Submitted:
02 January 2024
Posted:
03 January 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
3D printing and sensors, in retrospect
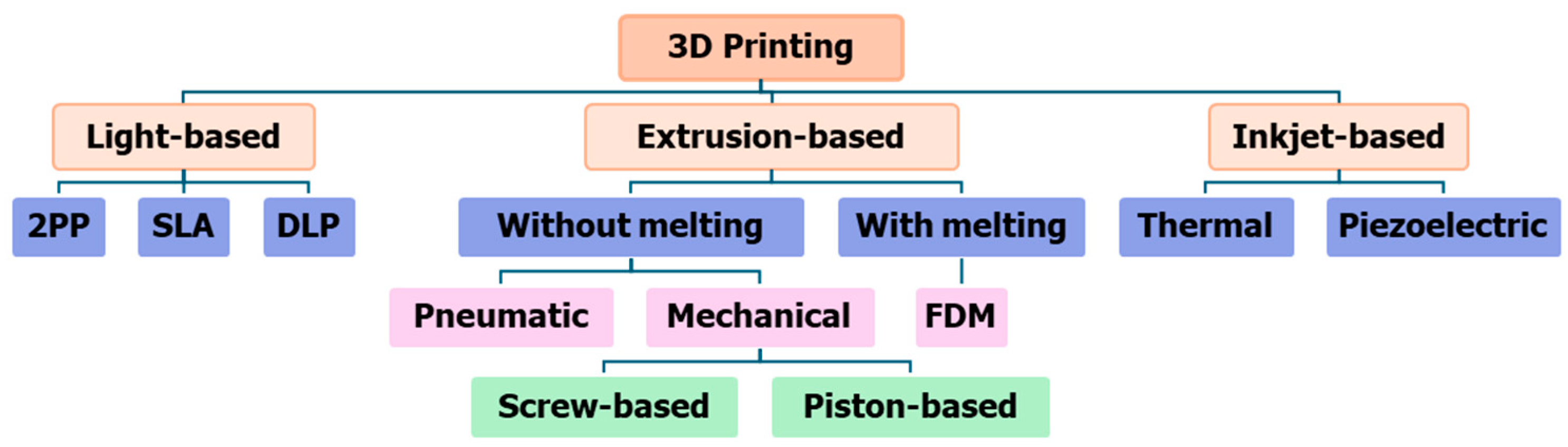
2. An overview of 3D printing technologies
2. Different 3D printing methods and materials
2.1. Vat photopolymerization
2.2. Powder bed fusion
2.3. Material Jetting
2.4. Extrusion-based system
2.5. Inkjet printing
3.1. 3D printers and bioprinters
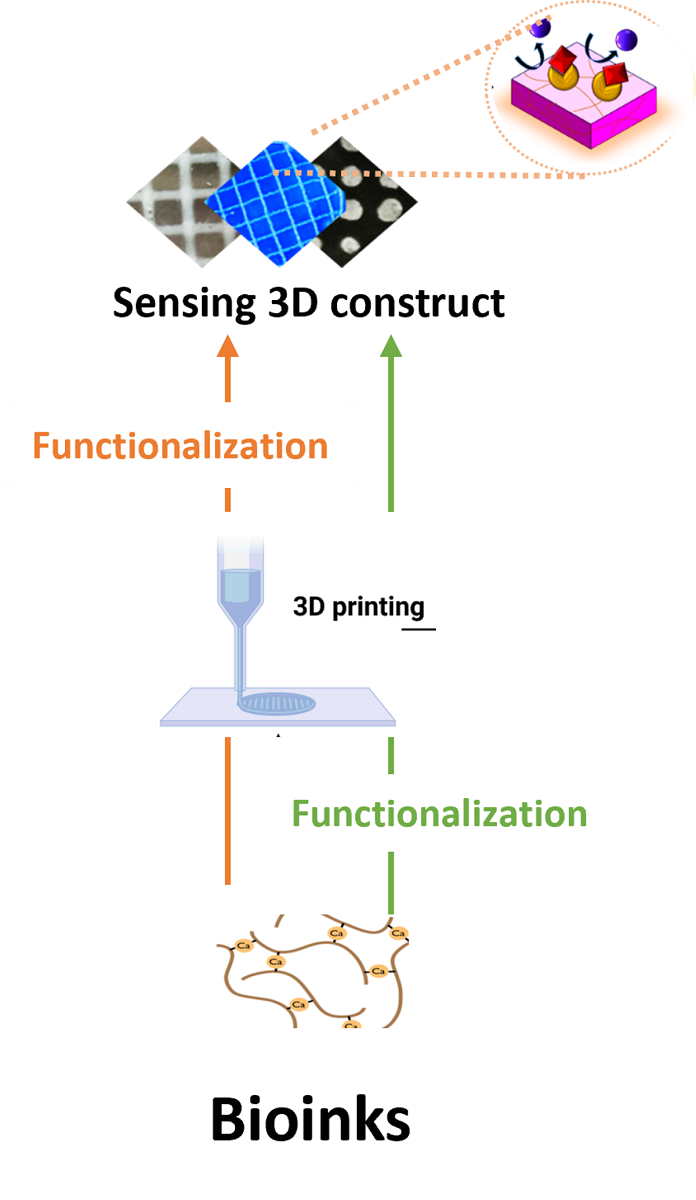
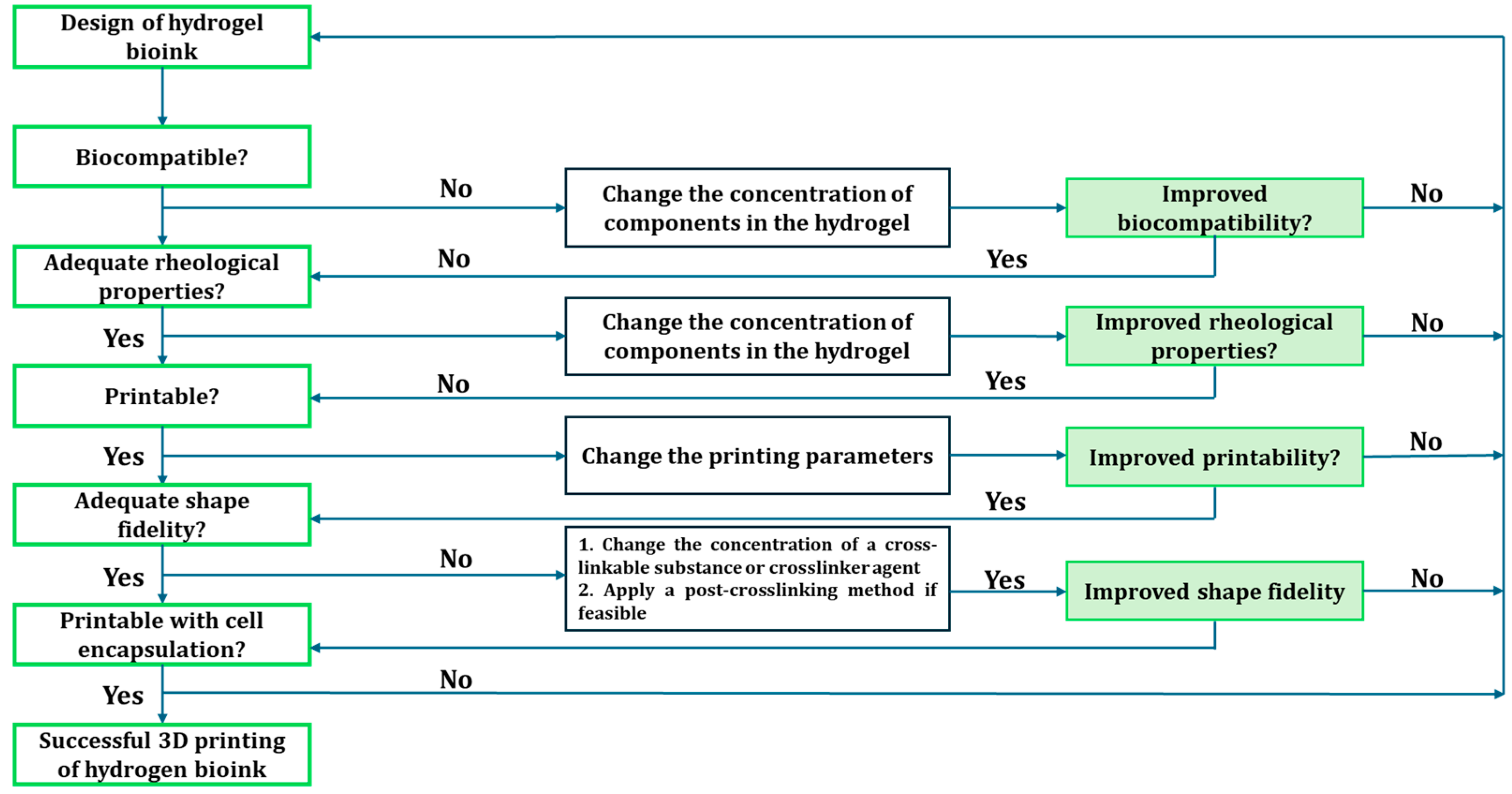
3.2. Bio-inks
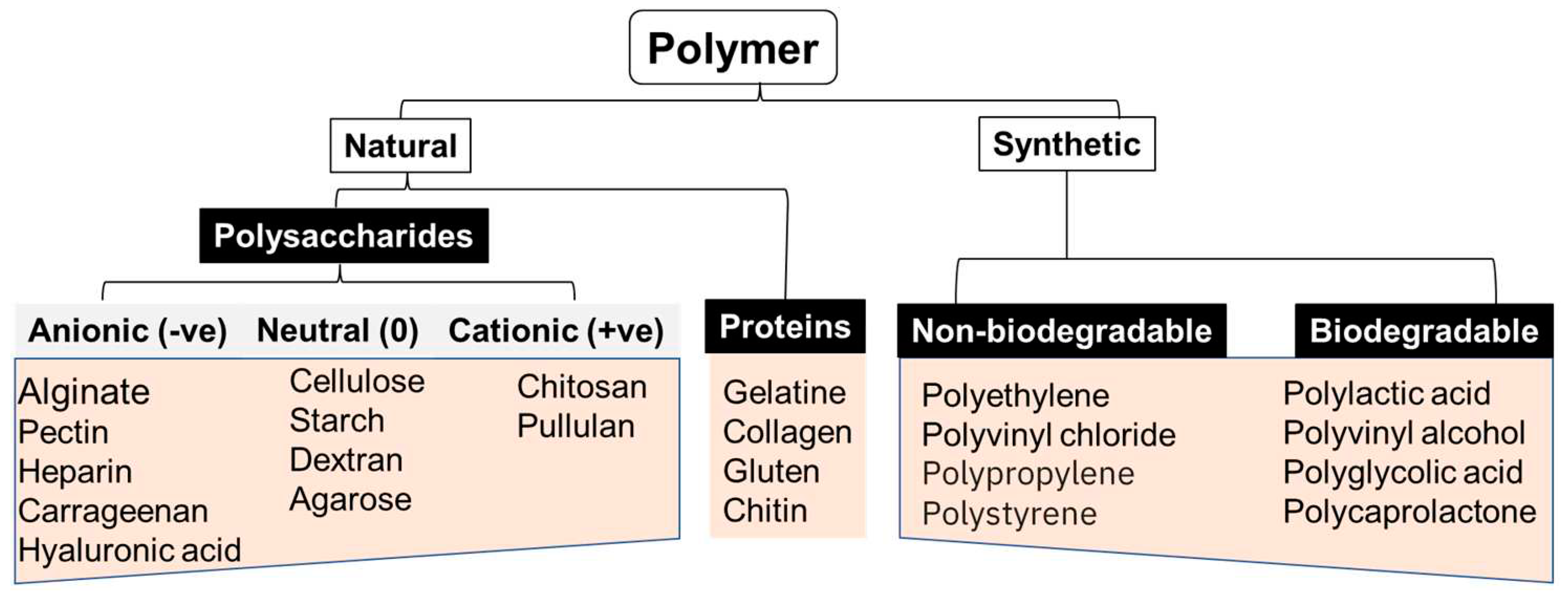
3.3. Different (bio)materials used in 3D printing.
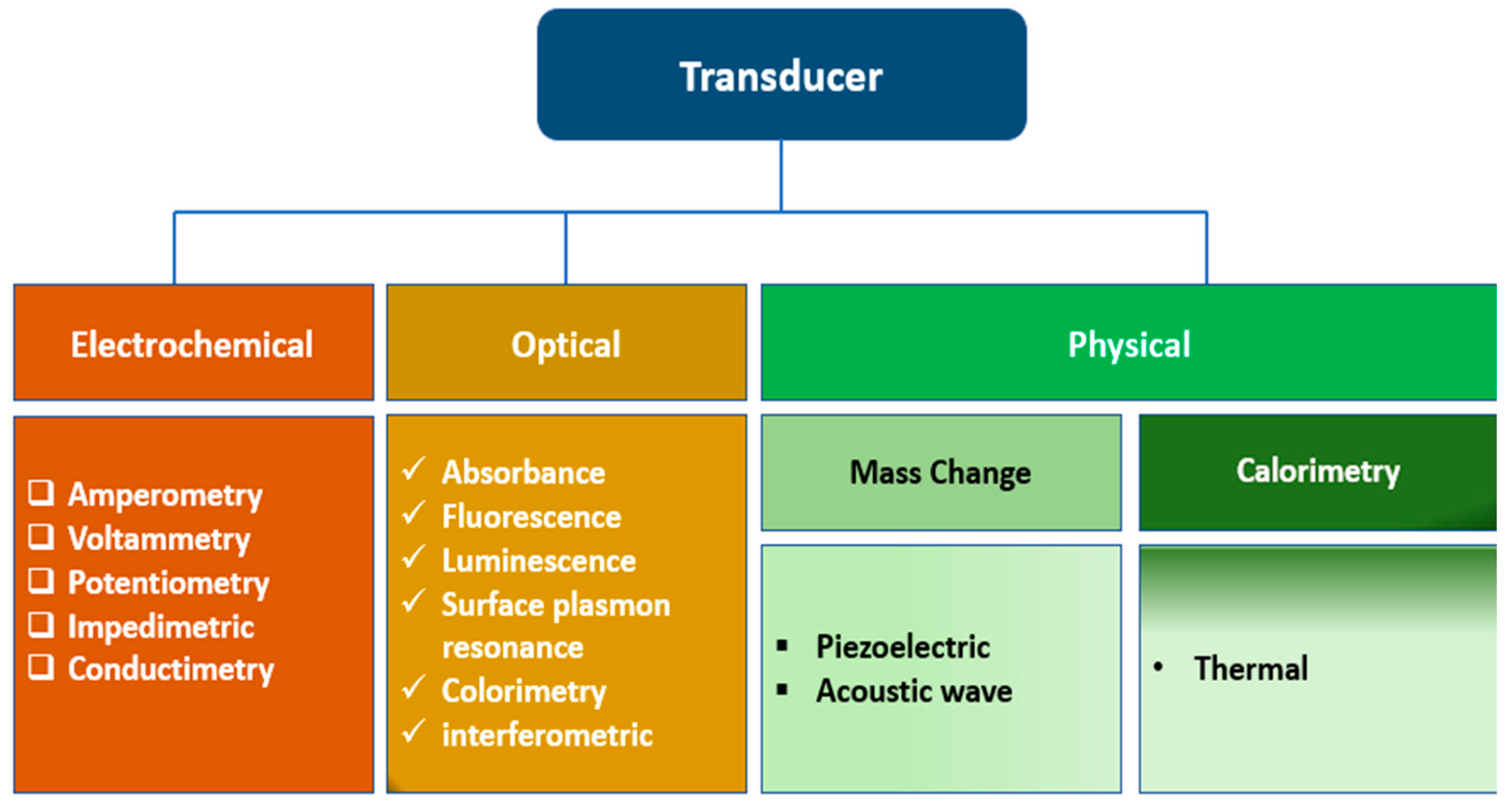
Biosensors: an overview
5. Bioprinting method applications in Biosensors
Approaches of introducing biosensors 3D Bio-printed Biosensors
6. Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yu, C.; Schimelman, J.; Wang, P.; Miller, K.L.; Ma, X.; You, S.; Guan, J.; Sun, B.; Zhu, W.; Chen, S.J.C.r. Photopolymerizable biomaterials and light-based 3D printing strategies for biomedical applications. 2020, 120, 10695-10743. [CrossRef]
- Mandon, C.l.A.; Blum, L.J.; Marquette, C.A.J.A.c. Adding biomolecular recognition capability to 3D printed objects. 2016, 88, 10767-10772. [CrossRef]
- Krujatz, F.; Lode, A.; Seidel, J.; Bley, T.; Gelinsky, M.; Steingroewer, J.J.N.b. Additive Biotech—Chances, challenges, and recent applications of additive manufacturing technologies in biotechnology. 2017, 39, 222-231. [CrossRef]
- Zhu, W.; Ma, X.; Gou, M.; Mei, D.; Zhang, K.; Chen, S.J.C.o.i.b. 3D printing of functional biomaterials for tissue engineering. 2016, 40, 103-112. [CrossRef]
- Reyes, D.R.; Iossifidis, D.; Auroux, P.-A.; Manz, A. Micro total analysis systems. 1. Introduction, theory, and technology. Analytical chemistry 2002, 74, 2623-2636. [CrossRef]
- Ali, M.A.; Hu, C.; Yttri, E.A.; Panat, R. Recent advances in 3D printing of biomedical sensing devices. Advanced functional materials 2022, 32, 2107671. [CrossRef]
- Beebe, D.J.; Mensing, G.A.; Walker, G.M. Physics and applications of microfluidics in biology. Annual review of biomedical engineering 2002, 4, 261-286. [CrossRef]
- Duffy, D.C.; McDonald, J.C.; Schueller, O.J.; Whitesides, G.M. Rapid prototyping of microfluidic systems in poly (dimethylsiloxane). Analytical chemistry 1998, 70, 4974-4984. [CrossRef]
- Whitesides, G.M.; Ostuni, E.; Takayama, S.; Jiang, X.; Ingber, D.E. Soft lithography in biology and biochemistry. Annual review of biomedical engineering 2001, 3, 335-373. [CrossRef]
- Xia, Y.; Whitesides, G.M. Soft lithography. Annual review of materials science 1998, 28, 153-184. [CrossRef]
- Raj M, K.; Chakraborty, S. PDMS microfluidics: A mini review. Journal of Applied Polymer Science 2020, 137, 48958. [CrossRef]
- Monserrat Lopez, D.; Rottmann, P.; Fussenegger, M.; Lörtscher, E. Silicon-Based 3D Microfluidics for Parallelization of Droplet Generation. Micromachines 2023, 14, 1289. [CrossRef]
- Palmara, G.; Frascella, F.; Roppolo, I.; Chiappone, A.; Chiadò, A. Functional 3D printing: Approaches and bioapplications. Biosensors and Bioelectronics 2021, 175, 112849. [CrossRef]
- Jammalamadaka, U.; Tappa, K. Recent advances in biomaterials for 3D printing and tissue engineering. Journal of functional biomaterials 2018, 9, 22. [CrossRef]
- Becker, H. Hype, hope and hubris: the quest for the killer application in microfluidics. Lab on a Chip 2009, 9, 2119-2122. [CrossRef]
- Aladese, A.D.; Jeong, H.-H. Recent developments in 3D printing of droplet-based microfluidics. BioChip Journal 2021, 15, 313-333. [CrossRef]
- Standard, A. Standard terminology for additive manufacturing technologies. ASTM International F2792-12a 2012, 1-9.
- Rajabi, M.; McConnell, M.; Cabral, J.; Ali, M.A.J.C.P. Chitosan hydrogels in 3D printing for biomedical applications. 2021, 260, 117768. [CrossRef]
- Shirazi, S.F.S.; Gharehkhani, S.; Mehrali, M.; Yarmand, H.; Metselaar, H.S.C.; Kadri, N.A.; Osman, N.A.A.J.S.; materials, t.o.a. A review on powder-based additive manufacturing for tissue engineering: selective laser sintering and inkjet 3D printing. 2015. [CrossRef]
- Pagac, M.; Hajnys, J.; Ma, Q.-P.; Jancar, L.; Jansa, J.; Stefek, P.; Mesicek, J.J.P. A review of vat photopolymerization technology: Materials, applications, challenges, and future trends of 3d printing. 2021, 13, 598. [CrossRef]
- Wang, Y.; McAninch, I.M.; Delarue, A.P.; Hansen, C.J.; Robinette, E.J.; Peterson, A.M.J.M.A. Additively manufactured thermosetting elastomer composites: small changes in resin formulation lead to large changes in mechanical and viscoelastic properties. 2023, 4, 607-615. [CrossRef]
- Hull, C. StereoLithography: Plastic prototypes from CAD data without tooling. Modern Casting 1988, 78, 38.
- Waldbaur, A.; Rapp, H.; Länge, K.; Rapp, B.E. Let there be chip—towards rapid prototyping of microfluidic devices: one-step manufacturing processes. Analytical Methods 2011, 3, 2681-2716. [CrossRef]
- Maruo, S.; Kawata, S. Two-photon-absorbed photopolymerization for three-dimensional microfabrication. In Proceedings of the Proceedings IEEE The Tenth Annual International Workshop on Micro Electro Mechanical Systems. An Investigation of Micro Structures, Sensors, Actuators, Machines and Robots, 1997; pp. 169-174. [CrossRef]
- Shallan, A.I.; Smejkal, P.; Corban, M.; Guijt, R.M.; Breadmore, M.C. Cost-effective three-dimensional printing of visibly transparent microchips within minutes. Analytical chemistry 2014, 86, 3124-3130. [CrossRef]
- Bertsch, A.; Zissi, S.; Jezequel, J.; Corbel, S.; Andre, J. Microstereophotolithography using a liquid crystal display as dynamic mask-generator. Microsystem technologies 1997, 3, 42-47. [CrossRef]
- Chan, H.N.; Shu, Y.; Xiong, B.; Chen, Y.; Chen, Y.; Tian, Q.; Michael, S.A.; Shen, B.; Wu, H. Simple, cost-effective 3D printed microfluidic components for disposable, point-of-care colorimetric analysis. Acs Sensors 2016, 1, 227-234. [CrossRef]
- Au, A.K.; Bhattacharjee, N.; Horowitz, L.F.; Chang, T.C.; Folch, A. 3D-printed microfluidic automation. Lab on a Chip 2015, 15, 1934-1941. [CrossRef]
- Hietala, M.; Rautio, T.; Mäkikangas, J.; Järvenpää, A. Mechanical properties of the laser powder deposition and laser powder bed fusion printed 316L. In Proceedings of the IOP Conference Series: Materials Science and Engineering, 2023; p. 012018. [CrossRef]
- Deckard, C.R. Method and apparatus for producing parts by selective sintering. 1991.
- Yan, X.; Gu, P. A review of rapid prototyping technologies and systems. Computer-aided design 1996, 28, 307-318. [CrossRef]
- Ho, E.H.Z.; Ambrosi, A.; Pumera, M. Additive manufacturing of electrochemical interfaces: simultaneous detection of biomarkers. Applied Materials Today 2018, 12, 43-50. [CrossRef]
- Gothait, H. Apparatus and method for three dimensional model printing. 2001.
- Erkal, J.L.; Selimovic, A.; Gross, B.C.; Lockwood, S.Y.; Walton, E.L.; McNamara, S.; Martin, R.S.; Spence, D.M. 3D printed microfluidic devices with integrated versatile and reusable electrodes. Lab on a Chip 2014, 14, 2023-2032. [CrossRef]
- Herbert, R.; Mishra, S.; Lim, H.R.; Yoo, H.; Yeo, W.H. Implantable Electronics: Fully Printed, Wireless, Stretchable Implantable Biosystem toward Batteryless, Real-Time Monitoring of Cerebral Aneurysm Hemodynamics (Adv. Sci. 18/2019). Advanced Science 2019, 6, 1970110. [CrossRef]
- Chua, C.K.; Leong, K.F.; Lim, C.S. Rapid prototyping: principles and applications (with companion CD-ROM); World Scientific Publishing Company: 2010.
- Crump, S.S. Apparatus and method for creating three-dimensional objects. 1992.
- Nguyen, T.N.; Nolan, J.K.; Park, H.; Lam, S.; Fattah, M.; Page, J.C.; Joe, H.-E.; Jun, M.B.; Lee, H.; Kim, S.J. Facile fabrication of flexible glutamate biosensor using direct writing of platinum nanoparticle-based nanocomposite ink. Biosensors and Bioelectronics 2019, 131, 257-266. [CrossRef]
- Cesewski, E.; Haring, A.P.; Tong, Y.; Singh, M.; Thakur, R.; Laheri, S.; Read, K.A.; Powell, M.D.; Oestreich, K.J.; Johnson, B.N. Additive manufacturing of three-dimensional (3D) microfluidic-based microelectromechanical systems (MEMS) for acoustofluidic applications. Lab on a Chip 2018, 18, 2087-2098. [CrossRef]
- Shah, M.A.; Lee, D.-G.; Lee, B.-Y.; Hur, S.J.I.A. Classifications and applications of inkjet printing technology: A review. 2021, 9, 140079-140102. [CrossRef]
- Yang, P.; Fan, H.J.J.A.M.T. Inkjet and extrusion printing for electrochemical energy storage: a minireview. 2020, 5, 2000217. [CrossRef]
- Sowade, E.; Polomoshnov, M.; Willert, A.; Baumann, R.R.J.A.E.M. Toward 3D-printed electronics: inkjet-printed vertical metal wire interconnects and screen-printed batteries. 2019, 21, 1900568. [CrossRef]
- Peng, X.; Lu, A.; Sun, Q.; Xu, N.; Xie, Y.; Wu, J.; Cheng, J.J.M. Design of H-shape chamber in thermal bubble printer. 2022, 13, 194. [CrossRef]
- Pu, Z.; Tu, J.; Han, R.; Zhang, X.; Wu, J.; Fang, C.; Wu, H.; Zhang, X.; Yu, H.; Li, D. A flexible enzyme-electrode sensor with cylindrical working electrode modified with a 3D nanostructure for implantable continuous glucose monitoring. Lab on a Chip 2018, 18, 3570-3577. [CrossRef]
- Bihar, E.; Wustoni, S.; Pappa, A.M.; Salama, K.N.; Baran, D.; Inal, S. A fully inkjet-printed disposable glucose sensor on paper. npj Flexible Electronics 2018, 2, 30. [CrossRef]
- Finny, A.S.; Jiang, C.; Andreescu, S.J.A.a.m.; interfaces. 3D printed hydrogel-based sensors for quantifying UV exposure. 2020, 12, 43911-43920. [CrossRef]
- Chimene, D.; Miller, L.; Cross, L.M.; Jaiswal, M.K.; Singh, I.; Gaharwar, A.K.J.A.a.m.; interfaces. Nanoengineered osteoinductive bioink for 3D bioprinting bone tissue. 2020, 12, 15976-15988. [CrossRef]
- Kim, J.; Choi, H.S.; Kim, Y.M.; Song, S.C.J.S. Thermo-Responsive Nanocomposite Bioink with Growth-Factor Holding and its Application to Bone Regeneration. 2023, 19, 2203464. [CrossRef]
- Kim, G.-J.; Kim, L.; Kwon, O.S.J.A.S.; Technology, C. Application of 3D Bioprinting Technology for Tissue Regeneration, Drug Evaluation, and Drug Delivery. 2023, 32, 1-6. [CrossRef]
- Puri, A.; Sahai, N.; Ahmed, T.; Saxena, K.J.M.T.P. 3D bioprinting for diagnostic and therapeutic application. 2023. [CrossRef]
- Kim, J.J.B. Characterization of Biocompatibility of Functional Bioinks for 3D Bioprinting. 2023, 10, 457. [CrossRef]
- Hennink, W.E.; van Nostrum, C.F.J.A.d.d.r. Novel crosslinking methods to design hydrogels. 2012, 64, 223-236. [CrossRef]
- Ning, L.; Sun, H.; Lelong, T.; Guilloteau, R.; Zhu, N.; Schreyer, D.J.; Chen, X.J.B. 3D bioprinting of scaffolds with living Schwann cells for potential nerve tissue engineering applications. 2018, 10, 035014. [CrossRef]
- Chimene, D.; Lennox, K.K.; Kaunas, R.R.; Gaharwar, A.K.J.A.o.b.e. Advanced bioinks for 3D printing: a materials science perspective. 2016, 44, 2090-2102. [CrossRef]
- Gao, T.; Gillispie, G.J.; Copus, J.S.; Pr, A.K.; Seol, Y.-J.; Atala, A.; Yoo, J.J.; Lee, S.J.J.B. Optimization of gelatin–alginate composite bioink printability using rheological parameters: A systematic approach. 2018, 10, 034106. [CrossRef]
- Senior, J.J.; Cooke, M.E.; Grover, L.M.; Smith, A.M.J.A.F.M. Fabrication of complex hydrogel structures using suspended layer additive manufacturing (SLAM). 2019, 29, 1904845. [CrossRef]
- Dani, S.; Ahlfeld, T.; Albrecht, F.; Duin, S.; Kluger, P.; Lode, A.; Gelinsky, M.J.G. Homogeneous and reproducible mixing of highly viscous biomaterial inks and cell suspensions to create bioinks. 2021, 7, 227. [CrossRef]
- Baranwal, J.; Barse, B.; Fais, A.; Delogu, G.L.; Kumar, A.J.P. Biopolymer: A sustainable material for food and medical applications. 2022, 14, 983. [CrossRef]
- Soltan, N.; Ning, L.; Mohabatpour, F.; Papagerakis, P.; Chen, X.J.A.B.S.; Engineering. Printability and cell viability in bioprinting alginate dialdehyde-gelatin scaffolds. 2019, 5, 2976-2987. [CrossRef]
- Legett, S.A.; Stockdale, J.R.; Torres, X.; Yeager, C.M.; Pacheco, A.; Labouriau, A.J.P. Functional Filaments: Creating and Degrading pH-Indicating PLA Filaments for 3D Printing. 2023, 15, 436. [CrossRef]
- Marquez, C.; Mata, J.J.; Renteria, A.; Gonzalez, D.; Gomez, S.G.; Lopez, A.; Baca, A.N.; Nuñez, A.; Hassan, M.S.; Burke, V.J.S. Direct Ink-Write Printing of Ceramic Clay with an Embedded Wireless Temperature and Relative Humidity Sensor. 2023, 23, 3352. [CrossRef]
- Cleetus, C.M.; Alvarez Primo, F.; Fregoso, G.; Lalitha Raveendran, N.; Noveron, J.C.; Spencer, C.T.; Ramana, C.V.; Joddar, B.J.I.j.o.n. Alginate hydrogels with embedded ZnO nanoparticles for wound healing therapy. 2020, 5097-5111. [CrossRef]
- Garg, M.; Mehrotra, S. Biosensors. In Principles and applications of environmental biotechnology for a sustainable future; Springer: 2017; pp. 341-363. [CrossRef]
- Cui, F.; Yue, Y.; Zhang, Y.; Zhang, Z.; Zhou, H.S.J.A.s. Advancing biosensors with machine learning. 2020, 5, 3346-3364. [CrossRef]
- Long, F.; Zhu, A.; Shi, H.J.S. Recent advances in optical biosensors for environmental monitoring and early warning. 2013, 13, 13928-13948. [CrossRef]
- Arlett, J.; Myers, E.; Roukes, M.J.N.n. Comparative advantages of mechanical biosensors. 2011, 6, 203-215. [CrossRef]
- GUNDOGDU, A.; GAZOGLU, G.; KAHRAMAN, E.; YİLDİZ, E.; CANDİR, G.; YALCİN, D.; Atakan, K.; Fatih, Ş.J.J.o.S.R.-A. BIOSENSORS: TYPES, APPLICATIONS, AND FUTURE ADVANTAGES. 2023, 457-481. [CrossRef]
- Kumar, A.J.M.L. Methods and materials for smart manufacturing: additive manufacturing, internet of things, flexible sensors and soft robotics. 2018, 15, 122-125. [CrossRef]
- Trampe, E.; Koren, K.; Akkineni, A.R.; Senwitz, C.; Krujatz, F.; Lode, A.; Gelinsky, M.; Kühl, M.J.A.F.M. Functionalized bioink with optical sensor nanoparticles for O2 imaging in 3D-bioprinted constructs. 2018, 28, 1804411. [CrossRef]
- Ammam, M.; Fransaer, J.J.S.; Chemical, A.B. Two-enzyme lactose biosensor based on β-galactosidase and glucose oxidase deposited by AC-electrophoresis: Characteristics and performance for lactose determination in milk. 2010, 148, 583-589. [CrossRef]
- Poortinga, A.T.; Bos, R.; Busscher, H.J.J.B.; bioengineering. Controlled electrophoretic deposition of bacteria to surfaces for the design of biofilms. 2000, 67, 117-120.
- Mateen, R.; Ali, M.M.; Hoare, T.J.N.c. A printable hydrogel microarray for drug screening avoids false positives associated with promiscuous aggregating inhibitors. 2018, 9, 1-9. [CrossRef]
- Bruen, D.; Delaney, C.; Chung, J.; Ruberu, K.; Wallace, G.G.; Diamond, D.; Florea, L.J.M.R.C. 3D Printed Sugar-Sensing Hydrogels. 2020, 41, 1900610. [CrossRef]
- Finny, A.S.; Jiang, C.; Andreescu, S. 3D printed hydrogel-based biosensors for wearable applications. In Proceedings of the Electrochemical Society Meeting Abstracts 237, 2020; pp. 1973-1973. [CrossRef]
- Muller, R.; Le, H.-P.; Li, W.; Ledochowitsch, P.; Gambini, S.; Bjorninen, T.; Koralek, A.; Carmena, J.M.; Maharbiz, M.M.; Alon, E. 24.1 A miniaturized 64-channel 225μW wireless electrocorticographic neural sensor. In Proceedings of the 2014 IEEE International Solid-State Circuits Conference Digest of Technical Papers (ISSCC), 2014; pp. 412-413. [CrossRef]
- Chung, H.-J.; Sulkin, M.S.; Kim, J.-S.; Goudeseune, C.; Chao, H.-Y.; Song, J.W.; Yang, S.Y.; Hsu, Y.-Y.; Ghaffari, R.; Efimov, I.R.J.A.h.m. Ultrathin, Stretchable, Multiplexing pH Sensor Arrays on Biomedical Devices With Demonstrations on Rabbit and Human Hearts Undergoing Ischemia. 2014, 3, 59. [CrossRef]
- Jiménez, M.; Romero, L.J.C. M. d. M. Espinosa and M. Domínguez. 2019, 2019, 1-30.
- Ali, M.A.; Hu, C.; Yttri, E.A.; Panat, R.J.A.f.m. Recent advances in 3D printing of biomedical sensing devices. 2022, 32, 2107671. [CrossRef]
- Gao, W.; Zhang, Y.; Ramanujan, D.; Ramani, K.; Chen, Y.; Williams, C.B.; Wang, C.C.; Shin, Y.C.; Zhang, S.; Zavattieri, P.D.J.C.-A.D. The status, challenges, and future of additive manufacturing in engineering. 2015, 69, 65-89. [CrossRef]
- Polyak, B.; Geresh, S.; Marks, R.S.J.B. Synthesis and characterization of a biotin-alginate conjugate and its application in a biosensor construction. 2004, 5, 389-396. [CrossRef]
- Abu-Rabeah, K.; Polyak, B.; Ionescu, R.E.; Cosnier, S.; Marks, R.S.J.B. Synthesis and characterization of a pyrrole− alginate conjugate and its application in a biosensor construction. 2005, 6, 3313-3318. [CrossRef]
- Niţă, I.I.; Abu-Rabeah, K.; Tencaliec, A.M.; Cosnier, S.; Marks, R.S.J.S.m. Amperometric biosensor based on the electro-copolymerization of a conductive biotinylated-pyrrole and alginate-pyrrole. 2009, 159, 1117-1122. [CrossRef]
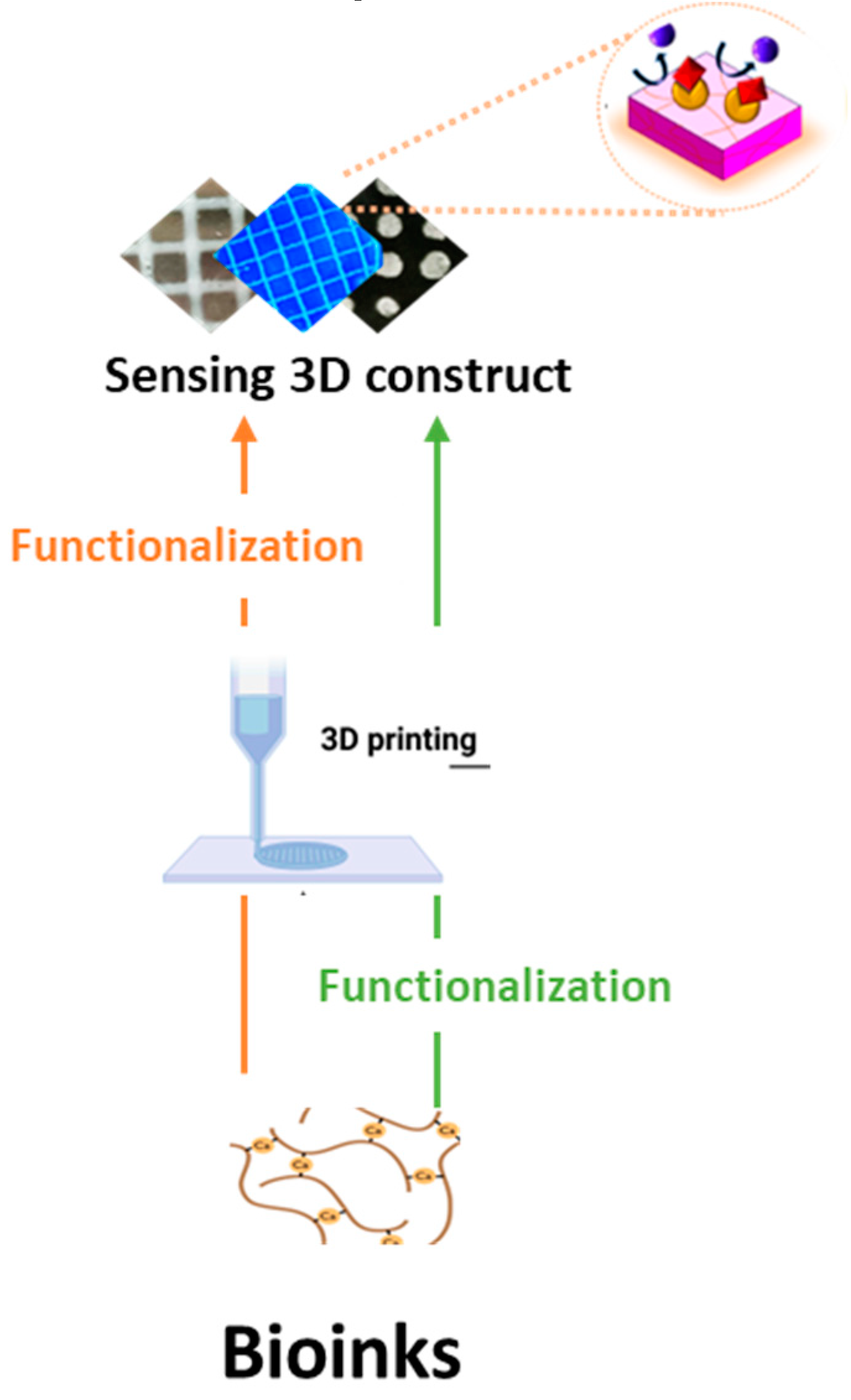
| Polymer (composite) | Source | 3D printing method | Application | Reference |
|---|---|---|---|---|
| Alginate dialdehyde-gelatine | Semi-synthetic | Extrusion | Tissue engineering | [59] |
| poly(lactic acid)/ poly(ethylene glycol) | Semi-synthetic | fused filament fabrication (FFF) | pH-sensor | [60] |
| Gelatine-alginate | Natural | Extrusion | Printability optimization | [55] |
| Ceramic clay | Natural | Direct ink-writing (DIW) | Temperature and humidity sensor | [61] |
| Alginate/ZnO nanoparticles | Natural | Extrusion | Wound healing | [62] |
| Alginate/gelatine/TiO2 | Natural | Extrusion | UV sensor | [46] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





