1. Introduction
Nanotechnology is like the wizardry of the scientific world, dealing with the manipulation and utilization of matter at the nanoscale—generally 1 to 100 nanometers (nm) [
1]. This realm is so tiny that it deals with building blocks on an atomic level [
2]. The similarity in size between nanoparticles and biological molecules, such as proteins and DNA, as well as bacteria and viruses, has sparked considerable interest in utilizing nanoparticles (NPs) for diverse biomedical purposes [
3]. These properties make nanoparticles versatile for a wide range of biomedical applications. The essence of nanotechnology lies in the size and shape of these particles, crucial factors that significantly influence their properties [
4,
5]. In recent decades, nanotechnology has emerged as a highly promising field, focusing on nanoparticles that exhibit distinct optical, electronic, chemical, photoelectrochemical, or magnetic properties [
2,
6].
NPs can be broadly classified by their composition and category. They are single component materials, composites, or core-shell forms, and can be classified into three general categories: polymeric, inorganic, and lipid-based [
7]. Examples include liposomes, lipid nanoparticles, polymeric nanoparticles, dendrimers, metallic, quantum dots, carbon nanotubes, and magnetic nanoparticles [
7]. Metallic inorganic nanomaterials include gold, silver, palladium, iron, copper, and platinum. Gold nanoparticles (GNPs) are widely used in the biomedical field as therapeutic and imaging agents [
8]. The unique optical and thermal properties of gold nanoparticles, coupled with their biocompatibility and ease of functionalization, make them valuable tools in biomedical research and clinical applications, ranging from advanced imaging techniques to targeted therapeutic interventions [
9,
10].
Nanoparticles can be designed and synthesized to be biocompatible by optimizing their properties such as size, shape, surface charge, and composition. Moreover, the body’s defense mechanisms might see these particles as invaders, triggering immune responses or causing inflammation [
11]. Concerted efforts are being made to fabricate nanoparticles to improve their targeting and biocompatibility, allowing them to break down safely after efficiently delivering the payload to the target site [
12]. The key factor is to engineer the nanoparticles with disease targeted ligands such as antibodies, peptides and small molecules for specific drug delivery and diagnostics [
12,
13].
Nanomedicine has experienced rapid growth in the treatment of various diseases such as brain cancer, lung cancer, breast cancer, cardiovascular, infection, and other diseases [
14]. These innovative treatments aim to enhance drug bioavailability and absorption time, minimize release time, prevent drug aggregation, and improve drug solubility in the bloodstream [
15]. The field of nanomedicine has ushered in a new era of drug delivery by optimizing the therapeutic pathways of active pharmaceutical ingredients encapsulated within nanoparticles. Among the diseases and disorders for which nanomedicine is being investigated, cancer encompasses a diverse group characterized by the uncontrolled growth and spread of abnormal cells [
16]. It is pervasive and can affect nearly any tissue or organ in the body [
17]. The mortality rate of the glioma Glioblastoma multiforme (GBM) is notably high, and its aggressive nature distinguishes it from many other cancers such as lung, liver, breast, and prostate. The challenges posed by GBM underscore the critical need for continued research and innovative therapeutic strategies to enhance the prognosis and quality of life for individuals affected by this formidable malignancy [
17,
18].
The therapeutic options for glioblastoma are constrained relative to the challenges posed by the disease. The standard of care, which includes radiation therapy, is associated with side effects. Therefore, the need for more effective and less toxic treatments remains a pressing concern in the scientific and clinical communities. Current and continuous research endeavors hold the promise of uncovering novel strategies to address the complexities of GBM treatment [
19,
20,
21,
22,
23,
24,
25]. Researchers are actively engaged in exploring novel strategies that could provide more effective and advanced solutions for managing the complex and aggressive GBM tumor, such as boron neutron capture therapy (BNCT). This active exploration of innovative methods, including emerging therapies, targeted interventions, and advancements in medical science, has the goal of improving the outcomes and quality of life for those diagnosed with GBM.
The present review article focuses on a detailed discussion of GNPs, their physicochemical properties, and their applications in the treatment of diseases like cancer with a focus on gliomas including GBM. Furthermore, we propose an alternative treatment approach for GBM using targeted GNP-based therapy.
2. Gold Nanoparticles
Nanoscale materials have been used since ancient times. Nanoparticles, in particular GNPs, and their medicinal applications are very attractive because of their desirable and manipulable physicochemical properties including composition, size, shape, and surface chemistry. Here we give a brief overview and historical record of gold nanoparticles and their biomedical applications.
2.1. Overview and History of Nanoparticles and Gold Nanoparticles
The inorganic metal, gold, has long been of interest historically, both for its beauty/longevity and its utility in pre-industrialization medicine [
26,
27]. Bulk gold is inert, extremely oxidation-resistant, and biocompatible, and thus very much favorable as a nanoparticle constituent [
28]. In contrast to bulk or molecular gold, nanoscale gold, when suspended in a solvent such that sub-micron gold particles are distributed throughout it, usually in water, is called colloidal gold. Indeed, preserved documents on the Chinese “golden solution” and Indian “liquid gold” medicines are among the earliest reports on such GNPs [
29,
30]. However, GNPs, as we know them today, would not have broken out in popularity were it not for the respective seminal work of both the English scientist Michael Faraday and the German physicist Gustav Mie, where the former demonstrated the range of brilliant colors of GNP colloidal solutions while the latter demonstrated that light absorption and scattering by the spherical GNPs contained within Faraday’s solutions were what created the colors described [
31]. Since then, many synthesis strategies, shapes, and biomedical/industrial/optical applications for GNPs have been developed, including the Turkevich, Frens, and Schiffrin-Brust methods.
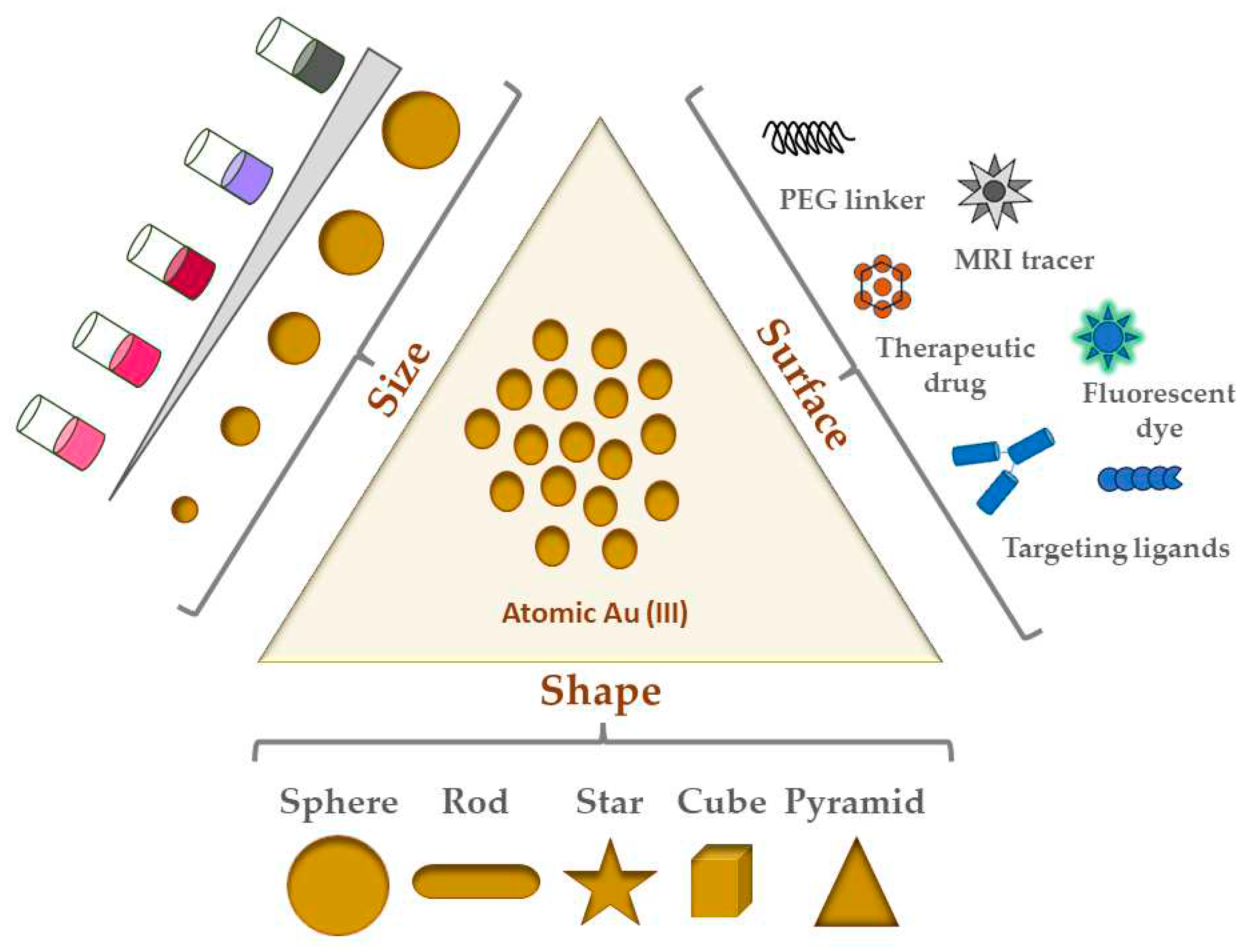
Figure 1 shows an illustrative view of the most common shapes/types of GNPs and their surface modifications.
2.2. GNP Shapes
Although GNPs come in many shapes and sizes, the following are the most common shapes of GNPs studied in the literature:
Most biomedical applications of GNPs for cancer therapeutic research possesses some variation of these shapes [
32]. Of the five shapes listed here, nanospheres are the most common and popular to work with. Synthesized via chloroauric acid reduction, sizes range between 5 to 100+ nm in diameter [
33]. Nanospheres have historically been most used for imaging and radiation dose enhancement applications. They are also related to nanoshells, which have a silica core surrounded by a thin layer shell of gold, hence their name. Given that nanoshell thickness and core diameter can be adjusted, their corresponding optical properties can also be similarly tuned. Furthermore, hollow nanospheres have been employed elsewhere because of their excellent plasmonic photothermal activities (detailed later in the next section) [
33]. The spherical shape of nanospheres lends itself to not only high surface curvature, but also a lower number of ligands as a result. Moreover, flocculation, or aggregation of small particles into larger and larger aggregates, is reduced in such smaller capped nanospheres due to this curvature and ligand-forming capacity. A vital part of ligand functionalization is the formation of a monolayer on these nanospheres, which in the case of thiol ligands has been shown to lead to self-assembling monolayer (SAM) coverage [
33,
34]. Interestingly in terms of possible biomedical applications, Chithrani and colleagues found that cellular uptake of GNPs (rods and spheres) in HeLa cells was inversely proportional to their aspect ratio, indicating that spheres in their study had overall greater uptake in this cell line than rods [
35].
Unlike their spherical counterpart, nanorods are elongated cylindrical-like GNPs [
33]. Made via a gold seed-mediated growth method (see Section 1.4), nanorod synthesis also often involves the cationic surfactant cetyltrimethylammonium bromide (CTAB) acting as a stabilizing agent. These GNPs have been used to conjugate a broad spectrum of materials to yield novel and improved plasmonic properties [
36]. Nanorods demonstrate two absorption bands (1. Transverse, and 2. Longitudinal) due to the electronic oscillation unique to the width and length of the particle (see 1Section .3 for more) [
37].
The other three shapes, namely nanostars, nanocubes, and nanopyramids (or nanotriangles) are gaining in popularity. Gold nanostars, which are anisotropic with multiple branches from the particle core, are known for their greater aspect ratio and thus higher surface area and reactivity compared to their counterparts [
38]. This high reactivity is attractive for photothermal therapy applications for cancer [
39]. Gold nanocubes, meanwhile, can serve as building blocks for plasmonic applications, particularly because of the LSPR foci at their vertices and edges [
40]. Unlike nanorods, their CTAB layer is thick and thus much more difficult to replace by ligand exchange with citrate. For similar reasons as both nanostars and nanocubes, nanopyramids/nanotriangles, by virtue of their anisotropic nature and surface area afforded by their shape, are increasingly looked at as dual photodynamic and photothermal therapy agents for cancer [
39,
41]. Campu and colleagues showed great preclinical promise in terms of melanoma cell death before and after irradiation with the pyramid LSPR wavelength for their dual photothermal and photodynamic therapy treatment [
41].
3. Physicochemical Properties of GNPs
Many aspects of GNPs as illustrated in
Figure 1 also affect their properties, some of which are crucial determinants of biocompatibility, biodistribution, and cytotoxicity. The physicochemical properties of GNPs include size, shape, coating, functional group/ligand attached, and/or surface charge (zeta potential). These properties enable high drug-loading capacity (via their immense surface area), storage stability (i.e., colloidal stability), and adaptability in the mode of administration [
42]. Because of their facile and well-defined synthesis and functionalization methods, GNP formulations of any combination of the above are theoretically possible for virtually any desired application while importantly remaining biocompatible and non-toxic.
3.1. How GNP Size and Shape Affect Their Physicochemical Properties
GNP size is extremely important for not only determining optical properties, but also for its renal clearance (and by extension its blood half-life and whole-body half-life). NPs less than 10 nm in diameter are easily filtered by the kidneys, and those larger than 200 nm are removed by the reticuloendothelial system (phagocytosing cells) [
43]. Other sources of NP removal from circulation also involve phagocytosis or similar filtration activity in the liver or spleen. Thus, size is a vital determinant of retention time and bioavailability, especially since NPs less than 100 nm demonstrate longer circulation periods in the bloodstream before being cleared. NPs in general are very prone to both aggregation and immune opsonization, the latter involving the deposition of markers (usually proteins) that signal phagocytes to engulf and eventually eliminate pathogens and foreign substances. These interactions of NPs with serum proteins form what has been dubbed the protein corona because of the crown-like arrangement of serum proteins that bind to the surface of NPs [
43,
44,
45]. Formation of the protein corona adds to the “base” diameter of the NP, leading to a separate but related and physiologically relevant size called the hydrodynamic diameter (HD) [
46]. Since globular proteins with an HD of 5-6 nm are known to be renally filtered into the urine, this size range and below (more specifically, an HD ≤ 5.5 nm) is associated with being the renal clearance limit [
47].
The shape of GNPs also governs their physicochemical and optical properties[
48,
49]. Optical properties are particularly dependent on GNP shape [
38,
50]. Mie’s pioneering work building off Faraday’s findings, originated the use of Maxwell’s equations for solving the absorption and scattering of electromagnetic radiation (
i.e., light) by the nanospheres in the colloidal solution [
51]. The vibrant and variable colors of GNPs are thought to be created by the enormous amount of light absorption (
i.e., extinction) that occurs during oscillation of the gold atom’s conduction band electrons when they encounter an electromagnetic field (
i.e., light) [
52]. These oscillations produce surface plasmons (a collective “resonance” of oscillations of the conduction band electrons) in a phenomenon known as localized surface plasmon resonance (LSPR). This LSPR is unique to nanoscale materials and thus does not occur with bulk gold. LSPR for GNPs depends on their size, shape, dielectric properties, aggregation, surface modifications, and medium refractive index [
51,
53]. Because of this LSPR, visible light absorption spectra of GNPs will exhibit a characteristic strong LSPR peak around 520 nm, but this peak position shifts based on GNP size and surface chemistry [
54]. The LSPR of nanostars can be adjusted from the visible to the near-infrared region via size and branching adjustments, thus posing highly favorable customization for biomedical applications [
38]. The optical properties of nanospheres can be tuned in the visible region as desired by modulating their synthesized size [
51]. Another important shape-dependent optical property of GNPs is surface-enhanced Raman scattering (SERS), in which the focalization of a localized SPR (LSPR) on GNP surfaces leads to enhanced Raman scattering of light at these focal points, producing a unique and highly-enhanced light scattering profile and local electric field, thus increasing the Raman signal of molecules nearby the GNP [
51,
54,
55]. In particular, nanorods and nanostars have been extensively studied as GNP shapes for biosensing, owing to their respective LSPR foci at the nanorod surface and nanostar tips. Interestingly, these LSPRs mediate GNP applications in the biomedical field since their enhanced electric fields can be transformed into heat in light-stimulated drug delivery and photothermal therapies [
56,
57].
3.2. Surface Charge and GNP Stablity
The high surface area of GNPs is important for their resistance to agglomeration. Agglomeration refers to the undesirable phenomenon of particles clumping together, which can affect their dispersion and performance in various applications. To understand and evaluate the potential for agglomeration, scientists often use zeta potential analysis. Zeta potential is a measure of the electric charge around the nanoparticles in a solution. It provides valuable information about the physical stability of the particles in the solution. A high positive or negative zeta potential value indicates a strong repulsion between particles, making them less likely to agglomerate. When particles repel each other due to a high zeta potential, the suspension remains stable, and the individual particles remain dispersed rather than forming clusters. On the other hand, NPs with poor zeta-potential values are more prone to flocculation, which is the process of particles coming together to form larger aggregates or flocs. The general criterion for determining the stability of a solution is often set at ±30 mV. If the zeta potential of a particle suspension is greater than +30 mV or more electronegative than -30 mV, it is considered stable. Outside these general ranges, the likelihood of agglomeration or flocculation increases. It is important to maintain a stable solution in various industries and applications, such as pharmaceuticals, cosmetics, and materials science. Researchers and engineers aim to design and produce nanoparticles with high zeta potential to ensure their stability and effective performance in different environments [
10,
58,
59].
4. Physicochemical Characterization Techniques for GNPs
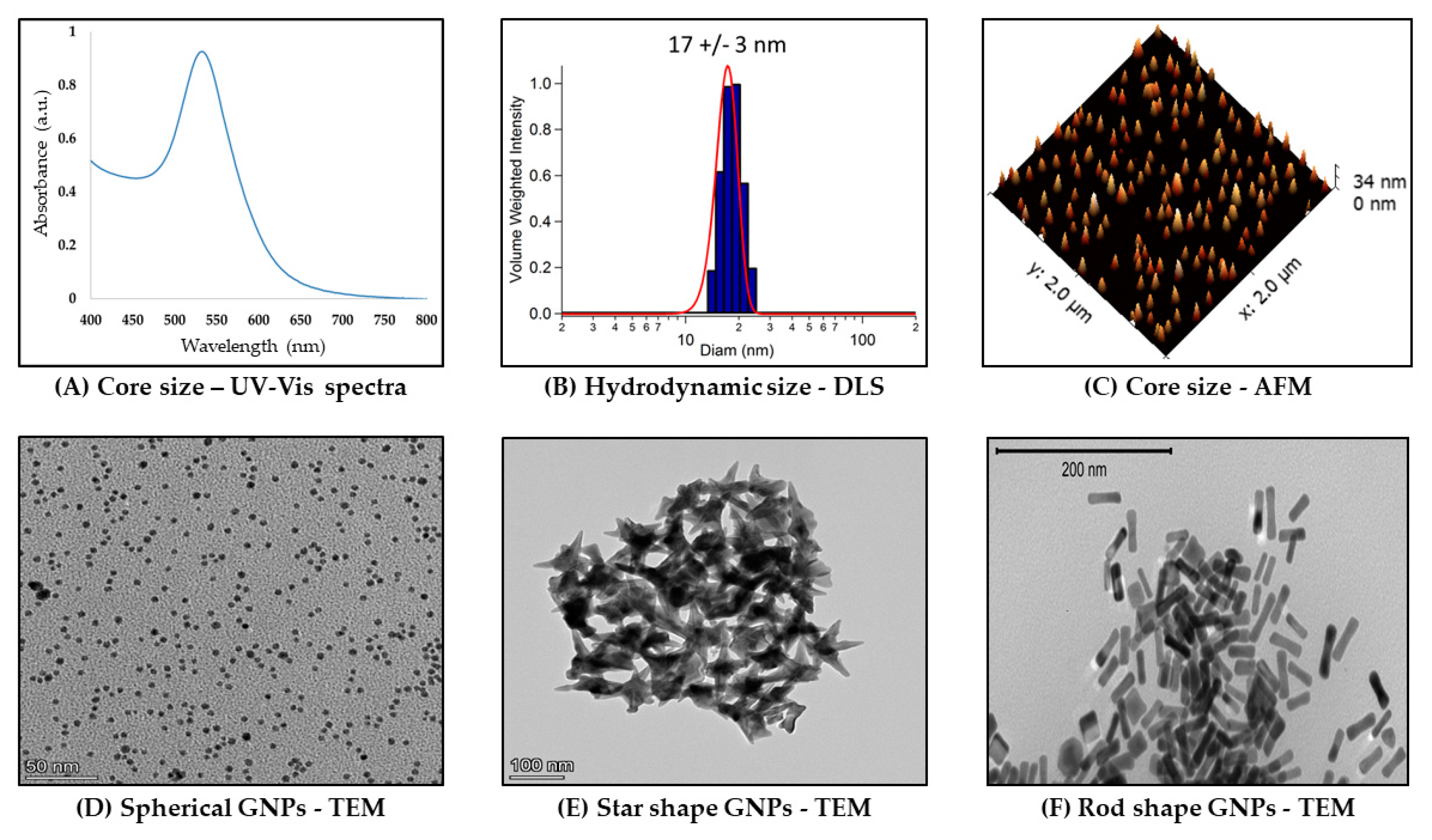
Characterization techniques are central to fully understand the composition of synthesized nanomaterials. There are a variety of analytical methods that have been successfully applied to the characterization of nanomaterials. Typically, spectroscopic and scattering characterization techniques span the electromagnetic spectrum and are key to confirming the physicochemical properties desired in GNP preparations (SPR/LSPR, core size, and hydrodynamic size). These methods can be sub-divided into spectroscopic, scattering, and microscopy techniques with origins in the field of colloidal chemistry.
Figure 2 summarizes these characterization techniques.
4.1. Ultraviolet-Visible (UV-vis) Spectrophotometry
Ultraviolet-visible spectrophotometry is an optical technique that exploits the interaction of electromagnetic radiation with a sample resulting in the wavelength-dependent extinction (absorption + scattering) spectrum. Noble metal nanoparticles, especially gold and silver, exhibit strong absorption bands in the visible region known as LSPRs. The LSPR bands are strongly dependent on GNP size, shape, and composition [
60,
61,
62,
63]. These properties and others influence important aspects of GNPs, including concentration, size, and aggregation state (
Figure 2A) [
64,
65]. Importantly, gold nanostars and nanorods both exhibit characteristic and strongly shape-dependent dual UV-vis LSPR bands due to the former’s core and spikes [
66,
67,
68] as well as the latter’s transverse and longitudinal particle dimensions/plasmon foci [
69]. This dual band is not present in spectra of gold nanospheres, which instead demonstrate a single SPR band around 510-520 nm, depending on their size [
10]. Since these properties are important in characterizing GNPs to ensure desired plasmon peaks, UV-vis is widely used as a basic tool to characterize GNPs.
4.2. Dynamic Light Scattering (DLS)
Dynamic Light Scattering (DLS) is a light scattering technique to determine hydrodynamic size (NP and solvent cage) (
Figure 2B). In this technique, the intensity fluctuations in the scattered laser light (Tyndall effect) due to the Brownian motion of the GNPs are used to determine the particle diffusion coefficient, which is then related to its hydrodynamic radius [
70,
71]. Brownian motion is the random walk/motion of colloidal particles, here GNPs, because of collisions with water or other solvent molecules in solution. The hydrodynamic radius will increase in biological media (and hence so too in physiological conditions as well as in the body) because of protein, particularly serum, adsorption on GNPs (see
Section 3.1 for more discussion). Thus, DLS has the advantage of determining an ensemble average of NP sizes and relative polydispersity [
71].
4.3. Atomic Force Microscopy (AFM)
Different in important respects from the in-suspension measurements of UV-vis and DLS, Atomic Force Microscopy (AFM) is a scanning probe technique that can be done under ambient conditions [
72,
73]. AFM can be used to image matter at the atomic scale through raster scanning the instrument stylus over its surface. The contouring of an air-exposed dried sample with the molecularly-sharp stylus in this technique also allows measuring their physical as well as chemical and biological properties via the reflection of an applied laser beam by a sample. The vertical distance between the stylus and the sample as a consequence of the force applied by the stylus on the sample is used to reconstruct the sample’s topography [
73].
Figure 2C shows an example of GNP core size topographic measurements using AFM. As part of the big-picture goal of GNP characterization for confirming desired physicochemical properties, this technique can be used to corroborate UV-vis and transmission electron microscopy measurements of GNP core size, albeit with the variables of context either in-buffer or in-air.
4.4. Transmission Electron Microscopy (TEM)
GNP core size can also be measured using transmission electron microscopy (TEM) [
74]. TEM imaging depends in principle on differential electron absorption and scattering by thinly-sectioned plastic-embedded samples. The contrast it provides in sample imaging because of the short wavelength of the electron beam is part of why the technique can get down to sub nanometer resolution. TEM is best performed with conducting or semiconducting samples under vacuum. The direct imaging capability of TEM is particularly useful for nanomaterials with non-spherical shapes since it allows for direct measurement of the aspect ratio.
Figure 2D–F shows examples of TEM images captured of three GNP shapes (nanospheres, nanostars, and nanorods).
5. GNP Synthesis Strategies
GNPs can be prepared using so-called top-down and bottom-up approaches [
75]. As its name implies, the top-down method involves breaking apart bulk gold until the desired dimensions are achieved, usually through assembling and forming via a pre-made pattern or matrix. However, the final GNP size and shape are not able to be finely tuned using this method. In comparison, the bottom-up method starts with gold ions and subjects them to either chemical or biological reduction into single atoms of gold [
76]. We will focus on chemical reduction for this review.
5.1. GNP Synthesis by Chemical Au(III) Reduction
Reduction in GNP synthesis is a two-step process. The first is nucleation, which is essentially the formation/coalescence of Au(0) into nuclei. Nucleation is initiated by the reduction of Au(III) to Au(0) by a reducing agent (
i.e., borohydrides, aminoboranes, hydrazine, or citric and oxalic acids, to name a few) until the Au(0) concentration surpasses a solubility threshold (at which point nucleation proceeds). Nucleation ends when that critical threshold is no longer met as the Au(0) concentration decreases over time. Nucleation requires the presence of an electrostatic or steric stabilization agent (
i.e., surfactant), such as citrate, the cationic surfactant cetyltrimethylammonium bromide (CTAB), or else a carboxylic acid, amine, or thiol [
77]. Once Au(0) nuclei form, a growth step then occurs, where more Au(0) nuclei and Au(0) itself further coalesce to form the final GNPs as a colloidal solution. Importantly, the growth step must have a passivator/stabilizer present (also known as a capping agent), which similarly to the nucleation step acts as an electrostatic/steric stabilizer for mediating colloidal stability (
i.e., preventing aggregation of GNPs via either electrostatic or steric stabilization). If both steps are performed in one pot, this is called in situ synthesis, or else seed-mediated growth if not [
75]. In the latter case, in situ synthesized GNPs are used as seeds for seed-mediated growth, where the GNPs grow larger and larger step by step so that size control is optimized. This two-step process first involves synthesis of GNP seeds followed by the second growth step in a solution containing Au(III) salt along with passivating and reducing agents. Here, the reduced Au(0) deposits and grows on the seeds to ultimately produce larger GNPs as desired. Importantly, the reducing agents for the growth step must be mild so that Au(0) is only deposited on the surface of existing seeds while preventing any new nucleation events [
75].
5.2. Common Methods for GNP Synthesis
There are three main GNP synthesis methods, some variation or combination of which is a part of most of the GNP research articles that we have found. They all rely in principle on a similar chemical reduction mechanism. The first and most popular of these synthesis methods was developed by its namesake, John Turkevich and colleagues [
75,
78]. Here, trisodium citrate dihydrate is quickly added with stirring to boiling gold Au(III) salt solution, after which a wine-red colloidal GNP solution of the order of 20 nm should be produced. A modification of this method developed by Frens modulates the trisodium citrate:Au(III) ratio to synthesize 15-150 nm particles, though 20+ nm syntheses using it are polydisperse [
79]. The third common synthesis method is known as the Brust-Schiffrin method, which was first to result in thiolate-stabilized GNPs as an in situ synthesis [
75,
80]. This approach has evolved over the years from a two-phase synthesis to a much simpler method with several upsides compared to the Turkevich method. Its advantages include ambient reaction temperature, excellent thermal and air stability, low aggregation or decomposition, sub-5 nm size range (2-5 nm) with high monodispersity, and simple functionalization/modification methods via ligand substitution. Because of the gold-S bonds, the GNP stability is fairly high, and the resulting shapes are cuboctahedral and icosahedral. Here, smaller GNP core sizes can be achieved by increasing the sulfur:gold (S/Au) molar ratios.
6. GNP Applications in Biomedicine
Several lines of recent biomedical research have utilized GNPs. The properties of the GNPs used in these studies impacted their corresponding biological efficacy in various ways and will be discussed. Furthermore, an exploration of some of the latest optical and biosensing applications of GNPs will be presented. Moreover, cancer treatment applications of GNPs are provided.
6.1. GNP Applications in Optics and Biosensing
GNPs have several real-world and developing applications, including biomedical imaging, diagnostics, nanobiosensing, nanotheranostics, nanomedicine, dentistry, and photothermal/photodynamic therapy [
39,
54,
81]. The unique and tunable optical properties of GNPs lend them to popular use in biomedical imaging techniques like x-ray computed tomography, photo-acoustic imaging, dark field microscopy, magnetic resonance imaging, and fluorescence imaging. Not only do GNPs have an LSPR that is tunable from the visible to the NIR wavelengths of the electromagnetic spectrum, but they also avoid the issues of photobleaching and blinking. Furthermore, GNPs also have significantly greater scattering efficiency than traditional fluorophores, and their sensitivity in dark field imaging is exceptional. The aforementioned SERS effect of GNPs enable their use in Raman signal-based imaging of biological cells [
54]. Moreover, GNPs prisms, rods, and shells can be utilized for photoacoustic imaging because of their intrinsic exogenous contrast as part of photoacoustic tomography (PAT), particularly nanocages. PAT imaging generates a three-dimensional cross-sectional image of biological structures while avoiding/minimizing the problems of light diffusion in other deep-tissue modalities. Other imaging techniques in which GNPs are applied include immune-electron microscopy, reflectance microscopy, and magnetic resonance imaging (MRI) [
54]. Biosensing via the unique optical properties of GNPs is also a rich and popular area of GNP research. Colorimetric, electrochemical, enzymatic, fluorescence resonance energy transfer (FRET), SERS, and optical sensing applications have all benefited from GNP innovations. Several other biomedical GNP applications specific to drug delivery are provided in detail in the next section.
Table 1.
Effect of various shapes and sizes of targeted gold nanoparticles in tumor treatment.
Table 1.
Effect of various shapes and sizes of targeted gold nanoparticles in tumor treatment.
| Shape (size nm) |
Surface Coating |
Application |
Reference |
| Nanosphere (~35) |
Cyclic RGD peptides |
Sarcoma treatment |
[82] |
| Nanosphere (89.7 ± 2) |
Aptamer-conjugated PTX (Paclitaxel) |
Breast cancer |
[83] |
| Nanosphere (18.5 ± 2) |
Silica coating |
Ductal mammary carcinoma |
[84] |
| Nanosphere (80) |
EGF peptides, Doxorubicin |
Brain cancer |
[85] |
| Nanosphere (26.5±1.1) |
Prostate-specific membrane antigen (PSMA-1) |
Prostate cancer |
[86] |
| Nanosphere (62.9±0.7) |
small-interfering RNA (siRNA) & RGD peptide |
Lung cancer |
[87] |
Nanosphere (56.37 ± 3.04)
Rods (22.41 ± 1.01)
|
Cyclic RGD peptides |
Non-small cell lung cancer (NSCLC) |
[88] |
| Nanostar (78.0 ±2.9) |
Chlorin e6-func-tionalized |
Breast cancer |
[89] |
| Nanosphere (29 ± 2) |
Doxorubicin or Varlitinib and PEG |
Pancreatic Adenocarcinoma |
[90] |
6.2. GNP Applications in Cancer Therapeutics
GNPs are increasingly gaining popularity for cancer therapeutic applications/development [
48,
91,
92,
93]. Five cancers which have recently documented applications in a research context are breast, colon, lung, prostate, and pancreatic cancers [
36].
Table 1 summarizes the applications of GNPs for various cancers. The MCF-7 breast cancer cell line treated with GNPs conjugated with extract from the Anacardiaceae showed greater dose-dependent cytotoxicity compared to control [
94]. Another study targeted GNPs to breast cancer cell lines using CD44 as a ligand conjugate and found high and specific cancer-cell-specific accumulation and reduced tumor growth in vivo [
95]. Furthermore, GNPs loaded with a radiolabeled version of the antioxidative polyphenol Resveratrol were used to treat HT29 colon cancer cells both in vitro and in vivo significantly reduced cancer cell viability [
96]. Moreover, treatment of colon cancer cell lines with GNPs electrostatically-conjugated to the candidate cytotoxic compound SN38 resulted in high red-LED-stimulated drug release and cytotoxicity [
97]. As for lung cancer, treatment of lung cancer cell lines with GNPs loaded with anti-cancer docetaxel and tumor-targeting folic acid resulted in highly specific and effective cancer cell death [
98]. Wang and colleagues loaded GNP nanocages with the anti-cancer paclitaxel and a micelle for green synthesis of GNPs successfully induced tumor cell death and dysfunction [
99]. Lastly, chitosan-capped GNP nanorods loaded with gemcitabine and coated with silica were fabricated by another group to treat pancreatic cancer cell lines via acidic-pH-sensitive drug release, finding greater cytotoxicity than with drug alone [
100].
7. GNPs as a Drug Delivery Vehicle
Systemic drug delivery should ideally be targeted and specific to avoid the issues with diffusion and off-target side effects as discussed earlier. While this review is focused on cancer applications of GNPs, there are also several examples in the literature of GNP systemic delivery of therapeutics which have been demonstrated to show efficacy [
101,
102,
103]. However, these untargeted therapeutics rely on passive accumulation in diseased tissues in vivo through the leakiness that occurs in the surrounding vasculature. Importantly, targeted brain and central nervous system delivery via GNPs is surveyed and discussed with regards to their specific accumulation and bioefficacy.
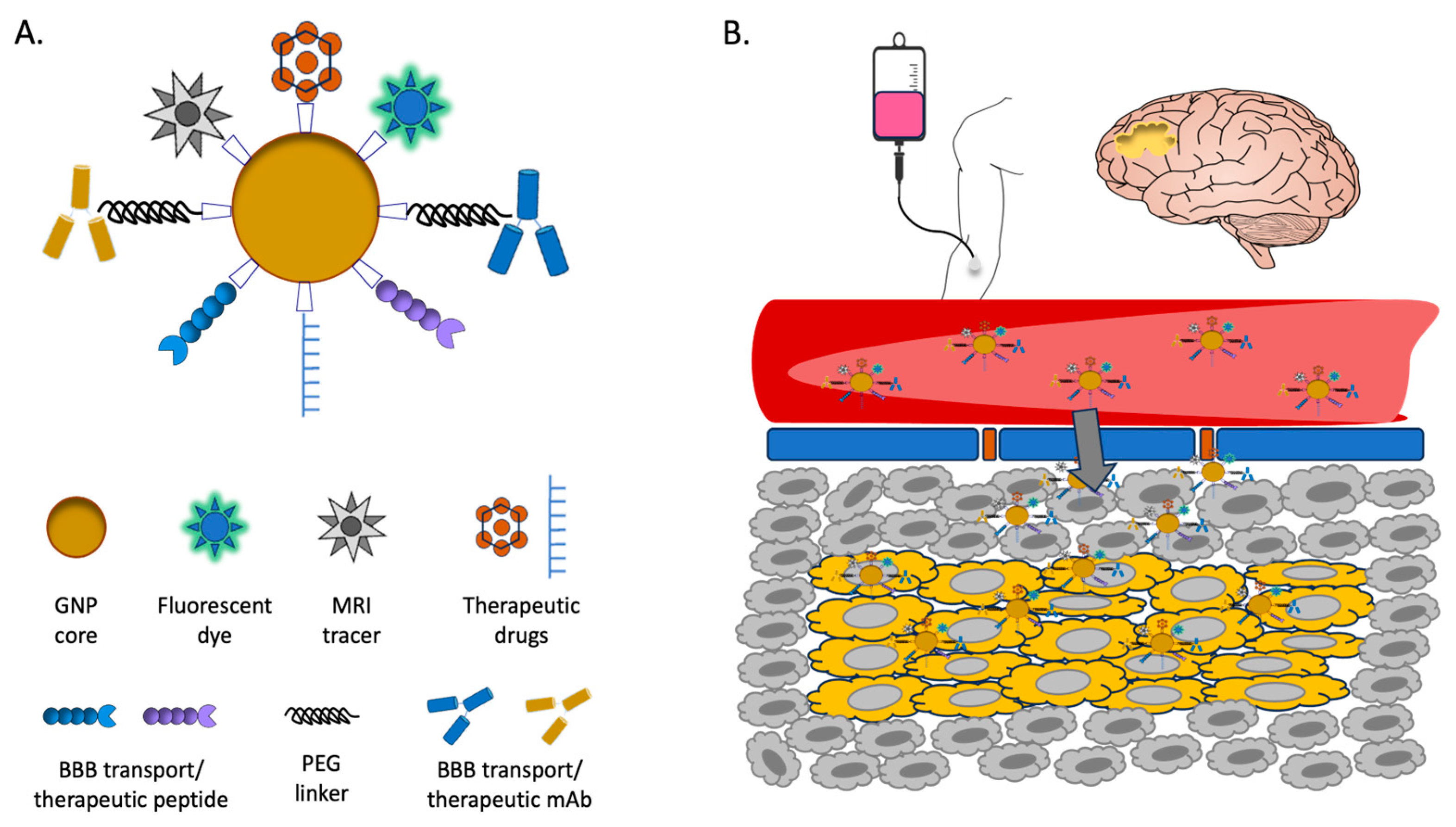
7.1. Strategies of GNP Drug Delivery in the Brain
Barriers to effective drug delivery in the brain are manifold, but the most important and pervasive is the blood-brain barrier (BBB) [
104]. Made mainly of tightly packed cerebral capillary endothelial cells (CCECs), the BBB is flanked by pericytes and presynaptic astrocytes through the basement membrane [
105]. This multilayered barrier, though semipermeable, effectively blocks the free movement of molecules (and particularly toxic chemicals) between the blood and brain parenchyma, which significantly limits the entry of therapeutics to the brain. Schematics of BBB is shown in
Figure 4B.
Most NP-based drug delivery mechanisms for the brain utilize one of three pathways: passive diffusion, carrier-mediated influx, and vesicular transport including receptor-mediated transport [
106]. Other mechanisms are either too invasive and carry major risks associated with them (
e.g., neurosurgery) or else too limited to lipid-soluble small molecule delivery. Other means of penetrating the BBB include intrathecal injection, osmotic destruction, ultrasound or magnetic interference, and nasal/olfactory administration, though they have detrimental effects on the BBB and biosafety in general [
106]. Wheat germ agglutinin linked to horse radish peroxidase (WGA-HRP) has been previously shown to mediate absorptive endocytic transport across the olfactory cerebrospinal fluid (CSF) barrier via WGA binding neural membrane lectin sites [
107].
7.2. Examples of GNP Drug Delivery in the Brain
Zhang and colleagues synthesized WGA-HRP conjugated to GNPs as targeting and imaging moieties to improve central nervous system delivery of therapeutic [
106]. The targeted GNPs were loaded with several polymer-linked drugs for the treatment of spinal-cord-injury-induced diaphragm paralysis [
106]. This system takes advantage of the WGA lectin recognized by N-acetylglucosamine and sialic acid receptors found on neuronal cell membranes. The specific accumulation of the GNP-delivered respiratory-neuron-targeted therapeutic drugs in the cervical spinal cord and medulla would be vital to avoid the detrimental side-effects of systemic non-targeted administration (
i.e., nausea and seizures associated with drug diffusion and it’s resulting off-target effects). Importantly, a biodegradable bond between the drugs and the GNP-mediated drug release at the target tissue. The final three GNP nanoconjugates (two conjugated to the prodrugs before intracellular activation, and one with only WGA-HRP-GNP) were evaluated by a single injection of each into the diaphragm muscles of separate C2Hx model rats with induced left hemidiaphragm paralysis (confirmed by electromyography, or EMG). All the rats treated with the drug-loaded WGA-HRP-GNPs exhibited restoration of diaphragm muscle function as evaluated by EMG. Immunohistochemical analyses confirmed the specific as-targeted accumulation of the GNPs at the tissue sites.
Although nanocluster-size GNPs could benefit from passive diffusion mechanisms, larger GNPs must be functionalized to utilize an active transport pathway [
104]. Nanocluster GNPs of the size 0.9-1.5 nm can cross the BBB directly through ion channels of the same order of dimension, although they also through their high concentration can exert osmotic pressure or else depend on transient changes in BBB membrane fluidity sufficient to allow GNP penetration [
108,
109]. A study by Gao and colleagues synthesized GNP nanoclusters for the delivery of novel anti-Parkinson’s-Disease (PD) drugs [
110]. Their N-isobutyryl-L-cysteine (L-NIBC) protected GNP nanoclusters administered to both in vitro PD cell line models and a murine PD model effectively ameliorated alpha-synuclein (alpha-Syn) fibrillation and reduced behavioral disorder phenotypes. Importantly, GNP nanocluster treatment in their study was shown to reverse dopaminergic neuron loss and decrease tyrosine hydroxylase expression in their chemically induced PD animal model. Regarding larger GNPs, several receptors known to be overexpressed on the membranes of the BBB have been leveraged to functionalize GNPs for receptor-mediated endocytosis entry. These receptors include those for transferrin, low-density lipoprotein, and insulin. Moreover, electrostatic interactions have been used to maximize the entry of positively charged GNPs (via their coating and/or ligands) into anionic CCECs [
104].
7.3. GNP Therapeutic Biosafety
GNP synthesis requires the use of potentially cytotoxic chemicals and compounds. Therefore, standardized removal/filtering/dialysis of these unreacted or excess chemicals and compounds is needed during drug development and especially before preclinical animal and finally clinical studies. For example, two independent studies in 2006 [
35] and 2010 [
111] evaluated the toxicity of the cationic surfactant cetyl trimethylammonium bromide (CTAB) coated GNPs. Chithrani and colleagues in 2006, coated nanorods with the CTAB [
35]. This positively-charged coat makes the GNPs prone to “sticking” to the negatively-charged cell membranes of human cells [
29]. Therefore, before exposing the HeLa cell line they used to nanorods of varying size, the authors importantly replaced the CTAB passivating coat with citric acid by chemical exchange as a ligand. The Chithrani paper found no indication of toxicity (measured by cell viability) in the HeLa cells exposed to the citrated nanorod formulations. Despite CTAB’s stickiness, twin studies published in 2010 by Bartneck and colleagues demonstrated that neither CTAB-passivated nor polyethylene oxide (PEO) polymer-passivated GNPs reduced cell viability when incubated with primary human blood phagocytes in both the short-term (2 h) and the long-term (7 days) [
111].
As was true for GNP properties, GNP biosafety strongly hinges on their size and surface ligands/coating. For example, the gold cluster/nanocluster and nanodots, varying in size from subnanometers to 20 nm, also vary widely in their respective consensus definition across the field of synthetic chemistry [
112]. However, the work of Tsoli and colleagues in 2005 on cell culture of eleven cell lines supplemented with gold cluster GNPs resulted in varying degrees of sensitivity to the treatment, though the metastatic melanoma cell line BLM demonstrated a dose-dependent reduction in cell viability after 72 hr gold cluster treatment. Here, 0.4 µM gold clusters were sufficient for 100% cell death, whereas the cisplatin-treated group still exhibited about 90% viability. After analyzing the intracellular distribution of the clusters, the group hypothesized that the gold cluster binds to the major groove of DNA selectively and stably, and thus poses a potentially potent transcriptional block reflected by their data. Pernodet and colleagues similarly showed in their studies that incubation of dermal fibroblasts with ~13 nm citrated gold nanoparticles for six days at 0.1 and 0.6 mg/mL resulted in major effects on cell morphology [
113]. These effects included narrowing of the cell footprint (and thus less cell adhesion) and reduced proliferation (related to shrinking of actin fiber diameter and formation of actin “dots” instead of normal stress fibers, which point to depolymerization and/or actin-fiber modulation). The group further performed a GNP treatment time-course with TEM, finding that three hours was the minimum time for GNPs to be found in cellular images, specifically in vacuoles. In terms of additional mechanisms of cytotoxicity, after six days of incubation, these vacuoles became pervasive and extremely large to the point of enlarging the cells and disturbing normal cell structure and function (including protein production) as a result.
In sharp contrast, Shukla and colleagues found that gold nanoparticles from 3-8 nm in size incubated with a macrophage cell line for 3-72 h are not cytotoxic, reduce reactive oxygen species (ROS) and nitrite species, and do not induce proinflammatory cytokine production [
114]. Connor and colleagues investigated GNPs ranging in size from 4-18 nm and capping agent (cysteine, citrate, glucose, and CTAB), finding that endocytosis by a leukemia cell line did not result in any apparent cytotoxicity measured by MTT and proliferation assays at GNP concentrations of up to 25 µM over 2-5 days [
115]. That said, surface coatings and free unbound ligands are a potential source of cytotoxicity if GNPs are not prepared properly. In related work, Goodman and colleagues provided evidence for moderate toxicity of cationic GNPs and low to no toxicity of anionic GNPs (the cores of both being 2 nm in size) in fibroblast-like and red blood cell (as well as interestingly in the bacteria model organism
E. coli) over the course of 1-24 h [
116]. The work of Pan and colleagues used gold nanocluster treatments (ranging in size from 0.8-15 nm) to test GNP cytotoxicity in the following cell types: epithelial and endothelial cells, macrophages, and connective tissue fibroblasts [
112]. When the cancer cell lines among the cell types listed above were exposed to the 1.4 nm nanoclusters for one hour, membrane blebbing and vesicle formation accompanied swelling and loss of adherence. These effects worsened at the 12-h timepoints into strong evidence of apoptosis and secondary necrosis. 48-h and 72-h treatments demonstrated clear and significant reductions in viability, but importantly only for the nanocluster-sized (1-2 nm) GNP preparations since their effects were compared to the 15 nm GNP preparations and untreated control cultures. Although larger GNPs possibly have improved BBB uptake because of active transport mechanisms, they also stay longer in circulation and preferentially accumulate in the liver and/or spleen, posing possible toxicity risks [
117]. Interestingly, the study of Ding and colleagues described earlier in the Introduction (
Section 1) importantly demonstrated no measurable negative effects of the Apt-Au@MSL treatment in the nude mice tumor xenograft model, despite the very large (150 nm) core and hydrodynamic sizes of the final GNP [
118]. Of note, both the anti-cancer effects and toxicity portions of the study evaluated these aspects 24-and 30-days post-treatment, indicating at least acute biosafety
in vivo.
8. Gliomas, Glioblastoma Multiforme (GBM), and the Promise of Nanoparticles
8.1. Glioblastoma Multiforme (GBM) Overview
Glioblastoma multiforme (GBM) is a grade IV glioma arising from glial and neuronal cells. It is the most common malignant tumor in the central nervous system [
119]. The location of GBM is dependent upon the amount of white matter present. The majority of GBM is found in supratentorial (31% temporal, 24% parietal, 23% frontal, 16% occipital) which is about more than 75% of tumors, <20% multifocal, 2% to 7% multicentric [
17,
120]. GBM accounts for nearly 80% of all malignant brain tumors and occurs with an incidence of 3-6.4 cases per 100,000 people annually. Nearly 13,000 new cases of GBM are diagnosed annually in the United States alone [
120]. Despite standard combined care, the mean overall survival period from diagnosis is less than 20 months. Moreover, the 5-year survival rate is only about 5.8% after standard combined treatment [
16]. GBMs are highly diffusive and infiltrative in nature, often growing a few centimeters from the core, thus making it extremely difficult to achieve complete remission.
For most of the patients with GBM, there is no known causative factors. Few risk factors have been determined to date to be linked to GBM tumorigenesis. They include age (incidence increases with age such that it peaks at 85 years and decreases after 85 years), environmental factors (such as exposure to ionizing radiation, smoking where tobacco specific nitrosamines and aromatic hydrocarbons penetrate BBB and affect the development of GBM), nutritional risk factors (such as obesity), pesticide exposure, familial history, and many more. Some studies also suggested the effect of Ovarian steroid hormones in the development of GBM. Interestingly, allergy and infection appear to be protective against GBM [
17,
121,
122]. Antihistamines have been shown to reduce the risk of developing gliomas. Aspirin and its metabolites have been shown to reduce the risk of GBM by blocking the attachment of FGF to its receptor and thus inhibiting the growth of GBM cells. Surprisingly, Cannabinoids have demonstrated tumor volume reduction effects in animal models. Various genes have been identified to contribute to tumorigenesis, including MGMT gene with promoter methylation, IDH1 gene mutation, EGFRv3 gene variant, BRAF gene V600E mutation, and ATRX [
17,
120].
WHO has classified GBM by 2 different categories. It’s redundant by cell type and they include Astrocytoma, Glioblastoma and Oligodendroglioma and also by malignancy grade as WHO Stage I, II, III, IV. In 2021, The WHO CNS5 has taken a new approach and has classifies Glioneural tumors into 6 families: 1) Adult-type diffuse gliomas, 2) pediatric-type diffuse low-grade gliomas, 3) pediatric-type diffuse high-grade gliomas, 4) circumscribed astrocytic gliomas, 5) Glioneural and Neuronal Tumors, and 6) Ependymoma [
123,
124].
GBM patients present with a variety of neurological symptoms, headaches being the most common and are seen in about 50% of patients. Papilledema is seen in patients with increased ICP due to tumor volume. Cognitive difficulties and personality changes may occur depending on the location of the tumor. Some may present with gait imbalances and incontinence. Few cases of GBM present so abruptly, they may resemble like a stroke. Various modalities are used to diagnose GBM and Magnetic resonance Imaging (MRI) being the gold standard for diagnostic imaging. CT also provides very useful information and is reserved for patients who are not able to undergo an MRI (like patients with pacemakers). MRI scans of glioma. Patients show positive enhancement with gadolinium contrast with an area of central necrosis surrounded by white matter oedema. Tumors can be unifocal and multifocal and can mimic various non-cancerous lesions in the brain such as brain abscess, stroke or MS. The diagnosis of gliomas has hindered due to various obstacles like tumor heterogenicity, the BBB and tumor complexity [
17,
123,
125].
8.2. Current treatment Modalities and Their Limitations
Various modalities are used to treat GBM. Developed by Stupp and colleagues in 2005, the gold-standard standard of care for GBM patients involves maximal surgical resection (surgical removal of as much of the tumor as possible (also known as tumor debulking) followed by combined radio-chemotherapy (photon/proton irradiation of the tumor cavity and concomitant treatment with the alkylating chemotherapeutic drug temozolomide) [
126,
127]. Various tools have been implemented to improve safety of resection such as Intra-operative ultrasound/MRI, functional mapping. Contrast enhanced MRI, 72 h before the procedure increases the chances of evaluation for the surgical resection. The presumed progression of tumor cells along neuronal fibers without macroscopic changes leads to failure to achieve clear surgical margins [
17,
120]. However, this clinical standard and the multitudes of novel treatments currently in or that have gone through clinical trials for safety and efficacy face several hurdles, including tumor heterogeneity, the BBB, and tumor complexity [
119]. One experimental therapy which cleared trials and is now included in the standard of care is the use of tumor-treating fields, which have improved survival in GBM patients [
124].
Despite the standard of care and decades of research into alternative therapies, GBM patient prognosis remains dismal [
121]. Factors which are associated with poor prognosis include low pre-operative Karnofsky performance status, age, tumor location, and the presence of molecular factors like methylation of the MGMT gene promoter and mutation of the IDH-1 gene. Extent of resection is one of the most important prognostic factors since GBM is characterized by diffuse infiltration into brain parenchyma (functional tissue). Thus, resection success is directly related to increasing overall survival and prolonging time until the inevitable tumor relapse [
123]. Though the scientific community have made new discoveries and treatment advancement, GBM still remains an incurable cancer. The goal of medical management is to make a diagnosis and to prolong and improve the quality of life of the patient [
17].
Compared to other malignant tumors, the BBB presents a unique and challenging barrier to effective drug delivery for treating GBM. Thus, chemotherapeutic drugs which are commonly used are not effective in GBM due its poor penetration across the BBB. All these factors lead to poor prognosis and tumor recurrence [
128]. That said, in 2002, the FDA approved the nitrosourea-containing BCNU (carmustine)-polymer wafers (marketed as Gliadel). These carmustine-containing polymer wafers deliver the alkylating agent, which crosslinks DNA and thus blocks replication and transcription [
129]. Xing and colleagues reviewed the literature in 2015 to determine if GBM overall survival significantly improved with carmustine wafer treatment of newly-diagnosed GBM, finding that there was no statistically significant increase in survival for the treatment in two randomized clinical trials compared to control. However, the four cohort studies also examined in the review did exhibit a significantly increased survival with treatment [
130]. Approximately 1600 clinical trials are currently categorized as “Glioblastoma” on “ClinicalTrials.gov”. Despite decades of such research, the long-term prospects for patients with GBM remain extremely poor. Currently, no screening tool or test is available for detecting GBM in early stages before the onset of symptoms [
17,
131].
8.3. Treatment Modalities for Glioma
Numerous novel treatments for GBM have been investigated. So-called GBM stem cells (GSCs) located in the perivascular and hypoxic zones of the tumor are believed to underlie tumor initiation, radiochemoresistance, and recurrence/relapse [
132,
133]. Targeting these GSCs through their unique stem-like surface markers in conjunction with other treatments and the standard of care pose an exciting area of research currently being pursued. Immune system therapies have also been a hot-topic research item, though none have yet yielded a clinically-approved treatment. These include immune checkpoint blockade therapies, co-administration of convection-enhanced delivery of temozolomide and whole-cell immunization with irradiation-inactivated tumor cells, sex-specific differences in myeloid cell populations in GBM murine models leading to differential immunosuppressive properties, and immunization with specific virus antigens to elicit local immune activation and instigate antineoplastic effects [
134,
135,
136,
137,
138,
139,
140,
141,
142]. Other examples include anti-angiogenic therapy with monoclonal antibodies to block angiogenic receptor binding and thus abrogate GBM tumor vascularization; epigenetic therapy involving histone deacetylase and methyltransferase inhibitors; oncolytic virus therapy with oncolytic viruses specific for entering followed by replicating in and then killing GBM tumor cells; and gene therapy wherein therapeutic genes or modifications of GBM tumorigenesis-related genes are introduced into tumor cells.
A long-studied but only recently resurged GBM treatment modality is boron neutron capture, or BNCT [
143]. First conceived in 1936, BNCT is a dual-arm therapy for several cancer types where a boron compound is infused into patients intravenously, followed by neutron irradiation of the tumor site. The “capture” or uptake of a neutron by boron-10 (a normally stable isotope) forms boron-11, which is now unstable and decays into three parts: an alpha particle (helium-4), lithium-7, and gamma rays. Theoretically, because the release radius (path length) of alpha particles and lithium-7 is about the size of a cell (5-9 µm, or microns), only tumor cells that have taken up boron-10 (and no or at least few normal healthy cells) will experience the major effects of BNCT (
i.e., DNA damage) and ideally die because of them [
129]. BNCT treatment of GBM and other gliomas have shown some promise in clinical trials, but the results are mixed or at most comparable or only slightly better than the standard of care [
144,
145]. Two key barriers to better efficacy (and which result in systemic toxicity and off-target necrosis) have already been discussed earlier: penetration of the BBB by boron-containing compounds and their tumor-specific accumulation. Interestingly, BNCT has been shown in a previous case to prolong a GBM patient’s life very significantly to approximately five years after initial diagnosis while affording excellent ability to perform activities of daily living independently [
145]. Although the tumor did ultimately recur, this case study points to the high potential for BNCT to extend GBM patients’ lives without its side-effects significantly reducing quality of life.
Here, the technology of nanomedicine comes into play which has revolutionized GBM therapies [
124]. With increasing GBM resistance to chemotherapy and development of delivery strategies, nanomedicine can be further optimized to achieve more effective GBM treatment, including potentially as a delivery vehicle for boron (16). With their many advantages, easy fabrication, and relative biosafety, GNPs are an ideal drug delivery vehicle for a wide array of ligands and therapeutics/theranostics, including boron-containing compounds for BNCT (
Figure 4). Thus, we propose that GNPs be at the forefront of future GBM therapeutics/theranostics research and development.
9. Conclusions
Gold nanoparticles (GNPs) are widely applied in the optical and biomedical industries. Because of their relative biosafety, well-defined synthesis methods, and favorable physicochemical properties. GNPs hold immense promise across the sciences, especially for cancer therapeutics/theranostics. Their potential applications for treatment of the malignant glioma Glioblastoma multiforme (GBM) are manifold. The extremely poor prognosis because of the inevitable relapse of GBM despite the standard of care requires continued intensive research into alternative therapies. Of the many therapies investigated, GNPs hold many advantages and few downsides, particularly in their potential for mediating boron neutron capture therapy (BNCT). Given the tumor-targeting issues of current boron carriers, we propose that using GNPs as delivery vehicles/platforms for GBM therapeutics/theranostics has excellent potential for successful development of a clinical treatment. We especially point to GNPs as a delivery vehicle for boron-10-containing compounds to mediate more effective and biosafe BNCT in glioma/GBM patients.
Author Contributions
Conceptualization, RP and CCP; writing, original draft preparation, CKL, MK, SR and RJ; writing, review and editing, RP, CCP and MK; All authors have read and agreed to the published version of the manuscript.
Funding
This review was funded by NIH/NCI grant 1R1CA259911-01A1 and funds from the Division of Cancer Science (LLU).
Acknowledgments
We would like to thank the Department of Basic Science, Loma Linda University for providing the facilities in which the manuscript was conceptualized, written, and discussed.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Amadi, E.V.; Venkataraman, A.; Papadopoulos, C. Nanoscale self-assembly: concepts, applications and challenges. Nanotechnology 2022, 33, 132001. [CrossRef]
- Baig, N.; Kammakakam, I.; Falath, W. Nanomaterials: a review of synthesis methods, properties, recent progress, and challenges. Mater. Adv. 2021, 2, 1821–1871. [CrossRef]
- Wang, E.C.; Wang, A.Z. Nanoparticles and their applications in cell and molecular biology. Integr. Biol. 2013, 6, 9–26. [CrossRef]
- Tao, L.; Hu, W.; Liu, Y.; Huang, G.; Sumer, B.D.; Gao, J. Shape-specific polymeric nanomedicine: emerging opportunities and challenges. Exp. Biol. Med. 2011, 236, 20–29. [CrossRef]
- Rasool, M.; Malik, A.; Waquar, S.; Arooj, M.; Zahid, S.; Asif, M.; Shaheen, S.; Hussain, A.; Ullah, H.; Gan, S.H. New challenges in the use of nanomedicine in cancer therapy. Bioengineered 2021, 13, 759–773. [CrossRef]
- Mabrouk, M.; Das, D.B.; Salem, Z.A.; Beherei, H.H. Nanomaterials for Biomedical Applications: Production, Characterisations, Recent Trends and Difficulties. Molecules 2021, 26, 1077. [CrossRef]
- Jeyaraj, M.; Gurunathan, S.; Qasim, M.; Kang, M.-H.; Kim, J.-H. A Comprehensive Review on the Synthesis, Characterization, and Biomedical Application of Platinum Nanoparticles. Nanomaterials 2019, 9, 1719. [CrossRef]
- Sarangi, M.K.; Padhi, S.; Rath, G.; Nanda, S.S.; Yi, D.K. Advances in immunological and theranostic approaches of gold nanoparticles – A review. Inorg. Chem. Commun. 2023, 153. [CrossRef]
- Zhang, R.; Kiessling, F.; Lammers, T.; Pallares, R.M. Clinical translation of gold nanoparticles. Drug Deliv. Transl. Res. 2022, 13, 378–385. [CrossRef]
- Khoobchandani, M., et al., Green nanotechnology of MGF-AuNPs for immunomodulatory intervention in prostate cancer therapy. Sci Rep, 2021. 11(1): p. 16797. [CrossRef]
- Sharma, S.; Parveen, R.; Chatterji, B.P. Toxicology of Nanoparticles in Drug Delivery. Curr. Pathobiol. Rep. 2021, 9, 133–144. [CrossRef]
- Barua, S.; Mitragotri, S. Challenges associated with penetration of nanoparticles across cell and tissue barriers: A review of current status and future prospects. Nano Today 2014, 9, 223–243. [CrossRef]
- Wolfram, J.; Zhu, M.; Yang, Y.; Shen, J.; Gentile, E.; Paolino, D.; Fresta, M.; Nie, G.; Chen, C.; Shen, H.; et al. Safety of Nanoparticles in Medicine. Curr. Drug Targets 2015, 16, 1671–1681. [CrossRef]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; del Pilar Rodriguez-Torres, M.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 71. [CrossRef]
- Song, Y.; Ding, Y.; Dong, C. Stimuli-responsive polypeptide nanoassemblies: Recent progress and applications in cancer nanomedicine. WIREs Nanomed. Nanobiotechnology 2021, 14, e1742. [CrossRef]
- Jiang, S.; Chai, H.; Tang, Q.; Shi, Z.; Zhou, L. Clinical advances in oncolytic virus therapy for malignant glioma: a systematic review. Discov. Oncol. 2023, 14, 1–13. [CrossRef]
- Grochans, S.; Cybulska, A.M.; Simińska, D.; Korbecki, J.; Kojder, K.; Chlubek, D.; Baranowska-Bosiacka, I. Epidemiology of Glioblastoma Multiforme–Literature Review. Cancers 2022, 14, 2412. [CrossRef]
- Ostrom, Q.T., et al., CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2016-2020. Neuro-Oncology, 2023. 25. [CrossRef]
- Mohaghegh, N.; Ahari, A.; Zehtabi, F.; Buttles, C.; Davani, S.; Hoang, H.; Tseng, K.; Zamanian, B.; Khosravi, S.; Daniali, A.; et al. Injectable hydrogels for personalized cancer immunotherapies. Acta Biomater. 2023, 172, 67–91. [CrossRef]
- Patil, R.; Galstyan, A.; Grodzinski, Z.B.; Shatalova, E.S.; Wagner, S.; Israel, L.L.; Ding, H.; Black, K.L.; Ljubimova, J.Y.; Holler, E. Single- and Multi-Arm Gadolinium MRI Contrast Agents for Targeted Imaging of Glioblastoma. Int. J. Nanomed. 2020, ume 15, 3057–3070. [CrossRef]
- Patil, R.; Galstyan, A.; Sun, T.; Shatalova, E.S.; Butte, P.; Mamelak, A.N.; Carico, C.; Kittle, D.S.; Grodzinski, Z.B.; Chiechi, A.; et al. Polymalic acid chlorotoxin nanoconjugate for near-infrared fluorescence guided resection of glioblastoma multiforme. Biomaterials 2019, 206, 146–159. [CrossRef]
- Patil, R., et al., Curcumin Targeted, Polymalic Acid-Based MRI Contrast Agent for the Detection of Abeta Plaques in Alzheimer’s Disease. Macromol Biosci, 2015. 15(9): p. 1212-7. [CrossRef]
- Patil, R.; Ljubimov, A.V.; Gangalum, P.R.; Ding, H.; Portilla-Arias, J.; Wagner, S.; Inoue, S.; Konda, B.; Rekechenetskiy, A.; Chesnokova, A.; et al. MRI Virtual Biopsy and Treatment of Brain Metastatic Tumors with Targeted Nanobioconjugates: Nanoclinic in the Brain. ACS Nano 2015, 9, 5594–5608. [CrossRef]
- Patil, R.; Sun, T.; Rashid, M.H.; Israel, L.L.; Ramesh, A.; Davani, S.; Black, K.L.; Ljubimov, A.V.; Holler, E.; Ljubimova, J.Y. Multifunctional Nanopolymers for Blood–Brain Barrier Delivery and Inhibition of Glioblastoma Growth through EGFR/EGFRvIII, c-Myc, and PD-1. Nanomaterials 2021, 11, 2892. [CrossRef]
- Sun, T.; Patil, R.; Galstyan, A.; Klymyshyn, D.; Ding, H.; Chesnokova, A.; Cavenee, W.K.; Furnari, F.B.; Ljubimov, V.A.; Shatalova, E.S.; et al. Blockade of a Laminin-411–Notch Axis with CRISPR/Cas9 or a Nanobioconjugate Inhibits Glioblastoma Growth through Tumor-Microenvironment Cross-talk. Cancer Res 2019, 79, 1239–1251. [CrossRef]
- Sasidharan, A.; Monteiro-Riviere, N.A. Biomedical applications of gold nanomaterials: opportunities and challenges. WIREs Nanomed. Nanobiotechnology 2015, 7, 779–796. [CrossRef]
- Freestone, I.; Meeks, N.; Sax, M.; Higgitt, C. The Lycurgus Cup — A Roman nanotechnology. Gold Bull. 2007, 40, 270–277. [CrossRef]
- Longmire, M.; Choyke, P.L.; Kobayashi, H.; El-Boubbou, K.; Tang, X.; Loc, W.S.; Dong, C.; Matters, G.L.; Butler, P.J.; Kester, M.; et al. Clearance properties of nano-sized particles and molecules as imaging agents: considerations and caveats. Nanomedicine 2008, 3, 703–717. [CrossRef]
- Minakshi Das, K.H.S. and S.S.A.A.D.K. Yi, Review on Gold Nanoparticles and Their Applications. Toxicol. Environ. Health. Sci, 2011. 3(4): p. 193-205. [CrossRef]
- Dykman, L.A. and N.G. Khlebtsov, Gold Nanoparticles in Biology and Medicine: Recent Advances and Prospects. Acta Naturae, 2011. 3(2): p. 34-55.
- Dreaden, E.C.; Alkilany, A.M.; Huang, X.; Murphy, C.J.; El-Sayed, M.A. The golden age: Gold nanoparticles for biomedicine. Chem. Soc. Rev. 2012, 41, 2740–2779. [CrossRef]
- Golombek, S.K.; May, J.-N.; Theek, B.; Appold, L.; Drude, N.; Kiessling, F.; Lammers, T. Tumor targeting via EPR: Strategies to enhance patient responses. Adv. Drug Deliv. Rev. 2018, 130, 17–38. [CrossRef]
- Zhang, X. Gold Nanoparticles: Recent Advances in the Biomedical Applications. Cell Biochem. Biophys. 2015, 72, 771–775. [CrossRef]
- Kanaras, A.G.; Kamounah, F.S.; Schaumburg, K.; Kiely, C.J.; Brust, M. Thioalkylated tetraethylene glycol: a new ligand for water soluble monolayer protected gold clusters. Chem. Commun. 2002, 2294–2295. [CrossRef]
- Chithrani, B.D.; Ghazani, A.A.; Chan, W.C.W. Determining the Size and Shape Dependence of Gold Nanoparticle Uptake into Mammalian Cells. Nano Lett. 2006, 6, 662–668. [CrossRef]
- Kesharwani, P.; Ma, R.; Sang, L.; Fatima, M.; Sheikh, A.; Abourehab, M.A.S.; Gupta, N.; Chen, Z.-S.; Zhou, Y. Gold nanoparticles and gold nanorods in the landscape of cancer therapy. Mol. Cancer 2023, 22, 1–31. [CrossRef]
- Nejabat, M.; Samie, A.; Ramezani, M.; Alibolandi, M.; Abnous, K.; Taghdisi, S.M. An Overview on Gold Nanorods as Versatile Nanoparticles in Cancer Therapy. J. Control. Release 2023, 354, 221–242. [CrossRef]
- Favi, P.M.; Gao, M.; Arango, L.J.S.; Ospina, S.P.; Morales, M.; Pavon, J.J.; Webster, T.J. Shape and surface effects on the cytotoxicity of nanoparticles: Gold nanospheres versus gold nanostars. J. Biomed. Mater. Res. Part A 2015, 103, 3449–3462. [CrossRef]
- Anik, M.I.; Mahmud, N.; Al Masud, A.; Hasan, M. Gold nanoparticles (GNPs) in biomedical and clinical applications: A review. Nano Sel. 2021, 3, 792–828. [CrossRef]
- Lee, D. and S. Yoon, Gold Nanocube-Nanosphere Dimers: Preparation, Plasmon Coupling, and Surface-Enhanced Raman Scattering. Journal of Physical Chemistry C, 2015. 119(14): p. 7873-7882. [CrossRef]
- Campu, A.; Focsan, M.; Lerouge, F.; Borlan, R.; Tie, L.; Rugina, D.; Astilean, S. ICG-loaded gold nano-bipyramids with NIR activatable dual PTT-PDT therapeutic potential in melanoma cells. Colloids Surfaces B: Biointerfaces 2020, 194, 111213. [CrossRef]
- Jahan, S.T.; Sadat, S.M.A.; Walliser, M.; Haddadi, A. Targeted Therapeutic Nanoparticles: An Immense Promise to Fight against Cancer. J. Drug Deliv. 2017, 2017, 1–24. [CrossRef]
- Yetisgin, A.A.; Cetinel, S.; Zuvin, M.; Kosar, A.; Kutlu, O. Therapeutic Nanoparticles and Their Targeted Delivery Applications. Molecules 2020, 25, 2193. [CrossRef]
- Rampado, R.; Crotti, S.; Caliceti, P.; Pucciarelli, S.; Agostini, M. Recent Advances in Understanding the Protein Corona of Nanoparticles and in the Formulation of “Stealthy” Nanomaterials. Front. Bioeng. Biotechnol. 2020, 8, 166. [CrossRef]
- Park, S.J. Protein–Nanoparticle Interaction: Corona Formation and Conformational Changes in Proteins on Nanoparticles. Int. J. Nanomed. 2020, ume 15, 5783–5802. [CrossRef]
- Adhipandito, C.F.; Cheung, S.-H.; Lin, Y.-H.; Wu, S.-H. Atypical Renal Clearance of Nanoparticles Larger Than the Kidney Filtration Threshold. Int. J. Mol. Sci. 2021, 22, 11182. [CrossRef]
- Choi, H.S.; Liu, W.; Misra, P.; Tanaka, E.; Zimmer, J.P.; Ipe, B.I.; Bawendi, M.G.; Frangioni, J.V. Renal clearance of quantum dots. Nat. Biotechnol. 2007, 25, 1165–1170. [CrossRef]
- Xie, X.; Liao, J.; Shao, X.; Li, Q.; Lin, Y. The Effect of shape on Cellular Uptake of Gold Nanoparticles in the forms of Stars, Rods, and Triangles. Sci. Rep. 2017, 7, 1–9. [CrossRef]
- Yang, L.; Zhou, Z.; Song, J.; Chen, X. Anisotropic nanomaterials for shape-dependent physicochemical and biomedical applications. Chem. Soc. Rev. 2019, 48, 5140–5176. [CrossRef]
- Elahi, N.; Kamali, M.; Baghersad, M.H. Recent biomedical applications of gold nanoparticles: A review. Talanta 2018, 184, 537–556. [CrossRef]
- Zeng, S.; Yong, K.-T.; Roy, I.; Dinh, X.-Q.; Yu, X.; Luan, F. A Review on Functionalized Gold Nanoparticles for Biosensing Applications. Plasmonics 2011, 6, 491–506. [CrossRef]
- Link, S.; El-Sayed, M.A. Size and Temperature Dependence of the Plasmon Absorption of Colloidal Gold Nanoparticles. J. Phys. Chem. B 1999, 103, 4212–4217. [CrossRef]
- Njoki, P.N.; Lim, I.-I.S.; Mott, D.; Park, H.-Y.; Khan, B.; Mishra, S.; Sujakumar, R.; Luo, J.; Zhong, C.-J. Size Correlation of Optical and Spectroscopic Properties for Gold Nanoparticles. J. Phys. Chem. C 2007, 111, 14664–14669. [CrossRef]
- Bansal, S.A.; Kumar, V.; Karimi, J.; Singh, A.P.; Kumar, S. Role of gold nanoparticles in advanced biomedical applications. Nanoscale Adv. 2020, 2, 3764–3787. [CrossRef]
- Rodríguez-Lorenzo, L.; Álvarez-Puebla, R.A.; de Abajo, F.J.G.; Liz-Marzán, L.M. Surface Enhanced Raman Scattering Using Star-Shaped Gold Colloidal Nanoparticles. J. Phys. Chem. C 2009, 114, 7336–7340. [CrossRef]
- Hong, S.; Zheng, D.-W.; Zhang, C.; Huang, Q.-X.; Cheng, S.-X.; Zhang, X.-Z. Vascular disrupting agent induced aggregation of gold nanoparticles for photothermally enhanced tumor vascular disruption. Sci. Adv. 2020, 6, eabb0020. [CrossRef]
- Zhang, X. Gold Nanoparticles: Recent Advances in the Biomedical Applications. Cell Biochem. Biophys. 2015, 72, 771–775. [CrossRef]
- Dheyab, M.A.; Aziz, A.A.; Oladzadabbasabadi, N.; Alsaedi, A.; Braim, F.S.; Jameel, M.S.; Ramizy, A.; Alrosan, M.; Almajwal, A.M. Comparative Analysis of Stable Gold Nanoparticles Synthesized Using Sonochemical and Reduction Methods for Antibacterial Activity. Molecules 2023, 28, 3931. [CrossRef]
- Khoobchandani, M., et al., Laminin Receptor-Avid Nanotherapeutic EGCg-AuNPs as a Potential Alternative Therapeutic Approach to Prevent Restenosis. Int J Mol Sci, 2016. 17(3): p. 316. [CrossRef]
- Chen, H.; Kou, X.; Yang, Z.; Ni, W.; Wang, J. Shape- and Size-Dependent Refractive Index Sensitivity of Gold Nanoparticles. Langmuir 2008, 24, 5233–5237. [CrossRef]
- Grzelczak, M.; Pérez-Juste, J.; Mulvaney, P.; Liz-Marzán, L.M. Shape control in gold nanoparticle synthesis. Chem. Soc. Rev. 2008, 37, 1783–1791. [CrossRef]
- Link, S.; El-Sayed, M.A. Spectral Properties and Relaxation Dynamics of Surface Plasmon Electronic Oscillations in Gold and Silver Nanodots and Nanorods. J. Phys. Chem. B 1999, 103, 8410–8426. [CrossRef]
- Sosa, I.O.; Noguez, C.; Barrera, R.G. Optical Properties of Metal Nanoparticles with Arbitrary Shapes. J. Phys. Chem. B 2003, 107, 6269–6275. [CrossRef]
- Amendola, V.; Meneghetti, M. Size Evaluation of Gold Nanoparticles by UV−vis Spectroscopy. J. Phys. Chem. C 2009, 113, 4277–4285. [CrossRef]
- Haiss, W.; Thanh, N.T.K.; Aveyard, J.; Fernig, D.G. Determination of Size and Concentration of Gold Nanoparticles from UV−Vis Spectra. Anal. Chem. 2007, 79, 4215–4221. [CrossRef]
- Shao, L.; Susha, A.S.; Cheung, L.S.; Sau, T.K.; Rogach, A.L.; Wang, J. Plasmonic Properties of Single Multispiked Gold Nanostars: Correlating Modeling with Experiments. Langmuir 2012, 28, 8979–8984. [CrossRef]
- Hao, F.; Nehl, C.L.; Hafner, J.H.; Nordlander, P. Plasmon Resonances of a Gold Nanostar. Nano Lett. 2007, 7, 729–732. [CrossRef]
- Le, N.T.; Boskovic, T.J.M.; Allard, M.M.; Nick, K.E.; Kwon, S.R.; Perry, C.C. Gold Nanostar Characterization by Nanoparticle Tracking Analysis. ACS Omega 2022, 7, 44677–44688. [CrossRef]
- Mayer, K.M. and J.H. Hafner, Localized Surface Plasmon Resonance Sensors. Chemical Reviews, 2011. 111(6): p. 3828-3857. [CrossRef]
- Stetefeld, J.; McKenna, S.A.; Patel, T.R. Dynamic light scattering: a practical guide and applications in biomedical sciences. Biophys. Rev. 2016, 8, 409–427. [CrossRef]
- Hassan, P.A.; Rana, S.; Verma, G. Making Sense of Brownian Motion: Colloid Characterization by Dynamic Light Scattering. Langmuir 2014, 31, 3–12. [CrossRef]
- Krieg, M.; Fläschner, G.; Alsteens, D.; Gaub, B.M.; Roos, W.H.; Wuite, G.J.L.; Gaub, H.E.; Gerber, C.; Dufrêne, Y.F.; Müller, D.J. Atomic force microscopy-based mechanobiology. Nat. Rev. Phys. 2018, 1, 41–57. [CrossRef]
- Alsteens, D.; Gaub, H.E.; Newton, R.; Pfreundschuh, M.; Gerber, C.; Müller, D.J. Atomic force microscopy-based characterization and design of biointerfaces. Nat. Rev. Mater. 2017, 2. [CrossRef]
- Winey, M.; Meehl, J.B.; O’Toole, E.T.; Giddings, T.H., Jr. Conventional transmission electron microscopy. Mol. Biol. Cell 2017, 25, 319–426. [CrossRef]
- Zhao, P.; Li, N.; Astruc, D. State of the art in gold nanoparticle synthesis. Coord. Chem. Rev. 2013, 257, 638–665. [CrossRef]
- Parab, H.; Jung, C.; Woo, M.-A.; Park, H.G. An anisotropic snowflake-like structural assembly of polymer-capped gold nanoparticles. J. Nanoparticle Res. 2010, 13, 2173–2180. [CrossRef]
- Suárez-López, R.; Puntes, V.F.; Bastús, N.G.; Hervés, C.; Jaime, C. Nucleation and growth of gold nanoparticles in the presence of different surfactants. A dissipative particle dynamics study. Sci. Rep. 2022, 12, 1–12. [CrossRef]
- John Turkevich, P.C.S.a.J.H., A study of the nucleation and growth processes in the synthesis of colloidal gold. Discussions of the Faraday Society, 1951. 11: p. 55-75. [CrossRef]
- Frens, G. Controlled Nucleation for the Regulation of the Particle Size in Monodisperse Gold Suspensions. Nat. Phys. Sci. 1973, 241, 20–22. [CrossRef]
- Mathias Brust, M.W., Donald Bethell, David J. Schiffrin and Robin Whyman Synthesis of thiol-derivatised gold nanoparticles in a two-phase Liquid–Liquid system. J,. Chem. Soc., Chem. Commun, 1994: p. 801-802. [CrossRef]
- Hammami, I.; Alabdallah, N.M.; Al Jomaa, A.; Kamoun, M. Gold nanoparticles: Synthesis properties and applications. J. King Saud Univ. - Sci. 2021, 33, 101560. [CrossRef]
- Ashton, J.R.; Castle, K.D.; Qi, Y.; Kirsch, D.G.; West, J.L.; Badea, C.T. Dual-Energy CT Imaging of Tumor Liposome Delivery After Gold Nanoparticle-Augmented Radiation Therapy. Theranostics 2018, 8, 1782–1797. [CrossRef]
- Kadkhoda, J.; Aghanejad, A.; Safari, B.; Barar, J.; Rasta, S.H.; Davaran, S. Aptamer-conjugated gold nanoparticles for targeted paclitaxel delivery and photothermal therapy in breast cancer. J. Drug Deliv. Sci. Technol. 2021, 67, 102954. [CrossRef]
- Kobal, M.; Camacho, S.; Moreira, L.; Toledo, K.; Tada, D.; Aoki, P. Unveiling the mechanisms underlying photothermal efficiency of gold shell-isolated nanoparticles (AuSHINs) on ductal mammary carcinoma cells (BT-474). Biophys. Chem. 2023, 300, 107077. [CrossRef]
- Feng, Q.; Shen, Y.; Fu, Y.; Muroski, M.E.; Zhang, P.; Wang, Q.; Xu, C.; Lesniak, M.S.; Li, G.; Cheng, Y. Self-Assembly of Gold Nanoparticles Shows Microenvironment-Mediated Dynamic Switching and Enhanced Brain Tumor Targeting. Theranostics 2017, 7, 1875–1889. [CrossRef]
- Mangadlao, J.D.; Wang, X.; McCleese, C.; Escamilla, M.; Ramamurthy, G.; Wang, Z.; Govande, M.; Basilion, J.P.; Burda, C. Prostate-Specific Membrane Antigen Targeted Gold Nanoparticles for Theranostics of Prostate Cancer. ACS Nano 2018, 12, 3714–3725. [CrossRef]
- Conde, J.; Tian, F.; Hernández, Y.; Bao, C.; Cui, D.; Janssen, K.-P.; Ibarra, M.R.; Baptista, P.V.; Stoeger, T.; de la Fuente, J.M. In vivo tumor targeting via nanoparticle-mediated therapeutic siRNA coupled to inflammatory response in lung cancer mouse models. Biomaterials 2013, 34, 7744–7753. [CrossRef]
- Zhang, L.; Su, H.; Wang, H.; Li, Q.; Li, X.; Zhou, C.; Xu, J.; Chai, Y.; Liang, X.; Xiong, L.; et al. Tumor Chemo-Radiotherapy with Rod-Shaped and Spherical Gold Nano Probes: Shape and Active Targeting Both Matter. Theranostics 2019, 9, 1893–1908. [CrossRef]
- Wang, S.; Huang, P.; Nie, L.; Xing, R.; Liu, D.; Wang, Z.; Lin, J.; Chen, S.; Niu, G.; Lu, G.; et al. Single Continuous Wave Laser Induced Photodynamic/Plasmonic Photothermal Therapy Using Photosensitizer-Functionalized Gold Nanostars. Adv. Mater. 2013, 25, 3055–3061. [CrossRef]
- Coelho, S.C.; Reis, D.P.; Pereira, M.C.; Coelho, M.A.N. Doxorubicin and Varlitinib Delivery by Functionalized Gold Nanoparticles Against Human Pancreatic Adenocarcinoma. Pharmaceutics 2019, 11, 551. [CrossRef]
- Cho, E.C.; Au, L.; Zhang, Q.; Xia, Y. The Effects of Size, Shape, and Surface Functional Group of Gold Nanostructures on Their Adsorption and Internalization by Cells. Small 2010, 6, 517–522. [CrossRef]
- Fytianos, K.; Rodriguez-Lorenzo, L.; Clift, M.J.; Blank, F.; Vanhecke, D.; von Garnier, C.; Petri-Fink, A.; Rothen-Rutishauser, B. Uptake efficiency of surface modified gold nanoparticles does not correlate with functional changes and cytokine secretion in human dendritic cells in vitro. Nanomedicine: Nanotechnology, Biol. Med. 2015, 11, 633–644. [CrossRef]
- Nambara, K.; Niikura, K.; Mitomo, H.; Ninomiya, T.; Takeuchi, C.; Wei, J.; Matsuo, Y.; Ijiro, K. Reverse Size Dependences of the Cellular Uptake of Triangular and Spherical Gold Nanoparticles. Langmuir 2016, 32, 12559–12567. [CrossRef]
- Pechyen, C.; Ponsanti, K.; Tangnorawich, B.; Ngernyuang, N. Biogenic synthesis of gold nanoparticles mediated by Spondias dulcis (Anacardiaceae) peel extract and its cytotoxic activity in human breast cancer cell. Toxicol. Rep. 2022, 9, 1092–1098. [CrossRef]
- Liu, R.; Xiao, W.; Hu, C.; Xie, R.; Gao, H. Theranostic size-reducible and no donor conjugated gold nanocluster fabricated hyaluronic acid nanoparticle with optimal size for combinational treatment of breast cancer and lung metastasis. J. Control. Release 2018, 278, 127–139. [CrossRef]
- Kamal, R., V.D. Chadha, and D.K. Dhawan, Physiological uptake and retention of radiolabeled resveratrol loaded gold nanoparticles ((99m)Tc-Res-AuNP) in colon cancer tissue. Nanomedicine, 2018. 14(3): p. 1059-1071. [CrossRef]
- Hosseinzadeh, H.; Atyabi, F.; Varnamkhasti, B.S.; Hosseinzadeh, R.; Ostad, S.N.; Ghahremani, M.H.; Dinarvand, R. SN38 conjugated hyaluronic acid gold nanoparticles as a novel system against metastatic colon cancer cells. Int. J. Pharm. 2017, 526, 339–352. [CrossRef]
- Thambiraj, S.; Shruthi, S.; Vijayalakshmi, R.; Shankaran, D.R. Evaluation of cytotoxic activity of docetaxel loaded gold nanoparticles for lung cancer drug delivery. Cancer Treat. Res. Commun. 2019, 21, 100157. [CrossRef]
- Wang, Q.; Zhang, X.; Sun, Y.; Wang, L.; Ding, L.; Zhu, W.-H.; Di, W.; Duan, Y.-R. Gold-caged copolymer nanoparticles as multimodal synergistic photodynamic/photothermal/chemotherapy platform against lethality androgen-resistant prostate cancer. Biomaterials 2019, 212, 73–86. [CrossRef]
- Zeiderman, M.R., et al., Acidic pH-targeted chitosan capped mesoporous silica coated gold nanorods facilitate detection of pancreatic tumors via multispectral optoacoustic tomography. ACS Biomater Sci Eng, 2016. 2(7): p. 1108-1120. [CrossRef]
- Ahangari, A., et al., Development of gentamicin-gold nanospheres for antimicrobial drug delivery to Staphylococcal infected foci. Drug Deliv, 2013. 20(1): p. 34-9. [CrossRef]
- Bagga, P.; Ansari, T.M.; Siddiqui, H.H.; Syed, A.; Bahkali, A.H.; Rahman, A.; Khan, M.S. Bromelain capped gold nanoparticles as the novel drug delivery carriers to aggrandize effect of the antibiotic levofloxacin. 2016, 15, 772–780. [CrossRef]
- Jia, C.; Chen, H.; Wei, M.; Chen, X.; Zhang, Y.; Cao, L.; Yuan, P.; Wang, F.; Yang, G.; Ma, J. Gold nanoparticle-based miR155 antagonist macrophage delivery restores the cardiac function in ovariectomized diabetic mouse model. Int. J. Nanomed. 2017, 12, 4963–4979. [CrossRef]
- Tu, L.; Luo, Z.; Wu, Y.-L.; Huo, S.; Liang, X.-J. Gold-based nanomaterials for the treatment of brain cancer. Cancer Biol. Med. 2021, 18, 372–387. [CrossRef]
- Abbott, N.J., L. Rönnbäck, and E. Hansson, Astrocyte–endothelial interactions at the blood–brain barrier. Nature Reviews Neuroscience, 2006. 7(1): p. 41-53. [CrossRef]
- Zhang, Y.; Walker, J.B.; Minic, Z.; Liu, F.; Goshgarian, H.; Mao, G. Transporter protein and drug-conjugated gold nanoparticles capable of bypassing the blood-brain barrier. Sci. Rep. 2016, 6, 25794. [CrossRef]
- Moreno, D.E.; Yu, X.-J.; Goshgarian, H.G. Identification of the axon pathways which mediate functional recovery of a paralyzed hemidiaphragm following spinal cord hemisection in the adult rat. Exp. Neurol. 1992, 116, 219–228. [CrossRef]
- Cardoso, F.L.; Brites, D.; Brito, M.A. Looking at the blood–brain barrier: Molecular anatomy and possible investigation approaches. Brain Res. Rev. 2010, 64, 328–363. [CrossRef]
- Woźniak, A.; Malankowska, A.; Nowaczyk, G.; Grześkowiak, B.F.; Tuśnio, K.; Słomski, R.; Zaleska-Medynska, A.; Jurga, S. Size and shape-dependent cytotoxicity profile of gold nanoparticles for biomedical applications. J. Mater. Sci. Mater. Med. 2017, 28, 92. [CrossRef]
- Gao, G.; Chen, R.; He, M.; Li, J.; Wang, L.; Sun, T. Gold nanoclusters for Parkinson's disease treatment. Biomaterials 2018, 194, 36–46. [CrossRef]
- Bartneck, M.; Keul, H.A.; Singh, S.; Czaja, K.; Bornemann, J.; Bockstaller, M.; Moeller, M.; Zwadlo-Klarwasser, G.; Groll, J. Rapid Uptake of Gold Nanorods by Primary Human Blood Phagocytes and Immunomodulatory Effects of Surface Chemistry. ACS Nano 2010, 4, 3073–3086. [CrossRef]
- Pan, Y.; Neuss, S.; Leifert, A.; Fischler, M.; Wen, F.; Simon, U.; Schmid, G.; Brandau, W.; Jahnen-Dechent, W. Size-Dependent Cytotoxicity of Gold Nanoparticles. Small 2007, 3, 1941–1949. [CrossRef]
- Pernodet, N.; Fang, X.; Sun, Y.; Bakhtina, A.; Ramakrishnan, A.; Sokolov, J.; Ulman, A.; Rafailovich, M. Adverse Effects of Citrate/Gold Nanoparticles on Human Dermal Fibroblasts. Small 2006, 2, 766–773. [CrossRef]
- Shukla, R.; Bansal, V.; Chaudhary, M.; Basu, A.; Bhonde, R.R.; Sastry, M. Biocompatibility of Gold Nanoparticles and Their Endocytotic Fate Inside the Cellular Compartment: A Microscopic Overview. Langmuir 2005, 21, 10644–10654. [CrossRef]
- Connor, E.E.; Mwamuka, J.; Gole, A.; Murphy, C.J.; Wyatt, M.D. Gold Nanoparticles Are Taken Up by Human Cells but Do Not Cause Acute Cytotoxicity. Small 2005, 1, 325–327. [CrossRef]
- Goodman, C.M.; McCusker, C.D.; Yilmaz, T.; Rotello, V.M. Toxicity of Gold Nanoparticles Functionalized with Cationic and Anionic Side Chains. Bioconjugate Chem. 2004, 15, 897–900. [CrossRef]
- Li, S.-D.; Huang, L. Nanoparticles evading the reticuloendothelial system: Role of the supported bilayer. Biochim. et Biophys. Acta (BBA) - Biomembr. 2009, 1788, 2259–2266. [CrossRef]
- Ding, X., et al., Designing Aptamer-Gold Nanoparticle-Loaded pH-Sensitive Liposomes Encapsulate Morin for Treating Cancer. Nanoscale Res Lett, 2020. 15(1): p. 68. [CrossRef]
- Olivet, M.M.; Brown, M.C.; Reitman, Z.J.; Ashley, D.M.; Grant, G.A.; Yang, Y.; Markert, J.M. Clinical Applications of Immunotherapy for Recurrent Glioblastoma in Adults. Cancers 2023, 15, 3901. [CrossRef]
- Juloori, A., J.S. Yu, and S.T. Chao, Glioblastoma, M.C. Ward, R.D. Tendulkar, and G.M.M. Videtic, Editors., Springer Publishing Company: New York. p. 2-10.
- Rong, L.; Li, N.; Zhang, Z. Emerging therapies for glioblastoma: current state and future directions. J. Exp. Clin. Cancer Res. 2022, 41, 1–18. [CrossRef]
- Yalamarty, S.S.K.; Filipczak, N.; Li, X.; Subhan, A.; Parveen, F.; Ataide, J.A.; Rajmalani, B.A.; Torchilin, V.P. Mechanisms of Resistance and Current Treatment Options for Glioblastoma Multiforme (GBM). Cancers 2023, 15, 2116. [CrossRef]
- Jose, L.; Liu, S.; Russo, C.; Cong, C.; Song, Y.; Rodriguez, M.; Di Ieva, A. Artificial Intelligence–Assisted Classification of Gliomas Using Whole Slide Images. Arch. Pathol. Lab. Med. 2022, 147, 916–924. [CrossRef]
- Yu, S.; Chen, L.; Xu, H.; Long, S.; Jiang, J.; Wei, W.; Niu, X.; Li, X. Application of nanomaterials in diagnosis and treatment of glioblastoma. Front. Chem. 2022, 10, 1063152. [CrossRef]
- Manea, A.J.; Ray, S.K. Advanced Bioinformatics Analysis and Genetic Technologies for Targeting Autophagy in Glioblastoma Multiforme. Cells 2023, 12, 897. [CrossRef]
- Stupp, R., et al., Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol, 2009. 10(5): p. 459-66. [CrossRef]
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.B.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus Concomitant and Adjuvant Temozolomide for Glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [CrossRef]
- Omuro, A. and L.M. DeAngelis, Glioblastoma and Other Malignant Gliomas A Clinical Review. Jama-Journal of the American Medical Association, 2013. 310(17): p. 1842-1850. [CrossRef]
- Zhang, H.; Wang, R.; Yu, Y.; Liu, J.; Luo, T.; Fan, F. Glioblastoma Treatment Modalities besides Surgery. J. Cancer 2019, 10, 4793–4806. [CrossRef]
- Qi, Z.-Y.; Xing, W.-K.; Shao, C.; Yang, C.; Wang, Z. The role of Gliadel wafers in the treatment of newly diagnosed GBM: a meta-analysis. Drug Des. Dev. Ther. 2015, 9, 3341–3348. [CrossRef]
- van Solinge, T.S., et al., Advances in local therapy for glioblastoma - taking the fight to the tumour. Nature Reviews Neurology, 2022. 18(4): p. 221-236. [CrossRef]
- Loras, A.; Gonzalez-Bonet, L.G.; Gutierrez-Arroyo, J.L.; Martinez-Cadenas, C.; Marques-Torrejon, M.A. Neural Stem Cells as Potential Glioblastoma Cells of Origin. Life 2023, 13, 905. [CrossRef]
- Wang, Z.; Zhang, H.; Xu, S.; Liu, Z.; Cheng, Q. The adaptive transition of glioblastoma stem cells and its implications on treatments. Signal Transduct. Target. Ther. 2021, 6, 1–13. [CrossRef]
- Datta, M.; Chatterjee, S.; Perez, E.M.; Gritsch, S.; Roberge, S.; Duquette, M.; Chen, I.X.; Naxerova, K.; Kumar, A.S.; Ghosh, M.; et al. Losartan controls immune checkpoint blocker-induced edema and improves survival in glioblastoma mouse models. Proc. Natl. Acad. Sci. 2023, 120. [CrossRef]
- Pérez, J.E.; Kopecky, J.; Visse, E.; Darabi, A.; Siesjö, P. Convection-enhanced delivery of temozolomide and whole cell tumor immunizations in GL261 and KR158 experimental mouse gliomas. BMC Cancer 2020, 20, 1–12. [CrossRef]
- Ning, J.; Gavil, N.V.; Wu, S.; Wijeyesinghe, S.; Weyu, E.; Ma, J.; Li, M.; Grigore, F.-N.; Dhawan, S.; Skorput, A.G.J.; et al. Functional virus-specific memory T cells survey glioblastoma. Cancer Immunol. Immunother. 2022, 71, 1863–1875. [CrossRef]
- Ochocka, N.; Segit, P.; Wojnicki, K.; Cyranowski, S.; Swatler, J.; Jacek, K.; Grajkowska, W.; Kaminska, B. Specialized functions and sexual dimorphism explain the functional diversity of the myeloid populations during glioma progression. Cell Rep. 2023, 42, 111971. [CrossRef]
- Sener, U.; Ruff, M.W.; Campian, J.L. Immunotherapy in Glioblastoma: Current Approaches and Future Perspectives. Int. J. Mol. Sci. 2022, 23, 7046. [CrossRef]
- Tritz, Z.P.; Ayasoufi, K.; Johnson, A.J. Anti-PD-1 checkpoint blockade monotherapy in the orthotopic GL261 glioma model: the devil is in the detail. Neuro-Oncology Adv. 2021, 3, vdab066. [CrossRef]
- Yin, S.; Chen, Z.; Chen, D.; Yan, D. Strategies targeting PD-L1 expression and associated opportunities for cancer combination therapy. Theranostics 2023, 13, 1520–1544. [CrossRef]
- Yu, M.W.; Quail, D.F. Immunotherapy for Glioblastoma: Current Progress and Challenges. Front. Immunol. 2021, 12. [CrossRef]
- Zhang, H.; Wang, R.; Yu, Y.; Liu, J.; Luo, T.; Fan, F. Glioblastoma Treatment Modalities besides Surgery. J. Cancer 2019, 10, 4793–4806. [CrossRef]
- Malouff, T.D.; Seneviratne, D.S.; Ebner, D.K.; Stross, W.C.; Waddle, M.R.; Trifiletti, D.M.; Krishnan, S. Boron Neutron Capture Therapy: A Review of Clinical Applications. Front. Oncol. 2021, 11. [CrossRef]
- Schaff, L.R. and I.K. Mellinghoff, Glioblastoma and Other Primary Brain Malignancies in Adults: A Review. JAMA, 2023. 329(7): p. 574-587. [CrossRef]
- Shimizu, S.; Nakai, K.; Li, Y.; Mizumoto, M.; Kumada, H.; Ishikawa, E.; Yamamoto, T.; Matsumura, A.; Sakurai, H. Boron Neutron Capture Therapy for Recurrent Glioblastoma Multiforme: Imaging Evaluation of a Case With Long-Term Local Control and Survival. Cureus 2023, 15, e33898. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).