1. Introduction
The spring water of an area can reveal evidence of the past and provide evidence of very slow geological changes and human intervention in the present. In this sense, by looking back and using historical data of hydrochemical analyzes and comparing them with today's data, we can understand today's geological processes and contrasts, but also anthropogenic interventions. Thermal springs are defined as a location where the water flowing out is above 20 degrees or above the average annual ambient temperature of the area. Thermal spring water has various uses such as power generation, spas, agriculture, washing, and aquaculture. Despite the numerous thermal springs worldwide, there is limited scientific understanding of their hydrogeochemistry. The temperature of thermal springs may be elevated due to geothermal gradient in the area, exothermic reactions, and radioactive decay. Physiochemical parameters like pH, temperature, electrical conductivity, total dissolved solids, ions, and heavy metals affect thermal spring quality. Elevated temperatures accelerate interaction with minerals of the rocks which may result in elevated levels of dissolved solids, minerals, and gases depending on geology of the area [
1,
2].
In Lentas, southern Crete, within a sequence of exotic rocks (gneisses, ophiolites, granitoids, lavas, etc.) that structure the wider area, small fissured aquifers were formed that are discharged from scattered sources. The main source in the area is hot and has properties that were recognized and exploited for the establishment of the well-known homonymous Asclepieio (or Asklepieio) in the area. Asclepius was one of the most important healers of Ancient Greece. As a deity, his worship spread through several regions in the classical era and has enjoyed exceptional popularity, especially during the Hellenistic and Greco-Roman eras. Moreover, in the 4th century BC but also during the Roman and Byzantine eras, Levinaion was a famous centre for hydrotherapy, physiotherapy and a psychiatric hospital [
3]. At the beginning of the previous century, the archaeological dig found the remains of the ancient Asklepiion [
4]. The therapeutical importance of the sacred spring in the Lentas area with the ancient Asclepius remains has survived to present times in Crete's cultural tradition.
Literature studies show that major water parameters in thermal springs vary significantly due to differences in primary geochemistry, water temperature, and the intense anthropogenic pressures observed worldwide. Compared to other water sources, the levels of electrical conductivity (EC), total dissolved solids (TDS), major anions and cations, and trace elements are higher. Warm water with high mineral content and low dissolved oxygen supports therapeutic applications, including skin therapy, bone healing, soothing body aches, and gastrological diseases [
2,
5,
6].
The paper deals with the holistic approach to managing reserves and hydrochemistry of an important archaeological site of global scope. The paper proceeds as follows: In the next section, we discuss the archaeological environment of Asclepieia and also the diverse nature of archaeological evidence that brought to light the sacred spring of Asclepieion of Lenta. In section 3, we present a geological outline of the Lenta area, and the hydrogeochemical status of water resources in this area with emphasis on the sacred spring of Asclepieion in the past and in the present. In section 4, we describe our methodology for investigating both the physicochemical and hydrochemical status of the water sources in Lenta. In section 5, we are examining the impact of urban modernization on Lentas' spring water resources by comparing hydrochemical data from current spring and borehole with sacred spring data from the past. The remainder of the paper centres around our findings, future perspectives of research, and a proposal to bring back into operation the sacred spring of Lentas.
3. The Geological and Hydrogeological Outline
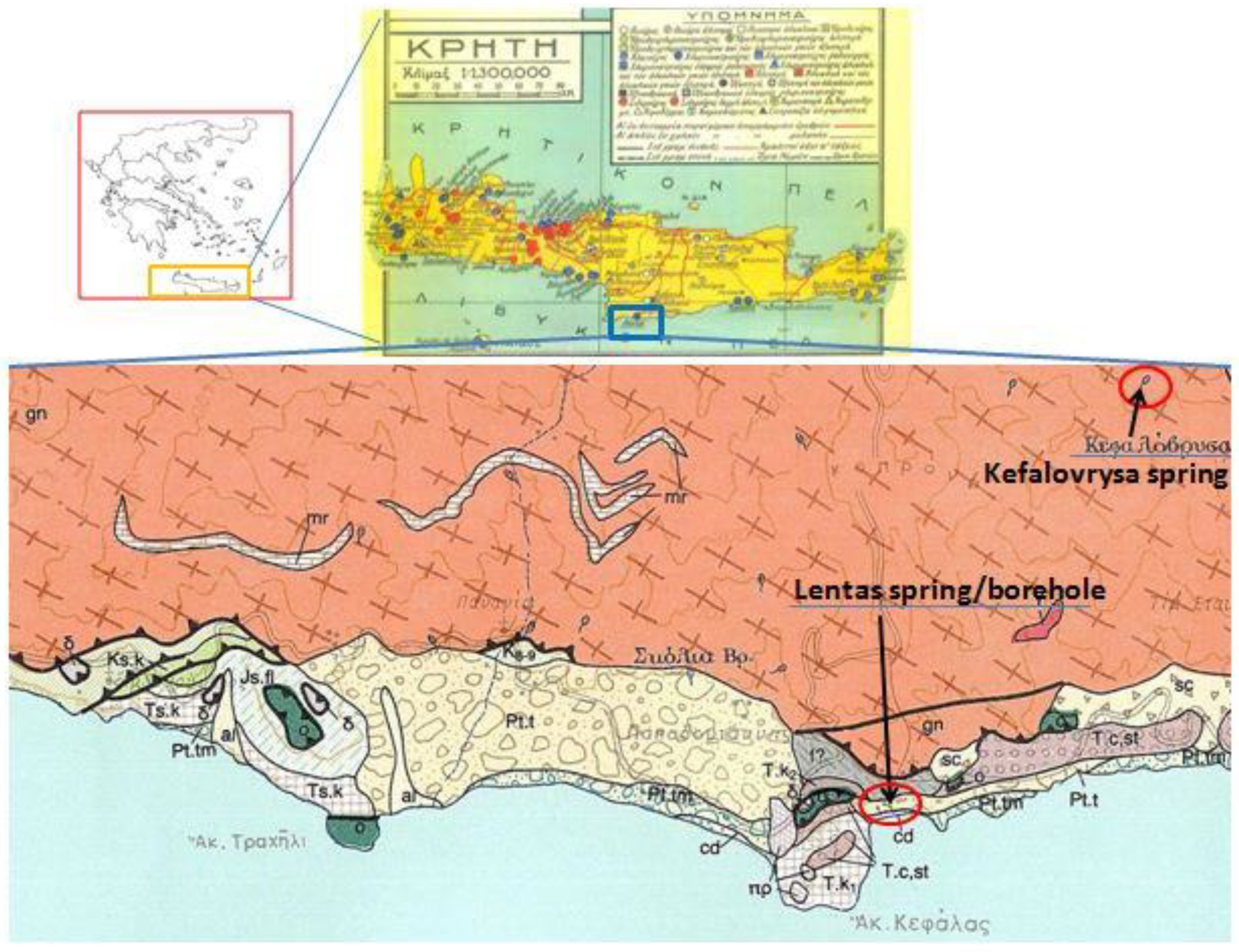
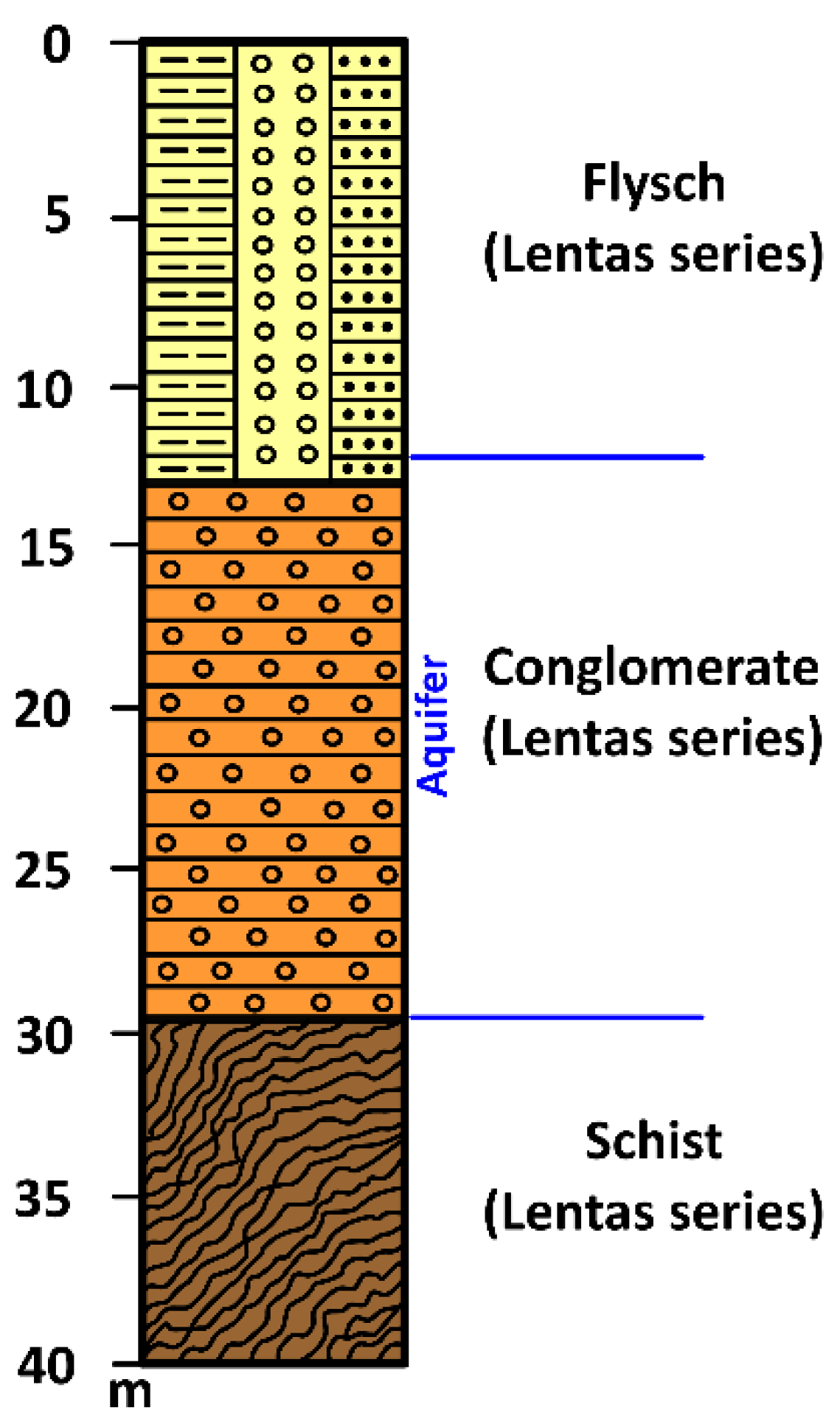
Greece, like many other Mediterranean countries, is rich in thermal or/and mineral waters. Such wealth is because the greater part of this country is located in an area geo-dynamically very active, and where both the high mountain ranges and active fault systems allow the precipitation, circulation and rapid rise of meteoric originated deep waters, mainly along the boundaries of the main tectonic structures. As is well known, these dynamics are a consequence of crustal shortening caused by the northward movement of the African plate towards the Eurasian plate. Compressive tectonic causing the thrusting of predominantly sedimentary nappes, as well as the overlapping of relatively stable crystalline basemen and tensional tectonic phases that followed during the Neogene and Quaternary led, in combination with the alternating lithostratigraphy, to the creation of hundreds of aquifers and thousands of springs scattered throughout the country. The External Hellenides almost exclusively constitute the island of Crete without missing parts of the internal Hellenides referred to as the Uppermost Nappe and expose a complex nappe structure as a whole (
Figure 2). The lack of recent volcanism in Crete led to the absence of hot springs on the island without ruling out the creation of hypothermic mineral springs. One of them is located in the area of Lentas and is, as has been described, connected with the sanctuary of Asclepios in the area for thousands of years [
14].
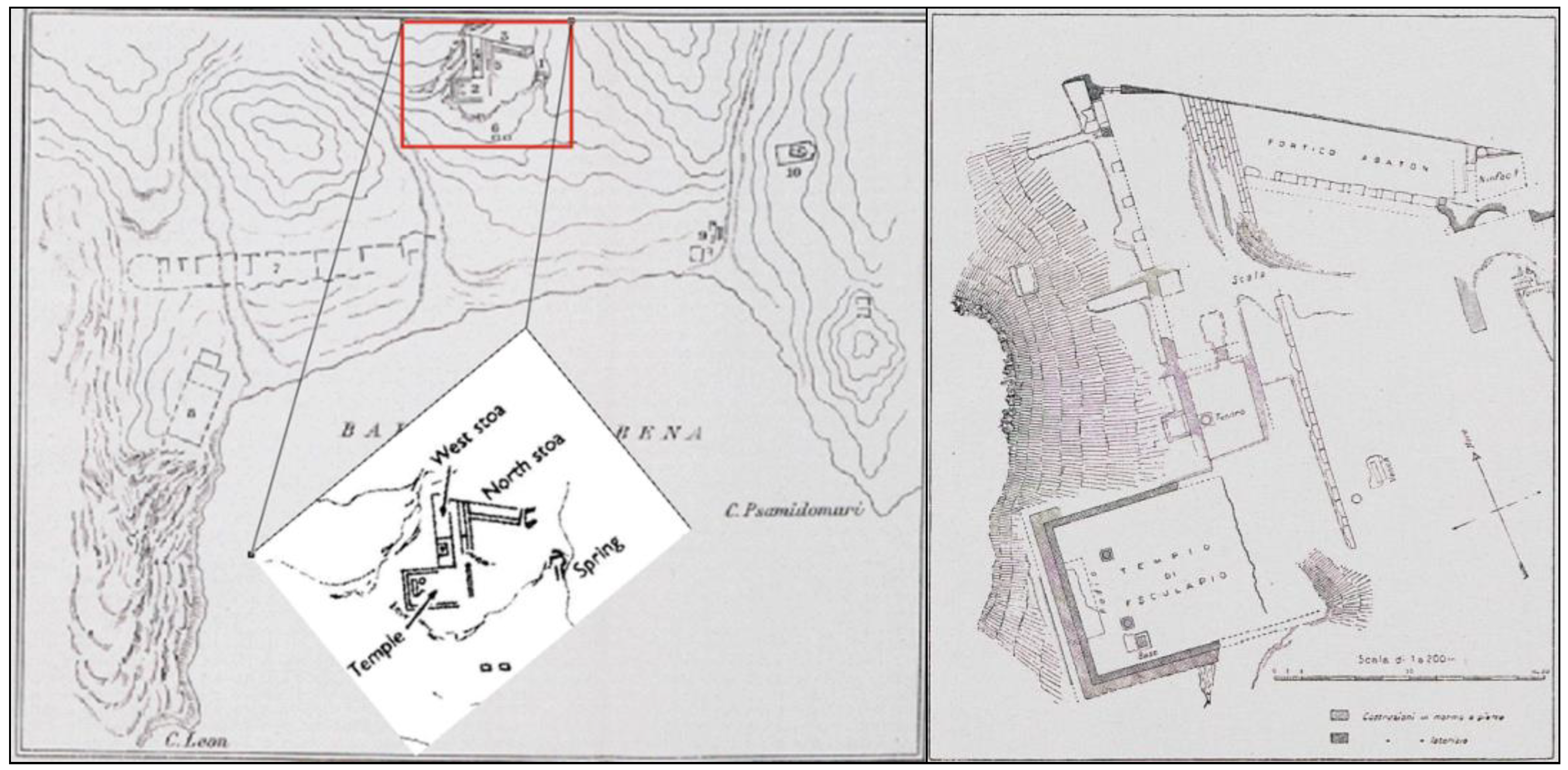
The Asclepieio of Lentas is located in the southern part of Crete, specifically on the outskirts of Asterousia in the south-central region (
Figure 3). Taramelli described that the area between Cape Leo (now Kefalas) and Cape Psammidomouri had three waterways - the eastern, western, and central rivers - during the initial excavations of the Italian archaeological mission. It is probable that the first two rivers existed in ancient times. The central river, which flows from north to south and divides the holy structures, was not present during the city's occupation and probably formed after the city was deserted due to seasonal water flow [
13].
Nowadays, in the area, there are no rivers that flow constantly, but water streams do appear from time to time. The geological map shows that there are small discharges or springs scattered around, with names like Skolia and Kefalovrysa. Kefalovrysa springs are known to vanish after appearing on the surface and reappear at lower altitudes, typically near the sea coast [193]. Nevertheless, the most significant spring in the area is the sacred spring (of Asclepieion). Lenta experiences a transitional climate from Mediterranean to desert, with dry summers and mild winters. This area receives an average annual rainfall of 400 mm making it a suitable location for the establishment.
3.1. Outline of the geology of the area
In the study area, the origin and significance of the Lentas Unit/Series is still subject to controversy. A polygenic conglomerate with reworking middle Permian limestones that evolves into dm- to m-bedded breccias plays a decisive role in interpreting the Lentas unit. Davi and Bonneau [
15] were the first to describe the Lentas unit and proposed a Triassic age for the limestones, but it is possible that they are from an older age (Permian). In the geological section of the existing basic geological map for the area, on a scale of 1:50,000, along the line A-A1, which is located in the broader area of interest (
Figure 4), it is considered that the impermeable formations that allow the formation of aquifers are either the flysch of the Tripoli zone or flysch of the Pindos zone (
Supplementary Material S1) [
15]. Thorbecke [
16] states that the Lentas unit is an Upper Jurassic-Lower Cretaceous olistolith within the Pindos second flysch unit (Oligocene). Vachard et al. [
17] focus on the Misellina Zone (latest Kungurian) identified in reworked blocks within a conglomerate in the Lentas unit and suggest that the Lentas unit could represent the base of the Pindos sequence.
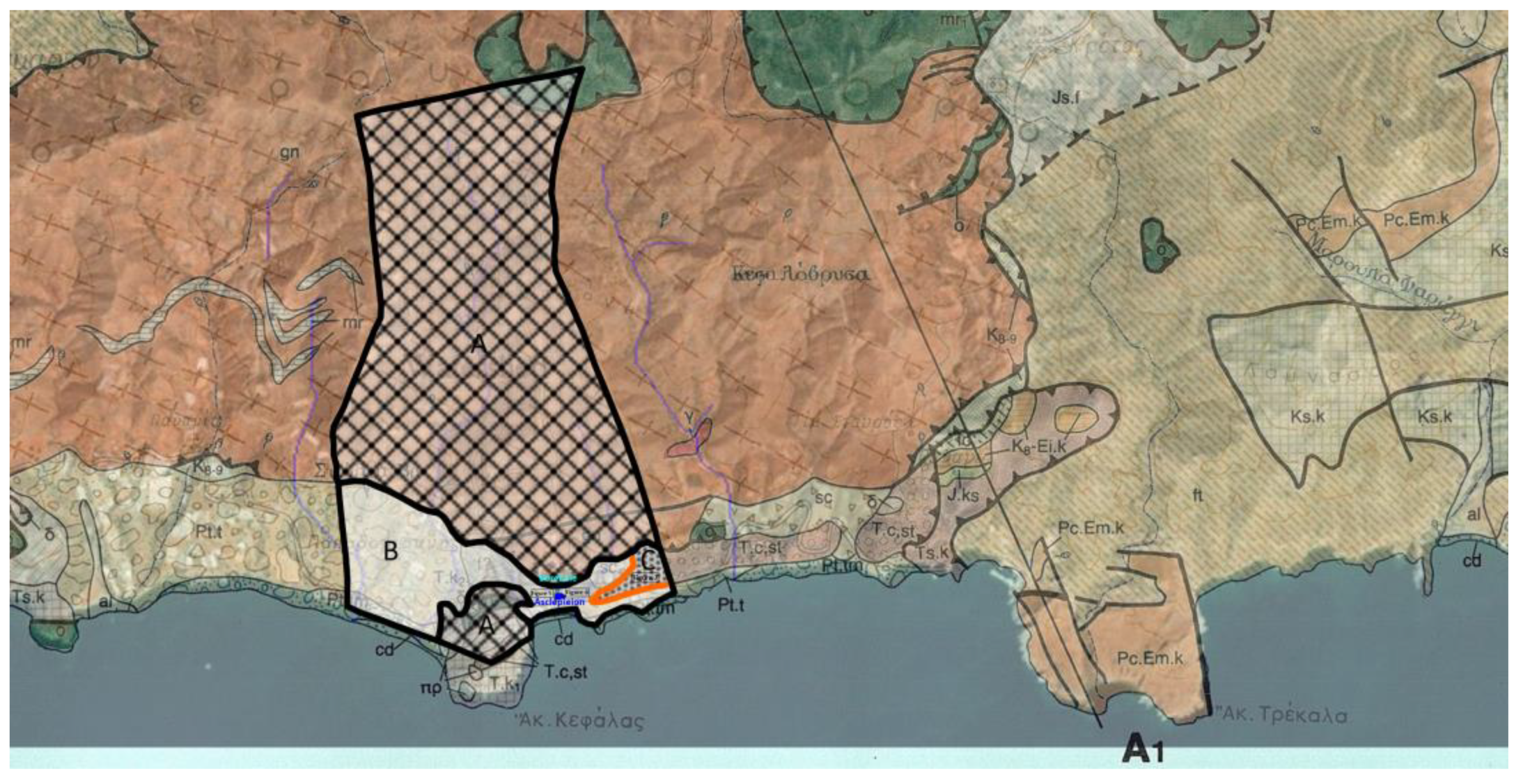
Apart from the intersections along the main roads that lead to the settlement of Lentas, there are no other natural or artificial intersections in the broader area. In addition, a strong topographical relief prevails. The combination of the superior leads to a limitation of observation and collection of geological data. As a result of this, the proposed views are interpretations. The sediments that make up the hill on which the Asklepieion structures are built can be seen in the small natural and technical sections that exist north and south of the hill (
Figure 5,
Figure 6 and
Figure 7). Photographs have been taken at the same level along the road, approximately 350 meters left and right, north of the entrance to Asklepiion (
Figure 5a,b and
Figure 6a,b, respectively). Two different research groups have characterized the sediments in photos 5a and 5b as either a possible flyschoid formation [
15] or a breccia with intercalated tuffs [
17]. In contrast, in photos 6a and 6b, the sediments have been designated as Quaternary sediments [
15]. About one kilometre east of the entrance of Asklepiion (
Figure 7a,b), a formation has been characterized as a cobblestone appears to be part of the Quaternary sediments.
Lebena was constructed on an alluvial fan developed on a terrace. In the wider area, there are scattered fragments of gneiss of Asterousia Nappe and ophiolites of the upper tectonic units of the inner zones of Hellenides and rocks of the Lentas series. Within the alluvial fan is a conglomerate formation with a thickness of more than 10 meters. The conglomerate and pebbles are from quartz, limestone, granite, ophiolite and schist. This formation continues with thick polygenic breccias. The volcanic rework clastic matrix is dark grey with the alteration from red to green. The breccia clasts are comparable to those from the conglomerate, and the distinction between the two lithologies is impossible. The main difference is the larger size of the clasts (up to several metres in diameter) in the breccias. Another interpretation has recently been proposed [
18]. This conglomerate can be interpreted as being created within the terrace, as part of an alluvial slope, or from the two ephemeral rivers that ran through the area and were mentioned by the first excavators.
3.2. The springs and the hydrogeological regime in the area
As can be seen from the geological map of the study area, there is small aquifers within the breached gneissic rocks that discharges water through multiple springs located on the northern perimeter of the settlement within the Alpine Formations. The aquifer within the gneisses communicates with an aquifer created within the Quaternary and feeds the spring of a continuous flow of the Asclepieion. Besides this spring, until a century ago, there was a second spring south of the place of the sacred spring, about eight meters from the beach. The spring, referred to as the “small spring”, always had a minimal supply and ran dry first. Its temperature was measured in 1915 at 21 C. She was also hypothermic. In the studies that followed, no reference is made to this source [
3,
19].
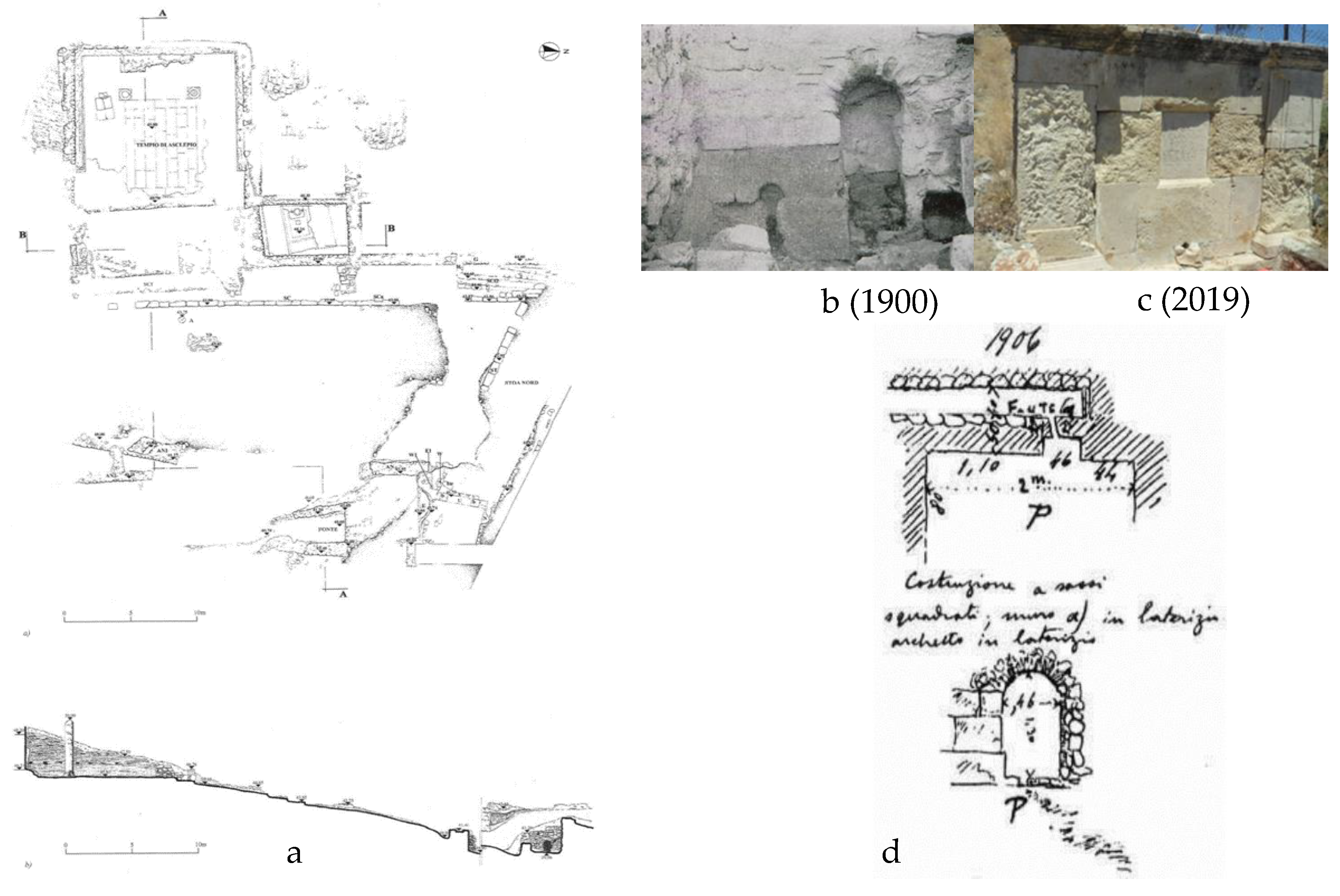
The outlet of the sacred spring (
Figure 8) was found directly in front of the nymphaeum, 3.50 meters below its level. It was an old cult nucleus that was wholly redesigned during Roman times and roofed with a brick arch. Perhaps it was only later transferred to this place from another; according to an inscription, the spring had dried up at a particular time but was rediscovered through God's vision and with a sacred serpent's help. Further east of the retaining wall, at an altitude of 39.96 m, is the access to the sacred spring with masonry, built directly into the ground leading to an underground compartment, dug into the slope, and now completely inaccessible. It is believed that the spring water collection channels were placed in this compartment, as shown in
Figure 8b. The facade of the source has a general east-west orientation and measures 2.7 meters in length and 2 meters in maximum height. Two thick walls, oriented towards the north and slightly diverging from each other, flank the facade and define an access corridor (
Figure 8d).
The preserved west wall stands at a maximum height of 1.40 meters and is over 1 meter thick. Its construction technique is a mix of styles, indicating multiple interventions over time. It appears that only the first row of the original building to the west of the access arch has been preserved. The construction consists of regular-shaped blocks of yellowish limestone, which were placed without adhesive mortar. Based on photographs taken during the initial excavations conducted by the Italian expedition, it appears that the structure was constructed atop the same limestone that was buried beneath the ground level. The upper section of the masonry was constructed using the typical method of laying blocks with a cement centre, in contrast to the rest of the arch's eastern side which shares the same characteristic. The spring could be accessed through a small brick arch that was added at a later time.
In the 1960s, a small tank with dimensions of 0.90 x 1.20 meters continued to be fed by the historic source that had previously supplied thermal water to the Lebena healing centre. The source was located at an altitude of 25 meters and approximately 200 meters away from the coast [
3]. Apart from the sacred spring in ancient Lebena, the settlement also had access to another water source called Kefalovrysa, which is still known by that name today (as shown in
Figure 2,
Figure 4 and
Figure 9b).
The water was transported from about 4 kilometres from the settlement by a stone-built pipeline, traces are still preserved today in various places [
3]. The spring is located in the middle of a ravine called “the Minas”, at an elevation of 312 meters above sea level. It's located within metamorphic and tectonically cataclastic rocks with an ultra-basic composition. Water is available all the time, but the amount is limited, especially during summer. This indicates that the source might discharge part of the larger aquifer in the area.
In the 1980s and, more specifically, in March 1989, a borehole (
Figure 9a and
Figure 10) was dug to meet the needs of the local community and the tourist stream that began to visit the beautiful beach and the ancient Asklepiion. Drilling penetrated Quaternary clastic formations and stopped at schists in the Lentas Series. The drilling of the borehole started at 35 meters elevation, and the total drilling length is 33 meters. The aquifer was located within the permeable conglomerate. After 18 hours of pumping, the flow of the borehole was Q=20m3/h, and the pumping level was 16 meters. Today, the well has an exploitable flow of Q=10m3/h and pumps water from 25 meters (accompanying material 2).
4. Materials and Methods
The physicochemical characteristics and ion variations of water samples from two springs and one borehole in Lentas area were examined from 1915 to 2021. In this study, hydrochemical types were presented by the Piper diagram, Schoeller and Durov diagram, as effective tools for determining differentiations in chemical composition of water samples.
4.1. Sampling site and in situ analysis of water samples
4.1.1. Sampling site
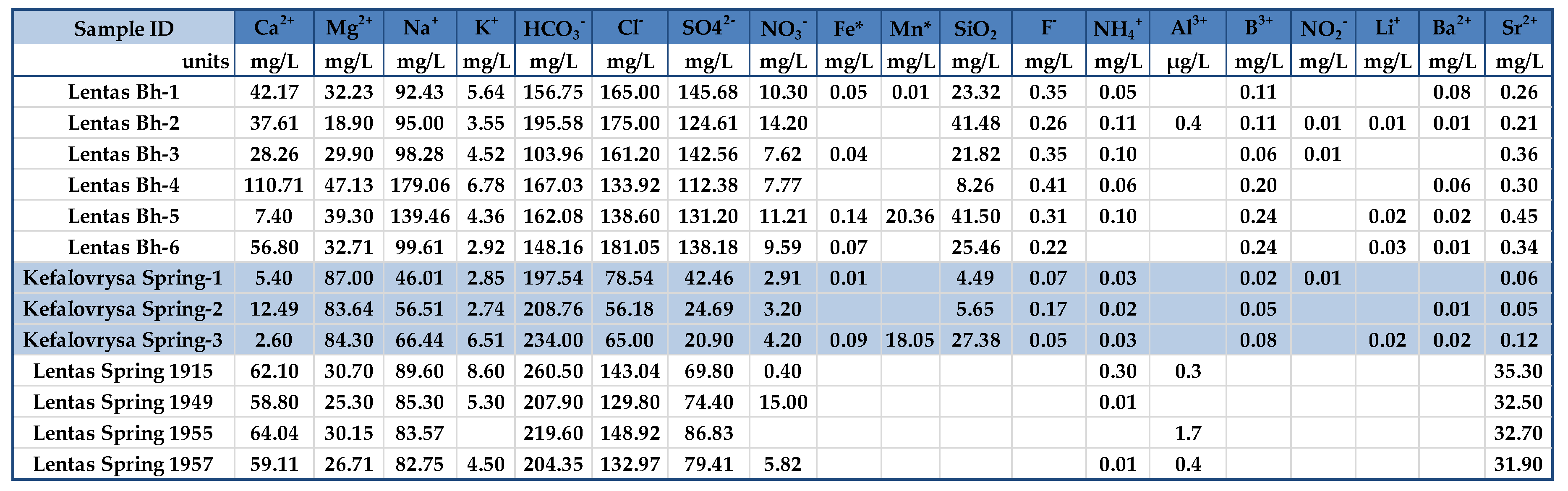
To track and analyze changes in both physical attributes and ion content, water samples were gathered during both wet and dry periods. Water samples were collected from one spring and one borehole from March 2018 to May 2021. A dry and clean polyethylene plastic bottle was used to hold spring and borehole water. Before sampling, the sampling bottles was cleaned and rinsed 2–3 times with the water to be taken and then filled to the top to minimize entrapment of air in water samples. To prepare for analysis, the samples underwent filtration using 0.45 μm filters and were then stored in HDPE bottles. To preserve the aliquots for major and trace metal analyses, 2% HNO3 acidification was used. Water samples were stored in a portable fridge until they were transported to the laboratory HersLab (School of Chemical and Environmental Engineering, Technical University of Crete) for inorganic, main and trace element analysis of water. Samples numbers and samples names are shown in
Table 1.
For this study, we gathered water samples from two sites:
the borehole that has been providing water to Lentas since the 1989, and
the Kefalovrysa spring, which was used for drinking water in the settlement during historical times.
Unfortunately, we were unable to collect water from the sacred spring (
Figure 9a light blue arrow) due to its lack of discharge, resulting from the drilling that occurred in the 1989. The Kefalovrysa spring can be found in Mina's ravine, east of Lenta, at an altitude of 312 meters (
Figure 9b), while the Lentas borehole is situated 70 meters north of the sacred spring, at an altitude of 35 meters (
Figure 9b, blue arrow).
In addition, some historical hydrochemical data, from 1915 to 1957, were included from previous studies (
Table 1) [
3].
4.1.2. In situ measurements of water samples
We measured the temperature (T), pH, electrical conductivity (EC), and total dissolved solids (TDS) of water samples using portable instruments on site. The above parameters have been measured in the field using the HI-9811-5 Portable Meter (HANNA instruments). Calibration was always carried out before measurement using standards buffer solutions of pH 4.00 and 7.00 respectively.
4.2. Analytical methods for elemental composition
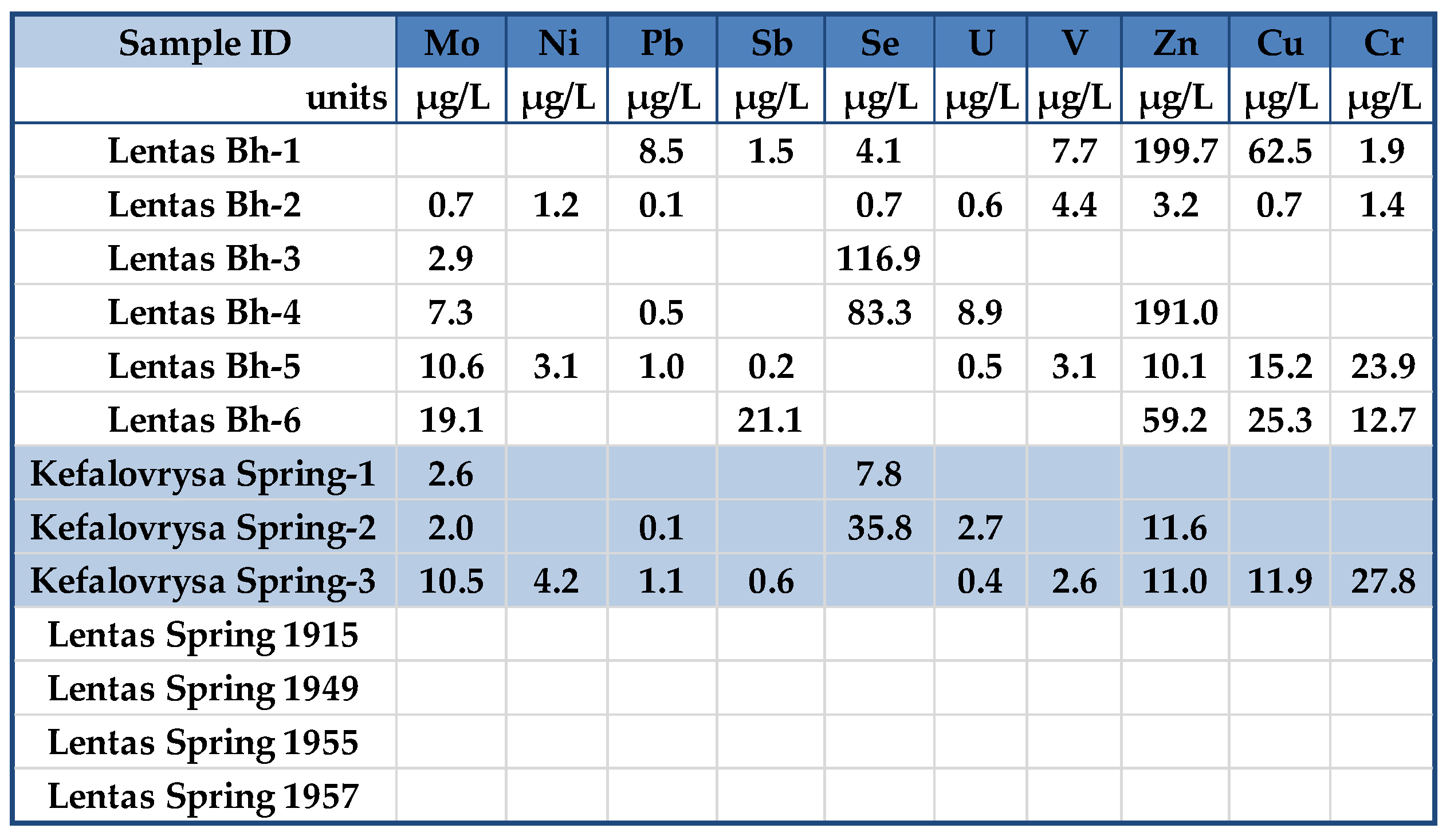
The laboratory conducted measurements on various water ions, including Na, Mg, Al, K, Cl, Ca, Fe, Li, F, B, Ba, Sr, V, Cr, Mn, Pb, Mo, Sb, Ni, U, Cu, Zn, As, Se, and, along with nutrients like N-NO3, N-NH3, and P-PO4. They also measured total organic carbon (TOC), and other elements such as SiO2, SO4, NO2, NO3, CO3, NH4, and HCO3.
The metals underwent analysis through inductively coupled plasma mass spectrometry (ICP-MS) using Agilent 7500-CX equipment (Agilent Technologies, Santa Clara, CA 95051, USA). The amount of nutrients was measured through UV-VIS spectroscopy, which involved using a HACH 2800DR spectrophotometer (Methods: LCK311, LCK153, LCK339, 8051, 8038 developed by HACH LANGE, Düsseldorf, Germany). The laboratory follows standardized quality assurance and quality control (QAQC) procedures with 10 % of the samples as duplicates, standard additions where necessary, and blank correction procedures. Aq.QA 1.5.0., 2020, (RockWare Inc.) was used for data analysis and visualization. Of note, the software Aq.QA 1.5.0 calculates the predominant water type.
4.3. Radon estimation in borehole water and spring water
As part of our current study, we conducted an analysis of the radon levels found in water samples collected from two distinct areas in Lenta: the Lenta borehole and Kefalovrysa spring. To measure these levels, we utilized an electronic Radon detector (RTM-1688-2, SARAD GmbH). The RTM-1688-2 is an active detector and belongs to the category of instruments with a continuous recording method, as it enables its user to monitor in real-time the variation of the concentration of
222Rn in the air passing through it. For the examination of water samples, the measuring arrangement of the instrument includes a pump to extract the radon by disturbing the water and driving the air current into the meter. The sample is aspirated by a pump built into the device (0.25 l/min), and the measurement is made in a chamber with a semiconductor detector. The measured quantity is the content in Bq/m3. At the same time, the readings from the temperature and atmospheric pressure sensors, also integrated into the device, are measured and recorded in the device's memory. The water samples were obtained from flowing water, as seen in
Figure 9a,9b.
4. Discussion
The study's scope is to present in a holistic approach archaeological, geological, and hydrochemical evidence of the area's water resource management, compare hydrochemical data from 1915 to 2021, highlight incipient groundwater salinization along the Lentas coast, and propose a sustainable solution through the management of sporadic small water springs in the region.
Almost a century ago, the thermometallic springs in Greece were recorded in an attempt to highlight their healing properties in a simplistic, empirical way. Out of the 750 sources that were documented, over a hundred were found in Crete [
19]. In this first record, a special mention was given to the spring of the ancient Asclepius in Lentas, also known as the ancient Lebena. According to Marinatos, even before archaeologists discovered the Lebenaion, Lentas (formerly Lebenaion) was renowned in Crete as a destination for individuals seeking treatment for stomach ailments (Marinatos in [
3]). This spring has sodium chloride at a temperature of 22°C and was widely used for medicinal purposes during the ancient, Roman and Byzantine eras. According to the historical text of Lekkas [
19], it is crucial to prioritize the study and monitoring of the source in Lentas, particularly the six sources in Crete, including the Lentas spring of Miamos.
To this end, we examine water samples from various sources in the Lentas region at different times. The goal is to compare the results with previous analyses conducted since the early 1900s (
Table 1). However, the most recent water sample was not taken from the sacred spring as in the past. Instead, it was retrieved from a borehole dug in the 1989, located about 70 meters north of the spring (
Figure 9, upper panel). Comparing the measurements and analyses made over time (1915 - 2021,
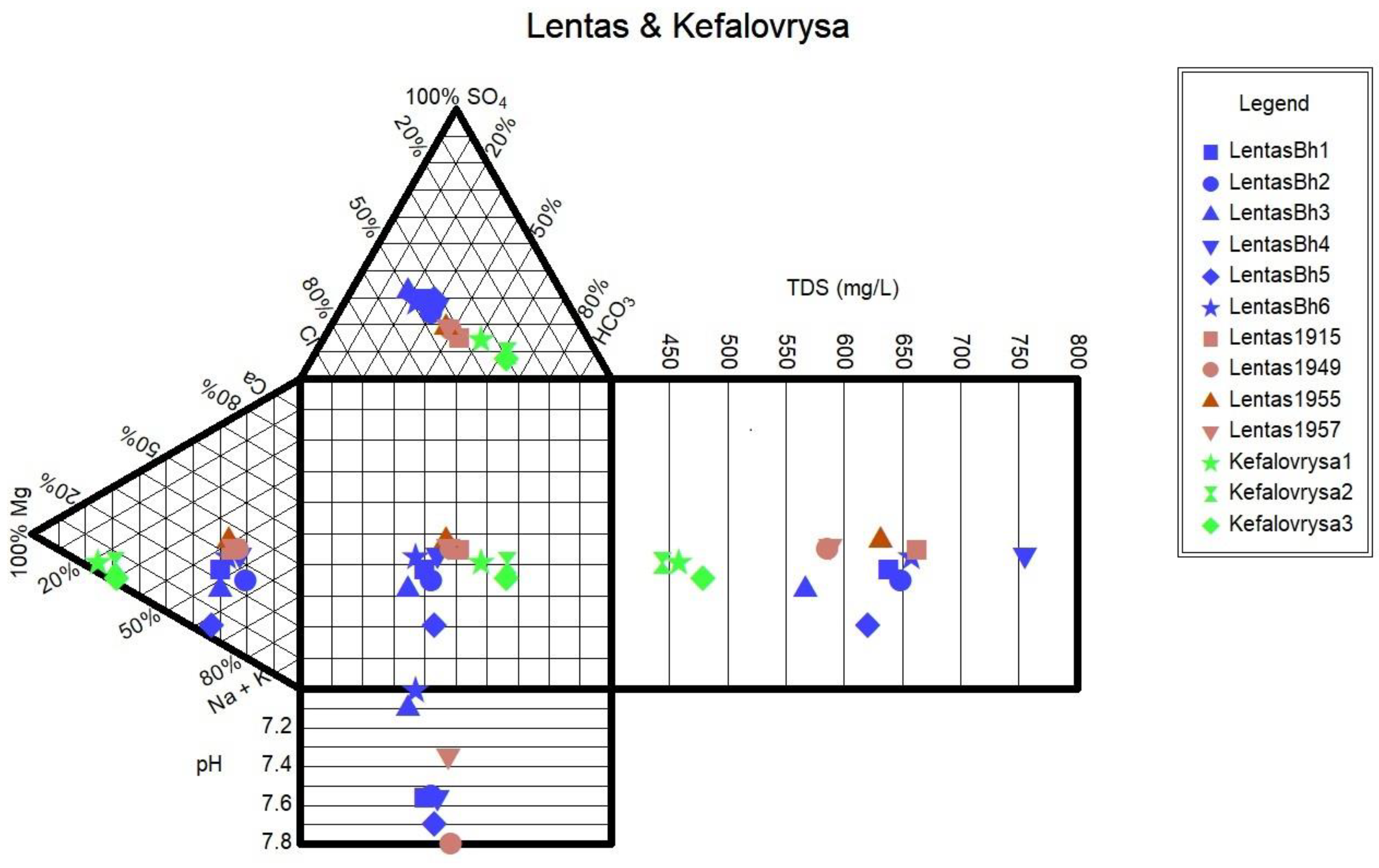
Table 1), the water from the borehole/spring has a neutral to alkaline pH ranging from 6.9 to 7.8.
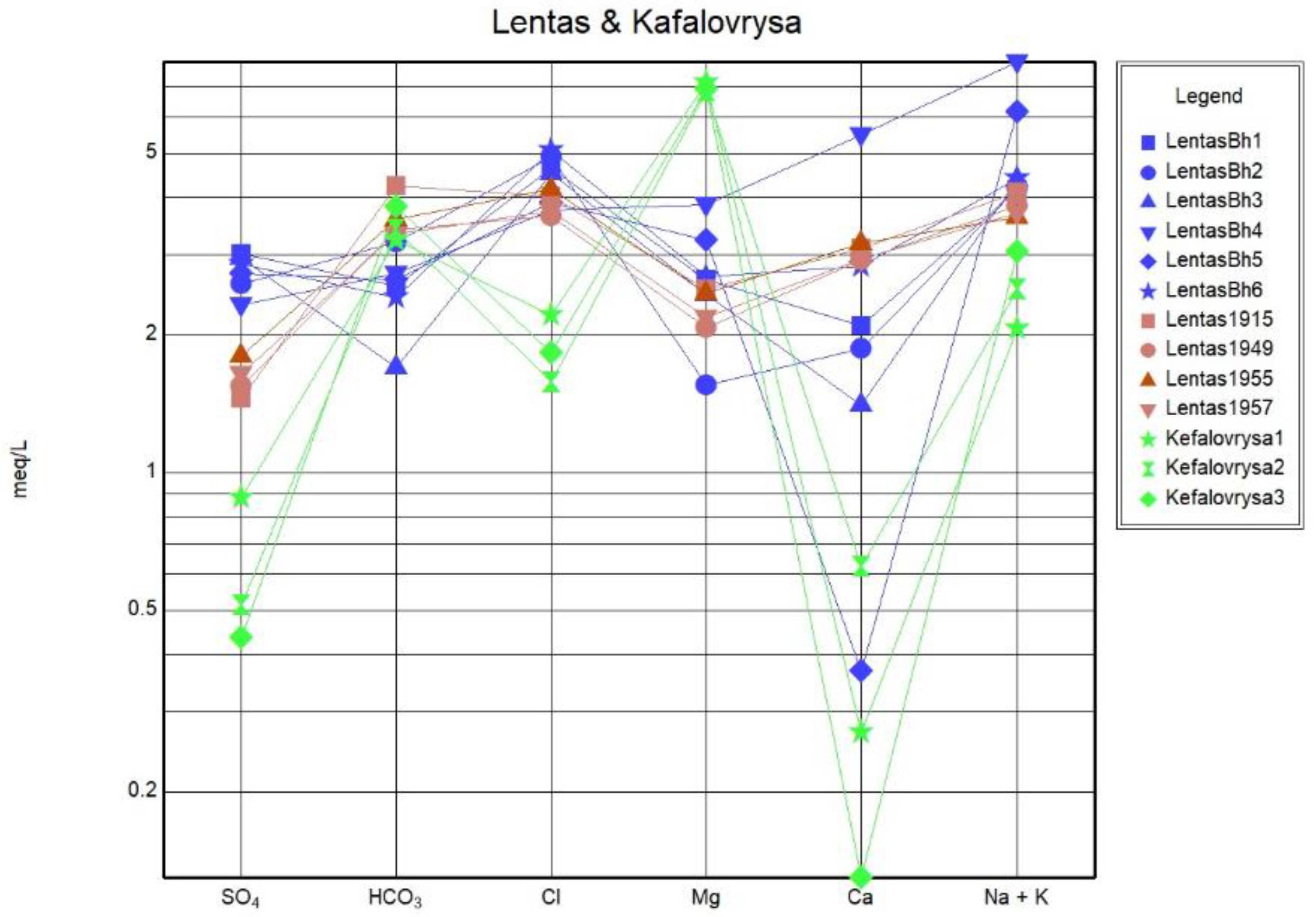
Since the beginning of the previous century, measurements have consistently shown that the spring of Lentas water is hypothermic. The chemical composition of the water shows that the source is alkaline, determined by the content of calcium and magnesium bicarbonates, whose ratio is about 2:1. That is, magnesium is found in a greater proportion than typical drinking water. The sodium chloride content is relatively higher. The results of the analyzes made over 42 years (1915-1957) showed that the composition of the spring water was relatively stable. A newer result added by the above study is the presence of traces of arsenic (0.00097 mg As=0.0018 mg HAsO4=0.0014 mg HAsO2). In their opinion, the Lentas spring should be classified as a healing spring because of its alkaline properties that produce calcium and magnesium hydrocarbonate salts, as well as the presence of arsenic traces [
3].
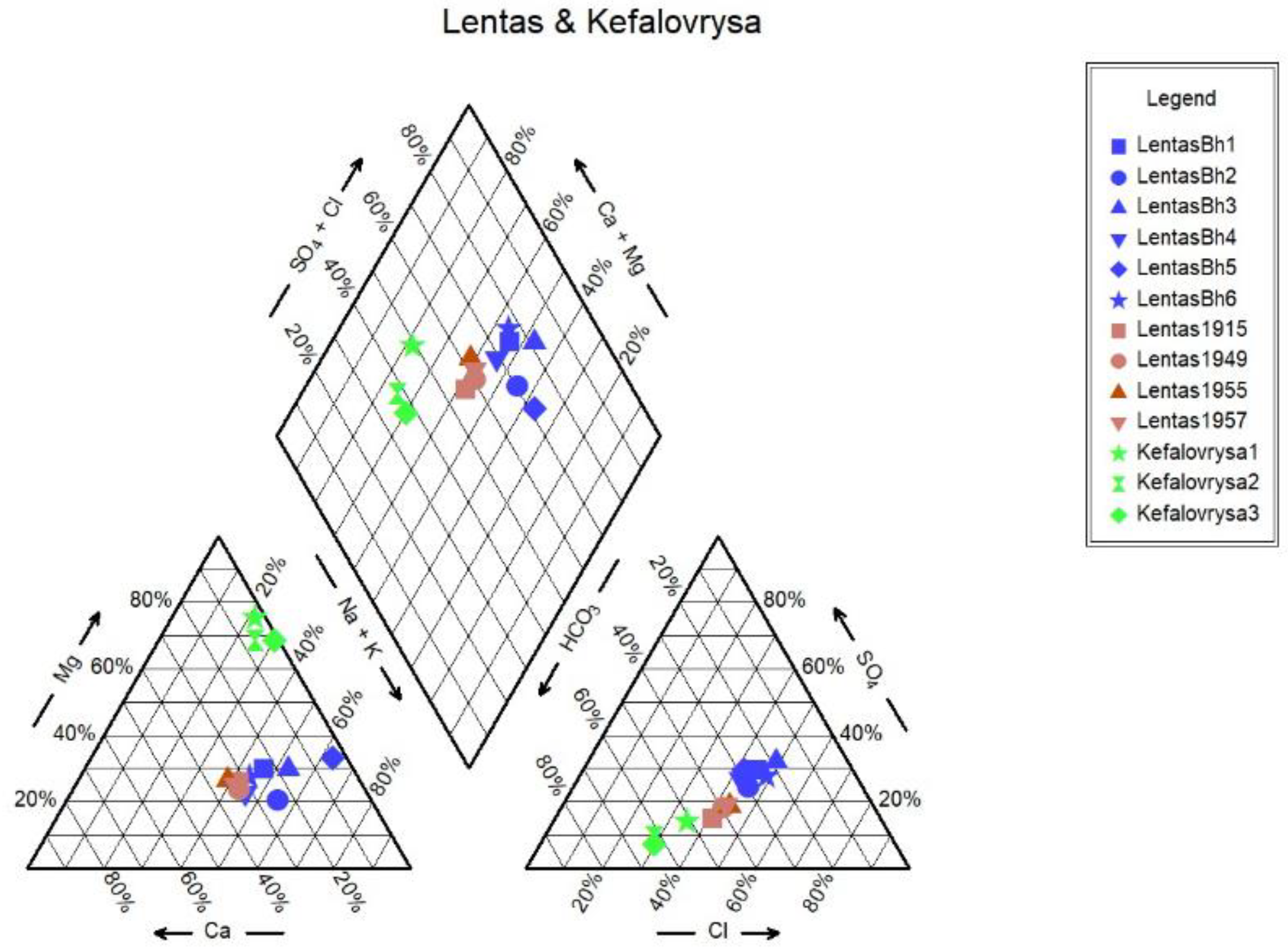
Monitoring of the physicochemical characteristics of main and trace elements in the springs of Lentas was conducted in a recent study [
18] in order to enable a comparison of recent measurements of water data with historical ones. Thus, in this study, we present the updated results of the water monitoring in the Lentas area. According to this, two hydrogeochemical water types were identified among the samples using the Piper diagram. Samples are classified into a) the Mg-HCO3 water type for the Kefalovrysa spring; and b) the Mixed Na-HCO3-Cl and subsequently to Na-Cl water type initially for the waters of the sacred spring and then for the borehole waters in Lentas, i.e. there is a shift from bicarbonate to chloride type. The sacred spring water's original mixed composition Na-HCO3-Cl from 1915 has not reappeared, and there is not enough evidence for comparison. Therefore, the second water type is Na-Cl, which dominates in porous aquifer within the Quarternary sediments. The Mg-HCO3 water type dominates groundwater found within the gneisses and overlying ophiolites. The water–ultramafic rock interaction typically produces slightly alkaline to strongly alkaline pH conditions because of the absorption of dissolved CO2 from atmospheric water in the serpentine and olivine. The pH values that characterized the studied Kefalovrysa spring varied from 7.6 to 8.4, indicating slightly alkaline conditions typical of groundwater interacting with ultramafic and carbonate rocks [
34]. As can be seen in the Schoeller diagram, the contents of SO
42-, Ca
2+ and Cl
- ions in the waters of Kefalovrysa spring are lower than in the waters of the sacred spring and also in the borehole in Lentas. In contrast, the content in Mg
2+ is higher. When groundwater from a fractured-bedrock aquifer flows through clay-rich alluvial fan deposits, it undergoes several geochemical processes. The chemical composition of this groundwater is heavily influenced by water-rock interactions that occur along its flow paths. Factors such as the flow path length, the presence of organic matter, the reactivity of soluble salts, the rate of chemical weathering of silicates, and ion-exchange rates all impact groundwater chemistry. Factors suggested to explain the source of salt include evaporation, evapotranspiration, cation-exchange reactions on clays, solution of existing salt and wetting/drying processes [
35]. This natural process is slow and long-term and is not affected on the time scale of decades without significant anthropogenic influence. In the area, there are no intensive crops but only tourist activity in the broader area of the settlement (
Figure 3).
Groundwaters in clastic formations show an increasing trend of calcium against sodium. This trend may be explained by the dissolution of calcium carbonate minerals in calcareous rocks and shale and reactions in the alluvium fan deposits. The excess of sodium over chloride by ion exchange could be explained similarly. However, these values are not certified in the analyses of waters in Lentas. So, another reason should be sought for the variation of the TDS value over time in the sacred spring and the borehole in Lentas.
The measurement of groundwater quality in coastal regions is complex due to the various input sources, including precipitation, seawater, and domestic and agricultural influences. Among the sources mentioned above of influence, seawater intrusion is the most widespread problem that makes groundwater resources unfit for domestic utilities. In order to gain insight into seawater intrusion and similar concerns, it is crucial to uncover the fundamentals of salinity through hydrogeochemical analysis [
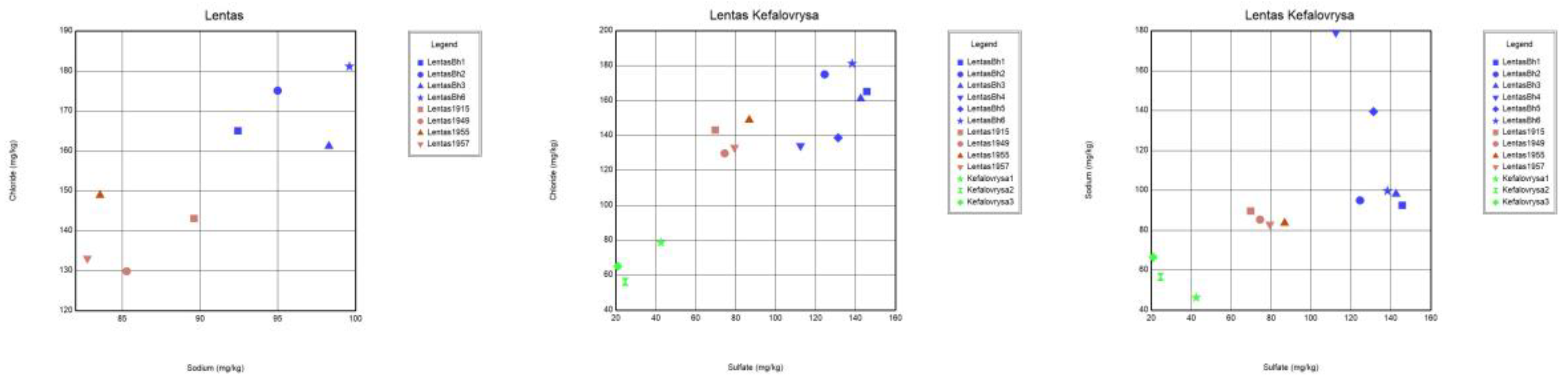
36]. Na-Cl ratios in samples from coastal aquifers have been used effectively to probe the mechanisms controlling water pollution in coastal areas worldwide. If the Na/Cl ratio relative to the EC value of the sample is constant (i.e. no fluctuation), this is displayed as a horizontal line [
35]. In this case, horizontal lines indicate the effective contribution of evaporation and evapotranspiration. The Na/Cl ratio plot in
Figure 13 suggests that samples analyzed nearly a few decades apart have apparent differences that cannot be attributed to evaporation effects. Additionally, a simultaneous increase in the content of Ca, Na, Cl, SO4, and F ions is interpreted as a transient but beginning salinization in the area. A possible interpretation could be the incipient seawater intrusion in the area. This may be due to the intensification of pumping with what this may entail. In addition to the differences between the chemistry of the two springs, the list of temperatures measured over time shows the hypothermic nature of the sacred spring, which does not exist today due to drilling. The presence of traces of arsenic was not verified during recent sampling. Nevertheless, it turns out that the historically sacred thermal spring is rich in other inorganic and beneficial trace elements, such as fluoride concentrations in mg/L and zinc in µg/L. However, there are substantial differences between the chemistry of these waters and those of Kefalovrysa. It is evident from these early data that the springs discharge aquifers located in a completely different geological environment. These new elements, combined with the historical ones, highlight the necessity to reoperate the sacred spring of the Asclepius of Lentas, which will add historical, social, and environmental value to the archaeological site.
Coastal aquifers are prone to pollution and, in particular, to salinization. The Eu-ropean Environment Agency has acknowledged that groundwater over-exploitation causing saltwater intrusion is a major threat to freshwater resources in European coastal areas [
37,
38]. Bearing in mind the definition of NBL as “the concentration of a substance or the value of an indicator in a body of groundwater corresponding to no, or only very minor, anthropogenic alterations to undisturbed conditions” according to the EU Groundwater Directive, the first step in assessing human impact on groundwater quality and identifying potentially contaminated groundwater in aquifer systems is establishing natural background levels (NBLs) of groundwater composition. The NBL of Cl, determined through different methods (e.g. simple interpolation/geostatistical methods, density-dependent solutions for groundwater flow investigations), can vary significantly from one aquifer to another. For instance, the natural background level (NBL) of chloride (Cl) in aquifers varies greatly in different countries. It can be as low as 10 mg/L in Finland or 13.2 mg/L in Latvia, but can reach up to 200 mg/L in Portugal [
39,
40]. Pulido-Velazquez performed and compared different statistical approaches to derive chloride NBLs from different hydrogeological settings across Europe based on five case studies conducted in coastal aquifers in the Atlantic Sea, the Mediterranean Sea, the North Sea and the Baltic Sea; the estimated NBLs were below 85 mg/L, with one exception [
39]. In our case, the historical data could be used as NBL reference for the coastal aquifer of Lentas.
Groundwater resources are crucial for economic and social development. Unfortunately, urbanization can lead to pollution and damage of spring water resources [
41,
42,
43,
44]. Most of the groundwater is used for irrigated agriculture. However, there is also significant urban water supply abstraction for both public and private supply [
29], and inadequate attention is paid to the overuse of water in coastal regions due to enhanced tourism needs. Based on geological observation and as can be seen from the geological map (
Figure 4,
Supplementary Material S1), there are several places with marked small water springs, some of which also have names (Kefalovrysa, Skolia) on the broader study area that have yet to be systematically studied nor exploited. What is certain is that these aquifers also feed the coastal aquifer, from which the settlement of Lenda is supplied. Alternatively, to serve the needs of the settlement, the small springs in the broader area could be used and supply the settlement after a systematic study of the spatial distribution and water capacity of the aquifers they discharge. This step could also mark the restarting of the sacred spring of Lentas.
Modern tourism began with thermalism in the interwar period, and now it is making a global comeback. In Europe, thermal springs with curative powers have maintained a high reputation for their success in healing through thermalism [
45,
46]. As far as we know, a limited number of studies have explored the hydrological and hydrochemical changes in the aquifers of holly and sacred springs [
47,
48,
49]. While it is known that mineral waters have been historically used to treat various ailments, there is a lack of understanding about the biological function of the components present in these waters that contribute to their therapeutic properties [
50,
51,
52]. Recently, there has been a growing interest in utilizing preclinical models such as animal or
in vitro studies to explore the various biological effects of balneotherapy on inflammation, immunity, cartilage and bone metabolism, as well as drinking cures on digestion [
51]. As an example, the experimental animal study carried out by Crespo et al. [
52] aimed to examine the impact of Lanjarón-Capuchina mineral water on the intestinal epithelium. This experimental model served as a prototype of sodium chloride-rich mineral waters that are used to treat digestive disorders. They observed through microscopic analysis that the intake of mineral water high in sodium chloride has a hypertonic effect on the intestinal epithelium [
52]. It is crucial to note that the experimental outcomes observed are not necessarily universally applicable to all mineral waters rich in sodium chloride. Acknowledging that thermal waters are complex systems made up of a combination of various organic and inorganic compounds, more studies are necessary to reveal and understand the mechanisms of action of drinking cure and balneotherapy. One way to approach this is by using computational tools to analyze large databases and generate evidence-based hypotheses on the impact of important elements in digestive, bone, and skin disorders. This can help guide further experimental research in these areas [
53].
Limitations of the study
The main limitations of our study are the small number of water samples, the lack of long-term follow-up of the borehole/spring, and the complete lack of other drillings in the area, which all limits the use of additional methodological approaches.