Submitted:
03 January 2024
Posted:
04 January 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. SOX4 structure and function
3. SOX4 single nucleotide variants
3.1. Heterozygous pathogenic variants
3.2. Variants of uncertain significance and possible bi-allelic inheritance
3.3. Co-occurrence of variants in other genes
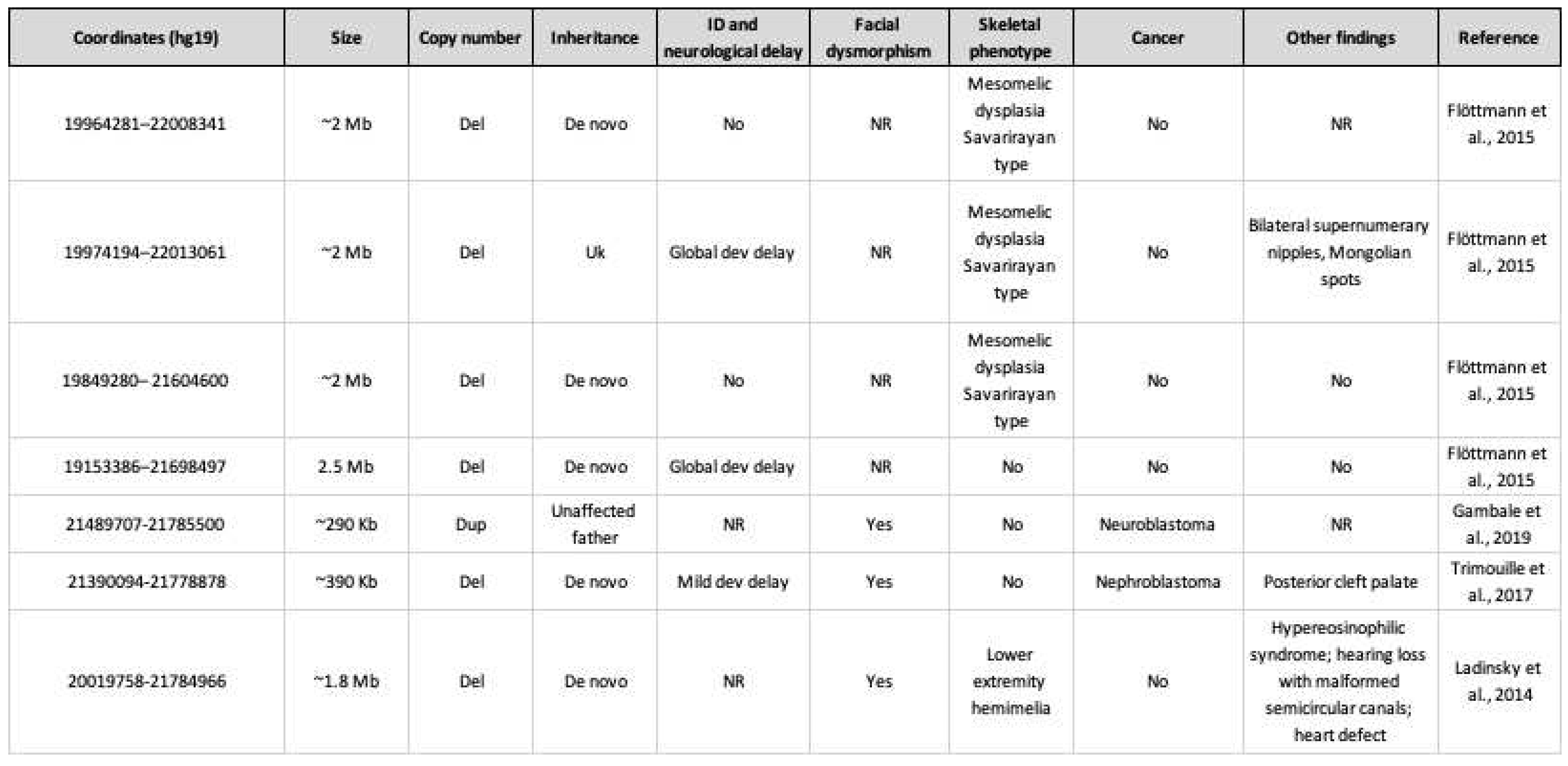
4. Copy number variants involving SOX4
5. Phenotype
6. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Schepers, G.E.; Teasdale, R.D.; Koopman, P. Twenty Pairs of Sox: Extent, Homology, and Nomenclature of the Mouse and Human Sox Transcription Factor Gene Families. Developmental Cell 2002, 3, 167–170. [Google Scholar] [CrossRef] [PubMed]
- Harley, V.R.; Clarkson, M.J.; Argentaro, A. The Molecular Action and Regulation of the Testis-Determining Factors, SRY (Sex-Determining Region on the Y Chromosome) and SOX9 [SRY-Related High-Mobility Group (HMG) Box 9]. Endocrine Reviews 2003, 24, 466–487. [Google Scholar] [CrossRef] [PubMed]
- Sutton, E.; Hughes, J.; White, S.; Sekido, R.; Tan, J.; Arboleda, V.; Rogers, N.; Knower, K.; Rowley, L.; Eyre, H.; et al. Identification of SOX3 as an XX Male Sex Reversal Gene in Mice and Humans. J Clin Invest 2011, 121, 328–341. [Google Scholar] [CrossRef] [PubMed]
- Fantes, J.; Ragge, N.K.; Lynch, S.-A.; McGill, N.I.; Collin, J.R.O.; Howard-Peebles, P.N.; Hayward, C.; Vivian, A.J.; Williamson, K.; van Heyningen, V.; FitzPatrick, D.R. Mutations in SOX2 Cause Anophthalmia. Nat Genet 2003, 33, 462–463. [Google Scholar] [CrossRef] [PubMed]
- Wagner, T.; Wirth, J.; Meyer, J.; Zabel, B.; Held, M.; Zimmer, J.; Pasantes, J.; Bricarelli, F.D.; Keutel, J.; Hustert, E.; et al. Autosomal Sex Reversal and Campomelic Dysplasia Are Caused by Mutations in and around the SRY-Related Gene SOX9. Cell 1994, 79, 1111–1120. [Google Scholar] [CrossRef] [PubMed]
- Lamb, A.N.; Rosenfeld, J.A.; Neill, N.J.; Talkowski, M.E.; Blumenthal, I.; Girirajan, S.; Keelean-Fuller, D.; Fan, Z.; Pouncey, J.; Stevens, C.; et al. Haploinsufficiency of SOX5 at 12p12.1 Is Associated with Developmental Delays with Prominent Language Delay, Behavior Problems, and Mild Dysmorphic Features. Hum Mutat 2012, 33, 728–740. [Google Scholar] [CrossRef]
- Pingault, V.; Zerad, L.; Bertani-Torres, W.; Bondurand, N. SOX10: 20 Years of Phenotypic Plurality and Current Understanding of Its Developmental Function. J Med Genet 2022, 59, 105–114. [Google Scholar] [CrossRef]
- Angelozzi, M.; Lefebvre, V. SOXopathies: Growing Family of Developmental Disorders Due to SOX Mutations. Trends Genet 2019, 35, 658–671. [Google Scholar] [CrossRef] [PubMed]
- Kavyanifar, A.; Turan, S.; Lie, D.C. SoxC Transcription Factors: Multifunctional Regulators of Neurodevelopment. Cell Tissue Res 2018, 371, 91–103. [Google Scholar] [CrossRef]
- Schilham, M.W.; Oosterwegel, M.A.; Moerer, P.; Ya, J.; de Boer, P.A.J.; van de Wetering, M.; Verbeek, S.; Lamers, W.H.; Kruisbeek, A.M.; Cumano, A.; Clevers, H. Defects in Cardiac Outflow Tract Formation and Pro-B-Lymphocyte Expansion in Mice Lacking Sox-4. Nature 1996, 380, 711–714. [Google Scholar] [CrossRef]
- Sock, E.; Rettig, S.D.; Enderich, J.; Bösl, M.R.; Tamm, E.R.; Wegner, M. Gene Targeting Reveals a Widespread Role for the High-Mobility-Group Transcription Factor Sox11 in Tissue Remodeling. Mol Cell Biol 2004, 24, 6635–6644. [Google Scholar] [CrossRef] [PubMed]
- Hoser, M.; Potzner, M.R.; Koch, J.M.C.; Bösl, M.R.; Wegner, M.; Sock, E. Sox12 Deletion in the Mouse Reveals Nonreciprocal Redundancy with the Related Sox4 and Sox11 Transcription Factors. Mol Cell Biol 2008, 28, 4675–4687. [Google Scholar] [CrossRef]
- Tsurusaki, Y.; Koshimizu, E.; Ohashi, H.; Phadke, S.; Kou, I.; Shiina, M.; Suzuki, T.; Okamoto, N.; Imamura, S.; Yamashita, M.; et al. De Novo SOX11 Mutations Cause Coffin–Siris Syndrome. Nat Commun 2014, 5, 4011. [Google Scholar] [CrossRef]
- Hempel, A.; Pagnamenta, A.T.; Blyth, M.; Mansour, S.; McConnell, V.; Kou, I.; Ikegawa, S.; Tsurusaki, Y.; Matsumoto, N.; Lo-Castro, A.; et al. Deletions and de Novo Mutations of SOX11 Are Associated with a Neurodevelopmental Disorder with Features of Coffin–Siris Syndrome. J Med Genet 2016, 53, 152–162. [Google Scholar] [CrossRef] [PubMed]
- Al-Jawahiri, R.; Foroutan, A.; Kerkhof, J.; McConkey, H.; Levy, M.; Haghshenas, S.; Rooney, K.; Turner, J.; Shears, D.; Holder, M.; et al. SOX11 Variants Cause a Neurodevelopmental Disorder with Infrequent Ocular Malformations and Hypogonadotropic Hypogonadism and with Distinct DNA Methylation Profile. Genet Med 2022, 24, 1261–1273. [Google Scholar] [CrossRef]
- Zawerton, A.; Yao, B.; Yeager, J.P.; Pippucci, T.; Haseeb, A.; Smith, J.D.; Wischmann, L.; Kühl, S.J.; Dean, J.C.S.; Pilz, D.T.; Holder, S.E.; McNeill, A.; Graziano, C.; Lefebvre, V. De Novo SOX4 Variants Cause a Neurodevelopmental Disease Associated with Mild Dysmorphism. Am J Hum Genet 2019, 104, 246–259. [Google Scholar] [CrossRef]
- Van de Wetering, M.; Oosterwegel, M.; van Norren, K.; Clevers, H. Sox-4, an Sry-like HMG Box Protein, Is a Transcriptional Activator in Lymphocytes. EMBO J 1993, 12, 3847–3854. [Google Scholar] [CrossRef] [PubMed]
- Bhattaram, P.; Penzo-Méndez, A.; Sock, E.; Colmenares, C.; Kaneko, K.J.; Vassilev, A.; DePamphilis, M.L.; Wegner, M.; Lefebvre, V. Organogenesis Relies on SoxC Transcription Factors for the Survival of Neural and Mesenchymal Progenitors. Nat Commun 2010, 1, 1–12. [Google Scholar] [CrossRef]
- Shim, S.; Kwan, K.Y.; Li, M.; Lefebvre, V.; Šestan, N. Cis-Regulatory Control of Corticospinal System Development and Evolution. Nature 2012, 486, 74–79. [Google Scholar] [CrossRef]
- Bhattaram, P.; Penzo-Méndez, A.; Kato, K.; Bandyopadhyay, K.; Gadi, A.; Taketo, M.M.; Lefebvre, V. SOXC Proteins Amplify Canonical WNT Signaling to Secure Nonchondrocytic Fates in Skeletogenesis. J Cell Biol 2014, 207, 657–671. [Google Scholar] [CrossRef]
- Sun, B.; Mallampati, S.; Gong, Y.; Wang, D.; Lefebvre, V.; Sun, X. Sox4 Is Required for the Survival of Pro-B Cells. J Immunol 2013, 190, 2080–2089. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Llamas, J.; Trecek, T.; Shi, T.; Tao, L.; Makmura, W.; Crump, J.G.; Segil, N.; Gnedeva, K. SoxC Transcription Factors Shape the Epigenetic Landscape to Establish Competence for Sensory Differentiation in the Mammalian Organ of Corti. Proceedings of the National Academy of Sciences 2023, 120, e2301301120. [Google Scholar] [CrossRef] [PubMed]
- Scharer, C.D.; McCabe, C.D.; Ali-Seyed, M.; Berger, M.F.; Bulyk, M.L.; Moreno, C.S. Genome-Wide Promoter Analysis of the SOX4 Transcriptional Network in Prostate Cancer Cells. Cancer Res 2009, 69, 709–717. [Google Scholar] [CrossRef] [PubMed]
- Angelozzi, M.; Karvande, A.; Molin, A.N.; Ritter, A.L.; Leonard, J.M.M.; Savatt, J.M.; Douglass, K.; Myers, S.M.; Grippa, M.; Tolchin, D.; et al. Consolidation of the Clinical and Genetic Definition of a SOX4-Related Neurodevelopmental Syndrome. J Med Genet 2022, 59, 1058–1068. [Google Scholar] [CrossRef] [PubMed]
- Grosse, M.; Kuechler, A.; Dabir, T.; Spranger, S.; Beck-Wödl, S.; Bertrand, M.; Haack, T.B.; Grasemann, C.; Manka, E.; Depienne, C.; Kaiser, F.J. Novel Variants of SOX4 in Patients with Intellectual Disability. Int J Mol Sci 2023, 24, 3519. [Google Scholar] [CrossRef]
- Ghaffar, A.; Rasheed, F.; Rashid, M.; van Bokhoven, H.; Ahmed, Z.M.; Riazuddin, S.; Riazuddin, S. Biallelic In-Frame Deletion of SOX4 Is Associated with Developmental Delay, Hypotonia and Intellectual Disability. Eur J Hum Genet 2022, 30, 243–247. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, R.E.; Vincent, V.; Spellicy, C.J.; Friez, M.J.; Chaubey, A. Biallelic Deletions of the Waardenburg II Syndrome Gene, SOX10, Cause a Recognizable Arthrogryposis Syndrome. American Journal of Medical Genetics Part A 2018, 176, 1968–1971. [Google Scholar] [CrossRef]
- Irrthum, A.; Devriendt, K.; Chitayat, D.; Matthijs, G.; Glade, C.; Steijlen, P.M.; Fryns, J.-P.; Van Steensel, M.A.M.; Vikkula, M. Mutations in the Transcription Factor Gene SOX18 Underlie Recessive and Dominant Forms of Hypotrichosis-Lymphedema-Telangiectasia. Am J Hum Genet 2003, 72, 1470–1478. [Google Scholar] [CrossRef] [PubMed]
- Posey, J.E.; Harel, T.; Liu, P.; Rosenfeld, J.A.; James, R.A.; Coban Akdemir, Z.H.; Walkiewicz, M.; Bi, W.; Xiao, R.; Ding, Y.; et al. Resolution of Disease Phenotypes Resulting from Multilocus Genomic Variation. N Engl J Med 2017, 376, 21–31. [Google Scholar] [CrossRef]
- Smith, E.D.; Blanco, K.; Sajan, S.A.; Hunter, J.M.; Shinde, D.N.; Wayburn, B.; Rossi, M.; Huang, J.; Stevens, C.A.; Muss, C.; et al. A Retrospective Review of Multiple Findings in Diagnostic Exome Sequencing: Half Are Distinct and Half Are Overlapping Diagnoses. Genet Med 2019, 21, 2199–2207. [Google Scholar] [CrossRef]
- Sobering, A.K.; Bryant, L.M.; Li, D.; McGaughran, J.; Maystadt, I.; Moortgat, S.; Graham, J.M.; van Haeringen, A.; Ruivenkamp, C.; Cuperus, R.; et al. Variants in PHF8 Cause a Spectrum of X-Linked Neurodevelopmental Disorders and Facial Dysmorphology. HGG Adv 2022, 3, 100102. [Google Scholar] [CrossRef] [PubMed]
- Weiss, K.; Terhal, P.A.; Cohen, L.; Bruccoleri, M.; Irving, M.; Martinez, A. F.; Rosenfeld, J.A.; Machol, K.; Yang, Y.; Liu, P.; et al. De Novo Mutations in CHD4, an ATP-Dependent Chromatin Remodeler Gene, Cause an Intellectual Disability Syndrome with Distinctive Dysmorphisms. Am J Hum Genet 2016, 99, 934–941. [Google Scholar] [CrossRef] [PubMed]
- Ladinsky, H.T.; Elizalde, A.; Schickler, R.; Dees, P.B.; Crenshaw, M.L.; Sleasman, J.W. Hypereosinophilic Syndrome and Hemimelia in a Patient with Chromosome 6p22.3 Deletion. Pediatr Allergy Immunol 2014, 25, 500–503. [Google Scholar] [CrossRef] [PubMed]
- Flöttmann, R.; Wagner, J.; Kobus, K.; Curry, C.J.; Savarirayan, R.; Nishimura, G.; Yasui, N.; Spranger, J.; Esch, H.V.; Lyons, M.J.; et al. Microdeletions on 6p22.3 Are Associated with Mesomelic Dysplasia Savarirayan Type. Journal of Medical Genetics 2015, 52, 476–483. [Google Scholar] [CrossRef] [PubMed]
- Trimouille, A.; Barouk-Simonet, E.; Charron, S.; Bouron, J.; Bernhard, J.-C.; Lacombe, D.; Fergelot, P.; Rooryck, C. Deletion of the Transcription Factor SOX4 Is Implicated in Syndromic Nephroblastoma. Clinical Genetics 2017, 92, 449–450. [Google Scholar] [CrossRef] [PubMed]
- Gambale, A.; Russo, R.; Andolfo, I.; Quaglietta, L.; De Rosa, G.; Contestabile, V.; De Martino, L.; Genesio, R.; Pignataro, P.; Giglio, S.; et al. Germline Mutations and New Copy Number Variants among 40 Pediatric Cancer Patients Suspected for Genetic Predisposition. Clinical Genetics 2019, 96, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Hanieh, H.; Ahmed, E.A.; Vishnubalaji, R.; Alajez, N.M. SOX4: Epigenetic Regulation and Role in Tumorigenesis. Seminars in Cancer Biology 2020, 67, 91–104. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, T.-C.; Lesmann, H.; Krawitz, P.M. Facilitating the Molecular Diagnosis of Rare Genetic Disorders Through Facial Phenotypic Scores. Current Protocols 2023, 3, e906. [Google Scholar] [CrossRef] [PubMed]
- Forwood, C.; Ashton, K.; Zhu, Y.; Zhang, F.; Dias, K.-R.; Standen, K.; Evans, C.-A.; Carey, L.; Cardamone, M.; Shalhoub, C.; et al. Integration of EpiSign, Facial Phenotyping, and Likelihood Ratio Interpretation of Clinical Abnormalities in the Re-Classification of an ARID1B Missense Variant. American Journal of Medical Genetics Part C: Seminars in Medical Genetics 2023, 193, e32056. [Google Scholar] [CrossRef]
- Innoceta, A.M.; Olivucci, G.; Parmeggiani, G.; Scarano, E.; Pragliola, A.; Graziano, C. Chromosomal Microarray Analysis Identifies a Novel SALL1 Deletion, Supporting the Association of Haploinsufficiency with a Mild Phenotype of Townes–Brocks Syndrome. Genes (Basel) 2023, 14, 258. [Google Scholar] [CrossRef]
- Innella, G.; Greco, D.; Carli, D.; Magini, P.; Giorgio, E.; Galesi, O.; Ferrero, G.B.; Romano, C.; Brusco, A.; Graziano, C. Clinical Spectrum and Follow-up in Six Individuals with Lamb–Shaffer Syndrome (SOX5). American J of Med Genetics Pt A 2021, 185, 608–613. [Google Scholar] [CrossRef] [PubMed]
- Severi, G.; Bonora, E.; Perri, A.; Scarano, E.; Mazzanti, L.; Isidori, F.; Zuntini, R.; Menabò, S.; Graziano, C. HDAC8 Loss of Function and SHOX Haploinsufficiency: Two Independent Genetic Defects Responsible for a Complex Phenotype. Cytogenetic and Genome Research 2018, 157, 135–140. [Google Scholar] [CrossRef] [PubMed]

 |
 |
 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





