variation in human neurons. Science, 342(6158). [CrossRef]
- Rodin, R. E., Dou, Y., Kwon, M., Sherman, M. A., D’Gama, A. M., Doan, R. N., … Walsh, C. A. (2021). The landscape of somatic mutation in cerebral cortex of autistic and neurotypical individuals revealed by ultra-deep whole-genome sequencing. Nature Neuroscience, 24(2). [CrossRef]
- Sun, C., Kathuria, K., Emery, S. B., Kim, B., Burbulis, I. E., Shin, J. H., … Mcconnell, M. J. (2023). Mapping the Complex Genetic Landscape of Human Neurons. BioRxiv.
- Rehen, S. K., Yung, Y. C., McCreight, M. P., Kaushal, D., Yang, A. H., Almeida, B. S. V., … Chun, J. (2005). Constitutional aneuploidy in the normal human brain. Journal of Neuroscience, 25(9). [CrossRef]
- Westra, J. W., Rivera, R. R., Bushman, D. M., Yung, Y. C., Peterson, S. E., Barral, S., & Chun, J. (2010). Neuronal DNA content variation (DCV) with regional and individual differences in the human brain. Journal of Comparative Neurology, 518(19). [CrossRef]
- Hellman, K., Aadal Nielsen, P., Ek, F., & Olsson, R. (2016). An ex Vivo Model for Evaluating Blood-Brain Barrier Permeability, Efflux, and Drug Metabolism. ACS Chemical Neuroscience, 7(5). [CrossRef]
- Soong, N. W., Hinton, D. R., Cortopassi, G., & Arnheim, N. (1992). Mosaicism for a specific somatic mitochondrial DNA mutation in adult human brain. Nature Genetics, 2(4). [CrossRef]
- Piotrowski, A., Bruder, C. E. G., Andersson, R., De Ståhl, T. D., Menzel, U., Sandgren, J., … Dumanski, J. P. (2008). Somatic mosaicism for copy number variation in differentiated human tissues. Human Mutation, 29(9).
- O’Huallachain, M., Karczewski, K. J., Weissman, S. M., Urban, A. E., & Snyder, M. P. (2012). Extensive genetic variation in somatic human tissues. Proceedings of the National Academy of Sciences of the United States of America, 109(44). [CrossRef]
- Žilina, O., Koltšina, M., Raid, R., Kurg, A., Tõnisson, N., & Salumets, A. (2015). Somatic mosaicism for copy-neutral loss of heterozygosity and DNA copy number variations in the human genome. BMC Genomics, 16(1). [CrossRef]
- Watson, C. J., & Blundell, J. R. (2023). Mutation rates and fitness consequences of mosaic chromosomal alterations in blood. Nature Genetics, 55(10). [CrossRef]
- Fraga, M. F., Ballestar, E., Paz, M. F., Ropero, S., Setien, F., Ballestar, M. L., … Esteller, M. (2005). Epigenetic differences arise during the lifetime of monozygotic twins. Proceedings of the National Academy of Sciences of the United States of America, 102(30). [CrossRef]
- Hordyjewska, A., Popiołek, Ł., & Horecka, A. (2015). Characteristics of hematopoietic stem cells of umbilical cord blood. Cytotechnology. [CrossRef]
- Lee MW, Yang MS, Park JS, Kim HC, Kim YJ, Choi J (2005). Isolation of mesenchymal stem cells from cryopreserved human umbilical cord blood. Int J Hematol. 81:126–130. doi: 10.1532/IJH97.A10404.
- Wang, Z. gang, He, Z. yi, Liang, S., Yang, Q., Cheng, P., & Chen, A. min. (2020). Comprehensive proteomic analysis of exosomes derived from human bone marrow, adipose tissue, and umbilical cord mesenchymal stem cells. Stem Cell Research and Therapy, 11(1). [CrossRef]
- Molloy, C. A., Morrow, A. L., Meinzen-Derr, J., Schleifer, K., Dienger, K., Manning-Courtney, P., … Wills-Karp, M. (2006). Elevated cytokine levels in children with autism spectrum disorder. Journal of Neuroimmunology, 172(1–2).
- Gesundheit, B., Rosenzweig, J. P., Naor, D., Lerer, B., Zachor, D. A., Procházka, V., … Ashwood, P. (2013). Immunological and autoimmune considerations of Autism Spectrum Disorders. Journal of Autoimmunity. [CrossRef]
- Hughes, H. K., R.J.Moreno, & Ashwood, P. (2023). Innate immune dysfunction and neuroinflammation in autism spectrum disorder (ASD). Brain, Behavior, and Immunity. [CrossRef]
- Vargas, D. L., Nascimbene, C., Krishnan, C., Zimmerman, A. W., & Pardo, C. A. (2005). Neuroglial activation and neuroinflammation in the brain of patients with autism. Annals of Neurology, 57(1). [CrossRef]
- Usui, N., Kobayashi, H., & Shimada, S. (2023). Neuroinflammation and Oxidative Stress in the Pathogenesis of Autism Spectrum Disorder. International Journal of Molecular Sciences. [CrossRef]
- Betjes, M. G. H. (2013). Immune cell dysfunction and inflammation in end-stage renal disease. Nature Reviews Nephrology. [CrossRef]
- Vaziri, N. D., Pahl, M. V., Crum, A., & Norris, K. (2012). Effect of Uremia on Structure and Function of Immune System. Journal of Renal Nutrition, 22(1). [CrossRef]
- Liu, M., Lü, Y. tao, Huan, Y., Ge, R. cun, Zhang, J., Jiang, S., … An, L. (2011). Safety and efficacy of cord blood mononuclear cells and umbilical cord mesenchymal stem cells therapy for childhood autism. Journal of Clinical Rehabilitative Tissue Engineering Research, 15(23). [CrossRef]
- Dawson, G., Sun, J. M., Baker, J., Carpenter, K., Compton, S., Deaver, M., … Kurtzberg, J. (2020). A Phase II Randomized Clinical Trial of the Safety and Efficacy of Intravenous Umbilical Cord Blood Infusion for Treatment of Children with Autism Spectrum Disorder. Journal of Pediatrics, 222. [CrossRef]
- Adnan, M., Motiwala, F., Trivedi, C., Chaudhari, G., Mansuri, Z., & Jain, S. (2022). Human Umbilical Cord Blood Infusions in the Management of Autism Spectrum Disorder. Primary Care Companion for CNS Disorders.
- Kuçi, S., Kuçi, Z., Kreyenberg, H., Deak, E., Pütsch, K., Huenecke, S., … Bader, P. (2010). CD271 antigen defines a subset of multipotent stromal cells with immunosuppressive and lymphohematopoietic engraftment-promoting properties. Haematologica, 95(4). [CrossRef]
- McGuckin, C., Forraz, N., Baradez, M. O., Basford, C., Dickinson, A. M., Navran, S., & Hartgerink, J. D. (2006). Embryonic-like stem cells from umbilical cord blood and potential for neural modeling. Acta Neurobiologiae Experimentalis.
- McGuckin, C., Jurga, M., Ali, H., Strbad, M., & Forraz, N. (2008). Culture of embryonic-like stem cells from human umbilical cord blood and onward differentiation to neural cells in vitro. Nature Protocols, 3(6). [CrossRef]
- Abu-Elneel, K., Liu, T., Gazzaniga, F. S., Nishimura, Y., Wall, D. P., Geschwind, D. H., … Kosik, K. S. (2008). Heterogeneous dysregulation of microRNAs across the autism spectrum. Neurogenetics, 9(3). [CrossRef]
- Mundalil Vasu, M., Anitha, A., Thanseem, I., Suzuki, K., Yamada, K., Takahashi, T., … Mori, N. (2014). Serum microRNA profiles in children with autism. Molecular Autism, 5(1). [CrossRef]
- Kichukova, T. M., Popov, N. T., Ivanov, I. S., & Vachev, T. I. (2017). Profiling of Circulating Serum MicroRNAs in Children with Autism Spectrum Disorder using Stem-loop qRT-PCR Assay. Folia Medica, 59(1). [CrossRef]
- Huang, Z. X., Chen, Y., Guo, H. R., & Chen, G. F. (2021). Systematic Review and Bioinformatic Analysis of microRNA Expression in Autism Spectrum Disorder Identifies Pathways Associated With Cancer, Metabolism, Cell Signaling, and Cell Adhesion. Frontiers in Psychiatry. [CrossRef]
- Delpire, E., & Ben-Ari, Y. (2022). A Wholistic View of How Bumetanide Attenuates Autism Spectrum Disorders. Cells. [CrossRef]
- Doğan, M., Albayrak, Y., & Erbaş, O. (2023). Torasemide Improves the Propionic Acid-Induced Autism in Rats: A Histopathological and Imaging Study. Alpha Psychiatry, 24(1), 22–31.
- Price, M. Z., & Price, R. L. (2023). Extended-Release Viloxazine Compared with Atomoxetine for Attention Deficit Hyperactivity Disorder. CNS Drugs, 37(7). [CrossRef]
- Narzisi, A. (2022). Haste Makes Waste: There Is No Solid Evidence to Translate the Use of Stem Cells into Clinical Practice for Children with Autism Spectrum Disorder. Brain Sciences. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).