Submitted:
03 January 2024
Posted:
05 January 2024
You are already at the latest version
Abstract
Keywords:
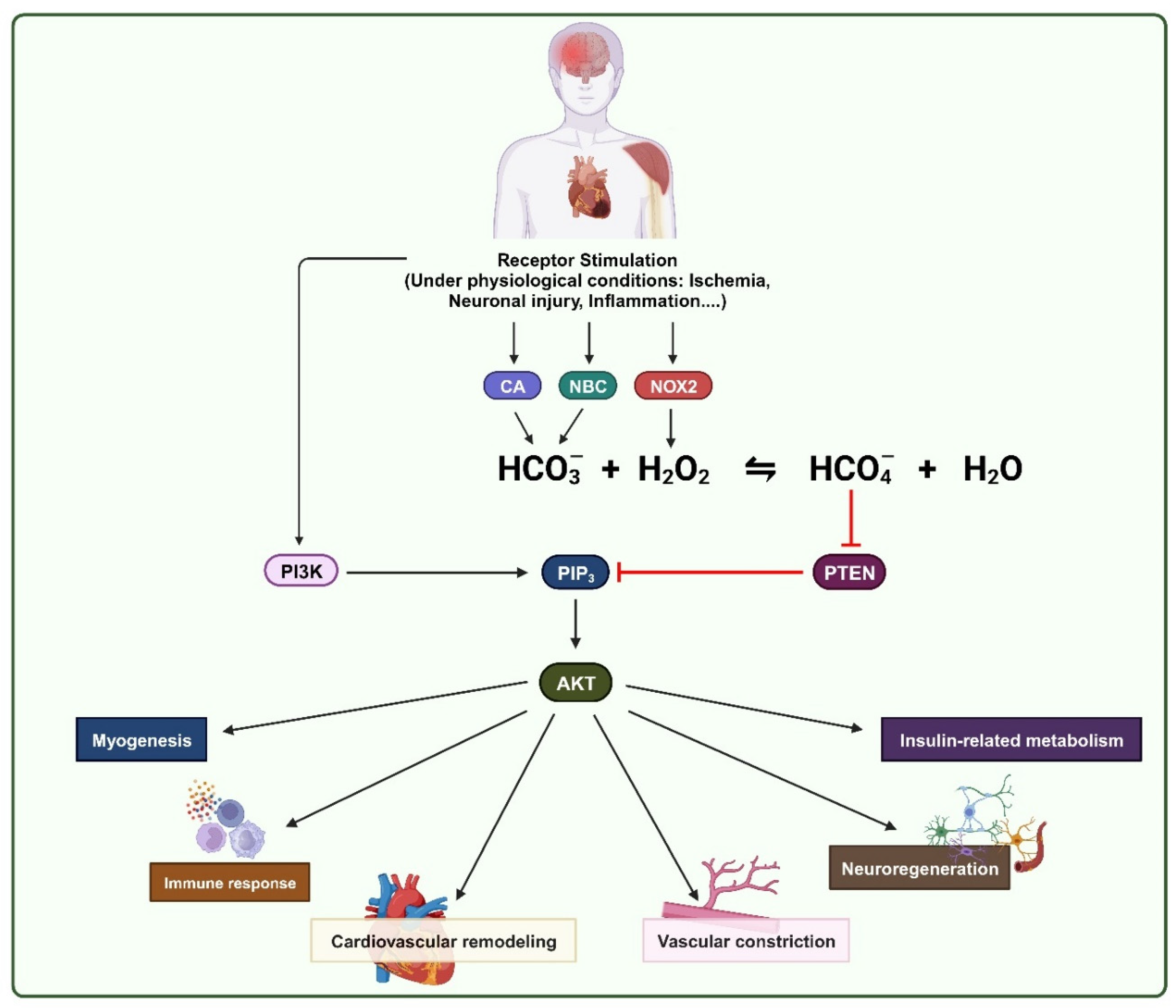
1. Introduction
2. Oxidative inhibition of PTEN by ROS in physiological processes
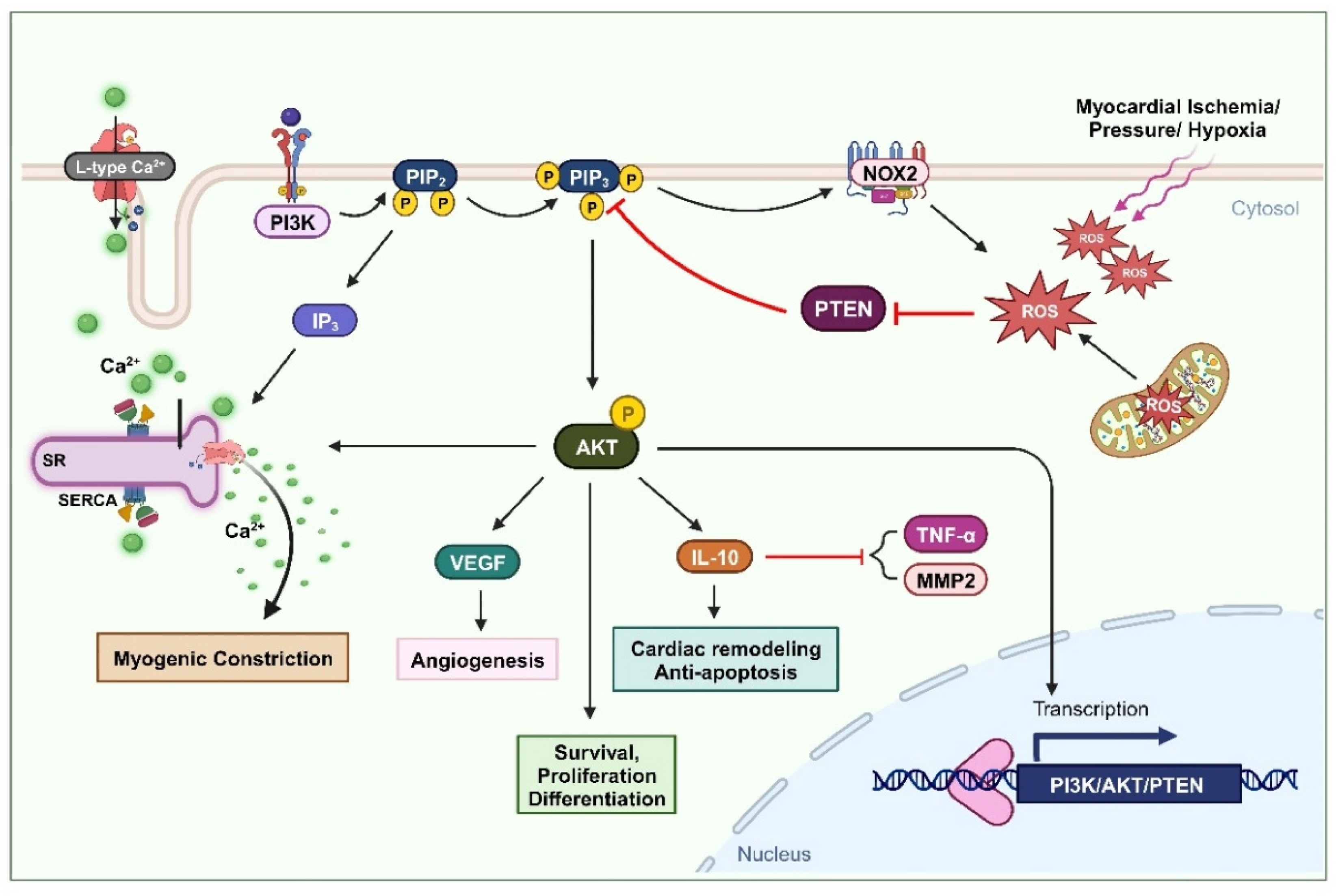
2.1. Cardiovascular remodeling
2.2. Vascular constriction
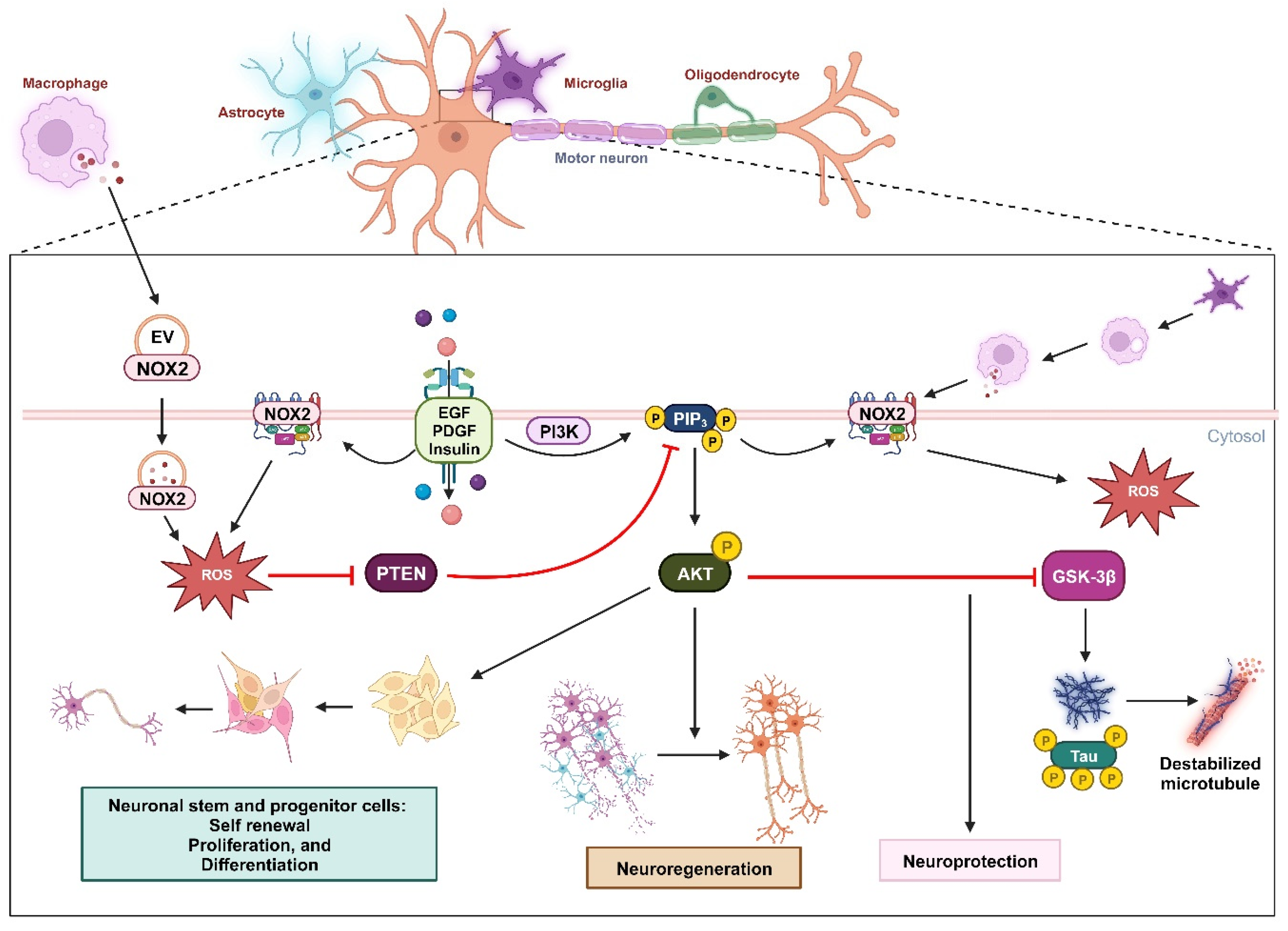
2.3. Neuro-regeneration and neuro-survival
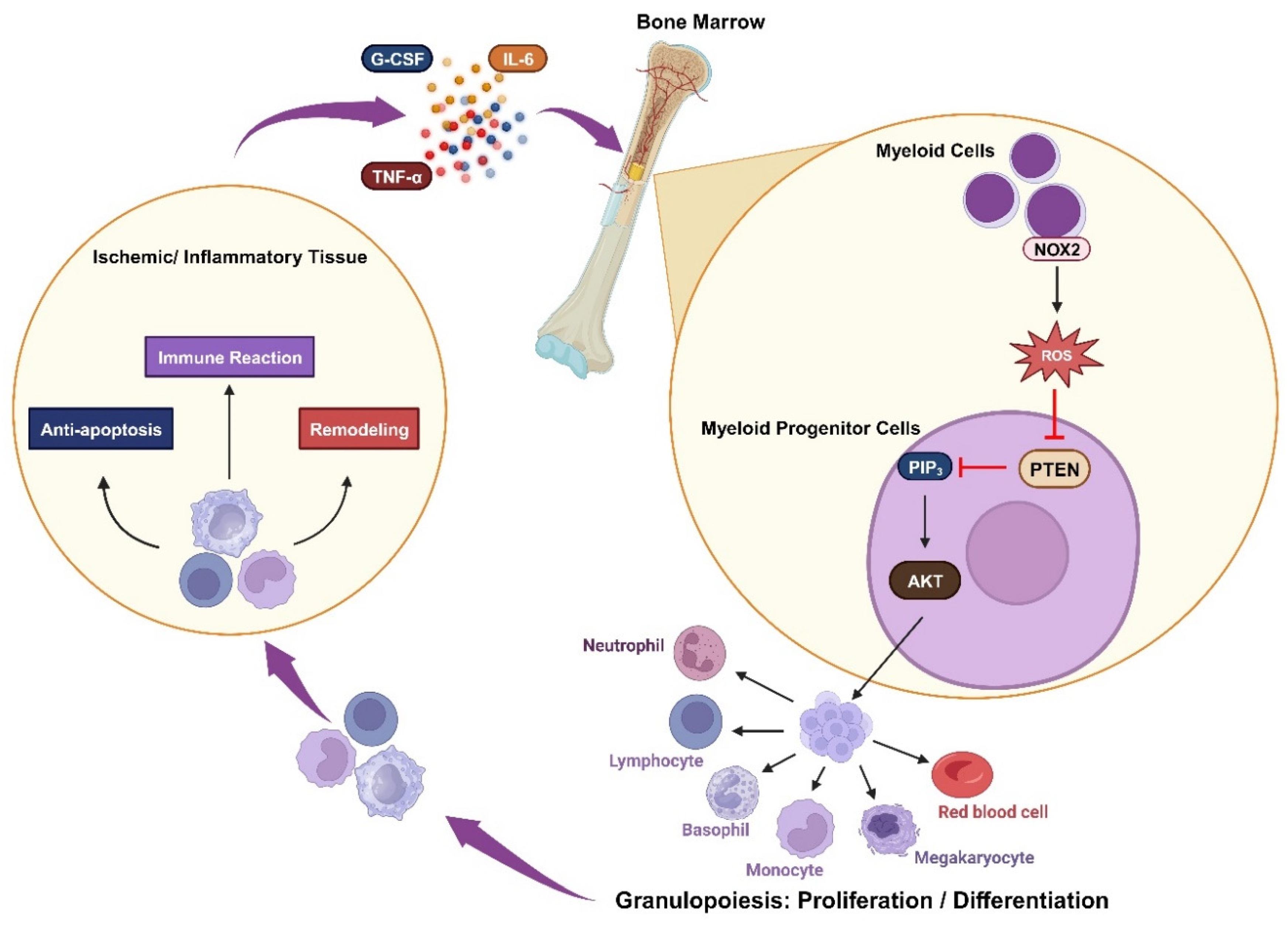
2.4. Immune response
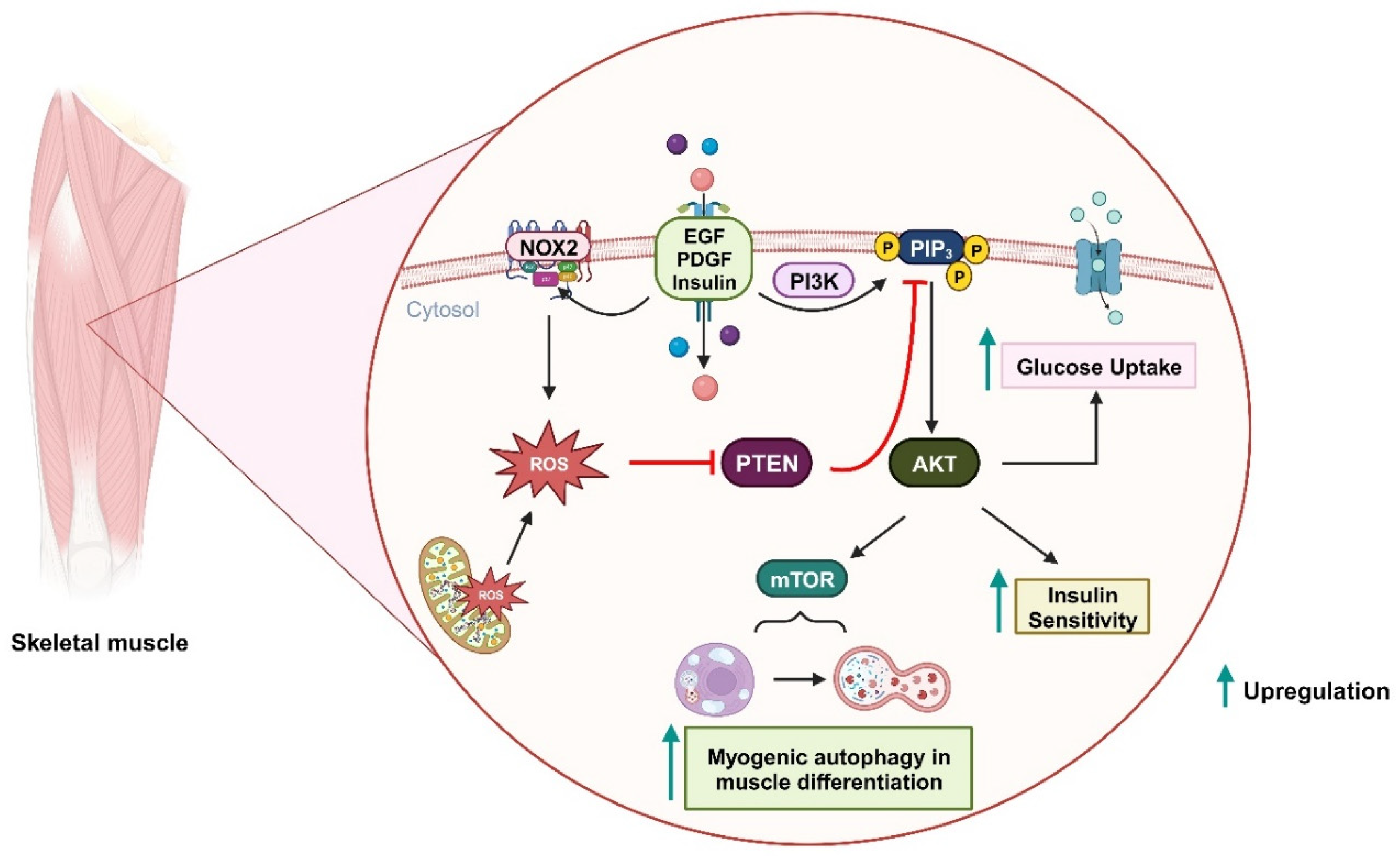
2.5. Insulin-related metabolism
2.6. Myogenic autophagy in muscle differentiation
3. Role of bicarbonate in the oxidation of PTPs by H2O2
4. Concluding remarks
Author Contributions
Acknowledgments
Conflicts of interest
References
- Zhang, Y.; et al., Redox regulation of tumor suppressor PTEN in cell signaling. Redox biology 2020, 34, 101553.
- Zhang, Y.; et al., Redox regulation of the tumor suppressor PTEN by hydrogen peroxide and tert-butyl hydroperoxide. International journal of molecular sciences 2017, 18, 982. [CrossRef] [PubMed]
- Han, S.-J.; et al., Redox regulation of the tumor suppressor PTEN by the thioredoxin system and cumene hydroperoxide. Free Radical Biology and Medicine 2017, 112, 277–286. [CrossRef] [PubMed]
- Han, S.-J.; et al., Assay of the redox state of the tumor suppressor PTEN by mobility shift. Methods 2015, 77, 58–62.
- Boosani, C.S., P. Gunasekar, and D.K. Agrawal, An update on PTEN modulators–a patent review. Expert opinion on therapeutic patents 2019, 29, 881–889. [CrossRef] [PubMed]
- Lee, Y.-R., M. Chen, and P.P. Pandolfi, The functions and regulation of the PTEN tumour suppressor: New modes and prospects. Nature reviews Molecular cell biology 2018, 19, 547–562. [CrossRef] [PubMed]
- Blumenthal, G.M. and P.A. Dennis, PTEN hamartoma tumor syndromes. European journal of human genetics 2008, 16, 1289–1300. [CrossRef] [PubMed]
- Baig, R.M.; et al., Genetic changes in the PTEN gene and their association with breast cancer in Pakistan. Asian Pac J Cancer Prev 2011, 12, 2773–2778.
- Liaw, D.; et al., Germline mutations of the PTEN gene in Cowden disease, an inherited breast and thyroid cancer syndrome. Nature genetics 1997, 16, 64–67. [CrossRef]
- Norimatsu, Y.; et al., Immunohistochemical expression of PTEN and β-catenin for endometrial intraepithelial neoplasia in Japanese womenomen. Annals of diagnostic pathology 2007, 11, 103–108. [CrossRef]
- Patel, R.; et al. Sprouty2, PTEN, and PP2A interact to regulate prostate cancer progression. The Journal of clinical investigation 2013, 123, 1157–1175. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; et al., Combined PTEN mutation and protein expression associate with overall and disease-free survival of glioblastoma patients. Translational oncology 2014, 7, 196–205. [CrossRef] [PubMed]
- Romano, C. and C. Schepis, PTEN gene: A model for genetic diseases in dermatology. The Scientific World Journal 2012, 2012.
- Pulido, R., PTEN inhibition in human disease therapy. Molecules 2018, 23, 285. [CrossRef] [PubMed]
- Denu, J.M. and J.E. Dixon, Protein tyrosine phosphatases: Mechanisms of catalysis and regulation. Curr Opin Chem Biol 1998, 2, 633–641. [CrossRef] [PubMed]
- Nguyen Huu, T.; et al., The Role of Oxidative Inactivation of Phosphatase PTEN and TCPTP in Fatty Liver Disease. Antioxidants (Basel) 2023, 12.
- Sun, Y., Oxidative stress and cardiac repair/remodeling following infarction. The American journal of the medical sciences 2007, 334, 197–205. [CrossRef]
- Meng, T.-C., T. Fukada, and N.K. Tonks, Reversible oxidation and inactivation of protein tyrosine phosphatases in vivo. Molecular cell 2002, 9, 387–399. [CrossRef]
- Rhee, S.G.; et al., Intracellular messenger function of hydrogen peroxide and its regulation by peroxiredoxinss. Curr Opin Cell Biol 2005, 17, 183–189. [CrossRef]
- Rhee, S.G.; et al., Cellular regulation by hydrogen peroxide. J Am Soc Nephrol 2003, 14 Suppl. S3, S211–S215. [CrossRef]
- Rhee, S.G., Redox signaling: Hydrogen peroxide as intracellular messenger. Experimental & Molecular Medicine 1999, 31, 53–59.
- Zhang, L.; et al., Biochemical basis and metabolic interplay of redox regulation. Redox Biol 2019, 26, 101284.
- Gupta, S.C.; et al., Upsides and downsides of reactive oxygen species for cancer: The roles of reactive oxygen species in tumorigenesis, prevention, and therapy. Antioxid Redox Signal 2012, 16, 1295–1322. [CrossRef] [PubMed]
- Veal, E.A., A.M. Day, and B.A. Morgan, Hydrogen peroxide sensing and signaling. Molecular cell 2007, 26, 1–14. [CrossRef] [PubMed]
- Rhee, S.G., H2O2, a necessary evil for cell signaling. Science 2006, 312, 1882–1883. [CrossRef] [PubMed]
- Lambeth, J.D.; et al., Novel homologs of gp91phox. Trends Biochem Sci 2000, 25, 459–461. [CrossRef]
- Lambeth, J.D., NOX enzymes and the biology of reactive oxygen. Nature Reviews Immunology 2004, 4, 181–189. [CrossRef] [PubMed]
- Lee, S.-R.; et al., Reversible inactivation of the tumor suppressor PTEN by H2O2. Journal of Biological Chemistry 2002, 277, 20336–20342. [CrossRef] [PubMed]
- Leslie, N.R.; et al., Redox regulation of PI 3-kinase signalling via inactivation of PTEN. The EMBO journal 2003, 22, 5501–5510. [CrossRef]
- Takakura, K.; et al., Rapid and irreversible inactivation of protein tyrosine phosphatases PTP1B, CD45, and LAR by peroxynitrite. Arch Biochem Biophys 1999, 369, 197–207. [CrossRef]
- Pacher, P., J.S. Beckman, and L. Liaudet, Nitric oxide and peroxynitrite in health and disease. Physiological reviews 2007, 87, 315–424. [CrossRef] [PubMed]
- Leslie, N.R., The redox regulation of PI 3-kinase-dependent signaling. Antioxid Redox Signal 2006, 8, 1765–1774. [CrossRef] [PubMed]
- Downes, C.P.; et al., Stimulation of PI 3-kinase signaling via inhibition of the tumor suppressor phosphatase, PTEN. Adv Enzyme Regul 2007, 47, 184–194. [CrossRef] [PubMed]
- Dagnell, M.; et al., Bicarbonate is essential for protein-tyrosine phosphatase 1B (PTP1B) oxidation and cellular signaling through EGF-triggered phosphorylation cascades. Journal of Biological Chemistry 2019, 294, 12330–12338. [CrossRef] [PubMed]
- Winterbourn, C.C.; et al., Carbon dioxide/bicarbonate is required for sensitive inactivation of mammalian glyceraldehyde-3-phosphate dehydrogenase by hydrogen peroxide. Proc Natl Acad Sci U S A 2023, 120, e2221047120. [CrossRef] [PubMed]
- Radi, R., Interplay of carbon dioxide and peroxide metabolism in mammalian cells. Journal of Biological Chemistry 2022, 298. [CrossRef] [PubMed]
- Murphy, E., Primary and secondary signaling pathways in early preconditioning that converge on the mitochondria to produce cardioprotection. Circulation research 2004, 94, 7–16. [CrossRef] [PubMed]
- Jonassen, A.K.; et al., Myocardial protection by insulin at reperfusion requires early administration and is mediated via Akt and p70s6 kinase cell-survival signaling. Circulation research 2001, 89, 1191–1198. [CrossRef] [PubMed]
- Matsui, T.; et al., Adenoviral gene transfer of activated phosphatidylinositol 3′-kinase and Akt inhibits apoptosis of hypoxic cardiomyocytes in vitro. Circulation 1999, 100, 2373–2379. [CrossRef]
- Uchiyama, T.; et al. Role of Akt signaling in mitochondrial survival pathway triggered by hypoxic preconditioning. Circulation 2004, 109, 3042–3049. [Google Scholar] [CrossRef]
- Tong, H.; et al. Ischemic preconditioning activates phosphatidylinositol-3-kinase upstream of protein kinase C. Circulation research 2000, 87, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Mocanu, M.M., R.M. Bell, and D.M. Yellon, PI3 kinase and not p42/p44 appears to be implicated in the protection conferred by ischemic preconditioning. Journal of molecular and cellular cardiology 2002, 34, 661–668. [CrossRef]
- Mocanu, M. and D. Yellon, PTEN, the Achilles' heel of myocardial ischaemia/reperfusion injury? British journal of pharmacology 2007, 150, 833–838. [CrossRef]
- Parajuli, N.; et al. Phosphatase PTEN is critically involved in post-myocardial infarction remodeling through the Akt/interleukin-10 signaling pathway. Basic Research in Cardiology 2012, 107, 1–15. [Google Scholar] [CrossRef]
- Burchfield, J.S.; et al. Interleukin-10 from transplanted bone marrow mononuclear cells contributes to cardiac protection after myocardial infarction. Circulation research 2008, 103, 203–211. [Google Scholar] [CrossRef]
- Krishnamurthy, P.; et al. IL-10 inhibits inflammation and attenuates left ventricular remodeling after myocardial infarction via activation of STAT3 and suppression of HuR. Circulation research 2009, 104, e9–e18. [Google Scholar] [CrossRef] [PubMed]
- Stumpf, C.; et al. Interleukin-10 improves left ventricular function in rats with heart failure subsequent to myocardial infarction. European journal of heart failure 2008, 10, 733–739. [Google Scholar] [CrossRef]
- Yang, Z., B. Zingarelli, and C. Szabó, Crucial role of endogenous interleukin-10 production in myocardial ischemia/reperfusion injury. Circulation 2000, 101, 1019–1026. [CrossRef] [PubMed]
- Keyes, K.T.; et al. Pharmacological inhibition of PTEN limits myocardial infarct size and improves left ventricular function postinfarction. American Journal of Physiology-Heart and Circulatory Physiology 2010, 298, H1198–H1208. [Google Scholar] [CrossRef]
- Ruan, H.; et al. Inducible and cardiac specific PTEN inactivation protects ischemia/reperfusion injury. Journal of molecular and cellular cardiology 2009, 46, 193–200. [Google Scholar] [CrossRef]
- Fukui, T.; et al. Expression of p22-phox and gp91-phox, essential components of NADPH oxidase, increases after myocardial infarction. Biochem Biophys Res Commun 2001, 281, 1200–1206. [Google Scholar] [CrossRef]
- Krijnen, P.; et al. Increased Nox2 expression in human cardiomyocytes after acute myocardial infarction. Journal of clinical pathology 2003, 56, 194–199. [Google Scholar] [CrossRef]
- Sirker, A.; et al. Cell-specific effects of Nox2 on the acute and chronic response to myocardial infarction. J Mol Cell Cardiol 2016, 98, 11–17. [Google Scholar] [CrossRef]
- Nguyen Huu, T.; et al. Redox Regulation of PTEN by Peroxiredoxins. Antioxidants (Basel) 2021, 10. [Google Scholar] [CrossRef]
- Cai, Z. and G.L. Semenza, PTEN activity is modulated during ischemia and reperfusion: Involvement in the induction and decay of preconditioning. Circulation research 2005, 97, 1351–1359. [Google Scholar] [CrossRef]
- Xiang, M.; et al., Role of oxidative stress in reperfusion following myocardial ischemia and its treatments. Oxidative medicine and cellular longevity 2021, 2021. [CrossRef] [PubMed]
- Lee, S.H.; et al. Early expression of angiogenesis factors in acute myocardial ischemia and infarction. New England Journal of Medicine 2000, 342, 626–633. [Google Scholar] [CrossRef] [PubMed]
- Kanazawa, M.; et al. Angiogenesis in the ischemic core: A potential treatment target? Journal of Cerebral Blood Flow & Metabolism 2019, 39, 753–769. [Google Scholar]
- Zaitone, S.A. and N.M. Abo-Gresha, Rosuvastatin promotes angiogenesis and reverses isoproterenol-induced acute myocardial infarction in rats: Role of iNOS and VEGF. European journal of pharmacology 2012, 691, 134–142. [Google Scholar] [CrossRef]
- Connor, K.M.; et al. Mitochondrial H2O2 regulates the angiogenic phenotype via PTEN oxidation. Journal of Biological Chemistry 2005, 280, 16916–16924. [Google Scholar] [CrossRef]
- Latronico, M.V.; et al. Regulation of cell size and contractile function by AKT in cardiomyocytes. Annals of the New York Academy of Sciences 2004, 1015, 250–260. [Google Scholar] [CrossRef]
- Saward Peter Zahradka, L., Angiotensin II activates phosphatidylinositol 3-kinase in vascular smooth muscle cells. Circulation research 1997, 81, 249–257. [CrossRef] [PubMed]
- Sugden, P.H., Ras, Akt, and mechanotransduction in the cardiac myocyte. Circulation research 2003, 93, 1179–1192. [CrossRef] [PubMed]
- McDowell, S.A.; et al. Phosphoinositide 3-kinase regulates excitation-contraction coupling in neonatal cardiomyocytes. American Journal of Physiology-Heart and Circulatory Physiology 2004, 286, H796–H805. [Google Scholar] [CrossRef] [PubMed]
- Goncharova, E.A.; et al. PI3K is required for proliferation and migration of human pulmonary vascular smooth muscle cells. American Journal of Physiology-Lung Cellular and Molecular Physiology 2002, 283, L354–L363. [Google Scholar] [CrossRef] [PubMed]
- Perrino, C.; et al. Dynamic regulation of phosphoinositide 3-kinase-γ activity and β-adrenergic receptor trafficking in end-stage human heart failure. Circulation 2007, 116, 2571–2579. [Google Scholar] [CrossRef] [PubMed]
- Namgaladze, D. and B. Brüne, Phospholipase A2–modified low-density lipoprotein activates the phosphatidylinositol 3-kinase-akt pathway and increases cell survival in monocytic cells. Arteriosclerosis, thrombosis, and vascular biology 2006, 26, 2510–2516. [Google Scholar] [CrossRef] [PubMed]
- Northcott, C.A., J.S. Hayflick, and S.W. Watts, PI3-Kinase upregulation and involvement in spontaneous tone in arteries from DOCA-salt rats: Is p110δ the culprit? Hypertension 2004, 43, 885–890. [CrossRef] [PubMed]
- Wu, K.L.; et al. Redox-sensitive oxidation and phosphorylation of PTEN contribute to enhanced activation of PI3K/Akt signaling in rostral ventrolateral medulla and neurogenic hypertension in spontaneously hypertensive rats. Antioxidants & Redox Signaling 2013, 18, 36–50. [Google Scholar]
- Gebremedhin, D.; et al. Redox signaling via oxidative inactivation of PTEN modulates pressure-dependent myogenic tone in rat middle cerebral arteries. PLoS ONE 2013, 8, e68498. [Google Scholar] [CrossRef]
- Carnevale, D.; et al. PI3Kγ inhibition reduces blood pressure by a vasorelaxant Akt/L-type calcium channel mechanism. Cardiovascular research 2012, 93, 200–209. [Google Scholar] [CrossRef] [PubMed]
- Ning, K.; et al. Dual neuroprotective signaling mediated by downregulating two distinct phosphatase activities of PTEN. J Neurosci 2004, 24, 4052–4060. [Google Scholar] [CrossRef] [PubMed]
- Mao, L.; et al. Delayed administration of a PTEN inhibitor BPV improves functional recovery after experimental stroke. Neuroscience 2013, 231, 272–281. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; et al. PTEN inhibition prevents rat cortical neuron injury after hypoxia–ischemia. Neuroscience 2013, 238, 242–251. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; et al. Rho-kinase inhibitor, fasudil, prevents neuronal apoptosis via the Akt activation and PTEN inactivation in the ischemic penumbra of rat brain. Cellular and molecular neurobiology 2012, 32, 1187–1197. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.-Y.; et al. Dose-dependent protective effect of bisperoxovanadium against acute cerebral ischemia in a rat model of ischemia/reperfusion injury. International journal of molecular sciences 2013, 14, 12013–12022. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.-N.; et al. Down-regulation of PTEN by sodium orthovanadate inhibits ASK1 activation via PI3-K/Akt during cerebral ischemia in rat hippocampus. Neuroscience letters 2006, 404, 98–102. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H., R.M. Sapolsky, and G.K. Steinberg, Phosphoinositide-3-kinase/akt survival signal pathways are implicated in neuronal survival after stroke. Molecular neurobiology 2006, 34, 249–269. [CrossRef] [PubMed]
- Christie, K.J. and D. Zochodne, Peripheral axon regrowth: New molecular approaches. Neuroscience 2013, 240, 310–324. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Junco-Clemente, P. and P. Golshani, PTEN: A master regulator of neuronal structure, function, and plasticity. Commun Integr Biol 2014, 7, e28358. [Google Scholar] [CrossRef]
- Knafo, S. and J.A. Esteban, PTEN: Local and Global Modulation of Neuronal Function in Health and Disease. Trends Neurosci 2017, 40, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Ohtake, Y., U. Hayat, and S. Li, PTEN inhibition and axon regeneration and neural repair. Neural Regen Res 2015, 10, 1363–1368. [PubMed]
- Park, K.K.; et al. Promoting axon regeneration in the adult CNS by modulation of the PTEN/mTOR pathway. Science 2008, 322, 963–966. [Google Scholar] [CrossRef] [PubMed]
- Park, K.K.; et al. PTEN/mTOR and axon regeneration. Exp Neurol 2010, 223, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; et al. Therapeutic Targets for Cerebral Ischemia Based on the Signaling Pathways of the GluN2B C Terminus. Stroke 2015, 46, 2347–2353. [Google Scholar] [CrossRef] [PubMed]
- Soltoff, S.P.; et al. Nerve growth factor promotes the activation of phosphatidylinositol 3-kinase and its association with the trk tyrosine kinase. J Biol Chem 1992, 267, 17472–17477. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; et al. PTEN deletion enhances the regenerative ability of adult corticospinal neurons. Nat Neurosci 2010, 13, 1075–1081. [Google Scholar] [CrossRef] [PubMed]
- Christie, K.J.; et al. PTEN inhibition to facilitate intrinsic regenerative outgrowth of adult peripheral axons. J Neurosci 2010, 30, 9306–9315. [Google Scholar] [CrossRef]
- Little, D.; et al. PTEN depletion decreases disease severity and modestly prolongs survival in a mouse model of spinal muscular atrophy. Mol Ther 2015, 23, 270–277. [Google Scholar] [CrossRef]
- Ning, K.; et al. PTEN depletion rescues axonal growth defect and improves survival in SMN-deficient motor neurons. Hum Mol Genet 2010, 19, 3159–3168. [Google Scholar] [CrossRef]
- Le Belle, J.E.; et al. Proliferative neural stem cells have high endogenous ROS levels that regulate self-renewal and neurogenesis in a PI3K/Akt-dependant manner. Cell stem cell 2011, 8, 59–71. [Google Scholar] [CrossRef]
- Giridharan, S.S. and S. Caplan, MICAL-family proteins: Complex regulators of the actin cytoskeleton. Antioxid Redox Signal 2014, 20, 2059–2073. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; et al. Implication of PTEN in production of reactive oxygen species and neuronal death in in vitro models of stroke and Parkinson's disease. Neurochem Int 2007, 50, 507–516. [Google Scholar] [CrossRef] [PubMed]
- Hervera, A.; et al. Reactive oxygen species regulate axonal regeneration through the release of exosomal NADPH oxidase 2 complexes into injured axons. Nat Cell Biol 2018, 20, 307–319. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Isla, T.; et al. Neuronal loss correlates with but exceeds neurofibrillary tangles in Alzheimer's disease. Ann Neurol 1997, 41, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Trinczek, B.; et al. Domains of tau protein, differential phosphorylation, and dynamic instability of microtubules. Mol Biol Cell 1995, 6, 1887–1902. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, M., V.M. Lee, and J.Q. Trojanowski, Tau and axonopathy in neurodegenerative disorders. Neuromolecular Med 2002, 2, 131–150. [CrossRef] [PubMed]
- Cavallini, A.; et al. An unbiased approach to identifying tau kinases that phosphorylate tau at sites associated with Alzheimer disease. J Biol Chem 2013, 288, 23331–23347. [Google Scholar] [CrossRef]
- Hernandez, F., J.J. Lucas, and J. Avila, GSK3 and tau: Two convergence points in Alzheimer's disease. J Alzheimers Dis 2013, 33 Suppl 1, S141–S144.
- Matsuda, S.; et al. Implications of PI3K/AKT/PTEN Signaling on Superoxide Dismutases Expression and in the Pathogenesis of Alzheimer's Disease. Diseases 2018, 6. [Google Scholar] [CrossRef]
- Kwon, J.; et al. Reversible oxidation and inactivation of the tumor suppressor PTEN in cells stimulated with peptide growth factors. Proc Natl Acad Sci U S A 2004, 101, 16419–16424. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Esteban, M.; et al. Inhibition of PTEN by peroxynitrite activates the phosphoinositide-3-kinase/Akt neuroprotective signaling pathway. J Neurochem 2007, 102, 194–205. [Google Scholar] [CrossRef]
- Manz, M.G. and S. Boettcher, Emergency granulopoiesis. Nature Reviews Immunology 2014, 14, 302–314. [Google Scholar] [CrossRef] [PubMed]
- Walker, F.; et al. IL6/sIL6R complex contributes to emergency granulopoietic responses in G-CSF–and GM-CSF–deficient mice. Blood, The Journal of the American Society of Hematology 2008, 111, 3978–3985. [Google Scholar] [CrossRef] [PubMed]
- Gwechenberger, M.; et al. Cardiac myocytes produce interleukin-6 in culture and in viable border zone of reperfused infarctions. Circulation 1999, 99, 546–551. [Google Scholar] [CrossRef]
- Kleinbongard, P., G. Heusch, and R. Schulz, TNFα in atherosclerosis, myocardial ischemia/reperfusion and heart failure. Pharmacology & therapeutics 2010, 127, 295–314.
- Frangogiannis, N.G., The mechanistic basis of infarct healing. Antioxid Redox Signal 2006, 8, 1907–1939. [CrossRef]
- Kwak, H.-J.; et al. Myeloid cell-derived reactive oxygen species externally regulate the proliferation of myeloid progenitors in emergency granulopoiesis. Immunity 2015, 42, 159–171. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; et al. Constitutively activated Akt-1 is vital for the survival of human monocyte-differentiated macrophages: Role of Mcl-1, independent of nuclear factor (NF)-κB, Bad, or caspase activation. The Journal of experimental medicine 2001, 194, 113–126. [Google Scholar] [CrossRef]
- Tiganis, T., Reactive oxygen species and insulin resistance: The good, the bad and the ugly. Trends in pharmacological sciences 2011, 32, 82–89. [CrossRef]
- Loh, K.; et al. Reactive oxygen species enhance insulin sensitivity. Cell metabolism 2009, 10, 260–272. [Google Scholar] [CrossRef]
- Seo, J.H.; et al. The major target of the endogenously generated reactive oxygen species in response to insulin stimulation is phosphatase and tensin homolog and not phosphoinositide-3 kinase (PI-3 kinase) in the PI-3 kinase/Akt pathway. Molecular biology of the cell 2005, 16, 348–357. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.Z., A. Di Cristofano, and M. Woo, Metabolic role of PTEN in insulin signaling and resistance. Cold Spring Harbor Perspectives in Medicine 2020, 10. [CrossRef] [PubMed]
- Rosivatz, E., Inhibiting PTEN. Biochem Soc Trans 2007, 35 Pt 2, 257–259. [CrossRef]
- Mizushima, N. and M. Komatsu, Autophagy: Renovation of cells and tissues. Cell 2011, 147, 728–741. [Google Scholar] [CrossRef]
- Harris, H. and D.C. Rubinsztein, Control of autophagy as a therapy for neurodegenerative disease. Nat Rev Neurol 2011, 8, 108–117. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; et al. Mitochondrial ROS-derived PTEN oxidation activates PI3K pathway for mTOR-induced myogenic autophagy. Cell Death Differ 2018, 25, 1921–1937. [Google Scholar] [CrossRef]
- Lee, S.-R.; et al. Reversible inactivation of protein-tyrosine phosphatase 1B in A431 cells stimulated with epidermal growth factor. Journal of Biological Chemistry 1998, 273, 15366–15372. [Google Scholar] [CrossRef]
- Tonks, N.K., Protein tyrosine phosphatases: From genes, to function, to disease. Nature reviews Molecular cell biology 2006, 7, 833–846. [CrossRef]
- Tanner, J.J.; et al. Redox regulation of protein tyrosine phosphatases: Structural and chemical aspects. Antioxidants & redox signaling 2011, 15, 77–97. [Google Scholar]
- Zhou, H.; et al. The biological buffer bicarbonate/CO2 potentiates H2O2-mediated inactivation of protein tyrosine phosphatases. Journal of the American Chemical Society 2011, 133, 15803–15805. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; et al. Prdx1 inhibits tumorigenesis via regulating PTEN/AKT activity. Embo j 2009, 28, 1505–1517. [Google Scholar] [CrossRef] [PubMed]
- Bakhmutova-Albert, E.V.; et al. Kinetics and mechanism of peroxymonocarbonate formation. Inorganic chemistry 2010, 49, 11287–11296. [Google Scholar] [CrossRef]
- Trindade, D.F., G. Cerchiaro, and O. Augusto, A role for peroxymonocarbonate in the stimulation of biothiol peroxidation by the bicarbonate/carbon dioxide pair. Chem Res Toxicol 2006, 19, 1475–1482. [CrossRef] [PubMed]
- Dorai, T.; et al. The role of carbonic anhydrase IX overexpression in kidney cancer. European journal of cancer 2005, 41, 2935–2947. [Google Scholar] [CrossRef]
- Aalkjaer, C.; et al. Cation-coupled bicarbonate transporters. Comprehensive Physiology 2014, 4, 1605. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




