1. Introduction
Pseudomyogenic hemangioendothelioma (PMHE) is a distinctive mesenchymal vascular neoplasm quiet often arising in superficial &/or deep soft tissue. PMHE presents with a male predilection and wide age ranging from 14 to 80 years old with a mean of 31 years old [
1]. PMHE predominantly presents as multiple painful or painless nodules with no distinctive clinical manifestation, and occasionally bone pain, blurred vision, or joint weakness is reported depending on the location of the lesion [
2]. Extremities are the most common site, occupying for approximately 80% of PMHE cases with the lower extremities predominance comprising of ~54%, followed by trunk, and rarely head and neck region [
1,
2]. In a series of 50 cases, only 2 cases (4%) affected the head and neck region: one case presenting as multifocal lesion only on the nose in 47-year old male and the other one presenting as a solitary lesion on the forehead in a 34-year old male [
1].
The following case presented below is the first reported case in a patient aged more than 50 years old, to the best of our knowledge, of multifocal PMHE in the head and neck area with wide-spread distribution from right calvarium to right mandible.
2. Case presentation
A 59-year-old male presented to the NeuroSurgery Clinic of University of California Irvine Medical Center on 3/31/2023 with a right temporal mass. About 6 months ago he did complain of pain in the right jay when he opened his mouth of yawned, and noticed the painless lump about 2 months ago. Subsequent MRI imaging showed a heterogeneous lesion measuring 1.6 x 1.1 cm centered in the right temporalis muscle (
Figure 1A). Furthermore, additional smaller 9 x 7 mm lesion within the right frontoparietal calvarium, 9 mm lesion within the right pterygoid muscles, and multiple small foci of signal abnormality within the right mandible (
Figure 1B). PET CT showed increased uptake in these lesions concerning for neoplasm (
Figure 1C). Laboratory values and past medical history was unremarkable except for hypertension, type II diabetes mellitus, hypercholesterolemia and arthritis. Past surgery history included endoscopic bilateral knee surgery and abdominal hernia surgery. No smoking, alcohol use or known allergy history. The patient eventually underwent a punch biopsy of right temporal mass.
Histological examination of the right temporal mass showed a multinodular biphasic lesion composed of sheets and fascicles of elongated spindle cells, and epithelioid small round to ovoid cells with abundant eosinophilic cytoplasm infiltrating into the adjacent skeletal muscle admixed with abundant neutrophilic infiltration (
Figure 2). Rhabdomyoblast-like cells, and prominent nuclear pleomorphism and mitosis were not identified. IHC staining revealed that the tumor cells were diffusely and strongly positive for FOSB, pan-cytokerain (AE1/AE3), CD31, ERG, and negative for CD34 (
Figure 3), as well as for HHV8, EMA, TLE1, squamous markers (p40, p63), melanocytic markers (S100, SOX10), and muscular markers (Desmin, SMA). INI-1 was retained, and Ki67/Mib1 highlighted 15-20% tumor cell in the highest proliferating areas. DNA and RNA next-generation sequencing (NGS) performed by Genomic Testing Coorperative further revealed a t(9:19) EGFL7::FOSB fusion mRNA.
In this constellation of histological, IHC, and molecular findings, the pathologic diagnosis was consistent with pseudomyogenic hemangioendothelioma with EGFL7::FOSB fusion. This is the first case of PMHE in a patient aged more than 50 years old in the head and neck area, as well as the first case with EGFL7::FOSB fusion in the head and neck area.
Figure 1.
(A) Axial images of the patient's MRI with and without contrast demonstrate a heterogeneous, peripherally enhancing 1.8 x 1.2 x 2.1 cm (AP x ML x CC) lesion centered within the right temporalis muscle (solid yellow arrow). The lesion demonstrates T1 isointensity to surrounding muscle tissue (dotted yellow arrow). On diffusion weighted sequences, the lesion demonstrates heterogeneous hyperintensity on diffusion restriction sequence (dotted blue arrow), suggesting areas of high cellularity of the lesion. FLAIR (fluid-attenuated inversion recovery) sequences also demonstrate heterogeneously increased signal of the lesion (solid blue arrow). Findings are suggestive of a neoplastic process, with possible cystic change or central necrosis due to the lack of central enhancement. (B) Multiple axial images of the patient's brain MRI (all T1 post-contrast enhanced sequences) demonstrate additional lesions with similar imaging features as the right temporalis muscle lesion in figure 1, including T2/FLAIR (fluid-attenuated inversion recovery) hyperintensity, diffusion restriction, and enhancement. Of note, these lesions demonstrate slight differences in enhancement pattern compared to the right temporalis muscle lesion, with more homogeneous enhancement compared to the right temporalis muscle which demonstrated more heterogeneous and peripheral enhancement. These additional lesions are centered in the right frontoparietal calvarium (red solid arrow), right lateral pterygoid/temporalis muscle (dotted red arrow), superficial aspect of the right masseter muscle with possible extension into the right zygomatic arch (solid orange arrow), and the right mandibular condyle (dotted orange arrow). These lesions were also avid on subsequent PET-CT (shown in figure 3), with the exception of the masseter/zygomatic arch lesion which demonstrated FDG uptake slightly above background activity at approximately 2.7 SUV. (C) PET-CT (positron emission tomography computed tomography) scan in the same patient demonstrates increased FDG (fluorodeoxyglucose 18F) radiotracer uptake with maximal uptake of 9.3 SUV in the right frontoparietal calvarial lesion which corresponds to a lytic lesion on the accompanying CT (blue arrow), concerning for neoplasm. There are additional FDG avid lesions in the right mandibular condyle with maximal uptake of 5.1 SUV (white arrow) and in the right temporalis/ lateral pterygoid muscle with maximal uptake of 8.7 SUV (green arrow), also concerning for neoplastic process. Previously noted right temporal lesion is not seen on this PET-CT as it was excised, although was shown to be FDG avid on prior PET (not shown).
Figure 1.
(A) Axial images of the patient's MRI with and without contrast demonstrate a heterogeneous, peripherally enhancing 1.8 x 1.2 x 2.1 cm (AP x ML x CC) lesion centered within the right temporalis muscle (solid yellow arrow). The lesion demonstrates T1 isointensity to surrounding muscle tissue (dotted yellow arrow). On diffusion weighted sequences, the lesion demonstrates heterogeneous hyperintensity on diffusion restriction sequence (dotted blue arrow), suggesting areas of high cellularity of the lesion. FLAIR (fluid-attenuated inversion recovery) sequences also demonstrate heterogeneously increased signal of the lesion (solid blue arrow). Findings are suggestive of a neoplastic process, with possible cystic change or central necrosis due to the lack of central enhancement. (B) Multiple axial images of the patient's brain MRI (all T1 post-contrast enhanced sequences) demonstrate additional lesions with similar imaging features as the right temporalis muscle lesion in figure 1, including T2/FLAIR (fluid-attenuated inversion recovery) hyperintensity, diffusion restriction, and enhancement. Of note, these lesions demonstrate slight differences in enhancement pattern compared to the right temporalis muscle lesion, with more homogeneous enhancement compared to the right temporalis muscle which demonstrated more heterogeneous and peripheral enhancement. These additional lesions are centered in the right frontoparietal calvarium (red solid arrow), right lateral pterygoid/temporalis muscle (dotted red arrow), superficial aspect of the right masseter muscle with possible extension into the right zygomatic arch (solid orange arrow), and the right mandibular condyle (dotted orange arrow). These lesions were also avid on subsequent PET-CT (shown in figure 3), with the exception of the masseter/zygomatic arch lesion which demonstrated FDG uptake slightly above background activity at approximately 2.7 SUV. (C) PET-CT (positron emission tomography computed tomography) scan in the same patient demonstrates increased FDG (fluorodeoxyglucose 18F) radiotracer uptake with maximal uptake of 9.3 SUV in the right frontoparietal calvarial lesion which corresponds to a lytic lesion on the accompanying CT (blue arrow), concerning for neoplasm. There are additional FDG avid lesions in the right mandibular condyle with maximal uptake of 5.1 SUV (white arrow) and in the right temporalis/ lateral pterygoid muscle with maximal uptake of 8.7 SUV (green arrow), also concerning for neoplastic process. Previously noted right temporal lesion is not seen on this PET-CT as it was excised, although was shown to be FDG avid on prior PET (not shown).
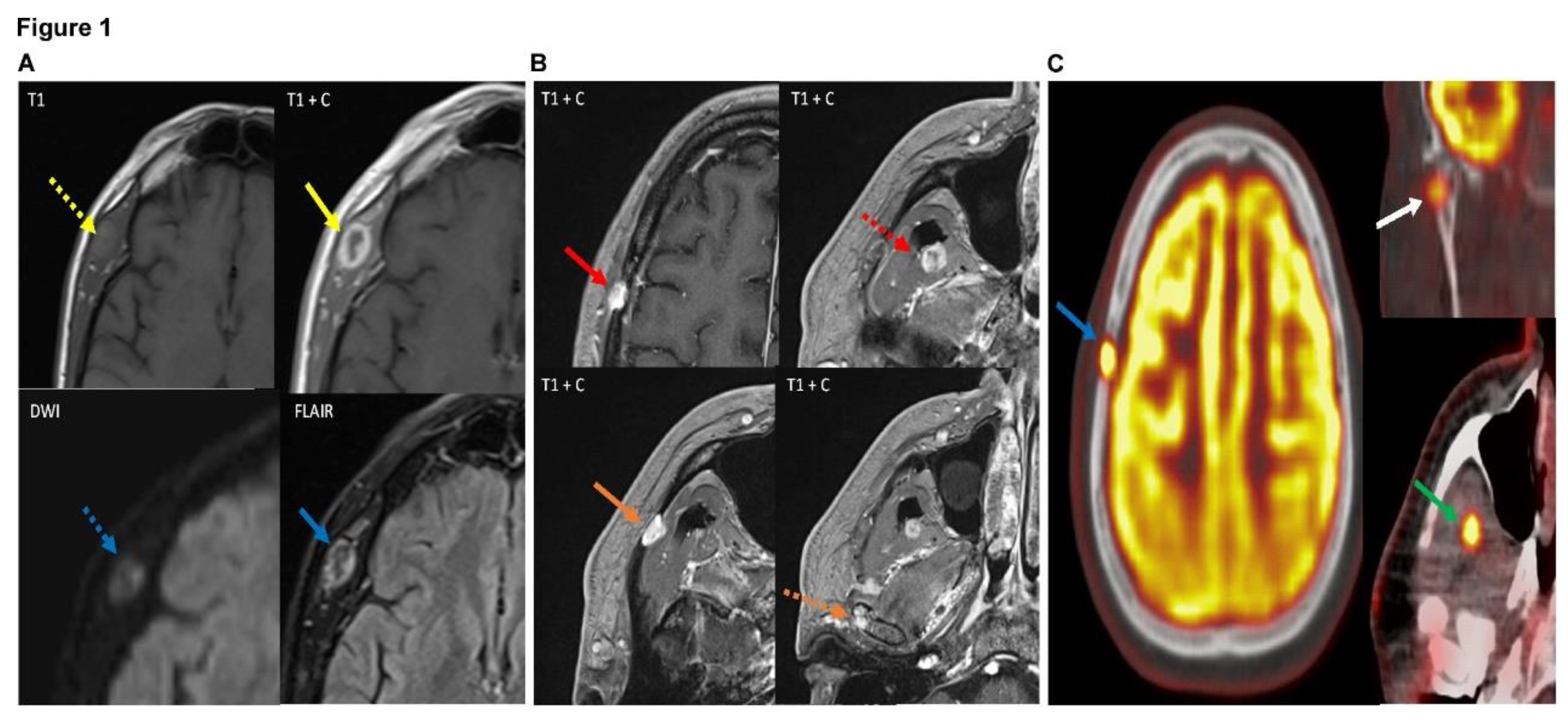
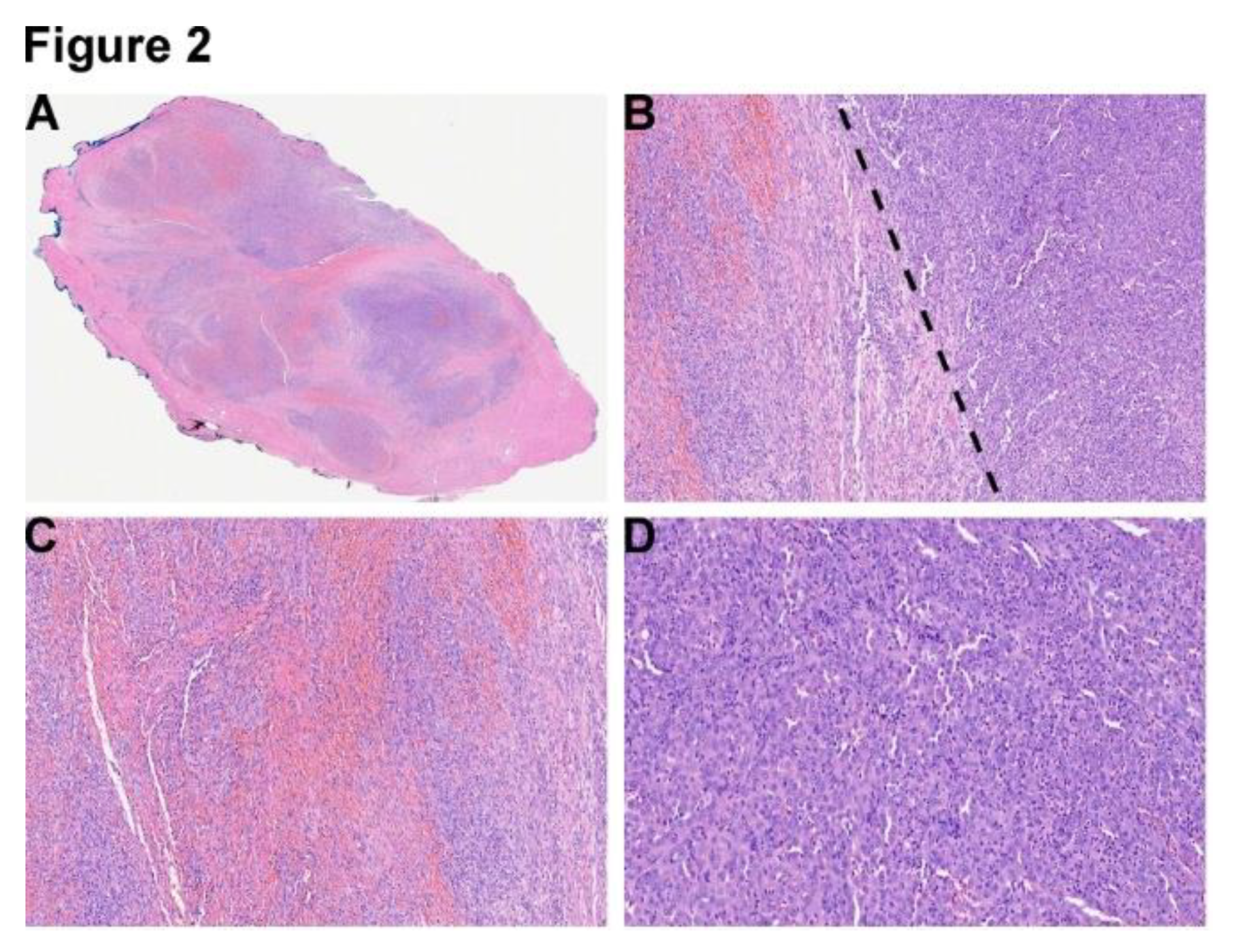
Figure 2.
(A) Section of the final resection specimen from right temporal mass shows a multinodular lesion separated by fibrous septa, 4X. (B) A well-demarcated biphasic lesion is composed of composed of sheets and fascicles of elongated spindle cells (spindle cell component-left side of the dotted line), and epithelioid small round to ovoid cells with abundant eosinophilic cytoplasm (epithelioid component-right side of the dotted line), 20X. (C) Representative section of spindle cell component with neutrophilic infiltration, 20X. (D) Representative section of spindle cell component with neutrophilic infiltration, 20X.
Figure 2.
(A) Section of the final resection specimen from right temporal mass shows a multinodular lesion separated by fibrous septa, 4X. (B) A well-demarcated biphasic lesion is composed of composed of sheets and fascicles of elongated spindle cells (spindle cell component-left side of the dotted line), and epithelioid small round to ovoid cells with abundant eosinophilic cytoplasm (epithelioid component-right side of the dotted line), 20X. (C) Representative section of spindle cell component with neutrophilic infiltration, 20X. (D) Representative section of spindle cell component with neutrophilic infiltration, 20X.
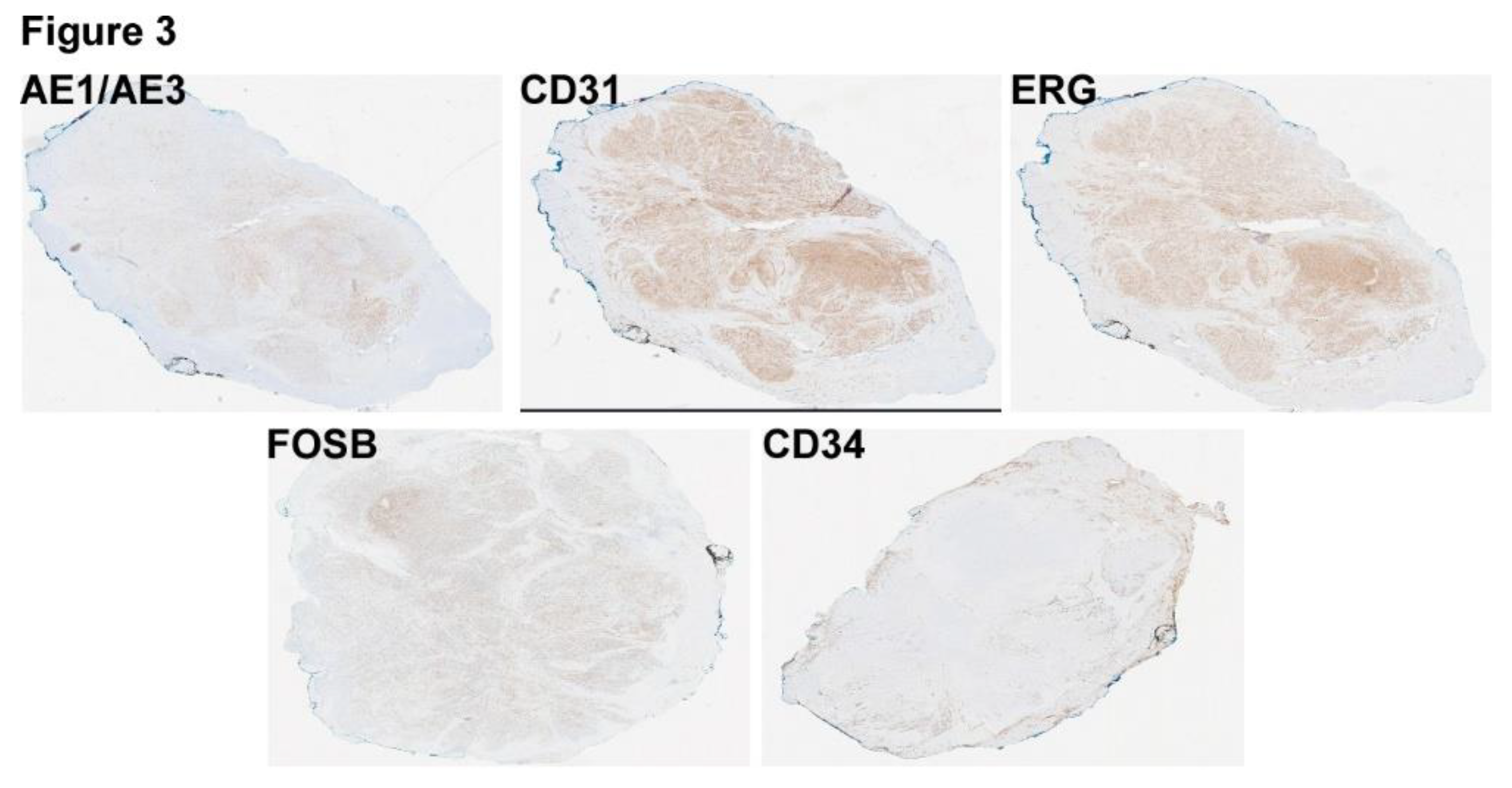
Figure 3.
Immunostains of the lesion reveal that the tumor cells are diffusely positive forAE1/AE3, CD31, ERG, and FOSB, but negative for CD34.
Figure 3.
Immunostains of the lesion reveal that the tumor cells are diffusely positive forAE1/AE3, CD31, ERG, and FOSB, but negative for CD34.
3. Discussion
Histologically, PMHE is predominantly composed of sheets and fascicles of elongated spindle cells with abundant eosinophilic cytoplasm, vesicular nuclei and variable nucleoli. Mild to moderate cytologic atypia can be present, but rarely marked. Epithelioid cells may be present in PMHE and intimately intermingled with spindle cell component. In a series of 50 PMHE cases, at least focal epithelioid cells were identified in all cases [
1,
2]. Rarely, prominent biphasic spindle and epithelioid components are clearly demarcated from each other, such as in this case. Rhabdomyoblast-like cells and neutrophils infiltration were variable present. Foci of necrosis has been reported in 14.5 % of a series of PMHE cases (8/55) [
1,
3]. Therefore, a broad spectrum of differential diagnoses is recognized for the diagnosis of PMHE based on the non-specific morphological features, including benign and malignant tumors. Benign entities mainly include nodular fasciitis and fibrous histiocytoma, and malignant tumors include epithelioid sarcoma, leiomyosarcoma, spindle cell squamous cell carcinoma, spindle cell melanoma, monophasic spindle cell synovial sarcoma, spindle cell rhabdomyosarcoma, angiosarcoma, epithelioid hemangioendothelioma (EHE). Therefore, a comprehensive IHC and molecular testing panel is needed to rule out all, but not limited to, the differential diagnosis mentioned above before arriving at the correct diagnosis.
Immunohistochemically, all the PMHE cases have been reported to show strong, diffuse positivity to pan-cytokeratin (AE1/AE3), FLI-1 and INI-1, and negativity to CD34, 40-50 % positive to CD31 and variable reactivity to CAM5.2, EMA and SMA [
1,
2]. Histological examination of this case showed a multinodular well-demarcated biphasic lesion composed of the sheets and fascicles of elongated spindle cells, and epithelioid cells with round to ovoid morphology. Abundant neutrophilic infiltration was identified. Based on the histologic findings, further IHC staining revealed that the tumor cells were diffuse positive for AE1/AE3, CD31, ERG and INI-1 and negative for EMA, p63, p40, CD34, S100, SOX10, Desmin, SMA and TLE1. This constellation of IHC pattern was against the diagnosis of nodular fasciitis, fibrous histiocytoma epithelioid sarcoma, leiomyosarcoma, spindle cell squamous cell carcinoma, spindle cell melanoma, monophasic spindle cell synovial sarcoma, spindle cell rhabdomyosarcoma, and supportive of the diagnosis of vascular tumor, but was supportive of vascular neoplastic process.
Immunostaining for HHV-8 for Kaposi sarcoma and FOSB for PMHE, as well as molecular testing were performed to determine the specific vascular tumor. Positivity for FOSB and negativity for HHV-8 were more supportive of PMHE and against Kaposi sarcoma. Molecular testing further demonstrated that the tumor harbored a t(9:19) EGFL7::FOSB fusion mRNA, supporting PMHE as the final diagnosis.
The most distinct form of molecular signature in PMHE is a genetic fusion between FOSB (19q) and SERPINE (7q22) [
4,
5]. Furthermore, WWTR1(3q25) that is a common partner fusion gene with CAMTA1 in epithelioid hemangioendothelioma [
6,
7] has also been reported to be a partner fusion gene of FOSB in PMHE [
8]. Other gene fusions, such as t(7:19) ACTB::FOSB and t(2:19) POTEI-FOSB, have been reported in PMHE as well [
9,
10]. All these fusions will upregulate FOSB expression, which is positive in several diseases, including PMHE [
11], epithelioid hemangioma [
12] and osteoblastomas [
13]. In the current study, our molecular testing for the first time revealed a rare t(9:19) EGFL7::FOSB fusion mRNA in PMHE in the head and neck region. Importantly, although metastasis is extremely uncommon in PMHE and only reported in two cases so far (the molecular alterations were not mentioned in the studies) [
1,
14], this novel gene fusion of EGFL7::FOSB was first identified in PMHE from multifocal hand lesions with widely metastatic disease, including numerous masses in the lungs, liver, soft tissues, and axial and appendicular skeleton, at the initial presentation [
15], suggesting that EGFL7::FOSB fusion may be associated with or drive the metastatic potential of PMHE. Interestingly, MRI showed additional three lesions, including in right pterygoid muscles, right mandible and right calvarium, shared similar radiologic features as the lesion at right temporalis muscle, in combination with the finding of EGFL7::FOSB fusion identified in this case, the metastatic possibility from right temporal area to the other three areas should be carefully considered and excluded at the molecular level if the other three lesions become available.
As mentioned above, PMHE is characterized by young age (94% < 50 years old) and extremity location (approximately 80%) [
1,
2,
16]. Head and neck region is one of the rarest sites, and no more than 15 cases, to the best of our knowledge, have been reported in this area till date [
1,
11,
16,
17,
18,
19,
20], including 2 cases in the skull, 2 cases in the scalp, 2 cases in the face, 2 cases in the neck, 2 case in the forehead, 2 cases in the nose, 1 case in the anterior maxillary gingiva, 1 case in perioral. Among them, the age of all the patients was less than 50 years old and the eldest one aged 47. In this case, we presented a 59-year-old male with multifocal lesion in the head and neck region, and pathologic, IHC and molecular examination of the specimen from the largest mass at right temporal was consistent with the diagnosis of PHME. So far, this is the first reported case of PMHE with EGFL7::FOSB fusion in the head and neck region, as well as the first case in patient aged more than 50 years old in the head and neck region.