1. Introduction
Energy is a fundamental aspect of human life and the progress of civilization. Consequently, in discussions and gatherings worldwide focusing on sustainable energy development, energy takes center stage. These discussions often encompass various green energy sources, such as solar electricity, wind power, wave power, and tidal power[
1]. Over the coming decades, there is a projected increase in the demand for hydrogen fuel[
2], which has garnered attention both as a potential energy source and as a means of energy storage[
3].
Hydrogen fuel offers several advantages over traditional fossil fuels, serving as a cleaner alternative while mitigating the depletion of finite fossil fuel resources. Fossil fuels, due to their combustion byproducts like carbon dioxide, nitrogen, sulfur, and others contributing significantly to global warming, have detrimental effects on the environment [
4]. Hydrogen fuel, on the other hand, presents a low-carbon alternative derived from renewable resources. Despite most hydrogen currently being sourced from fossil fuels; recent studies indicate a growing potential for hydrogen production through water electrolysis using renewable energy sources. This positions hydrogen fuel as a long-term alternative to hydrocarbon fuels, offering benefits and versatility [
5]. Notably, hydrogen's combustion byproduct is water, making it one of the most efficient and cleanest energy forms [
6].
Numerous studies have explored the challenges associated with transitioning to a hydrogen-based economy [
7]. They have examined the essential steps required for the implementation of a hydrogen economy and outlined perspectives on hydrogen energy to address climate change concerns. These studies have also scrutinized the rationale behind hydrogen energy systems and technologies, comparing them to existing energy systems and their environmental impact [
8]. Hydrogen, being the cleanest form of energy, holds the potential for diverse applications, including power generation and transportation. The future energy landscape is expected to be dominated by hydrogen fuel, offering advantages like minimal environmental impact, long-term storage capabilities, and enhanced mobility options [
9].
This comprehensive study has a multifaceted approach. It first investigates the pivotal role of solar energy technology in the generation of hydrogen, specifically for the purpose of sustainable energy systems. Simultaneously, it embarks on the exploration and development of environmentally friendly techniques designed to enhance hydrogen production. The primary objective of this research is to actively promote the advancement of affordable and low-carbon technologies, with a specific focus on the production of green hydrogen. To provide practical insights into sustainable hydrogen production, this study endeavors to showcase a self-contained microgrid model. Within this model, the operational dynamics integral to the production of green hydrogen are thoroughly evaluated. Additionally, the study aims to assess the unique characteristics of hydrogen generation, particularly in terms of its utilization of solar energy and energy storage systems.
Recognizing the complexity of green hydrogen production, the study aspires to implement a comprehensive multi-domain framework. This framework encompasses various aspects, including electrical, thermal liquid, and thermal gas components, facilitating an in-depth exploration of the intricate processes underlying the green hydrogen production system. To further enhance the practical applicability of the research, the study endeavors to simulate and optimize the feasibility and efficiency of renewable energy sources. This optimization specifically addresses off-grid hydrogen production scenarios, contributing valuable insights to the field of sustainable energy solutions. Ultimately, the overarching goal of this study is to make significant contributions to the advancement of sustainable energy solutions. Through rigorous research and a focus on green hydrogen, the study aims to support and drive the transition towards cleaner and more environmentally responsible energy systems.
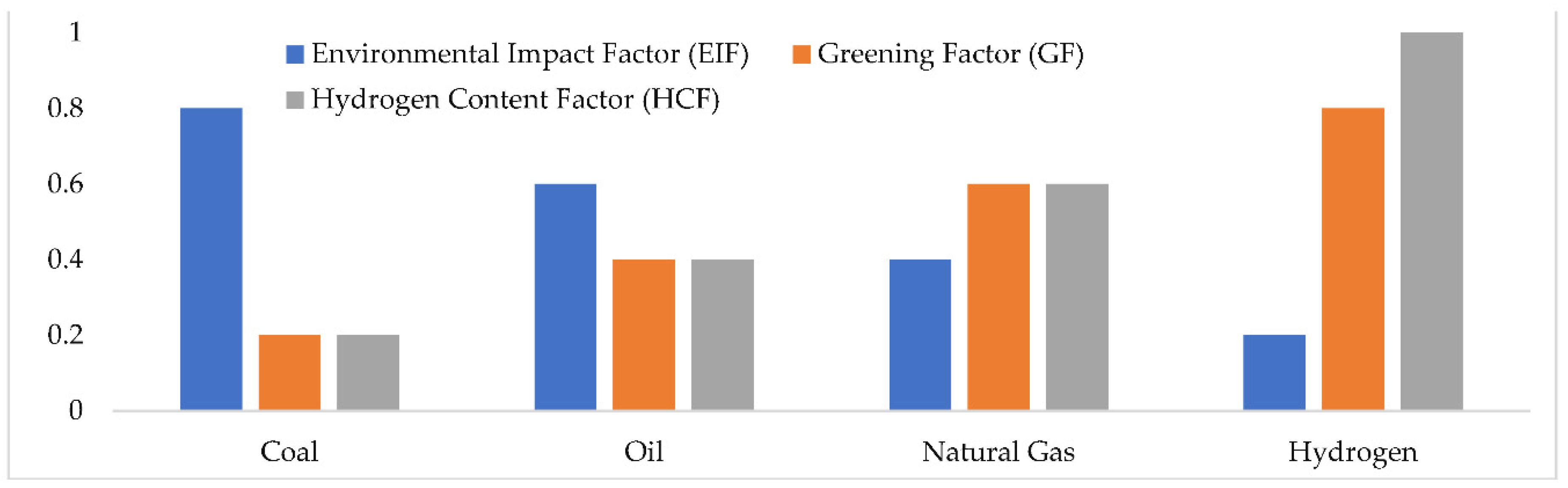
Figure 1 illustrates how various energy carriers, including coal, oil, natural gas, and hydrogen, differ in their Greening Factors (GF), Environmental Impact Factors (EIF), and Hydrogen Content Factors (HCF) [
10]. The focus on the hydrogen economy underscores its significance in hydrogen production to harness the benefits of hydrogen fuel. Therefore, cost-effective hydrogen production must rely on renewable energy sources [
11]. A methodology for assessing the environmental impact of hydrogen production processes has been implemented [
12]. Hydrogen fuel has been identified as the cleanest and most renewable energy source for the future. A comparative study was conducted to reduce overall environmental emissions from shipping by utilizing hydrogen as a fuel [
13].
To emphasize the unique value of hydrogen as a distinct choice, the following formulas are utilized to make comparisons between hydrogen and conventional fuels in terms of Environmental Impact Factor (EIF), Greenization Factor (GF), and Hydrogen Content Factor (HCF).):
where
is max is the highest value of EIF among the choices that were analyzed. Coal is chosen as the
in this specific instance with a 3.6. When shown in
Figure 1 the energy sources grow greener (increasing GF) and have less of an influence on the environment (EIF) as the hydrogen content (HCF) increases [
14].
This is an obvious benefit of hydrogen in terms of lowering emissions related to carbon. The hydrogen economy must be produced from abundant or renewable sources at cheap costs to fully benefit from it. In the literature, there are several studies emphasizing how hydrogen can be one of the most efficient options, significantly contributing to greater sustainability and the environment. Natural gas steam reforming is the most widely utilized process of the potential hydrogen production techniques examined in the literature, and it produces significant GHG emissions [
15].
Natural gas steam reforming provides over 50% of the world's hydrogen needs, followed by oil reforming at 30%, coal gasification at 18%, water electrolysis at 3.9%, and other sources at 0.1%. Hydrogen should be produced from clean and abundant sources using ecologically friendly processes to mitigate the negative impacts of fossil fuel use on the environment, human health, and the climate. The phrase "green hydrogen production" refers to this idea [
16].
2. Green hydrogen
The production of hydrogen from renewable sources is a critical step towards achieving zero-carbon hydrogen, often referred to as "green hydrogen." Utilizing renewable enreduceurces not only reduces dependence on oil and gas, thereby easing geopolitical tensions in the short term, but also opens new possibilities for hydrogen production. This is particularly relevant given the increasing generation of electricity from renewable sources and the temporary affordability of excess capacity resulting from the variability of solar and wind energy. To address the challenge of long-term electricity storage, finding sustainable solutions is becoming increasingly important [
17].
Yadav and Banerjee emphasize the necessity of leveraging renewable energy for hydrogen production to establish a sustainable hydrogen economy. Currently, two relatively established methods for renewable energy-based hydrogen production are water electrolysis and biogas steam reforming.
Renewable electricity sources, such as solar, hydro, and wind power, or biofuels like biogas, can be used to generate green hydrogen. Among these methods, electrolysis powered by hydro or wind sources stands out as one of the most promising hydrogen production processes, particularly in terms of a Life Cycle Assessment [
18].
While water electrolysis offers a cost-effective means of producing hydrogen compared to synthetic natural gas production, it still requires a significant amount of energy. However, projections suggest that by 2030, the cost of production may become competitive with existing methods. There are three types of electrolysis cells: solid oxide electrolysis cells (SOEC), proton exchange membrane electrolysis cells (PEMEC), and alkaline electrolysis cells (AEC). SOEC is particularly effective for hydrogen production and co-electrolysis of carbon dioxide, thanks to its superior energy conversion efficiency.
Various reforming techniques, such as steam reforming (SR), dry reforming (DR), dry oxidation reforming (DOR), partial oxidation reforming (POR), and autothermal reforming (ATR), can be employed to produce low-carbon hydrogen from biogas. Technologies for hydrogen production from renewable sources that are still in the maturation phase include biomass gasification, pyrolysis, thermochemical water decomposition, and biomass supercritical water gasification. Solar-powered hydrogen production is an environmentally friendly approach, and thermochemical cycles offer another avenue for green hydrogen production. Thermochemical pyrolysis and gasification processes are the most likely candidates for large-scale applications soon, as they are commercially viable. While biological approaches hold promise, further research is needed to enhance their output [
19].
3. Hydrogen's chemical and physical properties
Since the early 1800s, hydrogen has been used as a form of energy. In the 1970s, when it started to become significant as the driving force behind the Space Race, the potential for it to become the primary energy carrier in contemporary energy systems emerged [
20].
The most abundant element on the planet, hydrogen, is extremely important. It is found mostly in demineralized form and only as part of a molecule in nature, primarily in hydrocarbons and water. Under normal temperatures and pressures, it exists as a gas (293.15 K and 1 atm). At ambient pressure, it has a very low boiling point of -252.76 °C (20.3 K). Hydrogen is also colorless, odorless, and non-toxic under normal conditions, making it environmentally friendly. Hydrogen changes states over a tight temperature and pressure range. Liquefaction is thus primarily accomplished through cooling rather than compression, creating opportunities for heat recovery schemes [
21]. Liquefaction increases the density of hydrogen by an order of magnitude. Natural gas has a factor of 600, while LPG has a factor of 250. This makes hydrogen liquefaction an appealing mode of long-distance transportation. During transport, the temperature is extremely low (-253°C) [
22].To keep hydrogen from evaporating, you must use a highly efficient insulation system [
23].
Under moderate or high pressure, hydrogen can also be transported in gaseous form. A promising method of hydrogen storage referred to as adsorption on solids and liquids, is currently being researched. Even at atmospheric pressures, this intriguing storage method can hold significant amounts of H2 [
24].
The lowest molecular weight of any substance is hydrogen (2.0016 g/mol). It is combustible over a very wide concentration range, which results in a wide variety of ignition conditions, as indicated by the lower and upper explosive limits [
25]. Extremely lean air/gas combinations are made possible by this wide ignition range, which reduces fuel use and promotes more effective combustion [
26].
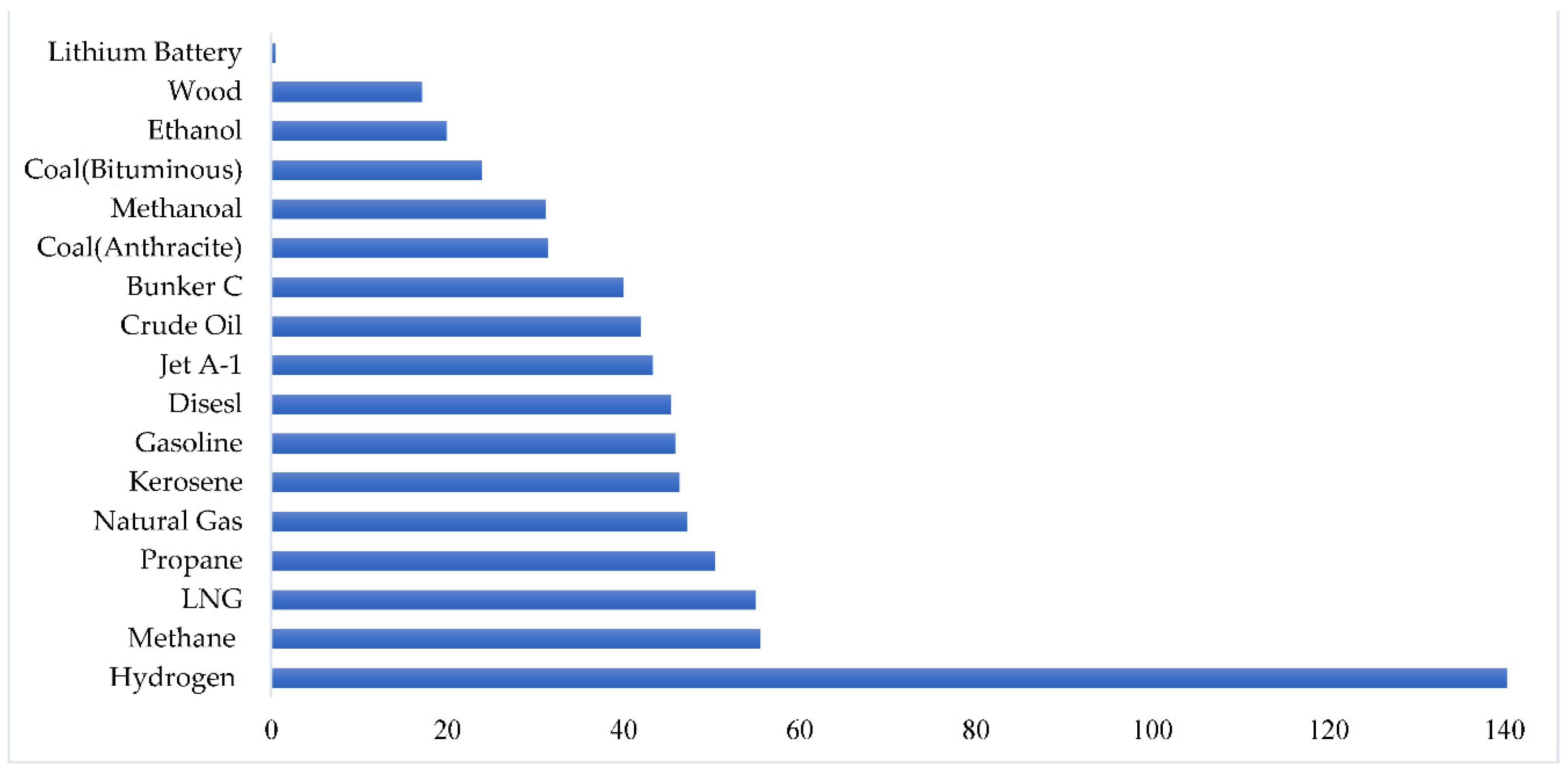
Overall, as shown in
Figure 2, hydrogen has a high mass-based or gravimetric energy density of 120 MJ/kg or 33 kWh/kg, which is nearly three times greater than the energy density of today's conventional fuels. The calorific value or lower heating value of a fuel determines its mass-based energy density [
22].
4. Demand for hydrogen
Energy and hydrogen have a long history together; more than 200 years ago, hydrogen powered the first internal combustion engines and is now a crucial component of the modern refining sector [
27]. It emits no greenhouse gases or pollutants directly and is light, storable, and energy dense. But the adoption of hydrogen in areas where it is virtually nonexistent, like transportation, buildings, and power generation, is necessary for it to significantly contribute to clean energy transitions [
28].
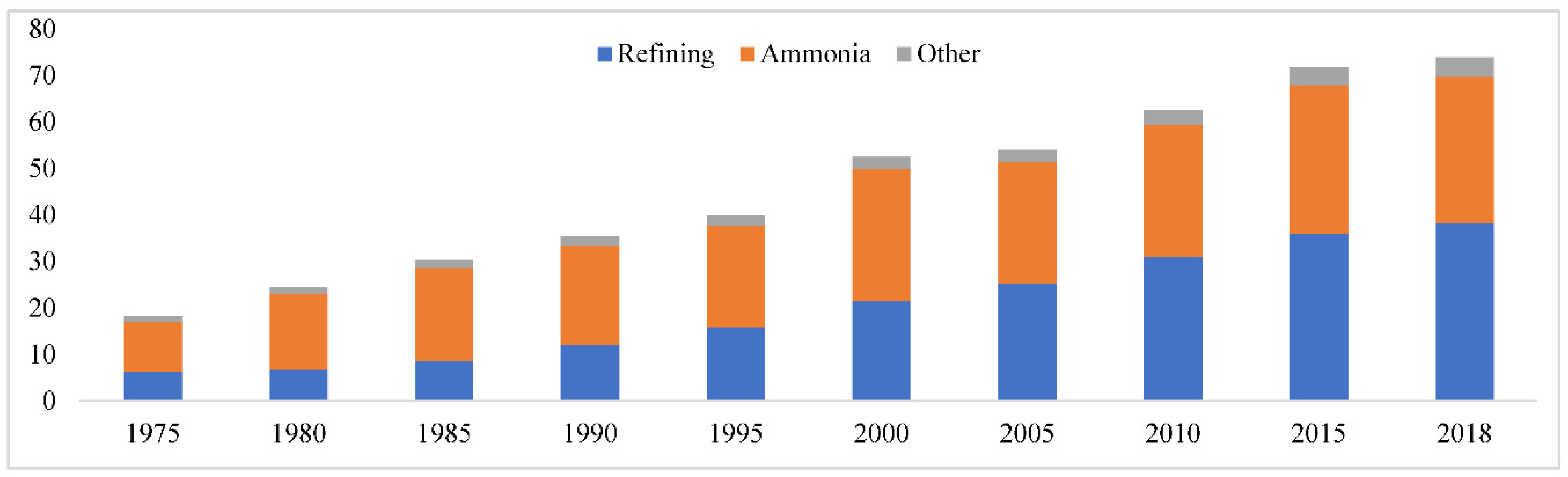
In
Figure 3 of Hydrogen is a thorough, unbiased examination of hydrogen that outlines the current situation, how hydrogen might contribute to the development of a clean, secure, and economical energy future, and how to go about realizing its potential [
29].
The supply of hydrogen to industrial customers has grown significantly in recent years [
30]. Since 1975, the demand for hydrogen has more than tripled and is still growing [
31]. The manufacture of hydrogen uses 6% of the natural gas and 2% of the coal in the globe [
32].
As a result, the creation of hydrogen generates about 830 million tons of CO2, which is comparable to the emissions of Indonesia and the United Kingdom put together [
33].
5. Technology for Hydrogen Production
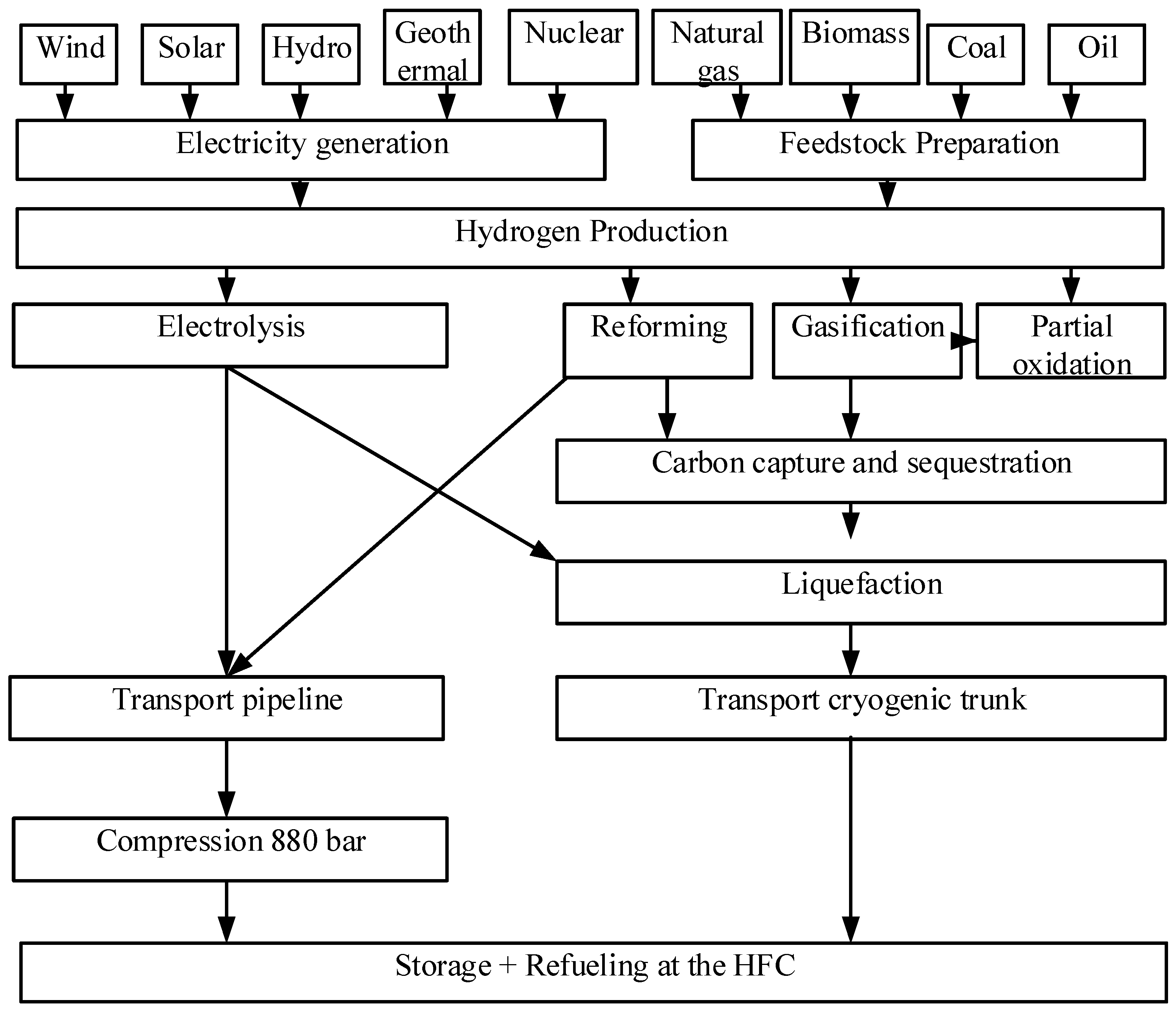
The following happens when water is divided into O2 and H2 gases by electricity produced by renewable energy sources (RSE) in
Figure 4 such as biomass, geothermal, wind, and solar energy [
34].
F is the constant of Faraday, which is equal to 96485 °C for 1 mol of electricity [
35]. The opposites of reaction (1) can be used to create electricity from hydrogen using HFC or combustion processes [
36].
Because the cost of water in reaction (1) is negligible, the price of hydrogen production from the electrolysis process is heavily influenced by the cost of electricity. from the electrolysis of water [
37]. At 80°C, commercial electrolytes use 30% KOH (alkaline electrolysis) that can be recovered and reused [
38]. When using an alkaline electrolysis system (AES), the efficiency is approximately 55-75%. The electrodes are nickel, and the cathode and anode are platinum or manganese oxide coated. Pure hydrogen is typically produced [
39]. Commercial AES with a power consumption of 4.49 kWh/m3.
Although the electrolysis of water has shown promise in the production of hydrogen, this technology still faces challenges [
40]. The electrodes used in today's water electrolysis technology are generally coated with expensive platinum, making the process uneconomical [
42]. As a result, the use of nanomaterials to remove such noble metals in the water electrolysis process has attracted attention [
41], and a cobalt phosphate catalyst has been proposed as a promising method to split water molecules into their pressurized atmosphere by doubling photosynthesis. A conventional indium tin oxide anode is used in this system for water splitting needed. The use of abundant cobalt phosphate, which is much cheaper than expensive platinum, makes electrolysis technology less expensive.
6. Green H2 Production from Renewable Sources
Currently, hydrocarbons are the primary source of H2 production, but the integration of renewable options is becoming crucial as fossil fuels deplete and concern grows about the greenhouse effect [
42]. Over time, renewable technology is predicted to surpass traditional methods, particularly as the demand for more sustainable solutions grows [
43]. There are numerous methods for obtaining hydrogen from renewable resources, and this section gives a quick rundown of some of the ways and technologies used for biomass-based water splitting [
44].
a. Biomass process
Biomass energy production releases CO2, which is balanced by the amount consumed by living organisms. Biomass can be converted into hydrogen via thermochemical or biological processes. While biological processes are environmentally friendly and use less energy, they have low hydrogen rates and yields based on the feedstock used. Thermochemical processes are quicker and produce more hydrogen, with gasification being a feasible choice considering cost and environmental impact [
45].
-
i
Thermochemical processes
Thermochemical methods convert biomass into hydrogen and hydrogen-rich gases [
45]. This technology is a step toward a sustainable future with reduced greenhouse gas emissions [
46]. Techniques include pyrolysis and gasification, which produce CH4, CO, and other gases that can further be transformed into hydrogen. Incineration and liquefaction are less favorable options in this process [
47].
Low hydrogen generation, the former produces environmentally damaging byproducts, while the latter demands running under difficult circumstances of 5 to 20 MPa without air [
3]. Heat is used in the thermochemical process of biomass pyrolysis to create solid carbon, liquid oils, and gaseous chemicals. biomass at a pressure of 0.1 to 0.5 MPa and a temperature of 650 to 800 K. except for incomplete situations [
48]. When combustion is allowed to supply the process's thermal energy needs, it does so in the total absence of oxygen. Methane and other hydrocarbon gases can be created, and these gases can then be steam reformed and used in the WGS process to create more hydrogen. After the CO is transformed into CO2 and H2, the PSA can produce the necessary amount of pure H2.
The following equations depict the individual steps of the biomass transformation process depicted in
Figure 5.
The feedstock, catalyst employed, temperature, and residence time all affect the yield of hydrogen generated during biomass pyrolysis. According to performance length and biomass type, the price of hydrogen produced by biomass pyrolysis is predicted to range from
$8.86/GJ to
$15.52/GJ (or
$1.25/kg at second
$20/kg) [
49].
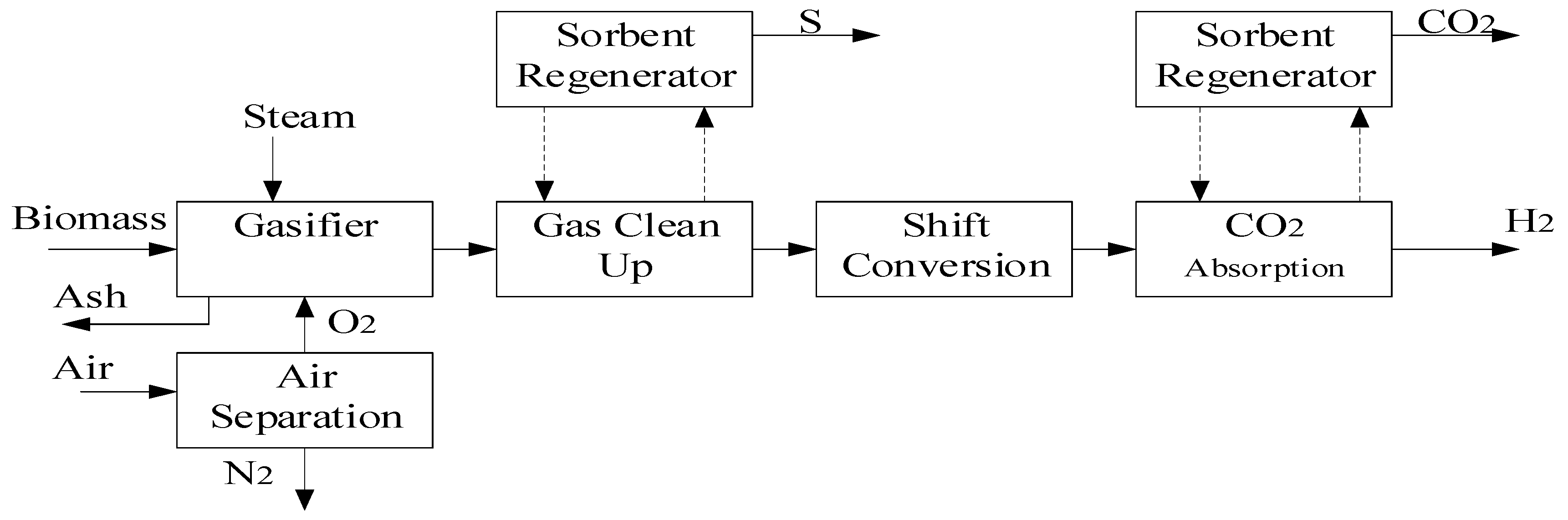
The thermochemical process of turning biomass into a gaseous fuel (syngas) in a gasification medium like air, oxygen, or steam is known as biomass gasification. Reactor types, plant scale, and the eventual use of the produced syngas are all classified according to the flow and velocity of the gasification agent. The three main reactor types used for biomass gasification are fixed cushion, fluidized bed, and inclined cushion gasifiers:
Their maximal space configurations are shown in
Figure 5 Eqs. 6 and 7, it is seen how biomass combines with air or steam to create synthesis gas.
The mixture of gases is regarded as the pyrolysis process product gas after biomass has been transformed into synthesis gas, as shown in
Figure 6. However, the particle size, temperature, steam-to-biomass ratio, and biomass type all affect the kind of biomass. The catalyst that is utilized has the biggest impact on hydrogen production. In comparison to flash pyrolysis, steam gasification yields a lot more hydrogen, and its overall efficiency (thermal to hydrogen) can reach up to 52%, making it a useful technique for generating renewable hydrogen. It is seen as typical [
50].
It is necessary to use the PSA gasification pathway of biomass steam reforming. Primary energy consumption is 2.4 TJ per TJ of hydrogen for a plant with an expected hydrogen production of 139,700 kg/day and biomass costs of $46-80/ton of hydrogen production. The price is expected to range between $1.77 and $2.05 per kilogram.
-
ii
Biological Reactions
Recent years have seen a considerable increase in analysis on the synthesis of biological chemical elements because of a greater focus on property development and waste reduction [
51]. Low temperatures and pressures are used by most biological processes, which use less energy [
52].
They may employ a variety of waste items as raw materials, which helps them use renewable energy sources that are unlimited and support trash recycling [
53]. The main biological methods used to produce hydrogen gas include direct and indirect bio photolysis, photo, and dark fermentation, as well as sequential or multi-stage dark fermentations and photo fermentations.[
54] Two examples of biohydrogen feeds include water for photolysis, in which some bacteria or algae make hydrogen directly through their hydrogenase or nitrogenase enzyme systems, and biomass for fermentative processes, in which materials containing carbohydrates are transformed into organic matter. These feeds are then used to create acids utilizing bioprocessing methods, which are ultimately transformed into hydrogen gas.
To make hydrogen gas, a biological process known as bio photolysis applies the same principles of plant and algae photosynthesis. Only because green plants lack the enzymes that catalyze the production of hydrogen does CO2 reduction occur. On the other hand, algae can produce hydrogen and enzymes under specific conditions in addition to containing hydrogen. Green and blue-green algae may split water molecules into hydrogen and oxygen ions by direct and indirect bio photolysis.
The only reason CO2 is reduced in green plants is that the enzymes that catalyze hydrogen synthesis aren't present. Algae, however, may produce hydrogen and enzymes in specific conditions and contain hydrogen themselves [
55]. Green and blue-green algae can split water molecules into ions of hydrogen and oxygen by direct and indirect bio photolysis. In indirect bio photolysis, all hydrogen reactions [
56].
Table 1 provide a method for producing hydrogen according to primary energy and material sources The following reactions can be used to illustrate how cyanobacteria or blue-green algae create water:
-
iii
Hydrogen Production Through Methane Catalytic Steam
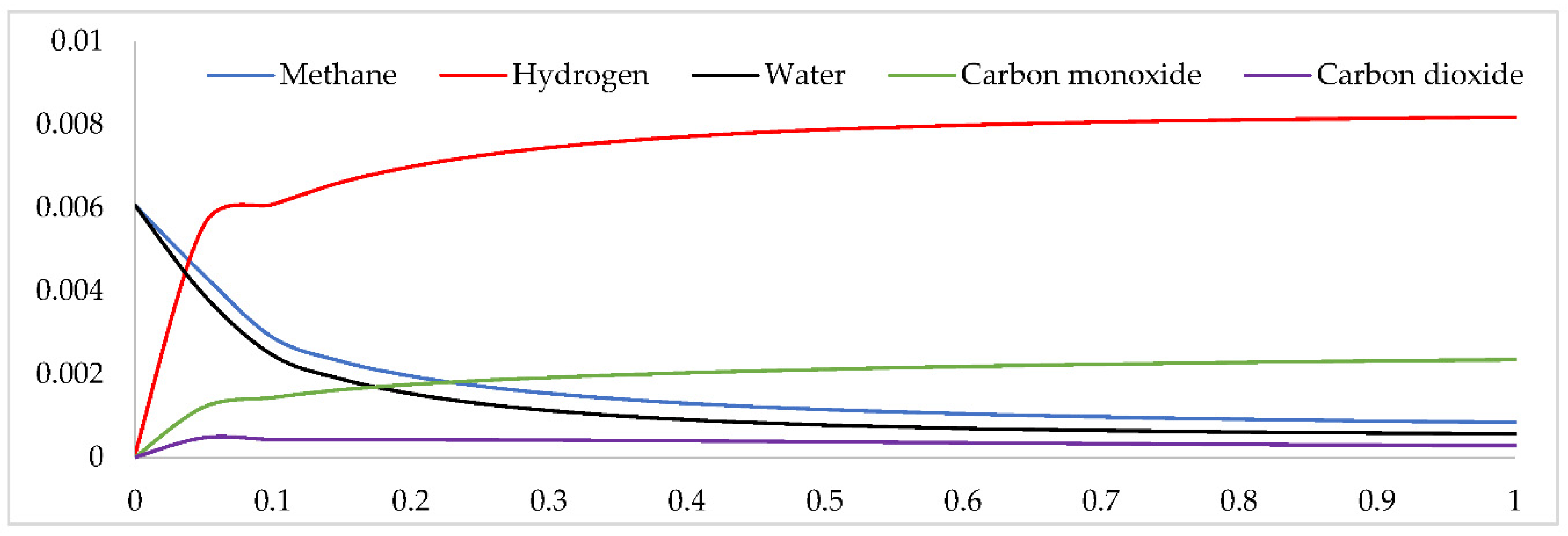
Figure 7 provide an Example of Steam Reforming Reactor, these samples simulate a PFR for producing hydrogen by steam reforming methane [
57]. Methane (CH4) and water (H2O) as well as carbon dioxide (CO2), carbon monoxide (CO), and hydrogen are fed into the reactor as educts. (H2) is generated over a nickel catalyst on an alumina support [
58].
b. Hydrogen Production from Solar Energy
The creation of sustainable energy systems relies heavily on the manufacturing of hydrogen using solar energy technology. Hydrogen can traditionally be produced using a wide range of techniques from traditional sources such as natural gas, coal, and oil. However, their use results in the release of ozone-depleting chemicals like CO2. This essay examines the most recent advancements in solar hydrogen-generating techniques for use in remote areas. Water is the primary raw material used in the thermochemical, photoelectrochemical, and electrochemical processes that are covered [
59].
-
i
Thermochemical
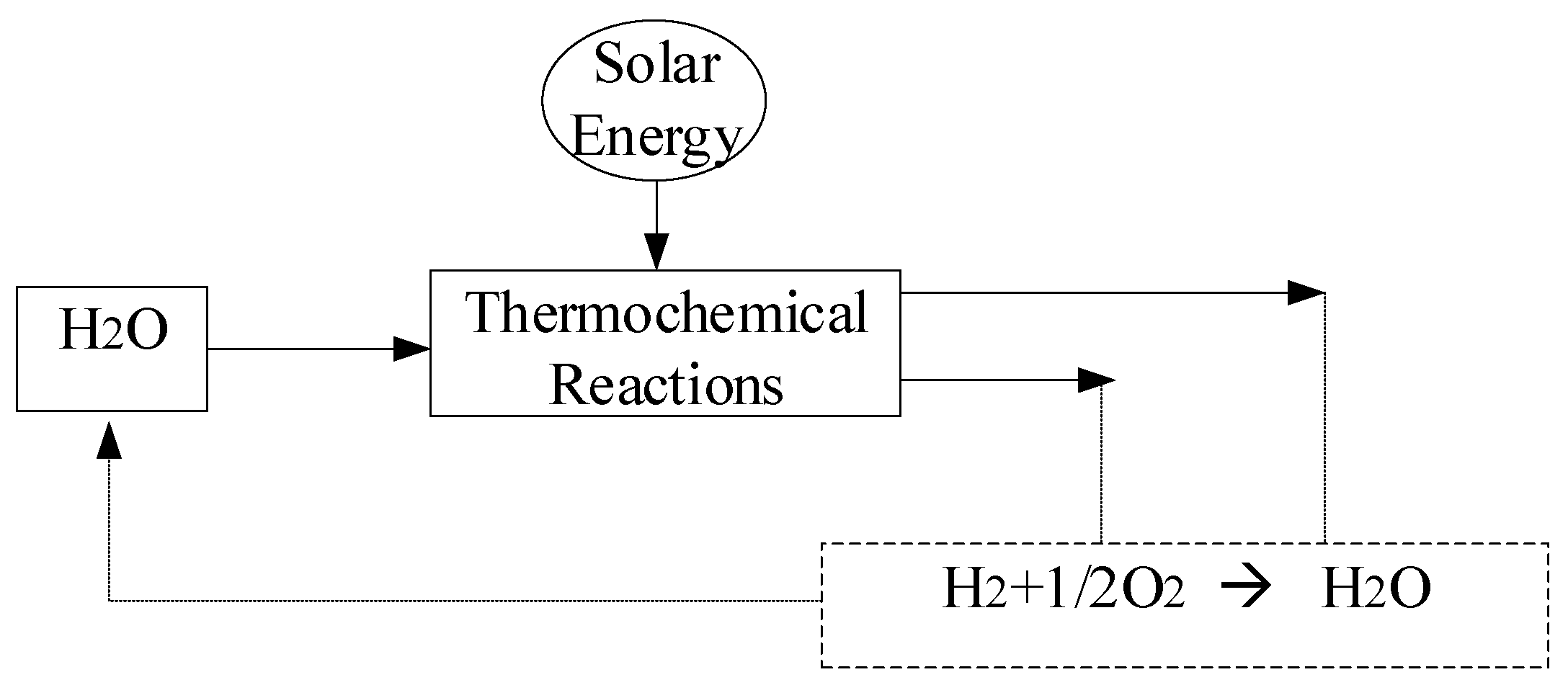
Thermolysis, also referred to as thermochemical water splitting, is a method for producing hydrogen utilizing heat and solar energy. At temperatures around 2500 °C, water is converted into hydrogen and oxygen. Even though efficiencies of 50n are attained, it is difficult to maintain material stability at this high temperature, and renewable heat sources are few. The splitting of water directly or indirectly through chemical intermediaries can provide a solution. Thermolysis, thermochemical water splitting, or thermal decomposition are all terms used to describe the heat-driven separation of water into hydrogen and oxygen. All these processes depend on the same type of energy input, regardless of the temperature needed or the direct versus indirect breakdown process. The solar thermochemical process of water splitting is seen in
Figure 8. Concentrated solar energy is focused to produce high-temperature heat. radiation from appliances like solar cookers, parabolic reflectors, and tower systems.
The thermochemical process in
Figure 8 comprises approximately 300 thermochemical cycles, which can be categorized as either entirely thermal or hybrid in nature. One drawback of this method is the necessity for extremely high temperatures, exceeding 2000 °C, to achieve direct water decomposition without the aid of additional chemicals. This high thermolysis temperature poses challenges in terms of material selection for refractory components and equipment construction [
60]. The outcome of the thermolysis process is a gaseous mixture of hydrogen and oxygen, which raises concerns regarding reaction rates, materials durability, and retroreflection losses, along with the elevated risk of explosion.
To address these challenges, various studies have proposed the use of chemical reagents or catalysts within two-step water separation cycles based on metal oxide oxidation/reduction processes. These approaches aim to achieve lower operating temperatures [
61]. The general cycle involves the following two distinct steps:
A metal oxide molecule has x and y metal and oxygen atoms, respectively[
62]. M is metal. There weren't many temperature variations. The temperature is still between 15000 and 25000 degrees Celsius [
63]. As a result, many researchers are reluctant to investigate thermochemical technologies for hydrogen production in remote locations [
64].
-
ii
Photoelectrochemical
Traditionally, the process of using sunlight to perform water hydrolysis through photo electrolysis has been employed and is also commonly referred to as photoelectrochemical [
65]. This process involves the photoelectrochemical dissociation of water, which describes the separation of water molecules into hydrogen and oxygen under the influence of an electric current generated by light [
66].
The fundamental setup for photo electrochemistry typically includes two electrodes submerged in an aqueous electrolyte within a container. When exposed to sunlight, either one or both electrodes have the capability to break down water into its constituent gases, hydrogen, and oxygen. These photoelectrochemical electrodes are constructed from semiconductor materials, like the materials used in photovoltaic electrodes. When two doped semiconductor materials, specifically an n-type and a p-type, are brought together, they form a p-n junction. This junction creates a constant electric field due to the rearrangement of charges on the p- and n-type materials [
19].
Photoelectrochemical techniques are employed when at least one light-absorbing electrode is utilized, and minimal to no current is required for reduction reactions [
8]. The oxidation reaction at the electrode is powered by either internal or external energy sources. When a photon with energy greater than the semiconductor material's band gap is absorbed at the junction, a hole is generated, and an electron is released [
33]. In the presence of an external charge and an electric field, these hole and electron pairs are compelled to move in opposite directions., producing an electric current [
8]. The outcome of the photoelectrochemical reaction is:
where h represents Planck's constant, and n denotes frequency [
26]. The materials used for the sunlight-absorbing electrode in photoelectrochemical processes are prone to corrosion during the process. Researchers are actively exploring new materials to improve process efficiency and minimize corrosion [
28].
To improve performance, hybrid systems are being incorporated that use external power supplies to increase the potential of the electrode. A photoelectrochemical system is a combination of photoelectrochemical and water electrolysis processes, utilizing a material that absorbs sunlight as the electrode. Researchers have suggested that optimizing the photoanode, such as adjusting its amount and electronic structure, can enhance the performance of the photoelectrochemical process. Furthermore, increasing the amount of light that reaches the system can also result in improved performance [
13].
-
iii
Electrochemical
where h represents Planck's constant, and n is the frequency. The materials used for the sunlight-absorbing electrode in photoelectrochemical processes are prone to corrosion during the process [
36]. Researchers are actively investigating new materials to improve process efficiency and reduce corrosion [
23]. To improve performance, hybrid systems that use external power supplies to raise the potential of the electrode at the anode are being implemented:
The general electrolysis reaction:
The electrolysis process can be conducted at both low and high temperatures [
44]. When electricity is exclusively generated from sources like photovoltaic panels or turbines powered by solar energy or other gases, water electrolysis becomes a viable option. An electrolytic conductor, which separates ionic particles, facilitates the movement of ions between the electrodes [
12]. Once received by the system, electrical energy is converted into chemical energy in the form of hydrogen. There are two primary methods for electrolyzing water to produce hydrogen using solar energy [
15]. The widely utilized technique is alkaline electrolysis, which employs a liquid electrolyte. However, alternative proton exchange membrane (PEM) electrolysis systems are currently under development [
67]
It's important to note that water electrolysis is economically feasible only when the electricity source is renewable, such as solar or wind energy [
15]. The efficiency of current solar panels typically ranges from 10% to 20%.
Table 2 examines the potential of hydrogen production using water as the starting material and solar energy, considering factors such as efficiency, applicability, operating pressure, temperature, energy input, fundamental components, and water-splitting methods. In comparison to thermochemical and photoelectrochemical techniques, electrochemical hydrogen synthesis exhibits higher efficiency. Moreover, it operates at lower temperatures and under reduced atmospheric pressure, which enhances its technical suitability for various applications.
However, the primary limitation of electrolysis as a hydrogen production method lies in its dependence on electrical power. To make it feasible in remote locations, it must be coupled with either solar or wind energy sources to ensure a consistent power supply. The utilization of renewable energy sources addresses the primary challenge of energy supply and reduces operational costs, making it a more financially viable option, even if the production rate may be lower than that of thermochemical and photoelectrochemical processes. Achieving further enhancements in efficiency and hydrogen yield requires advancements in photovoltaic cells and proton-exchange membrane (PEM) electrolyzer [
52].
7. Simulation and results
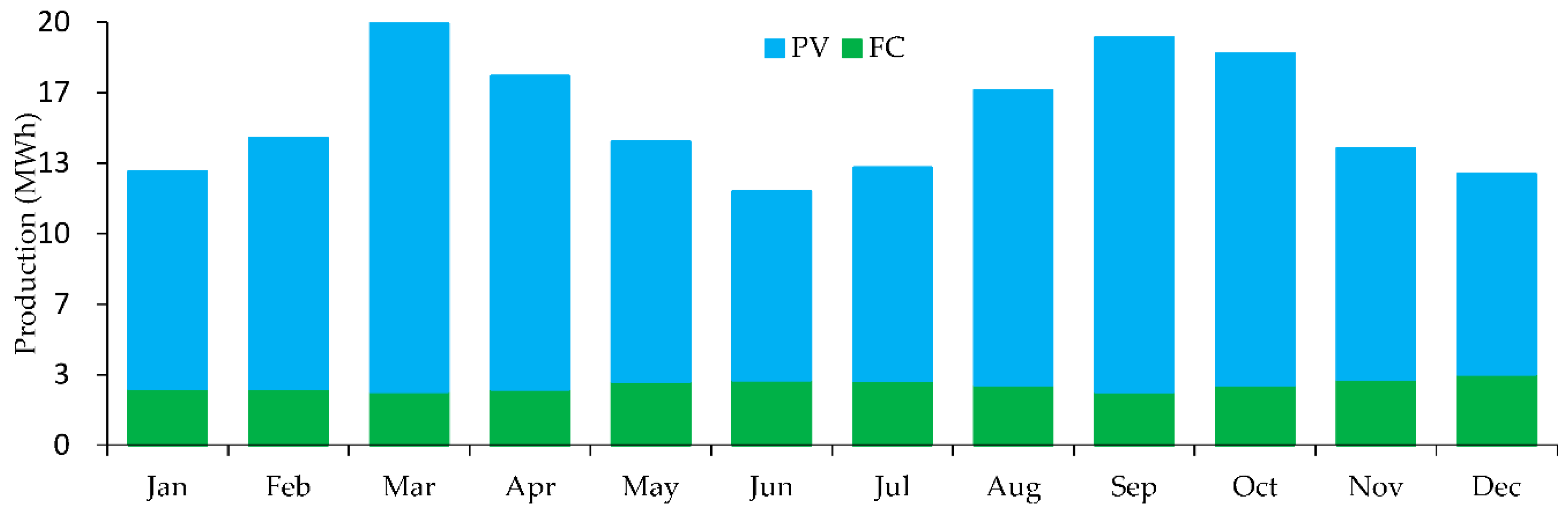
This microgrid requires 460 kWh/day and has a peak of 63 kW. In the proposed system, the following generation sources serve the electrical load as in
Figure 9.
The system architecture includes a 20.0 kW fuel cell generator, 120 kW PV, 50.0 kW electrolyzer, and a hydrogen tank with a capacity of 120 kg, all managed by the HOMER Load Following dispatch strategy as shown in
Table 3.
-
i
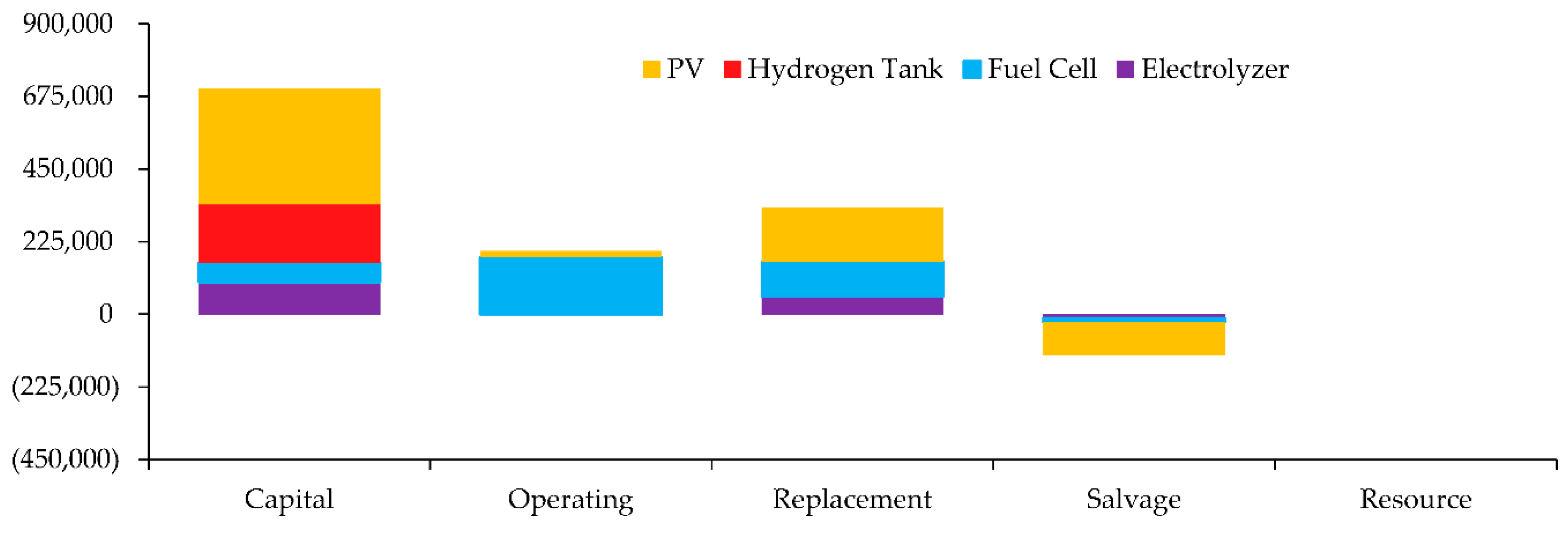
Cost Summary
The Cost Summary for various components related to a system. Each component has different cost elements such as Capital, Operating, Replacement, Salvage, and Resource costs. Here's a breakdown of the information in the
Figure 10.
The Net Present Costs for various components related to a system. Each component has different cost elements such as Capital, Operating, Replacement, Salvage, and Resource costs. Here's a breakdown of the information in the
Table 4.
The Annualized Costs for various components related to a system. Each component has different Annualized Costs such as Capital, Operating, Replacement, Salvage, and Resource costs. Here's a breakdown of the information in the
Table 5
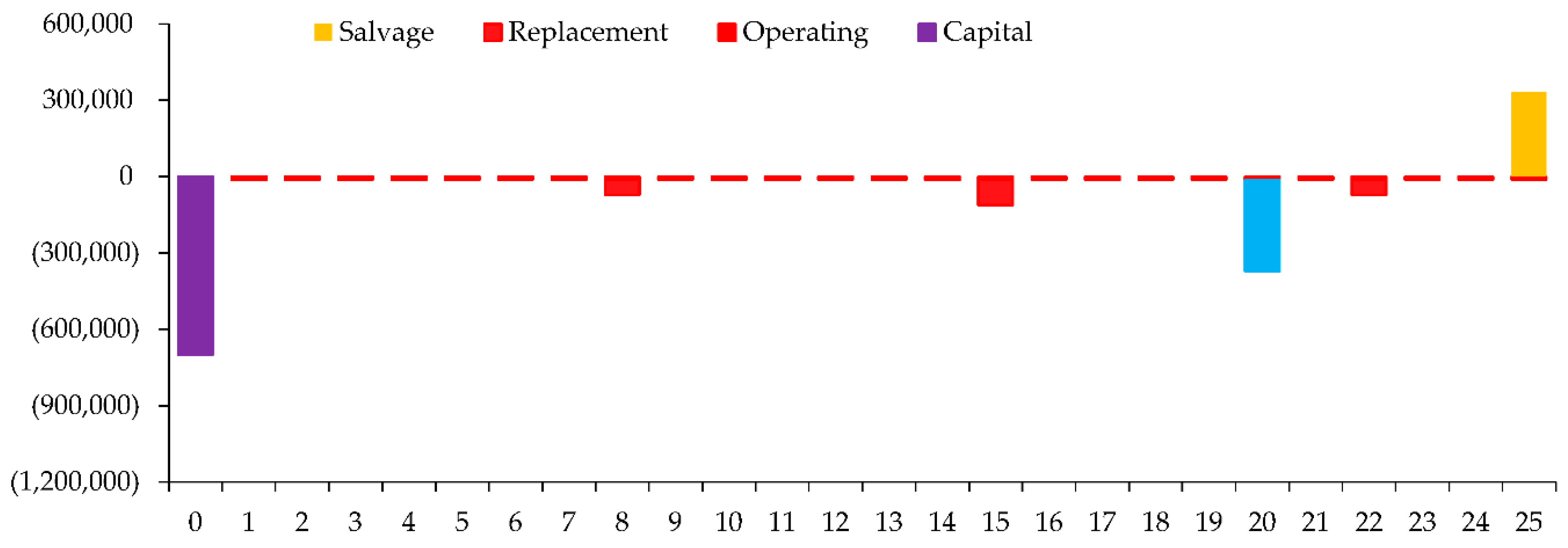
The Cash Flow for various components related to a system. Each component has different Cash Flow such as Capital, Operating, Replacement, Salvage, and Resource costs. Here's a breakdown of the information in the
Figure 11
-
ii
Electrical Summary
Table 6 summarizing information related to excess and unmet quantities of the system.
Table 7 provided a production summary table for different components, specifying the production quantities in kilowatt-hours per year (kWh/Year) and the corresponding percentage contribution.
Table 8 provided a consumption summary table for different components, specifying the consumption quantities in kilowatt-hours per year (kWh/Year) and the corresponding percentage contribution.
Table 9 provides a comprehensive summary of various metrics related to the electrical performance of a Fuel Cell
Table 10 provides a summary of various metrics related to the electrical performance of a Photovoltaic (PV) system.
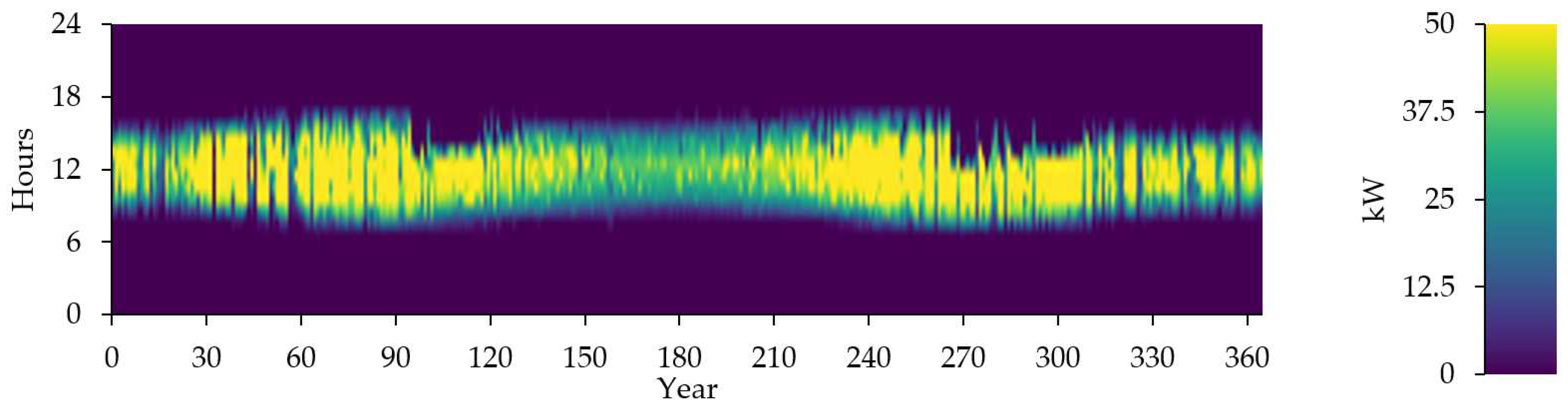
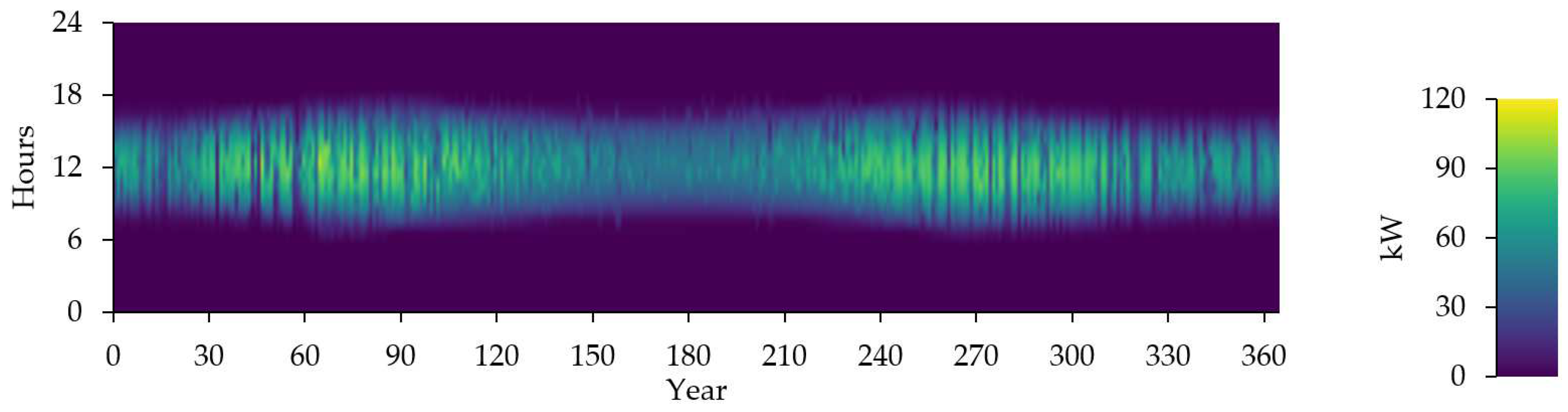
Figure 14 shows the photovoltaic output in kW.
Table 11 presents a summary of various metrics related to the performance of an electrolyzer.
Figure 14 represent of Electrolyzer Input Power
Figure 14.
Electrolyzer Input Power (kW).
Figure 14.
Electrolyzer Input Power (kW).
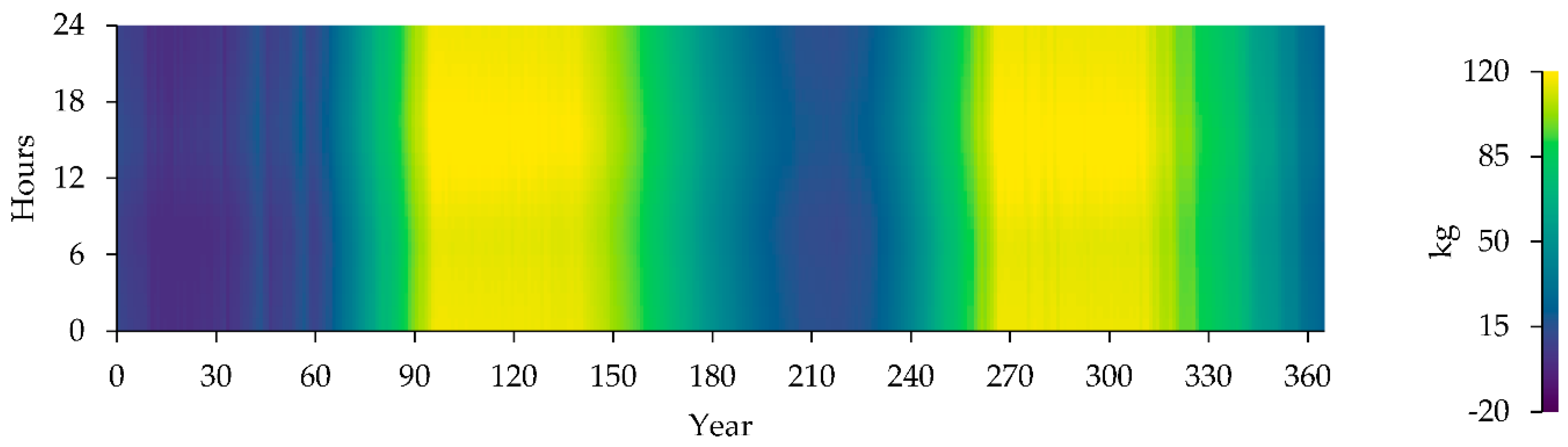
Table 12 provides information on the properties and statistics of a hydrogen tank, including its storage capacities, autonomy, and hydrogen content at the beginning and end of the year.
Figure 15 shows the level of hydrogen fuel tank over year.
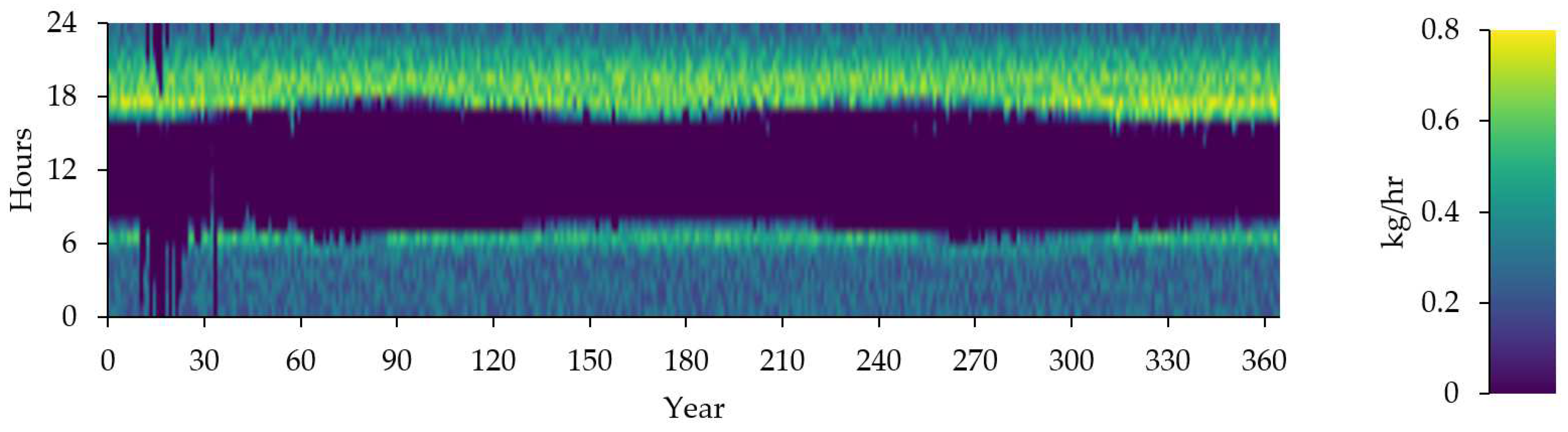
Table 13 provides statistics on the consumption of stored hydrogen, including the total amount consumed, average daily consumption, and average hourly consumption.
Figure 16 provide the state of Stored hydrogen Consumption.
Table 14 provides information on the emissions of various pollutants, including carbon dioxide, carbon monoxide, unburned hydrocarbons, particulate matter, sulfur dioxide, and nitrogen oxides.
Renewable Summary
Table 15 provides various metrics related to renewable energy, including capacity-based metrics, energy-based metrics, and peak values.
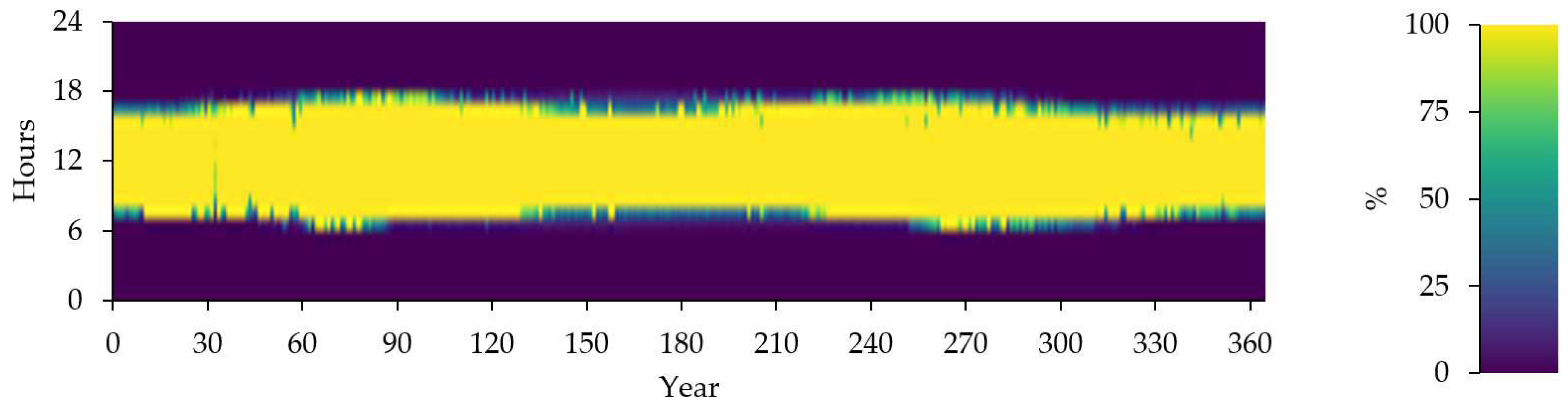
Figure 17 provide the Instantaneous Renewable Output Percentage of Total Generation
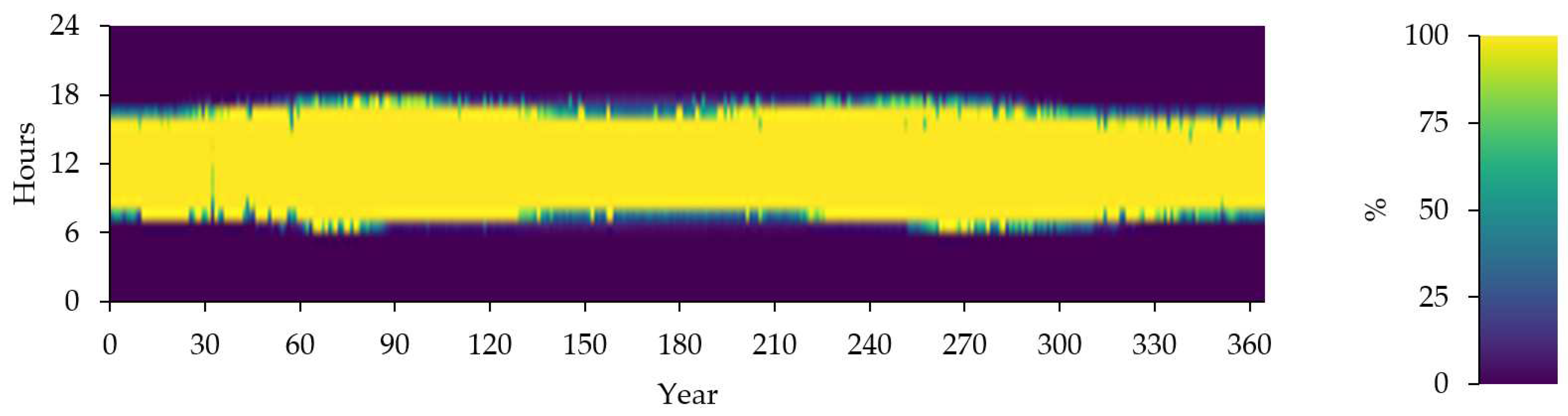
Figure 18 shows the 100% Minus Instantaneous Nonrenewable Output as Percentage of Total Load in one year.
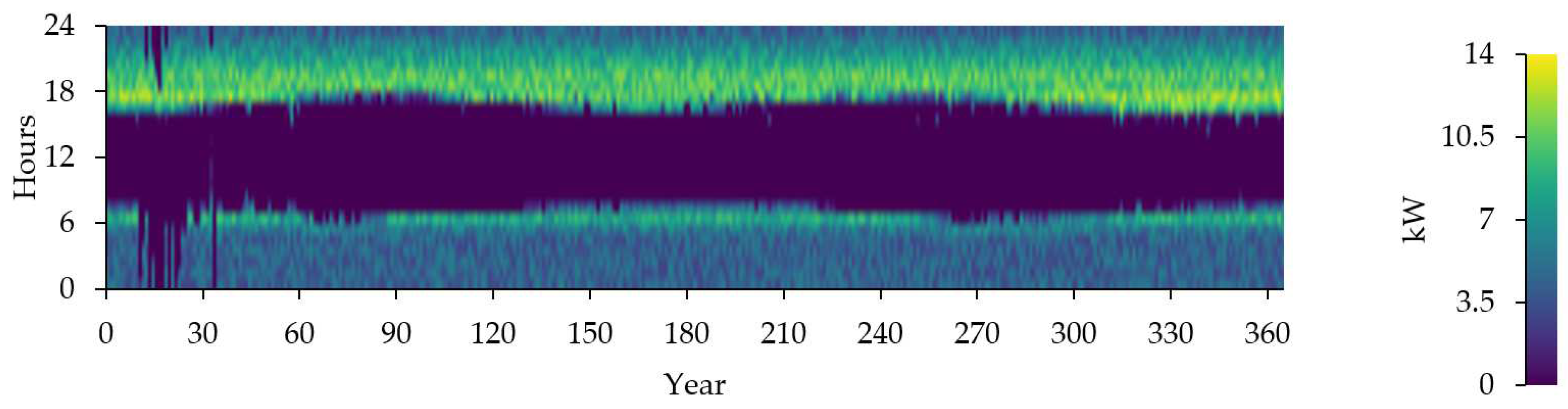
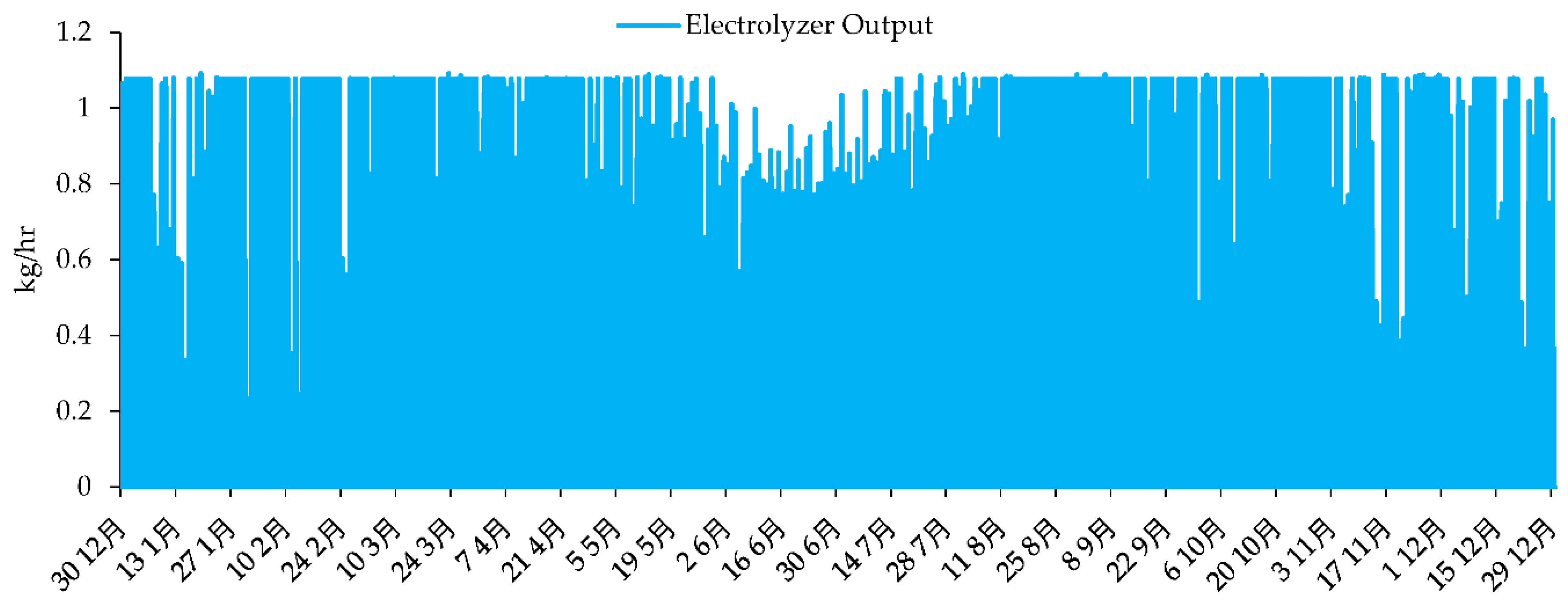
Figure 19 represent the time series of electrolyzer output.
8. Conclusion
This study underscores the critical role of solar energy technology in the sustainable production of hydrogen. Traditional methods relying on fossil fuels for hydrogen production are environmentally detrimental, making it imperative to explore greener alternatives. Green hydrogen, while initially more expensive, holds the promise of affordable and low-carbon technologies, driving innovation in the field. The development of a self-contained microgrid model, integrating solar energy and energy storage, provides a versatile platform for evaluating the intricacies of green hydrogen production. This multi-domain framework allows for a holistic exploration of the processes involved, paving the way for optimized off-grid hydrogen production using renewable sources. The microgrid system presents a robust solution to the energy requirements, requiring 460 kWh/day with a peak demand of 63 kW. The system integrates a 20.0 kW fuel cell generator, 120 kW PV system, 50.0 kW electrolyzer, and a 120 kg hydrogen tank, all managed by the HOMER Load
The analysis of solar hydrogen production methods, with an emphasis on electrochemical production using photovoltaic technology, reveals its high efficiency and potential. However, cost limitations may restrict its broader adoption, necessitating further advancements in technology to enhance affordability and reliability. With the global push for carbon reduction and the urgency to combat climate change, hydrogen emerges as a clean and dependable energy carrier capable of replacing fossil fuels. However, the environmental friendliness of hydrogen production greatly depends on the primary energy source employed, highlighting the significance of transitioning to low-carbon hydrogen production methods.
As we look ahead, the maturity of low-carbon hydrogen production technologies becomes paramount. Investing in research and development, expanding production infrastructure, and providing long-term policies to reduce uncertainties are crucial steps in realizing the potential of low-carbon hydrogen as a key player in decarbonizing our future energy systems. The transition to sustainable hydrogen production is not only desirable but also necessary to achieve a cleaner and more sustainable energy future.
Author Contributions
Conceptualization, M.G.O., G.L., A.C.L., C.P, and C.-V.S.; Methodology, G.L. and M.G.O.; Data curation, G.L., A.C.L., and M.G.O.; Writing—review and editing, G.L., M.G.O., C.P, and C.-V.S. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by a grant from the Ministry of Research, Innovation, and Digitization, CNCS-UEFISCDI, project number PN-III-P4-PCE-2021-0777, within PNCDI III, contract PCE 5/2022 and by a grant of the Ministry of Research, Innovation and Digitalization, project number PNRR-C9-I8-760111/23.05.2023, code CF 48/14.11.2022.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- M. Yu, K. Wang, and H. Vredenburg, “Insights into low-carbon hydrogen production methods: Green, blue and aqua hydrogen,” Int J Hydrogen Energy, vol. 46, no. 41, pp. 21261–21273, 2021. [CrossRef]
- S. Dorel, M. Gmal Osman, C.-V. Strejoiu, and G. Lazaroiu, “Exploring Optimal Charging Strategies for Off-Grid Solar Photovoltaic Systems: A Comparative Study on Battery Storage Techniques,” Batteries, vol. 9, no. 9, p. 470, 2023. [CrossRef]
- G. Lazaroiu, M. Gmal Osman, and C.-V. Strejoiu, “Performance Evaluation of Renewable Energy Systems: Photovoltaic, Wind Turbine, Battery Bank, and Hydrogen Storage,” Batteries, vol. 9, no. 9, p. 468, 2023. [CrossRef]
- A. Poullikkas, “A comparative overview of large-scale battery systems for electricity storage,” Renewable and Sustainable energy reviews, vol. 27, pp. 778–788, 2013. [CrossRef]
- E. Vartiainen, G. Masson, C. Breyer, D. Moser, and E. Román Medina, “Impact of weighted average cost of capital, capital expenditure, and other parameters on future utility-scale PV levelised cost of electricity,” Progress in photovoltaics: research and applications, vol. 28, no. 6, pp. 439–453, 2020. [CrossRef]
- B. Lee, C. S. Yoon, H. R. Lee, K. Y. Chung, B. W. Cho, and S. H. Oh, “Electrochemically induced reversible transition from the tunneled to layered polymorphs of manganese dioxide,” Sci Rep, vol. 4, no. 1, p. 6066, 2014. [CrossRef]
- D. Kundu et al., “Organic cathode for aqueous Zn-ion batteries: taming a unique phase evolution toward stable electrochemical cycling,” Chemistry of materials, vol. 30, no. 11, pp. 3874–3881, 2018. [CrossRef]
- S. Verhelst and T. Wallner, “Hydrogen-fueled internal combustion engines,” Prog Energy Combust Sci, vol. 35, no. 6, pp. 490–527, 2009. [CrossRef]
- F. Feng, M. Geng, and D. O. Northwood, “Electrochemical behaviour of intermetallic-based metal hydrides used in Ni/metal hydride (MH) batteries: A review,” Int J Hydrogen Energy, vol. 26, no. 7, pp. 725–734, 2001. [CrossRef]
- M. Guezgouz, J. Jurasz, B. Bekkouche, T. Ma, M. S. Javed, and A. Kies, “Optimal hybrid pumped hydro-battery storage scheme for off-grid renewable energy systems,” Energy Convers Manag, vol. 199, p. 112046, 2019. [CrossRef]
- T. Ohta, Energy carriers and conversion systems with emphasis on hydrogen-volume II, vol. 8. EOLSS Publications, 2009.
- M. G. Osman, D.-A. Ciupagenau, G. Lazaroiu, and I. Pisa, “Increasing Renewable Energy Participation in Sudan,” in 2022 11th International Conference on Renewable Energy Research and Application (ICRERA), 2022, pp. 169–173.
- I. Yüksel, “Hydropower for sustainable water and energy development,” Renewable and Sustainable Energy Reviews, vol. 14, no. 1, pp. 462–469, 2010. [CrossRef]
- T. da Silva Veras, T. S. Mozer, and A. da Silva César, “Hydrogen: trends, production and characterization of the main process worldwide,” Int J Hydrogen Energy, vol. 42, no. 4, pp. 2018–2033, 2017. [CrossRef]
- T. da Silva Veras, T. S. Mozer, and A. da Silva César, “Hydrogen: trends, production and characterization of the main process worldwide,” Int J Hydrogen Energy, vol. 42, no. 4, pp. 2018–2033, 2017. [CrossRef]
- M. G. Osman, D. Ciupageanu, and A. Stan, “Analysis of Solar Radiation in Sudan and Optimal Location of Photovoltaic Panels,” UPB Sci. Bull. Series C, vol. 84, pp. 387–401, 2022.
- T. da Silva Veras, T. S. Mozer, and A. da Silva César, “Hydrogen: trends, production and characterization of the main process worldwide,” Int J Hydrogen Energy, vol. 42, no. 4, pp. 2018–2033, 2017. [CrossRef]
- B. Bhandari, K.-T. Lee, C. S. Lee, C.-K. Song, R. K. Maskey, and S.-H. Ahn, “A novel off-grid hybrid power system comprised of solar photovoltaic, wind, and hydro energy sources,” Appl Energy, vol. 133, pp. 236–242, 2014. [CrossRef]
- A. Borgschulte, A. Züttel, and U. Wittstadt, “Hydrogen production,” Hydrogen as a future energy carrier, pp. 149–164, 2008.
- B. H. E. Outlook, “Hydrogen Economy Outlook: Key messages.” 2020.
- M. Yu, K. Wang, and H. Vredenburg, “Insights into low-carbon hydrogen production methods: Green, blue and aqua hydrogen,” Int J Hydrogen Energy, vol. 46, no. 41, pp. 21261–21273, 2021. [CrossRef]
- I. Dincer, “Green methods for hydrogen production,” Int J Hydrogen Energy, vol. 37, no. 2, pp. 1954–1971, 2012. [CrossRef]
- H. Balat and E. Kırtay, “Hydrogen from biomass–present scenario and future prospects,” Int J Hydrogen Energy, vol. 35, no. 14, pp. 7416–7426, 2010. [CrossRef]
- A. Kovač, M. Paranos, and D. Marciuš, “Hydrogen in energy transition: A review,” Int J Hydrogen Energy, vol. 46, no. 16, pp. 10016–10035, 2021. [CrossRef]
- D. Iribarren, M. Martín-Gamboa, J. Manzano, and J. Dufour, “Assessing the social acceptance of hydrogen for transportation in Spain: An unintentional focus on target population for a potential hydrogen economy,” Int J Hydrogen Energy, vol. 41, no. 10, pp. 5203–5208, 2016. [CrossRef]
- L. Schlapbach and A. Borgschulte, Hydrogen as a Future Energy Carrier. Wiley-VCH, 2008.
- R. W. Coughlin and M. Farooque, “Hydrogen production from coal, water and electrons,” Nature, vol. 279, no. 5711, pp. 301–303, 1979. [CrossRef]
- K. Nagasawa, F. T. Davidson, A. C. Lloyd, and M. E. Webber, “Impacts of renewable hydrogen production from wind energy in electricity markets on potential hydrogen demand for light-duty vehicles,” Appl Energy, vol. 235, pp. 1001–1016, 2019. [CrossRef]
- J. Ivy, “Summary of electrolytic hydrogen production: milestone completion report,” National Renewable Energy Lab., Golden, CO (US), 2004.
- H. K. Abdel-Aal, M. Sadik, M. Bassyouni, and M. Shalabi, “A new approach to utilize hydrogen as a safe fuel,” Int J Hydrogen Energy, vol. 30, no. 13–14, pp. 1511–1514, 2005. [CrossRef]
- S. A. Sherif, F. Barbir, and T. N. Veziroglu, “Wind energy and the hydrogen economy—review of the technology,” Solar energy, vol. 78, no. 5, pp. 647–660, 2005. [CrossRef]
- P. Fragiacomo and M. Genovese, “Modeling and energy demand analysis of a scalable green hydrogen production system,” Int J Hydrogen Energy, vol. 44, no. 57, pp. 30237–30255, 2019. [CrossRef]
- G. L. Juste, “Hydrogen injection as additional fuel in gas turbine combustor. Evaluation of effects,” Int J Hydrogen Energy, vol. 31, no. 14, pp. 2112–2121, 2006. [CrossRef]
- C.-L. Tseng et al., “Numerical analysis of the solar reactor design for a photoelectrochemical hydrogen production system,” Int J Hydrogen Energy, vol. 37, no. 17, pp. 13053–13059, 2012. [CrossRef]
- A. Midilli and I. Dincer, “Hydrogen as a renewable and sustainable solution in reducing global fossil fuel consumption,” Int J Hydrogen Energy, vol. 33, no. 16, pp. 4209–4222, 2008. [CrossRef]
- M. Balat, “Potential importance of hydrogen as a future solution to environmental and transportation problems,” Int J Hydrogen Energy, vol. 33, no. 15, pp. 4013–4029, 2008. [CrossRef]
- A. V. Abad and P. E. Dodds, “Green hydrogen characterisation initiatives: Definitions, standards, guarantees of origin, and challenges,” Energy Policy, vol. 138, p. 111300, 2020. [CrossRef]
- D. J. Jovan and G. Dolanc, “Can green hydrogen production be economically viable under current market conditions,” Energies (Basel), vol. 13, no. 24, p. 6599, 2020. [CrossRef]
- C. Zamfirescu and I. Dincer, “Ammonia as a green fuel and hydrogen source for vehicular applications,” Fuel processing technology, vol. 90, no. 5, pp. 729–737, 2009. [CrossRef]
- J. Manna, P. Jha, R. Sarkhel, C. Banerjee, A. K. Tripathi, and M. R. Nouni, “Opportunities for green hydrogen production in petroleum refining and ammonia synthesis industries in India,” Int J Hydrogen Energy, vol. 46, no. 77, pp. 38212–38231, 2021. [CrossRef]
- M. Yue, H. Lambert, E. Pahon, R. Roche, S. Jemei, and D. Hissel, “Hydrogen energy systems: A critical review of technologies, applications, trends and challenges,” Renewable and Sustainable Energy Reviews, vol. 146, p. 111180, 2021. [CrossRef]
- S. Kar, R. C. Bindal, S. Prabhakar, and P. K. Tewari, “The application of membrane reactor technology in hydrogen production using S–I thermochemical process: A roadmap,” Int J Hydrogen Energy, vol. 37, no. 4, pp. 3612–3620, 2012. [CrossRef]
- S. Z. Baykara, “Hydrogen production by direct solar thermal decomposition of water, possibilities for improvement of process efficiency,” Int J Hydrogen Energy, vol. 29, no. 14, pp. 1451–1458, 2004. [CrossRef]
- M. Momirlan and T. N. Veziroglu, “The properties of hydrogen as fuel tomorrow in sustainable energy system for a cleaner planet,” Int J Hydrogen Energy, vol. 30, no. 7, pp. 795–802, 2005. [CrossRef]
- E. Gupta et al., “Hydrogen as a renewable and sustainable solution in reducing global fossil fuel consumption,” Energy Policy, vol. 36, no. 16, pp. 4209–4222, 2008. [CrossRef]
- K. Meier, “Hydrogen production with sea water electrolysis using Norwegian offshore wind energy potentials: Techno-economic assessment for an offshore-based hydrogen production approach with state-of-the-art technology,” International Journal of Energy and Environmental Engineering, vol. 5, pp. 1–12, 2014. [CrossRef]
- P. G. Panah, X. Cui, M. Bornapour, R.-A. Hooshmand, and J. M. Guerrero, “Marketability analysis of green hydrogen production in Denmark: Scale-up effects on grid-connected electrolysis,” Int J Hydrogen Energy, vol. 47, no. 25, pp. 12443–12455, 2022. [CrossRef]
- A. Ozawa, Y. Kudoh, A. Murata, T. Honda, I. Saita, and H. Takagi, “Hydrogen in low-carbon energy systems in Japan by 2050: The uncertainties of technology development and implementation,” Int J Hydrogen Energy, vol. 43, no. 39, pp. 18083–18094, 2018. [CrossRef]
- R. S. El-Emam and H. Özcan, “Comprehensive review on the techno-economics of sustainable large-scale clean hydrogen production,” J Clean Prod, vol. 220, pp. 593–609, 2019. [CrossRef]
- R. Rath, P. Kumar, S. Mohanty, and S. K. Nayak, “Recent advances, unsolved deficiencies, and future perspectives of hydrogen fuel cells in transportation and portable sectors,” Int J Energy Res, no. May, pp. 8931–8955, 2019. [CrossRef]
- A. K. M. Tarigan and S. B. Bayer, “Temporal change analysis of public attitude, knowledge and acceptance of hydrogen vehicles in Greater Stavanger, 2006–2009,” Renewable and Sustainable Energy Reviews, vol. 16, no. 8, pp. 5535–5544, 2012. [CrossRef]
- C.-C. Chen, H.-H. Tseng, Y.-L. Lin, and W.-H. Chen, “Hydrogen production and carbon dioxide enrichment from ethanol steam reforming followed by water gas shift reaction,” J Clean Prod, vol. 162, pp. 1430–1441, 2017. [CrossRef]
- K. Im-orb, N. Visitdumrongkul, D. Saebea, Y. Patcharavorachot, and A. Arpornwichanop, “Flowsheet-based model and exergy analysis of solid oxide electrolysis cells for clean hydrogen production,” J Clean Prod, vol. 170, pp. 1–13, 2018. [CrossRef]
- M. Babayan, A. E. Mazraeh, M. Yari, N. A. Niazi, and S. C. Saha, “Hydrogen production with a photovoltaic thermal system enhanced by phase change materials, Shiraz, Iran case study,” J Clean Prod, vol. 215, pp. 1262–1278, 2019. [CrossRef]
- F. Ali, A. Bennui, S. Chowdhury, and K. Techato, “Suitable Site Selection for Solar-Based Green Hydrogen in Southern Thailand Using GIS-MCDM Approach,” Sustainability, vol. 14, no. 11, p. 6597, 2022. [CrossRef]
- E. Haghi, K. Raahemifar, and M. Fowler, “Investigating the effect of renewable energy incentives and hydrogen storage on advantages of stakeholders in a microgrid,” Energy Policy, vol. 113, pp. 206–222, 2018. [CrossRef]
- O. Nematollahi, P. Alamdari, M. Jahangiri, A. Sedaghat, and A. A. Alemrajabi, “A techno-economical assessment of solar/wind resources and hydrogen production: A case study with GIS maps,” Energy, vol. 175, pp. 914–930, 2019. [CrossRef]
- B. Liu, S. Liu, S. Guo, and S. Zhang, “Economic study of a large-scale renewable hydrogen application utilizing surplus renewable energy and natural gas pipeline transportation in China,” Int J Hydrogen Energy, vol. 45, no. 3, pp. 1385–1398, 2020. [CrossRef]
- Y. Zhao, Y. Zhang, W. Li, Y. Hao, and H. Jin, “Experimental investigation and thermodynamic analysis of effective hydrogen production driven by mid-and low-temperature solar heat,” J Clean Prod, vol. 176, pp. 758–769, 2018. [CrossRef]
- S. K. Ngoh, L. M. A. Ohandja, A. Kemajou, and L. Monkam, “Design and simulation of hybrid solar high-temperature hydrogen production system using both solar photovoltaic and thermal energy,” Sustainable Energy Technologies and Assessments, vol. 7, pp. 279–293, 2014. [CrossRef]
- K. Hashimoto et al., “The use of renewable energy in the form of methane via electrolytic hydrogen generation using carbon dioxide as the feedstock,” Appl Surf Sci, vol. 388, pp. 608–615, 2016. [CrossRef]
- O. M. Babatunde, J. L. Munda, and Y. Hamam, “Off-grid hybrid photovoltaic–micro wind turbine renewable energy system with hydrogen and battery storage: Effects of sun tracking technologies,” Energy Convers Manag, vol. 255, p. 115335, 2022. [CrossRef]
- S. Fukaume, Y. Nagasaki, and M. Tsuda, “Stable power supply of an independent power source for a remote island using a Hybrid Energy Storage System composed of electric and hydrogen energy storage systems,” Int J Hydrogen Energy, vol. 47, no. 29, pp. 13887–13899, 2022.
- R. Abejón et al., “Hydrogen recovery from waste gas streams to feed (High-temperature PEM) fuel cells: Environmental performance under a life cycle thinking approach,” Applied Sciences, vol. 10, no. 21, p. 7461, 2020. [CrossRef]
- B. Olateju and A. Kumar, “Techno-economic assessment of hydrogen production from underground coal gasification (UCG) in Western Canada with carbon capture and sequestration (CCS) for upgrading bitumen from oil sands,” Appl Energy, vol. 111, pp. 428–440, 2013. [CrossRef]
- I. Abadlia, T. Bahi, and H. Bouzeria, “Energy management strategy based on fuzzy logic for compound RES/ESS used in stand-alone application,” Int J Hydrogen Energy, vol. 41, no. 38, pp. 16705–16717, 2016. [CrossRef]
- M. S. Yazici, H. A. Yavasoglu, and M. Eroglu, “A mobile off-grid platform powered with photovoltaic/wind/battery/fuel cell hybrid power systems,” Int J Hydrogen Energy, vol. 38, no. 26, pp. 11639–11645, 2013. [CrossRef]
Figure 1.
Greening Factor (GF), Hydrogen Content Factor (HCF), and Environmental Impact Factor (EIF) of hydrogen and other fossil fuels [
12].
Figure 1.
Greening Factor (GF), Hydrogen Content Factor (HCF), and Environmental Impact Factor (EIF) of hydrogen and other fossil fuels [
12].
Figure 2.
Energy Density of some Combustibles (in MJ/kg) [
25] .
Figure 2.
Energy Density of some Combustibles (in MJ/kg) [
25] .
Figure 3.
Global demand for pure hydrogen, 1975-2018 [
8].
Figure 3.
Global demand for pure hydrogen, 1975-2018 [
8].
Figure 4.
Hydrogen Production Process [
28].
Figure 4.
Hydrogen Production Process [
28].
Figure 5.
Process flow diagram for biomass pyrolysis.
Figure 5.
Process flow diagram for biomass pyrolysis.
Figure 6.
Process flow diagram for biomass gasification.
Figure 6.
Process flow diagram for biomass gasification.
Figure 7.
Hydrogen production through methane catalytic steam.
Figure 7.
Hydrogen production through methane catalytic steam.
Figure 8.
The basics of the thermochemical separation process of water.
Figure 8.
The basics of the thermochemical separation process of water.
Figure 9.
system Production (MWh).
Figure 9.
system Production (MWh).
Figure 12.
Fuel Cell Output (kW).
Figure 12.
Fuel Cell Output (kW).
Figure 13.
PV Output (kW).
Figure 13.
PV Output (kW).
Figure 15.
Hydrogen Tank Level (kg).
Figure 15.
Hydrogen Tank Level (kg).
Figure 16.
Stored hydrogen Consumption (kg/Hours).
Figure 16.
Stored hydrogen Consumption (kg/Hours).
Figure 17.
Instantaneous Renewable Output Percentage of Total Generation.
Figure 17.
Instantaneous Renewable Output Percentage of Total Generation.
Figure 18.
100% Minus Instantaneous Nonrenewable Output as Percentage of Total Load.
Figure 18.
100% Minus Instantaneous Nonrenewable Output as Percentage of Total Load.
Figure 19.
Time series charts of electrolyzer output.
Figure 19.
Time series charts of electrolyzer output.
Table 1.
Methods for producing hydrogen according to primary energy and material sources [
63].
Table 1.
Methods for producing hydrogen according to primary energy and material sources [
63].
| Method |
Source |
Brief description |
| Primary energy |
Material |
| M1 Electrolysis |
Electrical |
Water |
Direct current divides water into O2 and H2 (electrochemical reaction) |
| M2 Plasma arc decomposition |
|
Fossil fuels |
The plasma arc is used to create H2 and carbon soot from cleaned natural gas. |
| M3 Thermolysis |
Thermal |
Water |
Thermal breakdown of water (steam) occurs above 2500 K. |
| M4 Thermochemical processes |
Water splitting |
Water |
Chemical reactions that occur in cycles (net reaction: water splitting into H2). |
| M5 Biomass conversion |
|
Biomass |
Thermostatic conversion. |
| M6 Gasification |
|
|
Synthesis of gas from biomass |
| M7 Reforming |
|
|
H2 production from liquid biomass (biofuels). |
| M8 PV electrolysis |
photonic |
Water |
Electricity is produced using PV panels. |
| M9 Photocatalysis |
|
|
The photocatalyst produces an electron-hole pair that is utilized to convert water into H2. |
| M10 Photoelectrochemical method |
|
|
When light is absorbed by a hybrid cell, current and voltage are produced simultaneously. |
| M11 Dark fermentation |
Biochemical |
Biomass |
Without light, biological mechanisms are used to produce H2. |
| M12 High-temperature electrolysis |
Electrical + Thermal |
Water |
Water splitting is fueled by the combination of thermal and electrical energy at high temperatures. |
| M13 Hybrid thermochemical cycles |
|
|
Cycles of chemical reactions are driven by the combination of electrical and thermal energy. |
| M14 Coal gasification |
|
|
Conversion of coal into syngas |
| M15 Fossil fuel reforming |
|
|
The conversion of fossil fuels into H2 and CO2 |
| M16 Bio photolysis |
Photonic + Biochemical |
Biomass + Water |
H2 is produced by biological processes (bacteria, microorganisms, etc.). |
| M17 Photo fermentation |
|
|
The light activates the fermentation process. |
| M18 Artificial photosynthesis |
|
|
To generate H2, chemically engineered systems mimic photosynthesis. |
| M19 Photo electrolysis |
Electrical +Photonic |
Water |
Water electrolysis is powered by photoelectrodes and external electricity. |
Table 2.
Comparison of hydrogen production processes.
Table 2.
Comparison of hydrogen production processes.
| Method |
Thermochemical |
Photoelectrochemical |
Electrochemical |
| Efficiency [%] |
40% - 50% depending on the thermochemical cycle and temperature |
12.7% -18.2% Slope of solar irradiance and forbidden energy band for electrode material |
73% -85% depending on the type of electrolysis process and LHV or HHV basis |
| Application |
applications for stationary large-scale generation of thermal electricity. |
Hydrogen refueling stations are advantageous because they require fewer procedures, do not require external power sources, and do not require additional hydrogen distribution systems. |
Mobile applications for transportation Mobile and fixed applications for both large- and small-scale hydrogen production using a distance |
| Operation pressure |
20 - 50 bar |
atmospheric |
atmospheric |
| Temperature [0C] |
>500oC |
374 oC |
70 oC |
| Energy Input |
Thermal |
Electrical |
Electrical |
| Basic components |
More than 3 thermals
reactors |
2 electrodes and electrolyte |
Electrodes, electrolytes, and sunlight |
| Methods of water splitting |
Thermal splitting |
Potential |
Potential |
Table 3.
Cost Summary.
| Component |
Name |
Size |
Unit |
| Generator |
Fuel Cell |
20.0 |
kW |
| PV |
PV |
120 |
kW |
| Electrolyzer |
Electrolyzer |
50.0 |
kW |
| Hydrogen tank |
Hydrogen Tank |
120 |
kg |
| Dispatch strategy |
HOMER Load Following |
|
|
Table 4.
Net Present Costs.
Table 4.
Net Present Costs.
| Name |
Capital |
Operating |
Replacement |
Salvage |
Resource |
Total |
| Electrolyzer |
$100,000 |
$0.00 |
$56,158 |
-$12,742 |
$0.00 |
$143,416 |
| Fuel Cell |
$60,000 |
$176,395 |
$106,523 |
-$11,482 |
$0.00 |
$331,436 |
| Hydrogen Tank |
$180,000 |
$0.00 |
$0.00 |
$0.00 |
$0.00 |
$180,000 |
| PV |
$360,000 |
$18,903 |
$166,797 |
-$103,210 |
$0.00 |
$442,490 |
| System |
$700,000 |
$195,298 |
$329,478 |
-$127,434 |
$0.00 |
$1.10M |
Table 5.
Annualized Costs.
Table 5.
Annualized Costs.
| Name |
Capital |
Operating |
Replacement |
Salvage |
Resource |
Total |
| Electrolyzer |
$6,348 |
$0.00 |
$3,565 |
-$808.89 |
$0.00 |
$9,104 |
| Fuel Cell |
$3,809 |
$11,198 |
$6,762 |
-$728.91 |
$0.00 |
$21,040 |
| Hydrogen Tank |
$11,427 |
$0.00 |
$0.00 |
$0.00 |
$0.00 |
$11,427 |
| PV |
$22,854 |
$1,200 |
$10,589 |
-$6,552 |
$0.00 |
$28,090 |
| System |
$44,438 |
$12,398 |
$20,916 |
-$8,090 |
$0.00 |
$69,662 |
Table 6.
Excess and Unmet.
Table 6.
Excess and Unmet.
| Quantity |
Value |
Units |
| Excess Electricity |
17,752 |
kWh/Year |
| Unmet Electric Load |
637 |
kWh/Year |
| Capacity Shortage |
739 |
kWh/Year |
Table 7.
Production Summary.
Table 7.
Production Summary.
| Component |
Production (kWh/Year) |
Percent |
| PV |
151,514 |
81.6 |
| Fuel Cell |
34,169 |
18.4 |
| Total |
185,683 |
100 |
Table 8.
Consumption Summary.
Table 8.
Consumption Summary.
| Component |
Consumption (kWh/Year) |
Percent |
| AC Primary Load |
0 |
0 |
| DC Primary Load |
72,363 |
43.1 |
| Deferrable Load |
0 |
0 |
| Total |
167,932 |
100 |
Table 9.
Fuel Cell Electrical Summary.
Table 9.
Fuel Cell Electrical Summary.
| Quantity |
Value |
Units |
| Electrical Production |
34,169 |
kWh/Year |
| Mean Electrical Output |
6.10 |
kW |
| Minimum Electrical Output |
0.00459 |
kW |
| Maximum Electrical Output |
13.0 |
kW |
| Fuel Consumption |
2,050 |
kg |
| Specific Fuel Consumption |
0.0600 |
kg/kWh |
| Fuel Energy Input |
68,338 |
kWh/Year |
| Mean Electrical Efficiency |
50.0 |
% |
| Hours of Operation |
5,599 |
Hourss/Year |
| Number of Starts |
369 |
starts/Year |
| Operational Life |
7.14 |
Year |
| Capacity Factor |
19.5 |
% |
| Fixed Generation Cost |
3.50 |
$/Hours |
| Marginal Generation Cost |
0 |
$/kWh |
Table 10.
PV Electrical Summary.
Table 10.
PV Electrical Summary.
| Quantity |
Value |
Units |
| Minimum Output |
0 |
kW |
| Maximum Output |
106 |
kW |
| PV Penetration |
208 |
% |
| Hours of Operation |
4,210 |
Hourss/Year |
| Levelized Cost |
0.185 |
$/kWh |
| Rated Capacity |
120 |
kW |
| Mean Output |
17.3 |
kW |
| Mean Output |
415 |
kWh/d |
| Capacity Factor |
14.4 |
% |
| Total Production |
151,514 |
kWh/Year |
Table 11.
Electrolyzer Summary.
Table 11.
Electrolyzer Summary.
| Quantity |
Value |
Units |
| Mean output |
0.235 |
kg/Hours |
| Minimum Output |
0 |
kg/Hours |
| Maximum Output |
1.08 |
kg/Hours |
| Total production |
2,059 |
kg/Year |
| Specific consumption |
46.4 |
kWh/kg |
| Rated capacity |
50.0 |
kW |
| Mean input |
10.9 |
kW |
| Minimum input |
0 |
kW |
| Maximum input |
50.0 |
kW |
| Total input energy |
95,568 |
kWh/Year |
| Capacity Factor |
21.8 |
% |
| Hours of operation |
3,106 |
Hours/Year |
Table 12.
Properties and Statistics of Hydrogen Tank.
Table 12.
Properties and Statistics of Hydrogen Tank.
| Quantity |
Value |
Units |
| Hydrogen storage capacity |
120 |
kg |
| Energy storage capacity |
4,000 |
kWh |
| Tank autonomy |
480 |
Hours |
| Content at beginning of year |
12.0 |
kg |
| Content at end of year |
21.3 |
kg |
Table 13.
Stored hydrogen Consumption Statistics.
Table 13.
Stored hydrogen Consumption Statistics.
| Quantity |
Value |
Units |
| Total fuel consumed |
2,050 |
kg |
| Avg fuel per day |
5.62 |
kg/day |
| Avg fuel per hour |
0.234 |
kg/hour |
Table 14.
Gas Emissions.
| Pollutant |
Quantity |
Unit |
| Carbon Dioxide |
-20.9 |
kg/Year |
| Carbon Monoxide |
13.3 |
kg/Year |
| Unburned Hydrocarbons |
1.48 |
kg/Year |
| Particulate Matter |
1.00 |
kg/Year |
| Sulfur Dioxide |
0 |
kg/Year |
| Nitrogen Oxides |
119 |
kg/Year |
Table 15.
Renewable Summary.
Table 15.
Renewable Summary.
| Capacity-based metrics |
Value |
Unit |
| Nominal renewable capacity divided by total nominal capacity |
85.7 |
% |
| Usable renewable capacity divided by total capacity |
84.4 |
% |
| Energy-based metrics |
Value |
Unit |
| Total renewable production divided by load |
90.2 |
% |
| Total renewable production divided by generation |
81.6 |
% |
| One minus total nonrenewable production divided by load |
79.7 |
% |
| Peak values |
Value |
Unit |
| Renewable output divided by load (HOMER standard) |
953 |
% |
| Renewable output divided by total generation |
100 |
% |
| One minus nonrenewable output divided by total load |
100 |
% |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).