1. Introduction
Maize is one of the three most important cereals for food security at the global level and is of particular importance in the diets of the poor in Africa and Latin America [
1]. Maize is a key income earner for farmers and a source of foreign exchange for the Government of Uganda. Uganda earns about US
$75m annually from maize exports for the last decade [
2]. Productivity growth has not been in line with the ever-increasing population and the demand for maize for food, feed and industrial material due to biotic and abiotic pressures. The pressures include drought, heat, poor soil fertility, and waterlogging/excess moisture [
3], often coupled with diseases [
4] and insect pests. Arthropod pests are among the key factors contributing to low yields in today’s maize production. These include the maize stalk borers
Busseola fusca (Fuller, 1901, Lepidoptera: Noctuidae) and
Chilo partellus (Swinhoe, 1885, Lepidoptera: Crambidae), cutworms and weevils [
5].
Spodoptera frugiperda (J.E. Smith, Lepidoptera: Noctuidae), commonly referred to as fall armyworm (FAW), is now a major insect pest that was first reported in Africa in early 2016 [
6].
Spodoptera frugiperda has a high potential for rapid spread and poses a real threat to the food security and livelihoods of millions of smallholder maize farmers in Africa. A study by Day et al. [
7] showed that without effective control, the pest has the potential to cause annual maize losses of 80 – 200 million tonnes in 12 maize-producing countries in SSA. In Uganda, it has the potential to cause 50% yield losses (Otim et al., Unpublished).
Spodoptera frugiperda attacks all crop stages and causes severe leaf damage and direct damage to maize ears. The larvae defoliate and can kill young plants, or the young whorl of plants, resulting in a dead heart [
8].
Host plant resistance, cultural, biological, botanical, chemical, and biotechnological approaches have been used to manage
S. frugiperda [
9]. The agronomic and cultural approaches include early planting, adequate nutrient supply through mineral fertilizer, intercropping, frequent weeding, proper tillage, and the use of pheromone traps. Farmers also use their innovations such as using ash, chilli and sand, sugar solutions, and fish soup [
10,
11].
In response to the enormous threat of crop yield losses by the invasive
S. frugiperda, the government of Uganda promoted the use of synthetic insecticides e.g Striker (Lamba Cyahalothrin and Thiomethoxam), Roket (Profenos and Cypermethrin) for its control on Maize. However, the results of the effectiveness of the pesticides are variable and inconclusive, and the chemical insecticides present a hazard to users, the environment, and consumers. Also, the farmers’ use of insecticides has not been guided by proper ecological considerations related to population dynamics, particularly knowledge of factors affecting population density, damage and abundance. This information is not yet available in Uganda. Nonetheless, many factors, including farmers’ agricultural practices such as pesticide use, fertilizer use, weeding frequency, cropping and tillage system [
12], and environmental factors such as rainfall and temperature are key to understanding the population dynamics of
S. frugiperda [
13]. Also, there has not been any systematic season-long-follow up of the abundance of FAW in Uganda. Therefore, the aim of this study was; 1. to assess population dynamics and damage by
S. frugiperda as influenced by farmers’ practices and weather conditions in three districts of Uganda.
2. Materials and Methods
The study was conducted in Nakaseke, Kole and Kiryandongo, which lie in three different agro-ecologies of Uganda. Nakaseke, Kole, and Kiryandongo lie in Western savannah grasslands, North Eastern savannah grasslands and North Western savannah grasslands, respectively (
Table 1). The districts are one of the major producers of maize in the respective agro-ecologies [
14]. Additionally, unpublished results of a survey carried out in 2020 showed these districts as having high
S. frugiperda damage (Otim et al., unpublished).
2.2. Study design
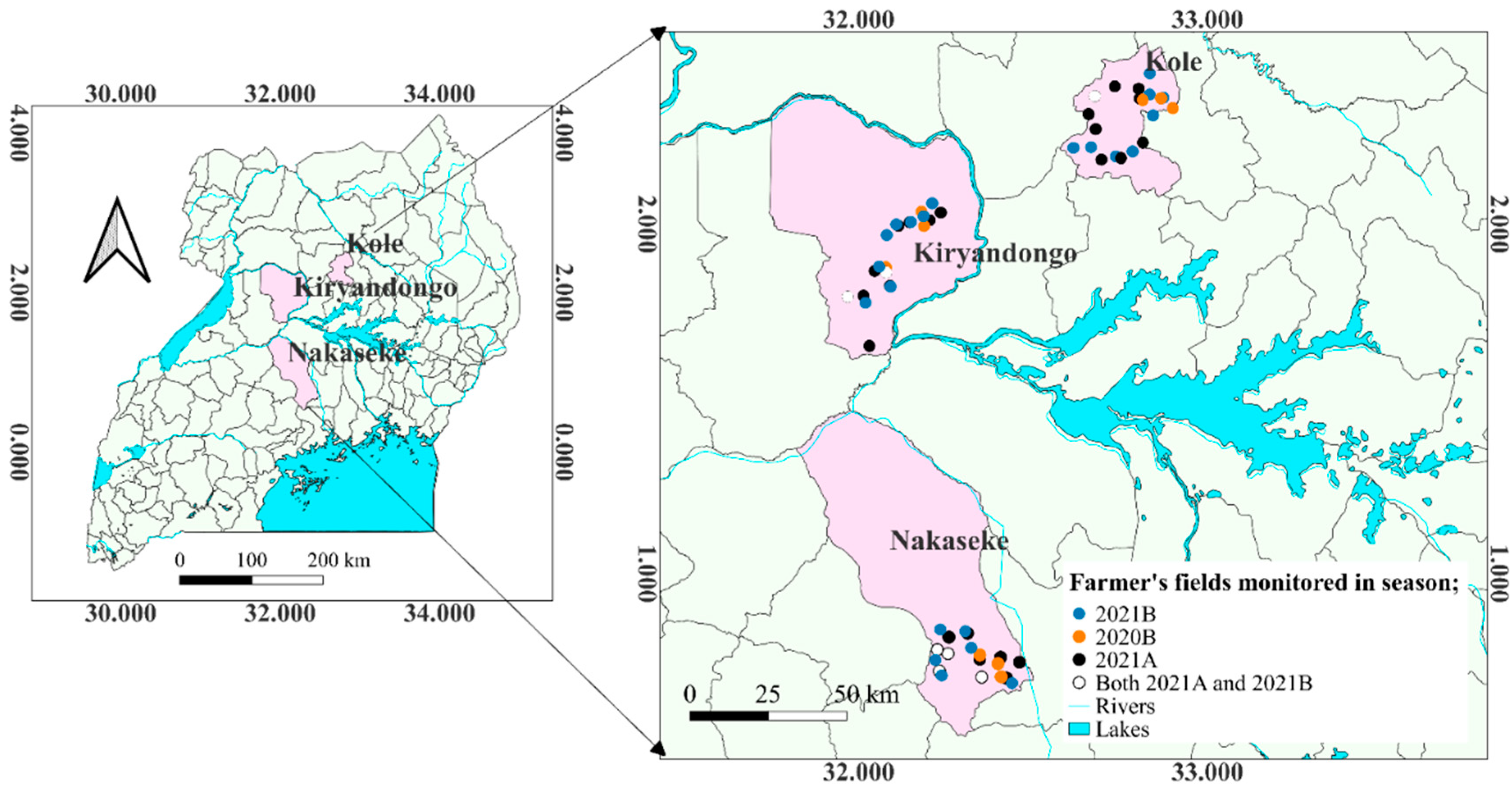
A longitudinal monitoring survey was conducted in Kole, Kiryandongo and Nakaseke districts (
Figure 1) for three seasons; 2020B (September to December), 2021A (March to June) and 2021B (September to December). In each of the districts, ten maize fields (20221A and 2021B) and three fields earlier in 2020B were selected purposively based on the date of planting and farmers’ willingness to allow access. A total of 69 fields were monitored. The differences in the number of fields in the different seasons were because of initial operational costs. The fields measured at least one acre and were separated by at least 5 Km. The GPS location of each field was recorded using GPS Test App version 1.6.3. All fields were managed entirely by the farmers. The daily minimum and maximum temperature, and rainfall of the study period in the different districts were obtained from Uganda Meteorological Authority (UMA).
2.2 Abundance and damage of Spodoptera frugiperda life stages
Data collection began approximately three weeks after planting (WAP) and continued every two weeks till harvest. On every visit, the phenological stage of maize plants was obtained using the ‘leaf collar method’ [
15] and recorded. To determine the abundance of
S. frugiperda in the field, each field was divided into four quadrants measuring approximately 0.125 acres and 15 maize plants were sampled randomly in each, making a total of 60 plants per field. Plants within five meters from the edge were not sampled to avoid edge effects. Each sampled plant was scored for
S. frugiperda leaf damage and examined for the presence of
S. frugiperda eggs, larvae, and pupae. The number of life stages on each plant was counted and recorded. Leaf damage was scored on a scale of 0 – 9 according to [
16]; where 0 = No visual leaf injury and 9 = Whorl and furl leave almost destroyed.
Leaf damage incidence (percentage damage) for
S. frugiperda was calculated using the formula below;
Adult populations were also monitored using the pheromone traps deployed in universal bucket traps (
Figure 3) set up in each farmer’s field a month after planting. The P061 pheromone containing Z11-hexadecenyl acetate and Z9-tetradecenyl acetate (4.35g a.i/kg) manufactured by Chemtica Internacional S.A was used. The traps were hung in an upright orientation on a long pole at 1.2 m off the ground. The pheromone lure was placed on the top section of the bucket trap and replaced every four weeks based on the manufacturer’s recommendation. The trapped adults were counted and recorded every two weeks until harvest.
2.3 Farmers’ practices
We interviewed owners of the selected farms to obtain information on fertilizer use – (yes or no) and pesticide use frequency, tillage system, weeding, and maize variety planted. Conservational tillage was defined as zero tillage where herbicides were used to kill weeds before planting maize while conventional tillage was land opened and fine-tilled using ox plough, hoe, or tractor. The cropping system was observed and recorded. The cropping system was defined as sole or intercropped.
2.4 Environmental parameters
Mean daily minimum and maximum temperature and total rainfall were sourced from the Uganda Meteorological Authority. This is because temperature and rainfall are reported to affect the damage and abundance of S. frugiperda (Observation from the field).
2.5 Yield
Yield data was taken at physiological maturity. Fifteen maize plants were randomly sampled per quadrant and their cob weight after removing the husks was measured and recorded. Grain yield (t/ha) was determined from field weight [kg] (FW) per plot and corrected to 13.5% moisture content as:
Where mc is the field moisture content of grain per plot, 0.8 is the shelling coefficient, 10,000 m2 is the area of a hectare, and 2.81 m2 is the area of the 20 plants per plot.
2.6. Data analysis
The means of leaf damage, damage incidence (proportion of damaged plants), and the mean number of egg batches, larvae and trapped adults were tested for normality using the Shapiro-Wilk test and Levene test for equal variance using the ‘car’ package of R Studio version 4.0.4. Data on the number of egg batches and adults trapped was transformed by powers 0.375 and 0.38, respectively using Tukey’s Ladder of Powers procedure. Two-way ANOVA was done on leaf damage, leaf damage incidence, and the number of egg batches and larvae to compare different seasons and districts (locations). Graphs on mean leaf damage and mean number of larvae per 20 plants over the different growth stages were plotted however for mean number of egg batches was not done because of the very low numbers. Three-way ANOVA was performed on leaf damage and the number of trapped adults to compare different seasons, management practices, and locations. Mean separations were done using Fisher’s LSD. Linear regression was done using ‘The R stats’ package in R Studio version 4.0.4 to establish the relationships between yield and leaf damage at the late vegetative stage. The late vegetative stage was used because it gave the clearest relationship. The relationship between the life stages of FAW (egg batches and larvae) and grain yield was not carried out because their numbers were very low.
The categorical data on the different management practices were coded using the dummy coding method. Their dummy variables were used to perform the multiple regression analysis in R studio using the lm() function to establish the relationship between management practices and mean leaf damage and mean number of larvae per twenty plants. The management practices except pesticide application frequency were coded and the following were baseline variables for each management practice, fertilizer use; no – 0, tillage system; conventional – 0, cropping system; sole – 0. These baseline variables were used because they occurred most often. Maize varieties were grouped into different categories, hybrid, local and OPV. These were then subjected to one-way ANOVA to determine differences in damage and number of larvae per twenty plants between these categories in the different districts.
Linear regression was done using ‘The R stats’ package in R Studio version 4.0.4 to establish the relationships between weather factors (daily maximum temperature and rainfall) and leaf damage. The relationship between temperature (minimum, maximum and mean daily temperature) was explored and only maximum temperature gave some significant relationships and trends. Relationships between weather factors and the number of egg batches and larvae were not done because they were very low to establish this relationship.
3. Results
3.1 Abundance and damage of Spodoptera frugiperda life stages in the study districts
The number of egg batches was low on all sampling dates in the three districts. Kole had significantly more egg batches per plant in season 202IB, whilst the number of batches did not differ significantly between the seasons in the other two districts (
Table 2). There were significant (P<0.01) interactions between location and season in the mean number of larvae per plant (
Table 2). Kiryandongo had significantly higher larval abundance in all seasons when compared with the other two districts (
Table 2).
Maize in all investigated fields was damaged by
S. frugiperda. Leaf damage differed significantly between districts (P<0.05) in all seasons (
Table 3). There were significant interactions (P<0.001) in leaf damage between seasons and districts. Kiryandongo had significantly the highest damage in all seasons and Nakaseke had the lowest damage (
Table 3). The incidence of leaf damage was high (>70%) in all locations and differed significantly between the locations in the three seasons (
Table 2). It ranged from 72% in Kole in 2020B to 90% in Nakaseke in 2021A.
3.2 Variation in Spodoptera frugiperda abundance and damage with maize growth stage
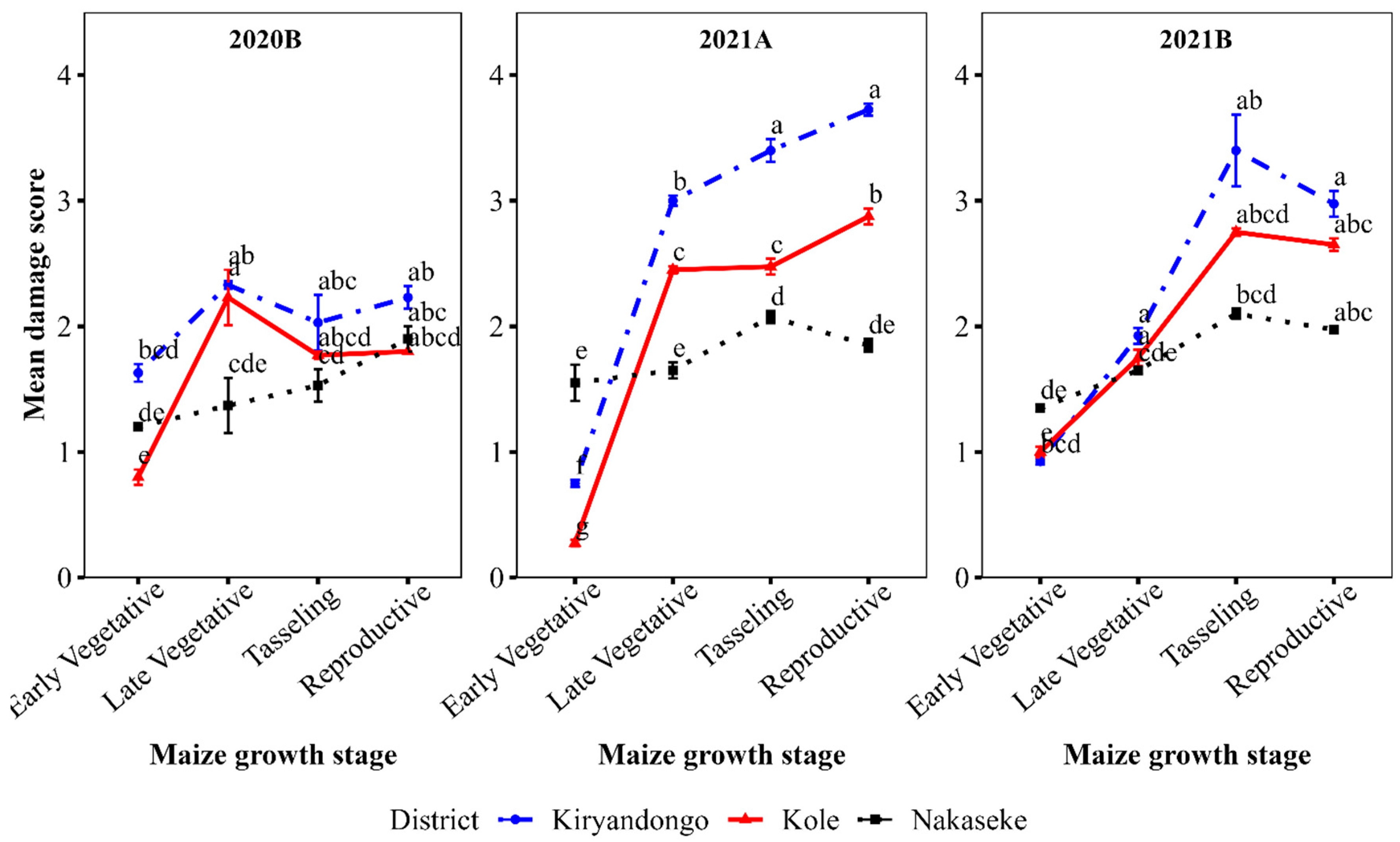
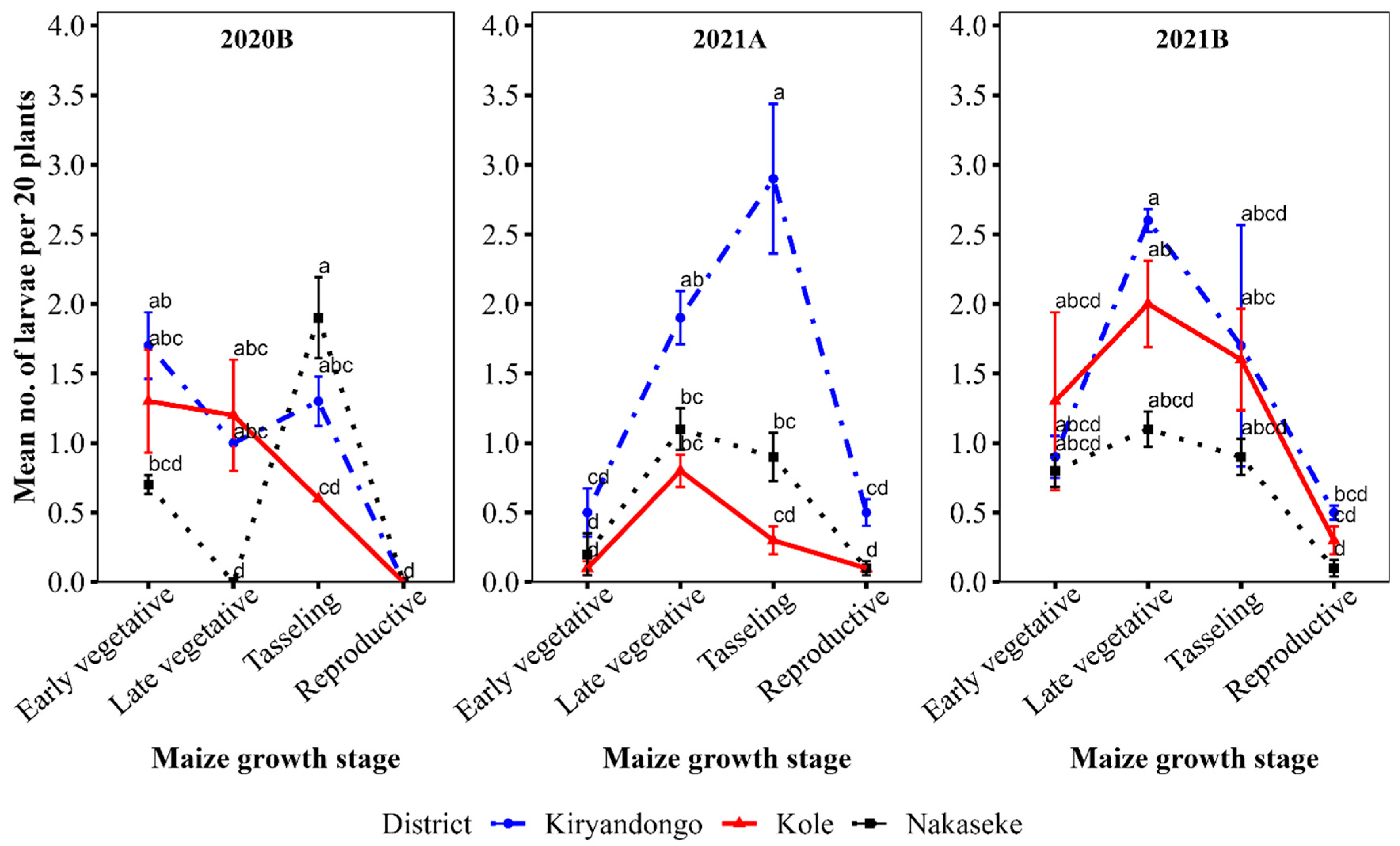
The mean number of larvae was significantly different in the different growth stages in all districts (P<0.001). The mean number of larvae per plant was very variable with the most larvae counted at tasselling stage in 2020B and 2021A and at the late vegetative stage in 2021B (
Figure 2).
Leaf damage generally varied significantly in the different growth stages in all seasons and districts (
Figure 3). In 2020B, the peak was at the late vegetative stage in Kole; in Kiryadongo damage peaked at the late vegetative and again at the reproductive stage, while in Nakaseske there was a steady increase in the reproductive stage. In 2021A, which had the highest damage levels, damage increased up to the reproductive stage in Kiryadongo and Kole, but peaked at tasselling in Nakaseke, whereas in 2021B, damage in all the districts peaked at tasselling (
Figure 3).
Figure 3.
Mean leaf damage at different growth stages in the surveyed seasons and districts (Mean separations for mean damage score for growth stage*district in a given season).
Figure 3.
Mean leaf damage at different growth stages in the surveyed seasons and districts (Mean separations for mean damage score for growth stage*district in a given season).
The mean number of
S. frugiperda adults trapped per field was significantly different (p<0.05) growth stages in season 2021A (
Table 4). In this season, the highest number of FAW adults were mostly trapped in the reproductive stage of maize; and Kiryandongo had the highest catches, followed by Kole and the least were in Nakaeske (
Table 4).
3.3. The relationship between management practices and leaf damage/larval abundance
Regression analyses were conducted on the relationship between different management practices with leaf damage and the abundance of
S. frugiperda larvae (
Table 5). The tillage system was the only significant predictor for
S. frugiperda leaf damage (P = 0.002), where damage was higher in the conventional tillage than in the conservational tillage (
Figure 4). A shift from conventional tillage to conservational reduced mean damage by 0.6 (
Table 5). Fertilizer use, cropping system, weeding frequency and pesticide use frequency were not significant predictors of leaf damage. The cropping system and tillage system were the significant predictors for larval abundance (P = 0.027, and P = 0.013, respectively). A shift from sole to intercropping reduced the abundance of larvae per plant by 0.04. Likewise, a shift from conventional tillage to conservational reduced the mean number of larvae per plant by 0.04 as per the fitted regression lines below.
Where; FU= Fertilizer Use, CS = Cropping system, TS = Tillage system, WF =Weeding Frequency and PF = Pesticides Application frequency.
3.4. Relationship between maize varieties and leaf damage
Maize variety was analyzed separately. No significant differences (p>0.05) in leaf damage were observed among maize varieties in the three districts (
Table 5). The majority of the farmers in Kiryandongo and Nakaseke planted hybrid varieties while in Kole majority planted local varieties.
3.5 Effect of weather factors on the damage and abundance of Spodoptera frugiperda
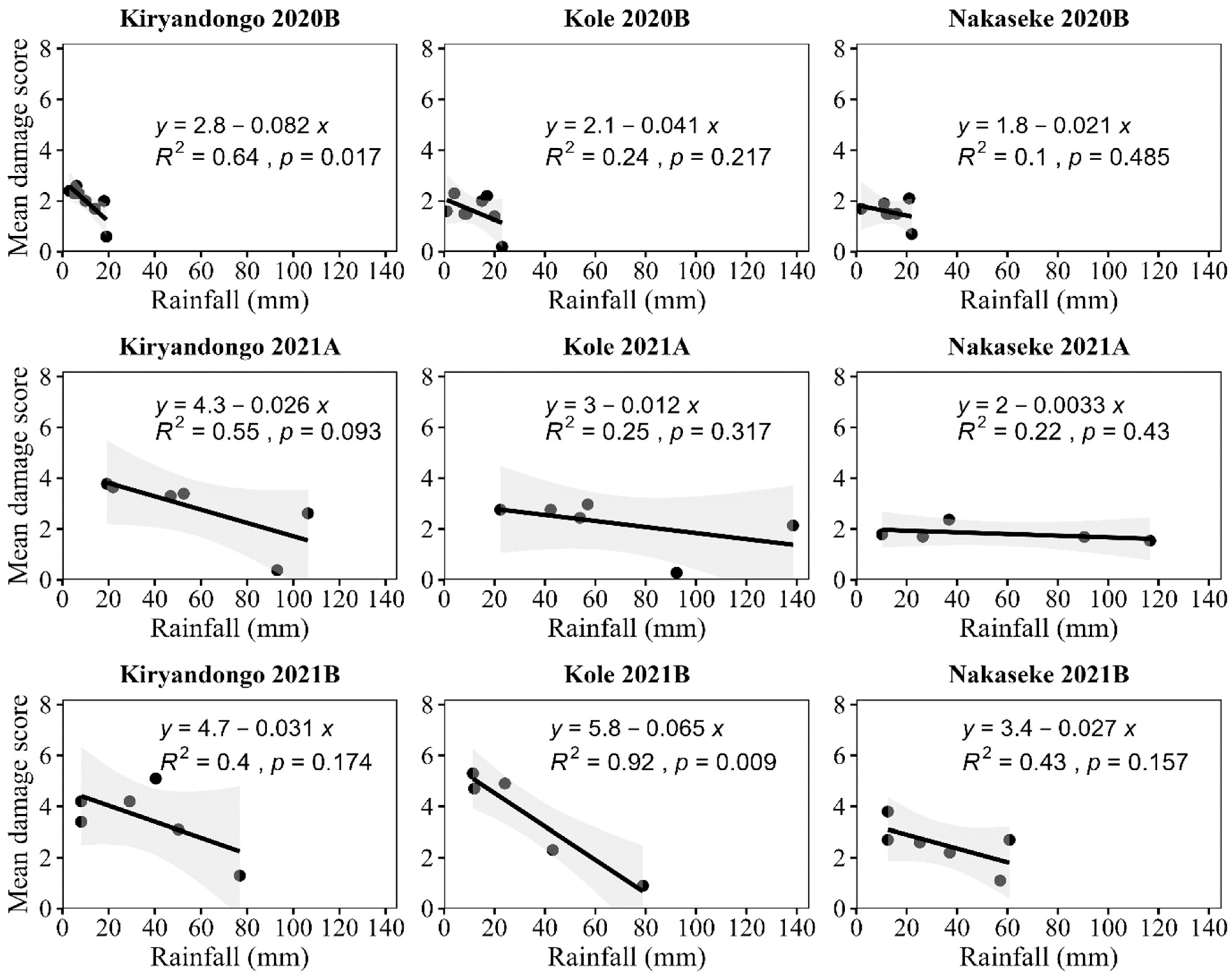
There were negative relationships between leaf damage scores and rainfall in all seasons and locations but this was only significant for Kiryadongo in 2020B and Kole in 2021B; and marginally so for Kole in 2021A (
Figure 5). When there was a significant relationship, the regression equation accounted for 64% of the variation in 2020B in Kiryandongo, and 92% of the variation in 2021B in Kole.
The only significant relationship between
S. frugiperda damage and maximum temperature was in 2020B in Nakaseke, where it was negative and maximum temperature accounted for 72% of the variation (
Figure 6).
3.6. Relationship between grain yield and leaf damage
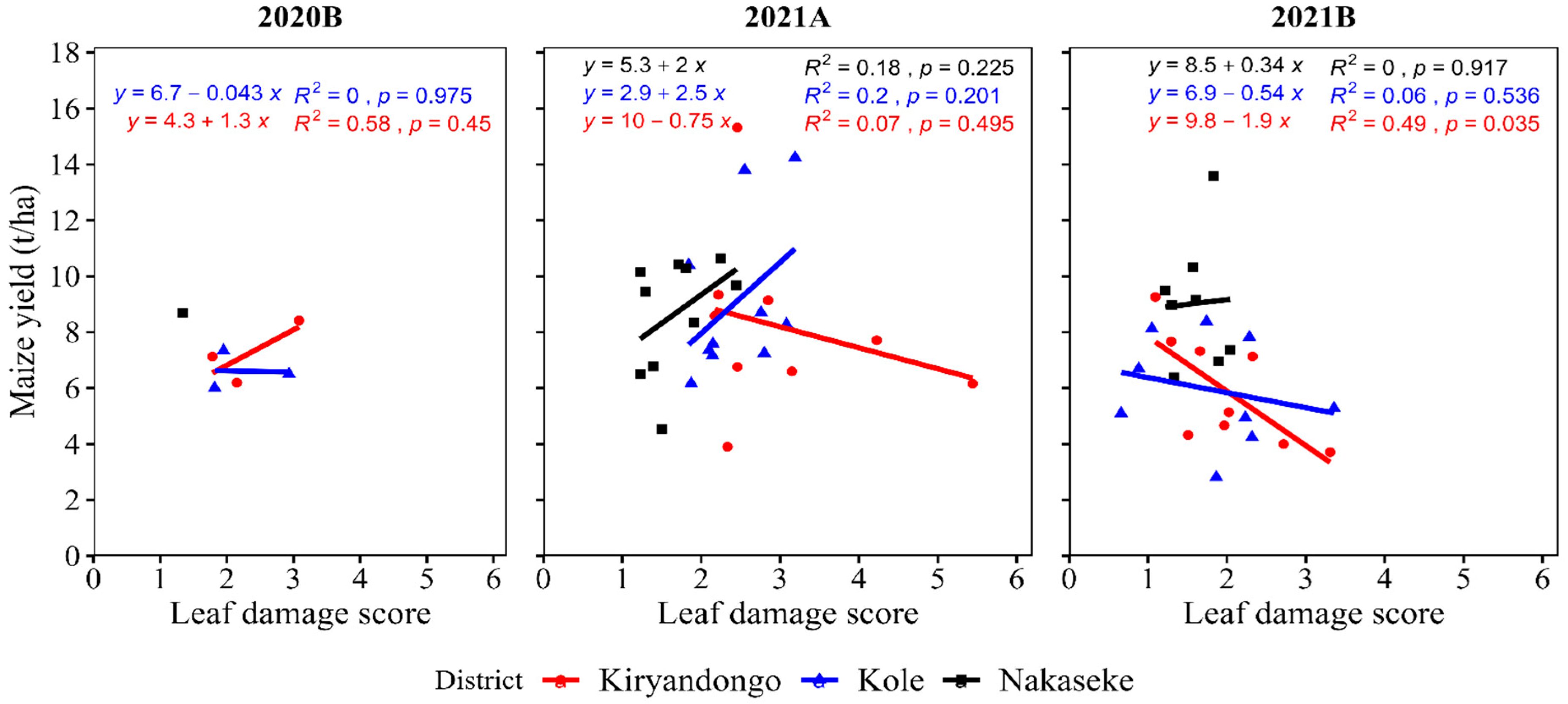
The relationship between maize yield and leaf damage was variable but was only significant (negatively) in Kiryadongo in 2021B where an increase in mean damage by 1 caused a decrease in yield by 1.9 t/ha (Figure 7).
4. Discussion
The objectives of this study were to assess population dynamics and damage by S. frugiperda as influenced by farmers’ practices and environmental conditions in three districts of Uganda. The results showed that S. frugiperda leaf damage was dependent on location, season, crop growth stage, tillage system, cropping system; rainfall, and temperature. The larval numbers were dependent on location, season, crop growth stage, cropping and tillage system.
The differences in locations are attributed to differences in weather factors (rainfall and temperature) and the main agronomic practices. Whereas the season differences are attributed to differences in weather factors such as rainfall and temperature.
There were significant negative relationships between rainfall and
S. frugiperda damage, in some cases. Heavy downpours are reported to be unfavorable for
S. frugiperda population build-up because of egg dislodgement [
17,
18]. Heavy rainfall could also have washed and drowned the first and second instar larvae. Kiryandongo had significantly the highest
S. frugiperda damage in all seasons. The low of an average of 110mm per month and sometimes non-existent rain may explain the high damage in Kiryandongo during the seasons. The negative impact of rainfall could mean that farmers who plant early can exploit the Nitrogen flush for high vigour, and also avoid severe damage by
S. frugiperda. Exploiting the period of much rainfall also results in a reduction in pesticide use and therefore a reduction in the cost of production and negative effects associated with pesticide use. Kiryandongo having the highest damage can also be explained by the high numbers of larvae. There was a positive highly significant relationship between leaf damage and larvae in this district
.
There was no significant influence of temperature (maximum) on
S. frugiperda leaf damage. There was one isolated case in Nakaseke season 2021B with a negative significant relationship between maximum temperature and leaf damage. This was unexpected as temperature rises are reported to favor
S. frugiperda multiplication. For example, Anandhi et al. [
19] indicated that every 1°C increase in maximum temperature increased the larval population
of S. frugiperda by 1.56 in India. Temperature between 18 °C and 30 °C, was inversely related to the development time for the entire larval development period and the optimal temperature for larval development is 30 °C [
20]. The length of the entire developmental period (egg to adult) increased from 20.27 days at 32 °C to 71 days at 18 °C [
21]. Low temperature is known to delay the development of
S. frugiperda and hence low damage.
4.1. Spodoptera frugiperda abundance and damage as influenced by maize growth stage
The results of this study have shown that larval numbers were generally higher at the late vegetative and tasseling stages. A fairly similar pattern was observed in the populations of adult moths. An increase in larval numbers in late vegetative and tasseling stages may in part be due to additional immigration into the fields, and an increase in the populations of the individuals existing in the fields. These observations are similar to those reported by Niassy et al. [
22], who found that larval numbers were mainly influenced by the maize stages.
Leaf damage generally peaked in the late vegetative stage, and occasionally in the tasseling and reproductive stages. The increase in leaf damage with the growth stage may be because of the gradual build-up in the population of larval numbers, and the increase in the abundance of bigger larvae that are reported to eat proportionate to their weight [
23]. Also, increased damage with crop age may be a result of an influx of new moths and multiplication in the same fields. It was also reported in Egypt, that
S. frugiperda damage increased with an increase in maize age [
23]. Gross et al, [
24] mentioned that the sensitivity of maize growth stages to
S. frugiperda attack varied based on the plant growth and development.
Spodoptera frugiperda larvae mainly consume a large leaf mass during vegetative growth stages. These results imply that farmers need to regularly and closely monitor maize fields in order to intervene and prevent the population of
S. frugiperda from reaching economically damaging levels at the vegetative stages, or reproductive stage.
4.2. The abundance and damage by Spodoptera frugiperda as influenced by management practices
Fields with conservational tillage had a lower
S. frugiperda damage and larval abundance than those using conventional tillage practices in all seasons. This was also observed in Zimbabwe where maize farms under minimum or no tillage had significantly reduced
S. frugiperda infestation [
25]. Maize production under zero or minimum tillage was reported to reduce
S. frugiperda damage in the Americas because it favored population build-up for predatory species. Most farmers in Nakaseke practice conservational tillage and had the highest larval parasitism rates (3.3%) (Ajam et. al., Unpublished) hence this explains why their fields had the lowest
S. frugiperda damage.
Intercropping significantly reduced
S. frugiperda larval abundance but not damage. The fact that maize intercropped with a non-host reduced the pest abundance is in line with the stipulation that the presence of non-host plants disrupts the movement of
S. frugiperda larvae from one maize plant to another [
26]. In our study, intercropping only reduced larval abundance. This concurs with research done by Yigezu and Wakgari [
27] where non-host legumes, such as beans, when intercropped with maize, significantly reduced
S. frugiperda infestation. Also in support of the findings of this study, intercropping of maize with legumes, such as cowpea (
Vigna unguiculata L.), groundnut (
Arachis hypogaea L.), and common bean (
Phaseolus vulgaris L.), was ineffective in reducing
S. frugiperda damage [
25,
28]. This seems to show that intercropping can reduce numbers but not damage. The crops intercropped with maize included beans, soybean, peas, groundnuts, sim sim and cassava, with the majority of the fields being intercropped with soybeans.
Insecticides are the most popular management option for the control of
S. frugiperda. In our study, however, we did not realize a difference in leaf damage under the different pesticide spray frequencies. Pesticide application also did not cause a significant reduction in larval numbers. This may be because of the timing of the pesticide application whereby fields could have been sprayed late when the damage was already high, or because the application rate of the pesticides was below recommended as was the case from most farmers’ narratives in Kiryandongo. An average of 8mls/20ltrs of Roket (Profenos and Cypermethrin) was used, which is below the recommended rate of 30mls/20ltrs. Most farmers applied Roket and Striker (Lambacyahalothrin and thiomethoxam). Other studies also reported that pesticides were not effective in the control of
S. frugiperda, since fields sprayed had even higher damage than those sprayed [
29].
This study showed that there were no significant differences between fields where fertilizers were applied and those that did not apply them. This finding is contrary to recent reports by farmers that reported higher
S. frugiperda severity where fertilizer application was done than where it was not applied [
29]. This could have been because the farmers that applied fertilizer used urea (top dress) and foliar fertilizers like Super grow and MaizePlus in their fields which are majorly nitrogen-based fertilizers. A total of 18 out of the 69 monitored fields applied fertilizers. Nitrogen fertilizers change the C/N ratio and make plants more susceptible to
S. frugiperda damage [
30].
Leaf damage was not significantly different among the different weeding frequencies. However, studies have shown that repeated weeding reduced
S. frugiperda damage, probably due to areas where weeds were
S. frugiperda hosts of the Graminaceous family [
25]. These inconsistencies in the results could also be because there were varying numbers of farmers doing different weeding frequencies and differences in the effectiveness of how weeding was done. The lack of significant differences between maize varieties may be due to the lack of varieties with known resistance to
S. frugiperda. These observations are however contrary to the ones reported by Ntare et. al in Eastern Uganda [
31]. The possible reasons for this contraditions could include among others location, environment and management practices.
The relationship between yield and Spodoptera frugiperda leaf damage
In this study, though the relationship between grain yield and
S. frugiperda leaf damage was mostly non-significant; in Kiryadongo, the most hit district, there was a significant negative relationship between grain yield and leaf damage in 2021B. This indicates that the level of leaf damage was high enough to result in a significant effect on grain yield. Reports from Zimbabwe and Ethiopia also showed
S. frugiperda leaf damage to be significantly negatively associated with yield [
25,
32]. The lack of significant difference between leaf damage and grain yield in the other cases of this study agrees in part with the results by Britz, [
33], who reported that plants with low to moderate damage scores did not suffer significant yield losses. Plant response to damage is influenced not only by the severity of symptoms, but also by factors such as drought conditions [
34], soil nutrient status [
35], the length of the larval feeding period, and the inherent level of plant resistance to larval feeding damage [
36]. These factors, not documented in this study, solely or interactively could have influenced the results of this study.
5. Conclusions and recommendations
The study showed that there were significant differences between districts in S. frugiperda abundance and damage. Kiryandongo had significantly the highest leaf damage and Nakaseke the lowest. There were significant differences in the abundance of S. frugiperda larvae and damage between the growth stages, with the tasselling stage having the highest abundance of S. frugiperda larvae and the late vegetative to tasseling stages having the highest mean leaf damage.
With regard to management practices, conversation tillage was associated with reduced damage of S. frugiperda. On the other hand, pesticide application frequencies, weeding frequency and maize varieties were not associated with S. frugiperda damage. Tillage and cropping systems were significant predictors of the abundance of S. frugiperda.
There was a negative relationship between leaf damage and rainfall in all seasons and locations, where increased rainfall reduced S. frugiperda damage. There was no significant relationship between the mean number of larvae per twenty plants and rainfall in all seasons and locations. There were no significant relationships between leaf damage with mean daily maximum temperature in all seasons and locations. Similarly, there were no significant relationships between the mean number of larvae per twenty plants with the mean daily maximum temperature in all seasons and locations.
Monitoring and scouting of maize fields should start immediately after maize crop emergence since S. frugiperda infestation was recorded from early vegetative to reproductive stages.
Sensitization of farmers to be more vigilant in monitoring and scouting for S. frugiperda when there is less or no rain, which conditions promote pest buildup. In addition, exploiting the use of natural controls through the integration of weather information in S. frugiperda management could help reduce unnecessary pesticide applications, save costs for farmers and reduce heavy environmental hazards.
There were largely insignificant relationships between grain yield and damage. This indicates that, when there are low to moderate damage scores, a farmer may apply less costly cultural and biological and natural control methods to realize profits.
Recommendations
For management of S. frugiperda
Monitoring and scouting of maize fields should start immediately after maize crop emergence since S. frugiperda infestation was recorded from early vegetative to reproductive stages.
Sensitization of farmers to be more vigilant in monitoring and scouting for S. frugiperda when there is less or no rain, which conditions promote pest buildup. In addition, the integration of weather information in S. frugiperda management could help reduce unnecessary pesticide applications, and save costs for farmers and reduce heavy environmental hazards.
There is a need to promote conservation tillage to reduce S. frugiperda abundance in maize fields.
For further research
There is a need to evaluate the different varieties used by farmers in Uganda for resistance to S. frugiperda damage. The use of resistant varieties is cost-effective to farmers and it will reduce the use of pesticides which are harmful to humans and the environment.
Controlled studies on the effect of management practices on the incidence of S. frugiperda and since the farmers’ fields were heterogeneous.
Author Contributions
Conceptualization, M.H.O.; methodology, A.L.A., J.K., S.A.A., M.H.O.; formal analysis, A.L.A., G.O., J.K., M.H.O; investigation, A.L.A., G.O., S.A.A., M.H.O; resources, M.H.O.; writing—original draft preparation, A.L.A., G.O., J.K., P.P., M.H.O; writing—review and editing, A.L.A., J.K., G.O., S.A.A., P.P., M.H.O; visualization, G.O., A.L.A., M.H.O.; supervision, M.H.O., S.A.A.; project administration, M.H.O.; funding acquisition, P.P., M.H.O. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Korea - Africa Food & Agriculture Cooperation Initiative (KAFACI), Project No. KAB20200113.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. Acknowledgments:.
Acknowledgments
We are grateful to Tonny Amodoi, Peter Wasswa, George Osenduru, Florence Aryenyo, and Onen Denish Oyaro for assistance in the laboratory.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Shiferaw, B.; Prasanna, B.M.; Hellin, J.; Bänziger, M. Crops that feed the world 6. Past successes and future challenges to the role played by maize in global food security. Food Secur. 2011, 3, 307–327. [Google Scholar] [CrossRef]
- Bank of Uganda. Composition of exports values and volumes. Available online: https://www.bou.or.ug/bouwebsite/Statistics/ (accessed on 29 December 2023).
- Cairns, J.E.; Hellin, J.; Sonder, K.; Araus, J.L.; MacRobert, J.F.; Thierfelder, C.; Prasanna, B.M. Adapting maize production to climate change in sub-Saharan Africa. Food Secur. 2013, 5, 345–360. [Google Scholar] [CrossRef]
- Osunga, M.; Mutua, F.; Mugo, R. Spatial modelling of maize lethal necrosis disease in Bomet County, Kenya. J. Geosci. Geomatics, Vol. 5, 2017, Pages 251-258 2017, 5, 251–258. [Google Scholar] [CrossRef]
- Wainaina Nyong’o, A. Major constraints limiting maize productivity in smallholder (SH) farming sector of sub-Saharan Africa. Glob. J. Pests, Dis. Crop Prot. 2016, 4, 197–205. [Google Scholar]
- Goergen, G.; Kumar, P.L.; Sankung, S.B.; Togola, A.; Tamò, M. First report of outbreaks of the fall armyworm Spodoptera frugiperda (J E Smith) (Lepidoptera, Noctuidae), a new alien invasive pest in West and Central Africa. PLoS One 2016, 11, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Day, R.; Abrahams, P.; Bateman, M.; Beale, T.; Clottey, V.; Cock, M.; Colmenarez, Y.; Corniani, N.; Early, R.; Godwin, J.; et al. Fall armyworm: Impacts and implications for Africa. Outlooks Pest Manag. 2017, 28, 196–201. [Google Scholar] [CrossRef]
- Capinera, J.L.; Fall armyworm, Spodoptera frugiperda (J.E. Smith) (Insecta: Lepidoptera: Noctuidae). Available online: http://edis.ifas.ufl.edu/in255 (accessed on 10 October 2022).
- Kumar, R.M.; Gadratagi, B.G.; Paramesh, V.; Kumar, P.; Madivalar, Y.; Narayanappa, N.; Ullah, F. Sustainable management of invasive fall armyworm, Spodoptera frugiperda. Agronomy 2022, 12, 1–17. [Google Scholar] [CrossRef]
- Murray, K.; Jepson, P.C.; Chaola, M. Fall armyworm management for maize smallholders in Malawi; USAID and CIMMYT: Mexico, CDMX, CIMMYT, 2019. [Google Scholar]
- Kalyebi, A.; Otim, M.H.; Walsh, T.; Tay, W.T. Farmer perception of impacts of fall armyworm (Spodoptera frugiperda J. E. Smith) and transferability of its management practices in Uganda. CABI Agric. Biosci. 2023; 1–14. [Google Scholar] [CrossRef]
- Mutyambai, D.M.; Niassy, S.; Calatayud, P.A.; Subramanian, S. Agronomic factors influencing fall armyworm (Spodoptera frugiperda) infestation and damage and its co-occurrence with stemborers in maize cropping systems in Kenya. Insects 2022, 13, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Alam, T.; Prasad, R.; Narayan, A. Studies on the population dynamics of fall armyworm, Spodoptera frugiperda (J.E. Smith) on maize in relation to weather parameters. Pharma Innov. J. 2022, 11, 971–975. [Google Scholar]
- Uganda Bureau of Statistics Statistical Abstract; Kampala, 2020.
- Mark, L. Corn growth stages. Iowa State Univ. Ext. Outreach. 2016, 18–21. [Google Scholar]
- Davis, F. M, and Williams, W.P. Visual Rating scales for screening whorl- stage corn for resistance to fall armyworm. Mississippi Agricultural & Forestry Experiment Station, Technical Bulletin 186, Mississippi State University, MS39762, USA. 1992. [Google Scholar] [CrossRef]
- Baltzer, J.L.; Davies, S.J. Rainfall seasonality and pest pressure as determinants of tropical tree species ’ distributions. Ecol. Evol. 2012, 2, 2682–2694. [Google Scholar] [CrossRef]
- Obori, Y.K.; Mano, H.A. Effect of rainfall on a population of the diamondback moth, Plutella xylostella (Lepidoptera: Plutellidae). Appl. Entomol. Zool. 2003, 38, 249–253. [Google Scholar]
- Anandhi, S.; Saminathan, V.R.; Yasodha, P.; Roseleen, S.S.J.; Sharavanan, P.T.; Rajanbabu, V. Correlation of fall armyworm Spodoptera frugiperda (J.E. Smith) with weather parameters in maize ecosystem. Int. J. Curr. Microbiol. Appl. Sci. 2020, 9, 1213–1218. [Google Scholar] [CrossRef]
- Díaz-Álvarez, E.A.; Martínez-Zavaleta, J.P.; López-Santiz, E.E.; de la Barrera, E.; Larsen, J.; del-Val, E. Climate change can trigger fall armyworm outbreaks: a developmental response experiment with two mexican maize landraces. Int. J. Pest Manag. 2020, 0, 1–9. [Google Scholar] [CrossRef]
- Du Plessis, H.; Schlemmer, M.L.; Van den Berg, J. The effect of temperature on the development of Spodoptera frugiperda (Lepidoptera: Noctuidae). Insects 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Niassy, S.; Agbodzavu, M.K.; Kimathi, E.; Mutune, B.; Abdel-Rahman, E.F.M.; Salifu, D.; Hailu, G.; Belayneh, Y.T.; Felege, E.; Tonnang, H.E.Z.; et al. Bioecology of fall armyworm Spodoptera frugiperda (J. E. Smith), Its management and potential patterns of seasonal spread in Africa. PLoS One 2021, 16, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Bakrya, M.M.S. and Abdel-Baky. Population density of the fall armyworm, Spodoptera frugiperda (Smith) (Lepidoptera: Noctuidae) and its response to some ecological phenomena in maize crop. Brazilian J. Biol. 2023, 83, 1–17. [Google Scholar]
- Gross, H. R.; Young, J.R. Relative susceptibility of a summer-planted dent and tropical flint corn variety to whorl stage damage by the fall armyworm (Lepidoptera: Noctuidae) Econ. Entomol. 1982, 75, 1153–1156. [Google Scholar] [CrossRef]
- Baudron, F.; Zaman-Allah, M.A.; Chaipa, I.; Chari, N.; Chinwada, P. Understanding the factors influencing fall armyworm (Spodoptera frugiperda J.E. Smith) damage in African smallholder maize fields and quantifying its impact on yield. A case study in Eastern Zimbabwe. Crop Prot. 2019, 120, 141–150. [Google Scholar] [CrossRef]
- Harrison, R.D.; Thierfelder, C.; Baudron, F.; Chinwada, P.; Midega, C.; Schaffner, U.; van den Berg, J. Agro-Ecological options for fall armyworm (Spodoptera frugiperda J. E smith)management: providing low-cost, smallholder friendly solutions to an invasive pest. J. Environ. Manage. 2019, 243, 318–330. [Google Scholar] [CrossRef]
- Yigezu, G.; Wakgari, M. Local and indigenous knowledge of farmers management practice against fall armyworm (Spodoptera frugiperda) (J.E. Smith) (Lepidoptera: Noctuidae): A Review. J. Entomol. Zool. Stud. 2020, 8, 765–770. [Google Scholar]
- Hailu, G.; Niassy, S.; Zeyaur, K.R.; Ochatum, N.; Subramanian, S. Maize–legume intercropping and push–pull for management of fall armyworm, stemborers, and striga in Uganda. Agron. J. 2018, 110, 2513–2522. [Google Scholar] [CrossRef]
- Kansiime, M.K.; Mugambi, I.; Rwomushana, I.; Nunda, W.; Lamontagne-Godwin, J.; Rware, H.; Phiri, N.A.; Chipabika, G.; Ndlovu, M.; Day, R. Farmer perception of fall armyworm (Spodoptera frugiderda J.E. Smith) and farm-level management practices in Zambia. Pest Manag. Sci. 2019, 75, 2840–2850. [Google Scholar] [CrossRef]
- Zhang, B.; Liu, H.; Helen, H.S.; Wang, J.J. Effect of host plants on development, fecundity and enzyme activity of Spodoptera exigua (Hübner) (Lepidoptera: Noctuidae). Agric. Sci. China 2011, 10, 1232–1240. [Google Scholar] [CrossRef]
- Ntare, J.G. , Karungi, J., Muhumuza, J.B. and Talwana, H. Influence of farmers’ practices on fall armyworm infestation levels in maize fields. Makerere Univ. J. Agric. Environ. Sci. 2022, 11, 74–89. [Google Scholar]
- Kassie, M.; Wossen, T.; De Groote, H.; Tefera, T.; Sevgan, S.; Balew, S. Economic impacts of fall armyworm and its management strategies: Evidence from Southern Ethiopia. Eur. Rev. Agric. Econ. 2020, 47, 1473–1501. [Google Scholar] [CrossRef]
- Britz, C. Relationship between Spodoptera frugiperda (Lepidoptera: Noctuidae) Damage and yield loss in maize, North-West University, 2020.
- Chávez-Arias, C.C.; Ligarreto-Moreno, G.A.; Ramírez-Godoy, A.; Restrepo-Díaz, H. Maize responses challenged by drought, elevated daytime temperature and arthropod herbivory stresses: A physiological, biochemical and molecular view. Front. Plant Sci. 2021, 12, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Braimoh, A.K.; Vlek, P.L.G. Soil quality and other factors influencing maize yield in Northern Ghana. Soil Use Manag. 2006, 22, 165–171. [Google Scholar] [CrossRef]
- Soujanya, P.L.; Sekhar, J.C.; Yathish, K.R.; Karjagi, C.G.; Rao, K.S.; Suby, S.B.; Jat, S.L.; Kumar, B.; Kumar, K.; Vadessery, J.; et al. Leaf damage based phenotyping technique and its validation against fall armyworm, Spodoptera frugiperda (J. E. Smith), in Maize. Front. Plant Sci. 2022, 13, 1–14. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).