1. Introduction
Plants produce more than 200,000 distinct specialized metabolites [
1], constituting the principal reservoir of bioactive compounds combating diseases in numerous countries [
2,
3]. Nevertheless, the complexity of working with plant extracts lies in discerning the specific specialized metabolites responsible for eliciting bioactivity in bioassays, preclinical
in vivo models, or, ultimately, in humans. The traditional bioassay-guided fractionation approach is tedious and time-consuming [
4,
5]. This approach requires the separation of certain phytochemicals based on physicochemical properties such as polarity, charge or size, and assessing the bioactivity in a step-by-step methodology. Sequential steps of purification and assay may ultimately result in the isolation of the bioactive compound, only to find that the compound had been previously discovered [
4,
5,
6,
7,
8]. Additionally, there are risks associated with traditional exhaustive fractionation that compounds degrade or become lost during the process [
9]. There is an urgent need to accelerate the discovery of bioactive natural products and to remove the dereplication bottleneck. Methods capable of addressing this need are emerging. The Global Natural Product Social Molecular Networking (GNPS) platform can assist in the dereplication and annotation of specialized metabolites [
10,
11]. Statistical models, such as Partial Least Squares (PLS) models, utilize spectral information to predict bioactive metabolites in complex natural product mixtures [
12]. The selectivity ratio method is another well-established tool for assisting in the discovery of biomarkers which utilizes chromatographic and mass spectral profiles [
13,
14,
15]. The selectivity ratio method has been recently applied to the discovery of bioactive constituents in botanical extracts. Recently, our research group showed that Elastic Net (EN), a regularized regression model [
16], was capable of correctly predicting the anti-inflammatory bioactive constituents in hop extracts utilizing high-resolution mass spectrometry
m/z profiles of extract fractions [
17].
The objective of the present study was to find and annotate the bioactives in
C. asiatica extracts that ameliorate cytotoxicity caused by amyloid β in MC65 cells, a cell culture model amenable to high throughput screening [
18,
19]. Aqueous extracts of
C. asiatica are recognized as enhancing memory and mental health [
20,
21,
22,
23]. The use of
C. asiatica preparations in complementary medicine has been associated with ameliorating cognitive decline due to ageing and Alzheimer’s disease [
22,
24,
25]. For this purpose, we utilized the Elastic Net method to correlate molecular features (unknown phytochemicals) derived from high-resolution mass spectrometry with bioactivity levels observed for
C. asiatica fraction in the MC65 assay. We selected the Elastic Net as it allows accurate computation of the contribution of each bioactive phytochemical towards the total bioactivity of the fraction without limiting the number of phytochemicals being used for the prediction [
26]. Importantly, we validated the output of the Elastic Net method using the well-established selectivity ratio method. We created a GNPS network that visualizes the association of fraction, bioactivity and mass spectral data. This case study revealed that mono- and di-caffeoylquinic acids (CQAs) are associated with protecting against amyloid-β toxicity in a MC65 cell model.
2. Results and Discussion
2.1. Chemical Diversity and Viability in C. asiatica Fractions
The plant-based bioactive compound discovery field is challenged by the need to purify and identify specialized metabolites that exhibit bioactivity in various assays. To address these challenges, our research group developed an innovative approach combining fractionation, high-resolution mass spectrometry, and advanced computational models to rapidly screen plant extracts [
17]. An adaptation of this workflow was applied to
C. asiatica extracts. A critical aspect of our approach involved simplifying the chemical diversity of the plant extract by creating a set of 21 impure fractions, producing distinct compound concentrations across them (Fractions A1-A21,
Figure S1). The initial liquid-liquid extractions served as a pivotal step in isolating different components of the
Centella asiatica water (CAW) extract based on their solubility in various solvents. The sub-fractionation approach uses LH-20 chromatography which allows the separation of phytochemical constituents into low numbers of fractions, thus allowing mass spectral analysis of the chemical constituents while minimizing the matrix effects when analyzing complex mixtures.
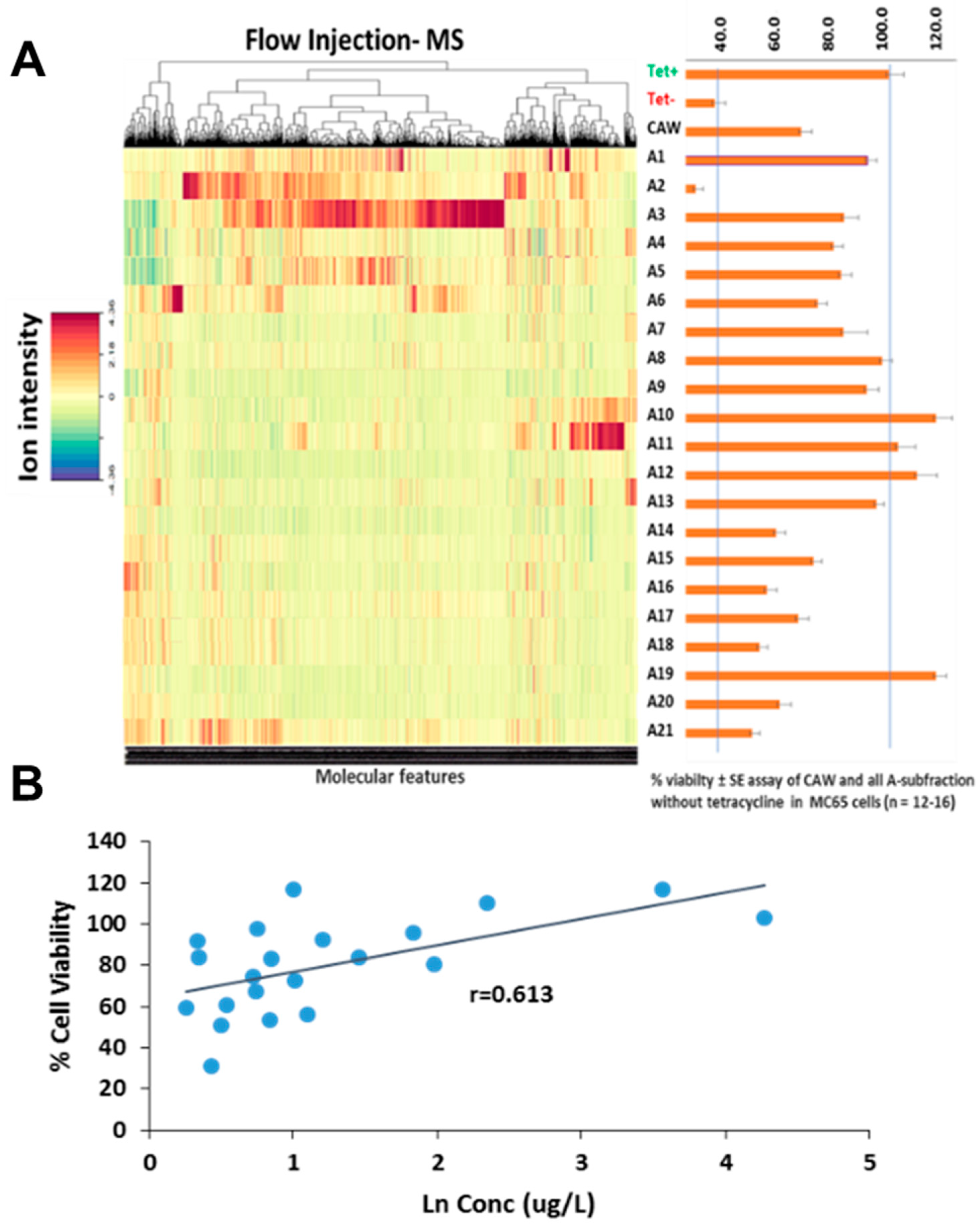
Mass spectral profiles for the 21 fractions were obtained by flow-injection HRMS (
Figure 1). Data were acquired in positive and negative electrospray ionization mode. Over 1500 molecular features were recorded across all fractions, and the gradient of concentrations was computed against the % of proliferation of MC65 cells using two computational models, namely Elastic Net and selectivity ratio (SR). Removing the use of the analytical column in flow-injections has the advantage of shortening the analysis time (under 2 min per run) with only 30 seconds of equilibration time before the next injection. Flow-injections increase the potential of the ion suppression phenomenon since all compounds elute together, including the sodium and other cations producing several adducts for each compound. However, the sub-fractionation of the plant extracts lessens this problem. Sub-fractionation in conjunction with flow-injection has the methodological advantage of minimizing matrix effects due to matrix simplification, allowing detection of additional molecular features. Nevertheless, careful processing and adduct analysis and deconvolution is needed to obtain mass spectral fingerprints of sufficient quality [
27] to feed the computational analysis.
MC65 cells, a neuroblastoma line, express the C-terminal fragment of the amyloid precursor protein (APP CTF) regulated by a tetracycline-responsive promoter. Upon tetracycline withdrawal from the medium, the C99 fragment of APP is expressed which is then cut by -secretase to form Aβ peptides. The accumulation of intracellular, endogenous Aβ leads to cell death within 72 hours [
28]. In the absence of tetracycline, cells treated with each of the 21 fractions exhibit cell viability levels ranging from 5% (A2) to 117% (A10). Remarkably, cells treated with fractions A10, A11, A12, and A19 not only displayed a notable absence of cell death but also exhibited a proliferation surpassing that of the control containing tetracycline, reaching >100% viability. This observation would typically trigger additional analyses to find the compound present in those fractions. However, this is not needed under this methodology. This computational approach has the capability to uncover correlations between variations in the levels of molecular features (phytochemicals) across the fractions and the percentage of viability, thereby identifying the most probable compounds influencing cell viability.
2.2. Correlation between Phytochemical Profiles and Neuroprotective Effect
The correlation of the HRMS profiles of the 21 CAW fractions with the neuroprotective activity levels of each fraction was achieved using Elastic Net as previously described [
17]. In addition, we applied selectivity ratio analysis to confirm independently the Elastic Net analysis results of putative bioactive compounds acting as inhibitors of Aβ cytotoxicity in the MC65 cell culture model. Selectivity ratio is established by determining the ratio between the explained and residual variances of the spectral variables on the target projected component [
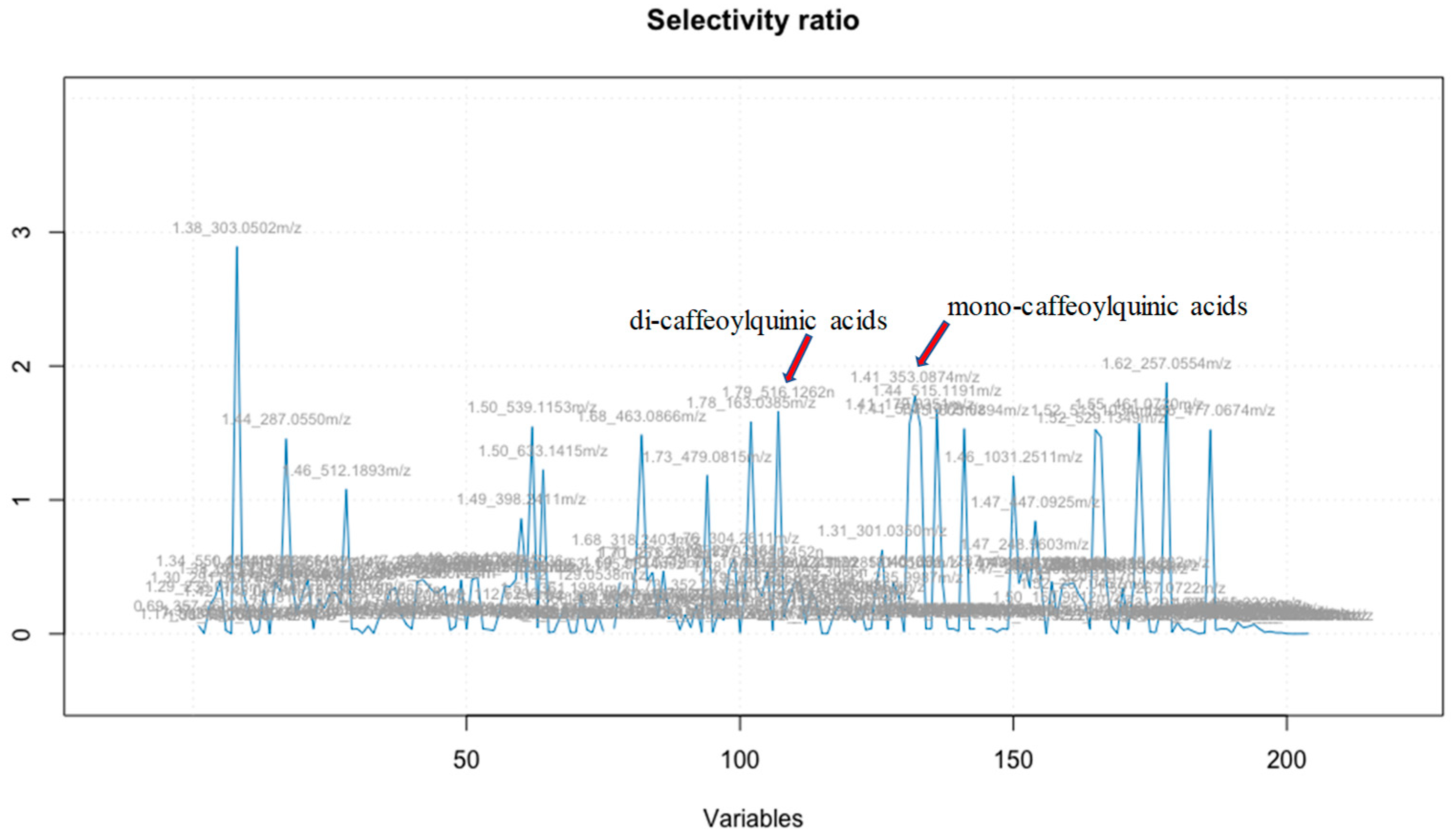
29]. This ratio serves as a useful tool for selecting variables in the analysis (
Figure 2).
The 21 fractions were tested for their protective effect in the MC65 cell culture model of Aβ toxicity, which uses % cell viability as a measure of cell protection. The Elastic Net model identified
m/z 515.1191 and
m/z 353.0874 as the top two bioactives, while the selectivity ratio pinpointed
m/z 303.0502 and
m/z 257.0554 as the top two bioactive compounds, respectively. Despite discrepancies in the top 10 bioactive compounds between the two models, six compounds emerged as consistent top candidates in both (
Table 1). Notably, the selectivity ratio emphasized the significance of
m/z 303.0502 [M+H]
+ and
m/z 257.0554 as the top bioactives; it also confirmed the identification of
m/z 515.1191 and
m/z 353.0874 as the next most important compounds. This validation highlights the effectiveness of Elastic Net in identifying the most likely compounds among the 1500 molecular features.
In predictive modelling for neuroprotective effects, the Elastic Net algorithm presents notable advantages and limitations when compared to other models. Elastic Net's incorporation of L1 (Lasso regression) and L2 regularization (Ridge regression) facilitates variable selection, making it adept at handling multicollinearity and providing flexibility through parameter tuning. However, Elastic Net's performance may be compromised when confronted with many irrelevant features and is sensitive to variable scaling [
17]. On the other hand, selectivity ratio excels in capturing linear relationships, proving robust against overfitting and capable of handling missing data. The choice between Elastic Net and the selectivity ratio hinges on the specific characteristics of the dataset and the interpretability requirements of the neuroprotective modelling effort. While
m/z 515.1191 acids exhibit a high correlation in both models, other compounds in
Table 1 showcase varying degrees of correlation, highlighting the complexity of the botanical extract's composition. This diversity opens avenues for further exploration in a resource-focused way. In this case study, both systems provide a reduction of the candidates from 1500 molecular features to a handful of them.
Despite the potential limitation of encountering antagonist compounds within the same subfraction, it is noteworthy that this aspect simultaneously highlights a strength of our current strategy, namely the potential of revealing synergies among compounds. This capability to uncover synergistic interactions is critical in investigating bioactive compounds [
30]. The intricacies of exploring synergy and antagonism present significant challenges, especially in natural product complex chemistry [
31]. The conventional approach in this field focuses on simplifying complexity and isolating single active constituents for drug development, thereby making the comprehensive study of synergistic and antagonistic interactions notably challenging.
Finally, quite often, one of the struggles of using traditional approaches with exhaustive fractionation and purification leads to the rediscovery of compounds already used in different studies, wasting resources. In this case, both models add helpful information to suggest consistent candidates. Furthermore, another advantage of this approach relies on suggesting the molecular features as candidate(s) for structural elucidation leveraging the MS/MS data of the crude used for the fractionation and described in the following section.
Table 1.
Selectivity ratio and Elastic Net ranks for the experiment flow injection-TOF acquisition ion correlated with MC65 bioactivity assay.
Table 1.
Selectivity ratio and Elastic Net ranks for the experiment flow injection-TOF acquisition ion correlated with MC65 bioactivity assay.
| Feature1
|
SR2
|
Variable importance in Elastic Net model |
Annotation3
|
Ion mode |
| 1.38_303.0502 m/z
|
2.89 |
N/A |
Quercetin |
POS |
| 1.62_257.0554 m/z
|
1.88 |
N/A |
N/A |
NEG |
| 1.41_353.0874 m/z
|
1.78 |
1.58 |
MCQAs |
NEG |
| 1.79_515.1191 m/z
|
1.66 |
2.44 |
DCQA's |
NEG |
| 1.78_163.0385 m/z
|
1.58 |
0.8 |
Hydroxycoumarin |
POS |
| 1.55_461.0720 m/z
|
1.57 |
N/A |
Myricetin 3-glucoside |
NEG |
| 1.41_179.0351 m/z
|
1.57 |
N/A |
Caffeic Acid |
NEG |
| 1.50_539.1153 m/z
|
1.55 |
N/A |
N/A |
POS |
| 1.41_537.1012 m/z
|
1.54 |
N/A |
N/A |
NEG |
| 1.45_605.0894 m/z
|
1.53 |
0.73 |
Bisdihydroquercetin |
NEG |
| 1.52_513.1034 m/z
|
1.52 |
0.69 |
N/A |
NEG |
| 1.66_477.0674m/z
|
1.52 |
0.66 |
Quercetin 7-glucuronide |
NEG |
2.3. Identification of Neuroprotective Phytochemicals
The mass spectral feature, m/z 353.0874 and m/z 515.1191 were predicted as bioactive compounds associated with the neuroprotective effects using the multivariate regression Elastic Net model as well as the selectivity ratio (
Table 1,
Figure 2). The molecular features with a selectivity ratio greater than 1 indicate that the molecular features explain 50% of the original variance and can be translated into potential neuroprotective activity. The m/z 303.0502 held a selectivity ratio SR 2.89, m/z 353.0874 [M-H]- SR 1.78 and m/z 515.1191 [M-H]- SR 1.67 (
Figure 2). By using our in-house Oregon Natural Products (ONAP) MS library containing 331 plant NPs, the m/z 353.0874 ion was assigned to monocaffeoylquinic acid(s) and the m/z 515.1191 ion to dicaffeoylquinic acid(s) [
17] and their identities verified by LC-MS/MS comparison with authentic standards as previously described [
32]. These results, indicating heightened activity for di-CQAs compared to mono-CQAs, align with earlier reports emphasising the increased bioactivity of CQAs corresponding to an increased number of caffeic acid moieties [
28,
33]. Additionally, m/z 305.0502 was also reported as one of the consituent in C. asiatica corresponding to quercetin [
32]. Among the highly active compounds, tentatively annotated constituents included hydroxycoumarin, myricetin 3-glucoside, caffeic acid, bisdihydroquercetin, and quercetin 7-glucuronide (
Table 1). Here, we investigated the correlation of % cell viability with the concentration of diCQAs (sum of isomers, [M-H]-, m/z 515.12) present in the 21 CA subfractions obtaining a positive correlation (r=0.613,
Figure 1B).
The discovery of mono- and dicaffeoylquinic acids as active compounds supports previous findings of their neuroprotective effects in both in vitro and in vivo models. For instance, evidence across various neural models such as MC65 [
28,
33], SH-SY5Y [
34,
35] and PC-12 cells [
36] highlights the neuroprotective role of CQAs. Additionally, pretreatment with CQAs resulted in a significant reduction in neuronal death in rats after ischemic insult [
37]. Furthermore, mono-CQAs exhibited the ability to mitigate synaptic dysfunction by either enhancing the restoration of synaptic transmission upon re-oxygenation or alleviating the aberrant alteration in hippocampal synaptic plasticity linked to memory impairment induced by exposure to β-amyloid peptides in mice models [
38]. Our study further reveals that fractions A10-A12, exhibiting the highest levels of DCQAs, correlated with enhanced cell viability. This finding corroborates our previous reports, demonstrating the protective effects of caffeoylquinic acids in C. asiatica against amyloid-β toxicity [
28]. Furthermore, caffeoylquinic acids were found able to mitigate the cognitive deficits in 5XFAD Alzheimer's disease mice model [
39]. As for quercetin, its anti-inflammatory and neuroprotective effects, modulating AMPK and influencing the NF-kB and NLRP3 inflammasome pathways have been well-documented [
40]. Additionally, quercetin has been implicated in promoting neuronal survival and synaptic plasticity, potentially influencing cognitive function [
41,
42,
43]. While these preclinical studies provide promising insights into the neuroprotective properties of CQAs and quercetin, further well-designed clinical trials are essential to establish their efficacy and safety in the context of neurological disorders in human populations.
2.4. Molecular Networking for Analyzing Chemical Diversity
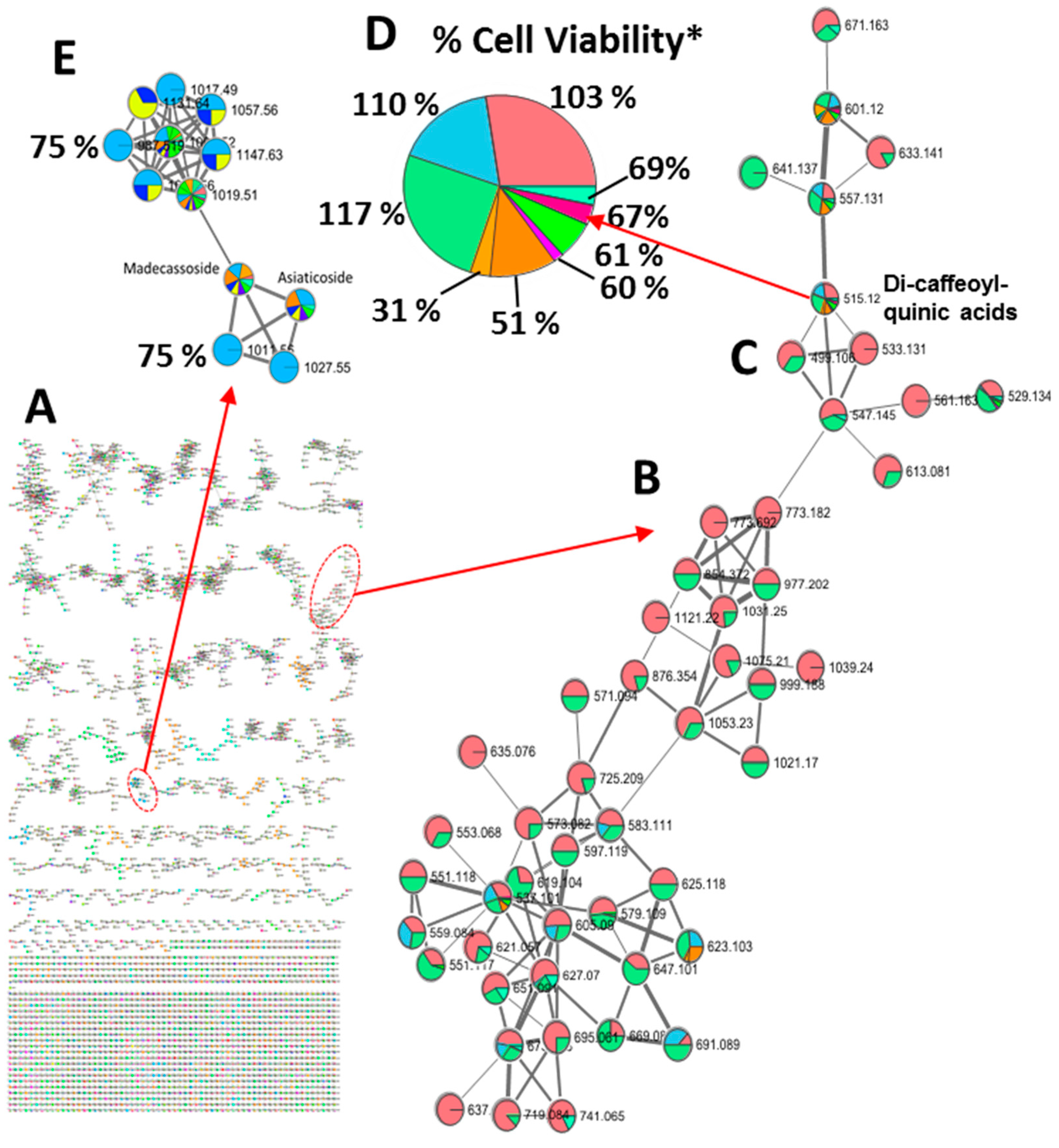
Molecular networking is a useful tool to propagate annotations of compounds sharing more than 70% of the spectral fragmentation. In addition to employing full-scan TOF-MS analysis on the 21 fractions, the crude extract containing all the components underwent an additional analysis via LC-QTOF-MS/MS to expand the characterization of the chemical diversity in the fractions. This process yielded required mass fragment (MS/MS) data for constructing a molecular network through GNPS [
44]. GNPS constructs these molecular networks by aligning MS/MS spectra, where node assignment corresponds to associated precursor ions. The edges between nodes are established based on the cosine score, representing the similarity between nodes. We adopted a cut-off value of 0.70 to identify nodes with significant similarity, with the matching relying on MS/MS fragmentation information. The resultant network was visually represented using Cytoscape V3.6.1 (
Figure 3). The organized MS/MS dataset comprised 5500 nodes clustered into 193 distinct groups containing three or more nodes within the network (
Figure 3 A). The generation of this extensive network enabled the association of molecular features with sub-fraction-specific biological activities. Our bioactivity mapping revealed the presence of dicaffeoylquinic acids in fractions that exhibited complete protection against Aβ toxicity (
Figures 3B–D), contrasting with the identification of triterpene glycosides (
Figure 3E) in fractions providing partial protection (bioactivity level, 75%). This integrated approach provides a powerful strategy for the rapid and resource-efficient discovery of bioactive compounds in complex plant extracts, advancing the understanding of their bioactive potential.
3. Materials and Methods
3.1. Associating chemical diversity of C. asiatica with % viability from MC65 bioassay
Centella asiatica water (CAW) extract was prepared as previously described [
32,
45]. For this study, the fractionation scheme depicted in
Figure S1 was used; initially, liquid-liquid extractions were performed, and the organic fractions were further sub-fractionated using LH-20 chromatography. CAW powder (140.5g) was sonicated in MeOH (3 x 200 mL); MeOH insoluble materials were separated, pooled and assigned as fraction A4 (41.3 g). The MeOH soluble fractions were pooled and dried and subjected to liquid-liquid partitioning between dichloromethane (DCM; 200 mL) and water (2 x 200 mL). The DCM layer was dried under vacuum (fraction A3; 7.8 g). The combined water layers were then partitioned with n-butanol (3 x 600 mL). The butanol and water layers were dried to give fractions A1 (15.9 g) and A2 (68.2 g) respectively. Sephadex LH-20 chromatography (length 40 cm, diameter 5 cm) with methanol was used to fractionate the BuOH-derived A1 and DCM-derived A3 residues, resulting in fractions A5-A13 and A14-A21, respectively. Overall, CAW constituents were distributed in 21 fractions (A1 – A21;
Figure S1).
3.2. Biological activity in MC65 cellular line
MC65 cells were used because of the ability to conditionally express the C99-terminal fragment of amyloid precursor protein (APP CTF) [
46]. In the absence of tetracycline, the cells are able to generate endogenous Aβ that results in cell death within 3 days. There has been evidence that links Aβ aggregates and resulting cytotoxicity with oxidative stress [
47]. The maintenance of MC65 cells was performed in MEMα supplemented with 10% FBS (Gibco-BRL, Carlsbad, CA) and 1 μg/mL tetracycline (Sigma-Aldrich, St. Louis, MO) using the procedure described in [
47,
48]. Confluent cells were treated with trypsin followed by washing in PBS. The cells were resuspended in OptiMEM without phenol red (Gibco/BRL, Carlsbad, CA). Cells were treated with vehicle with or without tetracycline, or treated with fractions and without tetracycline, and then plated at 25,000 cells/well in 96-well plates. Cell viability was measured at 3 days with CellTiter 96 Aqueous Non-Radioactive Cell Proliferation Assay (Promega Corporation, Madison, WI). For statistical significance and repeatability, the experiments were performed in triplicate wells for each of the CAW fractions and repeated two times [
25].
3.3. Profiling of fractions using flow-injection-HRMS
Flow-injection combined with high-resolution accurate mass spectrometry (HRMS) was conducted using a Shimadzu Nexera UHPLC system connected to an AB SCIEX TripleTOF® 5600 (Concord, Ontario, Canada) mass spectrometer equipped with a Turbo V ionization source operated in positive and negative electrospray ion mode. For negative ion mode acquisition, the following parameter settings were used to operate the mass spectrometer: spray voltage -4,200 V; source temperature 550 °C and a period cycle time of 150 ms was used. For positive ion mode acquisitions, the instrument settings were the same as used in the negative ion mode except that the spray voltage was set to 4500 V. The mass spectrometer was equipped with a calibrant delivery system.
For the flow-injection analysis, the flow rate was set at 0.2 mL/min utilizing aqueous methanol (20% v/v). A 3 µL injection volume was used. The total run time per sample was 3 minutes. The acquired data was aligned, deconvoluted and normalized using Progenesis QI
TM V2.4 (Nonlinear Dynamics, Waters Corporation, Milford, MA, USA). This deconvolution step assembles isotopologues and adducts from the same molecular species into one molecular feature [
17]. For creating a GNPS network for CAW and derived fractions, MS/MS data was acquired in data-dependent acquisition mode as previously described [
32].
3.4. Predicting protective biological activity with mass spectral data
Elastic Net penalized logistic regression multivariate analysis was performed to determine the phytochemicals responsible for the neuroprotective effect. The linear_model package from scikit-learn library V 0.24.2 was used to fit a regression model across molecular features obtained from 21 fractions and CAW crude extract. Elastic Net offered the advantage of shrinking some of the parameters to zero offering variable selection during the model fitting step. The penalization factor for elastic net was chosen using 10000 iterations and 5-fold cross-validation. The highest lambda from minimum standard error was selected for each iteration and median of the 10000 lambda values was computed to determine the final penalization factor. The selectivity ratio was computed using get SelectivityRatio from
mdatools package v 0.11.5 [
49] in R to identify discriminating
m/z molecular features. The selectivity ratios for the molecular features were plotted using Excel.
3.5. Compound identification
The molecular features identified as leads were queried by their exact mass in our
in-house database as well as online databases such as the Human Metabolome Database (HMDB) (
http://www.hmdb.ca/) and the METLIN database (
https://metlin.scripps.edu) as previously described [
32] with the following modifications. The exact mass was queried in the range of 10 ppm, and the annotations for molecular features were validated by comparing MS/MS fragments within the range of 50 ppm.
3.6. Molecular Networking
The MS/MS spectral data were used to create the GNPS network. MS/MS data have been deposited to the GNPS repository (
http://gnps.ucsd.edu). The
C. asiatica fractions’ chemical diversity and associated % viability were used to create molecular networks using the online workflow described for Global Natural Products Social molecular networking. MSCluster was used to cluster the identical MS/MS spectra into a single spectrum. The precursor and fragment ions in the spectra were compared to the spectral libraries with mass tolerance values of ±0.01 Da for the precursor ions and ±0.05 Da for fragment ions. The cosine score was used to compare similarities and differences of spectra with spectral libraries. The cosine score of 0.7 was used as a threshold for spectral match with libraries and the threshold for minimum matching peaks for annotating the spectral peaks was set at 6. The network was imported and visualized using Cytoscape version 3.7.
4. Conclusions
We have demonstrated the use of partial fractionation, HRMS and the computational Elastic Net tool for the discovery of neuroprotective bioactives in an aqueous extract of C. asiatica. The predicted bioactive compounds resulting from the Elastic Net method were also in the top list of candidates resulting from Selective Ratio method underscoring the usefulness of Elastic Net as a machine learning method for bioactive component discovery. Our strategy resulted in the discovery of mono- and dicaffeoylquinic acids. Our com-putational approaches correctly predicted compounds previously recognized for their bi-oactivity using traditional approaches; Dicaffeoylquinic acids have shown cognitive ben-efits in a preclinical in vitro and
in vivo models [
28,
33,
39]. To conclude, we report on an experimental strategy in conjunction with computational methods, which streamlines the discovery and identification of bioactive constituents in botanical extracts and minimizes the need of using time-consuming traditional bioassay-guided fractionation as a primary strategy for the discovery of bioactive compounds in natural product mixtures.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org, Figure S1. Fractionation scheme. 21 subfractions of CAW extract generated by solvent: solvent partitioning and LH-20 column chromatography. We analyzed each subfraction by flow-injection HRMS and correlated the features found with cytoprotective activity in an amyloid β-toxicity MC65 neuroblastoma cell model. In addition, CAW was analyzed by LC-HRMS/MS for obtaining precursor and fragment ion information for GNPS molecular network analysis. Relative polarity across fractions is indicated by “–“ and “+”.
Author Contributions
Conceptualization, J.F.S.; C.S.M.; A.S.; J.Q. and A.A.M.; methodology, J.F.S.; C.S.M.; A.S.; K.S.B. and A.A.M; computation, K.S.B.; Y.J.; and P.C.; formal analysis, M.N.A.; M.C.; N.G.; and A.A.M; investigation, A.A.M; A.V.; M.N.A.; C.S.M.; N.G.; and C.S.M.; resources, J.F.S.; C.S.M.; and A.S.; writing—original draft preparation, A.A.M and A.V.; writing—review and editing, all authors; funding acquisition, J.F.S.; C.S.M.; and A.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by The National Institutes of Health grants R01AT008099, S10RR022589, S10RR027878, and U19AT010829.
Data Availability Statement
Data and software are available from the authors upon request.
Acknowledgments
The authors acknowledge the BENFRA Botanical Dietary and Supplement Research Center (NIH/NCCIH U19AT010829) and the Oregon State University Mass Spectrometry Center.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Pyne, M.E.; Narcross, L.; Martin, V.J.J. Engineering Plant Secondary Metabolism in Microbial Systems. Plant Physiol 2019, 179, 844-861. [CrossRef]
- Mahady, G.B. Global harmonization of herbal health claims. J Nutr 2001, 131, 1120s-1123s. [CrossRef]
- Commisso, M.; Strazzer, P.; Toffali, K.; Stocchero, M.; Guzzo, F. Untargeted metabolomics: an emerging approach to determine the composition of herbal products. Comput Struct Biotec 2013, 4. [CrossRef]
- Weller, M.G. A Unifying Review of Bioassay-Guided Fractionation, Effect-Directed Analysis and Related Techniques. Sensors-Basel 2012, 12, 9181-9209. [CrossRef]
- Nothias, L.F.; Nothias-Esposito, M.; da Silva, R.; Wang, M.; Protsyuk, I.; Zhang, Z.; Sarvepalli, A.; Leyssen, P.; Touboul, D.; Costa, J.; et al. Bioactivity-Based Molecular Networking for the Discovery of Drug Leads in Natural Product Bioassay-Guided Fractionation. Journal of natural products 2018, 81, 758-767. [CrossRef]
- Stagliano, M.C.; DeKeyser, J.G.; Omiecinski, C.J.; Jones, A.D. Bioassay-directed fractionation for discovery of bioactive neutral lipids guided by relative mass defect filtering and multiplexed collision-induced dissociation. Rapid Commun Mass Sp 2010, 24, 3578-3584. [CrossRef]
- Abbas-Mohammadi, M.; Farimani, M.M.; Salehi, P.; Ebrahimi, S.N.; Sonboli, A.; Kelso, C.; Skropeta, D. Acetylcholinesterase-inhibitory activity of Iranian plants: Combined HPLC/bioassay-guided fractionation, molecular networking and docking strategies for the dereplication of active compounds. J Pharmaceut Biomed 2018, 158, 471-479. [CrossRef]
- Shine, V.J.; Anuja, G.I.; Suja, S.R.; Raj, G.; Latha, P.G. Bioassay guided fractionation of Cyclea peltata using in vitro RAW 264.7 cell culture, antioxidant assays and isolation of bioactive compound tetrandrine. Journal of Ayurveda and integrative medicine 2018. [CrossRef]
- Atanasov, A.G.; Waltenberger, B.; Pferschy-Wenzig, E.M.; Linder, T.; Wawrosch, C.; Uhrin, P.; Temml, V.; Wang, L.; Schwaiger, S.; Heiss, E.H.; et al. Discovery and resupply of pharmacologically active plant-derived natural products: A review. Biotechnol Adv 2015, 33, 1582-1614. [CrossRef]
- Sidebottom, A.M.; Johnson, A.R.; Karty, J.A.; Trader, D.J.; Carlson, E.E. Integrated metabolomics approach facilitates discovery of an unpredicted natural product suite from Streptomyces coelicolor M145. ACS Chem Biol 2013, 8, 2009-2016. [CrossRef]
- Yang, J.Y.; Sanchez, L.M.; Rath, C.M.; Liu, X.T.; Boudreau, P.D.; Bruns, N.; Glukhov, E.; Wodtke, A.; de Felicio, R.; Fenner, A.; et al. Molecular Networking as a Dereplication Strategy. Journal of natural products 2013, 76, 1686-1699. [CrossRef]
- Ali, K.; Iqbal, M.; Yuliana, N.D.; Lee, Y.J.; Park, S.; Han, S.; Lee, J.W.; Lee, H.S.; Verpoorte, R.; Choi, Y.H. Identification of bioactive metabolites against adenosine A1 receptor using NMR-based metabolomics. Metabolomics 2013, 9, 778-785. [CrossRef]
- Rajalahti, T.; Arneberg, R.; Berven, F.S.; Myhr, K.M.; Ulvik, R.J.; Kvalheim, O.M. Biomarker discovery in mass spectral profiles by means of selectivity ratio plot. Chemometr Intell Lab 2009, 95, 35-48. [CrossRef]
- Rajalahti, T.; Arneberg, R.; Kroksveen, A.C.; Berle, M.; Myhr, K.-M.; Kvalheim, O.M. Discriminating Variable Test and Selectivity Ratio Plot: Quantitative Tools for Interpretation and Variable (Biomarker) Selection in Complex Spectral or Chromatographic Profiles. Analytical Chemistry 2009, 81, 2581-2590. [CrossRef]
- Kellogg, J.J.; Todd, D.A.; Egan, J.M.; Raja, H.A.; Oberlies, N.H.; Kvalheim, O.M.; Cech, N.B. Biochemometrics for Natural Products Research: Comparison of Data Analysis Approaches and Application to Identification of Bioactive Compounds. Journal of natural products 2016, 79, 376-386. [CrossRef]
- Zou, H.; Hastie, T. Regularization and variable selection via the elastic net (vol B 67, pg 301, 2005). J R Stat Soc B 2005, 67, 768-768. [CrossRef]
- Brown, K.S.; Jamieson, P.; Wu, W.; Vaswani, A.; Alcazar Magana, A.; Choi, J.; Mattio, L.M.; Cheong, P.H.; Nelson, D.; Reardon, P.N.; et al. Computation-Assisted Identification of Bioactive Compounds in Botanical Extracts: A Case Study of Anti-Inflammatory Natural Products from Hops. Antioxidants (Basel) 2022, 11. [CrossRef]
- Gray, N.E.; Sampath, H.; Zweig, J.A.; Quinn, J.F.; Soumyanath, A. Centella asiatica Attenuates Amyloid-beta-Induced Oxidative Stress and Mitochondrial Dysfunction. J Alzheimers Dis 2015, 45, 933-946. [CrossRef]
- Gray, N.E.; Zweig, J.A.; Kawamoto, C.; Quinn, J.F.; Copenhaver, P.F. STX, a Novel Membrane Estrogen Receptor Ligand, Protects Against Amyloid-beta Toxicity. J Alzheimers Dis 2016, 51, 391-403. [CrossRef]
- Brinkhaus, B.; Lindner, M.; Schuppan, D.; Hahn, E.G. Chemical, pharmacological and clinical profile of the East Asian medical plant Centella asiatica. Phytomedicine 2000, 7, 427-448. [CrossRef]
- G.K, S.; Muralidhara; M.S. Bharath, M. Exploring the Role of “Brahmi” (Bacopa monnieri and Centella asiatica) in Brain Function and Therapy. Recent Patents on Endocrine, Metabolic & Immune Drug Discovery 2011, 5, 33-49. [CrossRef]
- Gray, N.E.; Alcazar Magana, A.; Lak, P.; Wright, K.M.; Quinn, J.; Stevens, J.F.; Maier, C.S.; Soumyanath, A. Centella asiatica - Phytochemistry and mechanisms of neuroprotection and cognitive enhancement. Phytochem Rev 2018, 17, 161-194. [CrossRef]
- Kapoor, L.D. CRC handbook of ayurvedic medicinal plants; CRC Press: Boca Raton, Fla., 1990; p. 416 p.
- Kumar, A.; Dogra, S.; Prakash, A. Neuroprotective Effects of Centella asiatica against Intracerebroventricular Colchicine-Induced Cognitive Impairment and Oxidative Stress. Int J Alzheimers Dis 2009, 2009. [CrossRef]
- Soumyanath, A.; Zhong, Y.-P.; Henson, E.; Wadsworth, T.; Bishop, J.; Gold, B.G.; Quinn, J.F. <i>Centella asiatica</i> Extract Improves Behavioral Deficits in a Mouse Model of Alzheimer's Disease: Investigation of a Possible Mechanism of Action. International Journal of Alzheimer’s Disease 2012, 2012, 381974. [CrossRef]
- Kirkpatrick, C.L.; Broberg, C.A.; McCool, E.N.; Lee, W.J.; Chao, A.; McConnell, E.W.; Pritchard, D.A.; Hebert, M.; Fleeman, R.; Adams, J.; et al. The "PepSAVI-MS" Pipeline for Natural Product Bioactive Peptide Discovery. Analytical Chemistry 2017, 89, 1194-1201. [CrossRef]
- Clark, T.N.; Houriet, J.; Vidar, W.S.; Kellogg, J.J.; Todd, D.A.; Cech, N.B.; Linington, R.G. Interlaboratory Comparison of Untargeted Mass Spectrometry Data Uncovers Underlying Causes for Variability. Journal of natural products 2021, 84, 824-835. [CrossRef]
- Gray, N.E.; Morré, J.; Kelley, J.; Maier, C.S.; Stevens, J.F.; Quinn, J.F.; Soumyanath, A. Caffeoylquinic acids in Centella asiatica protect against amyloid-β toxicity. J Alzheimers Dis 2014, 40, 359-373. [CrossRef]
- Rajalahti, T.; Arneberg, R.; Berven, F.S.; Myhr, K.-M.; Ulvik, R.J.; Kvalheim, O.M. Biomarker discovery in mass spectral profiles by means of selectivity ratio plot. Chemometr Intell Lab 2009, 95, 35-48. [CrossRef]
- Liu, R.H. Potential synergy of phytochemicals in cancer prevention: mechanism of action. J Nutr 2004, 134, 3479s-3485s. [CrossRef]
- Caesar, L.K.; Cech, N.B. Synergy and antagonism in natural product extracts: when 1 + 1 does not equal 2. Natural product reports 2019, 36, 869-888. [CrossRef]
- Magana, A.A.; Wright, K.; Vaswani, A.; Caruso, M.; Reed, R.L.; Bailey, C.F.; Nguyen, T.; Gray, N.E.; Soumyanath, A.; Quinn, J.; et al. Integration of mass spectral fingerprinting analysis with precursor ion (MS1) quantification for the characterisation of botanical extracts: application to extracts of Centella asiatica (L.) Urban. Phytochem Analysis 2020, 31, 722-738. [CrossRef]
- Alcázar Magaña, A.; Kamimura, N.; Soumyanath, A.; Stevens, J.F.; Maier, C.S. Caffeoylquinic acids: chemistry, biosynthesis, occurrence, analytical challenges, and bioactivity. The Plant Journal 2021, 107, 1299-1319. [CrossRef]
- Han, J.; Miyamae, Y.; Shigemori, H.; Isoda, H. Neuroprotective effect of 3,5-di-O-caffeoylquinic acid on SH-SY5Y cells and senescence-accelerated-prone mice 8 through the up-regulation of phosphoglycerate kinase-1. Neuroscience 2010, 169, 1039-1045. [CrossRef]
- Gao, H.; Jiang, X.-W.; Yang, Y.; Liu, W.-W.; Xu, Z.-H.; Zhao, Q.-C. Isolation, structure elucidation and neuroprotective effects of caffeoylquinic acid derivatives from the roots of Arctium lappa L. Phytochemistry 2020, 177, 112432. [CrossRef]
- Lee, S.G.; Lee, H.; Nam, T.G.; Eom, S.H.; Heo, H.J.; Lee, C.Y.; Kim, D.O. Neuroprotective effect of caffeoylquinic acids from Artemisia princeps Pampanini against oxidative stress-induced toxicity in PC-12 cells. Journal of food science 2011, 76, C250-256. [CrossRef]
- Liberato, J.L.; Rosa, M.N.; Miranda, M.C.R.; Lopes, J.L.C.; Lopes, N.P.; Gobbo-Neto, L.; Fontana, A.C.K.; Dos Santos, W.F. Neuroprotective Properties of Chlorogenic Acid and 4,5-Caffeoylquinic Acid from Brazilian arnica (Lychnophora ericoides) after Acute Retinal Ischemia. Planta medica 2023, 89, 183-193. [CrossRef]
- Fernandes, M.Y.D.; Dobrachinski, F.; Silva, H.B.; Lopes, J.P.; Gonçalves, F.Q.; Soares, F.A.A.; Porciúncula, L.O.; Andrade, G.M.; Cunha, R.A.; Tomé, A.R. Neuromodulation and neuroprotective effects of chlorogenic acids in excitatory synapses of mouse hippocampal slices. Scientific Reports 2021, 11, 10488. [CrossRef]
- Matthews, D.G.; Caruso, M.; Alcazar Magana, A.; Wright, K.M.; Maier, C.S.; Stevens, J.F.; Gray, N.E.; Quinn, J.F.; Soumyanath, A. Caffeoylquinic Acids in Centella asiatica Reverse Cognitive Deficits in Male 5XFAD Alzheimer's Disease Model Mice. Nutrients 2020, 12. [CrossRef]
- Chiang, M.-C.; Tsai, T.-Y.; Wang, C.-J. The Potential Benefits of Quercetin for Brain Health: A Review of Anti-Inflammatory and Neuroprotective Mechanisms. International Journal of Molecular Sciences 2023, 24, 6328.
- Khan, H.; Ullah, H.; Aschner, M.; Cheang, W.S.; Akkol, E.K. Neuroprotective Effects of Quercetin in Alzheimer's Disease. Biomolecules 2019, 10. [CrossRef]
- Ma, Z.-X.; Zhang, R.-Y.; Rui, W.-J.; Wang, Z.-Q.; Feng, X. Quercetin alleviates chronic unpredictable mild stress-induced depressive-like behaviors by promoting adult hippocampal neurogenesis via FoxG1/CREB/ BDNF signaling pathway. Behavioural Brain Research 2021, 406, 113245. [CrossRef]
- Chiang, M.C.; Tsai, T.Y.; Wang, C.J. The Potential Benefits of Quercetin for Brain Health: A Review of Anti-Inflammatory and Neuroprotective Mechanisms. Int J Mol Sci 2023, 24. [CrossRef]
- Wang, M.; Carver, J.J.; Phelan, V.V.; Sanchez, L.M.; Garg, N.; Peng, Y.; Nguyen, D.D.; Watrous, J.; Kapono, C.A.; Luzzatto-Knaan, T.; et al. Sharing and community curation of mass spectrometry data with Global Natural Products Social Molecular Networking. Nature biotechnology 2016, 34, 828-837. [CrossRef]
- Gray, N.E.; Morre, J.; Kelley, J.; Maier, C.S.; Stevens, J.F.; Quinn, J.F.; Soumyanath, A. Caffeoylquinic acids in Centella asiatica protect against amyloid-beta toxicity. J Alzheimers Dis 2014, 40, 359-373. [CrossRef]
- Sopher, B.L.; Fukuchi, K.; Kavanagh, T.J.; Furlong, C.E.; Martin, G.M. Neurodegenerative mechanisms in Alzheimer disease - A role for oxidative damage in amyloid beta protein precursor-mediated cell death. Mol Chem Neuropathol 1996, 29, 153-168. [CrossRef]
- Woltjer, R.L.; McMahan, W.; Milatovic, D.; Kjerulf, J.D.; Shie, F.S.; Rung, L.G.; Montine, K.S.; Montine, T.J. Effects of chemical chaperones on oxidative stress and detergent-insoluble species formation following conditional expression of amyloid precursor protein carboxy-terminal fragment. Neurobiol Dis 2007, 25, 427-437. [CrossRef]
- Woltjer, R.L.; Maezawa, I.; Ou, J.J.; Montine, K.S.; Montine, T.J. Advanced glycation endproduct precursor alters intracellular amyloid- beta/A beta PP carboxy-terminal fragment aggregation and cytotoxicity. J Alzheimers Dis 2003, 5, 467-476. [CrossRef]
- Kucheryayskiy, S. mdatools - R package for chemometrics. Chemometr Intell Lab 2020, 198. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).