1. Introduction
Rice blast disease, caused by the fungus
Magnaporthe oryzae, is a highly destructive disease that affects rice production around the world. It is estimated that this disease causes economic damages of approximately
$66 billion annually, which is enough to feed 60 million people [
1]. The disease begins when the conidia of the pathogen come into contact with the hydrophobic surface of the rice leaf. These conidia then germinate and develop the infection structures called appressoria. The appressoria accumulate high internal turgor pressure, allowing them to mechanically penetrate the rice cells by forming a hyphal penetration peg [
2,
3]. Once the pathogen has successfully colonized the rice tissue, multiple infection hyphae expand both within and between cells, leading to the formation of characteristic blast lesions within 3 to 5 days [
4]. From these lesions, new conidia are produced and released, initiating new infection cycles. In addition to its impact on rice,
M. oryzae has the ability to infect other important cereal crops such as wheat, barley, finger millet, and foxtail millet [
5]. This fungus recently caused a devastating outbreak of wheat blast in Bangladesh, resulting in significant economic losses [
6,
7]. Due to its economic importance, genetic tractability, and availability of genome sequence,
M. oryzae has emerged as a valuable model for studying fungal pathogenesis and its interaction with host plants [
8,
9]. Since TFs are considered as pivotal regulators necessary for fitness and virulence in
M. oryzae [
10], further understanding the cellular or biological functions mediated by these proteins should be beneficial to develop novel and practical strategies to control the blast disease and ensure global food security.
TFs are proteins that have DNA-binding domains. They can be classified into various families, including bZIP, bHLH, C2H2 zinc finger, homeobox, Zn2Cys6, and Myb, according their specific DNA-binding domains [
11]. The Myb family is known for its highly conserved Myb DNA-binding domain. This domain typically consists of 1 to 3 imperfect amino acid repeats, each containing 50 to 53 amino acids, which form a helix turn helix structure [
12,
13,
14,
15,
16]. The name "Myb" originated from v-Myb, the oncogenic motif of avian myeloblastosis virus (AMV), where it was initially identified [
15]. The Myb family is present in all eukaryotes including plants, animals and Fungi [
17]. In plants, Myb family proteins are predominantly known as transcription factors or repressors [
18,
19,
20,
21,
22,
23,
24]. However, proteins that are not classical transcription factors can contain Myb DNA binding domains [
23,
25,
26,
27,
28,
29,
30,
31,
32,
33]. In animal genomes, the Myb family is relatively small, consisting of only 4 to 5 proteins [
13]. In contrast, the Myb family has expanded in plants, with a range of 100 to 200 members [
13]. The fungal Myb family size is smaller than in plants but more extensive than in animals, with 10 to 50 members per genome [
11]. Animal Myb proteins have been reported to regulate cell division and a discrete subset of cellular differentiation events [
15,
34,
35]. In plants, Myb proteins bind DNA and regulate many metabolic, cellular, and developmental processes as transcription factors or in other ways [
13,
14,
20,
21,
22,
23,
24]. Fungal Myb proteins have been implicated in stress response regulation and are thought to play a role in the pathogenicity of plant pathogens [
36,
37,
38]. However, compared to Myb proteins in plants and animals, fungal Myb proteins have received less research attention, and their biological roles are largely unknown.
We aimed to investigate the biological roles of the Myb family TFs in the rice blast fungus,
M. oryzae. The Fungal Transcription Factor Database (FTFD,
http://ftfd.snu.ac.kr/) predicted 19 Myb family TFs in
M. oryzae. Prior to our study, 1 Myb TF was previously reported as MoMyb1 [
39] and 9 more recently identified as MoMyb2-10 [
40]. In this study, we attempted to knock out three additional genes, with gene IDs MGG_05099, MGG_01130, and MGG_14558, which were respectively named MoMyb11-13. However, we were only successful in obtaining knockout mutants for MoMyb13. Therefore, this paper will solely focus on investigating the function of MoMyb13. Our findings reveal that MoMyb13 is a key regulator for growth, conidiation and pathogenicity of
M. oryzae.
2. Materials and Methods
Organisms and media used
Magnaporthe oryzae B. Couch anamorph of the teleomorph
Pyricularia oryzae Cavara was used for this research. As background strain, we used Ku80 (generated from the WT strain 70-15) to minimize random integration events when transformed [
41]. The susceptible Indica rice (cv. CO-39) and barley (cv. Golden Promise) used for the fungal pathogenicity tests were from the seed bank of our laboratory. The CM (complete medium), MM (minimal medium), and RBM (rice bran medium) used for growing the fungus were prepared as described [
42]. The
Escherichia coli strain DH-5α used for routine bacterial transformations and maintenance of various plasmid vectors was bought from Solarbio Life Sciences, China. All primers used in this study are listed (
Table S1).
Knockouts, complementations, and verifications
The
MoMYB13 knockout vector was constructed in the plasmid pBS-HYG by inserting 1 kb up- and down-stream fragments of the
MoMYB13 coding region as flanking regions of the HPH (hygromycin phosphotransferase) gene [
43]. To perform gene deletion transformations, at least 2 μg of the vector DNA was introduced to Ku80 protoplasts, and transformants were selected for hygromycin resistance [
44]. Southern blotting was conducted to confirm the correct knockout mutants using the digoxigenin (DIG) high prime DNA labelling and detection starter Kit I (11745832910 Roche Germany). The complementation vector of
MoMYB13 was constructed by cloning the entire length of
MoMYB13 with the native promoter region (about 1.5-kb) to the pCB1532 plasmid. During making the complementation vector, GFP was linked to the C-terminal of
MoMYB13 to study the sub-cellar localization of MoMyb13. The constructed vector DNA was introduced into the knockout mutant protoplast for gene complementation, and resulting transformants were screened using 50 μg/ml chlorimuron-ethyl to select successful complementary strains. The detailed fungal protoplast preparation and transformation methods have been described previously [
45]. The sub-cellar localization of MoMyb13 was observed by confocal microscopy (Nikon A1). GFP and RFP excitation wavelengths were 488 nm and 561 nm, respectively.
Mutant phenotype measurements
Vegetative growth was tested by measuring the colony diameter after ten days of growth in 9 cm Petri dishes at 25℃ under 12h-to-12h light and dark periods. Conidia production was evaluated by flooding the 12-day-old colony with double distilled water, filtering out the mycelia with gauze, and counting the conidia using a hemacytometer. The conidiophore induction assay was performed by excising one thin agar block from the fungal colony and then incubating it in a sealed chamber for 24 h with constant light [
46]. Hyphal appressoria were induced by placing a suspension of hyphal fragments on a hydrophobic surface in a humid environment at 25℃ for 24h. Conidial appressoria were induced by placing the conidia suspension of 10
4 spores/ml in the same environment. The pathogenicity assay on excised barley and rice leaves was performed by cutting a small block from the agar culture of the fungus and placing it on excised leaves for five days in a moist chamber for disease development [
47]. The wounded leaves were created by gently scraping the leaf surface at the inoculation site with a blade.
RT-qPCR assay
Total RNA was extracted using Eastep
®Super Total RNA Extraction Kit (Promega (Beijing) Biotechnology, LS1040) to perform RT-qPCR. 5 mg of RNA was reverse-transcribed to cDNA using the Evo M-MLV RT kit with gDNA to clean before the qPCR (Accurate Biotechnology (Hunan), AG11705) according to the manufacturer’s instructions. The resulting cDNA was diluted ten times and used as the template for the qPCR. The qPCR reactions were performed using an Applied Biosystems 7500 Real-Time PCR System. Each reaction contained 25 μl of SuperRealPreMix Plus SYBR Green (Tiangen Biotechnology, Beijing, FP205-02), one μl of cDNA, and 1.5 μl of each primer solution. The thermal cycling conditions were 15 min at 95 ℃ followed by 40 cycles of 10 s at 95 ℃ and 20 s at 60 ℃. The threshold cycle (Ct) values were obtained by analyzing amplification curves with a normalized reporter threshold of 0.1. The relative expression value was calculated using the 2
-ΔΔCT method [
48].
3. Results
3.1. Sequence Identification and Evolution Analysis of MoMyb13
According to the NCBI database, MoMyb13 is a large protein consisting of 2305 amino acids (aa) (
Figure 1A). It has two Myb domains at positions 824-864 aa and 1119-1157 aa, and a transcription termination factor Rho domain at position 1849-2097 aa (
Figure 1A). Additionally, it has two DNA polymerase III subunit γ/τ domains at 385-603 aa and 1192-1381 aa (
Figure 1A). A similarity search conducted in the NCBI database (E value <10
-6) revealed that Momyb13 only has homologues in 17 genera of ascomycete fungi, but no homologues found in plants, animals, or fungi outside of the ascomycetes. We selected one homologue from each genus to construct a phylogenetic tree, and found that Momyb13 is most closely related to the homologues from Diaporthaceae sp. and Diaporthe eres (
Figure 1A).
3.2. MoMyb13 Is Located in the Nuclei of M. oryzae
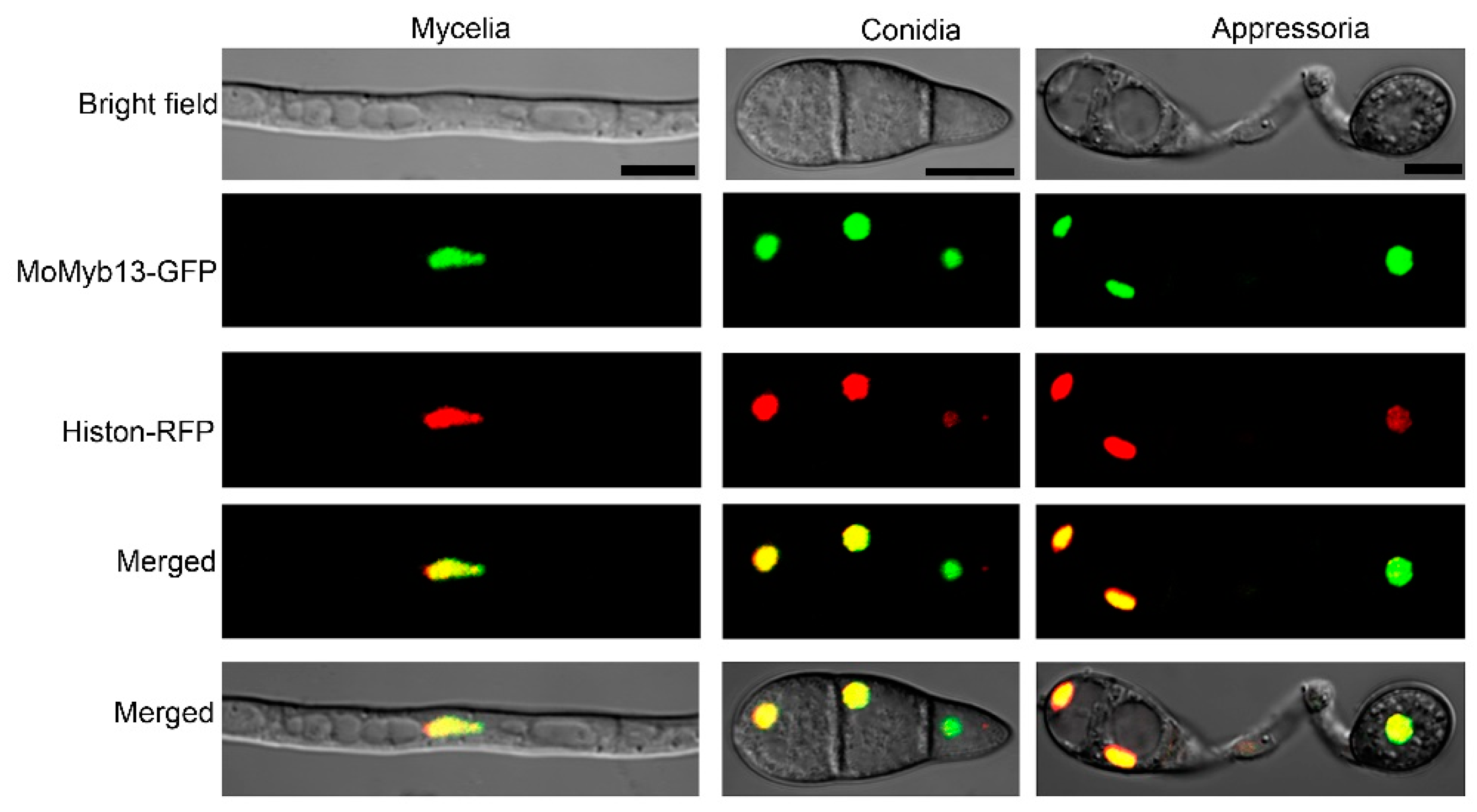
Given that MoMyb13 is predicted to be a TF, we confirmed its nuclear localization through subcellular protein localization experiments. We fused the GFP protein to the C-terminus of MoMyb13 and co-transformed it with the nuclear marker protein Histon-RFP into M. oryzae. By observing the fluorescent signals of the transformants, we found complete overlap of the red and green signals in the nuclei of hyphae, conidia, and appressoria of M. oryzae (
Figure 2), indicating that MoMyb13 is also localized to the nucleus in M. oryzae, consistent with its role as a transcription factor.
3.3. MoMyb13 Is Required for the Growth of M. oryzae
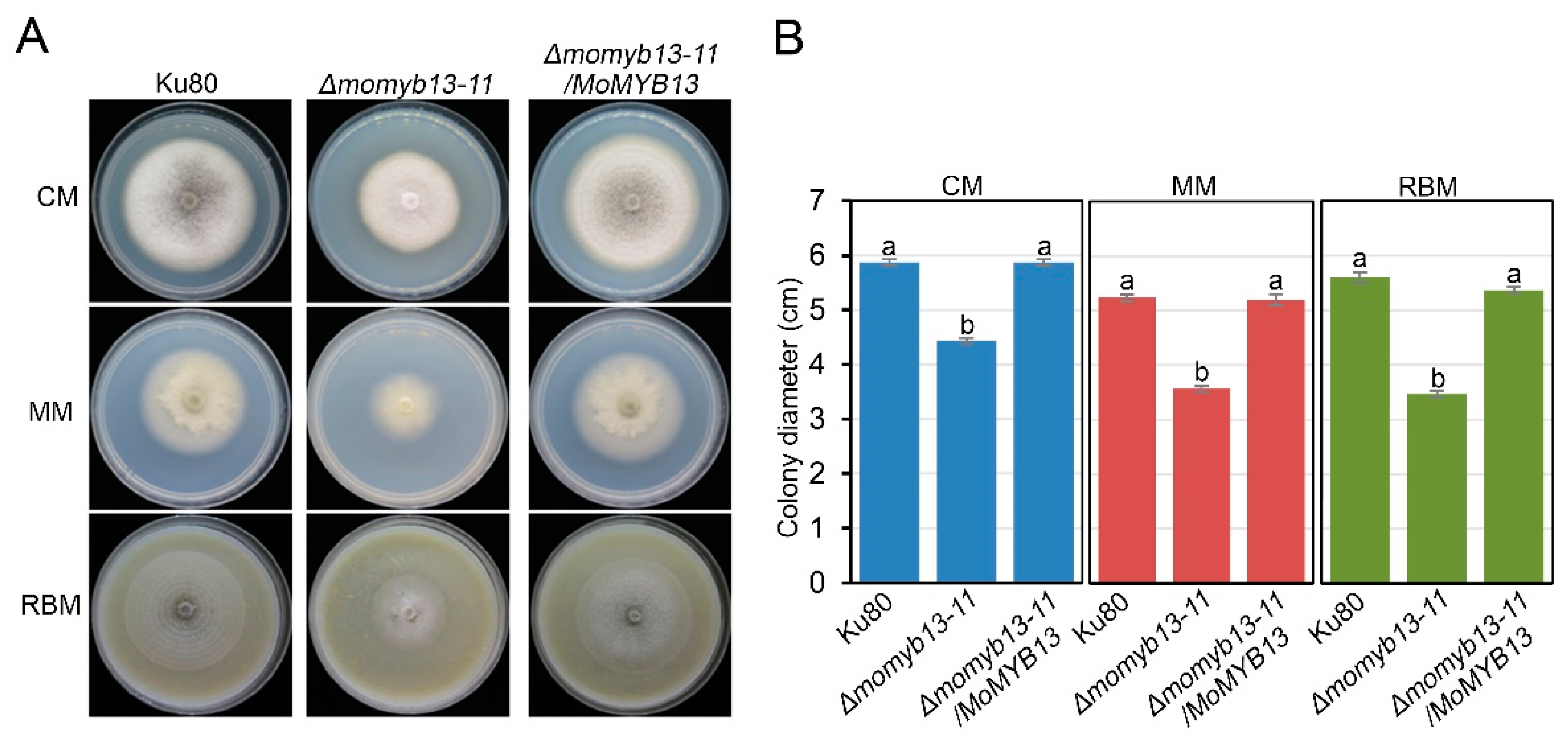
To study the function of MoMyb13, we conducted gene knockout experiments and obtained two mutants, Δmomyb13-11 and Δmomyb13-16, which were confirmed by Southern blot (
Figure S1). Since the two mutants showed the same phenotypes, we only exhibited the data of Δmomyb13-11 in the text. The complementary strain Δmomyb13-11/MoMYB13 was generated by reintroducing the MoMYB13 to Δmomyb13-11. Growth analysis of the mutant Δmomyb13-11 revealed a significant reduction in growth rate compared to the control strain ku80 and the complementary strain Δmomyb13-11/MoMYB13 on all three culture media, complete medium (CM), minimal medium (MM), and rice bran medium (RBM) (
Figure 3A and 3B). Additionally, the mutant Δmomyb13-11 exhibited a completely white colony color on CM (
Figure 3A). RT-qPCR analysis showed that two pigment synthesis genes, MoBUF1 and MoALB1, were significant downregulated in the mutant Δmomyb13-11 (
Figure S2), which may explain the white mutant colony. These results suggest that MoMyb13 is required for the vegetative growth and and normal colony color of M. oryzae.
3.4. MoMyb13 Is Essential to the Conidiation of M. oryzae
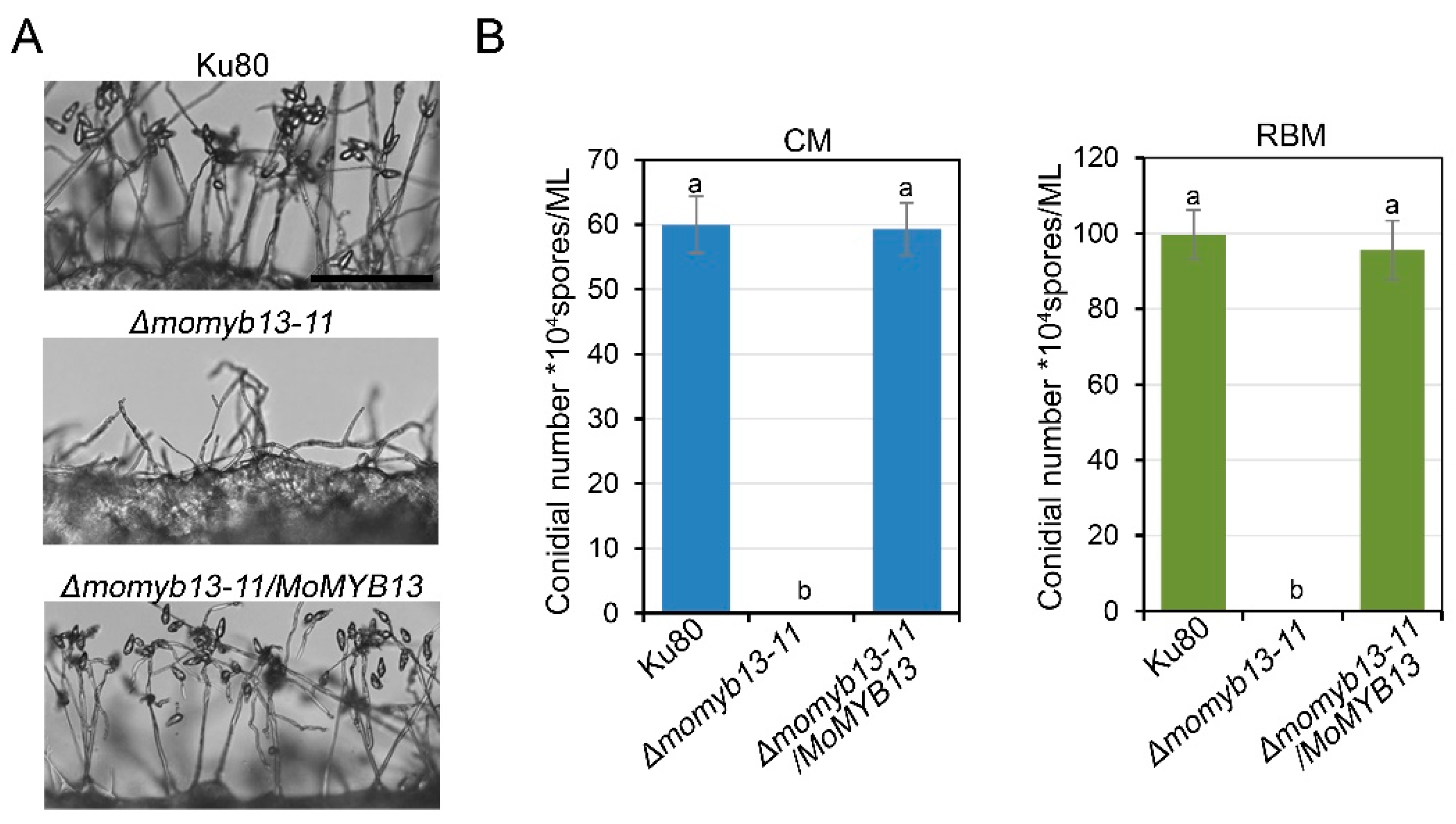
Since conidia are essential for the fungus to spread disease, the conidiation of mutants was investigated [
4]. We first observed the conidiophore formation, and found that the mutant only formed sparsely distributed conidiophores, whereas the control strain ku80 and complementary strain Δmomyb13-11/MoMYB13 exhibited dense conidiophores (
Figure 4A). Statistical analysis of the number of conidia showed that the mutant did not produce any conidia on CM or RBM, while the control strain ku80 and complementary strain Δmomyb13-11/MoMYB13 produced a large number of conidia (
Figure 4B). Determination of the expression of six conidiation-related genes revealed that three genes, MoCOS1, MoCON7 and MoCOM1, were significantly downregulated in the mutant (
Figure S3). These results suggest that MoMyb13 is essential for the conidiation of M. oryzae.
3.5. MoMyb13 Is Essential to the Pathogenicity of M. oryzae
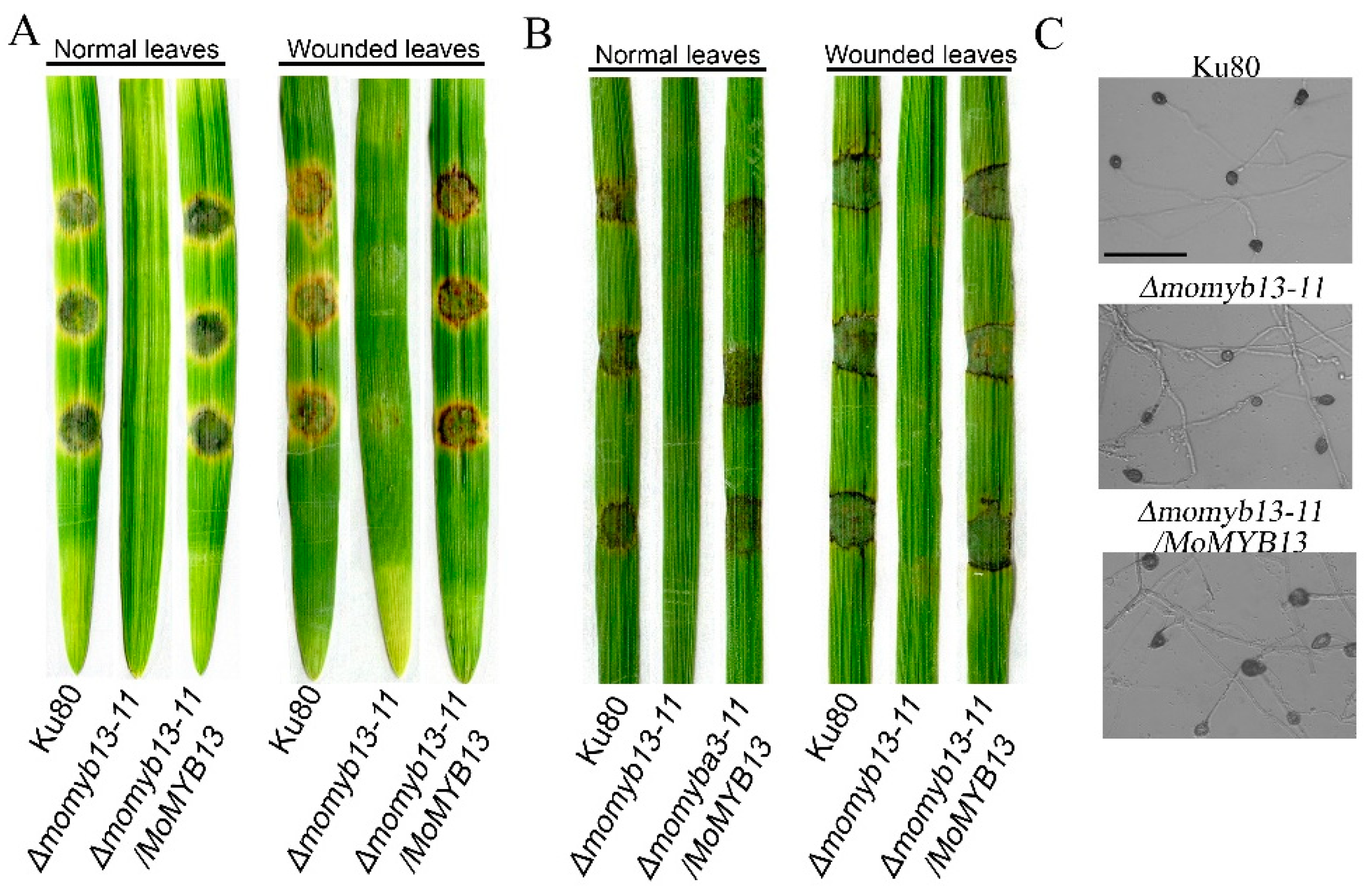
As a pathogenic fungus, its pathogenicity is the most important, so we analyzed the pathogenicity of the MoMyb13 mutant Δmomyb13-11. Since the mutant does not produce conidia, we used mycelial blocks for plant inoculation. This was done by placing them onto detached plant leaves and incubating for 7 days. We found that the mutant completely lost pathogenicity to both barley and rice, while the control strain and complemented strain showed normal pathogenicity (
Figure 5A and B). The mutant did not cause disease even on woundes leaves, (
Figure 5A and B). Considering that the hyphae of M. oryzae can develop into the infection structure, appressoria, we were particularly interested in whether the hyphae of mutant could produce appressoria. By incubating crushed hyphae on hydrophobic surface, we found that the mutant could produce appressoria normally (
Figure 5C). Since the mutant is unable to cause disease, these appressoria may however be non-functional. These results indicate that MoMyb13 is essential for pathogenicity but does not affect hyphal appressorium formation.
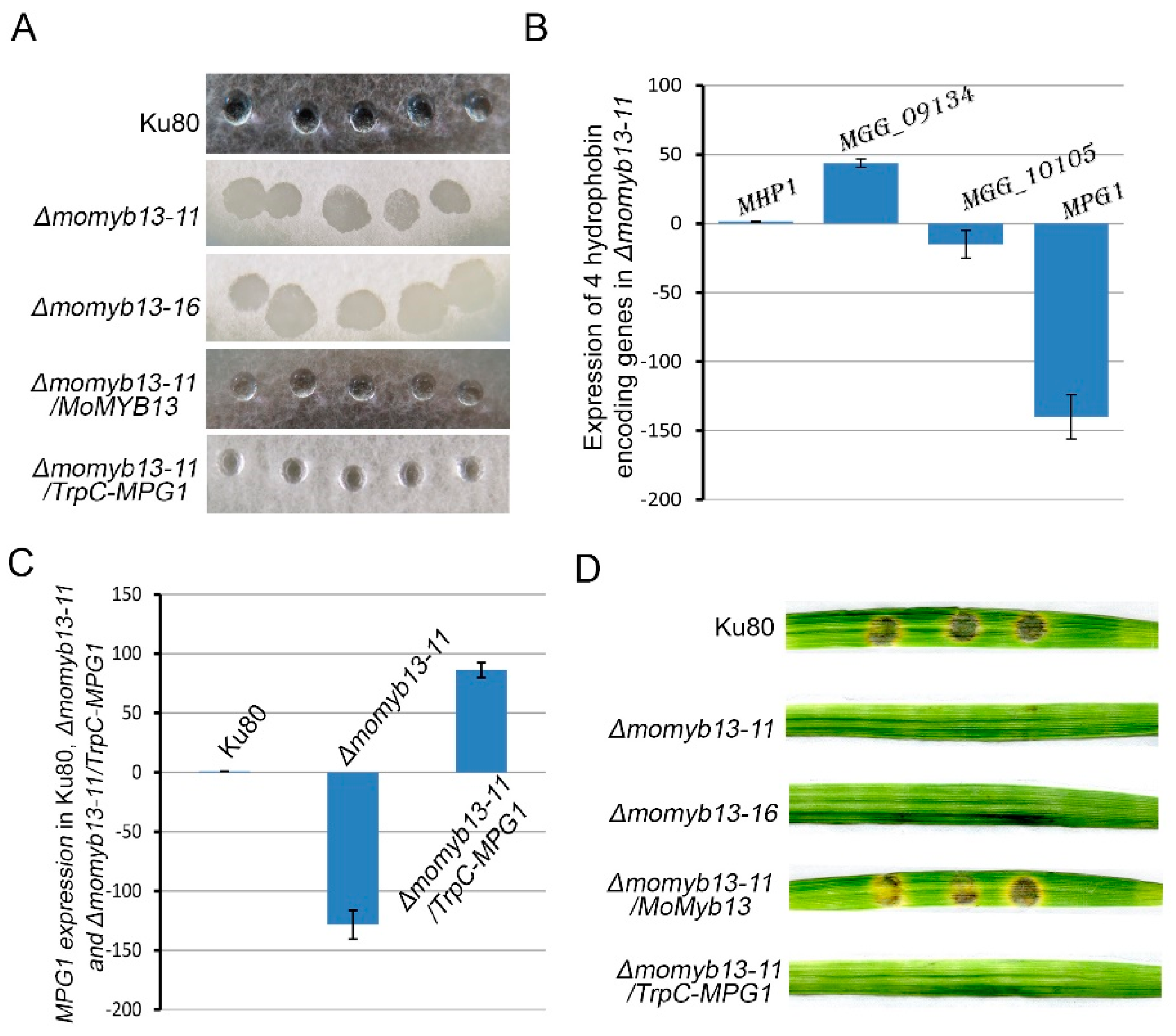
3.6. MoMyb13 Is Involved in Regulating Hydrophobins
The aerial mycelia of many ascomycetes are covered by small hydrophobic proteins known as hydrophobins making the colony hydrophobic [
49,
50]. We noticed that the MoMyb13 mutants had a more wettable colony (
Figure 6A), leading us to investigate if any hydrophobin genes were downregulated in mutant. Our results showed that one of the four tested hydrophobin related genes, MPG1 [
51], was strongly downregulated (
Figure 6B), prompting us to upregulate MPG1 in the MoMyb13 mutant (
Figure 6C). However, the upregulation of MPG1 only restored the hydrophobicity of the mutant, but not the pathogenicity (
Figure 6D). These results indicate that MoMyb13 regulates more genes necessary for rice pathogenicity than just hydrophobins.
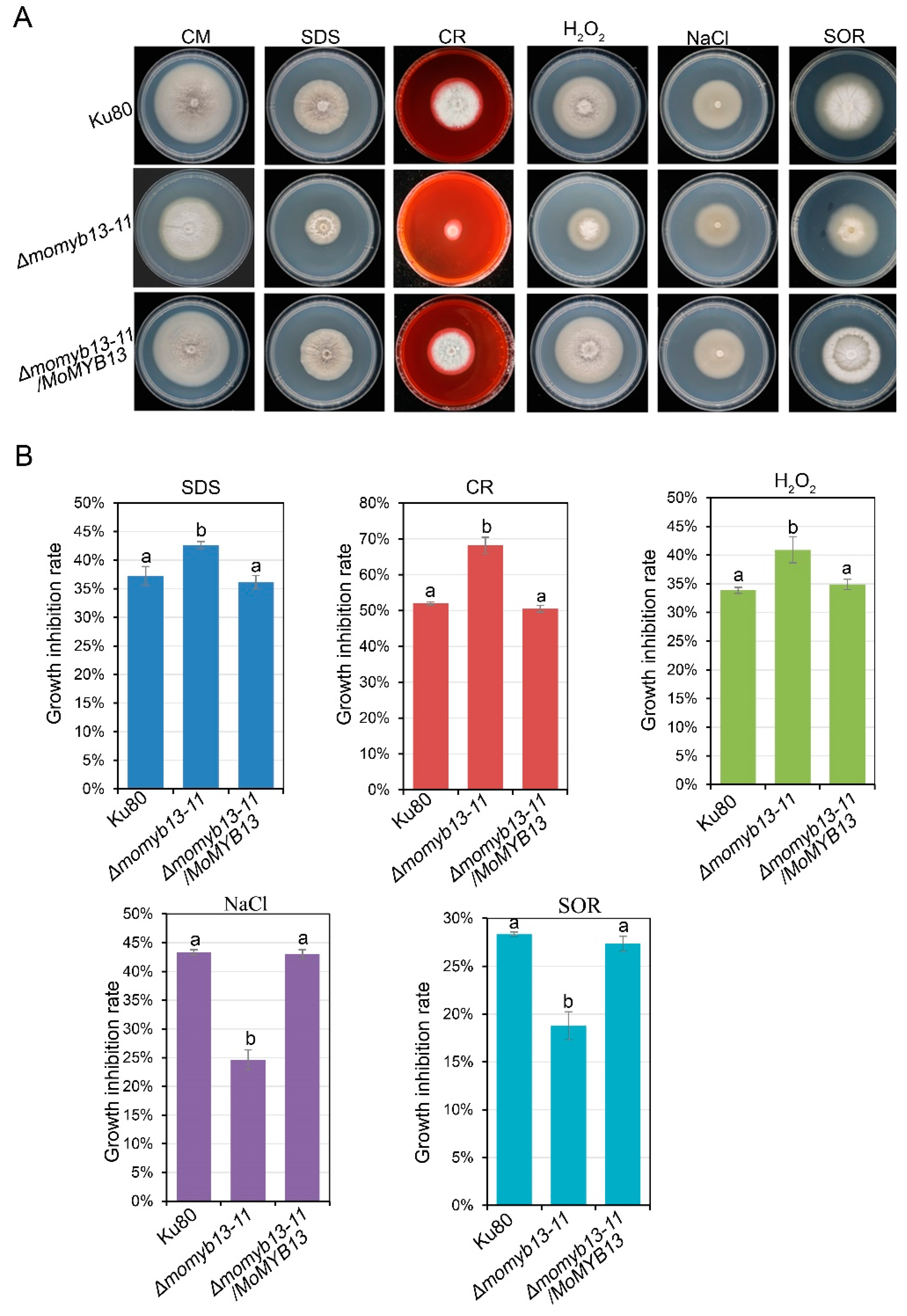
3.7. MoMyb13 Is Involved in Stress Response
A stress aasay was performed to assess the response of the MoMyb13 mutants to different stressors including sodium dodecyl sulfate (SDS), which affects membrane integrity; congo red (CR), which affects cell wall integrity; sodium chloride (NaCl), influencing ionic strength, water potential, and potential sodium toxicity; sorbitol (SOR), affecting osmotic strength; and hydrogen peroxide (H
2O
2), inducing oxidative stress. The mutants displayed an increased inhibition rate to SDS, CR and H
2O
2 (
Figure 7A and 7B), indicating a high sensitivity to cell membrane and wall damage, and oxidative stress. Conversely, the mutants exihibited a decreased inhibition rate to NaCl and SOR (
Figure 7A and 7B), suggesting a low sensitivity to salt and osmotic stress. Since melanin can act as an antioxidant [xx], the diminished melanin content in the mutant may contribut to its increased sensitivity to oxidative stress.
4. Discussion
MoMYB13 affected both growth and infection phenotypes. The MoMyb13 protein is large (
Figure 1A) and has no orthologs outside Ascomycota in the NCBI database. We investigated this further and found similarly large proteins with similar predicted aa sequences in 19 other published fungal genomes. Three of these hits were in different strains of
Pyricularia oryzae (teleomorph of
M. oryzae). Maybe more interesting, common to all hits were in that they where in fungal genomes of of fungi known to produce melanized hydrophobic structures [
52,
53,
54,
55,
56,
57,
58,
59]. That and our results (
Figure 6) suggest a possible involvement of these similar protein only found in Sordariomycetes in regulating hydrophobin and melanin production. Both MoMyb1 and MoMyb13 thus seems involved. Hovever, artificial regulation of the main hydrophobin negatively affected by mutation of
MoMyb13 did not restore conidiation indicating that the mutation ave affected more than just the formation of hydrophobins. Consequently, it is apparent that MoMyb13 does not on its own regulate all melanin production in the fungus since appressorium formation still takes place in the mutant. Similarly, MoMyb1 appear to mainly regulate MPG1 hydrophobin although no compensatory regulation was seen as for Myb13 as for MoMyb1 [
40].
The melanization of conidia and appressoria might be differently controlled. MoMyb1 is known to control HAD (Hyphae-driven appressoria formation) melanization and hydrophobin production [
40] pointing to that MoMyb1 and MoMyb13 might control melanization pigment formation differently in conidia and mycelia appressoria. Melanin production genes Alb1, Rsy1 and Buf1 have been extensively studied and these 3 genes are important for melanin production and appressoria formation in the strain 70-15 and Guy11 and deletion of any of them stopped infection even if some dark pigment was formed even in the Buf1 mutant [
60]. The main gene involved in producing pigmented colonies, Alb1, was downregulated in the MoMyb13 mutant bat that could as well be the consequence of MoMyb13 regulating MoMyb1 that in its turn regulates Alb1 (
Figure S1).
Interestingly, all the melanized fungi with orthologues to the MoMyb13 protein (
Figure 1B and
Table S1) are known to grow endophytically as
M. oryzae do in the first biotrophic stages of infection [
3] that overlaps with the stages when MoMyb13 is specifically up-regulated. If these genes in the other fungi have similar roles in the other fungi are unknown but could become a focus for future studies on the roles of melanins and hydrophobins in plant infection of these fungi with biotrophic initial stages.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on
Preprints.org.
Author Contributions
YL, domain prediction and evolutionary analysis, gene deletion, phenotype tests, protein localization, RT-qPCR, data collection and analysis, figure making, manuscript preparation, and writing. XZ, MoMyb13 gene deletion, MoMyb13 mutant phenotype analysis, MoMyb13 protein localization, RT-qPCR of hydrophobin gene. MC, MP, CL, LG, PH, SZ, Myb-gene deletions. SO work connected with comparing the found Myb proteins with orthologues in other fungi, manuscript preparation, and writing. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Natural Science Foundation of Fujian province (2022J01125), Fujian Key Laboratory for Monitoring and Integrated Management of Crop Pests (MIMCP-202301), Fujian Provincial Science and Technology Key Project (2022NZ030014) and the National Natural Science Foundation of China (NSFC31871914).
Data Availability Statement
Acknowledgments
The strain Ku80 used in this study was obtained initially from the lab of Nicholas J. Talbot, University of Exeter, UK.
Conflicts of Interest
The authors declare that the research was conducted without any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Pennisi, E. Armed and Dangerous. Science 2010, 327, 804–805. [Google Scholar] [PubMed]
- Howard, R.J.; Ferrari, M.A.; Roach, D.H.; Money, N.P. Penetration of hard Substrates by a Fungus employing Enormous Turgor Pressures. Proceedings of the National Academy of Sciences 1991, 88, 11281–11284. [Google Scholar] [CrossRef] [PubMed]
- Kankanala, P.; Czymmek, K.; Valent, B. Roles for Rice Membrane Dynamics and Plasmodesmata during Biotrophic Invasion by the Blast Fungus. The Plant Cell 2007, 19, 706–724. [Google Scholar] [CrossRef] [PubMed]
- Sakulkoo, W.; Osés-Ruiz, M.; Oliveira Garcia, E.; Soanes, D.M.; Littlejohn, G.R.; Hacker, C.; et al. A Single Fungal MAP Kinase controls Plant Cell-to-cell invasion by The Rice Blast Fungus. Science 2018, 359, 1399–1403. [Google Scholar] [CrossRef] [PubMed]
- Gladieux, P.; Condon, B.; Ravel, S.; Soanes, D.; Maciel, J.L.N.; Nhani, A.; et al. Gene Flow between Divergent Cereal- and Grass-Specific Lineages of the Rice Blast Fungus Magnaporthe oryzae. mBio 2018, 9, e01219-17. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.T.; Croll, D.; Gladieux, P.; Soanes, D.M.; Persoons, A.; Bhattacharjee, P.; et al. Emergence of Wheat Blast in Bangladesh was caused by a South American Lineage of Magnaporthe oryzae. BMC Biol 2016, 14, 84. [Google Scholar] [CrossRef] [PubMed]
- Malaker, P.K.; Barma, N.C.D.; Tiwari, T.P.; Collis, W.J.; Duveiller, E.; Singh, P.K.; et al. First Report of Wheat Blast Caused by Magnaporthe oryzae Pathotype triticum in Bangladesh. Plant Disease 2016, 100, 2330–2330. [Google Scholar] [CrossRef]
- Dean, R.A.; Talbot, N.J.; Ebbole, D.J.; Farman, M.L.; Mitchell, T.K.; Orbach, M.J.; et al. The Genome Sequence of the Rice Blast Fungus Magnaporthe grisea. Nature 2005, 434, 980–986. [Google Scholar] [CrossRef]
- Ebbole, D.J. Magnaporthe as a Model for Understanding Host-Pathogen Interactions. Annu Rev Phytopathol 2007, 45, 437–456. [Google Scholar] [CrossRef]
- Huang, P.Y.; Wang, J.; Li, Y.; Wang, Q.; Huang, Z.C.; Qian, H.; Liu, X.H.; Lin, F.C.; Lu, J.P. Transcription factors Vrf1 and Hox7 coordinately regulate appressorium maturation in the rice blast fungus Magnaporthe oryzae. Microbiol Res 2022, 263, 127141. [Google Scholar] [CrossRef]
- Verma, S.; Gazara, R.K.; Verma, P.K. Transcription Factor Repertoire of Necrotrophic Fungal Phytopathogen Ascochyta rabiei: Predominance of MYB Transcription Factors As Potential Regulators of Secretome. Front Plant Sci 2017, 8, 1037. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Zhang, L.; Liu, L.; Tang, X.F.; Yang, W.J.; Wu, Y.M.; et al. Biochemical and Molecular Characterization of Plant MYB Transcription Factor Family. Biochemistry Moscow 2009, 74, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Prouse, M.B. and Campbell, M.M. The Interaction Between MYB Proteins and their Target DNA binding Sites. Biochimica et Biophysica Acta (BBA) - Gene Regulatory Mechanisms 2012, 1819, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Roy, S. Function of MYB Domain Transcription Factors in Abiotic Stress and Epigenetic Control of Stress Response in Plant Genome. Plant Signaling & Behavior 2016, 11, e1117723. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Gao, W.; Wu, X.; Zhao, M.; Qu, J.; Huang, C.; Zhang, J. Genome-Wide Characterization and Expression Analyses of Pleurotus ostreatus MYB Transcription Factors during Developmental Stages and under Heat Stress Based on de novo Sequenced Genome. International journal of molecular sciences 2018, 19, 2052. [Google Scholar] [CrossRef] [PubMed]
- Ambawat, S.; Sharma, P.; Yadav, N.R.; Yadav, R.C. MYB transcription factor genes as regulators for plant responses: an overview. Physiology and molecular biology of plants 2013, 19, 307–321. [Google Scholar] [CrossRef] [PubMed]
- Eme, L.; Spang, A.; Lombard, J.; Stairs, C.W.; Ettema, T.J.G. Archaea and the Origin of Eukaryotes. Nature reviews. Microbiology 2017, 15, 711–723. [Google Scholar] [CrossRef] [PubMed]
- Allan, A.C. and Espley, R.V. MYBs Drive Novel Consumer Traits in Fruits and Vegetables. Trends in Plant Science 2018, 23, 693–705. [Google Scholar] [CrossRef] [PubMed]
- Baldoni, E.; Genga, A.; Cominelli, E. Plant MYB Transcription Factors: Their Role in Drought Response Mechanisms. International journal of molecular sciences 2015, 16, 15811–15851. [Google Scholar] [CrossRef]
- Cao, Y.; Li, K.; Li, Y.; Zhao, X.; Wang, L. MYB Transcription Factors as Regulators of Secondary Metabolism in Plants. Biology 2020, 9, 61. [Google Scholar] [CrossRef]
- Dubos, C.; Stracke, R.; Grotewold, E.; Weisshaar, B.; Martin, C.; Lepiniec, L. MYB Transcription Factors in Arabidopsis. Trends in Plant Science 2010, 15, 573–581. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Han, G.; Sun, C.; Sui, N. Research Advances of MYB Transcription Factors in Plant Stress Resistance and Breeding. Plant Signaling & Behavior 2019, 14, 1613131. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Osbourn, A.; Ma, P. MYB Transcription Factors as Regulators of Phenylpropanoid Metabolism in Plants. Molecular Plant 2015, 8, 689–708. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.; Constabel, C.P. Repressors as Regulators of Phenylpropanoid Metabolism in Plants. Trends in Plant Science 2019, 24, 275–289. [Google Scholar] [CrossRef] [PubMed]
- Yu, E.Y.; Yen, W.F.; Steinberg-Neifach, O.; Lue, N.F. Rap1 in Candida albicans: an Unusual Structural Organization and a Critical Function in Suppressing Telomere Recombination. Mol Cell Biol 2010, 30, 1254–1268. [Google Scholar] [CrossRef]
- Hogues, H.; Lavoie, H.; Sellam, A.; Mangos, M.; Roemer, T.; Purisima, E.; et al. Transcription Factor Substitution during the Evolution of Fungal Ribosome Regulation. Molecular Cell 2008, 29, 552–562. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Sánchez, L.; Rodríguez-López, M.; García, Z.; Tenorio-Gómez, M.; Schvartzman, J.B.; Krimer, D.B.; Hernández, P. The fission yeast rDNA-binding protein Reb1 regulates G1 phase under nutritional stress. Journal of Cell Science 2011, 124, 25–34. [Google Scholar] [CrossRef]
- Mukherjee, K.; Pandey, D.M.; Vidyarthi, A.S. Molecular Dynamics Simulation of Rap1 Myb-type domain in Saccharomyces cerevisiae. Bioinformation 2012, 8, 881–885. [Google Scholar] [CrossRef]
- Ramos-Sáenz, A.; González-Álvarez, D.; Rodríguez-Galán, O.; Rodríguez-Gil, A.; Gaspar, S.G.; Villalobo, E.; et al. Pol5 is an essential ribosome biogenesis factor required for 60S ribosomal subunit maturation in Saccharomyces cerevisiae. RNA 2019, 25, 1561–1575. [Google Scholar] [CrossRef]
- Matheis, S.; Yemelin, A.; Scheps, D.; Andresen, K.; Jacob, S.; Thines, E.; Foster, A.J. Functions of the Magnaporthe oryzae Flb3p and Flb4p transcription factors in the regulation of conidiation. Microbiological Research 2017, 196, 106–117. [Google Scholar] [CrossRef]
- Valsecchi, I.; Sarikaya-Bayram, Ö.; Wong Sak Hoi, J.; Muszkieta, L.; Gibbons, J.; Prevost, M.C.; et al. MybA, a transcription factor involved in conidiation and conidial viability of the human pathogen Aspergillus fumigatus: A transcription factor regulating conidiation in Aspergillus. Molecular Microbiology 2017, 105, 880–900. [Google Scholar] [CrossRef]
- Wieser, J.; Adams, T.H. flbD encodes a Myb-like DNA-binding protein that coordinates initiation of Aspergillus mdulans conidiophore development. Genes & Development 1995, 9, 491–502. [Google Scholar] [CrossRef]
- Mateos, L.; Jiménez, A.; Revuelta, J.L.; Santos, M.A. Purine Biosynthesis, Riboflavin Production, and Trophic-Phase Span Are Controlled by a Myb-Related Transcription Factor in the Fungus Ashbya gossypii. Appl Environ Microbiol 2006, 72, 5052–5060. [Google Scholar] [CrossRef]
- Mitra, P. Transcription Regulation of MYB: a Potential and Novel Therapeutic Target in Cancer. Ann Transl Med 2018, 6, 443–443. [Google Scholar] [CrossRef]
- Pattabiraman, D.R.; Gonda, T.J. Role and Potential for Therapeutic targeting of MYB in Leukemia. Leukemia 2013, 27, 269–277. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, H.; Son, H.; Choi, G.J.; Kim, J.C.; Lee, Y.W. MYT3, A Myb-Like Transcription Factor, Affects Fungal Development and Pathogenicity of Fusarium graminearum. PloS one 2014, 9, e94359. [Google Scholar] [CrossRef]
- Lin, Y.; Son, H.; Lee, J.; Min, K.; Choi, G.J.; Kim, J.C.; Lee, Y.W. A Putative Transcription Factor MYT1 Is Required for Female Fertility in the Ascomycete Gibberella zeae. PloS one 2011, 6, e25586. [Google Scholar] [CrossRef]
- Lin, Y.; Son, H.; Min, K.; Lee, J.; Choi, G.J.; Kim, J.C.; Lee, Y.W. A Putative Transcription Factor MYT2 Regulates Perithecium Size in the Ascomycete Gibberella zeae. PloS one 2012, 7, e37859. [Google Scholar] [CrossRef]
- Dong, Y.; Zhao, Q.; Liu, X.; Zhang, X.; Qi, Z.; Zhang, H.; et al. MoMyb1 is Required for Asexual Development and Tissue-specific Infection in the Rice Blast Fungus Magnaporthe oryzae. BMC Microbiol 2015, 15, 37. [Google Scholar] [CrossRef]
- Lee, S.; Völz, R.; Song, H.; Harris, W.; Lee, Y.H. Characterization of the MYB Genes Reveals Insights Into Their Evolutionary Conservation, Structural Diversity, and Functional Roles in Magnaporthe oryzae. Front Microbiol 2021, 12, 721530. [Google Scholar] [CrossRef] [PubMed]
- Villalba, F.; Collemare, J.; Landraud, P.; Lambou, K.; Brozek, V.; Cirer, B.; et al. Improved gene targeting in Magnaporthe grisea by inactivation of MgKU80 required for non-homologous end joining. Fungal Genetics and Biology 2008, 45, 68–75. [Google Scholar] [CrossRef]
- Li, Y.; Que, Y.; Liu, Y.; Yue, X.; Meng, X.; Zhang, Z.; Wang, Z. The Putative Gγ Subunit Gene MGG1 is required for Conidiation, Appressorium Formation, mating and Pathogenicity in Magnaporthe oryzae. Curr Genet 2015, 61, 641–651. [Google Scholar] [CrossRef]
- Li, Y.; Yue, X.; Que, Y.; Yan, X.; Ma, Z.; Talbot, N.J.; Wang, Z. Characterisation of Four LIM Protein-Encoding Genes Involved in Infection-Related Development and Pathogenicity by the Rice Blast Fungus Magnaporthe oryzae. PLoS ONE 2014, 9, e88246. [Google Scholar] [CrossRef]
- Li, Y.; Yan, X.; Wang, H.; Liang, S.; Ma, W.B.; Fang, M.Y.; et al. MoRic8 Is a Novel Component of G-Protein Signaling During Plant Infection by the Rice Blast Fungus Magnaporthe oryzae. Molecular plant-microbe interactions 2010, 23, 317–331. [Google Scholar] [CrossRef]
- Li, Y.; Zheng, X.; Zhu, M.; Chen, M.; Zhang, S.; He, F.; Chen, X.; Lv, J.; Pei, M.; Zhang, Y.; Zhang, Y.; Wang, W.; Zhang, J.; Wang, M.; Wang, Z.; Li, G.; Lu, G. MoIVD-Mediated Leucine Catabolism Is Required for Vegetative Growth, Conidiation and Full Virulence of the Rice Blast Fungus Magnaporthe oryzae. Frontiers in microbiology 2019, 10, 444. [Google Scholar] [CrossRef]
- Cao, Y.; Chen, J.; Xie, X.; Liu, S.; Jiang, Y.; Pei, M.; Wu, Q.; Qi, P.; Du, L.; Peng, B.; Lan, J.; Wu, F.; Feng, K.; Zhang, Y.; Fang, Y.; Liu, M.; Jaber, M.Y.; Wang, Z.; Olsson, S.; Lu, G.; Li, Y. Characterization of two infection-induced transcription factors of Magnaporthe oryzae reveals their roles in regulating early infection and effector expression. Molecular plant pathology 2022, 23, 1200–1213. [Google Scholar] [CrossRef]
- Li, Y.; Liang, S.; Yan, X.; Wang, H.; Li, D.; Soanes, D.M.; et al. Characterization of MoLDB1 Required for Vegetative Growth, Infection-Related Morphogenesis, and Pathogenicity in the Rice Blast Fungus Magnaporthe oryzae. Molecular plant-microbe interactions 2010, 23, 1260–1274. [Google Scholar] [CrossRef]
- Livak, K.J. and Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Berger, B.W. and Sallada, N.D. Hydrophobins: Multifunctional Biosurfactants for Interface Engineering. Journal of biological engineering 2019, 13, 10. [Google Scholar] [CrossRef]
- Bayry, J.; Aimanianda, V.; Guijarro, J.I.; Sunde, M.; Latgé, J.P. Hydrophobins—Unique Fungal Proteins. PLoS Pathog 2012, 8, e1002700. [Google Scholar] [CrossRef]
- Beckerman, J.L. MPG1, a Gene Encoding a Fungal Hydrophobin of Magnaporthe grisea, Is Involved in Surface Recognition. Molecular plant-microbe interactions 1996, 9, 450. [Google Scholar] [CrossRef] [PubMed]
- Kou, Y.; Qiu, J.; Tao, Z. Every Coin Has Two Sides: Reactive Oxygen Species during Rice—Magnaporthe oryzae Interaction. International journal of molecular sciences 2019, 20, 1191. [Google Scholar] [CrossRef] [PubMed]
- Collado, J.; Gonzalez, A.; Platas, G.; Stchigel, A.M.; Guarro, J.; Pelaez, F. Monosporascus ibericus sp. nov., an Endophytic Ascomycete from Plants on Saline Soils, with Observations on the Position of the Genus based on Sequence Analysis of the 18S rDNA. Mycological Research 2002, 106, 118–127. [Google Scholar] [CrossRef]
- Liu, K.; Ding, X.; Deng, B.; Chen, W. Isolation and Characterization of Endophytic Taxol-producing Fungi from Taxus chinensis. Journal of industrial microbiology & biotechnology 2009, 36, 1171–1177. [Google Scholar] [CrossRef]
- Al-Khawaldeh, M.M.; Araj, S.-E.; Alananbeh, K.M.; Antary, T.M.A. Wheat Cultivable Fungal Endophytes in Jordan. Fresenius Environmental Bulletin 2020, 29, 13. [Google Scholar]
- Geisen, S.; Hooven, F.C.; Kostenko, O.; Snoek, L.B.; Putten, W.H. Fungal Root Endophytes Influence Plants in a Species-specific Manner that depends on Plant’s Growth Stage. J Ecol 2021, 109, 1618–1632. [Google Scholar] [CrossRef]
- Sarsaiya, S.; Jain, A.; Fan, X.; Jia, Q.; Xu, Q.; Shu, F.; et al. New Insights into Detection of a Dendrobine Compound From a Novel Endophytic Trichoderma longibrachiatum Strain and Its Toxicity Against Phytopathogenic Bacteria. Frontiers in microbiology 2020, 11, 337. [Google Scholar] [CrossRef]
- Gao, H.; Pan, M.; Tian, C.; Fan, X. Cytospora and Diaporthe Species Associated with Hazelnut Canker and Dieback in Beijing, China. Frontiers in cellular and infection microbiology 2021, 11, 664366. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Mao, L.; Zhao, N.; Xia, C.; Liu, J.; Kubicek, C.P.; et al. Verification of TRI3 Acetylation of Trichodermol to Trichodermin in the Plant Endophyte Trichoderma taxi. Frontiers in microbiology 2021, 12, 731425. [Google Scholar] [CrossRef]
- Zhu, S.; Yan, Y.; Qu, Y.; Wang, J.; Feng, X.; Liu, X.; Lin, F.; Lu, J. Role refinement of melanin synthesis genes by gene knockout reveals their functional diversity in Pyricularia oryzae strains. Microbiological Research 2021, 242, 126620. [Google Scholar] [CrossRef]
Figure 1.
Domain identification and evolution analysis of MoMyb13. A. The domain structures of Momyb13 were predicted by NCBI database. The figure was drawn by the soft DOG 2.0. B. Evolutionary relationships of MoMyb13 homologues were analyzed by MEGA7 using the Neighbor-Joining method. The optimal tree with the sum of branch length = 4.48740141 is shown. The tree is drawn to scale, with branch lengths in the same units as those of the evolutionary distances used to infer the phylogenetic tree. The evolutionary distances were computed using the Poisson correction method and are in the units of the number of amino acid substitutions per site.
Figure 1.
Domain identification and evolution analysis of MoMyb13. A. The domain structures of Momyb13 were predicted by NCBI database. The figure was drawn by the soft DOG 2.0. B. Evolutionary relationships of MoMyb13 homologues were analyzed by MEGA7 using the Neighbor-Joining method. The optimal tree with the sum of branch length = 4.48740141 is shown. The tree is drawn to scale, with branch lengths in the same units as those of the evolutionary distances used to infer the phylogenetic tree. The evolutionary distances were computed using the Poisson correction method and are in the units of the number of amino acid substitutions per site.
Figure 2.
MoMyb13-GFP colocalizes with Histon-RFP. The gree signal of MoMyb13-GFP and red signal of Histon-RFP overlapped in the the nuclei of hyphae, conidia, and appressoria of M. oryzae. The signals were tested by confocal microscopy (Nikon A1). The appressoria from the conidia were obtained by inducating the conidia on hydrophobic surfaces for 8 hours at 25 ℃. Bar, 5 μm.
Figure 2.
MoMyb13-GFP colocalizes with Histon-RFP. The gree signal of MoMyb13-GFP and red signal of Histon-RFP overlapped in the the nuclei of hyphae, conidia, and appressoria of M. oryzae. The signals were tested by confocal microscopy (Nikon A1). The appressoria from the conidia were obtained by inducating the conidia on hydrophobic surfaces for 8 hours at 25 ℃. Bar, 5 μm.
Figure 3.
The MoMyb13 mutant Δmomyb13-11 was defective in growth. A. Δmomyb13-11 showed rdeuced colony size on CM, MM and RBM. The colony color of muant on CM was completely white, in comparision with Ku80 and Δmomyb13-11/MoMYB13. B. Bar chart analysis of the Δmomyb13-11 growth rate. The data indicates the colony diameter of each strain growing for 7 days, showing mean + standard errors (SE, n=3). The t-test was performed (P<0.05). The experiments were repeated 3 times.
Figure 3.
The MoMyb13 mutant Δmomyb13-11 was defective in growth. A. Δmomyb13-11 showed rdeuced colony size on CM, MM and RBM. The colony color of muant on CM was completely white, in comparision with Ku80 and Δmomyb13-11/MoMYB13. B. Bar chart analysis of the Δmomyb13-11 growth rate. The data indicates the colony diameter of each strain growing for 7 days, showing mean + standard errors (SE, n=3). The t-test was performed (P<0.05). The experiments were repeated 3 times.
Figure 4.
The MoMyb13 mutant Δmomyb13-11 produced no conidia. A. Δmomyb13-11 formed sparsely distributed conidiophores. Bar, 50 μm. B. Bar chart analysis of conidial production. The data indicates the conidial number of each strain growing for 12 days in 9 cm plate, showing the mean + standard errors (SE, n=3). The t-test was performed (P<0.05). The experiments were repeated 3 times.
Figure 4.
The MoMyb13 mutant Δmomyb13-11 produced no conidia. A. Δmomyb13-11 formed sparsely distributed conidiophores. Bar, 50 μm. B. Bar chart analysis of conidial production. The data indicates the conidial number of each strain growing for 12 days in 9 cm plate, showing the mean + standard errors (SE, n=3). The t-test was performed (P<0.05). The experiments were repeated 3 times.
Figure 5.
The MoMyb13 mutant Δmomyb13-11 lost pathogenicity, but produced appressoria. (A) Δmomyb13-11 is unable to cause lesions on both normal and wounded leaves of barley. (B) Δmomyb13-11 is unable to cause lesions on both normal and wounded leaves of rice. (C) The hyphae of Δmomyb13-11 were able to produce appressoria as normally as the control strain ku80 and complementary strain Δmomyb13-11/MoMYB13. Bar, 20 μm.
Figure 5.
The MoMyb13 mutant Δmomyb13-11 lost pathogenicity, but produced appressoria. (A) Δmomyb13-11 is unable to cause lesions on both normal and wounded leaves of barley. (B) Δmomyb13-11 is unable to cause lesions on both normal and wounded leaves of rice. (C) The hyphae of Δmomyb13-11 were able to produce appressoria as normally as the control strain ku80 and complementary strain Δmomyb13-11/MoMYB13. Bar, 20 μm.
Figure 6.
The MoMyb13 mutant Δmomyb13-11 has a wettable phenotype. (A) Colonies of Δmomyb13 are easily wettable compared to the surface of the control strain (Ku80), the complementary strain, or the Δmomyb13 strain containing the overexpressed hydrophobin MPG1 (Δmomyb13-11/TrpC-MPG1) is not wettable. (B) Percent change in expression of 4 hydrophobin genes in Δmomyb13 compared to Ku80 shows that MPG1 is severely downregulated while the other hydrophobins are less affected. (C) Percent change in MPG1 expression in Ku80, Δmomyb13 and Δmomyb13/TrpC-MPG1 strains compared to Ku80. (D) Leaf lesions formed by Ku80, Δmomyb13, and Δmomyb13/TrpC-MPG1 strains using the agar block inoculation. Error bars (B) and (C) show 95% confidence intervals. Bars with confidence intervals not overlapping with other bars’ confidence intervals or levels (as zero) are significantly different (P<0.05 for the null hypothesis that they are the same).
Figure 6.
The MoMyb13 mutant Δmomyb13-11 has a wettable phenotype. (A) Colonies of Δmomyb13 are easily wettable compared to the surface of the control strain (Ku80), the complementary strain, or the Δmomyb13 strain containing the overexpressed hydrophobin MPG1 (Δmomyb13-11/TrpC-MPG1) is not wettable. (B) Percent change in expression of 4 hydrophobin genes in Δmomyb13 compared to Ku80 shows that MPG1 is severely downregulated while the other hydrophobins are less affected. (C) Percent change in MPG1 expression in Ku80, Δmomyb13 and Δmomyb13/TrpC-MPG1 strains compared to Ku80. (D) Leaf lesions formed by Ku80, Δmomyb13, and Δmomyb13/TrpC-MPG1 strains using the agar block inoculation. Error bars (B) and (C) show 95% confidence intervals. Bars with confidence intervals not overlapping with other bars’ confidence intervals or levels (as zero) are significantly different (P<0.05 for the null hypothesis that they are the same).
Figure 7.
The MoMyb13 mutant Δmomyb13-11 showed responses to different stress. (A) Colony size and morphology of the mutant Δmomyb13-11, control strain ku80 and complementary strain Δmomyb13-11/MoMYB13 after 10 days growing on CM or CM with additions of SDS, CR, NaCl SOR, or H2O2 as stresses (see methods). (B) Bar chart analysis of the sensitivity of Δmomyb13-11 to different stressors. The data indicates the growth inhibition rate of each strain growing on CM with stressors, showing the mean + standard errors (SE, n=3). The t-test was performed (P<0.05). The experiments were repeated at least 3 times.
Figure 7.
The MoMyb13 mutant Δmomyb13-11 showed responses to different stress. (A) Colony size and morphology of the mutant Δmomyb13-11, control strain ku80 and complementary strain Δmomyb13-11/MoMYB13 after 10 days growing on CM or CM with additions of SDS, CR, NaCl SOR, or H2O2 as stresses (see methods). (B) Bar chart analysis of the sensitivity of Δmomyb13-11 to different stressors. The data indicates the growth inhibition rate of each strain growing on CM with stressors, showing the mean + standard errors (SE, n=3). The t-test was performed (P<0.05). The experiments were repeated at least 3 times.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).