1. Introduction
The phylum Apicomplexa includes approximately 5000 obligate intracellular parasites, many of which are pathogens of high veterinary and medical importance [
1]. Prominent members of the phylum include species in the genus
Plasmodium that infect humans causing malaria, a life threatening disease [
2];
Eimeria, a poultry and cattle pathogen [
3,
4];
Cryptosporidium, an opportunistic human and animal pathogen [
5]; and
Besnoitia,
Babesia and
Theileria, cattle parasites [
6,
7,
8].
Toxoplasma gondii is a tissue cyst forming coccidian [
9] that affects domestic and sylvatic animals and in humans represents a common cause of congenital neurological and ocular defects [
10], being a devastating opportunistic pathogen in immuno-compromised patients [
11]. Its ability to be transmitted by water and food has resulted in its classification as a category B priority pathogen by the National Institute for Allergy and Infectious Diseases (NIAID) [
12].
Understanding how parasites enter host cells and multiply is essential to understand disease and may contribute to the identification of targets for the design of novel therapeutic strategies. The invasion process of Apicomplexa zoites and the molecular mechanisms involved in it seem to be conserved. To penetrate host cells, apicomplexans use a system of adhesion-based motility called gliding, which has been described to be actin/myosin dependent [
13]. In this process, the apical complex, a microtubule (MT)-based cytoskeletal structure localized in the anterior region of the cell, plays a crucial role in the interaction with the host cell [
14]. Importantly, the molecular composition of this structure is not fully known, but it is likely enriched in proteins involved in MT assembly and dynamics, as well as in proteins participating in MT-associated processes and their crosstalk with other cellular systems (e.g., actin, vesicles). As a key component of the apical complex, it is critical to understand how this specialized class of MTs is assembled and maintained, and how it functionally interacts with other cellular structures. The existing knowledge on the biology of the apical complex suggests that tubulin post-translational modifications (PTMs) and the machineries involved in their generation and removal play key roles in this structure's assembly and functions during host cell invasion.
2. Microtubule cytoskeleton
Throughout evolution, eukaryotic cells developed highly sophisticated and specialised cytoskeleton systems namely, intermediate filaments, actin filaments and MTs. Despite their specific roles, these structures crosstalk and cooperate, for example, in supporting membrane structures (e.g., nuclear and plasma membranes), thus providing shape and mechanical resistance to the cell. In addition, the eukaryotic cytoskeletons are implicated in processes such as the organisation of the cytoplasm, organelle assembly and maintenance, cell division, cell polarity, cell migration, intracellular transport, and cell signalling. In multicellular organisms, the cytoskeleton also plays critical roles in the establishment of cell-cell contacts, and cell-extracellular matrix, and consequently in the integrity of tissues.
MTs are dynamic polymers made of heterodimers of the structurally and functionally conserved α- and β-tubulins, both of which are GTP-binding proteins and are present in all eukaryotes studied so far. The genomes of higher organisms, such as human and mouse, present large gene families coding for multiple α and β-tubulin isotypes [
15]. Importantly, some of the tubulin isotypes are expressed in a tissue-specific manner, being important for the assembly of specialized functional classes of MTs. For example, the correct expression of tubulin isotypes (e.g., β3-tubulin) is important for neuronal differentiation and survival in mammals, and mutations in their coding genes are associated with neurological diseases [
16,
17,
18]. On the other hand, lower eukaryotes like
Saccharomyces [
19,
20] and
Tetrahymena [
21,
22] present one or two α- and two β-tubulin coding genes.
For the assembly of the α/β-tubulin heterodimer, tubulins undergo a complex folding process assisted by molecular chaperones (prefoldin and CCT) [
23,
24,
25,
26] and the tubulin cofactors (TBCA-E). In addition to participating in heterodimer assembly, tubulin cofactors also play a role in quality control and recycling of heterodimers released from depolymerized MTs. Thus, by regulating the available pool of free tubulin dimers competent to polymerize, the tubulin folding pathway controls MT dynamics [
27,
28,
29,
30,
31]. Once folded and assembled, the tubulin heterodimers polymerize in a polarized head-to-tail manner to form protofilaments, which then assemble the MT hollow structure typically composed of 13 protofilaments. During the polymerization process, β-tubulin hydrolyses its GTP to GDP, and after MT depolymerization the GDP is exchanged to GTP so that β-tubulin can polymerize again. In contrast, the α-tubulin-bound GTP is not hydrolysed during polymerization [
32,
33].
Given the polar nature of MTs, one of its ends (minus end) presents α-tubulin subunits and the other (plus end) β-tubulin. Moreover, the two MT extremities have distinct dynamic properties, with the minus end presenting slow growth and the plus end fast polymerization. The minus end is usually associated with MT organizing centers (MTOCs) such as spindle pole bodies in fungi, and centrosomes and the Golgi apparatus in animal cells. MTOCs are structurally variable in different groups of eukaryotes, but are always enriched in proteins that promote MT nucleation (e.g., gamma-tubulin) and anchoring [
34,
35,
36,
37].
MTs can present distinct dynamic properties and stability. These different properties can be conferred by the preferential incorporation of specific tubulin isotypes (products of different tubulin genes) and by tubulin PTMs such as acetylation, detyrosination, glutamylation, and glycylation. Tubulin PTMs can differentially and reversibly mark MT subpopulations [
38]. These modifications are evolutionarily conserved and generate what is known as the tubulin code.
For example, α-tubulin can be acetylated on K40, the unique known PTM that localizes in the luminal surface of microtubules, and its C-terminal tail can be reversibly modified by deletion of the terminal tyrosine (detyrosination) or irreversibly modified by deletion of the last two residues (Δ2). The C-terminal tails of both α- and β-tubulins can be reversibly modified by glutamylation and glycylation. Although the K40 PTM has been associated with MT stability [
39], C-terminal PTMs alter MT interactions with associated proteins to influence sensitivity to MT targeting drugs.
Tubulin PTMs influence the binding of MT-associated proteins (MAPs) such as MT motors and MT severing proteins to MTs. Therefore, they are paramount for the assembly and maintenance of MT-based organelles such as centrioles, cilia and flagella, and MT cortical structures present in unicellular organisms such as
T. thermophila and
T. gondii [
38,
40]. For example, centriolar and axonemal MTs are highly acetylated and glutamylated, in contrast to cytoplasmic ones. In cilia, although it is not completely understood how tubulin PTMs affect intra-flagellar transport, tubulin glutamylation impacts intra-flagellar transport velocity [
41] and the localization of MT motor proteins [
42]. Importantly, tubulin PTMs can be modulated in response to environmental cues. In
Caenorhabditis elegans, for instance, tubulin glutamylation was shown to be upregulated in sensory cilia in response to temperature, osmolality, and dietary conditions [
41].
3. The specialized microtubule structures of Toxoplasma gondii
Apicomplexans are alveolate organisms because, underling the plasma membrane, they present a system of flattened vesicles (alveoli) – the pellicle structure [
43,
44,
45]. The pellicles are divided in three subdomains (apical, central, and basal) with different properties conferred by distinct cytoskeleton components. The plasma membrane associated with alveoli form the inner membrane complex (IMC). The IMC extends from the apical polar ring (APR) to the basal pole, leaving the extreme apical region of the parasite enclosed only by plasma membrane. Although γ-tubulin does not localize to the APR [
46], this structure has been considered a MTOC since the minus ends of subpellicular MTs (SPMTs) are attached there, by projections that resemble a cogwheel [
47,
48], and plus ends are distal to this structure. Until now, the proteins identified as being part of the APR are: TgRNG1, TgRNG2, TgAPR1, and TgKinesin A [
47,
49,
50,
51]. TgRNG1 appears at the mature APR only after completion of nuclear division [
49]. TgAPR1 is also a marker of the mature APR structure, and TgAPR1-null parasites exhibit a defect in the lytic cycle (Leung et al., 2017). The MT motor TgKinesin A is not an essential gene, and parasites lacking this protein present a modest reduction in growth rate. TgKinesin A labels emergent daughter buds and localizes immediately apical to APR1 at the APR of mature parasites [
47].
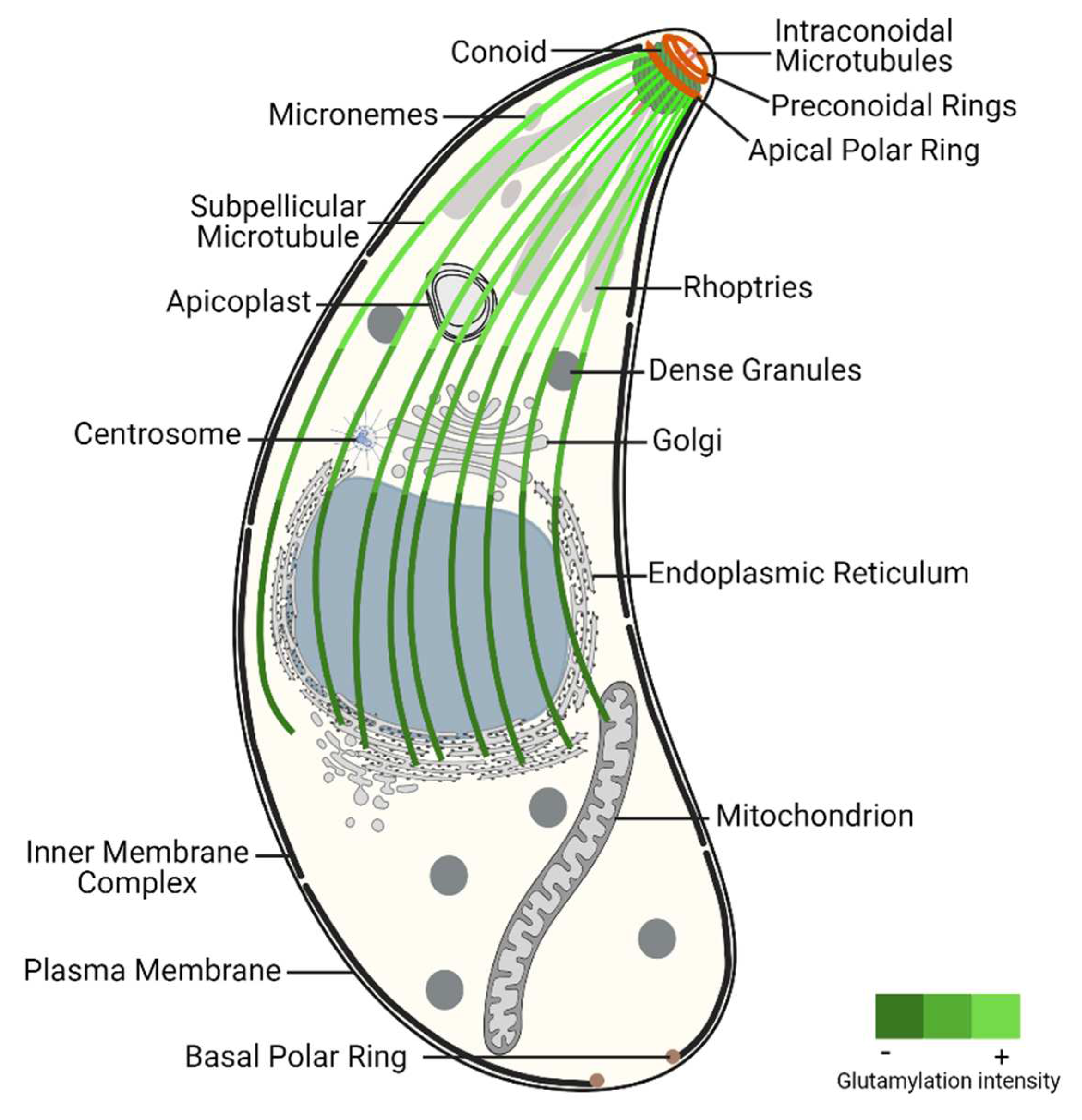
SPMTs extend in a gentle spiral from the APR to a region posterior to the position of the nucleus, being responsible for the elongated shape, rigidity, and the maintenance of the highly polarized cell organization [
52] (
Figure 1). These SPMTs are coated with MAPs [
53], including Subpellicular Microtubule Proteins 1 and 2 (SPM1 and SPM2), which are specific to apicomplexan parasites [
54].
The apical complex is built around the extensible and retractable conoid that is actively motile during host invasion [
55]. The conoid is mainly composed of tubulin arranged into a novel polymer form that is quite different from that of typical MTs [
56]. The conoid is part of a complex composed by two preconoidal rings (above the conoid) and two intraconoidal MTs [
57]. Although related alveolates present incomplete conoids or pseudoconoids, most apicomplexans lost the conoid structure. However, coccidian parasites, like
T. gondii, present a closed conoid [
58]. The proteomics of the
T. gondii conoid/apical complex led to the identification of ~200 proteins that represent 70% of
T. gondii cytoskeleton proteins, including several key cytoskeletal proteins such as actin and actin-binding proteins, varied myosin heavy and light chains, and the three isoforms of β-tubulin [
14].
At the apical pole, the rhoptries and micronemes are specialized secretory organelles that transit within the conoid to secrete their contents across the plasma membrane at the apical tip of the parasite [
59].
As part of the MT cytoskeleton,
T. gondii tachyzoites also present centrioles – a barrel-shaped structure formed by nine singlet MTs [
52]. Non-coccidian apicomplexans, like Plasmodium, lack asexual centrioles but this structure occurs in other coccidians. Coccidian centrioles are quite short and are arranged in a parallel rather than orthogonal configuration [
58]. Despite the lack of pericentrin and ninein genes, the term “centrosome” has been used in
T. gondii due to its nucleating activity and capacity to work as a signaling platform [
60].
Nuclear division depends on the centrocone and occurs without breaking the nuclear envelope. Chromosome segregation occurs without chromosome condensation. Spindle MTs originate in the cytoplasm and pass through the nuclear pores of the centrocone (a domain of the nuclear envelope), being essential for connecting centrosomes with the centrocone, and for segregating chromosomes into daughter nuclei [
61,
62]. EB1 proteins bind to the positive ends of dynamic MTs, promoting stability and consequently elongation of the MTs. In
T. gondii, the EB1 homolog is a nuclear protein that localizes to the centrocone after spindle assembly [
63]. In addition to the nuclear protein, which remains in the centrocone until cytokinesis, there is a small pool of cytoplasmic TgEB1 that associates transiently with the tips of SPMT daughter buds after nuclear division is complete [
63]. Also, studies in T.
gondii demonstrated that SPMTs are continuously polymerizing during daughter cell assembly. However, during later stages of cell division the SPMTs lose their dynamic behavior being relatively static (with very low tubulin exchange or incorporation rate). It was also described that SPMTs are highly resistant to distinct conditions that lead to MT depolymerization, e.g., cold, antimitotic agents, detergents, and high pressure [
52]. MAPs influence MT stability and endow MT populations with different properties. While MT motors (dyneins and kinesins), centriole components (SAS-6, centrin, CEP250) and regulatory proteins (EB1) are highly conserved with other eukaryotes, proteins associated with SPMTs and the conoid are predominantly specific to these organisms, reflecting the specialized role of these structures (Morrissette and Gubbels, 2020). SPMTs are heavily coated with MAPs such as TgSPM1, TgSPM2, TgTrxL1, TgTrxL2, TgTLAP1, TgTLAP2, TgTLAP3 and TgTLAP4. While some of these proteins are located throughout SPMTs, others are in distinct subregions [
52].
At the basal end,
T. gondii presents a basal complex, without tubulin, responsible for completing cytokinesis and consequently parasite replication [
64,
65,
66].
4. T. gondii tubulin post-translational modifications
The Toxoplasma genome contains genes for three α-tubulin isotypes (α1 - TGME49_316400, α2 - TGME49_231770, and α3 - TGME49_231400) and three β-tubulin isotypes (β1 - TGME49_266960, β2 - TGME49_221620, and β3 - TGME49_212240) [
67,
68,
69]. A genome-wide CRISPR screen indicates that α1-, α2-, β1-, and β2-tubulins are essential for in vitro tachyzoites, while α3- and β3-tubulins are not [
70]. The amino acid sequences of the three β-tubulin isotypes share 96.4-96.9% identity and 98.0-98.7% similarity, with most of the differences affecting seven of the last eight amino acid residues. Concerning the amino acid sequences of the three α-tubulin isotypes, they present 35.5-68.3% identity and 52.7-79.4% of similarity. Interestingly, among the three isotypes, the α3 isotype presents significantly lower percent identity/similarity compared with the other two isotypes. Peptides compatible with the three α- and the three β-tubulin isotypes have been previously detected in
T. gondii proteomes (evidence available at
www.ToxoDB.org, Proteomics section). Notably, the most divergent α3-tubulin isotype has only been detected in proteomes analyzing the tachyzoite stage, whereas the other five α- and β-tubulin isotypes have been identified in
T. gondii proteomes analyzing the tachyzoite, bradyzoite, and oocyst stages (available at
www.ToxoDB.org, Proteomics section). The differences in amino acid sequences between the α- and the β-tubulin isotypes correlate to specialized functions and differential expression throughout the
T. gondii life cycle [
68]. Some of these distinct features may be related to specialized tubulin structures, such as the conoid and the flagellar axoneme [
68]. Like in other eukaryotes, the most divergent region in the amino acid sequences of the different Toxoplasma tubulin isotypes is localized at their C-terminal ends [
67,
69]. This region is exposed to the outer surface of MTs when tubulin dimers polymerize [
71] providing binding sites for several MAPs and molecular motors [
72]. Also, tubulin C-terminal ends are subjected to various PTMs, most of them occurring on tubulin subunits after their polymerization into MTs. Therefore, most probably, distinct C-terminal amino acid sequences indicate different patterns of tubulins PTMs and distinct associations with MAPs and motor proteins.
In
T. gondii, post-translational modifications (PTMs) were identified on α-tubulin, including acetylation at K40 and removal of the last C-terminal amino acid residue, Y453 (resulting in detyrosinated tubulin), as well as truncation of the last five amino acid residues (ΔYGDEY). The acetylation of K40 on α1-tubulin is catalyzed by α-tubulin acetyltransferase (ATAT), an enzyme crucial for completing nuclear division. Acetylated α-tubulin is notably enriched during the formation of daughter buds [
73]. An antibody targeting mammalian α-tubulin, lacking the last two C-terminal residues (Δ2-tubulin), labeled the apical region of the parasite. Detyrosinated tubulin was diffusely present in SPMTs and exhibited an apparent accumulation at their posterior end. Polyglutamylation was also detected on both α- and β-tubulins. Glycylation, a modification specific to ciliated cells and enriched in axonemes and basal bodies, was not observed, consistent with the absence of flagellar structures in tachyzoites. Additionally, methylation, a PTM not previously described on tubulin, was identified on both tubulins, suggesting it might be a specific modification in Apicomplexa [
40]. However, a comprehensive understanding of the association between these various PTMs and their functional impact on the parasite's life cycle is still lacking.
In
T. gondii, secretion plays a crucial role in the successful invasion of host cells. Research has demonstrated that vesicles, within epithelial cells, traverse from the trans-Golgi network to the plasma membrane via polyglutamylated MTs [
74], suggesting a connection between tubulin glutamylation and vesicle transport. These findings propose that polyglutamylated MTs may function as a "fast track" in vesicle transport. Indeed, as mentioned above, in cilia tubulin glutamylation impacts the speed of transport and the localization of MT motor proteins [
41,
42]. In conclusion, gaining a deeper understanding of the regulation of tubulin glutamylation in
T. gondii is imperative.
5. T. gondii tubulin glutamylation
In
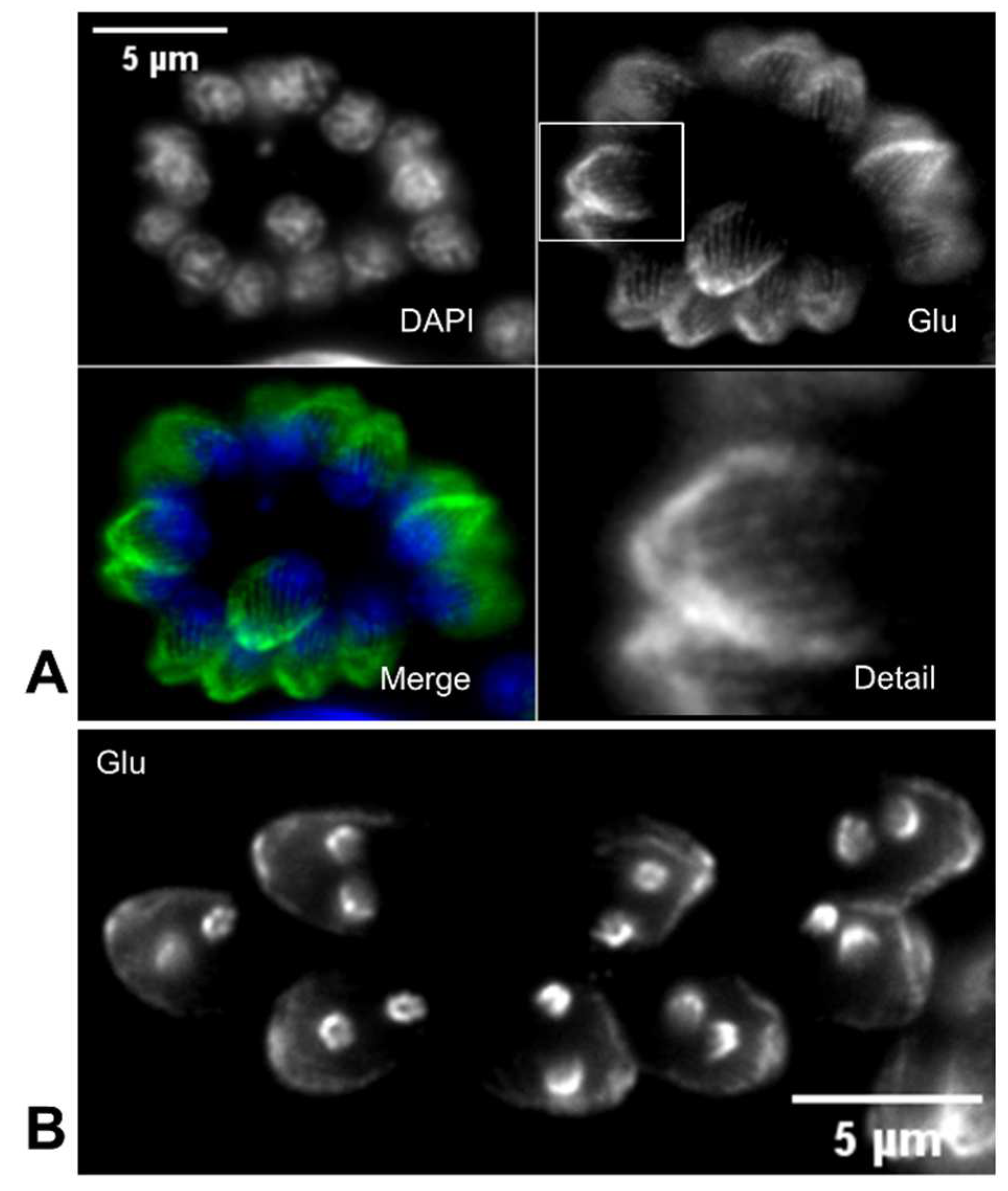
T. gondii, the use of antibodies against glutamylated tubulin revealed that glutamylation occurs close to the conoid and progressively decreases towards the distal end of the MTs (
Figure 2A); similar gradients were observed along cilia axonemal MTs in various species [
75,
76]. Curiously, it has been proposed that the apical complex of the
T. gondii tachyzoite originated from a repurposed flagellum [
77]. Like acetylated α-tubulin [
73], glutamylated tubulin is also enriched during the formation of daughter buds (
Figure 2B).
Tubulin polyglutamylation can act as a direct regulator of MT functions, for example it regulates MT-dynein interactions [
78,
79] and long glutamate side chains on tubulin provide a signal for MT severing by spastin [
80]. Tubulin polyglutamylation is a completely reversible PTM. In mammalian cells, the length controlling of the polyglutamate side chains on tubulin is critical for neural survival [
81].
Polyglutamylation is a strictly regulated PTM. The polyglutamylase enzymes have been identified as belonging to the tubulin tyrosine ligase-like (TTLL) protein family [
82]. Deglutamylase enzymes are members of the cytosolic carboxipeptidases (CCP) family [
83,
84]. The maintenance of the correct levels of tubulin polyglutamylation is crucial for the cell and results from the coordinated action of polyglutamylases and deglutamylating enzymes.
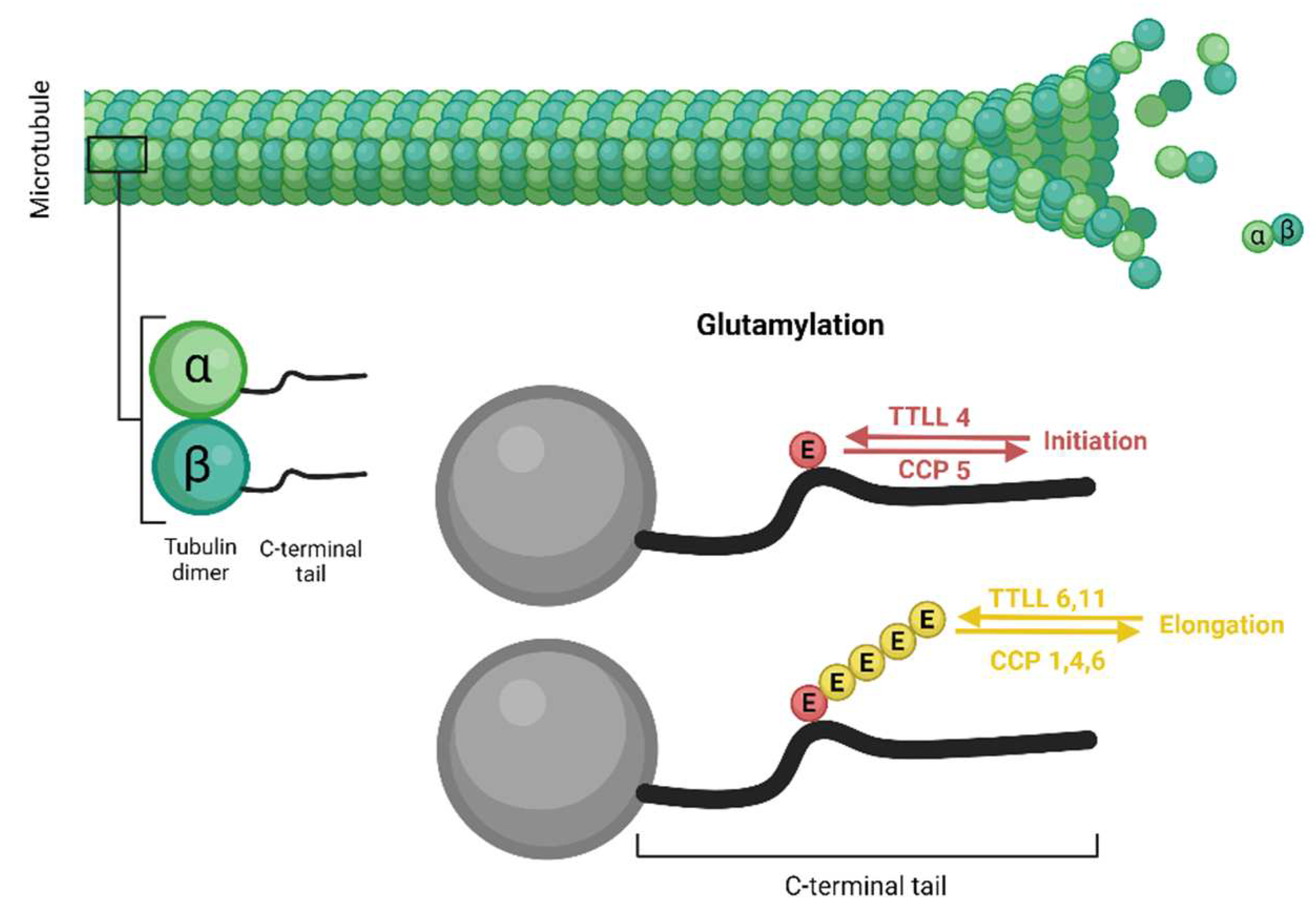
The polymodification side chain is formed in two biochemically distinct steps: initiation and elongation. These two steps are often, but not always, mediated by distinct TTLLs. These enzymes have distinct enzymatic characteristics; there are TTLLs that generate short side chains (e.g. TTLL4) and others that add long side chains (e.g. TTLL6, TTLL11) [
82] (
Figure 3).
A computational analysis searching for TTLLs orthologs of
Homo sapiens in the
T. gondii genome at
www.ToxoDb.org, revealed that the genome of this parasite contains five putative genes encoding the tubulin tyrosine ligase-like (TTLL) enzymes, one putative gene encoding a carboxypeptidase (CCP), one gene encoding spastin, and one gene encoding katanin p60 subunit (
Table 1). However, it is important to consider that glutamylation can occur in non-tubulin proteins [
82], and the same TTLL enzyme can glutamylate both a tubulin and a non-tubulin substrate [
82,
85]. Additionally, CCPs also deglutamylate non-tubulin proteins [
81,
86].
Interestingly, from the five putative
T. gondii TTLLs orthologs indicated in table 1 only the TTLL6 and TTLL11 (TGME49_244500) are predicted to be essential in the
T. gondii tachyzoite stage [
70]. The corresponding proteins in mammals are responsible for extending the glutamylation chains [
82]. Until now, proteomic analysis only supports the existence of the two orthologs of TTLL11, suggesting these could be key enzymes involved in the regulation of polyglutamylation in
T. gondii. Furthermore, the presence of two orthologs of mammalian TTLL11 and the putative absence of TTLLs responsible for short side chains raises the hypothesis that one of these orthologs may be involved in the production of short glutamylation chains. The presence of non-essential genes without protein evidence raises questions about why these candidate genes are retained in the genome and whether they fulfill any function. Even in the absence of protein evidence, it is significant to note that these genes undergo transcription. The question of whether these transcripts play a regulatory role remains unanswered.
Like TTLLs, CCPs differ in their enzymatic specificities. There are CCPs that catalyze the shortening of polyglutamate chains (CCP1, CCP4 and CCP6) and there is one CCP (CCP5) that specifically removes the branching point glutamates [
83,
84] (
Figure 3). In addition, CCP1, CCP4, and CCP6 also remove gene-encoded glutamates from the carboxyl termini of proteins, converting detyrosinated tubulin into α2-tubulin [
81]. Furthermore, these enzymes can present high specificity towards the activity of the members of the other class, for example, CCP1 is able to shorten the polyglutamate side chains and removes the branching point glutamates when the glutamylation is generated by TTLL6, but not by TTLL4 or by TTLL1 [
87].
T. gondii presents a single ortholog to CCP1 that probably works in coordination with the orthologs of TTLL6 and TTLL11 and is a candidate for both CCP functions described in mammals, namely removing glutamate branching points and shortening glutamate chains.
Interestingly, tubulin glutamylation has an increasingly well-documented role in the assembly and function of complex microtubular organelles, like cilia, in which tubulin polyglutamylation is observable [
38]. Additionally, tubulin glutamylation can also have a MT destabilizing effect through the regulation of MT-severing factors as katanin [
88] and spastin [
80]. Katanin and spastin are members of closely related MT-severing enzyme families that are widely distributed throughout eukaryotes [
89]. MT severing is a reaction that generates an internal break in a MT and may increase polymer mass by generating shorter MTs that can serve as seeds for nucleating new ones. Generally, both severing enzymes are much more activated by long glutamate side chains than by short side chains. However, they present different patterns of activation in response to specific TTLLs; TTLL11 showed a weaker impact on katanin activation as compared with its strong effect on spastin-mediated severing. Thus, although spastin is insensitive to these differences, katanin is more efficiently activated by glutamylation generated by TTLL6. This fact raises the exciting possibility that fine-tuning of these side chains could be implicated in a differential activation of these proteins [
80]. In
T. gondii, although we identified a candidate ortholog to the katanin p60 subunit, this gene is not essential, there is no protein evidence from proteomic analysis, and no candidate ortholog for the katanin p80 subunit was identified, suggesting that most probably there is no katanin-like functional protein. On the other hand, there is a strong candidate ortholog of spastin that is an essential gene and presents protein evidence. These data suggest that the spastin ortholog should be the enzyme responsible for MT severing in
T. gondii. Interestingly, mammalian TTLL11 triggers a strong MT severing response by spastin [
80] which suggest that the two activities may operate together in
T. gondii to modulate MT structures’ remodeling and dynamics.
While T. gondii exhibits specific and intricate MT structures, such as those found in the conoid, the polyglutamylation process is likely less complex compared to that in human cells. This distinction may arise from more structural functions leading to more stable MTs in T. gondii, whereas the greater tubulin glutamylation complexity in mammals may be associated with more regulatory functions, resulting in more dynamic MTs across various cell types with distinct functions. However, the generated patterns in conjunction with other PTMs (including specific methylation) are sufficient to ensure distinct features. These patterns influence the interactions with MAPs, impacting functions that may be related to microneme and rhoptry secretion, as well as conoid extension/retraction.
6. Concluding remarks
Polyglutamylation is a strictly regulated tubulin PTM. So, the identification and the characterization of the enzymes involved in the generation and removal of the glutamate side chains are key steps to understand the role of glutamylation in MT dynamics regulation in T. gondii. As expected, this parasite appears to present a less complex set of enzymes involved in polyglutamylation regulation, but still possess the key components of a similar regulation process, expressing enzymes that participate in each step, namely glutamylation, deglutamylation, and microtubule severing responsive to glutamylation. Experimental data is needed to assess and validate the functions of these enzymes in T. gondii.
Author Contributions
Conceptualization, S.N.; formal analysis, I.D.; investigation, I.D., J.G. and S.N.; writing—original draft preparation, I.D., J.G. and S.N.; writing—review and editing, I.D., J.G., R.F., S.Z., A.B., A.L., H.S., S.N.; visualization, I.D. and R.F.; supervision, I.D. and S.N.; funding acquisition, I.D., S.Z., A.B., A.L., H.S., S.N. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by FCT-Fundação para a Ciência e Tecnologia, I.P. (Portugal) and CIISA - Centro de Investigação Interdisciplinar em Sanidade Animal, Faculdade de Medicina Veterinária, Universidade de Lisboa, grants numbers UIDB/00276/2020 and LA/P/0059/2020 – AL4AnimalS.
Data Availability Statement
No new data were created during this study. The data analyzed referred throughout the text are publicly available at
www.ToxoDB.org.
Acknowledgments
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Levine, N.D. Progress in Taxonomy of the Apicomplexan Protozoa. J. Protozool. 1988, 35, 518–20. [CrossRef]
- [WHO] World Malaria Report 2020: 20 Years of Global Progress and Challenges; Geneva, 2020.
- Blake, D.P.; Tomley, F.M. Securing Poultry Production from the Ever-Present Eimeria Challenge. Trends Parasitol. 2014, 30, 12–19. [CrossRef]
- Chartier, C.; Paraud, C. Coccidiosis Due to Eimeria in Sheep and Goats, a Review. Small Rumin. Res. 2012, 103, 84–92. [CrossRef]
- Tzipori, S.; Ward, H. Cryptosporidiosis: Biology, Pathogenesis and Disease. Microbes Infect. 2002, 4, 1047–1058. [CrossRef]
- Álvarez-García, G.; García-Lunar, P.; Gutiérrez-Expósito, D.; Shkap, V.; Ortega-Mora, L.M. Dynamics of Besnoitia Besnoiti Infection in Cattle. Parasitology 2014, 141, 1419–1435. [CrossRef]
- Jacob, S.S.; Sengupta, P.P.; Paramanandham, K.; Suresh, K.P.; Chamuah, J.K.; Rudramurthy, G.R.; Roy, P. Bovine Babesiosis: An Insight into the Global Perspective on the Disease Distribution by Systematic Review and Meta-Analysis. Vet. Parasitol. 2020, 283, 109136. [CrossRef]
- Mans, B.J.; Pienaar, R.; Latif, A.A. A Review of Theileria Diagnostics and Epidemiology. Int. J. Parasitol. Parasites Wildl. 2015, 4, 104–118. [CrossRef]
- Delgado, I.L.S.; Zúquete, S.; Santos, D.; Basto, A.P.; Leitão, A.; Nolasco, S. The Apicomplexan Parasite Toxoplasma Gondii. Encyclopedia 2022, 2, 189–211. [CrossRef]
- Khan, K.; Khan, W. Congenital Toxoplasmosis: An Overview of the Neurological and Ocular Manifestations. Parasitol. Int. 2018, 67, 715–721. [CrossRef]
- Wang, Z.-D.; Liu, H.-H.; Ma, Z.-X.; Ma, H.-Y.; Li, Z.-Y.; Yang, Z.-B.; Zhu, X.-Q.; Xu, B.; Wei, F.; Liu, Q. Toxoplasma Gondii Infection in Immunocompromised Patients: A Systematic Review and Meta-Analysis. Front. Microbiol. 2017, 8. [CrossRef]
- Slifko, T.R.; Smith, H. V; Rose, J.B. Emerging Parasite Zoonoses Associated with Water and Food. Int. J. Parasitol. 2000, 30, 1379–1393. [CrossRef]
- Frénal, K.; Dubremetz, J.-F.; Lebrun, M.; Soldati-Favre, D. Gliding Motility Powers Invasion and Egress in Apicomplexa. Nat. Rev. Microbiol. 2017, 15, 645–660. [CrossRef]
- Hu, K.; Johnson, J.; Florens, L.; Fraunholz, M.; Suravajjala, S.; DiLullo, C.; Yates, J.; Roos, D.S.; Murray, J.M. Cytoskeletal Components of an Invasion Machine—The Apical Complex of Toxoplasma Gondii. PLoS Pathog. 2006, 2, e13. [CrossRef]
- Findeisen, P.; Mühlhausen, S.; Dempewolf, S.; Hertzog, J.; Zietlow, A.; Carlomagno, T.; Kollmar, M. Six Subgroups and Extensive Recent Duplications Characterize the Evolution of the Eukaryotic Tubulin Protein Family. Genome Biol. Evol. 2014, 6, 2274–88. [CrossRef]
- Jaglin, X.H.; Poirier, K.; Saillour, Y.; Buhler, E.; Tian, G.; Bahi-Buisson, N.; Fallet-Bianco, C.; Phan-Dinh-Tuy, F.; Kong, X.P.; Bomont, P.; et al. Mutations in the Beta-Tubulin Gene TUBB2B Result in Asymmetrical Polymicrogyria. Nat. Genet. 2009, 41, 746–52. [CrossRef]
- Tischfield, M.A.; Baris, H.N.; Wu, C.; Rudolph, G.; Van Maldergem, L.; He, W.; Chan, W.-M.; Andrews, C.; Demer, J.L.; Robertson, R.L.; et al. Human TUBB3 Mutations Perturb Microtubule Dynamics, Kinesin Interactions, and Axon Guidance. Cell 2010, 140, 74–87. [CrossRef]
- Tischfield, M.A.; Engle, E.C. Distinct Alpha- and Beta-Tubulin Isotypes Are Required for the Positioning, Differentiation and Survival of Neurons: New Support for the “multi-Tubulin” Hypothesis. Biosci. Rep. 2010, 30, 319–30. [CrossRef]
- Neff, N.F.; Thomas, J.H.; Grisafi, P.; Botstein, D. Isolation of the Beta-Tubulin Gene from Yeast and Demonstration of Its Essential Function in Vivo. Cell 1983, 33, 211–9. [CrossRef]
- Schatz, P.J.; Pillus, L.; Grisafi, P.; Solomon, F.; Botstein, D. Two Functional Alpha-Tubulin Genes of the Yeast Saccharomyces Cerevisiae Encode Divergent Proteins. Mol. Cell. Biol. 1986, 6, 3711–21. [CrossRef]
- Barahona, I.; Soares, H.; Cyrne, L.; Penque, D.; Denoulet, P.; Rodrigues-Pousada, C. Sequence of One Alpha- and Two Beta-Tubulin Genes of Tetrahymena Pyriformis. Structural and Functional Relationships with Other Eukaryotic Tubulin Genes. J. Mol. Biol. 1988, 202, 365–82. [CrossRef]
- Gaertig, J.; Thatcher, T.H.; McGrath, K.E.; Callahan, R.C.; Gorovsky, M.A. Perspectives on Tubulin Isotype Function and Evolution Based on the Observation That Tetrahymena Thermophila Microtubules Contain a Single Alpha- and Beta-Tubulin. Cell Motil. Cytoskeleton 1993, 25, 243–53. [CrossRef]
- Vainberg, I.E.; Lewis, S.A.; Rommelaere, H.; Ampe, C.; Vandekerckhove, J.; Klein, H.L.; Cowan, N.J. Prefoldin, a Chaperone That Delivers Unfolded Proteins to Cytosolic Chaperonin. Cell 1998, 93, 863–73. [CrossRef]
- Hansen, W.J.; Cowan, N.J.; Welch, W.J. Prefoldin-Nascent Chain Complexes in the Folding of Cytoskeletal Proteins. J. Cell Biol. 1999, 145, 265–77. [CrossRef]
- Gestaut, D.; Zhao, Y.; Park, J.; Ma, B.; Leitner, A.; Collier, M.; Pintilie, G.; Roh, S.-H.; Chiu, W.; Frydman, J. Structural Visualization of the Tubulin Folding Pathway Directed by Human Chaperonin TRiC/CCT. Cell 2022, 185, 4770-4787.e20. [CrossRef]
- Liu, C.; Jin, M.; Wang, S.; Han, W.; Zhao, Q.; Wang, Y.; Xu, C.; Diao, L.; Yin, Y.; Peng, C.; et al. Pathway and Mechanism of Tubulin Folding Mediated by TRiC/CCT along Its ATPase Cycle Revealed Using Cryo-EM. Commun. Biol. 2023, 6, 531. [CrossRef]
- Serna, M.; Zabala, J.C. Tubulin Folding and Degradation. In Encyclopedia of Life Sciences; Wiley, 2016; pp. 1–9.
- Gonçalves, J.; Tavares, A.; Carvalhal, S.; Soares, H. Revisiting the Tubulin Folding Pathway: New Roles in Centrosomes and Cilia. Biomol. Concepts 2010, 1, 423–34. [CrossRef]
- Nolasco, S.; Bellido, J.; Serna, M.; Carmona, B.; Soares, H.; Zabala, J.C. Colchicine Blocks Tubulin Heterodimer Recycling by Tubulin Cofactors TBCA, TBCB, and TBCE. Front. cell Dev. Biol. 2021, 9, 656273. [CrossRef]
- Francis, J.W.; Goswami, D.; Novick, S.J.; Pascal, B.D.; Weikum, E.R.; Ortlund, E.A.; Griffin, P.R.; Kahn, R.A. Nucleotide Binding to ARL2 in the TBCD∙ARL2∙β-Tubulin Complex Drives Conformational Changes in β-Tubulin. J. Mol. Biol. 2017, 429, 3696–3716. [CrossRef]
- Francis, J.W.; Newman, L.E.; Cunningham, L.A.; Kahn, R.A. A Trimer Consisting of the Tubulin-Specific Chaperone D (TBCD), Regulatory GTPase ARL2, and β-Tubulin Is Required for Maintaining the Microtubule Network. J. Biol. Chem. 2017, 292, 4336–4349. [CrossRef]
- Spiegelman, B.M.; Penningroth, S.M.; Kirschner, M.W. Turnover of Tubulin and the N Site GTP in Chinese Hamster Ovary Cells. Cell 1977, 12, 587–600. [CrossRef]
- Kristensson, M.A. The Game of Tubulins. Cells 2021, 10. [CrossRef]
- Chabin-Brion, K.; Marceiller, J.; Perez, F.; Settegrana, C.; Drechou, A.; Durand, G.; Poüs, C. The Golgi Complex Is a Microtubule-Organizing Organelle. Mol. Biol. Cell 2001, 12, 2047–60. [CrossRef]
- Yukawa, M.; Ikebe, C.; Toda, T. The Msd1-Wdr8-Pkl1 Complex Anchors Microtubule Minus Ends to Fission Yeast Spindle Pole Bodies. J. Cell Biol. 2015, 209, 549–62. [CrossRef]
- Sanchez, A.D.; Feldman, J.L. Microtubule-Organizing Centers: From the Centrosome to Non-Centrosomal Sites. Curr. Opin. Cell Biol. 2017, 44, 93–101. [CrossRef]
- Jaspersen, S.L. Anatomy of the Fungal Microtubule Organizing Center, the Spindle Pole Body. Curr. Opin. Struct. Biol. 2021, 66, 22–31. [CrossRef]
- Wloga, D.; Gaertig, J. Post-Translational Modifications of Microtubules. J. Cell Sci. 2010, 123, 3447–55. [CrossRef]
- Janke, C.; Montagnac, G. Causes and Consequences of Microtubule Acetylation. Curr. Biol. 2017, 27, R1287–R1292. [CrossRef]
- Xiao, H.; El Bissati, K.; Verdier-Pinard, P.; Burd, B.; Zhang, H.; Kim, K.; Fiser, A.; Angeletti, R.H.; Weiss, L.M. Post-Translational Modifications to Toxoplasma Gondii Alpha- and Beta-Tubulins Include Novel C-Terminal Methylation. J. Proteome Res. 2010, 9, 359–72. [CrossRef]
- Kimura, Y.; Tsutsumi, K.; Konno, A.; Ikegami, K.; Hameed, S.; Kaneko, T.; Kaplan, O.I.; Teramoto, T.; Fujiwara, M.; Ishihara, T.; et al. Environmental Responsiveness of Tubulin Glutamylation in Sensory Cilia Is Regulated by the P38 MAPK Pathway. Sci. Rep. 2018, 8, 8392. [CrossRef]
- Power, K.M.; Akella, J.S.; Gu, A.; Walsh, J.D.; Bellotti, S.; Morash, M.; Zhang, W.; Ramadan, Y.H.; Ross, N.; Golden, A.; et al. Mutation of NEKL-4/NEK10 and TTLL Genes Suppress Neuronal Ciliary Degeneration Caused by Loss of CCPP-1 Deglutamylase Function. PLoS Genet. 2020, 16, e1009052. [CrossRef]
- Leander, B.S.; Keeling, P.J. Morphostasis in Alveolate Evolution. Trends Ecol. Evol. 2003, 18, 395–402. [CrossRef]
- Leander, B.S.; Kuvardina, O.N.; Aleshin, V. V; Mylnikov, A.P.; Keeling, P.J. Molecular Phylogeny and Surface Morphology of Colpodella Edax (Alveolata): Insights into the Phagotrophic Ancestry of Apicomplexans. J. Eukaryot. Microbiol. 2003, 50, 334–40. [CrossRef]
- Goodenough, U.; Roth, R.; Kariyawasam, T.; He, A.; Lee, J.-H. Epiplasts: Membrane Skeletons and Epiplastin Proteins in Euglenids, Glaucophytes, Cryptophytes, Ciliates, Dinoflagellates, and Apicomplexans. MBio 2018, 9. [CrossRef]
- Suvorova, E.S.; Francia, M.; Striepen, B.; White, M.W. A Novel Bipartite Centrosome Coordinates the Apicomplexan Cell Cycle. PLoS Biol. 2015, 13, e1002093. [CrossRef]
- Leung, J.M.; He, Y.; Zhang, F.; Hwang, Y.-C.; Nagayasu, E.; Liu, J.; Murray, J.M.; Hu, K. Stability and Function of a Putative Microtubule-Organizing Center in the Human Parasite Toxoplasma Gondii. Mol. Biol. Cell 2017, 28, 1361–1378. [CrossRef]
- Russell, D.G.; Burns, R.G. The Polar Ring of Coccidian Sporozoites: A Unique Microtubule-Organizing Centre. J. Cell Sci. 1984, 65, 193–207. [CrossRef]
- Tran, J.Q.; de Leon, J.C.; Li, C.; Huynh, M.-H.; Beatty, W.; Morrissette, N.S. RNG1 Is a Late Marker of the Apical Polar Ring in Toxoplasma Gondii. Cytoskeleton (Hoboken). 2010, 67, 586–98. [CrossRef]
- Gould, S.B.; Kraft, L.G.K.; van Dooren, G.G.; Goodman, C.D.; Ford, K.L.; Cassin, A.M.; Bacic, A.; McFadden, G.I.; Waller, R.F. Ciliate Pellicular Proteome Identifies Novel Protein Families with Characteristic Repeat Motifs That Are Common to Alveolates. Mol. Biol. Evol. 2011, 28, 1319–31. [CrossRef]
- Katris, N.J.; van Dooren, G.G.; McMillan, P.J.; Hanssen, E.; Tilley, L.; Waller, R.F. The Apical Complex Provides a Regulated Gateway for Secretion of Invasion Factors in Toxoplasma. PLoS Pathog. 2014, 10, e1004074. [CrossRef]
- Morrissette, N.S.; Sibley, L.D. Cytoskeleton of Apicomplexan Parasites. Microbiol. Mol. Biol. Rev. 2002, 66, 21–38; table of contents. [CrossRef]
- Morrissette, N.S.; Murray, J.M.; Roos, D.S. Subpellicular Microtubules Associate with an Intramembranous Particle Lattice in the Protozoan Parasite Toxoplasma Gondii. J. Cell Sci. 1997, 110 ( Pt 1), 35–42. [CrossRef]
- Tran, J.Q.; Li, C.; Chyan, A.; Chung, L.; Morrissette, N.S. SPM1 Stabilizes Subpellicular Microtubules in Toxoplasma Gondii. Eukaryot. Cell 2012, 11, 206–216. [CrossRef]
- Mondragon, R.; Frixione, E. Ca(2+)-Dependence of Conoid Extrusion in Toxoplasma Gondii Tachyzoites. J. Eukaryot. Microbiol. 1996, 43, 120–7. [CrossRef]
- Hu, K.; Roos, D.S.; Murray, J.M. A Novel Polymer of Tubulin Forms the Conoid of Toxoplasma Gondii. J. Cell Biol. 2002, 156, 1039–50. [CrossRef]
- Nichols, B.A.; Chiappino, M.L. Cytoskeleton of Toxoplasma Gondii. J. Protozool. 1987, 34, 217–26. [CrossRef]
- Morrissette, N.; Gubbels, M.-J. The Toxoplasma Cytoskeleton: Structures, Proteins, and Processes. Toxoplasma gondii 2020, 743–788. [CrossRef]
- Mageswaran, S.K.; Guérin, A.; Theveny, L.M.; Chen, W.D.; Martinez, M.; Lebrun, M.; Striepen, B.; Chang, Y.-W. In Situ Ultrastructures of Two Evolutionarily Distant Apicomplexan Rhoptry Secretion Systems. Nat. Commun. 2021, 12, 4983. [CrossRef]
- Francia, M.E.; Dubremetz, J.-F.; Morrissette, N.S. Basal Body Structure and Composition in the Apicomplexans Toxoplasma and Plasmodium. Cilia 2015, 5, 3. [CrossRef]
- Naumov, A.; Kratzer, S.; Ting, L.-M.; Kim, K.; Suvorova, E.S.; White, M.W. The Toxoplasma Centrocone Houses Cell Cycle Regulatory Factors. MBio 2017, 8. [CrossRef]
- Farrell, M.; Gubbels, M.-J. The Toxoplasma Gondii Kinetochore Is Required for Centrosome Association with the Centrocone (Spindle Pole). Cell. Microbiol. 2014, 16, 78–94. [CrossRef]
- Chen, C.-T.; Kelly, M.; Leon, J. de; Nwagbara, B.; Ebbert, P.; Ferguson, D.J.P.; Lowery, L.A.; Morrissette, N.; Gubbels, M.-J. Compartmentalized Toxoplasma EB1 Bundles Spindle Microtubules to Secure Accurate Chromosome Segregation. Mol. Biol. Cell 2015, 26, 4562–76. [CrossRef]
- Hu, K. Organizational Changes of the Daughter Basal Complex during the Parasite Replication of Toxoplasma Gondii. PLoS Pathog. 2008, 4, e10. [CrossRef]
- Gubbels, M.-J.; White, M.; Szatanek, T. The Cell Cycle and Toxoplasma Gondii Cell Division: Tightly Knit or Loosely Stitched? Int. J. Parasitol. 2008, 38, 1343–58. [CrossRef]
- Blader, I.J.; Coleman, B.I.; Chen, C.-T.; Gubbels, M.-J. Lytic Cycle of Toxoplasma Gondii: 15 Years Later. Annu. Rev. Microbiol. 2015, 69, 463–85. [CrossRef]
- Nagel, S.D.; Boothroyd, J.C. The Alpha- and Beta-Tubulins of Toxoplasma Gondii Are Encoded by Single Copy Genes Containing Multiple Introns. Mol. Biochem. Parasitol. 1988, 29, 261–73. [CrossRef]
- Morrissette, N. Targeting Toxoplasma Tubules: Tubulin, Microtubules, and Associated Proteins in a Human Pathogen. Eukaryot. Cell 2015, 14, 2–12. [CrossRef]
- Morrissette, N.; Abbaali, I.; Ramakrishnan, C.; Hehl, A.B. The Tubulin Superfamily in Apicomplexan Parasites. Microorganisms 2023, 11, 706. [CrossRef]
- Sidik, S.M.; Huet, D.; Ganesan, S.M.; Huynh, M.-H.; Wang, T.; Nasamu, A.S.; Thiru, P.; Saeij, J.P.J.; Carruthers, V.B.; Niles, J.C.; et al. A Genome-Wide CRISPR Screen in Toxoplasma Identifies Essential Apicomplexan Genes. Cell 2016, 166, 1423-1435.e12. [CrossRef]
- Nogales, E.; Wolf, S.G.; Downing, K.H. Structure of the Alpha Beta Tubulin Dimer by Electron Crystallography. Nature 1998, 391, 199–203. [CrossRef]
- Wang, Z.; Sheetz, M.P. The C-Terminus of Tubulin Increases Cytoplasmic Dynein and Kinesin Processivity. Biophys. J. 2000, 78, 1955–64. [CrossRef]
- Varberg, J.M.; Padgett, L.R.; Arrizabalaga, G.; Sullivan, W.J. TgATAT-Mediated α-Tubulin Acetylation Is Required for Division of the Protozoan Parasite Toxoplasma Gondii. mSphere 2016, 1. [CrossRef]
- Spiliotis, E.T.; Hunt, S.J.; Hu, Q.; Kinoshita, M.; Nelson, W.J. Epithelial Polarity Requires Septin Coupling of Vesicle Transport to Polyglutamylated Microtubules. J. Cell Biol. 2008, 180, 295–303. [CrossRef]
- Plessmann, U.; Reiter-Owona, I.; Lechtreck, K.-F. Posttranslational Modifications of Alpha-Tubulin of Toxoplasma Gondii. Parasitol. Res. 2004, 94, 386–9. [CrossRef]
- Tosetti, N.; Dos Santos Pacheco, N.; Bertiaux, E.; Maco, B.; Bournonville, L.; Hamel, V.; Guichard, P.; Soldati-Favre, D. Essential Function of the Alveolin Network in the Subpellicular Microtubules and Conoid Assembly in Toxoplasma Gondii. Elife 2020, 9. [CrossRef]
- de Leon, J.C.; Scheumann, N.; Beatty, W.; Beck, J.R.; Tran, J.Q.; Yau, C.; Bradley, P.J.; Gull, K.; Wickstead, B.; Morrissette, N.S. A SAS-6-like Protein Suggests That the Toxoplasma Conoid Complex Evolved from Flagellar Components. Eukaryot. Cell 2013, 12, 1009–19. [CrossRef]
- Kubo, T.; Yanagisawa, H.; Yagi, T.; Hirono, M.; Kamiya, R. Tubulin Polyglutamylation Regulates Axonemal Motility by Modulating Activities of Inner-Arm Dyneins. Curr. Biol. 2010, 20, 441–5. [CrossRef]
- Suryavanshi, S.; Eddé, B.; Fox, L.A.; Guerrero, S.; Hard, R.; Hennessey, T.; Kabi, A.; Malison, D.; Pennock, D.; Sale, W.S.; et al. Tubulin Glutamylation Regulates Ciliary Motility by Altering Inner Dynein Arm Activity. Curr. Biol. 2010, 20, 435–40. [CrossRef]
- Lacroix, B.; van Dijk, J.; Gold, N.D.; Guizetti, J.; Aldrian-Herrada, G.; Rogowski, K.; Gerlich, D.W.; Janke, C. Tubulin Polyglutamylation Stimulates Spastin-Mediated Microtubule Severing. J. Cell Biol. 2010, 189, 945–54. [CrossRef]
- Rogowski, K.; van Dijk, J.; Magiera, M.M.; Bosc, C.; Deloulme, J.-C.; Bosson, A.; Peris, L.; Gold, N.D.; Lacroix, B.; Bosch Grau, M.; et al. A Family of Protein-Deglutamylating Enzymes Associated with Neurodegeneration. Cell 2010, 143, 564–78. [CrossRef]
- van Dijk, J.; Rogowski, K.; Miro, J.; Lacroix, B.; Eddé, B.; Janke, C. A Targeted Multienzyme Mechanism for Selective Microtubule Polyglutamylation. Mol. Cell 2007, 26, 437–48. [CrossRef]
- Berezniuk, I.; Vu, H.T.; Lyons, P.J.; Sironi, J.J.; Xiao, H.; Burd, B.; Setou, M.; Angeletti, R.H.; Ikegami, K.; Fricker, L.D. Cytosolic Carboxypeptidase 1 Is Involved in Processing α- and β-Tubulin. J. Biol. Chem. 2012, 287, 6503–17. [CrossRef]
- Kimura, Y.; Kurabe, N.; Ikegami, K.; Tsutsumi, K.; Konishi, Y.; Kaplan, O.I.; Kunitomo, H.; Iino, Y.; Blacque, O.E.; Setou, M. Identification of Tubulin Deglutamylase among Caenorhabditis Elegans and Mammalian Cytosolic Carboxypeptidases (CCPs). J. Biol. Chem. 2010, 285, 22936–41. [CrossRef]
- Xia, P.; Ye, B.; Wang, S.; Zhu, X.; Du, Y.; Xiong, Z.; Tian, Y.; Fan, Z. Glutamylation of the DNA Sensor CGAS Regulates Its Binding and Synthase Activity in Antiviral Immunity. Nat. Immunol. 2016, 17, 369–378. [CrossRef]
- Ye, B.; Li, C.; Yang, Z.; Wang, Y.; Hao, J.; Wang, L.; Li, Y.; Du, Y.; Hao, L.; Liu, B.; et al. Cytosolic Carboxypeptidase CCP6 Is Required for Megakaryopoiesis by Modulating Mad2 Polyglutamylation. J. Exp. Med. 2014, 211, 2439–2454. [CrossRef]
- Janke, C.; Rogowski, K.; Wloga, D.; Regnard, C.; Kajava, A. V; Strub, J.-M.; Temurak, N.; van Dijk, J.; Boucher, D.; van Dorsselaer, A.; et al. Tubulin Polyglutamylase Enzymes Are Members of the TTL Domain Protein Family. Science 2005, 308, 1758–62. [CrossRef]
- Sharma, N.; Bryant, J.; Wloga, D.; Donaldson, R.; Davis, R.C.; Jerka-Dziadosz, M.; Gaertig, J. Katanin Regulates Dynamics of Microtubules and Biogenesis of Motile Cilia. J. Cell Biol. 2007, 178, 1065–79. [CrossRef]
- Roll-Mecak, A.; McNally, F.J. Microtubule-Severing Enzymes. Curr. Opin. Cell Biol. 2010, 22, 96–103. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).