1. Introduction
Enzalutamide (ENZ) targets multiple steps in the androgen receptor signaling pathway, which are the key drivers of prostate cancer growth. It inhibits androgen signaling by binding to receptors, inhibiting nuclear translocation, binding to androgen response elements, and recruiting coactivators [
1]. ENZ has been reported to prolong progression-free survival (PFS) and overall survival (OS) rates in patients with metastatic castration-resistant prostate cancer (mCRPC), regardless of previous administration of chemotherapy [
2,
3]. It has been available in Japan for the treatment of mCRPC since 2014.
Biomarkers for predicting the prognosis and efficacy of ENZ treatment for mCRPC have not been widely established. Various prognostic models have been developed to estimate long-term survival using common clinical factors, such as prostate-specific antigen (PSA) levels [
5,
6,
7].
Antonarakis et al. reported that the detection of circulating tumor cells (CTCs) using liquid biopsy is a promising biomarker [
8]. Liquid biopsies allow carrying out analyses using non-solid biological tissues, and are thereby less invasive, painless, and inexpensive compared to tissue biopsies and can be carried out repeatedly [
9]. CTC-negative patients at the time of treatment administration demonstrate superior clinical outcomes to those of CTC-positive patients [
8]. In patients with mCRPC, the presence of androgen receptor splice variant 7 (AR-V7) in CTCs is a potential biomarker to predict the development of drug resistance to ENZ and abiraterone [
8,
10]. Unlike antiandrogen treatments, taxane-based chemotherapies are effective for mCRPC, irrespective of AR-V7 status [
11,
12]. Data from our previous study have also confirmed these findings in the Asian population [
13]. Therefore, to optimize treatment selection and avoid debilitating side effects, genetic analysis of CTCs has the potential to individualize treatment for mCRPC.
Since CTCs can be collected from a patient's peripheral blood, CTC analyses are minimally invasive and can be performed relatively easily in any laboratory. Therefore, we conducted this study to evaluate the usefulness of CTC analysis in the clinical setting of mCRPC. In a previous study conducted at our facilities and affiliated hospitals, we analyzed PFS in mCRPC patients with bone metastases treated with ENZ and demonstrated that a negative CTC status at baseline before ENZ treatment was a significant predictor for ENZ efficacy [
14]. It has already been reported in our laboratory that chronological monitoring of CTCs is very effective for evaluating possible treatments and their efficacy in mCRPC [
15,
16]. The purpose of the current study is to establish the relationship between the chronological detection of CTCs during ENZ treatment and OS by setting a longer observation period and performing CTC detection at 3 months after ENZ introduction
2. Materials and Methods
2.1. Patients and Study Design
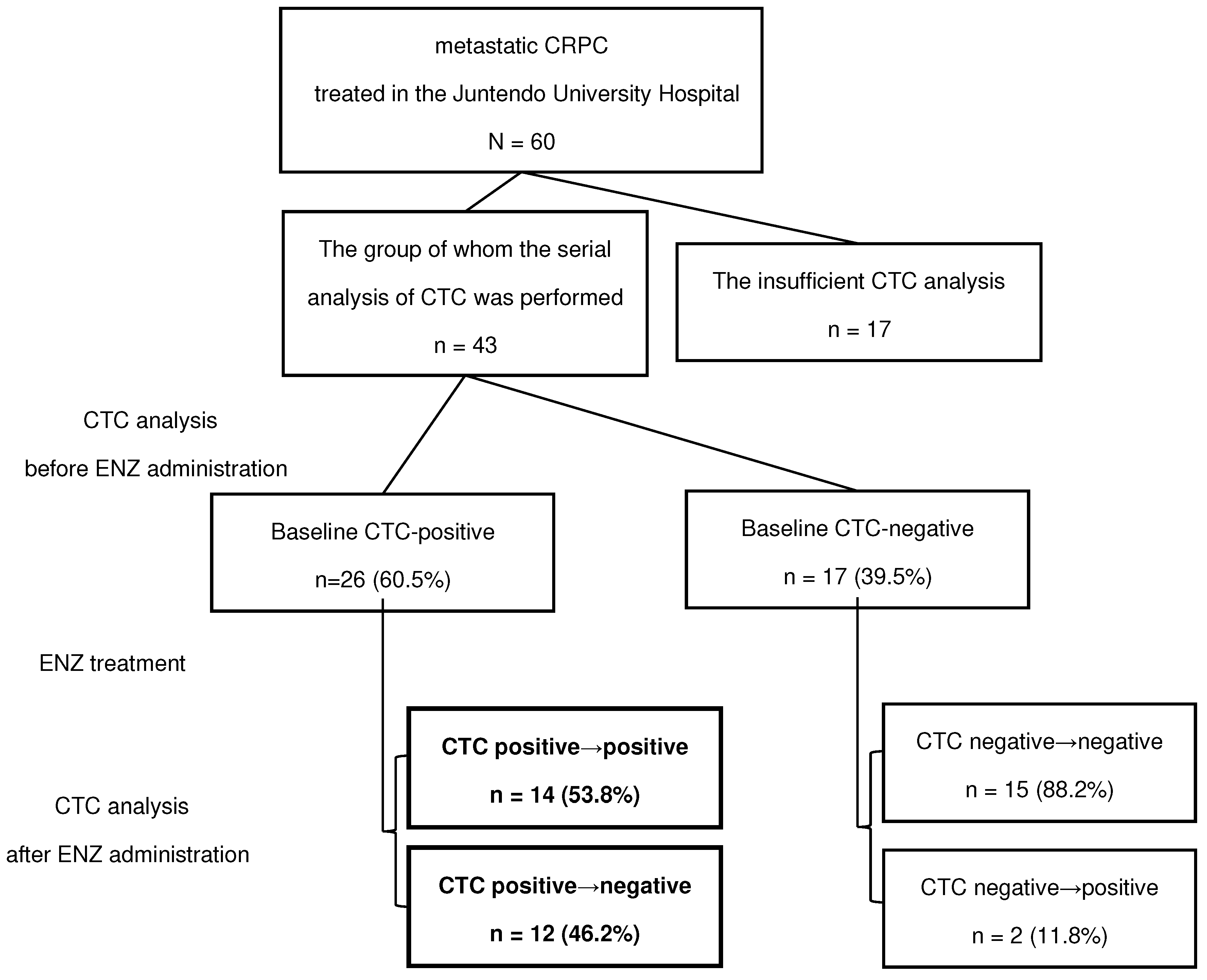
Sixty mCRPC patients with bone metastases treated at Juntendo University Hospital between 2015 and 2022 were included in the study. All patients were administered 160 mg ENZ daily. Blood samples and CTC analyses were performed regularly before and every three months after treatment at Juntendo University Hospital. Patients were grouped based on their CTC status prior to ENZ administration. As shown in the flowchart in
Figure 1, among patients who were CTC-positive at baseline, we compared patients who showed negative reversion of CTCs during ENZ treatment with those who remained CTC-positive despite treatment. Negative reversion was defined as a phenomenon in which CTCs dropped to undetectable levels during the course of treatment, although they were detected at pretreatment baseline. The primary endpoint was OS, a measure of the time from the date of ENZ administration to the date of death or loss of follow-up. Statistically significant clinical factors were identified in these two groups using univariate analysis. Specifically, patients' clinical background factors, such as age, Gleason Score (GS), previous treatment, time-to-CRPC, laboratory data, changes in bone scan index (BSI), and the presence of CTCs, were analyzed.
2.2. CTC Analyses
We used the AdnaTest (QIAGEN, Hilden, Germany) to detect CTCs according to the manufacturer’s protocol [
8,
10,
17]. Five milliliters of the patient’s blood was drawn into EDTA-3K collection tubes, followed by RNA extraction with antibody-conjugated magnetic beads using AdnaTest ProstateCancerSelect. mRNA was extracted using the AdnaTest Prostate CancerDetect kit. The extracted mRNA was subjected to reverse transcription using the Sensiscript Reverse Transcriptase Kit (QIAGEN). The expression of PSA, AR-V7, and AR in CTCs was examined by reverse transcription-polymerase chain reaction (RT-PCR). The AdnaTest PrimerMix ProstateDetect was used for the amplification of PSA (PCR condition for PSA: 95 °C for 15 min, 42 cycles of 94 °C for 30 s, 61 °C for 30 s, 72 °C for 30 s, followed by 10 min of extension). The AdnaTest PrimerMix AR-Detect was used to amplify AR (PCR conditions for AR: 95 °C for 15 min, 35 cycles of 94 °C for 30 s, 60 °C for 30 s, 72 °C for 60 s, followed by 10 min of extension).
Our experiments confirmed that the samples tested positive for PSA. Thus, we concluded that PSA positivity is a common denominator and defined successful CTC detection as positive PSA expression. The primer set and a PCR condition for AR-V7 RT-PCR is as follows; AR-V7 primer set designed to yield 125-bp AR-V7-specific band: 5′-CCATCTTGTCGTCTTCGGAAATGTTA-3′ and 5′-TTTGAATGAGGCAAGTCAGCCTTTCT-3′ (PCR condition for AR-V7: 95 °C for 5 min, 39 cycles of 95 °C for 10 s, 58 °C for 30 s, 72 °C for 30 s, followed by 10 min of extension). The amplified PCR products were electrophoresed and visualized using the DNA 1 K Experion Automated Electrophoresis System (Bio-Rad, Hercules, CA, USA). To evaluate gene expression, the fluorescence intensity scale was set to “scale to local” (default setting), and under this condition, any visible bands with detectable peaks were considered positive.
2.3. Statistical Analyses
Statistical analyses were performed using the Fisher’s exact test for categorical variables. The student’s t-test was used to analyze normally distributed continuous variables. A t-test with the natural logarithm of the variables was used for non-normally distributed continuous variables. OS analyses were performed using Kaplan-Meier plots, and differences were compared using the log-rank test. Univariate analyses were performed using log-rank tests. The cut-off value for each factor was the median value. Statistical significance was defined as p < 0.05.
2.4. Ethics STATEMENT
The study was approved by the Institutional Review Board of Juntendo Hospital (approval number: 14-052, 15-060), and all experiments were performed in accordance with approved guidelines. All participants provided written informed consent.
3. Results
This section may be divided by subheadings. It should provide a concise and precise description of the experimental results, their interpretation, as well as the experimental conclusions that can be drawn.
Total 43 of the 60 mCRPC patients were treated with ENZ without dose reduction or discontinuation of ENZ and underwent serial CTC analyses before and during treatment with ENZ. The baseline characteristics of patients before ENZ administration are shown in
Table 1. The median age at diagnosis of prostate cancer was 73.0 (interquartile range (IQR); 69.0–78.0) and the median initial PSA value was 119.2 (IQR; 36.3–658.4) ng/mL. The Gleason score of the biopsy when they were diagnosed with prostate cancer was 8 or more in 77% of cases (33/43). The median time-to-CRPC, time from diagnosis of prostate cancer to CRPC, was 16.0 (IQR; 10.0 – 39.0) months and median PSA value at baseline before ENZ administration was 9.6 (IQR; 4.1 – 36.6) ng/mL. ENZ was used as the first line treatment for mCRPC in 84% (36/43) of cases.
The baseline characteristics of the groups classified based on the absence or presence of CTCs before ENZ administration are shown in
Table 1. Two factors, PSA level and BSI upon bone scintigraphy before ENZ administration, were significantly higher in the CTC-positive group (p = 0.002 and p = 0.002, respectively).
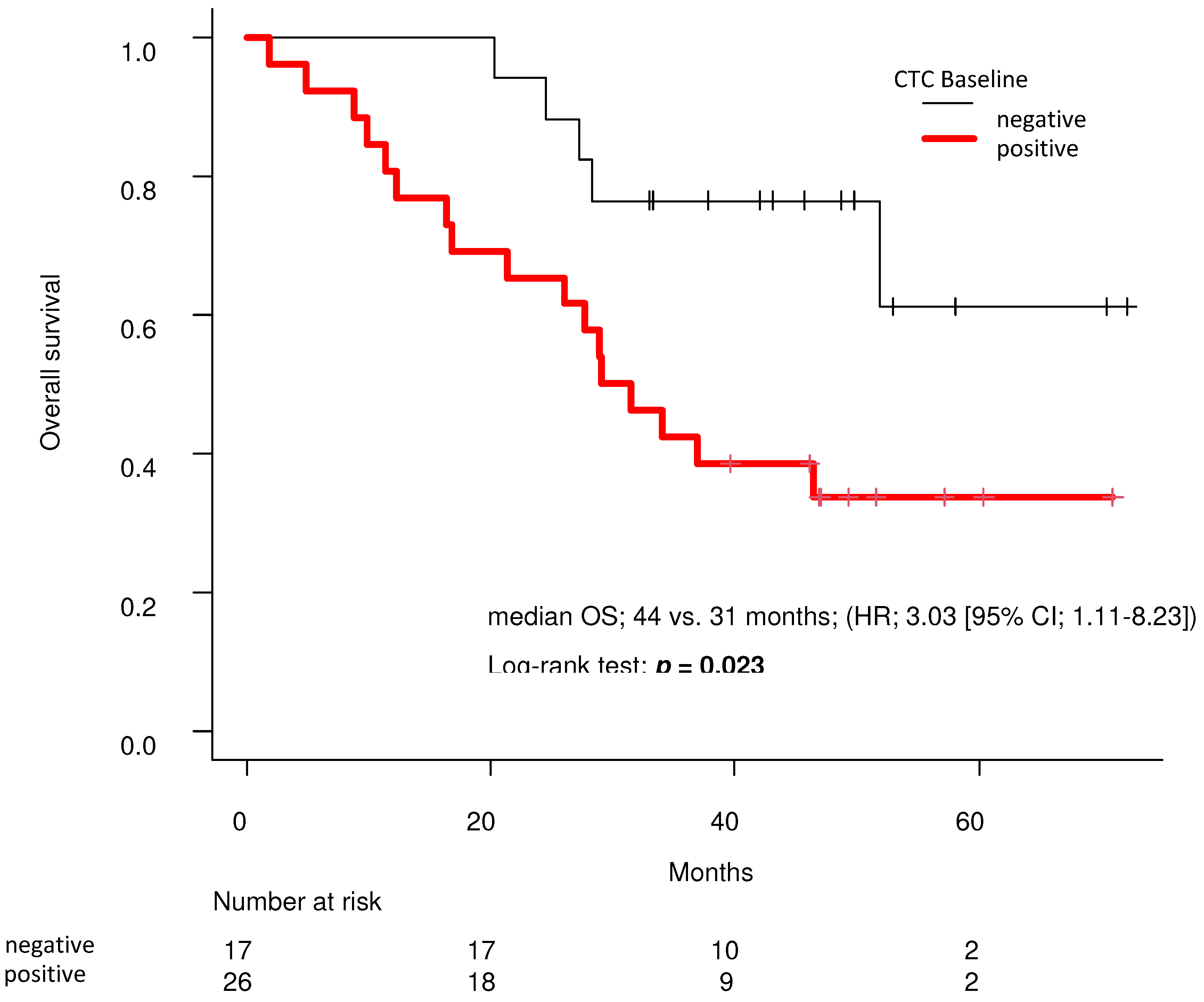
In the Kaplan-Meier curve analyzed by the log-rank test, the baseline CTC-negative group had significantly longer OS than the baseline CTC-positive group (median 44 vs. 31 months; (HR, 3.03 [95% CI; 1.11–8.23]; p = 0.023;
Figure 2). The number of CTC-positive patients at baseline was 60.5% (26/43) of cases (
Figure 1,
Table 1), while 46.2% of patients showed “negative reversion” of CTCs during treatment with ENZ (12/26). In addition, 53.8% (14/26) of CTC-positive patients remained as such during ENZ treatment. Only two cases converted from negative to positive despite ENZ treatment. Regarding the presence or absence of AR-V7, AR-V7 was positive in 4/14 cases in the continuous CTC positive group and 0/12 cases in the CTC negative conversion group.
Table 2 shows the differences in each background factor between the group that showed negative reversion of CTCs during ENZ treatment and the group that continued positive CTC detection during ENZ treatment. All cases that achieved negative reversion of CTCs during ENZ treatment were negative for lymph node metastasis at baseline (0/12), which was a statistically significant difference compared to those that remained positive for CTC detection (p = 0.017).
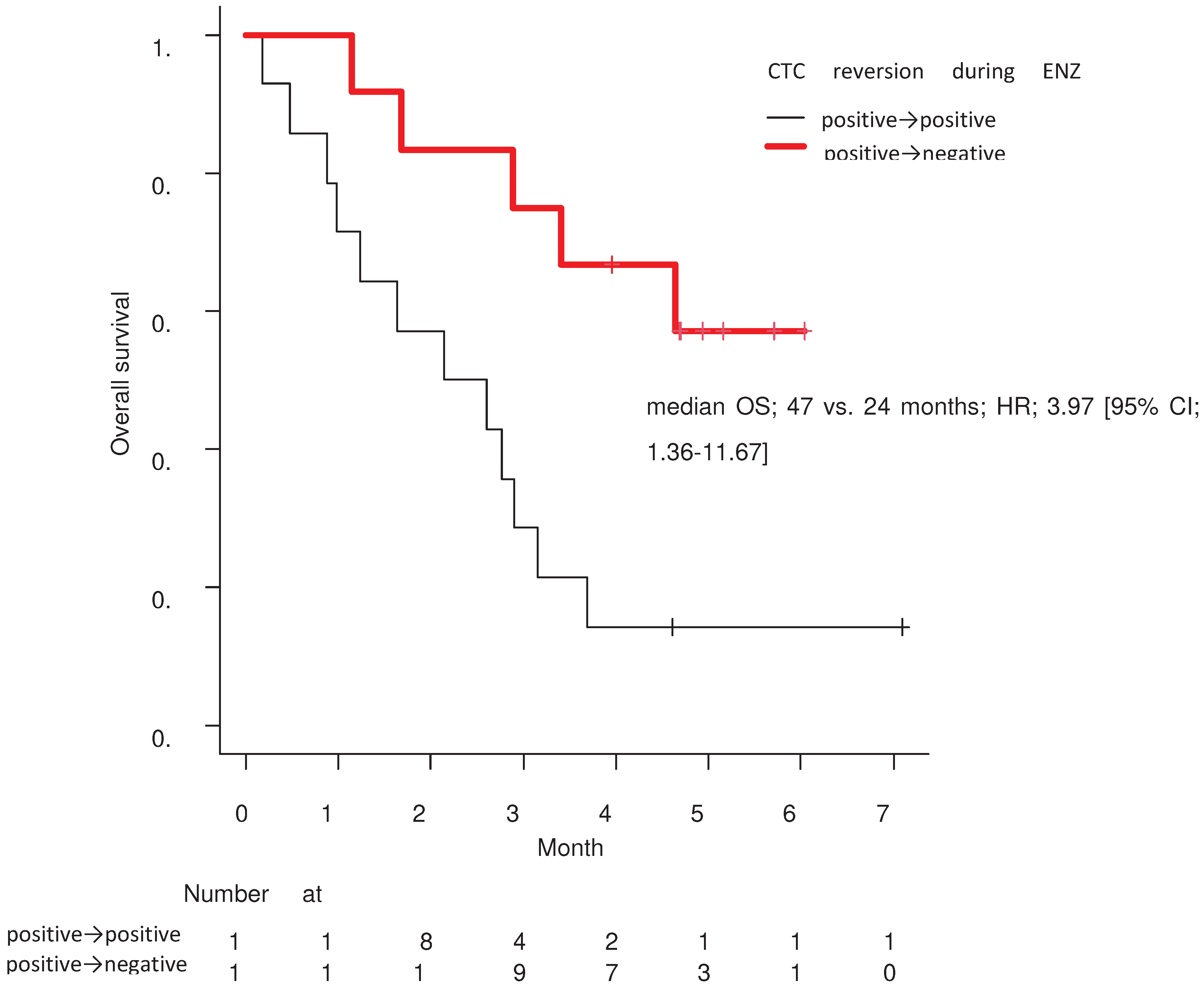
In the Kaplan-Meier curve analyzed by the log-rank test, the group that achieved negative reversion of CTCs during treatment with ENZ had significantly prolonged OS compared to the group that remained CTC-positive (median 47 vs. 24 months, HR; 3.97 [95% CI; 1.36–11.67]; p = 0.007;
Figure 3).
Each factor that significantly contributed to OS prolongation was identified using univariate analysis in the CTC-positive group at baseline (
Table 3). According to the log-rank test, two statistically significant clinical factors were identified: higher hemoglobin levels at baseline (p = 0.026) and negative reversion status of CTCs during ENZ treatment (p = 0.007).
4. Discussion
In our previous study, we demonstrated the efficacy of ENZ in treating mCRPC with bone metastases and showed that the presence or absence of CTCs at baseline correlated with the prolongation of PFS by ENZ treatment [
14]. In this study, by analyzing a subgroup from the previous study, we first demonstrated that the absence of CTCs detection at baseline might contribute to prolonged OS (
Figure 2) and showed the efficacy of chronological analyses of CTC in 43 patients with mCRPC. As a result, we showed that negative reversion in CTC is a strong prognostic biomarker for longer OS (
Figure 3,
Table 3).
Our analysis showed that CTC detection at baseline was significantly correlated with a high BSI upon bone scintigraphy and high PSA levels (
Table 1). All patients had bone metastasis, and since bone metastasis is generally considered a hematogenous metastasis, in cases with a large amount of bone metastasis, we expected to detect more prostate cancer cells in the blood. In contrast, a significant factor associated with negative reversion during ENZ administration was the absence of lymph node metastasis at baseline (0/12 cases) (
Table 2). It remains unclear why negative reversion of CTC cannot be achieved in patients with lymph node metastasis. Lymph node metastases, in principle, spread through the lymphatics; however, CTC-positive cases with lymph node metastases at baseline are thought to progress through two pathways, hematogenous and lymphatic, which might contribute to treatment resistance. In patients with lymph node cancer, it has been reported that the number of CTCs at diagnosis is significantly higher in patients with metastatic lymph node cancer compared to those with non-metastatic lymph node cancer, and lymphatic cancer progression might be proportional to hematogenous cancer progression, which might lead to treatment resistance [
18,
19]. The elimination of cancer cells from the blood during treatment had the strongest positive correlation with OS (
Table 3). At baseline, higher hemoglobin levels were significantly correlated with prolonged OS (
Table 3). This result is consistent with previous reports showing that pain, visceral metastasis, anemia, and bone scan progression are risk factors for poor prognosis in patients with mCRPC [
20].
CTC analysis has potential for practical clinical application in CRPC treatment. The presence or absence of CTCs and the number of CTCs before treatment can predict treatment resistance [
21]. Furthermore, CTC-positive patients have a significantly shorter overall survival than CTC-negative patients [
22]. In the present study, the CTC-negative group had a significantly longer OS than the CTC-positive group did at baseline. Furthermore, Heller et al. reported that a decrease or negative reversion of CTCs three months after ENZ treatment was a stronger biomarker for predicting therapeutic effects and OS than a decrease in PSA [
23]. The group analyzed and reported on five randomized phase 3 clinical trials. In contrast, early PSA decline at three months (67% of patients) was significant for predicting ENZ treatment effectiveness, as has been reported in the PREVAIL trial, in which PSA decline within three months was associated with a greater likelihood of 5-year survival [
24]. In this study, achieving a negative reversion of CTC during ENZ treatment gave a statistically significant prediction of OS prolongation (
Table 3). In other words, the results of large-scale studies in Europe and the United States were well reflected in the validation analysis of Asian subjects at our institution. The results from this study suggest that this method of chronological CTC analysis might more accurately predict the therapeutic effect of various treatments in patients with mCRPC than the previously widely used PSA and response to imaging.
Compared to tissue biopsy, which is a relatively invasive procedure, CTC analysis can be performed in a less invasive and time-series manner; therefore, monitoring CTCs during the treatment process, as in this study, may be more clinically practical in the future. According to recent findings, the number of CTCs should be considered when describing the relationship between AR-V7 status and prognosis. Where the AdnaTest method was negative for CTCs, the CellSearch method detected 69.5% of the CTCs, suggesting that the AdnaTest method could be less sensitive than the CellSearch method. However, in this study, we used the AdnaTest as a point-of-care testing (POCT) method, focusing on a simple and feasible way to carry out CTC analysis. AdnaTest may also be useful in clinical practice as a POCT tool, even if the sensitivity for CTC detection is slightly inferior to that of the CellSearch method.
This study had several limitations. First, the observation period was short, and this was a small-scale, single-center clinical study; thus, the number of fatal events was small. Therefore, patients whose CTCs were positive at baseline had a median OS that did not reach fatal events within the observation period. Further studies with longer observation periods are warranted. Although we classified 60 patients based on the absence or presence of CTCs before ENZ administration and further analyzed the groups that showed negative reversion of CTCs during ENZ treatment, only 12 patients were included in this cohort. Multivariate analysis was not possible because of the lack of Events Per Variable. A larger sample size is required to support the conclusions of this study.
Second, this was a single-arm study that did not include a control group, and it is necessary to apply this method to other CRPC medicines for comparison. In addition, androgen receptor splice variant-7 (AR-V7)-positive cases have also been demonstrated to develop significant ENZ treatment resistance, which is consistent with previous reports [
10]. It is also necessary to analyze and compare additional details of AR abnormalities, such as AR-V7, using liquid biopsies. Third, and most importantly, only the AdnaTest was used for CTC collection and analysis. If a highly sensitive method, such as CellSearch, was used to count CTCs, the number of CTC-negative groups would be considerably lower. The AdnaTest method may also have contributed to the higher survival rate in the CTC-negative group. Finally, although ENZ was the first-line treatment in most cases, the treatment lines were not standardized; thus, the analysis of OS could be biased by variations in the line of treatment.
5. Conclusions
Our study showed that patients with mCRPC and bone metastases without CTC detection at baseline had a significantly longer OS than those with positive CTC at baseline. Moreover, patients who achieved CTC-negative reversion during treatment showed long-term OS. Thus, chronological CTC analysis might predict the therapeutic effect of various treatments in patients with mCRPC more accurately than the previously widely used PSA levels and imaging responses. The AdnaTest kit would be useful in clinical practice as a POCT tool for evaluating CTC status. Furthermore, the timely evaluation CTC status might help physicians make a more reliable prognosis of mCRPC.
To further verify the conclusion, future studies may be designed with longer observation periods, larger sample sizes, more sensitive detection methods, and, if possible, a control group and standardized treatment.
Author Contributions
All authors contributed significantly to the study and agree with the contents of the manuscript. The contributions of each author are as follows: Conceptualization, S.N., M.N., and S.H.; methodology, S.N., H.H., A.T., and M.N.; patient recruitment, treatment, and follow-up, S.N., H.H., A.T., and M.N.; writing—original draft preparation, S.N., N.N., and M.N.; writing—review and editing, H.I. and S.H. All authors have read and agreed to the published version of the manuscript.
Funding
This clinical research was funded by Astellas Pharma Inc. (Tokyo, Japan).
Institutional Review Board Statement
This study was approved by the Institutional Review Board of Juntendo Hospital (approval number: 14-052, 15-060), and all experiments were performed in accordance with approved guidelines.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study. Written informed consent has been obtained from the patient(s) to publish this paper.
Data Availability Statement
All data analyzed in this study can be provided by applying to the corresponding author, S Horie.
Acknowledgments
We would like to thank
Editage [
http://www.editage.com] editing and reviewing this manuscript for English language.
Conflicts of Interest
Regarding the competing interest statement to be declared, this clinical research was funded by Astellas Pharma Inc. (Tokyo, Japan). Regarding other items, the authors have no conflict of interest.
References
- Tran, C.; Ouk, S.; Clegg, N.J.; Chen, Y.; Watson, P.A.; Arora, V.; et al. Development of a second-generation antiandrogen for treatment of advanced prostate cancer. Science. 2009, 324, 787–90. [Google Scholar] [CrossRef]
- Scher, H.I.; Fizazi, K.; Saad, F.; Taplin, M.E.; Sternberg, C.N.; Miller, K.; et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012, 367, 1187–97. [Google Scholar] [CrossRef]
- Beer, T.M.; Armstrong, A.J.; Rathkopf, D.E.; Loriot, Y.; Sternberg, C.N.; Higano, C.S.; et al. Enzalutamide in metastatic prostate cancer before chemotherapy. N Engl J Med. 2014, 371, 424–33. [Google Scholar] [CrossRef]
- Antonarakis, E.S.; Lu, C.; Luber, B.; Wang, H.; Chen, Y.; Zhu, Y.; et al. Clinical significance of androgen receptor splice variant-7 mRNA detection in circulating tumor cells of men with metastatic castration-resistant prostate cancer treated with first- and second-line Abiraterone and Enzalutamide. J Clin Oncol. 2017, 35, 2149–2156. [Google Scholar] [CrossRef]
- Armstrong, A.J.; Garrett-Mayer, E.S.; Yang, Y.C.; de Wit, R.; Tannock, I.F.; Eisenberger, M. A contemporary prognostic nomogram for men with hormone-refractory metastatic prostate cancer: a TAX327 study analysis. Clin Cancer Res. 2007, 13, 6396–403. [Google Scholar] [CrossRef]
- Armstrong, A.J.; Lin, P.; Higano, C.S.; Sternberg, C.N.; Sonpavde, G.; Tombal, B.; et al. Development and validation of a prognostic model for overall survival in chemotherapy-naive men with metastatic castration-resistant prostate cancer. Ann Oncol. 2018, 29, 2200–7. [Google Scholar] [CrossRef] [PubMed]
- Halabi, S.; Kelly, W.K.; Ma, H.; Zhou, H.; Solomon, N.C.; Fizazi, K.; et al. Meta-analysis evaluating the impact of site of metastasis on overall survival in men with castration resistant prostate cancer. J Clin Oncol. 2016, 34, 1652–9. [Google Scholar] [CrossRef] [PubMed]
- Marrugo-Ramirez, J.; Mir, M.; Samitier, J. Blood-based cancer biomarkers in liquid biopsy: A promising non-invasive alternative to tissue biopsy. Int J Mol Sci. 2018, 19, 2877. [Google Scholar] [CrossRef]
- Antonarakis, E.S.; Lu, C.; Wang, H.; Luber, B.; Nakazawa, M.; Roeser, J.C.; et al. AR-V7 and resistance to enzalutamide and abiraterone in prostate cancer. N Engl J Med. 2014, 371, 1028–38. [Google Scholar] [CrossRef] [PubMed]
- Onstenk, W.; Sieuwerts, A.M.; Kraan, J.; Van, M.; Nieuweboer, A.J.; Mathijssen, R.H.; et al. Efficacy of cabazitaxel in castration-resistant prostate cancer is independent of the presence of AR-V7 in circulating tumor cells. Eur Urol. 2015, 68, 939–45. [Google Scholar] [CrossRef] [PubMed]
- Antonarakis, E.S.; Lu, C.; Luber, B.; Wang, H.; Chen, Y.; Nakazawa, M.; et al. Androgen receptor splice variant 7 and efficacy of taxane chemotherapy in patients with metastatic castration-resistant prostate cancer. JAMA Oncol. 2015, 1, 582–91. [Google Scholar] [CrossRef] [PubMed]
- Ashizawa, T.; Nagata, M.; Nakamura, S.; Hirano, H.; Nagaya, N.; Lu, Y.; et al. Efficacy of cabazitaxel and androgen splicing variant-7 status in circulating tumor cells in Asian patients with metastatic castration-resistant prostate cancer. Sci Rep. 2022, 12, 18016. [Google Scholar] [CrossRef] [PubMed]
- Hirano, H.; Nagata, M.; Nagaya, N.; Nakamura, S.; Ashizawa, T.; Lu, Y. Bone Scan Index (BSI) scoring by using bone scintigraphy and circulating tumor cells (CTCs): predictive factors for enzalutamide effectiveness in patients with castration-resistant prostate cancer and bone metastases. Sci Rep. 2023, 13, 8704. [Google Scholar] [CrossRef] [PubMed]
- Nakazawa, M.; Lu, C.; Chen, Y. Serial blood-based analysis of AR-V7 in men with advanced prostate cancer. Ann Oncol. 2015, 26, 1859–1865. [Google Scholar] [CrossRef] [PubMed]
- Nagaya, N.; Kanayama, M.; Nagata, M.; Horie, S. Abiraterone rechallenge based on sequential testing of androgen receptor splice variant 7 expression in circulating tumor cells: A case report. Front Oncol. 2020, 10, 495-8. [Google Scholar] [CrossRef]
- Nagaya, N.; Nagata, M.; Lu, Y.; Kanayama, M.; Hou, Q.; Hotta, Z.; et al. Prostate-specific membrane antigen in circulating tumor cells is a new poor prognostic marker for castration-resistant prostate cancer. PLOS One. 2020, 15, e0226219. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Tang, W.; Huang, B. Longitudinal change of circulating tumour cell count and its relation to prognosis in advanced intrahepatic cholangiocarcinoma patients. Scand J Clin Lab Invest. 2023, 83, 234–240. [Google Scholar] [CrossRef]
- Hristozova, T.; Konschak, R.; Stromberger, C.; Fusi, A.; Liu, Z.; Weichert, W.; et al. The presence of circulating tumor cells (CTCs) correlates with lymph node metastasis in nonresectable squamous cell carcinoma of the head and neck region (SCCHN). Ann Oncol 2011, 22, 1878–1885. [Google Scholar] [CrossRef]
- Armstrong, A.J.; Tannock, I.F.; de Wit, R.; George, D.J.; Eisenberger, M.; Halabi, S.; et al. The development of risk groups in men with metastatic castration-resistant prostate cancer based on risk factors for PSA decline and survival. Eur J Cancer. 2010, 46, 517–25. [Google Scholar] [CrossRef]
- Danila, D.C.; Heller, G.; Gignac, G.A.; Gonzalez-Espinoza, R.; Anand, A.; Tanaka, E.; et al. Circulating tumor cell number and prognosis in progressive castration-resistant prostate cancer. Clin Cancer Res. 2007, 13, 7053–8. [Google Scholar] [CrossRef] [PubMed]
- Cattrini, C.; Rubagotti, A.; Zinoli, L.; Cerbone, L.; Zanardi, E.; Capaia, M.; et al. Role of circulating tumor cells (CTC), androgen receptor full length (AR-FL) and androgen receptor splice variant 7 (AR-V7) in a prospective cohort of castration-resistant metastatic prostate cancer patients. Cancers (Basel). 2019, 11, 1365. [Google Scholar] [CrossRef] [PubMed]
- Heller, G.; McCormack, R.; Kheoh, T.; Molina, A.; Smith, M.R.; Dreicer, R.; et al. Circulating tumor cell number as a response measure of prolonged survival for metastatic castration-resistant prostate cancer: a comparison with prostate-specific antigen across five randomized phase III clinical trials. J Clin Oncol. 2018, 36, 572–580. [Google Scholar] [CrossRef]
- Armstrong, A.J.; Lin, P.; Tombal, B.; Saad, F.; Higano, C.S.; Joshua, A.M.; et al. Five-year survival prediction and safety outcomes with enzalutamide in men with chemotherapy-naïve metastatic castration-resistant prostate cancer from the PREVAIL Trial. Eur Urol. 2020, 78, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Sharp, A.; Welti, J.C.; Lambros, M.B.; Dolling, D.; Rodriguez, D.N.; Pope, L.; et al. Clinical utility of circulating tumour cell androgen receptor splice Variant-7 status in metastatic castration-resistant prostate cancer. Eur Urol. 2019, 76, 676–685. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).