Submitted:
03 January 2024
Posted:
04 January 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Main features of camelids
3. Sarcocystis infecting OWC and SAC
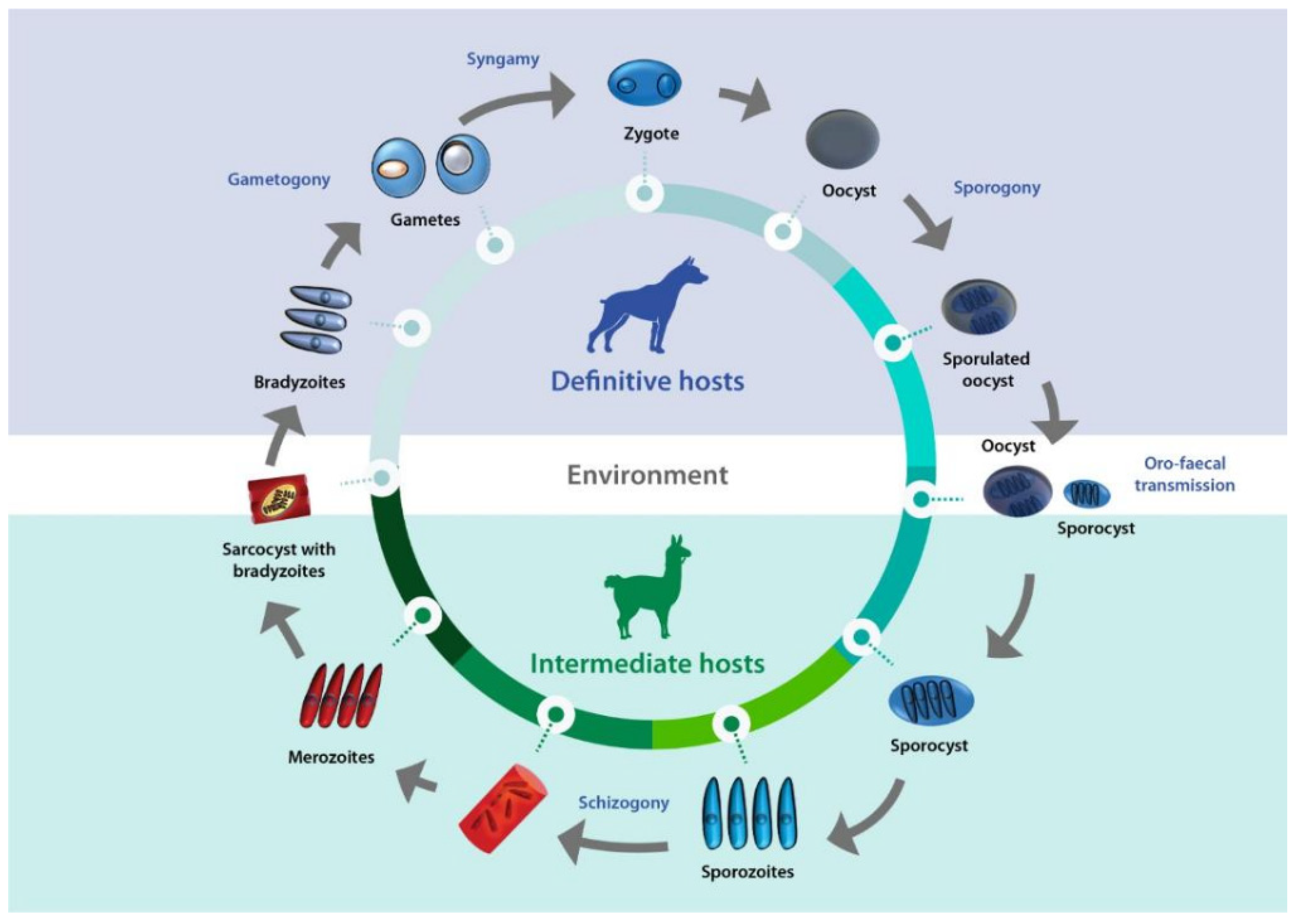
4. Definitive hosts
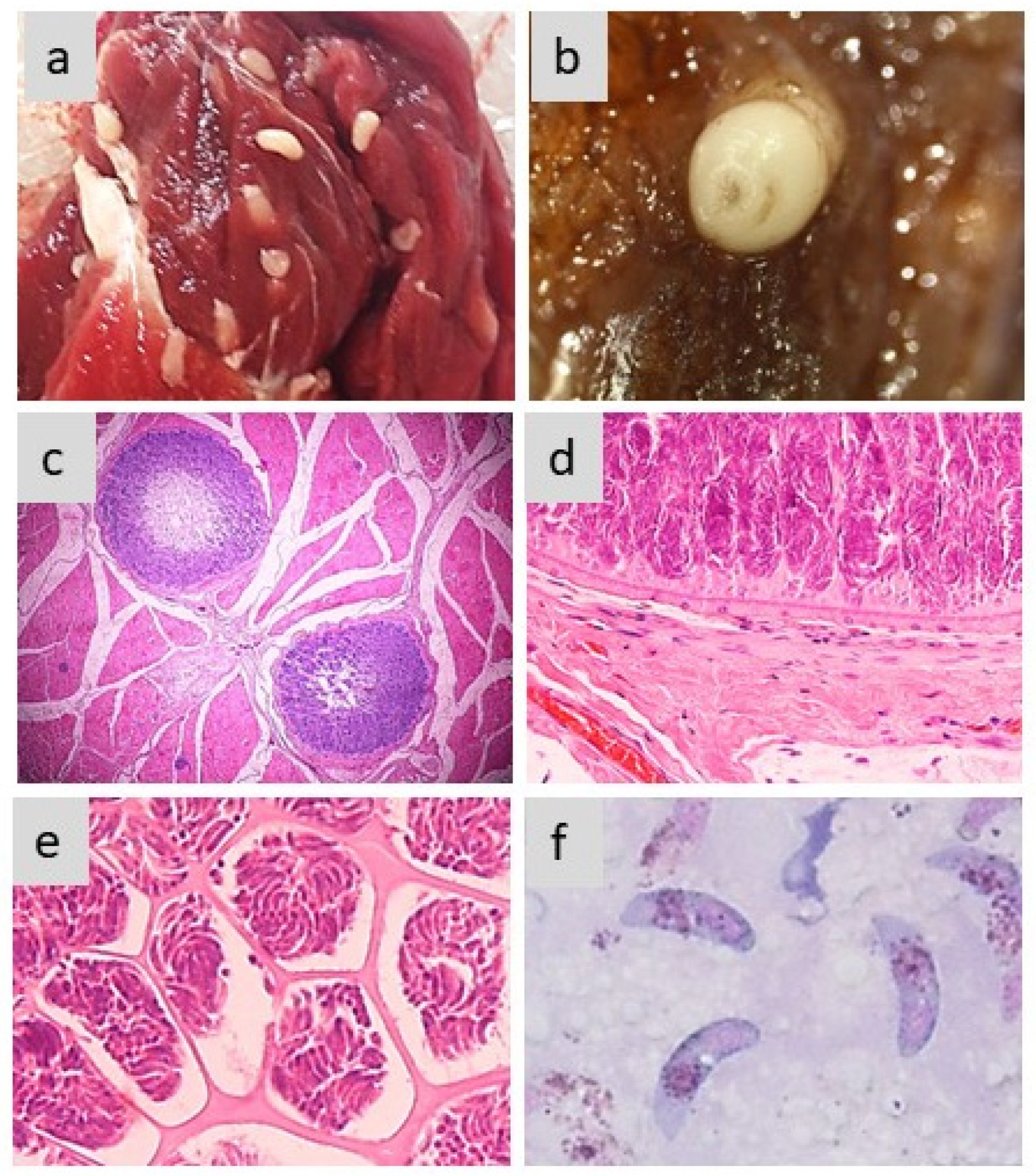
5. Pathogenesis of sarcocystosis in camelids
6. Diagnosis
7. Epidemiology and risk factors
8. Parasite biology and host-pathogen interaction
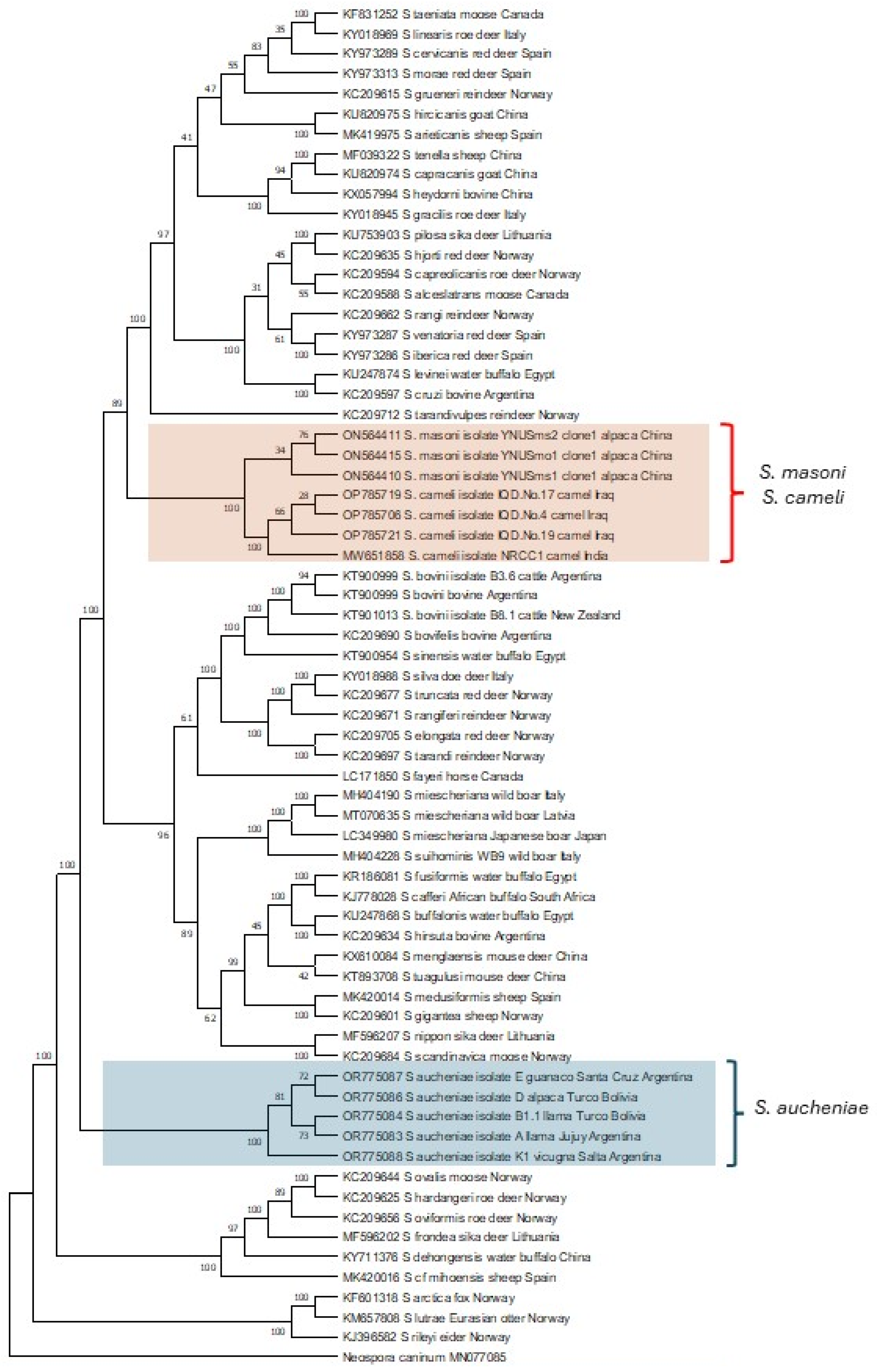
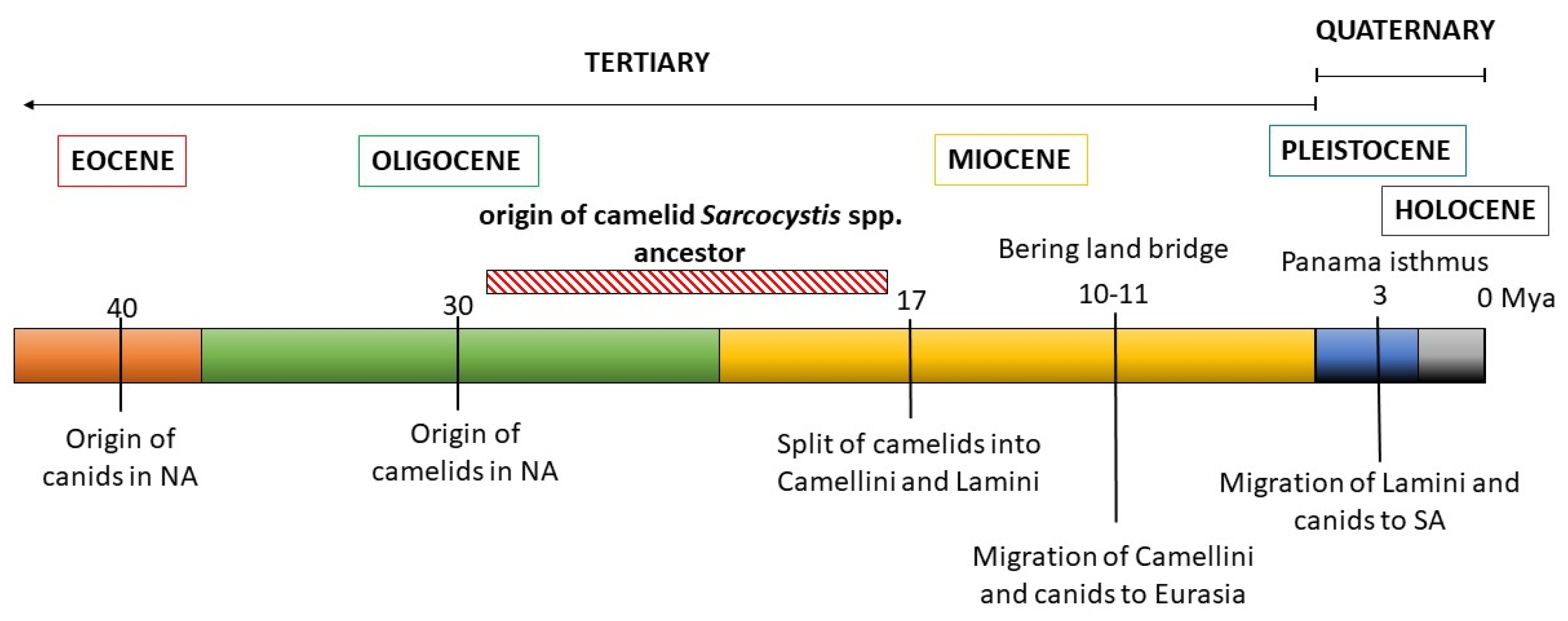
9. Phylogeny of camelids and camelid-infecting Sarcocystis spp.
10. Conclusions and perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Decker-Franco, C.; Schnittger, L.; Florin-Christensen, M., Sarcocystis, in: Parasitic Protozoa of Farm Animals and Pets. 2018a. Springer International Publishing, Cham, pp. 103–124. [CrossRef]
- Fayer, R. Sarcocystis spp. in human infections. Clin Microbiol Rev 2004, 17, 894-902. [CrossRef]
- Dubey, J.P.; Calero-Bernal, R.; Rosenthal, B.M.; Speer, C.A.; Fayer, R. Sarcocystosis of animals and humans, second edition, 2015. [CrossRef]
- Poulsen, C.S.; Stensvold, C.R. Current status of epidemiology and diagnosis of human sarcocystosis. J Clin Microbiol. 2014, 52, 3524-30. [CrossRef]
- Saeed, M.A.; Rashid, M.H.; Vaughan, J.; Jabbar, A. Sarcocystosis in South American camelids: The state of play revisited. Parasit Vect 2018, 11, 146. [CrossRef]
- Saeed, M.A.; Vaughan, J.L.; Jabbar, A. An update on sarcocystosis in one-humped camels (Camelus dromedarius). Parasitol 2018, 145, 1367–1377. [CrossRef]
- Giuliano, S. M.; Reategui Ordoñez, J.; Barriga Marcapuda X.; Florin-Christensen M. Situación actual de la calidad de carne de camélidos sudamericanos (llama y alpaca) en Argentina y Perú y su relación con la infestación con Sarcocystis aucheniae. Proceedings of the XXIII Reunión Nacional de la Asociación Boliviana de Producción Animal – ABOPA 2023. https://www.cifumss.agro.bo/abopa/index.html.
- Fowler, M.E. Camelids are not ruminants. Zoo Wild Animal Med 2008, 375–85. [CrossRef]
- FAOSTAT. https://www.fao.org/faostat/en/#data/QCL.
- Midagri. https://www.midagri.gob.pe/portal/datero/40-sector-agrario/situacion-de-las-actividades-de-crianza-y-producci/298-camelidos-sudamericanos?start=1.
- Zarrin, M.; Riveros, J.L.; Ahmadpour, A.; De Almeida, A.M.; Konuspayeva, G.; Vargas-Bello-Pérez, E.; Faye, B.; Hernández-Castellano, L.E.. Camelids: new players in the international animal production context, Trop Anim Health Prod 2020, 52, 902-913. [CrossRef]
- Fowler, M.E. Husbandry and diseases of camelids. Rev Sci Tech 1996, 15, 155-69. [CrossRef]
- Yacobaccio, H.D. The domestication of South American camelids: a review. Anim Front 2021, 11, 43-51. [CrossRef]
- Mamani-Linares, L.W.; Gallo, C.B. Meat quality attributes of the Longissimus lumborum muscle of the Kh'ara genotype of llama (Lama glama) reared extensively in northern Chile. Meat Sci 2013, 94, 89–94.
- Mamani-Linares, L.W.; Gallo, C.B. Meat quality, proximate composition and muscle fatty acid profile of young llamas (Lama glama) supplemented with hay or concentrate during the dry season. Meat Sci 2014, 96, 394–399. [CrossRef]
- Kadim, I.T.; Al-Amri, I. S.; Alkindi, A.Y.; Quazi Haq, M. I. Nutritional values and health benefits of dromedary camel meat. Anim Front 2022, 12, 61–70. [CrossRef]
- Dubey, J.P.; Hilali, M.; Van Wilpe, E.; Calero-Bernal, R.; Verma, S.K.; Abbas, I.E. A review of sarcocystosis in camels and redescription of Sarcocystis cameli and Sarcocystis ippeni sarcocysts from the one-humped camel (Camelus dromedarius). Parasitology 2015, 142, 1481–1492. [CrossRef]
- Dubey, J.P.; A’aji, N.N.; Mowery, J.D.; Verma, S.K.; Calero-Bernal, R. Identification of Macroscopic Sarcocysts of Sarcocystis cameli from One-Humped Camel (Camelus dromedarius) in Iraq. J Parasitol 2017, 103, 168–169. [CrossRef]
- Brumpt, E. (1913). Precis de Parasitologie, 2nd Edn. Masson et cie, Paris, France.
- Quiroga, D.; Lombardero, O.; Zorrilla, R.,. Sarcocystis tilopodi N sp. en guanacos (Lama guanicoe) de la Repdblica Argentina. Gac Vet 1969, 31, 67--70.
- Gorman, T. R.; Alcaino, H. A.; Munoz, H.; Cunazza, C. Sarcocystis sp. in guanaco (Lama guanicoe) and effect of temperature on its viability. Vet Parasitol 1984, 15, 95–101.
- Leguía, G.; Casas, E. Enfermedades parasitarias y atlas parasitológico de camélidos sudamericanos 1999, Primera edn. De Mar, Lima, Perú.
- Leguía, G. The epidemiology and economic impact of llama parasites. Parasitol Today 1991, 7, 54–56. [CrossRef]
- Carletti, T.; Martin, M.; Romero, S.; Morrison, D.A.; Marcoppido, G.; Florin-Christensen, M.; Schnittger, L. Molecular identification of Sarcocystis aucheniae as the macrocyst-forming parasite of llamas. Vet Parasitol 2013, 198, 396–400. [CrossRef]
- Moré, G.; Regensburger, C.; Gos, M.L.; Pardini, L.; Verma, S.K.; Ctibor, J.; Serrano-Martínez, M.E.; Dubey, J.P.; Venturini, M.C. Sarcocystis masoni, n. sp. (Apicomplexa: Sarcocystidae), and redescription of Sarcocystis aucheniae from llama (Lama glama), guanaco (Lama guanicoe) and alpaca (Vicugna pacos). Parasitology 2016, 143, 617–626. [CrossRef]
- Metwally, D.M.; Al-Otaibi, T.T.; Al-Turaiki, I.M.; El-Khadragy, M.F.; Alajmi, R.A. Identification of Sarcocystis spp. in one-humped camels (Camelus dromedarius) from Riyadh and Dammam, Saudi Arabia, via histological and phylogenetic approaches. Animals 2020, 10, 1108. [CrossRef]
- Motamedi, G.R.; Dalimi, A.; Nouri, A.; Aghaeipour, K. Ultrastructural and molecular characterization of Sarcocystis isolated from camel (Camelus dromedarius) in Iran. Parasitol Res 2011, 108, 949-954. [CrossRef]
- Regensburger, C.; Gos, M.L.; Ctibor, J.; Moré, G. Morphological and molecular characteristics of Sarcocystis aucheniae isiolated from meat of guanaco (Lama guanicoe). J Food Qual Haz Cont 2015, 2, 118-121.
- Schnieder, T.; Kaup, F.J.; Drommer, W.; Thiel, W.; Rommel, M. [Fine structure and development of Sarcocystis aucheniae in llamas]. Z Parasitenkd 1984, 70, 451–458. [CrossRef]
- Cornejo, R.B.; Chávez, A.V.; Leyva, V.V.; Falcón, N.P.; Panez S.L.; Ticona D.S. Relationship between the size of macrocysts of Sarcocysistis aucheniae and its viability in Canis familiaris. Rev Inv Vet Perú 2007, 18, 76-83.
- Zacarías, F.S.; Sam, R. T.; Ramos, D.D.; Lucas. O.A.; Lucas, J.L. Techniques for the isolation and purification of Sarcocystis aucheniae oocysts from small intestine of experimentally infected dogs. Rev Inv Vet Perú 2013., 24, 396-403.
- Wu, Z.; Sun, J.; Hu, J.; Song, J.; Deng, S;, Zhu, N.; Yang, Y.; Tao, J. Morphological and molecular characterization, and demonstration of a definitive host, for Sarcocystis masoni from an alpaca (Vicugna pacos) in China. Biology 2022, 11, 1016. [CrossRef]
- Hilali, M.; El Sayed Imam, B.; Hassan, A. The Endogenous Stages of Sarcocystis cameli (Mason, 1910) Vet Parasitol 1982, 11, 127—129.
- Hilali, M.; Fatani, A.; Al-Atiya, S. Isolation of tissue cysts of Toxoplasma, Isospora, Hammondia and Sarcocystis from camel (Camelus dromedarius) meat in Saudi Arabia. Vet Parasitol 1995, 58, 353–356.
- Hilali, M.; Mohamed A. The dog (Canis familiaris) as the final host of Sarcocystis cameli (Mason, 1910). Tropenmed Parasitol 1982, 31, 213-214.
- Hilali, M.; Nassar, A.M.; EI-Ghaysh A.,. Camel (Camelus dromedarius) and sheep (Ovis aries) meat as a source of dog infection with some coccidian parasites. Vet Parasitol 1992, 43, 37-43.
- Abdel-Ghaffar, F.; Mehlhorn, H.; Bashtar, A.R.; Al-Rasheid, K.; Sakran, T.; El-Fayoumi, H. Life cycle of Sarcocystis camelicanis infecting the camel (Camelus dromedarius) and the dog (Canis familiaris), light and electron microscopic study. Parasitol Res 2009, 106, 189–195. [CrossRef]
- Ishag, M.Y.; Majid, A.; Magzoub, A. Isolation of a new Sarcocystis species from Sudanese camels (Camelus dromedarius). Int J Trop Med 2006, 1, 167–169.
- Godoy, Z.; Vilca, L.; Gonzáles, Z.; Leyva, V.; Sam, T. Saneamiento y detoxificación de carne de llama (Lama glama) infectada con Sarcocystis aucheniae mediante cocción, horneado, fritura y congelado. Rev Invest Vet Perú 2007, 18, 51–56.
- Vilca, M.; Durán, J.; Ramos, D.; Lucas, J. Saneamiento y eliminación de la toxicidad de carne de alpaca (Vicugna pacos) con sarcocistiosis mediante ahu mado y curado. Rev Invest Vet Perú 2013, 24, 537–54.
- Saito, M.; Taguchi, K.; Shibata, Y.; Kobayashi, T.; Shimura, K.; Itagaki, H. Toxicity and properties of the extract from Sarcocystis cruzi cysts. J Vet Med Sci 1995, 57, 1049-51. [CrossRef]
- Irikura, D.; Saito, M.; Sugita-Konishi, Y.; Ohnishi, T.; Sugiyama, K.I. et al. Characterization of Sarcocystis fayeri's actin-depolymerizing factor as a toxin that causes diarrhea. Genes Cells 2017, 22, 25-835. [CrossRef]
- Kamata Y, Saito M, Irikura D, Yahata Y, Ohnishi T, Bessho T, Inui T, Watanabe M, Sugita-Konishi Y. A toxin isolated from Sarcocystis fayeri in raw horsemeat may be responsible for food poisoning. J Food Prot 2014, 77:814-9. [CrossRef]
- Honda, M.; Sawaya; M.; Taira, K.; Yamazaki, A.; Kamata, Y.; Shimizu, H.; Kobayashi, N.; Sakata, R.; Asakura, H.; Sugita-Konishi, Y. Effects of temperature, pH and curing on the viability of Sarcocystis, a Japanese sika deer (Cervus Nippon centralis) parasite, and the inactivation of their diarrheal toxin. J Vet Med Sci 2018, 80, 1337-1344. [CrossRef]
- La Perle, K.M.D.D.; Silveria, F.; Anderson, D.E.; Blomme, E.A.G.G.. Dalmeny disease in an alpaca (Lama pacos): Sarcocystosis, eosinophilic myositis and abortion. J Comp Pathol 1999, 121, 287–293. [CrossRef]
- Gabor, M.; Gabor, L.J.; Srivastava, M.; Booth, M.; Reece, R. Chronic myositis in an australian alpaca (Lama pacos) associated with sarcocystis spp. J Vet Diagn Invest 2010, 22, 966-9 . [CrossRef]
- Fatani, A.; Elsebaie, A.; Hilali, M. Clinical and haematobiochemical changes in camels (Camelus dromedarius) experimentally inoculated with Sarcocystis cameli. J Camel Pract Res 1996, 3, 11–15.
- Manal, Y.I.; El Amin, E.; Osman, A (2001) Camels experimentally infected with Sarcocystis. Sudan J Vet Res 2014, 17, 27–33.
- Valinezhad, A.; Oryan, A.; Ahmadi, N. Sarcocystis and its complications in camels (Camelus dromedarius) of Eastern Provinces of Iran. Korean J Parasitol 2008, 46, 229–234. [CrossRef]
- Metwally, D.M.; Al-Otaibi, T.T.; Semlali, A.; Alajmi, R.A. Sarcocystis camelicanis increases interleukin (IL)-6 expression in one-humped camels (Camelus dromedarius) from Riyadh and Al Qassim, Saudi Arabia. Biosci Rep 2021 41, BSR20203140. [CrossRef]
- Rooney, A.L.; Limon, G.; Vides, H.; Cortez, A.; Guitian, J. Sarcocystis spp. in llamas (Lama glama) in Southern Bolivia: A cross sectional study of the prevalence, risk factors and loss in income caused by carcass downgrades. Prev Vet Med 2014, 116, 296-304. [CrossRef]
- Gareh, A.; Soliman, M.; Saleh, A.A.; El-Gohary, F.A.; El-Sherbiny, H.M.M.; Mohamed, R.H.; Elmahallawy, E.K.; Kotb Elmahallawy, E. Epidemiological and histopathological investigation of Sarcocystis spp. in slaughtered dromedary camels (Camelus dromedarius) in Egypt. Vet Sci 2020, 7, 162. [CrossRef]
- Martin, M.; Decker Franco, C.; Romero, S.; Carletti, T.; Schnittger, L.; Florin-Christensen, M. Molecular detection of Sarcocystis aucheniae in the blood of llamas from Argentina. Rev Argent Microbiol 2016, 48, 200–205. [CrossRef]
- Decker Franco, C.; Romero, S.; Ferrari, A.; Schnittger, L.; Florin-Christensen, M. Detection of Sarcocystis aucheniae in blood of llama using a duplex semi-nested PCR assay and its association with cyst infestation. Heliyon. 2018, 4(11), e00928. [CrossRef]
- Moré, G.; Pardini, L.; Basso, W.; Marín, R.; Bacigalupe, D.; Auad, G.; Venturini, L.; Venturini, M.C. Seroprevalence of Neospora caninum, Toxoplasma gondii and Sarcocystis sp. in llamas (Lama glama) from Jujuy, Argentina. Vet Parasitol 2008, 155, 158-60. [CrossRef]
- Romero, S.; Carletti, T.; Decker Franco, C.; Moré, G.; Schnittger, L.; Florin-Christensen, M. Seropositivity to Sarcocystis infection of llamas correlates with breeding practices. Vet Parasitol Reg Stud Reports 2017, 10, 65-70. [CrossRef]
- Decker Franco, C.; Wieser, S.N.; Soria, M.; de Alba, P.; Florin-Christensen, M.; Schnittger, L. In silico identification of immunotherapeutic and diagnostic targets in the glycosylphosphatidylinositol metabolism of the coccidian Sarcocystis aucheniae. Transbound Emerg Dis. 2020, 67 Suppl 2, 165-174. [CrossRef]
- Wieser, S.N.; Decker-Franco, C.; de Alba, P.; Romero, S.; Ferrari, A.; Schnittger, L.; Florin-Christensen, M. Discovery of antigens and cellular mechanisms in the protozoan parasite Sarcocystis aucheniae using immunoproteomics. Parasitologia 2023, 3, 349-363. [CrossRef]
- Holmdahl, O.J.M.; Morrison, D.A.; Ellis, J.T.; Huong, L.T.T. Evolution of ruminant Sarcocystis (Sporozoa) parasites based on small subunit rDNA sequences. Mol Phylogenet Evol 1999, 11, 27-37. [CrossRef]
- Wieser, S.N.; Cafrune, M.W.; Romero, S.R.; Schnittger, L.; Florin-Christensen, M. Molecular identification of Sarcocystis aucheniae infesting Vicugna vicugna,.2023, submitted.
- Beldomenico, P.M.; Uhart, M.M.; Beldomenico, P.M.; Uhart, M.; Bono, M.F.; Marull, C.; Baldi, R.; Peralta, J.L. Internal Parasites of free-ranging guanacos from Patagonia. Vet Parasitol 2003, 118, 71–77. [CrossRef]
- Lucas, J.R.; Barrios-Arpi, M.; Rodríguez, J.; Balcázarnakamatsu, S.; Zarria, J.; Namiyama, G.; Taniwaki, N.; Gonzales-Viera, O. Ultrastructural description of Sarcocystis sp. in cardiac muscle of naturally infected alpacas (Vicugna pacos). Iran J Parasitol 2019, 14, 174–179.
- Jiang, N.; Xin, S.; Zhu, N.; Yang, L.; Huang, W.; Hu, J.; Zhu, X.; Yang, Y. First report of Sarcocystis Masoni in a captive alpaca (Vicugna pacos) from China. Front Vet Sci 2021, 8, 759252. [CrossRef]
- Fernandez-F, F.; Gutiérrez-A, R.; Pacheco-S, V.; Chirinos-T, J.; Lombardo, D.M.; Olivera, L.V.M.; Bernabe-Ortiz, J.C.; López-Casaperalta, P. Determination of Sarcocystis lamacanis microcysts in the cardiac muscle of alpacas (Vicugna pacos) and their correlation with troponin CTnI. A Study performed in the High Andean region of Southern Peru. Vet Anim Sci 2022, 18, 100270. [CrossRef]
- Rodríguez, A.; Quispe-Solano, M.; Rodríguez, J.L.; Lucas, J.R. The occurrence of Sarcocystis spp. in the myocardium of alpacas (Vicugna pacos) with associated risk factors in the Peruvian Andes. Trop Anim Health Prod 2023, 55, 66. [CrossRef]
- Latif, B.M.A.; Al-Delemi, J.K.; Mohammed, B.S.; Al-Bayati, S.M.; Al-Amiry, A.M. Prevalence of Sarcocystis spp. in meat-producing animals in Iraq. Vet Parasitol 1999, 84, 85–90. [CrossRef]
- Woldemeskel, M.; Gumi, B. Prevalence of Sarcocysts in one-humped camel (Camelus dromedarius) from Southern Ethiopia. J Vet Med 2001, 48, 223–226. [CrossRef]
- Fukuyo, M.; Battsetseg, G.; Byambaa, B. Prevalence of Sarcocystis Infection in Meat-Producing Animals in Mongolia. Southeast Asian J Trop Med Pub Health 2002, 33, 490–495.
- Shekarforoush, S.S.; Shakerian, A.; Hasanpoor, M. M. Prevalence of Sarcocystis in slaughtered one-humped camels (Camelus dromedarius) in Iran. Trop Anim Health Prod 2006, 38, 301–303. [CrossRef]
- Hamidinejat, H.; Hekmatimoghaddam, S.; Jafari, H.; Sazmand, A.; Haddad Molayan, P.; Derakhshan, L.; Mirabdollahi, S. Prevalence and distribution patterns of Sarcocystis in camels (Camelus dromedarius) in Yazd Province, Iran. J Parasit Dis 2013, 37, 163–165. [CrossRef]
- Omer, S.A.; Alzuraiq, A.A.; Mohammed, O.B. Prevalence and molecular detection of Sarcocystis spp. infection in the dromedary camel (Camelus dromedarius) in Riyadh City, Saudi Arabia. Biomed Res 2017, 28, 4962–4965.
- Al-Ani, F.K.; Amr, Z. Sarcocystis Spp Prevalence in Camel Meat in Jordan . Dairy and Vet Sci J. 2017, 4, 555643. [CrossRef]
- Castro, E.; Sam, R.; López, T.; González, A.; Silva, M. Evaluaciòn de la edad como factor de riesto de seropositividad a Sarcocystis sp. en alpacas. Rev Inv Vet Perú 2004, 15, 83–86.
- Silva Rochefort, B.; Root-Bernstein, M. History of canids in Chile and impacts on prey adaptations. Ecol Evol 2021, 11, 9892-9903. [CrossRef]
- Blazejewski, T.; Nursimulu, N.; Pszenny, V.; Dangoudoubiyam, S.; Namasivayam, S.; Chiasson, M.A.; Chessman, K.; Tonkin, M.; Swapna, L.S.; Hung, S.S.; et al. Systems-Based Analysis of the Sarcocystis neurona neurona genome identifies pathways that contribute to a heteroxenous life cycle. mBio 2015, 6. [CrossRef]
- Rodríguez, A.E.; Couto, A.; Echaide, I.; Schnittger, L.; Florin-Christensen, M. Babesia bovis contains an abundant parasite-specific protein-free glycerophosphatidylinositol and the genes predicted for its assembly. Vet Parasitol 2010, 167, 227-35. [CrossRef]
- Delorenzi, M.; Sexton, A.; Shams-Eldin, H.; Schwarz, R. T.; Speed, T.; Schofield, L. Genes for glycosylphosphatidylinositol toxin biosynthesis in Plasmodium falciparum. Infect Immun 2002, 70, 4510–4522. [CrossRef]
- Rodriguez, A.E.; Florin-Christensen, M.; Flores, D.A.; Echaide, I.; Suarez, C.E.; Schnittger, L. The glycosylphosphatidylinositol-anchored protein repertoire of Babesia bovis and its significance for erythrocyte invasion. Ticks Tick Borne Dis 2014, 5, 343-8. [CrossRef]
- Deroost, K.; Pham, T. T.; Opdenakker, G.; Van den Steen, P. E. The immunological balance between host and parasite in malaria. FEMS Microbiol Rev 2016, 40, 208–257. [CrossRef]
- Martins, V.P.; Pinheiro; C.S.; Figueiredo, B.C.; Assis, N.R.; Morais, S.B.; Caliari, M.V.; Azevedo, V.; Castro-Borges, W.; Wilson, R.A.; Oliveira, S.C. Vaccination with enzymatically cleaved GPI-anchored proteins from Schistosoma mansoni induces protection against challenge infection. Clin Dev Immunol. 2012, 962538. [CrossRef]
- Hegazy-Hassan, W.; Zepeda-Escobar, J. A.; Ochoa-García, L.; et al. TcVac1 vaccine delivery by intradermal elec troporation enhances vaccine induced immune protection against Trypanosoma cruzi infection in mice. Vaccine 2018, 37, 248–257. [CrossRef]
- Moubri, K.; Kleuskens, J.; Van de Crommert, J.; et al. Discovery of a recombinant Babesia canis supernatant antigen that protects dogs against virulent challenge infection. Vet Parasitol 2018, 249, 21–29. [CrossRef]
- Florin-Christensen, M.; Sojka, D.; Ganzinelli, S.; Šnebergerová, P.; Suarez, C.E.; Schnittger, L. Degrade to survive: the intricate world of piroplasmid proteases. Trends Parasitol 2023, 39, 532-546. [CrossRef]
- Kimura, M. A simple method for estimating evolutionary rate of base substitutions through comparative studies of nucleotide sequences. J Mol Evol 1980, 16:111-120.
- Tamura, K.; Stecher, G.; Kumar, S. MEGA 11: Molecular Evolutionary Genetics Analysis Version 11. Mol Biol Evol 2021, 38, 3022-3027. [CrossRef]
- Wu, H.; Guang, X.; Al-Fageeh, M. B.; Cao, J.; Pan, S. Camelid genomes reveal evolution and adaptation to desert environments. Nat Commun 2014, 21, 5188. [CrossRef]
- Diaz-Maroto, P.; Rey-Iglesia, A.; Cartajena, I.; Núñez L.; et al. Ancient DNA reveals the lost domestication history of South American camelids in Northern Chile and across the Andes. Elife 2021 10, e63390. [CrossRef]
- Wang, X.; Tedford, R.H.; Van Valkenburgh, B.; Wayne, R.K. Evolutionary history, molecular systematics and evolutionary ecology of Canidae. In: The Biology of Conservation of World Canids (D.W. Macdonald and C. Sillero-Zubrin, eds.) 2004, Chapter 2, pp. 39-54. Oxford University Press, Oxford.
- Chavez, D.E.; Gronau, I.; Hains, T.; Dikow, R.B. et al. Comparative genomics uncovers the evolutionary history, demography, and molecular adaptations of South American canids. Proc Natl Acad Sci U S A. 2022, 119, e2205986119. [CrossRef]
- van Asch, B.; Zhang, A.B.; Oskarsson, M.C.; Klütsch, C.F.; Amorim, A.; Savolainen, P. Pre-Columbian origins of Native American dog breeds, with only limited replacement by European dogs, confirmed by mtDNA analysis. Proc Biol Sci 2013, 280, 20131142. [CrossRef]
| Intermediate host | Species | Sarcocyst | Ref. | ||||
|
Shape, size (length x width) |
Cyst wall | Location | |||||
| Type | Thickness (µm) |
Villar protrusions (vp) size and aspect | |||||
| OWC | S. cameli |
Fusiform, microscopic (700 x 100 µm) and macroscopic (1.5-5.0 x 0.2-0.4 mm) |
9j | < 2 | 3.0 x 0.5 µm finger-like vp |
cardiac and skeletal muscle | 3,17,18,26, 27 |
| S. ippeni | Globular, microscopic (100-120 x 50-100 µm) |
32 | 2.3-3.0 | 1.0 x 0.25 -1.2 µm thorn-like vp |
skeletal muscle | 3,17 | |
| SAC | S. aucheniae | Oval, macroscopic (0.5 -2.0 x 0.2 cm) |
21 | 6-10 |
3-4.5 x 2.5-3.5 µm branched vp, cauliflower-like wall |
skeletal muscle | 1,24,25, 28,29 |
| S. masoni | Fuisform, microscopic (800 x 95 µm) |
9j | 2.5-3.5 | 2-2.8 x 0.5-0.7 µm conical to cylindrical vp |
cardiac and skeletal muscle | 25 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





