1. Introduction
In traditional and complementary (alternative) medicine, plant materials are extensively employed for healing purposes while a wide range of conventional (pharmaceutical) drugs derive from wild plants and a large part of human population rely on wild plants for remedies [
1,
2]. Furthermore, an increasing demand for a broad variety of natural products is also noted in perfumery, cosmetics, and food industry [
3,
4] coupled with intense quests globally for new ornamental plants originating from rich biodiversity hotspots [
5].
Due to limited public awareness and ineffective control mechanisms combined with ever-increasing commercial demand and modern consumerism trends, a substantial segment of the traded plant material globally still originates from wild phytogenetic resources, thus depleting wild-growing populations [
6]. To satisfy the multi-sectorial growing demand for novel products without further exhausting natural resources, recruiting focal neglected and underutilized species of economic interest to introduce them into cultivation systems, stands out as promising alternative and sustainable strategy for the development of new crops [
5]. To be operative such an endeavor, a well-documented initial plant material should be employed coupled with extant propagation protocols and cultivation guidelines applied in well-managed cultivation settings. From this perspective, fertilization schemes tailor-made to the species in concern ought to be established and applied [
7,
8].
The key challenge for agriculture is to increase crop yield and quality in a sustainable way for present and future. To support optimal plant growth, nutrients are conventionally added to the soil of any crop by means of chemical (inorganic) fertilizers. However, their over-application or insufficient use has been associated with a range of negative effects on the environment [
9]. Major offsets include soil quality degradation, and nutrient leaching [
10]. These nutrients, which are lost from agricultural lands, contaminate water bodies globally, and, in this way, adversely affect aquatic systems and biodiversity [
9,
11]. Accumulative evidence suggests that the respective environmental impact is comparatively limited when alternative fertilization schemes are employed instead [
10], such as biostimulants which may trigger root nutrient uptake [
12], or integrated nutrient management (INM) potentially promoting plant growth and productivity with a low environmental footprint [
13]. Generally, it is well established that fertilization schemes determine not only plant growth and productivity, but also quality aspects of the cultivated plant material [
10,
14,
15]. An increasing number of studies indicates that application of organic fertilizers and INM strategies may upgrade the antioxidant level of medicinal plant material, as compared to chemical fertilizers [
12,
16,
17,
18]. Organic fertilizers and INM schemes have also been previously related to increased chlorophyll content [
19], or to the intensity and homogeneity of leaf greenness [
20] or plants of medicinal interest [
7,
8,
21]. Antioxidants are important sets of compounds that deactivate free radicals and reactive oxygen species within cells [
23]. The plant antioxidant profiles represent one of the utmost attention-grabbing parameters in agriculture. As free radicals play a central role in the development of various chronic diseases, among others, cardiovascular syndromes, aging, heart diseases, anemias, cancers and inflammations, the plant antioxidants used in nutrition/pharmacology offer strong defense against injuries triggered by these free radicals [
24].
Species-wise, this study is focused on
Carlina diae (Rech. f.) Meusel and A. Kástner (Asteraceae) due to its ornamental value [
5] and medicinal interest [
21], its uniqueness as ancient, Tertiary relict, range-restricted species (local endemic of Dia Island off-shore from Crete Island) coupled with contemporary rarity and Endangered IUCN (International Union for the Conservation of Nature) status due to overgrazing of its small-sized wild-growing populations under protection (covered by Appendix I of the Bern Convention and the Greek Presidential Decree 67/1981) [
22]. Context-wise, this investigation builds on previous studies exploring its sustainable exploitation potential in the ornamental sector [
5] and the medicinal sector, as well as on the development of effective species-specific propagation protocols for integrated conservation actions [
22]. Herein, this combined field and laboratory study deals with the establishment of a pilot cultivation of
C. diae in Crete and aimed to the development of a species-specific fertilization protocol promoting optimally, not only plant growth, but also key herbal quality aspects, such as antioxidant metabolite levels.
2. Materials and Methods
2.1. Origin of Plant Material
The authorized collection data regarding the focal
C. diae GR-1-BBGK-19,006 (
Figure 1A,B) as well as the species-specific propagation protocols used to produce ex-situ enough plant individuals for field experimentation are reported in previous own study [
22]. The plants used in the experimental procedure were transplanted in 2 L plastic pots by the company of AFI GLAVAKI KE SIA OE Tree & Plant Nurseries, Aridea, PELLAS, GR-58400, Greece.
2.2. Establishment of Field Experiment and Soil Analysis
The general description of the established field experiment (
Figure 1C) in the campus of the Hellenic Mediterranean University, Heraklion, Crete (Greece) and the soil properties of the research field are reported in previous publications [
7,
8] and are provided briefly in
Supplementary Materials Method S1.
2.3. Fertilization Schemes
Details on the fertilization regime used in this experimentation are given briefly in
Supplementary Materials Method S1 and are provided by previous own studies [
7,
8]. The fertilization treatments of the pilot cultivation of
C. diae involved:
A. INM by foliar application (INM-fa): The nutrient solution consisted of THEORUN at 7 ml L-1, THEOCAL at 1.5 g L-1, THEOFAST at 5 ml L-1, 10-47-10 (AGRI.FE.M. LTD Fertilizers, Greece) at 3.2 g L-1, K2SO4 (0-0-52, AGRI.FE.M. LTD Fertilizers, Greece) at 2.07 g L-1, micronutrients (Plex Mix, AGRI.FE.M. LTD Fertilizers, Greece) at 1.5 ml L-1 and MgSO4 (Mg 25.6 %, AGRI.FE.M. LTD Fertilizers, Greece) at 0.6 g L-1.
Β. Chemical (inorganic) fertilization by foliar application (ChF-fa): The nutrient solution consisted of NH4NO3 (34,4-0-0, Neofert®, Neochim PLC, Bulgaria) at 2.7 g L-1, Ca(NO3)2 (NITROCAL, Agrohimiki, Greece) at 1.7 g L-1, 10-47-10 at 3.2 g L-1, K2SO4 (0-0-52) at 2.27 g L-1, micronutrients Plex Mix at 1.5 ml L-1 and MgSO4 (Mg 25.6 %) at 0.6 g L-1.
C. Control, with foliar and soil applications with tap water.
D. INM by soil application (INM-sa): The nutrient solution consisted of THEORUN at 7 ml L-1, THEOCAL at 1.5 g L-1, THEOMASS at 10 ml L-1, 10-47-10 at 3.2 g L-1, K2SO4 (0-0-52) at 2.1 g L-1, micronutrients Plex Mix at 1.5 ml L-1 and MgSO4 (Mg 25.6 %) at 0.3 g L-1.
E. Chemical (inorganic) fertilization by soil application (ChF-sa): The nutrient solution was consisted of NH4NO3 (34,4-0-0) at 2.7 g L-1, Ca(NO3)2 (NITROCAL) at 1.7 g L-1, 10-47-10 at 3.2 g L-1, K2SO4 (0-0-52) at 2.3 g L-1, micronutrients Plex Mix at 1.5 ml L-1 and MgSO4 (Mg 25.6 %) at 0.3 g L-1.
F. Mixture of plant extracts as bioreactor by soil application (MPE-sa): The nutrient solution was consisted of THEOMASS at 10 ml L-1.
2.4. Plant Measurements
Several plant and leaf level measurements were regularly conducted until May 25, 2021, ending with completion of flowering of C. diae. All sampled leaves were fully expanded, grown under direct light and devoid of visual symptoms of either pathogen infection or insect damage. In all instances, the time between sampling and the initiation of the evaluation was shorter than 15 min. When this was not feasible, samples were placed in vials, flash-frozen in liquid nitrogen and were transferred to a freezer (-80oC) for storage. Replicate leaves were sampled from separate plant individuals.
Details on the each of the following plant measurements are given briefly in Supplementary MaterialS Method S2 and are provided by previous own studies [
7,
8].
2.4.1. Non-Invasive Evaluation of Leaf Coloration in Growth Stages
In this study, leaf coloration was assessed by three different methods (for instruments used see
Supplementary Materials Method S2 and [
7,
8]): (i) Leaf SPAD value, approximating chlorophyll content; (ii) The index of absorbance difference (IAD) computed as the difference between the absorbance values at 670 and 720 nm, near the chlorophyll absorbance peak; (iii) Quantified leaf colour by using a Chroma Meter. These measurements were in situ conducted in attached leaves of intact plant individuals at vegetative, early flowering, and full flowering stages. Three points were recorded per replicate leaf and were further averaged. Three replicate leaves were assessed per treatment.
2.4.2. Non-Invasive Evaluation of Photosynthetic Performance in Growth Stages
As a valid indicator of leaf photosynthetic performance [
25], the ratio of variable to maximum chlorophyll fluorescence (Fv/Fm) was assessed (for instruments and conditions see
Supplementary Materials Method S2 and [
7,
8]). These measurements were in situ conducted in dark-adapted attached leaves of intact plant individuals at vegetative, early flowering, and full flowering stages. Three points were recorded per replicate leaf and were further averaged. Three replicate leaves were assessed per treatment.
2.4.3. Leaf Shape Indicators
A morphometric analysis was performed by analyzing leaf form in terms of aspect ratio, circularity, roundness, and solidity (for instruments, metrics and values see
Supplementary Materials Method S2 and [
7,
8]). Thirty leaves (5 per plant individual × 6 plant individuals) were analyzed per treatment.
2.4.4. Plant Growth and Biomass Partitioning to Generative Organs
Above-ground plant and inflorescence (fresh and dry) masses were determined (± 0.01 g; MXX-412; Denver Instruments, Bohemia, NY, USA). For measuring dry weight, samples were placed in a forced-air drying oven for 72 h at 80°C. Six replicate plants were evaluated per treatment.
2.4.5. Leaf Chlorophyll and Carotenoid Contents
Chlorophyll content is important for leaf coloration and photosynthetic performance. Carotenoids are key non-enzymatic antioxidants [
26]. The effect of growth conditions on chlorophyll and carotenoid contents was therefore assessed (see details in
Supplementary Materials Method S2 and [
7,
8]). Three leaves were assessed per treatment. For each replicate, four samples collected from different plant individuals were pooled, and the assay was performed twice.
2.4.6. Leaf Total Phenolic and Total Flavonoid Contents
Phenols and flavonoids are critical non-enzymatic antioxidants [
26,
27]. The contents of total phenolic and total flavonoid were determined by using the Folin-Ciocalteu assay and aluminum chloride colorimetric assay, respectively (see details in
Supplementary Materials Method S2 and [
7,
8]), following [
27]. Three leaves were assessed per treatment. For each replicate, four samples from different plant individuals were pooled and the assay was performed twice.
2.4.7. Leaf Soluble Sugar Content
Since carbohydrate status is related to photosynthetic activity, the effect of fertilization scheme on carbohydrate status was assessed (see details in
Supplementary Materials Method S2 and [
7,
8]). These measurements were conducted on three replicates per treatment. For each replicate, four samples from different plant individuals were pooled and the assay was performed twice.
2.4.8. Leaf and Inflorescence Mineral Analysis
To assess the role of fertilization regime on mineral uptake, leaf and inflorescence mineral analysis was conducted (see details in
Supplementary Materials Method S2 and [
7,
8]). Three replicates were evaluated per treatment. For each replicate, four samples from different plant individuals were pooled and the assay was performed twice.
2.5. Statistical Analysis
Data analyses were carried out using the SAS statistical software (SAS Institute, Cary, NC, USA). Data were tested for homogeneity of variances (Duncan’s test) and one way analysis of variance (ANOVA) was conducted. Subsequently, estimated least significant differences (LSD) of treatment effects were determined (P = 0.05).
3. Results
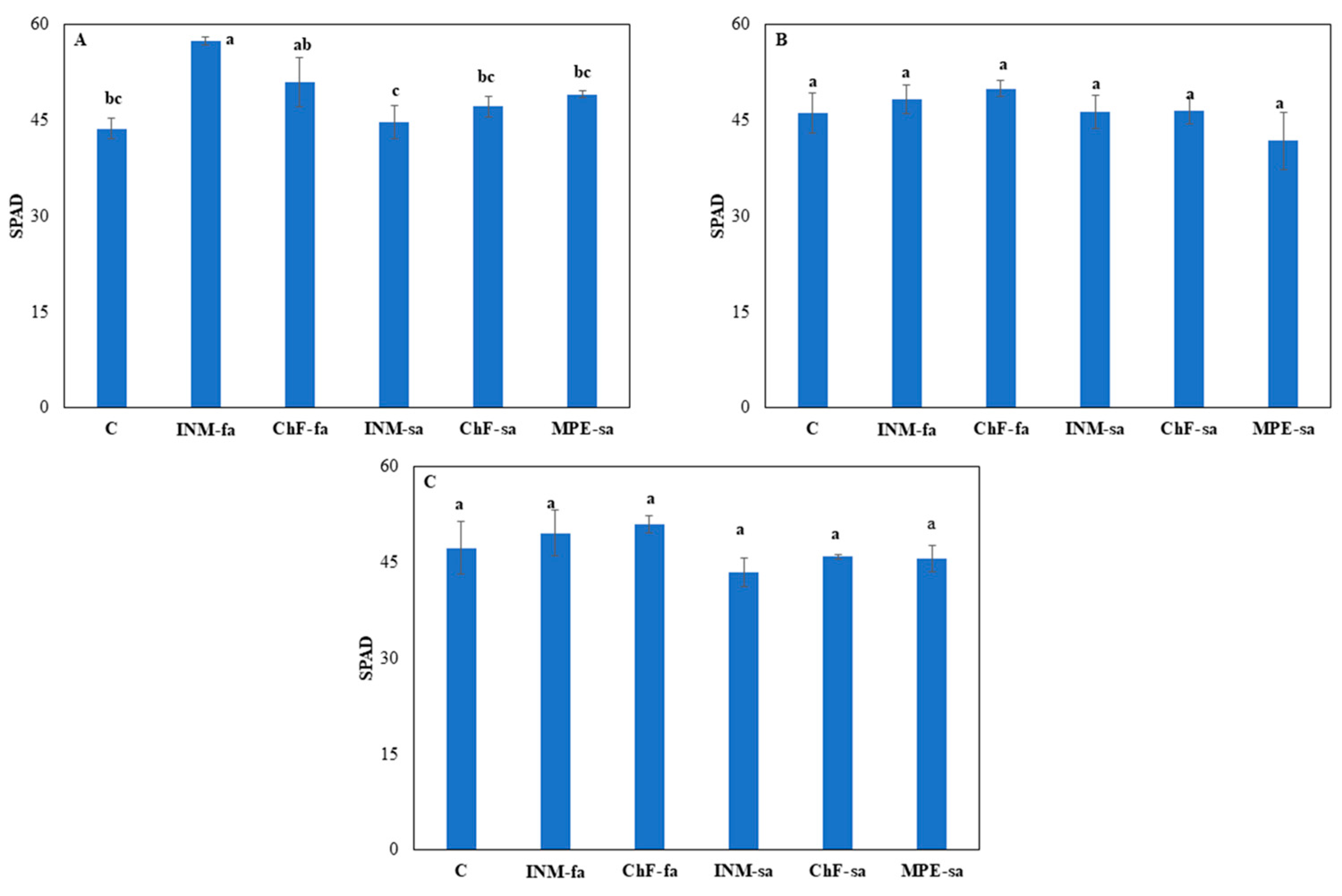
3.1. The Fertilization Scheme Exerted Minor Effects on Leaf Colour
At vegetative stage, foliar fertilization (INM-fa or ChF-fa) was associated with higher leaf SPAD value, as compared to INM-sa (
Figure 2A). At early and full flowering stages, the fertilization scheme did not affect the leaf SPAD value (
Figure 2B,C). At vegetative stage, chemical fertilization regardless of application method (ChF-fa or ChF-sa) was related to lower leaf IAD, as compared to the rest of the treatments (
Supplementary Figure S1A). At early and full flowering stages, the fertilization scheme did not affect leaf IAD (
Supplementary Figure S1B,C).
INM-fa resulted to higher leaf L value as compared to the control plants at vegetative stage, while it led to higher leaf L value as compared to INM-sa at full flowering stage (
Supplementary Figure S2A,C). With these two minor exceptions, leaf L value was not affected by the fertilization scheme (
Supplementary Figure S2). The fertilization scheme did not also affect leaf a and b values at all three growth stages (
Supplementary Figures S3 and S4).
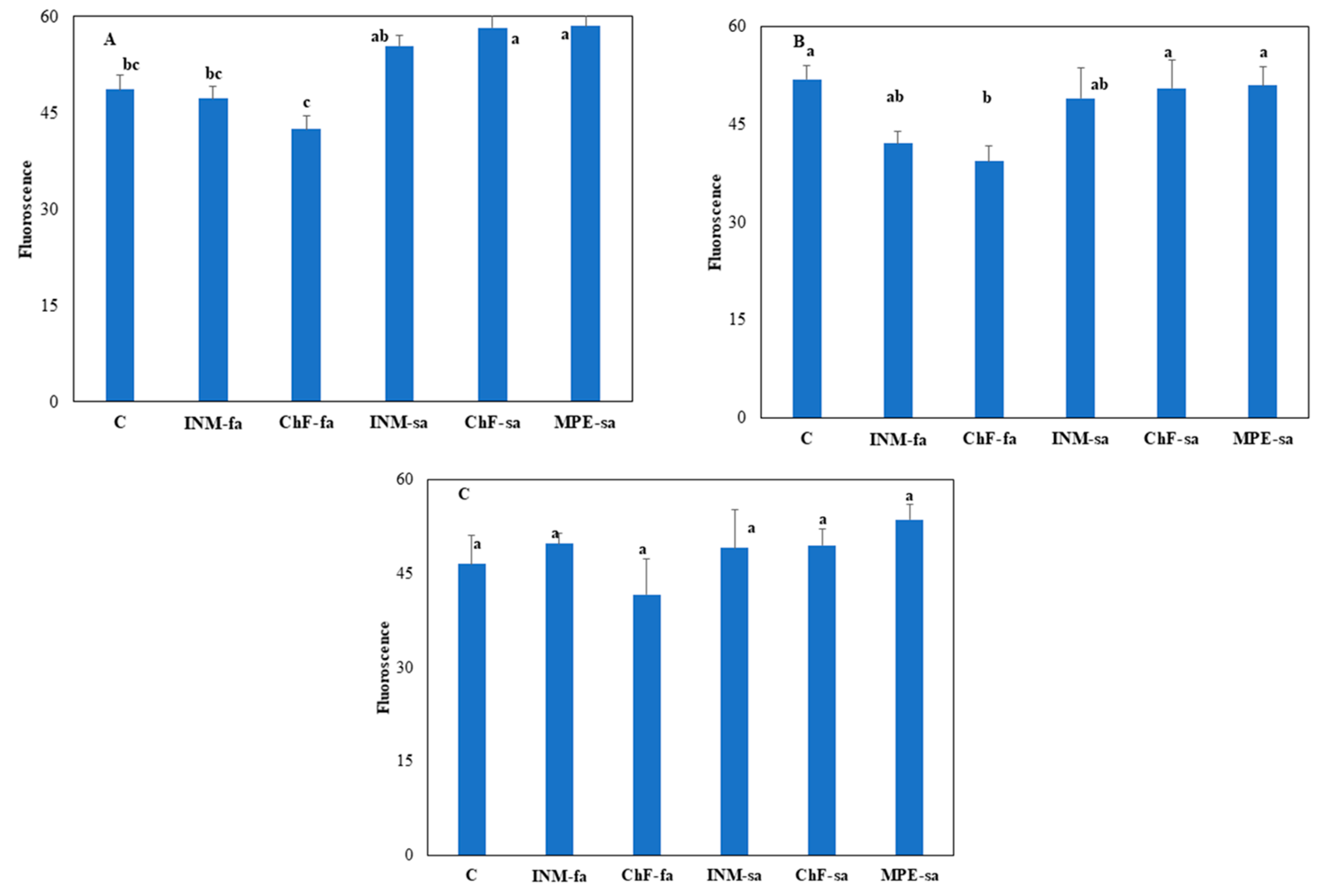
3.2. The Fertilization Scheme Induced Limited Effects on Leaf Photosynthetic Performance
At vegetative stage, ChF-sa and MPE-sa resulted to higher leaf chlorophyll fluorescence value as compared to controls plants and plants receiving foliar fertilization (INM-fa or ChF-fa;
Figure 3A). At early flowering stage, these two regimes (ChF-sa and MPE-sa) showed a significant difference when compared to ChF-fa (
Figure 3B). Fertilization scheme did not affect leaf chlorophyll fluorescence value at full flowering stage (
Figure 3C).
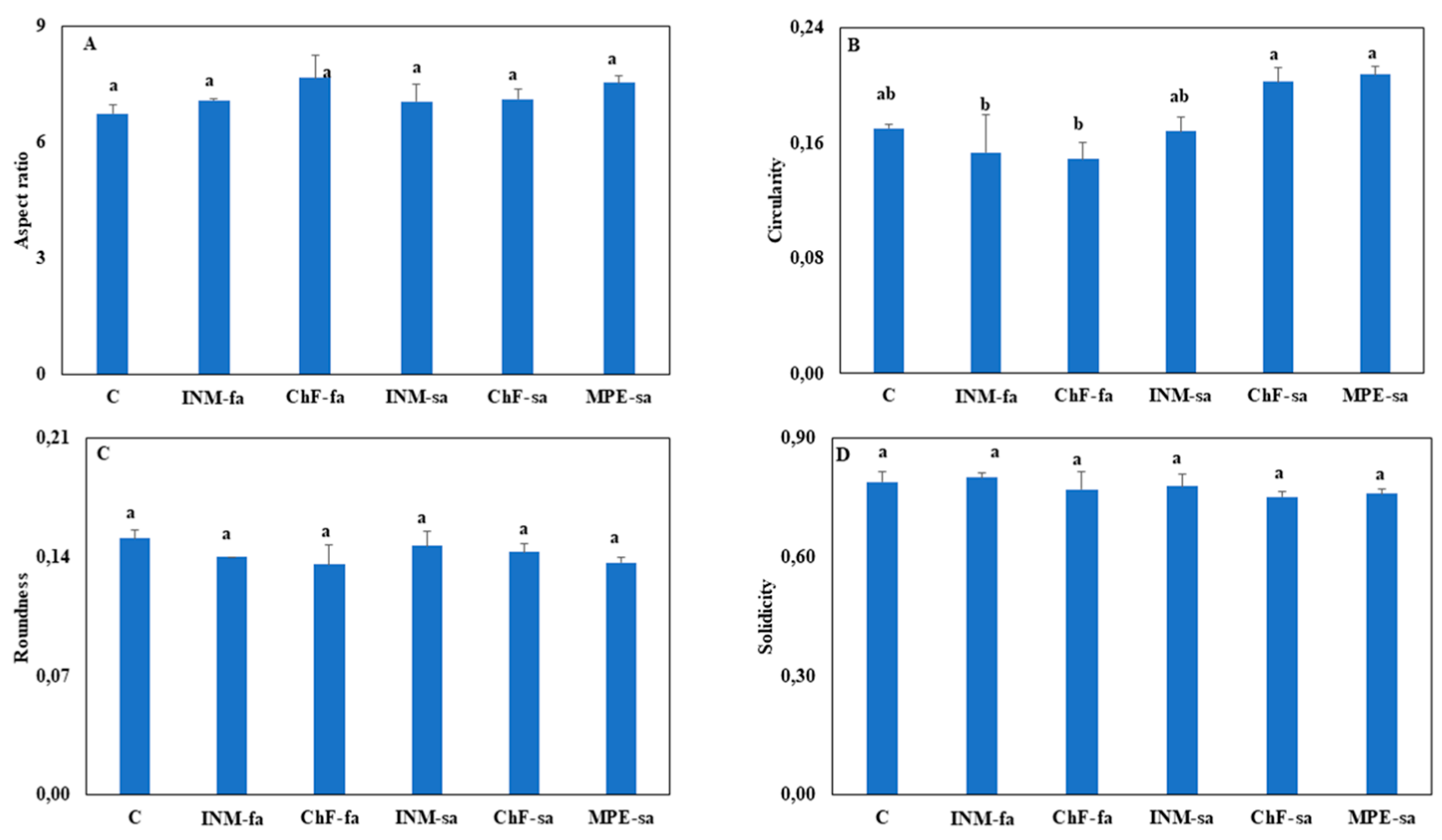
3.3. Leaf Shape Was Generally Not Affected by the Fertilization Scheme
A morphometric analysis was conducted by determining four leaf shape factors (aspect ratio, circularity, roundness, solidity). Aspect ratio, roundness, and solidity were not affected by the fertilization scheme (
Figure 4A,C,D). Plant cultivated under ChF-sa and MPE-sa had higher leaf circularity as compared to plants receiving foliar fertilization (INM-fa or ChF-fa;
Figure 4B).
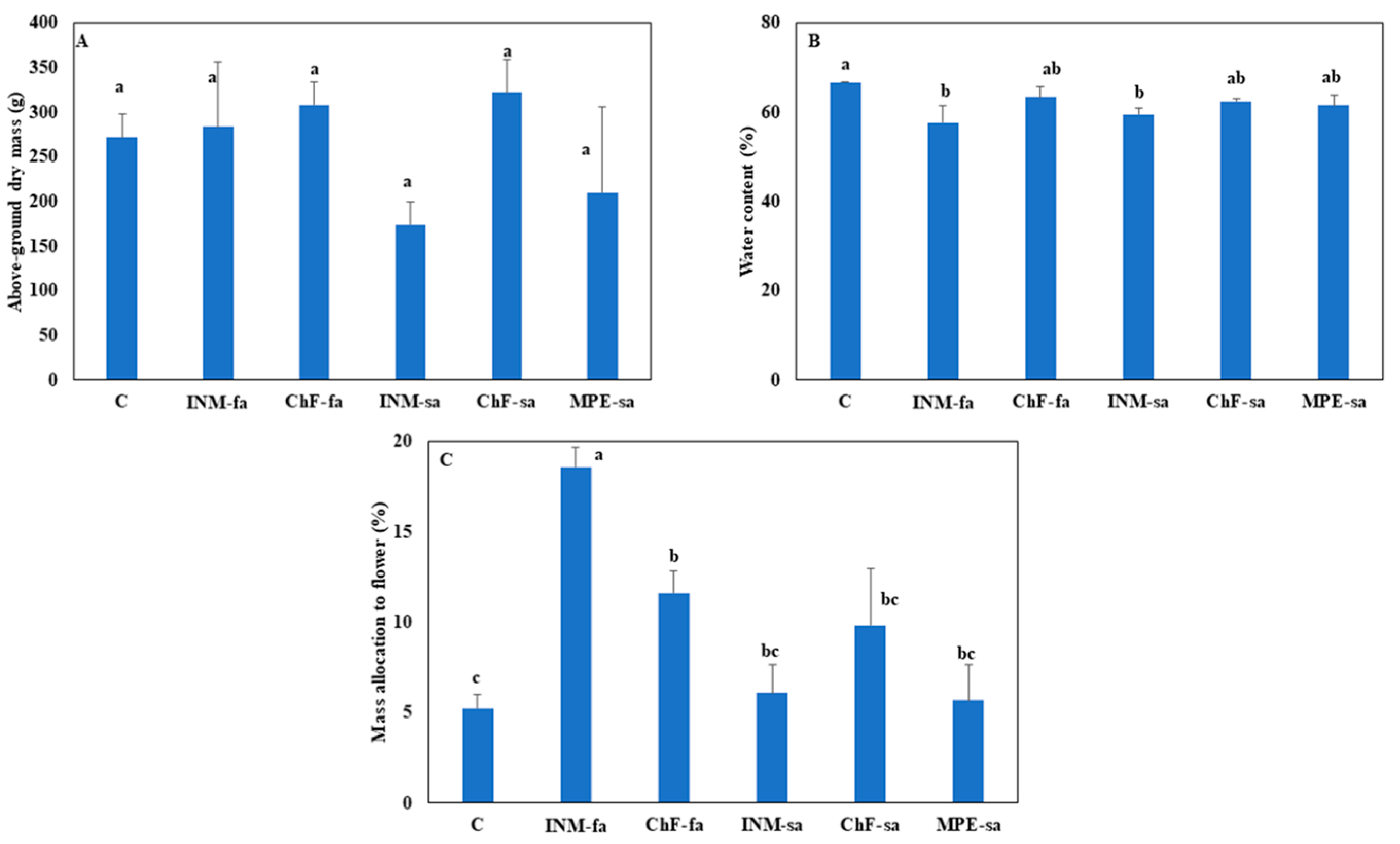
3.4. Fertilization Scheme Did Not Affect Plant Growth, though Foliar Fertilization Stimulated Biomass Allocation to Generative Organs
Above-ground plant biomass was not affected by the fertilization scheme (
Figure 5A). INM by either application method (INM-fa, INM-sa) was associated with lower water content, as compared to controls (
Figure 5B). Plants receiving foliar fertilization (INM-fa or ChF-fa) had increased biomass partitioning to the inflorescences as compared to controls, while plants cultivated under INM-fa had the highest partitioning to the inflorescences as compared to the remaining regimes (
Figure 5C).
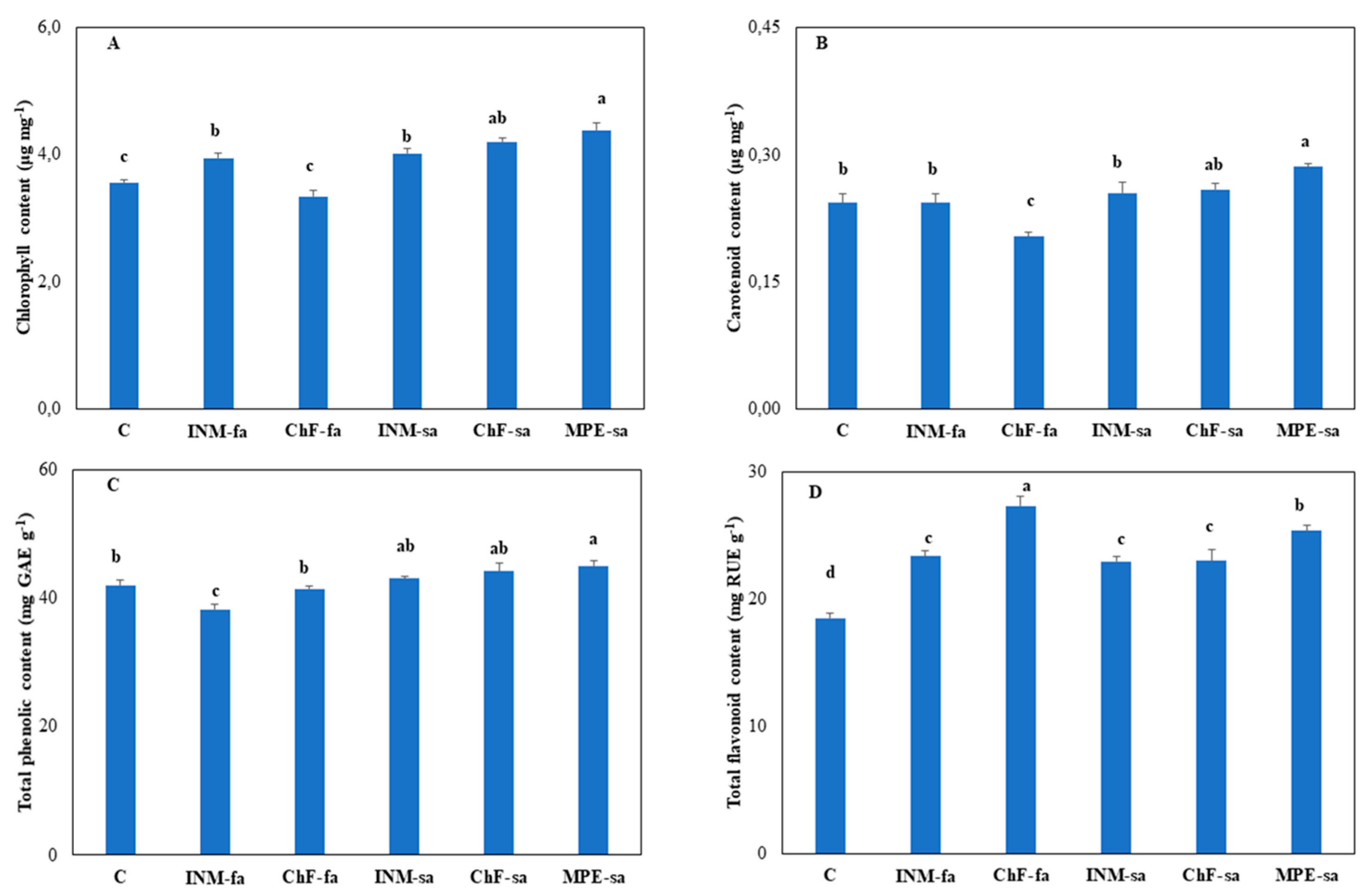
3.5. Fertilization Generally Improved Leaf Chlorophyll Content
Control plants and plants receiving chemical fertilization through foliar application (ChF-fa) had the lowest leaf chlorophyll content (
Figure 6A). Plants under MPE-sa had the highest leaf chlorophyll content as compared to all remaining regimes, besides ChF-sa (
Figure 6A).
3.6. Leaf Antioxidant Compound Content
Carotenoids, flavonoids, and phenols are critical non enzymatic antioxidants. Plants under MPE-sa had the highest leaf carotenoid content as compared to all remaining regimes, besides ChF-sa (
Figure 6B). The lowest leaf carotenoid content was noted in plants receiving ChF-fa (
Figure 6B).
Plants under MPE-sa had the highest leaf phenolic content as compared to the remaining regimes, besides ChF-sa and INM-sa (
Figure 6C). The lowest leaf phenolic content was noted in plants receiving INM-fa (
Figure 6C).
In all cases, fertilization stimulated leaf flavonoid content (
Figure 6D). The highest leaf flavonoid content was noted under ChF-fa, followed by MPE-sa (
Figure 6D).
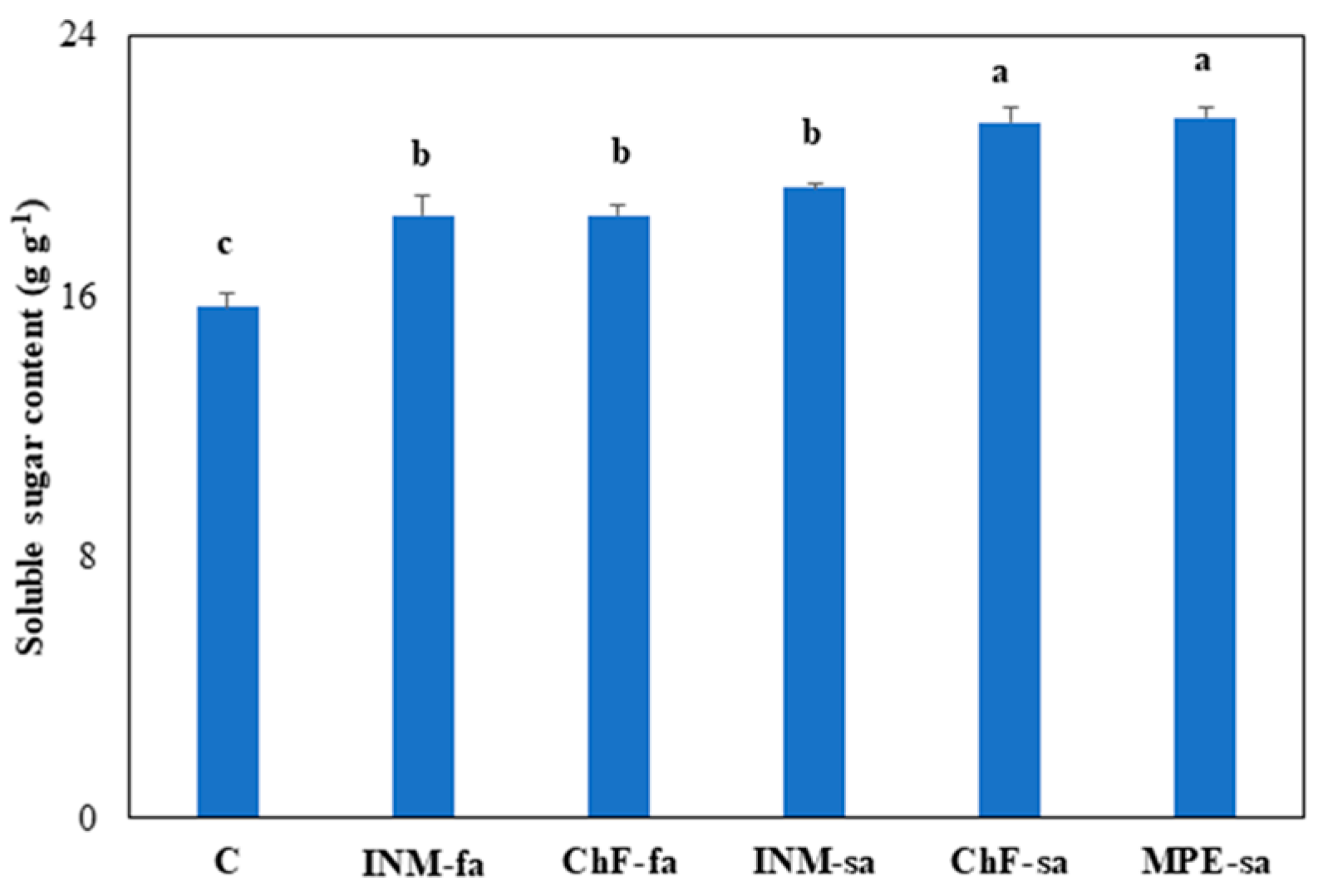
3.7. Fertilization Stimulated Leaf Soluble Sugar Content
In all cases, fertilization was associated with enhanced leaf soluble sugar content (
Figure 7). The highest leaf soluble sugar content was noted under the MPE-sa and ChF-fa schemes (
Figure 7).
3.8. Leaf and Floral Mineral Analysis
Fertilization scheme and mode of application affected significantly the nutrients’ leaf content of plants in certain cases (
Table 1 and
Table 2). Specifically, INM-fa and ChF-fa treatments showed the highest N and P content compared to all other treatments, besides C (control) in the case of N. Furthermore, K concentrations in all fertilization treatments were higher than control. No significant differences were observed among treatments regarding Ca and Mg (
Table 1).
As far as micronutrients’ leaf content is concerned, ChF-fa treatment showed the highest Cu, Zn, Fe and Mn concentrations comparing to all other treatments (including control). Moreover, concentrations of Cu in MPE-sa and Fe in INM-sa, ChF-sa and MPE-sa treatments were higher than C (control). Boron concentrations in the INM-fa, INM-sa, ChF-sa and MPE-sa treatments were higher than C (control), with the MPE-sa treatment showing the highest value (
Table 2).
Inflorescence mineral analysis showed significant differences among treatments in certain cases of nutrients (
Table 3 and
Table 4). MPE-sa showed the lowest N content and C (control) the highest P and Mg content. No significant differences were observed among treatments regarding K and Ca content (
Table 3).
Regarding inflorescence micronutrient content of
C. diae (
Table 4), the significantly highest Zn content was observed in C (control) and ChF-sa treatments and the lowest value in MPE-sa. No significant differences were observed among treatments in terms of Fe and B content (
Table 4).
4. Discussion
This pivotal field study presents the first steps of introducing
Carlina diae – an Endangered local endemic to Dia Island offshore from Crete, Greece with ornamental [
5] and medicinal interest [
21] - into sustainable cultivation, and assesses the fertilization regime which is suitable for stimulating both plant growth and herbal quality in terms of ornamental and/or medicinal interest. In this regard, the potential of employing INM instead conventional (inorganic) fertilizers was explored owing not only to the reduced environmental footprint, but also to the benefit of respective certification, which is greatly valued in the markets [
28].
Fertilization scheme did not affect plant biomass production in
C. diae (
Figure 5A). INM by either application method (INM-fa, INM-sa) was associated with lower water content, as compared to controls (
Figure 5B). This reduced water content owing to INM schemes is expected to facilitate processing, which reduces moisture content (through drying) to establish a shelf-stable herb product [
3]. Foliar fertilization (INM-fa or ChF-fa) was associated with increased biomass partitioning to the inflorescences as compared to controls, while plants under INM-fa had the highest inflorescence partitioning as compared to all treatments (
Figure 5C). Since inflorescence tissue represents the harvestable organ for ornamental purposes, foliar fertilization appears to be advantageous especially when employing INM in
C. diae, since it increases yield.
Macroscopic grading of herbal materials is based on visual inspection of leaf shape, and colour. The intensity and homogeneity of greenness is commonly employed as a quality criterion across the supply chain [
20]. In
C. diae, the fertilization scheme did not generally affect leaf coloration, as determined by three different instruments (SPAD meter, DA meter, Chroma Meter;
Figure 2, and Supplementary
Figures S1–S4). In other taxa, instead, organic fertilizers have been associated with enhanced leaf greenness [
19]. Through analysis of four diverse metrics, it became apparent that the fertilization scheme also exerted a rather limited influence on leaf shape (
Figure 4). Considering both leaf color and shape, it is concluded that the fertilization scheme did not alter the visual herbal quality aspects in
C. diae.
Since consumers are currently placing more emphasis on the positive aspects of antioxidant intake, the value of antioxidant content of the herbal medicinal material is continuously rising [
11,
27]. In this study, three key non enzymatic antioxidants (carotenoids, flavonoids, phenols) were determined in
C. diae which has also medicinal interest. The optimum fertilization scheme varied depending on the metabolite (
Figure 6B,C,D). Considering all three antioxidants together, MPE-sa appears the more stimulatory scheme, since it gives the highest [carotenoids (along with ChF-sa;
Figure 6B), phenolics (along with ChF-sa, INM-sa;
Figure 6C)] or the second highest [flavonoid (after ChF-fa);
Figure 6D] content. The promotive effect of INM on the antioxidant compound content has also been earlier shown in other taxa [
17,
29]. Previous own research lines employing other local endemic plants of Crete such as
Verbascum arcturus L. [
7] and
Origanum microphyllum (Benth.) Vegel [
8] offer first-time reports regarding the evaluation of the total phenolic and flavonoid contents which may further inspire the usage of fertilization in stimulating the herbal antioxidant content without compromised optical quality or yield in medicinal crops. Such investigations are supplementary to the current study dealing with
C. diae as they offer insight into several cases of exemplary neglected and underutilized local endemic species of Crete responding in similar ways to fertilization. Furthermore, the fertilization strategies employed in such studies incorporate a multidisciplinary approach dealing with biological, physiological, phytochemical, and agronomical aspects of different species with promising potential in different economic sectors, all being useful for the establishment of distinct species-specific value chains [
5].
With the exception of K, which increased in plant leaves upon application of both fertilization schemes and modes of application, only P among macronutrients responded to the foliar application of the integrated nutrient management or conventional fertilizers. The picture was quite differerent regarding the micronutrients, which responded to both fertilization schemes and modes of application in most cases. As far as micronutrients’ leaf content is concerned, ChF-fa treatment showed the highest Cu, Zn, Fe and Mn concentrations comparing to all other treatments (including control). Despite the variations among treatments, it is concluded that the conventional or integrated nutrient management fertilization did not cause increase in any of the macro- or micro-nutrient concentrations in the inflorescences of C. diae. By contrast, lower values were observed in all treatments for P and Mg concentrations compared to the control (C). Moreover, lower content compared to the control was found in INM-fa, INM-sa and MPE-sa with regards to K, and lower INM-fa, and MPE-sa for Zn. Considering, also, the lack of effect of fertilization management on above-ground dry biomass as shown here, these findings may suggest the effect of the initial macro- and micro-nutrients availabilities in the soil [
7,
8], which have probably masked or reduced any potential effect on inflorescence nutrients contents. Besides the above-mentioned, interestingly, the MPE-sa treatment showed lower Ca values compared to control and other treatments, which also needs further research given the significance of Ca on physiology of plant species; Ca is important for the stability of cell wall and membrane, being also a second messenger for specific developmental and physiological processes [
30].
Since the results reported in the study were obtained from only one season of field experimentation, longer period of research is needed to shed light into the optimum fertilization schemes and practices for C. diae in respect to both quantitative and qualitative characteristics of the plant. However, the current investigation provided for the first time baseline information for several herbal quality aspects in C. diae. Considering yield foliar fertilization, employing INM appears optimal, whereas MPE-sa is the best option when antioxidant compound content is the primary standard.
5. Conclusions
Carlina diae is an Endangered local endemic of Dia Island offshore from Crete (Greece) with high ornamental and medicinal potential. This pilot field study highlights the ameliorative effects of fertilization strategies on crop productivity and antioxidant profile of C. diae. Foliar fertilization employing INM increased biomass partitioning to inflorescences (harvestable organ), and decreased tissue water content (facilitating processing). The optimum fertilization scheme varied depending on the metabolite. Considering all three antioxidants together (carotenoids, flavonoids, phenols), INM with biostimulant appears the optimum scheme, since it was associated with the highest (carotenoids, phenolics) or the second highest (flavonoid) content. The present results in C. diae indicate that INM fertilization was optimal for upgrading yield (foliar application) or herbal quality in terms of antioxidant profiles (INM with biostimulant), thus facilitating the way towards its use in ornamental sector and/or medicine.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org, Method S1. Experimental design, soil analysis and fertilization treatments; Method S2. Plant measurements performed; Figure S1. Effect of fertilization regime through different (root/foliar) application methods on leaf index of absorbance difference of Carlina diae; Figure S2. Effect of fertilization regime through different (root/foliar) application methods on leaf L value of Carlina diae; Figure S3. Effect of fertilization regime through different (root/foliar) application methods on leaf “a” value of Carlina diae; Figure S4. Effect of fertilization regime through different (root/foliar) application methods on leaf “b” value of Carlina diae; Figures S1-S4 fertilization regimes. C: Control (water); INM-fa: Integrated nutrient management (INM) by foliar application; ChF-fa: Chemical fertilization by foliar application; INM-sa: INM by soil application; ChF-sa: Chemical fertilization by soil application; MPE-sa: Mixture of plant extracts as bioreactor by soil application (THEOMASS). Values represent the mean of three replicates ± SEM. Within each plot, different letters indicate significant differences.
Author Contributions
Conceptualization, G.T. (Georgios Tsoktouridis), and N.K.; Methodology, K.P., D.F., V.T., G.T. (Georgios Tsaniklidis), V.A.T., I.T., F.B., E.S., K.K., F.J.D., I.I., G.T. (Georgios Tsoktouridis), K.G., and T.M.; Software, K.P., D.F., V.T., G.T. (Georgios Tsaniklidis), V.A.T., I.I., and T.M.; Validation, D.F., V.A.T., I.T., F.B., E.S., K.K., F.J.D., I.I., T.M., K.G., and N.K.; Formal Analysis, K.P., D.F., V.T., S.V., I.K., G.T. (Georgios Tsaniklidis), V.A.T., I.T., F.B., E.S., I.I, and T.M.; Investigation, K.P., D.F., V.T., G.T. (Georgios Tsoktouridis), V.A.T., I.T., F.B., E.S., K.K., F.J.D., I.I., K.G., G.T. (Georgios Tsaniklidis), T.M.; Resources, K.P., T.M., I.I., and G.T. (Georgios Tsoktouridis); Data Curation, K.P., D.F., G.T. (Georgios Tsaniklidis), V.A.T., I.T., F.B., E.S., K.K., F.J.D., I.I., G.T. (Georgios Tsoktouridis), and T.M.; Writing—Original Draft Preparation, K.P., D.F., G.T. (Georgios Tsoktouridis), V.A.T., and N.K.; Writing—Review & Editing, K.P., D.F., V.T., G.T. (Georgios Tsoktouridis), V.A.T., F.B., E.S., K.K., F.J.D., I.I., G.T. (Georgios Tsaniklidis), T.M., and N.K.; Visualization, V.A.T., D.F., and N.K.; Supervision, T.M., and G.T. (Georgios Tsoktouridis); Project Administration, G.T. (Georgios Tsoktouridis); Funding Acquisition, K.P., K.G., N.K., T.M., and G.T. (Georgios Tsoktouridis). All authors have read and agreed to the published version of the manuscript.
Funding
This research has been co-financed by the European Union and Greek national funds through the Operational Program Competitiveness, Entrepreneurship, and Innovation, under the call RESEARCH—CREATE—INNOVATE, entitled “Conservation and sustainable utilization of rare threatened endemic plants of Crete for the development of new products with innovative precision fertilization” (Project code: T1EDK-05380; Acronym: PRECISE-M).
Data Availability Statement
All data supporting the results of this study are included in the manuscript and the datasets are available upon request.
Acknowledgments
We are grateful to the laboratory staff and the undergraduate students at the Hellenic Mediterranean University for their contributions, continued diligence, and dedication to their craft.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
a*, green to red range intensity; b*, blue to yellow range intensity; Fv/Fm, ratio of variable to maximum chlorophyll fluorescence; GAE, gallic acid equivalent; IAD, index of absorbance difference; INM, integrated nutrient management; L*, lightness; RUE, rutin equivalent; SAR, sodium absorption ratio.
References
- Santacroce, L.; Topi, S.; Haxhirexha, K.; Hidri, S.; Charitos, I.A.; Bottalico, L. Medicine and healing in the pre-socratic thought - a brief analysis of magic and rationalism in ancient herbal therapy. Endocr. Metab. Immune Disord. Drug Targets 2021, 21, 282–287. [Google Scholar] [CrossRef] [PubMed]
- Jain, D.; Chaudhary, P.; Kotnala, A.; Hossain, R.; Bisht, K.; Hossain, M.N. Hepatoprotective activity of medicinal plants: A mini review. J. Med. Plants Stud. 2020, 8, 183–188. [Google Scholar] [CrossRef]
- Taheri-Garavand, A.; Mumivand, H.; Fanourakis, D.; Fatahi, S.; Taghipour, S. An artificial neural network approach for non-invasive estimation of essential oil content and composition through considering drying processing factors: A case study in Mentha aquatica. Ind. Crops Prod. 2021, 171, 113985. [Google Scholar] [CrossRef]
- Hassanvand, F.; Rezaei Nejad, A.; Fanourakis, D. Morphological and physiological components mediating the silicon-induced enhancement of geranium essential oil yield under saline conditions. Ind. Crops Prod. 2019, 134, 19–25. [Google Scholar] [CrossRef]
- Krigas, N.; Tsoktouridis, G.; Anestis, I.; Khabbach, A.; Libiad, M.; Megdiche-Ksouri, W.; Ghrabi-Gammar, Z.; Lamchouri, F.; Tsiripidis, I.; Tsiafouli, M.A.; et al. Exploring the potential of neglected local endemic plants of three mediterranean regions in the ornamental sector: Value chain feasibility and readiness timescale for their sustainable exploitation. Sustainability 2021, 13, 2539. [Google Scholar] [CrossRef]
- Cheminal, A.; Kokkoris, I.P.; Strid, A.; Dimopoulos, P. Medicinal and aromatic Lamiaceae plants in Greece: Linking diversity and distribution patterns with ecosystem services. Forests 2020, 11, 661. [Google Scholar] [CrossRef]
- Paschalidis, K.; Fanourakis, D.; Tsaniklidis, G.; Tzanakakis, V.A.; Bilias, F.; Samara, E.; Kalogiannakis, K.; Debouba, F.J.; Ipsilantis, I.; Tsoktouridis, G.; et al. Pilot cultivation of the Vulnerable Cretan endemic Verbascum arcturus L. (Scrophulariaceae): Effect of fertilization on growth and quality features. Sustainability 2021, 13, 14030. [Google Scholar] [CrossRef]
- Fanourakis, D.; Paschalidis, K.; Tsaniklidis, G.; Tzanakakis, V.A.; Bilias, F.; Samara, E.; Liapaki, E.; Jouini, M.; Ipsilantis, I.; Maloupa, E.; et al. Pilot cultivation of the local endemic Cretan marjoram Origanum microphyllum (Benth.) Vogel (Lamiaceae): Effect of fertilizers on growth and herbal quality features. Agronomy 2022, 12, 94. [Google Scholar] [CrossRef]
- Ninou, E.G.; Paschalidis, K.A.; Mylonas, I.G.; Vasilikiotis, C.; A.G., M. The effect of genetic variation and nitrogen fertilization on productive characters of greek oregano. Acta Agric. Scandinavica, Soil Plant Sci 2017, 67, 372–379. [Google Scholar] [CrossRef]
- Kakar, K.; Xuan, T.D.; Noori, Z.; Aryan, S.; Gulab, G. Effects of organic and inorganic fertilizer application on growth, yield, and grain quality of rice. Agriculture 2020, 10, 544. [Google Scholar] [CrossRef]
- Ibrahim, M.H.; Jaafar, H.Z.; Karimi, E.; Ghasemzadeh, A. Impact of organic and inorganic fertilizers application on the phytochemical and antioxidant activity of kacip fatimah (Labisia pumila Benth). Molecules (Basel, Switzerland) 2013, 18, 10973–10988. [Google Scholar] [CrossRef]
- Krupa, M.; Witkowicz, R. Biostimulants as a response to the negative impact of agricultural chemicals on vegetation indices and yield of common buckwheat (Fagopyrum esculentum Moench). Agriculture 2023, 13, 825. [Google Scholar] [CrossRef]
- Gezahegn, A.M. Role of integrated nutrient management for sustainable maize production. Int. J. Agronomy 2021, 2021, 9982884. [Google Scholar] [CrossRef]
- Krigas, N.; Lazari, D.; Maloupa, E.; Stikoudi, M. Introducing dittany of crete (Origanum dictamnus L.) to gastronomy: A new culinary concept for a traditionally used medicinal plant. Int. J. Gastron. Food Sci. 2015, 2, 112–118. [Google Scholar] [CrossRef]
- Krigas, N.; Lykas, C.; Ipsilantis, I.; Matsi, T.; Weststrand, S.; Havstrom, M.; Tsoktouridis, G. Greek tulips: Worldwide electronic trade over the internet, global ex situ conservation and current sustainable exploitation challenges. Plants (Basel) 2021, 10, 580. [Google Scholar] [CrossRef] [PubMed]
- Asami, D.K.; Hong, Y.J.; Barrett, D.M.; A. E., M. Comparison of the total phenolic and ascorbic acid content of freeze-dried and air-dried marionberry, strawberry, and corn using conventional, organic, and sustainable agricultural practices. J. Agric. Food Chem. 2003, 51, 1237–1241. [Google Scholar] [CrossRef] [PubMed]
- Mrid, R.B.; Benmrid, B.; Hafsa, J.; Boukcim, H.; Sobeh, M.; Yasri, A. Secondary metabolites as biostimulant and bioprotectant agents: A review. Sci. Total Environ. 2021, 146204. [Google Scholar] [CrossRef]
- Rymuza, K.; Radzka, E.; Cała, J. The effect of applied biostimulants on the yielding of three non-genetically modified soybean cultivars. Agriculture 2023, 13, 900. [Google Scholar] [CrossRef]
- Amujoyegbe, B.J.; Opabode, J.T.; Olayinka, A.; Moench, *!!! REPLACE !!!*. Effect of organic and inorganic fertilizer on yield and chlorophyll content of maize (Zea mays L.) and sorghum (Sorghum bicolor L.). Afr. J. Biotechnol. 2007, 6, 1869–1873. [Google Scholar]
- Thamkaew, G.; Sjöholm, I.; Galindo, F. A review of drying methods for improving the quality of dried herbs. Crit. Rev. Food Sci. Nutr. 2020, 19, 1–24. [Google Scholar] [CrossRef]
- Bourgou, S.; Ben Haj Jilani, I.; Karous, O.; Megdiche-Ksouri, W.; Ghrabi-Gammar, Z.; Libiad, M.; Khabbach, A.; El Haissoufi, M.; Lamchouri, F.; Greveniotis, V.; et al. Medicinal-cosmetic potential of the local endemic plants of crete (greece), northern morocco and tunisia: Priorities for conservation and sustainable exploitation of neglected and underutilized phytogenetic resources. Biology (Basel) 2021, 10, 1344. [Google Scholar] [CrossRef]
- Grigoriadou, K.; Sarropoulou, V.; Krigas, N.; Maloupa, E.; Tsoktouridis, G. Gis-facilitated effective propagation protocols of the endangered local endemic of Crete Carlina diae (Rech. f.) Meusel and Kästner (asteraceae): Serving ex situ conservation needs and its future sustainable utilization as an ornamental. Plants (Basel) 2020, 9, 1465. [Google Scholar] [CrossRef] [PubMed]
- Paschalidis, K.A.; Moschou, P.N.; Aziz, A.; Toumi, T.; Roubelakis-Angelakis, K.A. Polyamines in grapevine: An update. In Grapevine Molecular Physiology & Biotechnology; Roubelakis-Angelakis, K.A., Ed.; Springer: Dordrecht, Switzerland, 2009; pp. 207–228. [Google Scholar]
- Manganaris, G.A.; Drogoudi, P.; Goulas, V.; Tanou, G.; Georgiadou, E.C.; Pantelidis, G.E.; Paschalidis, K.A.; Fotopoulos, V.; Manganaris, A. Deciphering the interplay among genotype, maturity stage and low-temperature storage on phytochemical composition and transcript levels of enzymatic antioxidants in Prunus persica fruit. Plant Physiol Biochem 2017, 119, 189–199. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Fanourakis, D.; Tsaniklidis, G.; Li, K.; Li, T. Contrary to red, blue monochromatic light improves the bioactive compound content in broccoli sprouts. Agronomy Res. 2021, 11, 2139. [Google Scholar] [CrossRef]
- Ahmadi-Majd, M.; Rezaei Nejad, A.; Mousavi-Fard, S.; Fanourakis, D. Postharvest application of single, multi-walled carbon nanotubes and graphene oxide stimulates rose keeping quality. J. Hortic. Sci. Biotechnol. 2021, 97, 346–360. [Google Scholar] [CrossRef]
- Chen, Y.; Fanourakis, D.; Tsaniklidis, G.; Aliniaeifard, S.; Yang, Q.; Li, T. Low uva intensity during cultivation improves the lettuce shelf-life, an effect that is not sustained at higher intensity. Postharvest Biol. Technol. 2021, 172, 111376. [Google Scholar] [CrossRef]
- Laird, S.A.; Pierce, A.R. Promoting sustainable and ethical botanicals: Strategies to improve commercial raw material sourcing, results from the sustainable botanicals pilot project industry surveys, case studies and standards collection; Rainforest Alliance: New York, NY, USA, 2002. [Google Scholar]
- Serri, F.; Souri, M.K.; Rezapanah, M. Growth, biochemical quality and antioxidant capacity of coriander leaves under organic and inorganic fertilization programs. Chem. Biol. Technol. Agric. 2021, 8, 33. [Google Scholar] [CrossRef]
- Kuronuma, T.; Watanabe, H. Identification of the causative genes of calcium deficiency disorders in horticulture crops: A systematic review. Agriculture 2021, 11, 906. [Google Scholar] [CrossRef]
Figure 1.
Wild-growing individual of Carlina diae as a rock-dweller in Dia Island offshore from Crete, Greece (A), harvested inflorescences from the wild utilized as domestic dried ornamental (B) and cultivated plant individual of C. diae (C) in the pilot field experiment at the campus of the Hellenic Mediterranean University, Heraklion, Crete (D). Photos A and B: M. Avramakis, Natural History Museum of Crete (reproduced with permission).
Figure 1.
Wild-growing individual of Carlina diae as a rock-dweller in Dia Island offshore from Crete, Greece (A), harvested inflorescences from the wild utilized as domestic dried ornamental (B) and cultivated plant individual of C. diae (C) in the pilot field experiment at the campus of the Hellenic Mediterranean University, Heraklion, Crete (D). Photos A and B: M. Avramakis, Natural History Museum of Crete (reproduced with permission).
Figure 2.
Effect of fertilization regime through different (root/foliar) application methods on leaf SPAD value of Carlina diae at vegetative (A), early flowering (B), and full flowering (C) stage. C: Control (water); INM-fa: Integrated nutrient management (INM) by foliar application; ChF-fa: Chemical fertilization by foliar application; INM-sa: INM by soil application; ChF-sa: Chemical fertilization by soil application; MPE-sa: Mixture of plant extracts as biostimulant by soil application (THEOMASS). Values represent the mean of three replicates ± SEM. Within each plot, different letters indicate significant differences.
Figure 2.
Effect of fertilization regime through different (root/foliar) application methods on leaf SPAD value of Carlina diae at vegetative (A), early flowering (B), and full flowering (C) stage. C: Control (water); INM-fa: Integrated nutrient management (INM) by foliar application; ChF-fa: Chemical fertilization by foliar application; INM-sa: INM by soil application; ChF-sa: Chemical fertilization by soil application; MPE-sa: Mixture of plant extracts as biostimulant by soil application (THEOMASS). Values represent the mean of three replicates ± SEM. Within each plot, different letters indicate significant differences.
Figure 3.
Effect of fertilization regime through different (root/foliar) application methods on chlorophyll fluorescence value of Carlina diae at vegetative (A), early flowering (B), and full flowering (C) stage. C: Control (water); INM-fa: Integrated nutrient management (INM) by foliar application; ChF-fa: Chemical fertilization by foliar application; INM-sa: INM by soil application; ChF-sa: Chemical fertilization by soil application; MPE-sa: Mixture of plant extracts as biostimulant by soil application (THEOMASS). Values represent the mean of three replicates ± SEM. Within each plot, different letters indicate significant differences.
Figure 3.
Effect of fertilization regime through different (root/foliar) application methods on chlorophyll fluorescence value of Carlina diae at vegetative (A), early flowering (B), and full flowering (C) stage. C: Control (water); INM-fa: Integrated nutrient management (INM) by foliar application; ChF-fa: Chemical fertilization by foliar application; INM-sa: INM by soil application; ChF-sa: Chemical fertilization by soil application; MPE-sa: Mixture of plant extracts as biostimulant by soil application (THEOMASS). Values represent the mean of three replicates ± SEM. Within each plot, different letters indicate significant differences.
Figure 4.
Effect of fertilization regime through different (root/foliar) application methods on four leaf shape factors of Carlina diae. C: Control (water); INM-fa: Integrated nutrient management (INM) by foliar application; ChF-fa: Chemical fertilization by foliar application; INM-sa: INM by soil application; ChF-sa: Chemical fertilization by soil application; MPE-sa: Mixture of plant extracts as biostimulant by soil application (THEOMASS). Values represent the mean of six replicates ± SEM. Within each plot, different letters indicate significant differences.
Figure 4.
Effect of fertilization regime through different (root/foliar) application methods on four leaf shape factors of Carlina diae. C: Control (water); INM-fa: Integrated nutrient management (INM) by foliar application; ChF-fa: Chemical fertilization by foliar application; INM-sa: INM by soil application; ChF-sa: Chemical fertilization by soil application; MPE-sa: Mixture of plant extracts as biostimulant by soil application (THEOMASS). Values represent the mean of six replicates ± SEM. Within each plot, different letters indicate significant differences.
Figure 5.
Effect of fertilization regime through different (root/foliar) application methods on above-ground dry weight (A), water content (B) and dry weight partitioning to inflorescences (C) of Carlina diae. C: Control (water); INM-fa: Integrated nutrient management (INM) by foliar application; ChF-fa: Chemical fertilization by foliar application; INM-sa: INM by soil application; ChF-sa: Chemical fertilization by soil application; MPE-sa: Mixture of plant extracts as biostimulant by soil application (THEOMASS). Values represent the mean of six replicates ± SEM. Within each plot, different letters indicate significant differences.
Figure 5.
Effect of fertilization regime through different (root/foliar) application methods on above-ground dry weight (A), water content (B) and dry weight partitioning to inflorescences (C) of Carlina diae. C: Control (water); INM-fa: Integrated nutrient management (INM) by foliar application; ChF-fa: Chemical fertilization by foliar application; INM-sa: INM by soil application; ChF-sa: Chemical fertilization by soil application; MPE-sa: Mixture of plant extracts as biostimulant by soil application (THEOMASS). Values represent the mean of six replicates ± SEM. Within each plot, different letters indicate significant differences.
Figure 6.
Effect of fertilization regime through different (root/foliar) application methods on leaf chlorophyll (A), carotenoid (B), total phenol (C) and total flavonoid (D) content of Carlina diae. C: Control (water); INM-fa: Integrated nutrient management (INM) by foliar application; ChF-fa: Chemical fertilization by foliar application; INM-sa: INM by soil application; ChF-sa: Chemical fertilization by soil application; MPE-sa: Mixture of plant extracts as biostimulant by soil application (THEOMASS). Values represent the mean of three replicates ± SEM. Within each plot, different letters indicate significant differences.
Figure 6.
Effect of fertilization regime through different (root/foliar) application methods on leaf chlorophyll (A), carotenoid (B), total phenol (C) and total flavonoid (D) content of Carlina diae. C: Control (water); INM-fa: Integrated nutrient management (INM) by foliar application; ChF-fa: Chemical fertilization by foliar application; INM-sa: INM by soil application; ChF-sa: Chemical fertilization by soil application; MPE-sa: Mixture of plant extracts as biostimulant by soil application (THEOMASS). Values represent the mean of three replicates ± SEM. Within each plot, different letters indicate significant differences.
Figure 7.
Effect of fertilization regime through different (root/foliar) application methods on leaf soluble sugar content of Carlina diae. C: Control (water); INM-fa: Integrated nutrient management (INM) by foliar application; ChF-fa: Chemical fertilization by foliar application; INM-sa: INM by soil application; ChF-sa: Chemical fertilization by soil application; MPE-sa: Mixture of plant extracts as biostimulant by soil application (THEOMASS). Values represent the mean of three replicates ± SEM. Within each plot, different letters indicate significant differences.
Figure 7.
Effect of fertilization regime through different (root/foliar) application methods on leaf soluble sugar content of Carlina diae. C: Control (water); INM-fa: Integrated nutrient management (INM) by foliar application; ChF-fa: Chemical fertilization by foliar application; INM-sa: INM by soil application; ChF-sa: Chemical fertilization by soil application; MPE-sa: Mixture of plant extracts as biostimulant by soil application (THEOMASS). Values represent the mean of three replicates ± SEM. Within each plot, different letters indicate significant differences.
Table 1.
Effect of fertilization scheme through different (root/foliar) application methods on leaf essential macronutrient content of Carlina diae. C: Control (water); INM-fa: Integrated nutrient management (INM) by foliar application; ChF-fa: Chemical fertilization by foliar application; INM-sa: INM by soil application; ChF-sa: Chemical fertilization by soil application; MPE-sa: Mixture of plant extracts as biostimulant by soil application (THEOMASS). Mineral content was expressed per dry weight basis. Values represent the mean of three replicates ±SEM.
Table 1.
Effect of fertilization scheme through different (root/foliar) application methods on leaf essential macronutrient content of Carlina diae. C: Control (water); INM-fa: Integrated nutrient management (INM) by foliar application; ChF-fa: Chemical fertilization by foliar application; INM-sa: INM by soil application; ChF-sa: Chemical fertilization by soil application; MPE-sa: Mixture of plant extracts as biostimulant by soil application (THEOMASS). Mineral content was expressed per dry weight basis. Values represent the mean of three replicates ±SEM.
| Treatment |
N |
P |
K |
Ca |
Mg |
| |
(g kg-1) |
| C |
9.8±0.5a* |
1.1±0.0c |
21.9±0.2b |
18.2±0.8a |
2.3±0.1a |
| INM-fa |
12.3±1.1a |
2.7±0.1b |
35.1±1.7a |
26.4±1.5a |
2.6±0.1a |
| ChF-fa |
11.4±0.5a |
3.3±0.1a |
33.7±1.0a |
27.8±1.4a |
2.6±0.1a |
| INM-sa |
5.9±0.2b |
1.2±0.0c |
30.1±1.1a |
27.0±1.5a |
2.6±0.1a |
| ChF-sa |
6.0±0.1b |
1.3±0.0c |
32.1±0.1a |
24.8±0.9a |
2.8±0.1a |
| MPE-sa |
5.5±0.2b |
1.5±0.1c |
30.3±1.6a |
24.8±1.9a |
2.5±0.1a |
|
pF-test
|
< 0.001 |
< 0.001 |
0.014 |
NS# |
NS |
Table 2.
Effect of fertilization scheme through different (root/foliar) application methods on leaf essential micronutrient content of Carlina diae. C: Control (water); INM-fa: Integrated nutrient management (INM) by foliar application; ChF-fa: Chemical fertilization by foliar application; INM-sa: INM by soil application; ChF-sa: Chemical fertilization by soil application; MPE-sa: Mixture of plant extracts as biostimulant by soil application (THEOMASS). Mineral content was expressed per dry weight basis. Values represent the mean of three replicates ±SEM.
Table 2.
Effect of fertilization scheme through different (root/foliar) application methods on leaf essential micronutrient content of Carlina diae. C: Control (water); INM-fa: Integrated nutrient management (INM) by foliar application; ChF-fa: Chemical fertilization by foliar application; INM-sa: INM by soil application; ChF-sa: Chemical fertilization by soil application; MPE-sa: Mixture of plant extracts as biostimulant by soil application (THEOMASS). Mineral content was expressed per dry weight basis. Values represent the mean of three replicates ±SEM.
| Treatment |
Cu |
Zn |
Fe |
Mn |
B |
| |
(mg kg-1) |
| C |
10.5±0.2c* |
36.3±0.3b |
439±18b |
114±2bc |
29.5±0.5c |
| INM-fa |
12.3±0.4abc |
41.3±2.0ab |
721±2ab |
136±8ab |
41.8±1.9ab |
| ChF-fa |
13.8±0.6a |
48.4±0.4a |
932±64a |
156±3a |
36.4±1.0bc |
| INM-sa |
11.3±0.2bc |
36.2±0.8b |
886±74a |
113±4bc |
39.7±1.3ab |
| ChF-sa |
11.4±0.3bc |
42.1±1.9ab |
865±43a |
115±1bc |
38.9±1.2ab |
| MPE-sa |
13.3±0.3ab |
44.0±1.5ab |
778±81a |
107±2c |
46.9±1.8a |
|
pF-test
|
0.028 |
0.037 |
0.059 |
0.005 |
0.007 |
Table 3.
Effect of fertilization regime through different (root/foliar) application methods on inflorescence essential macronutrient content of Carlina diae. C: Control (water); INM-fa: Integrated nutrient management (INM) by foliar application; ChF-fa: Chemical fertilization by foliar application; INM-sa: INM by soil application; ChF-sa: Chemical fertilization by soil application; MPE-sa: Mixture of plant extracts as biostimulant by soil application (THEOMASS). Values represent the mean of three replicates ± SEM.
Table 3.
Effect of fertilization regime through different (root/foliar) application methods on inflorescence essential macronutrient content of Carlina diae. C: Control (water); INM-fa: Integrated nutrient management (INM) by foliar application; ChF-fa: Chemical fertilization by foliar application; INM-sa: INM by soil application; ChF-sa: Chemical fertilization by soil application; MPE-sa: Mixture of plant extracts as biostimulant by soil application (THEOMASS). Values represent the mean of three replicates ± SEM.
| Treatment |
N |
P |
K |
Ca |
Mg |
| |
|
|
(mg kg-1) |
|
|
| C |
10.46±0.49a* |
2.11±0.35a |
15.08±0.05a |
3.88±0.05a |
1.59±0.05a |
| INM-fa |
9.42±0.03a |
1.32±0.11b |
12.70±1.03a |
3.04±0.70a |
1.02±0.10b |
| ChF-fa |
8.66±0.32a |
1.75±0.07b |
14.22±0.77a |
3.97±0.32a |
1.38±0.19b |
| INM-sa |
9.62±1.60a |
1.08±0.27b |
12.99±0.64a |
2.94±0.42a |
1.32±0.15b |
| ChF-sa |
9.29±1.02a |
1.23±0.22b |
13.82±0.57a |
3.33±0.07a |
1.30±0.13b |
| MPE-sa |
4.71±0.27b |
1.65±0.27b |
12.89±0.05a |
2.83±0.14a |
1.08±0.00b |
|
pF-test
|
0.007 |
0.076 |
NS |
NS |
0.06 |
Table 4.
Effect of fertilization regime through different (root/foliar) application methods on floral essential micronutrient content of Carlina diae. C: Control (water); INM-fa: Integrated nutrient management (INM) by foliar application; ChF-fa: Chemical fertilization by foliar application; INM-sa: INM by soil application; ChF-sa: Chemical fertilization by soil application; MPE-sa: Mixture of plant extracts as biostimulant by soil application (THEOMASS). Values represent the mean of three replicates ± SEM.
Table 4.
Effect of fertilization regime through different (root/foliar) application methods on floral essential micronutrient content of Carlina diae. C: Control (water); INM-fa: Integrated nutrient management (INM) by foliar application; ChF-fa: Chemical fertilization by foliar application; INM-sa: INM by soil application; ChF-sa: Chemical fertilization by soil application; MPE-sa: Mixture of plant extracts as biostimulant by soil application (THEOMASS). Values represent the mean of three replicates ± SEM.
| Treatment |
Zn |
Fe |
B |
| |
|
mg kg-1
|
|
| C |
19.65±1.29a* |
72.54±15.21a |
13.05±0.14a |
| INM-fa |
15.86±0.14b |
52.70±0.45a |
12.86±0.16a |
| ChF-fa |
17.78±0.89ab |
58.74±6.52a |
12.85±0.54a |
| INM-sa |
17.67±1.42ab |
45.95±8.20a |
25.51±12.47a |
| ChF-sa |
20.58±1.46a |
34.19±6.92a |
19.52±5.27a |
| MPE-sa |
16.11±0.50b |
51.30±12.79a |
16.73±1.58a |
|
pF-test
|
0.051 |
NS |
NS |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).