Submitted:
03 January 2024
Posted:
04 January 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Participant Recruitment
2.2. Study Protocol
2.3. Imaging Procedures
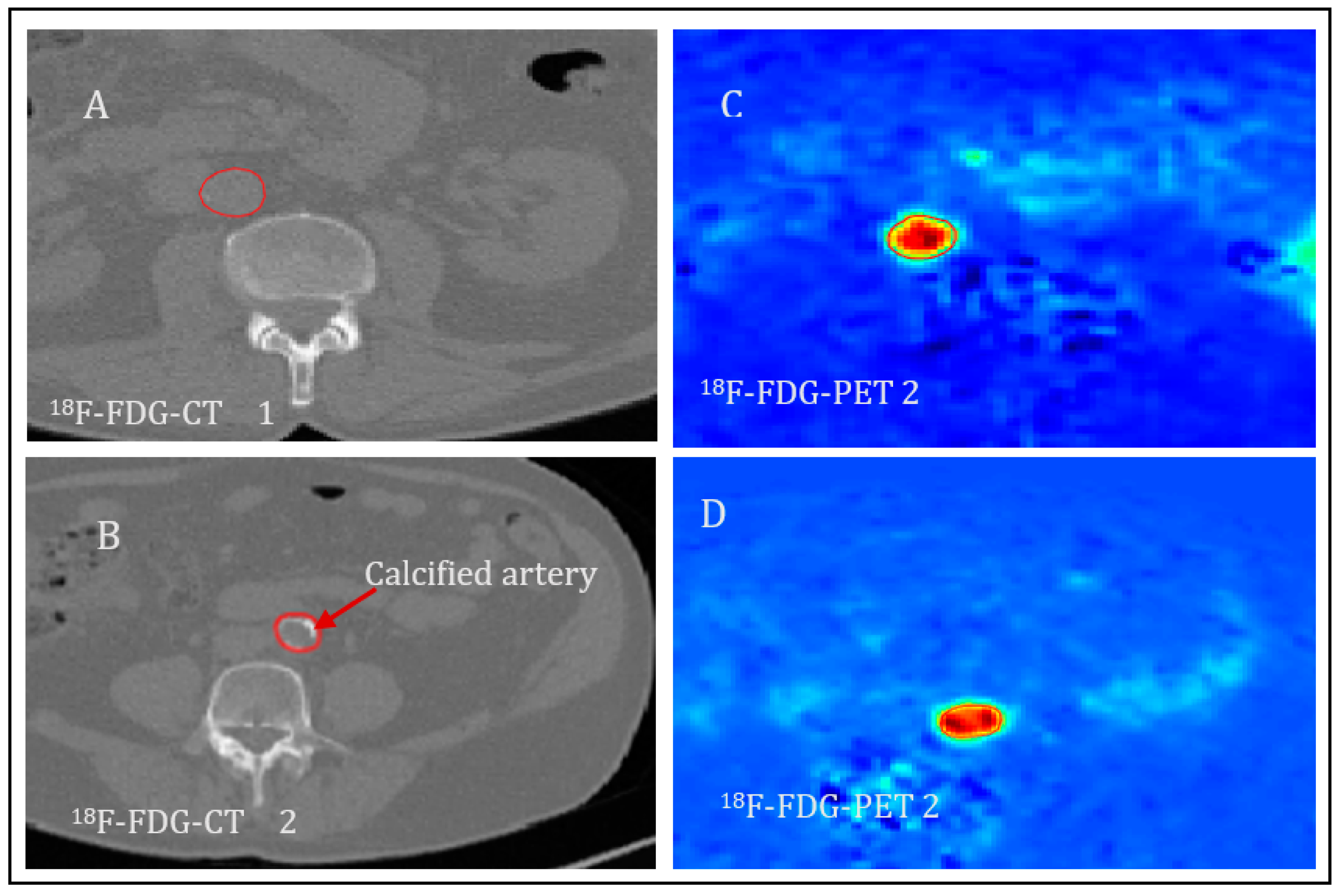
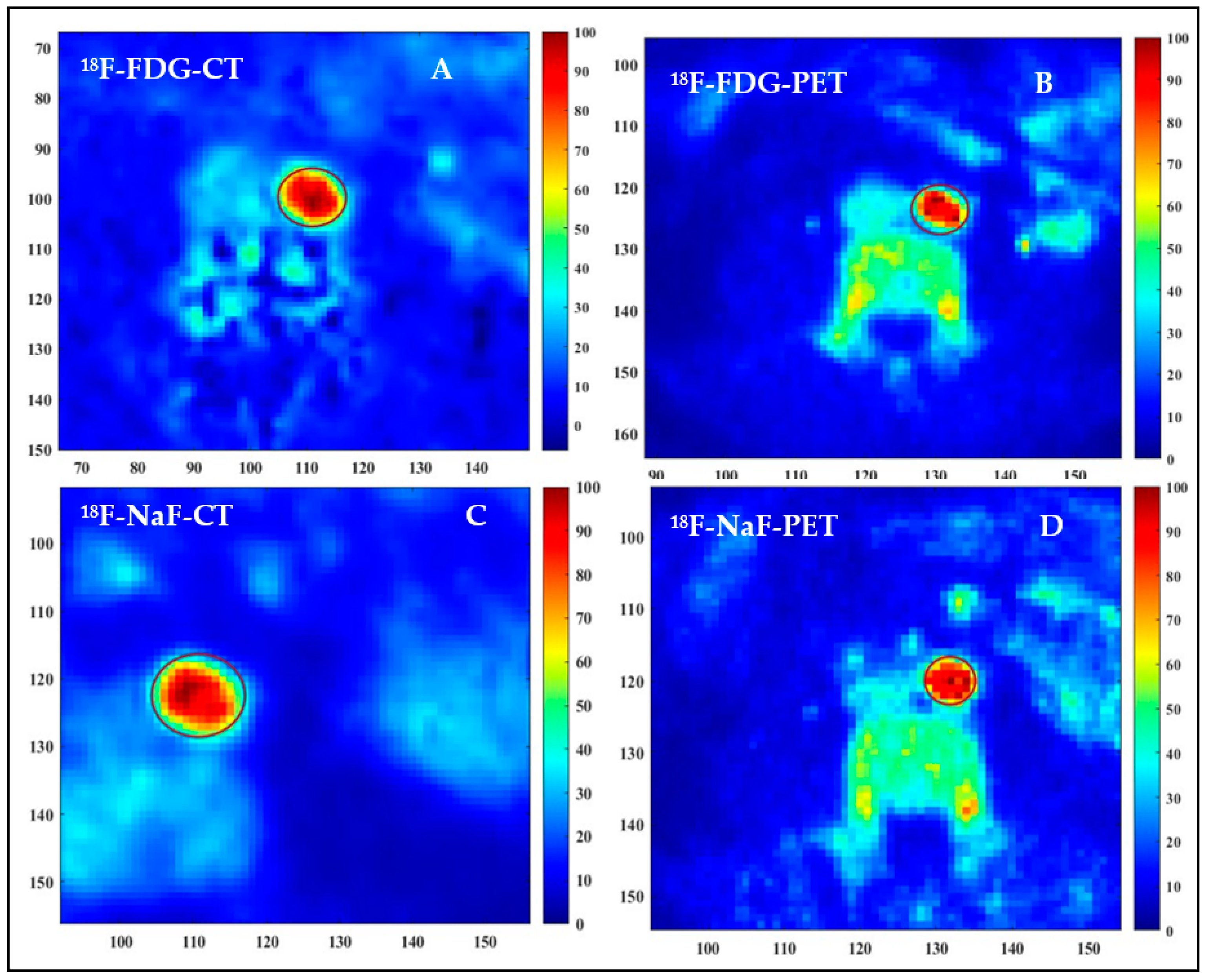
2.4. Image Analyses
2.5. Olive Oil Supplementation and Olive Oil Polyphenol Measurements
2.6. Statistical Analysis
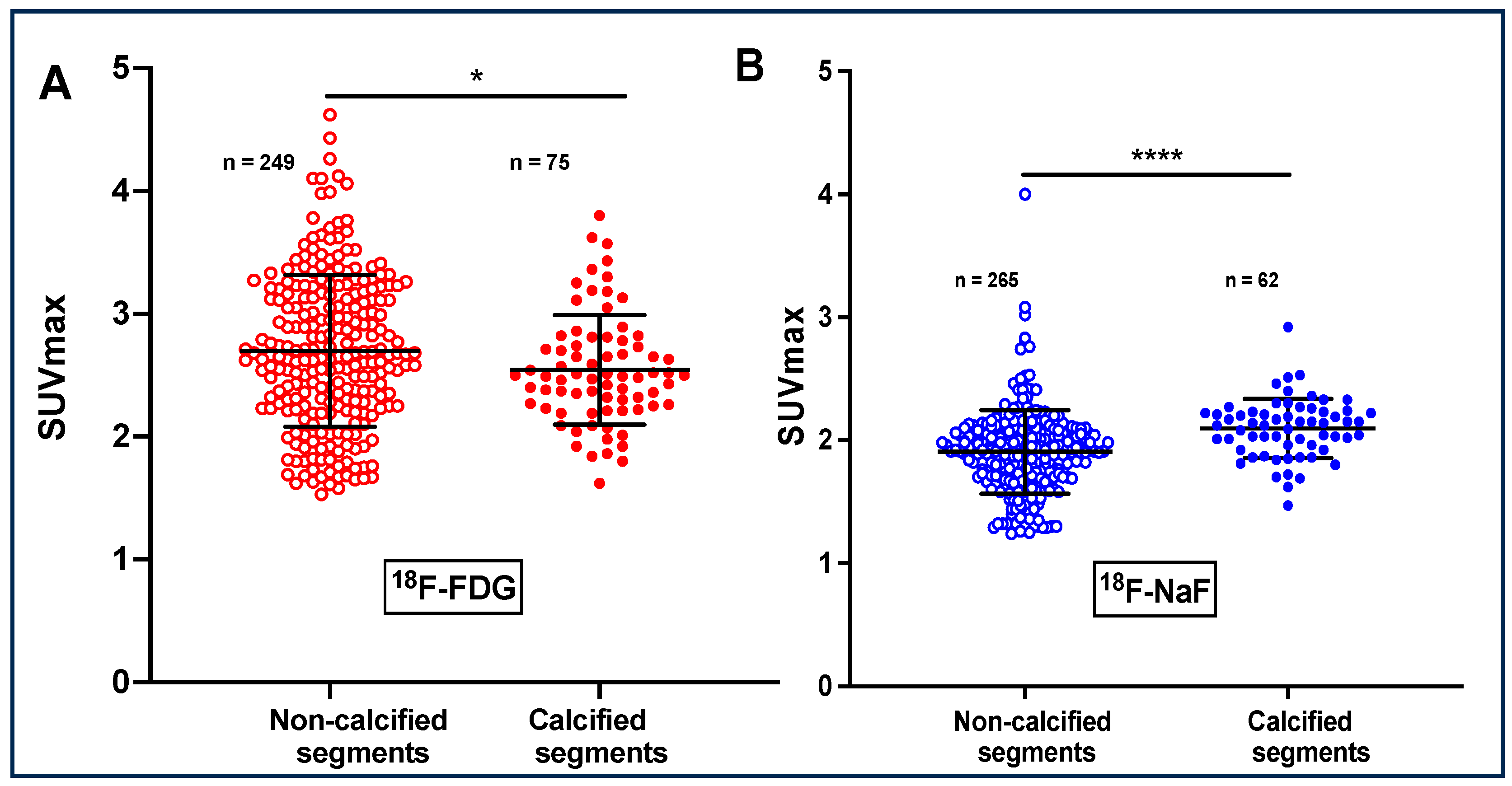
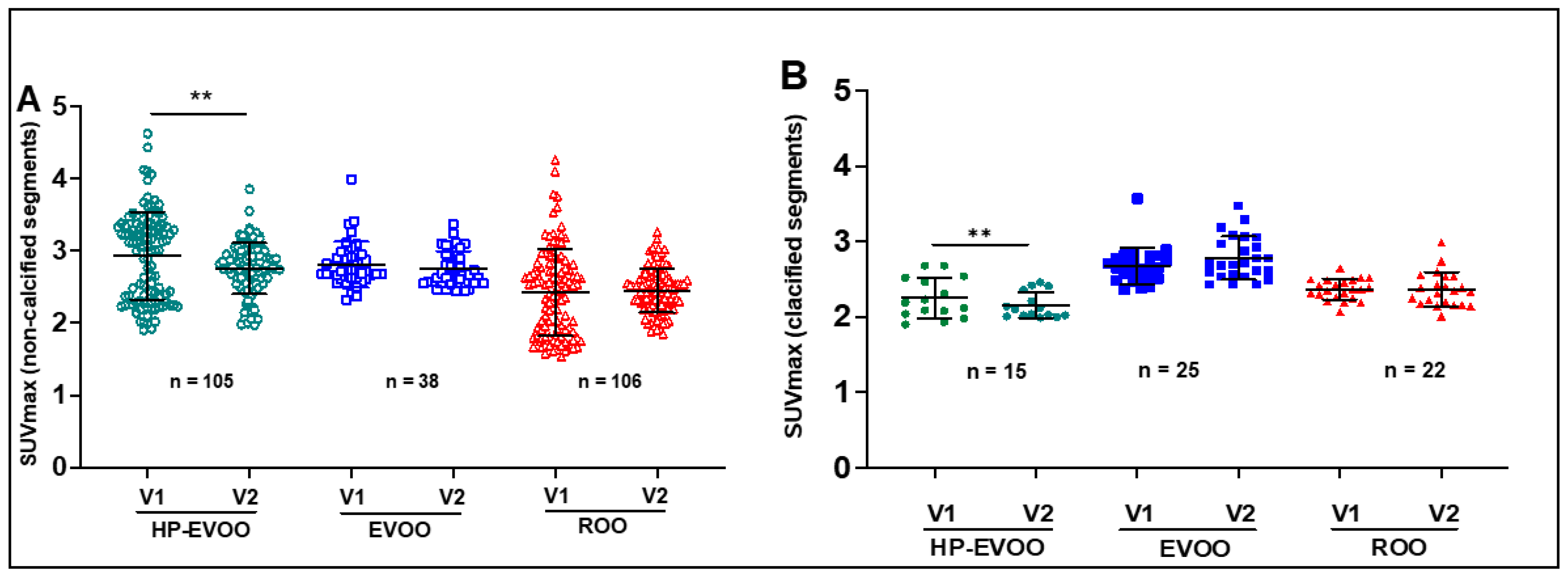
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Yazdanyar, A.; Newman, A.B. The burden of cardiovascular disease in the elderly: morbidity, mortality, and costs. Clin Geriatr Med. 2009, 25, 563–577. [Google Scholar] [CrossRef] [PubMed]
- Weintraub, H.S. Identifying the vulnerable patient with rupture-prone plaque. Am J Cardiol. 2008, 101, 3F–10F. [Google Scholar] [CrossRef] [PubMed]
- Soehnlein, O.; Libby, P. Targeting inflammation in atherosclerosis - from experimental insights to the clinic. Nat Rev Drug Discov. 2021, 20, 589–610. [Google Scholar] [CrossRef]
- Pedro-Botet, J.; Climent, E.; Benaiges, D. Atherosclerosis and inflammation. New therapeutic approaches. Med Clin (Barc). 2020, 155, 256–262. [Google Scholar] [CrossRef] [PubMed]
- Thomas, J.C.; Vohra, R.S.; Beer, S.; Bhatti, K.; Ponnambalam, S.; Homer-Vanniasinkam, S. Biomarkers in peripheral arterial disease. Trends Cardiovasc Med. 2009, 19, 147–151. [Google Scholar] [CrossRef] [PubMed]
- Kawtharany, L.; Bessueille, L.; Issa, H.; Hamade, E.; Zibara, K.; Magne, D. Inflammation and Microcalcification: A Never-Ending Vicious Cycle in Atherosclerosis? J Vasc Res. 2022, 59, 137–150. [Google Scholar] [CrossRef]
- Andrews, J.P.M.; Fayad, Z.A.; Dweck, M.R. New methods to image unstable atherosclerotic plaques. Atherosclerosis 2018, 272, 118–128. [Google Scholar] [CrossRef] [PubMed]
- Tawakol, A.; et al. In vivo 18F-fluorodeoxyglucose positron emission tomography imaging provides a noninvasive measure of carotid plaque inflammation in patients. J Am Coll Cardiol. 2006, 48, 1818–1824. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, S.F.; Graebe, M.; Fisker Hag, A.M.; Højgaard, L.; Sillesen, H.; Kjaer, A. Gene expression and 18FDG uptake in atherosclerotic carotid plaques. Nucl Med Commun. 2010, 31, 423–429. [Google Scholar] [CrossRef]
- Hutcheson, J.D.; Maldonado, N.; Aikawa, E. Small entities with large impact: microcalcifications and atherosclerotic plaque vulnerability. Curr Opin Lipidol. 2014, 25, 327–332. [Google Scholar] [CrossRef]
- Orellana, M.R.; Bentourkia, M.; Sarrhini, O.; Fulop, T.; Paquet, N.; Lavallee, E.; Turcotte, E.; Khalil, A. Assessment of inflammation in large arteries with 18F-FDG-PET in elderly. Comput Med Imaging Graph. 2013, 37, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Fiz, F.; Piccardo, A.; Morbelli, S.; Bottoni, G.; Piana, M.; Cabria, M.; Bagnasco, M.; Sambuceti, G. Longitudinal analysis of atherosclerotic plaques evolution: an (18)F-NaF PET/CT study. J Nucl Cardiol. 2022, 29, 1713–1723. [Google Scholar] [CrossRef] [PubMed]
- Khalil, A.; Orellana, M.R.; Fulop, T.; Turcotte, E.E.; Bentourkia, M. Positron emission tomography imaging for vascular inflammation evaluation in elderly subjects with different risk factors for cardiovascular diseases. American journal of nuclear medicine and molecular imaging 2014, 4, 283–292. [Google Scholar]
- Estruch, R.; et al. Primary Prevention of Cardiovascular Disease with a Mediterranean Diet Supplemented with Extra-Virgin Olive Oil or Nuts. N Engl J Med. 2018, 378, e34. [Google Scholar] [CrossRef] [PubMed]
- Otrante, A.; Trigui, A.; Walha, R.; Berrougui, H.; Fulop, T.; Khalil, A. Extra Virgin Olive Oil Prevents the Age-Related Shifts of the Distribution of HDL Subclasses and Improves Their Functionality. Nutrients 2021, 13. [Google Scholar] [CrossRef] [PubMed]
- Zimmer, A.; Otrante, A.; Zoubdane, N.; Nguyen, M.; Fülöp, T.; Khalil, A. The Immunomodulatory Effects of a 6-Month Extra Virgin Olive Oil Intervention on Monocyte Cytokine Secretion and Plasma Cytokine Levels in Dyslipidemic and Post-Infarct Patients: A Clinical Pilot Study. Nutrients 2023, 15. [Google Scholar] [CrossRef]
- Rietjens, S.J.; Bast, A.; de Vente, J.; Haenen, G.R. The olive oil antioxidant hydroxytyrosol efficiently protects against the oxidative stress-induced impairment of the NObullet response of isolated rat aorta. Am J Physiol Heart Circ Physiol. 2007, 292, H1931–H1936. [Google Scholar] [CrossRef] [PubMed]
- Gabbia, D.; et al. The phenolic compounds tyrosol and hydroxytyrosol counteract liver fibrogenesis via the transcriptional modulation of NADPH oxidases and oxidative stress-related miRNAs. Biomed Pharmacother. 2023, 157, 114014. [Google Scholar] [CrossRef]
- Bender, C.; Candi, I.; Rogel, E. Efficacy of Hydroxytyrosol-Rich Food Supplements on Reducing Lipid Oxidation in Humans. Int J Mol Sci. 2023, 24. [Google Scholar] [CrossRef]
- Santarelli, R.; Pompili, C.; Gilardini Montani, M.S.; Evangelista, L.; Gonnella, R.; Cirone, M. 3,4-Dihydroxyphenylethanol (DPE or Hydroxytyrosol) Counteracts ERK1/2 and mTOR Activation, Pro-Inflammatory Cytokine Release, Autophagy and Mitophagy Reduction Mediated by Benzo[a]pyrene in Primary Human Colonic Epithelial Cells. Pharmaceutics 2022, 14. [Google Scholar] [CrossRef]
- Zhang, X.; Qin, Y.; Wan, X.; Liu, H.; Iv, C.; Ruan, W.; Lu, L.; He, L.; Guo, X. Hydroxytyrosol Plays Antiatherosclerotic Effects through Regulating Lipid Metabolism via Inhibiting the p38 Signal Pathway. Biomed Res Int. 2020, 2020, 5036572. [Google Scholar] [CrossRef]
- Ferrucci, L.; Fabbri, E. Inflammageing: chronic inflammation in ageing, cardiovascular disease, and frailty. Nat Rev Cardiol. 2018, 15, 505–522. [Google Scholar] [CrossRef] [PubMed]
- Douhi, A.; Al-Enezi, M.S.; Berrahmoune, N.; Khalil, A.; Fulop, T.; Nguyen, M.; Turcotte, E.; Croteau, É.; Bentourkia, M. Non-calcified active atherosclerosis plaque detection with 18F-NaF and 18F-FDG PET/CT dynamic imaging. Phys Eng Sci Med. 2023. [Google Scholar] [CrossRef]
- Agatston, A.S.; Janowitz, W.R.; Hildner, F.J.; Zusmer, N.R.; Viamonte, M., Jr.; Detrano, R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990, 15, 827–832. [Google Scholar] [CrossRef] [PubMed]
- Croteau, E.; et al. Image-derived input function in dynamic human PET/CT: methodology and validation with 11C-acetate and 18F-fluorothioheptadecanoic acid in muscle and 18F-fluorodeoxyglucose in brain. Eur J Nucl Med Mol Imaging 2010, 37, 1539–1550. [Google Scholar] [CrossRef] [PubMed]
- Head, T.; Daunert, S.; Goldschmidt-Clermont, P.J. The Aging Risk and Atherosclerosis: A Fresh Look at Arterial Homeostasis. Front Genet. 2017, 8, 216. [Google Scholar] [CrossRef]
- Li, Z.; Lin, C.; Cai, X.; Hu, S.; Lv, F.; Yang, W.; Zhu, X.; Ji, L. Anti-inflammatory therapies were associated with reduced risk of myocardial infarction in patients with established cardiovascular disease or high cardiovascular risks: A systematic review and meta-analysis of randomized controlled trials. Atherosclerosis 2023, 379, 117181. [Google Scholar] [CrossRef]
- Tatsumi, M.; Cohade, C.; Nakamoto, Y.; Wahl, R.L. Fluorodeoxyglucose uptake in the aortic wall at PET/CT: possible finding for active atherosclerosis. Radiology 2003, 229, 831–837. [Google Scholar] [CrossRef]
- Bucerius, J.; et al. Arterial and fat tissue inflammation are highly correlated: a prospective 18F-FDG PET/CT study. Eur J Nucl Med Mol Imaging 2014, 41, 934–945. [Google Scholar] [CrossRef]
- He, Y.; Chen, Y.; Yao, L.; Wang, J.; Sha, X.; Wang, Y. The Inflamm-Aging Model Identifies Key Risk Factors in Atherosclerosis. Front Genet. 2022, 13, 865827. [Google Scholar] [CrossRef]
- Malik, D.; Verma, R.; Gupta, V.; Belho, E.S.; Drolia, B.; Seniaray, N.; Mahajan, H. Semiquantitative Interpretation Criteria for Fluorodeoxyglucose-Positron Emission Tomography/Computed Tomography in Large-Vessel Vasculitis: Pattern Recognition and Correlation with Polymyalgia Rheumatica. Indian J Nucl Med. 2020, 35, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; et al. Oncostatin M receptor β deficiency attenuates atherogenesis by inhibiting JAK2/STAT3 signaling in macrophages. J Lipid Res. 2017, 58, 895–906. [Google Scholar] [CrossRef]
- Shi, X.; Gao, J.; Lv, Q.; Cai, H.; Wang, F.; Ye, R.; Liu, X. Calcification in Atherosclerotic Plaque Vulnerability: Friend or Foe? Front Physiol. 2020, 11, 56. [Google Scholar] [CrossRef] [PubMed]
- Reith, S.; Milzi, A.; Dettori, R.; Marx, N.; Burgmaier, M. Predictors for target lesion microcalcifications in patients with stable coronary artery disease: an optical coherence tomography study. Clin Res Cardiol. 2018, 107, 763–771. [Google Scholar] [CrossRef] [PubMed]
- Creager, M.D.; et al. (18)F-Fluoride Signal Amplification Identifies Microcalcifications Associated With Atherosclerotic Plaque Instability in Positron Emission Tomography/Computed Tomography Images. Circ Cardiovasc Imaging 2019, 12, e007835. [Google Scholar] [CrossRef] [PubMed]
- Xia, M.; Zhong, Y.; Peng, Y.; Qian, C. Olive oil consumption and risk of cardiovascular disease and all-cause mortality: A meta-analysis of prospective cohort studies. Front Nutr. 2022, 9, 1041203. [Google Scholar] [CrossRef] [PubMed]
- Martínez-González, M.A.; Sayón-Orea, C.; Bullón-Vela, V.; Bes-Rastrollo, M.; Rodríguez-Artalejo, F.; Yusta-Boyo, M.J.; García-Solano, M. Effect of olive oil consumption on cardiovascular disease, cancer, type 2 diabetes, and all-cause mortality: A systematic review and meta-analysis. Clin Nutr. 2022, 41, 2659–2682. [Google Scholar] [CrossRef] [PubMed]
- Muriana, F.J.G.; Montserrat-de la Paz, S.; Lucas, R.; Bermudez, B.; Jaramillo, S.; Morales, J.C.; Abia, R.; Lopez, S. Tyrosol and its metabolites as antioxidative and anti-inflammatory molecules in human endothelial cells. Food Funct. 2017, 8, 2905–2914. [Google Scholar] [CrossRef]
- Karković Marković, A.; Torić, J.; Barbarić, M.; Jakobušić Brala, C. Hydroxytyrosol, Tyrosol and Derivatives and Their Potential Effects on Human Health. Molecules 2019, 24. [Google Scholar] [CrossRef]
- Rietjens, S.J.; Bast, A.; Haenen, G.R. New insights into controversies on the antioxidant potential of the olive oil antioxidant hydroxytyrosol. J Agric Food Chem. 2007, 55, 7609–7614. [Google Scholar] [CrossRef]
- Scoditti, E.; Nestola, A.; Massaro, M.; Calabriso, N.; Storelli, C.; De Caterina, R.; Carluccio, M.A. Hydroxytyrosol suppresses MMP-9 and COX-2 activity and expression in activated human monocytes via PKCα and PKCβ1 inhibition. Atherosclerosis 2014, 232, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Menotti, A.; Puddu, P.E. How the Seven Countries Study contributed to the launch and development of cardiovascular epidemiology in Italy. A historical perspective. Nutr Metab Cardiovasc Dis. 2020, 30, 368–383. [Google Scholar] [CrossRef] [PubMed]
- Perez-Jimenez, F.; et al. International conference on the healthy effect of virgin olive oil. Eur J Clin Invest. 2005, 35, 421–424. [Google Scholar] [PubMed]
- Lou-Bonafonte, J.M.; Arnal, C.; Navarro, M.A.; Osada, J. Efficacy of bioactive compounds from extra virgin olive oil to modulate atherosclerosis development. Mol Nutr Food Res. 2012, 56, 1043–1057. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zhou, D.; Shahidi, F. Antioxidant properties of tyrosol and hydroxytyrosol saturated fatty acid esters. Food Chem. 2018, 245, 1262–1268. [Google Scholar] [CrossRef] [PubMed]
- Elmaksoud, H.A.A.; Motawea, M.H.; Desoky, A.A.; Elharrif, M.G.; Ibrahimi, A. Hydroxytyrosol alleviate intestinal inflammation, oxidative stress and apoptosis resulted in ulcerative colitis. Biomed Pharmacother. 2021, 142, 112073. [Google Scholar] [CrossRef] [PubMed]
- Velotti, F.; Bernini, R. Hydroxytyrosol Interference with Inflammaging via Modulation of Inflammation and Autophagy. Nutrients 2023, 15. [Google Scholar] [CrossRef] [PubMed]
- Bellumori, M.; Cecchi, L.; Innocenti, M.; Clodoveo, M.L.; Corbo, F.; Mulinacci, N. The EFSA Health Claim on Olive Oil Polyphenols: Acid Hydrolysis Validation and Total Hydroxytyrosol and Tyrosol Determination in Italian Virgin Olive Oils. Molecules 2019, 24. [Google Scholar] [CrossRef] [PubMed]
- Vilaplana-Pérez, C.; Auñón, D.; García-Flores, L.A.; Gil-Izquierdo, A. Hydroxytyrosol and potential uses in cardiovascular diseases, cancer, and AIDS. Front Nutr. 2014, 1, 18. [Google Scholar] [PubMed]
- Romero, C.; Brenes, M.; Yousfi, K.; García, P.; García, A.; Garrido, A. Effect of cultivar and processing method on the contents of polyphenols in table olives. J Agric Food Chem. 2004, 52, 479–484. [Google Scholar] [CrossRef]
- Pirro, M.; Simental-Mendía, L.E.; Bianconi, V.; Watts, G.F.; Banach, M.; Sahebkar, A. Effect of Statin Therapy on Arterial Wall Inflammation Based on 18F-FDG PET/CT: A Systematic Review and Meta-Analysis of Interventional Studies. J Clin Med. 2019, 8. [Google Scholar] [CrossRef] [PubMed]
- Nicholls, S.J.; et al. Effect of two intensive statin regimens on progression of coronary disease. N Engl J Med. 2011, 365, 2078–2087. [Google Scholar] [CrossRef] [PubMed]
| Parameters | HP-EVOO | EVOO | ROO | |||
|---|---|---|---|---|---|---|
| V1 | V2 | V1 | V2 | V1 | V2 | |
| Numbers of participants | (2M ; 1F) | (2M ; 1F) | (2M ; 3F) | |||
| Age | 72 ± 5.48 | 79 ± 0.58 | 78 ± 5.66 | |||
| Weight (kg) | 94.36 ± 34.73 | 103.37 ± 38.55 | 65.80 ± 18.54 | 66.40 ± 16.44 | 80.36 ± 13.98 | 83.6 ± 10.16 |
| BMI | 32.28 ±10 | 34.78 ± 11.42 | 24.86 ± 3.10 | 25.17 ± 2.32 | 28.55 ± 5.10 | 29.77 ± 4.37 |
| PA systolique | 139.67 ± 16.17 | 140.67 ± 15.04 | 125.50 ± 19.09 | 123.33 ± 17.21 | 149.80 ± 24.01 | 145.40 ± 32.47 |
| PA diastolique | 83.33 ± 3.21 | 83.67 ± 3.79 | 74.00 ± 11.31 | 72.67 ± 14.50 | 82.12 ± 7.18 | 80.60 ± 12.97 |
| Total cholesterol (mmol/L) | 5.06 ± 0.54 | 4.9 ± 0.76 | 4.64 ± 0.24 | 5.15 ± 0.41 | 4.67 ± 0.74 | 4.74 ± 1.35 |
| C-HDL (mmol/L) | 1.64 ± 0.43 | 1.59 ± 0.37 | 1.46 ± 0.59 | 1.57 ± 0.72 | 1.67 ± 0.43 | 1.69 ± 0.51 |
| C-LDL (mmol/L) | 2.9 ± 0.37 | 2.83 ± 0.53 | 2.68 ± 0.53 | 2.97 ± 0.35 | 2.59 ± 0.59 | 2.65 ± 0.97 |
| CRP (mg/L) | 2.20 ± 2.34 | 2.95 ± 4.10 | 1.80 ± 0.66 | 2.50±1.47 | 1.24 ± 0.61 | 1.31 ± 0.42 |
| Lp(a) (nmol/L) | 21.03 ± 24.48 | 41.57 ± 7.72 | 82.60 ± 130.51 | 135.25 ± 180.10 | 90.48 ± 50.64 | 88.66 ± 43.07 |
| Hémoglobine A1c (%) | 5.50 ± 0.35 | 5.53 ± 0.23 | 5.93 ± 0.06 | 6.10 ± 0.10 | 5.50 ± 0.27 | 5.50 ± 0.22 |
| Troponine T (ng/L) | 7.33 ± 3.06 | 10.12 ± 4.58 | 10.67 ± 7.23 | 12.33 ± 8.39 | 12 ± 1.22 | 13.60 ± 0.55 |
| TSH (mUI/L) | 2.06 ± 0.03 | 2.12 ± 0.23 | 1.89 ±1.07 | 2.72 ± 0.71 | 3.81 ± 4.59 | 2.18 ± 0.93 |
| Fibrinogène (g/L) | 2.94 ± 0.99 | 2.95 ± 0.68 | 3.30 ± 0.66 | 3.03 ± 0.28 | 2.75 ± 0.13 | 3.27 ± 0.59 |
| ROO(mg/kg) | EVOO(mg/kg) | HP-EVOO(mg/kg) | |
|---|---|---|---|
| Total phenolic compounds | 2.7 | 255 | 1 249 |
| Tyrosol | n.d | 6.3 | 123.1 |
| Hydroxytyrsol | n.d | 7.8 | 233.6 |
| Parameters | HP-EVOO | P | EVOO | P | ROO | P | |||
|---|---|---|---|---|---|---|---|---|---|
| V1 | V2 | V1 | V2 | V1 | V2 | ||||
| Non-calcified segments of the aorta | |||||||||
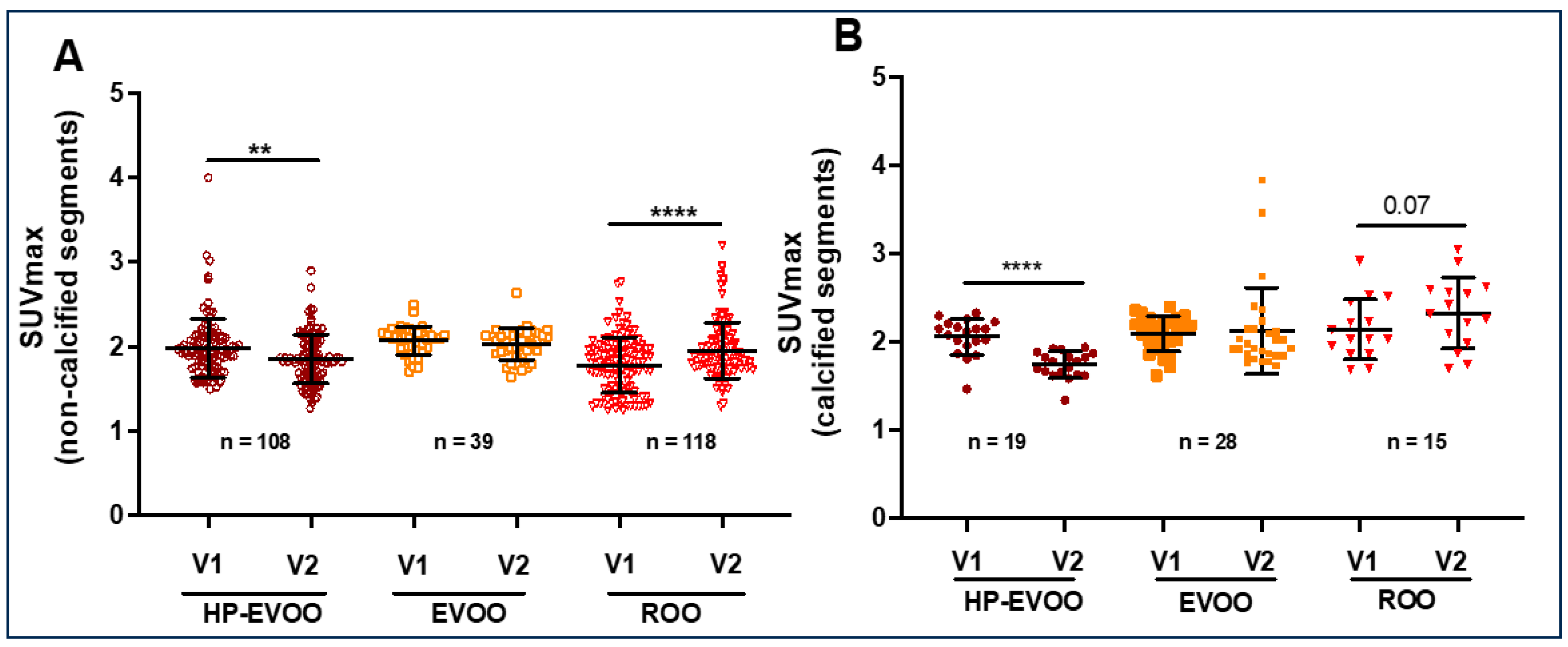
| SUVmax 18F-FDG | 2.93 ± 0.23 | 2.75 ± 0.38 | 0.004 | 2.81 ± 0.40 | 2.74 ± 0.31 | ns | 2.41 ± 0.66 | 2.44 ± 0.28 | ns |
| SUVmax 18F-NAF | 1.98 ± 0.33 | 1.85 ± 0.28 | 0.004 | 2.06 ± 0.21 | 2.02 ± 0.20 | ns | 1.78 ± 0.34 | 1.95 ± 0.34 | <0.0001 |
| Calcified segments of the aorta | |||||||||
| SUVmax 18F-FDG | 2.25 ± 0.29 | 2.15 ± 0.19 | 0.004 | 2.67 ± 0.27 | 2.78 ± 0.30 | ns | 2.36 ± 0.10 | 2.36 0.23 |
ns |
| SUVmax 18F-NAF | 2.06 ± 0.25 | 1.74 ± 0.15 | 0.0001 | 2.09 ± 0,17 | 2.12 ± 0.49 | ns | 2.13 ± 0.35 | 2.32 ± 0.40 | <0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





