1. Introduction
Recently, there has been increasing interest in the use of electrospinning to generate continuous nanofibers owing to its high efficiency and cost-effectiveness. The unique advantages of electrospun nanofibers, such as their large specific surface area and high porosity, make them a popular choice in the fields of biomedicine [
1], environmental filtration [
2], functional textiles [
3], and active food packaging [
4].
Electrospun nanofibers have the advantage of being able to load active functional substances. However, their application in cosmetics, particularly facial mask products, is limited. Most currently available sheet masks are soaked in liquid solutions containing a variety of essential ingredients, which are highly popular in the market. However, concerns have been raised regarding the presence of artificial additives and preservatives in these products [
5]. In addition, a limited amount of dry mask is produced using a low-temperature or microwave freeze-drying process [
6]. The process of producing a dry facial mask using electrospinning technology and nanofiber films is more time-consuming and costly than the traditional methods. In addition, the high temperature used in the preparation of this dry mask can result in inactivation of certain ingredients. However, by incorporating electrospinning technology into the cosmetic field, it is possible to directly add effective ingredients to dry mask fabrics without the use of preservatives. This dry mask eliminates the residual waste of active ingredients in the packaging of traditional sheet masks and the skin irritation caused by preservatives [
7].
Hyaluronic acid (HA), a natural glycosaminoglycan, possesses abundant carboxyl and hydroxyl functional groups. These groups facilitate the formation of intramolecular hydrogen bonds and three-dimensional structures, which in turn contribute to their exceptional solubility in water. Additionally, HA can effectively bind water, resulting in the formation of a gel-like substance [
8]. HA is extensively used in various disciplines, including medicine, nutrition, and cosmetic medicine, owing to its exceptional biocompatibility and biodegradation properties [
9]. The application of HA electrospinning is limited by its high viscosity and surface tension under conventional conditions. However, this disadvantage can be mitigated by blending it with other polymers [
8].
Collagen serves as the principal structural component of the extracellular matrix (ECM) in natural systems. Owing to its excellent biocompatibility and straightforward processing, collagen is frequently employed as a scaffold matrix in tubular tissue engineering [
10]. Collagen is typically extracted from natural sources, such as various animal tissues, or through recombinant protein production systems employing yeast, bacteria, mammalian cells, insects, plants, or artificial fibrils that emulate collagen properties [
11]. Collagen, a material that can be economically obtained from leather solid waste because of its prevalence as the main component of leather, exhibits exceptional biocompatibility and biodegradability. Despite these advantages, studies have revealed certain limitations of utilizing collagen fibers as adsorbent materials, such as their low adsorption capacity, poor selectivity, and inability to withstand high temperatures. These issues were addressed in a comprehensive review paper authored by Qiang et al. that focused on the technological aspects of research on collagen-based adsorbents [
12]. In recent years, there has been a notable trend towards the use of fish collagen in cosmetic applications, as it has been demonstrated to be a suitable alternative to mammalian collagen. Collagen derived from fish skin and scales is not only effective in scaffold preparation and wound healing but also has applications in pharmaceutical formulations [
13,
14].
The development of a collagen/hyaluronic acid (HA) blend in the form of nanofibers represents a significant advancement in the field of biomaterial science, particularly in applications related to biomedicine and skin care. This blend combines the structural benefits of collagen with the hydration capabilities of HA, which is encapsulated within the unique physical structure of the nanofibers. This blend helps maintain the structural integrity of various tissues. Collagen, owing to its mechanical strength and ability to support cell adhesion, is ideal for tissue engineering and wound-healing applications because of its biocompatibility and biodegradability. On the other hand, HA enhances the viscoelastic properties of nanofibers, making them highly suitable for skin hydration applications because of their ability to retain moisture. The process of creating collagen/HA blend nanofibers typically involves electrospinning, a technique that uses an electric field to draw a polymer solution into fine fibers. This method allows for precise control of the fiber diameter, porosity, and alignment, which are critical parameters that influence the properties of the material. The resulting nanofibers exhibited a high surface-area-to-volume ratio, which improved the delivery and sustained release of the active biomolecules.
This article discusses the use of electrospun collagen/hyaluronic acid nanofibers for skincare applications, particularly facial masks. The authors proposed using collagen and hyaluronic acid as the main components of the nanofiber film, which has excellent mechanical strength and moisture retention capacity and is highly suitable for skin hydration applications. The process of creating a nanofiber film involves electrospinning, which uses an electric field to draw a polymer solution into fine fibers. The authors suggested that the collagen/hyaluronic acid blend nanofiber film has great potential for use in skincare applications and can solve some of the problems existing in traditional sheet masks, such as the residual waste of active ingredients in packaging and skin irritation caused by preservatives.
2. Materials and Methods
2.1. Methods
Hyaluronic acid (HA) (Mw 25000 g/mol) was obtained from Sigma Aldrich, and CZ. Hydrolyzed Collagen (Mw 2000 Da) was obtained from Darling Instruments, USA. Distillated water was used in all experiments.
2.2. Electrospinning Solutions Preparation
Collagen 16.32 wt.% in 160 ml distilled water with heating and magnetic stirring was used. The prepared solutions were mixed at 280 rpm (RT10; IKA, Germany) and 39 °C. Pure Collagen (16.32 w.t.), Pure HA ( 2.5 wt. %) and Collagen/HA (18.4 wt.%) polymeric solutions were prepared. HA (2.5 wt.%) was dissolved in the collagen solution (
Figure 2). The resulting solution was then stirred at room temperature for 5 h.
2.3. Nanofiber Preparation
Electrospinning was performed using an electrospinning machine (Nanospider Lab, Elmarco, Czech Republic). The spinning parameters were as follows: temperature 21.4 °C, relative humidity, 44 %, electrode–substrate distance, 50 mm, nozzle diameter 0.9 mm.
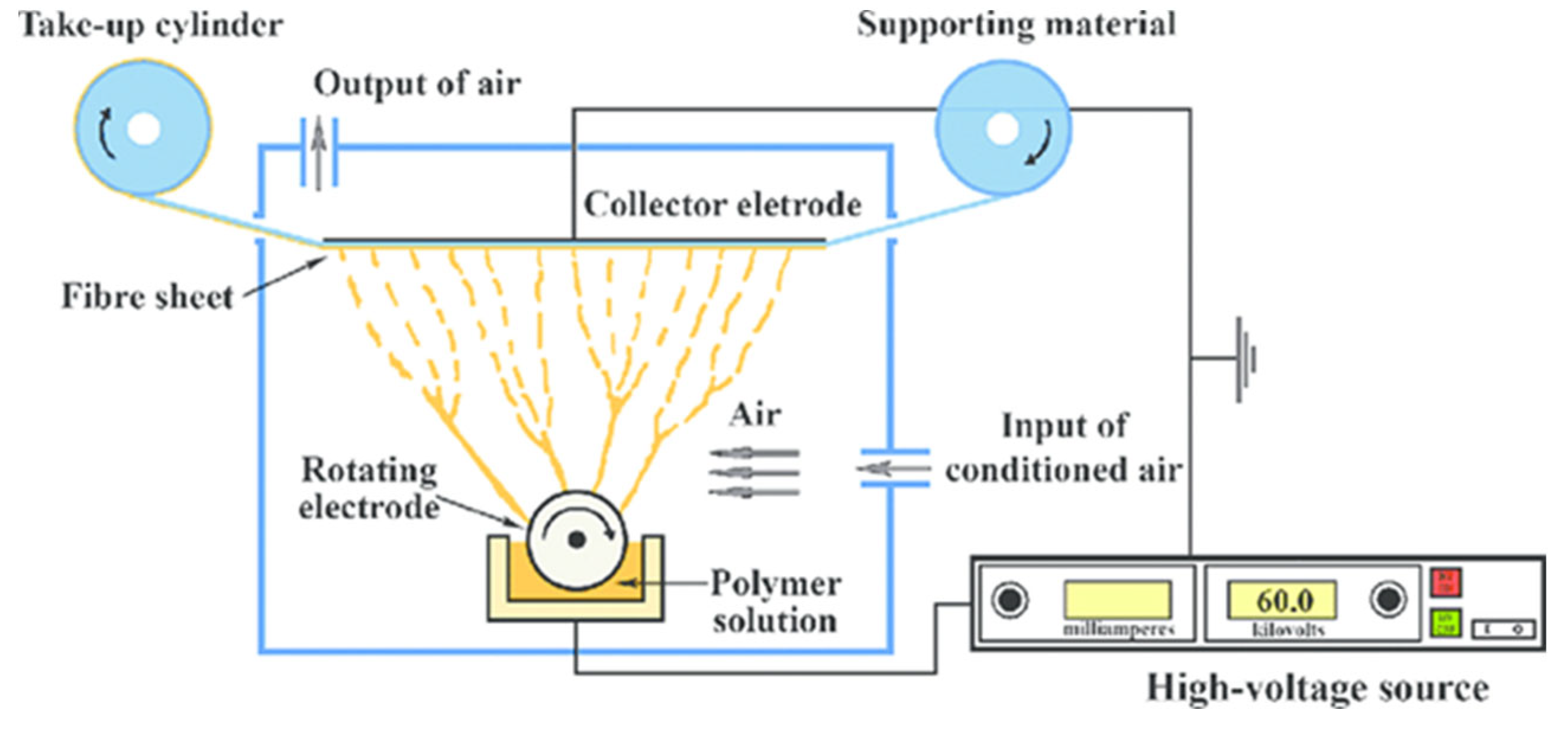
Advancements in needleless electrospinning techniques have resolved the limitations of conventional needle-based electrospinning. One significant advantage of needleless electrospinning is its ability to generate considerable quantities of polymeric droplets without the use of a needle through the implementation of diverse structural techniques [
15]. When a high DC voltage is applied between the polymeric droplets and the conductive collector, the electrostatic forces cause each droplet to assume a Taylor cone shape and form thin polymeric jets. Subsequently, a nonwoven nanofiber web is produced on the conductive collector once the jet reaches it. In contrast to conventional needle-based electrospinning systems, which generate numerous polymeric droplets without actual needles and may be impaired by charge repulsion between needles, which can damage nanofiber morphologies, this system is not affected by such artifacts [16].
The creation of highly functional nanofibers is facilitated by needleless electrospinning. This method relies on the formation of tiny polymeric droplets on surfaces of specific shapes. When an electric field is applied, these droplets produce a narrow polymeric jet that dissipates. Because multiple droplets are ready to jet in succession, this process is classified as a sequential (or multiple) batch process rather than a continuous process. Compared to continuous processes, the complexity of the experimental parameters is significantly reduced in this sequential batch process [17].
Figure 1.
Schematic presentation of needle free electrospinning [
15].
Figure 1.
Schematic presentation of needle free electrospinning [
15].
Figure 2.
Collagen/HA polymeric solution.
Figure 2.
Collagen/HA polymeric solution.
3. Characterization of Collagen/HA Nanofibers
3.1. Scanning Electrone Microscopy (SEM) Analysis
The morphology and characterization of nanofibers were reported via scanning electron microscope (SEM) Phenom ProX (Prague,CZ) scanning electron microscope operating at 15 kV. The average diameter of the electrospun fibers was measured from the SEM images using the ImageJ software. The average diameter and diameter distribution of the nanofibers were determined by selecting 50 random nanofibers from SEM images.
3.2. Atomic Force Microscopy (ATM) Analysis
The surface properties of the nanofibers were examined using a nanoscope atomic force microscope (Digital Instruments) in the tapping mode and expressed as height and phase images.
3.3. Fourier Transform Infrared Spectroscopy (FTIR)
The analysis was conducted using Fourier Transform Infrared (FTIR) spectroscopy with a range of 500–4000 cm-1 and a resolution of 4 × and 128 × scanning, as measured by Nicolet IZ10 (TUL,CZ).
3.4. Determination of the Mechanical Properties of Nanofibers
The mechanical properties of the fibers were evaluated using a Universal Testing Machine (UTM) strograph VGS S-E Toyoseiki, in accordance with the American Standard Testing and Materials (ASTM) D-1822L standard. The objective of this test was to determine the mechanical properties of the fibers, including their strength, elasticity, stiffness, and plasticity.
3.5. Cell Proliferation
Cell adhesion and proliferation in human keratinocytes and fibroblasts within the scaffold were assessed using the standard MTT assay to evaluate the cell number and viability. The surfaces of the polystyrene wells on the plates were used as the targets. Non-cross-linked and cross-linked scaffolds with CDI and glutaraldehyde were placed in the wells, and the cells were seeded onto the matrix. The cells that adhered to the surface of the wells were quantified by removing the scaffold at the time of measurement and placing it in a clean well, excluding those that adhered to the scaffold. The scaffold was then washed with phosphate-buffered saline (PBS) to eliminate cells with poor adhesion. Cell culture was carried out using normal adult human dermal fibroblasts (ATCCR PCS-201- 012) and normal primary human adult epidermal keratinocytes (ATCCR PCS-200-011), which were obtained from the suppliers of the cell lines with minor modifications following the procedures described by the suppliers (Primary Dermal Fibroblasts; Normal, Human, Adult (ATCCPCS201-012™); Primary Epidermal Keratinocytes: Normal, Human, Adult (ATCCPCS-200-011™)).
4. Results and Discussion
4.1. SEM Analysis
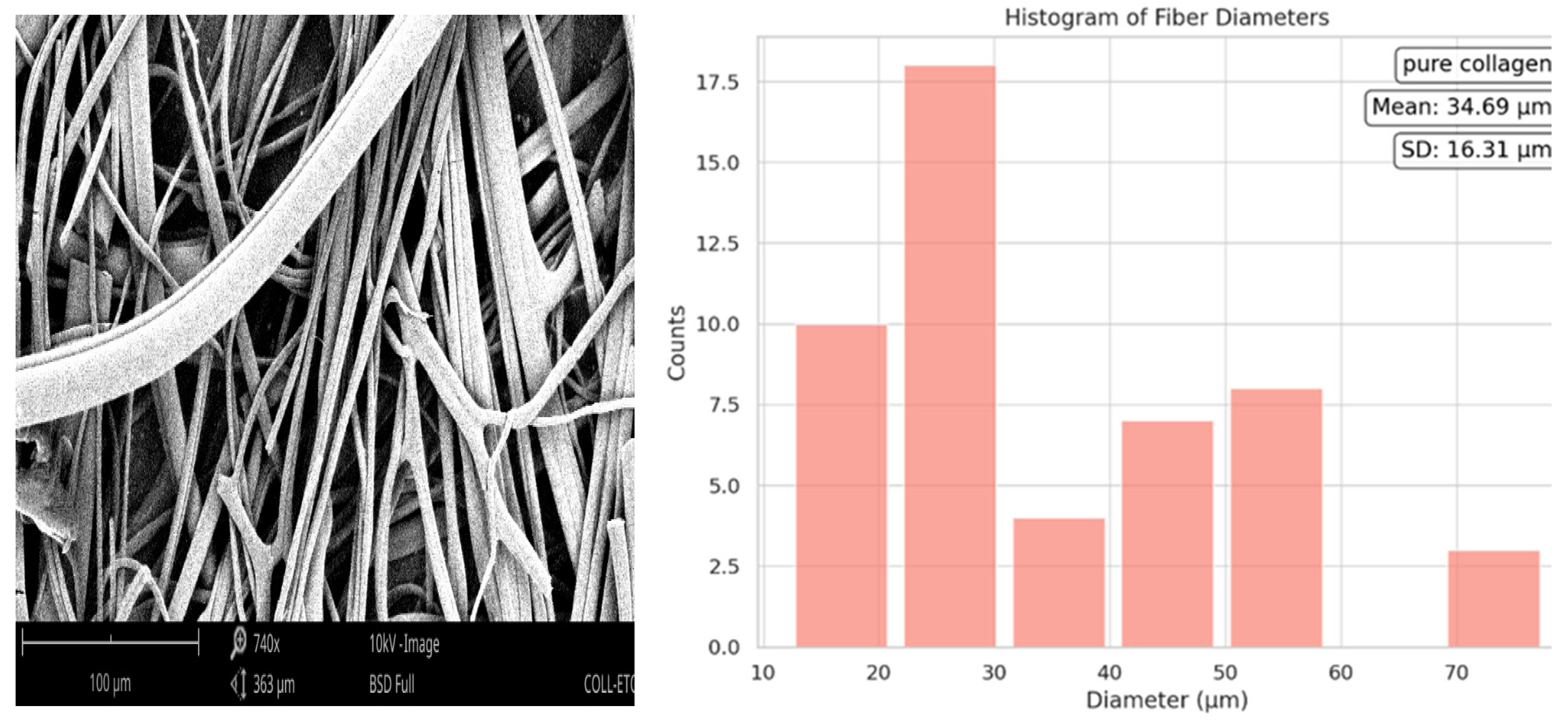
The histogram of the mean diameter of pure collagen was 34.69 µm with a standard deviation of ±16.31 µm. The distribution was right-skewed, suggesting a predominance of fibers with diameters less than the mean. The wide range of sizes implies the diverse nature of the fiber formation process for pure collagen.
Figure 3.
Fiber diameter of Pure Collagen.
Figure 3.
Fiber diameter of Pure Collagen.
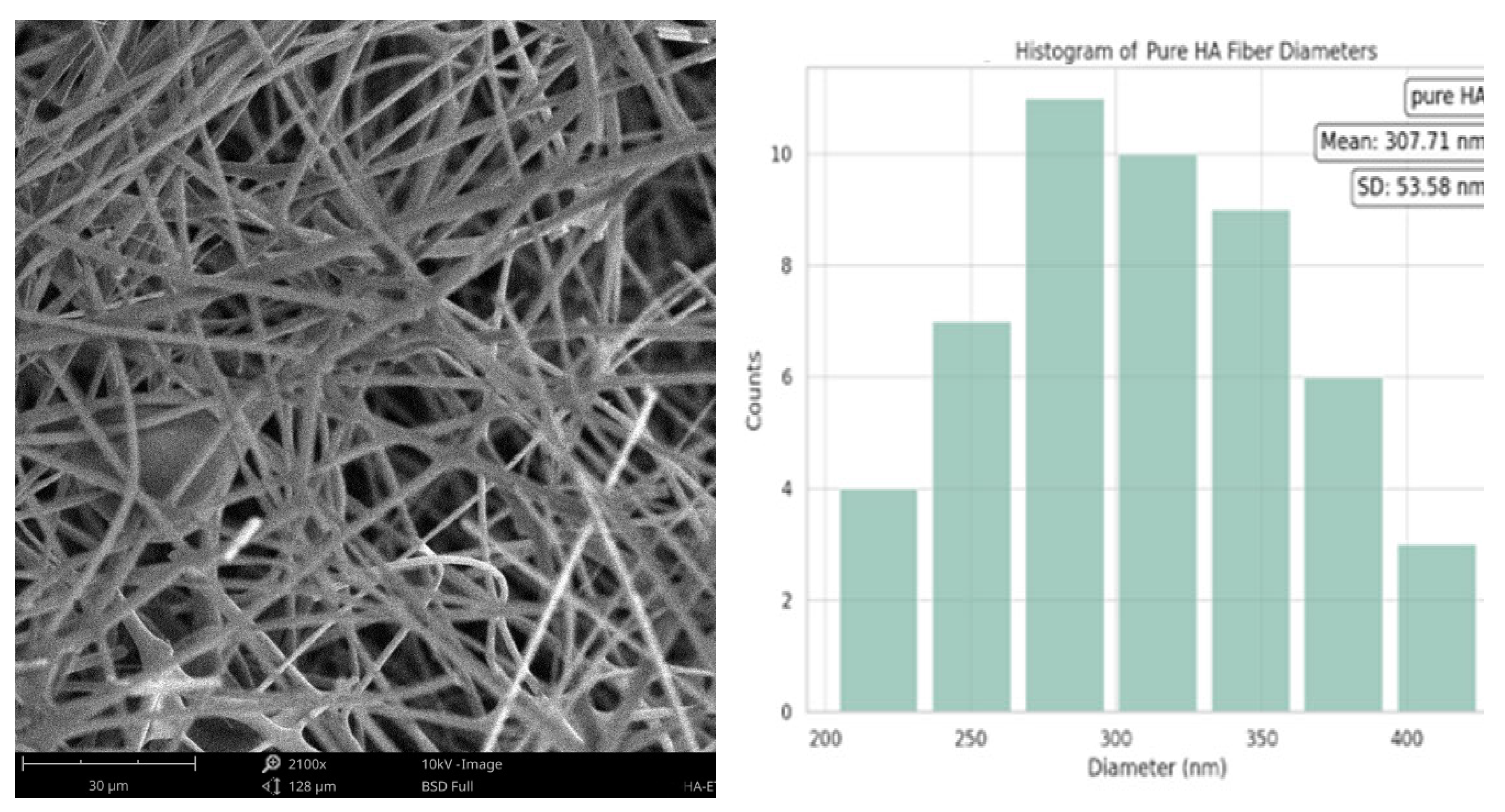
The mean diameter of the HA fibers was significantly smaller (307.71 nm, with a standard deviation of ±53.58 nm. This is consistent with the typical size of HA fibers in electrospun matrices. Additionally, the distribution of the fiber diameters was more symmetrical around the mean, indicating a more uniform fiber diameter than that of the pure collagen sample.
Figure 4.
Fiber diameter of Pure HA.
Figure 4.
Fiber diameter of Pure HA.
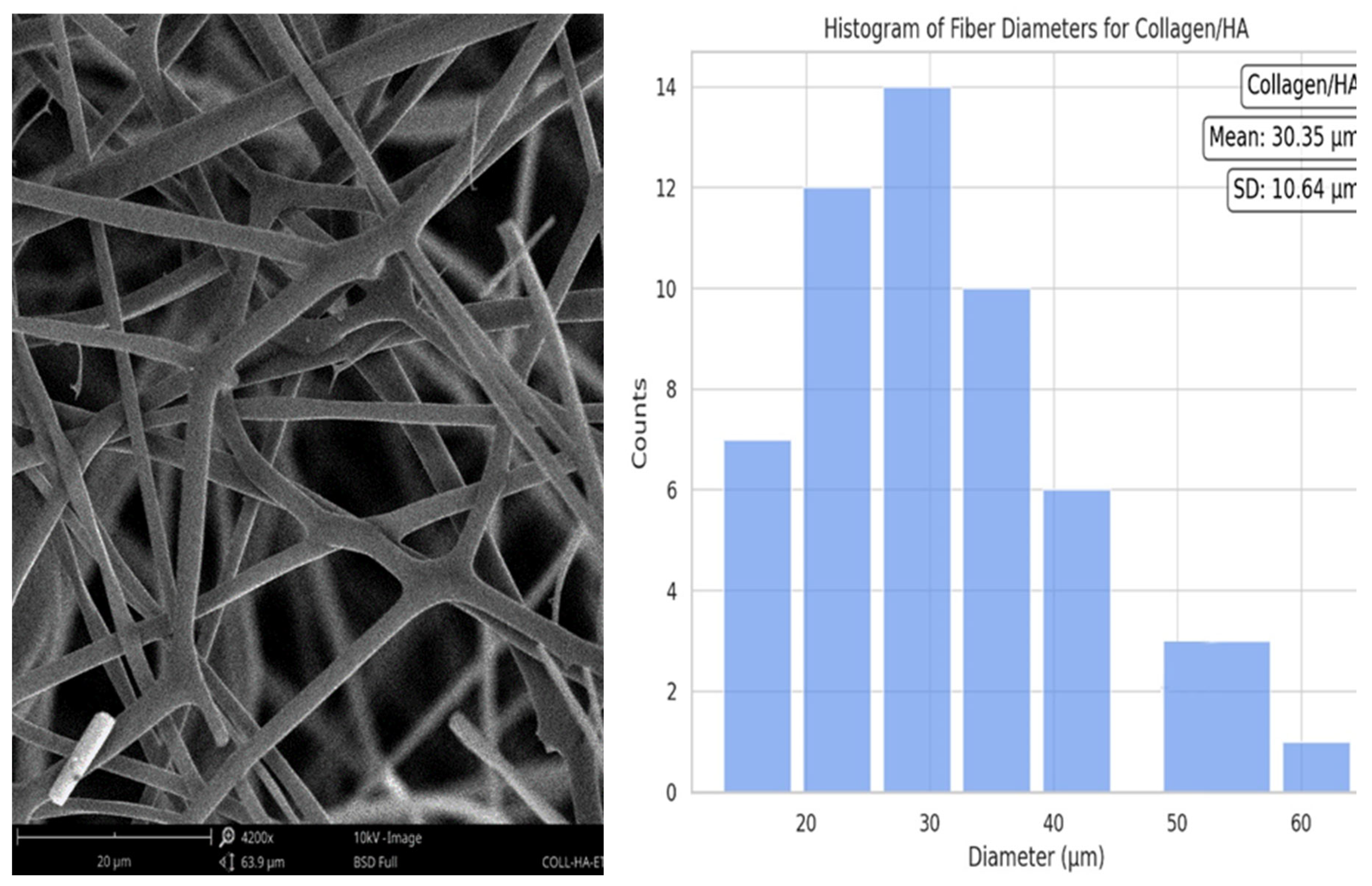
The collagen/HA composite histogram presented a mean fiber diameter of 30.35 µm, accompanied by a standard deviation of ±10.64 µm. The distribution exhibited a right-skewed pattern, albeit less pronounced than that of the pure collagen. This suggests that the incorporation of HA into collagen leads to a more uniform fiber diameter, closer to the mean, and with reduced variability.
Figure 5.
Collagen/HA nanofiber diameter.
Figure 5.
Collagen/HA nanofiber diameter.
4.2. AFM Analysis
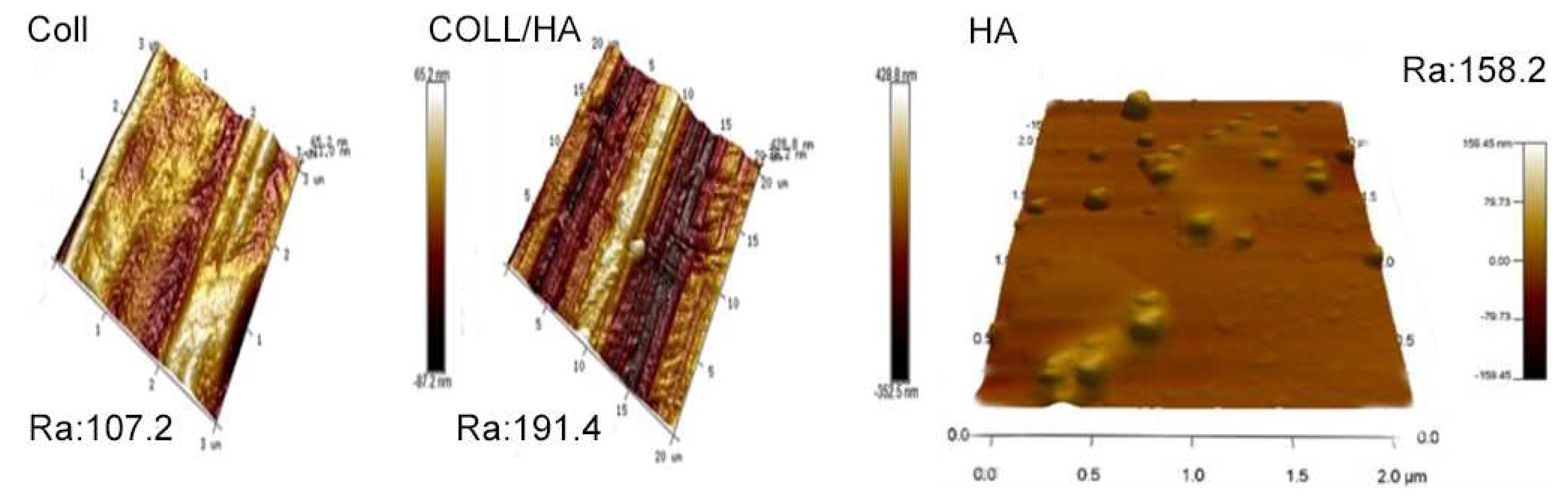
The topography of the surface revealed a significant texture, which is a hallmark of the collagen fibrils. Collagen often displays a banded arrangement owing to its natural triple-helix formation. The lowest Ra value recorded here was 107.2 nm, which is still relatively high, indicating a rough surface. However, this roughness is attributable to the presence of spherical particulates or aggregates on an otherwise smoother background. Compared to the pure collagen nanofibers, the average surface roughness (Ra) of the collagen/HA nanofibers showed a slight increase. This may be attributed to the formation of a thin HA nanofiber on top of each collagen nanofiber surface.
Figure 6.
AFM surface morphology of pure Collagen nanofiber, Collagen/HA nanofiber and pure HA nanofiebr.
Figure 6.
AFM surface morphology of pure Collagen nanofiber, Collagen/HA nanofiber and pure HA nanofiebr.
4.3. FTIR Analysis
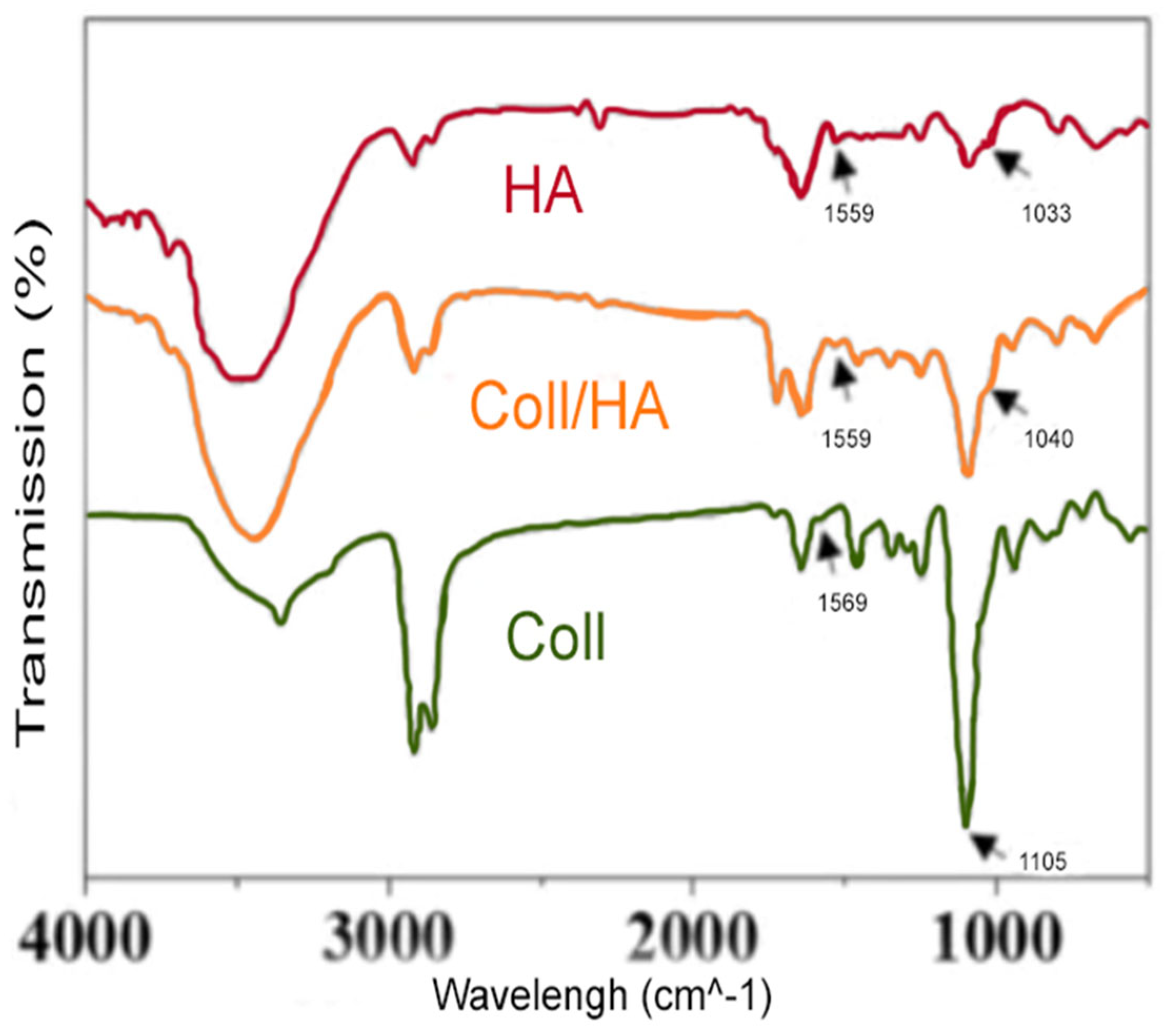
Fourier Transform Infrared (FTIR) spectroscopic analysis of the sample revealed several prominent peaks, which provided valuable insights into the chemical composition and structural characteristics of the material. The peak observed at 1559 cm-1 is believed to be associated with the N-H bending vibrations of amide II, a common feature of proteins. Moreover, the peak at 1033 cm-1 could be attributed to the C-O stretching vibrations typically found in polysaccharides, such as hyaluronic acid. The sharp peak at 1105 cm-1 may correspond to C-O stretching, which suggests the presence of polysaccharides or C-N stretching vibrations within the protein structure. The presence of peaks at 1559 cm-1 and 1040 cm-1 suggests a combination of the characteristics of both collagen and hyaluronic acid in the composite material. The slight shift from 1033 cm-1 in hyaluronic acid to 1040 cm-1 in the collagen/HA nanofibers may indicate some level of interaction or bonding between collagen and hyaluronic acid, causing a slight alteration in the vibrational frequencies.
Figure 7.
FTIR spectral examination of Collagen, Collagen/HA and HA nanofibers.
Figure 7.
FTIR spectral examination of Collagen, Collagen/HA and HA nanofibers.
Compared with collagen nanofbers, HA-collagen nanofber have obvious absorption peaks at 1559 and 1045 cm−1, which may be due to the redshift caused by in-plane bending of amide N-H and Tensile vibration of C-N, indicating the presence of the HA coating.
4.4. Analysis of the Mechanical Properties of Nanofibers
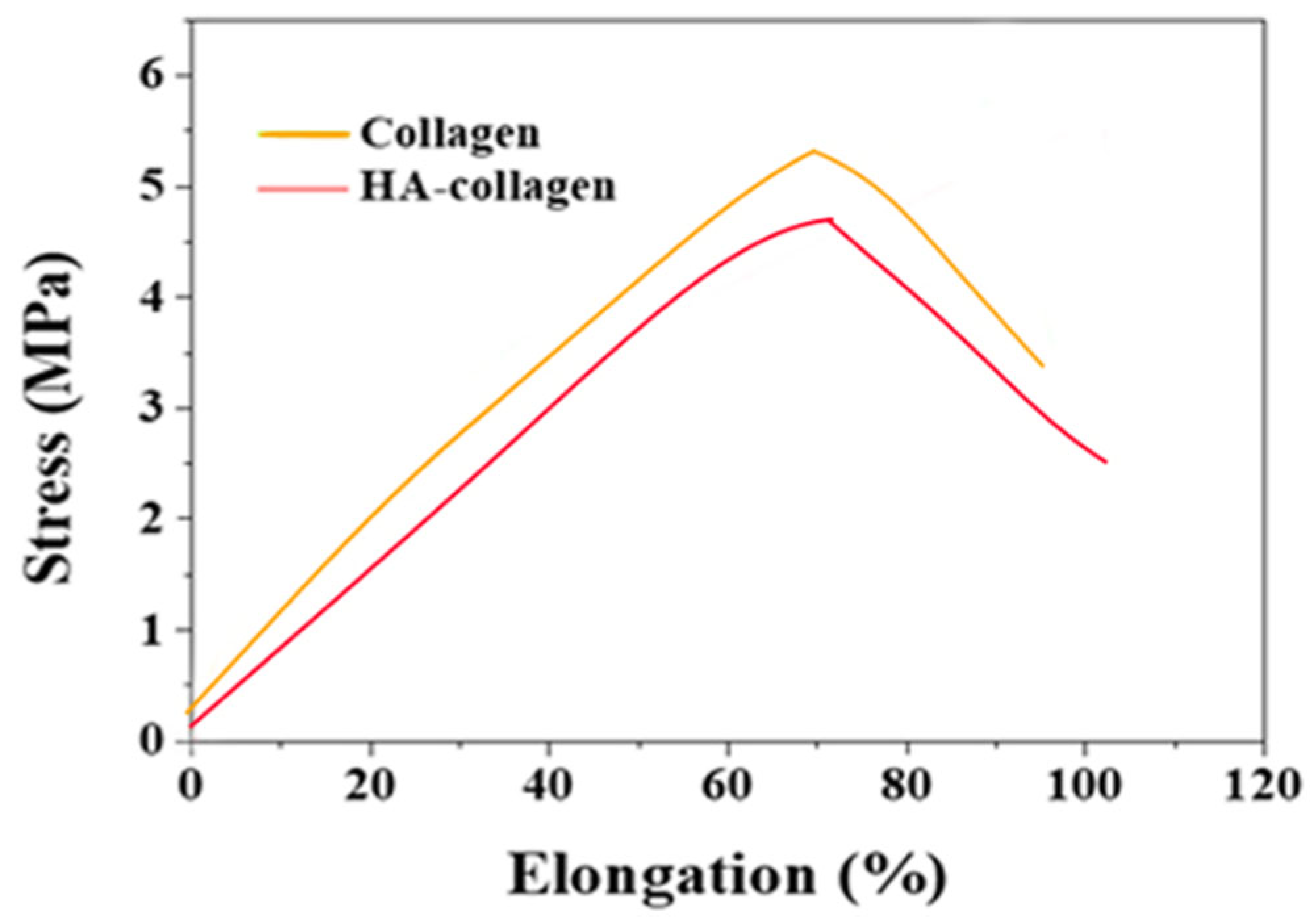
The mechanical properties of collagen and hydroxyapatite collagen/HA nanofibers were determined using uniaxial tensile testing to generate stress-strain curves and derive tensile properties. The ultimate tensile strength and Young's modulus of collagen were found to be (0.13±0.3) MPa and (0.35±0.3) MPa, respectively.
Figure 8.
Comparative Stress-Strain Analysis of Collagen and Hyaluronic Acid-Collagen Composites in Tensile Strength Testing.
Figure 8.
Comparative Stress-Strain Analysis of Collagen and Hyaluronic Acid-Collagen Composites in Tensile Strength Testing.
The level of stress experienced by collagen at its highest point was notably lower than that of the collagen/HA nanofibers, suggesting that the incorporation of HA into collagen results in a composite material with increased tensile strength. Furthermore, collagen/HA nanofibers demonstrated a greater capacity to maintain their structural integrity under substantial levels of elongation than collagen alone.
4.5. Cell Proliferation
The viability and proliferation of cells within the scaffolds were investigated using a standard 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay (Sigma-Aldrich, Czech Republic) at 1, 4, 7, and 15 days after culture initiation. Human fibroblasts and keratinocytes were seeded at a density of 104 cells/cm2 onto scaffolds in 24-well plates and cultured in DMEM HAMS F12 medium at 37 °C and 5% CO2. The medium was changed every two days. Non-adherent and poorly adherent cells were removed by washing the wells with PBS (pH 7.2–7.6), and then MTT solution in DMEM medium (1:9) was added to each well and incubated for 3 h at 37 °C in 5% CO2. The MTT solution was then removed and replaced with dimethyl sulfoxide (DMSO) (Sigma-Aldrich) to solubilize the formazan crystals. The absorbance of the released MTT-formazan product was measured at 570 nm using a microplate reader. As a control, the cells were seeded in standard polystyrene 24-well plates.
Figure 9.
Cell adhesion of Collagen/HA in human fibroblasts.
Figure 9.
Cell adhesion of Collagen/HA in human fibroblasts.
The collagen/HA composite demonstrated superior performance as a substrate for human fibroblast cell adhesion and growth compared to the control, suggesting its potential utility in applications such as tissue engineering and wound healing, where cell-material interactions are of paramount importance. Furthermore, temporal data indicate that the effectiveness of the composite is sustained over an extended period, which is crucial for long-term applications in regenerative medicine.
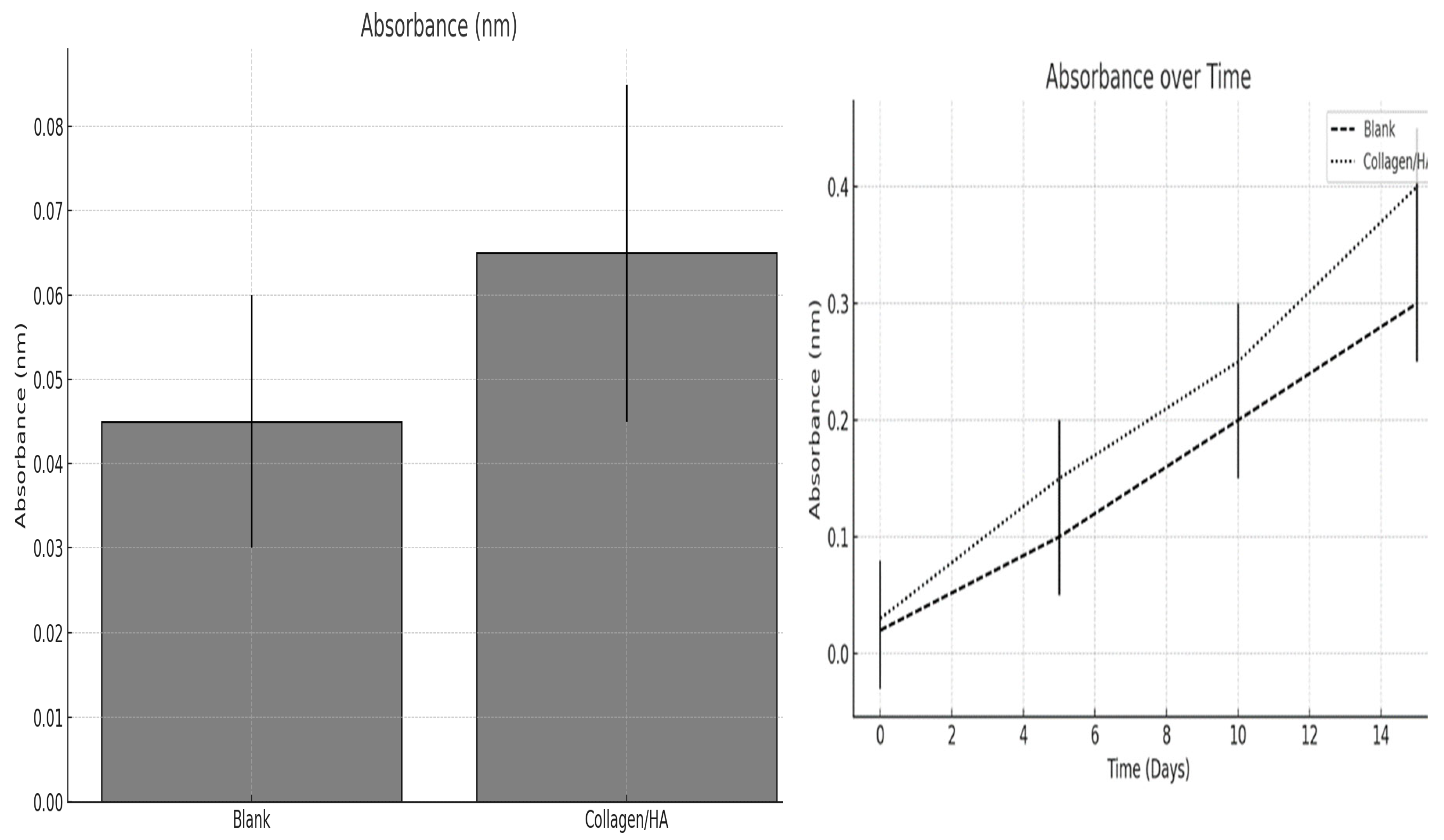
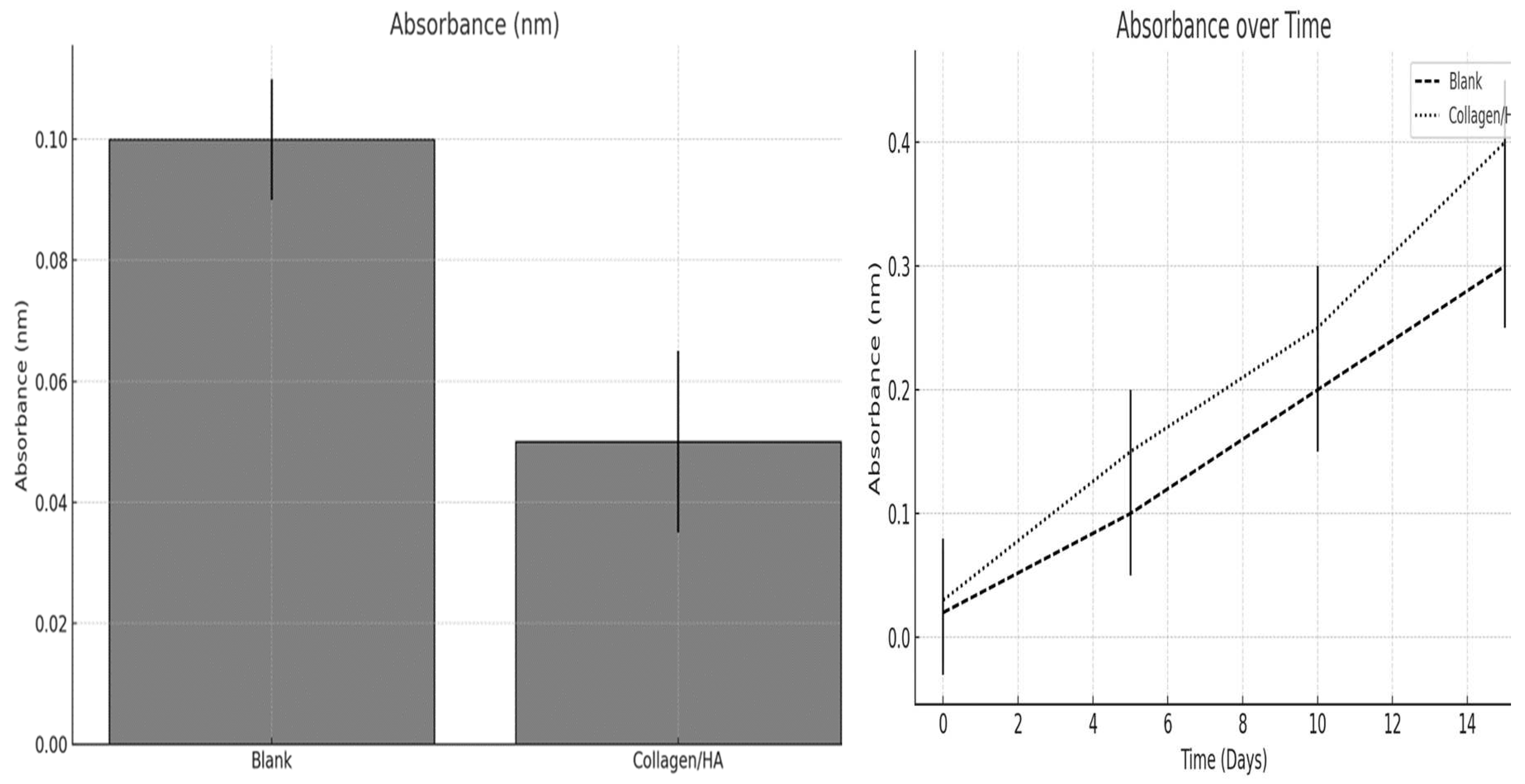
Figure 10.
The proliferation of human keratinocytes within the scaffolds was evaluated after one day of culture, and the growth kinetics were monitored over a period of 15 days.
Figure 10.
The proliferation of human keratinocytes within the scaffolds was evaluated after one day of culture, and the growth kinetics were monitored over a period of 15 days.
Figure 10 displays a bar graph illustrating the absorbance measurements after a day of culture. Absorbance is typically correlated with the number of cells, as it reflects their metabolic activity, which is often directly proportional to cell count. The blank scaffold exhibited a higher absorbance than the Collagen /HA scaffold, which appears counterintuitive given that Collagen /HA should provide a more bioactive substrate conducive to cell growth. One possible explanation for this discrepancy is that the blank scaffold initially supports a greater number of cells, resulting in increased metabolic activity. However, it is also possible that the cells on the blank scaffold were more metabolically active because of factors unrelated to the scaffold composition, such as variations in surface properties or experimental variability. It is important to consider that other factors, such as the presence of substances with inherently higher absorbance in the blank scaffold, may have influenced the measurement.
The line graph presented in
Figure 10 depicts the changes in absorbance over a 15-day period for both blank and collagen/HA scaffolds. Both the blank and Collagen /HA scaffolds exhibited a linear increase in absorbance over time, indicating cell growth. However, the Collagen/HA scaffold demonstrated a steeper slope, indicating that, after the initial day, the cells on the Collagen/HA scaffold proliferated more rapidly than those on the blank scaffold. This suggests that although the initial cell adhesion or metabolic activity was lower on the Collagen/HA scaffold, it provided better support for cell proliferation over time.
These findings indicate that while the blank scaffold exhibited greater initial metabolic activity, the collagen/HA scaffold exhibited more favorable cell growth dynamics over time. This observation implies that the collagen/HA scaffold may be a more appropriate choice for applications that require long-term cell proliferation, such as tissue engineering and wound healing models. Additional research is necessary to enhance the initial cell seeding and adhesion process on the collagen/HA scaffold as its lower metabolic activity at the outset merits attention.
5. Conclusions
This research provides a promising outlook for Collagen/HA nanofibers, which have the potential to revolutionize the skincare and biomedical industries by offering biocompatible, effective, environmentally sustainable, and cell-friendly materials for biomedical and cosmetic applications. The Collagen/HA nanofibers exhibited a uniform fiber diameter and distribution as well as mechanical properties, indicating an improved tensile strength and elongation capacity compared to collagen alone, suggesting a synergistic effect of the blend on the mechanical reinforcement of the nanofibers. The Collagen/HA scaffolds demonstrated superior cell adhesion and proliferation compared to the control scaffolds over an extended period, as evidenced by the MTT assay results, highlighting the biocompatibility and cell-friendly nature of the Collagen/HA composite, which is crucial for tissue engineering applications. The ability of the Collagen/HA nanofibers to sustain cell growth and maintain cell viability without the need for preservatives presents an advantage over traditional skincare products, offering a healthier and more efficient means of delivering active ingredients. The use of needleless electrospinning methods for the fabrication of Collagen/HA nanofibers constitutes a significant technological advancement, enabling extensive production without the limitations inherent in needle-based systems. The formulation of the Collagen/HA blend presents an eco-friendly alternative to conventional methods by eradicating the necessity for preservatives and diminishing residual waste, thereby addressing environmental concerns and potential health hazards associated with preservatives. Despite the higher initial production costs and time requirements, the long-term advantages of Collagen/HA nanofibers, particularly in terms of durability and reduced waste, may eventually justify these initial investments, rendering it a feasible option for the cosmetic industry. Further exploration into optimizing the initial cell seeding and adhesion process on Collagen/HA scaffolds is advised, as the initial lower metabolic activity suggests room for improvement. Additional research on the large-scale production and economic viability of this technology is crucial for its integration into commercial applications.
Data Availability
The datasets generated and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Declarations Ethics Approval
Not applicable.
Consent for Publication
All authors are happy with this publication.
Acknowledgments
The authors extend their gratitude to the technological support essential to this study. All figures presented in this research were meticulously generated using analysis of variance (ANOVA) in Excel 2019. Additionally, scanning electron microscopy (SEM) images were rigorously analyzed using ImageJ software. These tools were instrumental in the analysis and presentation of our findings, and their contribution to the rigour of our research is gratefully acknowledged.
Competing Interests
The authors declare no competing interests.
References
- Cheng H, Yang X, Che X, Yang M, Zhai G. Biomedical application and controlled drug release of electrospun fibrous materials. Materials Science and Engineering: C 2018;90:750–63. [CrossRef]
- Kim J, Chan Hong S, Bae GN, Jung JH. Electrospun Magnetic Nanoparticle-Decorated Nanofiber Filter and Its Applications to High-Efficiency Air Filtration. Environ Sci Technol 2017;51:11967–75. [CrossRef]
- Wang J, Xu J, He Y. Novel smart textile with ultraviolet shielding and thermo-regulation fabricated via electrospinning. Journal of Energy Storage 2021;42:103094. [CrossRef]
- Zhang C, Li Y, Wang P, Zhang H. Electrospinning of nanofibers: Potentials and perspectives for active food packaging. Comprehensive Reviews in Food Science and Food Safety 2020;19:479–502. [CrossRef]
- Velasco MVR, Vieira RP, Fernandes AR, Dario MF, Pinto C a. SO, Pedriali CA, et al. Short-term clinical of peel-off facial mask moisturizers. International Journal of Cosmetic Science 2014;36:355–60. [CrossRef]
- Amer SS, Mamdouh W, Nasr M, ElShaer A, Polycarpou E, Abdel-Aziz RTA, et al. Quercetin loaded cosm-nutraceutical electrospun composite nanofibers for acne alleviation: Preparation, characterization and experimental clinical appraisal. International Journal of Pharmaceutics 2022;612:121309. [CrossRef]
- Ali RS, Saad HA. Synthesis of Some Novel Fused Pyrimido[4″,5″:5′,6′]-[1,2,4]triazino[3′,4′:3,4] [1,2,4]triazino[5,6-b]indoles with Expected Anticancer Activity. Molecules 2018;23:693. [CrossRef]
- Juncan AM, Moisă DG, Santini A, Morgovan C, Rus L-L, Vonica-Țincu AL, et al. Advantages of Hyaluronic Acid and Its Combination with Other Bioactive Ingredients in Cosmeceuticals. Molecules 2021;26:4429. [CrossRef]
- Graça MFP, Miguel SP, Cabral CSD, Correia IJ. Hyaluronic acid—Based wound dressings: A review. Carbohydrate Polymers 2020;241:116364. [CrossRef]
- Zhu S, Yuan Q, Yin T, You J, Gu Z, Xiong S, et al. Self-assembly of collagen-based biomaterials: preparation, characterizations and biomedical applications. J Mater Chem B 2018;6:2650–76. [CrossRef]
- Suurs P, Barbut S. Collagen use for co-extruded sausage casings – A review. Trends in Food Science & Technology 2020;102:91–101. [CrossRef]
- Qiang T, Li X, Ren L, Wang X. Leather solidwaste: A review a low cost adsorption material based on collagen fibre. Journal of the Society of Leather Technologists and Chemists 2016;100:109–13.
- Suzuki A, Ogata K, Yoshioka H, Shim J, Wassif CA, Porter FD, et al. Disruption of Dhcr7 and Insig1/2 in cholesterol metabolism causes defects in bone formation and homeostasis through primary cilium formation. Bone Research 2020;8. [CrossRef]
- Shalaby M, Agwa M, Saeed H, Khedr SM, Morsy O, El-Demellawy MA. Fish Scale Collagen Preparation, Characterization and Its Application in Wound Healing. J Polym Environ 2020;28:166–78. [CrossRef]
- Sasithorn N, Martinová L, Horakova J, Mongkholrattanasit R. Fabrication of Silk Fibroin Nanofibres by Needleless Electrospinning, 2016, p. 95–113. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).