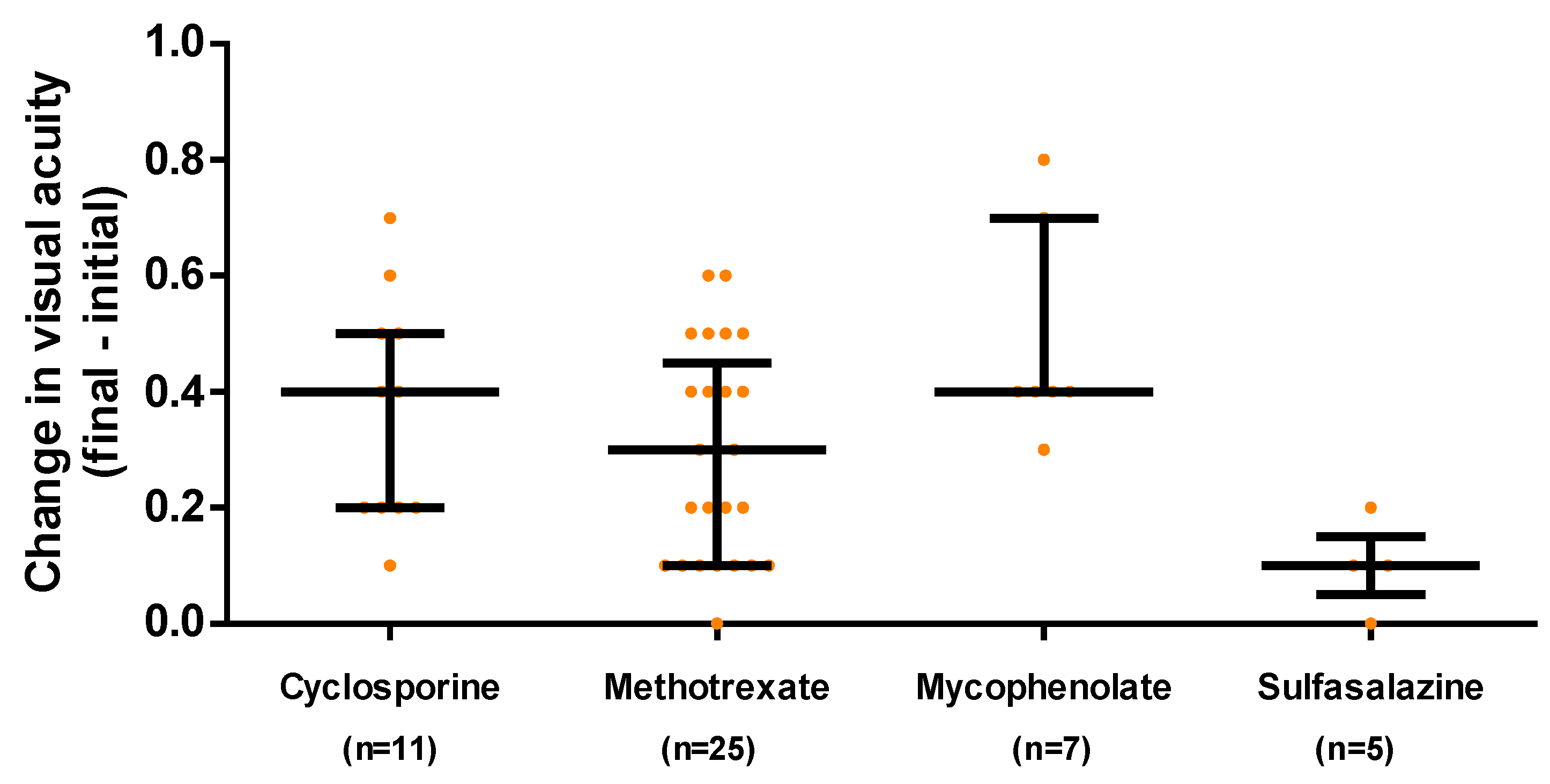1. Introduction
Uveitis is an ocular disease with a relatively wide list of causative agents (infectious, secondary to systemic disease, masquerade syndrome uveitis, purely ophthalmologic or idiopathic). It is a major cause of ocular morbidity and a frequent cause of blindness in the developed world (10-25%, depending on the series [
1]. Vision loss in patients with this disease seems to be most directly related to the duration, severity, location of inflammation and complications. The most frequent of which is cystoid macular edema (CME) [
1,
2]. This risk seems to decrease with early treatment, thus improving the quality of life as well as reducing the socioeconomic impact of the disease [
3].
The goals of therapy for noninfectious uveitis (NIU) are to reduce inflammation and achieve complete remission, thereby mitigating or avoiding ocular complications, permanent cumulative damage, and long-term vision loss. Rosenbaum et al reviewed the indicated treatments for uveitis [
4]. The therapeutic scale of treatment of NIU begins with corticosteroids (CS), which can be topical in anterior uveitis (AU) or systemic (oral or intravenous in more severe uveitis), immunosuppressive drugs (IS) (T-cell inhibitors or antimetabolites) and biologic therapy (BT) [
5]. Intravitreal CS is used to avoid adverse effects associated with systemic therapies while maintaining their anti-inflammatory effect limited to the eye for up to 3 years. Although their use has been associated with the appearance of increased intraocular pressure and the appearance or progression of cataracts [
6,
7].
Although systemic CS at high doses decreases or eliminates ocular inflammation immediately, IS is needed to control the inflammation to avoid the side effects and potential complications of steroids and to keep the patient stable [
8]. Although complete remission is not always possible, the main use of these drugs is to reduce exposure to CS and they are recommended as CS-sparing when inflammation is not under control within 3 months [
8,
9].
The IS used for this purpose include antimetabolites such as azathioprine, methotrexate (MTX) and mycophenolate mofetil (MFM), T-cell inhibitors such as cyclosporine (CyA), and tacrolimus and alkylating agents (cyclophosphamide, chlorambucil) [
4,
10].
Biologic therapy as tumor necrosis factor blocking (anti-TNF) drugs is an alternative to the use of IS. Adalimumab (ADA) is approved for the treatment of non-infectious non anterior uveitis [
11,
12]. Infliximab (INF) and ADA have shown similar efficacy in controlling inflammation in chronic non-infectious uveitis, recommending the use of INF for patients with Behcet's disease and in severe acute uveitis [
13,
14]. Tocilizumab (TOZ), another interleukin-6 inhibitor biologic therapy, showed improvement in visual acuity (VA) and a reduction of vitreous inflammation in patients with uveitis. It was well tolerated with no adverse effects and has demonstrated efficacy in severe and refractory uveitis as well as in the resolution of CME secondary to uveitis [
15,
16].
Although the efficacy of all these therapies in the treatment of uveitis has been demonstrated, there are no clear guidelines or protocols due to the variability in their presentation and the approaching difficulty of the NIU.
The objectives of this study are to describe the characteristics of patients requiring IS and/or BT treatment in a multidisciplinary uveitis practice and the response to these treatments in different diseases.
2. Materials and Methods
Observational, descriptive, longitudinal, and retrospective study of patients diagnosed with idiopathic uveitis or uveitis associated with autoimmune disease seen in the multidisciplinary uveitis consultation of a hospital. The hospital has a catchment area of 333,564 inhabitants. And during a period that spans from January 2011 to February 2022.
The research protocol for this study was approved by the research committee of the Hospital Universitario Infanta Sofía on the 15Th of March 2022.
For each patient included in the study, we recorded sex and age. Data about the characteristics of uveitis, such as anatomical location (anterior, intermediate, posterior, or panuveitis), number of episodes, laterality (unilateral or bilateral), and etiology [
17,
18].
Systemic treatment with CS, IS, BT, and markers of inflammatory activity (presence or absence of vitritis as well as CME findings detected on optical coherence tomography (OCT) and VA) were recorded [
18,
19,
20] before and after treatment to assess response.
Absolute and relative frequencies were used to describe qualitative variables. Means and standard deviations (or median and interquartile range, [Q1, Q3]) were calculated for quantitative variables, according to their behavior (assessed by Shapiro-Wilk tests). Demographic and clinical characteristics of patients who required treatment with IS and/or BT and those who did not were compared using Chi-square or independent samples Student's t-tests (or Mann Whitney U) as appropriate.
The responses to the different treatments with IS and/or BT and their respective confidence intervals were calculated and compared by square tests. Similarly, the proportion of patients with resolved vitritis and CME, and the change in VA after the different treatments were compared between treatments. A significance level of 5% was established and all analyses were performed with STATA BE v.17 (StataCorp) statistical tools.
3. Results
3.1. Characteristics of patients with non-infectious uveitis (NIU)
Table 1 summarizes the sociodemographic, clinical and treatment characteristics of the 365 patients with NIU seen at the multidisciplinary uveitis consultation at the Hospital Infanta Sofia between January 2011 and February 2022. Of these, 180 were women (50.6%) and the mean age at diagnosis was 42.8 ± 15.8 years. Of the total number of patients, 157 (44%) presented a single episode and in 271 (76%) the involvement was unilateral. In terms of anatomical location, 265 patients (74.4%) presented with an AU and in terms of etiology, the most frequent were idiopathic (43.3%) and those associated with HLA B27+ axial spondylarthritis (SA). Of the total number of patients, 313 (88%) were treated with local CS and 43 (12%) required systemic CS, 26 oral and 17 intravenous. In 66 patients (18.5%) treatment with IS and/or BT was required to achieve remission of uveitis. Of these, 34 were female (51.5%); the mean age at diagnosis was 39 ± 13 years and at the time of treatment initiation 42 ± 13 years. The time from uveitis diagnosis to initiation of treatment with IS and/or BT was 2 years [RIC: 2-4]. 23 patients-initiated treatment with IS and/or BT in the first episode (34.9%), 5 in the second and 38 (57.6%) in the third or more; 35 patients had unilateral involvement (53.0%).
3.2. Need for IS and/or BT treatment.
23 patients started treatment with IS and/or BT in the first disease episode (35%), 5 in the second and 38 (58%) from the third or more; 35 patients had unilateral involvement (47.0%) and 31 bilateral (53.0%).
Table 1 compares the characteristics of patients with NIU who were treated with IS and/or BT and those who did not receive IS or BT. The number of episodes, laterality, anatomic location, etiology, and use of systemic corticosteroids in all patients are described.
Significant differences were found between patients who required treatment with IS and/or BT and those who did not in age, laterality, anatomic location and need for systemic CS use. Patients with IS and/or BT were younger (38 [30-47] vs 42 [32-55] years; P=0.027), had more frequently bilateral uveitis (53% vs 19%; P<0.001), the location was in lower percentage anterior (48% vs 80%; P<0.001) and needed CS more frequently (36% vs 7%; P<0.001) than patients who did not receive IS or BT.
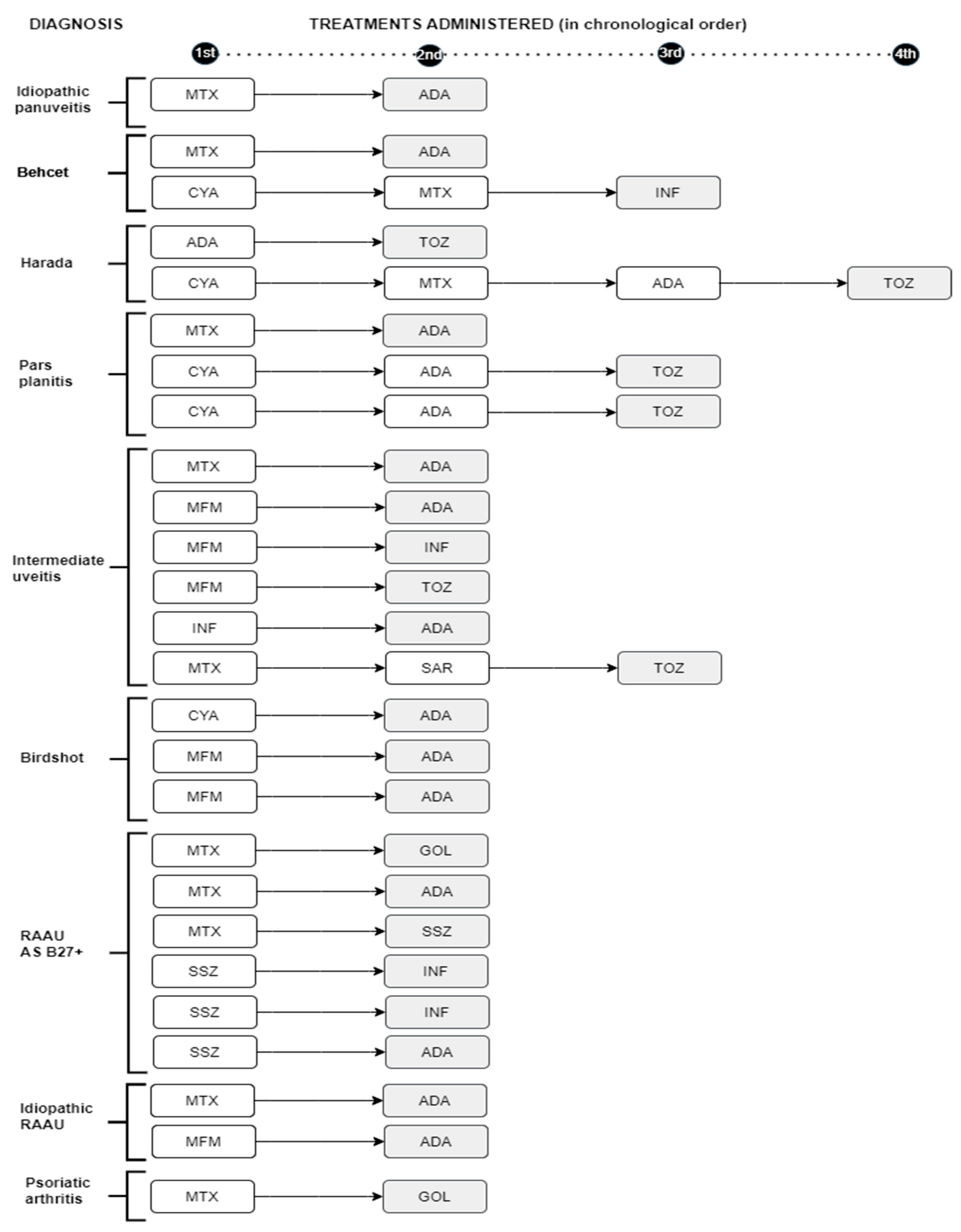
MTX was the most frequently used as first line of lS treatment (30 patients, 53.6%)
followed by CyA (11 patients, 19.6%), sulfasalazine (SSZ) (8 patients, 14%) and MFM (7 patients, 12.5%). In 3 of these patients, it was necessary to switch to a 2nd IS due to a lack of response to the first one (
Figure 1).
In 10 patients BT was the first line of treatment without previous IS. In 34 of the 66 patients (48%) BT was required at some point in the disease. A total of 20 patients (58.8%) received ADA as their first choice followed in frequency by INF (n=5 patients, 14.7%). Five patients needed to switch to another biologic due to a lack of response to the first biologic with a good response to it. In total, 35 patients (53%) needed to switch to another IS or BT in the absence of a response to the first drug used (
Figure 1).
Shaded: treatment that resolved the uveitis. RAAU: recurrent acute anterior uveitis AS: axial spondyloarthropathy MTX: Methotrexate. ADA: adalimumab MFM: mycophenolate mofetil
INF: Infliximab TOZ: tocilizumab SAR: sarilumab CYA: Cyclosporine. SSZ: sulfasalazine
3.3. Response to treatment
94% of the patients increased their visual acuity after treatment, changing vision after treatment by 0.3 [0.1, 0.4] units, with the change being different between the different IS
These results are shown in
Figure 2, which excludes those patients who started with a visual acuity of 1 and for whom the SI was used for other reasons such as frequent recurrences of uveitis.
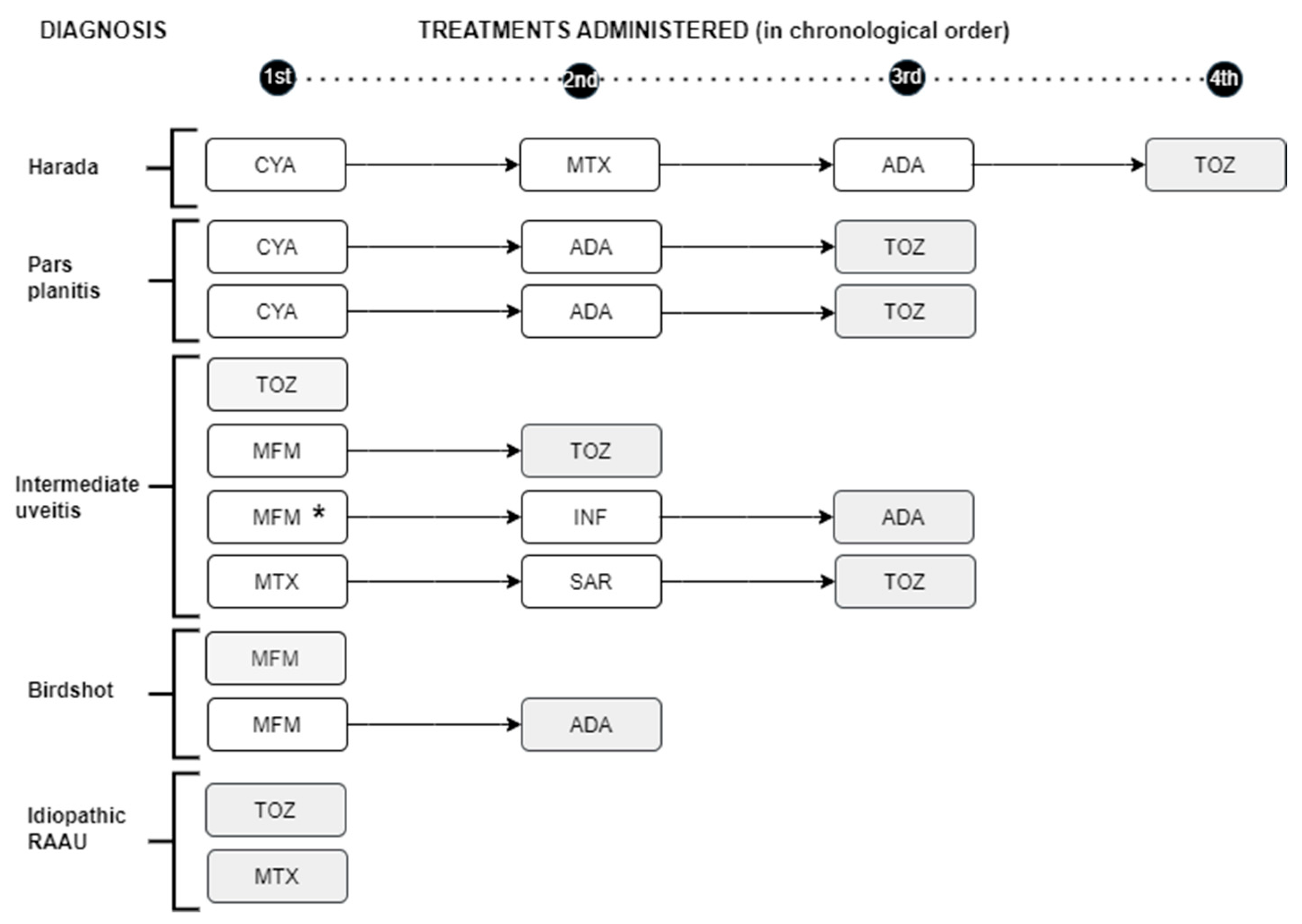
Of the 26 patients with vitritis who received initial IS (9 CyA, 42.3%, 11 MTX, 42.3% and 6 with MFMX, 23.1%), vitritis persisted in only 1 patient. Two patients with vitritis received BT initially (one ADA and one TOZ). Vitritis persisted in only 1 of these patients, with intermediate uveitis initially treated with MFM, whose treatment was changed to ADA and in this case the inflammation disappeared. Eleven patients presented MS. Nine of them were initially treated with IS (3 with CyA, 3 with MTX and 3 with MMF) and 2 were started directly with TOZ. Edema persisted in the 3 patients treated with CyA and resolved in 1 of the 3 and 2 of the 3 patients treated with MMF and MTX respectively. One patient with Harada syndrome required up to four treatments for the resolution of CME (1st CyA, 2nd MTX, 3rd ADA and 4th TOZ which led to the resolution of CME). We also found a patient with bilateral UI who developed CME despite being treated with MFM, switching first to INF with no response and finally to ADA which did resolve the inflammation (
Figure 3).
Macular edema resolved in all patients who were treated with TOZ (7 of 7) while in the rest of the treatments, the response was 0-50%. TOZ demonstrated a better response against CME than the rest of the treatments (100% vs. 29%, TOZ vs. rest; p-value= 0.005).
Shading: Treatment that resolved macular edema. *Patient who developed macular edema while on MFM treatment. RAAU: recurrent acute anterior uveitisCYA: cyclosporine MTX: methotrexate ADA: adalimumab TOZ: tocilizumab MFM: mycophenolate mofetil INF: infliximabSAR: sarilumab
4. Discussion
This work has been carried out to try to know the need and efficacy of treatment with IS and/or BT in patients with idiopathic or autoimmune disease-associated uveitis. The main problem of our study is the lack of references that have studied the overall efficacy of different treatments in large series of patients with idiopathic uveitis or uveitis associated with autoimmune disease. The indication for an IS associated with a CS is not known with certainty. There are not many such studies conducted and overall, there are a limited number of randomized clinical trials demonstrating the efficacy of immunosuppression in the treatment of uveitis. Although immunomodulators have demonstrated efficacy in patients with NIU in preventing recurrences and controlling ocular inflammation, further research is needed to adequately define the role of each immunomodulator in this population [
21,
22].
We conducted a descriptive study of the patients with NIU followed up in the multidisciplinary uveitis unit of our hospital. We first studied the characteristics of the 356 patients with NIU seen in this unit for 10 years. The results did not differ from other published series. [
23,
24,
25,
26]. There was no significant difference in sex, the mean age at diagnosis was 42.8 years, and the most frequent anatomical location was the anterior (74.4%) as were the unilateral cases (76.1%). 157 patients (44.1%) had presented during this time with only one outbreak of ocular inflammation. According to etiology, the most frequent were idiopathic (43.3%) followed by uveitis associated with HLA B27+ AE (76 patients, 21.4%). The time from uveitis diagnosis to initiation of treatment with immunosuppressants and/or biologics was 2 years [1, 4].
Of the uveitis that required treatment with CS via the general route, 19 were remitted without the need for subsequent immunosuppression as it was a single outbreak. Of the total, 66 patients were treated with IS and/or BT. The number of patients treated with IS was like or somewhat higher than in other studies [
26,
27] y less than in others [
24]. The etiology that most needed this type of treatment was idiopathic (21 patients, 31.4%). Ten patients started treatment directly with BT without previous IS while 56 (84.9%) had IS as their first treatment. A total of 35 patients (53%) needed to switch to another IS or to BT due to lack of response, with MMF being the one that needed it on more occasions (6 of 7, 85.7%). The proportion of males and females and the number of ocular inflammation flares is not different between patients treated with IMS and/or BT and untreated patients but treated patients were younger (perhaps because there is a higher number of posterior uveitis, intermediate uveitis or panuveitis in this age group, findings also found in the series of Millán et al [
24] and the proportion of patients with bilateral uveitis was also higher in this group.
In our study the most used drug was MTX (55.4%) followed by CyA (16.6%) like other series, being MTX considered a drug with good response and low cost [
24,
28,
29]. Compared to MFM, both have a high success rate (22,30), 63% and 64% respectively, although in our case MMF was used on 7 occasions, requiring a change due to lack of response in 6 of them (85.71%), 3 patients with intermediate uveitis, two birdshot and one idiopathic RAAU.
In general, most patients with AAU (anterior acute uveitis) are treated with topical ocular CS but a small percentage of them have frequent recurrences requiring systemic treatment. MTX significantly reduces the number of flare-ups and activity in patients with idiopathic or systemic-associated AU [
29]. We used MTX as the first IS in 30 patients, 16 of them with RAU either idiopathic or associated with the systemic disease: one patient was switched to SSZ due to poor tolerance and in 6 it was necessary to switch to BT due to lack of response. Etanercept (ETA) has demonstrated its clinical efficacy in the manifestations of AD with a lack of data on the efficacy of AU and even the paradoxical appearance of AAU after treatment with this drug [
31,
32] although in our series two patients with AAU associated with B27+ AS was in ocular remission with ETA.SSZ was used as the first immunosuppressant in 7 patients with RAASU associated with systemic conditions, requiring only 3 changes in treatment due to lack of response [
33].
In 34 of the 66 patients (48.5%) it was not possible to control the inflammatory activity with classic IS, so it was necessary to introduce BT treatment (mainly ADA) at some point in the disease, obtaining in general a very good response as in previous studies [
11,
12,
34].
Inflammatory activity, as well as the patient's improvement or not, was measured according to variables (VA, vitritis, MS) as other authors had already done [
2]. The presence of visual worsening or any of these markers of inflammation in the presence of CS treatment indicated the need to add an IS. If despite this, inflammatory activity or vision loss continued, it was switched to another, or BT was added. Regarding VA, excluding patients who previously presented maximum VA (in these patients the use of IS was due to other criteria such as a greater number of flare-ups and recurrences), 94% improved their vision. If we consider only those patients who initially presented vision loss due to uveitis, the proportion of them who improved VA is similar among the different IS. Regarding vitritis, present in 28 (42.4%) of the 66 patients who were treated with IS and/or BT, it persisted in only 1 of them (a case of idiopathic intermediate uveitis that did not respond to MFM but did respond to ADA). In the rest of the patients, there was a positive response to treatment with no differences between the different types of drugs. A patient with Harada syndrome needed up to four drugs to achieve remission of MS and recovery of VA, perhaps due to the delay in diagnosis and its chronicity [
35].
MS can be a complication of uveitis in any of its locations and etiologies and is the main condition associated with VA loss. [
1,
2] may benefit from early management including CS and IS and/or BT [
36]. Treatments with CyA, MTX or MMF have shown an 83% reduction in the development of MS in patients with birdshot as well as ADA and INF are effective in the treatment of MS in different uveitis entities. [
36]. Efficacy of TOZ, an antibody against the IL-6 receptor has been demonstrated in cases of refractory uveitis [
37,
38]. Subcutaneous sarilumab (SAR) may provide clinical benefit in the treatment of posterior NIU, especially in eyes with uveitic ME [
39]. In our study, SAR was used in one patient with IU and refractory ME without response and TOZ was used, which did achieve its disappearance. Of the 11 patients who presented with ME in our series, TOZ proved to be the most effective in the total number of times it was administered (7 out of 7), while the other treatments had a combined effectiveness of 29.4%.
5. Conclusions
The use of an immunosuppressant either conventional or biologic, alone or in combination, achieved, at least in our study, the objective of maintaining the patient with uveitis without inflammatory activity. It should be noted that in our series TOZ proved to be significantly more effective in the resolution of macular edema. Regarding the advantages of our study, we can highlight that we used a wide range of real clinical practices, being able to use different drugs and observing different degrees of response among them. On the other hand, the limitations of our study include its retrospective nature, which may lead to inaccurate and non-standardized data collected from the evaluation at irregular intervals and the presence of small groups of drugs having to be grouped, which may create biases regarding the individual effectiveness of each drug.
Author Contributions
Conceptualization, M.E and S.M.; methodology C.A and I.T.; software, C.A and I.T.; validation, C.A and I.T.; formal analysis, C.A and I.T.; investigation M.E, M.S and S.M.; resources, X.X.; data curation, X.X.; writing—original draft preparation, M.E.; writing—review and editing, A.D.; visualization, A.D.; supervision, M.E, S.M and M.S.; project administration, M.E.; All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The research protocol for this study was approved by the research committee of the Hospital Universitario Infanta Sofía on the 15Th of March 2022.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- E.Cunningham ET, Zierhut M. Vision Loss in Uveitis. Ocular Immunology and Inflammation. 2021;29(6):1037-9.
- Tomkins-Netzer O, Talat L, Bar A, Lula A, Taylor SRJ, Joshi L, et al. Long-term clinical outcome and causes of vision loss in patients with uveitis. Ophthalmology. 2014;121(12):2387-92.
- de Smet MD, Taylor SRJ, Bodaghi B, Miserocchi E, Murray PI, Pleyer U, et al. Understanding uveitis: the impact of research on visual outcomes. Prog Retin Eye Res. 2011;30(6):452-70.
- Rosenbaum JT, Bodaghi B, Couto C, Zierhut M, Acharya N, Pavesio C, et al. New observations and emerging ideas in diagnosis and management of non-infectious uveitis: A review. Semin Arthritis Rheum. 2019;49(3):438-45.
- van Laar JAM, Rothova A, Missotten T, Kuijpers RWAM, van Hagen PM, van Velthoven MEJ. Diagnosis and treatment of uveitis; not restricted to the ophthalmologist. J Clin Transl Res. 2015;1(2):94-9.
- Lowder C, Belfort R, Lightman S, Foster CS, Robinson MR, Schiffman RM, et al. Dexamethasone intravitreal implant for noninfectious intermediate or posterior uveitis. Arch Ophthalmol. 2011;129(5):545-53.
- Callanan DG, Jaffe GJ, Martin DF, Pearson PA, Comstock TL. Treatment of posterior uveitis with a fluocinolone acetonide implant: three-year clinical trial results. Arch Ophthalmol. 2008;126(9):1191-201.
- Jabs DA, Rosenbaum JT, Foster CS, Holland GN, Jaffe GJ, Louie JS, et al. Guidelines for the use of immunosuppressive drugs in patients with ocular inflammatory disorders: recommendations of an expert panel. Am J Ophthalmol. 2000;130(4):492-513.
- Nguyen QD, Hatef E, Kayen B, Macahilig CP, Ibrahim M, Wang J, et al. A cross-sectional study of the current treatment patterns in noninfectious uveitis among specialists in the United States. Ophthalmology. 2011;118(1):184-90.
- Dick AD, Rosenbaum JT, Al-Dhibi HA, Belfort R, Brézin AP, Chee SP, et al. Guidance on Noncorticosteroid Systemic Immunomodulatory Therapy in Noninfectious Uveitis: Fundamentals Of Care for UveitiS (FOCUS) Initiative. Ophthalmology. 2018;125(5):757-73.
- Suhler EB, Adán A, Brézin AP, Fortin E, Goto H, Jaffe GJ, et al. Safety and Efficacy of Adalimumab in Patients with Noninfectious Uveitis in an Ongoing Open-Label Study: VISUAL III. Ophthalmology. 2018;125(7):1075-87.
- Jaffe GJ, Dick AD, Brézin AP, Nguyen QD, Thorne JE, Kestelyn P, et al. Adalimumab in Patients with Active Noninfectious Uveitis. N Engl J Med. 2016;375(10):932-43.
- Takeuchi M, Kezuka T, Sugita S, Keino H, Namba K, Kaburaki T, et al. Evaluation of the long-term efficacy and safety of infliximab treatment for uveitis in Behçet’s disease: a multicenter study. Ophthalmology. 2014;121(10):1877-84.
- Levy-Clarke G, Jabs DA, Read RW, Rosenbaum JT, Vitale A, Van Gelder RN. Expert panel recommendations for the use of anti-tumor necrosis factor biologic agents in patients with ocular inflammatory disorders. Ophthalmology. 2014;121(3):785-796.e3.
- Sepah YJ, Sadiq MA, Chu DS, Dacey M, Gallemore R, Dayani P, et al. Primary (Month-6) Outcomes of the STOP-Uveitis Study: Evaluating the Safety, Tolerability, and Efficacy of Tocilizumab in Patients With Noninfectious Uveitis. Am J Ophthalmol. 2017;183:71-80.
- Leclercq M, Andrillon A, Maalouf G, Sève P, Bielefeld P, Gueudry J, et al. Anti-Tumor Necrosis Factor α versus Tocilizumab in the Treatment of Refractory Uveitic Macular Edema: A Multicenter Study from the French Uveitis Network. Ophthalmology. 2021;S0161-6420(21)00900-3.
- Deschenes J, Murray PI, Rao NA, Nussenblatt RB, International Uveitis Study Group. International Uveitis Study Group (IUSG): clinical classification of uveitis. Ocul Immunol Inflamm. 2008;16(1):1-2.
- Jabs DA, Nussenblatt RB, Rosenbaum JT, Standardization of Uveitis Nomenclature (SUN) Working Group. Standardization of uveitis nomenclature for reporting clinical data. Results of the First International Workshop. Am J Ophthalmol. 2005;140(3):509-16.
- Pistilli M, Joffe MM, Gangaputra SS, Pujari SS, Jabs DA, Levy-Clarke GA, et al. Visual Acuity Outcome over Time in Non-Infectious Uveitis. Ocul Immunol Inflamm. 2021;29(6):1064-71.
- Hunter RS, Skondra D, Papaliodis G, Sobrin L. Role of OCT in the diagnosis and management of macular edema from uveitis. Semin Ophthalmol. 2012;27(5-6):236-41.
- Gómez-Gómez A, Loza E, Rosario MP, Espinosa G, Morales JMGR de, Herreras JM, et al. Efficacy and safety of immunomodulatory drugs in patients with anterior uveitis: A systematic literature review. Medicine (Baltimore). 2017;96(42):e8045.
- Gómez-Gómez A, Loza E, Rosario MP, Espinosa G, de Morales JMGR, Herrera JM, et al. Efficacy and safety of immunomodulatory drugs in patients with non-infectious intermediate and posterior uveitis, panuveitis and macular edema: A systematic literature review. Seminars in Arthritis and Rheumatism. 2020;50(6):1299-306.
- García-Aparicio A, Alonso Martín L, López Lancho R, Quirós Zamorano R, Del Olmo Perez L, Sánchez Fernández S, et al. Epidemiology of Uveitis in a Spanish Region: Prevalence and Etiology. Ophthalmic Epidemiol. 2021;28(3):227-36.
- Millán-Longo C, Peiteado D, Schlincker A, Hidalgo V, Pieren A, Balsa A, et al. Use of Immunomodulatory Drugs at a Uveitis Clinic. Reumatol Clin (Engl Ed). 2019;15(5):271-6.
- Chang JHM, Wakefield D. Uveitis: a global perspective. Ocul Immunol Inflamm. 2002;10(4):263-79.
- Wang L, Guo Z, Zheng Y, Li Q, Yuan X, Hua X. Analysis of the clinical diagnosis and treatment of uveitis. Annals of Palliative Medicine. 2021;10(12):127822788-127812788.
- Bajwa A, Osmanzada D, Osmanzada S, Khan I, Patrie J, Xin W, et al. Epidemiology of uveitis in the mid-Atlantic United States. Clin Ophthalmol. 2015;9:889-901.
- Foster CS, Kothari S, Anesi SD, Vitale AT, Chu D, Metzinger JL, et al. The Ocular Immunology and Uveitis Foundation preferred practice patterns of uveitis management. Surv Ophthalmol. 2016;61(1):1-17.
- Muñoz-Fernández S, García-Aparicio AM, Hidalgo MV, Platero M, Schlincker A, Bascones ML, et al. Methotrexate: an option for preventing the recurrence of acute anterior uveitis. Eye (Lond). 2009;23(5):1130-3.
- Rathinam SR, Babu M, Thundikandy R, Kanakath A, Nardone N, Esterberg E, et al. A Randomized Clinical Trial Comparing Methotrexate and Mycophenolate Mofetil for Noninfectious Uveitis. Ophthalmology. 2014;121(10):1863-70.
- Guillot X, Prati C, Sondag M, Wendling D. Etanercept for treating axial spondyloarthritis. Expert Opin Biol Ther. 2017;17(9):1173-81.
- Wang F, Wang NS. Etanercept therapy-associated acute uveitis: a case report and literature review. Clin Exp Rheumatol. 2009;27(5):838-9.
- Benitez-Del-Castillo JM, Garcia-Sanchez J, Iradier T, Bañares A. Sulfasalazine in the prevention of anterior uveitis associated with ankylosing spondylitis. Eye (Lond). 2000;14 ( Pt 3A):340-3.
- Gómez-Gómez A, Loza E, Rosario MP, Espinosa G, de Morales JMGR, Herrera JM, et al. Efficacy and safety of immunomodulatory drugs in patients with non-infectious intermediate and posterior uveitis, panuveitis and macular edema: A systematic literature review. Semin Arthritis Rheum. 2020;50(6):1299-306.
- Shen E, Rathinam SR, Babu M, Kanakath A, Thundikandy R, Lee SM, et al. Outcomes of Vogt-Koyanagi-Harada Disease: A Subanalysis From a Randomized Clinical Trial of Antimetabolite Therapies. American Journal of Ophthalmology. 2016;168:279-86.
- Fardeau C, Champion E, Massamba N, LeHoang P. Uveitic macular edema. Eye (Lond). 2016;30(10):1277-92.
- Vegas-Revenga N, Calvo-Río V, Mesquida M, Adán A, Hernández MV, Beltrán E, et al. Anti-IL6-Receptor Tocilizumab in Refractory and Noninfectious Uveitic Cystoid Macular Edema: Multicenter Study of 25 Patients. Am J Ophthalmol. 2019;200:85-94.
- Suhler EB, Jaffe GJ, Fortin E, Lim LL, Merrill PT, Dick AD, et al. Long-Term Safety and Efficacy of Adalimumab in Patients with Noninfectious Intermediate Uveitis, Posterior Uveitis, or Panuveitis. Ophthalmology. 2021;128(6):899-909.
- Heissigerová J, Callanan D, de Smet MD, Srivastava SK, Karkanová M, Garcia-Garcia O, et al. Efficacy and Safety of Sarilumab for the Treatment of Posterior Segment Noninfectious Uveitis (SARIL-NIU): The Phase 2 SATURN Study. Ophthalmology. 2019;126(3):428-37.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).