Submitted:
02 January 2024
Posted:
03 January 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Shortbreads ingredients
2.1.2. Reagents
2.2. Methods
2.2.1. Experimental design
2.2.2. Shortbread preparation on the laboratory scale
2.2.3. Analytical determination
2.2.3.1. Moisture, pH, total nitrogen, protein, and ash contents were determined according to their respective Association of Official Analytical Chemists methods [13]. Protein content was calculated using the nitrogen conversion factors: 5.7 (for flour samples) and 6.25 (for shortbread samples) [11].
2.2.3.2. The aw was determined using an Aqualab® series 3 (Decagon, Pullman, Wash., U.S.A.) calibrated with lithium chloride solution (aw = 0.250 ± 0.003).
2.2.3.3. TDF was determined via a Megazyme assay kit (Megazyme International Ireland) following the AOAC method 985.29 [14].
2.2.3.4. Carbohydrates were calculated as the percentage difference with moisture, proteins, ashes, fats, And TDF [15].
2.2.3.5. An HPLC-ELSD system was used for the determination of free sugar content as reported by Sileoni et al. [11].
2.2.3.6. According to the conversion factors approved by the Food and Agriculture Organization of the United Nations (FAO) (4.0 kcal/g for protein, 9.0 kcal/g for fat, and 4.0 kcal/g for carbohydrates) [16], the shortbreads composition was used for the calculation of the energy values.
2.2.3.7. The total polyphenols content and antioxidant capacity were determined on flour and shortbreads’ extracts obtained by homogenizing 1 g of sample in 5 mL solution of methanol: water: hydrochloric acid (70:28:2; v/v/v) using an Ultra-Turrax homogenizer T25 (Ika Works Inc., USA) until uniform consistency (2 min). The homogenates were centrifuged at 3000 rpm for 10 min and the supernatants were recovered. The extraction was repeated two times and the extracts were collected into a 10 mL volumetric flask and taken to the final volume. The total polyphenols content was determined by using the Folin–Ciocalteau method [15], 2 mL of Folin-Ciocalteu reagent and 1.6 mL of Na2CO3 were added to 0.4 mL of sample. The obtained solution was incubated at room temperature, in the dark, for 120 min. The absorbance of the mixture was measured at 760 nm. The results were expressed as mg of GA equivalent (GAE) per g of sample dry matter (mg GAE g-1 dm) [15].
2.2.3.8. Free AAs determination was achieved by HPLC-FLD, 10 ml of 5% trichloroacetic acid was added to 1 g of flour or ground shortbread and extracted for 30 min under magnetic stirring. The sample, diluted ten times was filtered with a syringe filter (0.45 μm), derivatized with O-phtaldialdehyde (5 g/L in a ratio of 1 to 2 with the sample), and injected into an HPLC system consisting of a Kinetex EVO C18 column (5μ, 150x4.6 mm). The separation was carried out at 30 °C with a flow rate of 1 mL/min. Mobile phase A was potassium hydrogen phosphate (0.05 M, pH 7.5) and mobile phase B was methanol. The chromatographic separation was achieved in 45 min using the following elution gradient: mobile phase A 81% (0 min), 78% (6 min), 67% (7 min), 56% (30 min), 45% (32 min), 35% (40 min), 81% (42), 81% (45 min). The detector was an Agilent 1200 fluorescence detector (FLD), with excitation/emission wavelengths at 338/420 nm. The external standard method was used for the calibration and the calibration plots were constructed for standard compounds with a linearity between 0.1 and 1.7 µg ml-1. The AAs detected were aspartic acid, glutamic acid, asparagine, serine, glutamine, histidine, arginine, glycine, threonine, alanine, tyrosine, methionine, tryptophan, valine, phenylalanine, isoleucine, leucine, and lysine.
2.2.3.9. The fat extract was used for the determination of FA profiles by gas chromatography. The lipid extracts were trans-esterified by treatment with methanol/KOH solution, and the resulting fatty acid methyl esters were injected into the HRGC-FID system. The FAs were identified by comparing their retention times with those of commercial standards [17].
2.2.3.10. Shortbreads texture
2.2.3.11. According to the AACC, method 10–50.05 [18], the spread factor was calculated as the ratio between the diameter and thickness of baked biscuits. Ten shortbreads were analyzed for each parameter. A caliper determined the width (W) and thickness (T) after baking and the spread factor was calculated. The weight loss of shortbreads was calculated by the difference between the initial uncooked shortbreads’ weight and that of the same after the baking process. One hundred samples per shortbread group were used for the weight loss calculation [11].
2.2.4. Sensory analysis
2.2.4.1. Sensory discrimination and preference test
2.2.4.2. Quantitative descriptive analysis
2.2.5. Statistical analysis
3. Results and discussions
3.1. Chemical quality parameters of flour
3.2. Control and experimental shortbreads texture
3.2.1. Sensory discrimination and preference.
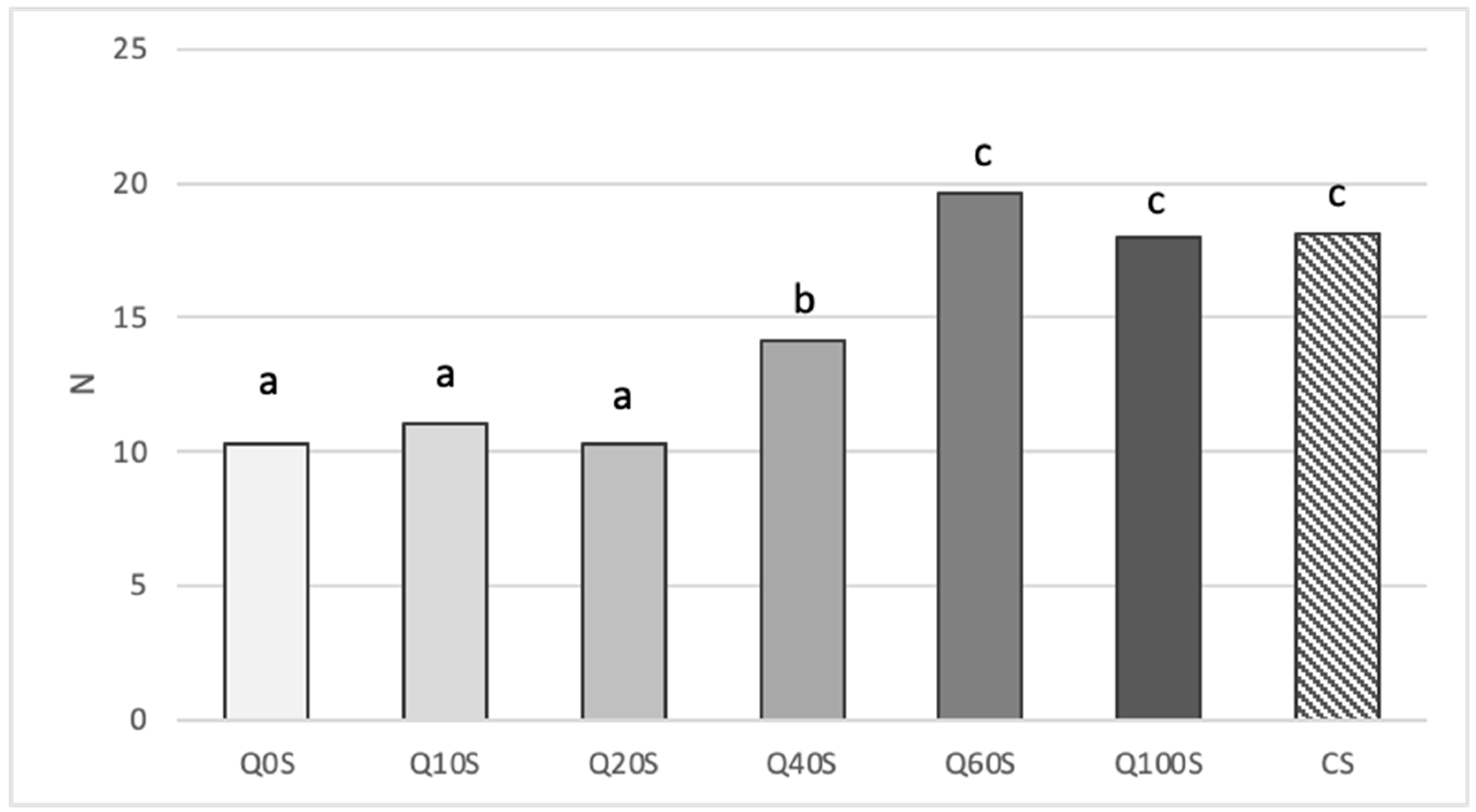
3.2.2. Quality parameters of shortbreads.
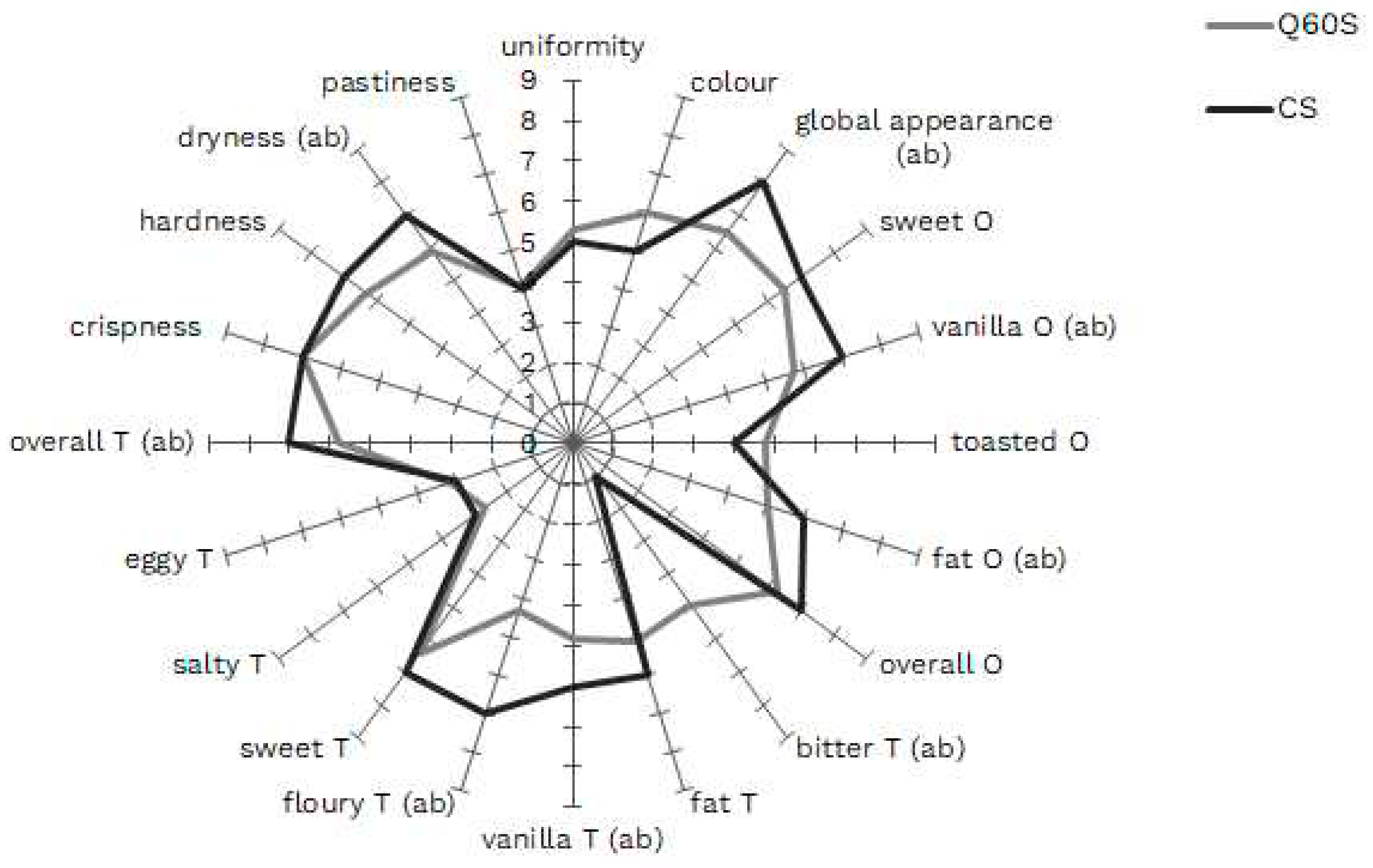
3.3. Sensory analysis of shortbreads
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Malik, R.A.; Srivastava, S.; Shahi, N.C. Formulation of quinoa incorporated protein-rich biscuits and numerical optimization of its process parameters. J Food Process Preserv 2022, 46, e16209. [Google Scholar] [CrossRef]
- Gordillo-Bastidas, E.; Díaz-Rizzolo, D.A.; Roura, E.; Massanés, T.; Gomis, R. Quinoa (Chenopodium quinoa Willd) from Nutritional Value to Potential Health Benefits: An Integrative Review. J Nutr Food Sci 2016, 6(3), 1000497. [Google Scholar]
- Angeli, V.; Silva, P.M.; Crispim Massuela, D.; Khan, M.W.; Hamar, A.; Khajehei, F.; Graeff-Hönninger, S.; Piatti, C. Quinoa (Chenopodium quinoa Willd.): An overview of the potentials of the “Golden Grain” and socio-economic and environmental aspects of its cultivation and marketization. Foods 2020, 9(2), 216. [Google Scholar]
- Bazile, D.; Pulvento, C.; Verniau, A.; Al-Nusairi, M.S.; Ba, D.; Breidy, J.; Hassan, L.; Mohammed, M.I.; Mambetov, O.; Otambekova, M.; Sepahvand, N.A.; Shams, A.; Souici, D.; Miri, K.; Padulosi, S. Worldwide Evaluations of Quinoa: Preliminary Results from Post International Year of Quinoa FAO Projects in Nine Countries. Front Plant Sci 2016, 7, 850. [Google Scholar]
- Lazíková, J.; Takáč, I.; Schneir, E.R.; Rumanovská, L. Legal Aspects of the Quinoa Imports Into the EU. EU agrarian Law 2022, 11(1), 13–21. [Google Scholar] [CrossRef]
- King, J.A.; Jeong, J.; Underwood, F.E.; Quan, J.; Panaccione, N.; Windsor, J.W.; Coward, S.; deBruyn, J.; Ronksley, P.E.; Shaheen, A.A.; Quan, H.; Godley, J.; Veldhuyzen van Zanten, S.; Lebwohl, B.; Ng, S.C.; Ludvigsson, J.F.; Kaplan, G.G. Incidence of Celiac Disease Is Increasing Over Time: A Systematic Review and Meta-analysis. Am J Gastroenterol 2020, 115(4), 507–525. [Google Scholar] [CrossRef]
- Savarese, M.; Wismer, W.; Graffign, G. Conceptualizing “free-from” food consumption determinants: A systematic integrative literature review focused on gluten and lactose. Food Qual Prefer 2021, 90, 104170. [Google Scholar] [CrossRef]
- Ahmad, S.; Ahmed, M. A review on biscuit, a largest consumed processed product in India, its fortification and nutritional improvement. IJSIT 2014, 3(2), 169–186. [Google Scholar]
- Agrahar-Murugkar, D.; Gulati, P.; Kotwaliwale, N.; Gupta, C. Evaluation of nutritional, textural and particle size characteristics of dough and biscuits made from composite flours containing sprouted and malted ingredients. J Food Sci Technol 2015, 52(8), 5129–5137. [Google Scholar] [CrossRef] [PubMed]
- Cayres, C.A.; Ascheri, J.L.R.; Peixoto-Gimenes Couto, M.A. Evaluation of nutritional characteristics and consumers’ acceptance of gluten-free sweet biscuits made from rice-based pregelatinized composite flours containing orange pomace and soy protein isolate. SN Appl Sci 2021, 3, 183. [Google Scholar] [CrossRef]
- Sileoni, V.; Alfeo, V.; Bravi, E.; Belardi, I.; Marconi, O. Upcycling of a by-product of the brewing production chain as an ingredient in the formulation of functional shortbreads. J. Funct. Foods 2022, 98, 105292. [Google Scholar] [CrossRef]
- Marconi, O.; Martini, R.; Mangione, A.; Falconi, C.; Pepe, C.; Perretti, G. Palatability and stability of shortbread made with low saturated fat content. J Food Sci 2014, 79(4), 469–475. [Google Scholar] [CrossRef]
- AOAC, Official Methods of Analysis. Association of Official Analytical Chemists. 14th ed., Arlington, VA, USA, 1984.
- AOAC, Official Methods of Analysis. Association of Official Analytical Chemists, 14th ed., Washington, WA, USA, 1986.
- Bravi, E.; Francesco, G.D.; Sileoni, V.; Perretti, G.; Galgano, F.; Marconi, O. Brewing by-product upcycling potential: Nutritionally valuable compounds and antioxidant activity evaluation. Antioxidants 2021, 10, 165. [Google Scholar] [CrossRef] [PubMed]
- Food and Agriculture Organization of the United Nations (FAO), Food energy - methods of analysis and conversion factors, Food and Nutrition paper 77, Rome, 2003.
- Bravi, E.; Perretti, G.; Buzzini, P.; Della Sera, R.; Fantozzi, P. Technological Steps and Yeast Biomass as Factors Affecting the Lipid Content of Beer during the Brewing Process. J Agric Food Chem 2009, 57, 6279–6284. [Google Scholar] [CrossRef] [PubMed]
- ACC, Approved Methods of the American Association of Cereal Chemists, 11th ed., AACC International, St. Paul, MN, U.S.A, 2000.
- Moawad, E.M.M.; Rizk, I.R.S.; Kishk, Y.F.M.; Youssif, M.R.G. Effect of substitution of wheat flour with quinoa flour on quality of pan bread and biscuit. Arab Univ J Agric Sci 2019, 26(7D), 2387–2400. [Google Scholar] [CrossRef]
- Jan, N.; Naik, H.R.; Gani, G.; Bashir, O.; Amin, T.; Wani, S.M.; Soi, S.A. Influence of replacement of wheat flour by rice flour on rheo-structural changes, in vitro starch digestibility and consumer acceptability of low-gluten pretzels. Food Prod Process Nutr 2022, 4, 9. [Google Scholar] [CrossRef]
- Kowalska, S.; Szłyk, E.; Jastrzębsk, A. Simple extraction procedure for free amino acids determination in selected gluten-free flour samples. Eur Food Res Technol 2022, 248, 507–517. [Google Scholar] [CrossRef]
- Bravi, E.; Sileoni, V.; Perretti, G.; Marconi, O. Accelerated shelf-life model of gluten-free rusks by using oxidation indices. Food Chem 2020, 326, 126971. [Google Scholar] [CrossRef] [PubMed]
- Regulation European Commission No 1924, of the European Parliament and the Council of 20 December 2006 on nutrition and health claims made on foods.
- Arslan, M.; Rakha, A.; Xiaobo, Z.; Arsalan Mahmood, M. Complimenting Gluten Free Bakery Products with Dietary Fiber: Opportunities and Constraints. Trends Food Sci Technol 2018, 83, 194–202. [Google Scholar] [CrossRef]
- Gasparre, N.; Pasqualone, A.; Mefleh, M.; Boukid, F. Nutritional Quality of Gluten-Free Bakery Products Labeled Ketogenic and/or Low-Carb Sold in the Global Market. Foods 2022, 11, 4095. [Google Scholar] [CrossRef]
- Beniwal, S.K.; Devi, A.; Sindhu, R. Effect of grain processing on nutritional and physicochemical, functional and pasting properties of amaranth and quinoa flours. IJTK 2019, 18(3), 500–507. [Google Scholar]
- Suárez-Estrella, D.; Marti, A.; Torri, L.; Pagani, M.A. Quinoa bitterness: causes and solutions for improving product acceptability. J Sci Food Agric 2018, 98, 4033–4041. [Google Scholar] [CrossRef]
- Melini, F.; Melini, V.; Galfo, M. A Cross-Sectional Survey of the Nutritional Quality of Quinoa Food Products Available in the Italian Market. Foods 2023, 12(8), 1562. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, K.B.; Biondi, S.; Oses, R.; Acuna-Rodriguez, I.S.; Antognoni, F.; Martinez-Mosqueira, E.A.; Coulibaly, A.; Canahua-Murillo, A.; Pinto, M.; Zurita-Silva, A.; Bazile, D.; Jacobsen, S.E.; Molina-Montenegro, M.A. Quinoa biodiversity and sustainability for food security under climate change. A review. Agron Sustain Dev 2014, 34, 349–359. [Google Scholar] [CrossRef]
| Ingredients (g) | CS | Q0S | Q10S | Q20S | Q40S | Q60S | Q100S |
| Wheat flour | 325 | - | - | 180 | - | - | - |
| Quinoa flour | - | - | 34,5 | 1,25 | 138 | 207 | 345 |
| Rice flour | - | 345 | 310,5 | 69 | 207 | 138 | - |
| Sucrose | 180 | 180 | 180 | 276 | 180 | 180 | 180 |
| NaCl | 1.25 | 1,25 | 1,25 | 180 | 1,25 | 1,25 | 1,25 |
| (NH4)HCO3 | - | 1 | 1 | 1 | 1 | 1 | 1 |
| NaHCO3 | 1 | 1,50 | 1,50 | 1,50 | 1,50 | 1,50 | 1,50 |
| Butter | 1.50 | 100 | 100 | 100 | 100 | 100 | 100 |
| Yolk | 100 | 24 | 24 | 24 | 24 | 24 | 24 |
| Egg white | 24 | 37 | 37 | 37 | 37 | 37 | 37 |
| Water | 37 | 30 | 30 | 30 | 30 | 30 | 30 |
| Quality parameter | WF | QF | RF |
|---|---|---|---|
| Moisture % | 10.60±0.25a | 9.22±0.11b | 2.59 ± 0.02c |
| Carbohydrates (% dm) | 87.26±0.38a | 68.9 ± 1.3b | 87.5 ± 0.5a |
| Sugars (% dm) | 1.00±0.01b | 3.5 ± 0.3a | nd |
| Ash (% dm) | 0.57±0.01b | 2.69 ± 0.01a | 0.40 ± 0.01c |
| Proteins (% dm) | 9.41±0.08b | 13.8 ± 0.1a | 8.1 ± 0.1c |
| Fat (% dm) | 1.01±0.01b | 7.0 ± 0.1a | 0.4 ± 0.1c |
| TDF (% dm) | 1.76±0.33c | 7.7 ± 1.4a | 3.6 ± 0.5b |
| aw | 0.57±0.01a | 0.44 ± 0.01a | 0.50 ± 0.01a |
| Total-Polyphenols (GA mg/g) | 0.16 ± 0.01c | 1.64 ± 0.08a | 0.76 ± 0.04b |
| ABTS (TE/g) | 0.15 ± 0.03c | 0.69 ± 0.08a | 0.29 ± 0.01b |
| DPPH (TE/g) | 10.68 ± 1.00c | 75.77 ± 0.80a | 70.10 ± 0.91b |
| FRAP (TE/g) | 0.98 ± 0.03c | 3.58 ± 0.05a | 1.12 ± 0.04b |
| AAs (mg/Kg dm) |
WF | QF | RF |
|---|---|---|---|
| Aspartic Acid | 105.8 ± 7.9b | 228.7 ± 15.9a | 17.6 ± 0.2c |
| Glutammic Acid | 60.9 ± 0.9b | 692.7 ± 0.7a | 43.6 ± 1.2c |
| Asparagine | 87.2 ± 0.3a | 50.0 ± 3.2b | 52.8 ± 1.1b |
| Serine | 11.8 ± 3.0b | 64.9 ± 11.4a | 8.7 ± 0.2b |
| Glutamine | 44.5 ± 0.1b | 163.1 ± 7.1a | 6.9 ± 0.4c |
| Histidine | 8.3 ± 0.6b | 254.1 ± 0.8a | 4.5 ± 0.1c |
| Arginine | 25.4 ± 0.2b | 736.1 ± 0.5a | 6.3 ± 0.5c |
| Glycine | 13.1 ± 0.9b | 69.4 ± 8.2a | 13.9 ± 0.2b |
| Alanine | 29.0 ± 1.1b | 146.4 ± 5.9a | 26.2 ± 1.5b |
| Tyrosine | 12.3 ± 0.5b | 56.1 ± 3.7a | 4.1 ± 0.4b |
| Threonine | 8.4 ± 0.7b | 37.6 ± 4.6a | 4.8 ± 0.1c |
| Methionine | 4.8 ± 0.5b | 27.6 ± 0.1a | 3.3 ± 0.2c |
| Tryptophan | 63.8 ± 0.7b | 74.4 ± 0.3a | 2.8 ± 0.1c |
| Valine | 13.8 ± 1.2b | 85.5 ± 0.3a | 6.7 ± 0.1c |
| Phenylalanine | 11.3 ± 0.5b | 45.2 ± 4.0a | 2.9 ± 0.2c |
| Isoleucine | 6.3 ± 0.3b | 28.8 ± 2.0a | 1.4 ± 0.1c |
| Leucine | 10.3 ± 0.3b | 32.6 ± 2.4a | 2.6 ± 0.2c |
| Lysine | 10.6 ± 1.0b | 42.9 ± 4.4a | 4.1 ± 0.3c |
| Ʃ AAs | 527.6 ± 16.3b | 2836.1 ± 40.7a | 213.1 ± 2.9c |
| Ʃ sweetness AAs | 62.2 ± 5.7b | 318.3 ± 30.1a | 53.6 ± 2.0c |
| Ʃ bitterness AAs | 103.2 ± 5.1b | 1308.9 ± 18.2a | 35.8 ± 2.1c |
| Ʃ neutral AAs | 365.2 ± 9.9b | 1208.9 ± 27.2a | 123.8 ± 3.9c |
| Fatty acids (% dm) |
WF | QF | RF |
|---|---|---|---|
| Butyric C4:0 | 0.21 ± 0.02a | nd | nd |
| Myristic C14:0 | 0.13 ± 0.01b | 0.12 ± 0.01b | 0.58 ± 0.01a |
| Palmitic C16:0 | 17.14 ± 0.01a | 8.72 ± 0.02b | 16.72 ± 0.01a |
| Palmitoleic C16:1 ω7 | 0.14 ± 0.01c | 0.05 ± 0.01a | 0.22± 0.01b |
| Stearic C18:0 | 1.17 ± 0.01b | 0.79 ± 0.01a | 2.25± 0.01b |
| Oleic C18:1 ω9 | 15.83 ± 0.01c | 29.75 ± 0.02b | 41.16 ± 0.01a |
| Linoleic C18:2 ω6 | 60.72 ± 0.08a | 47.65 ± 0.03b | 34.88 ± 0.01c |
| γ-linolenic C18:3 ω6 | 3.30 ± 0.02b | 7.57 ± 0.01b | 1.29 ± 0.01c |
| Eicosenoic C20:1 ω9 | 0.19 ± 0.01c | 0.59 ± 0.01b | 0.89 ± 0.02a |
| α-linolenic C18:3 ω3 | 0.74 ± 0.01b | 1.80 ± 0.01a | 0.70 ± 0.01b |
| Arachidonic C20:4 ω6 | 0.21 ± 0.01c | 0.78 ± 0.01a | 0.42 ± 0.01b |
| Trycosilic C23:0 | nd | 1.58 ± 0.05a | nd |
| Eicosapentaenoic C20:5 ω3 | 0.22 ± 0.01b | 0.30 ± 0.01b | 0.89 ± 0.01a |
| Nervonic C24:1 ω9 | nd | 0.30 ± 0.01a | nd |
| Ʃ Saturated | 18.65 ± 0.05a | 11,21 ± 0,09b | 19,55 ± 0,08a |
| Ʃ Unsaturated | 81.35 ± 0.16b | 88,79± 0,12a | 80,45 ± 0,01b |
| Ʃ Monounsaturated | 16.16 ± 0.03c | 30,69 ± 0,05b | 42,27 ± 0,03a |
| Ʃ Polyunsaturated | 65.19 ± 0.13a | 58,10 ± 0,07b | 38,18 ± 0,05c |
| Quality parameter | CS | Q60S | |
|---|---|---|---|
| Energy value | (kcal/100g) | 466 | 446 |
| (kJ/100g) | 1975 | 1866 | |
| Moisture (% dm) | 1.56 ± 0.02b | 2.51 ± 0.09a | |
| Carbohydrates (% dm) | 73.28 ± 0.13a | 67.3 ± 1.3b | |
| Sugars (% dm) | 24.77 ± 0.22a | 25.1 ± 0.1a | |
| Ash (% dm) | 1.01 ± 0.05a | 1.09 ± 0.03a | |
| Proteins (% dm) | 7.13 ± 0.19a | 7.6 ± 0.1a | |
| Fat (% dm) | 15.99 ± 0.17a | 16.3 ± 0.6a | |
| TDF (% dm) | 1.03 ± 0.02b | 5.2 ± 0.5a | |
| aw | 0.28 ±0.01a | 0.36 ± 0.05a | |
| Spread factor | 48.89 ±0.82b | 62.50 ± 1.08a | |
| Weight loss | 1.67 ± 0.03a | 1.33 ± 0.03b | |
| Weight loss (%) | 16.06 ± 0,23a | 13.4 ± 0.2b | |
| T-Polyphenols (GA mg/g) | 1.25 ± 0.01b | 5.73 ± 0.01a | |
| ABTS (TE/g) | 0.10 ± 0.01b | 0.37 ± 0.06a | |
| DPPH (TE/g) | 25.07 ± 0.12b | 70.03 ± 0.24a | |
| FRAP (TE/g) | 2.77 ± 0.03b | 8.37 ± 0.09 | |
|
AAs (mg/Kg dm) |
CS | Q60S |
| Aspartic Acid | 44.8 ± 0.1bO | 82.3 ± 6.0aL |
| Glutammic Acid | 32.5 ± 0.6bM | 239.0 ± 11.9aN |
| Asparagine | 40.0 ± 0.3aN | 31.7 ± 1.7bEFG |
| Serine | 9.6 ± 0.5bF | 30.0 ± 3.4aDEF |
| Glutamine | 22.1 ± 0.2bH | 40.2 ± 1.8aG |
| Histidine | 5.1 ± 0.3bC | 72.0 ± 3.4aI |
| Arginine | 26.9 ± 0.1bI | 217.8 ± 12.2aM |
| Glycine | 7.3 ± 0.1bD | 29.7 ± 0.9aDEF |
| Alanine | 28.0 ± 0.5bL | 59.6 ± 3.9aH |
| Tyrosine | 9.1 ± 0.5bF | 28.6 ± 1.8aCDEF |
| Threonine | 5.3 ± 0.2bC | 23.7 ± 2.6aBCDE |
| Methionine | 2.7 ± 0.1bA | 10.5 ± 0.1aA |
| Tryptophan | 21.6 ± 0.2aH | 21.0 ± 0.9aBCD |
| Valine | 13.7 ± 0.3bG | 35.4 ± 1.9aFG |
| Phenylalanine | 8.2 ± 0.3bE | 19.0 ± 0.6aABC |
| Isoleucine | 7.1 ± 0.2bD | 14.5 ± 0.7aAB |
| Leucine | 9.7 ± 0.3bF | 21.9 ± 1.3aBCD |
| Lysine | 4.0 ± 0.2bB | 23.4 ± 0.7aBCDE |
| Ʃ AAs | 297.7 ± 5.0b | 1000.3 ± 55.8a |
| Ʃ sweetness AAs | 50.2 ± 1.3bA | 143.0 ± 10.8aA |
| Ʃ bitterness AAs | 86.5 ± 2.3bB | 443.1 ± 22.7aB |
| Ʃ neutral AAs | 161.0 ± 1.4bC | 414.2 ± 22.3aB |
|
Fatty acids (% dm) |
CS | Q60S |
| Butyric C4:0 | 2.13 ± 0.03aI | 2.01 ± 0.05aH |
| Caproic C6:0 | 1.66 ± 0.01aG | 1.64 ± 0.01aG |
| Caprylic C8:0 | 1.13 ± 0.01aF | 1.10 ± 0.01aF |
| Capric C10:0 | 2.69 ± 0.01aL | 2.62 ± 0.01aI |
| Lauric C12:0 | 3.27 ± 0.01aM | 3.17 ± 0.02bL |
| Myristic C14:0 | 12.01 ± 0.04aP | 10.12 ± 0.08bO |
| Myristoleic C14:1 ω9 | 0.85 ± 0.01bE | 0.94 ± 0.01aE |
| Pentadecanoic C15:0 | 0.08 ± 0.01bA | 1.15 ± 0.01aF |
| Palmitic C16:0 | 31.08 ± 0.02aR | 29.51 ± 0.01bQ |
| Palmitoleic C16:1 ω7 | 1.79 ± 0.01aH | 1.57 ± 0.02bG |
| Heptadecanoic C17:0 | 0.1 ± 0.01bA | 0.58 ± 0.01aC |
| Heptadecenoic C17:1 | 0.1 ± 0.01bA | 0.24 ± 0.01aB |
| Stearic C18:0 | 10.37 ± 0.03aO | 9.16 ± 0.04bN |
| Oleic C18:1 ω9 | 25.55 ± 0.04aQ | 25.15 ± 0.13aP |
| Linoleic C18:2 ω6 | 5.14 ± 0.03bN | 8.36 ± 0.02aM |
| γ-linolenic C18:3 ω6 | 0.1 ± 0.01bA | 1.17 ± 0.01aF |
| Eicosenoic C20:1 ω9 | 0.65 ± 0.01aC | 0.12 ± 0.01bA |
| α-linolenic C18:3 ω3 | 0.77 ± 0.01bD | 0.85 ± 0.01aD |
| Behenic C22:0 | 0.10 ± 0.01aA | 0.11 ± 0.01aA |
| Eicosatrienoic C20:3 ω6 | nd | 0.11 ± 0.01A |
| Arachidonic C20:4 ω6 | 0.2 ± 0.01aB | 0.13 ± 0.01bA |
| Trycosilic C23:0 | 0.23 ± 0.01aB | 0.22 ± 0.04aB |
| Ʃ Saturated | 64.95 ± 0,20aA | 61.36 ± 0,30bD |
| Ʃ Unsaturated | 35.15± 0,14bB | 38.64 ± 0,23aC |
| Ʃ Monounsaturated | 28.94 ± 0,08aC | 28.02 ± 0,17bB |
| Ʃ Polyunsaturated | 6.21 ± 0.06bD | 10.62 ± 0.06aA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





